Significance
The relevance and biological activities of extracellular vesicles (EVs) from gram-positive bacteria are poorly understood. We report that EVs released by Staphylococcus aureus are internalized by human macrophages by an endocytic process, highlighting the role of EVs as a delivery system for bacterial virulence determinants. Macrophages incubated with S. aureus EVs undergo NLRP3 inflammasome activation that is dependent on EV cargo, including pore-forming toxins and lipoproteins. We provide evidence that S. aureus lipoproteins modulate EV content and biogenesis, revealing a previously unrecognized role for lipoproteins. This study advances our understanding of the biological activities of EVs from gram-positive bacteria and demonstrates their role as a vehicle for the delivery of microbial effector molecules into host cells.
Keywords: Staphylococcus aureus, extracellular vesicles, inflammasomes, lipoproteins, pore-forming toxins
Abstract
Release of extracellular vesicles (EVs) is a common feature among eukaryotes, archaea, and bacteria. However, the biogenesis and downstream biological effects of EVs released from gram-positive bacteria remain poorly characterized. Here, we report that EVs purified from a community-associated methicillin-resistant Staphylococcus aureus strain were internalized into human macrophages in vitro and that this process was blocked by inhibition of the dynamin-dependent endocytic pathway. Human macrophages responded to S. aureus EVs by TLR2 signaling and activation of NLRP3 inflammasomes through K+ efflux, leading to the recruitment of ASC and activation of caspase-1. Cleavage of pro–interleukin (IL)-1β, pro-IL-18, and gasdermin-D by activated caspase-1 resulted in the cellular release of the mature cytokines IL-1β and IL-18 and induction of pyroptosis. Consistent with this result, a dose-dependent cytokine response was detected in the extracellular fluids of mice challenged intraperitoneally with S. aureus EVs. Pore-forming toxins associated with S. aureus EVs were critical for NLRP3-dependent caspase-1 activation of human macrophages, but not for TLR2 signaling. In contrast, EV-associated lipoproteins not only mediated TLR2 signaling to initiate the priming step of NLRP3 activation but also modulated EV biogenesis and the toxin content of EVs, resulting in alterations in IL-1β, IL-18, and caspase-1 activity. Collectively, our study describes mechanisms by which S. aureus EVs induce inflammasome activation and reveals an unexpected role of staphylococcal lipoproteins in EV biogenesis. EVs may serve as a novel secretory pathway for S. aureus to transport protected cargo in a concentrated form to host cells during infections to modulate cellular functions.
Staphylococcus aureus is a primary cause of invasive human infections, such as bacteremia, endocarditis, pneumonia, and surgical wound infections, leading to morbidity, mortality, and excessive healthcare costs (1). Many S. aureus isolates have developed resistance to commonly used antibiotics (2). To establish a successful infection and survive in a hostile host environment, S. aureus employs a wide array of virulence determinants, including surface proteins and glycopolymers, as well as secreted proteins, such as pore-forming toxins (PFTs), superantigens, and proteases. Such factors are either associated with the cell surface to facilitate colonization or secreted to the environment to damage host cells and evade innate and adaptive host immune mechanisms (3–5).
Extracellular vesicles (EVs) are nano-sized, spherical particles that are enclosed by a bilayered membrane. Most cells secrete vesicles, including eukaryotes, archaea, and bacteria (6). Generation of EVs from gram-positive bacteria is a complex and poorly understood process since EVs released from the cytoplasmic membrane must traverse a thick peptidoglycan cell wall to reach the external environment (7–9). We demonstrated that S. aureus alpha-type phenol-soluble modulins and autolysins promote EV production whereas highly cross-linked peptidoglycan presents a barrier for EV release (8). However, specific mechanisms involved in EV biogenesis and the complex downstream effects of EV release on host cells remain poorly characterized.
S. aureus EVs package a diverse array of bacterial components, including cytosolic, surface, and membrane proteins, as well as adhesins, lipoproteins, and secreted PFTs (8, 10–12), and many of these components have been shown to play significant roles in bacterial virulence (13–16). S. aureus EVs were detected in vivo during experimental pneumonia (10), and EVs purified from S. aureus strains have been shown to contain biologically active toxins, exhibit cytotoxicity, and elicit proinflammatory mediators (8, 10, 11, 17, 18). Thus, EVs may play a previously unrecognized role in staphylococcal pathogenesis although the mechanisms whereby this occurs are unknown.
NLRP3 inflammasomes, multimeric cytosolic protein complexes formed in myeloid cells in response to various pathogenic or physiological stimuli, require two distinct steps for activation (19). A bacterial stimulus such as lipoprotein or lipopolysaccharide (LPS) triggers a priming step through TLR2- or TLR4-mediated nuclear factor κB (NF-κB) signaling, resulting in the production of pro–interleukin (IL)-1β and pro-IL-18 and transcription and posttranslational modification of NLRP3. A second stimulus, such as bacterial toxins or adenosine 5′-triphosphate (ATP), activates the inflammasome through K+ efflux, leading to the recruitment of ASC and activation of caspase-1, resulting in cleavage of pro–IL-1β and pro–IL-18. NLRP3 activation is characterized by cellular release of the mature cytokines IL-1β and IL-18 and induction of an inflammatory cell death termed pyroptosis, a host defense mechanism allowing removal of damaged or infected host cells (20, 21).
Inflammasome activation plays an essential role in protection against S. aureus infections (22, 23), particularly in mounting an effective innate immune response, which may determine the outcome of infection by controlling the bacterial burden and shaping the nature and magnitude of the adaptive immune response (24). Unregulated inflammasome activation, however, may result in an exaggerated innate immune response that leads to host tissue damage (25). S. aureus culture supernatants, containing both secreted PFTs and lipoproteins, activate inflammasomes in vitro by providing both the priming and secondary stimulus (26, 27), but culture supernatants are not representative of the in vivo environment. Of note, S. aureus cells, purified PFTs, or lipoproteins alone are not sufficient to activate the NLRP3 inflammasome (27). We postulate that EVs serve as a unique S. aureus secretion system that transports its protected cargo, including lipoproteins and PFTs, to host cells.
In this study, we provide evidence that EVs released by S. aureus cells are internalized by human macrophages (MΦs) via an endocytic pathway and induce NLRP3 inflammasome-dependent pyroptosis and IL-1β and IL-18 production. We demonstrate that EV-associated PFTs play a critical role in NLRP3 activation through K+ efflux whereas EV lipoproteins prime inflammasome activation through TLR2 signaling but also modulate the biogenesis and PFT content of EVs. Our study provides critical insights into the role of staphylococcal lipoproteins in EV production and characterizes mechanisms by which S. aureus EVs induce MΦ inflammasome activation.
Results
EVs Are Internalized by Human Macrophages by an Endocytic Process.
Although numerous studies have reported the release of EVs from S. aureus, the interactions between EVs and host cells remain poorly characterized. We predicted that EVs were internalized within MΦs and that internalization played a critical role in modulating the host innate immune response. To test this hypothesis, we labeled JE2 EVs with the 3,3′-dioctadecyloxacarbocyanine perchlorate (DiO), a highly fluorescent lipophilic dye that has been widely used for tracing the internalization of membrane vesicles (28). The uptake of 2 µg/mL DiO-labeled EVs by differentiated MΦ-like THP-1 cells after different incubation time points was assessed by confocal microscopy. The fluorescence of EVs, but not the sham control (SI Appendix, Fig. S1A), was detected within THP-1 cells after 60 min (Fig. 1A), and longer incubation times resulted in a greater distribution of fluorescent EVs within cells, peaking after ∼90 min. To determine how cellular uptake of EVs occurred, MΦs were pretreated with inhibitors of clathrin-mediated endocytosis (chlorpromazine [CPZ]), lipid raft-mediated endocytosis (methyl-β-cyclodextrin [MβCD]), the solvent dimethyl sulfoxide (DMSO), or inhibitors of dynamin-dependent endocytosis (dynasore) or actin-dependent endocytosis/macropinocytosis (cytochalasin D) before incubation with DiO-labeled EVs. Pretreatment of THP-1 cells (Fig. 1B) or monocyte-derived human MΦs (Fig. 1C) with dynasore, but not CPZ, MβCD, DMSO, or cytochalasin D, inhibited cellular entry of EVs. Quantification of EV-positive THP-1 cells (Fig. 1D) or cellular fluorescence of monocyte-derived human MΦs (Fig. 1E) confirmed a significant (>threefold) reduction in the DiO-EV signal in MΦs treated with dynasore.
Fig. 1.
Dynamin-dependent endocytosis of S. aureus EVs by human MΦs. (A) Confocal micrographs of THP-1 cells incubated with 2 µg/mL DiO-labeled EVs for different time periods. (Scale bars: 10 µm.) (B) Confocal micrographs of THP-1 cells pretreated with or without indicated inhibitors for 1 h, followed by incubation for 90 min with 2 µg/mL DiO-labeled S. aureus EVs. (Scale bars: 10 µm.) (C) Confocal micrographs of human monocyte-derived MΦs pretreated with or without indicated inhibitors for 1 h, followed by incubation for 90 min with 5 µg/mL DiO-labeled S. aureus EVs. (Scale bars: 10 µm.) (D) Percentage of EV uptake by THP-1 cells treated with or without the indicated inhibitors (n = 10). (E) Relative EV uptake by monocyte-derived human MΦs treated with or without the indicated inhibitors (n = 35 to 60). Image data are representative of two independent experiments. Data from D and E were analyzed by one-way ANOVA with Dunnett’s multiple comparison test. NS, not significant; **P < 0.01, ****P < 0.0001.
To validate this result, we labeled JE2 EVs with the lipophilic dye octadecyl rhodamine B chloride (R18) (29); a sham-treated dye control was incubated without EVs. R18 fluorescence is quenched at high concentrations in the cell membrane and “dequenched” when the probe is diluted by membrane fusion. Thus, R18 fluorescence reflects an increase in EV internalization or membrane fusion. The uptake of 5 µg/mL R18-labeled EVs by human monocyte-derived MΦs after different incubation time points was assessed by confocal microscopy. Fluorescence of R18-labeled EVs, but not the sham control, was detected within MΦs after 45 min (SI Appendix, Fig. S1B), and longer incubation of cells with EVs led to a greater distribution of fluorescent EVs within cells. The sham-treated control showed minimal fluorescence after 90 min (SI Appendix, Fig. S1B). Consistent with the results obtained from the DiO-labeling experiment, pretreatment of THP-1 cells or monocyte-derived human MΦs with dynasore, but not the other inhibitors, prevented cellular entry of EVs (SI Appendix, Fig. S1C). Quantification of cellular fluorescence confirmed a significant (>fivefold) reduction in the R18-EV signal in MΦs treated with dynasore (SI Appendix, Fig. S1D). Subsequently, THP-1 MΦs were pretreated with endocytosis inhibitors or DMSO before incubation with R18-labeled EVs, and fluorescence was measured over time. THP-1 cells fluoresced within 40 min after the addition of R18-labeled EVs (SI Appendix, Fig. S1E). Pretreatment of THP-1 cells with dynasore, but not DMSO or CPZ, resulted in a significant reduction in the fluorescent signal at each time point (SI Appendix, Fig. S1E). Together, these data indicate that internalization of EVs in MΦs is primarily dependent upon dynamin-mediated endocytosis.
S. aureus EVs Activate Caspase-1 in Human Macrophages and Induce the Release of IL-1β and IL-18.
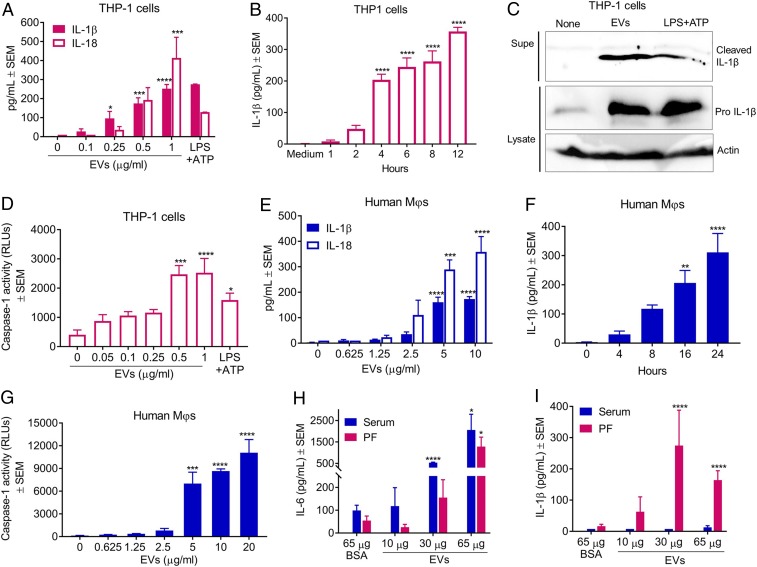
Previous studies demonstrated that S. aureus cutaneous infection induces IL-1β production by activation of NLRP3 inflammasomes in myeloid cells (22, 30). Furthermore, S. aureus EVs were shown to elicit skin barrier disruption in mice with characteristic atopic dermatitis-like skin inflammation (31). Because staphylococcal EV cargo includes both PFTs and lipoproteins (8), we postulated that EVs may play a critical role in inflammasome activation during infection. To test this hypothesis, THP-1 MΦs were incubated for 4 h with increasing concentrations of purified S. aureus JE2 EVs. As shown in Fig. 2A, EV concentrations ≥0.5 µg/mL resulted in cellular release of IL-1β and IL-18. In time course experiments, secretion of IL-1β by THP-1 MΦs was detected as soon as 4 h after treatment with 0.5 µg/mL EVs (Fig. 2B). To confirm proteolytic cleavage of the precursor pro–IL-1β, THP-1 MΦs were incubated with 0.8 µg/mL EVs for 12 h, and cleaved IL-1β from the culture supernatants and pro–IL-1β from cell lysates were detected by immunoblot. Cleaved IL-1β was induced by JE2 EVs or positive control LPS+ATP, but not by untreated cells (Fig. 2C). Because release of mature IL-1β and IL-18 depends on caspase-1–mediated cleavage of the precursor molecules, we measured caspase-1 activity in THP-1 supernatants. Activation of caspase-1 was consistently induced within 4 h by EV concentrations ≥0.5 µg/mL (Fig. 2D). Similar experiments performed with human monocyte-derived MΦs revealed that a higher concentration of EVs was necessary to induce secretion of IL-1β and IL-18 (Fig. 2E). Nonetheless, both IL-1β (Fig. 2F) and caspase-1 activity (Fig. 2G) were induced within 8 h after incubation of monocyte-derived MΦs with 5 µg/mL EVs.
Fig. 2.
Inflammasome activation in vitro and in vivo by S. aureus EVs. (A) Dose-dependent release of IL-1β and IL-18 in culture supernatants of THP-1 MΦs incubated for 4 h with purified EVs (n = 4). (B) Time course of IL-1β production by THP-1 MΦs treated with 1 µg/mL EVs (n = 4). (C) Immunoblot showing cleaved IL-1β in culture supernatants and pro-IL-1β and actin (control) in cell lysates of THP-1 MΦs treated for 12 h with 1 µg/mL EVs. Media alone and LPS+ATP were assay controls (n = 2). (D) Detection of cleaved caspase-1 in supernatants of THP-1 MΦs treated for 4 h with EVs (n = 4). RLUs, relative light units. (E) Dose-dependent release of IL-1β and IL-18 from monocyte-derived human MΦs incubated for 8 h with purified EVs (n = 4). (F) Time course of IL-1β production by monocyte-derived MΦs treated with 5 µg/mL EVs (n = 4). (G) Detection of cleaved caspase-1 in supernatants of monocyte-derived human MΦs that were incubated for 8 h with increasing concentrations of S. aureus EVs (n = 4). Data pooled from at least two independent experiments were analyzed by one-way ANOVA with Dunnett’s multiple comparison test. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. (H) IL-6 or (I) IL-1β levels in the sera and peritoneal fluids (PFs) collected at 4 h from C57BL/6 mice injected intraperitoneally with 10, 30, or 65 µg of purified JE2 EVs or 65 µg of BSA (n = 8).
To test whether EVs would induce IL-1β production in vivo, mice were injected intraperitoneally with 65 µg of purified JE2 EVs. Cytokine levels in mouse serum and peritoneal washes were measured by enzyme-linked immunosorbent assay (ELISA) from samples taken from 4 to 48 h postinjection. At the earliest time point (4 h), mice given EVs showed a strong proinflammatory signature characterized by elevated levels of IL-6 in both the serum and peritoneal fluid (SI Appendix, Fig. S2A), consistent with the lipoprotein content of S. aureus EVs (8). IL-1β was detected in the peritoneal fluid but not in mouse serum at the 4-h time point (SI Appendix, Fig. S2B). The in vivo response was rapid as cytokines were not elevated in samples collected at time points later than 4 h (SI Appendix, Fig. S2 A and B). In subsequent experiments, mice were challenged with either 65 µg of bovine serum albumin (BSA) or 10, 30, or 65 µg of S. aureus EVs, and samples were collected after 4 h. An IL-6 dose-dependent response was observed in both sera and peritoneal fluid samples (Fig. 2H) taken from mice given S. aureus EVs. An IL-1β response was detected in the peritoneal fluid, but not in the serum (Fig. 2I). These data suggest that S. aureus EVs activate inflammasomes in vivo in a rapid and dose-dependent manner.
NLRP3 Inflammasomes and Caspase-1 Are Essential for S. aureus EV-Mediated Release of IL-1β and IL-18 from Human Macrophages.
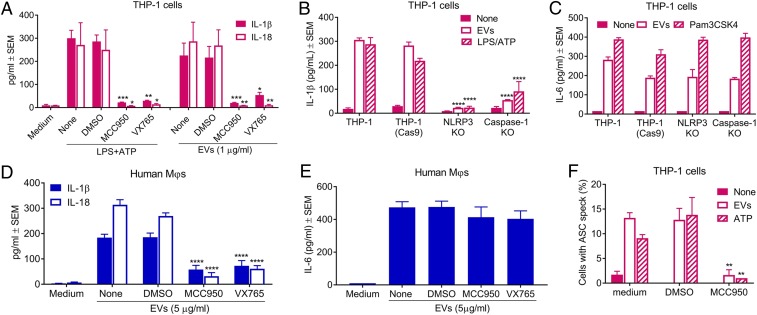
IL-1β and IL-18 release from THP-1 cells incubated with ATP+LPS or S. aureus EVs was significantly reduced when the cells were pretreated with the NLRP3 inhibitor MCC950 or the caspase-1 inhibitor VX765 (Fig. 3A). Likewise, THP-1 cells deficient in NLRP3 or caspase-1 abrogated IL-1β release induced by EVs (Fig. 3B) but had no effect on IL-6 release (Fig. 3C). IL-6 production is not dependent on NLRP3 activation since it is generated from the interactions of EV-associated lipoproteins (or the control TLR2 ligand Pam3CSK4) with TLR2 and either TLR1 or TLR6, resulting in downstream NF-κB pathway activation. These results were verified in monocyte-derived human MΦs by treating the cells with MCC950 or VX765 before incubation with 5 µg/mL EVs. Consistent with the results from THP-1 MΦs, the inhibitors significantly reduced release of IL-1β and IL-18 in monocyte-derived human MΦs (Fig. 3D) but had no effect on IL-6 production (Fig. 3E).
Fig. 3.
NLRP3 inflammasome activation by S. aureus EVs is essential for induction of cytokines IL-1β and IL-18 in human MΦs. (A) Levels of IL-1β and IL-18 in culture supernatants of THP-1 MΦs that were treated with or without NLRP3 inhibitor MCC950, caspase-1 inhibitor VX765, or DMSO for 1 h prior to incubation for 4 h with an LPS+ATP control or 1 µg/mL EVs (n = 4 to 8). Levels of IL-1β (B) and IL-6 (C) in culture supernatants of THP-1, THP-1 (Cas9), NLRP3-deficient THP-1 (NLRP3 KO), or caspase-1–deficient THP-1 (Caspase-1 KO) MΦs that were incubated for 4 h (IL-1β) or 12 h (IL-6) with 1 µg/mL EVs or LPS+ATP (n = 4 to 6). (D and E) Concentrations of IL-1β and IL-18 (D) or IL-6 (E) in culture supernatants of monocyte-derived human MΦs treated with or without MCC950, VX765, or DMSO for 1 h prior to incubation for 8 h (IL-1β and IL-18) or 2 h (IL-6) with 5 µg/mL EVs (n = 4 to 6). (F) Quantification of the percentage of THP-1 cells with detectable ASC specks. More than 400 cells from at least 10 different fields were counted for ASC speck formation in each treatment. Data pooled from three independent experiments were analyzed by one-way ANOVA with Dunnett’s multiple comparison test. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
ASC is a key adaptor molecule required for caspase-1 activation via NLR inflammasomes (19). Upon inflammasome activation, ASC is recruited from the nucleus to the cytosol where it self-aggregates and forms “specks” ∼1 μm in diameter (32, 33). Treatment of THP-1 MΦs with either S. aureus EVs or the positive control ATP induced the formation of ASC specks that were visualized by confocal microscopy (SI Appendix, Fig. S3). Blockage of NLRP3 inflammasome activation by MCC950 resulted in a significant reduction in EV-induced ASC speck formation in THP-1 MΦs (SI Appendix, Fig. S3 and Fig. 3F). Taken together, our results indicate that EV-induced secretion of the proinflammatory cytokines IL-1β and IL-18 by human MΦs is dependent upon NLRP3 inflammasome activation.
EV-Associated S. aureus Pore-Forming Toxins Play a Critical Role in NLRP3 Inflammasome Activation.
S. aureus EVs carry an array of PFTs, including alpha toxin (Hla) and a family of leukocidins (8), which are positively regulated by the accessory gene regulator (agr) quorum sensing system and the SaeR/S two-component system (34). Mutation of either regulator abrogates the expression of most PFT genes and reduces the cytotoxicity of EVs (8). Incubation of THP-1 MΦs with 1 µg/mL S. aureus JE2 EVs over 24 h resulted in significant cellular toxicity as measured by lactate dehydrogenase (LDH) release (SI Appendix, Fig. S4A). EVs purified from Δsae, Δagr, or ΔagrΔsae JE2 mutants showed reduced cytotoxicity toward THP-1 cells (SI Appendix, Fig. S4B), a result that was confirmed by dose-dependent treatment of THP-1 MΦs with EVs from the wild type (WT) or the ΔagrΔsae mutant (SI Appendix, Fig. S4C). Monocyte-derived human MΦs treated with WT JE2 EVs for 8 h also showed a dose-dependent release of LDH (SI Appendix, Fig. S4D), and EVs purified from JE2ΔagrΔsae showed reduced cytotoxicity toward these cells (SI Appendix, Fig. S4E). These results suggest that EVs purified from the ΔagrΔsae mutants packaged minimal amount of PFTs.
LDH release is a marker of pyroptosis, which is initiated by inflammasome-mediated caspase-1 activation and cleavage of gasdermin D (GSDMD) to its active form, resulting in pore formation and cell death (35). Inhibition of NLRP3 inflammasome activation by MCC950 resulted in significant reductions in LDH release from THP-1 MΦs treated with LPS+ATP or WT EVs (SI Appendix, Fig. S5A). Likewise, THP-1 cells deficient in NLRP3 or caspase-1 released lower levels of LDH induced by S. aureus EVs (SI Appendix, Fig. S5B). To investigate whether EV-associated PFTs are critical for pyroptosis, THP-1 cells were incubated with EVs from JE2 or the ΔagrΔsae mutant or LPS+ATP, and cleaved GSDMD was detected by immunoblot. Although cleaved GSDMD was detected in supernatants and lysates from THP-1 cells incubated with EVs purified from JE2 or JE2ΔagrΔsae (Fig. 4A), the levels of cleaved GSDMD in cells treated with the WT EVs were higher than that of cells treated with EVs from JE2ΔagrΔsae (SI Appendix, Fig. S5C). These findings suggest that S. aureus EVs induce inflammasome-mediated pyroptosis in MΦs and that EV-associated PFTs enhance this process.
Fig. 4.
Activation of NLRP3 inflammasomes by S. aureus EVs is dependent upon PFTs. (A) Immunoblot showing cleaved N-terminal GSDMD in culture supernatants and cell lysates of THP-1 cells incubated for 12 h with 1 µg/mL EVs from the WT or ΔagrΔsae mutant. Media alone and LPS+ATP were assay controls (n = 2). (B and C) Levels of IL-1β and IL-18 (B) or IL-6 (C) in culture supernatants of THP-1 MΦs incubated for 4 h (IL-1β and IL-18) or 12 h (IL-6) with or without EVs purified from the indicated S. aureus strains or positive controls LPS+ATP and Pam3CSK4 (TLR2 ligand) (n = 4 to 6). (D) Immunoblot showing cleaved IL-1β and cleaved caspase-1 in THP-1 culture supernatants and caspase-1 in cell lysates of THP-1 cells incubated for 12 h with 1 µg/mL EVs from the WT or ΔagrΔsae mutant. Media alone and LPS+ATP were assay controls. *, background immunoblot band (n = 2). (E–G) Levels of IL-1β and IL-18 (E), active caspase-1 (F), or IL-6 (G) in culture supernatants of human monocyte-derived MΦs incubated for 8 h (IL-1β and IL-18) or 2 h (IL-6) with 5 µg/mL EVs purified from indicated strains, LPS+ATP, or Pam3CSK4 (n = 4 to 6). (H [n = 11] and I [n = 6]) Levels of IL-1β in culture supernatants of THP-1 MΦs that were incubated for 4 h with or without EVs purified from indicated S. aureus strains. (J and K) Detection of IL-1β (J) or active caspase-1 (K) in culture supernatants of THP-1 MΦs treated with or without indicated concentrations of KCl for 1 h prior to incubation for 4 h with 1 µg/mL EVs or LPS+ATP (n = 4). (L) Concentrations of IL-1β and IL-18 in culture supernatants of human monocyte-derived MΦs that were treated with or without 130 mM KCl for 1 h prior to incubation for 8 h with 5 µg/mL EVs (n = 4). Data pooled from at least three independent experiments were analyzed by one-way ANOVA with Dunnett’s multiple comparison test. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
IL-1β and IL-18 release from THP-1 MΦs incubated for 4 h with 1 µg/mL EVs purified from Δsae, Δagr, or ΔagrΔsae mutants was significantly reduced compared to that of cells incubated with WT EVs (Fig. 4B). In contrast, IL-6 production resulting from TLR2 activation by EV-associated lipoproteins was similar for THP-1 cells incubated with EVs from the WT or mutant strains (Fig. 4C). To assess proteolytic cleavage of pro–IL-1β resulting from EV treatment, THP-1 MΦs were treated with 1 µg/mL EVs purified from JE2 or the ΔagrΔsae mutant. Cleaved IL-1β and caspase-1 in the THP-1 culture supernatants and pro-IL-1β and caspase-1 in cell lysates were detected by immunoblot. Although pro–IL-1β and caspase-1 were present in lysates from cells treated with EVs purified from JE2 and JE2ΔagrΔsae, cleaved IL-1β and cleaved caspase-1 were detected primarily in cells incubated with WT EVs (Fig. 4D). These results were validated by treatment of monocyte-derived human MΦs with EVs from the WT or ΔagrΔsae mutant. Only WT JE2 EVs induced MΦ release of IL-1β and IL-18 (Fig. 4E) and caspase-1 activation (Fig. 4F). In contrast, IL-6 release was induced by monocyte-derived MΦs treated with EVs from either the WT or the ΔagrΔsae mutant (Fig. 4G).
S. aureus JE2 produces five PFTs: LukAB, Hla, LukSF-PV, HlgAB, and HlgCB, and LukAB, Hla, and LukSF-PV are the most potent in inflammasome activation (36). THP-1 MΦs were treated for 4 h with 1 µg/mL EVs purified from WT JE2 or single PFT mutants (ΔlukAB, Δhla, or ΔlukSF-PV). As shown in Fig. 4H, release of IL-1β from THP-1 MΦs incubated with EVs purified from individual toxin mutants was significantly reduced compared to that of cells treated with WT EVs. As a follow-up experiment, THP-1 cells were incubated with EVs purified from the JE2ΔagrΔsae mutant alone or complemented with the genes encoding individual PFTs: LukAB, Hla, or LukSF-PV. Complementation with lukAB or hla partially restored the production of IL-1β from THP-1 MΦs treated with ΔagrΔsae EVs (Fig. 4I). Whereas individual PFTs contribute to NLRP3 inflammasome activation mediated by S. aureus EVs, our results indicate that the constellation of PFTs produced by this USA300 strain provides maximal NLRP3 inflammasome activation.
To assess whether EV-induced NLRP3 inflammasome activation was dependent on K+ efflux, THP-1 MΦs were pretreated with increasing concentrations of potassium chloride (KCl) for 1 h before a 4-h stimulation with S. aureus EVs. A dose-dependent inhibition of EV-mediated IL-1β release (Fig. 4J) and caspase-1 activity (Fig. 4K) was observed in THP-1 cells pretreated with KCl. Similarly, pretreatment of monocyte-derived human MΦs with 130 mM KCl blocked release of IL-1β and IL-18 induced by S. aureus EVs (Fig. 4L).
To rule out the possibility that the impaired activation of inflammasomes in MΦs was due to a defect in EV internalization, THP-1 MΦs were incubated for 90 min with DiO-labeled EVs purified from the ΔagrΔsae mutant. As shown in SI Appendix, Fig. S6, EVs purified from the mutant were efficiently internalized within THP-1 MΦs. When the THP-1 cells were pretreated with or without inhibitors (SI Appendix, Fig. S6A), only treatment of cells with dynasore significantly blocked internalization of EVs purified from the ΔagrΔsae mutant (SI Appendix, Fig. S6B), Thus, MΦ uptake of EVs purified from both the WT and the ΔagrΔsae mutant occurred through dynamin-dependent endocytosis.
Together, our findings reveal that S. aureus EVs induce NLRP3 inflammasome activation and that EV-induced release of IL-1β and IL-18 can be attributed to PFT-mediated K+ efflux. Moreover, we show that EV-associated PFTs play an essential role in caspase-1 activation and enhance NLRP3-mediated pyroptosis, but are not involved in TLR2 signaling nor in EV internalization within MΦs.
Localization of PFTs in S. aureus EVs.
It is not known whether S. aureus EV-associated PFTs are localized within the EV lumen or present at the EV surface. In the latter case, pore formation on the MΦ membrane could occur independent of EV internalization, inducing K+-mediated inflammasome activation. A proteinase K susceptibility assay was employed to detect surface localization of three PFTs that are abundant in purified EVs (8): Hla, LukS-PV, and LukAB. As shown by silver-stained sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS/PAGE) (SI Appendix, Fig. S7A), treatment of intact EVs with proteinase K for 60 min resulted in partial digestion of EV-associated proteins. In contrast, EV proteins were fully digested by treatment with proteinase K in the presence of 1% SDS, an anionic detergent that disrupts the EV membrane and exposes the EV cargo to the environment. Proteolytic digestion of purified Hla, LukS-PV, and LukAB occurred within 30 min, 90 min, and 60 min, respectively, as deduced from immunoblots (SI Appendix, Fig. S7B). When EVs were incubated with proteinase K for 60 min, most of the EV-associated Hla remained intact and was only digested in the presence of 1% SDS (SI Appendix, Fig. S7C). In contrast, proteinase K treatment of intact EVs in the presence or absence of 1% SDS completely digested EV-associated LukS-PV (SI Appendix, Fig. S7D) and LukAB (SI Appendix, Fig. S7E) after 90 min and 60 min, respectively. These data suggest that S. aureus PFTs are localized on the surface and within the lumen of EVs and that the latter was protected from proteolytic digestion.
To determine whether LukS-PV and LukAB surface receptor binding was essential for EV-induced cytokine production, we utilized murine J774A.1 MΦs. LukS-PV binds to human, but not mouse, C5aR, and LukAB binds to human, but not mouse, CD11b (37, 38). Whereas WT EVs stimulated IL-6 production by J774A.1 MΦs in a dose-dependent fashion (SI Appendix, Fig. S8A), IL-1β production was not detected with EV concentrations up to 5 µg/mL (SI Appendix, Fig. S8B). To rule out the possibility that impaired J774A.1 inflammasome activation was due to a defect in EV internalization, we incubated the J774A.1 cells for 90 min with DiO-labeled EVs purified from WT JE2. As shown in SI Appendix, Fig. S8C, EV-associated fluorescence was readily detectable within J774A.1 macrophages, indicating efficient EV internalization. These findings suggest that surface-associated PFT interactions with specific leukocidin receptors on MΦs are essential for EV-mediated NLRP3 activation.
EV-Associated Lipoproteins Are Critical for TLR2 Signaling and Caspase-1 Activation.
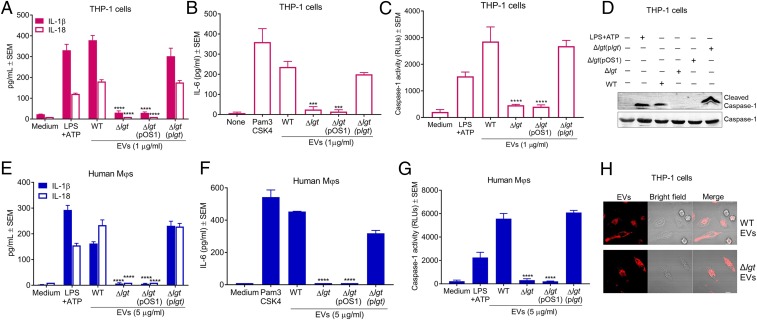
S. aureus lipoproteins are critical for TLR2 signaling and triggering host innate immune defenses against staphylococcal infections (39–41). The S. aureus lgt gene product catalyzes the transfer of a diacyl-glyceryl group to a cysteine residue in the lipobox of the lipoprotein signal peptide (42). An lgt mutant is deficient in the lipidation and maturation of lipoproteins although the proteins themselves are still produced (43). To investigate the role of lipoproteins in EV-induced NLRP3 inflammasome activation, THP-1 MΦs were incubated with 1 µg/mL EVs purified from the WT or lgt mutant, and cytokines in the MΦ culture supernatants were measured. EVs purified from the lgt mutant were defective in the induction of IL-1β, IL-18, and IL-6 from THP-1 MΦs, and this defect was complemented when lgt was provided in trans (Fig. 5 A and B). Unexpectedly, mutation of lgt also abrogated EV-induced cleavage of caspase-1 in THP-1 MΦs, as revealed by luminescent assays (Fig. 5C) and by immunoblot (Fig. 5D). To validate these findings, monocyte-derived human MΦs were similarly treated with 5 µg/mL EVs purified from JE2, the lgt mutant, or a complemented mutant. As expected, mutation of lgt abrogated the induction of cytokines IL-1β, IL-18, and IL-6 (Fig. 5 E and F), as well as caspase-1 activation (Fig. 5G). To rule out the possibility that the impaired activation of caspase-1 in MΦs was due to a defect in EV internalization, THP-1 MΦs were incubated for 1 h with R18-labeled EVs purified from the WT or lgt mutant strain. As shown in Fig. 5H, EV fluorescence within MΦs was similar for both strains, indicating that EVs harvested from the lgt mutant were efficiently internalized within human MΦs. We further determined the cellular internalization mechanism of EVs from the lgt mutant. Pretreatment of THP-1 MΦs with dynasore, but not other inhibitors, significantly inhibited the cellular entry of EVs (SI Appendix, Fig. S9 A and B), indicating that, like WT EVs, the EVs purified from the lgt mutant were internalized within MΦs through dynamin-dependent endocytosis. Together, these results demonstrate that EV-associated lipoproteins are critical for both TLR2 signaling and caspase-1 activation, but not for the internalization of EVs.
Fig. 5.
S. aureus EV-associated lipoproteins are critical for TLR2 signaling and caspase-1 activation. (A–C) Levels of IL-1β and IL-18 (A), IL-6 (B), and active caspase-1 (C) in culture supernatants of THP-1 MΦs incubated for 4 h (IL-1β, IL-18, and caspase-1) or 12 h (IL-6) with EVs purified from WT JE2, the lgt mutant, the complemented lgt mutant, or controls LPS+ATP and Pam3CSK4 (n = 4). (D) Immunoblot showing cleaved caspase-1 in culture supernatants and caspase-1 in cell lysates of THP-1 MΦs that were incubated for 12 h with 1 µg/mL EVs purified from WT JE2, the lgt mutant, the complemented lgt mutant, or LPS+ATP (n = 2). (E–G) Levels of IL-1β and IL-18 (E), IL-6 (F), and active caspase-1 (G) in culture supernatants of monocyte-derived human MΦs incubated for 8 h (IL-1β, IL-18, and caspase-1) or 2 h (IL-6) with 5 µg/mL EVs purified from WT JE2, the lgt mutant, the complemented lgt mutant, LPS+ATP, or Pam3CSK4 (n = 4). (H) Representative images of two independent experiments for THP-1 MΦs incubated for 90 min with 1 µg/mL R18-labeled EVs purified from the WT or lgt mutant (n = 2). (Scale bars: 10 µm.) Data pooled from at least two independent experiments were analyzed by one-way ANOVA with Dunnett’s multiple comparison test. ***P < 0.001, ****P < 0.0001.
Lipoproteins Modulate the Content and Biogenesis of EVs in S. aureus.
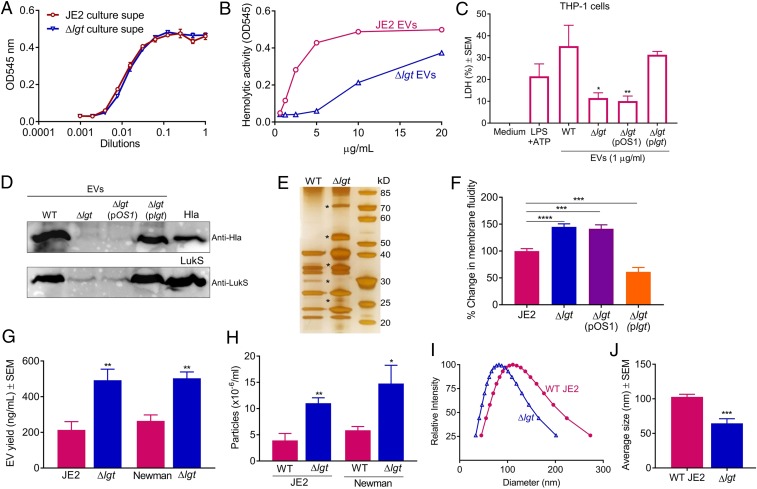
Because EVs purified from the lgt mutant were deficient in the activation of caspase-1, we investigated whether EVs from the mutant strain still packaged PFTs, which are important for EV-induced caspase-1 activation (Fig. 4 D and F). Culture supernatants of JE2 and its lgt mutant showed similar levels of hemolytic activity against rabbit erythrocytes (Fig. 6A), which are susceptible to Hla, phenol soluble modulins, and the leukocidins HlgAB and LukED (5, 44, 45). In contrast, EVs recovered from the lgt mutant exhibited reduced hemolytic activity compared to WT EVs (Fig. 6B). Likewise, THP-1 MΦs incubated with EVs from the lgt mutant released less LDH than WT EV-treated cells, and lgt complementation restored EV cytotoxicity (Fig. 6C). These data suggest that the lgt mutation resulted in reduced PFT cargo associated with S. aureus EVs. To validate this assumption, the Hla and LukS-PV content of EVs from the WT or lgt mutant was evaluated by immunoblot analysis. The Hla and LukS-PV content was dramatically reduced in EVs purified from the lgt mutant compared to WT EVs (Fig. 6D), and the toxin cargo was restored when the lgt gene was provided in trans to the mutant strain.
Fig. 6.
S. aureus lipoproteins modulate EV content and biogenesis. The hemolytic activity of S. aureus culture supernatants (A) and purified EVs (B) was assessed with rabbit erythrocytes (n = 4). (C) Release of LDH in culture supernatants of THP-1 MΦs that were incubated for 4 h with 1 µg/mL EVs purified from WT JE2, the lgt mutant, or the complemented lgt mutant (n = 4). (D) The Hla and LukS-PV content of EVs purified from the indicated strains was detected by immunoblot. Purified Hla and LukS-PV were used as controls (n = 2). (E) EVs purified from WT or lgt mutant strains were subjected to SDS/PAGE and silver staining (n = 3). kD, kilodalton. (F) The membrane fluidity of the indicated strains was determined using a pyrenedecanoic acid fluorescence probe, and the percent change in membrane fluidity was calculated (n = 14). (G) EV production from S. aureus strains JE2 and Newman and their isogenic lgt mutants was evaluated by quantification of total EV protein yield (n = 8 to 10) or (H) by EV quantification using nanoparticle tracking analysis (n = 4). (I) EV size distribution and (J), average size of EVs isolated from JE2 and its lgt mutant were measured by dynamic light scattering (n = 6). Membrane fluidity, EV protein yield, and EV particle quantification experiments were calculated from at least three independent experiments. The data were analyzed with a one-way ANOVA with Dunnett’s multiple comparison test (C and F) or with an unpaired, two-tailed Student’s t test (G, H, and J). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
To rule out the possibility that the reduced PFT content of lgt EVs was dependent on the bacterial growth phase, EVs were purified from cultures grown to log phase (4.5 h) or to late log phase (7 h), and the levels of Hla and LukS-PV in purified EVs were compared. Immunoblot analysis again revealed a similar toxin profile in the culture supernatants of WT and lgt mutant strains (SI Appendix, Fig. S10A). The remarkable reduction in the Hla and LukS content of EVs harvested from the lgt mutant was consistent for EVs prepared from cultures in the early or late phases of logarithmic growth (SI Appendix, Fig. S10A). Likewise, EVs prepared from both growth phases of the lgt mutant showed significant reductions in cytotoxicity compared to WT EVs when incubated with THP-1 MΦs (SI Appendix, Fig. S10B). Together these results demonstrate that lipoproteins modulate the PFT content of S. aureus EVs.
We predicted that, if the PFT content of EVs from lgt mutant was altered, the lipoprotein defect might also have an impact on the total protein content of EVs. This assumption was corroborated by silver-stained SDS/PAGE analysis that showed distinct protein profiles for EVs purified from the WT JE2 and its lgt mutant (Fig. 6E). To validate these data, a proteomic analysis of EVs purified from JE2 and its lgt mutant was performed by liquid chromatography–tandem mass spectrometry (LC–MS/MS). A total of 180 and 198 proteins were identified from EVs recovered from the WT and lgt mutant, respectively (Dataset S1). However, the lgt mutation resulted in significant alterations in the EV protein content. Compared to WT EVs, more cytosolic proteins and fewer extracellular proteins, including PFTs, were identified in EVs from the lgt mutant (SI Appendix, Fig. S11A). In addition, 49 of 180 (27.2%) and 67 of 198 (33.8%) identified proteins were unique to EVs recovered from the WT and lgt mutant, respectively (SI Appendix, Fig. S11B). These data suggest that S. aureus lipoproteins modulate EV biogenesis.
Membrane fluidity plays a fundamental role in governing the flexibility of membranes, which may affect membrane curvature (46). Because increases in membrane fluidity can promote vesicle budding (47), we measured the membrane fluidity of WT and lgt mutant strains using the excimer-forming lipid technique (48). Mutation of lgt resulted in a significant increase in S. aureus cytoplasmic membrane fluidity, and this increase was abrogated when the lgt gene was provided in trans to the mutant strain (Fig. 6F). Consistent with the lgt mutation resulting in heightened membrane fluidity, we observed significantly increased EV yield (Fig. 6G) and particle numbers (Fig. 6H) in lgt mutants of either strain JE2 or Newman. Moreover, EVs from the JE2 lgt mutant were smaller in size than WT EVs (Fig. 6 I and J).
Discussion
Outer membrane vesicles (OMVs) secreted by gram-negative bacteria package multiple virulence factors and are considered to be important mediators in bacterial–host cell interactions. OMVs taken up by host cells modulate innate and adaptive immune responses and may lead to cell injury (28, 49–52). In addition to OMV production, many gram-negative pathogens manipulate host cellular functions by utilizing dedicated secretion systems (T3SS, T4SS, and T6SS) to transport bacterial products into host cells (53). In contrast, gram-positive bacteria lack sophisticated secretion systems characteristic of gram-negative microbes. S. aureus EV cargo includes secreted proteins, cell wall-anchored proteins, and membrane proteins, but it is cytoplasmic proteins that represent the most abundant component of EVs (8). Because cytoplasmic proteins lack export signals, EVs clearly represent a unique secretory mechanism for gram-positive bacteria. The concept that EV production enables bacteria to encapsulate and deliver their products into host cells, while protecting the cargo from detection or destruction by external factors, has modified and shaped our understanding of the pathogenesis of infections caused by gram-positive pathogens. Nonetheless, the biological role of EVs in S. aureus infection, as well as the downstream fate of EVs following bacterial release, remains poorly characterized.
In this report, we demonstrate that S. aureus EVs are sensed in human MΦs by NLRP3 inflammasomes (SI Appendix, Fig. S12), a major signaling pathway of the innate immune system that is critical for host defense against bacterial infections (54). Consistent with previous reports indicating that activation of NLRP3 inflammasomes requires pore forming-mediated K+ efflux (26, 27, 55, 56), NLRP3 activation by S. aureus EVs was dependent on PFT cargo and K+ efflux. S. aureus EVs carry many virulence factors, including surface proteins and glycopolymers, as well as secreted proteins, such as PFTs, superantigens, phenol soluble modulins, and proteases, and EVs are toxic to a broad range of host cells (8, 10–12). Our data indicate that multiple PFTs such as LukAB, Hla, and LukSF-PV are involved in EV-induced NLRP3 inflammasome activation and that the combined action of PFTs was required for maximal activation by EVs. S. aureus produces a diverse array of PFTs, and individual PFTs target specific cellular receptors carried by a subset of human hemopoietic cells (5, 57). Whereas individual PFTs promote bacterial virulence, the synergistic action of multiple PFTs may be necessary to target sufficient numbers of host cells to result in inflammatory signaling and pore formation (57). Pyroptosis is a programmed lytic cell death process associated with inflammasome activation. Our data indicate that pyroptosis was activated upon incubation of human MΦs with S. aureus EVs. However, inhibition of NLRP3 inflammasomes or knockout of NLRP3 or caspase-1 did not fully abrogate the EV-induced LDH release, suggesting that other lytic cell death pathways are likely involved in EV-induced cell death in human MΦs. Necroptosis is another programmed cell death pathway (58), and PFT-mediated activation of necroptosis can occur during S. aureus infection (59, 60). It is likely that multiple lytic cell death pathways are involved in EV-induced MΦ cytotoxicity.
To gain a better understanding of how EV-associated PFTs interact with host cells, we investigated whether prototype S. aureus PFTs (Hla, LukS-PV, and LukAB) were surface associated or contained within the EV lumen. Because of its relative resistance to protease treatment, EV-associated Hla appears to be contained primarily within the EV lumen. In contrast, the bulk of LukS-PV and LukAB were protease sensitive, suggesting surface localization of these PFTs. EV surface-associated PFTs may activate inflammasomes extracellularly through pore formation, but whether there are additional mechanisms involved in this process remains unclear. Our data show that S. aureus EVs enter MΦs via a dynamin-dependent endocytic pathway, suggesting that EV-associated PFTs and other cargo may be presented to host cells within endosomal vesicles. S. aureus cytolysins, such as LukAB and delta-toxin, are capable of disrupting the membranes of subcellular compartments (61, 62). Membrane permeation, lysosomal damage, and mitochondrial disruption are cellular events that can elicit NLRP3 inflammasome activation (63–66). As such, it is possible that EV-associated PFTs may trigger NLRP3 activation by pore formation directly at the cell membrane or following the entry of EVs into cellular compartments. Although S. aureus is deemed a classic extracellular pathogen, it is capable of survival within host cells, including macrophages (67), where it presumably secretes EVs. Intracellular EV formation has been demonstrated for typical intracellular microbes, like Listeria monocytogenes (68) and Mycobacterium avium (69).
Lipoproteins, abundant in S. aureus EVs, represent a subset of bacterial membrane proteins that are anchored in the cytoplasmic membrane by a covalently linked N-terminal lipid moiety (70). S. aureus lipoproteins are critical for bacterial nutrient uptake (15), but they also play a role in host immune defense against infection by activating host innate immunity through TLR2 signaling (39, 41, 71). An S. aureus lgt mutant, deficient in the lipidation and maturation of lipoproteins, produces prelipoproteins that are not modified by lipidation and are unable to activate TLR2 signaling (43). These prelipoproteins may be retained transiently at the cytoplasmic membrane by the uncleaved signal sequence, including the characteristic lipobox, but eventually are released from S. aureus cells in high amounts due to proteolytic processing (43, 72). We characterized EVs from an lgt mutant of S. aureus because we suspected that they would be deficient in TLR2 signaling, which is essential for NLRP3 inflammasome activation. Predictably, EVs purified from the lgt mutant were deficient in stimulating human MΦs to secrete IL-6, IL-1β, and IL-18. However, we did not anticipate the loss of caspase-1 activity that occurred when THP-1 MΦs or monocyte-derived human MΦs were incubated with EVs purified from the lgt mutant. Upon further investigation, we observed that an S. aureus lgt mutant produced more EVs than the parental strain and that the EVs from the lgt mutant showed an altered protein composition with a marked reduction in PFT cargo. Thus, lipoproteins play a previously unrecognized role in S. aureus EV biogenesis. Membrane proteins maintain the integrity, organization, and flow of materials through the bacterial membrane (73). The WT S. aureus USA300 strain expresses ∼67 lipoproteins (15), and the absence of lipoproteins resulted in an increase in membrane fluidity of S. aureus, as well as alterations in the protein content of EVs, their yield, and size.
In summary, our work provides insights into the potential role of EVs in the molecular pathogenesis of gram-positive infections by serving as a secretory pathway for transporting protected bacterial cargo into host cells. We demonstrate the critical role that staphylococcal lipoproteins play in EV biogenesis and the essential role of S. aureus EV-associated PFTs in triggering inflammasome activation, which modulates the host innate immune response during infection.
Materials and Methods
The S. aureus strains used in this study are listed in SI Appendix, Table S1. The isolation, purification, and analysis of EV protein concentrations, particle numbers, and EV diameters were performed as described previously (8). Primary human monocytes were isolated from the peripheral blood of healthy volunteers as approved by the Institutional Review Board of Partners HealthCare System. Mouse experiments were approved by the Institutional Animal Care and Use Committee of the Brigham and Women’s Hospital. All other materials and details of experimental procedures involved in the generation of S. aureus mutants, cell culture, immunoblots, ELISAs, confocal microscopy, membrane fluidity assays, proteinase K assays, and animal studies are provided in SI Appendix.
Data Availability.
The data supporting the findings of this study are available within the main text and SI Appendix. Mass spectrometry proteomics data were deposited in the ProteomeXchange Consortium (http://proteomecentral.proteomexchange.org/cgi/GetDataset) via the PRIDE partner repository with the data set identifier PXD014888.
Supplementary Material
Acknowledgments
Research reported in this publication was supported by a Brigham Research Institute Pilot Funding Award from Brigham and Women’s Hospital and the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Awards R21AI135613 and R01AI141885 (to J.C.L.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We thank Drs. Romeo Ricci (Institut de Génétique et de Biologie Moléculaire et Cellulaire, Illkirch, France) for providing the NLRP3 knockout THP-1 cell line; Seth L. Masters (Inflammation Division, The Walter and Eliza Hall Institute of Medical Research, Parkville, Australia) for providing the THP-1 (Cas9) and Caspase-1 knockout THP-1 cell lines; and Simon Dove (Division of Infectious Diseases, Boston Children’s Hospital and Harvard Medical School, Boston, MA) for providing the J774A.1 cell line.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
Data deposition: Mass spectrometry proteomics data have been deposited in the ProteomeXchange Consortium, http://proteomecentral.proteomexchange.org/cgi/GetDataset via the PRIDE partner repository (dataset identifier PXD014888).
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1915829117/-/DCSupplemental.
References
- 1.Tong S. Y., Davis J. S., Eichenberger E., Holland T. L., Fowler V. G. Jr, Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 28, 603–661 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lakhundi S., Zhang K., Methicillin-resistant Staphylococcus aureus: Molecular characterization, evolution, and epidemiology. Clin. Microbiol. Rev. 31, e00020-18 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Foster T. J., Geoghegan J. A., Ganesh V. K., Höök M., Adhesion, invasion and evasion: The many functions of the surface proteins of Staphylococcus aureus. Nat. Rev. Microbiol. 12, 49–62 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Riordan K., Lee J. C., Staphylococcus aureus capsular polysaccharides. Clin. Microbiol. Rev. 17, 218–234 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reyes-Robles T., Torres V. J., Staphylococcus aureus pore-forming toxins. Curr. Top. Microbiol. Immunol. 409, 121–144 (2017). [DOI] [PubMed] [Google Scholar]
- 6.Deatherage B. L., Cookson B. T., Membrane vesicle release in bacteria, eukaryotes, and archaea: A conserved yet underappreciated aspect of microbial life. Infect. Immun. 80, 1948–1957 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown L., Wolf J. M., Prados-Rosales R., Casadevall A., Through the wall: Extracellular vesicles in gram-positive bacteria, mycobacteria and fungi. Nat. Rev. Microbiol. 13, 620–630 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang X., Thompson C. D., Weidenmaier C., Lee J. C., Release of Staphylococcus aureus extracellular vesicles and their application as a vaccine platform. Nat. Commun. 9, 1379 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Toyofuku M., et al. , Prophage-triggered membrane vesicle formation through peptidoglycan damage in Bacillus subtilis. Nat. Commun. 8, 481 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gurung M., et al. , Staphylococcus aureus produces membrane-derived vesicles that induce host cell death. PLoS One 6, e27958 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jeon H., et al. , Variation among Staphylococcus aureus membrane vesicle proteomes affects cytotoxicity of host cells. Microb. Pathog. 93, 185–193 (2016). [DOI] [PubMed] [Google Scholar]
- 12.Lee E. Y., et al. , Gram-positive bacteria produce membrane vesicles: Proteomics-based characterization of Staphylococcus aureus-derived membrane vesicles. Proteomics 9, 5425–5436 (2009). [DOI] [PubMed] [Google Scholar]
- 13.Clarke S. R., Foster S. J., Surface adhesins of Staphylococcus aureus. Adv. Microb. Physiol. 51, 187–224 (2006). [DOI] [PubMed] [Google Scholar]
- 14.Weidenmaier C., Lee J. C., Structure and function of surface polysaccharides of Staphylococcus aureus. Curr. Top. Microbiol. Immunol. 409, 57–93 (2017). [DOI] [PubMed] [Google Scholar]
- 15.Shahmirzadi S. V., Nguyen M. T., Götz F., Evaluation of Staphylococcus aureus lipoproteins: Role in nutritional acquisition and pathogenicity. Front. Microbiol. 7, 1404 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Otto M., Staphylococcus aureus toxins. Curr. Opin. Microbiol. 17, 32–37 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thay B., Wai S. N., Oscarsson J., Staphylococcus aureus α-toxin-dependent induction of host cell death by membrane-derived vesicles. PLoS One 8, e54661 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi S. J., et al. , Active immunization with extracellular vesicles derived from Staphylococcus aureus effectively protects against staphylococcal lung infections, mainly via Th1 cell-mediated immunity. PLoS One 10, e0136021 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elliott E. I., Sutterwala F. S., Initiation and perpetuation of NLRP3 inflammasome activation and assembly. Immunol. Rev. 265, 35–52 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jorgensen I., Miao E. A., Pyroptotic cell death defends against intracellular pathogens. Immunol. Rev. 265, 130–142 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jorgensen I., Rayamajhi M., Miao E. A., Programmed cell death as a defence against infection. Nat. Rev. Immunol. 17, 151–164 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cho J. S., et al. , Neutrophil-derived IL-1β is sufficient for abscess formation in immunity against Staphylococcus aureus in mice. PLoS Pathog. 8, e1003047 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maher B. M., et al. , Nlrp-3-driven interleukin 17 production by γδT cells controls infection outcomes during Staphylococcus aureus surgical site infection. Infect. Immun. 81, 4478–4489 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Evavold C. L., Kagan J. C., How inflammasomes inform adaptive immunity. J. Mol. Biol. 430, 217–237 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kebaier C., et al. , Staphylococcus aureus α-hemolysin mediates virulence in a murine model of severe pneumonia through activation of the NLRP3 inflammasome. J. Infect. Dis. 205, 807–817 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Craven R. R., et al. , Staphylococcus aureus alpha-hemolysin activates the NLRP3-inflammasome in human and mouse monocytic cells. PLoS One 4, e7446 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muñoz-Planillo R., Franchi L., Miller L. S., Núñez G., A critical role for hemolysins and bacterial lipoproteins in Staphylococcus aureus-induced activation of the Nlrp3 inflammasome. J. Immunol. 183, 3942–3948 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vanaja S. K., et al. , Bacterial outer membrane vesicles mediate cytosolic localization of LPS and caspase-11 activation. Cell 165, 1106–1119 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bomberger J. M., et al. , Long-distance delivery of bacterial virulence factors by Pseudomonas aeruginosa outer membrane vesicles. PLoS Pathog. 5, e1000382 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller L. S., et al. , Inflammasome-mediated production of IL-1beta is required for neutrophil recruitment against Staphylococcus aureus in vivo. J. Immunol. 179, 6933–6942 (2007). [DOI] [PubMed] [Google Scholar]
- 31.Hong S. W., et al. , Extracellular vesicles derived from Staphylococcus aureus induce atopic dermatitis-like skin inflammation. Allergy 66, 351–359 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beilharz M., De Nardo D., Latz E., Franklin B. S., Measuring NLR oligomerization II: Detection of ASC speck formation by confocal microscopy and immunofluorescence. Methods Mol. Biol. 1417, 145–158 (2016). [DOI] [PubMed] [Google Scholar]
- 33.Bryan N. B., Dorfleutner A., Rojanasakul Y., Stehlik C., Activation of inflammasomes requires intracellular redistribution of the apoptotic speck-like protein containing a caspase recruitment domain. J. Immunol. 182, 3173–3182 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Münzenmayer L., et al. , Influence of Sae-regulated and Agr-regulated factors on the escape of Staphylococcus aureus from human macrophages. Cell. Microbiol. 18, 1172–1183 (2016). [DOI] [PubMed] [Google Scholar]
- 35.Shi J., et al. , Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526, 660–665 (2015). [DOI] [PubMed] [Google Scholar]
- 36.Melehani J. H., Duncan J. A., Inflammasome activation can mediate tissue-specific pathogenesis or protection in Staphylococcus aureus infection. Curr. Top. Microbiol. Immunol. 397, 257–282 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spaan A. N., et al. , The staphylococcal toxin Panton-Valentine Leukocidin targets human C5a receptors. Cell Host Microbe 13, 584–594 (2013). [DOI] [PubMed] [Google Scholar]
- 38.DuMont A. L., et al. , Staphylococcus aureus LukAB cytotoxin kills human neutrophils by targeting the CD11b subunit of the integrin Mac-1. Proc. Natl. Acad. Sci. U.S.A. 110, 10794–10799 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bubeck Wardenburg J., Williams W. A., Missiakas D., Host defenses against Staphylococcus aureus infection require recognition of bacterial lipoproteins. Proc. Natl. Acad. Sci. U.S.A. 103, 13831–13836 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fournier B., The function of TLR2 during staphylococcal diseases. Front. Cell. Infect. Microbiol. 2, 167 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hanzelmann D., et al. , Toll-like receptor 2 activation depends on lipopeptide shedding by bacterial surfactants. Nat. Commun. 7, 12304 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sheldon J. R., Heinrichs D. E., The iron-regulated staphylococcal lipoproteins. Front. Cell. Infect. Microbiol. 2, 41 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stoll H., Dengjel J., Nerz C., Götz F., Staphylococcus aureus deficient in lipidation of prelipoproteins is attenuated in growth and immune activation. Infect. Immun. 73, 2411–2423 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spaan A. N., et al. , Staphylococcus aureus targets the Duffy antigen receptor for chemokines (DARC) to lyse erythrocytes. Cell Host Microbe 18, 363–370 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang R., et al. , Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nat. Med. 13, 1510–1514 (2007). [DOI] [PubMed] [Google Scholar]
- 46.Lipowsky R., Remodeling of membrane compartments: Some consequences of membrane fluidity. Biol. Chem. 395, 253–274 (2014). [DOI] [PubMed] [Google Scholar]
- 47.Schwechheimer C., Kuehn M. J., Outer-membrane vesicles from Gram-negative bacteria: Biogenesis and functions. Nat. Rev. Microbiol. 13, 605–619 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Galla H. J., Hartmann W., Excimer-forming lipids in membrane research. Chem. Phys. Lipids 27, 199–219 (1980). [DOI] [PubMed] [Google Scholar]
- 49.Santos J. C., et al. , LPS targets host guanylate-binding proteins to the bacterial outer membrane for non-canonical inflammasome activation. EMBO J. 37, e98089 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Losier T. T., et al. , AMPK promotes xenophagy through priming of autophagic kinases upon detection of bacterial outer membrane vesicles. Cell Rep. 26, 2150–2165.e5 (2019). [DOI] [PubMed] [Google Scholar]
- 51.Yang D., et al. , Dysregulated lung commensal bacteria drive interleukin-17B production to promote pulmonary fibrosis through their outer membrane vesicles. Immunity 50, 692–706 e7 (2019). [DOI] [PubMed] [Google Scholar]
- 52.Finethy R., et al. , Inflammasome activation by bacterial outer membrane vesicles requires guanylate binding proteins. MBio 8, e01188-17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Green E. R., Mecsas J., Bacterial secretion systems: An overview. Microbiol. Spectr. 4, 215–239 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vladimer G. I., Marty-Roix R., Ghosh S., Weng D., Lien E., Inflammasomes and host defenses against bacterial infections. Curr. Opin. Microbiol. 16, 23–31 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Holzinger D., et al. , Staphylococcus aureus Panton-Valentine leukocidin induces an inflammatory response in human phagocytes via the NLRP3 inflammasome. J. Leukoc. Biol. 92, 1069–1081 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Muñoz-Planillo R., et al. , K+ efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity 38, 1142–1153 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Seilie E. S., Bubeck Wardenburg J., Staphylococcus aureus pore-forming toxins: The interface of pathogen and host complexity. Semin. Cell Dev. Biol. 72, 101–116 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang H., et al. , Mixed lineage kinase domain-like protein MLKL causes necrotic membrane disruption upon phosphorylation by RIP3. Mol. Cell 54, 133–146 (2014). [DOI] [PubMed] [Google Scholar]
- 59.González-Juarbe N., et al. , Pore-forming toxins induce macrophage necroptosis during acute bacterial pneumonia. PLoS Pathog. 11, e1005337 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kitur K., et al. , Necroptosis promotes Staphylococcus aureus clearance by inhibiting excessive inflammatory signaling. Cell Rep. 16, 2219–2230 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Giese B., et al. , Expression of δ-toxin by Staphylococcus aureus mediates escape from phago-endosomes of human epithelial and endothelial cells in the presence of β-toxin. Cell. Microbiol. 13, 316–329 (2011). [DOI] [PubMed] [Google Scholar]
- 62.Melehani J. H., James D. B., DuMont A. L., Torres V. J., Duncan J. A., Staphylococcus aureus leukocidin A/B (LukAB) kills human monocytes via host NLRP3 and ASC when extracellular, but not intracellular. PLoS Pathog. 11, e1004970 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shimada K., et al. , Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity 36, 401–414 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lopez-Castejon G., et al. , P2X(7) receptor-mediated release of cathepsins from macrophages is a cytokine-independent mechanism potentially involved in joint diseases. J. Immunol. 185, 2611–2619 (2010). [DOI] [PubMed] [Google Scholar]
- 65.Orlowski G. M., et al. , Frontline Science: Multiple cathepsins promote inflammasome-independent, particle-induced cell death during NLRP3-dependent IL-1β activation. J. Leukoc. Biol. 102, 7–17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Campden R. I., Zhang Y., The role of lysosomal cysteine cathepsins in NLRP3 inflammasome activation. Arch. Biochem. Biophys. 670, 32–42 (2019). [DOI] [PubMed] [Google Scholar]
- 67.Jubrail J., et al. , Inability to sustain intraphagolysosomal killing of Staphylococcus aureus predisposes to bacterial persistence in macrophages. Cell. Microbiol. 18, 80–96 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Coelho C., et al. , Listeria monocytogenes virulence factors, including listeriolysin O, are secreted in biologically active extracellular vesicles. J. Biol. Chem. 294, 1202–1217 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chiplunkar S. S., Silva C. A., Bermudez L. E., Danelishvili L., Characterization of membrane vesicles released by Mycobacterium avium in response to environment mimicking the macrophage phagosome. Future Microbiol. 14, 293–313 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Buddelmeijer N., The molecular mechanism of bacterial lipoprotein modification–How, when and why? FEMS Microbiol. Rev. 39, 246–261 (2015). [DOI] [PubMed] [Google Scholar]
- 71.Schmaler M., et al. , Lipoproteins in Staphylococcus aureus mediate inflammation by TLR2 and iron-dependent growth in vivo. J. Immunol. 182, 7110–7118 (2009). [DOI] [PubMed] [Google Scholar]
- 72.Baumgärtner M., et al. , Inactivation of Lgt allows systematic characterization of lipoproteins from Listeria monocytogenes. J. Bacteriol. 189, 313–324 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kovacs-Simon A., Titball R. W., Michell S. L., Lipoproteins of bacterial pathogens. Infect. Immun. 79, 548–561 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings of this study are available within the main text and SI Appendix. Mass spectrometry proteomics data were deposited in the ProteomeXchange Consortium (http://proteomecentral.proteomexchange.org/cgi/GetDataset) via the PRIDE partner repository with the data set identifier PXD014888.