ZIKV emerged as a vector-borne pathogen capable of causing illness in infected adults and congenital birth defects in infants born to mothers infected during pregnancy. Despite the decrease in ZIKV cases since the 2015-2016 epidemic, questions concerning the prevalence and longevity of protective immunity have left vulnerable communities fearful that they may become the center of next ZIKV outbreak. Although preexisting herd immunity in regions of past outbreaks may dampen the potential for future outbreaks to occur, we currently do not know the longevity of protective immunity to ZIKV after a person becomes infected. Here, we establish a new experimentally defined minimal length of protective ZIKV immunity. We show that five rhesus macaques initially infected with ZIKV 22 to 28 months prior to rechallenge elicit a durable immune response that protected from detectable plasma viremia. This study establishes a new minimal length of protective immunity.
KEYWORDS: antibody, immunity, long-term immunity, Zika, Zika virus, arbovirus, flavivirus
ABSTRACT
By the end of the 2016 Zika virus (ZIKV) outbreak, it is estimated that there were up to 100 million infections in the Americas. In approximately one in seven infants born to mothers infected during pregnancy, ZIKV has been linked to microcephaly, developmental delays, or other congenital disorders collectively known as congenital Zika syndrome, as well as Guillain-Barré syndrome, in ZIKV-infected adults. It is a global health priority to develop a vaccine against ZIKV that elicits long-lasting immunity; however, the durability of immunity to ZIKV is unknown. Previous studies in mice and nonhuman primates have been crucial in vaccine development but have not defined the duration of immunity generated by ZIKV infection. In this study, we rechallenged five rhesus macaques with ZIKV 22 to 28 months after a primary ZIKV infection. We show that primary ZIKV infection generates high titers of neutralizing antibodies that protect from detectable plasma viremia following rechallenge and persist for at least 22 to 28 months. While additional longitudinal studies are necessary with longer time frames, this study establishes a new experimentally defined minimal length of protective ZIKV immunity.
IMPORTANCE ZIKV emerged as a vector-borne pathogen capable of causing illness in infected adults and congenital birth defects in infants born to mothers infected during pregnancy. Despite the decrease in ZIKV cases since the 2015-2016 epidemic, questions concerning the prevalence and longevity of protective immunity have left vulnerable communities fearful that they may become the center of next ZIKV outbreak. Although preexisting herd immunity in regions of past outbreaks may dampen the potential for future outbreaks to occur, we currently do not know the longevity of protective immunity to ZIKV after a person becomes infected. Here, we establish a new experimentally defined minimal length of protective ZIKV immunity. We show that five rhesus macaques initially infected with ZIKV 22 to 28 months prior to rechallenge elicit a durable immune response that protected from detectable plasma viremia. This study establishes a new minimal length of protective immunity.
INTRODUCTION
Zika virus (ZIKV) is a mosquito-borne flavivirus first isolated in 1947 in the Zika forest of Uganda (1). ZIKV is contracted through a mosquito bite or sexual contact and can be passed from mother to fetus during pregnancy (2). ZIKV infections are typically mild and are characterized by low-grade fever, maculopapular rash, arthralgia, and conjunctivitis (3). The recent outbreak of ZIKV in the Americas was associated with more severe disease. Guillain-Barré syndrome was found in a small proportion of ZIKV-positive adults in the French Polynesian and American outbreaks (4, 5). In addition, infants born to mothers infected with ZIKV during pregnancy have an approximately one in seven chance of having congenital Zika syndrome, a collection of fetal abnormalities and developmental disorders (6, 7). A retrospective analysis of infants, now aged 7 to 32 months, born to mothers infected with ZIKV during pregnancy, has shown that one-third of those infants have below average neurodevelopment (8). Currently, it is unknown whether ZIKV infection in humans confers lifelong immunity; however, it has been suggested that waning immunity over time leads to more severe ZIKV in the future (9, 10). It is thought that a single infection or vaccination that generates neutralizing antibody (nAb) titers above 1:10 PRNT50 (50% plaque reduction neutralization test) is enough to confer lifelong immunity in other flaviviruses, such as yellow fever virus (YFV) or dengue virus (DENV) (11, 12). Antibodies to YFV and DENV have been detected at least 60 and 35 years after infection, respectively (13–15). Despite long-term immunity to YFV and DENV, challenges remain in the eradication of these viruses due to evidence of circulation in sylvatic (forest) cycles in Asia and Africa and to urban human-mosquito cycles in South America (16–19). ZIKV circulates as one serotype while DENV circulates as four antigenically distinct serotypes (DENV-1 through DENV-4) (20, 21). No single serotype of DENV has been eradicated after an outbreak even though there is long-term population-level immunity (15). Similarly, there is a highly effective YFV vaccine that induces lifelong immunity but has not led to the eradication of YFV (14). Little is known about ZIKV transmission cycles in the Americas, but considering past flavivirus outbreaks, herd immunity and vector control alone will likely not be enough to eradicate the virus. Determining the longevity of immunity elicited from previous natural ZIKV infection will be important for assessing the risk of reinfection in future outbreaks, as well as for estimating the number of people who are at risk of future ZIKV infections.
The current understanding of ZIKV immunity in humans is derived primarily from vaccine clinical trials that are in progress (22). Recipients of a prM+E consensus DNA vaccine (GLS-5700) developed nAb titers ranging from 1:18 to 1:317 14 weeks after the first vaccination. Adoptive transfer of week 14 serum provided protection in 92% of mice challenged with a Puerto Rican isolate of ZIKV (23). Similar work with three purified inactivated virus (PIV) vaccines found that 92% of recipients generated nAb titers greater than 1:100, which corresponds to protection from viremia in mice that were challenged after adoptive antibody transfer of day 57 purified IgG (24). ZIKV vaccine studies have shown that a range of nAb titers can protect from viremia. However, these studies did not look past 14 weeks to examine the duration of protection of vaccine-derived nAbs.
Several animal models, including mice and rhesus macaques, have been used to explore vaccine protection against experimental ZIKV exposure (25). Immunocompromised mouse models have been used to advance the knowledge of ZIKV due to their cost effectiveness and availability. Published ZIKV challenge intervals in mice have varied from 3 to 30 weeks after vaccine administration and shown that nAb titers with an EC50 (half-maximal effective concentration) ranging from 1:75 to 1:100,000 were protective from viremia after ZIKV challenge (26–32). While immunodeficient mouse models are readily accessible, ZIKV infection is typically lethal because these mice lack necessary components of the antiviral response. Immunodeficient mice provide information on efficacy and safety of therapeutic interventions but fail to provide information on natural virus pathogenicity (25, 33). Nonhuman primates (NHPs) have also been used in ZIKV studies because they more closely mimic human ZIKV infections (25). For this reason, NHP studies have been used to assess the durability of immunity from 4 weeks to 1 year following vaccine administration (31, 32, 34, 35). Looking at the longest duration between vaccination and challenge, Abbink et al. (32) conducted a study using rhesus macaques to demonstrate the protective efficacy of a ZIKV PIV, DNA-M-Env, and RhAd52-M-Env vaccine candidates. At 52 weeks after administration, they challenged the macaques with Zika virus/H.sapiens-tc/BRA/2015/Brazil-ZKV2015 (ZIKV-BR). The PIV and RhAd52-M-Env vaccines generated log10 MN50 (50% reduction of microneutralization, comparable to the EC50 and PRNT50 values [36]) titers of 1.88 and 2.38 by week 4, but by week 8 after boost immunization the log10 MN50 titers increased to and fluctuated between 3.71 and 2.42 through week 52, respectively. The DNA-M-Env vaccine generated log10 MN50 titers of 2.23 at week 8 after boost immunization and rapidly declined to 1.43 by week 14 but remained stable until week 52. These titers of neutralizing antibodies showed that the PIV, RhAd52-M-Env, and DNA-M-Env vaccines protected 75% (6 of 8), 100% (4 of 4), and 29% (2 of 7), respectively, of macaques after ZIKV-BR challenge (32).
Relying on vaccine studies is not sufficient to determine the durability of immunity because vaccine studies rely on booster vaccinations to increase the level of immunity (37). Natural infections elicit a higher level of immunity that plateaus above the protective threshold thereby maintaining longer protection (38). For this reason, rechallenge experiments are useful to understand the durability of immunity elicited by natural infection.
Rechallenge experiments using animal models have been used to examine immune responses following natural ZIKV infection and to confirm that these infections protect from subsequent exposures (25). Mouse models have been used to rechallenge 3 weeks after primary infection (39–41). In rhesus macaques, challenge periods have varied from 6 to 10 weeks after primary challenge (42–45). We previously demonstrated that rhesus macaques were protected from homologous or heterologous rechallenge up to 10 weeks after primary infection (44, 45). These studies have shown that following ZIKV rechallenge, CD8 T cell and CD4 T cell responses are weak, but there is an increase in natural killer (NK) cells and nAbs, which corresponds to protection from disease but not sterilizing immunity (41, 42, 45). Although this is useful for establishing the potency of naturally elicited immunity, rapid rechallenge does not mimic the situation where someone infected in the 2015/2016 American outbreak is reexposed years later in a subsequent outbreak.
Here, we demonstrate a new minimum length of protection resulting from primary ZIKV infection using Indian-origin rhesus macaques from previously published studies (44, 46, 47).
(This article was submitted to an online preprint archive [48].)
RESULTS
No detectable ZIKV in plasma after rechallenge.
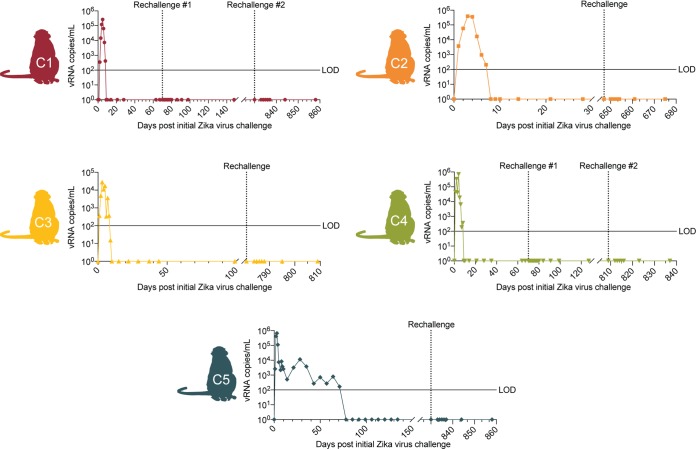
Five animals were infected with ZIKV as part of previously published studies (44–47). Animals C1 and C4 were previously used in rechallenge studies where the animals were rechallenged with 1 × 104 PFU Zika virus/H.sapiens-tc/PUR/2015/PRVABC59 (ZIKV-PR) 70 days after their initial ZIKV infection (44, 45). In addition, animals C1 and C4 were exposed to SHIV on 346 and 326 days after initial ZIKV challenge, respectively. However, neither animal became productively infected, and they have never had a positive viral load, suggesting that there would not be an effect on antigen-specific T cell levels. Animal C2 was previously challenged with dengue virus/H.sapiens-tc/IDN/1978/Sleman/78 (DENV-3) 164 days prior to initial ZIKV challenge. Our group previously showed that previous DENV-3 infection did not affect ZIKV pathogenesis or immunity upon ZIKV challenge (47). Animals C5 and C3 were previously infected with 104 PFU of Zika virus/H.sapiens-tc/FRA/2013/FrenchPolynesia-01_v1c1 (ZIKV-FP) during their first and late second/early third trimesters of pregnancy, respectively (46). Full animal demographics, infection history, and naming scheme used for each animal are presented in Table 1. Following these initial ZIKV infections, peak plasma vRNA load occurred between 2 and 6 days postinfection (dpi) and ranged from 2.8 × 104 to 7.0 × 105 vRNA copies per ml (44–47). In nonpregnant animals, vRNA load became undetectable by 10 to 14 dpi. In animals initially infected during pregnancy, vRNA load remained detectable for a longer period of time, ranging from 9 to 70 dpi (46). To determine the durability of immunity following primary ZIKV infection, we subcutaneously (s.c) rechallenged these five animals 22 to 28 months following initial ZIKV infection with 1 × 104 PFU Zika virus/H.sapiens-tc/PUR/2015/PRVABC59 (ZIKV-PR) (Table 1). This stock has been used to productively infect 40 animals from previous studies from our group, confirming infectivity (https://zika.labkey.com/project/OConnor/begin.view?). After rechallenge, ZIKV was measured in plasma by quantitative reverse transcription-PCR (qRT-PCR) daily on days 3 to 7 after rechallenge and on days 10, 14, and 28 days after rechallenge (Fig. 1). On all days throughout the study, plasma vRNA loads remained negative, indicating protection from plasma viremia after ZIKV rechallenge.
TABLE 1.
Animal demographicsa
| Animal | PAI | Initial |
Rechallenge |
Other infection, dpi | |||
|---|---|---|---|---|---|---|---|
| Statusb | ZIKV inoculation strain | Inoculation dose in PFU (s.c.) | ZIKV inoculation strain, dpi | Inoculation dose in PFU (s.c.) | |||
| C1 | 295022 | NP | ZIKV-MR766 | 104 | ZIKV-FP, 70 | 104 | SHIV, 347 |
| ZIKV-PR, 858 | 104 | ||||||
| C2 | 321142 | NP | ZIKV-FP | 106 | ZIKV-PR, 675 | 104 | DENV3, 164 |
| C3 | 598248 | P | ZIKV-FP | 104 | ZIKV-PR, 809 | 104 | None |
| C4 | 610107 | NP | ZIKV-FP | 106 | ZIKV-FP, 70 | 104 | SHIV, 326 |
| ZIKV-PR, 837 | 104 | ||||||
| C5 | 827577 | P | ZIKV-FP | 104 | ZIKV-PR, 858 | 104 | None |
Public animal identifiers (PAI) are used in studies on the Zika Open Research Portal (https://zika.labkey.com/project/OConnor/begin.view?). All animals were female. Animal C1 was previously infected with 104 PFU of ZIKV-MR766 and rechallenged with 104 PFU of ZIKV-FP 70 days after initial ZIKV infection (45). Animal C2 was previously infected with 106 PFU ZIKV-FP and Dengue virus 3 164 days prior to initial ZIKV infection (47). Animals C5 and C3 were previously infected with 104 PFU of ZIKV-FP during their first and late second/early third trimesters of pregnancy, respectively (46). Animal C4 was previously infected with 106 PFU of ZIKV-FP and rechallenged 70 days after initial infection with104 PFU of ZIKV-FP (44). All animals were rechallenged with 104 PFU of ZIKV-PR 675 to 858 days after initial infection. ZIKV-MR766, Zika virus/R.macaque- tc/UGA/1947/MR766-3329; ZIKV-FP, Zika virus/H.sapiens-tc/FRA/2013/FrenchPolynesia-01_v1c1; ZIKV-PR, Zika virus/H.sapiens-tc/PUR/2015/PRVABC59. dpi, days after initial ZIKV infection.
The pregnancy status at the time of initial infection: NP, nonpregnant; P, pregnant.
FIG 1.
ZIKV viral loads of animals rechallenged with ZIKV-PR from day 0 of primary infection. Schematic of animals included in this study next to their plasma vRNA loads measured by qRT-PCR before and after rechallenge with ZIKV-PR (indicated by an arrow). The limit of detection of ZIKV RNA is 100 copies/ml (indicated by the solid line).
Immune parameters do not change significantly following ZIKV rechallenge.
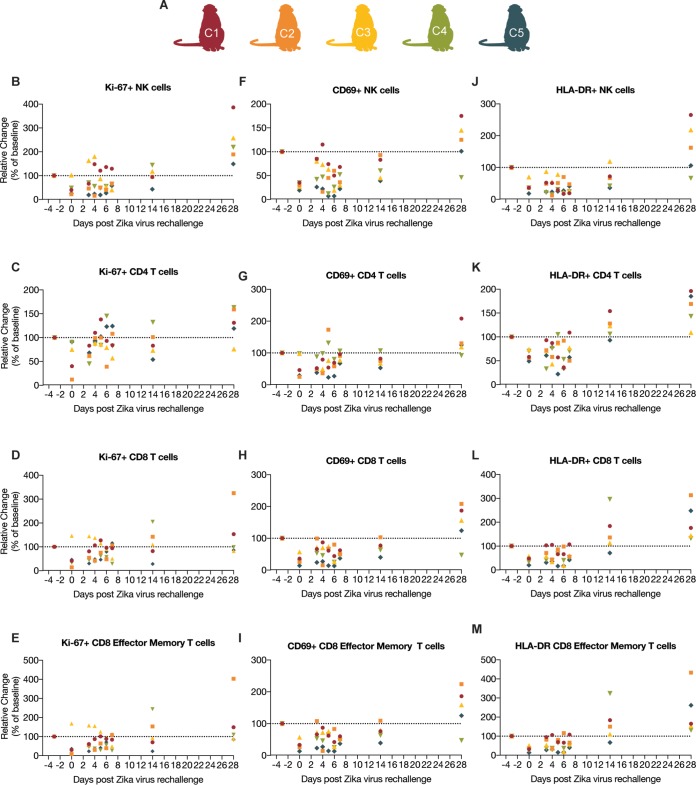
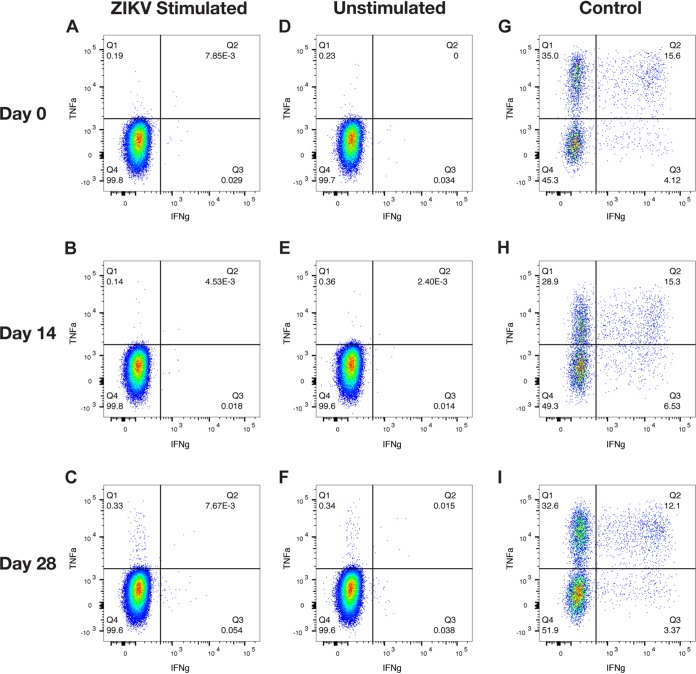
In order to characterize the immune responses following a long-term ZIKV rechallenge, we assessed immune activation by measuring Ki-67, CD69, and HLA-DR expression, as markers of activation, in natural killer (NK) cells and CD4, CD8, and CD8 effector memory (TEM) T cells by flow cytometry (Fig. 2 and 3). The percentages of activated cells per microliter of blood are presented as the fold change from the number of activated cells found 3 days before rechallenge (baseline) to determine the fold change in activation from baseline (Fig. 2). Repeated-measures analysis of variance (ANOVA) showed that there were no significant changes in the number of activated Ki-67+ CD4, CD8 TEM, CD8, and CD69+ CD4 T cell populations over time. In contrast, there was significant variation from baseline measurements in Ki-67+ NK cell populations [F(2.263, 9.051) = 10.36, P = 0.0039], CD69+ NK cell populations [F(2.381, 9.524) = 9.769, P = 0.0039], and HLA-DR+ NK cell populations [F(1.524, 6.097) = 11.54, P = 0.0104]. However, further analysis using Dunnett’s multiple-comparison test showed significant changes from baseline in the negative direction in NK cell populations when comparing number of activated NK cells each day after rechallenge to the number 3 days before rechallenge. Similar decreases from baseline were seen in CD69+ CD8 TEM and CD8 T cell populations and HLA-DR+ CD4, CD8 TEM, and CD8 T cell populations. To further define the cellular immune response following rechallenge, we performed intracellular cytokine staining assays on CD8 and CD4 T cells (Fig. 4 and 5) using whole ZIKV as the stimulating antigen on all animals on 0, 14, and 28 days after rechallenge. As expected, we did not see any ZIKV antigen-specific CD8 or CD4 T cell activation after rechallenge, except for animal C1 on day 14 (Fig. 4B). However, the response was weak and tailed from the original cell population, suggesting that this finding may be a false positive. This further suggests that there was a lack of a cellular immune response following rechallenge.
FIG 2.
Measurement of immune activation of animals rechallenged with ZIKV-PR. (A) Schematic of animals included in this study. (B to E) Spread of Ki-67+ NK cells, CD4 T cells, CD8 T cells, and CD8 effector memory (TEM) T cells. (F to I) Spread of CD69+ NK cells, CD4 T cells, CD8 T cells, CD8 effector memory (TEM) T cells. (J to M) Spread of HLA-DR+ NK cells, CD4 T cells, CD8 T cells, and CD8 effector memory (TEM) T cells. The percentage of activation in all cell populations was measured on −3, 0, 3 to 7, 10, 14, and 28 days after rechallenge. The percentages of activated cells per μl of blood are presented as relative to the baseline value set to 100% 3 days before rechallenge.
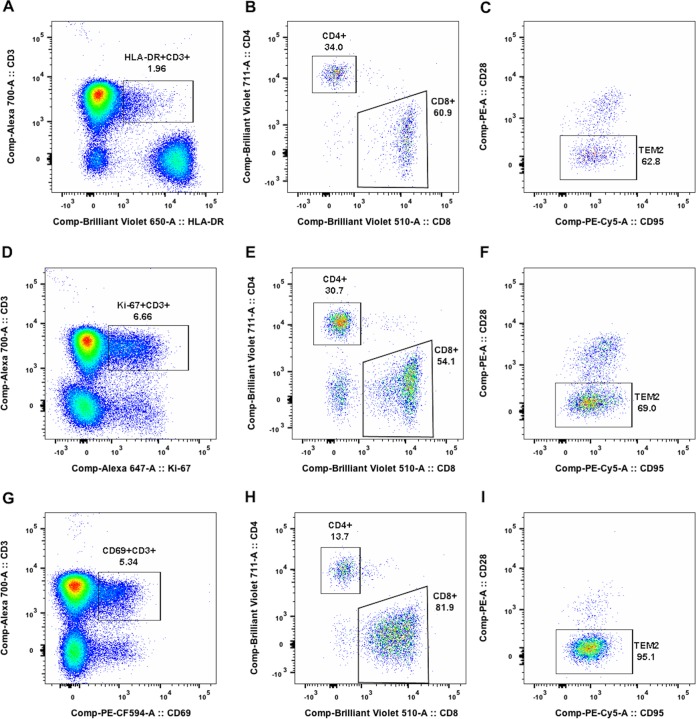
FIG 3.
Quantification of activated or proliferating T cells using HLA-DR, CD69 and Ki-67 markers. Panels A to I display the data of animal C2 on day 28 postchallenge. (A, D, and G) Expression of HLA-DR, Ki-67 and CD69 by CD3+ T cells within the CD45+ lymphocyte gate. (B, E, and H) Frequencies of CD4+ and CD8+ T cells within the HLA-DR+ CD3+, Ki-67+ CD3+, and CD69+ CD3+ gates, respectively. (C, F, and I) Frequencies of TEM2 (CD95+ CD28−) cells within the CD8-positive T cell populations.
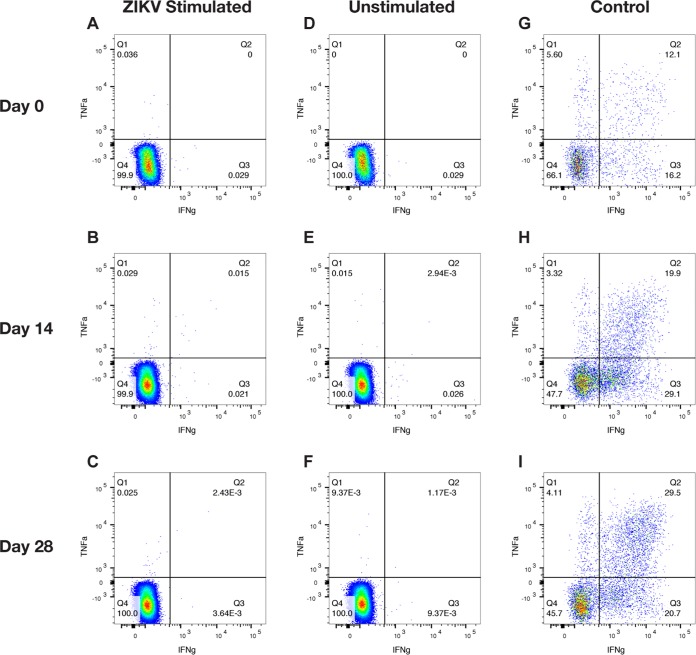
FIG 4.
Intracellular cytokine staining for CD8 T cells of ZIKV-rechallenged animals. Representative flow plots for animal C1 on days 0, 14, and 28 after rechallenge show the production of IFN-γ and TNF-α as measures of cellular activation. (A to C) Data for activated CD8 T cells following whole ZIKV stimulation. (D to F) Numbers of activated CD8 T cells when unstimulated. (G to I) Numbers of activated CD8 T cells when stimulated with a leukocyte activation cocktail as a positive control.
FIG 5.
Intracellular cytokine staining for CD4 T cells of ZIKV-rechallenged animals. Representative flow plots for animal C4 on days 0, 14, and 28 after rechallenge the production of IGN-γ and TNF-α as measures of cellular activation. (A to C) Data for activated CD4 T cells following whole ZIKV-PR stimulation. (D to F) Numbers of activated CD4 T cells when unstimulated. (G to I) Numbers of activated CD4 T cells when stimulated with a leukocyte activation cocktail as a positive control.
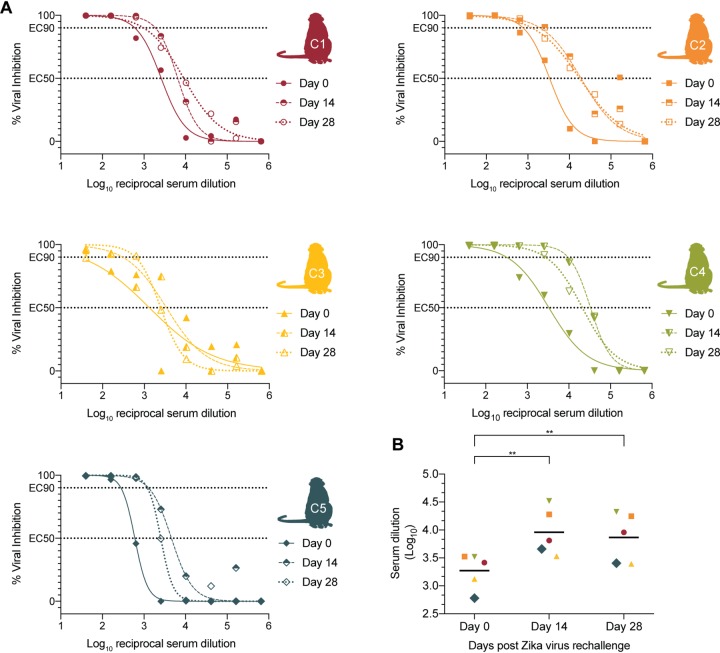
Serum nAb responses were measured using a flow cytometry-based ZIKV neutralization assay. Prior to rechallenge, all animals had high EC50 (50% effective concentration) values (Fig. 6A) that were greater than the 1:10 PRNT50 values recommended for other flavivirus vaccines (11, 12). Using a one-way paired t test, we compared the EC50 values on day 0 to the EC50 values on days 14 and 28 (Fig. 6B). All animals showed increased nAb titers following rechallenge, as noted by a greater EC50 value on day 14 compared to day 0 (P = 0.0025, t = 5.604, df = 4) and on day 28 (P = 0.0015, t = 6.446, df = 4). The increase in nAbs shows that initial infection may fail to provide sterilizing immunity. Alternatively, it is possible that the antigen present in the virus preparation was sufficient to boost preexisting antibody responses. Regardless, the combination of immunophenotyping data, neutralization assay data, and persistent negative plasma ZIKV vRNA loads following rechallenge suggest that primary ZIKV infection is sufficient to protect from detectable viremia in the blood.
FIG 6.
Neutralizing antibody curves. ZIKV neutralizing antibody titers were measured on days 0, 14, and 28. (A) The percent viral inhibitions corresponding to that log10 reciprocal serum dilution are plotted and fit with a nonlinear regression line in order to estimate the EC50 and EC90 values. (B) The log10 serum dilutions corresponding to EC50 nAb titers are plotted for days 0, 14, and 28. Increases in titers were measured by a one-way paired t test. Significance is indicated (**, P < 0.005).
Clinical evaluation of ZIKV rechallenged macaques.
After initial ZIKV challenge and rechallenge, all five animals were monitored daily for signs of disease (for example, inappetence, dehydration, diarrhea, injury, or psychological abnormalities), and complete blood counts (CBCs) were carried out. Reference intervals (RIs) developed for Wisconsin National Primate Research Center (WNPRC) animals for species, sex, and age were used to evaluate results (44).
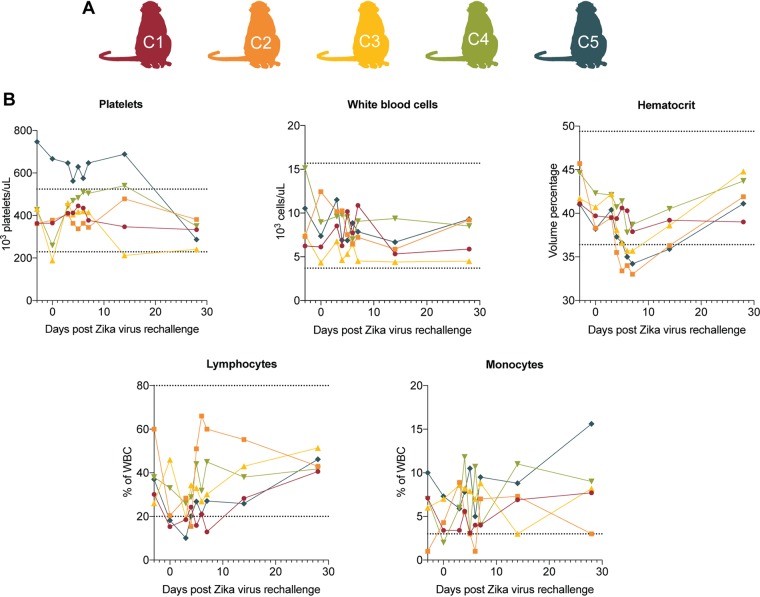
After primary ZIKV infection, animal C1 presented a rash at the injection site. No other clinical abnormalities were noted. CBCs were evaluated for all five animals 3 days prior to challenge, daily on days 0 through 10, on day 14, and again on days 21 and 28 dpi. Mild monocytosis with levels exceeding the RI (0 to 3% of white blood cells [WBC]) was reported for all animals before initial infection and persisted throughout the duration of the study (44–47). Animal C1 had lymphocyte levels dip below the RI (20 to 80% WBC) on days 4 and 7 but rebounded to within the normal range by day 8. Animal C5 had high platelet counts exceeding the RI (229,000 to 524,000 platelets/μl) present 3 days prior to rechallenge and persisted to be above the RI throughout the study period (44–47).
After rechallenge, animals C1 and C4 developed inappetence 6 days after rechallenge. Animal C1 also developed diarrhea at 6 days after rechallenge. All animals were relocated to new housing 1 day prior to rechallenge, which may have contributed to inappetence and diarrhea. No other clinical abnormalities were noted (Table 2). CBC tests were evaluated for all five animals 3 days prior to rechallenge, on the day of rechallenge, and on days 3 to 7, 10, 14, and 28 after rechallenge (Fig. 7). After rechallenge, all animals displayed persistent mild monocytosis, with levels exceeding the RI across all days that CBCs were evaluated. This persistence of monocytosis is consistent with that observed following primary ZIKV infection (44–47). The stress of frequent blood draws, sedation, experimental infections, and living in captivity have all been associated with an increased risk of monocytosis and lymphopenia, potentially explaining these findings in rechallenged animals (49–51).
TABLE 2.
Clinical remarks for each animal after rechallengea
| Animal | Days after rechallenge (dpi) | Clinical remark(s) |
|---|---|---|
| C1 | –1 | Relocated to new housing. |
| 0 | Possible chow inappetence. | |
| 3 | Third consecutive day of 50% or greater chow remaining; expect appetite to improve with acclimation; normal fecal production suggests adequate intake; weight stable, BCS 5/5 at last physical exam; attitude good. | |
| 6 | Chow inappetence; ninth consecutive report of 50% chow left over; diarrhea; overall weight stable; animal appears stable. | |
| 14 | Animal reported for redness at right temple over the weekend; did not observe reported erythema; animal appeared BARH and accepted treats. | |
| 17 | Animal reported for redness at right temple over the weekend; did not observe reported erythema; animal appeared BARH and accepted treats. | |
| C2 | –1 | Relocated to new housing. |
| 28 | Animal reported for a scab on the left brow; a 3-mm scab was observed on the lateral L brow; mild erythema was present; no swelling or discharge was seen; animal appeared BARH and accepted treats. | |
| C3 | –1 | Relocated to new housing. |
| C4 | –2 | Possible chow inappetence. |
| –1 | Relocated to new housing. | |
| 0 | During rounds, observed a mild amount of blood in cage; no wounds observed on animal; suspect minor trauma. | |
| 3 | Third consecutive day of 50% or greater chow remaining; expect appetite to improve with acclimation; normal fecal production suggests adequate intake; weight stable, BCS 5/5 at last physical exam; attitude good. | |
| 6 | Chow inappetence; ninth consecutive report of 50% chow left over; animal appears stable, BARH, and accepted treats. | |
| 10 | Confirmed chow inappetence; commonly reported in this animal; has unresolved problem of chow inappetence and is on supplemental diet; weight stable; animal appeared BARH and accepted treats. | |
| 13 | Confirmed chow inappetence; commonly reported in this animal; firm stool, first report; fas unresolved problem of chow inappetence and is on supplemental diet; weight stable; animal appeared BARH and accepted treats. | |
| 17 | Confirmed chow inappetence; commonly reported in this animal; possible persistent firm stool; currently on supplemental diet; weight stable; animal appears stable, BARH, and accepted treats. | |
| 18 | Chow inappetence; animal on supplemental diet for chow inappetence; possible firm stool, rarely reported in this animal; animal appears stable, BARH, and accepted treats. | |
| 24 | Chow inappetence; animal on supplemental diet for chow inappetence; possible firm stool, rarely reported in this animal; animal appears stable, BARH, and accepted treats. | |
| 25 | Mild chow inappetence; common report for this animal; most recent weight from one month ago was stable; animal appeared BARH and accepted treats. | |
| 26 | Animal reported for a scab above the brow; a 4-mm scab was seen above the brow on midline; mild erythema was observed, no swelling or discharge was seen; animal appeared BARH and accepted treats. | |
| C5 | –1 | Relocated to new housing. |
Abbreviations: BARH, bright, alert, responsive, and hydrated; BCS, body condition score.
FIG 7.
Complete blood count graphs of animals rechallenged with ZIKV-PR. (A) Schematic of animals included in this study. (B) Clinical hematology was performed by WNPRC on whole blood collected daily from macaques on days 3 to 7, as well as on days −3, 0, 10, 14, and 28 after ZIKV rechallenge. Horizontal dotted lines indicate the range of normal reference intervals (RI). Four of five animals had lymphocyte levels dip below the RI (20 to 80% of WBC) at 3 days after exposure but rebounded to the levels within the normal range by 4 days after exposure. Mild monocytosis exceeding the RI (0 to 3% of the WBC) was reported in animal C2 7 days prior to rechallenge. Animal C5 had platelet values exceeding the RI (229,000 to 524,000 platelets ths/μl) ranging from 575,000 to 747,000 platelets ths/μl present at day −3 and continuing until day 28. Thrombocytosis was also noted following the initial ZIKV challenge for this animal. No other abnormal CBC data were reported.
DISCUSSION
Since ZIKV could reemerge in areas affected by the 2015 ZIKV outbreak, it is important to evaluate the durability of ZIKV immunity after initial infection in order to understand the risk of reinfection, particularly among women. This study shows that initial ZIKV infection provides apparently protective immunity at least 22 to 28 months after initial ZIKV infection. As with many NHP studies, this was an opportunistic rechallenge study using previously ZIKV-infected animals that were available despite having different medical histories. Nonetheless, regardless of previous infection history, we show that primary ZIKV infection generated nAb titers above the 1:10 threshold deemed protective against reinfection for other flaviviruses (Fig. 6) (11, 12). This finding bodes well for those who have had different exposure histories; however, further work will need to corroborate this in humans.
These results showing protection from long-term rechallenge have important implications for vaccine design and testing. Protection from ZIKV infection following vaccination is thought to require lasting MN50 log10 titers of 2.0 to 2.1, equivalent to log10 EC50 titers. (32). In our study, prior to rechallenge, EC50 nAb titers were well above the log10 2.0 threshold that Abbink et al. (32), determined to be protective from ZIKV infection. Does the immunity observed in these animals provide sterilizing protection from reinfection? Although we did not detect any ZIKV RNA in the plasma following rechallenge, we cannot exclude the possibility that ZIKV RNA persisted in sanctuary tissues following initial ZIKV infection. Others have shown that while an increase of ZIKV nAbs rapidly controls blood viremia, sanctuary tissues were found to harbor persistent ZIKV RNA for up to 72 dpi (52, 53). The impact of virus that might be replicating in sanctuaries on subsequent immunity from reinfection would be of interest in future studies.
After rechallenge, we observed a modest increase in ZIKV nAb titers, which suggests that the antigen present in the challenge virus itself was sufficient to stimulate an expansion of preexisting nAb responses. However, it is possible that the inoculum virus was able to replicate below the limit of detection. Future studies could begin to discern if the antigen present in the rechallenge inoculum was sufficient to boost antibody titers or if the virus replicated below the limit of detection. This could be done by rechallenging again with an equivalent low dose of inactivated ZIKV and measuring the effects on neutralizing antibody levels. Others who have encountered similar results have defined empirical thresholds for sterilizing immunity. For example, Kirkpatrick et al. similarly observed a boost in nAb titers in DENV-vaccinated animals following DENV challenge (54). These researchers defined an increase of <4-fold nAb titer as sterilizing immunity and a ≥4-fold increase in nAb titer as nonsterilizing immunity. In our study, two animals, C1 and C3, exhibited 2.5× increases in nAb titer by 14 days after exposure. However, animals C2, C4, and C5 exhibited a 5.7×, 10.0×, and 7.6× increases, respectively, in nAb titer by 14 days postexposure. While we did not look specifically at B cell activation, we showed that ZIKV-specific nAbs became elevated following rechallenge, suggesting that there was ZIKV-specific activation in the humoral arm of the immune response. Additional work will need to be done to determine the effects of ZIKV rechallenge on antibody binding, as well as the isotypes recognizing ZIKV.
Two animals used in our rechallenge study, C3 and C5, were previously part of a published study that assessed fetal outcomes following ZIKV infection in utero (46). This study concluded that similar to human pregnancy, maternal-fetal transmission is highly efficient with variable vRNA levels detected in all fetuses assessed. Despite a small sample size in our rechallenge study (n = 2 animals initially infected during pregnancy), we show that these animals develop immune responses similar to nonpregnant animals. After primary ZIKV infection during pregnancy in 2016, animal C5 has gone on to give birth to two apparently healthy infants (in 2017 and 2018), and C3 has gone on to give birth to one healthy infant (in 2019). While this study did not look at the impacts of a ZIKV rechallenge during a second pregnancy, we show that subsequent infants born to mothers who were previously infected with ZIKV are in qualitatively good health. Future studies will need to determine whether immunity elicited by a primary infection is sufficient to protect a subsequent pregnancy from the effects of a ZIKV rechallenge during pregnancy. In addition, it will be important to characterize the extent of ZIKV-specific antibody responses in infants exposed to ZIKV in utero.
In summary, our study establishes a new experimentally defined minimal length of protective immunity against ZIKV of up to 22 to 28 months. Future longitudinal studies will need to assess ZIKV immunity of previously infected individuals with longer time frames than we have used here in order to continue to define the maximum length of immunity elicited by natural ZIKV infection. Establishing the true maximum length of ZIKV protective immunity will be critical for understanding outbreak dynamics, help elucidate the future transmission potential of ZIKV, and influence future vaccination strategies.
MATERIALS AND METHODS
Ethics statement.
All rhesus macaques in this study were cared for by the staff at the WNPRC in accordance with the regulations, guidelines, and recommendations outlined in the Animal Welfare Act, the Guide for the Care and Use of Laboratory Animals, and the Weatherall report (55–57). The University of Wisconsin—Madison College of Letters and Science and Vice Chancellor for Research and Graduate Education Centers Institutional Animal Care and Use Committee approved the NHP research covered under protocol G005401-R01. The University of Wisconsin—Madison Institutional Biosafety Committee approved this work under protocol B00000117.
All animals were housed in enclosures with required floor space and fed using a nutritional plan based on recommendations published by the National Research Council. Animals were fed a fixed formula, extruded dry diet with adequate carbohydrate, energy, fat, fiber, mineral, protein, and vitamin content. Macaque dry diets were supplemented with fruits, vegetables, and other edible objects (e.g., nuts, cereals, seed mixtures, yogurt, peanut butter, popcorn, marshmallows, etc.) to provide variety to the diet and to inspire species-specific behaviors such as foraging. To further promote psychological well-being, animals were provided with food enrichment, structural enrichment, and/or manipulanda. Environmental enrichment objects were selected to minimize chances of pathogen transmission from one animal to another and from animals to care staff. While on study, all animals were evaluated by trained animal care staff at least twice each day for signs of pain, distress, and illness by observing appetite, stool quality, activity level, physical condition. Animals exhibiting abnormal presentation for any of these clinical parameters were provided appropriate care by attending veterinarians. Prior to all minor/brief experimental procedures, macaques were anesthetized with an intramuscular dose of ketamine (10 ml kg−1) prior to virus inoculation and blood collection and monitored regularly until fully recovered from anesthesia. Per WNPRC standard operating procedures for animals assigned to protocols involving the experimental inoculation of an infectious pathogen, environmental enhancement included constant visual, auditory, and olfactory contact with conspecifics, the provision of feeding devices which inspire foraging behavior, the provision and rotation of novel manipulanda (e.g., Kong toys, nylabones, etc.), and enclosure furniture (i.e., perches and shelves).
Virus stocks.
Zika virus/H.sapiens-tc/FRA/2013/FrenchPolynesia-01_v1c1 (ZIKV-FP) (GenBank accession no. KJ776791) was obtained from Xavier de Lamballerie (European Virus Archive, Marseille, France) and passage history is described in Dudley et al. (44). African lineage Zika virus (ZIKV-MR766), and Zika virus/H.sapiens-tc/PUR/2015/PRVABC59 (ZIKV-PR) (GenBank accession no. KU501215) were provided by Brandy Russell (Centers for Disease Control and Prevention, Ft. Collins, CO). ZIKV-MR766 passage history has been described in Aliota et al. (45). Prior to rechallenge, animal C1 was previously infected with 104 PFU of ZIKV-MR766 and rechallenged with 104 PFU of ZIKV-FP 70 days after initial ZIKV infection, as described by Aliota et al. (45). Animal C4 was previously infected with 106 PFU of ZIKV-FP and rechallenged with 104 PFU of ZIKV-FP 70 days after initial ZIKV infection as described by Dudley et al. (44). In addition, animals C1 and C4 were exposed to SHIV on 346 and 326 days after the initial ZIKV challenge, respectively, but never had a positive viral load. Animals C5 and C3 were previously infected with 104 PFU of ZIKV-FP during their first and late second/early third trimesters of pregnancy, respectively, as described by Nguyen et al. (46). Animal C2 was previously infected with dengue virus/H.sapiens-tc/IDN/1978/Sleman/78 (DENV-3) 164 days prior to being infected with 106 PFU ZIKV-FP as previously described by Breitbach et al. (47). Full infection histories are presented in Table 1.
Inoculations.
Virus stocks were thawed and diluted in phosphate-buffered saline (PBS) to 1 × 104 PFU Zika virus/H.sapiens-tc/PUR/2015/PRVABC59 (ZIKV-PR) for each challenge. Prepared virus stocks were then loaded into a 1-ml syringe that was kept on ice until administration. Animals were anesthetized as described above, and 1 ml of inocula was administered s.c. over the cranial dorsum (44). Animals were monitored for adverse reactions and signs of disease by the veterinary and animal care staff of WNPRC.
Quantification of ZIKV RNA.
Plasma and PBMC were isolated from EDTA-anticoagulated whole blood by Ficoll density centrifugation at 1,860 × g for 30 min. Plasma was collected and centrifuged at 670 × g for an additional 8 min to remove remaining cells. Viral RNA was extracted from 300 μl of plasma using a viral total nucleic acid purification kit (Promega, Madison, WI) on a Maxwell 48 RSC instrument (Promega). The vRNA was then quantified using qRT-PCR previously as described in Dudley et al. (44). Briefly, the qRT-PCR was performed using SuperScript III Platinum One-Step quantitative RT-PCR system (Invitrogen, Carlsbad, CA) on a LightCycler 480 or LightCycler instrument (Roche Diagnostics, Indianapolis, IN). Primers and probes were used at final concentrations of 600 and 100 nM, respectively, with the addition of 150 ng of random primers (Promega). Cycling conditions were as follows: 37°C for 15 min, 50°C for 30 min, and 95°C for 2 min, followed by 50 cycles of 95°C for 15 s and 60°C for 1 min. Viral concentration was calculated by interpolation onto an internal standard curve composed of seven 10-fold serial dilutions of a ZIKV RNA fragment based on ZIKV-FP.
The limit of quantification for this assay was determined using methods adapted from Cline et al. (58). Briefly, half-dilutions of ZIKV RNA were prepared to generate standard curves from 3 to 1,000,000 copies. The overall positivity for each half dilution was assessed by qRT-PCR. The limit of quantification for this assay was established at 100 copies/ml.
Immunophenotyping.
The number of activated/proliferating NK cells, CD4 T cells, CD8 T cells, and CD8 TEM cells were quantified as described previously (59). Briefly, 100-μl portions of EDTA-anticoagulated whole-blood samples were incubated at room temperature for 15 min with an antibody master mix described by Dudley et al. (44). Red blood cells were lysed using BD Pharm Lyse (BD BioSciences, San Jose, CA), washed twice in media, and fixed with 125 μl of 2% paraformaldehyde for 15 min. After fixation, the cells were washed and permeabilized using bulk permeabilization reagent (Life Technologies, Madison, WI). While the permeabilizer was present, the cells were stained for 15 min with Ki-67 (clone B56, Alexa Fluor 647 conjugate). The cells were then washed twice in media and resuspended in 2% paraformaldehyde until they were run on a BD LSRII flow cytometer (BD BioSciences). Flow data were analyzed using FlowJo version 10.5.3.
CBC tests.
CBC tests were performed on EDTA-anticoagulated whole-blood samples using a SYsmex XS-1000i automated hematology analyzer (Sysmex Corporation, Kobe, Japan). CBC results were reported with species-, age-, and sex-specific reference ranges. When samples were outside the laboratory-defined criteria (increased total WBC counts, increased monocyte, eosinophil, and basophil percentages, decreased hemoglobin, hematocrit, and platelet values, and unreported differential values), manual slide evaluation was performed.
Flow cytometry-based neutralization assay.
Macaque serum samples were screened for ZIKV nAbs by a flow-based ZIKV neutralization assay adapted from Dowd et al. (20). Briefly, Vero cells (ATCC, CLR-1587) were plated at a concentration of 2.5 × 104 cells (100 μl/well) in a 96-well plate and incubated at 37°C for 24 h prior to ZIKV infection. Equal volumes of 1.9 × 102 PFU/ml ZIKV-PR and 1:4 serial serum dilutions were incubated for 30 min at 37°C before infection of duplicate wells. The plate was incubated for 72 h at 37°C. After incubation, the plates were washed five times with 1 × PBS, and treated with trypsin. After the cells separated from the plate, they were transferred to a cluster tube and stained with a Live/Dead Fixable Near-IR dead cell stain kit (Life Technologies, Madison, WI). The plate was incubated for 15 min at room temperature, and 1 ml of R10 medium was added, followed by incubation for 5 min at 530 × g. The medium was aspirated, and 125 μl of 2% paraformaldehyde (PFA) was added to fix the cells. The plates were incubated for 15 min at room temperature, 1 ml of R10 medium was added, and then the cells were centrifuged for 5 min at 530 × g. The medium was aspirated, and 100 μl of fix and permeabilization medium B (Life Technologies) was added to permeabilize the cell membrane. Next, 2 μl of a 1:10 dilution of the flavivirus-specific antibody (4G2-A647; Novus Biologicals, Centennial, CO) stock was added to each well. The plate was incubated at room temperature for 15 min. Then, 1 ml of R10 medium was added, and the plates were centrifuged for 5 min at 530 × g. The medium was aspirated, and 125 μl of 2% PFA was added to fix the cells. Infection was monitored by flow cytometry on the BD LSRII flow cytometer. Flow cytometry data were analyzed using FlowJo version 10.5.3. Neutralization curves were generated using GraphPad Prism v.8.0.1 (San Diego, CA). The dilutions required to inhibit 50 and 90% of infection (EC50 and EC90 values) were calculated using a nonlinear regression model.
Intracellular cytokine staining.
Cryo-stored peripheral blood mononuclear cells from rhesus macaques were screened for activated CD4 and CD8 T cells by intracellular cytokine staining assays using whole-virus ZIKV-PR as a stimulant on days 0, 14, and 28 after rechallenge. Approximately 1.5 × 106 to 2 × 106 cells were plated and stimulated with ZIKV-PR leukocyte activation cocktail (BD Biosciences) or tissue culture medium for 90 min at 37°C in a 5% CO2 incubator in the presence of 0.5 mg of anti-CD28 (clone L293), 0.5 mg of anti-CD49d (clone 9F10), and CD107a PE (clone H4A3) antibodies. After the 90-min incubation, 0.5 mg of brefeldin A (BioLegend, San Diego, CA) and 0.1 nmol of monensin solution (BioLegend) were added, and the samples were returned to the incubator overnight. The cells were stained for the surface expression of CD3 PE-CF594 (clone SP34-2), CD4 PerCP-Cy5.5 (clone L200), CD8 brilliant violet 711 (clone RPA-T8), and Live/Dead Fixable Near-IR dead cell stain kit (Life Technologies). They were then washed twice with fluorescence-activated cell sorting (FACS) buffer and fixed with 2% PFA. Cells were then permeabilized with 0.1% saponin buffer, intracellularly stained for gamma interferon (IFN-γ)/FITC (clone 4S.B3; BD Biosciences), tumor necrosis factor alpha (TNF-α))/Alexa Fluor 700 (clone MAb11; BD Biosciences), and interleukin-2 (IL-2)/APC (clone MQ1-17H12; BD Biosciences); the samples were then washed twice with saponin buffer and fixed with 2% paraformaldehyde. All antibodies were acquired from BD Biosciences.
Samples were acquired using FACS DIVA (v8.0.1) on a special-order research product BD LSR II apparatus equipped with a 50-mW 405-nm violet, a 100-mW 488-nm blue, and a 50-mW 640-nm red laser. We collected up to 300,000 events in the lymphocyte gate defined by forward- and side-scatter parameters.
Statistical analysis.
Changes in immunophenotyping and CBC parameters from baseline were analyzed with repeated-measures ANOVA, for the populations where ANOVA proved significant, we followed with Dunnett’s multiple-comparison test, comparing each day after rechallenge back to 3 days before rechallenge.
The log10 serum dilutions corresponding to EC50 nAb titers are plotted for days 0, 14, and 28. Increases in titers were measured by one-way paired t test. Significance is indicated by asterisks (*, P < 0.05; **, P < 0.005). All statistical analysis was performed using Prism v8.0.1 (GraphPad, San Diego, CA).
Data availability.
Complete data sets for these studies have been made publicly available in a manuscript-specific folder on the Zika Open Research Portal (https://go.wisc.edu/bn71k1). We declare that all other data for these study findings are available via this portal.
ACKNOWLEDGMENT
We thank the Veterinary Services, Colony Management, Scientific Protocol Implementation, and the Pathology Services staff at the Wisconsin National Primate Research Center for their contributions to this study.
REFERENCES
- 1.Dick GWA, Kitchen SF, Haddow AJ. 1952. Zika virus. I. Isolations and serological specificity. Trans R Soc Trop Med Hyg 46:509–520. doi: 10.1016/0035-9203(52)90042-4. [DOI] [PubMed] [Google Scholar]
- 2.Petersen LR, Jamieson DJ, Powers AM, Honein MA. 2016. Zika virus. N Engl J Med 374:1552–1563. doi: 10.1056/NEJMra1602113. [DOI] [PubMed] [Google Scholar]
- 3.Musso D, Nilles EJ, Cao-Lormeau V-M. 2014. Rapid spread of emerging Zika virus in the Pacific area. Clin Microbiol Infect 20:O595–O596. doi: 10.1111/1469-0691.12707. [DOI] [PubMed] [Google Scholar]
- 4.Parra B, Lizarazo J, Jiménez-Arango JA, Zea-Vera AF, González-Manrique G, Vargas J, Angarita JA, Zuñiga G, Lopez-Gonzalez R, Beltran CL, Rizcala KH, Morales MT, Pacheco O, Ospina ML, Kumar A, Cornblath DR, Muñoz LS, Osorio L, Barreras P, Pardo CA. 2016. Guillain-Barré syndrome associated with Zika virus infection in Colombia. N Engl J Med 375:1513–1523. doi: 10.1056/NEJMoa1605564. [DOI] [PubMed] [Google Scholar]
- 5.Cao-Lormeau VM, Blake A, Mons S, Lastere S, Roche C, Vanhomwegen J, Dub T, Baudouin L, Teissier A, Larre P, Vial AL, Decam C, Choumet V, Halstead SK, Willison HJ, Musset L, Manuguerra JC, Despres P, Fournier E, Mallet HP, Musso D, Fontanet A, Neil J, Ghawché F. 2016. Guillain-Barré syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet 387:1531–1539. doi: 10.1016/S0140-6736(16)00562-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moore CA, Staples JE, Dobyns WB, Pessoa A, Ventura CV, Fonseca EBD, Ribeiro EM, Ventura LO, Neto NN, Arena JF, Rasmussen SA. 2017. Characterizing the pattern of anomalies in congenital Zika syndrome for pediatric clinicians. JAMA Pediatr 171:288–295. doi: 10.1001/jamapediatrics.2016.3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rice ME, Galang RR, Roth NM, Ellington SR, Moore CA, Valencia-Prado M, Ellis EM, Tufa AJ, Taulung LA, Alfred JM, Pérez-Padilla J, Delgado-López CA, Zaki SR, Reagan-Steiner S, Bhatnagar J, Nahabedian JF, Reynolds MR, Yeargin-Allsopp M, Viens LJ, Olson SM, Jones AM, Baez-Santiago MA, Oppong-Twene P, VanMaldeghem K, Simon EL, Moore JT, Polen KD, Hillman B, Ropeti R, Nieves-Ferrer L, Marcano-Huertas M, Masao CA, Anzures EJ, Hansen RL, Pérez-Gonzalez SI, Espinet-Crespo CP, Luciano-Román M, Shapiro-Mendoza CK, Gilboa SM, Honein MA. 2018. Vital signs: Zika-associated birth defects and neurodevelopmental abnormalities possibly associated with congenital Zika virus infection—U.S. territories and freely associated states, 2018. MMWR Morb Mortal Wkly Rep 67:858–867. doi: 10.15585/mmwr.mm6731e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nielsen-Saines K, Brasil P, Kerin T, Vasconcelos Z, Gabaglia CR, Damasceno L, Pone M, Abreu de Carvalho LM, Pone SM, Zin AA, Tsui I, Salles TRS, da Cunha DC, Costa RP, Malacarne J, Reis AB, Hasue RH, Aizawa CYP, Genovesi FF, Einspieler C, Marschik PB, Pereira JP, Gaw SL, Adachi K, Cherry JD, Xu Z, Cheng G, Moreira ME. 2019. Delayed childhood neurodevelopment and neurosensory alterations in the second year of life in a prospective cohort of ZIKV-exposed children. Nat Med 25:1213–1217. doi: 10.1038/s41591-019-0496-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baud D, Gubler DJ, Schaub B, Lanteri MC, Musso D. 2017. An update on Zika virus infection. Lancet 390:2099–2109. doi: 10.1016/S0140-6736(17)31450-2. [DOI] [PubMed] [Google Scholar]
- 10.Shim BS, Kwon YC, Ricciardi MJ, Stone M, Otsuka Y, Berri F, Kwal JM, Magnani DM, Jackson CB, Richard AS, Norris P, Busch M, Curry CL, Farzan M, Watkins D, Choe H. 2019. Zika virus-immune plasmas from symptomatic and asymptomatic individuals enhance Zika pathogenesis in adult and pregnant mice. mBio 10:e00758-19. doi: 10.1128/mBio.00758-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tauber E, Kollaritsch H, Korinek M, Rendi-Wagner P, Jilma B, Firbas C, Schranz S, Jong E, Klingler A, Dewasthaly S, Klade CS. 2007. Safety and immunogenicity of a Vero-cell-derived, inactivated Japanese encephalitis vaccine: a non-inferiority, phase III, randomised controlled trial. Lancet 370:1847–1853. doi: 10.1016/S0140-6736(07)61780-2. [DOI] [PubMed] [Google Scholar]
- 12.Lindquist L, Vapalahti O. 2008. Tick-borne encephalitis. Lancet 371:1861–1871. doi: 10.1016/S0140-6736(08)60800-4. [DOI] [PubMed] [Google Scholar]
- 13.Amanna IJ, Slifka MK. 2016. Questions regarding the safety and duration of immunity following live yellow fever vaccination. Expert Rev Vaccines 15:1519–1533. doi: 10.1080/14760584.2016.1198259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.2013. Vaccines and vaccination against yellow fever. WHO position paper—June 2013. Releve Epidemiol Hebd 88:269–283. [PubMed] [Google Scholar]
- 15.Imrie A, Meeks J, Gurary A, Sukhbaatar M, Truong TT, Cropp CB, Effler P. 2007. Antibody to dengue 1 detected more than 60 years after infection. Viral Immunol 20:672–675. doi: 10.1089/vim.2007.0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Althouse BM, Lessler J, Sall AA, Diallo M, Hanley KA, Watts DM, Weaver SC, Cummings D. 2012. Synchrony of sylvatic dengue isolations: a multi-host, multi-vector SIR model of dengue virus transmission in Senegal. PLoS Negl Trop Dis 6:e1928. doi: 10.1371/journal.pntd.0001928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moreira-Soto A, Torres MC, Lima de Mendonça MC, Mares-Guia MA, Dos Santos Rodrigues CD, Fabri AA, Dos Santos CC, Machado Araújo ES, Fischer C, Ribeiro Nogueira RM, Drosten C, Sequeira PC, Drexler JF, Bispo de Filippis AM. 2018. Evidence for multiple sylvatic transmission cycles during the 2016-2017 yellow fever virus outbreak, Brazil. Clin Microbiol Infect 24:1019.e1. doi: 10.1016/j.cmi.2018.01.026. [DOI] [PubMed] [Google Scholar]
- 18.Vasilakis N, Cardosa J, Hanley KA, Holmes EC, Weaver SC. 2011. Fever from the forest: prospects for the continued emergence of sylvatic dengue virus and its impact on public health. Nat Rev Microbiol 9:532–541. doi: 10.1038/nrmicro2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gardner CL, Ryman KD. 2010. Yellow fever: a reemerging threat. Clin Lab Med 30:237–260. doi: 10.1016/j.cll.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dowd KA, DeMaso CR, Pelc RS, Speer SD, Smith ARY, Goo L, Platt DJ, Mascola JR, Graham BS, Mulligan MJ, Diamond MS, Ledgerwood JE, Pierson TC. 2016. Broadly neutralizing activity of Zika virus-immune sera identifies a single viral serotype. Cell Rep 16:1485–1491. doi: 10.1016/j.celrep.2016.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Calisher CH, Karabatsos N, Dalrymple JM, Shope RE, Porterfield JS, Westaway EG, Brandt WE. 1989. Antigenic relationships between flaviviruses as determined by cross-neutralization tests with polyclonal antisera. J Gen Virol 70:37–43. doi: 10.1099/0022-1317-70-1-37. [DOI] [PubMed] [Google Scholar]
- 22.Barrett A. 2018. Current status of Zika vaccine development: Zika vaccines advance into clinical evaluation. NPJ Vaccines 3:24. doi: 10.1038/s41541-018-0061-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tebas P, Roberts CC, Muthumani K, Reuschel EL, Kudchodkar SB, Zaidi FI, White S, Khan AS, Racine T, Choi H, Boyer J, Park YK, Trottier S, Remigio C, Krieger D, Spruill SE, Bagarazzi M, Kobinger GP, Weiner DB, Maslow JN. 2017. Safety and immunogenicity of an anti-Zika virus DNA vaccine: preliminary report. N Engl J Med doi: 10.1056/NEJMoa1708120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Modjarrad K, Lin L, George SL, Stephenson KE, Eckels KH, De La Barrera RA, Jarman RG, Sondergaard E, Tennant J, Ansel JL, Mills K, Koren M, Robb ML, Barrett J, Thompson J, Kosel AE, Dawson P, Hale A, Tan CS, Walsh SR, Meyer KE, Brien J, Crowell TA, Blazevic A, Mosby K, Larocca RA, Abbink P, Boyd M, Bricault CA, Seaman MS, Basil A, Walsh M, Tonwe V, Hoft DF, Thomas SJ, Barouch DH, Michael NL. 2018. Preliminary aggregate safety and immunogenicity results from three trials of a purified inactivated Zika virus vaccine candidate: phase 1, randomised, double-blind, placebo-controlled clinical trials. Lancet 391:563–571. doi: 10.1016/S0140-6736(17)33106-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dong S, Liang Q. 2018. Recent advances in animal models of Zika virus infection. Virol Sin 33:125–130. doi: 10.1007/s12250-018-0007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Richner JM, Himansu S, Dowd KA, Butler SL, Salazar V, Fox JM, Julander JG, Tang WW, Shresta S, Pierson TC, Ciaramella G, Diamond MS. 2017. Modified mRNA vaccines protect against Zika virus infection. Cell 168:1114–1125. doi: 10.1016/j.cell.2017.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shan C, Muruato AE, Nunes BTD, Luo H, Xie X, Medeiros DBA, Wakamiya M, Tesh RB, Barrett AD, Wang T, Weaver SC, Vasconcelos PFC, Rossi SL, Shi P-Y. 2017. A live-attenuated Zika virus vaccine candidate induces sterilizing immunity in mouse models. Nat Med 23:763–767. doi: 10.1038/nm.4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu K, Song Y, Dai L, Zhang Y, Lu X, Xie Y, Zhang H, Cheng T, Wang Q, Huang Q, Bi Y, Liu WJ, Liu W, Li X, Qin C, Shi Y, Yan J, Zhou D, Gao GF. 2018. Recombinant chimpanzee adenovirus vaccine AdC7-M/E protects against Zika virus infection and testis damage. J Virol 92:e01722-17. doi: 10.1128/JVI.01722-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brault AC, Domi A, McDonald EM, Talmi-Frank D, McCurley N, Basu R, Robinson HL, Hellerstein M, Duggal NK, Bowen RA, Guirakhoo F. 2017. A Zika vaccine targeting NS1 protein protects immunocompetent adult mice in a lethal challenge model. Sci Rep 7:14769. doi: 10.1038/s41598-017-15039-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kwek SS, Watanabe S, Chan KR, Ong EZ, Tan HC, Ng WC, Nguyen MTX, Gan ES, Zhang SL, Chan KWK, Tan JH, Sessions OM, Manuel M, Pompon J, Chua C, Hazirah S, Tryggvason K, Vasudevan SG, Ooi EE. 2018. A systematic approach to the development of a safe live attenuated Zika vaccine. Nat Commun 9:1031. doi: 10.1038/s41467-018-03337-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li X-F, Dong H-L, Wang H-J, Huang X-Y, Qiu Y-F, Ji X, Ye Q, Li C, Liu Y, Deng Y-Q, Jiang T, Cheng G, Zhang F-C, Davidson AD, Song Y-J, Shi P-Y, Qin C-F. 2018. Development of a chimeric Zika vaccine using a licensed live-attenuated flavivirus vaccine as backbone. Nat Commun 9:673. doi: 10.1038/s41467-018-02975-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abbink P, Larocca RA, Visitsunthorn K, Boyd M, De La Barrera RA, Gromowski GD, Kirilova M, Peterson R, Li Z, Nanayakkara O, Nityanandam R, Mercado NB, Borducchi EN, Chandrashekar A, Jetton D, Mojta S, Gandhi P, LeSuer J, Khatiwada S, Lewis MG, Modjarrad K, Jarman RG, Eckels KH, Thomas SJ, Michael NL, Barouch DH. 2017. Durability and correlates of vaccine protection against Zika virus in rhesus monkeys. Sci Transl Med 9:eaao4163. doi: 10.1126/scitranslmed.aao4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marzi A, Emanuel J, Callison J, McNally KL, Arndt N, Chadinha S, Martellaro C, Rosenke R, Scott DP, Safronetz D, Whitehead SS, Best SM, Feldmann H. 2018. Lethal Zika virus disease models in young and older interferon α/β receptor knockout mice. Front Cell Infect Microbiol 8:117. doi: 10.3389/fcimb.2018.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Medina LO, To A, Lieberman MM, Wong TAS, Namekar M, Nakano E, Andersen H, Yalley-Ogunro J, Greenhouse J, Higgs S, Huang Y-J, Vanlandingham DL, Horton JS, Clements DE, Lehrer AT. 2018. A recombinant subunit-based Zika virus vaccine is efficacious in non-human primates. Front Immunol 9:2464. doi: 10.3389/fimmu.2018.02464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abbink P, Larocca RA, De La Barrera RA, Bricault CA, Moseley ET, Boyd M, Kirilova M, Li Z, Ng’ang’a D, Nanayakkara O, Nityanandam R, Mercado NB, Borducchi EN, Agarwal A, Brinkman AL, Cabral C, Chandrashekar A, Giglio PB, Jetton D, Jimenez J, Lee BC, Mojta S, Molloy K, Shetty M, Neubauer GH, Stephenson KE, Peron JPS, Zanotto P. M d A, Misamore J, Finneyfrock B, Lewis MG, Alter G, Modjarrad K, Jarman RG, Eckels KH, Michael NL, Thomas SJ, Barouch DH. 2016. Protective efficacy of multiple vaccine platforms against Zika virus challenge in rhesus monkeys. Science 353:1129–1132. doi: 10.1126/science.aah6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu L, Wen K, Li J, Hu D, Huang Y, Qiu L, Cai J, Che X. 2012. Comparison of plaque- and enzyme-linked immunospot-based assays to measure the neutralizing activities of monoclonal antibodies specific to domain III of dengue virus envelope protein. Clin Vaccine Immunol 19:73–78. doi: 10.1128/CVI.05388-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Centers for Disease Control and Prevention. 2018. Understanding how vaccines work. Centers for Disease Control and Prevention, Atlanta, GA: https://www.cdc.gov/vaccines/hcp/conversations/downloads/vacsafe-understand-color-office.pdf. Accessed 12 July 2019. [Google Scholar]
- 38.Slifka MK, Amanna I. 2014. How advances in immunology provide insight into improving vaccine efficacy. Vaccine 32:2948–2957. doi: 10.1016/j.vaccine.2014.03.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scott JM, Lebratti TJ, Richner JM, Jiang X, Fernandez E, Zhao H, Fremont DH, Diamond MS, Shin H. 2018. Cellular and humoral immunity protect against vaginal Zika virus infection in mice. J Virol 92:e00038-18. doi: 10.1128/JVI.00038-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Turner LH, Kinder JM, Wilburn A, D’Mello RJ, Braunlin MR, Jiang TT, Pham G, Way SS. 2017. Preconceptual Zika virus asymptomatic infection protects against secondary prenatal infection. PLoS Pathog 13:e1006684. doi: 10.1371/journal.ppat.1006684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang H, Li S, Zhang Y, Han X, Jia B, Liu H, Liu D, Tan S, Wang Q, Bi Y, Liu WJ, Hou B, Gao GF, Zhang F. 2017. CD8+ T cell immune response in immunocompetent mice during Zika virus infection. J Virol 91:e00900-17. doi: 10.1128/JVI.00900-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Osuna CE, Lim S-Y, Deleage C, Griffin BD, Stein D, Schroeder LT, Omange RW, Best K, Luo M, Hraber PT, Andersen-Elyard H, Ojeda EFC, Huang S, Vanlandingham DL, Higgs S, Perelson AS, Estes JD, Safronetz D, Lewis MG, Whitney JB. 2016. Zika viral dynamics and shedding in rhesus and cynomolgus macaques. Nat Med 22:1448–1455. doi: 10.1038/nm.4206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rayner JO, Kalkeri R, Goebel S, Cai Z, Green B, Lin S, Snyder B, Hagelin K, Walters KB, Koide F, Rayner J, Kalkeri R, Goebel S, Cai Z, Green B, Lin S, Snyder B, Hagelin K, Walters K, Koide F. 2018. Comparative pathogenesis of Asian and African-lineage Zika virus in Indian rhesus macaque’s and development of a non-human primate model suitable for the evaluation of new drugs and vaccines. Viruses 10:229. doi: 10.3390/v10050229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dudley DM, Aliota MT, Mohr EL, Weiler AM, Lehrer-Brey G, Weisgrau KL, Mohns MS, Breitbach ME, Rasheed MN, Newman CM, Gellerup DD, Moncla LH, Post J, Schultz-Darken N, Schotzko ML, Hayes JM, Eudailey JA, Moody MA, Permar SR, O’Connor SL, Rakasz EG, Simmons HA, Capuano S, Golos TG, Osorio JE, Friedrich TC, O’Connor DH. 2016. A rhesus macaque model of Asian-lineage Zika virus infection. Nat Commun 7:12204. doi: 10.1038/ncomms12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aliota MT, Dudley DM, Newman CM, Mohr EL, Gellerup DD, Breitbach ME, Buechler CR, Rasheed MN, Mohns MS, Weiler AM, Barry GL, Weisgrau KL, Eudailey JA, Rakasz EG, Vosler LJ, Post J, Capuano S, Golos TG, Permar SR, Osorio JE, Friedrich TC, O’Connor SL, O’Connor DH. 2016. Heterologous protection against Asian Zika virus challenge in rhesus macaques. PLoS Negl Trop Dis 10:e0005168. doi: 10.1371/journal.pntd.0005168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nguyen SM, Antony KM, Dudley DM, Kohn S, Simmons HA, Wolfe B, Salamat MS, Teixeira LBC, Wiepz GJ, Thoong TH, Aliota MT, Weiler AM, Barry GL, Weisgrau KL, Vosler LJ, Mohns MS, Breitbach ME, Stewart LM, Rasheed MN, Newman CM, Graham ME, Wieben OE, Turski PA, Johnson KM, Post J, Hayes JM, Schultz-Darken N, Schotzko ML, Eudailey JA, Permar SR, Rakasz EG, Mohr EL, Capuano S, Tarantal AF, Osorio JE, O’Connor SL, Friedrich TC, O’Connor DH, Golos TG. 2017. Highly efficient maternal-fetal Zika virus transmission in pregnant rhesus macaques. PLoS Pathog 13:e1006378. doi: 10.1371/journal.ppat.1006378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Breitbach ME, Newman CM, Dudley DM, Stewart LM, Aliota MT, Koenig MR, Shepherd PM, Yamamoto K, Crooks CM, Young G, Semler MR, Weiler AM, Barry GL, Heimsath H, Mohr EL, Eichkoff J, Newton W, Peterson E, Schultz-Darken N, Permar SR, Dean H, Capuano S, Osorio JE, Friedrich TC, O’Connor DH. 2019. Primary infection with dengue or Zika virus does not affect the severity of heterologous secondary infection in macaques. PLoS Pathog 15:e1007766. doi: 10.1371/journal.ppat.1007766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moreno GK, Newman CM, Koenig MR, Mohns MS, Weiler AM, Rybarczyk S, Vosler LJ, Pomplun N, Schultz-Darken N, Rakasz E, Dudley DM, Friedrich TC, O’Connor DH. 2019. Long-term protection of rhesus macaques from Zika virus reinfection. bioRxiv 10.1101/712281. [DOI] [PMC free article] [PubMed]
- 49.Balcombe JP, Barnard ND, Sandusky C. 2004. Laboratory routines cause animal stress. Contemp Top Lab Anim Sci 43:42–51. [PubMed] [Google Scholar]
- 50.Dutta P, Nahrendorf M. 2014. Regulation and consequences of monocytosis. Immunol Rev 262:167–178. doi: 10.1111/imr.12219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ramaekers LH, Theunissen PM, Went K. 1975. Acute lymphopenia, stress, and plasma cortisol. Arch Dis Child 50:555–558. doi: 10.1136/adc.50.7.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aid M, Abbink P, Larocca RA, Boyd M, Nityanandam R, Nanayakkara O, Martinot AJ, Moseley ET, Blass E, Borducchi EN, Chandrashekar A, Brinkman AL, Molloy K, Jetton D, Tartaglia LJ, Liu J, Best K, Perelson AS, De La Barrera RA, Lewis MG, Barouch DH. 2017. Zika virus persistence in the central nervous system and lymph nodes of rhesus monkeys. Cell 169:610–620. doi: 10.1016/j.cell.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Figueiredo CP, Barros-Aragão FGQ, Neris RLS, Frost PS, Soares C, Souza INO, Zeidler JD, Zamberlan DC, de Sousa VL, Souza AS, Guimarães ALA, Bellio M, Marcondes de Souza J, Alves-Leon SV, Neves GA, Paula-Neto HA, Castro NG, De Felice FG, Assunção-Miranda I, Clarke JR, Da Poian AT, Ferreira ST. 2019. Zika virus replicates in adult human brain tissue and impairs synapses and memory in mice. Nat Commun 10:3890. doi: 10.1038/s41467-019-11866-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kirkpatrick BD, Whitehead SS, Pierce KK, Tibery CM, Grier PL, Hynes NA, Larsson CJ, Sabundayo BP, Talaat KR, Janiak A, Carmolli MP, Luke CJ, Diehl SA, Durbin AP. 2016. The live attenuated dengue vaccine TV003 elicits complete protection against dengue in a human challenge model. Sci Transl Med 8:330ra36. doi: 10.1126/scitranslmed.aaf1517. [DOI] [PubMed] [Google Scholar]
- 55.U.S. Department of Agriculture. 1970. Animal Welfare Act of 1970: PL 91–579. https://www.nal.usda.gov/awic/public-law-91-579-animal-welfare-act-amendments-1970. Approved 24 December 1970.
- 56.National Institutes of Health. 2011. Guide for the care and use of laboratory animals. National Institutes of Health, Bethesda, MD. [Google Scholar]
- 57.Academy of Medical Sciences, Weatherall DJ. 2006. The use of nonhuman primates in research: a working group report. Academy of Medical Sciences, London, United Kingdom. [Google Scholar]
- 58.Cline AN, Bess JW, Piatak M, Lifson JD. 2005. Highly sensitive SIV plasma viral load assay: practical considerations, realistic performance expectations, and application to reverse engineering of vaccines for AIDS. J Med Primatol 34:303–312. doi: 10.1111/j.1600-0684.2005.00128.x. [DOI] [PubMed] [Google Scholar]
- 59.Pomplun N, Weisgrau KL, Evans DT, Rakasz EG. 2015. OMIP-028: activation panel for rhesus macaque NK cell subsets. Cytometry A 87:890–893. doi: 10.1002/cyto.a.22727. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Complete data sets for these studies have been made publicly available in a manuscript-specific folder on the Zika Open Research Portal (https://go.wisc.edu/bn71k1). We declare that all other data for these study findings are available via this portal.