Significance
Increasing diversity within crops may be a powerful way to reduce agricultural declines from climate change. As such, it has garnered increasing attention, especially in documenting within-crop diversity through different cultivars or wild relatives. Yet, there are few tests of whether this diversity can mitigate losses with warming. Here, using European (predominantly French) databases to forecast winegrape phenology, we test if shifting cultivars changes predictions of future growing regions. We find that cultivar diversity halved potential losses of winegrowing regions under a 2 °C warming scenario and could reduce losses by a third if warming reaches 4 °C. Thus, diversity—if adopted by growers locally—can mitigate agricultural losses, but its effectiveness will depend on global decisions regarding future emissions.
Keywords: agrobiodiversity, resilience, phenology, agriculture, climate change adaptation
Abstract
Agrobiodiversity—the variation within agricultural plants, animals, and practices—is often suggested as a way to mitigate the negative impacts of climate change on crops [S. A. Wood et al., Trends Ecol. Evol. 30, 531–539 (2015)]. Recently, increasing research and attention has focused on exploiting the intraspecific genetic variation within a crop [Hajjar et al., Agric. Ecosyst. Environ. 123, 261–270 (2008)], despite few relevant tests of how this diversity modifies agricultural forecasts. Here, we quantify how intraspecific diversity, via cultivars, changes global projections of growing areas. We focus on a crop that spans diverse climates, has the necessary records, and is clearly impacted by climate change: winegrapes (predominantly Vitis vinifera subspecies vinifera). We draw on long-term French records to extrapolate globally for 11 cultivars (varieties) with high diversity in a key trait for climate change adaptation—phenology. We compared scenarios where growers shift to more climatically suitable cultivars as the climate warms or do not change cultivars. We find that cultivar diversity more than halved projected losses of current winegrowing areas under a 2 °C warming scenario, decreasing areas lost from 56 to 24%. These benefits are more muted at higher warming scenarios, reducing areas lost by a third at 4 °C (85% versus 58%). Our results support the potential of in situ shifting of cultivars to adapt agriculture to climate change—including in major winegrowing regions—as long as efforts to avoid higher warming scenarios are successful.
The potential adverse effects of climate change on agriculture, including shifts in growing areas, decreased yields, and crop failures (1–6), are a major concern to practitioners, policymakers, scientists, and consumers alike (7). Forecasts predict a future where regional climates will become increasingly mismatched with crops currently cultivated in those regions (e.g., ref. 8), unless there are large shifts in agricultural practices.
Practices that increase the resilience of agricultural regions would foster growing regions that can maintain normal processes and function—including in yields and quality—despite increases in stress or disturbance (9) from climate change. Research has especially focused on exploiting intraspecific crop diversity (10–15) because of its potential to increase resilience without requiring agricultural regions or the crops they grow to shift. As expansion of agriculture is one of the primary drivers of biodiversity loss, keeping agricultural regions in place and thereby preventing natural lands from being lost to new agricultural regions is a major international conservation goal (16, 17).
To increase resilience with climate change, intraspecific diversity must link to the traits most needed to adapt to future climate regimes (2). Such traits include a cultivar’s heat and drought tolerance and its phenology—the timing of recurring developmental stages, such as budburst and maturity. Variation in phenology may be a particularly important trait for developing agricultural systems resilient to climate change, as differences in cultivar phenology (e.g., an early versus late-ripening cultivar) can translate to very different climatic conditions during critical developmental phases, such as fruit maturation.
Given enough variation in traits—such as phenology—across cultivars, growers could select and plant cultivars suited to their current climate, then shift to more appropriate cultivars over time as the climate shifts, a process we refer to as “turnover.” Cultivar turnover is expected to increase the resilience of agricultural systems and thus lead to improved agricultural forecasts (18). Yet, this basic assumption, which underlies much of the current research, has rarely been tested.
Here, focusing on winegrapes (Vitis vinifera subspecies vinifera), we quantify how much in situ cultivar turnover affects forecasts of suitable growing regions with climate change. We selected winegrapes, given their high diversity and extensive records, which make testing the importance of intraspecific diversity to forecasts possible. Growers today plant over 1,100 different vinifera cultivars of winegrapes (19), called varieties, which are geographically and morphologically diverse. Different varieties possess important trait variation related to climate, including variation in phenology of 6 to 10 wk across varieties grown in the same climate (20).
Winegrape diversity is well documented, allowing us to combine winegrape phenology and global variety-level planting data with projections of daily temperature and precipitation from a large ensemble of a state-of-the-art climate model (Community Earth System Model [CESM]; SI Appendix, Fig. S5; ref. 21) to forecast climatic suitability of 11 globally planted varieties of winegrapes (Cabernet-Sauvignon, Chasselas, Chardonnay, Grenache, Merlot, Monastrell [synonym Mourvèdre], Pinot noir, Riesling, Sauvignon blanc, Syrah, and Ugni blanc). These varieties make up 35% of the area planted globally, reaching 64 to 87% in many important winegrowing countries (e.g., Australia, Chile, France, New Zealand, Switzerland, and the United States; ref. 22).
Our approach to model future winegrowing regions provides an important advance on previous efforts. Studies to date have generally ignored intraspecific diversity (forecasting only one or few varieties) and have used species-distribution models or simple linear phenological models, which fail to adequately include nonlinear developmental responses to temperature (23). Instead, our approach fits nonlinear process-based models for multiple varieties, which can predict expected phenological delays due to heat stress, and characterizes specific climatic conditions during maturation. Using predominantly French long-term phenology records (SI Appendix, Tables S1 and S2), we developed and validated models to forecast budbreak, flowering, and the onset of ripening (veraison) in each region for two warming scenarios, +2 °C and +4 °C, and a 0 °C reference scenario of no warming (SI Appendix, Fig. S5; see also Warming Scenarios for more details). Next, using global data on winegrape variety plantings (22), we predicted the climatic suitability of each region during the ensuing maturation stage—a period that controls whether a variety can be harvested in a particular region each year (24–26)—for our reference and warming scenarios.
To quantify the change (including gains and losses) in areas suitable for winegrowing, and resulting cultivar turnover, we compare our results relative to: 1) current winegrowing regions, and 2) areas identified as climatically suitable (estimated as supporting at least one of the 11 cultivars modeled to maturity in most model years) under our 0 °C reference scenario (Calculating Climatic Suitability).
Results
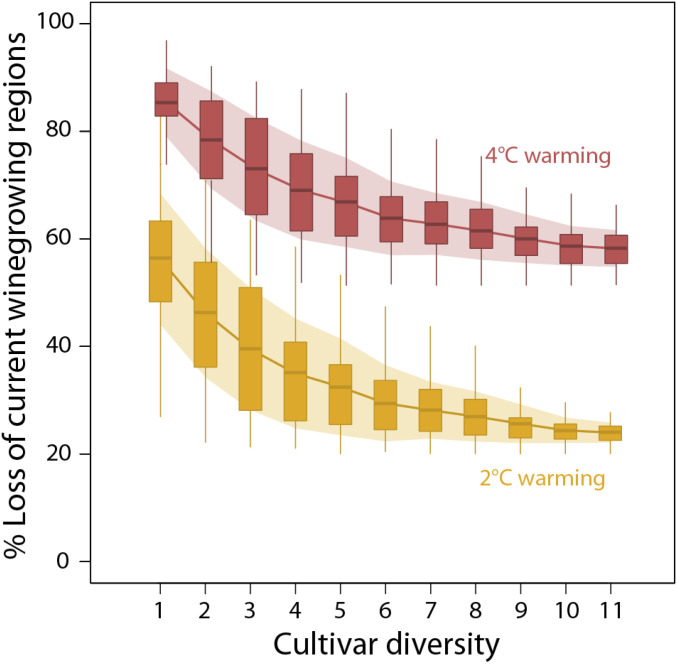
Without cultivar turnover, our results predict major global gains and losses in future winegrowing regions (Fig. 1). Under a 2 °C warming scenario, 51% of all areas we identified as climatically suitable for winegrowing under our 0 °C reference scenario would be lost. At 4 °C, losses reach 77% (SI Appendix, Fig. S14). Losses were higher when focusing only on regions that currently grow winegrapes: At 2 °C, 56% of current growing regions were lost; at 4 °C 85% were lost (Fig. 2).
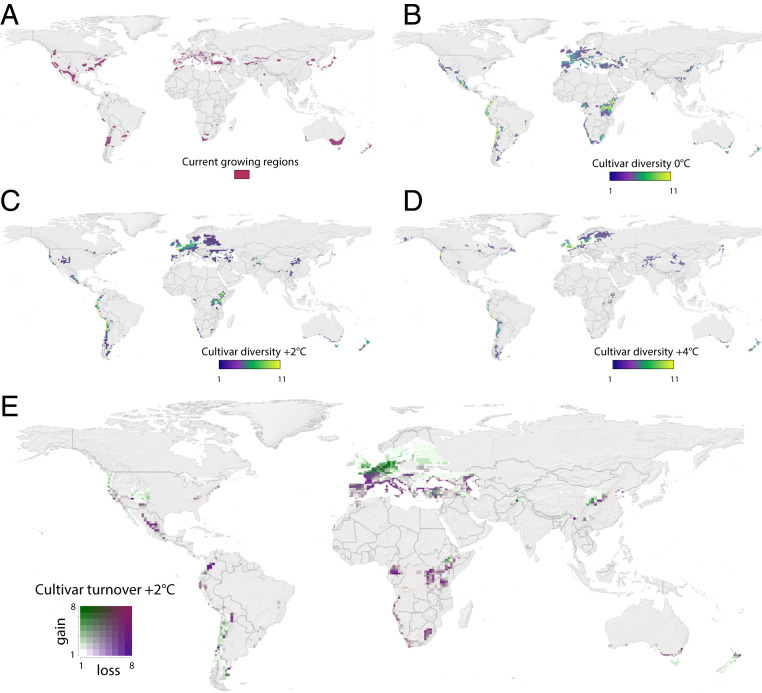
Fig. 1.
Global maps of winegrowing. (A) Current winegrowing regions (see SI Appendix, Fig. S1 for more detail). (B) Modeled predictions of cultivar diversity (total number of varieties) under our 0 °C reference scenario—all colored pixels show areas predicted as climatically suitable (Calculating Climatic Suitability). (C–E) Predicted effects of climate change on cultivar diversity and distribution under 2 °C warming (C), 4 °C warming (D), and cultivar turnover (E; cultivar gains and losses shown simultaneously via a bivariate color scale). See SI Appendix, Fig. S13 for 4 °C turnover.
Fig. 2.
Winegrape cultivar diversity can impact the loss of current winegrowing regions (see SI Appendix, Fig. S14 for losses within all climatically suitable areas). Predictions of loss are shown for scenarios of 2 °C warming (yellow bars) and 4 °C warming (red bars) relative to a 0 °C reference scenario. Shaded areas illustrate SD around the mean loss for each number of cultivars, combining two sources of uncertainty: 1) variability according to all possible combinations of n cultivars (e.g., at one cultivar, that cultivar could be any of the 11 considered and each covers a different area), and 2) modeled climatic suitability under each climate change scenario (e.g., one model member may predict suitability of an area, while another does not). These results are based on climatic suitability calculated with all eight climate variables (Modeling Maturity).
When we allowed turnover of cultivars, however, losses declined by up to 57% (Fig. 2). With cultivar turnover included under the 2 °C warming scenario, our models predicted a loss of 24% of current winegrowing regions (compared to 56% without cultivar turnover, yielding: (56 24)/56 = 57% decline in areas lost). Thus, exploiting cultivar diversity more than halved the potential losses with warming. Similar improvements were seen when considering all climatically suitable areas (losses declined by a quarter to 38% under the 2 °C warming scenario). However, the benefits of including cultivar diversity were muted at higher warming: Under the 4 °C scenario, loss of current winegrowing regions was 58%, including turnover, an improvement of 32% over predictions without cultivar turnover (considering all climatically suitable areas losses declined to 64%).
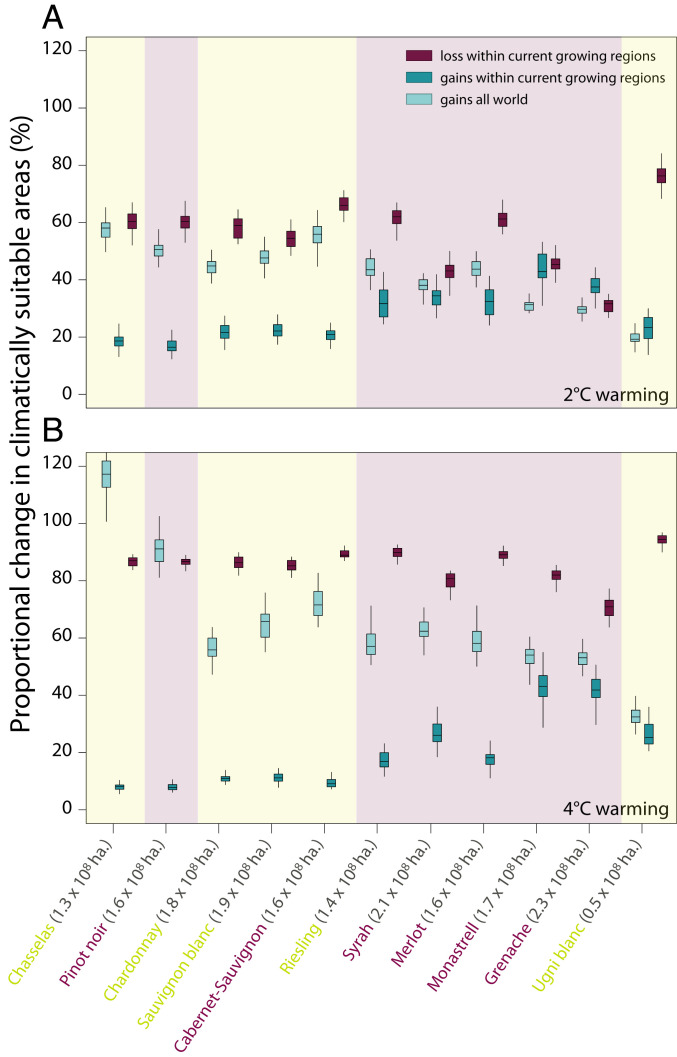
The importance of using different varieties to maintaining current winegrowing regions, versus expanding into new areas, was related to variation in phenology. Many later-ripening varieties—e.g. Grenache and Monastrell—were critical to maintaining current growing areas (Fig. 3), while early ripening varieties dominated new regions (Fig. 3); in particular, at 4 °C, Pinot noir and Chasselas showed large increases (Fig. 3B) as they moved far north into large Northern Hemisphere land masses (Fig. 1D).
Fig. 3.
Predicted gains (turquoise) and losses (purple) of climatically suitable growing areas for each of the 11 studied winegrape varieties (cultivars) under scenarios of 2 °C (A) and 4 °C (B) warming. To facilitate comparison of varieties and warming scenarios, we show gains and losses as the proportional change for each variety with warming (i.e., the area predicted with warming relative to the area predicted under our reference scenario of 0 °C). Gains are shown relative to both current winegrowing regions (darker turquoise) and all areas identified as climatically suitable under our reference scenario (pale turquoise). Background shading and variety name coloring differentiates red from white varieties; for each variety, we also give the total hectares predicted to be climatically suitable under our reference scenario of 0 °C within current winegrowing regions parenthetically. For a version of this figure showing gains and losses as absolute change in hectares, see SI Appendix, Fig. S23.
Geographical shifts were accompanied by phenological advances of veraison, which changes the timing of the ripening period for grapes and is important for winegrape quality potential (24–27). Early varieties—Pinot noir, Chasselas, and Riesling—advanced veraison date by over 15 d under a 4 °C warming scenario when averaged over all regions predicted as climatically suitable (SI Appendix, Fig. S12). This change, however, was smaller than shifts that could occur within regions if growers do not shift varieties. For example, at 4 °C warming, Pinot noir in the area including Burgundy was predicted to advance 24 d (SI Appendix, Fig. S11). Advances were similarly large for some later-ripening varieties (e.g., advance of 28 d for Cabernet-Sauvignon in the area including Bordeaux). However, other late-ripening varieties showed delays of up to 10 d with 4 °C warming (SI Appendix, Fig. S12). Such delays were generally caused by a nonlinear response to temperature: While higher temperatures usually accelerate phenology, too-high temperatures can produce heat stress and slow phenology (SI Appendix, Fig. S22 and ref. 28). Advances and delays in veraison date impacted the suitability of different cultivars by changing the timing, and, thus, climate, of the veraison-maturity window. These delays would likely impact wine quality, both through the high temperatures that produce the delays (29) and through later-season effects by changing the climate during maturity (24).
Losses of climatic suitability across regions and varieties were mainly due to shifting temperature regimes during the veraison-maturity window (SI Appendix, Figs. S16 and S17), particularly increases in minimum and maximum temperatures and greater accumulations of temperatures above 25 °C. In the 4 °C warming scenario, the number of days above 40 °C was also commonly related to the loss of climatic suitability. These results are in accordance with increasing evidence that higher temperatures can desynchronize the development of sugars, acid, and other berry components important to quality during ripening (29). Predicted shifts in precipitation (maximum and/or summed precipitation) were less frequently associated with a loss of suitability for winegrowing (SI Appendix, Figs. S16 and S17). Including precipitation in our predictions, however, did have an important moderating effect: Models that excluded precipitation variables yielded higher estimates of varieties lost and showed a reduced capacity of cultivar turnover to buffer regions from such losses (SI Appendix, Fig. S14 C and D). While our models integrate over diverse winegrowing regions, we expect that effects of precipitation may be particularly location-specific, as irrigation—which can decouple a region from local precipitation regimes—is prohibited in some winegrowing regions and widespread in others. Even in irrigated areas, however, irrigating vines my not be a sustainable practice due to increasingly scarce water resources.
Discussion
Our results show that cultivar diversity can decrease the loss of agricultural areas by over 50%—highlighting the critical role that human decisions play in building agricultural systems resilient to climate change. We show that cultivar turnover—if adopted by growers locally—can reduce the negative outcomes of climate change on winegrapes, with implications for other crops with high diversity. However, we also find that the benefits of cultivar turnover decline under greater warming. Without global efforts to reduce emissions sufficiently to stabilize temperatures at or below 2 °C, our results suggest that half of current global winegrowing regions would become climatically unsuitable for today’s major winegrapes.
These findings do not extend to all regions—in some regions, we find that cultivar diversity alone may not be enough to prevent declines. As seen in other studies (e.g., ref. 16), gains and losses of varieties are distributed unequally across the globe (Fig. 1), with warmer regions suffering the greatest losses and cooler regions seeing higher gains. Currently, even if growers exploit cultivar diversity, top-producing countries, particularly in Southern Europe, are predicted to sustain major declines of suitable winegrowing areas, with minimal gains (Fig. 1 C and D). For example, Spain and Italy are expected to lose 65% and 68%, respectively, of climatically suitable areas, under a 2 °C warming scenario (SI Appendix, Fig. S15), with gains of only 5% and 9% (respectively). France is projected to see balanced losses (22%) and gains (25%; SI Appendix, Fig. S15). In contrast, wine-producing regions in the Pacific Northwest (United States) or New Zealand expand in climatically suitable area for the latest-ripening varieties by 20 to 100% and 15 to 60%, respectively (SI Appendix, Fig. S15). Further, losses increase dramatically under a 4 °C warming scenario (SI Appendix and Figs. 1d and 2), while gains decrease. Losses at 4 °C are predicted to be particularly high in countries that are already warm; this includes losses reaching ∼90% in Spain and Italy (SI Appendix, Fig. S15).
For regions where our results suggest that cultivar diversity may be most critical, growers must choose to actively shift varieties—which requires overcoming legal and cultural hurdles. Currently, traditional practices, alongside regulations at local, regional, and higher levels, limit how much and where growers can shift varieties easily (19). This, coupled with other considerations, such as the temporal and related financial cost of replanting or regrafting a vineyard, may lead many growers to prefer alternative options that keep a particular variety tied to a region. For example, local management practices to reduce microclimatic temperatures or adjust the pace of development (e.g., shade cloth, reduced leaf area to fruit weight ratio, or longer-cycle rootstocks)—may help some growers (30–32), but generally work best for lower amounts of warming, especially compared to changing varieties.
Growers who want to exploit cultivar diversity would benefit from improved climate and crop-diversity data. For winegrapes, an immediate need is data on a greater number of varieties at a vineyard-relevant spatial scale. Our modeling approach requires projected climatic data at a high temporal resolution (e.g., simulated daily temperature values), which are only available at a low spatial resolution (e.g., circa 100 pixels), and thus cannot capture the unique microclimates of many vineyards. Our results could be expanded to finer spatial resolutions, given climate data downscaled with attention to the important climatic attributes of a particular viticultural region (e.g., coastal influences and/or cold air pools in complex terrains). Additionally, our approach requires sufficient phenological data, which we obtained for 11 varieties from a narrow geographical range (i.e., mainly France). These varieties span a diversity of phenologies (SI Appendix, Figs. S4 and S14E), yet they still represent less than 1% of known winegrape diversity, suggesting that benefits from cultivar diversity could be higher if more varieties were included.
Our results apply clearly to winegrowing regions, but have implications for many of the world’s agricultural regions. We focused on winegrapes given their diversity and extensive data resources: winegrape harvest dates are some of the longest written records on earth (33); major repositories collect, preserve, and document the crop’s diversity (20); and newly available data describe the planted geographic distribution of winegrape varieties across the globe (22). Such resources make winegrapes an excellent crop to test how intraspecific diversity may help agriculture adapt to a changing future, but many other crops also harbor high genotypic and phenotypic (e.g., morphological) diversity. Some of this diversity is obvious to consumers (e.g., historical and new cultivars of apples; ref. 13), while other diversity is hidden, present mainly in the wild or in research collections (e.g., banana and orange; refs. 34 and 35, respectively). Gathering sufficient data for tests similar to ours will be critical to identifying the full potential of cultivar turnover, but we expect that our results extend to many other crops, if growers have the flexibility and resources to shift in step with climate change.
Materials and Methods
Phenological Data for Parameterization and Validation.
We assembled historical data for 50% budbreak, 50% flowering, and 50% veraison dates from 62, mostly French, locations between 1956 and 2015 (see list of data sources in SI Appendix, Table S1). Most observations are for the 1995 to 2007 period and, secondarily, the 1975 to 1994 period. The dataset included 517 observations of budbreak, 757 observations of flowering, and 688 veraison observations. Requirements for phenological observations were as follows: Budbreak was identified as stage 4 on the modified Eichhorn and Lorenz (E-L) scale (36); flowering was identified as the 50% flowering date corresponding to stage 23 on the modified E-L scale; and veraison corresponded to stage 35 on the modified E-L scale, where 50% of berries softened or changed from green to translucent for white cultivars, or changed color for red cultivars. These data represent a portion of the original database collected in ref. 37, which was subsequently released for use within this project and includes matched local meteorological data. To conduct an independent validation of our phenological model predictions, we used further phenological observations from three locations in Europe and two in North America (SI Appendix, Table S2).
Viticulture Data—The Geography of Winegrowing.
To analyze climatic suitability in current winegrowing regions, we digitized the global distribution of the major winegrowing regions of the world according to a published atlas of winegrowing (38). We considered our results within this limited region (SI Appendix, Fig. S1) and, also, all areas that our models classified as suitable (see below).
Climate Data.
We used two different sources of climate data to build and validate our models. First, meteorological local data from weather stations (situated not more than 5 km away and not more than 100 m of difference in elevation from each vineyard), providing daily minimum and maximum temperatures (SI Appendix, Table S1). The average daily temperature was calculated as the arithmetic mean of the daily maximum and minimum temperature. Second, a gridded reconstruction of daily minimum and maximum land-surface air temperatures (Berkeley Earth Surface Temperatures, BEST; http://berkeleyearth.org/data/), based on 37,000 climate records spanning the period 1880 to 2013. We used both the BEST and local climate data to parameterize the phenological models and to test for any major parameter differences across the two datasets. We further used the BEST data to test our phenological models against validation datasets (see above in Phenological Data for Parameterization and validation), save for one site (Davis, CA), where BEST did not provide overlapping years and we instead used local station data (http://atm.ucdavis.edu/weather/uc-davis-weather-climate-station/). BEST data are very strongly correlated with weather-station data (r = 0.982; rms error [RMSE] = 1.829) and, where biased, tend to underestimate warming trends (39).
Warming Scenarios.
For our climate projections, we used output from the National Center for Atmospheric Research Large Ensemble (LENS; ref. 21). The LENS is a multimember ensemble of a single general circulation model (GCM), the CESM. Each member starts from its own unique initial condition in the atmosphere, and all members are simulated with the same scenario of historical climate forcings (1920 to 2005) and a high-emissions/high-warming scenario for the 21st century (2006 to 2100; Representative Concentration Pathway [RCP] 8.5). We chose this model and ensemble because it provided some of the highest spatial-temporal resolution output available from climate models (∼ 1° latitude/longitude, daily projections), similar to the resolution of the BEST data on which our models were ultimately calibrated.
The LENS ensemble is also well-designed for our warming threshold approach, allowing us to sample a large number of model-years (300) at different warming levels above preindustrial temperatures to force our phenology models: +2 °C (2039 to 2048) and +4 °C (2076 to 2085), in addition to a 0 °C (1970 to 1979) reference scenario that corresponds to a recent period where the temperature was the same as our preindustrial baseline (SI Appendix, Fig. S5). The median estimates for the +2 °C/+4 °C warming scenarios are +2.03/+3.99 °C, with interquartile ranges of 1.99 to 2.06 °C and 3.97 to 4.04 °C, respectively. We used this temperature-based approach because we believe that it is easier to interpret and link to current global agreements on climate change and that it provides relevant information on potential losses and gains of climatic suitability for winegrowing at different plausible future warming levels, without tying those predictions to any time horizon in the future. Our projections, therefore, do not depend on any singular future greenhouse-gas forcing scenario and are not intended to; however, we note that the +2 °C and +4 °C temperature thresholds we use in this study correspond closely with the broad warming estimates (relative to preindustrial) in surface air temperature for the end of the 21st century found for the RCP 4.5 (+2.55 K) and RCP 8.5 (+4.39 K) scenarios (40).
Data and Code Availability.
Raw data were generated at several large-scale facilities (see SI Appendix for details). Derived data supporting the findings of this study are available from the corresponding author upon request. Phenological parameterization and cross-validation was implemented with the software PMP (Version 5.0; ref. 41). All other analyses utilized custom computer R code, freely available at GitHub, https://github.com/MoralesCastilla/PhenoDiversity (42).
Phenological Modeling.
We modeled winegrape phenology for each of the 11 varieties according to a phenological process-based sequential model, considering only pixels where each 10-y scenario had no more than 2 d below −20 °C or 1 d below −30 °C, which represent lower lethal temperatures for most winegrape buds (43, 44). Our approach combined model estimates of three key stages of grapevine development: budbreak, flowering, and veraison. After comparison against alternative models (SI Appendix, Table S3), the budbreak stage was simulated by combining the Smoothed-Utah model (45, 46), to simulate dormancy break (accumulating chilling units), and the Wang and Engel model (47), to simulate the postdormancy phase until budbreak. Then, the Wang and Engel model was also used to simulate the accumulation of forcing units until flowering and from flowering until veraison. The curvilinear structure of both the Smoothed-Utah and Wang and Engel models reproduce the known effect of a developmental slowdown at high temperatures (48). We fixed the minimum- and maximum-temperature thresholds for development at 0 and 40 °C (respectively), based on physiological thresholds well established in the literature of winegrapes (49, 50) and other species (51, 52). We calculated a single optimum temperature for each phenological stage and parameterized the chilling and forcing units required to reach each stage for each variety. See SI Appendix for equations and further details, including a discussion of our 40 °C maximum.
To evaluate model accuracy and performance, we calculated 1) the RMSE as described by ref. 53 and 2) model efficiency; and 3) we performed a leave-one-out cross-validation calculating RMSE of prediction (RMSEP) as described by ref. 54 and 4) residual prediction deviation (RPD), which can increase comparability of metrics such as RMSEP (55).
Following this validation, we further validated our phenological models against independent observations of winegrape phenology recorded at other geographical locations (e.g., Germany, Portugal, Serbia, and the United States; SI Appendix, Tables S2 and S8). Our analyses show good accuracy of the fitted phenological models to other regions (SI Appendix, Figs. S6–S9).
Modeling Maturity.
We modeled the veraison to harvest phenophase using bioclimatic envelope models (56, 57) based on a suite of bioclimatic variables and the recorded climatic conditions experienced by each variety under pre-climate change conditions. We selected eight bioclimatic variables relevant to winegrape ripening (but necessarily excluded some potentially relevant variables, see also SI Appendix, Diurnal Temperature Range); more details on the selection of these variables and references are provided in SI Appendix, Modeling Maturity. Bioclimatic envelope models are often used to characterize the climatic niche or climatic conditions under which a studied species can survive. We chose bioclimatic envelope models over alternative algorithms—e.g., MaxEnt (58) or Random Forests (59)—because they allow for direct traceability of which climatic variables are responsible for changes in suitability, leading to either gains or losses as climate changes (SI Appendix, Figs. S16 and S17), which was a goal of our analysis.
We fitted these models according to the climatic conditions recorded during the veraison-harvest temporal window over a 30-y normal period (1950 to 1980), within existing winegrowing regions where each of the 11 modeled varieties is cultivated (22). We chose a 30-y normal period for fitting our models to capture a period before significant anthropogenic warming with robust global climate data; this period is longer than our reference scenario (0 °C), as a longer time series allows us to better characterize the climate envelope for each winegrape variety.
Calculating Climatic Suitability.
Our estimates of climatic suitability are the outcome of a multistep process, characterized, first, by sequential phenological models (explained above and in detail in SI Appendix), ensuring that veraison occurs within adequate dates—e.g., prior to October 1 in the Northern Hemisphere (this cutoff had a negligible effect on suitability; SI Appendix, Fig. S17); and, second, by comparison of the bioclimatic envelope forecasted under climate change with the envelope fitted during the 30-y normal period (1950 to 1979) for each variety (explained in detail above). Then, third, we considered the percentage of model-years (using 30 members from the CESM GCM, with 10 y each compiled into 300 model-years) for each grid cell and warming scenario where our models predicted suitability for a given variety (see also SI Appendix, Fig. S11). All figures and calculations—unless otherwise noted—used a cutoff of 75% or more of model-years predicting that a grid cell is climatically suitable for a given variety. Thus, some regions we suggest as climatically suitable may be suitable in only some years, and other regions we suggest are not suitable may be suitable, but in fewer model-years than this cutoff.
Our modeled estimates of climatically suitable winegrowing areas under our reference scenario overlap substantially with current regions (76%), but do not capture all growing regions, and include areas where winegrapes are not currently grown. This is expected given our modeling framework (e.g., we consider only 11 varieties) and its assumption that not all potential growing areas will be—or are currently—exploited.
Supplementary Material
Acknowledgments
We thank C. Marchal and S. Dedet, who helped with data from the Institut National de la Recherche Agronomique Domaine de Vassal Grape Repository; T. J. Davies, A. K. Ettinger, and two anonymous reviewers for comments that improved the manuscript; and all those who shared data. We thank Harvard Research Computing for computing facilities. I.M.-C. acknowledges funding from a postdoctoral fellowship by University of Alcalá and from the Spanish Ministry of Science and Innovation (Grant CGL2017-86926-P to M. Á. Rodríguez).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
Data deposition: Analyses other than phenological parameterization and cross-validation utilized custom computer R code, freely available at GitHub, https://github.com/MoralesCastilla/PhenoDiversity.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1906731117/-/DCSupplemental.
Change History
November 9, 2020: The SI Appendix has been updated to coincide with a formal Correction.
References
- 1.Food and Agriculture Organization of the United Nations , “The contribution of plant genetic resources for food and agriculture to food security and sustainable agricultural development” in The Second Report on the State of the World’s Plant Genetic Resources for Food and Agriculture (Food and Agriculture Organization of the United Nations, Rome, Italy: 2010), pp. 182–201; http://www.fao.org/docrep/013/i1500e/i1500e08.pdf. [Google Scholar]
- 2.Wood S. A., et al. , Functional traits in agriculture: Agrobiodiversity and ecosystem services. Trends Ecol. Evol. 30, 531–539 (2015). [DOI] [PubMed] [Google Scholar]
- 3.Hajjar R., Jarvis D. I., Gemmill-Herren B., The utility of crop genetic diversity in maintaining ecosystem services. Agric. Ecosyst. Environ. 123, 261–270 (2008). [Google Scholar]
- 4.Godfray H. C. J., et al. , Food security: The challenge of feeding 9 billion people. Science 327, 812–818 (2010). [DOI] [PubMed] [Google Scholar]
- 5.Lobell D. B., et al. , Prioritizing climate change adaptation needs for food security in 2030. Science 319, 607–610 (2008). [DOI] [PubMed] [Google Scholar]
- 6.Porter J. R., et al. , “Food security and food production systems” in Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part A: Global and Sectoral Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change (Intergovernmental Panel on Climate Change, Geneva, Switzerland, 2014), pp. 485–533 [Google Scholar]
- 7.Howden S. M., et al. , Adapting agriculture to climate change. Proc. Natl. Acad. Sci. U.S.A. 104, 19691–19696 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trnka M., et al. , Adverse weather conditions for European wheat production will become more frequent with climate change. Nat. Clim. Change 4, 637–643 (2014). [Google Scholar]
- 9.Folke C., et al. , Regime shifts, resilience, and biodiversity in ecosystem management. Annu. Rev. Ecol. Evol. Systemat. 35, 557–581 (2004). [Google Scholar]
- 10.Duchêne E., Huard F., Dumas V., Schneider C., Merdinoglu D., The challenge of adapting grapevine varieties to climate change. Clim. Res. 41, 193–204 (2010). [Google Scholar]
- 11.Himanen S. J., et al. , Cultivar diversity has great potential to increase yield of feed barley. Agron. Sustain. Dev. 33, 519–530 (2013). [Google Scholar]
- 12.Lopes M. S., et al. , Exploiting genetic diversity from landraces in wheat breeding for adaptation to climate change. J. Exp. Bot. 66, 3477–3486 (2015). [DOI] [PubMed] [Google Scholar]
- 13.Myles S., Improving fruit and wine: What does genomics have to offer? Trends Genet. 29, 190–196 (2013). [DOI] [PubMed] [Google Scholar]
- 14.Kahiluoto H., et al. , Cultivating resilience by empirically revealing response diversity. Global Environ. Change Hum. Policy Dimens. 25, 186–193 (2014). [Google Scholar]
- 15.Parent B., et al. , Maize yields over Europe may increase in spite of climate change, with an appropriate use of the genetic variability of flowering time. Proc. Natl. Acad. Sci. U.S.A. 115, 10642–10647 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hannah L., et al. , Climate change, wine, and conservation. Proc. Natl. Acad. Sci. U.S.A. 110, 6907–6912 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pecl G. T., et al. , Biodiversity redistribution under climate change: Impacts on ecosystems and human well-being. Science 355, eaai9214 (2017). [DOI] [PubMed] [Google Scholar]
- 18.Lin B. B. Resilience in agriculture through crop diversification: Adaptive management for environmental change. BioScience 61, 183–193 (2011). [Google Scholar]
- 19.Wolkovich E. M., García de Cortázar-Atauri I., Morales-Castilla I., Nicholas K. A., Lacombe T., From Pinot to Xinomavro in the world’s future winegrowing regions. Nat. Clim. Change 8, 29–37 (2018). [Google Scholar]
- 20.Boursiquot J. M., Dessup M., Rennes C., Distribution des principaux caractères phénologiques, agronomiques et technologiques chez Vitis vinifera L. Vitis, 34, 31–35 (1995). [Google Scholar]
- 21.Kay J. E., et al. , The Community Earth System Model (CESM) large ensemble project a community resource for studying climate change in the presence of internal climate variability. Bull. Am. Meteorol. Soc. 96, 1333–1349 (2015). [Google Scholar]
- 22.Anderson K., Aryal N. R., Which Winegrape Varieties Are Grown Where? A Global Empirical Picture (University of Adelaide Press, Adelaide, Australia, 2015). [Google Scholar]
- 23.García de Cortázar-Atauri I., Brisson N., Gaudillere J. P., Performance of several models for predicting budburst date of grapevine (Vitis vinifera L.). Int. J. Biometeorol. 53, 317–326 (2009). [DOI] [PubMed] [Google Scholar]
- 24.Jones G. V., White M. A., Cooper O. R., Storchmann K., Climate change and global wine quality. Clim. Change 73, 319–343 (2005). [Google Scholar]
- 25.Webb L. B., Whetton P. H., Barlow E. W. R., Climate change and winegrape quality in Australia. Clim. Res. 36, 99–111 (2008). [Google Scholar]
- 26.Ashenfelter O., Storchmann K., Climate change and wine: A review of the economic implications. J. Wine Econ. 11, 105–138 (2016). [Google Scholar]
- 27.van Leeuwen C, Seguin G., The concept of terroir in viticulture. J. Wine Res. 17, 1–10 (2006). [Google Scholar]
- 28.Wang E., et al. , The uncertainty of crop yield projections is reduced by improved temperature response functions. Nat. Plants 3, 17102 (2017). [DOI] [PubMed] [Google Scholar]
- 29.Rienth M., et al. , Temperature desynchronizes sugar and organic acid metabolism in ripening grapevine fruits and remodels their transcriptome. BMC Plant Biol. 16, 164 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nicholas K. A., Durham W. H., Farm-scale adaptation and vulnerability to environmental stresses: Insights from winegrowing in Northern California. Global Environ. Change Hum. Policy Dimens. 22, 483–494 (2012). [Google Scholar]
- 31.Parker A. K., Hofmann R. W., van Leeuwen C., McLachlan A. R. G., Trought M. C. T., Leaf area to fruit mass ratio determines the time of veraison in Sauvignon Blanc and Pinot Noir grapevines. Aust. J. Grape Wine Res. 20, 422–431 (2014). [Google Scholar]
- 32.van Leeuwen C., Destrac-Irvine A., Modified grape composition under climate change conditions requires adaptations in the vineyard. OENO One 51, 147–154 (2017). [Google Scholar]
- 33.Daux V., et al. , An open-access database of grape harvest dates for climate research: Data description and quality assessment. Clim. Past 8, 1403–1418 (2012). [Google Scholar]
- 34.Arias P., Dankers C., Pascal L., Pilkauskas P., The World Banana Economy: 1985-2002 (Food and Agriculture Organization of the United Nations, Rome, Italy, 2003). [Google Scholar]
- 35.Wu G. A., Sequencing of diverse Mandarin, pummelo and orange genomes reveals complex history of admixture during citrus domestication. Nat. Biotechnol. 32, 656–662 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coombe B. G., Dry P. R., Practices (Viniculture, Winetitles, Adelaide, Australia, 1992), vol. 2. [Google Scholar]
- 37.Parker A. K., García De Cortázar-Atauri I., van Leeuwen C., Chuine I., General phenological model to characterise the timing of flowering and veraison of Vitis vinifera L. Aust. J. Grape Wine Res. 17, 206–216 (2011). [Google Scholar]
- 38.Clarke O., Oz Clarke Wine Atlas: Wines and Wine Regions of the World (Anova Books, London, UK, 2007). [Google Scholar]
- 39.Way R. G., Oliva F., Viau A. E., Underestimated warming of northern Canada in the Berkeley Earth temperature product. Int. J. Climatol. 37, 1746–1757 (2017). [Google Scholar]
- 40.Schurer A. P., Mann M. E., Hawkins E., Tett S. F. B., Hegerl G. C., Importance of the pre-industrial baseline for likelihood of exceeding Paris goals. Nat. Clim. Change 7, 563–567 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chuine I., García de Cortázar-Atauri I., Kramer K., Hänninen H., “Plant development models” in Phenology: An Integrative Environmental Science, Schwartz M., Ed. (Springer, Berlin, Germany, 2013), pp. 275–293. [Google Scholar]
- 42.Morales-Castilla I., Wolkovich E. M., Forecasting phenology and climatic suitability of crop cultivar diversity. GitHub. https://github.com/MoralesCastilla/PhenoDiversity. Deposited 3 January 2020.
- 43.Mills L. J., Ferguson J. C., Keller M., Cold-hardiness evaluation of grapevine buds and cane tissues. Am. J. Enol. Vitic. 57, 194–200 (2006). [Google Scholar]
- 44.Davenport J. R., Keller M., Mills L. J., How cold can you go? Frost and winter protection for grape. HortScience 43, 1966–1969 (2008). [Google Scholar]
- 45.Richardson E. A., A model for estimating the completion of rest for ‘Redhaven’ and ‘Elberta’ peach trees. HortScience 9, 331–332 (1974). [Google Scholar]
- 46.Bonhomme M., Rageau R., Lacointe A., “Optimization of endodormancy release models, using series of endodormancy release data collected in France” in VIII International Symposium on Temperate Zone Fruits in the Tropics and Subtropics, Herter F. G., Leite G. B., do M., Raseira C. B., Eds. (Acta Horticulturae, International Society for Horticultural Science, Leuven, Belgium, 2010), vol. 872, pp. 51–60. [Google Scholar]
- 47.Wang E., Engel T., Simulation of phenological development of wheat crops. Agric. Syst. 58, 1–24 (1998). [Google Scholar]
- 48.García de Cortázar-Atauri I., Chuine I., Donatelli M., Parker A., van Leeuwen C., A curvilinear process-based phenological model to study impacts of climatic change on grapevine (Vitis vinifera L.). Proc. Agro 2010, 907–908 (2010). [Google Scholar]
- 49.Champagnol F., Éléments de Physiologie de la Vigne et de Viticulture Générale (Montpellier Imprimerie Dehan, Saint-Gely-du-Fesc, France, 1984). [Google Scholar]
- 50.Jones G. V., “Winegrape phenology” in Phenology: An Integrative Environmental Science, Schwartz M. D., Ed. (Springer, Dordrecht, the Netherlands, 2013), pp. 563–584. [Google Scholar]
- 51.Zaka S., et al. , How variable are non-linear developmental responses to temperature in two perennial forage species? Agric. For. Meteorol. 232, 433–442 (2017). [Google Scholar]
- 52.Parent B., Tardieu F., Temperature responses of developmental processes have not been affected by breeding in different ecological areas for 17 crop species. New Phytol. 194, 760–774 (2012). [DOI] [PubMed] [Google Scholar]
- 53.Janssen P. H. M., Heuberger P. S. C., Calibration of process-oriented models. Ecol. Model. 83, 55–66 (1995). [Google Scholar]
- 54.Wallach D., Evaluating Crop Models (Elsevier, Amsterdam, the Netherlands, 2006), pp. 6–37. [Google Scholar]
- 55.Luedeling E., chillR: Statistical methods for phenology analysis in temperate fruit trees. R package (Version 0.54, 2013). http://CRAN.R-project.org/package=chillR. Accessed 7 January 2020.
- 56.Pearson R. G., Dawson T. P., Predicting the impacts of climate change on the distribution of species: Are bioclimate envelope models useful? Glob. Ecol. Biogeogr. 12, 361–371 (2003). [Google Scholar]
- 57.Araújo M. B., Peterson A. T., Uses and misuses of bioclimatic envelope modeling. Ecology 93, 1527–1539 (2012). [DOI] [PubMed] [Google Scholar]
- 58.Phillips S. J., Dudík M., Modeling of species distributions with maxent: New extensions and a comprehensive evaluation. Ecography 31, 161–175 (2008). [Google Scholar]
- 59.Evans J. S., Murphy M. A., Holden Z. A., Cushman S. A., “Modeling species distribution and change using random forest” in Predictive Species and Habitat Modeling in Landscape Ecology, Drew C. A., Wiersma Y., Huettmann F., Eds. (Springer, Berlin, Germany, 2011) pp. 139–159. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw data were generated at several large-scale facilities (see SI Appendix for details). Derived data supporting the findings of this study are available from the corresponding author upon request. Phenological parameterization and cross-validation was implemented with the software PMP (Version 5.0; ref. 41). All other analyses utilized custom computer R code, freely available at GitHub, https://github.com/MoralesCastilla/PhenoDiversity (42).