Human astroviruses (HAstV) are thought to cause between 2 and 9% of acute, nonbacterial diarrhea cases in children worldwide. HAstV infection can be especially problematic in immunocompromised people and infants, where the virus has been associated with necrotizing enterocolitis and severe and persistent diarrhea, as well as rare instances of systemic and fatal disease. And yet, no antivirals have been identified to treat astrovirus infection. Our study provides the first evidence that nitazoxanide may be an effective therapeutic strategy against astrovirus disease.
KEYWORDS: antiviral agents, astrovirus, diarrhea, viral replication
ABSTRACT
Astroviruses (AstV) are a leading cause of diarrhea, especially in the very young, the elderly, and immunocompromised populations. Despite their significant impact on public health, no drug therapies for astrovirus have been identified. In this study, we fill this gap in knowledge and demonstrate that the FDA-approved broad-spectrum anti-infective drug nitazoxanide (NTZ) blocks astrovirus replication in vitro with a 50% effective concentration (EC50) of approximately 1.47 μM. It can be administered up to 8 h postinfection and is effective against multiple human astrovirus serotypes, including clinical isolates. Most importantly, NTZ reduces viral shedding in vivo, exhibiting its potential as a future clinical therapeutic.
IMPORTANCE Human astroviruses (HAstV) are thought to cause between 2 and 9% of acute, nonbacterial diarrhea cases in children worldwide. HAstV infection can be especially problematic in immunocompromised people and infants, where the virus has been associated with necrotizing enterocolitis and severe and persistent diarrhea, as well as rare instances of systemic and fatal disease. And yet, no antivirals have been identified to treat astrovirus infection. Our study provides the first evidence that nitazoxanide may be an effective therapeutic strategy against astrovirus disease.
INTRODUCTION
Diarrheal disease is the second leading cause of death in children under 5 years of age, with nearly 1.7 billion cases and 525,000 deaths each year (1). Since their discovery in 1975, human astroviruses (HAstV), which are positive single-stranded RNA viruses, have consistently ranked among the leading causes of diarrhea worldwide (2). However, human astrovirus infections can range from asymptomatic to mild diarrhea or rare instances of fatal systemic disease (3, 4). Infections in immunocompetent individuals typically present as watery diarrhea that resolves within 1 to 3 days postinfection without the need for hospitalization (2). Astrovirus outbreaks occur frequently in assisted living facilities, hospitals, and child care centers, where the young, elderly, and immunocompromised populations are at risk of persistent diarrhea, leading to wasting (5), and extragastrointestinal disease that may require medical intervention, including respiratory disease (6–10), fatal encephalitis, and meningitis (11).
Despite its high prevalence and the risk of severe disease, no vaccines or drug treatments exist for astrovirus. Only oral or parenteral fluids and electrolytes are available to prevent and treat dehydration caused by astrovirus-induced diarrhea. In these studies, we provide the first evidence that nitazoxanide (NTZ), an FDA-approved broad-spectrum antiparasitic and antiviral drug, inhibits the replication of multiple strains of human astrovirus in vitro even when administered up to 8 h postinfection and reduces viral shedding and diarrhea in vivo. This work highlights the potential use of NTZ as an effective therapeutic strategy against astrovirus infection.
(This article was submitted to an online preprint archive [12].)
RESULTS
Nitazoxanide blocks astrovirus replication in vitro.
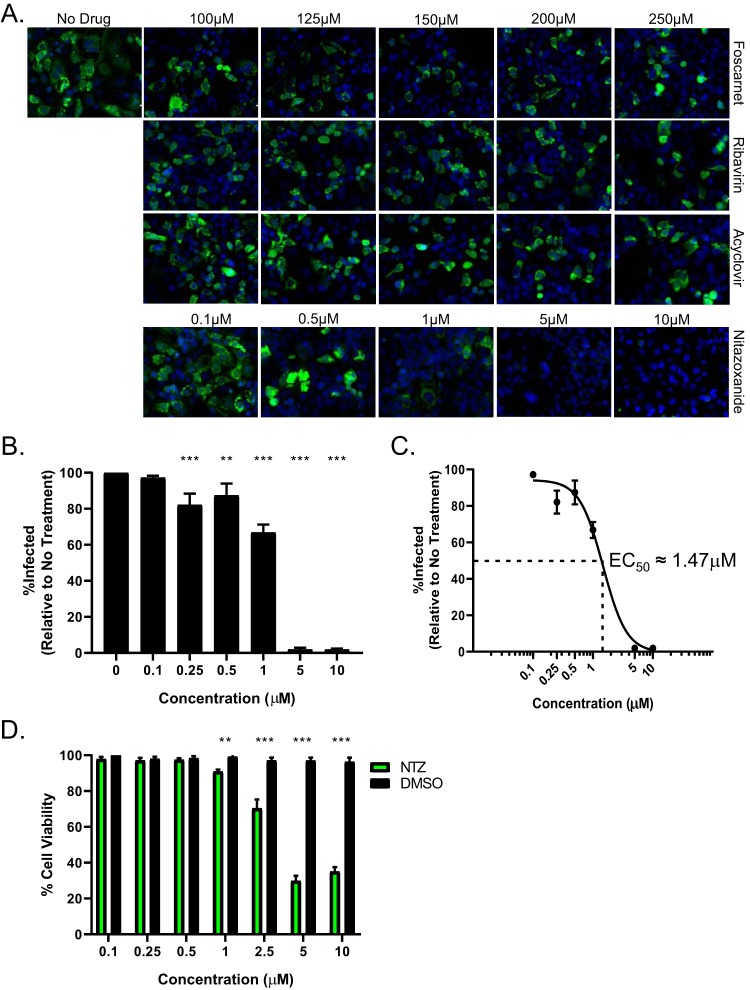
To identify an effective antiviral drug against astrovirus, human colon carcinoma (Caco-2) cells were infected with a laboratory strain of human astrovirus strain 1 (HAstV-1) at a multiplicity of infection (MOI) of 1 before increasing concentrations of antiviral compounds or dimethyl sulfoxide (DMSO; vehicle control) were added 1 h postinfection (hpi). The panel of antiviral compounds used in our study, foscarnet, ribavirin, acyclovir, and nitazoxanide, were chosen upon the recommendation of clinical colleagues, with acyclovir serving as a negative control. Viral capsid protein levels were quantitated at 24 hpi by immunofluorescence microscopy as described previously (13). Foscarnet, ribavirin, and acyclovir failed to inhibit HAstV-1 replication even at concentrations of 250 μM (Fig. 1A). In contrast, NTZ inhibited HAstV-1 replication in a dose-dependent manner, with the 5 μM treatment completely blocking virus replication (Fig. 1A and B). The 50% effective concentration (EC50) was calculated as approximately 1.47 μM (Fig. 1C). Concentrations of NTZ above 5 μM were associated with decreased cell viability compared to the cell viability with vehicle alone (DMSO) (Fig. 1D). Thus, subsequent studies were performed with NTZ at a concentration of 2.5 μM.
FIG 1.
Nitazoxanide inhibits HAstV-1 replication in Caco-2 cells. (A) Caco-2 cells were infected with HAstV-1 at an MOI of 1 and treated with a panel of antivirals (foscarnet, ribavirin, acyclovir, and nitazoxanide) at the indicated concentrations. At 24 hpi, cells were fixed and stained with DAPI (blue) and for the presence of astrovirus capsid protein (green). (B) The percentages of infected cells were calculated and compared to the results for nontreated cells. (C) Nonlinear regression analysis of percent infection data was used to determine the 50% effective concentration (EC50). (D) Cell viability of Caco-2 cells following 24 h of treatment with NTZ or vehicle alone (DMSO) at the indicated concentrations was determined by MTT assay. All error bars indicate standard errors of the means, and asterisks show statistical significance as measured by ordinary one-way ANOVA (B) and multiple t tests (D) as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
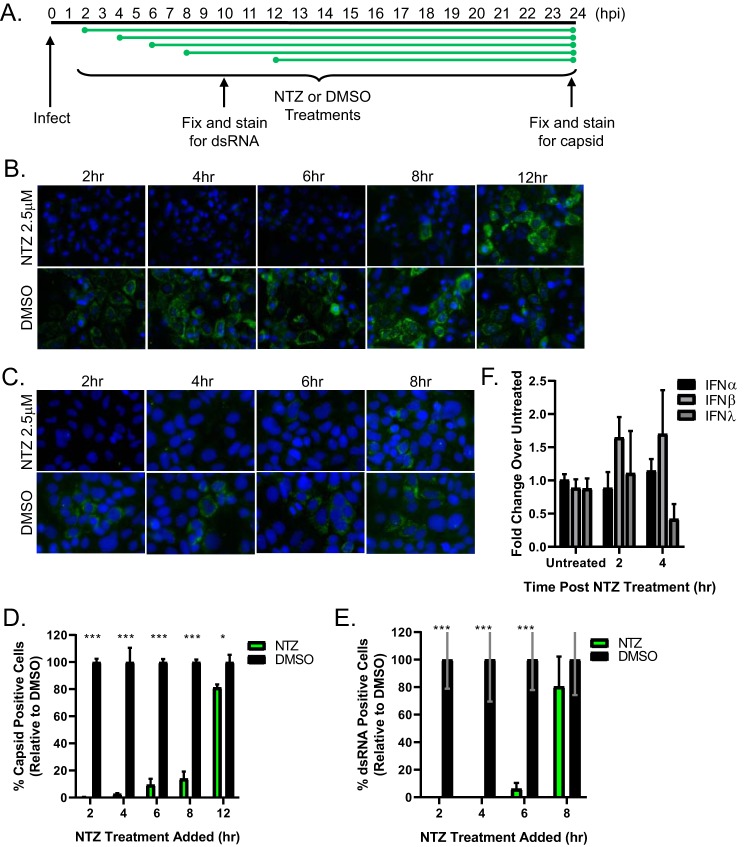
To determine the stage of the viral replication cycle blocked by NTZ, drug was added at 2, 4, 6, 8, and 12 h after HAstV-1 infection and viral capsid expression quantitated (Fig. 2A). The addition of NTZ up to 8 hpi completely inhibited HAstV-1 replication (Fig. 2B), suggesting it blocks a stage of the viral life cycle following entry and uncoating but before structural protein production. Indeed, NTZ reduced the formation of double-stranded RNA (dsRNA) that occurs when the astrovirus genome is generated via its RNA-dependent RNA polymerase (Fig. 2C). This method serves as a proxy for replication, given our lack of antibodies to the HAstV-1 nonstructural proteins.
FIG 2.
Nitazoxanide inhibits HAstV-1 replication in vitro when added up to 8 hpi. (A) Caco-2 cells were infected with HAstV-1, and at the indicated times postinfection, 2.5 μM NTZ or vehicle alone (DMSO) was added. (B) At 24 hpi, cells were fixed and stained with DAPI (blue) and for the presence of astrovirus capsid protein (green). (C) At 10 hpi, cells were fixed and stained with DAPI (blue) and for the presence of dsRNA (green). (D) Quantification of the percentages of cells with capsid staining from the experiment whose results are shown in panel B. (E) Quantification of the percentages of cells with dsRNA staining from the experiment whose results are shown in panel C. All error bars indicate standard errors of the means, and asterisks show statistical significance as measured by multiple t tests as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001. (F) Real-time RT-PCR for IFN-α, IFN-β, and IFN-λ was performed on RNA collected from Caco-2 cells treated with 2.5 μM NTZ and normalized to the value for GAPDH. Results are shown as fold increases over the results for untreated cells, and error bars indicate standard errors of the means.
Previous work demonstrated that thiazolide, the active form of NTZ, upregulates type I and II interferon (IFN) genes, which were critical for reducing HIV replication in human peripheral blood mononuclear cells (PBMCs) (14), Type I IFN limits HAstV replication (15). Thus, to determine whether NTZ increased type I or III IFN within the time frame it inhibits HAstV replication, we treated Caco-2 cells with NTZ or DMSO (untreated) and quantitated interferon alpha (IFN-α), IFN-β, or IFN-λ mRNA levels at 2 and 4 h. At 4 h post-NTZ treatment, the shortest duration of time NTZ can be added and still inhibit HAstV dsRNA production, IFN-α, IFN-β, and IFN-λ mRNA transcripts were not significantly upregulated compared to their levels in DMSO-treated cells (Fig. 2F), suggesting that NTZ inhibits HAstV independent of IFN induction. Future studies will explore the mechanism of action.
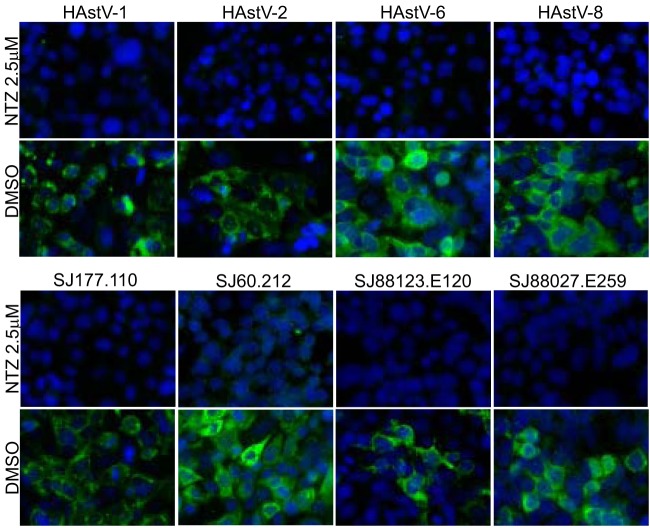
We next asked if NTZ was effective against multiple classical HAstV serotypes and clinical isolates. Briefly, Caco-2 cells were infected with four different laboratory-adapted HAstV serotypes (HAstV-1, -2, -6, and -8) and four clinical isolates obtained from patient samples (SJ177.110 [HAstV-2], SJ60.212 [HAstV-8], SJ88123.E120 [HAstV-1], and SJ88027.E259 [HAstV-1]) and treated with 2.5 μM NTZ or DMSO (vehicle control). NTZ completely inhibited all HAstV isolates, suggesting it is broadly protective against multiple HAstV serotypes (Fig. 3).
FIG 3.
Nitazoxanide inhibits the replication of multiple serotypes and clinical isolates of human astrovirus. Caco-2 cells were infected with laboratory-adapted virus serotypes (top) or clinical isolates (bottom) and treated with 2.5 μM NTZ or vehicle alone (DMSO). At 24 hpi, cells were fixed and stained with DAPI (blue) and for the presence of astrovirus capsid protein (green).
NTZ reduces viral replication and clinical disease in vivo.
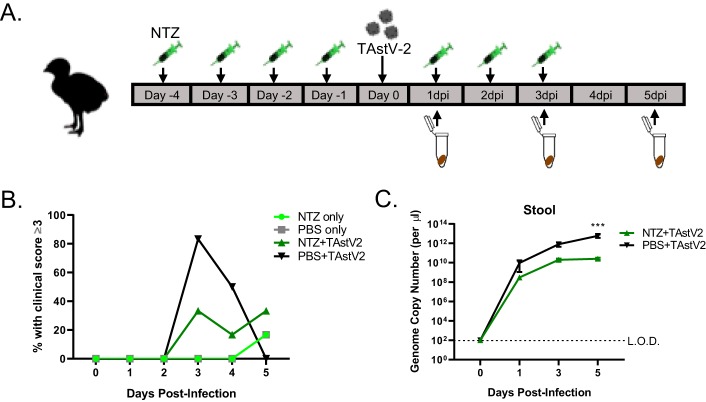
To determine if NTZ reduced disease in vivo, we used the only small animal model exhibiting astrovirus-induced diarrheal disease, turkey poults (16). Briefly, 5-day-old turkey poults were orally gavaged with 100 mg/kg of body weight NTZ once daily for 4 days prior to oral infection with intestinal filtrate containing between 1012 and 1013 genome copy units turkey astrovirus strain 2 (TAstV-2) in 500 μl phosphate-buffered saline (PBS) and then for 3 days postinfection (Fig. 4A). The dose of NTZ used to treat the turkey poults was selected based on previously published studies of NTZ treatment in mice (17–19). Stool samples were scored daily by four blinded volunteers as previously described (20). The scoring scale ranged from 1 to 4 based on color and consistency, with scores of 3 and 4 being considered diarrhea. Stool samples were also collected to quantitate viral titers. Although the difference was not statistically significant, NTZ treatment resulted in fewer poults with clinical disease (Fig. 4B). This likely results from the significant reduction (P = 0.005) in stool viral loads throughout the course of infection (Fig. 4C). The poults also showed no adverse symptoms from receiving NTZ alone. Excitingly, this gives evidence NTZ may be an effective therapeutic for astrovirus-induced diarrheal disease.
FIG 4.
Nitazoxanide reduces clinical symptoms and viral titers in turkey poults. (A) Turkey poults (n = 6 per group) were infected with turkey astrovirus (TAstV-2) from intestinal filtrate. For 4 days prior to infection and 3 days postinfection, poults were treated with NTZ. Poults were monitored for clinical score daily, and stool samples were collected to measure viral RNA titers every other day. (B) Percentages of poults with clinical scores of 3 or higher in the treatment groups indicated in the key. PBS, no antiviral treatment. (C) Viral RNA titers of stool samples collected from infected poults with or without NTZ treatment. All error bars indicate standard errors of the means, and dashed line represents the limit of detection. Asterisks show statistical significance as measured by two-way ANOVA as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
DISCUSSION
Our study provides the first evidence of an effective antiviral for astrovirus infection. We showed that NTZ is broadly protective against multiple classical HAstV serotypes and reduces the production of dsRNA during infection in vitro with an EC50 of approximately 1.47 μM. Additionally, we showed the potential NTZ has as a clinical therapeutic by its ability to reduce viral shedding and clinical disease in our symptomatic turkey poult model.
Nitazoxanide [2-acetyloxy-N-(5-nitro-2-thiazolyl) benzamide] (Alinia; Romark Laboratories) is a thiazolide compound for treatment of both intestinal protozoal and helminthic infections, specifically Giardia lamblia and Cryptosporidium parvum (21). Recently, this compound has been shown to have antiviral properties as well. The use of NTZ in vitro has been reported as an antiviral against influenza virus (22), rotavirus (23), norovirus (24), Japanese encephalitis virus (JEV) (18), rubella virus (25), Zika virus (26), hepatitis C virus (27), and hepatitis B virus (28). Successful clinical trials have demonstrated its effectiveness in treating influenza virus (29), norovirus and rotavirus (24, 30, 31), hepatitis B virus (32), and hepatitis C virus (27, 33). Its mechanism of action against protozoa is due to its interference with pyruvate:ferredoxin oxidoreductase (PFOR) enzyme-dependent electron transfer reactions (34). While its antiviral action is currently unknown, research suggests it may be through the induction of the interferon response via activation of protein kinase R or disruption of the unfolded protein response (21). We show that NTZ disrupts astrovirus infection early in the replication cycle, causing a significant decrease in the production of dsRNA. The inhibition by NTZ at an early stage of infection was also seen with JEV (18). Recent research has shown that thiazolides upregulate type I and II IFN (14), which modulate the immune system, and this could be how NTZ creates a broadly antiviral state. However, the rapid kinetics with which NTZ inhibits HAstV replication (Fig. 2) suggests that the induction of IFN is not responsible. Studies to uncover the exact mechanism will be difficult until further tools are available to investigate astrovirus replication.
Human astroviruses are classified into genotypes, and within the classical human genotype, comprising the genus Mamastrovirus 1 (MAstV1), strains are further divided into serotypes (HAstV-1 to -8) based on their antigenicities and genetic differences in the complete capsid sequences (2). These genetic differences between astrovirus serotypes can confer differences in replication kinetics and symptom severity (35). Thus, finding a compound that broadly inhibits astroviruses across genotypes and serotypes is crucial. We found that NTZ is broadly protective across multiple HAstV serotypes, including the dominant strain worldwide, HAstV-1 (36). Future studies will assess the ability of NTZ to inhibit the replication of the nonclassical HAstV genotypes, MLB and VA (MAstV6, -8, and -9), which have been associated with rare cases of encephalitis or meningitis in immunocompromised patients (11), although there has been 1 case of classical HAstV-associated encephalitis.
To evaluate efficacy in vivo, we turned to the turkey poult model. Turkey poults exhibit age-dependent diarrhea similar to that in humans when infected with TAstV, making them the only clinically relevant small animal model for astrovirus identified to date (16, 37). We found a significant decrease (P = 0.005) in stool viral titers, with NTZ-treated poults having nearly 2 log less virus at 5 days postinfection. We also showed that viral titers began to plateau in the NTZ-treated poults at 5 days postinfection, while untreated poults still showed increasing titers. This suggests NTZ treatment may lead to faster clearance of the virus. The decreased viral titers were associated with fewer poults having clinical disease. It is not surprising that NTZ did not completely prevent clinical disease. Viral titers are reduced but not completely inhibited. Furthermore, we demonstrated that administration of viral capsid alone is sufficient to induce diarrhea in turkey poults (20). Therefore, NTZ treatment may not be able to fully inhibit AstV-induced diarrhea. Finally, we administered between 1,012 and 1,013 genome copy units to each poult. While there have been reports of virus shedding in humans at this level (35), it is a large viral dose that may not be representative of natural infection. Overall, this work provides the first evidence that NTZ may be an effective antiviral option against human classical HAstV and turkey astrovirus infections.
MATERIALS AND METHODS
Cells and virus propagation.
The human intestinal adenocarcinoma cell line Caco-2 was obtained from ATCC (HTB-37). Cells were propagated in minimum essential medium (MEM; Corning) supplemented with 20% fetal bovine serum (FBS; Benchmark), GlutaMax-I (Gibco), 1 mM sodium pyruvate (Gibco), and penicillin-streptomycin (Gibco).
Laboratory-adapted human astrovirus stocks (HAstV-1, HAstV-2, HAstV-6, and HAstV-8) were propagated in Caco-2 cells, and the titers of the viruses were determined on Caco-2 cells by the fluorescent focus assay (focus-forming units [FFU]) as previously described (13).
Clinical isolates (SJ177.110, SJ60.212, SJ88123.E120, and SJ88027.E259) were derived from remnant fecal samples submitted for clinical diagnostic testing at St. Jude Children’s Research Hospital. All samples were deidentified before testing. The St. Jude Institutional Review Board approved this study with a waiver of consent. All isolates were propagated in Caco-2 cells. Briefly, a 10 to 20% dilution of stool extract, positive for HAstV by real-time reverse transcription-PCR (qRT-PCR), was filtered through a 0.22-μm filter. The extract was diluted 1:10 in MEM with 5 μg/ml porcine trypsin before adsorption onto Caco-2 cell monolayers. Following a 1-h adsorption period at 37°C, the inoculum was removed and replaced with MEM containing 10 μg/ml porcine trypsin and 0.3% bovine serum albumin (BSA). The titers of the viruses were again determined on Caco-2 cells by the fluorescent focus assay (13).
TAstV-2 stocks were prepared from intestines collected from infected turkey poults. Briefly, pieces of intestine were suspended in 0.5 ml PBS, homogenized using 2-mm zirconium oxide beads (Next Advance) for 4 min (at speed setting 4 in the Next Advance air cooling bullet blender), and pelleted by centrifugation at 12,000 rpm for 5 min. The supernatant was pooled and filtered through a 0.2-μm filter (fecal filtrate), and viral copy number was quantified by qRT-PCR.
In vitro HAstV infection and NTZ administration.
Briefly, 5 × 104 cells were seeded into 96-well tissue culture plates (Corning), and after 2 days, the cells were inoculated with virus (HAstV-1 or clinical isolates) in serum-free MEM for 1 h at 37°C, at which time the virus was replaced with MEM containing 0.3% BSA and infection was allowed to proceed until 24 hpi or as described in the figure legends. For NTZ treatment, NTZ or DMSO (0.3% to 3%) was added to the infection medium at the concentrations and times described in the figure legends.
Immunofluorescent staining.
Cells were fixed with 100% ice-cold methanol for 15 min and then blocked with 5% normal goat serum (NGS; Gibco) in PBS at room temperature. Cells stained for astrovirus capsid protein were incubated with HAstV mouse monoclonal antibody 8E7 (2 μg/ml; DakoCytomation), and cells stained for dsRNA were incubated with J2 mouse monoclonal antibody (Scicons) (38) for 1 h at room temperature. Following incubation with primary antibody, cells were incubated with anti-mouse IgG labeled with Alexa Fluor 488 (Invitrogen) as the secondary antibody and with DAPI (4′,6′-diamidino-2-phenylindole; Sigma) for 30 min at room temperature. Staining was imaged on the EVOS FL cell imaging system and analyzed using ImageJ 1.50i software.
MTT cell viability assay.
Cell viability was tested using an MTT [3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide] cell proliferation assay kit (Abcam) according to the manufacturer’s protocol. Briefly, cells were treated with various concentrations of nitazoxanide in serum-free medium for 24 h. The nitazoxanide-containing medium was removed and replaced with a 50:50 mixture of MTT reagent and serum-free medium. The cells were incubated with the mixture at 37°C for 3 h. Following incubation, an MTT solvent solution was added and the plate was placed on an orbital shaker for 15 min. The absorbance was then measured as optical density at 595 nm (OD595). Cell viability was calculated as the percentage compared to the cell viability in nontreated cells.
IFN qRT-PCR.
Caco-2 cells were treated with 2.5 μM NTZ or not treated, and RNA extracted at 2 and 4 h posttreatment using TRIzol (Ambion) according to the manufacturer’s specifications. Then, qRT-PCR was performed using the QuantiTect SYBR green kit (Qiagen) primer assays for IFNα1 (catalog number QT00201964; Qiagen), IFNβ1 (catalog number QT00203763; Qiagen), and IFNλ1 (catalog number QT00222495; Qiagen). The resulting cycle threshold (CT) values were normalized to the value for GAPDH (glyceraldehyde-3-phosphate dehydrogenase) (catalog number QT00079247; Qiagen). The log-transformed ΔΔCT values are reported as fold changes over the values for untreated cells.
Animals and NTZ treatment.
Broad-breasted white turkey poults were obtained from a commercial hatchery. Five-day-old poults were randomly assigned to groups (n = 6 per group) and housed in individual, temperature-controlled Horsfall units with HEPA-filtered inlet and exhaust air valves, where they were given free access to water and routine turkey starter feed. Poults were orally inoculated with 500 μl of TAstV-2 intestinal filtrate, containing approximately 1012 to 1013 genome copies, or PBS alone. Stool samples from individual birds were scored from 1 to 4. Scoring was performed daily postinfection. Scores of 3 (liquid or loose stool with some undigested food or solid material) and 4 (watery stool with no solids present) were defined as diarrhea, in accordance with previously published work from Meliopoulos et al. (20).
For NTZ treatment, poults were orally administered 100 mg/kg nitazoxanide in 500 μl of ultrapure water. Administration of NTZ was carried out for 4 days prior to infection and 3 days postinfection.
Turkey astrovirus qRT-PCR assay.
TAstV-2 genome copies were determined as previously described (20). Briefly, viral RNA was isolated from 10% stool by using the MagMAX-96 avian influenza (AI)/Newcastle disease (ND) viral RNA isolation kit (Applied Biosystems) according to the manufacturer’s protocol. PCR was performed on 3 μl of each sample using TaqMan fast virus 1-step master mix (Applied Biosciences) with 600 nM forward primer 5′GACTGAAATAAGGTCTGCACAGGT, 600 nM reverse primer 5′AACCTGCGAACCCTGCG, and 200 nM probe 6-carboxyfluorescein (6FAM)-ATGGACCCCCTTTTTCGGCGG-BHQ1 (black hole quencher 1) under the following conditions: 50°C for 5 min and 95°C for 20 s, followed by 45 cycles with one cycle consisting of 95°C for 3 s and 60°C for 30 s on a Bio-Rad CFX96 real-time PCR detection system. The number of genome copies/μl of total RNA was determined using a standard curve generated from a synthesized TAstV-2 DNA sequence, comprising nucleotides 4001 to 4201, with a known copy number (calculated using Thermo Fisher Scientific DNA Copy Number and Dilution Calculator). Log10 dilutions of the synthesized TAstV-2 DNA were used for qRT-PCR as described above.
Statistical analysis.
Data were analyzed by ordinary one-way analysis of variance (ANOVA) (EC50 and MTT assay), multiple t test (capsid and dsRNA staining time course), and two-way ANOVA (turkey stool titers) using GraphPad Prism version 8.
ACKNOWLEDGMENTS
We thank Rebekah Honce, Pamela Freiden, and Victoria Meliopoulos for their expert poop scoring abilities, Sean Offord and Sharon Lokey from the St. Jude Animal Resources Center for assistance with turkey studies, and David Wang for graciously providing VA1 for these studies.
These studies were funded by National Institute of Allergy and Infectious Diseases grant number R21 AI135254-01 and by ALSAC.
REFERENCES
- 1.World Health Organization. 2017. Diarrhoeal disease fact sheet. World Health Organization, Geneva, Switzerland: https://www.who.int/news-room/fact-sheets/detail/diarrhoeal-disease. [Google Scholar]
- 2.Bosch A, Pintó RM, Guix S. 2014. Human astroviruses. Clin Microbiol Rev 27:1048–1074. doi: 10.1128/CMR.00013-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Méndez-Toss M, Griffin DD, Calva J, Contreras JF, Puerto FI, Mota F, Guiscafré H, Cedillo R, Muñoz O, Herrera I, López S, Arias CF. 2004. Prevalence and genetic diversity of human astroviruses in Mexican children with symptomatic and asymptomatic infections. J Clin Microbiol 42:151–157. doi: 10.1128/jcm.42.1.151-157.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kurtz JB, Lee TW, Craig JW, Reed SE. 1979. Astrovirus infection in volunteers. J Med Virol 3:221–230. doi: 10.1002/jmv.1890030308. [DOI] [PubMed] [Google Scholar]
- 5.Bosch A, Guix S, Pintó RM. 2013. Epidemiology of human astroviruses, p 1–18. In Schultz-Cherry S. (ed), Astrovirus research: essential ideas, everyday impacts, future directions. Springer, New York, NY. [Google Scholar]
- 6.Lin H-C, Kao C-L, Chang L-Y, Hsieh Y-C, Shao P-L, Lee P-I, Lu C-Y, Lee C-Y, Huang L-M. 2008. Astrovirus gastroenteritis in children in Taipei. J Formos Med Assoc 107:295–303. doi: 10.1016/S0929-6646(08)60090-X. [DOI] [PubMed] [Google Scholar]
- 7.Tseng W-C, Wu F-T, Hsiung CA, Chang W-C, Wu H-S, Wu C-Y, Lin J-S, Yang S-C, Hwang K-P, Huang Y-C. 2012. Astrovirus gastroenteritis in hospitalized children of less than 5 years of age in Taiwan, 2009. J Microbiol Immunol Infect 45:311–317. doi: 10.1016/j.jmii.2011.12.017. [DOI] [PubMed] [Google Scholar]
- 8.Amaral MSC, Estevam GK, Penatti M, Lafontaine R, Lima ICG, Spada PKP, Gabbay YB, Matos NB. 2015. The prevalence of norovirus, astrovirus and adenovirus infections among hospitalised children with acute gastroenteritis in Porto Velho, state of Rondônia, western Brazilian Amazon. Mem Inst Oswaldo Cruz 110:215–221. doi: 10.1590/0074-02760140381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ueda Y, Nakaya S, Takagi M, Ushijima H. 1996. Diagnosis and clinical manifestations of diarrheal virus infections in Maizuru area from 1991 to 1994—especially focused on small round structured viruses. Kansenshogaku Zasshi 70:1092–1097. (In Japanese.) doi: 10.11150/kansenshogakuzasshi1970.70.1092. [DOI] [PubMed] [Google Scholar]
- 10.Cordey S, Zanella M-C, Wagner N, Turin L, Kaiser L. 2018. Novel human astroviruses in pediatric respiratory samples: a one-year survey in a Swiss tertiary care hospital. J Med Virol 90:1775–1778. doi: 10.1002/jmv.25246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vu D-L, Cordey S, Brito F, Kaiser L. 2016. Novel human astroviruses: novel human diseases? J Clin Virol 82:56–63. doi: 10.1016/j.jcv.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 12.Hargest V, Sharp B, Livingston B, Cortez V, Schultz-Cherry S. 2019. Astrovirus replication is inhibited by nitazoxanide in vitro and in vivo. bioRxiv doi: 10.1101/797316. [DOI] [PMC free article] [PubMed]
- 13.Marvin S, Meliopoulos V, Schultz-Cherry S. 2014. Human astrovirus propagation, purification and quantification. Bio Protoc 4:e1078. doi: 10.21769/BioProtoc.1078. [DOI] [Google Scholar]
- 14.Trabattoni D, Gnudi F, Ibba SV, Saulle I, Agostini S, Masetti M, Biasin M, Rossignol J-F, Clerici M. 2016. Thiazolides elicit anti-viral innate immunity and reduce HIV replication. Sci Rep 6:27148. doi: 10.1038/srep27148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marvin SA, Huerta CT, Sharp B, Freiden P, Cline TD, Schultz-Cherry S. 2016. Type I interferon response limits astrovirus replication and protects against increased barrier permeability in vitro and in vivo. J Virol 90:1988–1996. doi: 10.1128/JVI.02367-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koci MD, Moser LA, Kelley LA, Larsen D, Brown CC, Schultz-Cherry S. 2003. Astrovirus induces diarrhea in the absence of inflammation and cell death. J Virol 77:11798–11808. doi: 10.1128/jvi.77.21.11798-11808.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hong SK, Kim HJ, Song CS, Choi IS, Lee JB, Park SY. 2012. Nitazoxanide suppresses IL-6 production in LPS-stimulated mouse macrophages and TG-injected mice. Int Immunopharmacol 13:23–27. doi: 10.1016/j.intimp.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 18.Shi Z, Wei J, Deng X, Li S, Qiu Y, Shao D, Li B, Zhang K, Xue F, Wang X, Ma Z. 2014. Nitazoxanide inhibits the replication of Japanese encephalitis virus in cultured cells and in a mouse model. Virol J 11:10. doi: 10.1186/1743-422X-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El-Kowrany SI, El Ghaffar A-SA, Shoheib ZS, Mady RF, Gamea G. 2019. Evaluation of nitazoxanide as a novel drug for the treatment of acute and chronic toxoplasmosis. Acta Trop 195:145–154. doi: 10.1016/j.actatropica.2019.04.013. [DOI] [PubMed] [Google Scholar]
- 20.Meliopoulos VA, Marvin SA, Freiden P, Moser LA, Nighot P, Ali R, Blikslager A, Reddivari M, Heath RJ, Koci MD, Schultz-Cherry S. 2016. Oral administration of astrovirus capsid protein is sufficient to induce acute diarrhea in vivo. mBio 7:e01494-16. doi: 10.1128/mBio.01494-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rossignol J-F. 2014. Nitazoxanide: a first-in-class broad-spectrum antiviral agent. Antiviral Res 110:94–103. doi: 10.1016/j.antiviral.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rossignol JF, La Frazia S, Chiappa L, Ciucci A, Santoro MG. 2009. Thiazolides, a new class of anti-influenza molecules targeting viral hemagglutinin at the post-translational level. J Biol Chem 284:29798–29808. doi: 10.1074/jbc.M109.029470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rossignol J-F, Abu-Zekry M, Hussein A, Santoro MG. 2006. Effect of nitazoxanide for treatment of severe rotavirus diarrhoea: randomised double-blind placebo-controlled trial. Lancet 368:124–129. doi: 10.1016/S0140-6736(06)68852-1. [DOI] [PubMed] [Google Scholar]
- 24.Rossignol J-F, El Gohary YM. 2006. Nitazoxanide in the treatment of viral gastroenteritis: a randomized double-blind placebo-controlled clinical trial. Aliment Pharmacol Ther 24:1423–1430. doi: 10.1111/j.1365-2036.2006.03128.x. [DOI] [PubMed] [Google Scholar]
- 25.Perelygina L, Hautala T, Seppänen M, Adebayo A, Sullivan KE, Icenogle J. 2017. Inhibition of rubella virus replication by the broad-spectrum drug nitazoxanide in cell culture and in a patient with a primary immune deficiency. Antiviral Res 147:58–66. doi: 10.1016/j.antiviral.2017.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Z, Brecher M, Deng Y-Q, Zhang J, Sakamuru S, Liu B, Huang R, Koetzner CA, Allen CA, Jones SA, Chen H, Zhang N-N, Tian M, Gao F, Lin Q, Banavali N, Zhou J, Boles N, Xia M, Kramer LD, Qin C-F, Li H. 2017. Existing drugs as broad-spectrum and potent inhibitors for Zika virus by targeting NS2B-NS3 interaction. Cell Res 27:1046–1064. doi: 10.1038/cr.2017.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rossignol J, Elfert A, El–Gohary Y, Keeffe EB. 2009. Improved virologic response in chronic hepatitis C genotype 4 treated with nitazoxanide, peginterferon, and ribavirin. Gastroenterology 136:856–862. doi: 10.1053/j.gastro.2008.11.037. [DOI] [PubMed] [Google Scholar]
- 28.Korba BE, Montero AB, Farrar K, Gaye K, Mukerjee S, Ayers MS, Rossignol J-F. 2008. Nitazoxanide, tizoxanide and other thiazolides are potent inhibitors of hepatitis B virus and hepatitis C virus replication. Antiviral Res 77:56–63. doi: 10.1016/j.antiviral.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 29.Haffizulla J, Hartman A, Hoppers M, Resnick H, Samudrala S, Ginocchio C, Bardin M, Rossignol J-F. 2014. Effect of nitazoxanide in adults and adolescents with acute uncomplicated influenza: a double-blind, randomised, placebo-controlled, phase 2b/3 trial. Lancet Infect Dis 14:609–618. doi: 10.1016/S1473-3099(14)70717-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Teran CG, Teran-Escalera CN, Villarroel P. 2009. Nitazoxanide vs. probiotics for the treatment of acute rotavirus diarrhea in children: a randomized, single-blind, controlled trial in Bolivian children. Int J Infect Dis 13:518–523. doi: 10.1016/j.ijid.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 31.Siddiq DM, Koo HL, Adachi JA, Viola GM. 2011. Norovirus gastroenteritis successfully treated with nitazoxanide. J Infect 63:394–397. doi: 10.1016/j.jinf.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rossignol J-F, Keeffe EB. 2008. Thiazolides: a new class of drugs for the treatment of chronic hepatitis B and C. Future Microbiol 3:539–545. doi: 10.2217/17460913.3.5.539. [DOI] [PubMed] [Google Scholar]
- 33.Rossignol J-F, Elfert A, Keeffe EB. 2010. Treatment of chronic hepatitis C using a 4-week lead-in with nitazoxanide before peginterferon plus nitazoxanide. J Clin Gastroenterol 44:504–509. doi: 10.1097/MCG.0b013e3181bf9b15. [DOI] [PubMed] [Google Scholar]
- 34.Hoffman PS, Sisson G, Croxen MA, Welch K, Harman WD, Cremades N, Morash MG. 2007. Antiparasitic drug nitazoxanide inhibits the pyruvate oxidoreductases of Helicobacter pylori, selected anaerobic bacteria and parasites, and Campylobacter jejuni. Antimicrob Agents Chemother 51:868–876. doi: 10.1128/AAC.01159-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Caballero S, Guix S, El-Senousy WM, Calicó I, Pintó RM, Bosch A. 2003. Persistent gastroenteritis in children infected with astrovirus: association with serotype-3 strains. J Med Virol 71:245–250. doi: 10.1002/jmv.10476. [DOI] [PubMed] [Google Scholar]
- 36.Vu D-L, Bosch A, Pintó RM, Guix S. 2017. Epidemiology of classic and novel human astrovirus: gastroenteritis and beyond. Viruses 9:E33. doi: 10.3390/v9020033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schultz-Cherry S, Kapczynski DR, Simmons VM, Koci MD, Brown C, Barnes HJ. 2000. Identifying agent(s) associated with poult enteritis mortality syndrome: importance of the thymus. Avian Dis 44:256–265. doi: 10.2307/1592538. [DOI] [PubMed] [Google Scholar]
- 38.Weber F, Wagner V, Rasmussen SB, Hartmann R, Paludan SR. 2006. Double-stranded RNA is produced by positive-strand RNA viruses and DNA viruses but not in detectable amounts by negative-strand RNA viruses. J Virol 80:5059–5064. doi: 10.1128/JVI.80.10.5059-5064.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]