Significance
Acylceramide is essential for skin permeability barrier formation. However, its biosynthesis pathway has not yet been elucidated in its entirety. In the present study, we found that Fatp4 disruption substantially decreased the amount of acylceramides in mice, as did FATP4 knockdown in human keratinocytes. In addition, in vitro experiments demonstrated that FATP4 exhibited acyl-CoA synthetase activity toward an ω-hydroxy ultra-long-chain fatty acid, an intermediate of the acylceramide biosynthetic pathway. From these results, we conclude that FATP4 functions in skin barrier formation through acylceramide synthesis. Our findings not only reveal the pathogenic mechanism of ichthyosis prematurity syndrome, but also help to elucidate the molecular mechanism of the synthesis of the skin barrier lipid acylceramide.
Keywords: acylceramide, ceramide, lipid, skin, sphingolipid
Abstract
The epidermis-specific lipid acylceramide plays a pivotal role in the formation of the permeability barrier in the skin; abrogation of its synthesis causes the skin disorder ichthyosis. However, the acylceramide synthetic pathway has not yet been fully elucidated: Namely, the acyl-CoA synthetase (ACS) involved in this pathway remains to be identified. Here, we hypothesized it to be encoded by FATP4/ACSVL4, the causative gene of ichthyosis prematurity syndrome (IPS). In vitro experiments revealed that FATP4 exhibits ACS activity toward an ω-hydroxy fatty acid (FA), an intermediate of the acylceramide synthetic pathway. Fatp4 knockout (KO) mice exhibited severe skin barrier dysfunction and morphological abnormalities in the epidermis. The total amount of acylceramide in Fatp4 KO mice was reduced to ∼10% of wild-type mice. Decreased levels and shortening of chain lengths were observed in the saturated, nonacylated ceramides. FA levels were not decreased in the epidermis of Fatp4 KO mice. The expression levels of the FA elongase Elovl1 were reduced in Fatp4 KO epidermis, partly accounting for the reduction and shortening of saturated, nonacylated ceramides. A decrease in acylceramide levels was also observed in human keratinocytes with FATP4 knockdown. From these results, we conclude that skin barrier dysfunction observed in IPS patients and Fatp4 KO mice is caused mainly by reduced acylceramide production. Our findings further elucidate the molecular mechanism governing acylceramide synthesis and IPS pathology.
Skin possesses a powerful permeability barrier (the skin barrier), which functions to prevent infectious diseases and allergies by blocking the invasion of external substances such as pathogens, allergens, and chemicals. It also prevents water loss from the body, an essential function for terrestrial animals. The skin barrier is so strong that infections through the skin rarely occur under normal circumstances; but if it is damaged, such as by a burn or cut, the risk of infection increases dramatically. Ichthyosis is an inherited disease featuring skin barrier abnormalities and is characterized by dryness, thickening, and scaly skin (1). Similarly, atopic dermatitis patients suffer from reduced skin barrier function, allowing allergens to enter (2).
Ichthyosis prematurity syndrome (IPS) is one of the syndromic forms of ichthyosis (3, 4). IPS is characterized by premature birth, neonatal asphyxia, and ichthyosis. Ichthyosis improves during the first several weeks after birth but persist for life, often accompanying atopic dermatitis and recurrent infections. The epidermis of IPS patients is thickened, and hyperkeratosis and presence of droplets are observed in the stratum corneum (3, 5). Furthermore, curved multilamellar membranes exist in the stratum corneum and granular layer of such patients (3, 5, 6). The causative gene of IPS is FATP4 (fatty acid transporter, member 4; also known as ACSVL4 [acyl-CoA synthetase very-long-chain, member 4] or SLC27A4 [solute carrier family 27, member 4]) (3). As the alternative name of FATP4, ACSVL4, indicates, FATP4 is an acyl-CoA synthetase (ACS) that converts fatty acids (FAs) to acyl-CoAs. FAs are classified by chain length: Long-chain (LC) FAs have a chain length of C11–20 and very-long-chain (VLC) FAs have a length of ≥C21. VLCFAs with ≥C26 are sometimes denominated ultra-long-chain (ULC) FAs, since their functions and tissue distributions differ from shorter VLCFAs (7, 8). FATP4/ACSVL4 belongs to the very-long (VL) subfamily of ACSs and exhibits higher ACS activity toward VLCFAs than LCFAs (9). Several Fatp4 knockout (KO) mice have been reported, either generated spontaneously or by gene targeting (10–14). Except for one report indicating prenatal lethality (12), Fatp4 KO mice are neonatal lethal (10, 11, 13, 14). Newborn Fatp4 KO mice exhibit severe skin barrier abnormalities, hyperkeratosis, keratinocyte hyperproliferation, acanthosis, and small keratohyalin granules (10, 11, 13, 14). In addition, the rigid skin of Fatp4 KO mice causes facial dysmorphia, movement restriction, and respiratory failure. However, the mechanism by which mutations in the FATP4/Fatp4 gene cause ichthyosis in IPS patients and Fatp4 KO mice remains unclear.
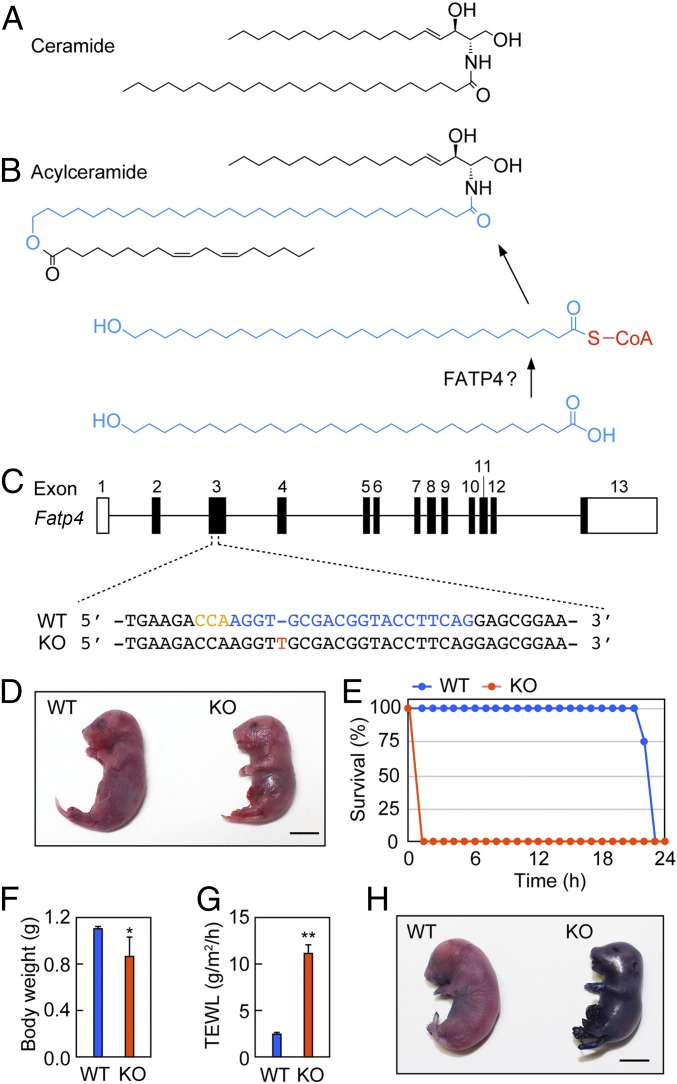
Lipids are suitable for the formation of permeability barriers due to their high hydrophobicity. The stratum corneum contains a multilayer lipid structure (the lipid lamellae) that plays a central role in skin barrier formation (8, 15). The lipid lamellae are mainly composed of ceramides, FAs, and cholesterol. Ceramide forms the backbone of sphingolipids (biological membrane lipids) consisting of two hydrophobic chains: a long-chain base and a FA (Fig. 1A). In addition to this normal-type ceramide, acylceramide (ω-O-acylceramide), a ceramide class specialized for skin barrier formation, exists in the epidermis (8, 16, 17). Acylceramide shows a unique three hydrophobic-chain structure, in which linoleic acid is esterified to the ω-position of the FA moiety of ceramide (Fig. 1B). As a C30–36 ULCFA, acylceramide also has a characteristic and much longer FA chain length than normal ceramide (C16–24). The unique structure of acylceramide is important for the formation and maintenance of lipid lamellae and skin barrier formation: Mutations in the human genes involved in its synthesis cause autosomal recessive congenital ichthyosis, the most severe type in the family of disorders (1, 8, 17, 18). In mice, these mutations lead to neonatal lethality due to skin barrier dysfunction (19–24).
Fig. 1.
Skin barrier dysfunction in Fatp4 KO mice. (A) Structure of ceramide. (B) Structure of acylceramide and reactions that introduce ω-OH ULCFA into acylceramide. (C) Genome structure of mouse Fatp4 gene and WT and mutant nucleotide sequences around CRISPR-Cas9 target sequence. The black boxes represent coding sequences. Red, inserted nucleotide in Fatp4 KO mouse; blue, target sequence of the guide RNA; orange, protospacer adjacent motif (PAM) sequence. (D–H) WT and Fatp4 KO mice were prepared by cesarean section at E18.5. (D) Photograph of specimens. (Scale bar, 1 cm.) (E) Measurement of survival rates (WT, n = 4; KO, n = 3). (F) Measurement of body weights (WT, n = 4; KO, n = 4). (G) Measurement of TEWL (WT, n = 4; KO, n = 4). Values represent the means ± SD. Statistically significant differences are indicated (two-tailed Student’s t test; *P < 0.05; **P < 0.01). (H) WT and Fatp4 KO mice were incubated with 0.1% toluidine blue solution for 2 h and photographed. (Scale bar, 1 cm.)
Despite the importance of acylceramide in skin barrier formation, most of the genes involved in acylceramide production have only recently been identified. The identified genes include the FA elongases ELOVL1 and ELOVL4 for elongation of FAs up to C30–36 (19, 21, 25), the ceramide synthase CERS3 for amide bond formation between a long-chain base and a ULCFA (20), the cytochrome P450 member CYP4F22 for ULCFA ω-hydroxylation (26), and the transacylase PNPLA1 for ester bond formation between a ULCFA and a linoleic acid (22–24, 27) (SI Appendix, Fig. S1).
We previously identified the above-mentioned FA ω-hydroxylase gene CYP4F22, which hydroxylates the ω-position of ULCFAs (26). CYP4F22 is one of the causative genes of autosomal recessive congenital ichthyosis (28–30), and the amount of acylceramide was greatly reduced in the patient examined in that study (26). We also found that the substrates of CYP4F22 are ULCFAs using biochemical and cell-based assays (26). The FA elongases ELOVL1 and ELOVL4 are responsible for the elongation of shorter FAs to ULCFAs (19, 21, 25). Since the elongation reactions proceed in acyl-CoA forms (7, 8), ULC acyl-CoAs produced by the FA elongases must be converted to ULCFAs in order to be catalyzed by CYP4F22. After ω-hydroxylation by CYP4F22, the resulting ω-hydroxy (ω-OH) ULCFAs need to be reconverted to acyl-CoA forms (Fig. 1B), the substrates of the subsequent reaction catalyzed by the ceramide synthase CERS3 (20). However, the ACS involved in this reaction has remained unknown.
Given the importance of acylceramide in skin barrier formation, mutations in the unidentified ACS gene in the acylceramide synthesis pathway are expected to cause congenital ichthyosis. Twenty-six ACSs exist in mammals (31). Of these, we speculate that FATP4 is the unidentified ACS gene that functions in the acylceramide synthesis pathway, based on the severe skin symptoms/phenotypes observed in human IPS patients and Fatp4 KO mice. Since no hypotheses about the involvement of FATP4 in acylceramide synthesis have been proposed so far, acylceramide levels have not yet been measured in Fatp4 KO mice. Shortening of the chain length of nonacylated (normal type) ceramides has been reported in Fatp4 KO mice, specifically, decreases in ≥C26 ceramides and increases in ≤C24 ceramides (10). In the present study, we performed several analyses of Fatp4 KO mouse epidermis to examine the function of Fatp4 in acylceramide synthesis and to reveal the causes of skin barrier dysfunction observed in IPS patients.
Results
Abnormalities in Skin Barrier Formation and Epidermal Morphology in Fatp4 KO Mice.
To date, several Fatp4 mutant mouse lines have been generated (10–14). To investigate the involvement of FATP4 in acylceramide synthesis in the present study, we created another Fatp4 KO mouse line by genome editing using CRISPR-Cas9. Setting the target sequence to exon 3 of the Fatp4 gene, we obtained Fatp4 KO mice having a single nucleotide insertion between the 196th thymine and the 197th guanine in the coding sequence (Fig. 1C). This gene product is predicted to contain the N-terminal 66 amino acids of wild-type (WT) protein (643 amino acids) followed by an unrelated 42 amino acids, due to a frameshift and its accompanying early stop codon. Since the translated region is very short, this mutated gene product is expected to be completely nonfunctional. As in the previously reported Fatp4 mutant mice (10, 11, 14), our Fatp4 KO mice also had more rigid, less wrinkled skin than WT mice (Fig. 1D) and were neonatal lethal. We then examined the time course of the survival rates of the mice obtained by cesarean section on embryonic day 18.5 (E18.5). All KO mice died within 1 h after cesarean section (Fig. 1E). Under the same conditions, where newborn mice were taken from their mothers and could not drink milk, even WT mice did not survive beyond 22 h after cesarean section. The body weight of E18.5 KO mice was lower than that of WT mice (Fig. 1F). The value of the transepidermal water loss (TEWL), an index of the skin permeability barrier function from inside the body to the outside, was 4.4-fold higher in Fatp4 KO mice than WT mice (Fig. 1G). The inverse skin permeability barrier function, from the outside to the body, was examined by toluidine blue staining. Little dye penetration was observed in WT mice, while strong penetration was observed in Fatp4 KO mice (Fig. 1H). These high TEWL values and enhanced dye staining have also been reported in the previous Fatp4 KO mice (10, 13), confirming the presence of impaired skin barrier function in our newly created Fatp4 KO mice.
Hematoxylin/eosin staining was conducted to examine morphological changes in the epidermis of Fatp4 KO mice. In the stratum corneum of WT mice, intercorneocyte gaps, which correspond to lipid lamellae, were observed (Fig. 2A). In Fatp4 KO mice, the existence of gaps was less obvious, indicating impaired lipid lamellae formation. Acanthosis was observed in the stratum spinosum of Fatp4 KO mice. A more detailed examination of the structure of the epidermis was carried out by transmission electron microscopy. The number of cell layers in the stratum corneum was increased in Fatp4 KO mice compared to WT mice (Fig. 2 B and C). The existence of keratohyalin granules filled with profilaggrin is a feature of the stratum granulosum. These granules in Fatp4 KO mice were smaller than those in WT mice (Fig. 2B). Hyperkeratosis, acanthosis, and small keratohyalin granules were also observed in the previously reported Fatp4 mutant mice (10, 11, 13, 14). We found vacuoles and curved membrane structures, which may represent deposits of lipids/membranes probably caused by impaired lamellar body release, in the stratum corneum of Fatp4 KO mice (Fig. 2 C and D). Similar structures have also been observed in IPS patients (3, 5, 6). These morphological abnormalities suggest that keratinocyte differentiation and lipid lamellae formation are impaired in the Fatp4 KO mouse epidermis.
Fig. 2.
Hyperkeratosis and impaired formation of lipid lamellae and keratohyalin granules in Fatp4 KO mice. (A) Paraffin sections of E18.5 WT and Fatp4 KO mouse skin were prepared and stained with hematoxylin and eosin, and bright field images were photographed. (Scale bars, 20 μm.) (B–D) Ultrathin sections of E18.5 WT and Fatp4 KO mouse skin were prepared and subjected to transmission electron microscopy. (B) Red arrowheads indicate keratohyalin granules. (Scale bars, 10 μm.) (C) Enlarged views of the yellow boxes in B. Magenta and light blue arrowheads indicate curved membrane structures and vacuoles in corneocytes, respectively. (Scale bars, 5 μm.) (D) Enlarged views of the curved membrane structures observed in Fatp4 KO mouse skin are shown. (Scale bars, 1 μm.) SC, stratum corneum; SG, stratum granulosum; SS, stratum spinosum; SB, stratum basale.
Decreased Amount of Acylceramide in Fatp4 KO Mouse Epidermis.
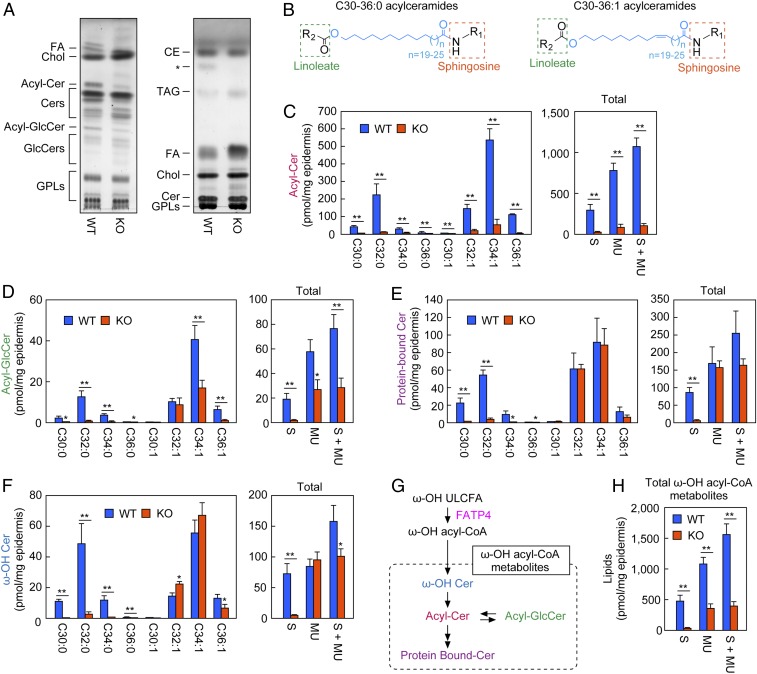
To examine the effect of Fatp4 disruption on epidermal lipid composition, lipids were extracted from the mouse epidermis, separated by thin-layer chromatography (TLC), and stained with copper sulfate/phosphoric acid solution. We observed decreases in the amounts of acylceramide and acyl-glucosylceramide in Fatp4 KO mice compared with WT mice, whereas FA and triacylglycerol (triglyceride; TAG) levels were increased (Fig. 3A). No apparent differences between WT and Fatp4 KO mice were found in the amounts of cholesterol and glycerophospholipids.
Fig. 3.
Decreased amounts of acylceramides in the epidermis of Fatp4 KO mice. (A) Lipids were extracted from the epidermis of WT and Fatp4 KO mice at postnatal day 0. Lipids (2.5 mg skin) were separated by TLC using the solvent system for separation of ceramide and glucosylceramide (Left) and that for separation of TAG, FA, and cholesterol (Right) and stained with copper sulfate/phosphoric acid solution. The asterisk represents unidentified lipid band. (B) Structures of saturated (Left) and monounsaturated (Right) acylceramides. The position of the double bond in the monounsaturated FA of acylceramide is n-9. Sphingosine is the most abundant long-chain base among mammalian long-chain bases. (C–F) Lipids were extracted from the epidermis of WT (n = 3) and Fatp4 KO (n = 3) mice at postnatal day 0. LC-MS/MS analyses were performed on acylceramides (C), acyl-glucosylceramides (D), protein-bound ceramides (E), and ω-OH ceramides (F). The Left shows the amount of each lipid species, and the Right shows the total of saturated (S) species, total of monounsaturated (MU) species, and total of all species. (G) The metabolic pathway of ω-OH ULCFA, an intermediate of acylceramide. (H) Sum of the amounts of ω-OH acyl-CoA metabolites [acylceramide (C), acyl-glucosylceramide (D), protein-bound ceramide (E), and ω-OH ceramide (F)]. Values represent the means ± SD. Statistically significant differences are indicated (two-tailed Student’s t test; *P < 0.05; **P < 0.01). Chol, cholesterol; Acyl-Cer, acylceramide; Cer, ceramide; Acyl-GlcCer, acyl-glucosylceramide; GlcCer, glucosylceramides; GPL, glycerophospholipid.
Quantitative analysis of acylceramide species was performed using liquid chromatography (LC) coupled with tandem mass spectrometry (MS/MS). In Fatp4 KO mice, the amounts of all of the acylceramide species, regardless of FA chain length and degree of unsaturation (saturated or monounsaturated), were substantially decreased compared with those in WT mice (Fig. 3 B and C). The total amount of acylceramides in Fatp4 KO mice was 9.8% of WT mice, with saturated and monounsaturated acylceramides being similarly reduced (saturated, 7.4%; monounsaturated, 11%). These results indicate that Fatp4 is indeed involved in the acylceramide synthesis.
We also measured the amounts of acyl-glucosylceramides, which are acylceramide metabolites (SI Appendix, Fig. S1). The total amount of saturated acyl-glucosylceramides was substantially reduced in Fatp4 KO mice (9.1% of WT mice; Fig. 3D), as was that of saturated acylceramides (Fig. 3C). On the other hand, the decrease in the total amount of monounsaturated acyl-glucosylceramides in KO mice was rather small (46% of WT mice; Fig. 3D), in contrast to that of monounsaturated acylceramides (Fig. 3C). The total amount of acyl-glucosylceramides (saturated plus monounsaturated) was 37% of that in WT mice (Fig. 3D).
We next measured the amounts of protein-bound ceramides in Fatp4 KO mice, into which a portion of the acylceramide is converted (SI Appendix, Fig. S1). Protein-bound ceramide is a crucial component of a unique membrane structure to corneocytes, termed the corneocyte lipid envelope, which is thought to function in connecting lipid lamellae and corneocytes (32–34). Again, a difference in the degree of reduction between saturated and monounsaturated types owing to Fatp4 disruption was observed. The amount of total saturated protein-bound ceramides in Fatp4 KO mice was reduced to 6.8% of that in WT mice, whereas the total amount of monounsaturated protein-bound ceramides remained comparable (Fig. 3E). In addition, the total amount of protein-bound ceramides (saturated plus monounsaturated) was 65% of that in WT mice.
The final step of acylceramide production is the ester bond formation between ω-OH ceramide and linoleic acid (SI Appendix, Fig. S1). This reaction is catalyzed by the transacylase PNPLA1, where PNPLA1 transfers the linoleic acid in TAG to ω-OH ceramide (27). We measured the amounts of ω-OH ceramides, which are precursors of acylceramides, and found a large decrease (6.5% of WT mice) in the total amount of saturated ω-OH ceramides in Fatp4 KO mice (Fig. 3F). In contrast, no difference was observed in the total amounts of monounsaturated ω-OH ceramides. The total amount of ω-OH ceramides (saturated plus monounsaturated) in Fatp4 KO mice was decreased to 64% of that in WT mice.
In the acylceramide synthetic pathway, ω-OH acyl-CoA, the product of FATP4, is converted to ω-OH ceramide, acylceramide, acyl-glucosylceramide, and protein-bound ceramide (Fig. 3G and SI Appendix, Fig. S1). The sum of all these ω-OH acyl-CoA metabolites in Fatp4 KO mice was reduced to 25.6% of WT mice (Fig. 3H). The decrease was large in saturated types (7.2%) but smaller in monounsaturated types (33.5%).
Fatp4 Gene Disruption Does Not Reduce the Uptake of FAs into the Epidermis.
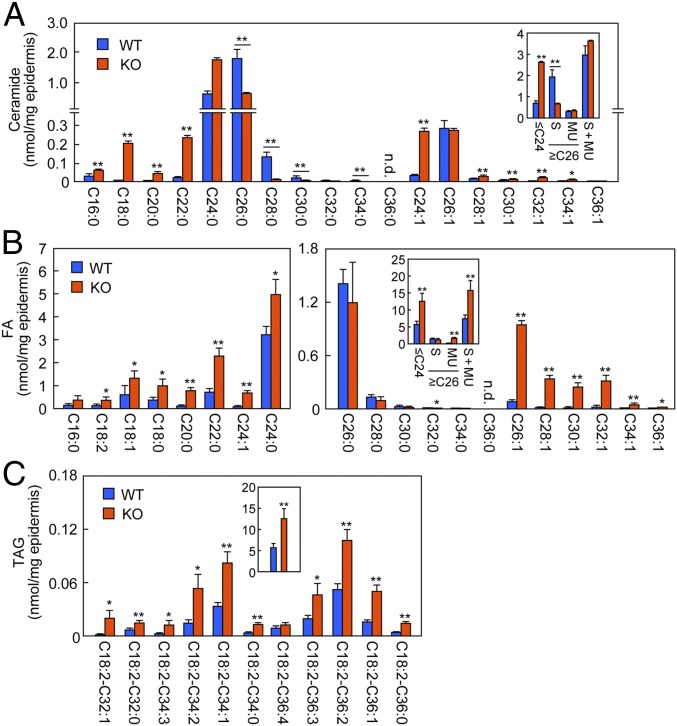
Previous studies measuring ceramides (nonacylated, normal type) reported that ≥C26 ceramides were decreased in Fatp4 KO epidermis compared with WT mice, whereas ≤C24 ceramides were increased (10). In the present study, we performed more detailed analyses. First, we confirmed the decreases in ≥C26 and increases in ≤C24 for saturated ceramides in the epidermis of the Fatp4 KO mice (Fig. 4A), consistent with the previous report (10). While that study measured only C18:1 and C24:1 ceramides for monounsaturated ceramides (10), we here expanded the analyses to ≥C24 monounsaturated species. As in the case of saturated ceramides, the amount of monounsaturated ceramide with a chain length of C24 was also increased in Fatp4 KO mice compared with WT mice (Fig. 4A). On the other hand, unlike the saturated types, the amount of C26 monounsaturated ceramide was unchanged, and those of ≥C28 monounsaturated ceramides were unexpectedly increased. In summary, the total amount of ≤C24 ceramides was increased approximately threefold in Fatp4 KO mice compared with WT mice, whereas that of ≥C26 saturated ceramides was decreased to 34%. The total amount of ≥C26 monounsaturated ceramides as well as total amount of ceramides (saturated plus monounsaturated) was almost unchanged. Thus, Fatp4 disruption had a greater effect on acylceramides than nonacylated ceramides. Similar changes in ceramide composition (increases in ≤C24 and decreases in ≥C26 ceramides, and large effects on saturated ceramides but mild effects on monounsaturated ceramides) were also observed in Elovl1 (a FA elongase)-KO mice (21), implying a reduced activity of Elovl1 in Fatp4 KO mice.
Fig. 4.
Effects of Fatp4 disruption on ceramide, FA, and TAG levels. (A–C) Lipids were extracted from the epidermis of WT (n = 3) and Fatp4 KO (n = 3) mice at postnatal day 0. LC-MS/MS analyses were performed on ceramides (A), FAs (B), and TAGs containing linoleic acid (C). (A and B) The graphs show the amount of each lipid species, and the Insets show the total of ≤C24 species, total of ≥C26 saturated (S) species, total of ≥C26 monounsaturated (MU) species, and total of all species. (C) The graph shows the amount of each TAG species containing linoleic acid (C18:2), while the Inset shows the total TAG quantities. The numbers after “C18:2-” represent the sum of two FAs other than linoleic acid. For example, C32:1 indicates that the summed chain length and degree of unsaturation of the two FA chains are 32 and 1, respectively. Values represent the means ± SD. Statistically significant differences are indicated (two-tailed Student’s t test; *P < 0.05; **P < 0.01). n.d., not detected.
Since the effect of Fatp4 disruption on the abundance of individual FA species has so far not been investigated using MS, we estimated species composition and abundance using epidermal samples. Similar to ceramides (Fig. 4A), the amounts of most ≤C24 FAs were increased in Fatp4 KO mice compared with WT mice (Fig. 4B). In contrast, the amounts of ≥C26 saturated FAs in Fatp4 KO mice were not significantly different from those in WT mice, and ≥C26 monounsaturated FAs were unexpectedly increased. The total amount of FAs in Fatp4 KO mice was 2.1-fold higher than that of WT mice. The substrates of FA elongases in the FA elongation process are acyl-CoAs rather than FAs (7). Therefore, the VLCFAs and ULCFAs in Fatp4 KO mice are most likely produced from the corresponding acyl-CoAs via hydrolysis of CoA by thioesterases after FA elongation. The increases in many FAs, especially monounsaturated FAs, in Fatp4 KO mice suggest that FATP4 has a function of converting the FAs generated by thioesterase back to acyl-CoAs. It is likely that the equilibrium between FAs and acyl-CoA shifts to FAs due to Fatp4 disruption. Although an FA transporter function of FATP4 has been hypothesized (4, 11), our results (specifically the increases in the amounts of FAs in Fatp4 KO mice) did not support this hypothesis. Since linoleic acid (C18:2), a component of acylceramide, is an essential FA that cannot be synthesized in mammals, keratinocytes must procure it from outside the cells. However, the amount of linoleic acid was also increased in Fatp4 KO epidermis (Fig. 4B).
The linoleic acid moiety in acylceramide is derived from TAG; Pnpla1 transfers linoleic acid in TAG to ω-OH ceramide by transacylation (27) (SI Appendix, Fig. S1). We examined the amounts of linoleic acid-containing TAGs in the epidermis of Fatp4 KO mice and found that all TAG species examined were increased compared with WT mice (Fig. 4C). These results indicate that the skin barrier dysfunction and the decreased acylceramide in Fatp4 KO mice are not due to reduced uptake of linoleic acid.
Decreased Expression of Elovl1 and Pnpla1 due to Fatp4 Disruption.
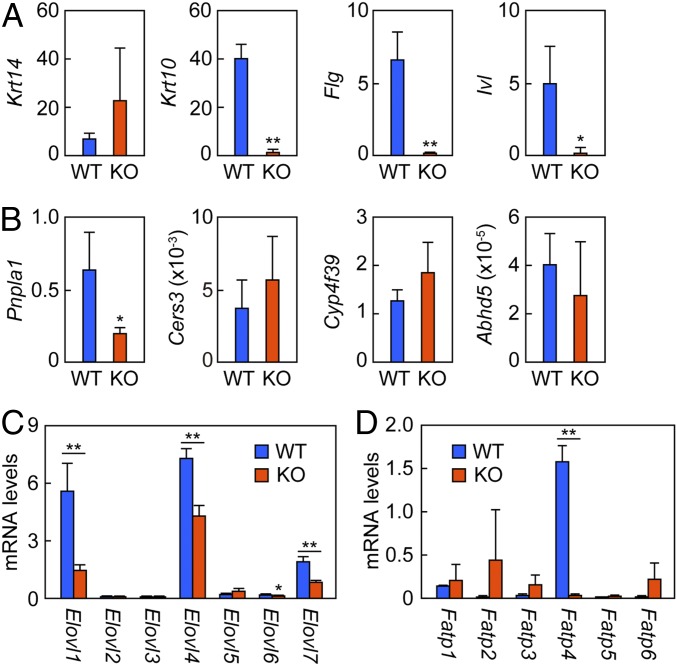
Abnormalities in epidermal morphology and changes in lipid metabolism were observed in Fatp4 KO mice (Figs. 2–4). To investigate the causes behind these changes, the expression levels of keratinocyte differentiation markers and lipid metabolism genes were analyzed by quantitative RT-PCR. Among keratinocyte differentiation markers, the stratum basale marker Krt14 (keratin 14) showed a tendency to increase in Fatp4 KO mice compared with WT mice, although this was not statistically significant (Fig. 5A). In contrast, Krt10 (keratin 10), a marker of the stratum spinosum and stratum granulosum, was reduced in the KO mice (8.3% of WT mice). The stratum granulosum markers Flg (filaggrin) and Ivl (involucrin) were also decreased (1.2% and 7.8% of WT mice, respectively). We confirmed decreases in the protein levels of filaggrin and keratin 10 by immunoblotting (SI Appendix, Fig. S2). These results indicate that differentiation into stratum spinosum and/or stratum granulosum was impaired in the Fatp4 KO mouse epidermis. Abnormal expression of differentiation markers has been observed in mice with KO of several genes involved in acylceramide synthesis (Pnpla1, Cers3, and Cyp4f39 KO mice) (20, 22, 23, 35). Although the exact reason is unknown, it is possible that keratinocytes sense and respond to impaired lamellar body and lipid lamella formation.
Fig. 5.
Effects of Fatp4 disruption on the expression levels of keratinocyte differentiation markers and acylceramide synthesis-related genes. Total RNA was prepared from the epidermis of WT (n = 3) and Fatp4 KO (n = 3) mice at postnatal day 0. Real-time quantitative RT-PCR was performed on Hprt1, keratinocyte differentiation markers (Krt14, Krt10, Flg, and Ivl) (A), acylceramide synthesis-related genes (Pnpla1, Cers3, Cyp4f39, and Abhd5) (B), Elovl family members (Elovl1–7) (C), and Fatp family members (Fatp1–6) (D). Values represent the means ± SD and indicate the relative amounts to Hprt1. Statistically significant differences are indicated (two-tailed Student’s t test; *P < 0.05; **P < 0.01).
Next, the expression levels of acylceramide synthesis-related genes were examined (Fig. 5B). The expression levels of the transacylase Pnpla1 were reduced in the Fatp4 KO mouse epidermis (32% of WT mice), while almost no changes were observed in the expression levels of the ceramide synthase Cers3, the FA ω-hydroxylase Cyp4f39 (a mouse ortholog of human CYP4F22), or Abhd5, a gene product that functions to promote utilization of TAGs by Pnpla1 (36).
Since changes in the chain lengths of ceramides and FAs were observed (Fig. 4 A and B), the expression levels of the FA elongase Elovl family members were examined. In Fatp4 KO mice, the expression levels of Elovl1 were reduced to 25% of WT mice (Fig. 5C). Elovl1 plays an important role in the elongation of C24:0-CoA to C26:0-CoA in epidermis (21). The expression levels of Elovl4, which is responsible for elongation of C26-CoA to ≥C28-CoAs (7, 8, 19), were reduced to 59% of WT mice. The expression levels of Elovl6 and Elovl7, which respectively exhibit activity toward C16:0-CoA and C18-CoAs (7, 8, 25), were reduced to about half the levels of WT mice.
We investigated the possibility of compensatory changes in the expression of other Fatp/Acsvl family members due to Fatp4 disruption. In WT mice, expression levels of Fatp4 were the highest among Fatp family members, as also observed in human keratinocytes (37). The expression levels of Fatp4 were substantially reduced in Fatp4 KO mice (Fig. 5D), possibly due to destabilization of Fatp4 mRNA by nonsense-mediated mRNA decay. In Fatp4 KO mice, the expression levels of Fatp1, Fatp2, Fatp3, Fatp5, and Fatp6 showed tendencies to increase, although these were not statistically significant. In summary, our results suggest that changes in ceramide/fatty acid chain lengths and decreased acylceramide levels observed in Fatp4 KO mice are at least partly attributable to decreased expression levels of Elovl1 and Pnpla1.
FATP4 Exhibits ACS Activity toward ω-OH ULCFA.
We speculate that FATP4 acts as an ACS that converts ω-OH ULCFAs to ω-OH ULC acyl-CoAs in the acylceramide synthetic pathway. However, it has not been examined whether FATP4 has ACS activity toward ω-OH ULCFAs, although activity toward LC and VLC nonhydroxylated FAs has been reported (9, 38, 39). Therefore, we performed in vitro ACS assays using ω-OH C30:0 FA as a substrate and membrane fractions prepared from HEK 293T cells overexpressing human FATP4 as enzyme source. Overexpression of FATP4 caused a 10.5-fold increase in ACS activity compared with control (Fig. 6). Furthermore, overexpression of FATP4 increased ACS activities toward C24:0 and C24:1 FAs by 6.6- and 3.5-fold, respectively. It is worthwhile to note that substrate preference for FATP4 among FA substrates cannot be evaluated by this method, since the water solubility of the substrate FAs, which affects the enzyme activity, and the ionization efficiency of the product acyl-CoAs are different. Our results indicate that FATP4 can indeed produce ω-OH ULC acyl-CoAs, which are required for acylceramide synthesis. In addition, our findings reveal that FATP4 is active toward a wide range of FAs from LC to ULC, regardless of nonhydroxylated or ω-hydroxylated status.
Fig. 6.
ACS activity of FATP4 toward ω-OH ULCFA. HEK 293T cells were transfected with vector (pCE-puro 3×FLAG-1) or a plasmid encoding 3xFLAG-FATP4 (pCE-puro 3×FLAG-FATP4), and membrane fractions were prepared 24 h after transfection. Membrane fractions (20 μg) were incubated with 10 μM FA (ω-OH C30:0 FA, C24:0 FA, or C24:1 FA) and 0.5 mM CoA, 2.4 mM ATP at 37 °C for 1 h. The generated acyl-CoAs were extracted and quantified by LC-MS/MS. Values represent the means ± SD obtained from three independently prepared samples. Statistically significant differences are indicated (two-tailed Student’s t test; **P < 0.01).
FATP4 Knockdown in Human Keratinocytes Decreases Acylceramide Production.
We next performed knockdown analyses to investigate whether FATP4 is involved in acylceramide production in human keratinocytes. Human keratinocytes were infected with lentiviral plasmids to express an shRNA targeting FATP4, and they then differentiated. Two shRNAs targeting different FATP4 sequences (shFATP4-1 and shFATP4-2) both showed a knockdown efficiency of over 90% (Fig. 7A). These FATP4 shRNAs reduced the amounts of all C30–36 acylceramide species, and they were decreased overall by ∼40% compared to the control (Fig. 7 B and C). On the other hand, the shRNAs did not affect the amounts of nonacylated ceramides (Fig. 7D). These results indicate that FATP4 is also involved in acylceramide production in human keratinocytes.
Fig. 7.
Reduction in acylceramide levels with FATP4 knockdown in human keratinocytes. Human keratinocytes were infected with lentiviral particles expressing control shRNA, shFATP4-1, or shFATP4-2, and then differentiation was induced for 7 d. (A) Total RNA was prepared, and real-time quantitative RT-PCR was performed for GAPDH and FATP4. Values represent the means ± SD obtained from three independent experiments and indicate the expression levels relative to GAPDH. (B and C) Lipids were extracted, and acylceramides (B and C) and (nonacylated) ceramides (D) were quantified by LC-MS/MS. The amount of each acylceramide species (B), total amount of acylceramides (C), and total amount of ceramides (D) are shown. Values represent the means ± SD obtained from three independent experiments. Statistically significant differences are indicated (Dunnett’s test; *P < 0.05; **P < 0.01). sh-1, shFATP4-1; sh-2, shFATP4-2.
Discussion
Acylceramide is a lipid that is essential for skin barrier formation, and the abrogation of its production causes ichthyosis in humans and neonatal lethal barrier defects in mice (1, 8, 17–24). However, not all genes involved in the production of acylceramide have been identified to date. Based on our previous finding that the substrates of the FA ω-hydroxylase CYP4F22 are ULCFAs (26), we speculate that the acylceramide synthesis pathway includes three successive reactions: ULC acyl-CoAs to ULCFAs by a thioesterase, ULCFAs to ω-OH ULCFAs by the FA ω-hydroxylase CYP4F22, and ω-OH ULCFAs to ω-OH ULC acyl-CoAs by an ACS (SI Appendix, Fig. S1). Of these, the thioesterase and ACS remained to be identified. In the present study, we found that acylceramide levels in Fatp4 KO mice are reduced to ∼10% of those in WT mice (Fig. 3C). Given this large decrease in acylceramide levels, it is reasonable to conclude that Fatp4 is directly involved in acylceramide production. We also confirmed the involvement of FATP4 in acylceramide production using human keratinocytes and knockdown analyses (Fig. 7). Furthermore, these results support the validity of our model, which assumes that ACS is involved in the acylceramide synthesis pathway. While the thioesterase(s) has not yet been identified, members of the ACOT (acyl-CoA thioesterase) family are considered candidates. Mammals have 15 ACOTs (ACOT1–13, THEM4, and THEM5), and it is possible that some of these redundantly function in acylceramide synthesis.
Several previous studies have generated Fatp4 mutant mice and reported the presence of severe skin barrier abnormalities (10, 11, 13, 14). However, the mechanism that causes the abnormalities has remained unknown. One hypothesis as to the cause of the skin barrier dysfunction is the loss of FA transporter activity by Fatp4 (4, 11); another is changes in (nonacylated) ceramide metabolism (10). In recent years, many acylceramide-related genes have been identified, and the respective KO mice were created and analyzed. These mice exhibited severe skin barrier abnormalities that resemble those observed in Fatp4 mutant mice (19–24). This phenotypic resemblance led to our hypothesis that the impaired skin barrier formation observed in IPS patients and Fatp4 mutant mice is caused by a defect in acylceramide synthesis. In the present study, we provide proof for this notion (Fig. 3C). With respect to the reduced FA uptake hypothesis, we found that the amounts of linoleic acid and linoleic acid-containing TAGs were not decreased but rather increased in the epidermis of Fatp4 KO mice (Fig. 4 B and C), contradicting the hypothesis. With respect to changes in (nonacylated) ceramide metabolism, the decreases in ≥C26 ceramides and increases in ≤C24 ceramides (10) (Fig. 4A) in Fatp4 KO mice likely partly contribute to the skin barrier dysfunction. However, the effect of Fatp4 disruption on the amounts of acylceramide was much greater than on ceramides. Furthermore, considering the severe skin barrier abnormality in Pnpla1 KO mice in which only the amounts of acylceramides (but not of ceramides) are decreased (23, 24), it is very possible that the decreases in acylceramide amounts are mainly responsible for the observed skin barrier dysfunction.
The FA elongase Elovl1 plays important roles in the elongation of saturated VLC acyl-CoA, especially of C24:0-CoA to C26:0-CoA, although its contribution to the elongation of monounsaturated VLC acyl-CoAs is lower (21, 40). Expression levels of Elovl1 were decreased in Fatp4 KO mouse epidermis (Fig. 5C). Therefore, we speculate that this decrease in Elovl1 expression causes the changes in ceramide metabolism—i.e., the decreases in ≥C26 saturated ceramides and increases in ≤C24 saturated ceramides—as observed in the epidermis of Elovl1 KO mice (21). The exact cause for the decreased Elovl1 expression is unknown. However, it is likely that impaired skin barrier formation due to reduced acylceramide production indirectly leads to expressional changes in various genes including Elovl1 (SI Appendix, Fig. S3A). Decreased expression of Elovl1 has also been observed in KO mice of Nipal4 (another ichthyosis-causative gene) (41).
The expression levels of Pnpla1 were also reduced in Fatp4 KO mice (Fig. 5B). Pnpla1 catalyzes the final step of acylceramide production, the conversion of ω-OH ceramide to acylceramide (27). The decreased expression of Pnpla1 in Fatp4 KO mice may be partly responsible for reduced acylceramide production. The monounsaturated ω-OH ceramide was unexpectedly not reduced in Fatp4 KO mice (Fig. 3F). We speculate that two effects—reduced production due to Fatp4 deficiency and substrate accumulation due to decreased expression of Pnpla1—acted in a mutually countervailing manner here.
The reaction products of Fatp4, ω-OH ULC acyl-CoAs, are rapidly metabolized to ω-OH ceramides, acylceramides, acyl-glucosylceramides, and protein-bound ceramides (Fig. 3G and SI Appendix, Fig. S1). Since ω-OH ULC acyl-CoAs are transient metabolic intermediates, the sum of the ω-OH ULC acyl-CoA metabolites, rather than the amounts of ω-OH ULC acyl-CoAs themselves, may represent the actual amounts of ω-OH ULC acyl-CoAs produced by Fatp4 (and other Fatp/Acsvl subfamily members having redundant functions). The sum of saturated ω-OH ULC acyl-CoA metabolites was reduced to ∼1/14 by Fatp4 disruption (Fig. 3H). We consider this to be caused by the combined effects of the decrease in the conversion step of ω-OH ULCFAs to ω-OH ULC acyl-CoAs due to Fatp4 disruption, and the decreased supply of ULC acyl-CoAs due to reduced expression levels of Elovl1 (SI Appendix, Fig. S3B). However, it is difficult to estimate the degree of contribution of the Fatp4 disruption. On the other hand, since the contribution of Elovl1 to the elongation of the monounsaturated VLCFAs is small, the decrease in the sum of monounsaturated ω-OH ULC acyl-CoA metabolites as well as total amount of monounsaturated acylceramides can be considered to be simply due to Fatp4 disruption (SI Appendix, Fig. S3B). Indeed, the amounts of nonacylated ceramides with ≥C30:1 were not reduced in Fatp4 KO mice compared with WT mice (Fig. 4A), indicating that the amounts of ≥C30:1 acyl-CoAs were not reduced by the Fatp4 disruption. In Fatp4 KO mice, the sum of monounsaturated ω-OH ULC acyl-CoA metabolites was reduced to ∼1/3 compared with WT mice (Fig. 3H). This implies that the contribution of Fatp4 to conversion of ω-OH ULCFAs to ω-OH ULC acyl-CoAs in the acylceramide production pathway is ∼2/3 of the total. This may even be an underestimate, considering that the expressions of other Fatp family members had tendencies to increase in Fatp4 KO mice (Fig. 5D). Among Fatp subfamily members, Fatp1 shares the highest amino acid sequence similarity with Fatp4, and forced expression of Fatp1 in the epidermis of Fatp4 KO mice can complement the skin barrier abnormalities (42).
In the present study, we elucidated one of the missing links in the acylceramide synthesis pathway. Furthermore, we revealed that the cause of IPS pathology is impaired acylceramide production, not reduction in FA uptake as assumed to date. Further research into the treatment of IPS with acylceramide or compounds with similar effects is thus required.
Methods
Extended details for all methods are available in SI Appendix, Materials and Methods.
Production and Breeding of Mice.
Fatp4 KO mice were generated by CRISPR-Cas9. Mice were housed at 23 ± 1 °C ambient temperature and at 50 ± 5% humidity with a 12-h light/dark cycle with food available ad libitum. All animal experiments were approved by the Institutional Animal Care and Use Committee of Hokkaido University and conducted in accordance with the institutional guidelines.
Skin Permeability Barrier Assay.
TEWL was measured on the dorsal skin of E18.5 mice as described previously (21), using a Vapo Scan AS-VT100RS evaporimeter (Asch Japan). Toluidine blue staining was performed by incubating E18.5 mice with methanol for 5 min, washing with phosphate buffered saline (PBS), and then immersing in 0.1% (wt/vol) toluidine blue in PBS solution at 4 °C for 2 h.
Histological Analyses.
Hematoxylin/eosin staining and transmission electron microscopy on E18.5 mouse skin were performed as described previously (43).
Plasmids.
A plasmid expressing 3×FLAG-tagged human FATP4 gene (pCE-puro 3×FLAG-FATP4) was constructed by cloning the FATP4 gene (39) into the BamHI–NotI site of the mammalian expression vector pCE-puro 3×FLAG-1 (44).
FATP4 Knockdown.
Lentiviral particles expressing a FATP4 shRNA were prepared as previously described (27).
Lipid Analyses.
LC-MS/MS analyses were performed as described previously (27). Separation of lipids by TLC using silica gel 60 (Merck) was conducted using two solvent systems. The solvent system for separation of ceramide and glucosylceramide species has been described previously (26); that for TAG, FA, and cholesterol species was hexane/diethyl ether/acetic acid (65:35:1, vol/vol). Lipids were detected by spraying a copper sulfate/phosphoric acid solution (3% [wt/vol] CuSO4 in 8% [vol/vol] aqueous phosphoric acid) onto TLC plates and heating at 180 °C for 10 min.
Statistical Analyses.
Data are presented as means ± SD. The significance of differences between groups was evaluated using nonpaired two-tailed Student’s t test or Dunnett’s test in Microsoft Excel or JMP13 (SAS Institute), respectively. A P value of <0.05 was considered significant.
Data Availability.
All data generated in this study are included in this published article and SI Appendix.
Supplementary Material
Acknowledgments
We thank Dr. Takayuki Sassa for technical support. This work was supported by funding from the Cosmetology Research Foundation (to A.K.); by the Advanced Research and Development Programs for Medical Innovation (Japan Agency for Medical Research and Development [AMED]–Core Research for Evolutional Science and Technology [CREST]) Grant JP19gm0910002 (to A.K.) from the AMED; and by KAKENHI Grants JP18H03976 (to A.K.), JP18H04664 (to A.K.), and JP15H05589 (to Y.O.) from the Japan Society for the Promotion of Science.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission. A.R.B. is a guest editor invited by the Editorial Board.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1917525117/-/DCSupplemental.
References
- 1.Oji V., et al. , Revised nomenclature and classification of inherited ichthyoses: Results of the First Ichthyosis Consensus Conference in Sorèze 2009. J. Am. Acad. Dermatol. 63, 607–641 (2010). [DOI] [PubMed] [Google Scholar]
- 2.Goleva E., Berdyshev E., Leung D. Y., Epithelial barrier repair and prevention of allergy. J. Clin. Invest. 129, 1463–1474 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klar J., et al. , Mutations in the fatty acid transport protein 4 gene cause the ichthyosis prematurity syndrome. Am. J. Hum. Genet. 85, 248–253 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khnykin D., Miner J. H., Jahnsen F., Role of fatty acid transporters in epidermis: Implications for health and disease. Dermatoendocrinol. 3, 53–61 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bueno E., et al. , Novel mutations in FATP4 gene in two families with ichthyosis prematurity syndrome. J. Eur. Acad. Dermatol. Venereol. 31, e11–e13 (2017). [DOI] [PubMed] [Google Scholar]
- 6.Bygum A., Westermark P., Brandrup F., Ichthyosis prematurity syndrome: A well-defined congenital ichthyosis subtype. J. Am. Acad. Dermatol. 59 (suppl. 5), S71–S74 (2008). [DOI] [PubMed] [Google Scholar]
- 7.Kihara A., Very long-chain fatty acids: Elongation, physiology and related disorders. J. Biochem. 152, 387–395 (2012). [DOI] [PubMed] [Google Scholar]
- 8.Kihara A., Synthesis and degradation pathways, functions, and pathology of ceramides and epidermal acylceramides. Prog. Lipid Res. 63, 50–69 (2016). [DOI] [PubMed] [Google Scholar]
- 9.Herrmann T., et al. , Mouse fatty acid transport protein 4 (FATP4): Characterization of the gene and functional assessment as a very long chain acyl-CoA synthetase. Gene 270, 31–40 (2001). [DOI] [PubMed] [Google Scholar]
- 10.Herrmann T., et al. , Mice with targeted disruption of the fatty acid transport protein 4 (Fatp 4, Slc27a4) gene show features of lethal restrictive dermopathy. J. Cell Biol. 161, 1105–1115 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moulson C. L., et al. , Cloning of wrinkle-free, a previously uncharacterized mouse mutation, reveals crucial roles for fatty acid transport protein 4 in skin and hair development. Proc. Natl. Acad. Sci. U.S.A. 100, 5274–5279 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gimeno R. E., et al. , Targeted deletion of fatty acid transport protein-4 results in early embryonic lethality. J. Biol. Chem. 278, 49512–49516 (2003). [DOI] [PubMed] [Google Scholar]
- 13.Lin M. H., Chang K. W., Lin S. C., Miner J. H., Epidermal hyperproliferation in mice lacking fatty acid transport protein 4 (FATP4) involves ectopic EGF receptor and STAT3 signaling. Dev. Biol. 344, 707–719 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tao J., et al. , A spontaneous Fatp4/Scl27a4 splice site mutation in a new murine model for congenital ichthyosis. PLoS One 7, e50634 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feingold K. R., Elias P. M., Role of lipids in the formation and maintenance of the cutaneous permeability barrier. Biochim. Biophys. Acta 1841, 280–294 (2014). [DOI] [PubMed] [Google Scholar]
- 16.Uchida Y., Holleran W. M., Omega-O-acylceramide, a lipid essential for mammalian survival. J. Dermatol. Sci. 51, 77–87 (2008). [DOI] [PubMed] [Google Scholar]
- 17.Hirabayashi T., Murakami M., Kihara A., The role of PNPLA1 in ω-O-acylceramide synthesis and skin barrier function. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1864, 869–879 (2019). [DOI] [PubMed] [Google Scholar]
- 18.Sugiura K., Akiyama M., Update on autosomal recessive congenital ichthyosis: mRNA analysis using hair samples is a powerful tool for genetic diagnosis. J. Dermatol. Sci. 79, 4–9 (2015). [DOI] [PubMed] [Google Scholar]
- 19.Vasireddy V., et al. , Loss of functional ELOVL4 depletes very long-chain fatty acids (≥C28) and the unique ω-O-acylceramides in skin leading to neonatal death. Hum. Mol. Genet. 16, 471–482 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jennemann R., et al. , Loss of ceramide synthase 3 causes lethal skin barrier disruption. Hum. Mol. Genet. 21, 586–608 (2012). [DOI] [PubMed] [Google Scholar]
- 21.Sassa T., et al. , Impaired epidermal permeability barrier in mice lacking Elovl1, the gene responsible for very-long-chain fatty acid production. Mol. Cell. Biol. 33, 2787–2796 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grond S., et al. , PNPLA1 deficiency in mice and humans leads to a defect in the synthesis of omega-O-acylceramides. J. Invest. Dermatol. 137, 394–402 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hirabayashi T., et al. , PNPLA1 has a crucial role in skin barrier function by directing acylceramide biosynthesis. Nat. Commun. 8, 14609 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pichery M., et al. , PNPLA1 defects in patients with autosomal recessive congenital ichthyosis and KO mice sustain PNPLA1 irreplaceable function in epidermal omega-O-acylceramide synthesis and skin permeability barrier. Hum. Mol. Genet. 26, 1787–1800 (2017). [DOI] [PubMed] [Google Scholar]
- 25.Ohno Y., et al. , ELOVL1 production of C24 acyl-CoAs is linked to C24 sphingolipid synthesis. Proc. Natl. Acad. Sci. U.S.A. 107, 18439–18444 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ohno Y., et al. , Essential role of the cytochrome P450 CYP4F22 in the production of acylceramide, the key lipid for skin permeability barrier formation. Proc. Natl. Acad. Sci. U.S.A. 112, 7707–7712 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ohno Y., Kamiyama N., Nakamichi S., Kihara A., PNPLA1 is a transacylase essential for the generation of the skin barrier lipid ω-O-acylceramide. Nat. Commun. 8, 14610 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lefèvre C., et al. , Mutations in a new cytochrome P450 gene in lamellar ichthyosis type 3. Hum. Mol. Genet. 15, 767–776 (2006). [DOI] [PubMed] [Google Scholar]
- 29.Sugiura K., et al. , Lamellar ichthyosis in a collodion baby caused by CYP4F22 mutations in a non-consanguineous family outside the Mediterranean. J. Dermatol. Sci. 72, 193–195 (2013). [DOI] [PubMed] [Google Scholar]
- 30.Hotz A., et al. , Mutation update for CYP4F22 variants associated with autosomal recessive congenital ichthyosis. Hum. Mutat. 39, 1305–1313 (2018). [DOI] [PubMed] [Google Scholar]
- 31.Watkins P. A., Maiguel D., Jia Z., Pevsner J., Evidence for 26 distinct acyl-coenzyme A synthetase genes in the human genome. J. Lipid Res. 48, 2736–2750 (2007). [DOI] [PubMed] [Google Scholar]
- 32.Candi E., Schmidt R., Melino G., The cornified envelope: A model of cell death in the skin. Nat. Rev. Mol. Cell Biol. 6, 328–340 (2005). [DOI] [PubMed] [Google Scholar]
- 33.Muñoz-Garcia A., Thomas C. P., Keeney D. S., Zheng Y., Brash A. R., The importance of the lipoxygenase-hepoxilin pathway in the mammalian epidermal barrier. Biochim. Biophys. Acta 1841, 401–408 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Elias P. M., et al. , Formation and functions of the corneocyte lipid envelope (CLE). Biochim. Biophys. Acta 1841, 314–318 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miyamoto M., Itoh N., Sawai M., Sassa T., Kihara A., Severe skin permeability barrier dysfunction in knockout mice deficient in a fatty acid ω-hydroxylase crucial to acylceramide production. J. Invest. Dermatol. 140, 319–326 (2020). [DOI] [PubMed] [Google Scholar]
- 36.Ohno Y., Nara A., Nakamichi S., Kihara A., Molecular mechanism of the ichthyosis pathology of Chanarin-Dorfman syndrome: Stimulation of PNPLA1-catalyzed ω-O-acylceramide production by ABHD5. J. Dermatol. Sci. 92, 245–253 (2018). [DOI] [PubMed] [Google Scholar]
- 37.Schmuth M., et al. , Differential expression of fatty acid transport proteins in epidermis and skin appendages. J. Invest. Dermatol. 125, 1174–1181 (2005). [DOI] [PubMed] [Google Scholar]
- 38.Nakahara K., et al. , The Sjögren-Larsson syndrome gene encodes a hexadecenal dehydrogenase of the sphingosine 1-phosphate degradation pathway. Mol. Cell 46, 461–471 (2012). [DOI] [PubMed] [Google Scholar]
- 39.Ohkuni A., Ohno Y., Kihara A., Identification of acyl-CoA synthetases involved in the mammalian sphingosine 1-phosphate metabolic pathway. Biochem. Biophys. Res. Commun. 442, 195–201 (2013). [DOI] [PubMed] [Google Scholar]
- 40.Sassa T., Tadaki M., Kiyonari H., Kihara A., Very long-chain tear film lipids produced by fatty acid elongase ELOVL1 prevent dry eye disease in mice. FASEB J. 32, 2966–2978 (2018). [DOI] [PubMed] [Google Scholar]
- 41.Honda Y., et al. , Decreased skin barrier lipid acylceramide and differentiation-dependent gene expression in ichthyosis gene Nipal4-knockout mice. J. Invest. Dermatol. 138, 741–749 (2018). [DOI] [PubMed] [Google Scholar]
- 42.Lin M. H., Miner J. H., Fatty acid transport protein 1 can compensate for fatty acid transport protein 4 in the developing mouse epidermis. J. Invest. Dermatol. 135, 462–470 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Naganuma T., et al. , Disruption of the Sjögren-Larsson syndrome gene Aldh3a2 in mice increases keratinocyte growth and retards skin barrier recovery. J. Biol. Chem. 291, 11676–11688 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kihara A., Anada Y., Igarashi Y., Mouse sphingosine kinase isoforms SPHK1a and SPHK1b differ in enzymatic traits including stability, localization, modification, and oligomerization. J. Biol. Chem. 281, 4532–4539 (2006). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated in this study are included in this published article and SI Appendix.