Significance
Chronic low-grade inflammation is a key driver of metabolic syndrome. This inflammatory response is mediated by immune-cell infiltration and the expression of inflammatory cytokines. IL6 is implicated in this response, but the physiological role of IL6 signaling is unclear. Here, we demonstrate that the source of IL6 influences the nature of the inflammatory response. We report that IL6 secreted by myeloid cells inhibits adipose tissue macrophage (ATM) accumulation by a canonical signaling mechanism, but IL6 secreted by adipocytes or muscle promotes ATM accumulation by a noncanonical mechanism. These observations provide physiological insight into the complexity of IL6-mediated regulation of inflammation.
Keywords: adipose tissue, inflammation, interleukin 6, insulin resistance
Abstract
Obesity is associated with a chronic state of low-grade inflammation and progressive tissue infiltration by immune cells and increased expression of inflammatory cytokines. It is established that interleukin 6 (IL6) regulates multiple aspects of metabolism, including glucose disposal, lipolysis, oxidative metabolism, and energy expenditure. IL6 is secreted by many tissues, but the role of individual cell types is unclear. We tested the role of specific cells using a mouse model with conditional expression of the Il6 gene. We found that IL6 derived from adipocytes increased, while IL6 derived from myeloid cells and muscle suppressed, macrophage infiltration of adipose tissue. These opposite actions were associated with a switch of IL6 signaling from a canonical mode (myeloid cells) to a noncanonical trans-signaling mode (adipocytes and muscle) with increased expression of the ADAM10/17 metalloprotease that promotes trans-signaling by the soluble IL6 receptor α. Collectively, these data demonstrate that the source of IL6 production plays a major role in the physiological regulation of metabolism.
Interleukin 6 (IL6) is a cytokine with many physiological actions that regulate metabolism (1). Indeed, studies using IL6 infusion in healthy humans demonstrate increased insulin-stimulated glucose disposal, increased lipolysis, increased glucose and fatty acid oxidation, and increased energy expenditure (2, 3). IL6 therefore targets multiple physiological processes that impact whole-body metabolism. The physiology of IL6 signaling is complex because the effects of IL6 on metabolism requires signal integration between cell types (4) that involve proinflammatory, anti-inflammatory, and noninflammatory mechanisms (5).
The molecular mechanism of IL6 signaling is mediated by IL6 receptor α (IL6-Rα) through the signaling protein IL6ST (also known as gp130). Canonical IL6 signaling involves binding of IL6 to an IL6-Rα/IL6ST complex at the plasma membrane (5). Noncanonical (trans) IL6 signaling is mediated by the interaction of IL6ST with a proteolytically processed (soluble) form of IL6-Rα bound to IL6 (5). Both forms of IL6 signaling lead to activation of the JAK1–STAT3 pathway (5). Targets of IL6-stimulated STAT3 signaling include the Il4ra promoter that leads to increased IL4-Rα expression by macrophages that promotes alternative (M2a) activation and suppression of obesity-induced insulin resistance (6). Similarly, IL6 signaling by hepatocytes causes increased tolerance to both glucose and insulin (7). Moreover, IL6 acts on adipose tissue to increase leptin secretion and suppress satiety (8), and IL6 increases adipose tissue lipolysis (8), which, in turn, promotes hepatic gluconeogenesis (9) and hepatic insulin resistance (9, 10). IL6 also increases the loss of adipose tissue following exercise (11) and in response to cancer cachexia (12). Furthermore, IL6 signaling in the paraventricular nucleus of the hypothalamus improves energy and glucose homeostasis in response to obesity (13). It has also established that IL6 can increase insulin secretion by an incretin-based mechanism (14). IL6 is therefore a potent cytokine that acts at key sites of metabolic regulation in multiple tissues. Indeed, tissue-specific knockout mice confirm that IL6 plays an important role in the obesity response (15–23).
While insight concerning the complex actions of IL6 on metabolism has been obtained, the consequences of IL6 production by specific tissues is poorly understood because the source of IL6 under many physiological conditions has not been established. It is known that adipose tissue represents a major source of increased circulating IL6 caused by obesity (24, 25), but the relevant adipose-tissue cell type is unclear because Il6 messenger RNA (mRNA) is expressed by adipocytes, adipose tissue macrophages (ATMs), and other adipose-tissue cell types (1). Here, we tested the relative roles of IL6 expression by ATMs and adipocytes by comparing mice with Il6 gene ablation selectively in adipocytes or myeloid cells. We found that adipocyte IL6 potently promotes ATM accumulation in the absence of major changes in glucose or insulin tolerance. In contrast, myeloid cell IL6 suppresses M1 macrophage polarization, reduces ATM accumulation, and improves tolerance to both glucose and insulin. These data demonstrate that the source of IL6 dramatically affects the physiological metabolic response to IL6 in vivo.
Results
Adipose Tissue Inflammation Associated with Diet-Induced Obesity.
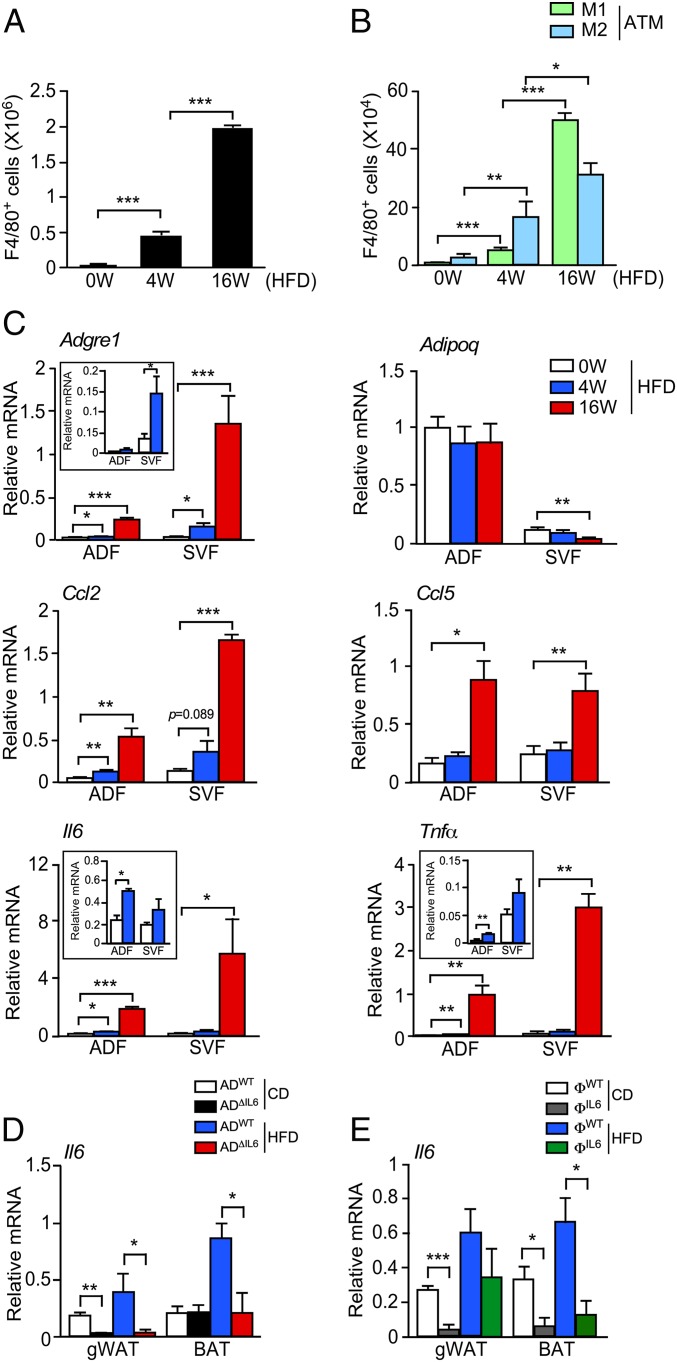
C57BL/6J male mice were fed a chow diet (CD) or a high-fat diet (HFD), and gonadal white adipose tissue (gWAT) macrophages (ATMs) were examined by flow cytometry. The number of F4/80+ ATMs was dramatically increased by feeding a HFD (Fig. 1A and SI Appendix, Fig. S1 A and B). Immunophenotyping demonstrated increased numbers of both F4/80+ CD11c+ CD206− ATM (M1-like) and F4/80+ CD11c− CD206+ ATM (M2-like) macrophages (Fig. 1B and SI Appendix, Fig. S1C). Gene-expression analysis demonstrated selective expression of Adipoq mRNA by the adipocyte fraction (ADF) and Adgre1 mRNA by the stromal vascular fraction (SVF) (Fig. 1C). Inflammatory gene expression was increased in both the ADF and SVF when mice were fed a HFD, including Ccl2, Ccl5, Il6, and Tnfα (Fig. 1C). The source of inflammatory cytokine/chemokine expression in the adipose tissue of HFD-fed mice is therefore unclear.
Fig. 1.
The HFD induced infiltration of macrophages and secretion of cytokines. (A and B) The SVF of gWAT was isolated from HFD-fed (0, 4, and 16 wk) mice and examined by flow cytometry to detect the total number of F4/80+ ATMs, the number of F4/80+ CD11c+ CD206− (M1-like) ATMs, and the number of F4/80+ CD11c− CD206+ (M2-like) ATMs per mouse (mean ± SEM; n = 2). *P < 0.05; **P < 0.01; ***P < 0.001. (C) Total RNA was isolated from ADF and SVF from HFD-fed (0, 4, and 16 wk) mice. The relative expression of mRNA associated with makers of macrophages (Adgre1), M1-polarized macrophages (Ccl2, Ccl5, Il6, and Tnfα), and adipocytes (Adipoq) was measured by qRT-PCR assays (mean ± SEM; n = 4 ∼ 6). *P < 0.05; **P < 0.01; ***P < 0.001. (D) Total RNA was isolated from gWAT and BAT from CD- and HFD-fed ADWT and ADΔIL6 mice. The relative expression of Il6 mRNA was measured by qRT-PCR assays (mean ± SEM; n = 5 ∼ 8). *P < 0.05; **P < 0.01. (E) Total RNA was isolated from gWAT and BAT in CD- and HFD-fed ФWT and ФΔIL6 mice. The relative expression of Il6 mRNA was measured by qRT-PCR assays (mean ± SEM; n = 4 ∼ 8). *P < 0.05; ***P < 0.001.
Further studies of inflammatory cytokine expression in adipose tissue were focused on IL6. Both adipocytes and macrophages represent potential sources of adipose tissue IL6. We therefore examined Il6 mRNA expression in control mice and mice with IL6 deficiency in adipocytes (Adipoq-Cre+/− Il6LoxP/LoxP) or myeloid cells (Lyz2-Cre+/− Il6LoxP/LoxP) (SI Appendix, Fig. S1 D and E). We found that the HFD-induced expression of Il6 mRNA in adipose tissue was suppressed by IL6 deficiency in either adipocytes or myeloid cells (Fig. 1 D and E). Moreover, the serum concentration of IL6 was reduced by IL6 deficiency in either adipocytes or myeloid cells (SI Appendix, Fig. S1 F and G). These data indicate that IL6 may be secreted by both adipocytes and macrophages in the adipose tissue of HFD-fed mice. However, the relative role of IL6 expression by these cell types is unclear.
Adipocyte IL6 Promotes HFD-Induced Adipose Tissue Inflammation.
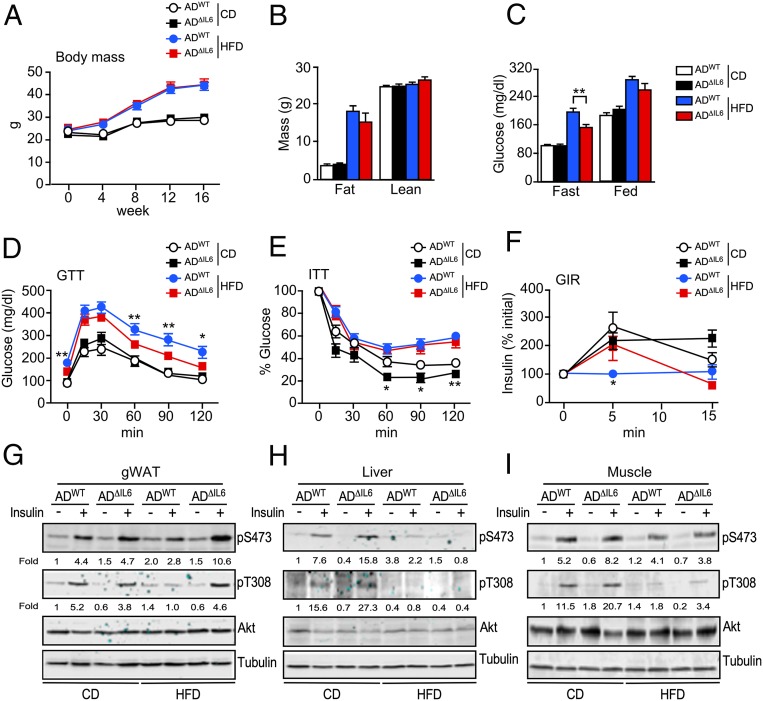
To examine the role of IL6 secreted by adipocytes, we compared control ADWT mice (Adipoq-Cre+/−) and mice with IL6-deficiency (AD∆IL6) in adipocytes (Adipoq-Cre+/− Il6LoxP/LoxP). No differences in body mass were detected in mice fed a CD or a HFD (Fig. 2 A and B). Metabolic cage analysis demonstrated that adipocyte IL6 deficiency in HFD-fed mice caused increased energy expenditure (SI Appendix, Fig. S2 A and B), increased food consumption (SI Appendix, Fig. S2C), increased core body temperature (SI Appendix, Fig. S2D), and reduced triglyceride accumulation by the liver and brown adipose tissue (BAT) (SI Appendix, Fig. S2 F–H) compared with HFD-fed control mice. The absence of a weight-gain difference between HFD-fed control and HFD-fed adipocyte IL6-deficient mice most likely represents a compound phenotype that balances increased food consumption with increased energy expenditure.
Fig. 2.
Adipocyte IL6 promotes obesity-induced insulin resistance. (A) The change in body mass of CD- and HFD-fed ADWT and ADΔIL6 mice is presented (mean ± SEM; n = 7 ∼ 14). (B) Body composition was examined by proton magnetic resonance spectroscopy (mean ± SEM; n = 7 ∼ 8). (C) The blood concentration of glucose in overnight fasted mice and the blood glucose concentration in fed mice were measured (mean ± SEM; n = 7 ∼ 14). **P < 0.01. (D) The CD- and HFD-fed (16 wk.) mice were examined by GTTs (mean ± SEM; n = 7 ∼ 14). *P < 0.05; **P < 0.01. (E) The CD- and HFD-fed (16 wk) mice were examined by ITTs (mean ± SEM; n = 5 ∼ 13). *P < 0.05; **P < 0.01. (F) GIR measurements were performed by i.p. injection of glucose (mean ± SEM; n = 6 ∼ 7). *P < 0.05. (G–I) The CD- and HFD-fed mice (16 wk) were fasted overnight, treated by i.p. injection with 1 U/kg insulin (15 min), and examined by immunoblot analysis of gWAT, liver, and gastrocnemius muscle by probing with antibodies to phospho-Akt (pThr308 and pSer473), Akt, and αTubulin. Relative quantitation of the phospho-Akt immunoblots is presented below each subpanel.
Analysis of the circulating concentration of hormones demonstrated that adipocyte IL6 deficiency caused no changes in HFD-induced hyperinsulinemia or hyperleptinemia, although a small reduction in the concentration of resistin was detected (SI Appendix, Fig. S2E). Measurement of blood-glucose concentration demonstrated that adipocyte IL6 deficiency partially suppressed HFD-induced hyperglycemia in overnight fasted mice (Fig. 2C), and this was associated with modestly improved tolerance to glucose (Fig. 2D). No changes in insulin tolerance were detected in the HFD-fed mice (Fig. 2E and SI Appendix, Fig. S2I), but we did detect slightly improved glucose-induced insulin release in HFD-fed IL6-deficient mice compared with control mice (Fig. 2F). Biochemical analysis of insulin signaling in AD∆IL6 mice demonstrated that HFD-induced suppression of insulin-stimulated phosphorylation of AKT on the activating sites pSer473 and pThr308 was reduced in gWAT, but not in liver or gastrocnemius muscle, compared with control ADWT mice (Fig. 2 G–I). Nevertheless, hyperinsulinemic–euglycemic clamp assays demonstrated no significant differences between control and adipocyte IL6-deficient mice (SI Appendix, Fig. S3A). Together, these data demonstrate that adipocyte IL6 deficiency caused only modest changes in the development of metabolic syndrome caused by the consumption of a HFD (23).
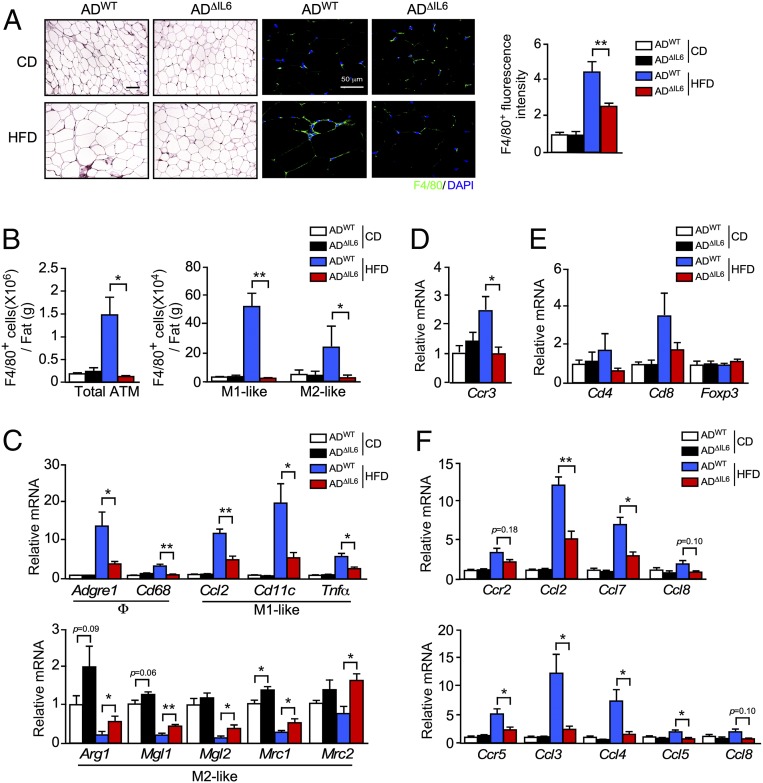
We found that adipocyte IL6 deficiency reduced the number of F4/80+ crown-like structures in sections prepared from gWAT of HFD-fed mice (Fig. 3A). Immunophenotyping analysis by flow cytometry demonstrated that the consumption of a HFD increased the number of ATMs in control mice and that this was suppressed by adipocyte IL6 deficiency (Fig. 3B and SI Appendix, Fig. S3B). The reduced immune-cell population included loss of both M1- and M2-like macrophages (Fig. 3 B and C and SI Appendix, Fig. S3B) together with reduced eosinophils and T cells (Fig. 3 D and E) and was associated with reduced HFD-induced expression of chemokine receptors CCR2 (and ligands CCL2, CCL7, and CCL8) and CCR5 (and ligands CCL3, CCL4, CCL5, and CCL8) (Fig. 3F).
Fig. 3.
Adipocyte IL6 promotes M1 polarization and an infiltration of ATMs. (A) ADWT and ADΔIL6 mice were fed a CD or a HFD for 16 wk. (A, Left) Sections of gWAT were stained with hematoxylin and eosin. (Scale bar, 100 µm.) (A, Center) Macrophages were stained by using an antibody to F4/80 (green), and nuclei were stained by using DAPI (blue) detection by fluorescence microscopy. (Scale bar, 50 µm.) (A, Right) ATMs were quantitated by measurement of F4/80 staining of tissue section using ImageJ software (mean ± SEM; n = 9 ∼ 15). **P < 0.01. (B) The SVF of gWAT was isolated from CD- and HFD-fed (16 wk) mice and examined by flow cytometry to detect the total number of ATMs (F4/80+), the number of M1-like ATMs (F4/80+ CD11c+ CD206−), and the number of M2-like ATMs (F4/80+ CD11c− CD206+) (mean ± SEM; n = 4). *P < 0.05; **P < 0.01. (C) Total RNA was isolated from gWAT from CD- and HFD-fed ADWT and ADΔIL6 mice. The relative expression of mRNA associated with markers of macrophages (Adgre1 and Cd68), M1-like polarized macrophages (Ccl2, Cd11c, and Tnfα), and M2-like polarized macrophages (Arg1, Mgl1, Mgl2, Mrc1, and Mrc2) was measured by qRT-PCR assays (mean ± SEM; n = 6 ∼ 8). *P < 0.05; **P < 0.01. (D and E) Total RNA was isolated from gWAT from CD- and HFD-fed ADWT and ADΔIL6 mice. The relative expression of the eosinophil marker (Ccr3) and T cell markers (Cd4, Cd8, and Foxp3) were measured by qRT-PCR assays of mRNA (mean ± SEM; n = 6 ∼ 8). *P < 0.05. (F) The relative expression of the receptor Ccr2 and the ligands Ccl2, Ccl7, and Ccl8; and the receptor Ccr5 and the ligands Ccl3, Ccl4, Ccl5, and Ccl8 was measured by qRT-PCR assays of mRNA (mean ± SEM; n = 6 ∼ 8). *P < 0.05; **P < 0.01.
Together, these data demonstrate that adipocyte IL6 in HFD-fed mice promotes adipose tissue inflammation by increasing the accumulation of ATMs in adipose tissue (Fig. 3) without causing major changes in glycemia and insulin resistance (Fig. 2). This conclusion is consistent with previous studies that have demonstrated a role for IL6 in ATM accumulation that does not markedly impact insulin resistance (21, 26).
Myeloid-Cell IL6 Suppresses HFD-Induced Adipose Tissue Inflammation.
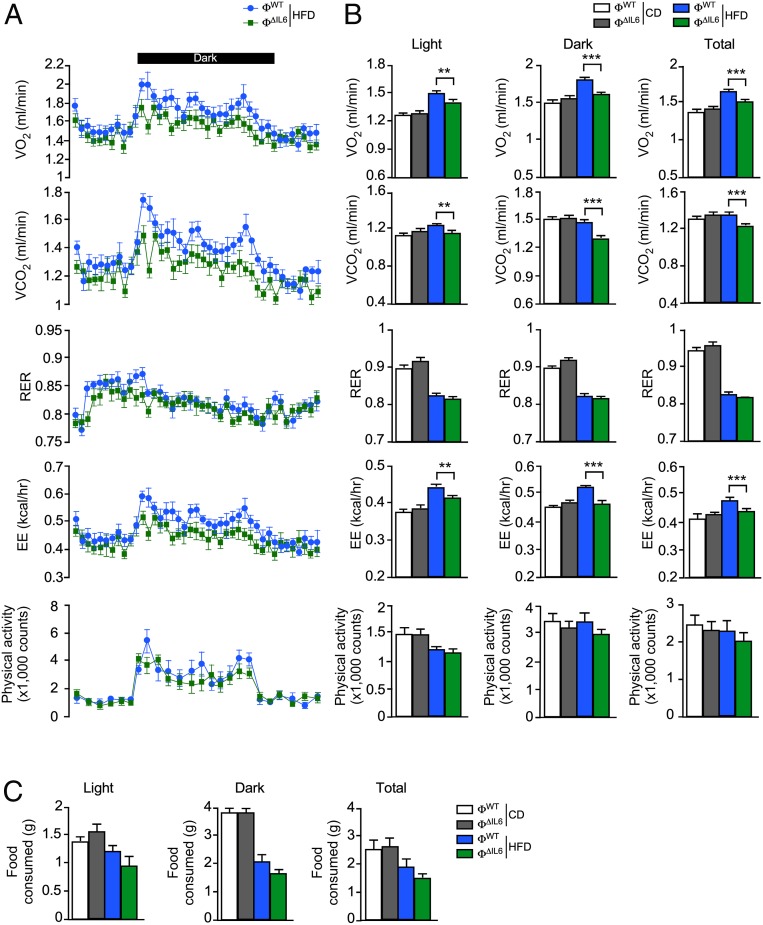
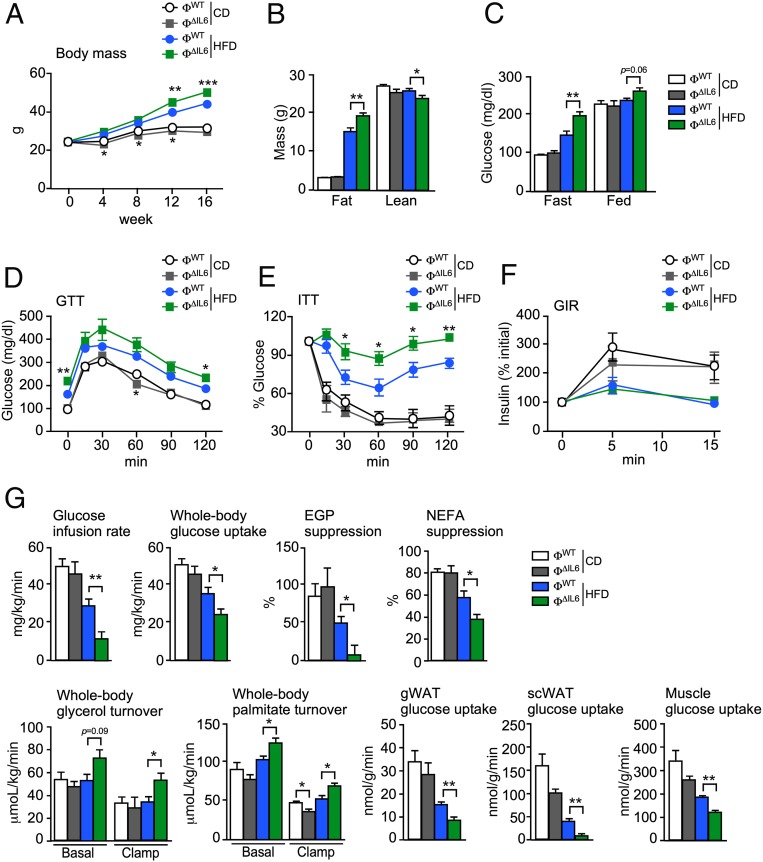
To examine the role of IL6 secreted by myeloid cells, we compared control ФWT (Lyz2-Cre+/−) mice and mice with IL6 deficiency (Ф∆IL6) in myeloid cells (Lyz2-Cre+/− Il6LoxP/LoxP). Metabolic cage analysis of CD-fed mice demonstrated no significant differences between ФWT and Ф∆IL6 mice (SI Appendix, Fig. S4). In contrast, IL6 deficiency in myeloid cells caused a significant reduction in energy expenditure, but no significant change in food consumption, when the mice were fed a HFD (Fig. 4). This reduction in energy expenditure caused by myeloid cell IL6 deficiency was associated with increased HFD-induced obesity (Fig. 5 A and B). These observations are consistent with prior studies that identify a role for circulating IL6 in HFD-fed mice to promote energy expenditure mediated by the paraventrical nucleus of the hypothalamus (13).
Fig. 4.
Myeloid-cell IL6 increases energy expenditure. Metabolic cage analysis of CD- and HFD-fed ФWT and ФΔIL6 mice (6 wk) to measure oxygen consumption (VO2), carbon dioxide production (VCO2), respiratory exchange rate (RER), energy expenditure (EE), and activity (A and B) and food intake per mouse (C) is presented (mean ± SEM; n = 8). **P < 0.01; ***P < 0.001.
Fig. 5.
Macrophage IL6 suppresses diet-induced insulin resistance. (A) The change in body mass of CD- and HFD-fed ФWT and ФΔIL6 mice is presented (mean ± SEM; n = 6 ∼ 8). *P < 0.05; **P < 0.01; ***P < 0.001. (B) Body composition was examined by proton magnetic resonance spectroscopy (mean ± SEM; n = 6 ∼ 8). *P < 0.05; **P < 0.01. (C) The blood concentration of glucose in overnight fasted mice and fed mice was measured (mean ± SEM; n = 6 ∼ 8). **P < 0.01. (D) CD- and HFD-fed (16 wk) mice were examined by GTTs (mean ± SEM; n = 5 ∼ 8). *P < 0.05; **P < 0.01). (E) CD- and HFD-fed (16 wk) mice were examined by ITTs (mean ± SEM; n = 5 ∼ 8). *P < 0.05; **P < 0.01. (F) CD- and HFD-fed (16 wk) mice were examined by GIR (mean ± SEM; n = 5 ∼ 7). (G) Hyperinsulinemic–euglycemic clamps of CD- and HFD-fed (8 wk) mice were used to measure the glucose infusion rate, whole-body glucose uptake, EGP, NEFA suppression, whole-body glycerol turnover, whole-body palmitate turnover, and glucose uptake in gWAT, subcutaneous WAT (scWAT), and gastrocnemius muscle (mean ± SEM; n = 3 ∼ 6). *P < 0.05; **P < 0.01.
We found that myeloid-cell IL6 deficiency was associated with increased hyperglycemia (Fig. 5C) and hyperinsulinemia (SI Appendix, Fig. S5A), but changes in the circulating concentration of the adipokines leptin or resistin were not detected (SI Appendix, Fig. S5A). Moreover, the HFD-fed IL6-deficient mice were found to be more glucose intolerant (Fig. 5D) and more insulin intolerant (Fig. 5E and SI Appendix, Fig. S5B) than control HFD-fed mice, but no significant changes in glucose-stimulated insulin secretion were detected (Fig. 5F). Hyperinsulinemic–euglycemic clamp studies confirmed that the HFD-fed myeloid-cell IL6-deficient mice exhibited increased insulin resistance, as monitored by reduced glucose infusion rate and whole-body glucose uptake, compared with HFD-fed control mice (Fig. 5G). This increased insulin resistance was associated with reduced suppression of endogenous glucose production (EGP), reduced suppression of the circulating concentration of nonesterified fatty acids (NEFAs), increased whole-body turnover of both glycerol and palmitate, and reduced glucose uptake by muscle and adipose tissue (Fig. 5G). Biochemical analysis of the AKT signaling pathway in adipose tissue, liver, and muscle demonstrated similar insulin-stimulated phosphorylation of AKT on the activating sites pThr308 and pSer473 in CD-fed control and IL6-deficient mice, but reduced insulin-stimulated AKT activation in HFD-fed IL6-deficient mice compared with HFD-fed control mice (SI Appendix, Fig. S5 C–E). These observations are consistent with increased hepatic steatosis and serum lipoproteins in the HFD-fed IL6-deficient mice compared with HFD-fed control mice (SI Appendix, Fig. S5 F–H). Collectively, these data demonstrate that myeloid-cell IL6 deficiency promotes increased insulin resistance in HFD-fed mice.
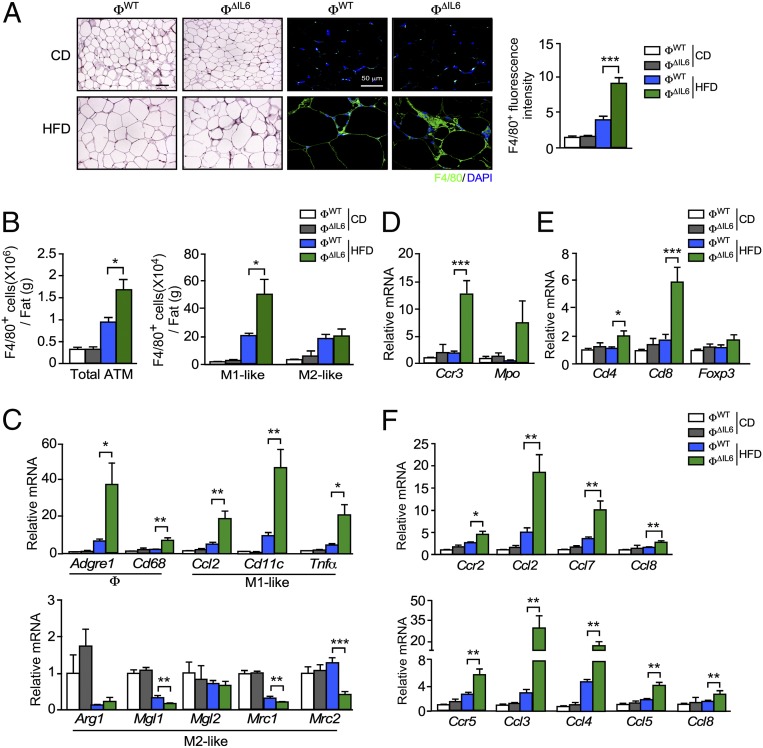
The increased HFD-induced insulin resistance of myeloid-cell IL6-deficient mice (Fig. 5) may be related to increased adipose tissue inflammation. Indeed, we found that myeloid-cell IL6 deficiency caused increased numbers of F4/80+ crown-like structures in sections prepared from the gWAT of HFD-fed mice (Fig. 6A). Immunophenotyping analysis by flow cytometry demonstrated that HFD consumption by control mice increased the number of ATMs and that this increase was further promoted by myeloid-cell IL6 deficiency (Fig. 6B and SI Appendix, Fig. S6). The increased inflammatory cell population included increased numbers of M1-like F4/80+ CD11c+ CD206− ATM macrophages, but not M2-like F4/80+ CD11c− CD206+ ATM (Fig. 6 B and C), together with increased eosinophils, neutrophils, and T cells (Fig. 6 D and E) and was associated with increased expression of chemokine receptors CCR2 (and ligands CCL2, CCL7, and CCL8) and CCR5 (and ligands CCL3, CCL4, CCL5, and CCL8) (Fig. 6F).
Fig. 6.
Macrophage IL6 suppresses the accumulation and M1-like polarization of ATMs. (A, Left) CD- and HFD-fed (16 wk) ФWT and ФΔIL6 mice were examined by staining sections of gWAT with hematoxylin and eosin. (Scale bar, 100 µm.) (A, Center) Macrophages were stained by using an antibody to F4/80 and detection by fluorescence microscopy. (Scale bar, 50 µm.) (A, Right) ATMs were quantitated by measurement of F4/80 staining of tissue sections using ImageJ software (mean ± SEM; n = 8 ∼ 11). ***P < 0.001. (B) The SVF of gWAT was isolated from ФWT and ФΔIL6 CD- and HFD-fed (16 wk) mice and examined by flow cytometry to detect the total number of ATMs (F4/80+), M1-like ATMs (F4/80+ CD11c+ CD206−), and M2-like ATMs (F4/80+ CD11c− CD206+) (mean ± SEM; n = 5). *P < 0.05. (C) Total RNA was isolated from gWAT of CD- and HFD-fed ФWT and ФΔIL6 mice (16 wk). The relative expression of mRNA associated with macrophages (Adgre1 and Cd68), M1-like polarized macrophages (Ccl2, Cd11c, and Tnfα), and M2-like polarized macrophages (Arg1, Mgl1, Mgl2, Mrc1, and Mrc2) was measured by qRT-PCR assays (mean ± SEM; n = 5 ∼ 8). *P < 0.05; **P < 0.01; ***P < 0.001. (D and E) The relative expression of the eosinophil marker (Ccr3), neutrophil marker (Mpo), and T cell markers (Cd4, Cd8, and Foxp3) were measured by qRT-PCR assays of mRNA (mean ± SEM; n = 5 ∼ 8). *P < 0.05, ***P < 0.001. (F) The relative expression of the receptor Ccr2 (and ligands Ccl2, Ccl7, and Ccl8) and the receptor Ccr5 (and ligands Ccl3, Ccl4, Ccl5, and Ccl8) were measured by qRT-PCR assays of mRNA (mean ± SEM; n = 5 ∼ 8). *P < 0.05; **P < 0.01.
Together, these data demonstrate that myeloid-cell IL6 in HFD-fed mice improves glycemia and insulin resistance (Fig. 5) and suppresses adipose tissue inflammation by reducing M1-like macrophage polarization and ATM accumulation in adipose tissue (Fig. 6).
IL6 Signaling Differentially Regulates Adipose Tissue Inflammation.
Our studies of macrophage and adipocyte IL6 deficiency lead to opposite conclusions concerning the role of IL6 in adipose tissue inflammation and metabolic syndrome. We show that adipocyte IL6 is required for ATM accumulation, but causes only modest changes in insulin resistance. In contrast, myeloid-cell IL6 suppresses ATM accumulation and protects against the development of insulin resistance. It is difficult to rationalize these two different actions of IL6 within a single conceptual framework. To gain some insight into this biological complexity, we examined an alternative physiological mechanism that promotes IL6 signaling in adipose tissue. For example, it is established that IL6 secreted by skeletal muscle after exercise targets adipose tissue (11). We therefore compared adipose tissue in control MWT mice (Mck-Cre+/−) and M∆IL6 mice with skeletal muscle deficiency of IL6 (Mck-Cre+/− Il6LoxP/LoxP) (SI Appendix, Fig. S7 A and B).
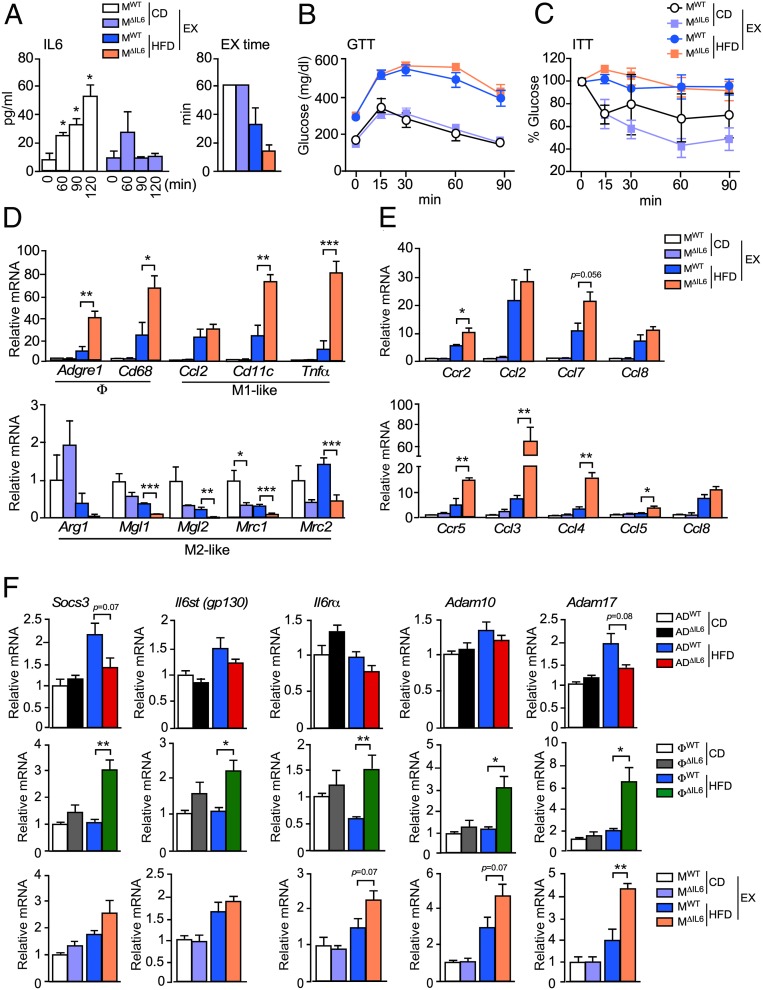
Examination of control and muscle IL6-deficient mice fed a CD or a HFD demonstrated similar body mass; similar blood concentrations of glucose, insulin, leptin, and resistin; and similar tolerance to glucose and insulin (SI Appendix, Fig. S7 C–H). Muscle IL6 therefore appeared to play no obvious metabolic role. However, the increased blood concentration of IL6 observed in control mice after exercise was reduced in muscle IL6-deficient mice (Fig. 7A). This suppression of circulating IL6 did not cause significant (P < 0.05) changes in tolerance to glucose or insulin (Fig. 7 B and C). However, the accumulation of ATMs (primarily M1-like macrophages) by gWAT was increased in the muscle IL6-deficient mice compared with control mice (Fig. 7D) and was associated with increased expression of chemokine receptors CCR2 (and ligands CCL2, CCL7, and CCL8) and CCR5 (and ligands CCL3, CCL4, CCL5, and CCL8) (Fig. 7E). These data indicate that muscle IL6 functions to suppress adipose tissue inflammation by decreasing the accumulation of ATMs.
Fig. 7.
Muscle IL6 suppresses the accumulation and M1-like polarization of ATMs. (A) The concentration of circulating IL6 in blood was measured from exercised CD- or HFD-fed (16 wk) MWT and MΔIL6 mice (mean ± SEM; n = 6). *P < 0.05. The exercise (EX) time is presented. (B) The exercised CD- and HFD-fed (16 wk) mice were examined by GTTs (mean ± SEM; n = 6). (C) The exercised CD- and HFD-fed (16 wk.) mice were examined by ITTs (mean ± SEM; n = 6). (D) Total RNA was isolated from gWAT from CD- and HFD-fed MWT and MΔIL6 mice (16 wk). The relative expression of mRNA associated with macrophages (Adgre1 and Cd68), M1-like polarized macrophages (Ccl2, Cd11c, and Tnfα), and M2-like polarized macrophages (Arg1, Mgl1, Mgl2, Mrc1, and Mrc2) were measured by qRT-PCR assays (mean ± SEM; n = 5 ∼ 7). *P < 0.05; **P < 0.01; ***P < 0.001. (E) The relative expression of the receptor Ccr2 (and ligands Ccl2, Ccl7, and Ccl8) and the receptor Ccr5 (and ligands Ccl3, Ccl4, Ccl5, and Ccl8) were measured by qRT-PCR assays of mRNA (mean ± SEM; n = 5 ∼ 7). *P < 0.05; **P < 0.01. (F) The relative expression of IL6 pathway-associated genes (Socs3, Il6st, Il6rα, Adam10, and Adam17) by gWAT was measured by qRT-PCR assays of mRNA (mean ± SEM; n = 5 ∼ 8). *P < 0.05; **P < 0.01.
Adipose Tissue Inflammation Is Determined by the Source of IL6 Expression.
Our analysis demonstrates that IL6 expression by adipocytes promotes the accumulation of ATMs (Fig. 3), while IL6 expression by myeloid cells and muscle suppresses ATM accumulation (Figs. 6 and 7). To identify mechanisms that might contribute to this difference in IL6 signaling, we profiled gWAT gene expression for differences between IL6 deficiency in adipocytes, myeloid cells, and muscle. This analysis demonstrated increased expression of the metalloproteases ADAM10 and ADAM17 by gWAT in mice with IL6 deficiency in muscle or myeloid cells that was not detected in gWAT from mice with adipocyte deficiency of IL6 (Fig. 7F). The increased expression of Adam10 and Adam17 was associated with increased expression of the IL6 receptor (Il6rα and Il6st) and increased expression of the IL6 target gene Socs3 (Fig. 7F). Interestingly, ADAM10 and ADAM17 control the formation of soluble IL6-Rα and, thus, the switch of IL6 signaling from a canonical mode to a noncanonical trans-signaling mode (5). This switch in IL6 signaling may contribute to the different phenotypes detected in mice with IL6 deficiency in adipocytes compared with myeloid cells and muscle (5). Indeed, it is established that while canonical IL6 signaling suppresses ATM accumulation (6), IL6 trans-signaling promotes ATM accumulation (26).
Discussion
Recent studies have provided significant insight into the metabolic actions of IL6, a potent cytokine that is secreted by several tissues (1). However, little is known about the source of IL6 expression under different physiological conditions. Nevertheless, it is established that exercise causes increased IL6 expression by muscle (27), insulin signaling on AgRP neurons in the hypothalamus causes increased hepatic expression of IL6 (28), and obesity increases IL6 expression by adipose tissue (24, 25).
Previous studies have demonstrated that the source of IL6 may influence the consequence of IL6 signaling. Thus, hepatic IL6 signaling is different when IL6 is secreted by resident Kupffer cells or adipose tissue depots (10, 29). Adipocyte-derived IL6 causes decreased hepatic expression of the adapter protein IRS1 (10). In contrast, Kupffer cell-derived IL6 promotes expression of the adapter protein IRS2 by hepatocytes (29). This difference in the signaling response to different physiological sources of IL6 represents one aspect of the complexity of IL6 function.
The purpose of this study was to examine IL6 expression by adipose tissue during the response to obesity. We tested the role of IL6 expression by adipocytes or myeloid cells by comparing mice with selective Il6 gene ablation. We found that adipocyte IL6 knockout mice manifested decreased ATM accumulation, increased energy expenditure, and relatively minor differences in whole-body glucose homeostasis when fed a HFD (Figs. 2 and 3). In contrast, HFD-fed myeloid cell IL6 knockout mice manifested decreased energy expenditure, which predisposed them to increased body weight, increased hepatic steatosis, and insulin resistance (Figs. 5 and 6), and HFD-fed muscle IL6 knockout mice manifested increased ATM accumulation following exercise (Fig. 7). These data demonstrate that the physiological metabolic response to IL6 depends on the specific source of IL6 in vivo, where adipocyte-derived IL6 promotes ATM accumulation in the absence of major changes in glucose or insulin tolerance, whereas myeloid cell-derived IL6 suppresses M1-like macrophage polarization and ATM accumulation and improves glucose and insulin tolerance, and exercise-induced muscle IL6 suppresses ATM accumulation.
Canonical IL6 signaling involves binding of IL6 to an IL6-Rα/IL6ST complex at the plasma membrane that activates the JAK1–STAT3 pathway (5). Targets of this signaling pathway include the IL4-Rα on macrophages that that promotes alternative (M2a) activation and suppression of obesity-induced insulin resistance (6). It is likely that a defect in this mechanism contributes to the inflammatory phenotype (increased ATMs, increased M1-like macrophages, and insulin resistance) of mice with IL6 deficiency in myeloid cells (Figs. 4–6).
Noncanonical (trans) IL6 signaling is mediated by the interaction of IL6ST with a proteolytically processed (soluble) form of IL6-Rα bound to IL6 (5). The formation of soluble IL6-Rα is largely mediated by the metalloproteases ADAM10 and ADAM17 (5). It is established that trans-signaling by IL6 potently promotes ATM recruitment (26). This trans-signaling mechanism most likely accounts for the effect of adipocyte IL6 to increase ATM recruitment (Fig. 6) because of increased ADAM10 and ADAM17 expression (Fig. 7F).
An intriguing aspect of trans signaling is that the promoted ATM recruitment does not cause major changes in insulin resistance (26), including in mice with adipocyte IL6 deficiency (Fig. 2). This is in contrast to canonical IL6 signaling that regulates insulin sensitivity (6), including in mice with myeloid-cell IL6 deficiency (Fig. 5). This difference in the insulin-resistance response to IL6 may relate to the direct actions of canonical IL6 signaling to promote anti-inflammatory M2a macrophage polarization (6) that have not been reported for noncanonical IL6 signaling (26). The mechanism that accounts for this difference in IL6 signaling is unclear. However, it is possible that autocrine IL6 signaling by myeloid cells triggers canonical signaling, while paracrine/endocrine signaling by adipocytes and muscle leads to noncanonical signaling.
In conclusion, this study has identified a layer of complexity in the physiology of the metabolic actions of IL6 by showing that the source of cytokine expression can determine the inflammatory signaling response. Thus, the physiological context of IL6 signaling is important for IL6 function. For example, muscle can secrete IL6 following exercise (27) and suppress ATM accumulation in adipose tissue. In contrast, adipocytes can secrete IL6 in response to obesity (1) and increase ATM accumulation. This mechanistic plasticity enables IL6 to promote an appropriate biological response within a specific physiological context.
Materials and Methods
Animals.
C57BL/6J mice (stock no. 000664), B6.129P2-Lyz2tm1(cre)Ifo/J mice (stock no. 004781) (30), and B6;FVB-Tg(Adipoq-Cre)1Evdr mice (stock no. 028020) (31) were obtained from The Jackson Laboratory. The Il6LoxP/LoxP mice have been described (32). Mice were back-crossed to the C57BL/6J strain (10 generations) and housed in a specific pathogen-free facility accredited by the American Association for Laboratory Animal Care using laminar flow cages. Male mice (age 8 wk.) were fed a CD or a HFD (33). The studies were approved by the Animal Care and Use Committees of the University of Massachusetts Medical School and Yale University Medical School.
Exercise.
Mice ran on a treadmill (Columbus Instruments model 1055-SRM-D58) at 10 m/min at a 0° inclination (15 min) followed by a gradual increase to 18 m/min with a 5° inclination (60 min or until exhaustion). Mice were acclimated to the treadmill running (8 m/min for 15 min) every other day for 1 wk before the test. Glucose tolerance tests (GTTs) (prefasted 5 h), insulin tolerance tests (ITTs), and gene-expression studies were performed 1 h after exercise.
Genotype Analysis.
Genomic DNA was genotyped by using a PCR-based method. Cre+ (450 bp) was detected by using amplimers 5′-TTACTGACCGTACACCAAATTTGCCTGC-3′ and 5′-CCTGGCAGCGATCGCTATTTTCCATGAGTG-3′. Il6LoxP (420 bp) and Il6WT (317 bp) were detected by using amplimers 5′-CCCACCAAGAACGATAGTCA-3′ and 5′-GGTATCCTCTGTGAAGTCCTC-3′. Il6Δ (260 bp), Il6LoxP (1,000 bp), and Il6WT (900 bp) were detected by using amplimers 5′-CCCACCAAGAACGATAGTCA-3′ and 5′-ATGCCCAGCCTAATCTAGGT-3′.
RNA Analysis.
These methods have been described (34). The expression of mRNA was examined by qPCR analysis using a Quantstudio PCR machine (Life Technologies). TaqMan assays were used to quantitate Adam10 (Mm00545742_m1), Adam17 (Mm00456428_m1), Adgre1 (Mm00802529_m1), Adipoq (Mm00456425_m1), Arg1 (Mm00475988_m1), Ccl2 (Mm00441242_m1), Ccl3 (Mm00441259_g1), Ccl4 (Mm0044311_m1), Ccl5 (Mm01302427_m1), Ccl7 (Mm01308393_g1), Ccl8 (Mm01297183_m1), Ccr2 (Mm00438270_m1), Ccr3 (Mm00515543_s1), Ccr5 (Mm01963251_s1), Cd11c (Mm00498698_m1), Cd4 (Mm00442754_m1), Cd68 (Mm00839639_g1), Cd8a (Mm01182107_g1), Foxp3 (Mm00475162_m1), Il6 (Mm00446190_m1), Il6rα(Mm00439653_m1), Il6st (Mm00439665_m1), Mgl1 (Mm00546125_g1), Mgl2 (Mm00460844_m1), Mpo (Mm01298424_m1), Mrc1 (Mm00485148_m1), Mrc2 (Mm00485184_m1), Socs3 (Mm00545913_s1), and Tnfα (Mm00443258_m1). The relative mRNA expression was normalized by measurement of the amount of 18S RNA in each sample using Taqman© assays (catalog no. 4352339E, Life Technologies).
Immunoblot Analysis.
These methods have been described (34). Intraperitoneal (i.p.) injection of mice with saline (control) or 1 U/kg insulin (15 min) was performed. Tissue extracts were prepared by using Triton Lysis Buffer (20 mM Tris [pH 7.4], 1% Triton X-100, 10% glycerol, 137 mM NaCl, 2 mM ethylenediaminetetraacetic acid, 25 mM β-glycerophosphate, 1 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride, and 10 µg/mL aprotinin plus leupeptin). Extracts (20 to 50 µg of protein) were examined by protein immunoblot analysis by probing with antibodies to AKT (catalog no. 9272; Cell Signaling Technology), pSer473 AKT (catalog no. 9271; Cell Signaling Technology), pThr308 AKT (catalog no. 4056; Cell Signaling Technology), and αTubulin (clone B-5-1-2; catalog no. T5168; Millipore Sigma). IRDye 680LT conjugated-donkey anti-mouse IgG antibody (catalog no. 926-68022; LI-COR Biosciences) and IRDye 800CW conjugated-goat anti-rabbit IgG (catalog no. 926-32211; LI-COR Biosciences) were used to detect and quantitate immune complexes with the Odyssey infrared imaging system (LI-COR Biosciences).
Measurement of Blood Glucose and Adipokine Concentration.
These methods have been described (34, 35). Blood glucose was measured with an Ascensia Breeze 2 glucometer (Bayer). Insulin, leptin, IL6, and resistin in plasma were measured by enzyme-linked immunosorbent assay using a Luminex 200 machine (Millipore).
GTTs and ITTs.
GTTs and ITTs were performed by using described methods (10).
Glucose-Induced Insulin Release.
Glucose-induced insulin release (GIR) measurements were performed by i.p. injection of glucose (2 g/kg) and measurement of blood insulin concentration at 0, 5, and 15 min.
Lipid Analysis.
Hepatic triglyceride was measured by using a kit purchased from Sigma (catalog no. TR0100). Blood lipoprotein analysis was performed by the University of Cincinnati Mouse Metabolic Phenotyping Center.
Body Temperature.
Biocompatible and sterile microchip transponders (IPTT-300 Extended Accuracy Calibration; Bio Medic Data Systems) were implanted, and core body temperature was measured by telemetry.
Hyperinsulinemic-Euglycemic Clamp Studies.
Basal infusion and hyperinsulinemic–euglycemic clamp studies were performed after an overnight fast to assess whole-body and hepatic insulin sensitivity (9).
Metabolic Cages.
The analysis was performed by the Mouse Metabolic Phenotyping Center at the University of Cincinnati (35). The mice were housed under controlled temperature and lighting with free accesses to food and water. Respiratory exchange ratio and energy exchange ratio were measured by using metabolic cages.
Analysis of Tissue Sections.
The methods used for the analysis of tissue sections have been described (34, 35). Histology was performed by using tissue fixed in 10% formalin for 24 h, dehydrated, and embedded in paraffin. Sections (7 µm) were cut and stained by using hematoxylin and eosin (American Master Tech Scientific). Immunohistochemistry was performed by using deparaffinized tissue sections that were incubated in blocking buffer for 1 h (1% bovine serum albumin [BSA] and 10% normal goat serum in phosphate-buffered saline [PBS]) and permeabilized with 0.1% Triton X-100. The sections were incubated in blocking buffer containing the primary antibody F4/80 (catalog no. ab6640; Abcam). The primary antibody was detected by incubation with goat-anti rabbit IgG-Alexa Fluor 488 (catalog no. A2704; ThermoFisher Scientific). DNA was detected by staining with DAPI (catalog no. D3571; Invitrogen). Fluorescence was visualized by using a Leica TCS SP2 confocal microscope equipped with a 405-nm diode laser.
Adipocyte and Stromal Vascular Cell Isolation.
The SVF of gonadal white fat (gWAT) was isolated (36). Briefly, gWAT was excised and minced into 10 mL of KRB solution (12.5 mM Hepes [pH 7.4], 120 mM NaCl, 6 mM KCl, 1.2 mM MgSO4, 1 mM CaCl2, 2% BSA, and 2.5 mM glucose). Collagenase II (catalog no. C6885; Sigma; 1 mg/mL) and DNase I (catalog no. DN-25; Sigma; 0.2 mg/mL) were added, and the tissue was incubated at 37 °C with shaking (40 min). Larger particles were removed by using a 100-µm nylon sieve, and the filtrates were centrifuged at 1,000 rpm (3 min). Floating adipocytes were washed twice with PBS prior to RNA isolation. Nonadipocyte layers were collected, centrifuged at 3,000 rpm (5 min), and washed with PBS supplemented with 2% fetal bovine serum (FBS). The cells were treated (10 min) with 500 µL of Ack lysis buffer (catalog no. A10492; ThermoFisher Scientific) and washed prior to RNA isolation and flow cytometry.
Flow Cytometry.
Cell-surface antigens were examined by using cells stained with the Invitrogen LIVE/DEAD Fixable Blue Dead Cell Stain Kit (catalog no. L23105; ThermoFisher Scientific), 2% FBS–PBS plus FcBlock (catalog no. 553142; BD Bioscience), and conjugated antibodies including F4/80-Phycoerythrin (catalog no. 123110; Biolegend), CD206-Alexa Fluor 488 (catalog no. MCA2235A488; AbD serotec), and CD11c-Alexa Fluor 700 (catalog no. 56-0114-82; eBioscience) (33). Isotype control antibodies for Cd11c (Alexa Fluor 700 isotype control. catalog no. 56-4888-80) and CD206 (Alex Fluor 488 isotype control; catalog no. 400233) were obtained from ThermoFisher Scientific. The cells were washed and subsequently fixed with 2% paraformaldehyde. Analysis was performed by using a LSR-II cytometer (Becton Dickenson). Data were processed by using FlowJo Software (Tree Star).
Statistical Analysis.
Data are presented as the mean and SE. Statistical analysis was performed by using GraphPad Prism (Version 7; GraphPad Software). ANOVA with Bonferroni’s test was used to determine significance with an assumed CI of 95%. Two-tailed, unpaired t test with Welch’s correction was used for pairwise comparisons. Statistical significance was defined as P < 0.05.
Material and Data Availability.
Sources for materials used in this study are described in Materials and Methods. The raw data obtained for this study are presented in Datasets S1 and S2.
Supplementary Material
Acknowledgments
We thank Heather Learnard, Siobhan M. Craige, and John F. Keaney Jr. for assistance with exercise studies; Armanda Roy, Ali Nasiri, and Wanling Zhu for technical assistance; and Kathy Gemme for administrative assistance. These studies were supported by American Heart Association Grant 19CDA34660270 (to M.S.H.); Ministerio de Economía y Competitividad y Fondo Europeo de Desarrollo Regional Grants SAF2014-56546-R and RTI2018-101105-B-I00 (to J.H.): and NIH Grants R01 DK107220, R01 DK112698, and R01 DK121545 (to R.J.D.) and R01 DK116774, R01 DK114793, and P30 DK045735 (to G.I.S.).
Footnotes
The authors declare no competing interest.
See QnAs on page 2732.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1920004117/-/DCSupplemental.
References
- 1.Mauer J., Denson J. L., Brüning J. C., Versatile functions for IL-6 in metabolism and cancer. Trends Immunol. 36, 92–101 (2015). [DOI] [PubMed] [Google Scholar]
- 2.Carey A. L., et al. , Interleukin-6 increases insulin-stimulated glucose disposal in humans and glucose uptake and fatty acid oxidation in vitro via AMP-activated protein kinase. Diabetes 55, 2688–2697 (2006). [DOI] [PubMed] [Google Scholar]
- 3.van Hall G., et al. , Interleukin-6 stimulates lipolysis and fat oxidation in humans. J. Clin. Endocrinol. Metab. 88, 3005–3010 (2003). [DOI] [PubMed] [Google Scholar]
- 4.Schmidt-Arras D., Rose-John S., IL-6 pathway in the liver: From physiopathology to therapy. J. Hepatol. 64, 1403–1415 (2016). [DOI] [PubMed] [Google Scholar]
- 5.Scheller J., Chalaris A., Schmidt-Arras D., Rose-John S., The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Biophys. Acta 1813, 878–888 (2011). [DOI] [PubMed] [Google Scholar]
- 6.Mauer J., et al. , Signaling by IL-6 promotes alternative activation of macrophages to limit endotoxemia and obesity-associated resistance to insulin. Nat. Immunol. 15, 423–430 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wunderlich F. T., et al. , Interleukin-6 signaling in liver-parenchymal cells suppresses hepatic inflammation and improves systemic insulin action. Cell Metab. 12, 237–249 (2010). [DOI] [PubMed] [Google Scholar]
- 8.Wueest S., Konrad D., The role of adipocyte-specific IL-6-type cytokine signaling in FFA and leptin release. Adipocyte 7, 226–228 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perry R. J., et al. , Hepatic acetyl CoA links adipose tissue inflammation to hepatic insulin resistance and type 2 diabetes. Cell 160, 745–758 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sabio G., et al. , A stress signaling pathway in adipose tissue regulates hepatic insulin resistance. Science 322, 1539–1543 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wedell-Neergaard A. S., et al. , Exercise-induced changes in visceral adipose tissue mass are regulated by IL-6 signaling: A randomized controlled trial. Cell Metab. 29, 844–855.e3 (2019). [DOI] [PubMed] [Google Scholar]
- 12.Petruzzelli M., Wagner E. F., Mechanisms of metabolic dysfunction in cancer-associated cachexia. Genes Dev. 30, 489–501 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Timper K., et al. , IL-6 improves energy and glucose homeostasis in obesity via enhanced central IL-6 trans-signaling. Cell Rep. 19, 267–280 (2017). [DOI] [PubMed] [Google Scholar]
- 14.Ellingsgaard H., et al. , Interleukin-6 enhances insulin secretion by increasing glucagon-like peptide-1 secretion from L cells and alpha cells. Nat. Med. 17, 1481–1489 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knudsen J. G., et al. , Skeletal muscle IL-6 and regulation of liver metabolism during high-fat diet and exercise training. Physiol. Rep. 4, e12788 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hidalgo J., et al. , Transgenic mice with astrocyte-targeted production of interleukin-6 are resistant to high-fat diet-induced increases in body weight and body fat. Brain Behav. Immun. 24, 119–126 (2010). [DOI] [PubMed] [Google Scholar]
- 17.Guerra J., et al. , Muscular interleukin-6 differentially regulates skeletal muscle adaptation to high-fat diet in a sex-dependent manner. Cytokine 74, 145–151 (2015). [DOI] [PubMed] [Google Scholar]
- 18.Ferrer B., et al. , Muscle-specific interleukin-6 deletion influences body weight and body fat in a sex-dependent manner. Brain Behav. Immun. 40, 121–130 (2014). [DOI] [PubMed] [Google Scholar]
- 19.Molinero A., et al. , Role of muscle IL-6 in gender-specific metabolism in mice. PLoS One 12, e0173675 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fernández-Gayol O., et al. , Different responses to a high-fat diet in IL-6 conditional knockout mice driven by constitutive GFAP-cre and synapsin 1-cre expression. Neuroendocrinology 109, 113–130 (2019). [DOI] [PubMed] [Google Scholar]
- 21.Navia B., et al. , Interleukin-6 deletion in mice driven by aP2-Cre-ERT2 prevents against high-fat diet-induced gain weight and adiposity in female mice. Acta Physiol. (Oxf.) 211, 585–596 (2014). [DOI] [PubMed] [Google Scholar]
- 22.Señarís R. M., et al. , Interleukin-6 regulates the expression of hypothalamic neuropeptides involved in body weight in a gender-dependent way. J. Neuroendocrinol. 23, 675–686 (2011). [DOI] [PubMed] [Google Scholar]
- 23.Whitham M., et al. , Adipocyte-specific deletion of IL-6 does not attenuate obesity-induced weight gain or glucose intolerance in mice. Am. J. Physiol. Endocrinol. Metab. 317, E597–E604 (2019). [DOI] [PubMed] [Google Scholar]
- 24.Carey A. L., et al. , Interleukin-6 and tumor necrosis factor-alpha are not increased in patients with type 2 diabetes: Evidence that plasma interleukin-6 is related to fat mass and not insulin responsiveness. Diabetologia 47, 1029–1037 (2004). [DOI] [PubMed] [Google Scholar]
- 25.Mohamed-Ali V., et al. , Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo. J. Clin. Endocrinol. Metab. 82, 4196–4200 (1997). [DOI] [PubMed] [Google Scholar]
- 26.Kraakman M. J., et al. , Blocking IL-6 trans-signaling prevents high-fat diet-induced adipose tissue macrophage recruitment but does not improve insulin resistance. Cell Metab. 21, 403–416 (2015). [DOI] [PubMed] [Google Scholar]
- 27.Fischer C. P., Interleukin-6 in acute exercise and training: What is the biological relevance? Exerc. Immunol. Rev. 12, 6–33 (2006). [PubMed] [Google Scholar]
- 28.Könner A. C., et al. , Insulin action in AgRP-expressing neurons is required for suppression of hepatic glucose production. Cell Metab. 5, 438–449 (2007). [DOI] [PubMed] [Google Scholar]
- 29.Awazawa M., et al. , Adiponectin enhances insulin sensitivity by increasing hepatic IRS-2 expression via a macrophage-derived IL-6-dependent pathway. Cell Metab. 13, 401–412 (2011). [DOI] [PubMed] [Google Scholar]
- 30.Clausen B. E., Burkhardt C., Reith W., Renkawitz R., Förster I., Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 8, 265–277 (1999). [DOI] [PubMed] [Google Scholar]
- 31.Eguchi J., et al. , Transcriptional control of adipose lipid handling by IRF4. Cell Metab. 13, 249–259 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quintana A., et al. , Astrocyte-specific deficiency of interleukin-6 and its receptor reveal specific roles in survival, body weight and behavior. Brain Behav. Immun. 27, 162–173 (2013). [DOI] [PubMed] [Google Scholar]
- 33.Han M. S., et al. , JNK expression by macrophages promotes obesity-induced insulin resistance and inflammation. Science 339, 218–222 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vernia S., et al. , Diet-induced obesity mediated by the JNK/DIO2 signal transduction pathway. Genes Dev. 27, 2345–2355 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vernia S., et al. , Excitatory transmission onto AgRP neurons is regulated by cJun NH2-terminal kinase 3 in response to metabolic stress. eLife 5, e10031 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Herrero L., Shapiro H., Nayer A., Lee J., Shoelson S. E., Inflammation and adipose tissue macrophages in lipodystrophic mice. Proc. Natl. Acad. Sci. U.S.A. 107, 240–245 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.