Abstract
Toscana virus (TOSV) is endemic in the Mediterranean basin, where it is transmitted by sand flies. TOSV can infect humans and cause febrile illness as well as neuroinvasive infections affecting the central and peripheral nervous systems. Although TOSV is a significant human pathogen, it remains neglected and there are consequently many gaps of knowledge. Recent seroepidemiology studies and case reports showed that TOSV’s geographic distribution is much wider than was assumed a decade ago. The apparent extension of the TOSV circulation area raises the question of the sandfly species that are able to transmit the virus in natural conditions. Phlebotomus (Ph.) perniciosus and Ph. perfiliewi were historically identified as competent species. Recent results suggest that other species of sand flies could be competent for TOSV maintenance and transmission. Here we organize current knowledge in entomology, epidemiology, and virology supporting the possible existence of additional phlebotomine species such as Ph. longicuspis, Ph. sergenti, Ph. tobbi, Ph. neglectus, and Sergentomyia minuta in TOSV maintenance. We also highlight some of the knowledge gaps to be addressed in future studies.
Keywords: Toscana virus, sand fly, Phlebotomus, Sergentomyia, Mediterranean area, Phenuiviridae, Bunyavirales, Sandfly fever Naples phlebovirus, arbovirus, arthropod-borne, sandfly, phlebotomine
1. Introduction
The genus Phlebovirus includes 58 viruses classified into a ten-species complex: Bujaru, Candiru, Chilibre, Frijoles, Punta Toro, Rift Valley fever, Salehabad, Sandfly fever Naples, Severe fever with thrombocytopenia syndrome, and Uukuniemi phleboviruses. The Sandfly fever Naples phlebovirus species comprises thirteen viruses, including Toscana virus (TOSV) [1]. TOSV has a tropism for central and peripheral nervous systems and is responsible for meningitis and encephalitis in the Mediterranean region [2].
Phleboviruses are transmitted to humans by the bite of an infected female sand fly during the blood meal. There is still little information on the development cycle of phleboviruses in the vector [3].
During the last decade, the known geographical area where TOSV circulates has increased considerably. With recent entomological studies, seroepidemiological studies, and case reports, the known distribution of TOSV is now extended in Central and Eastern Europe, North Africa, and Turkey.
We suggest that the existence of a larger diversity of sand fly vectors may explain the TOSV circulation in different geographical regions, which is supported by the recent identification of the virus in phlebotomine species that were not previously considered as TOSV vectors.
Here we present a comprehensive analysis of recent data suggesting that additional species of sand flies are involved in natural cycles to explain the recently revealed broader geographical distribution of TOSV in the Mediterranean region.
2. Toscana Virus
2.1. Overview on Toscana Virus
TOSV is an enveloped, tri-segmented RNA arbovirus which belongs to the genus Phlebovirus, the family Phenuiviridae, and the order Bunyavirales [4]. At the family level, genomes are comprised of three unique molecules of negative or ambisense single-stranded RNA, designated L (large), M (medium), and S (small) to a total of 11 to 19 kb. Within each genus, viruses share similar segment and structural protein sizes, as well as characteristic terminal sequences at the 3’ and 5’ ends of each of the three segments. TOSV distribution and abundance are highly dependent on its arthropod vector. Human cases are observed during the warm season, with a peak during the hottest months in relation with sand fly activity. This virus shows a peculiar neurotropism and causes central nervous system (CNS) diseases in infected individuals [2,5]. Like other arboviruses, most TOSV infections in humans are asymptomatic [6]. So far, 864 symptomatic human cases (834 autochthonous and 30 imported) have been reported in residents of different Mediterranean countries and in tourists and travelers returning from endemic regions [7,8,9,10].
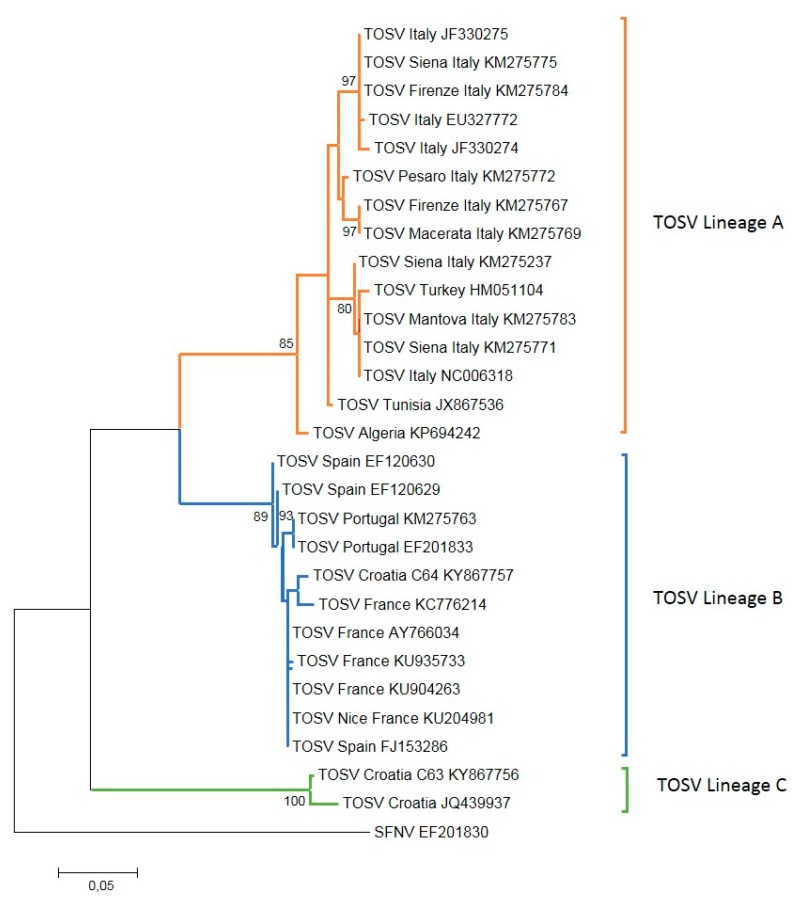
Genetic studies have identified the existence of three lineages of TOSV (A, B, and C) [11] (Figure 1). To date, there is no difference in virulence or clinical manifestations depending on the genetic lineage. At least two lineages co-circulate in France [12], Turkey [13], and Croatia [14]. Further studies are required in order to have more information about the genotype distribution and their association with vector distribution. The known geographical distribution of TOSV in the Mediterranean basin raises the question of whether the demonstrated vectors, Phlebotomus (Ph.) perniciosus and Ph. perfiliewi, can explain this distribution or whether other species could be implicated.
Figure 1.
Phylogenetic analysis of Toscana virus (TOSV) based on the nucleocapsid protein gene. Maximum likelihood analysis at nucleotide level was performed using the MEGA 6.06 software program [15]. Bootstrap values are indicated and correspond to 1000 pseudoreplications.
The knowledge of the vectors and geographical distribution of TOSV is increasing in parallel with the number of studies. The current TOSV distribution includes South European countries (Italy, Spain, France, and Portugal) [16,17,18,19], East Mediterranean countries (Turkey and Cyprus) [13,20], Balkan countries (Greece, Croatia, Bosnia and Herzegovina, Kosovo, and Bulgaria) [21], and North African countries (Tunisia, Morocco, and Algeria) [22]. The geographical distribution of TOSV in the Mediterranean basin raises the following question: Can the known vectors, Ph. perniciosus and Ph. perfiliewi, explain this distribution, or are other species implicated?
2.2. Epidemiology of Toscana Virus
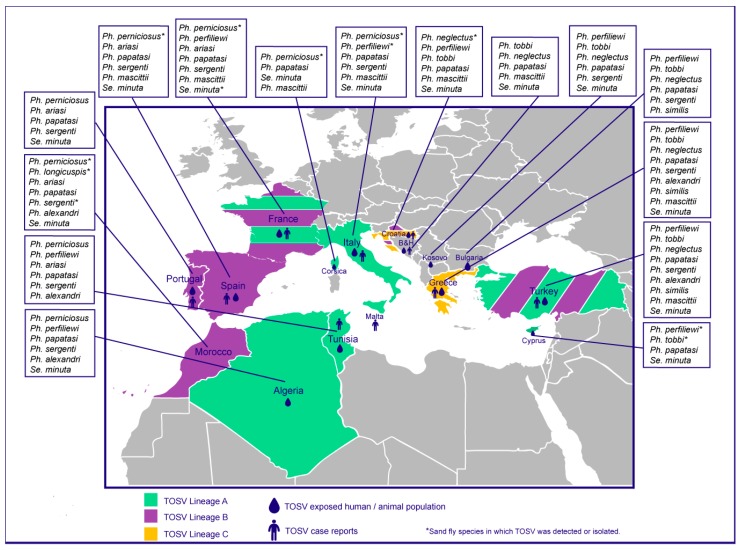
Seroprevalence studies demonstrate that human populations are exposed to TOSV in Italy, Spain, France, Portugal, Turkey, Malta, Cyprus, Greece, Croatia, Bosnia and Herzegovina, Kosovo, Bulgaria, Tunisia, and Algeria [7,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36]. Together, the results of serological surveillance studies and case reports showed that the TOSV geographic distribution is much wider than believed a decade ago; in particular, the presence of TOSV has been recently documented in North Africa and the Balkan countries [31,32,34,35,36] (Figure 2).
Figure 2.
Geographical distribution of Toscana virus (TOSV) and reported distribution of predominant sand fly species in the countries where TOSV is present.
High rates of TOSV-neutralizing antibodies (17.2–59.4%) have been identified in the healthy population of Tunisia, with geographical variation [35,36]. Similar results were obtained in Algeria with a prevalence up to 50% in Draa El Mizan [22]. Although the studies are geographically limited within Tunisia and Algeria, the high rates of neutralizing antibodies emphasize the massive circulation of TOSV in these countries (Figure 2).
In Kosovo, 5.5% of blood donors possess IgG against TOSV [32]. Higher rates were reported in healthy residents of Croatia, with the highest values in coastal areas and Croatian islands [31]. Another study identified the presence of TOSV antibodies in patients from Bosnia and Herzegovina [33]. Recently, anti-TOSV IgGs were found in 24.4% of healthy residents of Bulgaria [34] (Figure 2).
2.3. Pathways of TOSV Transmission and Maintenance
Transovarial and venereal pathways of TOSV transmission have been demonstrated experimentally with Ph. perniciosus colonies [37,38,39]. These experimental results were complemented by the observation in nature of equal rates of TOSV infection in male and female sand flies, suggesting the existence of transovarial (vertical) and/or venereal (horizontal) transmission during mating [40,41,42,43]. Additionally, the survival of TOSV in overwintering Ph. perniciosus larvae underlined the capability of TOSV for maintenance during diapause [37]. Co-feeding transmission of TOSV during sugar meal was also suggested experimentally in males and females since it was demonstrated in Ph. perniciosus with Massilia virus, a relative of TOSV also transmitted by Ph. perniciosus in nature [44]. Future studies need to be conducted with TOSV and other species belonging to the Phlebotomus genus in order to confirm that viral transmission is possible with other species.
3. Sand Flies
3.1. Overview on Sand Flies
Sand flies are tiny blood-feeding (hematophagous) insects belonging to the order Diptera, family Psychodidae, and subfamily Phlebotominae. Approximately 900 sand fly species are described, of which 70 have been identified as potential vectors of Leishmania [45] and a few species were associated with Phlebovirus and other viruses [46]. Each species can be identified by the characteristic shape of their cibarium, pharynx, and reproductive organs [47].
Both genders feed on honeydew, plant sap, and aphid secretions [48], but only females take a blood meal, necessary for egg maturation. Sand flies present a crepuscular and nocturnal activity [49]. They are very abundant in warm regions (e.g., Mediterranean basin, Asia, Africa, South America), but their range is very wide (between 50° N and 40° S). They occur on all continents but have not been reported in New Zealand or the Pacific Islands. Their altitudinal distribution ranges from sea level to 3500 m above sea level (Afghanistan: Ph. rupester) [50,51,52]. Modeling studies suggest an expansion of the range of different Phlebotomus species in Europe due to the influence of environmental and climate change (Ph. ariasi, Ph. mascittii, Ph. perniciosus, Ph. neglectus, and Ph. perfiliewi) allowing the colonization of new habitats [53,54].
However, this insect remains relatively unknown from a biological and ecological point of view (unknown breeding sites, poorly known food preferences, etc.).
3.2. Sandflies as TOSV Vectors
Sandfly activity is seasonal in the Mediterranean countries. They are present from May to October due to low temperatures in winter. The average annual temperatures and the latitude influence the emergence periods and the seasonal dynamics of these insects (with a peak in July and August) [49]. Their activity and abundance are dependent on environmental and climatic conditions and may vary depending on the species and the location of capture.
The first isolation of TOSV was obtained from Ph. perniciosus in central Italy, in 1971 [55]. Subsequently, other strains were isolated from Ph. perfiliewi [56]. The isolation or detection of TOSV in sand flies started to be reported not only in Italy but also in other Mediterranean countries. Ph. perniciosus was identified as vector species of TOSV in Southern France [12], the South-West region of Madrid, Spain [57], and Morocco [58]. Additionally to Ph. perniciosus, Ph. longicuspis and Ph. sergenti are identified as potential vectors of TOSV in Morocco [59]. Another study reported TOSV presence in Se. minuta in France which feeds preferentially on cold-blooded vertebrates [60]. More recently, TOSV was detected from two pools of Ph. neglectus in Croatia [14]. In Cyprus, TOSV was detected in one pool of Ph. perfiliewi and in tow pools of Ph. tobbi [61].
In some other studies, the infected vectors were identified at the genus level only, thus preventing implication of a given species. This was the case in Spain (where almost 70% of captured insects were Ph. perniciosus) [62], Corsica (where Ph. perniciosus and Se. minuta were largely predominant in equal proportions) [63], Algeria (where six species were morphologically identified: Ph. perfiliewi (51.4%), Ph. perniciosus (36.7%), Ph. longicuspis (2.6%), Ph. papatasi (6.5%), Ph. sergenti (0.5%), and Se. minuta (2.3%)) [22], and in Tunisia (where Ph. perniciosus was the most abundant species (71.74%)) [64] (Figure 2).
To date, only TOSV lineage A has been recorded in Ph. perfiliewi [56]. However, at this stage of knowledge, the correlation of TOSV genetic lineage and phlebotomine species is poorly supported (Figure 2). Ph. perniciosus can transmit TOSV strains belonging to lineage A and lineage B [12,55,57,58]. Lineage A was also detected once in Se. minuta [60]. In addition to Ph. perniciosus, Ph. longicuspis, Ph. sergenti, and Ph. neglectus are possible vectors of TOSV strains belonging to lineage B [11,59]. So far, TOSV strains belonging to lineage C have only been recorded in Croatia and Greece, where they are assumed to be transmitted by Ph. neglectus [14] (Figure 2).
In regions where known vector species (e.g., Ph. perniciosus and Ph. perfiliewi) are in the minority or even totally absent (e.g., Bosnia and Herzegovina), seroprevalence rates observed in humans and animals suggest that alternative species could be competent (e.g., Ph. neglectus in Bosnia and Herzegovina). It is therefore important to study the specificities for each country and species in order to better understand the current expansion of TOSV together with its demonstrated and putative vectors.
Climate change affects the geographical distribution of many sand fly species [65,66]. As a consequence, the geographical distribution of sand flies has expanded towards Northern Europe during the last decade. For instance, populations of Ph. perniciosus are now present in new regions in Germany [54,67]. Consequently, TOSV could also spread to these newly colonized regions where competent species are present [68].
Despite the lack of knowledge for many species of sand flies, those belonging to the genus Phlebotomus have benefited from recent efforts from the European Center for Disease Prevention and Control (ECDC) in terms of species inventory and mapping. However, these efforts should be pursued and completed by abundance studies [69]. Regarding Sergentomyia species, very little is known, nevertheless these species deserve more attention due to their possible association with TOSV transmission [60] and their putative role in Leishmania transmission [70,71,72,73].
4. Vertebrates as Possible Reservoir or Amplification Host
The conditions required for a given species to be an efficient reservoir or amplifying host are (i) to be able to generate high and/or sustained viremia for transmission to the competent sand fly species and (ii) to present a geographical distribution equal to, or larger than, that of the disease.
In humans the short duration of viremia together with the absence of persistent infection preclude any role in TOSV maintenance in nature. TOSV was isolated once from the brain of one wild caught bat (Pipistrellus kuhlii) in Italy where TOSV-infected Ph. perniciosus and Ph. perfiliewi were present [56]. This is the first and the only record of TOSV identification from bats.
Many seroprevalence studies testing vertebrate sera have described the presence of variable rates of antibodies directed against TOSV. These studies showed that TOSV circulates in the following countries: Greece [74], Spain [75], Portugal [76,77], France (Corsica island) [78], Tunisia [79], Algeria [80], and Kosovo [81], and can infect dogs, cats, cattle, and sheep. One study reported the presence of TOSV RNA detection in dogs in Portugal [76] and another study detected TOSV RNA in goat serum in southern Spain [75]. These results are very likely to be anecdotic. So far, there is no evidence for a vertebrate species acting as reservoir or amplifying host in the natural cycle of TOSV. Considering that dogs are a reservoir for sand-fly-borne Leishmania infantum, they are good candidates as natural reservoir hosts for TOSV. The fact that neutralizing antibodies against TOSV have been repeatedly described in dogs (4.3–8.4% in studies conducted in France, Portugal, Tunisia, Algeria, Greece, and Cyprus) is likely to reflect exposure rather than being indicative of a more peculiar role in the natural cycle [74,76,77,78,79,80]. However, two studies conducted in dogs from Mediterranean Anatolia (Turkey) reported the presence of TOSV RNA in the blood of approximately 3% and 10% of tested dogs, respectively, of which some were co-infected with Leishmania parasites [82,83]. Co-infection could contribute to this apparent active replication of TOSV. More recently, TOSV was detected in the brain and kidney from a greater flamingo (Phoenicopterus roseus), a great white pelican (Pelecanus onocrotalus), and a black stork (Ciconia nigra) in Turkey [84]. Nevertheless, the role of these animals in the life cycle of TOSV remains unknown. Currently, no data support the hypothesis that humans and/or any other vertebrates are the reservoirs of sand-fly-borne phleboviruses, due to the short duration of the viremia and the lack of persistent infection. The consideration of the vector as the reservoir of phleboviruses is currently under debate [60].
5. Conclusions
Here we highlight that there is very limited information on the biology and epidemiology of TOSV, its reservoirs, and its vectors. As there is neither a vaccine nor a specific treatment, the control of TOSV infections can only be achieved through sand fly control measures (indoor and outdoor residual spraying, attractive toxic sugar baits, etc.) or personal protection against bites (skin repellents, impregnated bed nets, etc.). In order to be able to target regions where these measures could be promoted, it is necessary to know the geographical distribution of populations at risk through seroepidemiological studies and the surveillance of neuro-invasive TOSV infection cases. The recent demonstration that TOSV circulates in a much broader area than suspected a decade ago has raised questions about the possibility that additional phlebotomine species (at least Ph. longicuspis, Ph. sergenti, Ph. tobbi, Ph. neglectus, and Sergentomyia minuta) may be involved in the TOSV natural cycle. Whether these species or others could be competent for the transmission of TOSV merit further study. Accordingly, it is necessary to set up specific studies to address this question (i) in the natural environment and (ii) under experimental conditions using sand fly colonies in insectarium.
Author Contributions
A.-L.B. and R.N.C.: concept of the review. N.A., J.P., L.L., A.-L.B., and R.N.C.: wrote, read, amended, and approved the final version of the manuscript. All authors have read and agree to the published version of the manuscript.
Funding
This research was funded by (i) IRD (Institut de Recherche pour le Développement), CNRS (Centre National de la Recherche Scientifique), (ii) INFRAVEC2 project (https://infravec2.eu/), and (iii) the European Virus Archive goes Global (EVAg) project funded from the European Union’s Horizon 2020 research and innovation program under grant agreement No 653316. N.A. was supported by postdoctoral fellowships from the Institute of Research for Development and from the Université de Corse Pasquale Paoli (UCPP), Corte, France. J.P. was financially supported by INFRAVEC2 project.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Maes P., Alkhovsky S.V., Bao Y., Beer M., Birkhead M., Briese T., Buchmeier M.J., Calisher C.H., Charrel R.N., Choi I.R., et al. Taxonomy of the family Arenaviridae and the order Bunyavirales: Update 2018. Arch. Virol. 2018;163:2295–2310. doi: 10.1007/s00705-018-3843-5. [DOI] [PubMed] [Google Scholar]
- 2.Charrel R., Bichaud L., De Lamballerie X. Emergence of Toscana virus in the mediterranean area. World J. Virol. 2012;1:135. doi: 10.5501/wjv.v1.i5.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tesh R.B., Modi G.B. Studies on the biology of phleboviruses in sand flies (Diptera: Psychodidae). I. Experimental infection of the vector. Am. J. Trop. Med. Hyg. 1984;33:1007–1016. doi: 10.4269/ajtmh.1984.33.1007. [DOI] [PubMed] [Google Scholar]
- 4.King A.M.Q., Lefkowitz E.J., Mushegian A.R., Adams M.J., Dutilh B.E., Gorbalenya A.E., Harrach B., Harrison R.L., Junglen S., Knowles N.J., et al. Changes to taxonomy and the International Code of Virus Classification and Nomenclature ratified by the International Committee on Taxonomy of Viruses (2018) Arch. Virol. 2018;163:2601–2631. doi: 10.1007/s00705-018-3847-1. [DOI] [PubMed] [Google Scholar]
- 5.Charrel R.N., Gallian P., Navarro-Mari J.M., Nicoletti L., Papa A., Sanchez-Seco M.P., Tenorio A., de Lamballerie X. Emergence of Toscana virus in Europe. Emerg. Infect. Dis. 2005;11:1657–1663. doi: 10.3201/eid1111.050869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Braito A., Corbisiero R., Corradini S., Marchi B., Sancasciani N., Fiorentini C., Ciufolini M.G. Evidence of Toscana virus infections without central nervous system involvement: A serological study. Eur. J. Epidemiol. 1997;13:761–764. doi: 10.1023/A:1007422103992. [DOI] [PubMed] [Google Scholar]
- 7.Schultze D., Korte W., Rafeiner P., Niedrig M. First report of sandfly fever virus infection imported from Malta into Switzerland, October 2011. Euro. Surveill. 2012;17 doi: 10.2807/ese.17.27.20209-en. [DOI] [PubMed] [Google Scholar]
- 8.Jaijakul S., Arias C.A., Hossain M., Arduino R.C., Wootton S.H., Hasbun R. Toscana meningoencephalitis: A comparison to other viral central nervous system infections. J. Clin. Virol. 2012;55:204–208. doi: 10.1016/j.jcv.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nougairede A., Bichaud L., Thiberville S.D., Ninove L., Zandotti C., de Lamballerie X., Brouqui P., Charrel R.N. Isolation of Toscana virus from the cerebrospinal fluid of a man with meningitis in Marseille, France, 2010. Vector Borne Zoonotic Dis. 2013;13:685–688. doi: 10.1089/vbz.2013.1316. [DOI] [PubMed] [Google Scholar]
- 10.Karunaratne K., Davies N. Toscana virus meningitis following a holiday in Elba, Italy. Br. J. Hosp. Med. 2018;79:292. doi: 10.12968/hmed.2018.79.5.292. [DOI] [PubMed] [Google Scholar]
- 11.Echevarria J.M., de Ory F., Guisasola M.E., Sanchez-Seco M.P., Tenorio A., Lozano A., Cordoba J., Gobernado M. Acute meningitis due to Toscana virus infection among patients from both the Spanish Mediterranean region and the region of Madrid. J. Clin. Virol. 2003;26:79–84. doi: 10.1016/S1386-6532(02)00041-0. [DOI] [PubMed] [Google Scholar]
- 12.Eitrem R., Vene S., Niklasson B. Incidence of sand fly fever among Swedish United Nations soldiers on Cyprus during 1985. Am. J. Trop. Med. Hyg. 1990;43:207–211. doi: 10.4269/ajtmh.1990.43.207. [DOI] [PubMed] [Google Scholar]
- 13.Hemmersbach-Miller M., Parola P., Charrel R.N., Paul Durand J., Brouqui P. Sandfly fever due to Toscana virus: An emerging infection in southern France. Eur. J. Intern. Med. 2004;15:316–317. doi: 10.1016/j.ejim.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 14.Ayhan N., Charrel R.N. Emergent Sand Fly–Borne Phleboviruses in the Balkan Region. Emerg. Infect. Dis. 2018;24:2324–2330. doi: 10.3201/eid2412.171626. [DOI] [Google Scholar]
- 15.Alkan C., Allal-Ikhlef A.B., Alwassouf S., Baklouti A., Piorkowski G., de Lamballerie X., Izri A., Charrel R.N. Virus isolation, genetic characterization and seroprevalence of Toscana virus in Algeria. Clin. Microbiol. Infect. 2015;21:1040. doi: 10.1016/j.cmi.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 16.Ayhan N., Charrel R.N. Of phlebotomines (sandflies) and viruses: A comprehensive perspective on a complex situation. Curr. Opin. Insect Sci. 2017;22:117–124. doi: 10.1016/j.cois.2017.05.019. [DOI] [PubMed] [Google Scholar]
- 17.Charrel R.N., Izri A., Temmam S., Delaunay P., Toga I., Dumon H., Marty P., de Lamballerie X., Parola P. Cocirculation of 2 genotypes of Toscana virus, southeastern France. Emerg. Infect. Dis. 2007;13:465–468. doi: 10.3201/eid1303.061086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ergunay K., Ayhan N., Charrel R.N. Novel and emergent sandfly-borne phleboviruses in Asia Minor: A systematic review. Rev. Med. Virol. 2017;27 doi: 10.1002/rmv.1898. [DOI] [PubMed] [Google Scholar]
- 19.Ayhan N., Alten B., Ivovic V., Martinkovic F., Kasap O.E., Ozbel Y., de Lamballerie X., Charrel R.N. Cocirculation of Two Lineages of Toscana Virus in Croatia. Front. Public Health. 2017;5:336. doi: 10.3389/fpubh.2017.00336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peyrefitte C.N., Devetakov I., Pastorino B., Villeneuve L., Bessaud M., Stolidi P., Depaquit J., Segura L., Gravier P., Tock F., et al. Toscana virus and acute meningitis, France. Emerg. Infect. Dis. 2005;11:778–780. doi: 10.3201/eid1105.041122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mendoza-Montero J., Gamez-Rueda M.I., Navarro-Mari J.M., de la Rosa-Fraile M., Oyonarte-Gomez S. Infections due to sandfly fever virus serotype Toscana in Spain. Clin. Infect. Dis. 1998;27:434–436. doi: 10.1086/514684. [DOI] [PubMed] [Google Scholar]
- 23.Anagnostou V., Papa A. Seroprevalence of Toscana virus among residents of Aegean Sea islands, Greece. Travel Med. Infect. Dis. 2013;11:98–102. doi: 10.1016/j.tmaid.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 24.De Lamballerie X., Tolou H., Durand J.P., Charrel R.N. Prevalence of Toscana virus antibodies in volunteer blood donors and patients with central nervous system infections in southeastern France. Vector Borne Zoonotic Dis. 2007;7:275–277. doi: 10.1089/vbz.2006.0637. [DOI] [PubMed] [Google Scholar]
- 25.Eitrem R., Stylianou M., Niklasson B. High prevalence rates of antibody to three sandfly fever viruses (Sicilian, Naples, and Toscana) among Cypriots. Epidemiol. Infect. 1991;107:685–691. doi: 10.1017/S0950268800049384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brisbarre N., Attoui H., Gallian P., Di Bonito P., Giorgi C., Cantaloube J.F., Biagini P., Touinssi M., Jordier F., de Micco P. Seroprevalence of Toscana virus in blood donors, France, 2007. Emerg. Infect. Dis. 2011;17:941–943. doi: 10.3201/eid1705.101052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ergunay K., Aydogan S., Ilhami Ozcebe O., Cilek E.E., Hacioglu S., Karakaya J., Ozkul A., Us D. Toscana virus (TOSV) exposure is confirmed in blood donors from Central, North and South/Southeast Anatolia, Turkey. Zoonoses Public Health. 2012;59:148–154. doi: 10.1111/j.1863-2378.2011.01436.x. [DOI] [PubMed] [Google Scholar]
- 28.Papa A., Andriotis V., Tzilianos M. Prevalence of Toscana virus antibodies in residents of two Ionian islands, Greece. Travel Med. Infect. Dis. 2010;8:302–304. doi: 10.1016/j.tmaid.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 29.Santos L., Cardoso M.J., Marinho A.S., Guimaraes T., Sarmento A. [Seroprevalence survey of Toscana virus infection in Oporto region] Acta Med. Port. 2011;24:479–482. [PubMed] [Google Scholar]
- 30.Terrosi C., Olivieri R., Bianco C., Cellesi C., Cusi M.G. Age-dependent seroprevalence of Toscana virus in central Italy and correlation with the clinical profile. Clin. Vaccine Immunol. 2009;16:1251–1252. doi: 10.1128/CVI.00376-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Punda-Polic V., Jeroncic A., Mohar B., Sisko Kraljevic K. Prevalence of Toscana virus antibodies in residents of Croatia. Clin. Microbiol. Infect. 2012;18:E200–E203. doi: 10.1111/j.1469-0691.2012.03840.x. [DOI] [PubMed] [Google Scholar]
- 32.Venturi G., Marchi A., Fiorentini C., Ramadani N., Quaglio G., Kalaveshi A., Bertinato L., Putoto G., Benedetti E., Rezza G., et al. Prevalence of antibodies to phleboviruses and flaviviruses in Peja, Kosovo. Clin. Microbiol. Infect. 2011;17:1180–1182. doi: 10.1111/j.1469-0691.2010.03445.x. [DOI] [PubMed] [Google Scholar]
- 33.Hukic M., Salimovic-Besic I. Sandfly—Pappataci fever in Bosnia and Herzegovina: The new-old disease. Bosn. J. Basic Med. Sci. 2009;9:39–43. doi: 10.17305/bjbms.2009.2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Christova I., Panayotova E., Trifonova I., Taseva E., Gladnishka T., Ivanova V. Serologic evidence of widespread Toscana virus infection in Bulgaria. J. Infect. Public Health. 2019 doi: 10.1016/j.jiph.2019.07.008. [DOI] [PubMed] [Google Scholar]
- 35.Fezaa O., Bahri O., Alaya Bouafif N.B., Triki H., Bouattour A. Seroprevalence of Toscana virus infection in Tunisia. Int. J. Infect. Dis. 2013;17:e1172–e1175. doi: 10.1016/j.ijid.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 36.Sakhria S., Bichaud L., Mensi M., Salez N., Dachraoui K., Thirion L., Cherni S., Chelbi I., De Lamballerie X., Zhioua E., et al. Co-circulation of Toscana virus and Punique virus in northern Tunisia: A microneutralisation-based seroprevalence study. PLoS Negl. Trop. Dis. 2013;7:e2429. doi: 10.1371/journal.pntd.0002429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tesh R.B., Lubroth J., Guzman H. Simulation of arbovirus overwintering: Survival of Toscana virus (Bunyaviridae: Phlebovirus) in its natural sand fly vector Phlebotomus perniciosus. Am. J. Trop. Med. Hyg. 1992;47:574–581. doi: 10.4269/ajtmh.1992.47.574. [DOI] [PubMed] [Google Scholar]
- 38.Maroli M., Ciufolini M.G., Verani P. Vertical transmission of Toscana virus in the sandfly, Phlebotomus perniciosus, via the second gonotrophic cycle. Med. Vet. Entomol. 1993;7:283–286. doi: 10.1111/j.1365-2915.1993.tb00689.x. [DOI] [PubMed] [Google Scholar]
- 39.Tesh R.B., Modi G.B. Maintenance of Toscana virus in Phlebotomus perniciosus by vertical transmission. Am. J. Trop. Med. Hyg. 1987;36:189–193. doi: 10.4269/ajtmh.1987.36.189. [DOI] [PubMed] [Google Scholar]
- 40.Peyrefitte C.N., Grandadam M., Bessaud M., Andry P.-E., Fouque F., Caro V., Diancourt L., Schuffenecker I., Pagès F., Tolou H., et al. Diversity of Phlebotomus perniciosus in Provence, Southeastern France: Detection of Two Putative New Phlebovirus Sequences. Vector Borne Zoonotic Dis. 2013;13:630–636. doi: 10.1089/vbz.2012.1169. [DOI] [PubMed] [Google Scholar]
- 41.Zhioua E., Moureau G., Chelbi I., Ninove L., Bichaud L., Derbali M., Champs M., Cherni S., Salez N., Cook S., et al. Punique virus, a novel phlebovirus, related to sandfly fever Naples virus, isolated from sandflies collected in Tunisia. J. Gen. Virol. 2010;91:1275–1283. doi: 10.1099/vir.0.019240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alkan C., Alwassouf S., Piorkowski G., Bichaud L., Tezcan S., Dincer E., Ergunay K., Ozbel Y., Alten B., de Lamballerie X., et al. Isolation, genetic characterization, and seroprevalence of Adana virus, a novel phlebovirus belonging to the Salehabad virus complex, in Turkey. J. Virol. 2015;89:4080–4091. doi: 10.1128/JVI.03027-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Remoli M.E., Fortuna C., Marchi A., Bucci P., Argentini C., Bongiorno G., Maroli M., Gradoni L., Gramiccia M., Ciufolini M.G. Viral isolates of a novel putative phlebovirus in the Marche Region of Italy. Am. J. Trop. Med. Hyg. 2014;90:760–763. doi: 10.4269/ajtmh.13-0457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jancarova M., Bichaud L., Hlavacova J., Priet S., Ayhan N., Spitzova T., Volf P., Charrel R.N. Experimental infection of sand flies by Massilia virus and viral transmission by co-feeding on sugar meal. Viruses. 2019;11:332. doi: 10.3390/v11040332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ready P. Biology of Phlebotomine Sand Flies as Vectors of Disease Agents. Annu. Rev. Entomol. 2013;58:227–250. doi: 10.1146/annurev-ento-120811-153557. [DOI] [PubMed] [Google Scholar]
- 46.Rueda L.M., Pecor J.E., Wolkoff M., Pecor D., Benyamin S., Bousses P., Debboun M. New records, distribution, and updated checklists of old world Phlebotomine sand flies, with emphasis on Africa, southwest Asia, and central Asia. US Army Med. Dep. J. 2017;1–17:6–85. [PubMed] [Google Scholar]
- 47.Abonnenc E. Les Phlébotomes de la Région Éthiopienne (Diptera, Psychodidae) Service Central de Documentation de l’ORSTOM; Bondy, France: 1972. pp. 1–290. [Google Scholar]
- 48.Muller G., Schlein Y. Nectar and honeydew feeding of Phlebotomus papatasi in a focus of Leishmania major in Neot Hakikar oasis. J. Vector Ecol. 2004;29:154–158. [PubMed] [Google Scholar]
- 49.Alten B., Maia C., Afonso O., Campino L., Jiménez M., González E., Molina R., Bañuls A.L., Prudhomme J., Vergnes B., et al. Seasonal dynamics of phlebotomine sand fly species proven vectors of Mediterranean leishmaniasis caused by Leishmania infantum. PLoS Negl. Trop. Dis. 2016;10:e0004458. doi: 10.1371/journal.pntd.0004458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Killick-Kendrick R. The biology and control of phlebotomine sand flies. Clin. Dermatol. 1999;17:279–289. doi: 10.1016/S0738-081X(99)00046-2. [DOI] [PubMed] [Google Scholar]
- 51.Lane R. Medical Insects and Arachnids. Springer; Dordrecht, Germany: 1993. Sandflies (Phlebotominae) pp. 78–119. [Google Scholar]
- 52.Alten B., Ozbel Y., Ergunay K., Kasap O., Cull B., Antoniou M., Velo E., Prudhomme J., Molina R., Bañuls A., et al. Sampling strategies for phlebotomine sand flies (Diptera: Psychodidae) in Europe. Bull. Entomol. Res. 2015;105:664–678. doi: 10.1017/S0007485315000127. [DOI] [PubMed] [Google Scholar]
- 53.Fischer D., Moeller P., Thomas S., Naucke T., Beierkuhnlein C. Combining Climatic Projections and Dispersal Ability: A Method for Estimating the Responses of Sandfly Vector Species to Climate Change. PLoS Negl. Trop. Dis. 2011;5:e1407. doi: 10.1371/journal.pntd.0001407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Naucke T., Menn B., Massberg D., Lorentz S. Sandflies and leishmaniasis in Germany. Parasitol. Res. 2008;103:65–68. doi: 10.1007/s00436-008-1052-y. [DOI] [PubMed] [Google Scholar]
- 55.Verani P., Lopes M., Nicoletti L., Balducci M. Studies on Phlebotomus-transmitted viruses in Italy. I. Isolation and characterization of a sandfly fever Naples-like virus. Arboviruses Mediterr. Ctries. Stuttg. Gustav Fischer Verl. 1980;(Suppl. 9):195–201. [Google Scholar]
- 56.Verani P., Ciufolini M.G., Caciolli S., Renzi A., Nicoletti L., Sabatinelli G., Bartolozzi D., Volpi G., Amaducci L., Coluzzi M., et al. Ecology of viruses isolated from sand flies in Italy and characterized of a new Phlebovirus (Arabia virus) Am. J. Trop. Med. Hyg. 1988;38:433–439. doi: 10.4269/ajtmh.1988.38.433. [DOI] [PubMed] [Google Scholar]
- 57.Remoli M.E., Jimenez M., Fortuna C., Benedetti E., Marchi A., Genovese D., Gramiccia M., Molina R., Ciufolini M.G. Phleboviruses detection in Phlebotomus perniciosus from a human leishmaniasis focus in South-West Madrid region, Spain. Parasit. Vectors. 2016;9:205. doi: 10.1186/s13071-016-1488-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Es-Sette N., Nourlil J., Hamdi S., Mellouki F., Lemrani M. First detection of Toscana virus RNA from sand flies in the genus Phlebotomus (Diptera: Phlebotomidae) naturally infected in Morocco. J. Med. Entomol. 2012;49:1507–1509. doi: 10.1603/ME12042. [DOI] [PubMed] [Google Scholar]
- 59.Es-Sette N., Ajaoud M., Anga L., Mellouki F., Lemrani M. Toscana virus isolated from sandflies, Morocco. Parasit. Vectors. 2015;8:205–208. doi: 10.1186/s13071-015-0826-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Charrel R.N., Izri A., Temmam S., de Lamballerie X., Parola P. Toscana virus RNA in Sergentomyia minuta files. Emerg. Infect. Dis. 2006;12:1299–1300. doi: 10.3201/eid1708.060345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ergunay K., Kasap O.E., Orsten S., Oter K., Gunay F., Yoldar A.Z., Dincer E., Alten B., Ozkul A. Phlebovirus and Leishmania detection in sandflies from eastern Thrace and Northern Cyprus. Parasit. Vectors. 2014;7:575. doi: 10.1186/s13071-014-0575-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sanbonmatsu-Gamez S., Perez-Ruiz M., Collao X., Sanchez-Seco M.P., Morillas-Marquez F., de la Rosa-Fraile M., Navarro-Mari J.M., Tenorio A. Toscana virus in Spain. Emerg. Infect. Dis. 2005;11:1701–1707. doi: 10.3201/eid1111.050851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bichaud L., Izri A., Lamballerie X., Moureau G., Charrel R. First detection of Toscana virus in Corsica, France. Clin. Microbiol. Infect. 2014;20:O101–O104. doi: 10.1111/1469-0691.12347. [DOI] [PubMed] [Google Scholar]
- 64.Bichaud L., Dachraoui K., Piorkowski G., Chelbi I., Moureau G., Cherni S., De Lamballerie X., Sakhria S., Charrel R.N., Zhioua E. Toscana virus isolated from sandflies, Tunisia. Emerg. Infect. Dis. 2013;19:322–324. doi: 10.3201/eid1902.121463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chalghaf B., Chemkhi J., Mayala B., Harrabi M., Benie G.B., Michael E., Ben Salah A. Ecological niche modeling predicting the potential distribution of Leishmania vectors in the Mediterranean basin: Impact of climate change. Parasit. Vectors. 2018;11:461–470. doi: 10.1186/s13071-018-3019-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Koch L.K., Kochmann J., Klimpel S., Cunze S. Modeling the climatic suitability of leishmaniasis vector species in Europe. Sci. Rep. 2017;7:13325. doi: 10.1038/s41598-017-13822-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mencke N. The importance of canine leishmaniosis in non-endemic areas, with special emphasis on the situation in Germany. Berl. Munch. Tierarztl. Wochenschr. 2011;124:434–442. [PubMed] [Google Scholar]
- 68.Depaquit J., Grandadam M., Fouque F., Andry P.-E., Peyrefitte C. Arthropod-borne viruses transmitted by Phlebotomine sandflies in Europe: A review. Euro. Surveill. 2010;15:19507. [PubMed] [Google Scholar]
- 69.European Centre for Disease Prevention and Control Phlebotomine Sand Flies Maps. [(accessed on 1 October 2019)]; Available online: https://www.ecdc.europa.eu/en/disease-vectors/surveillance-and-disease-data/phlebotomine-maps.
- 70.Senghor M.W., Niang A.A., Depaquit J., Ferte H., Faye M.N., Elguero E., Gaye O., Alten B., Perktas U., Cassan C., et al. Transmission of Leishmania infantum in the Canine Leishmaniasis Focus of Mont-Rolland, Senegal: Ecological, Parasitological and Molecular Evidence for a Possible Role of Sergentomyia Sand Flies. PLoS Negl. Trop. Dis. 2016;10:e0004940. doi: 10.1371/journal.pntd.0004940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jaouadi K., Ghawar W., Salem S., Gharbi M., Bettaieb J., Yazidi R., Harrabi M., Hamarsheh O., Ben Salah A. First report of naturally infected Sergentomyia minuta with Leishmania major in Tunisia. Parasit. Vectors. 2015;8:649. doi: 10.1186/s13071-015-1269-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Maia C., Depaquit J. Can Sergentomyia (Diptera, Psychodidae) play a role in the transmission of mammal-infecting Leishmania? Parasite. 2016;23:55. doi: 10.1051/parasite/2016062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bravo-Barriga D., Parreira R., Maia C., Afonso M.O., Blanco-Ciudad J., Serrano F.J., Perez-Martin J.E., Gomez-Gordo L., Campino L., Reina D., et al. Detection of Leishmania DNA and blood meal sources in phlebotomine sand flies (Diptera: Psychodidae) in western of Spain: Update on distribution and risk factors associated. Acta Trop. 2016;164:414–424. doi: 10.1016/j.actatropica.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 74.Alwassouf S., Christodoulou V., Bichaud L., Ntais P., Mazeris A., Antoniou M., Charrel R.N. Seroprevalence of Sandfly-Borne Phleboviruses Belonging to Three Serocomplexes (Sandfly fever Naples, Sandfly fever Sicilian and Salehabad) in Dogs from Greece and Cyprus Using Neutralization Test. PLoS Negl. Trop. Dis. 2016;10:e0005063. doi: 10.1371/journal.pntd.0005063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Navarro-Mari J.M., Palop-Borras B., Perez-Ruiz M., Sanbonmatsu-Gamez S. Serosurvey study of Toscana virus in domestic animals, Granada, Spain. Vector Borne Zoonotic Dis. 2011;11:583–587. doi: 10.1089/vbz.2010.0065. [DOI] [PubMed] [Google Scholar]
- 76.Alwassouf S., Maia C., Ayhan N., Coimbra M., Cristovao J.M., Richet H., Bichaud L., Campino L., Charrel R.N. Neutralization-based seroprevalence of Toscana virus and sandfly fever Sicilian virus in dogs and cats from Portugal. J. Gen. Virol. 2016;97:2816–2823. doi: 10.1099/jgv.0.000592. [DOI] [PubMed] [Google Scholar]
- 77.Maia C., Alwassouf S., Cristovao J.M., Ayhan N., Pereira A., Charrel R.N., Campino L. Serological association between Leishmania infantum and sand fly fever Sicilian (but not Toscana) virus in sheltered dogs from southern Portugal. Parasit. Vectors. 2017;10:92. doi: 10.1186/s13071-017-2023-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dahmani M., Alwassouf S., Grech-Angelini S., Marie J.L., Davoust B., Charrel R.N. Seroprevalence of Toscana virus in dogs from Corsica, France. Parasit. Vectors. 2016;9:381. doi: 10.1186/s13071-016-1665-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sakhria S., Alwassouf S., Fares W., Bichaud L., Dachraoui K., Alkan C., Zoghlami Z., De Lamballerie X., Zhioua E., Charrel R.N. Presence of sandfly-borne phleboviruses of two antigenic complexes (Sandfly fever Naples virus and Sandfly fever Sicilian virus) in two different bio-geographical regions of Tunisia demonstrated by microneutralisation-based seroprevalence study in dogs. Parasit. Vectors. 2014;7:476. doi: 10.1186/s13071-014-0476-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tahir D., Alwassouf S., Loudahi A., Davoust B., Charrel R.N. Seroprevalence of Toscana virus in dogs from Kabylia (Algeria) Clin. Microbiol. Infect. 2016;22:e16–e17. doi: 10.1016/j.cmi.2015.10.029. [DOI] [PubMed] [Google Scholar]
- 81.Ayhan N., Sherifi K., Taraku A., Berxholi K., Charrel R.N. High Rates of Neutralizing Antibodies to Toscana and Sandfly Fever Sicilian Viruses in Livestock, Kosovo. Emerg. Infect. Dis. 2017;23:989–992. doi: 10.3201/eid2306.161929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dincer E., Karapinar Z., Oktem M., Ozbaba M., Ozkul A., Ergunay K. Canine Infections and Partial S Segment Sequence Analysis of Toscana Virus in Turkey. Vector Borne Zoonotic Dis. 2016;16:611–618. doi: 10.1089/vbz.2016.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dincer E., Gargari S., Ozkul A., Ergunay K. Potential animal reservoirs of Toscana virus and coinfections with Leishmania infantum in Turkey. Am. J. Trop. Med. Hyg. 2015;92:690–697. doi: 10.4269/ajtmh.14-0322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hacioglu S., Dincer E., Isler C.T., Karapinar Z., Ataseven V.S., Ozkul A., Ergunay K. A Snapshot Avian Surveillance Reveals West Nile Virus and Evidence of Wild Birds Participating in Toscana Virus Circulation. Vector Borne Zoonotic Dis. 2017;17:698–708. doi: 10.1089/vbz.2017.2138. [DOI] [PubMed] [Google Scholar]