Abstract
The present study was carried out to investigate the beneficial role of exogenous application of salicylic acid (1 mM SA) and nitric oxide (100 μM NO) in preventing the oxidative damage in Vigna angularis triggered by salinity stress. Salinity (100 mM NaCl) stress reduced growth, biomass accumulation, chlorophyll synthesis, photosynthesis, gas exchange parameters, and photochemical efficiency (Fv/Fm) significantly. Exogenous application of SA and NO was affective in enhancing these growth and photosynthetic parameters. Salinity stress reduced relative water content over control. Further, the application of SA and NO enhanced the synthesis of proline, glycine betaine, and sugars as compared to the control as well as NaCl treated plants contributing to the maintenance of tissue water content. Exogenous application of SA and NO resulted in up-regulation of the antioxidant system. Activities of enzymatic antioxidants including superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), dehydroascorbate reductase (DHAR), and glutathione reductase (GR), as well as the content of non-enzymatic components, were more in SA + NO treated seedlings as compared to control and salinity stressed counterparts resulting in significant alleviation of the NaCl mediated oxidative damage. Content of nitrogen, potassium, and calcium increased due to SA and NO under normal conditions and NaCl stress conditions while as Na and Cl content reduced significantly.
Keywords: antioxidants, lipid peroxidation, osmolytes, salicylic acid, nitric oxide, Vigna angularis
1. Introduction
Salinity stress is considered one of the devastating abiotic stress factors affecting the growth and productivity of crop plants [1,2]. Excess availability of toxic salts including sodium, carbonates, etc., reduces plant growth by imparting osmotic and ionic stress [3]. It has been reported that salinity stress declines root growth, water and mineral uptake, enzyme activity, and assimilation of minerals [4]. Among the key salinity-sensitive metabolic pathways are photosynthesis, respiration, mineral assimilation, biomass accumulation, and yield productivity [5,6]. Stress-induced damaging effects are initiated by the excess accumulation of toxic free radicals commonly referred to as reactive oxygen species (ROS), which includes hydrogen peroxide, superoxide, and hydroxyl radicals [4,5]. These radicals are continuously produced at different sites within the cells. Plants are equipped with different tolerance mechanisms to counteract the damaging effects of salinity stress, which include antioxidant systems, efficient ion compartmentation, and improved accumulation of osmolytes [1,4]. It has been reported that salinity stressed plants exhibit up-regulation of antioxidant systems and osmolyte accumulation [5,7]. Nowadays, for strengthening the indigenously existing tolerance mechanisms, different strategies are being tested and adopted including conventional and biotechnological approaches [3]. The last few years have focused on the exogenous use of phytohormones for strengthening the tolerance mechanisms thus as to improve the growth and yield performance of plants.
Salicylic acid (SA) is a phenolic compound and has been reported to be involved in growth and developmental regulation of plants. As a phytohormone, SA vitally regulates growth and multiple developmental events like photosynthesis, uptake, and assimilation of essential mineral ions, enzyme activity, and stress tolerance [8,9,10]. The role of SA in improving the resistance against environmental stresses is well reported [8,11]. SA strengthens the plant immunity against toxic metals [12]. SA strengthens the stress withstanding potential by improving antioxidant functioning and glycine betaine accumulation, resulting in the protection of photosynthesis [13]. Nitric oxide is another important signaling molecule that has been reported to regulate growth and developmental events from germination to ripening [14]. However, the effects of NO can be beneficial as well as damaging depending on its concentration. Reports discussing its beneficial impact on the regulation of growth and stress tolerance in crop plants have witnessed concentration-dependent effects [15]. It has been reported that the optimal application of NO mitigates the damaging effects of salinity [16] and cadmium [17] stress by up-regulating the stress withstanding mechanisms, including antioxidant systems and osmolyte accumulation. Such beneficial effects of NO contribute significantly to the maintenance of growth by preventing damage to major cellular processes including photosynthesis. Both SA and NO are believed to crosstalk with other key phytohormones to fine tune the developmental processes and responses to stresses [11,14]. For example, Ahmad, et al. [7] have demonstrated beneficial interaction of NO with jasmonic acid reflecting in greater salinity tolerance in Solanum lycopersicum. However, reports discussing investigation of possible interaction, whether beneficial or damaging, between NO and SA are not available. Therefore, the present study was undertaken to understand this.
Vigna angularis is one of the important legume crops grown widely for its food value. It is rich in proteins, vitamins, and minerals. Its growth and productivity are affected by the biotic and abiotic stress factors like pests, fungal infection, drought, salinity etc. Therefore, the present study was carried to evaluate the role of exogenous application of SA and NO in preventing salinity stress-induced oxidative damage and growth reduction.
2. Materials and Methods
Healthy seeds of Vigna angularis were surface sterilized using 5% NaOCl for 5 minutes followed by washing with distilled water. Seeds were sown in pots filled with sand, soil, and compost in a 2:1:1 ratio. At the time of sowing, pots were irrigated with 200 mL full-strength Hoagland solution. After germination, 2 seedlings per pots were maintained and allowed to grow for 10 days. Thereafter, pots were divided into 2 groups, and to 1 group salinity stress was started by adding 100 mM NaCl to the Hoagland solution (150 mL), while the other group was maintained as normal and was irrigated with an equal amount of normal Hoagland solution. Application of SA (1 mM) and NO (100 μM, sodium nitroprusside) or SA + NO was done by a manual sprayer onto the foliage. Both salinity and SA and/ or NO treatment extended for another 20 days. Thirty-day-old seedlings were analyzed for different parameters, including oxidative stress measurement, photosynthesis, antioxidants, and osmolytes.
2.1. Plant Height and Dry Weight
Shoot height was measured manually, while dry weight was recorded after drying the samples in an oven for 48 h at 70 °C.
2.2. Estimation of Photosynthetic Pigments, Photosynthesis, and Relative Water Content
Chlorophyll and carotenoids were estimated by macerating fresh leaf tissues in acetone and the optical density of the supernatant was measured at 480, 645, and 663 nm [18] using a spectrophotometer.
Net photosynthetic rate (Pn), intercellular CO2 concentration (Ci), stomatal conductance (gs), and transpiration rate (E) were measured in fully expanded leaves using the portable photosynthetic system. For measurement of maximum photochemical efficiency (Fv/Fm), leaves were dark-adapted for 25 minutes, and measurements were carried out using Chlorophyll Fluorometer (PAM 2500; Walz, Germany).
RWC was estimated following Smart and Bingham [19], and the following formula was used for the calculation:
| (1) |
2.3. Estimation of Electrolyte Leakage, Hydrogen Peroxide, and Superoxide
The method described by Dionisio-Sese and Tobita [20] was used, with electrolyte leakage determination and the following formula was used for calculation:
| Percent electrolyte leakage = (EC1 − EC0) / (EC2 − EC0) × 100 | (2) |
For measurement of hydrogen peroxide (H2O2), the method described by Velikova et al. [21] was adopted and absorbance was measured at 390 nm. Concentration of H2O2 was calculated from the standard curve. Estimation of superoxide (O2−) concentrations was done following Yang et al. [22] and optical density was taken at 530 nm. For the calculation, the standard curve of NaNO2 was used.
2.4. Measurement of Lipid Peroxidation and Lipoxygenase Activity
For measuring lipid peroxidation formation of malonaldehyde content was estimated in line with the method of Madhava Rao and Sresty [23] and the extinction coefficient of 155 mM−1cm−1 was used for calculation. Lipoxygenase (LOX; EC, 1.13.11.12) activity in fresh leaf tissues was measured by monitoring the change in absorbance at 234 nm using linoleic acid as a substrate [24]. For calculation, the extinction coefficient of 25 mM−1 cm−1 was used.
2.5. Estimation of Proline, Glycine Betaine, and Sugars
Proline was extracted in sulphosalicylic acid according to the method of Bates et al. [25] and the absorbance was taken at 520 nm. Glycine betaine content was estimated following the Grieve and Grattan [26] method. Formation of periodide crystals with cold KI–I2 reagent were read at 365 nm and the standard curve of glycine betaine was used for calculation. Sugar content was estimated using the anthrone method described by Ahanger et al. [27]. After extraction in ethanol, supernatant was mixed with anthrone reagent and absorbance recorded at 620 nm.
2.6. Assay of Antioxidant Enzymes
Fresh 500 mg leaf tissue was macerated in ice-cold potassium phosphate buffer (100 mM, pH 7.0) supplemented with 1% PVP and 1 mM EDTA using a pre-chilled mortar and pestle. After centrifugation for 15 min at 12,000× g at 4 °C, supernatant was collected and used as enzyme source.
Activity of superoxide dismutase (SOD, EC1.15.1.1) was measured by estimating the ability of the enzyme (100 µL) to inhibit photochemical reduction of nitroblue tetrazolium (NBT) in an assay mixture containing 100 mM phosphate buffer (pH 7.4), 1.0 mM EDTA, 10 mM methionine, 50 µM riboflavin, and 75 µM NBT incubated under fluorescent light for 15 min. Optical density was measured spectrophotometrically at 560 nm and activity was expressed as EU mg−1 protein [28].
For assaying catalase (CAT, EC1.11.1.6) activity disappearance of H2O2 was recorded at 240 nm for 2 min in an assay mixture containing 50 mM phosphate buffer (pH 6.0), 0.1 mM EDTA, H2O2 and 100 µL enzyme extract in a final volume of 2 mL. Extinction coefficient of 39.4 mM−1cm−1 was used for the calculation [29].
Ascorbate peroxidase (APX, EC1.11.1.1) was assayed in an assay mixture containing 50 mM phosphate buffer (pH 7.5), 100 µL of each EDTA, ascorbate, enzyme, and H2O2. Change in absorbance was recorded at 290 nm for 2 min and the extinction coefficient of 2.8 mM−1cm−1 was used for calculation [30].
Activity of glutathione reductase (GR, EC1.6.4.2) was assayed by measuring the change in absorbance at 340 nm for 3 min in a reaction mixture containing 100 mM potassium phosphate buffer (pH 7.0), EDTA, 50 μM NADPH, 100 μM oxidized glutathione, and 100 μL enzyme [31].
Dehydroascorbate reductase (DHAR, EC: 1.8.5.1) activity was assayed by monitoring change in absorbance at 265 nm and extinction coefficient of 14 mM−1 cm−1 was used for the calculation [30].
2.7. Estimation of Ascorbate, Reduced Glutathione, and Tocopherol
For estimation of ascorbate (AsA) content, fresh leaf material was extracted in 6% TCA and supernatant was reacted with dinitrophenylhydrazine (2%) and thiourea (10%). Absorbance was taken at 530 nm and calculations were done using the standard curve of AsA [32]. For estimation of reduced glutathione (GSH), fresh tissue was homogenized in phosphate buffer (pH 8.0) and 500 µL supernatant was reacted with 5,5-dithiobis-2-nitrobenzoic acid. Optical density was taken at 412 nm and concentration of GSH was determined from the standard graph of GSH [33]. For estimation of tocopherol tissue was extracted in ethanol and petroleum ether (1.6:2 and supernatant was incubated with 2% of 2,2-dipyridyl in dark. Absorbance was recorded at 520 nm and the standard curve was used for calculation [34].
2.8. Estimation of N, K, Ca, Na, and Cl
K, Na, and Ca were estimated flame photometrically described by Ahanger et al. [27]. Micro-Kzeldahl’s method suggested by Jackson [35] was employed for estimation of N content. Chloride was estimated by titrating the extract against AgNO3 using K2CrO4 as an indicator.
2.9. Statistical Analysis
The mean (±SE) of 4 replicates were presented. Statistical analysis for a completely randomized design was carried using the analysis of variance and the least significant difference (LSD) was calculated at p < 0.05.
3. Results
Salinity stress declined the growth of Vigna angularis significantly over the control, and the application of NO or SA, individually or in combination, mitigated the decline to considerable levels. Relative to the control, height and weight declined by 30.45% and 41.37% due to salinity stress. Height and dry weight increased by 31.85% and 39.89% respectively in NaCl + SA + NO application over the NaCl stressed plants (Table 1).
Table 1.
Effect of exogenous application of salicylic acid (SA) and nitric oxide (NO) on shoot length (cm) and plant dry weight (g/plant) in Vigna angularis under salinity (100 mM NaCl) stress. Data are the mean of four replicates and different letters show a significant difference at p < 0.05.
| Treatments | Shoot Length | Dry Weight |
|---|---|---|
| Control | 24.3 ± 2.04c | 2.03 ± 0.063c |
| SA | 29.1 ± 2.11b | 2.86 ± 0.050b |
| NO | 27.9 ± 2.07b | 2.77 ± 0.054b |
| SA + NO | 32.0 ± 2.40a | 3.23 ± 0.065a |
| NaCl | 16.9 ± 1.14e | 1.19 ± 0.033e |
| NaCl + SA | 19.4 ± 1.08d | 1.66 ± 0.040d |
| NaCl + NO | 18.9 ± 1.62d | 1.59 ± 0.049d |
| NaCl + SA + NO | 24.8 ± 2.16c | 1.98 ± 0.053c |
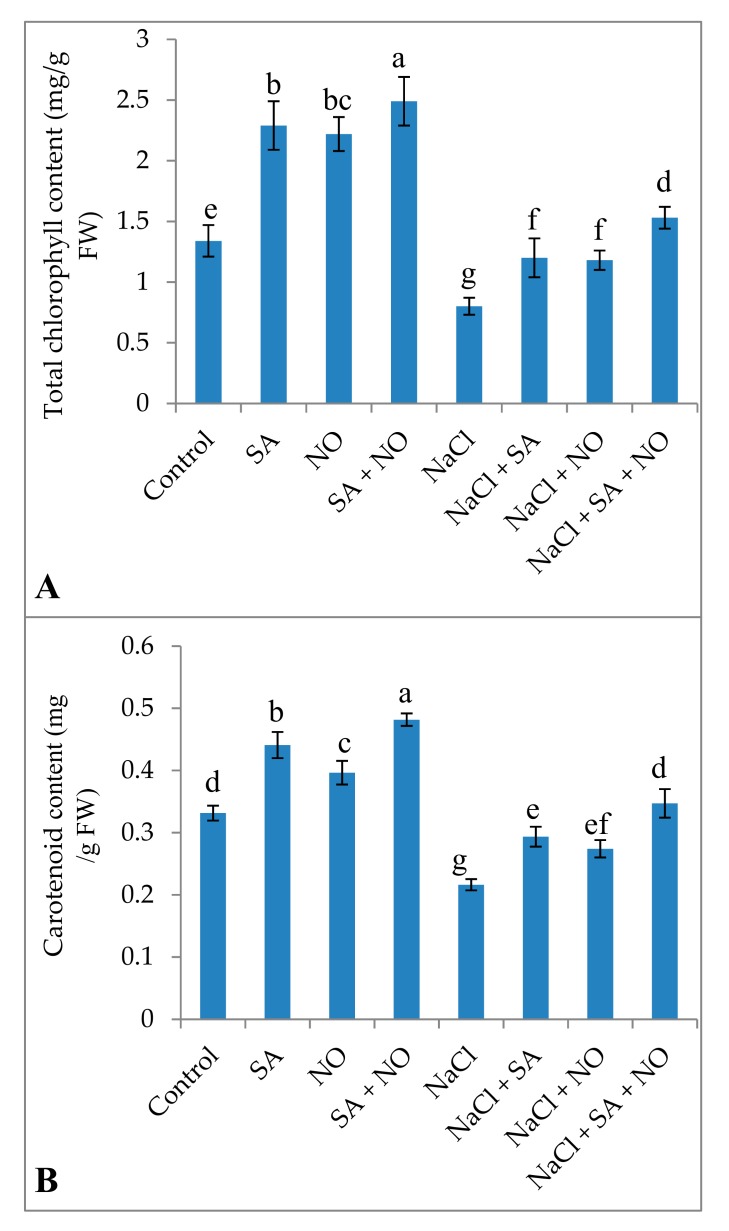
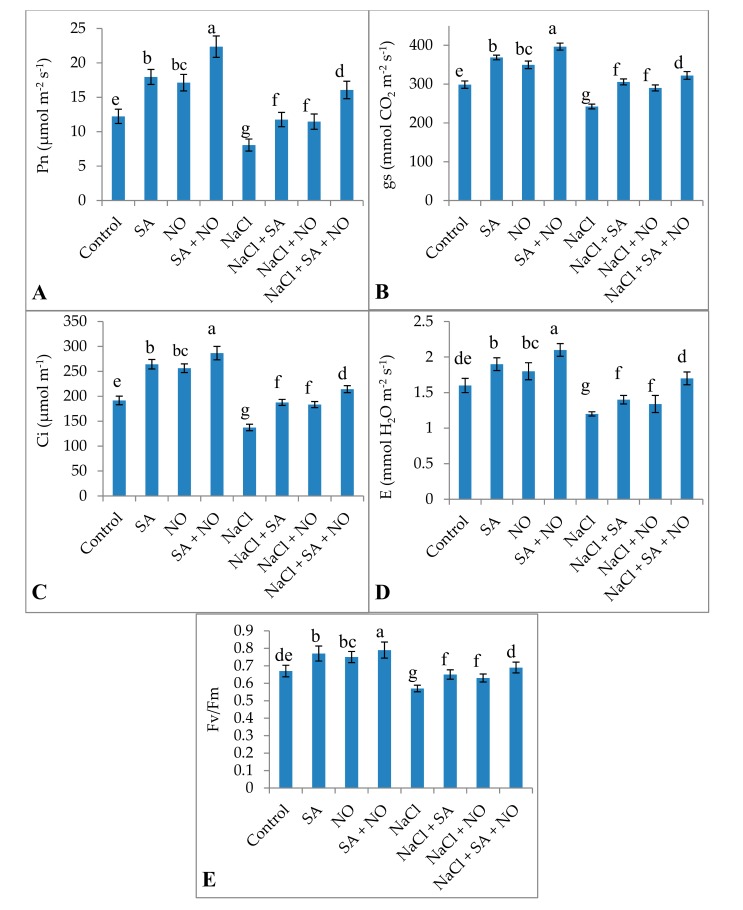
Exogenous application of SA or NO and SA + NO improved the total chlorophyll and carotenoid content, gas exchange attributes including net photosynthesis, stomatal conductance, transpiration rate, and intercellular CO2 significantly over the control. Maximal enhancement in total chlorophylls (46.18%), carotenoids (31.18%), Pn (45.30%), gs (24.71%), E (23.80%), Ci (33.40%), and Fv/Fm (15.18%) was observed in seedlings treated with SA + NO. However, salinity stress resulted in the decline of 40.22% for chlorophylls, 34.75% for carotenoids, 34.09% for Pn, 18.85% for gs, 25.05% for E, 28.34% for Ci, and 14.92% for Fv/Fm. Salinity stress declined these parameters significantly over the control and application of NO and /or SA ameliorated the decline maximally when applied combinedly. Relative to NaCl stressed plants total chlorophylls, carotenoids, Pn, gs, E, Ci and Fv/Fm increased by 47.64%, 37.68%, 49.81%, 24.82%, 29.41%, 35.93%, and 17.39% respectively in NaCl + SA + NO treated seedlings (Figure 1 and Figure 2).
Figure 1.
Effect of exogenous application of SA and NO on (A) total chlorophyll and (B) carotenoids in Vigna angularis under salinity (100 mM NaCl) stress. Data are the mean of four replicates and different letters show significant difference at p < 0.05.
Figure 2.
Effect of exogenous application of SA and NO on (A) net photosynthesis (Pn), (B) stomatal conductance (gs), (C) intercellular CO2 (Ci), (D) transpiration (E) and (E) PSII activity (Fv/Fm) in Vigna angularis under salinity (100 mM NaCl) stress. Data are the mean of four replicates and different letters show significant difference at p < 0.05.
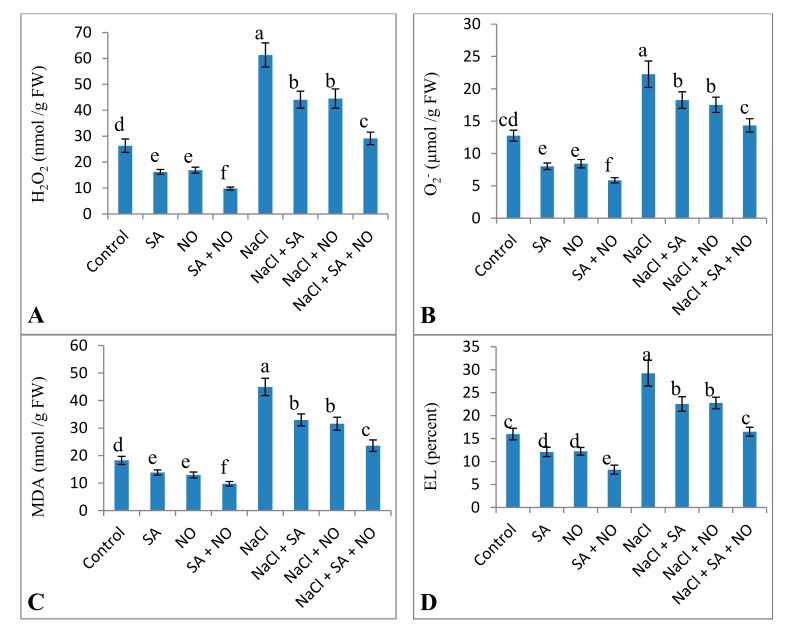
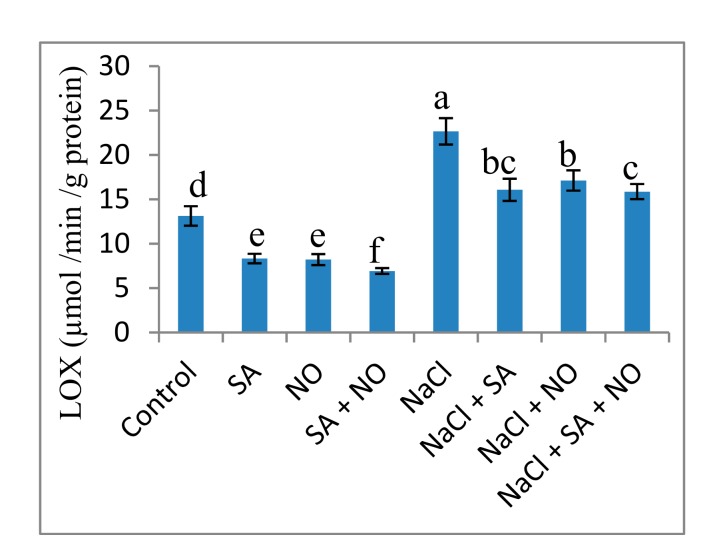
Exposure to salinity stress resulted in an increase in the oxidative parameters studied. Relative to the control, NaCl treatment increased H2O2 (57.04%), O2− (42.67%), MDA (58.45%), EL (45.33%) and LOX (42.05%) activity. Foliar application of NO and SA decreased these parameters significantly with maximal decline of 62.82%, 54.07%, 46.62%, 48.49%, and 47.29% in H2O2, O2−, MDA, EL and LOX activity respectively over the control. Application of NO and/or SA to NaCl stressed seedlings declined these parameters with a maximal decline of 52.47% for H2O2, 34.96% for O2−, 47.55% for MDA, 43.55% for EL and 29.96% for LOX activity attained with NaCl + SA + NO over the NaCl stressed plants (Figure 3 and Figure 4).
Figure 3.
Effect of exogenous application of SA and NO on (A) hydrogen peroxide, (B) superoxide, (C) lipid peroxidation (MDA), and (D) electrolyte leakage (EL) in Vigna angularis under salinity (100 mM NaCl) stress. Data are the mean of four replicates and different letters show significant difference at p< 0.05.
Figure 4.
Effect of exogenous application of SA and NO on activity of lipoxygenase in Vigna angularis under salinity (100 mM NaCl) stress. Data are the mean of four replicates and different letters show significant difference at p< 0.05.
Vigna angularis exposed to salinity stress exhibited increased accumulation of proline (21.92%), sugars (14.93%) and glycine betaine (23.49%) content over the control. Under normal growth conditions, supplementation of SA and NO individually increased the content of proline, sugars, and glycine betaine with maximal increase observed due to their combined application. When SA and /or NO were supplied to NaCl stressed seedlings content of proline, sugars, and glycine betaine exhibited a further increase of 37.61%, 41.89%, and 51.08% respectively over the NaCl stressed plants (Table 2). Salinity declined RWC by 23.02% over the control and application of SA + NO ameliorated the decline by 22.34% over NaCl stressed seedlings (Table 2).
Table 2.
Effect of exogenous application of SA and NO on proline, glycine betaine, sugars and relative water content in Vigna angularis under salinity (100 mM NaCl) stress. Data are the mean of four replicates and different letters show significant difference at p < 0.05.
| Treatments | Proline | Glycine Betaine | Sugars | RWC |
|---|---|---|---|---|
| Control | 41.3 ± 3.64f | 2.93 ± 0.32f | 4.33 ± 0.45f | 76.43 ± 4.10c |
| SA | 67.9 ± 4.50c | 4.86 ± 0.25d | 6.26 ± 0.45d | 83.23 ± 4.70b |
| NO | 62.8 ± 4.08d | 4.66 ± 0.47d | 6.16 ± 0.47d | 82.90 ± 5.60b |
| SA + NO | 81.0 ± 5.50a | 5.73 ± 0.50b | 7.83 ± 0.60b | 88.46 ± 4.07a |
| NaCl | 52.9 ± 4.91e | 3.83 ± 0.37e | 5.09 ± 0.27e | 58.83 ± 3.81e |
| NaCl + SA | 83.0 ± 4.92a | 5.73 ± 0.43b | 7.23 ± 0.83bc | 68.17 ± 4.29d |
| NaCl + NO | 77.4 ± 5.48b | 5.36 ± 0.41c | 6.96 ± 0.80c | 65.13 ± 3.94d |
| NaCl + SA + NO | 84.8 ± 5.26a | 7.83 ± 0.51a | 8.76 ± 0.76a | 75.76 ± 4.33c |
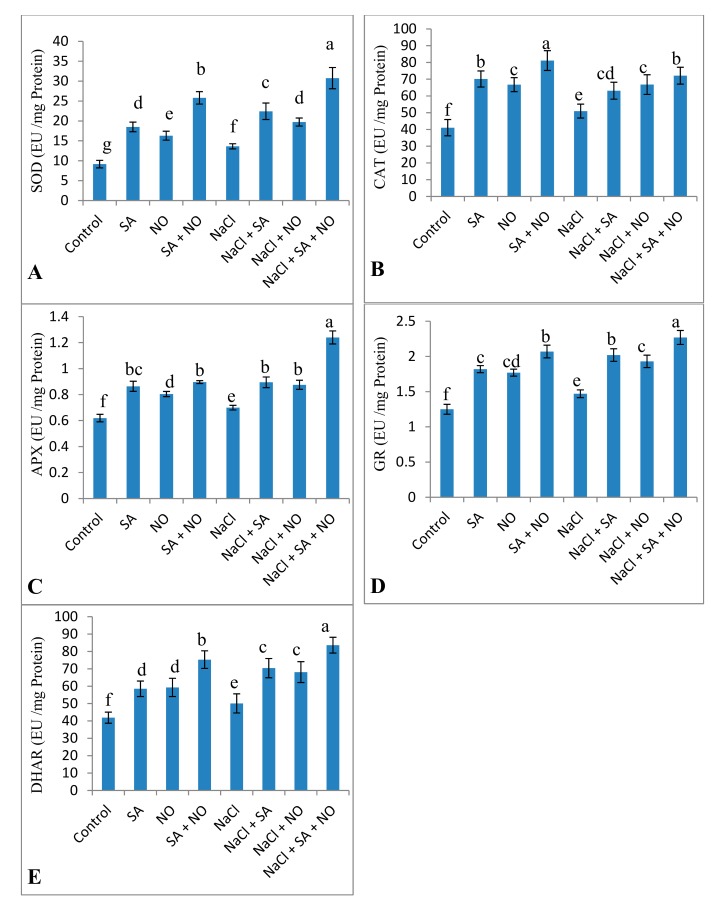
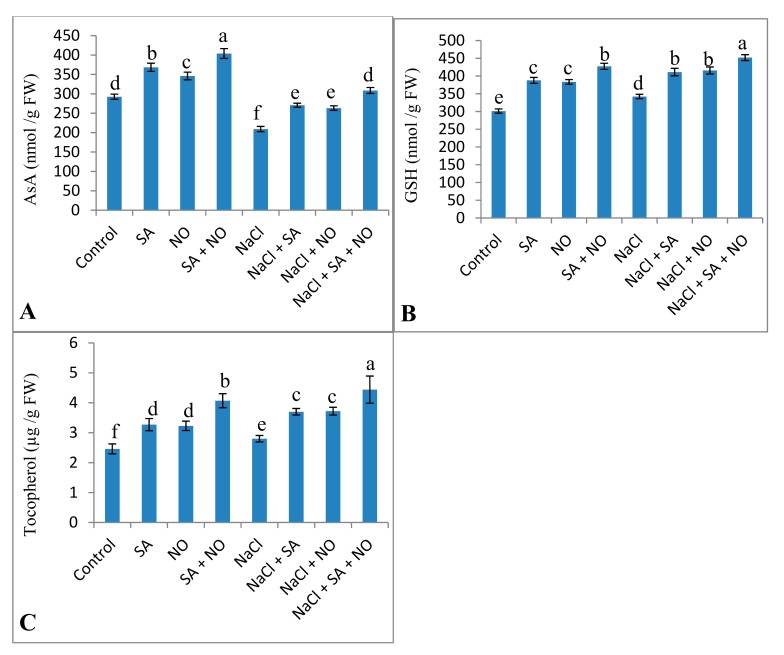
Activities of antioxidant enzymes, including SOD, CAT, APX, DHAR, and GR, increased due to the application of NO and SA under normal and salinity stress conditions. Percent increase was 32.79% for SOD, 19.42% for CAT, 11.59% for APX, 14.96% for GR, and 16.36% for DHAR due to NaCl stress, however, this was further increased by 55.68%, 29.32%, 43.58%, 35.24%, and 40.07% respectively due to combined application of SA + NO over the NaCl stressed ones (Figure 5). Salinity decreased AsA by 28.47% but increased the content of GSH and tocopherol by 11.98% and 12.14% over the control. Foliar spray of SA and NO resulted in increased AsA, GSH and tocopherol content under normal as well as salinity stressed conditions. GSH and tocopherol maximally increased by 33.32% and 44.59% in NaCl + SA + NO treated seedlings. Decline in AsA was mitigated by application of SA and NO with maximal mitigation of 32.20% in NaCl + SA+ NO treated seedlings (Figure 6).
Figure 5.
Effect of exogenous application of SA and NO on activity of (A) superoxide dismutase (SOD), (B) catalase (CAT), (C) ascorbate peroxidase (APX), (D) glutathione reductase (GR) and (E) DHAR in Vigna angularis under salinity (100 mM NaCl) stress. Data are the mean of four replicates and different letters show significant difference at p < 0.05.
Figure 6.
Effect of exogenous application of SA and NO on (A) ascorbic acid, (B) reduced glutathione and (C) tocopherol content in Vigna angularis under salinity (100 mM NaCl) stress. Data are the mean of four replicates and different letters show significant difference at p < 0.05.
Content of Na and Cl increased due to NaCl stress and application of SA and NO declined their accumulation significantly. Relative to control, Na and Cl increased by 61.05% and 68.78% due to NaCl treatment, however, the application of SA + NO reduced the content of Na and Cl by 50.07% and 47.77% over the NaCl stressed plants. N, K and Ca declined by 40.10%, 45.07%, and 33.33% due to NaCl treatment over the control. Application of SA and NO increased the content of N, K, and Ca significantly over the control with a maximal increase of 29.96%, 27.91%, and 50.58% in SA + NO treated seedlings. Relative to NaCl stressed plants, decline in N, K and Ca was ameliorated by 38.12, 43.88 and 33.33% in NaCl + SA + NO treated plants (Table 3).
Table 3.
Effect of exogenous application of SA and NO on uptake of Na, Cl, N, K, and Ca (mg g−1 DW) in Vigna angularis under salinity (100 mM NaCl) stress. Data are the mean of four replicates and different letters show a significant difference at p < 0.05.
| Treatments | Na | Cl | N | K | Ca |
|---|---|---|---|---|---|
| Control | 5.25 ± 0.27g | 3.43 ± 0.32e | 18.7 ± 1.3c | 14.2 ± 1.1c | 4.2 ± 0.081c |
| SA | 3.41 ± 0.20e | 2.86 ± 0.19d | 23.8 ± 2.1b | 17.1 ± 1.6b | 6.8 ± 0.085b |
| NO | 3.50 ± 0.13e | 2.82 ± 0.23d | 22.5 ± 1.5b | 16.8 ± 1.3b | 6.3 ± 0.043b |
| SA + NO | 3.04 ± 0.19f | 2.45 ± 0.25f | 26.7 ± 1.9a | 19.7 ± 2.1a | 8.5 ± 0.18a |
| NaCl | 13.48 ± 1.71a | 10.99 ± 1.08a | 11.2 ± 0.87e | 7.8 ± 0.63e | 2.8 ± 0.07e |
| NaCl + SA | 9.00 ± 0.15c | 8.10 ± 0.17b | 15.5 ± 1.1d | 10.4 ± 0.91d | 3.4 ± 0.093d |
| NaCl + NO | 9.77 ± 1.23b | 8.22 ± 0.36b | 15.3 ± 1.3d | 9.2 ± 0.84d | 3.0 ± 0.091d |
| NaCl + SA + NO | 6.73 ± 0.35d | 5.74 ± 0.64c | 18.1 ± 1.6c | 13.9 ± 1.0c | 4.2 ± 0.043c |
4. Discussion
In the contemporary era, a cumbersome challenge for plant scientists and agriculturalists has been to develop strategies to improve the stress tolerance potential in plants. Such beneficial strategies result in the protection of global food security [36]. In the present study, the role of exogenous supplementation of NO and SA individually or combinedly was studied, and it was observed that the combined application of SA and NO proved much more beneficial in ameliorating the growth decline under salinity stress. Considerable research reports have described the salinity mediated growth decline in terms of height and biomass accumulation [5,7,8]. The application of SA [37] and NO [7] have been reported to improve the growth and biomass accumulation in salinity stressed Brassica juncea and chickpea, respectively. Reduced growth and biomass accumulation under salinity stress is attributed to the osmotic and ionic effects resulting in decline cellular division and proliferation [38]. SA [11] and NO [39] are believed to interact with other important molecules to protect growth retardations under stress. In the present study combined application of SA and NO proved much more beneficial in mitigating the decline in growth and biomass accumulation. Further, increased growth under salinity stress due to SA and/or NO application was correlated with their positive influence on the photosynthetic parameters including gas exchange parameters and PSII functioning. Earlier salinity mediated reduction in chlorophyll synthesis [5] and photosynthesis [13] has been reported in wheat and mungbean, respectively. Reduced chlorophyll content due to salinity results from its effect on the uptake of magnesium and nitrogen [40]. Moreover, stresses trigger greater damage to chlorophyll by up-regulating chlorophyllase activity and down-regulating the enzymes mediating chlorophyll synthesis [41]. Exogenous SA and NO have been reported to improve photosynthetic functioning under salinity stress by enhancing chlorophyll synthesis and improving gas exchange [13,17]. Reduced mineral uptake under salinity directly influences photosynthetic functioning and NO application improves mineral uptake significantly [40]. Improved gas exchange and PSII activity in SA and NO treated seedlings determines their role in photosynthetic control and protection through their involvement in stomatal and non-stomatal attributes. Interaction of NO with JA [7] has been reported to increase chlorophyll synthesis under salinity stress. Exogenous application of SA has been reported to prevent salinity induced photosynthetic arrest in Brassica juncea [37]. From a recent study, it can be concluded that improved photosynthesis due to SA and NO application may have resulted due to up-regulation of antioxidant and osmolyte accumulation leading to lesser ROS accumulation and maintenance of tissue water content, respectively [16,42,43,44,45].
Greater generation of ROS, including H2O2 and O2 resulted due to salinity stress, has earlier been reported by several workers [16,46,47]. Reduced accumulation of ROS due to NO [16] and SA [37] has been reported. Recently, Ahanger et al. [6] have demonstrated that reduced ROS accumulation and lipoxygenase activity reflected in improved membrane functioning and growth performance under salinity stress. Reduction in oxidative damage under stress due to NO application has been reported due to its effects on the accumulation of H2O2 and O2 as well as reduced activity of lipoxygenase [48]. Excess accumulation of toxic radicals like H2O2 or O2 drastically affected the membrane functioning by initiating peroxidation of lipids and lessening their generation due to exogenous application of SA and /or NO could benefit plants to maximize the performance under stress conditions [8,13,17]. In corroboration with our results, reduced oxidative effects due exogenous application of SA [1,37] and NO [7,16,17] have been earlier reported, however, reports discussing their interactive role are scanty. Reduced accumulation of ROS in SA and NO treated seedlings may have directly contributed to the protection of key cellular organelles like chloroplast, mitochondria, thereby allowing their smooth functioning.
It was interesting to note that reduced oxidative damage in SA and NO treated seedlings was correlated with significant up-regulation of the antioxidant system in them. Plants potentially improve the antioxidant functioning to counter the deleterious effects of stress [43,45,46]. Antioxidant system constituted of enzymatic and non-enzymatic components operates in cells neutralize ROS to maintain their levels below toxic concentrations [7,17,49]. Up-regulation of the antioxidant system in repose to salinity stress has been reported earlier in mustard [8], wheat [5], and pepper [40]. Chen et al. [50] have demonstrated that an up-regulated antioxidant system due to SA application protects PSII activity in wheat. Exogenous application of NO up-regulates the activity of antioxidant enzymes in salt-stressed pepper seedlings leading to the prevention of oxidative effects of ROS on photosynthesis, mineral uptake, and assimilation. SOD acts on superoxide while H2O2 is neutralized by either CAT or APX in AsA-GSH cycle. APX, DHAR, and GR form enzymatic components of AsA-GSH cycle, while as ascorbate and glutathione form its non-enzymatic components [51]. AsA and GSH act as redox buffers besides their radical neutralizing function [51,52]. Up-regulation of the AsA-GSH cycle functioning due to exogenous application of SA [9] and NO [7,17] has been reported to prevent deleterious effects of stresses. Raman and Ravi [53] have demonstrated that the application of SA resulted in increased activities of SOD, CAT, and APX in Haematococcus pluvialis. Tocopherols exist in the chloroplast envelope, thylakoid membrane, and plastoglobuli, where it protects them from the damaging effects of ROS [54]. SA- and NO-mediated increase in tocopherol content contributed to strengthening of the antioxidant system thereby protecting the major cellular processes like photosynthesis, which was evident in the present study as well. Yusuf et al. [55] have demonstrated that the over-expression of tocopherol synthesis increases abiotic stress tolerance by protecting chlorophyll synthesis and chlorophyll fluorescence in Brassica juncea. It has been reported that GSH, SA, and ethylene interplay with each other to counteract the stress damage efficiently [56]. Improved GSH, AsA, and tocopherol content due to SA and NO application may have contributed to the regulation of such phytohormone dependent signaling networks for improved salinity tolerance. Further studies in this direction will be noteworthy.
Increased accumulation of proline, glycine betaine, and sugars due to SA and NO application was obvious in the present study. Compatible organic osmolytes prevent stress-induced damage to cellular organelles by protecting membrane structures, enzyme functioning, and maintaining water content [44,52]. Increased accumulation of proline, glycine betaine, sugars, and amino acids under salinity stress have been reported by several workers [5,8,57,58]. Plants accumulating significantly increased concentrations of osmolytes withstand the stressful conditions better [59]. Transgenic plants over-expressing proline biosynthesizing genes exhibit greater stress tolerance [60]. Greater accumulation of osmolytes like proline results from the up-regulation of their biosynthesizing pathways [4]. Proline and glycine betaine supplementation have been reported to prevent oxidative effects of salinity stress as well as lowered cell death [61]. Khan et al. [13] have demonstrated SA mediated improved salinity tolerance and photosynthetic protection in mungbean due to increased glycine betaine accumulation. Similarly, Ahmad et al. [7] have reported NO application resulting in greater accumulation of proline and glycine betaine resulting in enhanced salinity tolerance. However, reports discussing the interactive influence of SA and NO on osmolyte metabolism are rare. Increased accumulation of osmolytes like proline results from the improved mineral assimilation like N making the precursors for the synthesis of other amino acids available as well [43]. It has been proposed that improved stress in plants can be achieved by dissecting the osmolyte biosynthetic pathways [62]. Osmolytes help plants recover quickly after stress release [44].
The decline in uptake of mineral nutrients, including N, K, and Ca, was mitigated by the application of SA and/ or NO. Earlier increased mineral uptake due to SA [37,63] and NO [52,64] has been reported, however, reports discussing their interactive role in the regulation of mineral uptake are rare. Sheng et al. [65] have demonstrated increased uptake of K and Ca due to SA in wheat, resulting in the up-regulation of antioxidant system and mitigation of oxidative effects under manganese stress. Increased uptake of mineral nutrients influences the cellular functioning and whole plant performance by regulating processes like photosynthesis, antioxidant systems, and enzyme activity [42,66]. In salt-stressed cucumber, foliar application of SA has been reported to mitigate the reduction in uptake of key macro and microelements. Dong et al. [45] have also reported NO-mediated increased uptake of key mineral elements like K, Mg, Ca, and Zn, resulting in a significant enhancement in growth through modulations in the photosynthesis and antioxidant system. Therefore, it could be inferred from the present study that SA and/ or NO-mediated enhancement in the mineral uptake of Vigna angularis may have contributed to improved growth and salinity tolerance by regulating the major cellular processes. The content of Na and Cl was significantly declined due to SA and NO application.
5. Conclusions
Exogenous application of SA and NO considerably averted the deleterious effects of salinity stress in Vigna angularis by declining the generation of ROS and improving mineral ion uptake. The combined application of SA and NO proved more beneficial as compared to their individual application, resulting in greater protection against salinity stress. Up-regulation of the antioxidant system and increased accumulation of osmolytes in SA and NO-treated seedlings evidently contributed to improved growth and photosynthesis. The present study suggests beneficial interaction between SA and NO for growth protection under salinity stress.
Acknowledgments
The authors would like to extend their sincere appreciation to the Researchers Supporting Project Number (RSP-2019/116), King Saud University, Riyadh, Saudi Arabia.
Author Contributions
Conceptualization, M.A.A. and P.A.; methodology, M.A.A. and U.A.; software, M.N.A.; validation, M.A.A. and P.A.; formal analysis, P.A. and A.A.A.; investigation, M.A.A. and U.A.; resources, M.N.A. and A.A.A.; data curation, U.A. and P.A.; writing—original draft preparation, M.A.A., M.N.A. and P.A.; writing—review and editing, P.A.; visualization, M.A.A. and A.A.A.; supervision, M.A.A.; project administration, M.A.A.; funding acquisition, M.N.A., A.A.A. and P.A. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Yildirim E., Turan M., Guvenc I. Effect of Foliar Salicylic Acid Applications on Growth, Chlorophyll, and Mineral Content of Cucumber Grown Under Salt Stress. J. Plant Nutr. 2008;31:593–612. doi: 10.1080/01904160801895118. [DOI] [Google Scholar]
- 2.Yu X., Liang C., Chen J., Qi X., Liu Y., Li W. The effects of salinity stress on morphological characteristics, mineral nutrient accumulation and essential oil yield and composition in Mentha canadensis L. Sci. Hortic. 2015;197:579–583. doi: 10.1016/j.scienta.2015.10.023. [DOI] [Google Scholar]
- 3.Parihar P., Singh S., Singh R., Singh V.P., Prasad S.M. Effect of salinity stress on plants and its tolerance strategies: a review. Environ. Sci. Pollut. Res. Int. 2015;22:4056–4075. doi: 10.1007/s11356-014-3739-1. [DOI] [PubMed] [Google Scholar]
- 4.Elkelish A.A., Soliman M.H., Alhaithloul H.A., El-Esawi M.A. Selenium protects wheat seedlings against salt stress-mediated oxidative damage by up-regulating antioxidants and osmolytes metabolism. Plant Physiol. Biochem. 2019;137:144–153. doi: 10.1016/j.plaphy.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 5.Ahanger M.A., Agarwal R.M. Salinity stress induced alterations in antioxidant metabolism and nitrogen assimilation in wheat (Triticum aestivum L.) as influenced by potassium supplementation. Plant Physiol. Biochem. 2017;115:449–460. doi: 10.1016/j.plaphy.2017.04.017. [DOI] [PubMed] [Google Scholar]
- 6.Ahanger M.A., Qin C., Maodong Q., Dong X.X., Ahmad P., Abd_Allah E.F., Zhang L. Spermine application alleviates salinity induced growth and photosynthetic inhibition in Solanum lycopersicum by modulating osmolyte and secondary metabolite accumulation and differentially regulating antioxidant metabolism. Plant Physiol. Biochem. 2019;144:1–13. doi: 10.1016/j.plaphy.2019.09.021. [DOI] [PubMed] [Google Scholar]
- 7.Ahmad P., Abass Ahanger M., Nasser Alyemeni M., Wijaya L., Alam P., Ashraf M. Mitigation of sodium chloride toxicity in Solanum lycopersicum L. by supplementation of jasmonic acid and nitric oxide. J. Plant Interact. 2018;13:64–72. doi: 10.1080/17429145.2017.1420830. [DOI] [Google Scholar]
- 8.Ahmad P., Nabi G., Ashraf M. Cadmium-induced oxidative damage in mustard [Brassica juncea (L.) Czern. & Coss.] plants can be alleviated by salicylic acid. S. Afr. J. Bot. 2011;77:36–44. doi: 10.1016/j.sajb.2010.05.003. [DOI] [Google Scholar]
- 9.Nazar R., Iqbal N., Syeed S., Khan N.A. Salicylic acid alleviates decreases in photosynthesis under salt stress by enhancing nitrogen and sulfur assimilation and antioxidant metabolism differentially in two mungbean cultivars. J. Plant Physiol. 2011;168:807–815. doi: 10.1016/j.jplph.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 10.Moravcová Š., Tůma J., Dučaiová Z.K., Waligórski P., Kula M., Saja D., Słomka A., Bąba W., Libik-Konieczny M. Influence of salicylic acid pretreatment on seeds germination and some defence mechanisms of Zea mays plants under copper stress. Plant Physiol. Biochem. 2018;122:19–30. doi: 10.1016/j.plaphy.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 11.Khan M.I.R., Fatma M., Per T.S., Anjum N.A., Khan N.A. Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front. Plant Sci. 2015;6 doi: 10.3389/fpls.2015.00462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ali E., Maodzeka A., Hussain N., Shamsi I.H., Jiang L. The alleviation of cadmium toxicity in oilseed rape (Brassica napus) by the application of salicylic acid. Plant Growth Regul. 2014;75:641–655. doi: 10.1007/s10725-014-9966-0. [DOI] [Google Scholar]
- 13.Khan M.I.R., Asgher M., Khan N.A. Alleviation of salt-induced photosynthesis and growth inhibition by salicylic acid involves glycinebetaine and ethylene in mungbean (Vigna radiata L.) Plant Physiol. Biochem. 2014;80:67–74. doi: 10.1016/j.plaphy.2014.03.026. [DOI] [PubMed] [Google Scholar]
- 14.Sanz L., Albertos P., Mateos I., Sánchez-Vicente I., Lechón T., Fernández-Marcos M., Lorenzo O. Nitric oxide (NO) and phytohormones crosstalk during early plant development. J. Exp. Bot. 2015;66:2857–2868. doi: 10.1093/jxb/erv213. [DOI] [PubMed] [Google Scholar]
- 15.Fatma M., Khan N.A. Nitric Oxide Protects Photosynthetic Capacity Inhibition by Salinity in Indian Mustard. J. Funct. Environ. Bot. 2014;4:106. doi: 10.5958/2231-1750.2014.00009.2. [DOI] [Google Scholar]
- 16.Fatma M., Masood A., Per T.S., Khan N.A. Nitric Oxide Alleviates Salt Stress Inhibited Photosynthetic Performance by Interacting with Sulfur Assimilation in Mustard. Front. Plant Sci. 2016;7 doi: 10.3389/fpls.2016.00521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahmad P., Ahanger M.A., Alyemeni M.N., Wijaya L., Alam P. Exogenous application of nitric oxide modulates osmolyte metabolism, antioxidants, enzymes of ascorbate-glutathione cycle and promotes growth under cadmium stress in tomato. Protoplasma. 2018;255:79–93. doi: 10.1007/s00709-017-1132-x. [DOI] [PubMed] [Google Scholar]
- 18.Arnon D.I. Copper Enzymes in Isolated Chloroplasts. Polyphenoloxidase in Beta Vulgaris. Plant Physiol. 1949;24:1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smart R.E., Bingham G.E. Rapid Estimates of Relative Water Content. Plant Physiol. 1974;53:258–260. doi: 10.1104/pp.53.2.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dionisio-Sese M.L., Tobita S. Antioxidant responses of rice seedlings to salinity stress. Plant Sci. 1998;135:1–9. doi: 10.1016/S0168-9452(98)00025-9. [DOI] [Google Scholar]
- 21.Velikova V., Yordanov I., Edreva A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants. Plant Sci. 2000;151:59–66. doi: 10.1016/S0168-9452(99)00197-1. [DOI] [Google Scholar]
- 22.Yang H., Wu F., Cheng J. Reduced chilling injury in cucumber by nitric oxide and the antioxidant response. Food Chem. 2011;127:1237–1242. doi: 10.1016/j.foodchem.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 23.Madhava Rao K.V., Sresty T.V.S. Antioxidative parameters in the seedlings of pigeonpea (Cajanus cajan (L.) Millspaugh) in response to Zn and Ni stresses. Plant Sci. 2000;157:113–128. doi: 10.1016/S0168-9452(00)00273-9. [DOI] [PubMed] [Google Scholar]
- 24.Doderer A., Kokkelink I., van der Veen S., Valk B.E., Schram A., Douma A.C. Purification and characterization of two lipoxygenase isoenzymes from germinating barley. Biochim. Biophys. Acta. 1992;1120:97–104. doi: 10.1016/0167-4838(92)90429-H. [DOI] [PubMed] [Google Scholar]
- 25.Bates L.S., Waldren R.P., Teare I.D. Rapid determination of free proline for water-stress studies. Plant Soil. 1973;39:205–207. doi: 10.1007/BF00018060. [DOI] [Google Scholar]
- 26.Grieve C.M., Grattan S.R. Rapid assay for determination of water soluble quaternary ammonium compounds. Plant Soil. 1983;70:303–307. doi: 10.1007/BF02374789. [DOI] [Google Scholar]
- 27.Ahanger M.A., Agarwal R.M., Tomar N.S., Shrivastava M. Potassium induces positive changes in nitrogen metabolism and antioxidant system of oat (Avena sativa L. cultivar Kent) J. Plant Interact. 2013;10:211–223. doi: 10.1080/17429145.2015.1056260. [DOI] [Google Scholar]
- 28.Dhindsa R.S., Matowe W. Drought Tolerance in Two Mosses: Correlated with Enzymatic Defence Against Lipid Peroxidation. J. Exp. Bot. 1981;32:79–91. doi: 10.1093/jxb/32.1.79. [DOI] [Google Scholar]
- 29.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 30.Nakano Y., Asada K. Hydrogen-Peroxide Is Scavenged by Ascorbate-Specific Peroxidase in Spinach-Chloroplasts. Plant Cell Physiol. 1981;22:867–880. doi: 10.1093/oxfordjournals.pcp.a076232. [DOI] [Google Scholar]
- 31.Foster J.G., Hess J.L. Responses of Superoxide Dismutase and Glutathione Reductase Activities in Cotton Leaf Tissue Exposed to an Atmosphere Enriched in Oxygen. Plant Physiol. 1980;66:482–487. doi: 10.1104/pp.66.3.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mukherjee S.P., Choudhuri M.A. Implications of water stress-induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Physiol. Plant. 1983;58:166–170. doi: 10.1111/j.1399-3054.1983.tb04162.x. [DOI] [Google Scholar]
- 33.Ellman G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 34.Baker H., Frank O., DeAngelis B., Feingold S. Plasma tocopherol in man at various times after ingesting free or acetylated tocopherol. Nutr. Rep. Int. 1980;21:531–536. [Google Scholar]
- 35.Jackson M.L. Soil Chemical Analysis. Prentice Hall of India Pvt. Ltd.; New Delhi, India: 1973. [Google Scholar]
- 36.Yan K., Shao H., Shao C., Chen P., Zhao S., Brestic M., Chen X. Physiological adaptive mechanisms of plants grown in saline soil and implications for sustainable saline agriculture in coastal zone. Acta Physiol. Plant. 2013;35:2867–2878. doi: 10.1007/s11738-013-1325-7. [DOI] [Google Scholar]
- 37.Syeed S., Anjum N.A., Nazar R., Iqbal N., Masood A., Khan N.A. Salicylic acid-mediated changes in photosynthesis, nutrients content and antioxidant metabolism in two mustard (Brassica juncea L.) cultivars differing in salt tolerance. Acta Physiol. Plant. 2011;33:877–886. doi: 10.1007/s11738-010-0614-7. [DOI] [Google Scholar]
- 38.West G., Inzé D., Beemster G.T.S. Cell Cycle Modulation in the Response of the Primary Root of Arabidopsis to Salt Stress. Plant Physiol. 2004;135:1050–1058. doi: 10.1104/pp.104.040022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Asgher M., Per T.S., Masood A., Fatma M., Freschi L., Corpas F.J., Khan N.A. Nitric oxide signaling and its crosstalk with other plant growth regulators in plant responses to abiotic stress. Environ. Sci. Pollut. Res. 2017;24:2273–2285. doi: 10.1007/s11356-016-7947-8. [DOI] [PubMed] [Google Scholar]
- 40.Shams M., Ekinci M., Ors S., Turan M., Agar G., Kul R., Yildirim E. Nitric oxide mitigates salt stress effects of pepper seedlings by altering nutrient uptake, enzyme activity and osmolyte accumulation. Physiol. Mol. Biol. Plants. 2019;25:1149–1161. doi: 10.1007/s12298-019-00692-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dalal V.K., Tripathy B.C. Modulation of chlorophyll biosynthesis by water stress in rice seedlings during chloroplast biogenesis. Plant Cell Environ. 2012;35:1685–1703. doi: 10.1111/j.1365-3040.2012.02520.x. [DOI] [PubMed] [Google Scholar]
- 42.Ahanger M.A., Tittal M., Mir R.A., Agarwal R.M. Alleviation of water and osmotic stress-induced changes in nitrogen metabolizing enzymes in Triticum aestivum L. cultivars by potassium. Protoplasma. 2017;254:1953–1963. doi: 10.1007/s00709-017-1086-z. [DOI] [PubMed] [Google Scholar]
- 43.Ahanger M.A., Tomar N.S., Tittal M., Argal S., Agarwal R.M. Plant growth under water/salt stress: ROS production; antioxidants and significance of added potassium under such conditions. Physiol. Mol. Biol. Plants. 2017;23:731–744. doi: 10.1007/s12298-017-0462-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ahanger M.A., Tyagi S.R., Wani M.R., Ahmad P. Physiological Mechanisms and Adaptation Strategies in Plants Under Changing Environment. Springer; New York, NY, USA: 2014. Drought Tolerance: Role of Organic Osmolytes, Growth Regulators, and Mineral Nutrients; pp. 25–55. [DOI] [Google Scholar]
- 45.Dong Y., Xu L., Wang Q., Fan Z., Kong J., Bai X. Effects of exogenous nitric oxide on photosynthesis, antioxidative ability, and mineral element contents of perennial ryegrass under copper stress. J. Plant Interact. 2014;9:402–411. doi: 10.1080/17429145.2013.845917. [DOI] [Google Scholar]
- 46.Ahanger M.A., Alyemeni M.N., Wijaya L., Alamri S.A., Alam P., Ashraf M., Ahmad P. Potential of exogenously sourced kinetin in protecting Solanum lycopersicum from NaCl-induced oxidative stress through up-regulation of the antioxidant system, ascorbate-glutathione cycle and glyoxalase system. PLoS ONE. 2018;13:e0202175. doi: 10.1371/journal.pone.0202175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rasool S., Ahmad A., Siddiqi T.O., Ahmad P. Changes in growth, lipid peroxidation and some key antioxidant enzymes in chickpea genotypes under salt stress. Acta Physiol. Plant. 2012;35:1039–1050. doi: 10.1007/s11738-012-1142-4. [DOI] [Google Scholar]
- 48.Nahar K., Hasanuzzaman M., Alam M.M., Rahman A., Suzuki T., Fujita M. Polyamine and nitric oxide crosstalk: Antagonistic effects on cadmium toxicity in mung bean plants through upregulating the metal detoxification, antioxidant defense and methylglyoxal detoxification systems. Ecotox. Environ. Saf. 2016;126:245–255. doi: 10.1016/j.ecoenv.2015.12.026. [DOI] [PubMed] [Google Scholar]
- 49.Ahmad P., Jaleel C.A., Salem M.A., Nabi G., Sharma S. Roles of enzymatic and nonenzymatic antioxidants in plants during abiotic stress. Crit. Rev. Biotechnol. 2010;30:161–175. doi: 10.3109/07388550903524243. [DOI] [PubMed] [Google Scholar]
- 50.Chen Y.E., Cui J.M., Li G.X., Yuan M., Zhang Z.W., Yuan S., Zhang H.Y. Effect of salicylic acid on the antioxidant system and photosystem II in wheat seedlings. Biol. Plantarum. 2016;60:139–147. doi: 10.1007/s10535-015-0564-4. [DOI] [Google Scholar]
- 51.Noctor G., Foyer C.H. ASCORBATE AND GLUTATHIONE: Keeping Active Oxygen Under Control. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998;49:249–279. doi: 10.1146/annurev.arplant.49.1.249. [DOI] [PubMed] [Google Scholar]
- 52.Ahanger M.A., Gul F., Ahmad P., Akram N.A. Environmental Stresses and Metabolomics—Deciphering the Role of Stress Responsive Metabolites. In: Ahmad P., Ahnager M.A., Singh V.P., Tripathi D.K., Alyemeni M.N., editors. Plant Metabolites and Regulation Under Environmental Stress. Academic Press, Elsevier; New York, NY, USA: 2018. [DOI] [Google Scholar]
- 53.Raman V., Ravi S. Effect of salicylic acid and methyl jasmonate on antioxidant systems of Haematococcus pluvialis. Acta Physiol. Plant. 2011;33:1043–1049. doi: 10.1007/s11738-010-0623-6. [DOI] [Google Scholar]
- 54.Munné-Bosch S. The role of -tocopherol in plant stress tolerance. J. Plant Physiol. 2005;162:743–748. doi: 10.1016/j.jplph.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 55.Yusuf M.A., Kumar D., Rajwanshi R., Strasser R.J., Tsimilli-Michael M., Sarin N.B. Overexpression of γ-tocopherol methyl transferase gene in transgenic Brassica juncea plants alleviates abiotic stress: Physiological and chlorophyll a fluorescence measurements. Biochim. Biophys. Acta. 2010;1797:1428–1438. doi: 10.1016/j.bbabio.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 56.Ghanta S., Datta R., Bhattacharyya D., Sinha R., Kumar D., Hazra S., Mazumdar A.B., Chattopadhyay S. Multistep involvement of glutathione with salicylic acid and ethylene to combat environmental stress. J. Plant Physiol. 2014;171:940–950. doi: 10.1016/j.jplph.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 57.Ashrafijou M., Noori S.S., Darbandi A.I., Saghafi S. Effect of salinity and radiation on proline accumulation in seeds of canola (Brassica napus L.) Plant Soil Environ. 2010;56:312–317. doi: 10.17221/2/2010-PSE. [DOI] [Google Scholar]
- 58.Hussein M., Balbaa L., Gaballah M. Salicylic acid and salinity effects on growth of maize plants. Res. J. Agric. Biol. Sci. 2007;3:321–328. [Google Scholar]
- 59.Ahanger M.A., Agarwal R.M. Potassium up-regulates antioxidant metabolism and alleviates growth inhibition under water and osmotic stress in wheat (Triticum aestivum L) Protoplasma. 2017;254:1471–1486. doi: 10.1007/s00709-016-1037-0. [DOI] [PubMed] [Google Scholar]
- 60.Reddy P.S., Jogeswar G., Rasineni G.K., Maheswari M., Reddy A.R., Varshney R.K., Kavi Kishor P.B. Proline over-accumulation alleviates salt stress and protects photosynthetic and antioxidant enzyme activities in transgenic sorghum [Sorghum bicolor (L.) Moench] Plant Physiol. Biochem. 2015;94:104–113. doi: 10.1016/j.plaphy.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 61.Banu M.N.A., Hoque M.A., Watanabe-Sugimoto M., Matsuoka K., Nakamura Y., Shimoishi Y., Murata Y. Proline and glycinebetaine induce antioxidant defense gene expression and suppress cell death in cultured tobacco cells under salt stress. J. Plant Physiol. 2009;166:146–156. doi: 10.1016/j.jplph.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 62.Per T.S., Khan N.A., Reddy P.S., Masood A., Hasanuzzaman M., Khan M.I.R., Anjum N.A. Approaches in modulating proline metabolism in plants for salt and drought stress tolerance: Phytohormones, mineral nutrients and transgenics. Plant Physiol. Biochem. 2017;115:126–140. doi: 10.1016/j.plaphy.2017.03.018. [DOI] [PubMed] [Google Scholar]
- 63.Ghazijahani N., Hadavi E., Jeong B.R. Foliar sprays of citric acid and salicylic acid alter the pattern of root acquisition of some minerals in sweet basil (Ocimum basilicum L.) Front. Plant Sci. 2014;5 doi: 10.3389/fpls.2014.00573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bai X.Y., Dong Y.J., Xu L.L., Kong J., Liu S. Effects of exogenous nitric oxide on physiological characteristics of perennial ryegrass under cadmium and copper stress. Russ. J. Plant Phys. 2015;62:237–245. doi: 10.1134/S1021443715020028. [DOI] [Google Scholar]
- 65.Sheng H., Zeng J., Yan F., Wang X., Wang Y., Kang H., Fan X., Sha L., Zhang H., Zhou Y. Effect of exogenous salicylic acid on manganese toxicity, mineral nutrients translocation and antioxidative system in polish wheat (Triticum polonicum L.) Acta Physiol. Plant. 2015;37 doi: 10.1007/s11738-015-1783-1. [DOI] [Google Scholar]
- 66.Jan S., Alyemeni M.N., Wijaya L., Alam P., Siddique K.H., Ahmad P. Interactive effect of 24-epibrassinolide and silicon alleviates cadmium stress via the modulation of antioxidant defense and glyoxalase systems and macronutrient content in Pisum sativum L. seedlings. BMC Plant Biol. 2018;18 doi: 10.1186/s12870-018-1359-5. [DOI] [PMC free article] [PubMed] [Google Scholar]