Abstract
Gastrointestinal (GI) cancer is a prevailing global health disease with a high incidence rate which varies by region. It is a huge economic burden on health care providers. GI cancer affects different organs in the body such as the gastric organs, colon, esophagus, intestine, and pancreas. Internal and external factors like smoking, obesity, urbanization, genetic mutations, and prevalence of Helicobacter pylori and Hepatitis B and Hepatitis C viral infections could increase the risk of GI cancer. Phytochemicals are non-nutritive bioactive secondary compounds abundantly found in fruits, grains, and vegetables. Consumption of phytochemicals may protect against chronic diseases like cardiovascular disease, neurodegenerative disease, and cancer. Multiple studies have assessed the chemoprotective effect of selected phytochemicals in GI cancer, offering support to their potential towards reducing the pathogenesis of the disease. The aim of this review was to summarize the current knowledge addressing the anti-cancerous effects of selected dietary phytochemicals on GI cancer and their molecular activities on selected mechanisms, i.e., nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), detoxification enzymes, adenosine monophosphate activated protein kinase (AMPK), wingless-related integration site/β-catenin (wingless-related integration site (Wnt) β-catenin, cell apoptosis, phosphoinositide 3-kinases (PI3K)/ protein kinase B AKT/ mammalian target of rapamycin (mTOR), and mitogen-activated protein kinase (MAPK). In this review phytochemicals were classified into four main categories: (i) carotenoids, including lutein, lycopene, and β-carotene; (ii) proanthocyanidins, including quercetin and ellagic acid; (iii) organosulfur compounds, including allicin, allyl propyl disulphide, asparagusic acid, and sulforaphane; and (iv) other phytochemicals including pectin, curcumins, p-coumaric acid and ferulic acid. Overall, phytochemicals improve cancer prognosis through the downregulation of β-catenin phosphorylation, therefore enhancing apoptosis, and upregulation of the AMPK pathway, which supports cellular homeostasis. Nevertheless, more studies are needed to provide a better understanding of the mechanism of cancer treatment using phytochemicals and possible side effects associated with this approach.
Keywords: phytochemical, gastrointestinal cancer, intestinal cancer, apoptosis, anti-cancerous effects
1. Gastrointestinal Cancer and Phytochemicals
1.1. Gastrointestinal Cancer
Cancer is a leading cause of death worldwide, being responsible for approximately 7.9 million deaths (13% of all deaths) [1]. The rate of cancer-related death is expected to rise to an estimated 12 million deaths by 2030 [2]. Gastrointestinal cancer (GI) is the second most common cause of cancer-related death in the world [3]. Statistical results obtained in 2008 showed that GI cancer is the fourth most common cancer in men and the fifth most common cancer in women [4]. GI cancer is a malignant condition which affects the gastrointestinal tract and accessory organs such as the colon, esophagus, and intestine [5]. The carcinogenesis of GI cancer occurs due to the accumulation of genetic variation of multiple genes such as tumor suppressors, mismatch repair genes, and oncogenes [6]. Imbalance between cellular proliferation and apoptosis leads to the pathogenesis of GI cancer [7]. Internal and external factors such as genetic, obesity, alcohol consumption, and Helicobacter pylori infection contribute to the pathogenesis of GI cancer [8]. Although patients with GI cancer become symptomatic after they have advanced lesions with either local or distant metastasis, commonly presented findings include bloating, epigastric pain, and palpable epigastric mass [9]. Though the incident rate of GI cancer is declining, it remains a major health problem and a huge burden on health care providers [10]. The prognosis of GI cancer is variable between patients depending on its progression at the time of detection. Early detection of GI cancer improves the outcomes of patients. Treatments of the disease include surgery, radiation, and administration of chemotherapy components such as cisplatin, mitomycin, and docetaxel injection [11].
1.2. Colorectal Cancer
Colorectal cancer (CRC) is the fourth most common malignant tumor in the world, with an incidence of 1.2 million new cases and over 600,000 death cases [12]. CRC is the second most common cancer in women and the third most common cancer in men worldwide [10]. As CRC is a so-called westernized disease, the highest incidence rates are found in Australia, New Zealand, North America, and Europe [13]. Although advance treatments are available to improve the survival rate of the disease, CRC remains an incurable disease [14]. While the rate of CRC in adults aged 50 and above decreases, an increase in disease incidence is observed in adults younger than 50 [15]. This suggest that factors such as physical activity, gut microbiome composition, and diet may underline the development of the disease [16]. Like most cancers, CRC is driven by an accumulation of genetic mutations in tumor suppressors such as adenomatous polyposis coli (APC), Smad4 and p53, and oncogenes such as K-ras [17]. These mutagenic accumulations lead to a stepwise progression from normal intestinal epithelial cells to pre-malignant tumor development/adenoma to adenocarcinoma [18]. Etiologically, CRC may be sporadic (more than 80% of cases are sporadic), hereditary, or be related to a history of inflammatory bowel disease [19]. Signs of colon cancer include change in bowel dietary habits and blood in stools [20]. Although treatment of CRC depends on the time of diagnosis and the stage of the disease, common treatments used include surgery, radiation, immunotherapy, and chemotherapy [21].
1.3. Esophageal Cancer
Esophageal cancer is a serious malignancy which accounted for more than 400,000 deaths worldwide in 2005 [22]. Although the incidence rate of other types of cancer is expected to decrease by 2025, the prevalence of esophageal cancer is expected to increase by 140% [23]. The two predominant histological subtypes of esophageal cancer are adenocarcinoma and squamous cell carcinoma, with these having unreliable racial and geographical distribution [24]. Although squamous cell carcinoma remains the most common type of esophageal cancer globally, adenocarcinoma has become the leading type in Western countries due to the higher incidence of obesity and Barrett’s esophagus [25]. Treatment of esophageal cancer includes surgery, radiation, and chemotherapy [26].
1.4. Diet and Microbial Metabolites
The gastrointestinal tract in the human body has the highest population of different microbes, such as in the microbiome. They play a critical role in the well-being of the host [27]. It is estimated that the human gut contains between 30 trillion to 400 trillion micro-organisms [28]. The interaction between the microbiome with different parts of the human gut (mucus layer, epithelial cells, and immune cells) helps in determining the health or disease status of the host [29]. Changes in the gut microbiota due to environmental exposure, host genetics, and diet are known to affect human physiology, prevalence of disease, and nutrition [30]. The gut composition of people lacking Helicobacter pylori infection has identified 128 phylotypes within 8 bacterial phyla of which Proteobacteria, Firmicutes, Bacteroidetes, Fusobacteria, and Actinobacteria are the most abundant [31]. Epidemiological studies have indicated that a diet with high fiber and low red meat and fat content reduces the risk of CRC due to the presence of colonic microbiota [32]. They enhance the host’s health by promoting the metabolism of fiber to produce short chain fatty acids (SCFAs) such as butyrate which downregulate pro-inflammatory cytokines such as interleukin-6 (IL-6) and interleukin-12 (IL-12) [33].
1.5. Impact of Gastrointestinal Cancer on Selected Pathways
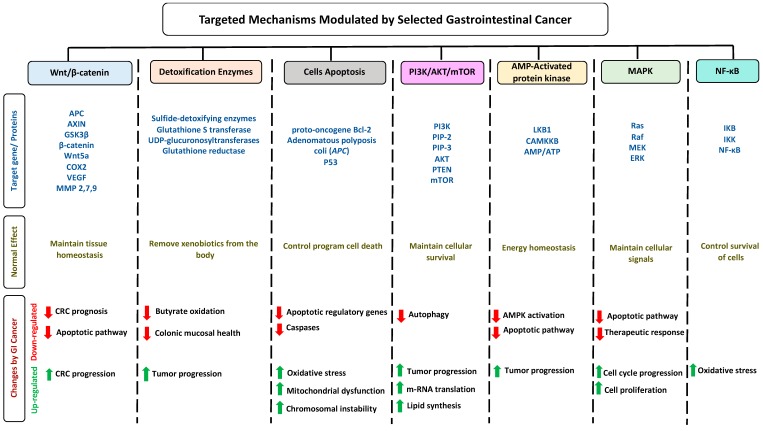
Cancer has become one of the leading causes of death due to the difficulty of treating the disease [34]. Complications of cancer can be divided into direct and indirect complications, such as invasion and immune suppression, respectively [35]. Cancer mutations affect several mechanisms and pathways in the body (Figure 1). The wingless-related integration site (Wnt) transduction signaling pathway is an important mediator to maintaining repair and tissue homeostasis [36]. In patients with GI cancer, the phosphorylation of β-catenin increases, reducing the apoptotic pathway in the body [37]. The expression of detoxification enzymes decreases during cancer, thus reducing butyrate expression [38]. GI cancer enhances the activation of apoptotic pathways by reducing the activity of apoptotic regulatory genes and caspases that upregulate oxidative stress, mitochondrial dysfunction, and chromosomal instability [39]. In addition, the phosphoinositide 3-kinases (PI3K)/AKT/mTOR intracellular pathway is activated during GI cancer; this pathway upregulates tumor progression, lipid synthesis, and m-RNA translation [40]. Adenosine monophosphate activated protein kinase (AMPK) plays a multiple beneficial role in gut health; it improves barrier function and intestinal absorption, suppresses colorectal carcinogenesis, and reduces intestinal inflammation [41]. The deactivation level of AMPK pathways reduces during GI cancer and as a result enhances tumor progression and reduces apoptotic pathway activation [42]. Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and mitogen-activated protein kinase (MAPK) pathways reduce during GI cancer, potentially leading to enhanced cell cycle progression, oxidative stress, and cellular proliferation [43,44]. High levels of NF-κB activity in early gastric carcinoma may enhance the prognosis of the disease [45]. Ninety-two genes have been tested to evaluate the relationship between NF-κB and CRC. The results have shown that out of 92 genes, 22 genes are significantly downregulated while nine genes are significantly upregulated, suggesting a strong correlation between NF-κB and colorectal cancer [46]. It has been shown that the inhibition of the NF-κB pathway leads to the reduction in vascular endothelial growth factor production, which leads to reducing angiogenesis [47].
Figure 1.
Schematic illustration of seven selected mechanisms modulated by GI cancer. The figure is divided into seven columns and three rows. The column headings represent the pathways while the row headings represent target genes/proteins for each pathway (blue), the overview physiological effect of these genes on pathways (dark yellow), and changes occurring on these pathways modulated by GI cancer.
1.6. Phytochemicals
Diet is considered a well-established risk determinant in the development of GI cancer [48]. Following a rich phytochemical diet abundantly found in fruits, vegetables, nuts, and whole grains possesses several health protective benefits [49]. Phytochemicals in food science refer to a variety of plant ingredients which have different structures capable of promoting health [50]. Phytochemicals, known as secondary metabolites, are non-nutritive bioactive chemical compounds produced by plants [51]. They are called non-nutritive since they are synthesized by plants only in specific cells and not by the energy metabolism nor by the catabolic or anabolic metabolisms [52]. So far, 10,000 phytochemicals have been identified, including pre- and pro-biotics, polyphenols, carotenoids, steroids, and thiosulfate, while many remain unknown [53]. Phytochemicals are important for plant metabolism as they repel pests and sunlight and regulate plants growth [54]. Recently, phytochemicals have emerged as modulators of critical cellular signaling pathways and health improvement [55].
Phytochemicals have multiple health benefits for metabolic disorders such as cancer, cardiovascular disease, neurodegenerative diseases, and obesity [56]. Plants with a higher concentration of phytochemicals play a role in protection against free radical damage [57]. Research and clinical studies have postulated the anti-carcinogenic effects of phytochemicals, including their inhibiting of mitosis, inducing apoptosis, and enhancing the excretion of carcinogens [58]. In addition, phytochemicals possess both antioxidant and anti-inflammatory activities, where they interfere with several proinflammatory mediators [59].
1.7. Metabolism of Phytochemicals
Phytochemicals show variations in metabolism and deposition due to the variability in absorption, distribution, and excretion of phytochemical pharmacokinetics [60]. Examples of variational sources of phytochemicals include phase 1 metabolism in the liver where hydroxylation of phytochemicals can occur, resulting in novel secondary oxidation products [61]. Another example is metabolism by gut bacteria where phytochemicals undergo reduction, dehydroxylation, and demethylation, resulting in more biologically active metabolites [62]. In general, most phytochemicals present as glycosides or other conjugates in plant food, which means hydrolyzation is an essential process for absorption [63]. The hydrolysis could be achieved either by gut bacterial β-glucosidases in the colon or lower small intestine or by brush border membrane bound β-glucosidases [64]. After absorption, aglycones undergo extensive metabolism in the liver or gut epithelium with multiple compounds being conjugated by sulfotransferases (SULT) and UDP-glucuronosyltransferases (UGT) with glucuronic acid, glutathione, or sulfate, being potentially excreted in the bile or urine [65]. Compounds that are re-excreted through bile duct are deconjugated through bacterial β-glucuronidase, where they undergo enterohepatic recycling [64].
1.8. Search Strategy and Selection Criteria
Medline, Scopus, and PubMed were searched for papers published from 2000 using the search terms “phytochemical”, “phytochemicals AND cancer”, “phytochemicals AND colon cancer”, “phytochemicals AND GI cancer”, “phytochemicals subclasses AND cancer”, “carotenoid AND GI cancer”, “polyphenol and cancer”, ”prebiotics AND probiotics AND GI cancer”, and “phytochemical AND metabolism”. The search yielded approximately 6000 articles, and for this review, 237 articles were selected and analyzed.
2. Anti-Cancerous Effects of Selected Phytochemicals
2.1. Carotenoids
Carotenoids are pigments found in plants, bacteria, algae, and fungi [66]. The family of carotenoids (tetraterpenes) contains 500 compounds, 50 of which exhibit provitamin A activity [67]. While only 40 carotenoids have been identified in the human diet, human blood and tissue contain 20 carotenoids [68]. Carotenoids are well recognized for their antioxidant activities, regulation of cellular growth, immune response, and modulation of gene expression [69]. Pre-dominant carotenoids include lutein, lycopene, and β-carotene, which are abundantly found in egg yolk, tomato, and carrot [70].
2.1.1. Lutein
Lutein in an abundant fat-soluble xanthophyll with a singular molecular formula (C40H56O2) [71]. It is found abundantly in egg yolk, oranges, yellow fruits, and green leafy vegetables [72]. Lutein is one of the two carotenoids that accumulates in fovea in the human retina [73]. It is a major constitute of macular pigment which is responsible for fine feature vision [74]. Recently, lutein has gained public health attention due to its putative role in protection against degenerative eye conditions and cancer [75]. A study performed on a Korean population showed an association between dietary lutein and the risk of colorectal cancer [76]. Lutein has considerable antioxidant function, which regulates apoptosis [77]. Administration of lutein in animal models has been observed to decrease the concentration of K-ras and AKT in tumors, resulting in cell cycle arrest [78]. Mice treated with lutein have been found to significantly inhibit aberrant crypt foci (ACF) development in the colon, reducing cellular proliferation [79]. Additionally, administration of lutein has been observed to reduce β-catenin concentration, hyperplasia, and adenocarcinoma in colonic samples [80]. It also acts as an effective blocking agent by reducing the concentration of specific protein-like β-catenin involved in cellular proliferation and apoptosis (Figure 2) [81]. Moreover, lutein plays a role in reducing reactive oxygen species and oxygen radicals while enhancing DNA damage repair (Table 1) [82].
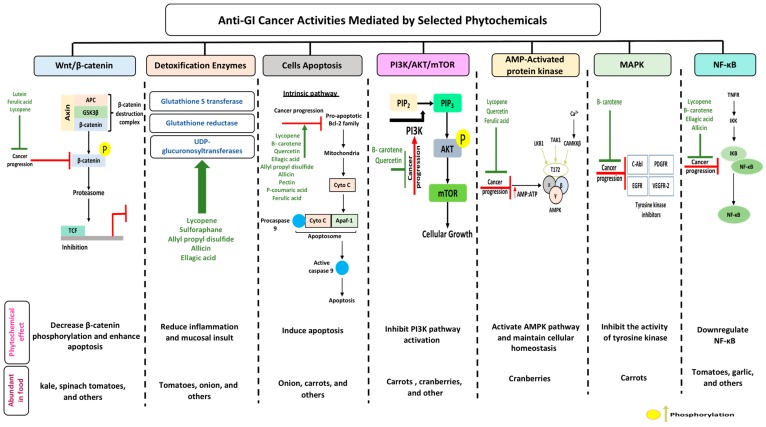
Figure 2.
Phytochemicals as anti-GI cancer agents: mode(s) of action, aberrant signaling pathways (Wnt/β-catenin, detoxification enzymes, cellular apoptosis, PI3K/AKT/mTOR, AMPK, MAPK, and NF-κB), and pathway components targeted by phytochemicals (highlighted in green). Phytochemicals have a wide range of anti-cancerous actions through which one could target multiple mechanisms. These phytochemicals can enhance or suppress (green and red lines, respectively) the mechanisms through several activities. (see text for detailed mode(s) of action for phytochemicals mentioned).
Table 1.
Representive Phytochemicals and Their Underlying Anti-Cancerous Effects.
| Phytochemical Subclass | Phytochemical and Structure | Dietary Source | Conversion Reaction | Metabolites Produced | Mechanism of Action | Model Used | References | |
|---|---|---|---|---|---|---|---|---|
| In Vivo | In Vitro | |||||||
| Carotenoids | Lutein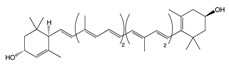
|
Egg yolk, kale, spinach, parsley, and peas | Oxidation | 3′-dehydro-lutein |
|
|
|
[74,78] |
Lycopene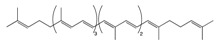
|
Tomato, guava, papaya, grapefruit, and watermelon | Auto-oxidation Radical-mediated oxidation | Apo-10′-lycopenoids |
|
|
|
[86,89] | |
β-Carotene
|
Carrot | Oxidation | Falcarindiol 6-methoxymellein |
|
|
|
[97,98,99] | |
| Pro-anthocy-anidins | Quercetin
|
Cranberry | Sulfation Conjugation | 3-(4hydroxyphenyl) -propionic acid hippuric acid catechol-O-sulfate |
|
|
|
[108,109] |
Ellagic Acid
|
Bilberry | Glucuronidation O-methylation |
Peonidin-3-galactoside |
|
|
|
[120,121] | |
| Organosulfur Compounds | Allicin
|
Garlic | Oxidation Hydrolysis | Allyl methyl sulfide (AMS) Allyl methyl sulfoxide (AMSO) Allyl methyl sulfone (AMSO2) |
|
|
|
[135,136] |
Allyl propyl disulfide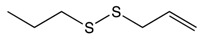
|
Onion | Reduction | Quercetin 3,4‘-diglucoside Quercetin 4‘-glucoside |
|
|
[146,147] | ||
Asparagusic acid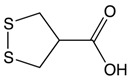
|
Asparagus | Sulfation | Asparagus polysaccharide dimethyl sulfide |
|
|
[152,153] | ||
Sulforaphane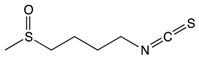
|
Broccoli, cabbage, Brussels sprout, and cauliflower | Hydrolysis | Thiocyanates Isothiocyanates Epithionitrile nitrile |
|
|
[164,165] | ||
| Other Phytochemicals | Pectin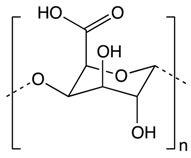
|
Apples, plums, oranges, and gooseberries | Colonic fermentation | Butyrate |
|
|
|
[169,170] |
Curcumin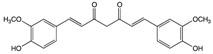
|
Ginger | Hydrolysis | Curcumin glucuronide Curcumin sulfate |
|
|
[183,184] | ||
p-Couramic acid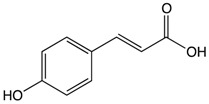
|
Navy beans | Hydrolysis | N-methylpipecolate 2-aminoadipate Piperidine Vanillate |
|
|
[189,190] | ||
Ferulic acid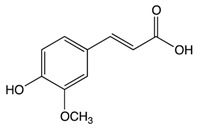
|
Rice bran | Colonic fermentation | Tryptophan α-ketoglutarate γ-tocopherol/β-tocopherol γ-tocotrienol |
|
|
|
[201,202] | |
2.1.2. Lycopene
Lycopene is a lipophilic pigment and the main component of-red colored fruits and vegetables such as tomatoes [83]. Lycopene is structurally similar to β-carotene with the molecular formula C50H56, a hydrocarbon chain, and no functional groups [84]. The concentration of lycopene in tomatoes ranges 0.9 to 9.27 mg/g [85]. Lycopene is a potent antioxidant which can counteract reactive oxygen species like peroxyl radicals [86]. The expression of lycopene’s antioxidant activity is due to (i) the detoxification process through the production of enzymes like glutathione peroxidase (GPx), glutathione-S-transferase (GST), and glutathione reductase (GR); (ii) the inhibition of cytochrome P450 2E1, which is critical for the conversion of xenobiotics in cancer; and (iii) the suppression of carcinogen progression (Figure 2) [87]. In addition, lycopene exerts both anti-inflammatory and anti-cancer activity specifically against colorectal cancer [88]. Administration of lycopene using gold nanoparticles as a vehicle has been found to reduce the expression of pro-caspase 3, 8, and 9 and enhance Bcl-2-associated X protein (BAX) expression, thus enhancing the apoptotic pathway [89]. A one-day cultured colon cancer cell with 10 μm of lycopene showed a reduction in cellular growth by reducing the expression of Hmg Co-A reductase and enhancement in Ras translocation from the plasma membrane to cytosol [90]. Lycopene is reported to inhibit the expression of NF-κB and c-Jun N-terminal kinases (JNK), which (i) leads to a decreased tumor necrosis factor α (TNF-α), interleukin-1 (IL-1), and IL-6 and (ii) inhibits the expression of cyclooxygenase 2 (COX-2) and NO production (Table 1) [91]. In a gastric-induced carcinogens model, lycopene has been found to block the activity of carcinogenic cells through the upregulation of a reduced glutathione (GSH) dependent hepatic detoxification system, thus protecting cells from oxidative damage [92].
2.1.3. β-Carotene
β-carotene, a core member of the carotenoid family, has been well documented for its natural antioxidant, anti-inflammatory, and anti-cancerous activity in recent years [93]. Carrot (Daucus carota L) is a popular root vegetable and an important dietary source of carotenoids [94]. The chemical composition of carrots includes moisture (86%), protein (0.9%), fat (0.2%), carbohydrate (10.6%), fiber (1.2%), total ash (1.1%), Ca (80 mg/100 g), p (53 mg/100 g), and Fe (2.2 mg/100 g) [95]. Carrots have been reported to inhibit the formation of neoplastic tumors in colonic cancer rat models, affecting the composition of low abundant gut microbiota like Lactobacillus reuteri [96]. Human cancer cells treated with 50 and 100 μg/mL or pentane fraction and 1:1 pentane: diethyl ether fraction have shown an inhibition in cellular proliferation due to induced sub G1 phase accumulation and enhanced apoptotic cell death [97]. Additionally, cancerous cells treated with carrot oil extract have reported an increase in BAX and P53 levels and a decrease in Bcl-2 levels (Table 1) [98]. Studies have shown that carrots inhibit cellular proliferation and induce apoptosis and cellular arrest through the suppression of the MAPK/ERK and PI3L/AKT pathways (Figure 2) [99]. A study has reported that consumption of carrots is more effective in the prevention of gastric cancer in people at risk of the disease (those with a family history of gastric cancer) compared to other people. This suggests that following a healthy lifestyle could prevent the development of gastric cancer in people with higher risk [100].
2.2. Proanthocyanidins
Proanthocyanidins, also known as condensed tannins, result from flavanol condensation [101]. They are abundantly found as polymers and oligomers in fruits, barriers, seeds, leaves, and flowers [102]. Recent interest in proanthocyanidins has been stimulated due to their potential health benefits which arise mainly from their antioxidant activity [103]. The effectiveness of proanthocyanidins are determined by gut microbiome composition [104]. Additionally, they have anticancer properties via the reduction of tumor development by inducing apoptosis or inhibiting cellular proliferation [105].
2.2.1. Quercetin
The cranberry (Vaccinium macrocarpon) is a fruit which has been used as a functional food due to its health benefits [106]. It is a rich source of polyphenols, which exerts anti-inflammatory, antiviral, antibacterial, antioxidant, anticarcinogenic, and antimutagenic activities [107]. It has a complex and rich phytochemical composition, consisting predominantly of A-type procyanidins (PACs), flavan-3-ols, anthocyanins, ursolic acid, quercetin, and benzoic acid [108]. Recently, the cranberry has received attention as a result of its effects related to lowering the risk of cancer [109]. Animal studies have reported the chemoprotective effect of cranberry to suppress the growth of several types of cancer cells, including colon, lung, prostate, oral, and ovarian [110]. Administration of 20% cranberry juice in water to rat models demonstrated a reduction in the total number of ACF [111]. Cranberry extracts have been reported to reduce proinflammatory interleukins and C-reactive protein [112]. APCmin/+ mice fed with 20% (w/w) freeze dried whole cranberry powder for 12 weeks showed a significant prevention of intestinal tumor formation (33.1%) due to induced cellular apoptosis and reduced cellular proliferation [113]. Also, it is reported that cranberry consumption inhibits the activation of the PI3K, AKT, and COX-2 signaling pathway (Table 1) [114]. Administration of cranberries has shown an activation in the AMPK pathway which helped maintain cellular homeostasis [115].
2.2.2. Ellagic Acid
The bilberry (Vaccinium myrtillus L) is a rich natural source of anthocyanins [116]. Total anthocyanin content in the bilberry ranges from 300–700 mg/100 g [117]. It is classified by the American Herbal Products Association as a class 1 herb, which means it can be safely consumed when used appropriately [118]. Ellagic acid is a phenolic compound found in bilberry extracts which has potent antioxidant properties and can chelate metal ions and scavenge free radicals [119]. Treatment of rats’ hepatocyte primary culture with bilberries has shown a protective effect against oxidative damage [120]. Bilberries have been reported to induce phase II xenobiotic detoxification enzymes, which are critical for cancer prevention [121]. Additionally, bilberry-rich extracts have been observed to inhibit the growth of colon cancer cells but to not affect normal colon cells, thus suggesting a possible protective effect against cancer [122]. Rats with genetic colon adenoma fed concentrated bilberry extract (10% w/w) have shown a significant reduction in intestinal adenoma by 15–30% [123]. In a pilot study on 25 patients with colorectal cancer who were given bilberry extract for 7 days, the results showed a significant reduction in tumor cellular proliferation by 7% compared to the results before bilberry administration [124]. Treatment of human monocytic THP-1 cells with bilberry extract showed reduction in pro-inflammatory gene expression, interferon γ (IFN-γ), and cytokine secretion [125]. Moreover, bilberry extract exerts the ability to induce apoptosis and arrest growth in GI cancer (Figure 2) [126]. Bilberry extract has been reported to diminish topoisomerase catalytic activity in colon carcinoma cells, showing a protective DNA effect [127].
2.3. Organosulfur Compounds
Organosulfur compounds are sulfur-containing organic compounds with beneficial anti-inflammatory, antioxidant, and anti-cancerous effects [128]. Animal and epidemiological studies have shown that administration of organosulfur compounds reduces the risk of colorectal cancer through the induction of mitotic arrest and apoptosis [129,130]. Garlic, onion, asparagus, and cruciferous vegetables are abundant in organosulfur compounds.
2.3.1. Allicin
Attention has been given recently to garlic due to its high content of flavonoids and organosulfur compounds like allicin [131]. Worldwide garlic (Allium sativum) has been frequently used as a dietary botanical supplement [132]. Ally sulfur compounds like allicin found in garlic (1% of garlic’s dye weight) seems to be responsible for the beneficial effects of garlic [133]. Animal studies have shown that administration of garlic reduces the formation of ACF [134]. The mechanisms by which garlic inhibits the growth of carcinogen cells include reduction of DNA adducts, regulation of cellular arrest, activation of metabolizing detoxification enzymes, and induction of differentiation and apoptosis [135,136]. Organosulfur compounds present in garlic have shown potential for an anti-cancer drug by the modulation of epithelial growth factor receptor (EGFR), which plays a role in cell division [137]. Results obtained from an induced colitis mouse model have shown that administration of diallyl disulfide extracted from garlic is able to prevent the development of colitis-induced colorectal cancer [138]. In addition, garlic has been observed to prevent prolonged inflammation in mice, which supports the chemoprotective effect of garlic in CRC [139]. Moreover, consumption of garlic suppresses the activity of NF-κB by inhibiting phosphorylated P65 translocation (Figure 2) [140]. In xenograft nude mice, administration of S-allylmercaptocysteine (SAMC) in combination with rapamycin (a macrolide compound) was found to enhance anticancer ability by suppressing tumor growth and inducing apoptosis (Table 1) [141]. Administration of aged garlic extract in rat tumor models has been shown to attenuate colon tumor progression effectively by reducing cellular proliferation through the attenuation of NF-κB activity [142]. A meta-analysis study has indicated that the consumption of garlic is associated with reduced gastric cancer with a 95% confidence interval and a 0.53 odd ratio [143].
2.3.2. Allyl Propyl Disulfide
Chemical groups found in onions such as flavonoids, alk(en)yl cysteine sulfoxides (ACSOs), and allyl propyl disulfide are associated with the health benefits of onions [144]. The consumption rate of onion (Allium cepa L.) has increased worldwide, leading to an increase in the national production of onion by 25% over the last decade [145]. Compounds from onions have been reported to have multiple health benefits, including having antiplatelet, anticarcinogenic, and antithrombogenic activities [146]. Onion extracts have been reported to significantly induce apoptosis and reduce cellular proliferation in colorectal cancer [147]. An in vivo study has indicated that administration of onion in a hyperlipidemic colorectal cancer model plays a similar role to capecitabine in a colorectal cancer model without hyperlipidemia by inhibiting CRC and reducing hyperlipidemia [148]. Human cancer adenocarcinoma cells treated with 200 μm Se-methyl-L-selenocysteine (MSeC) for 24 h have been found to trigger 80% apoptosis in cells through endoplasmic reticulum stress rather than reactive oxygen species stress (Table 1) [149]. The benefit of onions is not limited to reducing or treating GI cancer but also to detecting cancer. One study used carbon nano onion films to develop a capacitive immunosensor for a CA19-9 cancer biomarker detector which succeeded in detecting CA19-9 in whole lysate colorectal adenocarcinoma using the sensor combined with information visualization methods [150].
2.3.3. Asparagusic Acid
Asparagus species are native medical shrubs which have beneficial medical properties and which belong to the Liliaceae family [151]. Major bioactive compounds found in asparagus include steroidal saponins, asparagusic acid, vitamins (A, B1, B2, C, E, Mg, P, Ca, and Fe), folic acid, asparagine, tyrosine, arginine, essential oils, tannin, resin, and flavonoids. The health properties of asparagus include anti-microbial, antioxidant, and cytotoxic activities [152]. Asparagus extracts have illustrated a potent cytotoxic effect against colorectal cancer [153]. Treatment of Myeloid-derived suppressor cells (MDSCs) with asparagus polysaccharide have shown a significant increase in apoptosis through intrinsic pathways and a significant decrease in cellular proliferation [154]. Old stems of asparagus (SSA) tested on colon cancer cells have been found to suppress cellular viability and block cellular migration and invasion through Rho GTPase signaling pathway modulation [155]. In human colon adenocarcinoma, methanolic extracts from white asparagus have demonstrated TRAIL death receptor pathway activation leading to the activation of caspase-8 and caspase-3, and, finally, to cell death. In addition, asparagus extracts have been seen to inhibit cellular pro-inflammatory mediators like MMP7, MMP9, and TNF-α [156].
2.3.4. Sulforaphane
Cruciferous vegetables refer to those which belong to the Brassicaceae family and include cabbage, broccoli, and Brussel sprouts [157]. This family is known for the glucosinolate, a sulfur-containing compound synthesized endogenously in plants derived from amino acid and glucose residues [158]. Upon cellular rupture through vegetable consumption, glucosinolates are hydrolyzed by endogenous enzymes and produce potential compounds such as thiocyanates and nitriles [159]. Cruciferous vegetables contain several phytochemical compounds such as sulforaphane. Studies have shown the beneficial effects of cruciferous vegetables which have helped inhibit the development of GI cancer [160]. In vivo and in vitro studies have demonstrated the ability of cruciferous vegetables to defend healthy cells against radiation and chemically-induced carcinogenesis [161]. Additionally, these vegetables have been shown to inhibit cellular proliferation, migration, and survival of tumor cells [162]. Cruciferous vegetables demonstrate antioxidant activity as they widely show a protective effect against oxidative stress through the depletion of glutathione [163]. Additionally, these vegetables induce acute oxidative stress through the inhibition of P38 MAPK, which inhibits Nrf2-Keap 1 dissociation (Table 1) [164]. Cruciferous vegetables guard against colorectal cancer through several mechanisms: (i) the modulation of detoxification enzymes (Figure 2), (ii) the induction of cellular apoptosis, and (iii) the controlling of cancer cellular growth through cell cycle arrest [165,166,167]. A meta-analysis study has shown that cruciferous vegetables significantly reduce the risk of gastric and colorectal cancer by 19% and 8%, respectively [168].
2.4. Other Phytochemicals
The following four phytochemicals did not fit under any of the above categories, and, therefore, due to their beneficial anticancer effects, we decided to give them a section of their own.
2.4.1. Pectin
Pectin is a natural polysaccharide derived from the cell walls of plants like citrus fruits and apples consisting of a linear chain of and α-(1–4) linked D-galacturonic acid residues, with some of the galacturonic acid carboxyl group being methyl esterified [169]. Depending on the percentage of esterification, high methoxy pectin (more than 50%) or low methoxy pectin (less than 50%) can be formed [170]. Pectin has been proven to have multiple biological effects like the reduction of cholesterol, lipid, insulin, and sugar level in the body. In addition, pectin exerts anticancer activities [171]. Pectin has been used in the colon drug delivery carrier system as well as being conjugated with other drugs like cisplatin, thus enhancing the blood circulation of the drug in mice models [172,173]. Using pectin as a new anticancer oral drug delivery system will enhance antitumor efficacy and reduce the risk of systemic toxicity in colon cancer [174].
Gastric cancer cells treated with low molecular weight citrus pectin (LCP) have been found to suppress adhesion, aggregation, and metastasis of cancer cells [175]. Moreover, LCP decreases the activity of premetastatic protein GAL-3 resulting in the promotion of caspase-mediated apoptosis and inhibition of tumor cell growth [176]. Plant-based non-digestible carbohydrates like pectin show potential in reducing the risk of colorectal cancer through the inhibition of GAL-3 protein expression, thus enhancing apoptosis and inhibiting cancer cells migration [177]. Pectin extracted from potatoes has demonstrated a significant reduction in cellular proliferation of colon cancer cells through the suppression of intercellular adhesion molecule 1 (ICAM 1) expression [178,179].
2.4.2. Curcumin
Curcumin is an active phytochemical compound originating from the rhizomatous herbaceous perennial plant of the ginger family (Curcuma longa) [180,181]. Recently, curcumin has given more attention due to its multiple health benefits, especially with regard to the management of degenerative and inflammatory eye conditions [182]. Greater attention has been given to cancer and curcumin in recent years (21,098 articles have been published in PubMed in the last 10 years). Due to this huge number of articles, we decided to add the most important points in Table 1. In addition to this, we cite four articles we evaluated which discuss the correlation between GI cancer and curcumin and which have been published in the last two years [183,184,185,186].
2.4.3. p-Coumaric Acid
p-Coumaric acid is an aromatic phenolic compound found in navy beans (Phaseolus vulgaris), which are an economically and nutritionally important food crop worldwide [187]. Navy beans are rich in protein, carbohydrates, minerals, dietary fibers, vitamins, and many other polyphenolics structurally similar to p-coumaric acid [188]. They exhibit several beneficial health effects like anticancer and anti-microbial infection properties and anti-human immunodeficiency virus effects [189]. Rats fed with navy beans have shown a significant reduction in colon adenocarcinoma compared to controls. This significant reduction can be attributed to a (i) high level of butyrate production in the distal colon and (ii) a more controlled appetite, which reduces body fat significantly [190]. Dietary intervention in CRC survivors for four weeks has shown that the consumption of navy bean reduces the risk of CRC and other chronic conditions. In addition to this, results have measured how serum inflammatory markers can provide the chemoprotective effects of navy beans against CRC [191]. Navy beans and their metabolites (Table 1) have been implicated in the protection of CRC through the modulation of major metabolic pathways like lysine, sterol, amino and inositol, and fatty acid metabolism. Dietary intake of navy beans (35 g/day) has been found to increase the abundance of several amino acids in stools, showing a protective effect against CRC through the inhibition of cellular progression, reduction of oxidative stress, and induction of cellular apoptosis [192,193]. A study performed on 16 people (seven non-cancer subjects and nine CRC survivors) has shown that the addition of 35 g cooked navy bean powder in meals for 28 days is able to provide chemoprevention effects against CRC [194].
2.4.4. Ferulic Acid
Ferulic acid is a bioactive component found in rice bran [195]. Rice bran is one of the byproducts produced by the milling process of rice [196]. Great attention has been paid to rice bran recently due to its high nutritional value, potential to improve heath, low cost, and availability [197]. Bioactive and phytochemical compounds of rice bran, such as ferulic acid, have been reported to possess anti-inflammatory, anti-diabetic, anticancer, and antioxidant activities [198]. The oil present in rice bran contains specific bioactive compounds that are considered to have a cardiac friendly chemical profile [199]. Studies have shown that rice bran intake modifies intestinal microbiota, which helps to prevent CRC [200]. Additionally, rice bran exhibits antitumor activities through the modulation of multiple pathways like inhibition of oxidative stress and induction of apoptosis [201]. In vitro cancer studies have shown that rice bran and its bioactive compounds (Table 1) have been shown to inhibit carcinogenesis by suppressing chronic inflammation, blocking cell signaling, inhibiting proliferation, scavenging free radicals, and activating detoxification enzymes (Figure 2) [202,203,204]. The consumption of rice bran has been found to reduced the adenoma burden in APCmin mice through the modulation of adiponectin which was observed to be significantly altered, thus playing a role in tumor suppression [205]. Administration of inositol hexaphosphate extracted from rice bran has been reported to significantly suppress β-catenin and COX-2 expression, which could inhibit the development of CRC [206,207]. In addition, human colorectal adenocarcinoma HT-29 cells treated with inositol hexaphosphate have been shown to induce apoptosis through the upregulation of BAX, caspase-3, and caspase-8 expression, and through the downregulation of Bcl-xl [208].
3. Conclusions
Phytochemicals are biologically active compounds found in plants. They have illustrated anticancer activity against gastrointestinal cancer through the modulation of several mechanisms like inducing apoptosis, inhibition of oxidative stress and cellular progression, and blocking cellular signaling.
3.1. Challenges with Studying Phytochemicals
Although a lot of efforts have been spent to study the activities of phytochemicals as an anticancer agent, lots of limitations are linked to these studies. Firstly, the assessment of most of the studies performed has depended on an in vitro evaluation [209]. Most of the in vitro studies have been based on ethnopharmacological information where the selection of plants or phytochemicals occurs first, and then molecular-based approaches are used to emphasize the similarity between phytochemicals and approved drugs by comparing molecular structure and protein target cites and linking them to the potential health benefits [210,211]. These approaches are designed to study the anticancer effects of phytochemicals on specific phenotypes, which makes it difficult to analyze and generalize the health effects on the human body [212]. Secondly, lots of attention has been given to the positive effects of selective phytochemicals without any mention of the negative effects of other phytochemicals which could be potential carcinogens or tumor promoters [213].
3.1.1. Estimated Consumption Level of Phytochemicals
Phytochemicals are non-established nutrients with significant health promotion and protective effects [214]. When it comes to specifying the recommended phytochemical intake, several points need to be recognized: (i) the health benefits associated with phytochemicals cannot be attributed to a specific phytochemical compound, (ii) different biological activities make it hard to select a specific key biological role for phytochemicals and generalize this to the whole population, and (iii) there is no assigned disease or impaired function linked to one or any of the members of the phytochemical group [215]. Worldwide, efforts have been made to create an optimal consumption level for selected phytochemicals. A US study on twenty-six healthy participants showed that consumption of 13–22 g dietary fiber per day for 3 weeks reduces cholesterol levels by 7% [216]. The consumption level of phytochemicals varies between populations. For example, Italians consume 14.3 mg/day of total carotenoids while Americans consume 6.6–10.5 mg/day for men and 5.7–10.4 mg/day for women [217,218]. Studies have reported that consumption of lutein of up to 20 mg/day is safe and does not contribute to diverse side effects [219]. Limited information is available regarding the recommended dosage of phytochemicals due to many challenges. Firstly, there is limited information related to their bioavailability and heterogenicity. Secondly, there is limited information regarding the effects of food processing and dietary consumption on the effectivity of these flavonoids. Thirdly, differences in the absorbability of phytochemicals have been observed depending on the source of food [220].
3.1.2. Could Phytochemicals be Carcinogenic
Recently, more attention has been paid to secondary metabolites (phytochemicals) found in fruits and vegetables [221]. This has led to an increase in the consumption rate of these natural substances either in their natural form or as a supplement for medical purposes [222]. Many cancer patients use phytochemical supplements in combination with prescribed cancer treatments [223]. Despite the popular usage of phytochemicals, limited information is available regarding their toxicity and safety levels [224]. A number of phytochemicals found in people’s food and seasonings have shown potential carcinogenic effects [225]. One example is capsaicin, a principle pungent component belonging to the genus Capsicum which is responsible for the hotness or intensity of chili peppers [226]. Multiple epidemiological studies suggest the protective role of capsaicin in cancer treatment [227]. Results from animal studies have reported the opposite, stating the carcinogenic effect of capsaicin [228]. These controversial results which have been obtained suggest caution when consuming these phytochemicals and that normalize and control studies are needed to prevent result discrepancies. Other examples of phytochemicals as potential carcinogens include cycasin, phytoestrogens, ptaquiloside, and safrole [229,230,231,232]. So far, no specific data are available to address the short- and long-term effects of phytochemical consumption and their potential as tumor promoters or carcinogens.
3.1.3. Could Phytochemical Combinations Have Synergistic Effects
The consumption of phytochemicals triggers multiple selected mechanisms, as highlighted in Table 1. Studies have shown that administration of cancer treatments combined with phytochemicals may improve the prognosis of the disease, as it affects multiple pathways [233]. If we could trigger multiple mechanisms by combining the consumption of multiple phytochemicals, will this improve cancer manifestations? For example, administration of lycopene triggers five mechanisms, namely, it downregulates the NF-κB pathway and Wnt/β-catenin, upregulates MAP-mediated protein kinase, detoxifies enzymes, and induces apoptosis. Will the administration of carrots which activate the two other mechanisms not activated by lycopene (PI3K/AKT/mTOR and MAPK) improve the anti-cancerous activities mediated by these secondary metabolites (Figure 2)? Would this lead to a better outcome in which the action needed may be approached while the over-activation of mechanisms may be prevented?
Further research is needed to confirm the applicability of this suggestion, as well as the side effects of this combination, as there is little known about phytochemical/phytochemical interactions.
3.1.4. Phytochemicals in Cancer Drug Delivery
Cancer treatment with conventionally formulated anti-cancer drugs possesses multiple limitations like low solubility in water, short half-life in the body, poor specificity, and poor oral administration suitability [234]. Studies have shown enhancement effects when using phyto-nanotechnology on cancer cell lines [235]. This technology offers promising solutions for drug delivery systems by combining a phytochemical-based drug with a synthetic drug and then introducing the combination into the body [236]. Using nanotechnology in drug delivery increases bioavailability, decreases toxicity, prolongs circulation time, and improve efficacy [237]. More research is needed to confirm the positive effects of using this technology.
3.2. Final Thoughts
Phytochemicals found in fruits and vegetables have multiple beneficial effects on GI cancer. A diet rich in phytochemicals could improve the prognosis of GI cancer. Generally, the combination of phytochemicals could enhance anticancer effects by triggering multiple mechanisms, but more research is needed to support this promising means of enhancing cancer prognosis and possibly prevention. More attention needs to be paid to studying the bacteria involved in the biotransformation of phytochemicals to their metabolites, which could potentially help predict the health status of humans. Overall, a diet rich in fruits and vegetables is recommended.
Abbreviations
| GI | gastrointestinal |
| CRC | colorectal cancer |
| APC | adenomatous polyposis coli |
| SMAD | small mothers against decapentaplegic |
| IL | interleukins |
| SCFA | short chain fatty acid |
| Wnt | wingless-related integration site |
| TNF | tumor necrosis factor |
| AMPK | adenosine monophosphate activated protein kinase |
| PI3K | Phosphoinositide 3-kinases |
| STZ | streptozotocin |
| NF-κB | nuclear Factor kappa-light-chain-enhancer of activated B cells |
| Bcl-2 | B-cell lymphoma 2 |
| NO | nitric oxide |
| SULT | sulfotransferases |
| ACF | aberrant crypt foci |
| UGT | UDP-glucuronosyltransferase |
| GPx | glutathione peroxidase |
| GST | glutathione S-transferase |
| GR | glutathione reductase |
| BAX | Bcl-2-associated X protein |
| JNK | c-Jun N-terminal kinases |
| GSH | reduced glutathione |
| COX-2 | cyclooxygenase 2 |
| Nrf2 | nuclear factor erythroid 2-related factor 2 |
| PACs | predominant A- type procyanidins |
| EGFR | epithelial growth factor receptor |
| TBARS | thiobarbituric acid reactive substances |
| ROS | reactive oxygen species |
| SAMC | S-allylmercaptocysteine |
| ACSOs | alk(en)yl cysteine sulphoxides |
| MseC | Se-methyl-L-selenocysteine |
| MDSCs | myeloid-derived suppressor cells |
| MMP | matrix metallopeptidase |
| ICAM | intercellular adhesion molecules |
Author Contributions
Conceptualization, R.K.A.-I. and D.B.; literature review and resources, R.K.A.-I. and A.J.O.; writing—original draft preparation, R.K.A.-I.; writing—review and editing, D.B. and A.J.O.; figure preparation and editing, R.K.A.-I. and D.B.; visualization, R.K.A.-I., A.J.O. and D.B.; supervision, D.B.; project administration, D.B.; funding acquisition, D.B. All authors reviewed the results and approved the final version of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The review was funded by NPRP 11S-1214-170101 to D.B. The publication of this article was funded by the Qatar National Library.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Jemal A., Bray F., Center M.M., Ferlay J., Ward E., Forman D. Global cancer statistics. CA Cancer J. Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.National Cancer Institute Gastric cancer treatment PDQ. [(accessed on 8 July 2010)]; Available online: http://www.cancer.gov/cancertopics/pdq/treatment/gastric/HealthProfessional/page1.
- 3.Derakhshan M.H., Yazdanbod A., Sadjadi A.R., Shokoohi B., McColl K.E., Malekzadeh R. High incidence of adenocarcinoma arising from the right side of the gastric cardia in NW Iran. Gut. 2004;53:1262–1266. doi: 10.1136/gut.2003.035857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zali H., Rezaei-Tavirani M., Azodi M. Gastric cancer: Prevention, risk factors and treatment. Gastroenterol. Hepatol. Bed Bench. 2011;4:175–185. [PMC free article] [PubMed] [Google Scholar]
- 5.Sitarz R., Skierucha M., Mielko J., Offerhaus G.J.A., Maciejewski R., Polkowski W.P. Gastric cancer: Epidemiology, prevention, classification, and treatment. Cancer Manag. Res. 2018;10:239–248. doi: 10.2147/CMAR.S149619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holian O., Wahid S., Atten M.J., Attar B.M. Inhibition of gastric cancer cell proliferation by resveratrol: Role of nitric oxide. Am. J. Physiol. Gastrointest. Liver Physiol. 2002;282:G809–G816. doi: 10.1152/ajpgi.00193.2001. [DOI] [PubMed] [Google Scholar]
- 7.Zhou X.M., Wong B.C., Fan X.M., Zhang H.B., Lin M.C., Kung H.F., Lam S.K. Non-steroidal anti-inflammatory drugs induce apoptosis in gastric cancer cells through up-regulation of bax and bak. Carcinogenesis. 2011;22:1393–1397. doi: 10.1093/carcin/22.9.1393. [DOI] [PubMed] [Google Scholar]
- 8.Hundahl S.A., Phillips J.L., Menck H.R. The National Cancer Data Base Report on poor survival of U.S. gastric carcinoma patients treated with gastrectomy: Fifth Edition American Joint Committee on Cancer staging, proximal disease, and the “different disease” hypothesis. Cancer. 2000;88:921–932. doi: 10.1002/(SICI)1097-0142(20000215)88:4<921::AID-CNCR24>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 9.Correa P. Gastric cancer: Overview. Gastroenterol. Clin. North. Am. 2013:211–217. doi: 10.1016/j.gtc.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferlay J., Soerjomataram I., Dikshit R., Eser S., Mathers C., Rebelo M., Bray F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 11.Zali H., Rezaei-Tavirani M., Kariminia A., Yousefi R., Shokrgozar M.A. Evaluation of growth inhibitory and apoptosis inducing activity of human calprotectin on the human gastric cell line (AGS) Iran. Biomed. J. 2008;12:7–14. [PubMed] [Google Scholar]
- 12.Li Y.H., Niu Y.B., Sun Y., Zhang F., Liu C.X., Fan L., Mei Q.B. Role of phytochemicals in colorectal cancer prevention. World J. Gastroenterol. 2015;21:9262–9272. doi: 10.3748/wjg.v21.i31.9262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perdue D.G., Haverkamp D., Perkins C., Daley C.M., Provost E. Geographic variation in colorectal cancer incidence and mortality, age of onset, and stage at diagnosis among American Indian and Alaska Native people, 1990–2009. Am. J. Public Health. 2014;104:S404–S414. doi: 10.2105/AJPH.2013.301654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018, GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 15.Siegel R.L., Fedewa S.A., Anderson W.F., Miller K.D., Ma J., Rosenberg P.S., Jemal A. Colorectal Cancer Incidence Patterns in the United States, 1974–2013. J. Natl. Cancer Inst. 2017:109. doi: 10.1093/jnci/djw322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siegel R.L., Miller K.D., Fedewa S.A., Ahnen D.J., Meester R.G.S., Barzi A., Jemal A. Colorectal cancer statistics. CA Cancer J. Clin. 2017;67:177–193. doi: 10.3322/caac.21395. [DOI] [PubMed] [Google Scholar]
- 17.Tomasetti C., Marchionni L., Nowak M.A., Parmigiani G., Vogelstein B. Only three driver gene mutations are required for the development of lung and colorectal cancers. Proc. Natl. Acad. Sci. USA. 2015;112:118–123. doi: 10.1073/pnas.1421839112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Y., Zhang T., Chen G.Y. Flavonoids and Colorectal Cancer Prevention. Antioxid. 2018;7:187. doi: 10.3390/antiox7120187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao Y., Hu X., Zuo X., Wang M. Chemopreventive effects of some popular phytochemicals on human colon cancer: A review. Food Funct. 2018;9:4548–4568. doi: 10.1039/C8FO00850G. [DOI] [PubMed] [Google Scholar]
- 20.Marmol I., Sanchez-de-Diego C., Pradilla Dieste A., Cerrada E., Rodriguez Yoldi M.J. Colorectal Carcinoma: A General Overview and Future Perspectives in Colorectal Cancer. Int. J. Mol. Sci. 2017;18:197. doi: 10.3390/ijms18010197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuipers E.J., Grady W.M., Lieberman D., Seufferlein T., Sung J.J., Boelens P.G., Watanabe T. Colorectal cancer. Nat. Rev. Dis. Primers. 2015;1:15065. doi: 10.1038/nrdp.2015.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lambert R., Hainaut P. The multidisciplinary management of gastrointestinal cancer. Epidemiology of oesophagogastric cancer. Best Pr. Res. Clin. Gastroenterol. 2007;21:921–945. doi: 10.1016/j.bpg.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 23.Herszenyi L., Tulassay Z. Epidemiology of gastrointestinal and liver tumors. Eur. Rev. Med. Pharm. Sci. 2010;14:249–258. [PubMed] [Google Scholar]
- 24.Hongo M., Nagasaki Y., Shoji T. Epidemiology of esophageal cancer: Orient to Occident. Effects of chronology, geography and ethnicity. J. Gastroenterol. Hepatol. 2009;24:729–735. doi: 10.1111/j.1440-1746.2009.05824.x. [DOI] [PubMed] [Google Scholar]
- 25.Kubo A., Corley D.A. Marked multi-ethnic variation of esophageal and gastric cardia carcinomas within the United States. Am. J. Gastroenterol. 2004;99:582–588. doi: 10.1111/j.1572-0241.2004.04131.x. [DOI] [PubMed] [Google Scholar]
- 26.Haidry R.J., Butt M.A., Dunn J.M., Gupta A., Lipman G., Smart H.L., Registry U.R. Improvement over time in outcomes for patients undergoing endoscopic therapy for Barrett’s oesophagus-related neoplasia: 6-year experience from the first 500 patients treated in the UK patient registry. Gut. 2015;64:1192–1199. doi: 10.1136/gutjnl-2014-308501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hooper L.V., Macpherson A.J. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat. Rev. Immunol. 2010;10:159–169. doi: 10.1038/nri2710. [DOI] [PubMed] [Google Scholar]
- 28.Mokili J.L., Rohwer F., Dutilh B.E. Metagenomics and future perspectives in virus discovery. Curr. Opin Virol. 2012;2:63–77. doi: 10.1016/j.coviro.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ursell L.K., Haiser H.J., Van Treuren W., Garg N., Reddivari L., Vanamala J., Knight R. The intestinal metabolome: An intersection between microbiota and host. Gastroenterology. 2014;146:1470–1476. doi: 10.1053/j.gastro.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Turnbaugh P.J., Ley R.E., Hamady M., Fraser-Liggett C.M., Knight R., Gordon J.I. The human microbiome project. Nature. 2007;449:804–810. doi: 10.1038/nature06244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cho I., Blaser M.J. The human microbiome: At the interface of health and disease. Nat. Rev. Genet. 2012;13:260–270. doi: 10.1038/nrg3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang P.V., Hao L., Offermanns S., Medzhitov R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc. Natl. Acad. Sci. USA. 2014;111:2247–2252. doi: 10.1073/pnas.1322269111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wroblewski L.E., Peek R.M.Jr., Coburn L.A. The Role of the Microbiome in Gastrointestinal Cancer. Gastroenterol. Clin. North. Am. 2016;45:543–556. doi: 10.1016/j.gtc.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cervantes A., Chirivella I. Oncological emergencies. Ann. Oncol. 2004;15:iv299–iv306. doi: 10.1093/annonc/mdh943. [DOI] [PubMed] [Google Scholar]
- 35.Guimaraes M.D., Bitencourt A.G., Marchiori E., Chojniak R., Gross J.L., Kundra V. Imaging acute complications in cancer patients: What should be evaluated in the emergency setting? Cancer Imaging. 2014;14:18. doi: 10.1186/1470-7330-14-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Komiya Y., Habas R. Wnt signal transduction pathways. Organogenesis. 2008;4:68–75. doi: 10.4161/org.4.2.5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schatoff E.M., Leach B.I., Dow L.E. Wnt Signaling and Colorectal Cancer. Curr. Colorectal. Cancer Rep. 2017;13:101–110. doi: 10.1007/s11888-017-0354-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramasamy S., Singh S., Taniere P., Langman M.J., Eggo M.C. Sulfide-detoxifying enzymes in the human colon are decreased in cancer and upregulated in differentiation. Am. J. Physiol. Gastrointest. Liver Physiol. 2006;291:G288–G296. doi: 10.1152/ajpgi.00324.2005. [DOI] [PubMed] [Google Scholar]
- 39.Zhang L., Yu J. Role of apoptosis in colon cancer biology, therapy, and prevention. Curr. Colorectal. Cancer Rep. 2013;9:331–340. doi: 10.1007/s11888-013-0188-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tapia O., Riquelme I., Leal P., Sandoval A., Aedo S., Weber H., Roa J.C. The PI3K/AKT/mTOR pathway is activated in gastric cancer with potential prognostic and predictive significance. Virchows Arch. 2014;465:25–33. doi: 10.1007/s00428-014-1588-4. [DOI] [PubMed] [Google Scholar]
- 41.Walker J., Jijon H.B., Diaz H., Salehi P., Churchill T., Madsen K.L. 5-aminoimidazole-4-carboxamide riboside (AICAR) enhances GLUT2-dependent jejunal glucose transport: A possible role for AMPK. Biochem. J. 2005;385:485–491. doi: 10.1042/BJ20040694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun X., Zhu M.J. AMP-activated protein kinase: A therapeutic target in intestinal diseases. Open Biol. 2017;7:170104. doi: 10.1098/rsob.170104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sokolova O., Naumann M. NF-kappaB Signaling in Gastric Cancer. Toxins Basel. 2017;9:119. doi: 10.3390/toxins9040119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang M., Huang C.Z. Mitogen-activated protein kinase signaling pathway and invasion and metastasis of gastric cancer. World J. Gastroenterol. 2015;21:11673–11679. doi: 10.3748/wjg.v21.i41.11673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee B.L., Lee H.S., Jung J., Cho S.J., Chung H.Y., Kim W.H., Nam S.Y. Nuclear factor-kappaB activation correlates with better prognosis and Akt activation in human gastric cancer. Clin. Cancer Res. 2005;11:2518–2525. doi: 10.1158/1078-0432.CCR-04-1282. [DOI] [PubMed] [Google Scholar]
- 46.Slattery M.L., Mullany L.E., Sakoda L., Samowitz W.S., Wolff R.K., Stevens J.R., Herrick J.S. The NF-kappaB signalling pathway in colorectal cancer: Associations between dysregulated gene and miRNA expression. J. Cancer Res. Clin. Oncol. 2018;144:269–283. doi: 10.1007/s00432-017-2548-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xia Y., Shen S., Verma I.M. NF-kappaB, an active player in human cancers. Cancer Immunol. Res. 2014;2:823–830. doi: 10.1158/2326-6066.CIR-14-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gingras D., Beliveau R. Colorectal cancer prevention through dietary and lifestyle modifications. Cancer Microenviron. 2011;4:133–139. doi: 10.1007/s12307-010-0060-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee K.W., Bode A.M., Dong Z. Molecular targets of phytochemicals for cancer prevention. Nat. Rev. Cancer. 2011;11:211. doi: 10.1038/nrc3017. [DOI] [PubMed] [Google Scholar]
- 50.Leitzmann C. Characteristics and Health Benefits of Phytochemicals. Komplementmed. 2016;23:69–74. doi: 10.1159/000444063. [DOI] [PubMed] [Google Scholar]
- 51.Upadhyay S., Dixit M. Role of polyphenols and other phytochemicals on molecular signaling. Oxid. Med. Cell. Longev. 2015;2015:15. doi: 10.1155/2015/504253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Probst Y.C., Guan V.X., Kent K. Dietary phytochemical intake from foods and health outcomes: A systematic review protocol and preliminary scoping. BMJ Open. 2017;7:e013337. doi: 10.1136/bmjopen-2016-013337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang Y.J., Gan R.Y., Li S., Zhou Y., Li A.N., Xu D.P., Li H.B. Antioxidant Phytochemicals for the Prevention and Treatment of Chronic Diseases. Molecules. 2015;20:21138–21156. doi: 10.3390/molecules201219753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Petrovska B.B. Historical review of medicinal plants’ usage. Pharm. Rev. 2012;6:1–5. doi: 10.4103/0973-7847.95849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.González-Vallinas M., González-Castejón M., Rodríguez-Casado A., Ramírez de Molina A. Dietary phytochemicals in cancer prevention and therapy: A complementary approach with promising perspectives. Nutr. Rev. 2013;71:585–599. doi: 10.1111/nure.12051. [DOI] [PubMed] [Google Scholar]
- 56.Altemimi A., Lakhssassi N., Baharlouei A., Watson D.G., Lightfoot D.A. Phytochemicals: Extraction, Isolation, and Identification of Bioactive Compounds from Plant Extracts. Plants. 2017;6:42. doi: 10.3390/plants6040042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yoo S., Kim K., Nam H., Lee D. Discovering Health Benefits of Phytochemicals with Integrated Analysis of the Molecular Network, Chemical Properties and Ethnopharmacological Evidence. Nutrients. 2018;10:1042. doi: 10.3390/nu10081042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Johnson I.T. Phytochemicals and cancer. Proc. Nutr. Soc. 2007;66:207–215. doi: 10.1017/S0029665107005459. [DOI] [PubMed] [Google Scholar]
- 59.Cinzia F., Francesco F., Manuela B. Beneficial Role of Phytochemicals on Oxidative Stress and Age-Related Diseases. BioMed Res. Int. 2019;2019:16. doi: 10.1155/2019/8748253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Holst B., Williamson G. A critical review of the bioavailability of glucosinolates and related compounds. Nat. Prod. Rep. 2004;21:425–447. doi: 10.1039/b204039p. [DOI] [PubMed] [Google Scholar]
- 61.Piver B., Fer M., Vitrac X., Merillon J.M., Dreano Y., Berthou F. Involvement of cytochrome P450 1A2 in the biotransformation of trans-resveratrol in human liver microsomes. Biochem. Pharmacol. 2004;68:773–782. doi: 10.1016/j.bcp.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 62.Keppler K., Humpf H.U. Metabolism of anthocyanins and their phenolic degradation products by the intestinal microflora. Bioorg. Med. Chem. 2005;13:5195–5205. doi: 10.1016/j.bmc.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 63.Manach C., Williamson G., Morand C., Scalbert A., Remesy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005;81:230S–242S. doi: 10.1093/ajcn/81.1.230S. [DOI] [PubMed] [Google Scholar]
- 64.Lampe J.W., Chang J.L. Interindividual differences in phytochemical metabolism and disposition. Semin. Cancer Biol. 2007;17:347–353. doi: 10.1016/j.semcancer.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Burchell B. Genetic variation of human UDP-glucuronosyltransferase: Implications in disease and drug glucuronidation. Am. J. Pharm. 2003;3:37–52. doi: 10.2165/00129785-200303010-00006. [DOI] [PubMed] [Google Scholar]
- 66.Slattery M.L., Benson J., Curtin K., Ma K.N., Schaeffer D., Potter J.D. Carotenoids and colon cancer. Am. J. Clin. Nutr. 2000;71:575–582. doi: 10.1093/ajcn/71.2.575. [DOI] [PubMed] [Google Scholar]
- 67.Palozza P., Calviello G., Serini S., Maggiano N., Lanza P., Ranelletti F.O., Bartoli G.M. Beta-carotene at high concentrations induces apoptosis by enhancing oxy-radical production in human adenocarcinoma cells. Free Radic. Biol. Med. 2001;30:1000–1100. doi: 10.1016/S0891-5849(01)00488-9. [DOI] [PubMed] [Google Scholar]
- 68.Milani A., Basirnejad M., Shahbazi S., Bolhassani A. Carotenoids: Biochemistry, pharmacology and treatment. Br. J. Pharmacol. 2017;174:1290–1324. doi: 10.1111/bph.13625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Malila N., Virtamo J., Virtanen M., Pietinen P., Albanes D., Teppo L. Dietary and serum alpha-tocopherol, beta-carotene and retinol, and risk for colorectal cancer in male smokers. Eur. J. Clin. Nutr. 2002;56:615–621. doi: 10.1038/sj.ejcn.1601366. [DOI] [PubMed] [Google Scholar]
- 70.Smith-Warner S.A., Elmer P.J., Tharp T.M., Fosdick L., Randall B., Gross M., Potter J.D. Increasing vegetable and fruit intake: Randomized intervention and monitoring in an at-risk population. Cancer Epidemiol. Biomark. Prev. 2000;9:307–317. [PubMed] [Google Scholar]
- 71.Lim L.S., Mitchell P., Seddon J.M., Holz F.G., Wong T.Y. Age-related macular degeneration. Lancet. 2012;379:1728–1738. doi: 10.1016/S0140-6736(12)60282-7. [DOI] [PubMed] [Google Scholar]
- 72.Perry A., Rasmussen H., Johnson E.J. Xanthophyll (lutein, zeaxanthin) content in fruits, vegetables and corn and egg products. J. Food Compos. Anal. 2009;22:9–15. doi: 10.1016/j.jfca.2008.07.006. [DOI] [Google Scholar]
- 73.Ohnson E.J. Role of lutein and zeaxanthin in visual and cognitive function throughout the lifespan. Nutr. Rev. 2014;72:605–612. doi: 10.1111/nure.12133. [DOI] [PubMed] [Google Scholar]
- 74.Akuffo K.O., Nolan J., Stack J., Moran R., Feeney J., Kenny R.A., Peto T., Dooley C., O’Halloran A.M., Cronin H. Prevalence of age-related macular degeneration in the Republic of Ireland. Br. J. Ophthalmol. 2015;99:1037–1044. doi: 10.1136/bjophthalmol-2014-305768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ranard K.M., Jeon S., Mohn E.S., Griffiths J.C., Johnson E.J., Erdman J.W.J.r. Dietary guidance for lutein: Consideration for intake recommendations is scientifically supported. Eur. J. Nutr. 2017;56:37–42. doi: 10.1007/s00394-017-1580-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kim J., Lee J., Oh J., Chang H.J., Sohn D., Kwon O., Shin A., Kim J. Dietary Lutein Plus Zeaxanthin Intake and DICER1 rs3742330 A > G Polymorphism Relative to Colorectal Cancer Risk. Sci. Rep. 2019;9:3406. doi: 10.1038/s41598-019-39747-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Collins A.R., Harrington V. Antioxidants; not the only reason to eat fruit and vegetables. Phytochem. Rev. 2003;1:167–174. doi: 10.1023/A:1022514432233. [DOI] [Google Scholar]
- 78.Femia A.P., Tarquini E., Salvadori M., Ferri S., Giannini A. K-ras mutations and mucin profile in preneoplastic lesions and colon tumors induced in rats by 1,2-dimethylhydrazine. Int. J. Cancer. 2008;1:117–123. doi: 10.1002/ijc.23065. [DOI] [PubMed] [Google Scholar]
- 79.Gali-Muhtasib H.U., Younes I.H., Karchesy J.J., el-Sabban M.E. Plant tannins inhibit the induction of aberrant crypt foci and colonic tumors by 1,2-dimethylhydrazine in mice. Nutr. Cancer. 2001;39:108–116. doi: 10.1207/S15327914nc391_15. [DOI] [PubMed] [Google Scholar]
- 80.Reynoso-Camacho R., González-Jasso E., Ferriz-Martínez R., Villalón-Corona B., Salgado L., Ramos-Gómez M. Dietary Supplementation of Lutein Reduces Colon Carcinogenesis in DMH-Treated Rats by Modulating K-ras, PKB, and β-catenin Proteins. Nutr. Cancer. 2010;63:39–45. doi: 10.1080/01635581.2010.516477. [DOI] [PubMed] [Google Scholar]
- 81.Satia-Abouta J., Galanko J.A., Martin C.F., Potter J.D., Ammerman A., Sandler R.S. Associations of micronutrients with colon cancer risk in African Americans and whites: Results from the North Carolina Colon Cancer Study. Cancer Epidemiol. Biomark. Prev. 2003;12:747–754. [PubMed] [Google Scholar]
- 82.Santocono M., Zurria M., Berrettini M., Fedeli D., Falcioni G. Influence of astaxanthin, zeaxanthin and lutein on DNA damage and repair in UVA-irradiated cells. J. Photochem. Photobiol. B. 2006;85:205–215. doi: 10.1016/j.jphotobiol.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 83.Trejo-Solís C., Pedraza-Chaverrí J., Torres-Ramos M. Multiple molecular and cellular mechanisms of action of lycopene in cancer inhibition. Evid. Based Complement. Alternat. Med. 2013;2013:705121. doi: 10.1155/2013/705121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Story E.N., Kopec R.E., Schwartz S.J., Harris G.K. An update on the health effects of tomato lycopene. Annu. Rev. Food Sci. Technol. 2010;1:189–210. doi: 10.1146/annurev.food.102308.124120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bandeira A.C., da Silva T.P., de Araujo G.R. Lycopene inhibits reactive oxygen species production in SK-Hep-1 cells and attenuates acetaminophen-induced liver injury in C57BL/6 mice. Chem. Biol. Interact. 2016;263:7–17. doi: 10.1016/j.cbi.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 86.Boehm F., Edge R., Truscott T.G., Witt C. A dramatic effect of oxygen on protection of human cells against γ-radiation by lycopene. FEBS Lett. 2016;590:1086–1093. doi: 10.1002/1873-3468.12134. [DOI] [PubMed] [Google Scholar]
- 87.Slattery M.L., Lundgreen A., Welbourn B., Wollf R.K., Corcoran C. Oxidative balance and colon and rectal cancer: Interaction of lifestyle factors and genes. Mutat. Res. 2012;734:30–40. doi: 10.1016/j.mrfmmm.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Youn S.W. Systemic inflammatory response as a prognostic factor in patients with cancer. J. Korean Orient Oncol. 2012;17:1–7. [Google Scholar]
- 89.Lin M.C., Wang F.Y., Kuo Y.H., Tang F.Y. Cancer chemopreventive effects of lycopene: Suppression of MMP-7 expression and cell invasion in human colon cancer cells. J. Agric. Food Chem. 2011;59:11304–11318. doi: 10.1021/jf202433f. [DOI] [PubMed] [Google Scholar]
- 90.Palozza P., Colangelo M., Simone R. Lycopene induces cell growth inhibition by altering mevalonate pathway and Ras signaling in cancer cell lines. Carcinogenesis. 2010;31:1813–1821. doi: 10.1093/carcin/bgq157. [DOI] [PubMed] [Google Scholar]
- 91.Cha J.H., Kim W.K., Ha A.W., Kim M.H., Chang M.J. Anti-inflammatory effect of lycopene in SW480 human colorectal cancer cells. Nutr. Res. Pract. 2017;11:90–96. doi: 10.4162/nrp.2017.11.2.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bhuvaneswari V., Velmurugan B., Nagini S. Lycopene, an antioxidant carotenoid modulates glutathione-dependent hepatic biotransformation enzymes during experimental gastric carcinogenesis. Nutr. Res. 2001;8:1117–1124. [Google Scholar]
- 93.Kalt W. Effects of production and processing factor on major fruit and vegetable antioxidants. J. Food Sci. 2005;70:11–19. doi: 10.1111/j.1365-2621.2005.tb09053.x. [DOI] [Google Scholar]
- 94.Wally O.S., Punja Z.K. Carrot (Daucus carota L.) Methods Mol. Biol. 2015;1224:59–66. doi: 10.1007/978-1-4939-1658-0_6. [DOI] [PubMed] [Google Scholar]
- 95.Sharma K.D., Karki S., Thakur N.S., Attri S. Chemical composition, functional properties and processing of carrot—A review. J. Food Sci. Technol. 2012;49:22–32. doi: 10.1007/s13197-011-0310-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kobaek-Larsen M., Nielsen D.S., Kot W., Krych L., Christensen L.P., Baatrup G. Effect of the dietary polyacetylenes falcarinol and falcarindiol on the gut microbiota composition in a rat model of colorectal cancer. BMC Res. Note. 2018;11:411. doi: 10.1186/s13104-018-3527-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shebaby W.N., Bodman-Smith K.B., Mansour A., Mroueh M., Taleb R.I., El-Sibai M., Daher C.F. Daucus carota Pentane-Based Fractions Suppress Proliferation and Induce Apoptosis in Human Colon Adenocarcinoma HT-29 Cells by Inhibiting the MAPK and PI3K Pathways. J. Med. Food. 2005;18:745–752. doi: 10.1089/jmf.2014.3225. [DOI] [PubMed] [Google Scholar]
- 98.Purup S., Larsen E., Christensen L.P. Differential effects of falcarinol and related aliphatic C-polyacetylenes on intestinal cell proliferation. J. Agric. Food Chem. 2009;57:8290–8296. doi: 10.1021/jf901503a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pan M.H., Ho C.T. Chemopreventive effects of natural dietary compounds on cancer development. Chem. Soc. Rev. 2008;37:2558–2574. doi: 10.1039/b801558a. [DOI] [PubMed] [Google Scholar]
- 100.Huang X.E., Hirose K., Wakai K., Matsuo K., Ito H., Xiang J. Comparison of lifestyle risk factors by family history for gastric, breast, lung and colorectal cancer. Asian Pac. J. Cancer Prev. 2004;5:419–427. [PubMed] [Google Scholar]
- 101.De la Iglesia R., Milagro F.I., Campion J., Boque N., Martinez J.A. Healthy properties of proanthocyanidins. Biofactors. 2010;36:159–168. doi: 10.1002/biof.79. [DOI] [PubMed] [Google Scholar]
- 102.Blade C., Aragones G., Arola-Arnal A., Muguerza B., Bravo F.I., Salvado M.J., Suarez M. Proanthocyanidins in health and disease. Biofactors. 2016;42:5–12. doi: 10.1002/biof.1249. [DOI] [PubMed] [Google Scholar]
- 103.Cos P., De Bruyne T., Hermans N., Apers S., Berghe D.V., Vlietinck A.J. Proanthocyanidins in health care: Current and new trends. Curr. Med. Chem. 2004;11:1345–1359. doi: 10.2174/0929867043365288. [DOI] [PubMed] [Google Scholar]
- 104.Casanova-Marti A., Serrano J., Portune K.J., Sanz Y., Blay M.T., Terra X., Pinent M. Grape seed proanthocyanidins influence gut microbiota and enteroendocrine secretions in female rats. Food Funct. 2018;9:1672–1682. doi: 10.1039/C7FO02028G. [DOI] [PubMed] [Google Scholar]
- 105.Lee Y. Cancer Chemopreventive Potential of Procyanidin. Toxicol. Res. 2017;33:273–282. doi: 10.5487/TR.2017.33.4.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Neto C.C. Cranberries: Ripe for more cancer research? J. Sci. Food Agric. 2011;91:2303–2307. doi: 10.1002/jsfa.4621. [DOI] [PubMed] [Google Scholar]
- 107.Côté J., Caillet S., Doyon G., Sylvain J.F., Lacroix M. Bioactive compounds in cranberries and their biological properties. Crit. Rev. Food Sci. Nutr. 2010;50:666–679. doi: 10.1080/10408390903044107. [DOI] [PubMed] [Google Scholar]
- 108.Pappas E., Schaich K.M. Phytochemicals of cranberries and cranberry products: Characterization, potential health effects, and processing stability. Crit. Rev. Food Sci. Nutr. 2009;49:741–781. doi: 10.1080/10408390802145377. [DOI] [PubMed] [Google Scholar]
- 109.Duthie S.J., Jenkinson A.M., Crozier A., Mullen W., Pirie L., Kyle J., Yap L.S., Christen P., Duthie G.G. The effects of cranberry juice consumption on antioxidant status and biomarkers relating to heart disease and cancer in healthy human volunteers. Eur. J. Nutr. 2006;45:113–122. doi: 10.1007/s00394-005-0572-9. [DOI] [PubMed] [Google Scholar]
- 110.Wu X., Song M., Cai X., Neto C., Tata A., Han Y., Xiao H. Chemopreventive Effects of Whole Cranberry (Vaccinium macrocarpon) on Colitis-Associated Colon Tumorigenesis. Mol. Nutr. Food Res. 2018;62:e1800942. doi: 10.1002/mnfr.201800942. [DOI] [PubMed] [Google Scholar]
- 111.Boateng J., Verghese M., Shackelford L., Walker L.T., Khatiwada J., Ogutu S., Chawan C.B. Selected fruits reduce azoxymethane (AOM)-induced aberrant crypt foci (ACF) in Fisher 344 male rats. Food Chem. Toxicol. 2007;45:725–732. doi: 10.1016/j.fct.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 112.Xiao S.D., Shi T. Is cranberry juice effective in the treatment and prevention of Helicobacter pyloriinfection of mice? Chin. J. Dig. Dis. 2003;4:136–139. doi: 10.1046/j.1443-9573.2003.00127.x. [DOI] [Google Scholar]
- 113.Jin D., Liu T., Dong W., Zhang Y., Wang S., Xie R., Cao H. Dietary feeding of freeze-dried whole cranberry inhibits intestinal tumor development in Apc(min/+) mice. Oncotarget. 2017;8:97787–97800. doi: 10.18632/oncotarget.22081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Koosha S., Alshawsh M.A., Looi C.Y., Seyedan A., Mohamed Z. An Association Map on the Effect of Flavonoids on the Signaling Pathways in Colorectal Cancer. Int. J. Med. Sci. 2016;13:374–385. doi: 10.7150/ijms.14485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sun Q., Yue Y., Shen P., Yang J.J., Park Y. Cranberry Product Decreases Fat Accumulation in Caenorhabditis elegans. J. Med. Food. 2016;19:427–433. doi: 10.1089/jmf.2015.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Upton R. Bilberry Fruit Vaccinium myrtillus L. Standards of Analysis, Quality Control, and Therapeutics. AHP; Santa Cruz, CA, USA: 2001. [Google Scholar]
- 117.Chu W., Cheung S.C.M., Lau R.A.W., Benzie I.F.F. Bilberry (Vaccinium myrtillus L.) In: Benzie I.F.F., Wachtel-Galor Sissi, editors. Herbal Medicine: Biomolecular and Clinical Aspects. CRC Press; Boca Raton, FL, USA: 2011. [Google Scholar]
- 118.Mazza G., Kay C.D., Correll T., Holub B.J. Absorption of anthocyanins from blueberries and serum antioxidant status in human subjects. J. Agric. Food Chem. 2002;50:7731–7737. doi: 10.1021/jf020690l. [DOI] [PubMed] [Google Scholar]
- 119.Valentova K., Ulrichova J., Cvak L., Simanek V. Cytoprotective effect of a bilberry extract against oxidative damage of rat hepatocytes. Food Chem. 2006;101:912–917. doi: 10.1016/j.foodchem.2006.02.038. [DOI] [Google Scholar]
- 120.Hodges R.E., Minich D.M. Modulation of Metabolic Detoxification Pathways Using Foods and Food-Derived Components: A Scientific Review with Clinical Application. J. Nutr. Metab. 2015:760689. doi: 10.1155/2015/760689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lala G., Malik M., Zhao C., He J., Kwon Y., Giusti M.M., Magnuson B.A. Anthocyanin-rich extracts inhibit multiple biomarkers of colon cancer in rats. Nutr. Cancer. 2006;54:84–93. doi: 10.1207/s15327914nc5401_10. [DOI] [PubMed] [Google Scholar]
- 122.Mutanen M., Pajari A.M., Paivarinta E., Misikangas M., Rajakangas J., Marttinen M., Oikarinen S. Berries as preventive dietary constituents-a mechanistic approach with ApcMin+ mouse. Asia Pac. J. Clin. Nutr. 2008;17:123–125. [PubMed] [Google Scholar]
- 123.Wang L.S., Stoner G.D. Anthocyanins and their role in cancer prevention. Cancer Lett. 2008;269:281–290. doi: 10.1016/j.canlet.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lippert E., Ruemmele P., Obermeier F., Goelder S., Kunst C., Rogler G., Endlicher E. Anthocyanins Prevent Colorectal Cancer Development in a Mouse Model. Digestion. 2017;95:275–280. doi: 10.1159/000475524. [DOI] [PubMed] [Google Scholar]
- 125.Thomasset S., Berry D.P., Cai H., West K., Marczylo T.H., Marsden D., Gescher A.J. Pilot study of oral anthocyanins for colorectal cancer chemoprevention. Cancer Prev. Res. Phila. 2009;2:625–633. doi: 10.1158/1940-6207.CAPR-08-0201. [DOI] [PubMed] [Google Scholar]
- 126.Esselen M., Fritz J., Hutter M., Teller N., Baechler S., Boettler U., Marko D. Anthocyanin-rich extracts suppress the DNA-damaging effects of topoisomerase poisons in human colon cancer cells. Mol. Nutr. Food Res. 2011;55:S143–S153. doi: 10.1002/mnfr.201000315. [DOI] [PubMed] [Google Scholar]
- 127.Chau I., Cunningham D. Adjuvant therapy in colon cancer—what, when and how? Ann. Oncol. 2006;17:1347–1359. doi: 10.1093/annonc/mdl029. [DOI] [PubMed] [Google Scholar]
- 128.Xiao D., Pinto J.T., Gundersen G.G., Weinstein I.B. Effects of a series of organosulfur compounds on mitotic arrest and induction of apoptosis in colon cancer cells. Mol. Cancer Ther. 2005;4:1388–1398. doi: 10.1158/1535-7163.MCT-05-0152. [DOI] [PubMed] [Google Scholar]
- 129.Moriarty R.M., Naithani R., Surve B. Organosulfur compounds in cancer chemoprevention. Mini. Rev. Med. Chem. 2007;7:827–838. doi: 10.2174/138955707781387939. [DOI] [PubMed] [Google Scholar]
- 130.Nagini S. Cancer chemoprevention by garlic and its organosulfur compounds-panacea or promise? Anticancer Agents Med. Chem. 2008;8:313–321. doi: 10.2174/187152008783961879. [DOI] [PubMed] [Google Scholar]
- 131.El-Bayoumy K., Sinha R., Pinto J.T. Cancer chemoprevention by garlic and garlic-containing sulfur and selenium compounds. J. Nutr. 2016;136:S864–S869. doi: 10.1093/jn/136.3.864S. [DOI] [PubMed] [Google Scholar]
- 132.Hu J.Y., Hu Y.W., Zhou J.J., Zhang M.W., Li D., Zheng S. Consumption of garlic and risk of colorectal cancer: An updated meta-analysis of prospective studies. World J. Gastroenterol. 2014;20:15413–15422. doi: 10.3748/wjg.v20.i41.15413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ross S.A., Finley J.W., Milner J.A. Allyl sulfur compounds from garlic modulate aberrant crypt formation. J. Nutr. 2006;136:S852–S854. doi: 10.1093/jn/136.3.852S. [DOI] [PubMed] [Google Scholar]
- 134.Powolny A.A., Singh S.V. Multitargeted prevention and therapy of cancer by diallyl trisulfide and related allium vegetable-derived organosulfur compounds. Cancer Lett. 2008;269:305–314. doi: 10.1016/j.canlet.2008.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yi L., Su Q. Molecular mechanisms for the anticancer effects of diallyl disulfide. Food Chem. Toxicol. 2013;57:362–370. doi: 10.1016/j.fct.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 136.Roy N., Nazeem P.A., Babu T.D., Abida P.S., Narayanankutty A., Valsalan R., Raghavamenon A.C. EGFR gene regulation in colorectal cancer cells by garlic phytocompounds with special emphasis on S-Allyl-L-Cysteine Sulfoxide. Interdiscip Sci. 2008;10:686–693. doi: 10.1007/s12539-017-0227-6. [DOI] [PubMed] [Google Scholar]
- 137.Saud S.M., Li W., Gray Z., Matter M.S., Colburn N.H., Young M.R., Kim Y.S. Diallyl Disulfide (DADS), a Constituent of Garlic, Inactivates NF-kappaB and Prevents Colitis-Induced Colorectal Cancer by Inhibiting GSK-3beta. Cancer Prev. Res. Phila. 2016;9:607–615. doi: 10.1158/1940-6207.CAPR-16-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Janakiram N.B., Rao C.V. The role of inflammation in colon cancer. Advances in Experimental Medicine and Biology. Volume 816. Springer; Berlin, Germany: 2014. pp. 25–52. [DOI] [PubMed] [Google Scholar]
- 139.Erstad D.J., Cusack J.C. Targeting the NF-kappaB pathway in cancer therapy. Surg. Oncol. Clin. N. Am. 2013;22:705–746. doi: 10.1016/j.soc.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 140.Li S., Yang G., Zhu X., Cheng L., Sun Y., Zhao Z. Combination of rapamycin and garlic-derived S-allylmercaptocysteine induces colon cancer cell apoptosis and suppresses tumor growth in xenograft nude mice through autophagy/p62/Nrf2 pathway. Oncol. Rep. 2017;38:1637–1644. doi: 10.3892/or.2017.5849. [DOI] [PubMed] [Google Scholar]
- 141.Raghu R., Lu K.H., Sheen L.Y. Recent Research Progress on Garlic (da suan) as a Potential Anticarcinogenic Agent Against Major Digestive Cancers. J. Tradit. Complement Med. 2012;2:192–201. doi: 10.1016/S2225-4110(16)30099-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhou Y., Zhuang W., Hu W., Liu G.J., Wu T.X., Wu X.T. Consumption of large amounts of Allium vegetables reduces risk for gastric cancer in a meta-analysis. Gastroenterology. 2011;141:80–89. doi: 10.1053/j.gastro.2011.03.057. [DOI] [PubMed] [Google Scholar]
- 143.Griffiths G., Trueman L., Crowther T., Thomas B., Smith B. Onions--a global benefit to health. Phytother. Res. 2002;16:603–615. doi: 10.1002/ptr.1222. [DOI] [PubMed] [Google Scholar]
- 144.Suleria H.A., Butt M.S., Anjum F.M., Saeed F., Khalid N. Onion: Nature protection against physiological threats. Crit. Rev. Food Sci. Nutr. 2015;55:50–66. doi: 10.1080/10408398.2011.646364. [DOI] [PubMed] [Google Scholar]
- 145.Izzo A.A., Capasso R., Capasso F. Eating garlic and onion: A matter of life or death. Br. J. Cancer. 2004;91:194. doi: 10.1038/sj.bjc.6601918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Murayyan A.I., Manohar C.M., Hayward G., Neethirajan S. Antiproliferative activity of Ontario grown onions against colorectal adenocarcinoma cells. Food Res. Int. 2017;96:12–18. doi: 10.1016/j.foodres.2017.03.017. [DOI] [PubMed] [Google Scholar]
- 147.He Y., Jin H., Gong W., Zhang C., Zhou A. Effect of onion flavonoids on colorectal cancer with hyperlipidemia: An in vivo study. Onco Targets Ther. 2014;7:101–110. doi: 10.2147/OTT.S51835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Tung Y.C., Tsai M.L., Kuo F.L., Lai C.S., Badmaev V., Ho C.T., Pan M.H. Se-Methyl-L-selenocysteine Induces Apoptosis via Endoplasmic Reticulum Stress and the Death Receptor Pathway in Human Colon Adenocarcinoma COLO 205 Cells. J. Agric. Food Chem. 2015;63:5008–5016. doi: 10.1021/acs.jafc.5b01779. [DOI] [PubMed] [Google Scholar]
- 149.Ibanez-Redin G., Furuta R.H.M., Wilson D., Shimizu F.M., Materon E.M., Arantes L., Oliveira O.N. Screen-printed interdigitated electrodes modified with nanostructured carbon nano-onion films for detecting the cancer biomarker CA19-9. Mater. Sci. Eng. C Mater. Biol. Appl. 2019;99:1502–1508. doi: 10.1016/j.msec.2019.02.065. [DOI] [PubMed] [Google Scholar]
- 150.Negi J.S., Singh P., Joshi G.P., Rawat M.S., Bisht V.K. Chemical constituents of Asparagus. Pharm. Rev. 2010;4:215–220. doi: 10.4103/0973-7847.70921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Saxena V.K., Chaurasia S. A new isoflavone from the roots of Asparagus racemosus. Fitoterapia. 2001;72:307–309. doi: 10.1016/S0367-326X(00)00315-4. [DOI] [PubMed] [Google Scholar]
- 152.Hamdi A., Jaramillo-Carmona S., Srairi Beji R., Tej R., Zaoui S., Rodriguez-Arcos R., Guillen-Bejarano R. The phytochemical and bioactivity profiles of wild Asparagus albus L. plant. Food Res. Int. 2017;99:720–729. doi: 10.1016/j.foodres.2017.06.027. [DOI] [PubMed] [Google Scholar]
- 153.Jaramillo-Carmona S., Guillen-Bejarano R., Jimenez-Araujo A., Rodriguez-Arcos R., Lopez S. In Vitro Toxicity of Asparagus Saponins in Distinct Multidrug-Resistant Colon Cancer Cells. Chem. Biodivers. 2018;15:e1800282. doi: 10.1002/cbdv.201800282. [DOI] [PubMed] [Google Scholar]
- 154.Zhang W., He W., Shi X., Li X., Wang Y., Hu M., Qin Z. An Asparagus polysaccharide fraction inhibits MDSCs by inducing apoptosis through toll-like receptor 4. Phytother. Res. 2018;32:1297–1303. doi: 10.1002/ptr.6058. [DOI] [PubMed] [Google Scholar]
- 155.Wang J., Liu Y., Zhao J., Zhang W., Pang X. Saponins extracted from by-product of Asparagus officinalis L. suppress tumour cell migration and invasion through targeting Rho GTPase signalling pathway. J. Sci. Food Agric. 2013;93:1492–1498. doi: 10.1002/jsfa.5922. [DOI] [PubMed] [Google Scholar]
- 156.Bousserouel S., Le Grandois J., Gosse F., Werner D., Barth S.W., Marchioni E., Raul F. Methanolic extract of white asparagus shoots activates TRAIL apoptotic death pathway in human cancer cells and inhibits colon carcinogenesis in a preclinical model. Int. J. Oncol. 2013;43:394–404. doi: 10.3892/ijo.2013.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Tse G., Eslick G.D. Cruciferous vegetables and risk of colorectal neoplasms: A systematic review and meta-analysis. Nutr. Cancer. 2014;66:128–139. doi: 10.1080/01635581.2014.852686. [DOI] [PubMed] [Google Scholar]
- 158.Burow M., Bergner A., Gershenzon J., Wittstock U. Glucosinolate hydrolysis in Lepidium sativum—Identification of the thiocyanate-forming protein. Plant Mol. Biol. 2007;63:49–61. doi: 10.1007/s11103-006-9071-5. [DOI] [PubMed] [Google Scholar]
- 159.Koroleva O.A., Davies A., Deeken R., Thorpe M.R., Tomos A.D., Hedrich R. Identification of a New Glucosinolate-Rich Cell Type in Arabidopsis Flower Stalk. Plant Physiol. 2000;124:599–608. doi: 10.1104/pp.124.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Clarke J.D., Dashwood R.H., Ho E. Multi-targeted prevention of cancer by sulforaphane. Cancer Lett. 2008;269:291–304. doi: 10.1016/j.canlet.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ramos-Gomez M., Kwak M.K., Dolan P.M., Itoh K., Yamamoto M., Talalay P., Kensler T.W. Sensitivity to carcinogenesis is increased and chemoprotective efficacy of enzyme inducers is lost in nrf2 transcription factor-deficient mice. Proc. Natl. Acad. Sci. USA. 2001;98:3410–3415. doi: 10.1073/pnas.051618798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Gupta P., Kim B., Kim S.H., Srivastava S.K. Molecular targets of isothiocyanates in cancer: Recent advances. Mol. Nutr. Food Res. 2014;58:1685–1707. doi: 10.1002/mnfr.201300684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Higgins L.G., Kelleher M.O., Eggleston I.M., Itoh K., Yamamoto M., Hayes J.D. Transcription factor Nrf2 mediates an adaptive response to sulforaphane that protects fibroblasts in vitro against the cytotoxic effects of electrophiles, peroxides and redox–cycling agents. Toxicol. Appl. Pharmacol. 2009;237:267–280. doi: 10.1016/j.taap.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 164.Keum Y.S., Yu S., Chang P.P., Yuan X., Kim J.H., Xu C., Han J., Agarwal A., Kong A.N. Mechanism of Action of Sulforaphane: Inhibition of p38 Mitogen-Activated Protein Kinase Isoforms Contributing to the Induction of Antioxidant Response Element-Mediated Heme Oxygenase-1 in Human Hepatoma HepG2 Cells. Cancer Res. 2006;66:8804–8813. doi: 10.1158/0008-5472.CAN-05-3513. [DOI] [PubMed] [Google Scholar]
- 165.Kim J.K., Gallaher D.D., Chen C., Gallaher C.M., Yao D., Trudo S.P. Phenethyl isothiocyanate and indole-3-carbinol from cruciferous vegetables, but not furanocoumarins from apiaceous vegetables, reduced PhIP-induced DNA adducts in Wistar rats. Mol. Nutr. Food Res. 2016;60:1956–1966. doi: 10.1002/mnfr.201500790. [DOI] [PubMed] [Google Scholar]
- 166.Byun S., Shin S.H., Park J., Lim S., Lee E., Lee C., Sung D., Farrand L., Lee S.R., Kim K.H., et al. Sulforaphene suppresses growth of colon cancer-derived tumors via induction of glutathione depletion and microtubule depolymerization. Mol. Nutr. Food Res. 2016;60:1068–1078. doi: 10.1002/mnfr.201501011. [DOI] [PubMed] [Google Scholar]
- 167.Choi H.J., Lim D.Y., Park J.H. Induction of G1 and G2/M cell cycle arrests by the dietary compound 3,30-diindolylmethane in HT-29 human colon cancer cells. BMC Gastroenterol. 2009;9:39. doi: 10.1186/1471-230X-9-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Johnson I.T. Cruciferous Vegetables and Risk of Cancers of the Gastrointestinal Tract. Mol. Nutr. Food Res. 2018;62:e1701000. doi: 10.1002/mnfr.201701000. [DOI] [PubMed] [Google Scholar]
- 169.Sriamornsak P. Chemistry of pectin and its pharmaceutical uses: A Review. Silpakorn Univ. Int. J. 2003;3:206–228. [Google Scholar]
- 170.Ciriminna R., Fidalgo A., Delisi R., Tamburino A., Carnaroglio D., Cravotto G., Pagliaro M. Controlling the Degree of Esterification of Citrus Pectin for Demanding Applications by Selection of the Source. ACS Omega. 2017;2:7991–7995. doi: 10.1021/acsomega.7b01109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wikiera A., Irla M., Mika M. Health-promoting properties of pectin. Postepy Hig. Med. Dosw. Online. 2014;68:590–596. doi: 10.5604/17322693.1102342. [DOI] [PubMed] [Google Scholar]
- 172.Sriamornsak P. Application of pectin in oral drug delivery. Expert Opin Drug. Deliv. 2011;8:1009–1023. doi: 10.1517/17425247.2011.584867. [DOI] [PubMed] [Google Scholar]
- 173.Verma A.K., Sachin K. Novel hydrophilic drug polymer nano-conjugatesof cisplatin showing long blood retention profile—Its release kinetics, cellularuptake and bio-distribution. Curr. Drug. Deliv. 2008;5:120–126. doi: 10.2174/156720108783954806. [DOI] [PubMed] [Google Scholar]
- 174.Izadi Z., Divsalar A., Saboury A.A., Sawyer L. beta-lactoglobulin-pectin Nanoparticle-based Oral Drug Delivery System for Potential Treatment of Colon Cancer. Chem. Biol. Drug. Des. 2016;88:209–216. doi: 10.1111/cbdd.12748. [DOI] [PubMed] [Google Scholar]
- 175.Wang S., Li P., Lu S.M., Ling Z.Q. Chemoprevention of Low-Molecular-Weight Citrus Pectin (LCP) in Gastrointestinal Cancer Cells. Int. J. Biol. Sci. 2016;12:746–756. doi: 10.7150/ijbs.13988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Glinsky V.V., Raz A. Modified citrus pectin anti-metastatic properties: One bullet multiple targets. Carbohydr. Res. 2009;14:1788–1791. doi: 10.1016/j.carres.2008.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Zhang T., Miller M.C., Zheng Y., Zhang Z., Xue H., Zhao D. Macromolecular assemblies of complex polysaccharides with galectin-3 and their synergistic effects on function. Biochem. J. 2017;474:3849–3868. doi: 10.1042/BCJ20170143. [DOI] [PubMed] [Google Scholar]
- 178.Maxwell E.G., Colquhoun I.J., Chau H.K., Hotchkiss A.T., Waldron K.W., Morris V.J., Belshaw N.J. Rhamnogalacturonan I containing homogalacturonan inhibits colon cancer cell proliferation by decreasing ICAM1 expression. Carbohydr. Polym. 2015;132:546–553. doi: 10.1016/j.carbpol.2015.06.082. [DOI] [PubMed] [Google Scholar]
- 179.Odun-Ayo F., Mellem J., Naicker T., Reddy L. Chemoprevention of Azoxymethane-induced Colonic Carcinogenesis in Balb/c mice Using a Modified Pectin Alginate Probiotic. Anticancer Res. 2015;35:4765–4775. [PubMed] [Google Scholar]
- 180.Gupta S.C., Patchva S., Aggarwal B.B. Therapeutic Roles of Curcumin: Lessons Learned from Clinical Trials. AAPS J. 2013;15:195–218. doi: 10.1208/s12248-012-9432-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Priyadarsini K.I. The chemistry of curcumin: From extraction to therapeutic agent. Molecules. 2014;19:20091–20112. doi: 10.3390/molecules191220091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Mazzolani F., Togni S. Oral administration of a curcumin-phospholipid delivery system for the treatment of central serous chorioretinopathy: A 12-month follow-up study. Clin. Ophthalmol. 2013;7:939–945. doi: 10.2147/OPTH.S45820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Jalili-Nik M., Soltani A., Moussavi S., Ghayour-Mobarhan M., Ferns G.A., Hassanian S.M., Avan A. Current status and future prospective of Curcumin as a potential therapeutic agent in the treatment of colorectal cancer. J. Cell. Physiol. 2018;233:6337–6345. doi: 10.1002/jcp.26368. [DOI] [PubMed] [Google Scholar]
- 184.Ismail N.I., Othman I., Abas F., Lajis. N.H., Naidu R. Mechanism of Apoptosis Induced by Curcumin in Colorectal Cancer. Int. J. Mol. Sci. 2019;20:2454. doi: 10.3390/ijms20102454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Bahrami A., Majeed M., Sahebkar A. Curcumin: A potent agent to reverse epithelial-to-mesenchymal transition. Cell. Oncol. Dordr. 2019;42:405–421. doi: 10.1007/s13402-019-00442-2. [DOI] [PubMed] [Google Scholar]
- 186.Zhang Z., Chen Y., Xiang L., Wang Z., Xiao G.G., Hu J. Effect of Curcumin on the Diversity of Gut Microbiota in Ovariectomized Rats. Nutrients. 2017;9:1146. doi: 10.3390/nu9101146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Lin L.Z., Harnly J.M., Pastor-Corrales M.S., Luthria D.L. The polyphenolic profiles of common bean (Phaseolus vulgaris L.) Food Chem. 2008;107:399–410. doi: 10.1016/j.foodchem.2007.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Hayat I., Ahmad A., Masud T., Ahmed A., Bashir S. Nutritional and health perspectives of beans (Phaseolus vulgaris L.): An overview. Crit. Rev. Food Sci. Nutr. 2014;54:580–592. doi: 10.1080/10408398.2011.596639. [DOI] [PubMed] [Google Scholar]
- 189.He S., Simpson B.K., Sun H., Ngadi M.O., Ma Y., Huang T. Phaseolus vulgaris lectins: A systematic review of characteristics and health implications. Crit. Rev. Food Sci. Nutr. 2018;58:70–83. doi: 10.1080/10408398.2015.1096234. [DOI] [PubMed] [Google Scholar]
- 190.Hangen L., Bennink M.R. Consumption of black beans and navy beans (Phaseolus vulgaris) reduced azoxymethane-induced colon cancer in rats. Nutr. Cancer. 2002;44:60–65. doi: 10.1207/S15327914NC441_8. [DOI] [PubMed] [Google Scholar]
- 191.Borresen E.C., Brown D.G., Harbison G., Taylor L., Fairbanks A., O’Malia J., Ryan E.P. A Randomized Controlled Trial to Increase Navy Bean or Rice Bran Consumption in Colorectal Cancer Survivors. Nutr. Cancer. 2016;68:1269–1280. doi: 10.1080/01635581.2016.1224370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Baxter B.A., Oppel R.C., Ryan E.P. Navy Beans Impact the Stool Metabolome and Metabolic Pathways for Colon Health in Cancer Survivors. Nutrients. 2018;11:28. doi: 10.3390/nu11010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Zhang C., Monk J.M., Lu J.T., Zarepoor L., Wu W., Liu R., Power K.A. Cooked navy and black bean diets improve biomarkers of colon health and reduce inflammation during colitis. Br. J. Nutr. 2014;111:1549–1563. doi: 10.1017/S0007114513004352. [DOI] [PubMed] [Google Scholar]
- 194.Borresen E.C., Gundlach K.A., Wdowik M., Rao S., Brown R.J., Ryan E.P. Feasibility of Increased Navy Bean Powder Consumption for Primary and Secondary Colorectal Cancer Prevention. Curr. Nutr. Food Sci. 2014;10:112–119. doi: 10.2174/1573401310666140306005934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Perez-Ternero C., Werner C.M., Nickel A.G., Herrera M.D., Motilva M.J., Bohm M., Laufs U. Ferulic acid, a bioactive component of rice bran, improves oxidative stress and mitochondrial biogenesis and dynamics in mice and in human mononuclear cells. J. Nutr. Biochem. 2017;48:51–61. doi: 10.1016/j.jnutbio.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 196.Muthayya S., Sugimoto J.D., Montgomery S., Maberly G.F. An overview of global rice production, supply, trade, and consumption. Ann. N. Y. Acad. Sci. 2014;1324:7–14. doi: 10.1111/nyas.12540. [DOI] [PubMed] [Google Scholar]
- 197.Henderson A.J., Ollila C.A., Kumar A., Borresen E.C., Raina K., Agarwal R., Ryan E.P. Chemopreventive properties of dietary rice bran: Current status and future prospects. Adv. Nutr. 2012;3:643–653. doi: 10.3945/an.112.002303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Sohail M., Rakha A., Butt M.S., Iqbal M.J., Rashid S. Rice bran nutraceutics: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2017;57:3771–3780. doi: 10.1080/10408398.2016.1164120. [DOI] [PubMed] [Google Scholar]
- 199.Jolfaie N.R., Rouhani M.H., Surkan P.J., Siassi F., Azadbakht L. Rice Bran Oil Decreases Total and LDL Cholesterol in Humans: A Systematic Review and Meta-Analysis of Randomized Controlled Clinical Trials. Horm. Metab. Res. 2016;48:417–426. doi: 10.1055/s-0042-105748. [DOI] [PubMed] [Google Scholar]
- 200.So W.K., Law B.M., Law P.T., Chan C.W., Chair S.Y. Current Hypothesis for the Relationship between Dietary Rice Bran Intake, the Intestinal Microbiota and Colorectal Cancer Prevention. NutrIents. 2016;8:569. doi: 10.3390/nu8090569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Law B.M.H., Waye M.M.Y., So W.K.W., Chair S.Y. Hypotheses on the Potential of Rice Bran Intake to Prevent Gastrointestinal Cancer through the Modulation of Oxidative Stress. Int. J. Mol. Sci. 2017;18:1352. doi: 10.3390/ijms18071352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Ryan E.P., Heuberger A.L., Weir T.L., Barnett B., Broeckling C.D., Prenni J.E. Rice bran fermented with Saccharomyces boulardii generates novel metabolite profiles with bioactivity. J. Agric. Food Chem. 2011;59:1862–1870. doi: 10.1021/jf1038103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Fan H., Morioka T., Ito E. Induction of apoptosis and growth inhibition of cultured human endometrial adenocarcinoma cells (Sawano) by an antitumor lipoprotein fraction of rice bran. Gynecol. Oncol. 2000;76:170–175. doi: 10.1006/gyno.1999.5669. [DOI] [PubMed] [Google Scholar]
- 204.Kong C.K., Lam W.S., Chiu L.C., Ooi V.E., Sun S.S., Wong Y.S. A rice bran polyphenol, cycloartenyl ferulate, elicits apoptosis in human colorectal adenocarcinoma SW480 and sensitizes metastatic SW620 cells to TRAIL-induced apoptosis. Biochem. Pharmacol. 2009;77:1487–1496. doi: 10.1016/j.bcp.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 205.Norris L., Malkar A., Horner-Glister E., Hakimi A., Ng L.L., Gescher A.J., Jones D.J. Search for novel circulating cancer chemopreventive biomarkers of dietary rice bran intervention in Apc(Min) mice model of colorectal carcinogenesis, using proteomic and metabolic profiling strategies. Mol. Nutr. Food Res. 2015;59:1827–1836. doi: 10.1002/mnfr.201400818. [DOI] [PubMed] [Google Scholar]
- 206.Shafie N.H., Mohd Esa N., Ithnin H., Md Akim A., Saad N., Pandurangan A.K. Preventive inositol hexaphosphate extracted from rice bran inhibits colorectal cancer through involvement of Wnt/beta-catenin and COX-2 pathways. BioMed Res. Int. 2013:681027. doi: 10.1155/2013/681027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Pandurangan A.K., Ismail S., Esa N.M., Munusamy M.A. Inositol-6 phosphate inhibits the mTOR pathway and induces autophagy-mediated death in HT-29 colon cancer cells. Arch. Med. Sci. 2018;14:1281–1288. doi: 10.5114/aoms.2018.76935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Liu G., Song Y., Cui L., Wen Z., & Lu X. Inositol hexaphosphate suppresses growth and induces apoptosis in HT-29 colorectal cancer cells in culture: PI3K/Akt pathway as a potential target. Int. J. Clin. Exp. Pathol. 2015;8:1402–1410. [PMC free article] [PubMed] [Google Scholar]
- 209.Neto C.C. Cranberry and its phytochemicals: A review of in vitro anticancer studies. J. Nutr. 2007;137:186S–193S. doi: 10.1093/jn/137.1.186S. [DOI] [PubMed] [Google Scholar]
- 210.Aiyelaagbe O., Adeniyi B., Fatunsin O., Arimah B. In vitro antimicrobial activity and phytochemical analysis of jatropha curcas roots. Int. J. Pharmacol. 2007;3:106–110. [Google Scholar]
- 211.Broadhurst C.L., Polansky M.M., Anderson R.A. Insulin-like biological activity of culinary and medicinal plant aqueous extracts in vitro. J. Agric. Food Chem. 2000;48:849–852. doi: 10.1021/jf9904517. [DOI] [PubMed] [Google Scholar]
- 212.Kibble M., Saarinen N., Tang J., Wennerberg K., Mäkelä S., Aittokallio T. Network pharmacology applications to map the unexplored target space and therapeutic potential of natural products. Nat. Prod. Rep. 2015;32:1249–1266. doi: 10.1039/C5NP00005J. [DOI] [PubMed] [Google Scholar]
- 213.Russo M., Spagnuolo C., Tedesco I., Russo G.L. Phytochemicals in cancer prevention and therapy: truth or dare? Toxins. 2010;2:517–551. doi: 10.3390/toxins2040517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Budisan L., Gulei D., Zanoaga O.M., Irimie A.I., Sergiu C., Braicu C., Berindan-Neagoe I. Dietary Intervention by Phytochemicals and Their Role in Modulating Coding and Non-Coding Genes in Cancer. Int. J. Mol. Sci. 2017;18:1178. doi: 10.3390/ijms18061178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Dreosti I.E. Recommended dietary intake levels for phytochemicals: Feasible or fanciful? Asia Pac. J. Clin. Nutr. 2000;9:S119–S122. doi: 10.1046/j.1440-6047.2000.00167.x. [DOI] [PubMed] [Google Scholar]
- 216.Most M.M., Tulley R., Morales S., Lefevre M. Rice bran oil, not fiber, lowers cholesterol in humans. Am. J. Clin. Nutr. 2005;81:64–68. doi: 10.1093/ajcn/81.1.64. [DOI] [PubMed] [Google Scholar]
- 217.Lucarini M., Lanzi S., D’Evoli L., Aguizzi A., Lombardi-Boccia G. Intake of vitamin A and carotenoids from the Italian population–results of an Italian total diet study. Int. J. Vitam. Nutr. Res. 2006;76:103–109. doi: 10.1024/0300-9831.76.3.103. [DOI] [PubMed] [Google Scholar]
- 218.Porrini M., Riso P. What are typical lycopene intakes? J. Nutr. 2005;135:2042S–2045S. doi: 10.1093/jn/135.8.2042S. [DOI] [PubMed] [Google Scholar]
- 219.Shao A., Hathcock J.N. Risk assessment for the carotenoids lutein and lycopene. Regul. Toxicol. Pharmacol. 2006;45:289–298. doi: 10.1016/j.yrtph.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 220.Parada J., Aguilera J.M. Food microstructure affects the bioavailability of several nutrients. J. Food Sci. 2007;72:R21–R32. doi: 10.1111/j.1750-3841.2007.00274.x. [DOI] [PubMed] [Google Scholar]
- 221.Correa-Velez I., Clavarino A., Eastwood H. Surviving, relieving, repairing, and boosting up: Reasons for using complementary/alternative medicine among patients with advanced cancer: A thematic analysis. J. Palliat. Med. 2005;8:953–961. doi: 10.1089/jpm.2005.8.953. [DOI] [PubMed] [Google Scholar]
- 222.Fratamico P.M., Wasilenko J.L., Garman B., Demarco D.R., Varkey S., Jensen M. Evaluation of a multiplex real-time PCR method for detecting shiga toxin-producing Escherichia coli in beef and comparison to the U.S. Department of Agriculture Food Safety and Inspection Service Microbiology laboratory guidebook method. J. Food Prot. 2014;77:180–188. doi: 10.4315/0362-028X.JFP-13-248. [DOI] [PubMed] [Google Scholar]
- 223.Gaige S., Djelloul M., Tardivel C., Airault C., Felix B., Jean A. Modification of energy balance induced by the food contaminant T-2 toxin: A multimodal gut-to-brain connection. Brain Behav. Immun. 2014;37:54–72. doi: 10.1016/j.bbi.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 224.Yeung K.S., Gubili J., Cassileth B. Evidence-based botanical research: Applications and challenges. Hematol. Oncol. Clin. North Am. 2008;22:661–670. doi: 10.1016/j.hoc.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 225.Bode A.M., Dong Z. Toxic phytochemicals and their potential risks for human cancer. Cancer Prev. Res. Phila. 2015;8:1–8. doi: 10.1158/1940-6207.CAPR-14-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Perry L., Dickau R., Zarrillo S., Holst I., Pearsall D.M., Piperno D.R. Starch fossils and the domestication and dispersal of chili peppers (Capsicum spp. L.) in the Americas. Science. 2007;315:986–988. doi: 10.1126/science.1136914. [DOI] [PubMed] [Google Scholar]
- 227.Bode A.M., Dong Z. The two faces of capsaicin. Cancer Res. 2011;71:2809–2814. doi: 10.1158/0008-5472.CAN-10-3756. [DOI] [PubMed] [Google Scholar]
- 228.Serra I., Yamamoto M., Calvo A., Cavada G., Baez S., Endoh K. Association of chili pepper consumption, low socioeconomic status and longstanding gallstones with gallbladder cancer in a Chilean population. Int. J. Cancer. 2002;102:407–411. doi: 10.1002/ijc.10716. [DOI] [PubMed] [Google Scholar]
- 229.Kisby G.E., Fry R.C., Lasarev M.R., Bammler T.K., Beyer R.P., Churchwell M. The Cycad Genotoxin MAM Modulates Brain Cellular Pathways Involved in Neurodegenerative Disease and Cancer in a DNA Damage-Linked Manner. PLoS ONE. 2011;6:e20911. doi: 10.1371/journal.pone.0020911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Ward H.A., Kuhnle G.G., Mulligan A.A., Lentjes M.A., Luben R.N., Khaw K.T. Breast, colorectal, and prostate cancer risk in the European Prospective Investigation into Cancer and Nutrition-Norfolk in relation to phytoestrogen intake derived from an improved database. Am. J. Clin. Nutr. 2010;91:440–448. doi: 10.3945/ajcn.2009.28282. [DOI] [PubMed] [Google Scholar]
- 231.Masuda E.K., Kommers G.D., Martins T.B., Barros C.S., Piazer J.V. Morphological factors as indicators of malignancy of squamous cell carcinomas in cattle exposed naturally to bracken fern (Pteridium aquilinum) . J. Comp. Pathol. 2010;144:48–54. doi: 10.1016/j.jcpa.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 232.Liu T.Y., Chung Y.T., Wang P.F., Chi C.W., Hsieh L.L. Safrole-DNA adducts in human peripheral blood–an association with areca quid chewing and CYP2E1 polymorphisms. Mutat. Res. 2004;559:59–66. doi: 10.1016/j.mrgentox.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 233.Ho J.W., Cheung M.W. Combination of phytochemicals as adjuvants for cancer therapy. Recent. Pat. Anticancer Drug. Discov. 2014;9:297–302. doi: 10.2174/1574892809666140619154838. [DOI] [PubMed] [Google Scholar]
- 234.Cho K.J., Wang X., Nie S.M., Chen Z., Shin D.M. Therapeutic nanoparticles for drug delivery in cancer. Clin. Cancer Res. 2008;14:1310–1316. doi: 10.1158/1078-0432.CCR-07-1441. [DOI] [PubMed] [Google Scholar]
- 235.Maeda H. Tumor-selective delivery of macromolecular drugs via the EPR effect: Background and future prospects. Bioconjugate. Chem. 2010;21:797–802. doi: 10.1021/bc100070g. [DOI] [PubMed] [Google Scholar]
- 236.Mansoori G.A., Mohazzabi P., McCormack P. Nanotechnology in cancer prevention, detection and treatment: Bright future lies ahead. World Rev. Sci. Tech. Sust. Dev. 2007;2:226–257. doi: 10.1504/WRSTSD.2007.013584. [DOI] [Google Scholar]
- 237.Khan T., Gurav P. PhytoNanotechnology: Enhancing Delivery of Plant Based Anti-cancer Drugs. Front Pharmacol. 2017;8:1002. doi: 10.3389/fphar.2017.01002. [DOI] [PMC free article] [PubMed] [Google Scholar]