Abstract
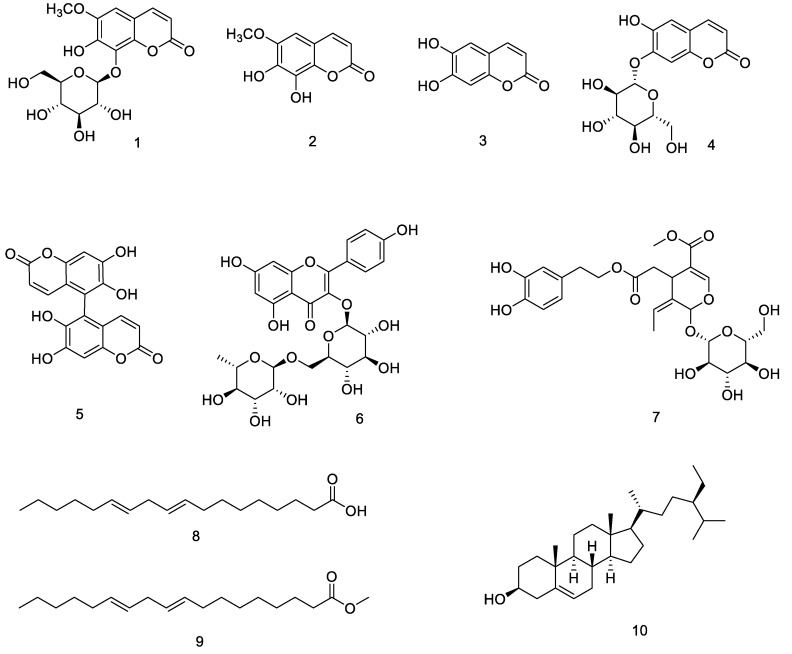
The phytochemical investigation of Fraxinus hupehensis led to the isolation and characterization of ten compounds which were identified as fraxin (1), fraxetin (2), esculetin (3), cichoriin (4), euphorbetin (5), kaempferol-3-O-β-rutinoside (6), oleuropein (7), linoleic acid (8), methyl linoleate (9), and β-sitosterol (10). Structures of the isolated constituents were characterized by 1H NMR, 13C NMR and HRMS. All the compounds, except compounds 3 and 4, were isolated for the first time from this plant. Further, this was the first report for the occurrence of compound 5 in the Fraxinus species. Antifungal activity evaluation showed that compound 2 exhibited significant inhibitory effects against Bipolaris maydis, Sclerotium rolfsii, and Alternaria solani with EC50 values of 0.31 ± 0.01 mmol/L, 10.50 ± 0.02 mmol/L, and 0.40 ± 0.02 mmol/L respectively, compared to the positive control, Carbendazim, with its EC50 values of 0.74 ± 0.01 mmol/L, 1.78 ± 0.01 mmol/L and 1.41 ± 0.00 mmol/L. Herbicidal activity tests showed that compounds 8–10 had strong inhibitory effects against the roots of Echinochloa crus-galli with EC50 values of 1.16 ± 0.23 mmol/L, 1.28 ± 0.58 mmol/L and 1.33 ± 0.35 mmol/L respectively, more potently active than that of the positive control, Cyanazine, with its EC50 values of 1.56 ± 0.44 mmol/L. However, none of the compounds proved to be active against the tested bacteria (Erwinia carotovora, Pseudomonas syringae, and Ralstonia solanacearum).
Keywords: Fraxinus hupehensis, isolation and characterization, phytochemical investigation, fungicide, herbicidal activity
1. Introduction
The genus Fraxinus includes more than 60 species in the world and 30 native species in China, many of which commonly used in Traditional Chinese Medicines. F. hupehensis, a member of Oleaceae family, is only distributed in Zhongxiang and Jingshan counties, Hubei province of China [1]. It grows slowly, lives long and often forms spines in pairs on their branches. Besides, due to its beautiful tree shape and twisted roots, it is considered to be an ideal bonsai and landscape greening plant which has been called Living fossil or King of bonsai [2]. In folk applications, it is very popular because of its delicate appearance and its lack of pests [3]. However, since it was discovered as a new species in 1979, F. hupehensis has been subjected to excessive resource exploitation. In addition, its seeds have long dormancy period (>1 year) which limits their dispersal, so the populations of this precious plant have been continuing to decline, and it was officially recognized as a rare and endangered plant in China in 1990 [4].
In traditional Chinese medicine, some species of the genus Fraxinus have been used and the barks of them called Cortex Fraxini [5], commonly known as ‘Qin-Pi’ in Chinese. At present, the origin of Chinese medicine ‘Qin-Pi’ mainly includes F. rhynchophylla Hance, F. chinensis Roxb, F. szaboana Lingelsh and F. stylosa Lingelsh. The bark of F. hupehensis is sometimes used as Cortex Fraxinus [6]. Previous studies have shown that they have anti-inflammatory [7], anti-tumor [8], anti-bacterial [9], anti-viral [10], and antioxidant [11] activities. In addition, some literature indicated the leaves extract exhibited antifungal activities [12,13,14]. However, there are only two previously reports [15,16] for the phytochemical investigation of F. hupehensis to the best of our knowledge. Based on the above information, it is necessary to systematically study its phytochemistry and examine its fungicidal, herbicidal and bactericidal activities.
2. Experimental
2.1. Plant Collection and Authentication
The leaves and bark of F. hupehensis were collected in the Bonsai garden of the western campus of Yangtze University, Hubei, China in May 2017, and identified by one of authors, Prof. Qing-Lai Wu. A voucher specimen was deposited in the herbarium of Yangtze University (YZU201705-FH).
2.2. Extraction, Isolation and Identification
The leaves of F. hupehensis (10.0 kg) were dried at 25 °C and pulverized by a plant grinder (Shanghai Heysu Pharmceutical Machinery co., LTD, Shanghai, China). They were extracted three times with 60 L of 80% MeOH at room temperature. After removal of the solvent under reduced pressure, the residue of crude extract was gained (1.02 kg). The crude extract was suspended in H2O (1.5 L) and partitioned successively with petroleum ether, EtOAc and n-butanol, respectively. The EtOAc extract (180 g, solid) was subjected to a silica gel column chromatography (CC), and eluted with CHCl3-MeOH (20:1, 18:1, 16:1, 14:1, 12:1, 10:1, 8:1, 6:1, 4:1, 2:1, 1:1) to obtain 26 fractions (A–Z). Fraction F was applied to silica gel CC and eluted with a gradient of chloroform-methanol (90:10–1:100) to give 18 sub-fractions (F1–F18). The fraction F1 was dissolved in a solvent of chloroform-methanol (4:1) and recrystallized at −10 °C to obtain compound 1. Fraction G was dissolved in methanol and filtered. The filtrate was evaporated, and then chromatographed on a silica gel CC with CHCl3-MeOH (20:1~5:1) to yield five sub-fractions (G1–G5). The sub-fraction G5 was recrystallized to give compounds 2 and 3. Fraction O was chromatographed on a silica gel CC eluting with CHCl3-MeOH (5:1~2:1) to obtain three sub-fractions (O1–O3). And then the O2 part was recrystallized to give compound 4. Fraction L was subjected to a Sephadex LH–20 CC (Pharmacia Biotech Ltd. Piscataway, NJ, USA), eluting with MeOH to yield compound 7. Fraction P was subjected to C18 reverse phase CC and eluted with MeOH-H2O (50:50–100:0) to gain ten sub-fractions (P1–P10). Sub-fraction P6 was separated by Sephadex LH–20 CC (MeOH) to give compound 6. Sub-fraction P8 was purified by C18 reverse column CC, eluting with MeOH-H2O (70:30) to give compound 5.
The bark of F. hupehensis (1 kg) was extracted with 95% EtOH twice, each time at 80 °C for 3 h. The EtOH extract was isolated on a silica gel column to give seven fractions (Fr.1–7), and Fr.1 was repeatedly purified by Sephadex LH–20 CC, eluting with CHCl3-MeOH (1:2) to give compound 8. Fr.2 was separated by silica gel CC (200–300 mesh) to obtain eight sub-fractions (Fr.2.1–2.8). Fr.2.3 was subjected on a Sephadex LH–20 CC to give compound 9. Fr.2.5 was recrystallized three times to give compound 10 (See Figure 1).
Figure 1.
Chemical structures of 1–10 isolated from F. hupehensis.
Chemicals and solvents were purchased from commercial suppliers and were used without further purification. Thin-layer chromatography (TLC) was performed on silica gel 60 F254 (Qingdao Marine Chemical Ltd., Qingdao, China). Column chromatography (CC) was performed over silica gel (200–300 mesh, Qingdao Marine Chemical Ltd.). 1H and 13C NMR spectrum were recorded in CDCl3 or DMSO-d6 solution on a Bruker 400 MHz spectrometer (Bruker Co., Fällanden, Switzerland), using tetramethyl silane (TMS) as an internal standard, and chemical shift values (δ) were given in parts per million (ppm). The following abbreviations were used to designate chemical shift multiplicities: s = singlet, d = doublet, t = triplet, q = quartet, m = multiple. MS data were obtained using an APEX IV Fourier-Transform Mass Spectrometry (Bruker). All spectra are shown in Supplementary Materials.
Compound 1: Yellow solid; m.p. 145–146 °C; HRESIMS: m/z 369.0827 [M − H]− (calcd for C16H17O10, 369.0822). 1H NMR (DMSO-d6; 400 MHz): δ ppm 7.93 (d, J = 9.6 Hz, 1H), 7.07 (s, 1H), 6.27 (d, J = 9.6 Hz, 1H), 5.18 (d, J = 4.7 Hz, 1H), 5.03 (d, J = 4.7 Hz, 1H), 4.97 (d, J = 7.7 Hz, 1H), 3.14–3.44 (overlapped ), 3.81 (s, 6–OCH3, 3H). 13C NMR (DMSO-d6; 101 MHz): δ ppm 160.66, 145.88, 145.22, 144.13, 143.13, 132.01, 112.70, 110.59, 105.39, 104.34, 77.81, 76.70, 74.31, 70.07, 61.21, 56.54. Comparing these NMR data with ref. [17], compound 1 was identified as fraxin.
Compound 2: Yellow solid; m.p. 228–229 °C; HRESIMS: m/z 209.0438 [M + H]+ (calcd for C10H9O5, 209.0450). 1H NMR (DMSO-d6; 400 MHz): δ ppm 9.52 (s, 2H), 7.89 (d, J = 9.6 Hz, 1H), 6.80 (s, 1H), 6.22 (d, J = 9.6 Hz, 1H), 3.86 (s, 3H). 13C NMR (DMSO-d6; 101 MHz): δ ppm 160.99, 145.80, 145.50, 139.77, 139.76, 133.31, 112.30, 110.70, 100.77, 56.50. Comparing these data with ref. [17], compound 2 was identified as fraxetin.
Compound 3: Yellow solid; m.p. 271–273 °C; HRESIMS: m/z 179.0333 [M + H]+ (calcd for C9H7O4, 179.0344). 1H NMR (DMSO-d6; 400 MHz): δ ppm 9.83 (s, 2H), 7.87 (d, J = 9.6 Hz, 1H), 6.98 (s, 1H), 6.75 (s, 1H), 6.17 (d, J = 9.6 Hz, 1H). 13C NMR (DMSO-d6; 101 MHz): δ ppm 161.25, 150.85, 148.95, 144.89, 143.33, 112.78, 111.96, 111.22, 103.10. Comparing these data with ref. [17], compound 3 was identified as esculetin.
Compound 4: White solid; m.p. 196–198 °C; HRESIMS: m/z 341.0862 [M + H]+ (calcd for C15H17O9, 341.0873). 1H NMR (DMSO-d6; 400 MHz): δ ppm 9.00 (s, 1H), 7.93 (d, J = 9.5 Hz, 1H), 7.14 (s, 1H), 7.10 (s, 1H), 6.31 (d, J = 9.5 Hz, 1H), 5.38 (s, 1H), 5.12 (d, J = 3.3 Hz, 1H), 5.10 (d, J = 5.1 Hz, 1H), 4.94 (d, J = 7.3 Hz, 1H), 4.66 (t, J = 5.3 Hz, 1H), 3.75 (dd, J = 9.4, 4.8 Hz, 1H), 3.46 (q, J = 5.7 Hz, 3H), 3.39–3.30 (m, 1H), 3.17 (d, J = 4.1 Hz, 1H). 13C NMR (DMSO-d6; 101 MHz): δ ppm 161.01, 149.27, 148.26, 144.62, 144.04, 113.91, 113.43, 113.10, 103.84, 101.46, 77.74, 76.33, 73.64, 70.27, 61.20. Comparing these data with ref. [17], compound 4 was identified as cichoriin.
Compound 5: Orange oil; HRESIMS: m/z 353.03.3 [M − H]− (calcd for C18H9O8, 353.0297). 1H NMR (DMSO-d6; 400 MHz): δ ppm 9.75 (s, 4H), 7.14 (d, J = 9.4 Hz, 2H), 6.89 (s, 2H), 6.14 (dd, J = 9.1 Hz, 2H). 13C NMR (DMSO-d6; 101 MHz): δ ppm 161.02, 150.75, 149.24, 143.22, 141.55, 118.13, 112.04, 110.81, 102.55. Comparing these data with ref. [18], compound 5 was identified as euphorbetin.
Compound 6: Yellow solid; m.p. 181–183 °C; HRESIMS: m/z 593.1515 [M − H]– (calcd for C27H29O15, 693.1506). 1H NMR (DMSO-d6; 400 MHz): δ ppm 12.56 (s, 1H), 7.99 (d, J = 8.8 Hz, 2H), 6.88 (d, J = 8.8 Hz, 2H), 6.41 (d, J = 2.0 Hz, 1H), 6.20 (dd, J = 2.0 Hz, 1H), 5.31 (d, J = 7.4 Hz, 1H), 4.38 (s, 1H), 4.03 (q, J = 7.1 Hz, 1H), 3.69 (d, J = 9.7 Hz, 1H), 3.63–2.89 (overlapped, 9H), 0.97 (d, J = 6.1 Hz, 3H),. 13C NMR (DMSO-d6; 101 MHz): δ ppm 177.80, 164.70, 161.65, 160.36, 157.26, 156.98, 133.66, 131.34, 131.34, 121.35, 115.56, 104.34, 101.82, 101.24, 99.26, 94.25, 76.81, 76.19, 74.64, 72.27, 71.05, 70.81, 70.37, 68.71, 60.23, 18.2. Comparing these data with ref. [19], compound 6 was identified as kaempferol–3–rutinoside.
Compound 7: Yellow solid; m.p. 89–90 °C; HRESIMS: m/z 539.1775 [M − H]– (calcd for C25H31O13, 539.1765). 1H NMR (DMSO-d6; 400 MHz): δ ppm 8.74 (s, 2H), 7.52 (d, J = 2.0 Hz, 1H), 6.64 (d, J = 8.0 Hz, 1H), 6.61 (d, J = 2.0 Hz, 1H), 6.48 (dd, J = 8.0, 2.0 Hz, 1H), 5.97 (q, J = 6.9 Hz, 1H), 5.87 (t, J = 1.8 Hz, 1H), 5.28–4.89 (m, 3H), 4.65 (d, J = 7.8 Hz, 1H), 4.51 (s, 1H), 4.08 (qt, J = 10.7, 7.2 Hz, 2H), 3.86 (dd, J = 9.1, 4.2 Hz, 1H), 3.70 (m, 1H), 3.65 (s, 3H), 3.46 (dd, J = 11.7, 6.3 Hz, 1H), 3.25–3.00 (m, 2H), 3.08 (td, J = 8.9, 4.2 Hz, 2H), 2.68 (t, J = 7.2 Hz, 2H), 2.50 (t, J = 1.9 Hz, 1H), 2.40 (dd, J = 14.4, 9.3 Hz, 1H), 1.65 (dd, J = 7.1, 1.4 Hz, 3H). 13C NMR (DMSO-d6; 101 MHz): δ ppm 171.15, 166.65, 153.90, 145.55, 144.22, 129.60, 128.86, 123.51, 120.01, 116.64, 115.99, 108.15, 99.45, 93.39, 77.82, 76.97, 73.73, 70.39, 65.52, 61.56, 51.73, 49.07, 34.16, 30.59, 13.47. Comparing these data with ref. [20], compound 7 was identified as oleuropein.
Compound 8: Pale yellow oil; HRESIMS: m/z 303.2297 [M + Na]+ (calcd for C18H32O2Na, 303.2300). 1H NMR (CDCl3, 400 MHz): δ ppm 5.46–5.24 (m, 4H), 2.77 (t, J = 6.3 Hz, 2H), 2.34 (t, J = 7.5 Hz, 2H), 2.05 (q, J = 6.9 Hz, 4H), 1.62 (p, J = 7.2 Hz, 2H), 1.46–1.19 (m, 14H), 0.89 (t, J = 6.9 Hz, 3H). 13C NMR (CDCl3, 101 MHz) δ ppm 180.50, 129.94, 129.75, 127.96, 127.79, 33.99, 31.44, 29.50, 29.27, 29.07, 28.99, 28.95, 27.10, 27.07, 25.52, 24.54, 22.49, 13.91. Comparing these data with ref. [21], compound 8 was similar to the data of linolenic acid.
Compound 9: Pale yellow oil; HRESIMS: m/z 317.2451 [M + Na]+ (calcd for C19H34O2Na, 317.2457). 1H NMR (CDCl3, 400 MHz): δ ppm 5.49–5.17 (m, 4H), 3.66 (s, 3H), 2.77 (t, J = 6.3 Hz, 2H), 2.30 (t, J = 7.5 Hz, 2H), 2.05 (dd, J = 6.8 Hz, 4H), 1.62 (t, J = 7.2 Hz, 2H),, 1.39–1.17 (m, 14H), 0.89 (t, J = 6.8 Hz, 3H). 13C NMR (CDCl3, 101 MHz): δ ppm 173.30, 129.60, 129.45, 127.68, 127.55, 50.74, 33.56, 31.20, 29.26, 29.03, 28.86, 28.80, 28.77, 26.84, 26.82, 25.26, 24.57, 22.25, 13.63. Comparing these data with ref. [22], compound 9 was similar to the data of methyl linoleate.
Compound 10: White solid; m.p. 140 °C; HRESIMS: m/z 437.3755 [M + Na]+ (calcd for C29H50ONa, 437.3759). 1H NMR (CDCl3, 400 MHz): δ ppm 5.35 (m, 1H), 3.51 (m, 1H), 1.02 (s, 3H), 0.93 (d, J = 6.6 Hz, 3H), 0.85 (t, J = 7.1 Hz, 3H), 0.83 (t, J = 6.3 Hz, 3H), 0.81 (t, J = 7.1 Hz, 3H), 0.68 (s, 3H). 13C NMR (DMSO–d6; 101 MHz): δ ppm 140.72, 121.68, 71.75, 56.73, 56.01, 50.09, 45.79, 42.28, 42.24, 39.74, 37.22, 36.46, 36.11, 33.90, 31.87, 31.87, 31.60, 29.11, 28.22, 26.02, 24.27, 23.04, 21.05, 19.79, 19.37, 19.00, 18.75, 11.95, 11.83. Comparing these data with ref. [18], compound 10 was identified as β–sitosterol.
2.3. Biological Activities
2.3.1. Determination of Antifungal Activities
All compounds were tested by the mycelium growth rate method [23] against six pathogenic fungi (Rhizoctonia solani, Fusarium graminearum, Bipolaris maydis, Botrytis cinema, Sclerotium rolfsii, and Alternaria solani) at the concentration of 0.2 mmol/L. The compounds were dissolved in acetone and the solutions were diluted with aqueous 0.1% Tween-80 and then added to sterile potato dextrose agar (PDA). After the PDA was solidified, the pathogenic fungi cakes (6 mm) were placed on the center of the culture plates and then incubated at 28 °C. The same concentration of the Carbendazim was used as the positive control, a common broad-spectrum fungicide. The same concentration of acetone and aqueous 0.1% tween-80 were used as negative control, repeating three times for each treat. When the mycelia of CK grew to 3/4 area of the diameter, it was measured by the cross-intersection method [24], and the inhibitory ratio was calculated by the Equation 1.
In order to accurately inspect the inhibitory effect of compound 2, EC50 values were tested against six pathogens fungi. The compound was formed the stock solution of 0.8 mmol/L and divided into 6 gradients. Different gradient concentrations were 0.8 mmol/L, 0.6 mmol/L, 0.4 mmol/L, 0.2 mmol/L, 0.1 mmol/L, 0.05 mmol/L, respectively. The method of inoculating pathogenic fungi was equal to the method for testing the antifungal activities, and the method of measuring the circle mycelium was equal to Equation (1).
| Relative inhibitory ratio (%) = [(CK − PT)/(CK − 6 mm)] × 100% | (1) |
CK is the diameter of the mycelium circle in the negative control group and PT is the diameter of the mycelium circle in the treatment group.
2.3.2. Determination of Herbicidal Activities
All the compounds were tested the herbicidal activities against Echinochloa crus-galli and Brassica napus by the seed germination method [25]. The method of dispensing was equal to that in the experiment of antifungal activities. Subsequently, 5 mL of the solution was placed in 75 mm medium with double-layer filter paper when the seeds were emerge-germinating, and each culture dish was sow 12 seeds. The same concentration of Cyanazine was used as positive control, a common broad-spectrum herbicide. The seeds in the medium were germinated in the light incubator with the temperature 28 ± 1 °C, the humidity 80% rH, the luminance 1100 lux, and the photoperiod 14 h/day. When the root or stalk of the CK group grew to 40–50 mm, the measurement started. And the inhibitory ratios were calculated by Equation (2).
In order to test the EC50 value of compound 8 against Barnyard grass, 200 mL of 8 mmol/L stock solution was prepared and divided into five gradients, which were 8 mmol/L, 4 mmol/L, 2 mmol/L, 1 mmol/L, and 0.5 mmol/L, respectively. The method of sowing, cultivating, and measuring was equal to the above herbicidal activities experiment.
| Relative inhibitory ratio (%) = [(CK − PT)/CK] × 100% | (2) |
CK is the root or stalk length of the negative control group and PT is the root or stalk length of the treatment group.
2.3.3. Determination of Antibacterial Activities
In this experiment, all the isolates were examined by the filter paper dispersion methods [26] against Erwinia carotovora, Pseudomonas syringae and Ralstonia solanacearum. Each compound was prepared with 0.2 mmol/L of the solution. 90 mL beef extract peptone agar medium was made (peptone (m): NaCl (m): glucose (m): agar (m): deionized water (v) = 0.6:2:1:2:3.6:200), which were sterilized at 121 °C for 30 min, and poured into the 90 mm plate, 30 mL for each dish. When the medium was concretionary, a ring of activated bacteria was picked up by the inoculating loop, and parallel lines were drawn on the medium, and the sealing film was sealed in a 37 °C biochemical incubator for 1–2 days. 150 mL beef extract peptone liquid medium was made (peptone (m): NaCl (m): glucose (m): deionized water (v) = 0.6:2:1:2:200), which were sterilized at 121 °C for 30 min. When the bacterial suspension was formed, a ring of growing colonies was placed in liquid medium and placed at 37 °C with 150 r/min incubator for 24 h. Using saline to dilute the bacterial suspension to a concentration of 6 × 109–6 × 1010 cfu/mL by double gradient dilution method. 0.2 mL of the diluted bacterial suspension were pipetted into 90 mL beef extract peptone agar medium (45–50 °C) and packed separately to solid. The filter paper was dipped into the extract solution for 3 s and pasted on the center of the delineated area of the bacteria-containing medium. The same concentration of tetracycline was used as positive control. Each treatment was repeated three times and placed in a 37 °C incubator for 24 h. The diameter of the inhibitory zone was measured by the cross method.
In order to ensure the validity of the results, the difference from two parallel measurements should not be greater than one step on the dilution scale. The experiments were conducted in duplicates. Each value is represented in terms of mean (n = 3) ± SD (Standard deviation). All activities were evaluated by statistical analysis. All statistical analysis was performed using EXCEL 2010 software. The log dose-response curves allowed determination of the EC50 for the fungi bioassay according to probit analysis. The 95% confidence limits for the range of EC50 values were determined by the least-square regression analysis of the relative growth rate (% control) against the logarithm of the compound concentration.
3. Results
3.1. Antifungal Activity
The result of antifungal activities was shown in Table 1. It can be seen the most of compounds have different levels of inhibitory effects against the tested fungi at the concentration of 0.2 mmol/L. Compounds 2, 3, and 4 have moderate inhibitory effects against all tested fungi, and compound 2 was the best, with the inhibitory effect of against Sclerotium rolfsii reaching 30.34 ± 8.43%, which was higher than positive control, Carbendazim, a broad-spectrum fungicide, whose inhibitory rate was 7.3 ± 4.59%. Comparing the bioassay results of compounds 1–5 belonging to the same skeleton of coumarin [27], the occurence of hydroxyl at C-8 could enhance the activity against Sclerotium rolfsii and the structure-activity relationship of these coumarin derivatives need be further studied later.
Table 1.
Inhibitory ratio of 10 compounds against six phytopathogenic fungi (Inhibitory ratio ± SD, %).
| Compd. | Rhizoctonia solani | Fusarium graminearum | Bipolaris maydis | Botrytis cinema | Sclerotium rolfsii | Alternaria solani |
|---|---|---|---|---|---|---|
| 1 | −1.14 ± 0.99 | 1.28 ± 2.22 | −0.72 ± 1.26 | 4.54 ± 1.07 | 9.46 ± 3.10 | 4.96 ± 0.07 |
| 2 | 36.37 ± 1.68 | 23.33 ± 2.89 | 37.39 ± 2.85 | 20.1 ± 1.82 | 30.34 ± 8.43 | 32.22 ± 2.09 |
| 3 | 27.78 ± 4.72 | −1.33 ± 3.00 | 10.08 ± 2.57 | 6.44 ± 4.80 | 13.52 ± 6.26 | 0.79 ± 3.75 |
| 4 | 17.64 ± 1.44 | 1.26 ± 2.98 | 6.46 ± 2.08 | 11.67 ± 1.73 | 14.19 ± 0.16 | 0.81 ± 1.41 |
| 5 | −1.63 ± 1.64 | −2.37 ± 1.04 | −2.48 ± 1.06 | 5.34 ± 3.29 | −1.15 ± 3.42 | 2.1 ± 2.08 |
| 6 | −3.24 ± 0.03 | −0.06 ± 1.03 | −0.31 ± 2.13 | −0.11 ± 4.60 | 0.94 ± 4.81 | 1.4 ± 1.22 |
| 7 | −1.62 ± 0.02 | 1.78 ± 1.79 | −3.73 ± 1.85 | 0.69 ± 3.46 | 5.20 ± 3.14 | 3.52 ± 1.20 |
| 8 | 18.91 ± 2.32 | −0.12 ± 2.71 | 0.31 ± 1.05 | 7.67 ± 3.21 | 2.58 ± 2.36 | 21.3 ± 2.13 |
| 9 | 18.94 ± 3.57 | 28.99 ± 1.93 | 0.37 ± 3.21 | −0.85 ± 3.57 | −1.67 ± 2.89 | 3.52 ± 1.21 |
| 10 | 32.43 ± 1.37 | 4.73 ± 1.00 | −2.49 ± 2.16 | −3.17 ± 2.75 | −0.6 ± 2.49 | 3.49 ± 4.33 |
| Carbendazim | 100.00 ± 0.00 | 100.00 ± 0.00 | 24.23 ± 0.26 | 36.35 ± 3.38 | 7.3 ± 4.59 | 11.29 ± 2.56 |
Note: The values represent the mean ± SD of three individual observations.
In addition, the inhibitory effect of compound 2 against Alternaria solani was also higher than the positive control, reaching 32.23 ± 0.49%. In order to further inspect the inhibitory activity of compound 2, EC50 values were obtained, as shown in Table 2. These results showed that it could be used as a lead compound for broad-spectrum fungicide and had great potential development value and prospects.
Table 2.
The EC50 values (mmol/L) of compound 2 against six pathogenic fungi.
| Compd. | Rhizoctonia solani | Fusa rium graminearum | Bipolaris maydis | Botrytis cinema | Sclerotium rolfsii | Alternaria solani |
|---|---|---|---|---|---|---|
| 2 | 0.33 ± 0.01 | 0.48 ± 0.02 | 0.31 ± 0.01 | 1.11 ± 0.02 | 0.50 ± 0.02 | 0.40 ± 0.02 |
| Carbendazim | 0.12 ± 0.00 | 0.13 ± 0.01 | 0.74 ± 0.01 | 0.32 ± 0.01 | 1.78 ± 0.01 | 1.41 ± 0.00 |
Note: The values represent the mean ± SD of three individual observations.
3.2. Herbicidal Activity
The results of herbicidal activities were shown in Table 3. Among them, compounds 8 and 10 showed excellent inhibitory effects against roots of Echinochloa crus-galli, which were higher than 90%. And the inhibitory activitis against stalk of E. crus-galli were also excellent, reaching 78.07 ± 0.32% and 63.16 ± 0.32%, respectively, which were both higher than the positive control (40.39 ± 0.21%). According to the results of EC50 values, compound 8 showed outstanding inhibitory effects against the tested weeds (Table 4). It is interesting to note that compound 8 is not generally considered a bioactive ingredient, due to its widespread nature and little structural novelty. Nonetheless, previous studies on herbicidal and phytotoxic activities of linoleic acid and similar fatty acid derivatives, have been reported [28,29,30,31,32,33]. So, the structural modification of linoleic acid and its mechanism of herbicidal activity, remain to be valuable to further study.
Table 3.
Inhibitory ratio of 10 compounds against Echinochloa crus-galli and Brassica napus (Inhibitory ratio ± SD, %).
| Compd. | Echinochloa crus-galli | Brassica napus | ||
|---|---|---|---|---|
| Root | Stalk | Root | Stalk | |
| 1 | 18.32 ± 0.29 | 4.13 ± 0.29 | 32.28 ± 0.82 | −28.93 ± 1.76 |
| 2 | 40.36 ± 0.24 | 5.63 ± 0.36 | 13.26 ± 1.94 | −45.62 ± 1.44 |
| 3 | 43.29 ± 0.23 | 17.56 ± 0.42 | −25.53 ± 4.08 | −33.06 ± 1.89 |
| 4 | 29.97 ± 0.27 | 18.66 ± 0.33 | −16.85 ± 2.32 | −51.37 ± 1.67 |
| 5 | 47.86 ± 0.27 | 13.14 ± 0.26 | 48.11 ± 1.22 | −10.91 ± 0.37 |
| 6 | 9.95 ± 0.21 | 2.69 ± 0.18 | −43.08 ± 4.16 | −27.20 ± 0.38 |
| 7 | 4.61 ± 0.29 | −7.92 ± 0.20 | −29.04 ± 1.96 | 4.85 ± 0.27 |
| 8 | 96.71 ± 0.06 | 78.07 ± 0.32 | 29.82 ± 1.78 | 14.35 ± 0.39 |
| 9 | 64.85 ± 0.41 | 71.56 ± 0.34 | −59.43 ± 4.03 | −35.07 ± 0.78 |
| 10 | 91.43 ± 0.10 | 63.16 ± 0.32 | 28.54 ± 1.52 | −10.77 ± 0.40 |
| Cyanazine | 66.52 ± 0.08 | 40.39 ± 0.21 | 48.83 ± 0.68% | 6.78 ± 0.32 |
Note: The values represent the mean ± SD of three individual observations.
Table 4.
The EC50 values (mmol/L) of compounds 8–10 against the root of E. crus-galli.
| Compd. | Root | Stalk |
|---|---|---|
| 8 | 1.16 ± 0.23 | 1.32 ± 0.27 |
| 9 | 1.28 ± 0.58 | 1.31 ± 0.46 |
| 10 | 1.33 ± 0.35 | 2.35 ± 0.98 |
| Cyanazine | 1.56 ± 0.44 | 2.84 ± 0.73 |
Note: The values represent the mean ± SD of three individual observations.
3.3. Antibacterial Activity
Regrettably, no compound showed any antibacterial activities against the tested bacteria (Erwinia carotovora, Pseudomonas syringae and Ralstonia solanacearum). We speculated that we have not found the right bacteria, considering that all the selected bacteria are Gram-negative [34]. However, due to the limitations of research conditions, we failed to carry out further research on the broader selection and specific mechanism.
4. Conclusions
The genus Fraxinus contains 30 native species in China and only four species of them previously can be used as the origin of Chinese medicine ‘Qin-Pi’ which based on their morphological characteristics and folk medicinal efficacy. In addition, previous phytochemistry and chemotaxonomic research on Fraxinus have shown coumarins are their characteristic ingredients. In this work, chemical constituents from F. hupehensis were isolated systematically for the first time, and five of ten compounds obtained were coumarins. To our best knowledge, known coumarins 1–5 were isolated for the first time from the leaves of F. hupehensis and compound 5 was the first report in genus Fraxinus, which indicated that F. hupehensis and ‘Qin-Pi’ ′s species have a close relationship in term of chemical taxonomy.
Furthermore, All the isolated compounds were evaluated for antifungal, herbicidal and antibacterial activities. The results showed compound 2 deserves favourable effect for fungicide. The structural modification of 2, belonging to the coumarin skeleton, and the structure-activity relationship of these coumarin derivatives have a very valuable prospect for the development of novel plant-derived pesticides in the future. More research is needed to probe into these activities and to explore the mechanisms of action of these active compounds.
Acknowledgments
Thanks for Tom Hsiang who revised the abstract and introduction for this article.
Supplementary Materials
The following are available online at https://www.mdpi.com/2218-273X/10/1/74/s1, Figure S1: Antifungal activity of 2 against Bipolaris maydis evaluated by the mycelium growth rate method; and Figure S2: Comparison of the effects of 8–10 and Cyanazine against Echinochloa crus-galli growth (CK reprents Echinochloa cr us-galli grown on regular medium.)
Author Contributions
Conceptualization, Q.-L.W. and J.-K.L.; software and formal analysis (spectral analysis and structure determination), X.-D.Z.; methodology and investigation (the extraction and isolation, bioactivities assay), C.-N.Z. and Z.-L.Y.; data curation, D.Y. and J.K.; writing—original draft preparation, C.-N.Z. and Z.-L.Y.; writing—review and editing, X.-D.Z.; visualization and supervision, Q.-L.W. and J.-K.L.; project administration and funding acquisition, Q.-L.W. and J.-K.L. All authors have read and agreed to the published version of the manuscript.
Funding
The authors gratefully acknowledge financial support by the National Natural Science Foundation of China (Nos. 31672069 and 81903514) and the National Key R&D Program of China (No. 2018YFD0200500).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Qu S.Z., Xiang Q.B., Su P.L. A new specie of the genus Fraxinus Linn. from Hubei province. J. Nanjing For. Univ. 1979;21:146–148. [Google Scholar]
- 2.Cheng J.R., Zhou M.Q. The distribution status and conservation strategy of Fraxinus hupehensis germplasm resources. J. Yangtze Univ. 2016;13:7–9. [Google Scholar]
- 3.Zeng X.B., Wang J.C., Wu W.X. Fraxinus hupehensis—A new specie of the genus Fraxinus Linn. For. Sci. Technol. 1979;12:6–7. [Google Scholar]
- 4.Ming J., Liao H.R. On the present situation of Fraxinus hupehensis and its sustainable utilization. J. Plant Resour. Environ. 1998;7:19–22. [Google Scholar]
- 5.Chinese Pharmacopoeia Commission . In: Fraxini Cortex. 4th ed. Zhao Y.Y., editor. The Pharmacopoeia of the People’s Republic of China; Beijing, China: 2015. p. 271. [Google Scholar]
- 6.Wu J.L., Fu G.L., Li F. Identification of Qin–Pi (Cortex fraxini) by ultraviolet spectrum. Tradit. Chin. Med. J. 1983;8:11–16. [PubMed] [Google Scholar]
- 7.Choi J.H., Kim D.Y., Yoon J.H., Youn H.Y., Yi J.B., Rhee H.I., Ryu K.H., Jung C.K., Han C.K., Kwak W.J., et al. Effects of SKI 306X, a new herbal agent, on proteoglycan degradation in cartilage explant culture and collagenase-induced rabbit osteoarthritis model. Osteoarthr. Cartil. 2002;10:471–478. doi: 10.1053/joca.2002.0526. [DOI] [PubMed] [Google Scholar]
- 8.Park C., Jin C.Y., Kwon H.J., Hwang H.J., Kim G.Y., Choi W., Kwon T.K., Kim B.W., Kim W.J., Choi Y.H. Induction of apoptosis by esculetin in human leukemia U937 cells: Roles of Bcl-2 and extracellular–regulated kinase signaling. Toxicol. In Vitro. 2010;24:486–494. doi: 10.1016/j.tiv.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 9.Wang H.T., Zhou D., Xie K.P., Xie M.J. Antibacterial mechanism of fraxetin against Staphylococcus aureus. Mol. Med. Rep. 2014;10:2341–2345. doi: 10.3892/mmr.2014.2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galabov A.S., Iosifova T., Vassileva E., Kostova I. Antiviral activity of some hydroxycoumarin derivativese. Z. Naturforsch. C Biosci. 1996;51:558–562. doi: 10.1515/znc-1996-7-815. [DOI] [PubMed] [Google Scholar]
- 11.Wu C.R., Huang M.Y., Lin Y.T., Ju H.Y., Ching H. Antioxidant properties of Cortex Fraxini and its simple coumarins. Food Chem. 2007;104:1464–1471. doi: 10.1016/j.foodchem.2007.02.023. [DOI] [Google Scholar]
- 12.Wu Q.L., Zhang L.J., Xu H.H., Xu Z.H., Li J.K. Study on fungicidal activities of Fraxinus hupehensis extracted from its leaves. Southwest Chin. J. Agric. Sci. 2017;30:978–980. [Google Scholar]
- 13.Zhang L.J., Xu H.H., Xu Z.H., Li J.K., Wu Q.L. GC–MS analysis and preliminary study on structures of extracts from Fraxinus hupehensis leaves. Nonwood For. Res. 2017;35:186–192, 228. [Google Scholar]
- 14.Zhang L.J., Xu H.H., Xu Z.H., Li J.K., Wu Q.L. Extraction, GC–MS analysis and fungicidal activities determination of volatile components from the leaves of Fraxinus hupehensis. J. Henan. Agric. Sci. 2017;46:80–83. [Google Scholar]
- 15.Ma L., Yan H.J., Qu J.J., Zhang Y. Method for determining the content of esculin and esculetin from the bark of Fraxinus hupehensis. Chin. J. Hosp. Pharm. 2010;30:1336–1337. [Google Scholar]
- 16.Ma L., Yan H.J., Qu J.J., Zhang Y. Extraction technics of total coumarins from the leaf of Fraxinus hupehensis. Herald Med. 2010;29:925–928. [Google Scholar]
- 17.Liu R.M., Sun Q.H., Sun A.L., Cui J.C. Isolation and purification of coumarin compounds from Cortex Fraxinus by high-speed counter-current chromatography. J. Chromatogr. A. 2005;1072:195–199. doi: 10.1016/j.chroma.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 18.Zhou H.Y., Qin M.J., Hong J.L., Ni Y.J., Wu G. Chemical constituents of Viola yedoensis. Chin. J. Nat. Med. 2009;7:290–292. doi: 10.3724/SP.J.1009.2008.00290. [DOI] [Google Scholar]
- 19.Fang W., Ruan J.L., Wang Z., Cai Y.L. Studies on chemrical constituents of Arachniodes rhcmboidea. China J. Chin. Mate. Med. 2008;6:649–650. [PubMed] [Google Scholar]
- 20.Shan F., Zhang J., Li T., Wang S., Ding W.J., Zhao M.M., Du Y.F., Wang Q., Jia J. Multi-responses extraction optimization based on response surface methodology combined with polarity switching HPLC–MS/MS for the simultaneous quantitation of 11 compounds in Cortex Fraxini: Application to four species of Cortex Fraxini and its 3 confusable species. J. Pharm. Biomed. Anal. 2014;91:210–221. doi: 10.1016/j.jpba.2013.12.033. [DOI] [PubMed] [Google Scholar]
- 21.Huang Y.L., Wei Y.Q., He R.J., Li Y., Tang P.D., Ruan J., Wang Y.F., Li D.P. Chemical constituents from Agriolimax agrestis. Chin. Trad. Patent Med. 2018;40:2471–2474. [Google Scholar]
- 22.Wei H., Liu L.L., Xu L.J., Peng Y., Xiao P.G. Chemical constituents in Tibetan medicine Dolomiaea souliei (Franch.) Shih. Chin. Pharm. 2017;20:785–787. [Google Scholar]
- 23.Niu J.F., Nie D.Y., Yu D.Y., Wu Q.L., Yu L.H., Yao Z.L., Du X.Y., Li J.K. Synthesis, fungicidal activities and phloem mobility of phenazine-1-carboxylic acid-alanine conjugates. Pestic. Biochem. Physiol. 2017;11:8–13. doi: 10.1016/j.pestbp.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 24.Lu X.L., Zhu X., Zhang M., Wu Q.L., Zhou X.D., Li J.K. Synthesis and fungicidal activities of 1, 3, 4–oxadiazol–2–ylthioether derivatives containing a phenazine–1–carboxylic acid scaffold. Nat. Prod. Res. 2019;33:2145–2150. doi: 10.1080/14786419.2018.1489389. [DOI] [PubMed] [Google Scholar]
- 25.Zhu X., Zhang M., Yu L.H., Xu Z.H., Yang D., Du X.Y., Wu Q.L., Li J.K. Synthesis and bioactivities of diamide derivatives containing a phenazine–1–carboxamide scaffold. Nat. Prod. Res. 2019;33:2453–2460. doi: 10.1080/14786419.2018.1451997. [DOI] [PubMed] [Google Scholar]
- 26.Xue Q.H., Cheng L.J. Microbiology Experiment Course. World Publishing Corporation; Xi’an, China: 2000. pp. 30–32. [Google Scholar]
- 27.Kostova I., Iossifova T. Chemical components of Fraxinus species. Fitoterapia. 2007;78:85–106. doi: 10.1016/j.fitote.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 28.Zhang T.T., Zheng C.Y., He M., Wu A.P., Nie L.W. The inhibitory mechanism of linoleic acid on Microcystis aeruginosa. China Environ. Sci. 2009;29:419–424. [Google Scholar]
- 29.Zhang C.J., Li Z.R., Bai L.Y. Extraction and identification of herbicidal active substances in cottonseed hulls. Chin. J. Pestic. Sci. 2019;21:146–150. [Google Scholar]
- 30.Li Z.R., Huang Q.Q., Peng Q., Zhou Y., Zhou X.M., Bai L.Y. Herbicidal activity and response mechanism of botanical caprylic acid. J. Plant Prot. 2018;45:1161–1167. [Google Scholar]
- 31.Kolar J.M., Konduri S., Chang T.N., Wang H.J., McNerlin C., Ohlsson L., Harrod M., Siegel D., Saghatelian A. Linoleic acid esters of hydroxy linoleic acids are anti-inflammatory lipids found in plants and mammals. J. Biol. Chem. 2019;294:10698–10707. doi: 10.1074/jbc.RA118.006956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fukuda M., Tsujino Y., Fujimori T., Wakabayashi K., Boger P. Phytotoxic activity of middle-chain fatty acids I: Effects on cell constituents. Pestic. Biochem. Physiol. 2004;80:143–150. doi: 10.1016/j.pestbp.2004.06.011. [DOI] [Google Scholar]
- 33.Qin Z.G., Shen G.H., Li T., Chai X.L., Wen G.Y. Study on the herbicidal activity and applied technology of botanical pelargonic acid. Acta Agric. Shanghai. 2010;26:1–4. [Google Scholar]
- 34.Liao H.B., Liu M.F., Cheng A.C. Structural features and functional mechanism of Ton B in some Gram-negative bacteria—A review. Acta Microbiol. Sin. 2015;55:529–536. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.