Abstract
Background and objectives: Adenosquamous cancer of the uterine cervix is a rare type of cervical cancer with both malignant squamous and glandular components. A very rare subtype is mucoepidermoid carcinoma (MEC), which was first described as a salivary gland tumor. It has been described as having the appearance of a squamous cell carcinoma without glandular formation and contains intracellular mucin. The postoperative evolution of this tumor and the potentially poorer prognosis may indicate an intensification of the follow-up. The objective of our study was to analyze the frequency of mucoepidermoid carcinoma in hospitalized women with cervical cancer, clinical characteristics and prognosis. Material and Methods: A retrospective study of all cases of mucoepidermoid carcinoma of the cervix at Department of Gynecologic Oncology, University Hospital—Pleven, Pleven Bulgaria between 1 January 2007 and 31 December 2016 was performed. All patients were followed-up till December 2019. We analyzed certain clinical characteristics of the patients; calculated the frequency of mucoepidermoid carcinoma of the cervix from all patients with stage I cervical cancer; and looked at the overall survival rate, correlation between overall survival, lymph node status and the size of the tumor. Results: The frequency of MEC was 1.12% of all patients with stage I cervical cancer in this study. The median age of the patients with MEC was 46.7 years (range 38–62). Four patients (57.1%) were staged as FIGO IB1, and three patients (42.8%) were FIGO IB2. The size of the primary tumor was <2 cm in 2 patients (28.57%), 2–4 cm in 2 patients (28.57%) and >4 cm in 3 patients (42.8%). Metastatic lymph nodes were found in two patients (28.57%), and nonmetastatic lymph nodes were found in five patients (71.43%). There were two (28.57%) disease-related deaths during the study period. The five-year observed survival in the MEC group was 85.7% and in the other subtypes of adenosquamous cancer group was 78.3%. Conclusions: MEC of the uterine cervix is a rare entity diagnosis. As a mucin-producing tumor, it is frequently regarded as a subtype with worse clinical behavior and patients’ outcomes. Nevertheless, our data did not confirm this prognosis. New molecular markers and better stratification are needed for better selection of patients with CC, which may benefit more from additional treatment and new target therapies.
Keywords: mucoepidermoid cervical carcinoma, adenosquamous carcinoma, survival rate, lymph node involvment
1. Introduction
Adenosquamous cancer (ASC) of the uterine cervix is a rare type of cervical cancer (CC), and its frequency is between 3% and 10% [1,2,3]. This tumor is a biphasic variant of CC featuring both malignant squamous and glandular components [4]. ASC of the cervix must be distinguished from large cell nonkeratinising squamous cell carcinomas and endometrioid adenocarcinomas with squamous metaplasia in which the squamous component is benign [3]. Still, it carries genetic and protein signatures of both squamous cell carcinoma (SSC) and adenocarcinoma AC [5]. For diagnosis, it is necessary that both squamous and glandular elements are recognized on routine H&E sections without applying special stains or immunohistochemistry [4]. ASC has a prognosis than SSC [1] and a trend for a worse prognosis than adenocarcinoma AC, which does not reach significance [6] and therefore is not placed in the independent prognostic category. It is usually poorly differentiated/undifferentiated, and is more frequently diagnosed in stage I [1].
ASC of the cervix is extremely rare and when it displays three cells types: epidermoid, mucin-producing and intermediate, it is named mucoepidermoid carcinoma (MEC) [7]. This subtype of adenocarcinoma was first described as a salivary gland tumor by Stewart et al. [8] and occurs in about 30% of all malignant tumors of the major or minor salivary gland [9]. It can also occur in other localizations such as lung and esophagus [10], and it is extremely rarely observed in uterine cervi [11]. It has been described as having the appearance of a squamous cell carcinoma without glandular formation and contains intracellular mucin [11]. There are authors suggesting that the postoperative evolution of this tumor and the potentially poorer prognosis may indicate an intensification of the follow-up [12]. We aimed to investigate the prevalence and the prognosis of MEC among the patients treated for a 10-year period of time in our institution.
2. Material and Methods
An ethical committee approval (number 414-KEHИД/31.03.2016) was obtained to perform a retrospective study of all patients who operated for cervical cancer at Clinic of Oncogynaecology, University Hospital—Pleven, Bulgaria for a 10-year period between 1 January 2007 and 31 December 2016. All mucoepidermoid histologies were centrally reviewed in order to reconfirm the diagnosis. The information regarding demographic characteristics consisted of age at diagnosis, date and type of surgery, and clinical staging according to TNM and FIGO classification; furthermore, postoperative management, postoperative staging, lymph node metastatic and follow-up were obtained from medical history records. Patients were followed up until 1 December 2019. The standard followed up included clinical examination and blood workup every 3 months during the first 2 years and then annually, as well as annual whole-body CT. We compared the investigated clinicopathological characteristics: age at diagnosis, type of surgery, clinical and pathological staging according to TNM and FIGO classification and lymph node status among the patients with MEC and other adenosquamous carcinoma subtypes. We investigated the OS among these two groups. Statistical analysis was performed on SPSS software.
3. Results
During the study period, 624 patients with cervical cancer FIGO I stage were operated on in our clinic. Thirty one patients (4.97%) were diagnosed with adenosquamous carcinoma. Seven of them (22.58% from the adenosquamous carcinoma and 1.12% of all) had histology for MEC, and 24 patients were with other subtypes of adenosquamous carcinoma (control group).
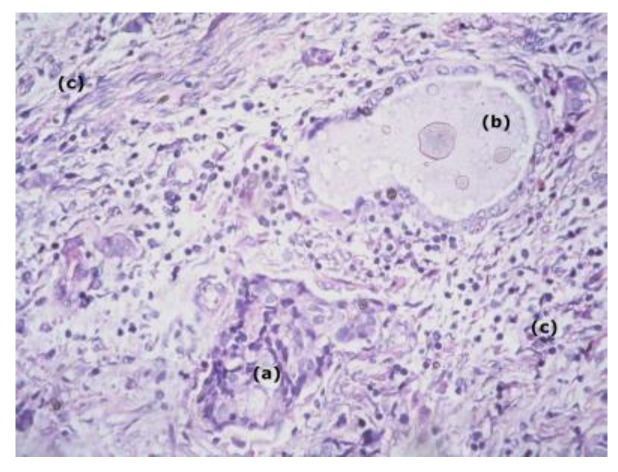
All patients had initially histologically confirmed diagnosis prior to surgery via biopsy or dilation and curettage, and had undergone radical hysterectomy with total pelvic lymph node dissection, followed by postoperative radiotherapy (Figure 1).
Figure 1.
Microscopic finding haematoxylin and eosin stain 200x. (a) epidermoid cells; (b) mucin-producing cells; (c) inmtermediate cells.
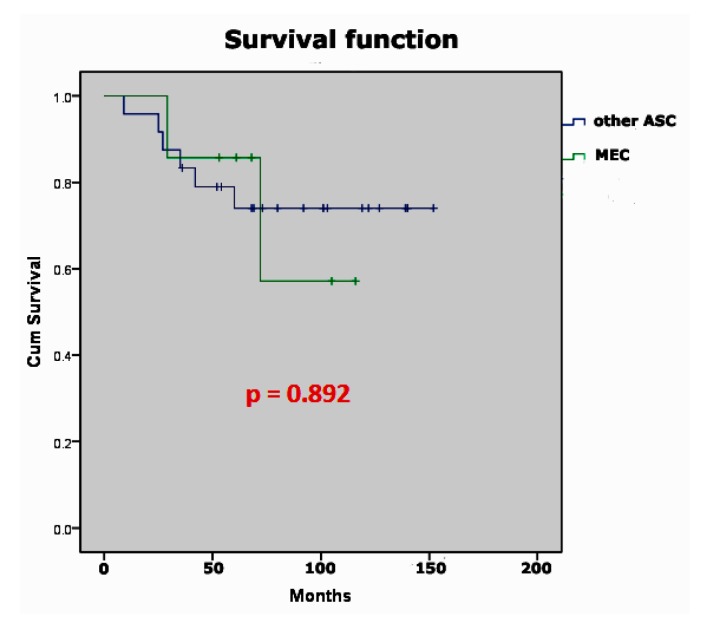
The median age of patients with MEC was 46.7 years (range 38–62). Four patients (57.1%) were staged as FIGO IB1, and three patients (42.8%) were FIGO IB2 stage. Patient’s characteristics are shown in Table 1; Table 2. There were two (28.57%) disease-related deaths during the study period (Table 1). It is ntable that the tumor in those diseased patients was >4 cm and they both had lymph node metastases. The five-year observed survival was higher in the MEC group, but the differences were not statistically significant (p0892, Figure 2).
Table 1.
Patient’s characteristics according to tumor histology.
| Patient’s Characteristics | Other Adenosquamous | MEC |
|---|---|---|
| Mean age at diagnosis | 48 | 47 |
| Size of primary tumor <2, n (%) | 11 (45.8%) | 2 (28.6%) |
| Size of primary tumor 2–4, n (%) | 3 (12.5%) | 2 (28.6%) |
| Size of primary tumor >4, n (%) | 10 (41.7%) | 3 (42.9%) |
| positive lymph nodes (N+) | 8 (33.3%) | 2 (28.6%) |
| Median OS | 70 | 76.5 |
| Five years observed survival | 78.3% | 85.7% |
| Total | 24 | 7 |
Table 2.
Clinical and pathological characteristics of the patients with mucoepidermoid carcinoma (MEC), outcome and survival.
| Case | Age (Years) | Pathological Stage | Size of Primary Tumor (cm) | Outcome | Survival (Months) |
|---|---|---|---|---|---|
| 41 | pT1b2pN1M0 | >4 | Dead | 72 | |
| 2 | 42 | pT1b1pN0M0 | <2 | Alive | 105 |
| 3 | 38 | pT1b2pN1M0 | >4 | Dead | 29 |
| 4 | 41 | pT1b1pN0M0 | B/n 2 and 4 | Alive | 68 |
| 5 | 55 | pT1b1pN0M0 | <2 | Alive | 61 |
| 6 | 48 | pT1b2pN0M0 | >4 | Alive | 116 |
| 7 | 62 | pT1b1pN0M0 | B/n 2 and 4 | Alive | 53 |
Figure 2.
Overall survival of mucoepidermoid cancer of the cervix and other subtypes of adenosquamous cancer of the cervix.
The mean age at diagnosis is almost the same in patients with adenosquamous carcinoma and MEC. Despite the small number of patients, we observed a trend for more lymph node metastasis in patients with pure adenosquamous cancer.
4. Discussion
Cervical cancer is the 4th most common cause of cancer-related death in the world [13]. Treatment for early stage CC includes surgery (such as cervical conization, total simple hysterectomy, or radical hysterectomy) with bilateral lymph node dissection and adjuvant therapy according to the presence of combination of risk factors such as lymph node involvement, and tumor differentiation, but independently of the histological type [14]. MEC of the uterine cervix is a rare subtype of ASC. Its prevalence was very low also among the patients treated in our hospital. There are specific criteria for this diagnosis: prevalence of epidermoid cells, scattered intermediate cells, and cells containing intracellular mucin with no glandular formation. Additionally, MEC tumors show specific genetic rearrangements [15], which could be used in the future development of target therapy [5]. They also show specific immunohistochemical profile (antiCEA stains are positive and antivimentin are negative in cervical MEC carcinoma, whereas in endometrial MEC it is the opposite: antiCEA is negative and antivimentin is positive) [16], which distinguishes them from other cervical cancers and endometrial MEC. It was interesting then for us to investigate if they have different metastatic pattern and survival outcomes.
MECs are mucin-producing tumors, and mucin production is considered to be a worse prognostic factor for those tumors [12]. These tumors were considered to have greater potential for metastatic spread to the regional lymph nodes [17,18,19]. Lymph node metastasis was stated to be an independent prognostic factor for overall survival [11,20].
In the literature, MEC of the uterine cervix is very rarely described [11,21,22] and its frequency is not well reported [21]. Recent data showed that tumors, diagnosed as MEC according to morphology, carry genetic rearrangements and mutations in the same genes, as MECs of the salivary glands [22]. These lead to the suggestion that MEC is a separate disease rather than a subtype of the cervical adenosquamous carcinoma [23], and that it might have more aggressive clinical behavior [11]. In a case series of 12 women, followed for 2–15 years, three of them died within 14 months and they all had lymphogenic and distant metastases. The incidence of lymph node metastases was reported in 33% of the cases.
Kim et al. [22] reported a case of MEC of cervix staged as 1B1. They did not prefer adjuvant therapy; however, the tumor recurred in the fourth month, and the patient died 19 months after surgery.
Different clinical behavior of cervical MEC was also reported by other groups, suggesting different clinical evolution from adenosquamous cancer with higher rates of lymph node metastases as well as comparable radiosensitivity [24].
Despite these differences between MEC and adenosquamous cancer, they are still classified as common conditions and their treatment is similar. Data about the treatment of MEC are scarce, and no management guidelines have been developed. Although data in the literature suggest more aggressive clinical behavior of MEC of the cervix, our data do not confirm this. Data from this study indicate more lymph node metastasis in other ASC sub-types than in the MEC subtype. Our survival data do not show a significant difference in overall survival between MEC and the other subtypes of adenosquamous cancer. The five-year observed survival was higher in MEC group, but not statistically significant.
5. Conclusions
MEC of the uterine cervix is a rare diagnosis. As a mucin-producing tumor, it is frequently regarded as a subtype with worse clinical behavior and patients’ outcomes. Nevertheless, our data did not confirm its poorer prognosis. Still, new molecular markers and better stratification are needed for better selection of patients with CC, who may benefit more from additional treatment and new targeted therapies.
Author Contributions
Conceptualization A.Y., L.T.; Methodology M.K., A.Y.; Formal Analysis A.K., S.S.; Investigation A.Y., L.T.; Resources M.V.-S., M.K.; Data Curation S.S., M.K.; Writing Original and Draft Preparation A.Y., M.K. and A.K.; Writing Review and Editing A.Y., M.V.-S.; Visualization M.V.-S., A.K.; Supervision A.Y., M.V.-S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no competing interests.
References
- 1.Zhou J., Wu S.G., Sun J.Y., Li F.Y., Lin H.X., Chen Q.H., He Z.Y. Comparison of clinical outcomes of squamous cell carcinoma, adenocarcinoma, and adenosquamous carcinoma of the uterine cervix after definitive radiotherapy: A population-based analysis. J. Cancer Res. Clin. Oncol. 2017;143:115–122. doi: 10.1007/s00432-016-2246-9. [DOI] [PubMed] [Google Scholar]
- 2.Cancer Research UK Types and Grades|Cervical Cancer|Cancer Research UK 2019. [(accessed on 12 January 2019)]; Available online: https://www.cancerresearchuk.org/about-cancer/cervical-cancer/stages-types-grades/types-and-grades.
- 3.Yoshida T., Sano T., Oyama T., Kanuma T., Fukuda T. Prevalence, viral load, and physical status of HPV 16 and 18 in cervical adenosquamous carcinoma. Virchows Arch. 2009;455:253–259. doi: 10.1007/s00428-009-0823-x. [DOI] [PubMed] [Google Scholar]
- 4.Perunovic B.S.A. Adenosquamous Carcinoma. PathologyOutlines.com Website 2019. [(accessed on 12 January 2019)]; Available online: http://www.pathologyoutlines.com/topic/cervixadenosquamous.html.
- 5.Imadome K., Iwakawa M., Nakawatari M., Fujita H., Kato S., Ohno T. Subtypes of cervical adenosquamous carcinomas classified by EpCAM expression related to radiosensitivity. Cancer Biol. Ther. 2010;10:1019–1026. doi: 10.4161/cbt.10.10.13249. [DOI] [PubMed] [Google Scholar]
- 6.Yokoi E., Mabuchi S., Takahashi R., Matsumoto Y., Kuroda H., Kozasa K., Kimura T. Impact of histological subtype on survival in patients with locally advanced cervical cancer that were treated with definitive radiotherapy: Adenocarcinoma/adenosquamous carcinoma versus squamous cell carcinoma. J. Gynecol. Oncol. 2017;28:e19. doi: 10.3802/jgo.2017.28.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurman R.J., Carcangiu M.L., Herrington C.S., Young R.H., editors. WHO Classification of Tumours of Female Reproductive Organs. 4th ed. WHO; Geneva, Switzerland: 2014. p. 6. [Google Scholar]
- 8.Stewart F.W., Foote F.W., Becker W.F. Muco-Epidermoid Tumors of Salivary Glands. Ann. Surg. 1945;122:820–844. doi: 10.1097/00000658-194511000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ellis G.L., Auclair P.L. Tumors of the Salivary Glands, Atlas of Tumor Pathology. J. Pathol. 2000;192:564–565. [Google Scholar]
- 10.Liu X., Adams A.L. Mucoepidermoid carcinoma of the bronchus: A review. Arch. Pathol. Lab. Med. 2007;131:1400–1404. doi: 10.5858/2007-131-1400-MCOTBA. [DOI] [PubMed] [Google Scholar]
- 11.Thelmo W.L., Nicastri A.D., Fruchter R., Spring H., DiMaio T., Boyce J. Mucoepidermoid carcinoma of uterine cervix stage IB. Long-term follow-up, histochemical and immunohistochemical study. Int. J. Gynecol. Pathol. 1990;9:316–324. doi: 10.1097/00004347-199010000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Selcuk I., Ozdal B., Turker M., Usubutun A., Gungor T., Meydanli M.M. Mucoepidermoid Carcinoma of Uterine Cervix: A Distinct Pathological and Clinical Entity. Case Rep. Obstet. Gynecol. 2015;2015 doi: 10.1155/2015/491875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 14.Marth C., Landoni F., Mahner S., McCormack M., Gonzalez-Martin A., Colombo N. Cervical cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017;28(Suppl. S4):iv72–iv83. doi: 10.1093/annonc/mdx220. [DOI] [PubMed] [Google Scholar]
- 15.Lennerz J.K., Perry A., Mills J.C., Huettner P.C., Pfeifer J.D. Mucoepidermoid carcinoma of the cervix: Another tumor with the t(11;19)-associated CRTC1-MAML2 gene fusion. Am. J. Surg. Pathol. 2009;33:835–843. doi: 10.1097/PAS.0b013e318190cf5b. [DOI] [PubMed] [Google Scholar]
- 16.Reich T. Gross omental metastasis of mucoepidermoid carcinoma of the uterine cervix: A case report. J. Gynecol. Cancer. 1998;8:349–351. doi: 10.1046/j.1525-1438.1998.09799.x. [DOI] [Google Scholar]
- 17.Husniye Dilek F., Kucukali T. Mucin production in carcinomas of the uterine cervix. Eur. J. Obstet. Gynecol. Reprod. Biol. 1998;79:149–151. doi: 10.1016/S0301-2115(97)00252-2. [DOI] [PubMed] [Google Scholar]
- 18.Benda JA P.C., Buchsbaum H., Lifshitz S. Mucin production in defining mixed carcinoma of the uterine cervix: A clinicopathologic study. Int. J. Gynecol. Pathol. 1985;4:314–327. doi: 10.1097/00004347-198512000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Ireland D., Cole S., Kelly P., Monaghan J.M. Mucin production in cervical intraepithelial neoplasia and in stage 1b carcinoma of cervix with pelvic lymph node metastases. Br. J. Obstet. Gynaecol. 1987;94:467–472. doi: 10.1111/j.1471-0528.1987.tb03127.x. [DOI] [PubMed] [Google Scholar]
- 20.Hale R.J., Wilcox F.L., Buckley C.H., Tindall V.R., Ryder W.D.J., Logueh J.P. Prognostic factors in uterine cervical carcinoma: A clinicopathological analysis. Int. J. Gynecol. Cancer. 1991;1:19–23. doi: 10.1111/j.1525-1438.1991.tb00034.x. [DOI] [Google Scholar]
- 21.Dinges H.P., Werner R., Waidecker F. Mucoepidermoid carcinoma of the cervix uteri. Zent. Gynakol. 1977;99:396–403. [PubMed] [Google Scholar]
- 22.Kim S.-H., Kim H.-J., Lee J.-W., Kim B.-G., Bae D.-S. Mucoepidermoid carcinoma of the cervix: A case report. Korean J. Obstet. Gynecol. 2012;55:791–794. doi: 10.5468/KJOG.2012.55.10.791. [DOI] [Google Scholar]
- 23.World Health Organization Classification of Tumours . In: Pathology and Genetics of Tumours of the Breast and Female Genital Organs. Tavassoli F.A., Devilee P., editors. IARC Press; Lyon, France: 2003. [Google Scholar]
- 24.Tsukahara Y., Kato J., Sakai Y., Ishii J., Fukuta T. Clinicopathologic study of adenosquamous carcinoma of the uterine cervix. Nihon Sanka Fujinka Gakkai Zasshi. 1983;35:2387–2394. [PubMed] [Google Scholar]