Abstract
Entomopathogenic fungi are the key regulators of insect populations and some of them are important biological agents used in integrated pest management strategies. Compared with their ability to become resistant to insecticides, insect pests do not easily become resistant to the infection by entomopathogenic fungi. In this study, we evaluated the mortality and immune response of the serious crop pest Locusta migratoria manilensis after exposure to a new entomopathogenic fungus strain, Metarhizium anisopliae CQMa421. M. anisopliae CQMa421 could effectively infect and kill the L. migratoria adults and nymphs. The locust LT50 under 1 × 108 conidia/mL concentration of M. anisopliae was much lower than that under conidial concentration 1 × 105 conidia/mL (i.e., 6.0 vs. 11.2 and 5.0 vs. 13.8 for adults and nymphs, respectively). The LC50 (log10) of M. anisopliae against locust adults and nymphs after 10 days was 5.2 and 5.6, respectively. Although the number of hemocytes in L. migratoria after exposure to M. anisopliae did not differ with that in the controls, the enzymatic activity of superoxide dismutase (SOD) and prophenoloxidase (ProPO) did differ between the two treatments. The activities of both SOD and ProPO under the M. anisopliae treatment were lower than that in the controls, except for the ProPO activity at 72 h and the SOD activity at 96 h. Further, the expression of the L. migratoria immune-related genes defensin, spaetzle, and attacin differed after exposure to M. anisopliae for 24 h to 96 h. Taken together, this study indicated that infection with M. anisopliae CQMa421 could cause the death of L. migratoria by interacting with the immune responses of the host, demonstrating that this fungal strain of M. anisopliae can be an efficient biocontrol agent against L. migratoria.
Keywords: Metarhizium anisopliae, mortality, Locusta migratoria, immune response, pest control
1. Introduction
Entomopathogenic fungi are typically present within natural insect populations and are often solely considered as effective microbial control agents in integrated pest management [1,2,3,4]. The use of fungal insect pathogens may have certain advantages over the use of parasitoids and insecticides such as efficiency and environmental safety [5]. Several fungal agents have been used to control pests, such as the rice planthopper Nilaparvata lugens, Haemaphysalis longicornis, and the oriental migratory locust Locusta migratoria manilensis Meyen, and have achieved good results [6,7,8,9]. Currently, chemical insecticides are commonly used for insect pest control [10]. However, the misuse of such insecticides has caused destructive damage to the environment and human health [11,12]. The side effects on nontargets and the resurgence of insect pests have received much attention, and consequently, there is a growing trend to reduce the use of these chemical insecticides [13]. Moreover, the enhanced resistance of insect pests to many chemical insecticides has resulted in long-standing and expanding problems for pest arthropod control [14]. Compared with their ability to become resistant to insecticides, insect pest hosts do not easily become resistant to fungal infection, and entomopathogenic fungi have been used to control a few insecticide-resistant insect species [15].
Entomopathogenic fungi can infect insects, namely, the fungal conidia attach and penetrate through the insect’s cuticle, causing death. Such processes involve several physiological or immune responses of hosts to these types of xenobiotics and pathogens [16]. Although all invertebrates lack an adaptive immune response, they may defend against pathogens by relying on their innate immunity (i.e., cellular and humoral immune responses) [17,18,19]. In hosts, these xenobiotics can be countered by phagocytosis, or by the activation of the host innate immunity. However, entomopathogenic fungi can mask their cell wells to evade the immune system of insect hosts [20] and release chitinase, chitosanase, and lipase to suppress the host regulatory system [21]. In addition, these fungi can produce a few compounds such as beauvericin compounds and destruxins to paralyze the hosts [22]. Thus, the immune responses of hosts are important processes activated in response to the functions of xenobiotics or pathogens.
In response to challenge with insecticides or pathogens, many metabolic processes or immune responses within insect hosts are activated. The antioxidant enzyme of superoxide dismutase (SOD) is a key modulator of host immunity function and is associated with the phagocytotic ability and melanization of insects [23]. The activity of enzymes such as SOD can be stimulated by different insecticides and is associated with the resistance to chemical insecticides [24]. Prophenoloxidase (ProPO) is a crucial factor in the defense against pathogen or insecticide challenge [25]. Increased ProPO activity can enhance the insect immune system ability in response to xenobiotic challenges and can promote healing [19,26].
In Drosophila melanogaster, the Toll and IMD signaling pathways regulate the synthesis of immune effectors [27,28,29]. Unlike its mammalian counterparts, insect Toll is activated through an endogenous ligand and nerve growth factor-related cytokine spaetzle (a gene encoding a Toll-activating protease), but not by direct interaction with microbial molecules [30]. Insect defensins are cationic, cysteine-rice peptides (ca. 4 kDa), inducible antibacterial peptides that may appear after pathogenic challenge or injury in the insect hemolymph [31]. Attacin is an important antimicrobial peptide that is related to the humoral immune system of insect hosts [32]. The expression level of these defensin genes may reflect the immune responses of hosts to the infection with microbial pathogens.
The oriental migratory locust Locusta migratoria manilensis Meyen is an important pest to many crops worldwide [33]. Neonicotinoids and organophosphate are two important types of insecticides for controlling this pest. However, resistance to such insecticides has become intense in some populations [34,35]. Metarhizium acridum and Beauveria bassiana have shown potential for the control of several insect pests such as, the cotton bollworm Helicoverpa zea and L. migratoria [33,36,37]. The mycopesticide “Green Muscle”, which specifically infects the short-horned grasshopper species, has been developed to save crops from locusts. However, the interactions of entomopathogenic fungi with insect hosts have been less frequently evaluated. Thus, we investigated the potential of the new fungal strain M. anisopliae CQMa421 for the mortality of L. migratoria. We further investigated the immune responses of L. migratoria after challenge with M. anisopliae. Infection with M. anisopliae could cause the immune responses of L. migratoria. This study also suggested that infection with the fungus M. anisopliae might result in the death of L. migratoria adults and nymphs, indicating that M. anisopliae might be a potential biocontrol agent to suppress this destructive pest.
2. Materials and Methods
2.1. M. anisopliae and Insect Culture
The entomopathogenic fungal strain, M. anisopliae CQMa421 was isolated from the rice leafroller Cnaphalocrocis medinalis and maintained at the China General Microbiological Culture Collection Center (CGMCC, No. 460). The strain used in this study was isolated and cultured in our laboratory (i.e., the Genetic Engineering Research Center, Chongqing University, Chongqing, China). Prior to the experiments, the conidia of M. anisopliae were collected after 14 days of growth in 1/4 SDAY medium, which comprises 18 g of agar, 5 g of yeast extract, 10 g of glucose and 2.5 g of peptone per liter of sterilized water. Mycelia were removed by filtration through sterile lens paper. Afterward, the conidia of M. anisopliae were diluted into serial concentrations in conjunction with 0.1% Tween 80 and used for subsequent experiments. The concentrations of M. anisopliae spores were verified using a Petroff-Hausser counting slide under a microscope. Then, serial conidial concentrations, 1 × 105 conidia/mL, 1 × 106 conidia/mL, 1 × 107 conidia/mL and 1 × 108 conidia/mL, of M. anisopliae, were prepared using 0.1% Tween 80.
The individuals of L. migratoria used in the study was obtained from the experimental colonies and reared for more than ten years at the Plant Experimental Base of Chongqing University. The locusts were maintained in cages at a temperature 30 ± 3 °C and relative humidity (RH) 50 ± 5% under a 14: 10 h (light: dark) photoperiod. Individual locusts were supplied the fresh maize leaves/ryegrass and wheat bran daily.
2.2. The Effect of M. anisopliae CQMa421 on L. migratoria Survival
To examine the potential effects of M. anisopliae on L. migratoria, the adults and the emerging fifth-instar nymphs of L. migratoria were collected for bioassay experiments. Each L. migratoria nymph or adult was treated from locust pronotum using 5 μL serial concentrations of M. anisopliae. A total of 20 larval or 20 adult individuals as a group were placed into a cage and provided maize leaves, and three replicates were included per treatment group. In the control counterpart, we used 5 μL of 0.1% Tween 80 to treat each individual locust. After treatment, all nymphs and adults were kept in the bioassay room, and their foods were replenished daily. The survival of the locusts was then checked daily and was monitored until the death of all individuals. Individual locusts found dead in the cages were removed and incubated for 10 days to check the conidial formation of locust corpses. We then determined the LT50 and the LC50 of M. anisopliae on locusts after 10 days on the basis of the results of a probit analysis.
2.3. Effects on Hemocyte Concentration
To further evaluate the effects of the fungus M. anisopliae on the hemocyte concentrations, L. migratoria individuals were selected for further examination after infection with the fungus M. anisopliae at a concentration 1 × 107 conidia/mL. The hemolymph cells of L. migratoria were collected according to the methods carried out by Gillespie et al. [38]. The arthrodial membrane of L. migratoria was first swabbed with 70% ethanol and then pierced with a sterile needle. The cells and an equal volume of anticoagulant solution were then immediately mixed together. In this treatment, the hemolymph from ten alive individuals L. migratoria was collected (10 μL for each), pooled after 24, 48, 72, and 96 h, and mixed with well-prepared anticoagulant solution. The anticoagulant solution was prepared by consisting of 30 mM sodium citrate, 26 mM citric acid, 100 mM d-glucose, 10 mM EDTA and 60 mM NaCl per 100 mL. The hemocyte concentrations of L. migratoria were quantified using a hemocytometer with 10 μL aliquots under a microscope, and five replicates were examined per treatment.
2.4. The Enzymatic Activities of ProPO and SOD
The enzymatic activity of ProPO and SOD were tested according to the manufacturer’s instructions after exposure to M. anisopliae at a concentration 1 × 107 conidia/mL (Suzhou Comin Biotech Co., Ltd., Suzhou, China). In brief, the hemolymph of 10 L. migratoria individuals was collected as described above and was diluted 10 times prior to subsequent experiments. To examine the activities of the ProPO and SOD, the diluted hemolymph was examined using a microplate reader (BioTek Instruments, Inc., Winooski, VT, USA). We evaluated the SOD activity according to the inhibition of the photochemical reduction of nitro blue tetrazolium (NBT) at a wavelength of 560 nm. Each unit of SOD activity was the amount of enzyme that caused a 50% inhibition of the NBT reduction. ProPO can catalyze the catechol to produce the quinones, which absorb light at a wavelength of 525 nm, so the ProPO activity was measured to be 525 nm. The activities of both SOD and ProPO were expressed as units/mg protein.
2.5. Immune-Related Gene Expression Induced by M. anispliae
We further evaluated the effects of M. anisopliae on the expression of immune-related genes (i.e., defensin, spaetzle and attacin) after fungal infection using quantitative real-time PCR (qRT-PCR). Individual locusts were collected randomly from the M. anisopliae treatment or control treatment group and analyzed. Briefly, the total RNA in the locust hemolymph of ten select individuals was extracted by using the Trizol reagent (Invitrogen, Shanghai, China). For this, 1 μg total RNA was reverse-transcribed in a 20-μL reaction using the RT-PCR Kit (TaKaRa, Beijing, China). Then, the iCycler iQ Real-time PCR System (Bio-Rad, Hercules, CA, USA) was selected to perform qRT-PCR with SYBR-Green. The cycling parameters were as follows: 95 °C for 3 min, and 40 cycles of 95 °C for 5 s and 60 °C for 15 s, followed by melting curve generation from 65 to 95 °C. The expression of β-actin was selected as to normalize the expression of the immune-related genes according to the 2−ΔΔCt method [39]. All qRT-PCR protocols used the least stringent criteria possible. The primers designed for qRT-PCR in this experiment are listed in Table 1, and three replicates were included per treatment.
Table 1.
PCR primers used in this study.
| Primers | Primer Sequences (5’-3’) |
|---|---|
| Spaetzle-F | AGCTTGTGGGTACGGAGAC |
| Spaetzle-R | GGGCGATGAATAGATGAAAC |
| Defensin-F | GCGTCTGTCTCCTCTG |
| Defensin-R | CCCTTGTAGCCCTTGTT |
| Attacin-F | GTGCTCCTCGTCGTTCTGA |
| Attacin-R | CCCACGCCTTTCTCTCTGT |
| β-actin-F | GCAGCCAGCAACCAGGAG |
| β-actin-R | ACCATCTGTCCACGGATAATAGC |
F, forward primer; R, reverse primer.
2.6. Data Analysis
The LT50 and LC50 of M. anisopliae against L. migratoria were analyzed using the probit analysis in SPSS 23.0 software. Prior to the analyses, the Shapiro-Wilk test and the Levene test were selected to evaluate the normality and homogeneity of variances, respectively. If the data were not normally distributed, they were normalized or analyzed by the Mann–Whitney U test. Afterward, one-way ANOVA with the least significant difference (LSD) test was applied to examine the effects of M. anisopliae on locust LT50. The LC50 of adult and larva was examined by t-test. The hemocyte concentration and the enzymatic activities of SOD and ProPO after the insects were exposed to L. anisopliae were analyzed via the t-test. The relative expression of the select genes was also analyzed via the t-test. The significance level was set at p < 0.05.
3. Results
3.1. Mortality of the Fungus M. anisopliae on L. migratoria
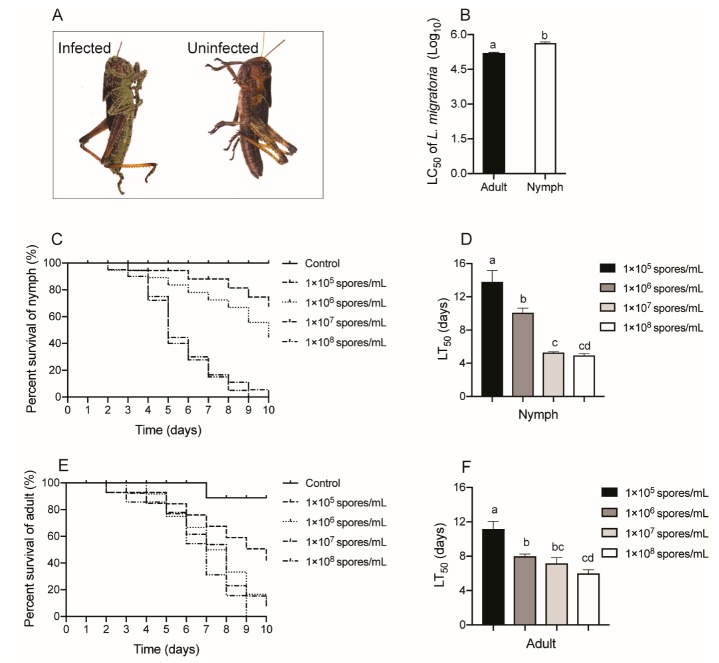
The adults and nymphs of L. migratoria responded differently to challenge with serial concentrations of the fungus M. anisopliae CQMa421. First, we noticed that the corpses of L. migratoria insects infected with M. anisopliae CQMa421 for 10 days covered the fungal conidia (Figure 1A) but not the controls. The LC50 of L. migratoria adults and nymphs after infection for 10 days also showed differences (p = 0.01, t-test; Figure 1B). The LT50 of locust nymphs treated with low concentrations of M. anisopliae, 1 × 105 conidia/mL or 1 × 106 conidia/mL (13.81 days and 10.09 days, respectively), was higher than that under high concentrations of M. anisopliae, 1 × 107 conidia/mL or 1 × 108 conidia/mL (5.30 days and 4.96 days, respectively). The LT50 of locust nymphs also showed significant differences after exposure to serial concentrations of M. anisopliae (F3,8 = 32.735, p < 0.001; Figure 1C,D). Similarly, the LT50 of L. migratoria adults after exposure to concentrations of 1 × 105 conidia/mL was 11.18 days, while the LT50 shortened to 6.00 days under 1 × 108 conidia/mL. The LT50 of adult locusts showed a significant difference between the serial concentrations of M. anisopliae (F3,8 = 13.527, p = 0.002; Figure 1E,F).
Figure 1.
Survival of L. migratoria after exposure to M. anisopliae CQMa421. (A) the corpses of L. migratoria insects infected with M. anisopliae CQMa421 or not; (B) LC50 of L. migratoria adults and nymphs; (C) Survival rate of L. migratoria nymphs treated with M. anisopliae CQMa421; (D) LT50 of L. migratoria nymphs after being challenged with M. anisopliae CQMa421. (E) Survival rate of L. migratoria adults treated with M. anisopliae CQMa421; (F) LT50 of L. migratoria adults after being challenged with M. anisopliae CQMa421. The different letters indicate significant differences, and the bars represent the means ± SEs.
3.2. Concentration of Hemocytes
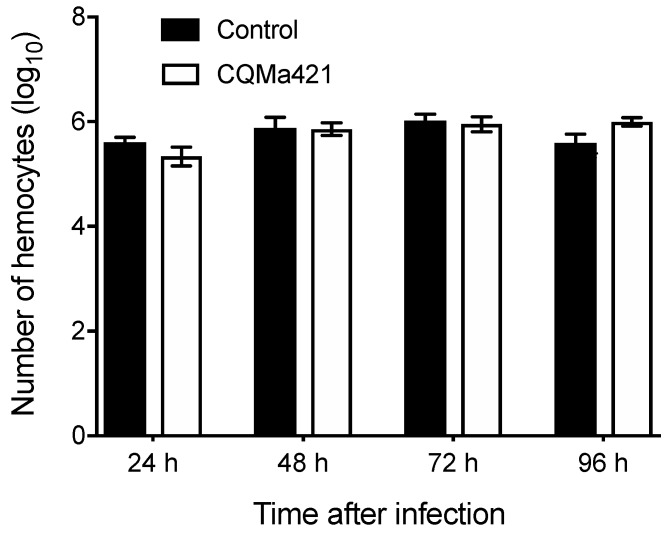
To further investigate the numbers of hemocytes after challenge with M. anisopliae, we counted the hemocyte concentration of L. migratoria hemolymphs. From 24 to 72 h, the number of hemocytes under the M. anisopliae treatment was similar to that under the control treatments (Figure 2). After 96 h of M. anisopliae infection, the number of hemocytes under the fungal treatment was greater than that under the controls, there was no statistical difference between the M. anisopliae and control treatment, with no significant difference (p = 0.061, t-test; Figure 2).
Figure 2.
Concentration of hemocytes after challenge with M. anisopliae CQMa421 from 24 to 96 h. The bars represent the means ± SEs.
3.3. Enzymatic Activity
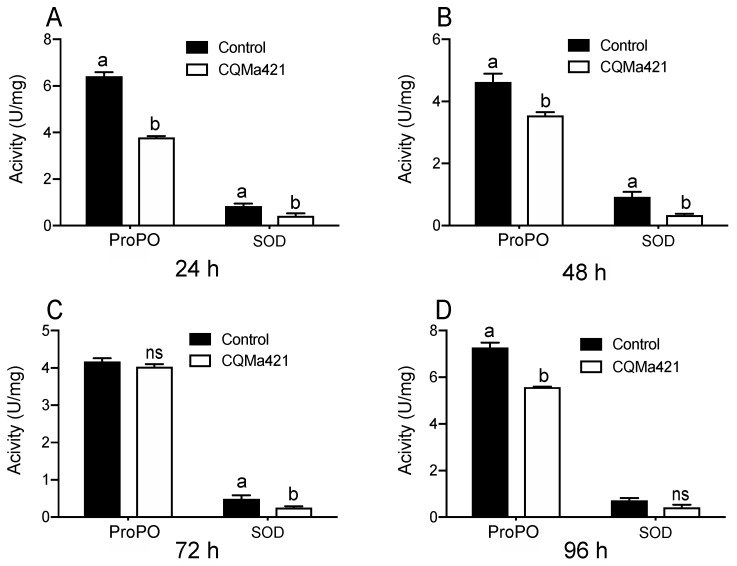
The enzymatic activities of the two enzymes ProPO and SOD varied after the locusts were treated with M. anisopliae and were lower than those under the control treatments from 24 h to 48 h (Figure 3A,B). The activities of ProPO at 72 h and SOD at 96 h did not differ from those under the control treatment (p = 2.44 and p = 0.60 for ProPO and SOD, t-test; Figure 3C,D). However, the activities of SOD at 72 h and ProPO at 96 h after exposure to the fungus displayed significant differences compared with those under the control treatment (p < 001 and p = 0.046 for SOD and ProPO, t-test; Figure 3C,D).
Figure 3.
ProPO and SOD activities of L. migratoria after challenge with M. anisopliae CQMa421. (A) Enzymatic activity of L. migratoria after treatment with M. anisopliae CQMa421 for 24 h; (B) Enzymatic activity of L. migratoria after treatment with M. anisopliae CQMa421 for 48 h; (C) Enzymatic activity of L. migratoria after treatment with M. anisopliae CQMa421 for 72 h; (D) Enzymatic activity of L. migratoria after treatment with M. anisopliae CQMa421 for 96 h. The different letters indicate significant differences, and the bars represent the means ± SEs.
3.4. Expression of Immune-Related Genes
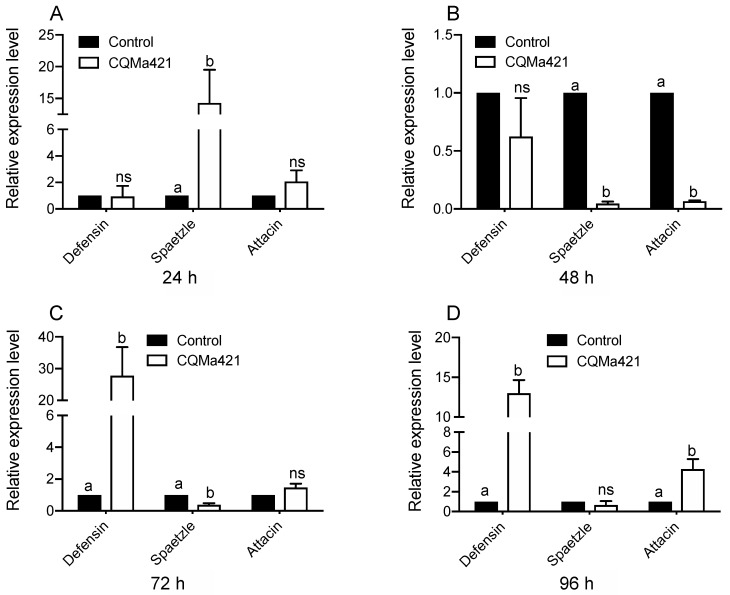
When L. migratoria was infected with the fungus M. anisopliae, the gene expression levels showed differences from 24 to 96 h. The expression of the gene spaetzle after exposure to M. anisopliae was greater than that under the control treatment at 24 h post infection (p < 0.001, t-test; Figure 4A), but the defensin and attacin expression did not differ from that under the control treatment. The expression levels of defensin, spaetzle and attacin after 48 h of M. anisopliae treatment were low (spaetzle: p < 0.001, t-test and attacin: p < 0.001, t-test; Figure 4B). However, after 72 h and 96 h of infection, defensin showed high expression levels, with a similar result observed in attacin at 96 h post infection (Figure 4C,D). In contrast, no significant difference was found in the attacin expression at 72 h and the spaetzli expression at 96 post infection (attacin: p = 0.451, t-test; spaetzle: 0.132, t-test; Figure 4C,D).
Figure 4.
Relative expression of genes after challenge with M. anisopliae CQMa421. (A) The relative expression of defensin, spaetzle and attacin 24-h post infection; (B) the relative expression of defensin, spaetzle, and attacin 48 h post infection; (C) the relative expression of defensin, spaetzle and attacin 72 h post infection; (D) the relative expression of defensin, spaetzle and attacin 96 h post infection. The different letters indicate significant differences, and the bars represent the means ± SEs.
4. Discussion
Locusta migratoria is one of the most persistent agricultural pests [40]. Among the methods used, the chemical pesticides are the most common way to suppress this pest. However, pest populations have become resistant to some insecticides due to their long-term application [41]. Moreover, effects on nontargets (i.e., natural enemies and pollinators) [42], and destructive environmental consequences, and threats to human health have resulted in a desire to decrease the use of such insecticides [43]. Thus, using alternative, environmentally friendly ways to control them and other insect pests is urgent. Fungal insecticides are promising alternatives for the control of insect pests given the lack of fungal-resistance and their environmental safety [44,45]. Several studies have investigated the use of entomopathogenic fungi for the controlling insect pests, including M. anisopliae [6,33,46].
Compared to chemical insecticides, entomopathogenic fungi are promising biological control agents for many insect pests and show efficient potential for insecticide-resistant pests with less environmental risk [15,47]. Our results found that M. anisopliae CQMa421 could effectively infected the adults and nymphs of the pest L. migratoria, suggesting the potential of this fungus for the pest control. Aspergillus oryzae (Eurotiales: Trichocomaceae) was also reported as an entomopathogenic fungus for the control of the locust L. migratoira [48]. The low LT50 of L. migratoria found under concentrations of 1 × 107 conidia/ml and 1 × 108 conidia/mL indicated high susceptibility of L. migratoria to M. anisopliae infection. Several other studies also reported the similar results, with an increased susceptibility under high conidial concentrations [49,50]. However, some of the infected locust individuals in our study were not dead after 10 days of treatment. The surviving locust individuals may be partially tolerant to the fungus M. anisopliae. In addition, the susceptibility of insect pests to entomopathogenic fungi may vary under different environmental conditions [51].
The hemocytes of insect hosts play important roles in mediating the production of cellular defenses and soluble effector molecules (i.e., encapsulation and phagocytosis) [52]. These organisms can produce different types of hemocytes when they face different invasions depending on the substances present [53,54]. Several studies have shown that hosts produce different numbers or types of hemocytes after infection with fungal strains of M. anisopliae and M. acridum [53,55]. Hosts also respond differently to different strengths of fungal infection [53]. The different responses of hosts to different external stimuli indicate that hosts have different response functions to such challenges [56]. Entomopathogenic fungi infect insect pests directly via the host cuticle [44], while the chemical thiamethoxam has different routes, including by physical contact, stomach action or systemic poison [57]. In addition, entomopathogenic fungi affect gut bacterial genera, which is one of the major factors leading to host death [58]. However, it is unknown whether the chemicals cause the death of the host due to changes in bacterial genera. In this study, we challenged L. migratoria with M. anisopliae to evaluate the immune responses of hosts. However, we noticed that the number of hemocytes in the M. anisopliae treatment was similar to that in the controls. In a previous study, the number of hemocytes also had no significant changes after 72 h of infection with the fungus M. acridum in L. migratoria, but a reduced number was observed after 96 h and 120 h [53].
The level of enzymatic activity in the host reflects the physiological activity of the host. Different stimuli may elicit responses from different enzymes and different levels of activity. Hosts may respond differently to the challenges with different substances, and even to the same enzymes [59]. Host protease and chitinase enzymes are usually initially expressed at high levels after exposure to entomopathogenic fungi [44]. During this process, the entomopathogenic fungi can penetrate the cuticle of the host via assistance from such related enzymes. However, the immune system of hosts is activated in response to fungal penetration, and the amount of phagocytes increases during this period [53]. In contrast, detoxifying enzymes become activated when hosts are challenged with chemical insecticides [6]. The immune responses of the cabbage looper Trichoplusiani host to baculovirus challenge suggest a dose- and time-dependent infection [60]. The integration of an insecticidal scorpion toxin (Bjα IT) gene into Metarhizium acridum can improve the fungal virulence to L. migratoria by growing quickly in the locust hemolymph, which may reduce the immune responses of the locust [61]. The results of this study showed that the enzymatic activities of ProPO and SOD differed after the insects were challenged with the fungus M. anisopliae for 24 h. The level of enzymatic activity tended to decrease in the fungal treatment, indicating that the ProPO of host L. migratoria might be inhibited during this period.
The expression levels of the genes defensin, spaetzle and attacin differed after challenge with M. anisopliae. Organismal immunity and antioxidants play important roles in defense against harmful chemicals, pathogenic microorganisms and parasites [55,62]. Studies of L. migratoria have identified 470 immune-related genes, 58 of which were differentially expressed in hemocytes and fat bodies after infection with the fungus Metarhizium acridum [63]. However, hosts may respond differently to different challenges. Our results showed that L. migratoria also presented different gene expression levels in response to challenge with fungus M. anisopliae, with high expression occurring from 24 h, 72 h and 48 h (for spaetzle). However, the expression level under the treatment varied with time, indicating that the host displayed different immune responses in terms of duration of exposure.
5. Conclusions
The fungus M. anisopliae could effectively infect L. migratoria and affect the immune responses of locust hosts. The study provides insights into the interactions of insect hosts and entomopathogenic fungi and suggests that the fungus M. anisopliae CQMa421 is a potential prospect for controlling this pest.
Acknowledgments
We thank Deyu Zeng, Chunfang Huang for assistance with rearing the insects used in this experiment.
Author Contributions
Conceptualization, J.X. and W.J.; methodology, W.J., Y.P., J.Y., Y.W., and G.L.; project administration, J.X. and Y.P.; investigation, W.J., J.Y., Y.W., and G.L.; writing-review and editing, J.X.; funding acquisition, J.X. and Y.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work received the grants from the National Key Research and Development Program of China (Project No. 2017YFD0201208), the National Natural Science Foundation of China (Project No. 31801798).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Shah P.A., Pell J.K. Entomopathogenic fungi as biological control agents. Appl. Microbiol. Biotechnol. 2003;61:413–423. doi: 10.1007/s00253-003-1240-8. [DOI] [PubMed] [Google Scholar]
- 2.Vega F.E., Goettel M.S., Blackwell M., Chandler D., Jackson M.A., Keller S., Koike M., Maniania N.K., Monzón A., Ownley B.H., et al. Fungal entomopathogens: New insights on their ecology. Fungal Ecol. 2009;2:149–159. doi: 10.1016/j.funeco.2009.05.001. [DOI] [Google Scholar]
- 3.De Faria M.R., Wraight S.P. Mycoinsecticides and Mycoacaricides: A comprehensive list with worldwide coverage and international classification of formulation types. Biol. Control. 2007;43:237–256. doi: 10.1016/j.biocontrol.2007.08.001. [DOI] [Google Scholar]
- 4.Mascarin G.M., Lopes R.B., Delalibera I., Fernandes E.K.K., Luz C., Faria M. Current status and perspectives of fungal entomopathogens used for microbial control of arthropod pests in Brazil. J. Invertebr. Pathol. 2019;165:46–53. doi: 10.1016/j.jip.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 5.Zimmermann G. Review on safety of the entomopathogenic fungus Metarhizium Anisopliae. Biocontrol Sci. Technol. 2007;17:879–920. doi: 10.1080/09583150701593963. [DOI] [Google Scholar]
- 6.Jia M., Cao G., Li Y., Tu X., Wang G., Nong X., Whitman D.W., Zhang Z. Biochemical basis of synergism between pathogenic fungus Metarhizium anisopliae and insecticide chlorantraniliprole in Locusta migratoria (Meyen) Sci. Rep. 2016;6:28424. doi: 10.1038/srep28424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zimmermann G. The entomopathogenic fungus Metarhizium anisopliae and its potential as a biocontrol agent. Pestic. Sci. 2010;37:375–379. doi: 10.1002/ps.2780370410. [DOI] [Google Scholar]
- 8.Tang J., Liu X., Ding Y., Jiang W., Xie J. Evaluation of Metarhizium anisopliae for rice planthopper control and its synergy with selected insecticides. Crop Prot. 2019;121:132–138. doi: 10.1016/j.cropro.2019.04.002. [DOI] [Google Scholar]
- 9.Lee M.R., Li D., Lee S.J., Kim J.C., Kim S., Park S.E., Baek S., Shin T.Y., Lee D.H., Kim J.S. Use of Metarhizum aniopliae s.l. to control soil-dwelling longhorned tick, Haemaphysalis longicornis. J. Invertebr. Pathol. 2019;166:107230. doi: 10.1016/j.jip.2019.107230. [DOI] [PubMed] [Google Scholar]
- 10.Larsen A.E., Gaines S.D., Deschênes O. Agricultural pesticide use and adverse birth outcomes in the San Joaquin Valley of California. Nat. Commun. 2017;8:302. doi: 10.1038/s41467-017-00349-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verger P.J.P., Boobis A.R. Reevaluate pesticides for food security and safety. Science. 2013;341:717–718. doi: 10.1126/science.1241572. [DOI] [PubMed] [Google Scholar]
- 12.Gerage J.M., Meira A.P.G., da Silva M.V. Food and nutrition security: Pesticide residues in food. Nutrire. 2017;42:3. doi: 10.1186/s41110-016-0028-4. [DOI] [Google Scholar]
- 13.Kohler H.R., Triebskorn R. Wildlife ecotoxicology of pesticides: Can we track effects to the population level and beyond? Science. 2013;341:759–765. doi: 10.1126/science.1237591. [DOI] [PubMed] [Google Scholar]
- 14.Whalon M.E., Mota-Sanchez D., Hollingworth R.M. Global Pesticide Resistance in Arthropods. CABI Publishing; Wallingford, UK: 2008. [Google Scholar]
- 15.Knols B.G., Bukhari T., Farenhorst M. Entomopathogenic fungi as the next-generation control agents against malaria mosquitoes. Future Microbiol. 2010;5:339–341. doi: 10.2217/fmb.10.11. [DOI] [PubMed] [Google Scholar]
- 16.Xu J., Xu X., Shakeel M., Li S., Wang S., Zhou X., Yu J., Xu X., Yu X., Jin F. The Entomopathogenic fungi Isaria fumosorosea plays a vital role in suppressing the immune system of Plutella xylostella: RNA-Seq and DGE analysis of immunity-related genes. Front. Microbiol. 2017;8:1421. doi: 10.3389/fmicb.2017.01421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Söderhäll K., Cerenius L. Role of the prophenoloxidase-activating system in invertebrate immunity. Curr. Opin. Immunol. 1998;10:23–28. doi: 10.1016/S0952-7915(98)80026-5. [DOI] [PubMed] [Google Scholar]
- 18.Arya B., Pier Adelchi R., Leifer C.A., Elena K., Alexander S., Oleg C., Shirakawa A.K., Farber J.M., Segal D.M., Oppenheim J.J. Toll-like receptor 4-dependent activation of dendritic cells by beta-defensin 2. Science. 2002;298:1025–1029. doi: 10.1126/science.1075565. [DOI] [PubMed] [Google Scholar]
- 19.Mullen L.M., Goldsworthy G.J. Immune responses of locusts to challenge with the pathogenic fungus Metarhizium or high doses of laminarin. J. Insect Physiol. 2006;52:389–398. doi: 10.1016/j.jinsphys.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 20.Wang C., Leger R.J.S. A collagenous protective coat enables Metarhizium anisopliae to evade insect immune responses. Proc. Natl. Acad. Sci. USA. 2006;103:6647–6652. doi: 10.1073/pnas.0601951103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ali S., Zhen H., Ren S. Production of cuticle degrading enzymes by Isaria fumosorosea and their evaluation as a biocontrol agent against diamondback moth. J. Pest Sci. 2010;83:361–370. doi: 10.1007/s10340-010-0305-6. [DOI] [Google Scholar]
- 22.Cerenius L., Thörnqvist P.O., Vey A., Johansson M.W., Söderhäll K. The effect of the fungal toxin destruxin E on isolated crayfish haemocytes. J. Insect Physiol. 1990;36:785–789. doi: 10.1016/0022-1910(90)90052-H. [DOI] [Google Scholar]
- 23.Gopalakrishnan S., Chen F.Y., Thilagam H., Qiao K., Xu W.F., Wang K.J. Modulation and interaction of immune-associated parameters with antioxidant in the immunocytes of Crab scylla paramamosain challenged with lipopolysaccharides. Evid. Based Complement. Altern. Med. 2011;2011:824962. doi: 10.1155/2011/824962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Müller P., Donnelly M.J., Ranson H. Transcription profiling of a recently colonised pyrethroid resistant Anopheles gambiae strain from Ghana. BMC Genom. 2007;8:36. doi: 10.1186/1471-2164-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsukamoto T., Ichimaru Y., Kanegae N., Watanabe K., Yamaura I., Katsura Y., Funatsu M. Identification and isolation of endogenous insect phenoloxidase inhibitors. Biochem. Biophys. Res. Commun. 1992;184:86–92. doi: 10.1016/0006-291X(92)91161-I. [DOI] [PubMed] [Google Scholar]
- 26.Cerenius L., Söderhäll K. The prophenoloxidase-activating system in invertebrates. Immunol. Rev. 2004;77:21–26. doi: 10.1111/j.0105-2896.2004.00116.x. [DOI] [PubMed] [Google Scholar]
- 27.Isabelle V.G., Bruno L., Frédéric B. Bacterial strategies to overcome insect defences. Nat. Rev. Microbiol. 2008;6:302–313. doi: 10.1038/nrmicro1870. [DOI] [PubMed] [Google Scholar]
- 28.Araújo J.P.M., Hughes D.P. Chapter one—Diversity of entomopathogenic fungi: Which groups conquered the insect body? Adv. Genet. 2016;94:1–39. doi: 10.1016/bs.adgen.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 29.Lu H.-L., Leger R.S. Insect immunity to entomopathogenic fungi. Adv. Genet. 2016;94:251–285. doi: 10.1016/bs.adgen.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 30.Kounatidis I., Ligoxygakis P. Drosophila as a model system to unravel the layers of innate immunity to infection. Open Biol. 2012;2:120075. doi: 10.1098/rsob.120075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cociancich S., Ghazi A., Hetru C., Hoffmann J.A., Letellier L. Insect defensin, an inducible antibacterial peptide, forms voltage-dependent channels in Micrococcus luteus. J. Biol. Chem. 1993;268:19239–19245. [PubMed] [Google Scholar]
- 32.Hultmark D., Engström A., Andersson K., Steiner H., Bennich H., Boman H.G. Insect immunity. Attacins, a family of antibacterial proteins from Hyalophora cecropia. EMBO J. 1983;2:571–576. doi: 10.1002/j.1460-2075.1983.tb01465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peng G., Wang Z., Yin Y., Zeng D., Xia Y. Field trials of Metarhizium anisopliae var. acridum (Ascomycota: Hypocreales) against oriental migratory locusts, Locusta migratoria manilensis (Meyen) in Northern China. Crop Prot. 2008;27:1244–1250. doi: 10.1016/j.cropro.2008.03.007. [DOI] [Google Scholar]
- 34.Yang M.L., Zhang J.Z., Zhu K.Y., Xuan T., Liu X.J., Guo Y.P., Ma E.B. Mechanisms of organophosphate resistance in a field population of oriental migratory locust, Locusta migratoria manilensis (Meyen) Arch. Insect Biochem. Physiol. 2010;71:3–15. doi: 10.1002/arch.20254. [DOI] [PubMed] [Google Scholar]
- 35.Yang H. Preliminary study on the resistance of Locusta migratoria manilensis to malathion. Plant Prot. Technol. Ext. 2002;8:11–16. [Google Scholar]
- 36.Delgado F.X., Britton J.H., Lobolima M.L. Field and laboratory evaluations of leading entomopathogenic fungi isolated from Locusta migratoria capito sauss in madagascar. Mem. Entomol. Soc. Can. 2012;129:323–328. doi: 10.4039/entm129171323-1. [DOI] [Google Scholar]
- 37.Lopez D.C., Sword G.A. The endophytic fungal entomopathogens Beauveria bassiana and Purpureocillium lilacinum enhance the growth of cultivated cotton (Gossypium hirsutum) and negatively affect survival of the cotton bollworm (Helicoverpa zea) Biol. Control. 2015;89:53–60. doi: 10.1016/j.biocontrol.2015.03.010. [DOI] [Google Scholar]
- 38.Gillespie J.P., Burnett C., Charnley A.K. The immune response of the desert locust Schistocerca gregaria during mycosis of the entomopathogenic fungus, Metarhizium anisopliae var acridum. J. Insect Physiol. 2000;46:429–437. doi: 10.1016/S0022-1910(99)00128-6. [DOI] [PubMed] [Google Scholar]
- 39.Pfaffl M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xie J., Li S., Zhang W., Xia Y. RNAi-knockdown of the Locusta migratoria nuclear export factor protein results in insect mortality and alterations in gut microbiome. Pest Manag. Sci. 2019;75:1383–1390. doi: 10.1002/ps.5258. [DOI] [PubMed] [Google Scholar]
- 41.Ahmad M. Potentiation between pyrethroid and organophosphate insecticides in resistant field populations of cotton bollworm Helicoverpa armigera (Lepidoptera: Noctuidae) in Pakistan. Pestic. Biochem. Physiol. 2008;91:24–31. doi: 10.1016/j.pestbp.2007.12.003. [DOI] [Google Scholar]
- 42.Whitehorn P.R., Goulson D. Neonicotinoid pesticide reduces bumble bee colony growth and queen production. Science. 2012;336:351. doi: 10.1126/science.1215025. [DOI] [PubMed] [Google Scholar]
- 43.Jin S.F., Feng M.G., Chen J.Q. Selection of global Metarhizium isolates for the control of the rice pest Nilaparvata lugens (Homoptera: Delphacidae) Pest Manag. Sci. 2008;64:1008–1014. doi: 10.1002/ps.1597. [DOI] [PubMed] [Google Scholar]
- 44.St Leger R., Screen S. Fungi as Biocontrol Agents: Progress, Problems and Potential. CABI Publishing; Wallingford, UK: 2001. [Google Scholar]
- 45.Jorgensen P.S., Aktipis A., Brown Z., Carriere Y., Downes S., Dunn R.R., Epstein G., Frisvold G.B., Hawthorne D., Grohn Y.T., et al. Antibiotic and pesticide susceptibility and the Anthropocene operating space. Nat. Sustain. 2018;1:632–641. [Google Scholar]
- 46.Wang C., Wang S. Insect pathogenic fungi: Genomics, molecular interactions, and genetic improvements. Annu. Rev. Entomol. 2017;62:73. doi: 10.1146/annurev-ento-031616-035509. [DOI] [PubMed] [Google Scholar]
- 47.Bahiense T.C., Fernandes E.K., Angelo Ida C., Perinotto W.M., Bittencourt V.R. Performance of Metarhizium anisopliae and Its combination with deltamethrin against a pyrethroid-resistant strain of Boophilus microplus in a stall test. Anim. Biodivers. Emerg. Dis. Predict. Prev. 2008;1149:242–245. doi: 10.1196/annals.1428.031. [DOI] [PubMed] [Google Scholar]
- 48.Zhang P.F., You Y.W., Song Y., Wang Y.Z., Zhang L. First record of Aspergillus oryzae (Eurotiales: Trichocomaceae) as an entomopathogenic fungus of the locust, Locusta migratoria (Orthoptera: Acrididae) Biocontrol Sci. Technol. 2015;25:1285–1298. doi: 10.1080/09583157.2015.1049977. [DOI] [Google Scholar]
- 49.Kirkland B.H., Cho E.M., Keyhani N.O. Differential susceptibility of Amblyomma maculatum and Amblyomma americanum (Acari: Ixodidea) to the entomopathogenic fungi Beauveria bassiana and Metarhizium anisopliae. Biol. Control. 2004;31:414–421. doi: 10.1016/j.biocontrol.2004.07.007. [DOI] [Google Scholar]
- 50.Shrestha G., Enkegaard A., Steenberg T. Laboratory and semi-field evaluation of Beauveria bassiana (Ascomycota: Hypocreales) against the lettuce aphid, Nasonovia ribisnigri (Hemiptera: Aphididae) Biol. Control. 2015;85:37–45. doi: 10.1016/j.biocontrol.2015.03.005. [DOI] [Google Scholar]
- 51.Wraight S.P., Ugine T.A., Ramos M.E., Sanderson J.P. Efficacy of spray applications of entomopathogenic fungi against western flower thrips infesting greenhouse impatiens under variable moisture conditions. Biol. Control. 2016;97:31–47. doi: 10.1016/j.biocontrol.2016.02.016. [DOI] [Google Scholar]
- 52.Strand M.R. The insect cellular immune response. Insect Sci. 2010;15:1–14. doi: 10.1111/j.1744-7917.2008.00183.x. [DOI] [Google Scholar]
- 53.Yu Y., Cao Y., Xia Y., Liu F. Wright-Giemsa staining to observe phagocytes in Locusta migratoria infected with Metarhizium acridum. J. Invertebr. Pathol. 2016;139:19–24. doi: 10.1016/j.jip.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 54.Duressa T.F., Vanlaer R., Huybrechts R. Locust cellular defense against infections: Sites of pathogen clearance and hemocyte proliferation. Dev. Comp. Immunol. 2015;48:244–253. doi: 10.1016/j.dci.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 55.Cao G., Jia M., Zhao X., Wang L., Tu X., Wang G., Nong X., Zhang Z. Different effects of Metarhizium anisopliae Strains IMI330189 and IBC200614 on enzymes activities and hemocytes of Locusta migratoria L. PLoS ONE. 2016;11:e0155257. doi: 10.1371/journal.pone.0155257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smith R.C., Carolina B.M., Marcelo J.L. Hemocyte differentiation mediates the mosquito late-phase immune response against Plasmodium in Anopheles gambiae. Proc. Natl. Acad. Sci. USA. 2015;112:3412–3420. doi: 10.1073/pnas.1420078112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Castle S.J., Byrne F.J., Bi J.L., Toscano N.C. Spatial and temporal distribution of imidacloprid and thiamethoxam in citrus and impact on Homalodisca coagulata populations. Pest Manag. Sci. 2010;61:75–84. doi: 10.1002/ps.949. [DOI] [PubMed] [Google Scholar]
- 58.Wei G., Lai Y., Wang G., Chen H., Li F., Wang S. Insect pathogenic fungus interacts with the gut microbiota to accelerate mosquito mortality. Proc. Natl. Acad. Sci. USA. 2017;114:5994–5999. doi: 10.1073/pnas.1703546114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li Z., Li M., He J., Zhao X., Chaimanee V., Huang W.F., Nie H., Zhao Y., Su S. Differential physiological effects of neonicotinoid insecticides on honey bees: A comparison between Apis mellifera and Apis cerana. Pestic. Biochem. Physiol. 2017;140:1–8. doi: 10.1016/j.pestbp.2017.06.010. [DOI] [PubMed] [Google Scholar]
- 60.Scholefield J.A., Shikano I., Lowenberger C.A., Cory J.S. The impact of baculovirus challenge on immunity: The effect of dose and time after infection. J. Invertebr. Pathol. 2019;167:107232. doi: 10.1016/j.jip.2019.107232. [DOI] [PubMed] [Google Scholar]
- 61.Peng G.X., Xia Y.X. Integration of an insecticidal scorpion toxin (Bj alpha IT) gene into Metarhizium acridum enhances fungal virulence towards Locusta migratoria manilensis. Pest Manag. Sci. 2015;71:58–64. doi: 10.1002/ps.3762. [DOI] [PubMed] [Google Scholar]
- 62.Dubovskiy I.M., Kryukov V.Y., Yaroslavtseva O.N., Levchenko M.V., Belgibaeva A.B., Adilkhankyzy A., Glupov V.V. The activity of nonspecific esterases and glutathione-S-transferase in Locusta migratoria larvae infected with the fungus Metarhizium anisopliae (Ascomycota, Hypocreales) Entomol. Rev. 2012;92:27–31. doi: 10.1134/S0013873812010022. [DOI] [Google Scholar]
- 63.Zhang W., Chen J., Keyhani N.O., Zhang Z., Li S., Xia Y. Comparative transcriptomic analysis of immune responses of the migratory locust, Locusta migratoria, to challenge by the fungal insect pathogen, Metarhizium acridum. BMC Genom. 2015;16:867. doi: 10.1186/s12864-015-2089-9. [DOI] [PMC free article] [PubMed] [Google Scholar]