There is a Blood Commentary on this article in this issue.
Key Points
Tisagenlecleucel for secondary CNS lymphoma has an acceptable safety profile.
Preliminary results using tisagenlecleucel demonstrate activity in a heavily pretreated population.
Abstract
Chimeric antigen receptor (CAR) T cells targeting CD19 have emerged as a leading engineered T-cell therapy for relapsed/refractory B-cell non-Hodgkin lymphoma. The phase 1/2 clinical trials that led to US Food and Drug Administration approval excluded patients with central nervous system (CNS) involvement, due to strict eligibility criteria. Here, we report on our institutional experience with 8 secondary CNS lymphoma patients treated with commercial tisagenlecleucel. No patient experienced greater than grade 1 neurotoxicity, and no patient required tocilizumab or steroids for CAR T-cell–mediated toxicities. Biomarker analysis suggested CAR T-cell expansion, despite the absence of systemic disease, and early response assessments demonstrated activity of IV infused CAR T cells within the CNS space.
Visual Abstract
Introduction
Chimeric antigen receptor (CAR) T-cell therapy has revolutionized the treatment of relapsed/refractory B-cell non-Hodgkin lymphoma. Axicabtagene ciloleucel and tisagenlecleucel are currently approved by the US Food and Drug Administration, having demonstrated response rates ≥80%.1,2 Because of concerns for CAR T-cell–related neurotoxicity (NT),3 patients with active central nervous system (CNS) involvement were excluded from both pivotal studies. Per the US Food and Drug Administration label, tisagenlecleucel is indicated for patients with relapsed or refractory large B-cell lymphoma, including diffuse large B-cell lymphoma (DLBCL) not otherwise specified, high grade B-cell lymphoma (HGBCL), and DLBCL arising from follicular lymphoma, with a specific exclusion for primary, but not secondary, CNS lymphoma. Here, we report the institutional experience at the Massachusetts General Hospital from a cohort of 8 patients treated between August of 2018 and March of 2019 with tisagenlecleucel for HGBCL with secondary CNS involvement.
Study design
Written informed consent for commercial treatment and biobanking was obtained. This retrospective analysis was approved by the Dana Farber/Harvard Cancer Center Institutional Review Board. Treatment consisted of a single infusion of tisagenlecleucel (0.6 × 108 to 6.0 × 108 CAR T cells) following lymphodepleting chemotherapy consisting of cyclophosphamide (250 mg/m2) and fludarabine (25 mg/m2) on days −5, −4, and −3. Patients had initial response assessments on day +28 following infusion (D+28; ±2 days). Luminex cytokine profiling was performed using banked samples. Grading for cytokine release syndrome (CRS) and NT were performed by the treating physician per Lee et al4 and Common Terminology Criteria for Adverse Events version 5.05, and they were retrospectively reviewed to compare with the recently released American Society for Transplantation and Cellular Therapy 2019 guidelines.6 Response assessments were determined by the treating physician in collaboration with neuro-oncology. Rapid autopsies of patients 1 and 5 were performed, and additional immunohistochemistry staining was performed in collaboration with neuro-pathology.
Results and discussion
Eight patients with CNS involvement were treated with tisagenlecleucel (Table 1). The median age was 50 years (range, 17-79), including 4 males and 4 females. The median number of prior therapies was 5 (range, 3-6), with 1 patient having a prior allogeneic stem cell transplant. Two of 8 patients had systemic disease in addition to CNS involvement at the time of infusion. All patients were receiving CNS-directed therapy for refractory disease up until lymphodepletion. At time of infusion, 3 patients had parenchymal involvement, 3 had leptomeningeal involvement, and 2 had parenchymal and leptomeningeal involvement. The performance status of 7 of 8 patients (88%) was Eastern Cooperative Oncology Group score 0-2 at time of infusion. Patient 5 was treated with 6 lines of therapy and experienced rapid progression following leukapheresis. All patients received prophylactic anticonvulsants, and no patient received growth factor support before D+7. Representative cases of treatment responses are summarized here.
Table 1.
Patient and treatment characteristics
| ID | Sex | Age, y | Disease pathology | Prior lines of therapy (most recent response) | Site of CNS disease (maximal dimension) | Systemic isease? | Maximum CRS/NT (Lee 20144) | Maximum CRS/NT (ASTCT 20196) | Required treatment for CRS/NT? | Concomitant maintenance during CAR T-cell therapy? | D+28 response | Subsequent follow-up |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 43 | DLBCL, GCB like | R-CHOP + HD-MTX with CNS relapse | Cerebral peduncles and midbrain, as well as the 3rd and 7/8th cranial nerves. | No | Grade 1 CRS. Grade 1 NT (headache). | Grade 1 CRS. No NT.* | No | Ibrutinib | Deceased, disease progression | |
| CYVE with relapse | ||||||||||||
| R-cytarabine with relapse | ||||||||||||
| Ibrutinib maintenance (progressive disease) | ||||||||||||
| 2 | F | 64 | HGBCL | DA-EPOCH-R + IT-MTX | CSF intermittently positive | Right lateral psoas (1.8 cm) | No CRS. No NT. | No CRS. No NT.* | No | None | CR, CSF (−) | Systemic relapse at D+90, CNS (−). RT to focal relapse with resolution on D+180 scans. No additional CNS-directed therapy. |
| HDC-ASCT with systemic/CNS relapse | ||||||||||||
| GemOx + HD-MTX | ||||||||||||
| RIC MUD with systemic/CNS relapse | ||||||||||||
| Cessation of immunosuppression & monthly IT-MTX maintenance for 6 mo (CNS: stable disease; systemic: progressive disease) | ||||||||||||
| 3 | M | 61 | DLBCL, GCB like | R-CHOP with CNS relapse | CSF+, ventral nerve root from T12 to L2, L1/L5 vertebral involvement | No | Grade 1 CRS. Grade 1 NT (neuropathy). | Grade 1 CRS. No NT.* | No | None | PR, CSF (−) | CR at D+180 |
| HD-MTX with CNS relapse | ||||||||||||
| CYVE with CNS relapse | ||||||||||||
| Twice weekly IT-MTX maintenance (PR) | ||||||||||||
| 4 | F | 56 | DLBCL, GCB like | R-CHOP with systemic relapse | Right superior frontal gyrus (1.4 cm) | Hepatic (4.5 cm) & mesenteric lymphadenopathy | Grade 1 CRS. No NT. | Grade 1 CRS. No NT.* | No | Ibrutinib | PD | WBI, lenalidomide |
| R-ICE with systemic/CNS relapse | ||||||||||||
| R-ibrutinib (CNS & systemic: PR) | ||||||||||||
| 5 | M | 24 | HGBCL | Modified McGrath regimen, CNS relapse | Left cerebellar (0.7 cm) and diffuse leptomeningeal involvement | No | Grade 1 CRS. NT.† | Grade 1 CRS. NT.† | No | Ibrutinib/steroids | Deceased; disease progression. | |
| HD-MTX with CNS progression | ||||||||||||
| CYVE with CNS progression | ||||||||||||
| IT cytarabine/MTX with CNS progression | ||||||||||||
| Pemetrexed with CNS progression | ||||||||||||
| Ibrutinib (progressive disease) | ||||||||||||
| 6 | M | 44 | DLBCL, GCB like | R-CHOP with systemic/CNS relapse | CSF+, intradural extramedullary nodule adjacent to the conus at L1 (2.5 cm). | No | Grade 1 CRS. No NT. | Grade 1 CRS. No NT. | No | None | PD, prior sites resolved, new PET-avid lesion, CSF+. | Ibrutinib maintenance, RT to residual disease. |
| HD-MTX–R with CNS progression | ||||||||||||
| CYVE with CNS progression | ||||||||||||
| Ibrutinib with CNS progression | ||||||||||||
| REVLIMID with CNS progression | ||||||||||||
| HiDAC (PR) | ||||||||||||
| 7 | M | 17 | PMBCL | DA-EPOCH-R with CNS relapse | Multiple sites, including right basal ganglia (2.3 cm), septum pellucidum and corpus callosum (2.1 cm), 9th-11th cranial nerves. | No | Grade 1 CRS. No NT. | Grade 1 CRS. No NT.* | No | None | PR | Ongoing response at D+90, decreased avidity of PET/MRI to adjacent brain parenchyma. |
| HD-MTX with CNS progression | ||||||||||||
| HiDAC with CNS progression | ||||||||||||
| Brentuximab and CYVE with partial response | ||||||||||||
| RT + pembro with CNS progression, craniospinal axis irradiation (PR) | ||||||||||||
| 8 | F | 79 | DLBCL, non-GCB | R-CHOP + HD-MTX with consolidative XRT with CNS relapse | Left Meckel’s cave and temporal lobe (5.0 cm) | No | Grade 1 CRS. Grade 1 NT (tremors). | Grade 1 CRS. No NT.* | No | None | CR | Ongoing response at D+90 |
| HD-MTX with progression | ||||||||||||
| R-HiDAC with PR | ||||||||||||
| Ibrutinib maintenance eventually held for rash (PD) |
ASTCT, American Society for Transplantation and Cellular Therapy; CR, complete response; CSF, cerebral spinal fluid; CYVE, cytarabine/etoposide; DA-EPOCH-R, dose adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab; F, female; GCB, germinal center B cell; GemOx, gemcitabine/oxaliplatin; HDC-ASCT, high-dose chemotherapy + autologous stem cell transplant; HD-MTX, high-dose methotrexate; HiDAC, high-dose cytarabine; IT, intrathecal; IT-MTX, intrathecal methotrexate; M, male; MTX, methotrexate; MUD, matched unrelated donor; PD, progressive disease; PET, positron emission tomography; PMBCL, primary mediastinal B-cell lymphoma; PR, partial response; R, rituxan; R-CHOP, rituxan/cyclophosphamide/doxorubicin/vincristine/prednisone; RIC, reduced intensity conditioning; R-ICE, rituxan/ifosfamide/carboplatin/etoposide; RT, radiotherapy; WBI, whole-brain irradiation; XRT, radiation therapy.
Immune Effector Cell-Associated Encephalopathy score not able to be calculated retrospectively.
Not evaluable because of disease progression.
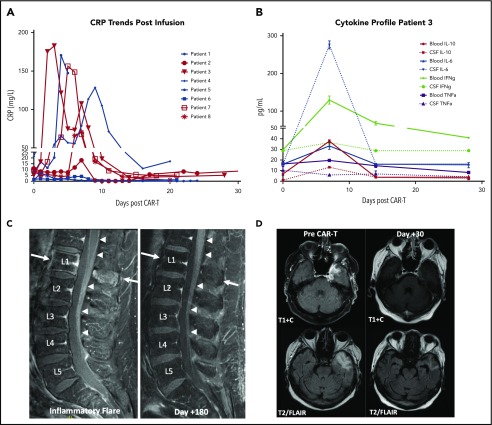
A 61-year-old male with stage IVA DLBCL achieved a complete response (CR) following rituxan/cyclophosphamide/doxorubicin/vincristine/prednisone but later relapsed in the CNS. His magnetic resonance imaging (MRI) at relapse showed a T2 hyperintense lesion at the L1 vertebral body with marrow edema and right L5 transverse process involvement. There was also subtle nodularity of the ventral L2 nerve root, as well as the ventral aspect of the cord at T12. Positron emission tomography/computed tomography imaging at that time revealed axillary and retroperitoneal disease. Cerebrospinal fluid (CSF) was positive for lymphomatous involvement. Treatment with high-dose methotrexate (HD-MTX), followed by cytarabine/etoposide resulted in a systemic CR, but only a partial CNS response. Bridging twice-weekly intrathecal methotrexate was administered during cellular therapy manufacturing, and CSF from his last intrathecal treatment demonstrated a residual clonal B-cell population. His CAR T-cell course was complicated by grade 1 CRS and grade 1 NT, and his C-reactive protein (CRP) peaked at 182 mg/L on D+3 (Figure 1A). Serum and CSF samples obtained weekly demonstrated marked increases in cytokines associated with CRS. Interestingly, interleukin-6 showed a greater than sevenfold increase in the CSF compared with matched serum (Figure 1B) on D+7. On D+6, the patient noted acute back pain and left leg radiculopathy. MRI at this time revealed linear enhancement along the lower thoracic cord and conus and cauda equina nerve roots, as well as sciatic neuritis (Figure 1C; inflammatory flare), that was not seen on imaging 6 months prior, despite positive CSF sampling. More recent spinal axis imaging was not available, emphasizing the importance of complete baseline neuroaxis imaging in this patient population. Following spontaneous resolution of his symptoms on D+10, he was discharged without recurrence. Subsequent MRI on D+28 showed a partial response, with CR seen at D+180 without additional therapy (Figure 1C; D+180 response).
Figure 1.
Biomarker and imaging correlates of patients treated with tisagenlecleucel. (A) CRP trends of secondary CNS lymphoma patients treated with tisagenlecleucel. Red, D+30 responders; blue, D+30 nonresponders/deceased. (B) Time-matched serum and CSF (via ommaya) on days 0, 7, 14, and 28 of tisagenlecleucel infusion, as measured by Luminex assay for patient 3. Error bars, SEM. Data are technical triplicates. (C) MRI of patient 3 during inflammatory flare on D+6 with linear enhancement along the lower thoracic cord, conus, and cauda equina nerve roots (left panel, denoted by arrows) with subsequent resolution by D+180 (right panel). (D) MRI of patient 8 demonstrating resolution of the left middle cranial fossa enhancing mass lesion with decreased expansile T2/fluid-attenuated inversion recovery (FLAIR) hyperintensity in the left anterior temporal white matter.
A 78-year-old female with DLBCL of the right maxillary sinus was treated with 6 cycles of rituxan/cyclophosphamide/doxorubicin/vincristine/prednisone and HD-MTX. At clinical progression, her brain MRI revealed a homogenously enhancing mass in the left medial temporal lobe. Biopsy confirmed recurrence of HGBCL; she was treated with HD-MTX, rituxan and high-dose cytarabine, and then ibrutinib, given the poor response. MRI prior to lymphodepleting chemotherapy showed a 5-cm enhancing lobular mass within the left temporal lobe (Figure 1D; pre–CAR T-cell therapy). Her CAR T-cell course was notable for grade 1 CRS and grade 1 NT. Inflammatory markers were notable for a peak CRP of 70 mg/L on D+7. No tocilizumab or steroids were administered. D+30 restaging scans demonstrated a complete metabolic response, as well as near-complete resolution of the temporal lobe lesion on MRI (Figure 1D; D+30). Her response was maintained at D+90.
Notably, no patients required tocilizumab or high-dose steroids for the management of CRS and/or NT. Autopsies were performed on 2 patients who died within 30 days of CAR T-cell infusion. Patient 1 passed on D+25 after presenting with 3 days of progressive nausea and vomiting. Her clinical course was notable for the absence of inflammatory markers suggestive of CAR T-cell expansion (peak CRP, 7.1 mg/L on D+1; ferritin, 72 μg/L on D+1) and grade 1 CRS with an isolated fever of 38.3°C on D+4. MRI imaging on D+23 noted diffuse disease progression without evidence of cerebral edema compared with D−28 (supplemental Figure 1A, available on the Blood Web site). Autopsy findings confirmed extensive disease involving the leptomeninges, cortex, brainstem, and pituitary and basal ganglia with a midbrain lesion measuring ≥3 × 2 × 2 cm with near-complete effacement of key structures. Immunohistochemistry stains confirmed extensive CD20+ B-cell involvement with admixed infiltrating CD3+ T cells (supplemental Figure 3E). Although CD68+ macrophages can be seen within the tumor mass, there was no significant extension into the surrounding brain parenchyma suggesting a reactive, as opposed to an inflammatory, infiltrate (supplemental Figure 3F). Patient 5 had rapidly progressive disease requiring aggressive management of elevated intracranial pressures prior to initiation of lymphodepleting chemotherapy. MRI performed the month preceding infusion revealed marked interval progression of disease with dural, diffuse leptomeningeal, and nodular involvement of the left cerebellar hemisphere despite ongoing CNS-directed therapy (supplemental Figure 1B). The patient died on D+3 from neurological demise resulting from uncal and downward tonsillar herniation, emphasizing the importance of earlier treatment during a window of clinical stability. Autopsy findings confirmed massive leptomeningeal and dural infiltration of CD20+ neoplastic B cells involving the entire cerebrum from the frontal to occipital lobes, with hemorrhagic transformation and significant mass effect (supplemental Figure 3A-D). Taken together, it was determined that both patients died of progressive disease.
Prior studies using CD19-directed CAR T-cell therapy have excluded CNS involvement because of concerns for potentially fatal NT. Only 1 case report has been published demonstrating the efficacy of CD19-directed CAR T-cell therapy in a patient with simultaneous CNS and systemic involvement.7 Tisagenlecleucel, a 4-1BB chimeric antigen receptor, was chosen based on lower rates of reported CRS/NT, evidence of CNS penetration in prior adult acute lymphoblastic leukemia and chronic lymphoblastic leukemia studies, and absence of grade 5 cerebral edema, which has been observed in CD28-containing constructs.2,8,9 In the present cohort, active systemic disease was not required for CAR T-cell expansion (as indicated by increases in inflammatory markers) and disease response, suggesting that IV CAR T-cell therapy can sufficiently traffic to the CNS. Although CAR T-cell expansion could not be directly assessed as commercial detection reagents are not available, robust CD3+ lymphocyte expansion was observed in patients without systemic disease (supplemental Figure 2). D+28 response assessments also suggest that the kinetics of CAR T-cell efficacy within the CNS may be similar to systemic disease, because treatment responses were seen as early as 3 to 4 weeks after infusion. Most importantly, higher rates of CRS and NT were not seen in this cohort, suggesting that CNS involvement may not be a risk factor for severe NT. This conclusion may be in line with recent data suggesting that CAR T-cell–mediated NT may be a CNS manifestation of an otherwise systemic inflammatory process involving blood–brain barrier breakdown and endothelial and macrophage activation.10-13
To our knowledge, this study represents the largest cohort of patients treated with CD19 CAR T cells for active secondary CNS lymphoma. Our findings are limited by the retrospective nature of the analysis, a short follow-up, and the use of CNS-directed bridging therapy. However, our findings suggest that the use of commercial tisagenlecleucel CAR T cells for isolated secondary CNS lymphoma may be a viable treatment option for this otherwise challenging patient population. Because of these promising results, we plan to proceed with a pilot study of tisagenlecleucel in primary CNS lymphoma this coming year. Further studies are warranted.
Supplementary Material
The online version of this article contains a data supplement.
Acknowledgments
The authors thank the patients and their families, as well as the Massachusetts General Hospital nurses, advanced practitioners, and support staff involved in their care.
This work was supported by the Susan M. Eid autopsy fund (rapid autopsy support) and National Institutes of Health/National Cancer Institute grant K12CA087723 (M.J.F.).
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: M.J.F., J.D., M.L., M.M.-L., and C.P. collected data; M.J.F., Y.-B.C., Z.D., B.D.C., J.D., J.A., J.C., P.A., L.N., and M.V.M. provided patient care; M.J.F., J.D., B.D.C., M.M.-L., K.G., and L.S.R. analyzed and interpreted data; and M.J.F., Y.-B.C., Z.D., B.D.C., J.D., J.A., J.C., P.A., M.M.-L., and M.V.M. wrote the manuscript.
Conflict-of-interest disclosure: M.J.F. has acted as a consultant for and received honoraria from Novartis, Kite/Gilead, Nkarta Therapeutics, Xenetic Biosciences, and Arcellx. Y.-B.C. has acted as a consultant for AbbVie, Magenta Therapeutics, Equillium, Takeda, Kiadis Pharma, and Incyte. J.A. has acted as a consultant for AbbVie, Bayer, Celgene, Genentech, Gilead Sciences, Janssen Pharmaceuticals, Juno Therapeutics, Karyopharm Therapeutics, Kite Pharma, Merck, and Novartis. P.A. has acted as a consultant for Merck, BMS, Pfizer, Affimed, Adaptive, Infinity, and ADC Therapeutics; has received institutional research funding from Merck, Bristol-Myers Squibb, Affimed, Adaptive Biotechnologies, Roche, Tensha Therapeutics, Otsuka, Sigma-Tau Pharnmaceuticals, and Genentech; and has received honoraria from Merck. L.N. has acted as a consultant for Bristol-Myers Squibb. M.V.M. has acted as a consultant for Novartis and is an inventor on unrelated patents licensed to Novartis. The remaining authors declare no competing financial interests.
Correspondence: Matthew J. Frigault, Massachusetts General Hospital Cancer Center, Zero Emerson Pl, Suite 118, Office 127, Boston, MA 02114; e-mail: mfrigault@partners.org.
REFERENCES
- 1.Neelapu SS, Locke FL, Bartlett NL, et al. . Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377(26):2531-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schuster SJ, Bishop MR, Tam CS, et al. ; JULIET Investigators . Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380(1):45-56. [DOI] [PubMed] [Google Scholar]
- 3.Karschnia P, Jordan JT, Forst DA, et al. . Clinical presentation, management, and biomarkers of neurotoxicity after adoptive immunotherapy with CAR T cells. Blood. 2019;133(20):2212-2221. [DOI] [PubMed] [Google Scholar]
- 4.Lee DW, Gardner R, Porter DL, et al. . Current concepts in the diagnosis and management of cytokine release syndrome [published correction appears in Blood. 2016;128(11):1533]. Blood. 2014;124(2):188-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.US Department of Health and Human Services Common Terminology Criteria for Adverse Events (CTCAE), Version 5.0. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf. Accessed 29 July 2019.
- 6.Lee DW, Santomasso BD, Locke FL, et al. . ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant. 2019;25(4):625-638. [DOI] [PubMed] [Google Scholar]
- 7.Abramson JS, McGree B, Noyes S, et al. . Anti-CD19 CAR T cells in CNS diffuse large-B-cell lymphoma. N Engl J Med. 2017;377(8):783-784. [DOI] [PubMed] [Google Scholar]
- 8.Mueller KT, Maude SL, Porter DL, et al. . Cellular kinetics of CTL019 in relapsed/refractory B-cell acute lymphoblastic leukemia and chronic lymphocytic leukemia. Blood. 2017;130(21):2317-2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.JCAR015 in ALL: a root-cause investigation. Cancer Discov. 2018;8(1):4-5. [DOI] [PubMed] [Google Scholar]
- 10.Giavridis T, van der Stegen SJC, Eyquem J, Hamieh M, Piersigilli A, Sadelain M. CAR T cell-induced cytokine release syndrome is mediated by macrophages and abated by IL-1 blockade. Nat Med. 2018;24(6):731-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Norelli M, Camisa B, Barbiera G, et al. . Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat Med. 2018;24(6):739-748. [DOI] [PubMed] [Google Scholar]
- 12.Teachey DT, Lacey SF, Shaw PA, et al. . Identification of predictive biomarkers for cytokine release syndrome after chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Cancer Discov. 2016;6(6):664-679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gust J, Hay KA, Hanafi LA, et al. . Endothelial activation and blood-brain barrier disruption in neurotoxicity after adoptive immunotherapy with CD19 CAR-T cells. Cancer Discov. 2017;7(12):1404-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.