Abstract
This article provides an overview of current research into the development, synthesis, photocatalytic bacterial activity, biocompatibility and cytotoxic properties of various visible-light active titanium dioxide (TiO2) nanoparticles (NPs) and their nanocomposites. To achieve antibacterial inactivation under visible light, TiO2 NPs are doped with metal and non-metal elements, modified with carbonaceous nanomaterials, and coupled with other metal oxide semiconductors. Transition metals introduce a localized d-electron state just below the conduction band of TiO2 NPs, thereby narrowing the bandgap and causing a red shift of the optical absorption edge into the visible region. Silver nanoparticles of doped TiO2 NPs experience surface plasmon resonance under visible light excitation, leading to the injection of hot electrons into the conduction band of TiO2 NPs to generate reactive oxygen species (ROS) for bacterial killing. The modification of TiO2 NPs with carbon nanotubes and graphene sheets also achieve the efficient creation of ROS under visible light irradiation. Furthermore, titanium-based alloy implants in orthopedics with enhanced antibacterial activity and biocompatibility can be achieved by forming a surface layer of Ag-doped titania nanotubes. By incorporating TiO2 NPs and Cu-doped TiO2 NPs into chitosan or the textile matrix, the resulting polymer nanocomposites exhibit excellent antimicrobial properties that can have applications as fruit/food wrapping films, self-cleaning fabrics, medical scaffolds and wound dressings. Considering the possible use of visible-light active TiO2 nanomaterials for various applications, their toxicity impact on the environment and public health is also addressed.
Keywords: antibacterial activity, photocatalyst, titania, nanomaterial, doping, Staphylococcus aureus, Escherichia coli, reactive oxygen species, silver nanoparticle, visible light
1. Introduction
The overuse of antimicrobials in humans, animal husbandry and aquafarming gives rise to the development of dangerous, antibiotic-resistant bacteria [1,2]. Infections caused by antibiotic-resistant bacteria are now emerging as worldwide public health challenges. Medicines find it harder to treat infections, increasing the risk of mortality and morbidity. For instance, Staphylococci such as Staphylococcus aureus (S. aureus) and Staphylococcus epidermidis (S. epidermidis), that cause orthopedic infections (e.g., osteomyelitis), have developed into methicillin-resistant S. aureus (MRSA) and methicillin-resistant S. epidermidis (MRSE). MRSA is capable of forming biofilms on medical devices, giving rise to antibiotic resistance [3,4]. Osteomyelitis is a bone infection induced by Staphylococci, leading to progressive bone loss and tissue damage. Moreover, multidrug-resistant (MDR) bacteria spread not only between hospital inpatients, but also through food chains and potable water [5]. Accordingly, researchers have concentrated on developing antimicrobial nanomaterials as alternatives to conventional antibiotics [6,7,8,9].
Current developments in nanoscience and nanotechnology have led to the creation of advanced functional nanomaterials with unique chemical, physical, and biological properties [9,10,11,12,13,14,15,16,17]. Nanomaterials with large, specific surface area-to-volume ratios enhance surface chemical reactivity due to the size reduction at the nanoscale. Thus, nanomaterials have opened up new opportunities for developing bactericidal agents to treat deadly microbial infections [18]. In particular, metal and metal oxide nanoparticles (NPs) have attracted great attention as promising candidates for antibacterial agents [19,20]. The main mechanisms of the antibacterial activities of those nanoparticles proposed in the literature include: (a) oxidative stress induction associated with the generation of reactive oxygen species (ROS) [21], where the oxidation process in bacterial cells causes peroxidation of the lipid membrane, thereby damaging proteins and DNA; (b) released metal ions from metal or metal oxide NPs penetrating through bacterial cell walls, directly interacting with the –SH, –NH and –COOH groups of nucleic acid and protein and eventually causing cell death [15,22]. For example, silver nanoparticles (AgNPs) have been employed as antibacterial agents for textile fabrics, healthcare products, cosmetics, coatings and wound dressings, because they exhibit relatively high bactericidal activity [15,23,24,25,26,27]. However, AgNPs are toxic for several human cell lines. This is because they induce a dose-, size- and time-dependent cytotoxicity, especially those with sizes of ≤10 nm [15].
Compared to other types of nanoparticles, titanium dioxide is particularly attractive for photocatalytic bactericidal activity because of its relatively low cost, natural abundance and superior chemical stability. Titanium dioxide (TiO2), generally known as titania, is an n-type semiconductor due to the presence of oxygen vacancies [28,29]. Those oxygen vacancies favor the formation of unpaired electrons or Ti3+ centers, thus acting as electron donors in the electronic structure of TiO2 [28]. Furthermore, oxygen vacancies can influence the charge transport and electron–hole recombination processes by trapping charge carriers in the defect sites [30,31,32,33]. Titania also has a high dielectric permittivity (κ = 50–80) that finds application as a gate insulator in the microelectronic industry. However, TiO2 with a bandgap of 3.2 eV suffers from a large leakage current and low dielectric breakdown field. In contrast, HfO2 with a larger bandgap (5.3–5.7 eV) is widely used as a high-κ gate dielectric material in the microelectronic sector [34].
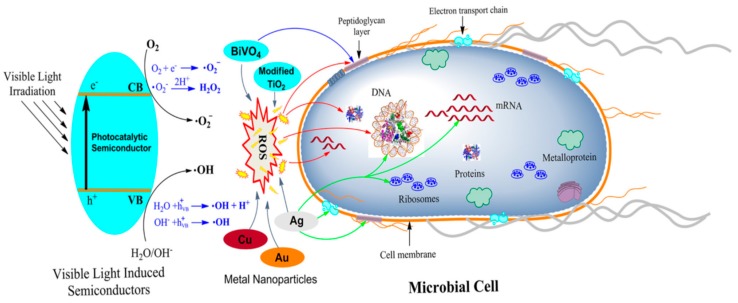
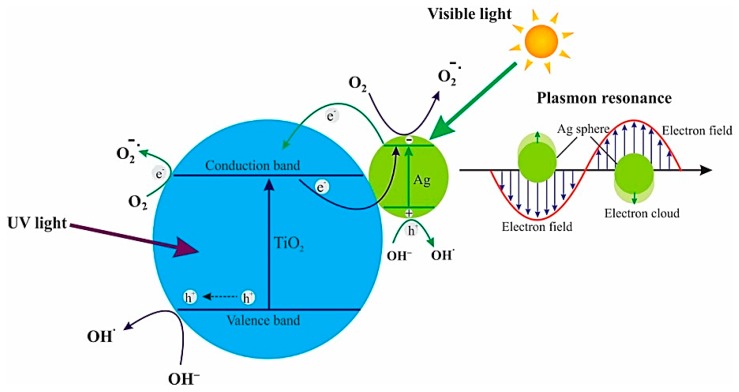
By irradiating photocatalytic semiconductors with a photon of sufficient energy (≥band gap energy), an electron in the valence band (VB) is excited to the conduction band (CB), leaving a positive hole in the VB. These charge carriers migrate to the photocatalyst surface and can generate highly reactive oxygen species (ROS) such as hydroxyl (•OH) and superoxide anion (O2−) radicals, and hydrogen peroxide (H2O2) through the oxidative or reductive path with surface-adsorbed water and oxygen (Figure 1). Hydroxyl and superoxide species are highly reactive due to the presence of unpaired valence shell electrons, and can cause oxidative damage to biomolecules such as proteins, lipids and nucleic acids [25,35,36].
Figure 1.
The possible mechanisms of antibacterial activities exhibited by different metal nanoparticles (NPs) and photocatalytic semiconductors. The activation of the photocatalytic semiconductor by visible light is depicted on the left-hand side of the figure. Reactive oxygen species created by various semiconductors destruct bacterial cell components, as indicated by red arrows. Ag, Cu, and Au nanoparticles also generate reactive oxygen species (ROS) for bacterial killing. The green arrow represents targets of Ag. Reproduced with permission from [22]. Copyright Frontiers, 2018.
Matsunaga et al. first reported the antimicrobial and photoelectrochemical activities of platinum-loaded titanium oxide (TiO2/Pt) powders for killing Lactobacillus acidophilus, Saccharomyces cerevisiae and Escherichia coli (E. coli) in 1985 [37]. Nano-TiO2 exhibits excellent photocatalytic bactericidal activity against viruses and MDR bacteria under UV irradiation [38]. Accordingly, extensive efforts have been carried out by researchers to improve the photocatalytic bactericidal activity of TiO2 nanomaterials. TiO2 nanostructures have a wide spectrum of industrial, environmental and energy applications, including water purification, food preservation, degradation of dyes, chemical sensors, dye-sensitized solar cells, and antimicrobial agents. [39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57]. In particular, visible light-responsive TiO2 doped with metals and non-metals exhibit bactericidal activity against a wide variety of bacterial species including Gram-negative E. coli, Acinetobacter baumannii, Shigella flexneri, and Gram-positive S. aureus, Bacillus subtilis, Listeria monocytogenes, as well as Bacillus anthracis spores [58]. Those photocatalysts can be used for the disinfection of pathogenic bacteria, thereby preventing the spread of microbe-related diseases. Recently, Markov and Vidaković reviewed antimicrobial testing methods of TiO2 photocatalysts, including thin-film technique, petri-dish system, and polytetrafluoroethylene membrane-separated system. They also addressed the calculation methods for assessing the antimicrobial efficacy of TiO2 photocatalysts [35]. To avoid mechanical damage to TiO2 NPs, they are embedded in the polymeric matrices to form antibacterial nanocomposites [59,60,61,62,63,64,65]. The beneficial effects of polymers as the matrix materials of functional composites include ease of processing and good moldability, and they are inexpensive with a low density [66,67,68,69,70,71,72].
Apart from bactericidal activity, TiO2 NPs also find attractive application in biomedical fields as photodynamic therapeutic agents for destroying human cancer cells from the skin to the internal organs under ultraviolet (UV) and visible light illumination [36]. This is due to the ROS created by TiO2. NPs can damage cellular respiration in mitochondria, thus releasing electron-transfer proteins and causing cell death. Moreover, light-activated TiO2 NPs can lead to DNA fragmentation as a result of the electron transfer mechanism. This approach shows promise for reprograming gene-coding either by deleting or by inserting gene codons. In addition, TiO2 nanotubes can be used for light-controlled delivery of drugs for treating the diseased tissues upon UV irradiation [36]. This article provides an update review on the current development, synthesis, photocatalytic bacterial inactivation, and cytotoxicity of TiO2 NPs and their nanocomposites, especially in a rapidly growing field of research, over the past five years.
2. Crystal Structure of Titania
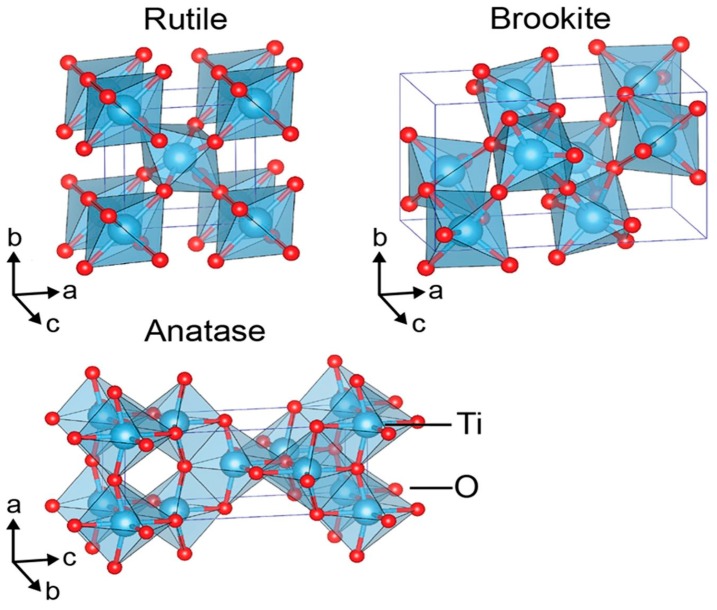
Titanium dioxide generally exists naturally in three crystalline structures, i.e., anatase, rutile, and brookite [42,43]. Anatase exhibits the tetragonal structure with a space group of I41/amd (I: body centered). Body-centered tetragonal anatase has lattice parameters of a = 3.7845 Å and c = 9.5143 Å. Rutile belongs to the P42/mnm (P: primitive) space group, with the primitive tetragonal lattice having lattice parameters a = 4.5937 Å, and c = 2.9587 Å. Brookite is orthorhombic with a space group of Pbca, having lattice parameters of a = 9.1819 Å, b = 5.4558 Å, and c = 5.1429 Å, as shown in Figure 2. [43,73,74]. These polymorphs are formed by linking the chains of distorted TiO6 octahedra through corner- and edge-sharing in different ways. In the TiO6 octahedra, titanium cations (Ti4+) are coordinated to six oxygen anions (O2−). The octahedron shares two, three, and four edges with adjacent octahedra to give rutile, brookite and anatase, respectively [42]. Anatase and brookite are metastable, and transform irreversibly to a stable rutile phase by heating at 500–700 °C. Moreover, anion fluorine dopant also stabilizes anatase at elevated temperatures (>1000 °C) [75]. Generally, anatase TiO2 is more photoactive than rutile and brookite. Anatase TiO2 absorbs ultraviolet light (UV) to create an electron–hole pair necessary for photocatalytic reaction. In the process, electron is excited from the valence band to the conduction band, leaving a positively charged hole in the valence band. This photogenerated electron–hole pair displays a high reducing and oxidizing capability. In this respect, the electron in the conduction band reacts with molecular oxygen to produce superoxide ion (O2−) via a reductive process, while the hole in the valence band oxidizes adsorbed water or hydroxyl ions at the titania surface into hydroxyl radicals (•OH) [76]. The photocatalytic activity of TiO2 depends mainly on the crystal structure, shape, particle size and surface area. The equilibrium shape of anatase consists of a truncated bipyramid constructed by {101} and {001} facets. According to the Wulff construction, the {001} facets constitute nearly 6% of the total exposed surface of anatase TiO2, while stable {101} facets contribute to more than 94% of the surface area [42]. However, the {001} facets of anatase TiO2 have a higher photocatalytic performance than {101} facets [77,78,79]. TiO2 NPs with a larger surface area and smaller size than their bulk counterparts generate more ROS during photoexcitation [80]. Xu et al. indicated that anatase TiO2 NPs exhibit a higher phototoxicity and cytotoxicity in human keratinocyte cells than rutile TiO2 NPs [81]. Recently, Bartlet et al. indicated that one-dimensional titania nanotubes prepared by electrochemical anodization exhibit superhydrophobic behavior with a large water contact angle of >150°. Such superhydrophobic titania nanotubes reduced bacterial adhesion on their surfaces [82].
Figure 2.
Connecting the chains of distorted TiO6 octahedra by sharing edges and corners in different ways to form rutile, brookite and anatase polymorphs. Titanium atoms are blue; oxygen atoms are red. Reproduced with permission from [42]. Copyright Nature Publishing Group, 2017.
3. Visible-Light Active TiO2
TiO2 NPs with a large bandgap (anatase = 3.2 eV and rutile = 3.0 eV) can only be activated by UV light, which accounts for less than 5% of the solar spectrum compared to 45% of visible light [83]. The low photocatalytic efficiency of titania under visible light limits its practical applications. Extending the utilization of solar energy to the visible region has motivated researchers to improve the visible-light photocatalytic performance of TiO2 NPs. Moreover, TiO2 NPs have another drawback, due to a rapid recombination of photogenerated electron–hole pairs. Recombination occurs when the excited electron returns to the valence band without interacting with the adsorbed species under UV irradiation. Accordingly, the energy of recombination is dissipated in the form of light or heat. Therefore, it deems necessary to enhance the photocatalytic activity of TiO2 NPs by reducing both the bandgap and the recombination of electron–hole pairs under visible light irradiation. Many attempts have been made by researchers to design and synthesize visible light-active TiO2 photocatalysts. These include metal and non-metal doping, coupling with semiconductors, and modification with graphene oxide or carbon nanotube [84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104]. The incorporation of those dopants into titania affects its electronic band structure greatly, thereby promoting visible light absorption and a red shift in the bandgap.
3.1. Metal Doping
The VB of titania is composed of hybridized states of O-2p and Ti-3d orbitals, while the CB consists of primarily Ti-3d orbitals. The electronic and optical properties of titania can be modified by doping. In this context, titanium or oxygen ions’ sites of titania lattice can be substituted with either metal or nonmetal dopants to alter their optical and photocatalytic properties. The cationic doping of titania with transition metals, rare earth metals and noble metals is typically used to improve its photocatalytic performance under visible light excitation. The presence of metal ion dopants can alter the charge transfer properties of TiO2, thus improving the separation efficiency of photogenerated carriers, and producing a shift in its absorption edge to the visible regime. The dopant energy level is located below the CB of TiO2, acting as an electron or hole trap, and thus allowing more carriers to transport to the surface. The photocatalytic activity of metal-doped titania depends on several factors, including the dopant concentration, type of metal dopant, d-electron configuration and energy band level of dopant in the titania lattice [105]. Although metal dopants facilitate a red shift in optical absorption edges of titania, they can induce defect states, acting as carrier recombination centers, especially at very high dopant contents. Thus, the occurrence of a rapid recombination rate of photogenerated charge carriers arises from a reduction in the distance between the trapping sites by increasing the number of dopant ions.
Doping TiO2 with transition metals influences its electronic energy levels and narrows the bandgap, resulting in a shift in the absorption spectrum of titania to longer wavelengths. Titania can be self-doped with Ti3+ ions to improve its visible-light absorption and avoid the incorporation of other impurities into its lattice. The introduction of Ti3+ energy level and the creation of an oxygen vacancy (Ovac) in the bandgap are responsible for the shift in optical adsorption of TiO2 into the visible light region. As such, the electrons in the VB can be excited to the Ovac–Ti3+ defect states, and electrons from these defect sites can be excited to the CB upon visible light illumination [106,107]. In this respect, the Ovac–Ti3+ sites can trap photogenerated electrons under visible light, thereby inhibiting the recombination of electron–hole pairs and improving photocatalytic activity accordingly. Generally, oxygen vacancy is not stable in air, and it remains a challenge to develop a stable Ti3+ self-doped titania with a high photocatalytic performance [108].
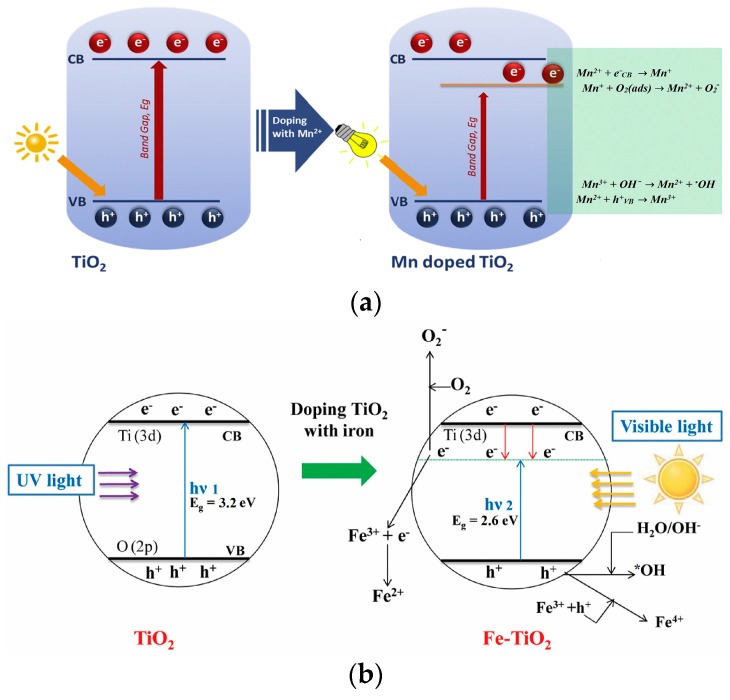
Apart from Ti3+ ions, other transition metals, such as copper (Cu), vanadium (V), chromium (Cr), manganese (Mn), iron (Fe) and nickel (Ni), are typically employed to enhance the visible-light photocatalytic activity of titania [88,89,90,91,92,93,94,95,109,110,111,112,113,114,115]. The redshift effectiveness takes the following order: V > Cr > Mn > Fe > Ni [86]. The substitution of Ti4+ in the TiO2 lattice by transition metal ions creates a new energy state in the bandgap of TiO2. Therefore, the localized d-electron state of transition metals introduced in the bandgap captures the excited electrons from the titania valence band, thereby suppressing the recombination of charge carriers. Figure 3a shows the typical charge transfer reactions involved during photocatalysis of Mn-doped TiO2. Mn2+ displays an electronic configuration of 3d5 and changes to 3d6 (Mn+) by trapping electrons, while it changes to 3d4 (Mn3+) as it traps the holes. Both Mn+ and Mn3+ species are unstable, and react with adsorbed O2 and surface hydroxyl molecules to yield ROS [92]. Similarly, Fe3+ ions of Fe-doped TiO2 can also act as hole and electron traps in prohibiting the recombination of the electron–hole pair and promoting ROS generation [84,85,86]. These result in a red shift in the absorption edge and thus enhance photocatalytic activity (Figure 3b) [95]. Doping TiO2 with vanadium, molybdenum (Mo) and tungsten can also shift its absorption edge to the visible region [111,112]. By doping TiO2 NPs with 1% and 2% Mo, the bandgap of TiO2 NPs decreases from 3.05 to 2.94 and 2.73 eV, respectively. The ionic radius of Mo6+ is 0.062 nm, while that of Ti4+ is 0.068 nm. As such, Mo ions can readily replace Ti4+ in the TiO2 lattice, as they have approximately the same ionic radii, resulting in a narrower bandgap [112]. This facilitates the charge transfer between the VB and Mo-3d orbitals, thereby promoting photocatalytic activity under visible light [112].
Figure 3.
The charge transfer processes between excited electrons from the valence band of TiO2 with (a) Mn2+ ions of Mn-doped TiO2, and (b) Fe3+ ions of Fe-doped TiO2. CB and VB are the conduction and valence bands of TiO2, respectively. Reproduced with permission from [92,95], respectively. Copyright Elsevier, 2017; Copyright American Chemical Society, 2013.
From the literature, rare earth metal ions are effective in extending the recombination time of charge carriers and improving their separation efficiency. Rare earth metals, such as cerium (Ce), lanthanide (La), erbium (Er) and ytterbium (Yb), with 4f, 5d, and 6s electrons are good dopants for modifying the electronic structure and optical properties of titania [116,117]. Rare earth dopants introduce several impurity energy levels due to the introduction of orbitals between the conduction and valence bands. Moreover, lattice defects are generated in titania as a result of a large mismatch of both the charge and ionic radius between the dopant and Ti cations. The impurity energy levels act as trapping centers for photogenerated electrons and holes, thereby favoring charge separation and reducing the electron–hole recombination [118,119,120,121]. Among these, the La dopant in titania is studied most frequently, followed by Ce doping, in recent years [116,117,118,119,120,121,122]. Kasinathan et al. reported that cerium doping suppresses the recombination of photogenerated electron–hole pairs in titania and promotes a red-shift in its band gap transition. As such, Ce-TiO2 had strong antibacterial activity against E. coli due to its strong oxidation activity and superhydrophilicity [121].
Generally, two or more types of metal cations can be incorporated into the TiO2 lattice to further improve its photocatalytic performance. This is typically termed the ‘co-doping’. The enhancement in the photocatalytic activity of co-doped TiO2 is attributed to the synergistic effect of the dopants in increasing visible light absorption, thus facilitating electron–hole generation and suppressing the recombination rate [90,113,114,115]. Very recently, Aviles-Garcia et al. synthesized W and Mo co-doped TiO2, and reported that the nanocomposite with the W:Mo = 1:1 ratio having a bandgap of 2.87 eV exhibits a synergistic effect between the dopants to generate more hydroxyl radicals for degrading 4-chlorophenol. This is because both the W6+ and Mo6+ ions are effective in trapping photogenerated electrons, thus extending the lifetime of electron–hole pairs and reducing their recombination rate. The holes can react with the adsorbed H2O or –OH groups on the TiO2 surface, giving rise to hydroxyl radicals. The photocatalytic reactions can be expressed as follows [114]
| W/Mo-TiO2 + hv → e− + h+ | (1) |
| W6+ + e− → W5+ | (2) |
| Mo6+ + e−→ Mo5+ | (3) |
| H2O + h+ → •OH + H+ | (4) |
| OH− + h+ → •OH. | (5) |
The visible light response of titania can also be achieved by doping with noble metals such as gold (Au), silver (Ag), platinum (Pt) and palladium (Pd) [123,124,125,126]. As recognized, a collective oscillation of conduction electrons can be induced in metal NPs by irradiating with light. This is because the collective oscillation of surface electrons resonates with the electromagnetic field of the incident light. This behavior is generally termed as the localized surface plasmon resonance (LSPR). LSPR covers a wide range of solar spectrum, particularly in the visible and near-infrared (NIR) regions [127,128]. After excitation, LSPR decays non-radiatively into hot electrons and holes through Landau damping, generating highly energetic charge carriers that are typically termed ‘hot carriers’ [129]. This ultrafast relaxation renders the hot carriers capable of rapidly separating and transferring into semiconductors to drive chemical reactions on adsorbed molecules [128,129,130,131]. The LSPR effect is more pronounced for Au and Ag nanoparticles compared with other metals.
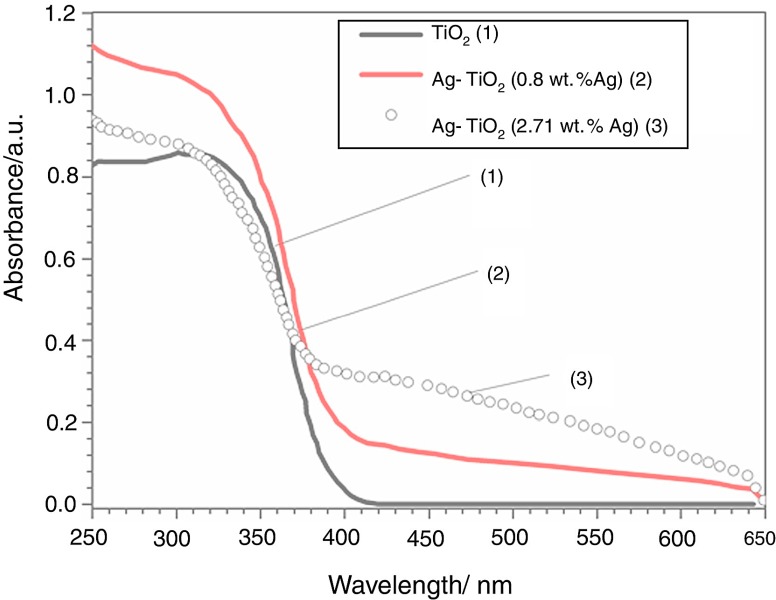
Employing plasmonic NPs on semiconductors is considered to be effective in improving their photocatalytic performance. In this respect, noble metal NPs act as electron donors for titania by injecting hot electrons into the conduction band of TiO2 under visible light [132]. The holes created in plasmonic AgNPs can capture conduction electrons of TiO2, thereby reducing the charge recombination in titania. Therefore, plasmonic oscillation from Au and Ag nanoparticles to TiO2 under visible light has received considerable attention in recent years [133,134,135,136,137,138]. Moreover, AgNPs with well-established antibacterial properties are particularly attractive dopants for titatia in addition to their LSPR effect [15]. Figure 4 shows the UV-visible spectra of pristine TiO2 and Ag/TiO2 nanocomposites with different AgNP contents [126]. Pristine TiO2 exhibits a strong UV light absorption band due to the excitation of the electron–hole pair across the bandgap. The Ag/TiO2 nanocomposites display higher absorption values in the UV region, and the absorption intensity increases with increasing AgNP concentrations. The introduction of AgNPs into TiO2 results in an increase in the absorption towards the visible light region, i.e., 400–650 nm wavelength. This arises from the LSPR effect of AgNPs that promotes the absorption of Ag/TiO2 nanocomposites in the visible regime.
Figure 4.
Ultraviolet-visible diffuse reflectance spectra of TiO2 and its nanocomposites. Reproduced with permission from [126]. Copyright Elsevier, 2019.
When a metal comes into contact with a semiconductor of a different work function, a large potential barrier is established at their interface, which is usually known as the Schottky barrier [137]. Such a barrier at the AgNPs/titania junction improves the charge separation or suppresses the charge recombination greatly [135,138]. Under UV irradiation and in the absence of plasmonic oscillation, electron transfer from the TiO2 conduction band to AgNPs is thermodynamically favorable, as the Fermi level of titania is higher than that of AgNPs. From this perspective, excited electrons are transferred from titania to AgNPs across the Schottky barrier at the Ag/TiO2 interface. Accordingly, AgNPs serve as an excellent electron accumulator, thus suppressing the charge recombination process. Under visible light irradiation, AgNPs experience the LSPR effect, and excite the conduction electrons for transfer to TiO2 to create ROS, as mentioned previously (Figure 5).
Figure 5.
The creation of reactive oxygen species in Ag/TiO2 nanomaterials due to the localized surface plasmon resonance (LSPR) effect of AgNPs under visible light. After excitation, LSPR decays into hot electrons and holes through Landau damping, creating highly energetic charge carriers. On the other hand, AgNPs serve as an excellent electron accumulator for TiO2 under UV irradiation. Reproduced with permission from [138]. Copyright MDPI, 2019.
3.2. Carbonaceous Nanomaterials Modified Titania
Pure and high-quality graphene is an excellent electrical conductor as it has no bandgap. Graphene consists of a monolayer of sp2-bonded carbon atoms that are tightly organized into a two-dimensional (2D) honeycomb structure. Graphene has been reported to possess an excellent electrical mobility of 2 × 105 cm2 V−1 s−1, a superior light transparency of 97.7%, a high specific surface area of 2600 m2 g−1, and good antibacterial activity [14,139,140]. In this context, graphene and its derivatives, such as graphene oxide (GO) and reduced graphene oxide (rGO), find attractive applications in electronic and optoelectronic devices, energy storage devices, chemical sensors and biomedical implants [141,142,143]. Moreover, a graphene sheet with a lateral dimension of several micrometers can serve as a template for anchoring TiO2 NPs onto its surface [102,144,145,146].
Large-area graphene sheets can be synthesized from chemical vapor deposition (CVD) [147]. However, the CVD approach is still an expensive process for manufacturing high-quality graphene sheets. To tackle this, GO can be prepared at a large scale by exposing the graphite flakes in a strong oxidizing solution, i.e., a mixture of sulfuric acid, sodium nitrate, and potassium permanganate, using a modified Hummers process [148]. As a result, GO bears oxygen functional groups having hydroxyl and epoxide on the graphene basal plane, with carboxyl and carbonyl groups at the edges [149]. Those oxygenated groups damage the conjugated structure of graphene, leading to poor electrical conductivity. To resume its electrical conducting properties, reducing agents, such as hydrazine and sodium borohydride, are used to reduce GO to form rGO [150]. The aforementioned reductants are toxic, so green reductants such as L-ascorbic acid, D-glucose and tea polyphenol can be used to reduce GO to rGO [151]. Generally, all chemical reductants cannot remove oxygenated groups of GO completely, rendering rGO with a certain degree of residual oxygen levels. Accordingly, GO would change from insulating to conducting behaviors by regulating the C/O ratios. GO and rGO with a tunable band gap of 4.3–2.4 eV is dependent upon the oxygen level; the bandgap generally increases with increasing O levels [152]. In contrast, pure graphene exhibits no bandgap with excellent electron mobility.
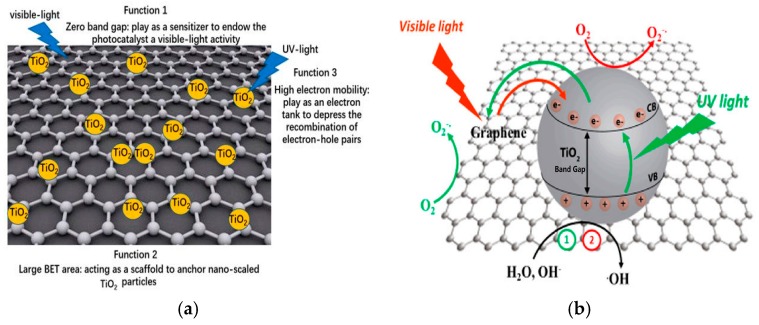
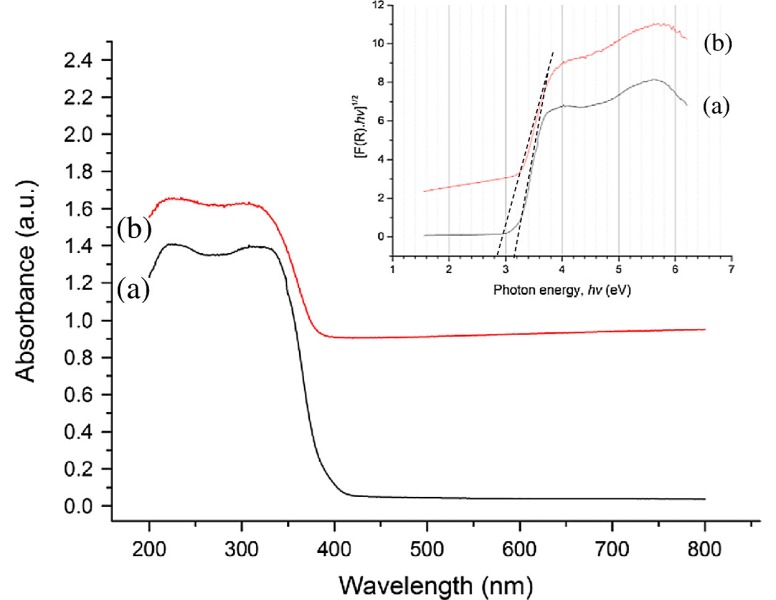
For novel graphene/TiO2 nanostructures, the migration of photogenerated charge carriers from TiO2 to graphene or vice-versa depends upon the interfacial contact between them, and the photon energy or wavelength [153]. As is known, uniformly dispersed TiO2 NPs on a large-area graphene sheet and a close interfacial interaction between them are essential for efficient charge transport across the interface (Figure 6a). Under UV irradiation, photoexcited electrons from titania are injected into graphene as the conduction band minimum of TiO2 is higher than the Fermi level of graphene [32]. As such, highly conductive graphene acts as an electron acceptor for titania, and provides a network to facilitate the rapid transfer of excited electrons. These promote the separation between electron–hole pairs and inhibit their recombination [154]. Under visible light illumination, electrons located in high-energy graphene states are delocalized into the conduction band of TiO2. Consequently, electrons react with oxygen adsorbed on the TiO2 surface to form superoxide anion (Figure 6b) [153]. In general, a few layer graphene sheets of rGO/TiO2 photocatalyst facilitate a red shift in the optical absorption, thereby narrowing its bandgap and enhancing its photocatalytic efficiency [144,146]. Figure 7 shows the UV-vis spectra of anatase TiO2 and rGO/TiO2 nanocomposite. The inset displays the Tauc plot of the modified Kubelka–Munk (KM) function with a linear extrapolation to produce respective bandgap values of TiO2 and rGO/TiO2 materials, i.e., 3.2 eV and 2.9 eV.
Figure 6.
Schematics displaying the (a) roles of graphene layers of graphene/TiO2 composite in photocatalysis, and (b) charge transfer mechanism under ultraviolet or visible light irradiation. Reproduced with permission from [145,153], respectively. Copyright MDPI, 2018 and 2017.
Figure 7.
UV–vis diffuse reflectance spectra of (a) anatase TiO2 and (b) nanostructured rGO/TiO2. Inset: plot of transformed KM function [F(R).hv]1/2 vs. hv for bandgap determination of anatase TiO2 and rGO/TiO2; R is reflectance and hv is photon energy. Reproduced with permission from [146]. Copyright Springer, 2013.
A single-walled carbon nanotube (SWNT) is formed by rolling-up a graphene sheet into a cylindrical or turbular shape, while several sheets rolls into a multi-walled nanotube (MWNT). The MWNTs with a large surface-area-to-volume ratio and remarkable electrical conductivity serve as the template for anchoring TiO2 NPs, facilitating the separation of electron–hole pairs and inhibiting the charge recombination by trapping photoexcited electrons from titania [154]. Accordingly, the bandgap of TiO2 NPs reduces from 3.25 to 2.71 eV with an increase in MWNTs content. This leads to a shift in the absorption edge into the visible region. The Ti–O–C bond extends the light absorption to longer wavelengths [155,156,157].
3.3. Non-Metal Doping
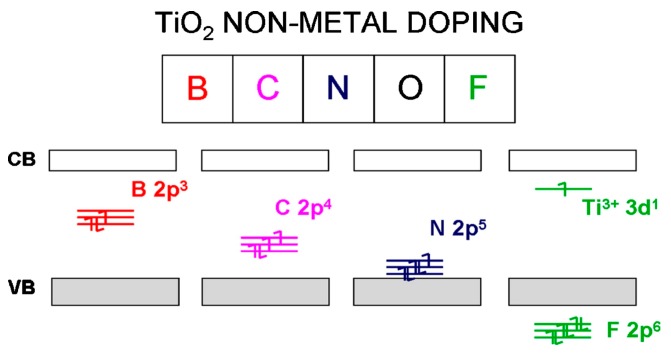
Metal doping has some drawbacks for enhancing the visible light response of titania. These include transition metals of high contents, which may serve as recombination sites for photogenerated charge carriers, the low thermal stability of photocatalysts, the formation of secondary phases and dopant insolubility [105,158,159]. Therefore, significant improvement in the photocatalytic performance of metal-doped titania can be achieved only at a low metal dopant concentration. Above an optimal dopant content, photocatalytic activity decreases owing to a higher recombination rate of charge carriers. Non-metal elements such as carbon, nitrogen and boron, with an atomic radius close to that of the O atom, can be utilized as anionic dopants for replacing lattice oxygen anions [76,97,159,160,161,162,163,164]. In this respect, non-metal doping appears to be an alternative route for enhancing visible light efficiency, due to the introduction of a new valence band associated with their localized 2p states lying above the valence band of TiO2 (Figure 8). As such, non-metal doping generates a hybridization of O-2p and N-2p orbitals, giving rise to an upshift in the valence band position. By irradiating with visible light, electrons are excited from the localized N-2p states to the CB, leaving behind holes on the localized states. The exception is fluorine with the highest electronegativity, having filled states below the O-2p valence band, leading to the formation of Ti3+ ions as of result of the charge compensation [165].
Figure 8.
Electronic band structure of titania due to non-metal doping. CB and VB represent conduction band and valence band, respectively. Reproduced with permission from [160]. Copyright Elsevier, 2013.
Among anionic dopants, nitrogen is widely employed to enhance the visible light response of titania. The N atom can occupy either the substitutional or interstitial site of the titania lattice. In the former case, the N atom substitute the O atom to yield TiO2−xNx, so that the doping energy state (N-2p) lies just above the valence band. Substitutional N doping reduces the bandgap of titania slightly, from 3.20 to ~3.06 eV. The interstitial N-doping reduces the bandgap to ~2.46 eV, in which the doping energy level lies in the midgap, i.e., at 0.74 eV above the valence band [166,167,168]. In an earlier study by Asahi et al., N-doping into substitutional sites of TiO2 is reported to be essential for bandgap reduction and efficient photocatalytic activity [166]. For the C-doped TiO2 photocatalyst, carbon dopant may replace oxygen or Ti in the substitutional lattice site. It may also occupy the interstitial site [169]. Therefore, C-doped TiO2 can have different photocatalytic behaviors, depending on the synthesis process employed. Density functional theory (DFT) calculations predict that substitutional (to oxygen) carbon and oxygen vacancies are formed at low carbon contents and oxygen-poor conditions. Under oxygen-rich conditions, interstitial and substitutional (to Ti) C atoms are favored [170]. Similarly, the B dopant can substitute for either the O or Ti atom, or can occupy the interstitial position. DFT simulations indicate that a B substitution for Ti is unlikely to take place. In contrast, the boron atom tends to either replace an oxygen atom or occupies the interstitial site [171]. From the X-ray photoelectron spectroscopic (XPS) results, Patel et al. reported that B preferentially occupies the interstitial site at low concentrations (up to 1%), while it occupies the substitutional O site as the concentration increases (≥2%) [172].
Recently, Sotelo-Vazquez et al. reported that phosphorus (P)-doping can result in the formation of both cationic (P5+) and anionic (P3−) states of anatase TiO2 films on the basis of XPS results. The P3− state of P-doped TiO2 exhibited inferior photocatalytic activity compared to undoped TiO2 film. Transient absorption spectroscopic results revealed that charge carrier concentrations increased by several orders of magnitude in films containing P5+ species [173]. From the XPS measurements, Gopal et al. demonstrated that the P dopant exists in a P5+ state which can replace part of Ti4+ through the formation of Ti–O–P bonds, i.e., forming P cation-doped TiO2 [174]. As a result, the photocatalytic activity of P-doped titania for degrading methylene blue was much enhanced and superior to undoped TiO2. Moreover, X-ray diffraction results indicated that P-dopant increases the thermal stability of TiO2 NPs, and retards the phase transition from anatase to rutile.
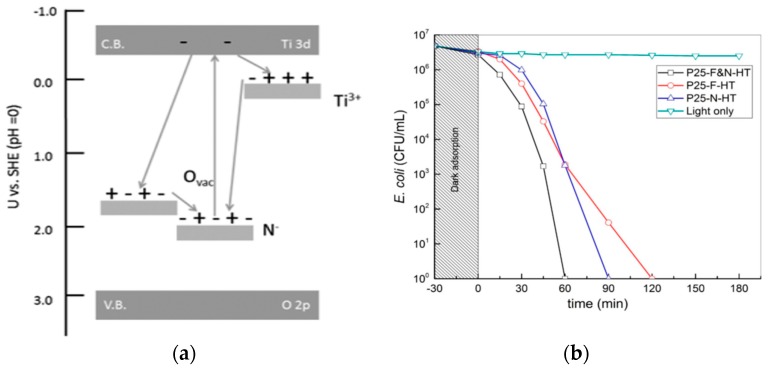
From the literature, fluorine doping stabilizes anatase TiO2 at elevated temperatures up to 1200 °C [75]. The substitution of fluorine for oxygen in TiO2 NPs leads to the creation of an oxygen vacancy [175]. Fluorine doping converts Ti4+ to Ti3+ in TiO2 NPs by charge compensation. The presence of Ti3+ suppresses the recombination of the electron–hole pairs and enhances the photocatalytic activity accordingly [165]. Co-doping TiO2 NPs with F and N is considered to be very effective in tuning the bandgap to further enhance visible-light photocatalytic activity. Multiple charge transfer transitions occur in the Ti3+ localized state, oxygen vacancy and N midgap state of the F–N, co-doped TiO2 NPs [176]. Table 1 summarizes visible-light active TiO2 NPs doped with metals and non-metals.
Table 1.
Visible-light active TiO2 NPs doped with metals and non-metals.
| Dopants | New Band (Gap) State Created | Reference |
|---|---|---|
| Metals | ||
| Ti | Ti3+, oxygen vacancy | [106,107] |
| Mn | Mn2+ | [92] |
| Fe | Fe3+ | [93,95] |
| Ni | Ni2+ | [110] |
| Cu | Cu2+ | [109] |
| V | V4+ | [88] |
| Mo | Mo6+ | [111,112] |
| Ce | Ce3+ | [121,122] |
| Mo and W | Mo6+, W6+ | [114] |
| V and Co | V4+, Co2+ | [90] |
| Fe and Co | Fe3+, Co2+ | [115] |
| Non-Metals | ||
| N | N midgap | [166] |
| P | P5+ | [173,174] |
| F | Ti3+, oxygen vacancy | [165,175] |
| F and N | Ti3+, oxygen vacancy, N midgap | [176] |
3.4. Coupling of Semiconductors
The poor photocatalytic efficiency of titania under visible light can be overcome through the formation of a heterojunction structure by coupling with other semiconductors with a suitable energy band level. Titania can be coupled with metal oxides (e.g., Cu2O, Fe2O3, WO3) and chalcogenides (e.g., CdS, MoS2 and WS2) to form a heterojunction for the charge separation in enhanced visible light absorption. Those coupled semiconductors acting as sensitizers should be nontoxic, and exhibit visible light photocatalytic activity, with a bandgap smaller than that of titania [177,178,179,180,181,182,183]. In this respect, photoexcited electrons in the CB and holes in the VB of a sensitizer semiconductor can be transferred to the CB and VB of TiO2 NPs [180]. Zinc oxide with good antimicrobial property is unsuitable to form visible-light active ZnO/TiO2 nanostructures due to its wide bandgap of 3.37 eV. Cadmium sulfide is toxic and carcinogenic, so it is unfavorable to form CdS/TiO2 heterojunction for practical applications. Nontoxic molybdenum disulfide (MoS2) with a direct band-gap of 1.9 eV can be coupled with titania to form MoS2/TiO2 nanocomposites, having excellent visible photocatalytic activity [181]. Oxide semiconductors, such as α-Fe2O3 and Cu2O with a respective small bandgap of 2.2 eV and 2.17 eV, can also form composite photocatalysts, with TiO2 having good antibacterial properties under visible light [177,179,182,183]. Inexpensive and nontoxic Cu2O, with its efficient electron injection to the conduction band of TiO2, is particularly suitable for forming heterojunction photocatalysts [182,183].
4. Synthesis of Titania Nanomaterials
Titania can be fabricated in the form of thin films, powders, or nanocrystals. Physical deposition techniques such as thermal evaporation, reactive sputtering and pulsed laser deposition, chemical gas-phase atomic layer deposition (ALD) process, and wet chemical deposition methods such as dip-coating, spin-coating, spray coating and sol-gel, have been employed by researchers to prepare TiO2 thin films [184,185,186,187,188,189]. Those homogeneous films deposited by physical deposition techniques are beneficial for use in dye-sensitized solar cells, microelectomechanical systems and electroluminescent devices [185,189]. In ALD, chemical precursors react sequentially on various substrate surfaces including carbon nanotubes, forming nanometer-sized films of metal oxides (e.g., TiO2 and HfO2) [190,191,192]. It offers the advantages of nanometer-level control of both thickness and film composition. For bactericidal applications, wet chemical processing is the most convenient, simple and effective synthesis route for preparing TiO2 NPs and nanocomposites. Moreover, the solution chemical synthesis process is capable of producing titania nanomaterials in larger quantities in comparison with the physical processing route. Solution processing techniques include the sol-gel, wet impregnation, photoreduction, hydrothermal and solvothermal processing, electrochemical anodization and electrospinning.
4.1. Solution Processing Route
4.1.1. Sol-Gel Method
Titania colloids can be synthesized through the hydrolysis and condensation reaction of titanium alkoxide in the presence of water, and these reactions are catalyzed by an acid [193,194,195,196]. The sol-gel process involves the transformation of metal alkoxide or metal salt into a solid by adding an excess of water to give a metal–oxo linkage (M–O–M). The hydrolysis facilitates the formation of original nuclei TiO2, and the subsequent condensation promotes the growth of a crosslinked network of TiO2 nuclei. This strategy allows the formation of TiO2 NPs with a high level of chemical purity [196,197]. From an earlier study of Padmanabhan et al., the sol-gel process involved the reaction of titanium tetraisopropoxide (TTIP) with trifluoroacetic acid (TFA), followed by hydrolysis, gelation, drying, and finally calcination at high temperatures. The sol was dried at 90 °C to obtain the gel, and then calcined at 500–900 °C to remove organic substances to form nano-TiO2 with a high photocatalytic activity [194]. In a recent study, Lusvard et al. employed different precursors and procedures for synthesizing TiO2 NPs with the preparation conditions compatible with the industrial scale for water purification [196]. Three different kinds of precursors were utilized for the synthesis of TiO2 NPs, including: titanium tetrachloride (TiCl4) and ethanol, titanium isopropoxide (C12H28O4Ti) and urea (CO(NH2)2), as well as titanium isopropoxide, isopropyl alcohol (C3H8O), acetic acid (CH3COOH) and methanol (CH3OH). They reported that TiO2 NPs, synthesized from molar TTIP: urea in a ratio of 2:1 at 50 °C, have the best photocatalytic activity for degrading methyl blue and bromothymol blue [196].
For fabricating metal-doped TiO2 nanopowders, an additional metal source reagent is needed, and added to titanium precursors during the sol-gel process [197,198,199,200]. For instance, Marami et al. prepared Fe-doped TiO2 powders by introducing FeSO4·7H2O into the TTIP, and the ethanol solution followed with the addition of acetic acid under vigorous stirring. Thereafter, the temperature of mixture was increased to 70 °C, and ethylene glycol was added, acting as a stabilizer. The product was dried and finally calcined at 600 °C for 4 h to yield Fe-doped TiO2 nanopowders [198]. In the case of Ag-doped TiO2, a desired amount of silver salt precursor, i.e., silver nitrate was added to the TTIP−methanol solution [50,199]. Reducing agents such as NaBH4 are employed to reduce silver ions to AgNPs. For the synthesis of N-doped TiO2, an organic compound with nitrogen (such as trimethylamine, 1,3-diaminopropane, ethylmethylamine), or ammonium salt bearing nitrogen (e.g., ammonium carbonate, ammonium chloride, ammonium nitrate), is added to the sol-gel solution during the synthesis process [200,201,202,203,204].
4.1.2. Hydrothermal/Solvothermal Synthesis
The hydrothermal/solvothermal method is a useful tool for fabricating TiO2 nanostructures involving chemical reactions in a solvent (water/nonaqueous) medium at an elevated temperature >100 °C and a pressure higher than 1 atm, within a closed system using an autoclave. As the sol-gel process generally produces amorphous or low crystalline materials, a subsequent annealing at high temperatures for crystallization is needed. In this context, hydrothermal or solvothermal processing is beneficial for improving the crystallinity of titania synthesized by the sol-gel technique. For example, Yanagizawa and Ovenstone investigated the effect of hydrothermal treatment on the crystallinity and phase structure of sol-gel prepared, TiO2 amorphous powders [205]. In their study, hydrothermal treatment was performed at 250 °C for 1 h in the presence of several inorganic salts under acidic and basic conditions. Acidic conditions led to the formation of anatase, brookite, and rutile, whereas basic conditions and/or the presence of sulfate ions favored the crystallization of anatase [205]. In addition, the hydrothermal approach can also be used to synthesize rGO/TiO2 nanocomposites [206].
The organic solvents in solvothermal treatment help to control the morphology of synthesized nanocrystals. Thus, this process enables better control of the shape, size distribution and crystallinity of TiO2 NPs in comparison with the hydrothermal method. TiO2 nanostructures of different morphologies can be obtained and tailored by manipulating several processing parameters, including the type of solvent and titanium precursor, molar ratio of reagents, addition of surfactant, reaction temperature and time [207,208,209,210,211,212]. For instance, TiO2 nanorods can be synthesized in TTIP, benzyl alcohol (BzOH) and acetic acid (AA) at 150 °C for 8 h. The molar ratio of TTIP/AA is kept at 1: 4 [208]. Recently, Falentin-Daudré et al. synthesized highly crystalline sphere and rod-shaped TiO2 nanostructures using TTIP, benzyl alcohol (BzOH) and AA reagents. The shape of the TiO2 nanostructure can be tuned by varying the concentration molar ratios of TTIP/BzOH and AA/BzOH (Figure 9) [209]. The X-ray diffraction patterns for TiO2 nanospheres and nanorods display well-defined peaks associated with pure anatase, thus revealing TiO2 nanospheres and nanorods with a high crystallinity.
Figure 9.
Transmission electron micrographs of solvothermally synthesized TiO2 with nanospheres (NP1–NP2) and nanorods (NP3–NP4) morphologies. Reproduced with permission from [209]. Copyright Elsevier, 2017.
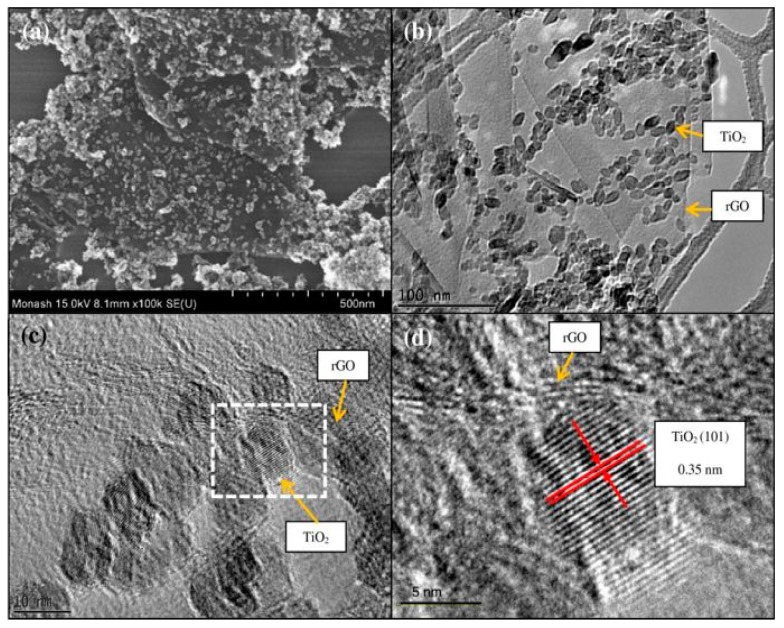
For preparing rGO/TiO2 nanocomposite, Tan et al. first obtained a mixed solution of tetrabutyl titanate, ethylene glycol and acetic acid, and then added it dropwise into a chilled GO solution under vigorous stirring. Thereafter, an autoclave filled with the GO–TiO2 solution was heated at 180 °C for 8 h. The greyish-black precipitate was obtained by centrifugation [146]. During the solvothermal synthesis, GO was reduced to rGO accordingly. Figure 10a,b show the respective field-emission scanning electron microscopic (FESEM) image and transmission electron micrograph (TEM) of the rGO/TiO2 nanocomposite. It is apparent that titania nanoparticles with an average size of 12 nm are dispersed and anchored on the rGO surface. The high-resolution TEM (HRTEM) images of a selected rGO-TiO2 heterojunction are shown in Figure 10c,d. The lattice fringes can be seen in titania nanoparticles, especially in the high magnification image shown in Figure 10d, implying that the TiO2 nanocrystals exhibit good crystallinity. The lattice spacing of TiO2 is determined to be 0.35 nm, which corresponds to the (101) plane of anatase TiO2. Moreover, the rGO–TiO2 interface is clean and free from the impurity products. The intimate connection enables photoinduced electrons to flow readily from rGO to TiO2 NPs, thereby enhancing the photocatalytic activity.
Figure 10.
(a) Field-emission scanning electron image and (b) transmission electron micrograph of the solvothermally synthesized rGO/TiO2 nanocomposite. (c,d) Enlarged images of high-resolution transmission electron micrographs showing the lattice fringes of TiO2 and a clean interface between TiO2 and rGO. Reproduced with permission from [146]. Copyright Springer, 2013.
4.1.3. Electrochemical Anodization
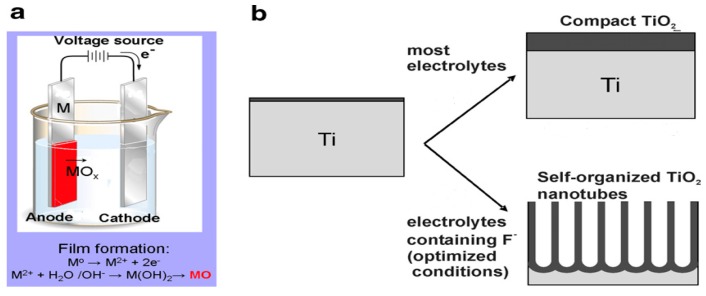
Transition metals like iron and chromium can form a thin oxide film on their surface upon electrochemical polarization in the anodic region [213,214]. Therefore, titanium and its alloys can also form anodic films on their surfaces during the anodizing process. Ti-based alloys are widely used as load-bearing bone prostheses and dental implants in clinical sectors. However, bacterial infection due to biofilm formation is the main cause of implant failures. In recent years, there has been a clinical demand for functional Ti-prostheses with enhanced bone cell adhesion/growth, and excellent antibacterial properties. To improve the biocompatibility of Ti-implants with the host-tissues, titania coating is formed on their surfaces through the anodization technique [215]. One-dimensional titania nanotubes’ (TNTs) high surface area to volume ratio and enhanced bone–cell adhesion ability makes them suitable for biomedical applications [216,217,218,219]. TNTs promote the osseointegration of bone implants more effectively than titanium alloys. Compared with TiO2 NPs, TNTs bear a stronger negative surface charge [55], enabling them to repel bacteria with a negatively charged membrane. Thus, TNTs show bactericidal effects to a lesser degree. By incorporating AgNPs into TNTs, the bactericidal performance of anodized Ti-alloys is improved significantly [218]. The TNTs fabricated from the sol-gel or hydrothermal methods are randomly oriented [220]. In contrast, ordered and self-organized titania nanotubes can be prepared by electrochemical anodization [219]. Anodization offers the additional advantages of simplicity, and ease of fabrication and scaling-up. The tube diameter, length and wall thickness can be properly manipulated by processing parameters including electrolyte composition, applied voltage, pH, temperature, and time [221]. In general, the applied voltage regulates the nanotube diameter, and the anodizing time controls the tube length.
Titanium anodization can be simply performed in a two-electrode cell system connected to a power supply (Figure 11a). The oxide film formation involves an anodic oxidation of metal at the metal surface, outward migration of Ti4+ ions toward the metal/oxide interface and field-assisted dissolution of oxide at the oxide/electrolyte interface [219]. The oxide layer generally has a low conductivity, which restricts the migration of oxygen and Ti ions accordingly. As such, continued oxide growth is assisted by an electric field, and a compact oxide layer is formed on the Ti surface. The electrochemical reactions occurring during anodization are given as follows
| Ti + 2H2O + 4e→ TiO2 + 4H+ Anodic oxidation, | (6) |
| 4H+ + 4e → 2H2 Cathodic reaction. | (7) |
Figure 11.
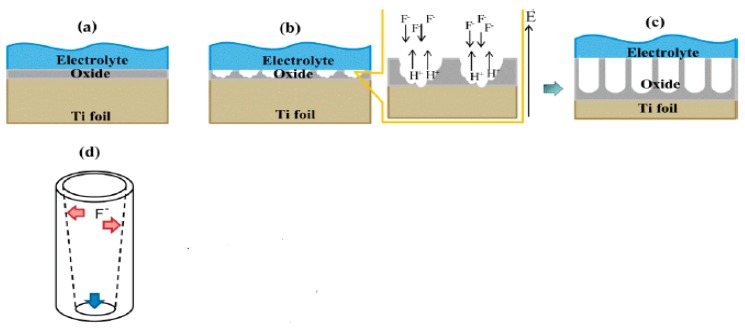
Schematic illustration displaying (a) the set-up for anodization and (b) frmation of compact titania layer on the Ti substrate in electrolytes without fluoride, and self-organized titania nanotube arrays in electrolytes with fluoride. Reproduced with permission from [222]. Copyright Elsevier, 2007.
To form TNTs on Ti foil substrate, aqueous fluoride-containing electrolytes such as (NH4)2HPO4/NH4F or (NH4)2SO4/NH4F, and organic electrolytes, e.g., ethylene glycol, formamide, or dimethylsulfoxide containing F− anions, are needed (Figure 11b). The presence of F− anions in the electrolyte results in the chemical dissolution of oxide at the electrolyte/oxide interface to yield [TiF6]2− and F−-rich layers. In other words, F− ions etch the oxide layer to form water-soluble [TiF6]2− complexes. The chemical reaction associated with the F− ions etching is given by [222]
| TiO2 + 6F− + 4H+ → [TiF6]2− +2H2O | (8) |
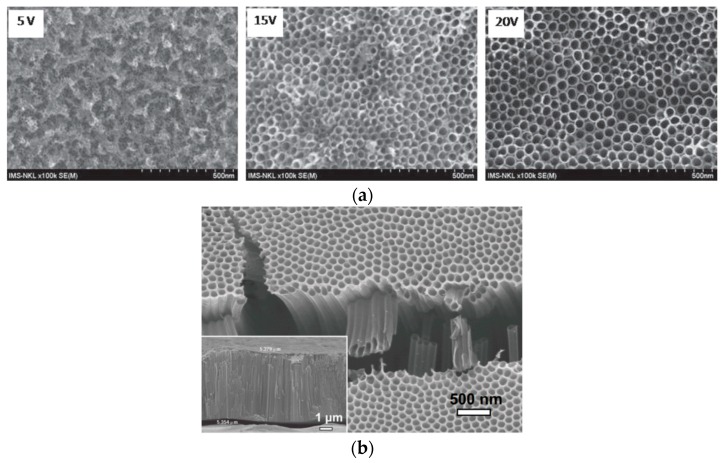
Accordingly, small pits are produced at the electrolyte/oxide interface due to the chemical dissolution of oxide. These pits gradually grow into nanopores, as shown in Figure 12a,b. The pores grow into tubular features and form TNT arrays as the anodizing process continues to its final stage (Figure 12c,d). The growth of TNT arrays is described as the competition between electrochemical oxide formation and chemical dissolution of oxide by F− ions of sufficient concentrations [222,223,224]. Figure 13a,b shows the formation of TNT arrays by anodizing Ti in a mixed ethylene glycol/NH4F and water solution [223,225]. At a low applied voltage of 5 V, an SEM image shows the formation of the rough Ti surface together with inhomogeneous TNTs. However, uniform and well-aligned TNTs are produced by increasing the applied voltage from 15 to 20 V (Figure 13a). The as-anodized TiO2 nanotubes generally exhibit an amorphous structure. Therefore, post-annealing treatment is typically performed to enhance their crystallinity.
Figure 12.
Schematic showing the formation of titania nanotube (TNT) arrays: (a) an initial development of a compact oxide layer on the surface of Ti, (b) small pits formation due to the etching of oxide by F− ions, (c) local growth of nanopores into well-aligned TNT arrays, and (d) the shape and wall thickness of a nanotube. Reproduced with permission from [223]. Copyright MDPI, 2019.
Figure 13.
(a) Top-view scanning electron micrographs of TNT arrays prepared by anodizing Ti in a mixed ethylene glycol/NH4F and water solution under applied voltages of 5, 15 and 20 V for 5 h. Reproduced with permission from [225]. Copyright IOP Publishing, 2014. (b) Cross-sectional SEM image (inset) and top view of TNTs fabricated by anodizing Ti in a mixed ethylene glycol/NH4F and water solution at 30 V for 1 h. Reproduced with permission from [223]. Copyright MDPI, 2019.
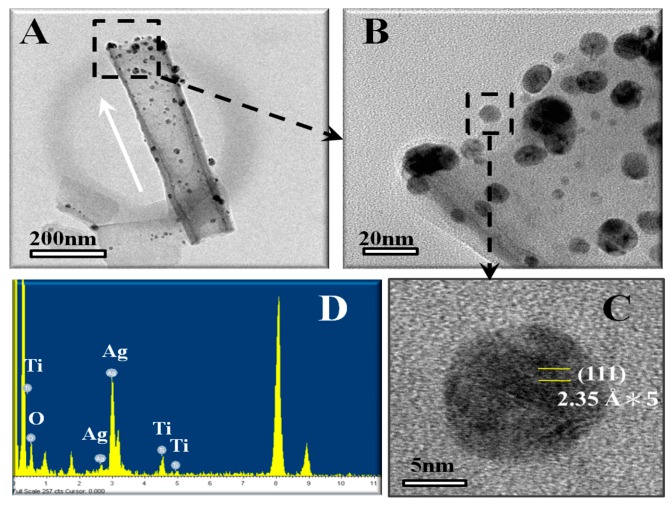
To introduce AgNPs into TNTs, Lan et al. deposited a thin Ag layer on anodized TNTs via electron-beam evaporation. AgNPs were directly coated onto inner- and outer-tube surfaces [226]. Figure 14A is the TEM image of AgNP-decorated TNTs, showing the uniform distribution of AgNPs along the tubes. High-magnification TEM images reveal that the sizes of AgNPs range from 5 to 20 nm (Figure 14B,C). The corresponding energy dispersive X-ray spectrum (EDS) reveals the presence of Ag in addition to Ti from the TNT (Figure 14D). Alternatively, the wet chemical synthesis route using TNT and silver nitrate mixed solution can yield Ag-decorated TNTs. Thereafter, UV illumination is employed to reduce Ag+ ions to AgNPs through the photoreduction process without using reducing agents. As such, photo-assisted deposition can bind AgNPs closely to TNTs [53].
Figure 14.
(A) TEM image of a single, Ag-decorated TiO2 nanotube with a diameter of 100 nm. The white arrow indicates the growth direction of a nanotube. (B) High-magnification image of the selected are, as marked by a dashed square in (A). (C) Enlarged view of a single AgNP, and (D) the corresponding EDS spectrum of AgNP and TiO2 nanotubes. Reproduced with permission from [226]. Copyright Public Library of Science, 2013.
4.1.4. Electrospinning
Electrospinning is a simple and versatile tool to form microfibers and nanofibers (NFs) from different materials including polymers, metal oxides and their nanocomposites. This process has been used extensively for fabricating nanofibers derived from polymers and polymer nanocomposites [27,72,227,228]. In the process, a high electric field is applied to the polymer/solvent solution. Beyond a critical voltage, the repulsive electrostatic force overcomes the surface tension of the polymer droplet, resulting in the ejection of a charged jet from the nozzle towards the collector. Several processing parameters affect the fiber diameter and porosity of electrospun polymer mats, including the type of solvent used, polymer concentration, applied voltage, flow rate, needle-to-collector distance, etc. [227,228]. Very recently, Feng et al. incorporated commercial Degussa P25 (70–80% anatase and 20–30% rutile) with a diameter of 20 nm into polylactic acid (PLA) using the electrospinning method [229]. They reported that the PLA/TiO2 composite nanofibers with 0.75 wt% TiO2 exhibit good bactericidal activity upon exposure to UV-A (360 nm) radiation.
In general, two approaches have been employed to prepare electrospun metal oxide NFs, i.e., a polymer-assisted spinning method and direct electrospinning without using a polymer [230,231,232,233,234]. The former strategy involves the mixing of metal alkoxide sol with a polymer solution in which the polymer controls the rheology during electrospinning. Without a polymer solution, the viscosity of the sol varies with time, causing a difficulty in controlling the rheological properties of a sol. In addition, the diameter of the as-spun ceramic fibers falls in the micrometer scale [232]. To improve solution spinnability, poly (vinyl pyrrolidone) (PVP) is added to the sol to obtain continuous ceramic NFs. As such, the diameter of titania fibers can be tuned from the micrometer to nanometer scale by regulating the concentration of PVP and the Ti alkoxide to PVP ratio. For example, Tekmen et al. electrospun TiO2 with a diameter of 54–78 nm, employing a mixture solution of PVP and TTIP [231].
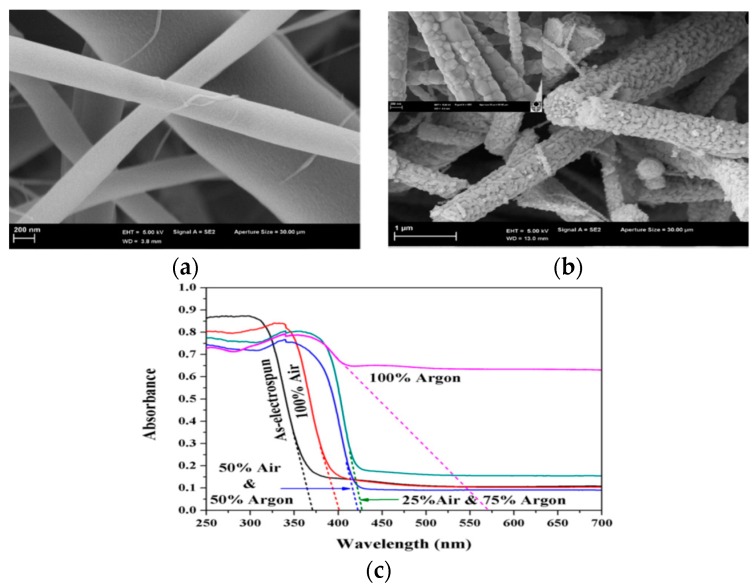
Albetran et al. studied the effect of calcination treatment on the bandgap reduction in electrospun titania nanofibers exposed to pure argon, air, and air–argon mixtures at 900 °C [233]. The spinning solution was prepared by mixing TTIP, ethanol, and acetic acid in a fixed volume ratio of 3:3:1, followed by the addition of 12 wt% PVP. The nanofibers heated in 100% argon exhibit an uneven or rough surface in comparison with the as-spun amorphous fibers due to the formation of crystalline grains of anatase and rutile (Figure 15a,b). In general, the anatase phase is stable in TiO2 up to 500–700 °C, and transforms to rutile with an increase in temperature [75]. Calcination at 900 °C led to a reduction in the diameter of NFs due to the removal of PVP and the densification of TiO2. The degree of crystallinity of calcined titania NFs was 73.4%. Moreover, calcination of the as-spun NFs in 100% argon induced the formation of a high amount of oxygen vacancies, thereby creating a localized state below the conduction band, and reducing the bandgap accordingly. The creation of oxygen vacancies was reported to be effective to enhance visible light absorption as the oxygen vacancy states were located 0.75 to 1.18 eV below the conduction band minimum of TiO2. Those oxygen vacancies were generated in titania by heating in an oxygen-poor environment, such as a N2, Ar or a vacuum at elevated temperatures (>400 °C) [235]. From Figure 15c, the as-spun mat calcined at 900 °C in a 100% argon atmosphere had the highest absorbance in the visible light region compared with the as-spun mats with and without calcination in air and air–argon gaseous mixtures. The bandgap of as-electrospun amorphous nanofibers determined from the UV-vis spectra reduced from 3.33 to 3.09, 2.91 and 2.18 eV through calcination in air, 25% air/75% argon and 100% argon, respectively. Nasr et al. electrospun (2%–7 wt%) rGO/TiO2 NFs, followed by annealing at 500 °C [236]. The rGO sheets reduced the bandgap of TiO2 NFs from 3.2 to 2.9 eV, thus suppressing the recombination of electron–hole pairs, and increasing visible-light photocatalytic activity for degrading methylene blue. Very recently, Chapman et al. successfully obtained TiO2 NFs with average diameters of ~70 nm without using a polymer through mixing an alkoxide precursor, solvent, water, and an acid [234]. They introduced TTIP in ethanol, aged under nitric acid condition, and then added N,N-dimethylformamide to obtain a sol needed for the continuous spinning of TiO2 NFs.
Figure 15.
Scanning electron micrographs of (a) electrospun titania nanofibers before heating, and (b) after heating in 100% argon atmosphere at 900 °C. (c) UV-visible spectra of as-spun titania nanofibers without calcination, and with calcination at 900 °C in 100% air, 50% air–50% argon, 25% air–75% argon and 100% argon. Reproduced with permission from [233]. Copyright Elsevier, 2016.
5. Bactericidal Activities
Titania NPs are negatively charged at the point of zero charge (pzc) at pH = 6.2. Therefore, they exhibit low bactericidal activity in neutral and alkaline solutions by repelling negatively charged bacteria in the absence of light. At acidic pH, positively charged TiO2 NPs strongly interact with the bacterial cells, resulting in bacterial membrane penetration and inducing oxidative damage accordingly [57]. Kiwi et al. reported that TiO2 NPs tend to kill E. coli by direct contact in the dark condition, thus damaging their cell walls, due to the electrostatic attraction between the TiO2 NPs and the negatively charged bacterial cell wall at a pH close to but below pzc [237]. On the contrary, the bactericidal effect is caused by the creation of ROS species on the TiO2 NPs under UV irradiation. This means that the bactericidal activity is due to the radiation itself and is not caused by the titania NPs [238]. TiO2 NPs can kill multidrug-resistant bacteria such as MRSA, vancomycin-resistant Enterococcus faecalis (VRE) and P. aeruginosa through the reactive radicals generated by electron–hole pairs upon UV excitation [239,240]. The photocatalytic inactivation of MRSA and VRE strains depends on the power and irradiation time of UV-A light [239]. Therefore, the disinfection process requires a high-power UV source to excite TiO2 NPs. Apparently, TiO2 NPs do not reach their full potential for bactericidal applications owing to their ineffective photoexcitation under visible light irradiation. As a result, TiO2 NPs have limited efficiency against microorganisms in indoor environments where the fraction of UV light is small. From this perspective, the development of a visible-light active TiO2 with excellent antibacterial performance is of crucial importance in medical and industrial sectors.
5.1. Metal Doping
Transition metals like Cr, Fe, Ni, Cu, and RE metals can be used to enhance the photocatalytic activity of nanocrystalline titania, and this in turn improves its bactericidal performance. Those metal cations substitute Ti4+ ions in the titania lattice, leading to a reduction in the bandgap and promoting the formation of charge carriers under visible light. As a result, ROS are generated on the titania surface, and they are very effective at killing bacteria through lipid peroxidation, the depletion of glutathione, DNA damage and the final disintegration of the cell membrane. This results in a leakage of cellular contents, thus causing cell lysis and eventual cell death [241,242]. Negatively charged superoxide and hydroxyl radicals generally reside on the membrane and do not penetrate into the bacterial cytoplasm, while electrically neutral H2O2 can pass through the cell membrane. Hydrogen radicals can abstract hydrogen atoms from the fatty acids of bacterial membrane lipids, causing lipid peroxidation and damaging the respiratory electron transport chain located in the membrane [242]. As is known, most transition and RE metals are toxic to humans. In terms of environmental and public health considerations, Cu is more suitable than other transition metals and RE metals for doping nanocrystalline titania. Copper metal is widely used in hospitals for preventing spread of bacteria among the patients because of its antimicrobial activity [243]. Therefore, copper can be used to dope titania for antibacterial purposes [244,245,246,247]. As an example, TiO2–Cu films exhibit bacterial inactivation for E. coli and MRSA under indoor visible light irradiation [244,245].
5.1.1. Doped Titania NPs
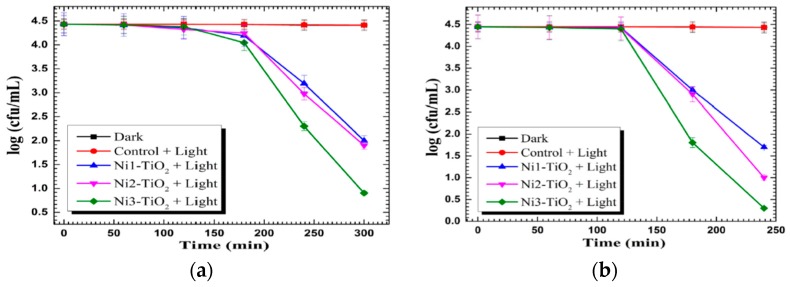
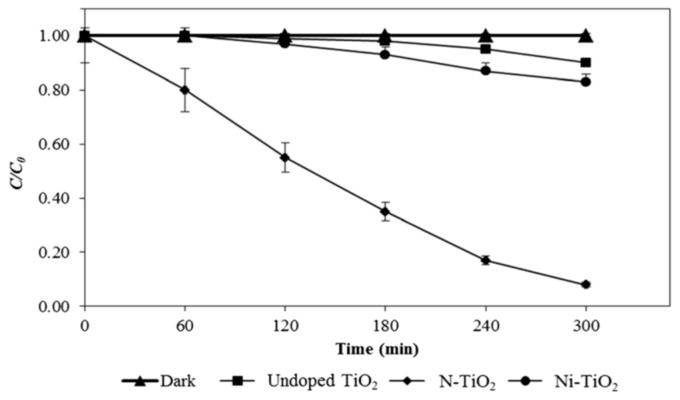
Yadav et al. fabricated Ni-doped TiO2 NPs using the sol-gel process through the addition of NiSO4(H2O)6 to a mixture solution containing TTIP, acetic acid and sodium dodecyl sulfate. The resulting powders were dried and calcined at 500 °C for 5 h [248]. Figure 16a,b shows the photocatalytic bactericidal activity against E. coli and S. aureus of Ni-doped TiO2 NPs with 1.0 mol %, 2.0 mol % and 3.0 mol % Ni dopants, denoting Ni1–TiO2, Ni2–TiO2 and Ni3–TiO2, respectively. In dark (with doped TiO2 NPs) and visible light (without doped TiO2 NPs) environments, both bacterial strains grow into a high density of cell populations, expressed as colony-forming units (CFU)/mL. The photocatalytic inactivation of E. coli and S. aureus takes place by illuminating Ni-doped TiO2 NPs with visible light. The Ni3–TiO2 sample shows the highest photocatalytic inactivation because it can generate a higher ROS level with an increase in Ni content. Moreover, the photocatalytic inactivation efficiency of Ni-doped TiO2 NPs toward Gram-positive S. aureus is somewhat faster than Gram negative E. coli. In the case of Gram-negative salmonella abony, complete inactivation takes 360 min of light irradiation (data not shown). The time required for the full inactivation of S. abony is higher than that for E. coli inactivation. Using the same approach, they also fabricated Cu-doped TiO2 NPs with 1.0 mol %, 2.0 mol % and 3.0 mol % Cu by adding different concentrations of CuSO4·5H2O to a solution containing TTIP and acetic acid. The catalysts were calcined in air at 500 °C for 5 h [247]. The 3%Cu/TiO2 NPs photocatalyst exhibits a higher bactericidal activity than those doped with 1.0 mol %, and 2.0 mol % Cu. The 3%Cu/TiO2 NPs catalyst shows 100% inhibition for S. aureus within 120 min, but it requires 240 min for the complete inactivation of E. coli. This implies that the rate of bacterial inactivation for E. coli is much slower than for S. aureus. This is caused by a difference in the cell wall structures between these two bacterial strains. As is known, bacteria exhibit a negative charge on their cell wall surface. The cell wall of Gram-positive bacteria is relatively porous and thick (20–80 nm) consisting of several layers of peptidoglycan, interspersed with teichoic and lipoteichoic acids. Peptidoglycan is negatively charged due to the presence of carboxyl and amino groups [249]. In contrast, the cell wall of Gram-negative bacteria is thinner (<10 nm) with a single peptidoglycan layer, surrounded by an outer membrane with a very complex structure. Lipopolysaccharides (LPS) and lipoproteins are located in the outer leaflet, while phospholipids are found in the inner leaflet of the outer membrane. The phosphate groups of LPS increase the overall negative charge. Thus Gram-negative bacteria have a higher negative charge than Gram-positive bacteria [250,251,252]. The structural variations in the cell walls between these two bacterial strains lead to their different interactions with photocatalysts. As such, Gram-negative bacteria is more resistant to attack from the superoxide anion and hydroxy radical with a negative charge. Moreover, LPS also creates a permeability barrier at the cell surface, thus contributing to its resistance against many antibiotics and substances [250,251,252].
Figure 16.
Inactivation of (a) E. coli and (b) S. aureus by Ni-doped TiO2 NPs as a function of time. Reproduced with permission from [248]. Copyright Elsevier, 2014.
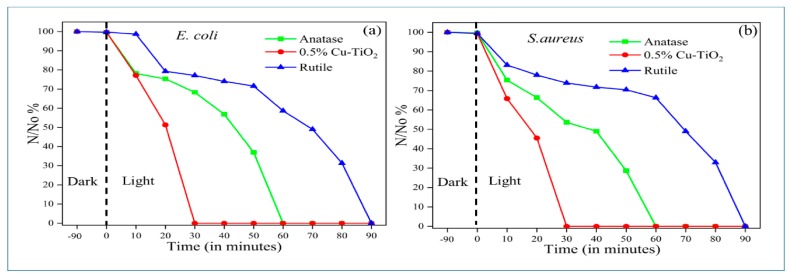
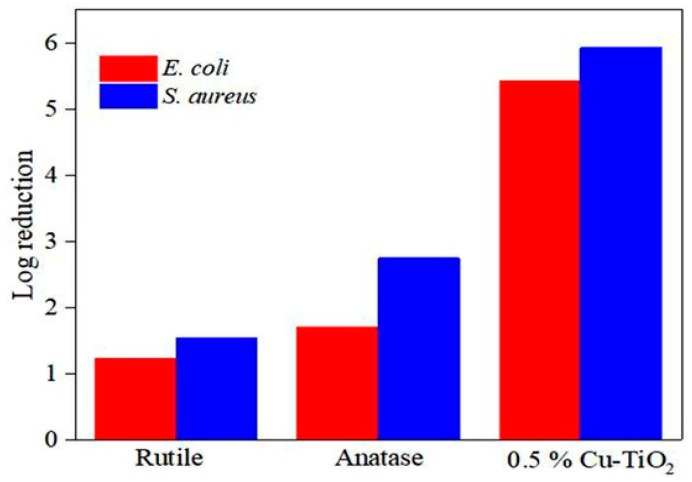
Very recently, Pillai and coworkers prepared Cu-doped TiO2 NPs by adding a copper sulfate solution to a mixture solution containing TTIP, and isopropanol [109]. The resulting gel was dried, and doped titania powders were calcined at 500, 600, 650 and 700 °C, respectively. Pure TiO2 powders were prepared from TTIP and isopropanol without copper sulphate addition. The obtained TiO2 powders were calcined at 500 and 700 °C to yield anatase and rutile, respectively. Their results showed that Cu doping is very effective to retain the anatase phase of TiO2 at calcined temperatures up to 650 °C. X-ray Photo-electron Spectroscopy (XPS) spectra reveals the presence of Cu+ and Cu2+ in Cu-doped TiO2 where the Cu+ state predominates. Figure 17a,b shows the photocatalytic bactericidal activity of 0.5 wt% Cu/TiO2, anatase TiO2 and rutile TiO2 against E. coli and S. aureus under dark and visible light illumination, respectively. In the dark, both bacteria strains grow quickly and their survival rate reaches a high plateau value. However, a 5-Log pathogen reduction (99.999%) is observed in both bacterial strains exposed to 0.5 wt% Cu/TiO2 following visible light irradiation for 30 min (Figure 18). In this respect, 0.5 wt% Cu/TiO2 photocatalyst exhibits a strong antibacterial effect against E. coli and S. aureous under visible light irradiation. The enhanced antibacterial performance of 0.5 wt% Cu/TiO2 photocatalyst calcined at 650 °C is attributed to the formation of a heterojunction between TiO2 and Cu2O, inducing hydroxyl radicals through the interfacial charge carrier transfer mechanism, to the copper ions killing effect. The replacement of Ti4+ with Cu2+ also induces the creation of oxygen vacancies. This gives rise to the high absorption rate of visible light as a result of a bandgap reduction from 3.17 to 2.8 eV. It is noted that some Cu+ ions may react with a transient metabolic byproduct of cellular respiration, i.e., H2O2 through the Fenton reaction, resulting in the formation of hydroxyl radicals and Cu2+ ions. The Fenton reaction for generating hydroxyl radicals due to the presence of Cu+ ions is given by [246]
| Cu+ + H2O2 → Cu2+ + OH− + •OH. | (9) |
Figure 17.
Photocatalytic inactivation of (a) E. coli and (b) S. aureus with 0.5% Cu/TiO2 calcined at 650 °C, pure anatase and rutile specimens. N/No is the reduction in the concentration of the bacteria. Reproduced with permission from [109]. Copyright MDPI, 2018.
Figure 18.
Photocatalytic bactericidal efficacy of 0.5% Cu/TiO2, pure anatase and rutile with E. coli and S. aureus upon visible light irradiation for 30 min. Reproduced with permission from [109]. Copyright MDPI, 2018.
Silver nanoparticles with an additional function as a bactericidal agent can be used to modify titania photocatalyst to further enhance its antibacterial performance. From the literature, AgNPs exhibit excellent antibacterial activity against various microorganisms, including S. aureus, MRSA, Bacillus subtilis, E. coli, Pseudomonas aeruginosa, Klebsiella pneumonia, and Acinetobacter baumanii [253]. Whether metallic Ag0 or ionic Ag+ released from AgNPs exerts killing effects on bacteria is still unknown [15,253,254,255]. The former mechanism involves the adhesion of AgNPs to the cell membrane, leading to membrane damage, the generation of oxidative stress and leakage of cellular contents. Moreover, AgNPs can move into the cytoplasm and interact with biomolecules such as protein and DNA. In some cases, they inactivate and destabilize ribosome, thus inhibiting protein synthesis and generating ROS accordingly. In the case of silver-ion induced toxic effects, released silver ions would interact with the thiol groups of respiratory chain proteins on the membrane, resulting in the disruption of the bacterial cell wall and the creation of ROS. The electron transport chain for bacterial respiration is located at the bacterial cytoplasmic membrane, since bacteria have no mitochondria (Figure 1). Silver ions can also penetrate into the cytoplasm and react with the thiol groups of cytoplasmic proteins [15,253,254,255].
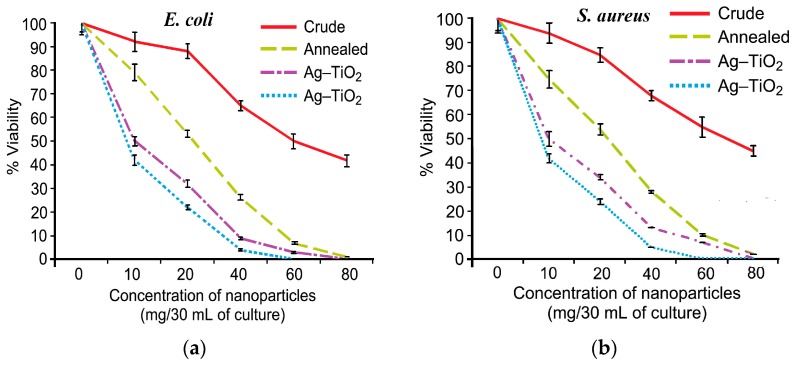
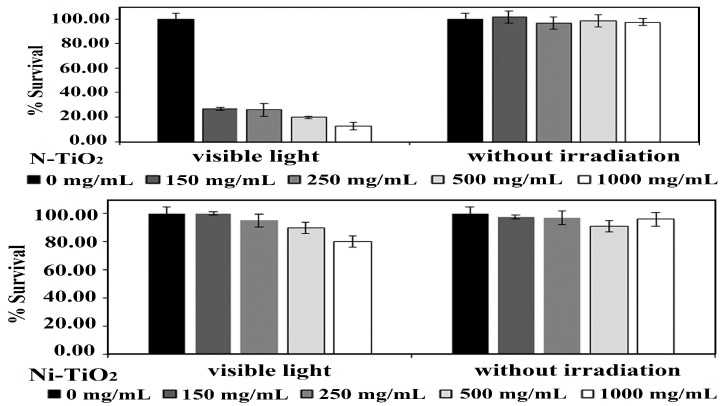
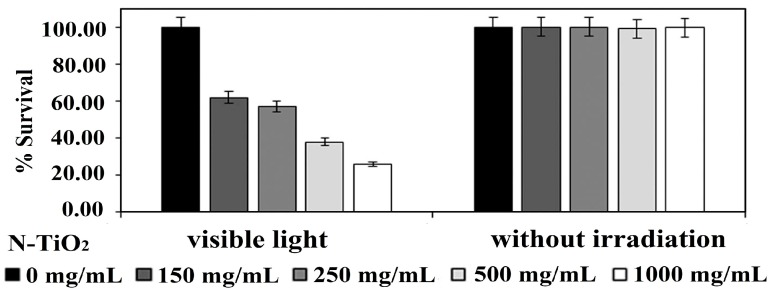
As mentioned, AgNPs can induce a collective oscillation of surface electrons under visible light irradiation, thereby creating a hot electron–hole pair and inducing ROS for bacterial inactivation. Thus, AgNPs serve as electron donors for titania, since plasmonic hot electrons are injected into the conduction band of TiO2 and trigger a photocatalytic disinfection reaction to generate superoxide anion, as shown in Figure 6. Accordingly, AgNPs play the dual role of antibacterial agent and electron donor for Ag-doped TiO2 NPs [124]. Gupta et al. fabricated Ag-doped TiO2 NPs with 3% and 7% AgNPs using the sol-gel process. The resulting powders were dried in an oven followed by annealing at 450 °C for 30 min [256]. The photocatalytic activities of the as-synthesized TiO2, annealed TiO2, and annealed Ag-doped TiO2 materials were assessed against Gram negative E. coli, Pseudomonas aeruginosa and Gram positive S. aureus under visible light. Figure 19a,b shows the viability of E. coli and S. aureus versus the concentration of catalyst nanoparticles, respectively. The as-synthesized TiO2 NPs with an amorphous structure inactivates some E. coli and S. aureus because their negatively charged surface can repel bacteria, resulting in a net negative charge on the cell wall [57]. Annealing treatment at 450 °C induces the crystallization of the anatase phase in TiO2 NPs. The bactericidal performance of annealed Ag-doped TiO2 NPs is markedly improved in comparison with the as-synthesized and annealed TiO2 NPs. The Ag-doped TiO2 NPs with 7% AgNPs exhibits toxicity to both bacterial strains at 60 mg/30 mL, and at 40 mg/30 mL culture in the case of P. aeruginosa.
Figure 19.
Viability of (a) E. coli and (b) S. aureus against the concentration of as-synthesized TiO2 NPs, annealed TiO2 NPs, and Ag-doped TiO2 NPs with 3% AgNPs (dash–dot curve) and 7% AgNPs (dot curve; blue). Reproduced with permission from [256]. Copyright Beilstein-Institut, 2013.
5.1.2. Doped Titania Nanotubes
The photocatalytic activity of one-dimensional TNTs is considerably higher than that of TiO2 NPs because of their large surface area, high aspect ratio, and good light-harvesting properties [257,258]. Recently, Podporska-Carroll et al. reported that TNTs exhibit very high bactericidal efficiency against E. coli (97.53%) and S. aureus (99.94%) under 24 h of UV irradiation [259]. Moreover, anodic TNTs exhibit a higher photocatalytic inactivation of bacteria than commercial Degussa P25 TiO2 powders. To extend the optical absorbance to the visible-light region and improve bactericial performance in this optical regime, noble metal dopants are added to TNTs accordingly. For instance, Viet et al. demonstrated that the 2 wt% Ag/TNTs photocatalyst exhibits higher bactericidal activity against S. aureus than pristine TNTs when exposed to sunlight at noontime [53].
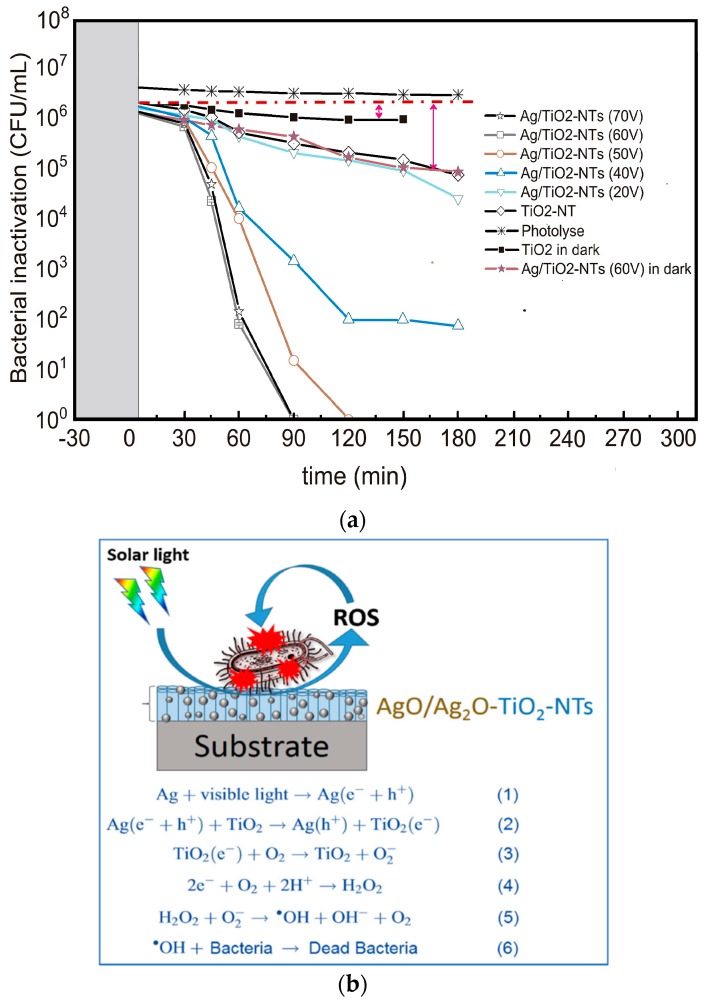
Rtimi and coworkers prepared TNTs of different diameters by varying applied voltages from 20 V–70 V during anodizing process. The anodized TNTs were air-dried, annealed for 3 h at 400 °C, and then immersed in a 0.1 M AgNO3; Ag+ ions were reduced to AgNPs on TNTs using the photoreduction method [260]. A low voltage of 20 V was not favorable for the formation of TNTs. The average diameters of TNTs under applied voltages of 40, 50, 60 and 70 V were 59.6, 93.6, 96.6 and 100. 9 nm, respectively. The tube diameter increased with increasing applied voltage. Figure 20a shows the bacterial survival rate of E. coli on pristine TNTs and Ag-decorated TNTs of different diameters upon exposure to solar-simulated light (50 mW/cm2). The used light intensity corresponds with the overcast daylight dose. Pristine TiO2–NTs inactivate 1.6log E coli within 180 min. Negatively charged TNTs tend to repel E. coli with a negative surface charge, giving rise to a low level of antibacterial activity. From this figure, a stronger E. coli inactivation can be achieved by increasing the diameter of TNTs. The Ag/TNTs with diameters of 96.6 nm and 100.9 nm exhibit excellent bacterial inactivation compared with neat TNTs. These two samples require 90 min for inactivating 99.99% E. coli upon exposure to solar-simulated light. The bacterial inactivation is attributed to the generation of ROS as a result of the plasmonic oscillation of surface electrons of AgNPs caused by solar-simulated light irradiation. This, in turn, leads to the generation of an electron–hole pair in TNTs to create ROS (Figure 20b). Free radicals abstract electrons from the lipid molecules of bacterial membrane, leading accordingly to lipid peroxidation and membrane damage.
Figure 20.
(a) Bacterial inactivation on neat TiO2–NT and Ag/TNTs photocatalysts exposed to solar-simulated light (50 mW/cm2, 310–800 nm). Error bars: standard deviation; n = 5. (b) Bacterial inactivation mechanism of Ag/TNTs, as described by Reaction (1–6). Reproduced with permission from [260]. Copyright Elsevier, 2018.
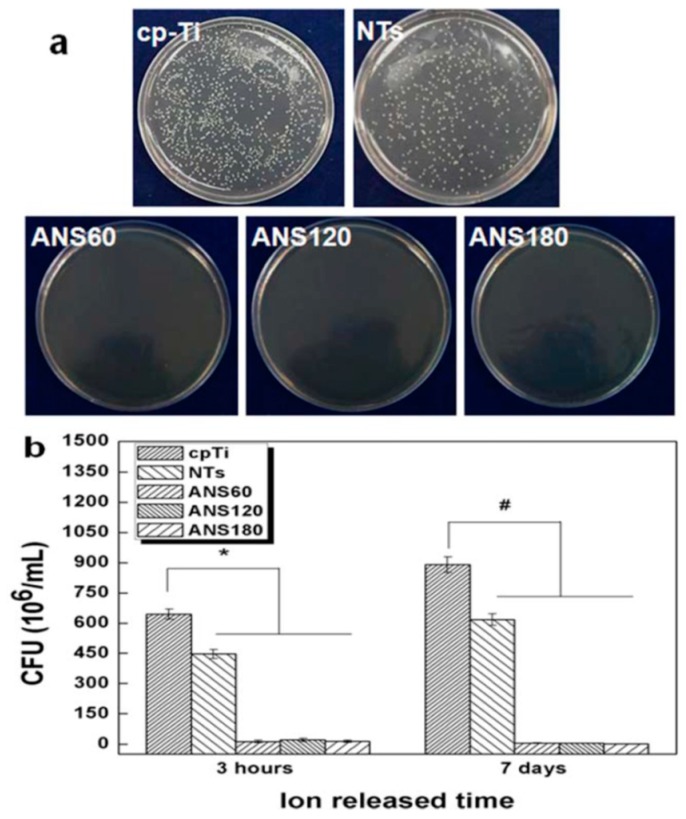
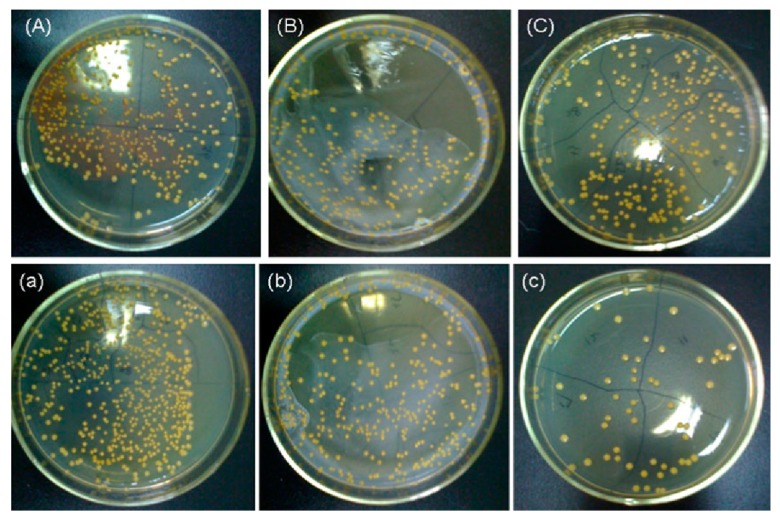
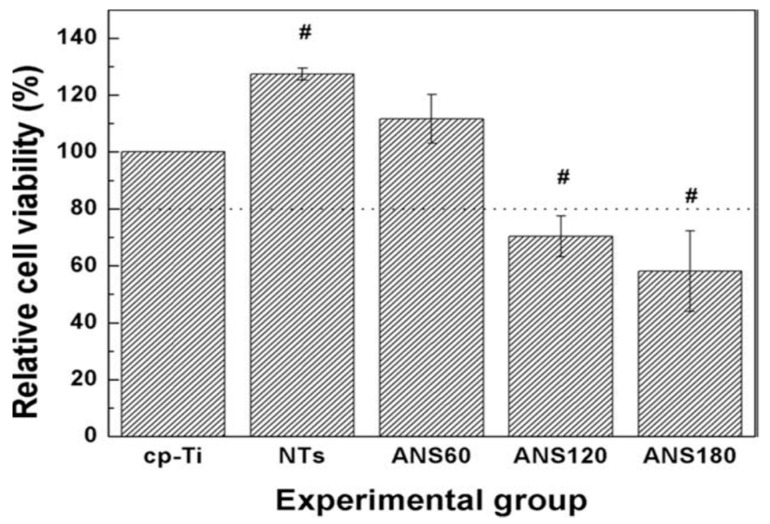
The AgNPs of silver-decorated TNTs also play the role of antibacterial agent through the released Ag+ ions. Uhm et al. fabricated Ag-doped TNTs by depositing a thin silver layer onto anodized TNTs via magnetron sputtering for different time periods [261]. The TNTs samples coated with silver for 60, 120 and 180 s were designated as ANS 60, ANS 120 and ANS 180, respectively. A longer sputtering time induced more AgNPs formation on the nanotubes, as expected. To assess the silver-ion induced toxic effect on the S. aureus, Ag+ ion, released in phosphate-buffered saline (PBS) and plate counting methods was employed in their study (Figure 21a,b). From Figure 21a, all Ag-doped TNTs samples showed excellent antibacterial activity compared to commercially pure Ti (cpTi) and pristine TNTs. This was attributed to the released Ag+ ions from the Ag-doped TNTs for bacterial inactivation (Figure 21b). Such an antibacterial effect was unrelated to photoactivity. A profound difference in bacterial reduction in terms of CFU was seen between neat TNTs and Ag-doped TNTs.
Figure 21.
(a) Photographs showing the spread of S. aureus on commercially pure titanium (cpTi), titanium nanotubes (NTs) and Ag-doped TNTs samples. (b) Antibacterial efficacy of all samples immersed in PBS for 3 h and 7 d. The error bars are the standard deviation (n = 5); * denotes p < 0.05 compared with cp-Ti at a 3 h ion extraction time, # denotes p < 0.05 compared with cp-Ti at 7 days ion extraction time. Reproduced with permission from [261]. Copyright Wiley, 2014.
5.2. Non-Metal Doping
Non-metal dopants such as nitrogen, carbon, and boron are typically employed to replace lattice oxygen anions of titania, in order to narrow its bandgap and extend the optical absorption edge to the visible regime, thereby increasing photocatalytic activity. This in turn leads to an improvement in its antibacterial properties under visible light [161,202,246,262,263,264,265]. The visible light response originates from the presence of localized energy levels of the dopant lying above the valence band, thus shifting the VB level upward (Figure 8) [97,159,160,161,162,163,164]. Among those dopants, nitrogen has a size comparable to oxygen, so it can be readily doped into the TiO2 lattice in either substitutional or interstitial sites. The N-2p orbital hybrids with the O-2p state, leading to the band gap narrowing. Recently, Ananpattarachai et al. prepared N-doped TiO2 NPs using the sol-gel technique with diethanolamine acting as the N source. For the purposes of comparison, they also prepared Ni-doped TiO2 NPs by adding NiSO4(H2O)6 to the Ti-sol. The as-synthesized N- and Ni- doped TiO2 NPs powders were calcined at 600 °C [161]. The bandgaps of N-doped TiO2 NPs and Ni-doped TiO2 NPs were determined to be 2.1 eV and 2.97 eV, respectively. The antibacterial activities of the photocatalysts were assessed using S. aureus and E. coli strains under visible light irradiation. Figure 22 shows the photocatalytic inactivation of S. aureus with neat TiO2, N-doped TiO2 NPs and Ni-doped TiO2 NPs. Apparently, N-doped TiO2 NPs are more effective than Ni-doped TiO2 NPs for S. aureus inactivation due to their smaller bandgap. Nearly 90% of S. aureus cells are inactivated by N-doped TiO2 NPs within 300 min. The complete inactivation time for S. aureus is 360 min. In contrast, the complete inactivation time for E. coli is 420 min (not shown). Figure 23 displays the photocatalytic inactivation of S. aureus with different concentrations of N-doped TiO2 NPs and Ni-doped TiO2 NPs. The survival of S. aureus with N-doped TiO2 NPs under visible light is smaller than with Ni-doped TiO2 NPs. Figure 24 shows the photocatalytic inactivation of E. coli with different concentrations of N-doped TiO2 NPs. The inactivation efficacy of Gram-positive S. aureus using N-doped TiO2 NPs is higher than that of Gram-negative E. coli under visible light illumination. According to the literature, carbon dopant is also beneficial in improving the visible light absorption of TiO2 NPs and photocatalytic inactivation of anthrax, a fatal bacterial disease that occurs in animals and can transmit to humans, and is caused by Gram-positive Bacillus anthracis [264].
Figure 22.
Survival ratio (C/Co) of S. aureus with neat TiO2, N-doped TiO2 NPs and Ni-doped TiO2 NPs under an 18 W visible light irradiation for different time periods. S. aureus without TiO2 in the dark is used as a control. Statistically significant at p < 0.05. Reproduced with permission from [161]. Copyright Springer, 2016.
Figure 23.
Photocatalytic inactivation of S. aureus with N- and Ni-doped TiO2 NPs of different contents under visible light. Statistically significant at p < 0.05. Reproduced with permission from [161]. Copyright Springer, 2016.
Figure 24.
Photocatalytic inactivation of E. coli with N-doped TiO2 NPs of different concentrations under an 18 W visible light. Statistically significant at p < 0.05. Reproduced with permission from [161]. Copyright Springer, 2016.
He et al. fabricated N-doped TiO2 NPs (30 nm) using the sol-gel technique. The photocatalytic and bactericidal behaviors of N-doped TiO2NPs against E. coli under dark and simulated-sunlight conditions were investigated [265]. The bacterial inactivation of this catalyst reaches 90% under simulated sunlight for 2 h, much higher than in the dark. Figure 25 displays photographs of neat TiO2 and N-doped TiO2 NPs treated with E. coli in the dark for 24 h, and under visible light for 2 h. Bacteria grows into colonies on these samples in the dark (top panel). However, N-doped TiO2 can inactivate E. coli by irradiating with simulated-sunlight for 2 h (bottom panel).
Figure 25.
Photographs of E. coli colonies developed on agar plates treated with (A) control, (B) TiO2 and (C) N-doped TiO2 samples in the dark for 24 h. (a–c) are the images of E. coli colonies under visible light irradiation for 2 h. (a): control, (b): neat TiO2, and (c) N-doped TiO2. Reproduced with permission from [265]. Copyright Springer, 2013.
From the literature, fluorine doping does not shift the bandgap of TiO2. Fluorine dopant stabilizes anatase TiO2 up to 1200 °C [75,266]. The replacement of lattice oxygen with fluorine in TiO2 converts Ti4+ to Ti3+ as a result of the charge compensation between F− and Ti4+ [165]. The Ti3+ ions suppress the recombination rate of photogenerated charge carriers, thereby enhancing photocatalytic activity. The substitution of fluorine for oxygen in the titania lattice gives rise to a dramatic increase in oxygen vacancy concentrations [175,267]. The visible-light photocatalytic activity of F-doped TiO2 can be further enhanced by co-doping with N [268]. Thus, the co-doping approach is an effective route to tune the energy band level of TiO2 NPs to enhance photocatalytic reactions. Figure 26a shows the charge-transfer mechanism for visible-light excitation of N−F co-doped TiO2. A series of charge transfer events take place during visible light irradiation. In the process, electrons are excited from the N midgap state to the conduction band, and the corresponding holes generated in the N-state are filled by electrons from the Ti3+ level. Oxygen vacancies (Ovac) also donate electrons to the empty N-state. Moreover, the conduction band can transfer electrons to the oxygen vacancies. This cascade effect facilitates the continuous generation of superoxide anion and hydroxide radical species [268,269]. The ROS then cause the destruction and death of microorganisms accordingly.
Figure 26.
(a) Visible light excitation of N−F codoped TiO2 and subsequent filling of empty N state by electron transfer from either Ti3+ or oxygen vacancies (Ovac). Reproduced with permission from [268]. Copyright American Chemical Society, 2014. (b) Survival rate of E. coli treated with N-doped P25 (P25-N-HT), F-doped P25 (P25-F-HT) and (3) F-N codoped P25 (P25-F&N-HT) under simulated light illumination. Reproduced with permission from [270]. Copyright MDPI, 2017.
Milosevic et al. fabricated N-doped and F-doped TiO2 by wet milling Aeroxide® P25 (21 nm) powders in the presence of glycine (N source) or polytetrafluoroethylene (PTFE; fluorine source), respectively [270]. For the preparation of F−N-co-doped P25, both glycine and PTFE were added during wet milling. The resulting N-doped P25 was heat-treated at 500 °C for 1 h, while F-doped P25 and F−N-doped P25 were calcined at 600 °C for 1 h. Figure 26b shows the survival rate of E. coli treated with F-doped P25, N-doped P25, and F−N-co-doped P25 under visible light irradiation. The complete bacterial inactivation times for F-doped P25, N-doped P25 and F−N-co-doped P25 catalysts are 120 min, 90 min and 60 min, respectively. Apparently, codoping P25 with F and N leads to the F−N-doped P25 catalyst with the best bactericidal performance. This is attributed to a synergistic effect between F and N dopants, creating a series of charge transfer reactions, thereby inducing ROS for bacterial inactivation (Figure 26a). In another study, Milosevic et al. prepared F-doped TiO2 NPs by means of solution precipitation through the hydrolysis of titanium oxychloride, using urea and ammonia as the precipitation agents, and potassium fluoride as the fluorine source. This was followed by wet milling in glycine, and the resulting F−N-doped TiO2 powders were calcined at 500 °C for 1 h [271]. The complete bacterial inactivation times for F-doped TiO2 and F-N codoped TiO2 are 75 min and 60 min, respectively.
5.3. Graphene and MWNT Modified Titania Nanocomposites
A graphene sheet with sharp edges can act as a ‘nanoknife’ for killing microorganisms during the direct contact of bacteria with the sheet edges. In addition, graphene with a lateral dimension of several micrometers can effectively wrap and isolate bacteria from the environment, thus stopping the supply of nutrients [272,273]. A direct contact of the bacterial cell wall with graphene may also lead to the induction of oxidative stress, resulting in the physical disruption of lipid bilayers and the generation of ROS [274,275]. In this respect, the inclusion of rGO to TiO2 NPs can produce novel photocatalysts with improved antibacterial performance. There are few studies in the literature reporting bacterial inactivation of rGO/TiO2 photocatalysts [101,102,276,277].
More recently, Wanag et al. fabricated (0.5–2.5 wt%) rGO/TiO2 NPs using hydrothermal process at 180 °C for 4 h. The resulting products were finally heated in a furnace at 100 °C for 4 h [101]. Figure 27A,B shows the survival rate of E. coli treated with (0.5–2.5 wt%) rGO/TiO2 NPs in the dark and under artificial solar light irradiation, respectively. Little change in the bacterial populations is seen in the dark condition. The complete bacterial inactivation times for TiO2 NPs, 0.5% rGO/TiO2 NPs, 1.5% rGO/TiO2 NPs, and 2.5% rGO/TiO2 NPs samples are 105, 90, 75 and 85 min, respectively. It appears that the 1.5% rGO/TiO2 NPs photocatalyst exhibits the best bacterial inactivation effect.
Figure 27.
Inactivation of E. coli in the presence of rGO, TiO2 and (0.5–2.5 wt%) rGO/TiO2 samples in (A) dark contition and (B) under artificial solar light irradiation. Reproduced with permission from [101]. Copyright Elsevier, 2018.
Nica et al. determined the minimum inhibitory concentration (MIC) and minimum biofilm eradication concentration (MBEC) values of rGO/1%Fe–N-doped TiO2 nanocomposites prepared by a hydrothermal synthesis of mixed 1%Fe–N-doped TiO2 and GO solutions at 150 °C for 2 h [277]. Two different precipitation strategies were adopted to yield 1%Fe–N-doped TiO2 powders. Sample A solution was prepared through a simultaneous precipitation of Ti3+ and Fe3+ ions by mixing desired amounts of TiCl3 and FeCl3.6H2O in water, with a subsequent addition of NH4OH to maintain an alkaline pH of 9. This solution was hydrothermally treated at 200 °C for 2 h, and the resulting powders were calcined at 400 °C for 2 h. Sample B solution was made via a sequential precipitation of these two cations. The Ti3+ ions were first precipitated and oxidized to Ti4+ followed by the addition of Fe3+ ions in an alkaline reaction medium. The purpose of this was to attain a higher iron concentration on the surfaces of synthesized powders [242]. The antibacterial activity of rGO/1%Fe–N-doped TiO2 nanocomposites was assessed with S. aureus, E. coli, P. aeruginosa and Candida albicans (a type of fungus). Figure 28 shows the the MIC and MBEC values of rGO/1%Fe–N-doped TiO2 nanocomposites irradiated with visible light. MIC is generally defined as the lowest antimicrobial agent concentration inhibiting visible growth of bacteria, whereas MBEC is the lowest concentration of an antimicrobial agent needed to kill a bacterial biofilm [278,279]. Compared with commercial P25, rGO/1%Fe–N-doped TiO2 nanocomposites made from the respective Sample A and Sample B solutions exhibit a much higher antibacterial activity under visible light exposure than Gram-positive S. aureus, Gram-negative E. coli, and P. aeruginosa. The MIC value of rGO/doped TiO2 nanocomposites for E. coli and S. aureus is 2.5 µg/mL, four times smaller than that of P25.
Figure 28.
MIC and MBEC values of rGO/1%Fe-N-doped TiO2 nanocomposites and commercial P25 TiO2 treated with S. aureus, E. coli, P. aeruginosa and fungal C. albicans under visible light at 37 °C for 24 h. Reproduced with permission from [277]. Copyright MDPI, 2017.
Akhavan et al. prepared MWNT/TiO2 thin films with 2–40 wt% MWNTs by sol-gel technique. The films were deposited on the glass slides by the dip coating method followed by annealing at 450 °C in air for 1 h to yield anatase phase, thereby forming Ti–C and Ti–O–C bonds [155]. The 20 wt% MWNT/doped TiO2 film inactivated E. coli completely under visible light irradiation for 60 min. They attributed bactericidal effects of 20 wt% MWNT/doped TiO2 nanocomposite to an efficient charge transfer between the MWNTs and TiO2 due to the formation of a Ti–C and Ti–O–C bond, and a reduction in the electron–hole recombination rate, leading to an increase in the production of hydroxyl radicals for photocatalytic inactivation. Very recently, Koli et al. fabricated (0.1–0.5 wt%) MWNT/doped TiO2 nanocomposites using the solution-mixing method. The final products were centrifuged and calcinated in air at 450 °C for 5 h [156]. The 0.5 wt% MWNT/doped TiO2 nanocomposite exhibited complete killing for S. aureus and E. coli under visible light irradiation for 180 and 300 min, respectively. However, nanocomposites with 0.3 wt% and 0.1 wt% MWNTs only showed 80% and 90% inhibition for S. aureus. In another study, Koli et al. prepared (0.1–0.5 wt%) MWNT/Fe-doped TiO2 nanocomposites using the sol gel process. The 0.5 wt% MWNT/Fe-doped TiO2 nanocomposite showed 100% inactivation for Gram-positive Bacillus subtilis under visible light illumination for 120 min. However, nanocomposites with 0.1 wt% and 0.3 wt% MWNTs exhibited complete inhibition at 180 min [157]. In the case of Gram-negative Pseudomonas aeruginosa, MWNT/Fe-doped TiO2 nanocomposites with 0.1, 0.3 and 0.5 wt% MWNTs required 240 and 300 min, respectively, for complete bacterial killing. The photocatalytic inactivation of these bacterial strains derived from the effective generation of ROS under visible light irradiation.
5.4. Coupled Semiconductors
Limited studies are available in the literature on the visible-light photocatalytic bactericidal activity of oxide semiconductor heterojunctions [177,179]. Recently, Janczarek et al. studied the antibacterial performance of cuprous oxide/titania nanocomposites prepared by mechanically mixing Cu2O with TiO2 powders of different structures including pure anatase TiO2 (8 nm), pure rutile TiO2 (16 nm) and Aeroxide® TiO2 P25 in an agate mortar [177]. Figure 29a–d shows the antibacterial performance of pure Cu2O and Cu2O/TiO2 nanocomposites against E. coli in the dark, under UV and visible light irradiation, respectively. Pure Cu2O displays high bactericidal activity under UV or visible light irradiation due to the intrinsic activity of Cu+ ions (Figure 29a). Pure anatase TiO2 NPs with a negative charge surface show little bacterial inactivation in the dark as they repel negatively charged bacteria to a lesser degree [44,57]. However, their antibacterial activity improves substantially under UV light irradiation as a result of the ROS generation. By coupling anatase TiO2 with Cu2O, the Cu2O/anatase shows enhanced antibacterial activity in the dark, under UV or visible light irradiation (Figure 29b; solid curve). The bactericidal activity of Cu2O/anatase in the dark is caused by the Cu+ ions in Cu2O. The enhanced bactericidal activity of this sample under visible light is attributed to the interfacial charge transfer of electrons from Cu2O to TiO2 across the heterojunction interface. This prolongs the lifetime of charge carriers, such that they can take part in photocatalytic reactions. Cu2O generates electron–hole pairs readily under visible light irradiation due to its small bandgap of 2.17 eV. Under UV light irradiation, the interfacial charge transfer from TiO2 to Cu2O, and the inhibition of charge carriers’ recombination contribute to an enhancement in bactericidal activity. In contrast, unmodified rutile and Cu2O/rutile show slower antibacterial activity under UV or visible light irradiation (Figure 29d).
Figure 29.
Survival of E. coli in CFU/mL treated with (a) Cu2O, (b) Cu2O/anatase TiO2, (c) Cu2O/P25, and (d) Cu2O/rutile TiO2 in solid curves with circle symbols; grey color (dark condition), violet (UV irradiation) and green (visible light). Umodified anatase TiO2, P25 and rutile TiO2 results are shown in dashed curves with diamond symbols in grey (dark condition), violet (UV irradiation) and green (visible light). Error bars in Cu2O: standard deviation determined from two or three independent measurements. Reproduced with permission from [177]. Copyright MDPI, 2018.
5.5. Polymer/Titania Nanocomposites
Novel polymer nanocomposites with enhanced chemical, thermal and mechanical properties can be designed and developed by adding functional nanofillers of unique properties [26,26,68,69,70,71,72,280,281]. Such polymer nanocomposites show great potential for structural, electronic, environmental and biomedical engineering applications. The polymeric matrix of nanocomposites immobilizes nanofillers and offers protection to the fillers from mechanical damage. The extent of property enhancement in the polymer nanocomposites depends greatly on the homogeneous dispersion of the fillers, and good interfacial bonding between the filler and polymer matrix [280]. The polymers employed for forming nanocomposites can be either degradable or nondegradable, depending on their intended application. Degradable polymers such as PLA, polyvinyl alcohol (PVA), poly(ε-caprolactone) (PCL) and chitosan are generally used to form scaffolds and wound dressings [26,65,282,283,284,285]. These polymers should have the ability to degrade with time and to heal the wounds.
Titania nanomaterials have been incorporated into polymers to form antibacterial coatings, food packaging materials, medical implants, wound dressings and scaffolds [59,60,61,62,63,64,65,229,286,287]. However, those studies are mainly focused on the bactericidal properties of the polymer nanocomposites with TiO2 nanomaterials under UV irradiation. However, it is impractical to use a UV source to excite electron–hole pairs in the polymer–TiO2 NP system for applications in theb medical sector and food industry. Therefore, the development of visible-light active polymer–TiO2 nanocomposites is considered of technological interest and practical importance [288,289,290,291,292,293].
The bactericidal activity of polymer–TiO2 nanocomposites under visible light also depends on the type of polymers employed. In particular, natural chitosan (CS) with biodegradable behavior can bind to TiO2 NPs through its amino and hydroxyl groups, thereby extending the optical absorption of TiO2 NPs into the visible region. The CS/TiO2 nanocomposites exhibit a red shift in absorption in their UV-vis spectra [289,292,294]. The bandgap of TiO2 NPs in CS/TiO2 nanocomposites is then reduced from 3.20 to 3.00 eV [292]. As such, CS/TiO2 nanocomposites exhibit the photocatalytic inactivation of microorganisms under visible light. Accordingly, CS/TiO2 films find useful application as antimicrobial wrapping films for vegetables and fruits under visible light. Thus, TiO2 nanofillers can delay the ripening process of fresh produce [289,295]. Very recently, Zhang et al. fabricated a CS/TiO2 nanocomposite film for wrapping red grapes. They assessed the antimicrobial activity of the film against food-borne pathogenic microbes, including E. coli, S. aureus, C. albicans, and Aspergillus niger (mold) [289]. They found that the film exhibited a good microbial inactivation effect, with complete sterilization for all microbial strains within 12 h. The composite film was very effective in protecting red grapes from microbial attack, thereby extending their shelf-life and improving the quality of fresh fruit accordingly (Figure 30a–c). Moreover, pure CS film also shows antibacterial effect to a certain degree. In contrast, grapes were spoiled and mouldy in the plastic wrap, as expected.
Figure 30.
Photographs showing the preservation of red grapes wrapped with (a) plastic film, (b) chitosan film, and (c) chitosan-TiO2 film at 37 °C for six days. Reproduced with permission from [289]. Copyright Elsevier, 2017.
It is noteworthy that CS/TiO2 nanocomposites also find medical applications as antibacterial scaffolds in the presence of visible light. Biodegradable chitosan has been used as a scaffold in orthopedics, particularly for bone tissue engineering. The hydrophilic behavior of CS facilitates the adhesion and growth of bone cells (osteoblasts) on its surface [296]. However, a pure chitosan scaffold suffers from poor mechanical strength, so it is unable to provide sufficient mechanical support for the proliferation of osteoblasts during the bone healing process. Therefore, TiO2 NPs and AgNPs are added to CS to improve mechanical strength [65]. Recently, Raut et al. enhanced the visible-light bacterial inactivation of the CS/TiO2 NPs nanocomposite by including a Cu dopant into TiO2 NPs (denoting as CT) through the sol-gel process. The CT nanofillers were then solution-mixed with CS to yield CS/Cu-doped TiO2, denoted as CS-CT [288]. Figure 31a shows the bactericidal activity of CS, CT and CS–CT samples against E. coli under visible light. A 100% bacterial inactivation time is achieved in 240 min by CT and 120 min by CS–CT. Therefore, a synergistic effect exists between Cu-doped TiO2 NPs (CT) and C, thereby giving rise to a faster bacterial reduction time of 120 min. Figure 31b shows the effect of an *OH radical, generated by the photoexcitation of an electron–hole pair, in destroying E. coli.
Figure 31.
(a) Antibacterial activity of CS, CT and CS–CT samples against E. coli under visible light illumination. (b) Mechanism of antibacterial activity of CS/Cu-doped TiO2 nanocomposite. Reproduced with permission from [288]. Copyright Elsevier, 2011.
Antibacterial polymer/TiO2 nanocomposites also find attractive applications in textile industries for producing odorless and self-cleaning fabrics. Clothing fabrics are prone to microbial contamination, and can spread infections accordingly. The clothes generate a warm and humid environment on human skin, causing the secretion of sweat and bacterial growth [297]. Cotton consists of natural cellulosic fibers that absorb more sweat than synthetic polymer fabrics. Thus, cotton clothes tend to keep the body dry while absorbing sweat. Very recently, Zahid et al. prepared Mn-doped TiO2 NPs (150 nm) by the sol-gel method, and then applied the spray coating technique to apply Mn-doped TiO2 NPs on cotton fabrics. NPs0, NPs10, NPs25 and NPs50 were designated to the cotton fabrics with zero, 10, 25 and 50 wt% Mn-doped TiO2 NPs, respectively [298]. The fabrics with and without Mn-doped TiO2 NPs in the dark exhibited no bactericidal effect because no photocatalytic excitation occurred in the absence of light. The NPs10 and NPs 25 reduced the S. aureus population by 80% and 90% within the first 60 min, respectively, while NPs50 and NPs50W reduced nearly ~100% S. aureus population in the same period under sunlight (Figure 32a). A lower bacterial inactivation rate was found for the fabrics with Mn-doped TiO2 NPs and treated with K. pneumoniae in the first 60 min (Figure 32b). The presence of Mn ions dopant promoted the generation of an electron–hole pair in TiO2 fillers for creating ROS under visible light, as shown in Figure 32a,c. Apart from its antimicrobial activity, the photocatalytic effect also removed or degraded color stains on the fabrics, thus performing self-cleaning, as shown in Figure 32c. The visible-light antibacterial activity of modified titania nanomaterials is summarized in Table 2.
Figure 32.
Bacterial reduction in percentage of (a) S. aureus and (b) K. pneumoniae on cotton fabrics with and without Mn-doped TiO2 NPs in the dark and under natural sunlight. NPs0, NPs10, NPs25 and NPs50 are cotton fabrics with zero, 10, 25 and 50 wt% Mn-doped TiO2 NPs; NPs50W is the NPS50 after 10 washing cycles. (c) Schematic of visible light induced the photocatalytic inactivation of bacteria and degradation of stain residues on contaminated fabric with Mn-doped TiO2 NPs. Reproduced with permission from [298]. Copyright American Chemical Society, 2018.
Table 2.
Bactericidal performance of modified titania nanoparticles under visible light.
| Material | Size, nm | Bacteria | Complete Inactivation Time | Ref. |
|---|---|---|---|---|
| (1–3 mol%) Ni/TiO2 NPs | 8–10 | E. coli | >300 min (3% Ni dopant) | [248] |
| S. aureus | >240 min (3% Ni dopant) | |||
| Samonella abony | >360 min (3% Ni dopant) | |||
| (1–3 mol%) Cu/TiO2 NPs | 9–10 | E. coli | 240 min (3% Cu dopant) | [247] |
| S. aureus | 120 min (3% Cu dopant) | |||
| 0.5%Cu/TiO2 NPs | 28.84 | E. coli | 30 min | [109] |
| S. aureus | 30 min | |||
| Ag/TiO2 NPs | AgNPs: 0.9; TiO2 NPs: 8 |
E. coli | 60 min | [124] |
| N/TiO2 NPs | 10–30 | E. coli | 420 min | [161] |
| S. aureus | 360 min | |||
| F-N doped P25 | 70 | E. coli | 60 min | [270] |
| F-N doped TiO2 | 21.3 | E. coli | 60 min | [271] |
| (0.5–2.5%) rGO/TiO2 NPs | 17–18 | E. coli | 75 min (1.5% rGO/TiO2 NPs) | [101] |
| (0.1–0.5%) MWNT/TiO2 NPs | TiO2 NPs: 8–15 MWNT diameter: 20–45 |
E. coli | 300 min (0.5% MWNT/TiO2) | [156] |
| S. aureus | 180 min (0.5% MWNT/TiO2) | |||
| 0.5 wt% MWNT/Fe-doped TiO2 | Fe-doped TiO2: 15–20 MWNT diameter: 20–45 |
B. subtilis | 120 min | [157] |
| P. aeruginosa | 240 min | |||
| Cu2O/TiO2 | TiO2 NPs: 8 | E. coli | 60 min | [177] |
| CS/Cu-doped TiO2 | 16 | E. coli | 120 min | [288] |
| Cotton/(10–50%) Mn-doped TiO2 | Mn-doped TiO2: 150 | S. aureus | 90 min (25 wt% Mn dopant) | [298] |
| K. pneumoniae | 90 min (25 wt% Mn dopant) | |||
| Cotton/(10–50%) Mn-doped TiO2 | Mn-doped TiO2: 150 | S. aureus | 60 min (50 wt% Mn dopant) | [298] |
| K. pneumoniae | 120 min (50 wt% Mn dopant) |
6. Biocompatibility and Cytotoxicity
Antibacterial materials and coatings have been a focus of global research topics for the past decade in orthopedics, due to an increasing incidence of implant-related infections caused by MDR bacteria [299]. Medical devices and implants are easily contaminated with microorganisms, leading to the formation of biofilms on their surfaces. Therefore, titania coating is an attractive solution for controlling implant infection by decreasing bacteria adhesion through the introduction of metal nanoparticles (e.g., AgNPs or CuNPs) to the coating [300,301]. Furthermore, titania coating formed on the medical devices should exhibit good biocompatibility with the host tissues. As an example, titania film deposited on polyetheretherketone (PEEK) spinal implant exhibits superior compatibility compared to uncoated PEEK in terms of osteoblastic adhesion, proliferation, and differentiation [302]. Similarly, anodic TNTs of large surface areas formed on Ti-based alloys have been reported to provide anchoring sites for osteoblasts and fibroblasts, thereby promoting cell proliferation effectively [217,218,219,303,304,305]. Ti-based alloys (e.g., Ti-6%Al-4%V and Ti-6%Al-7%Nb) are commonly used as the load-bearing prostheses in orthopedics and tooth implants in dentistry. The optimal diameter of TNTs for osteoblastic adhesion and growth is typically below 100 nm [217,218]. Recently, Radtke et al. reported that anodic TNTs formed on the Ti-6%Al-4%V alloy, especially at lower anodic potentials with diameters of 25–35 nm, were effective in promoting the growth of murine fibroblasts L929 [303]. Xu et al. investigated the biocompatibility of periodontal ligament cells on anodized TNTs of different diameters. Periodontal ligament cells (PDLCs) are the key cells responsible for periodontal tissue regeneration [304]. They demonstrated that the TNTs formed on thin Ti foil substrate favor the adhesion and growth of PDLCs. Furthermore, TNTs promoted the osseointegration of Ti substrate more effectively than untreated Ti foil, as evidenced by the high gene expression levels of alkaline phosphatase, osteocalcin and osteopontin.
6.1. Cytotoxicity
Advanced nanomaterials, prepared by emerging nanotechnology, pose toxicity to humans to a large/lesser extent depending on their structure, chemical composition, shape, distribution, etc. [14,15,306]. Wadhwa et al. reported that hydrothermally synthesized TNTs and TiO2 NPs exhibit no toxic effect towards the human alveolar carcinoma epithelial cell line (A549) [307]. Standalone and detached TNTs from Ti foil substrate were toxic to human dermal fibroblasts as a result of the ROS generation, leading to DNA damage and chromosomal aberration [308]. Allegri et al. reported that electrospun TiO2 nanofibers were toxic towards A549 and murine macrophage cell lines (Raw 264.7). The cytotoxic effects were dose-dependent, with larger effects on A549 than on Raw 264.7. However, TiO2 NPs exert no cytotoxic effect on these two cell lines [309].
As mentioned, TiO2 NPs have been produced commercially in large quantities for applications in paints, pharmaceutical, food, drug and cosmetic industries [39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56]. The synthesis and handling of TiO2 NPs during the production process can release a tremendous amount of these nanomaterials into the environment, including air, soil and water. The wastewater of titania production plants is the major source pollutant that leaks into the environment. The nanomaterials are discharged in the sewage sludge and can enter the soil and water ecosystem [310]. Therefore, the disposal and treatment of wasterwater, as well as the recycling of titania from water treatment plants, are considered of technological importance [311]. Moreover, the widespread use of products with TiO2 NPs would also release such nanoparticles into the environment. For instance, auto-manufacturing plants consume large quantities of titania pigment paints for coating car bodies. Therefore, plaint sludge is always found in the paint-bearing wastewaters. The recycling and reproduction of TiO2 NPs from the paint sludge are beneficial to both the environmental and industrial sectors for minimizing pollution [312]. Furthermore, embedded TiO2 NPs in the paint of buildings, bridges and traffic railings could be lost and released into the aquatic environment due to a lengthy outdoor weathering [313]. Some consumer titania products such as food-packages and textiles could also end up as waste, and are disposed of in incinerators and landfills [314]. The wastewater treatment plants remove most TiO2 NPs in the influent sewage; however, the residual nanoparticles would discharge to natural water ecosystems. In this respect, TiO2 NPs could potentially induce harmful effects to aquatic organisms, such as impaired metamorphosis and growth, teratogenicity, and mortality of fish larvae [315,316].
6.1.1. Neat TiO2 NPs
TiO2 NPs can enter the human body through exposure to workplace atmospheres, the use of commercial products, the ingestion of food and pharmaceuticals. The main routes of entry include skin contact, inhalation, ingestion and medical implants [317]. Accordingly, public concerns have been raised related to the safe use of TiO2 NPs, and their effects on human health and the environment. Conflicting results are reported in the literature regarding the biocompatibility and cytotoxicity of TiO2 NPs. Some studies indicate a good biocompatibility of TiO2 NPs with mammalian cells [166,201,309,318], while others reveal the toxic effects of TiO2 NPs [319,320,321,322,323,324,325]. The discrepancy is attributed to the biological and materials factors involved during in vitro studies. The former factors include the type of culture cells, cell cultivation time and cell-based assays used, while the latter factors include the size, shape, crystal structure (anatase or rutile), and dose of TiO2 NPs in the assay tests [326].
Wang et al. investigated cytotoxicity in A549 cells induced by TiO2 NPs (5 nm) using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), quantitative real-time PCR (qRT-PCR) and comet assays, as well as rhodamine 123 staining [323]. From the MTT results, the cytotoxic effect of TiO2 NPs was time- and concentration-dependent (in a range from 50 to 200 μg/mL). Comet assay revealed DMA damage in cells exposed to 50 to 200 μg/mL TiO2 NPs for 48 h. TiO2 NPs also decreased the mitochondrial membrane potential as determined by rhodamine 123 (Rh123) staining. The qRT-PCR analysis demonstrated that the expression of caspase-3 and caspase-9 messenger RNA (mRNA) increased dramatically upon exposure to 100 and 200 μg/mL TiO2 NPs for 48 h. As is known, caspases play the key role in executing apoptosis. Caspase-3 tends to induce DNA bond cleavage and inactivate cytoskeletal proteins. From this perpective, TiO2 NPs inhibit A549 proliferation, and induce DNA damage and eventual cell apoptosis through the activation of the intrinsic mitochondrial pathway [323].
TiO2 NPs can penetrate the human body through inhalation, then translocate from the lungs into the bloodstream, and the subsequent uptake in other organs like the heart, liver, spleen and brain. Huerta-Garcia et al. investigated the in vitro toxicity of TiO2 NPs on rat cardiomyoblasts (H9c2) [327]. They found that the cellular uptake of TiO2 NPs by H9c2 cells reduces their metabolic activity and cell growth, thus causing mitochondrial dysfunction due to a marked reduction in mitochondrial membrane potential. Furthermore, TiO2 NPs increase the ROS level and membrane permeability of H9c2 cells greatly, leading to final cell death. Therefore, the internalization and building up of TiO2 NPs in cardiomyoblasts would induce cardiac damage and pose threats to human health upon inhalation of those nanoparticles. More recently, Mottola et al. studied the genotoxic effect of TiO2 NPs on human amniotic fluid cells in vitro. The TiO2 NPs exposure caused DNA strand fragmentation, a loss of viability, and apoptosis of the cells [325].
Yin et al. studied the phototoxicity of TiO2 NPs (<25 nm, 31 nm, <100 nm, and 325 nm) in human skin keratinocytes (HaCaT) under UVA irradiation [328]. TiO2 NPs induced photocytotoxicity and cell membrane damage in a UVA dose- and TiO2 NPs dose-dependent manner. The smaller the size of TiO2 NPs, the higher the cell damage was. The induced photocytotoxic damage was attributed to the ROS generation during UVA irradiation, leading to lipid peroxidation of the plasma membrane. TiO2 NPs with a large surface-to-volume ratio enhanced biological reactivity by generating ROS. The degree of photocytotoxicity and cell membrane damage depends greatly on the level of ROS generated. More recently, Ren et al. also reported a similar finding on the phototoxicity of TiO2 NPs in HaCaT cells under UV irradiation [329].
The adverse effects of TiO2 NPs on mammalian cells, such as DNA damage, the generation of ROS, and apoptosis, addressed in in vitro studies, are supported by in vivo animal models [330,331,332,333,334]. Grassian et al. exposed mice to TiO2 NPs (2–5 nm; 8.88 mg/m3; 4 h/day for 10 days) through inhalation. Exposure for 1–2 weeks led to pulmonary inflammation with high cell counts of alveolar macrophages in bronchoalveolar lavage (BAL) fluid [330]. To evaluate the potential respiratory system toxicity, Liu et al. studied the biodistribution of TiO2 NPs (5, 21 and 50 nm) in rats via intratracheally instillation at doses of 0.5, 5, and 50 mg/kg body weight (bw). Rats were then sacrificed one week post-instillation [331]. Histopathological evaluation of lung tissues revealed a dose-dependent inflammatory lesion. At a specific dose, the pulmonary toxicity induced by 5 nm TiO2 NPs was more severe than that caused by 21 and 50 nm TiO2 NPs. In the case of dermal exposure, Wu et al. reported that TiO2 NPs can penetrate through the skin of hairless mice, and finally reach the liver following a prolonged exposure of 60 days. This led to a remarkable change in malondialdehyde (MDA) level [332]. MDA is a marker of liquid peroxidation and oxidation stress of cells. Therefore, an increase in free radicals produces high levels of MDA. Disdier et al. intravenously administrated P25 TiO2 NPs of 1 mg/kg into male Fisher F344 rats. They analyzed the biodistribution of Ti level in internal organs of rats using inductively coupled plasma mass spectrometry [333]. Biopersistence of Ti in the main target organs, i.e., the liver, lungs, spleen and kidneys, was observed after intravenous administration for up to 365 days (Figure 33a–d). Jia et al. intraperitoneally injected TiO2 NPs (5, 10, 60, 90 nm) and TiO2 microparticles at doses of 5, 10, 50, 100, 150, and 200 mg/kg (once a day for 14 days) into rats (half male and half female). TiO2 NPs were found to accumulate in the liver, kidney, spleen, lung, brain, and heart through the circulatory system [335]. The liver was damaged seriously due to mitochondrial dysfunction and the ROS generation at TiO2 NPs doses ≥10 mg/kg, leading to hepatocyte apoptosis. Furthermore, TiO2 NPs were more toxic than TiO2 microparticles, as expected. The distribution of TiO2 NPs in the brain tissue suggested that nanoparticles can enter directly into the central nervous system without crossing the blood–brain barrier. Finally, TiO2 NPs also caused genotoxicity in the ex vivo mouse embryo models [335].
Figure 33.
Biopersistence of titanium level in (a) liver, (b) lungs, (c) spleen and (d) kidneys after intravenous injection of 1 mg/kg TiO2 NPs in rats for 365 days. Grey and white bars are treated and control mice, respectively. Error bars represent the mean ± SD and n = 6. Statistical comparison is performed by two-way ANOVA, * p < 0.05; ** p < 0.01; *** p< 0.001. Reproduced with permission from [333]. Copyright BioMed Central, 2015.
6.1.2. Metal-Doped TiO2 NPs
We now consider the effect of metal doping on the cytotoxicity of TiO2 NPs in human cells. Doping TiO2 NPs with transition metals and noble metals is an efficient strategy for improving the photocatalytic inactivation of bacteria in the visible region. However, this approach has its own drawback as metal dopants can induce cytotoxicity in human cells. Therefore, metal-doped TiO2 NPs photocatalysts can fulfill the requirement of bactericidal performance, but they pose potential human health and safety hazards following long-term exposure. Recently, Ahamed et al. employed MTT and neutral red uptake (NRU) assays to assess the metabolic and lysosomal activities of human liver cancer (HepG2) cells exposed to Ag (0.5–5%)-doped TiO2 NPs [318]. Their results showed that Ag-doped TiO2 NPs reduce the cell viability in a dose-dependent manner, as shown in Figure 34a,b. Apparently, TiO2 NPs display no toxic effect to the HepG2 cell. In contrast, Ag (0.5–5%)-doped TiO2 NPs are toxic, as the Ag-dopant can release zero valent Ag0 or Ag+ ion to reduce cell viability through ROS generation [15]. This leads to the leakage of intracellular components at Ag (0.5–5%)-doped TiO2 NPs doses of ≥25 µg/mL, as evidenced by lactate dehydrogenase (LDH) assay (Figure 34c). Furthermore, cell viability decreases with increasing Ag content in Ag-doped TiO2 NPs, from 0.5% to 5%, while the ROS level increases with increasing Ag content (Figure 34d).
Figure 34.
(a) MTT, (b) NRU, and (c) lactase dehydrogenase leakage results of human liver cancer (HepG2) cells exposed to TiO2 NPs and Ag-doped TiO2 NPs of different concentrations. (d) ROS level of HepG2 cells exposed to pure TiO2 NPs and Ag-doped TiO2 NPs of 25, 50 and 100 µg/mL. Reproduced with permission from [318], Copyright Nature Publishing Group, 2017.
6.1.3. rGO-Modified TiO2 NPs
Jin et al. demonstrated that rGO/TiO2 NPs would separate independently into TiO2 NPs and GO after entering A549 cells. The rGO/TiO2 NPs composite could induce cytotoxicity in A549 due to the generation of oxidative stress [336]. More recently, Prakash et al. prepared rGO (10–50%)/TiO2 nanocomposites using hydrothermal synthesis, and studied their toxicity in zebrafish embryos and larvae [337]. The toxicity of rGO (10–50%)/TiO2 nanocomposites was highly dependent on the rGO concentrations and doses. At low concentration and dose levels (10%, 20%, and 30% rGO; 0.25–30 μg/mL), rGO (10–30%)/TiO2 nanocomposites exhibited no toxicity to the zebrafish embryos. At high doses of 0.125–1.0 mg/mL, all the rGO (10–50%)/TiO2 nanocomposites induced teratogenicity and cardiotoxicity due to the generation of ROS. Zebrafish (Danio rerio) are aquatic species commonly employed to detect toxicological effects due to their known physiology having a high degree of genetic similarity with mammals, and the optical transparency of the tissues.
7. Prospects and Challenges
Titania has been considered an inert and nontoxic material. It is typically used as a color additive for foods up to 1 wt%, and was approved by the Food and Drug Agency of the United States under Title 21 of the Code of Federal Regulations [338]. The additive contains TiO2 NPs as described by the E171, European Food Safety Authority (EFSA) of the European Union [339]. Titania is also widely used in toothpaste as a white pigment with a small fraction of TiO2 NPs. These nanoparticles are also detected in sweets containing E 171, e.g., chewing gum, colored candy, chocolate and cake-icing. The approximate oral ingestion of TiO2 NPs for the Dutch is 0.19 mg/kg bw/day for elderly, 0.55 mg/kg bw/day for 7–69 year old people, and 2.16 mg/kg bw/day for young children [340]. Very recently, Hwang et al. demonstrated that commercial TiO2 additive as outlined by the E171 contains particles with mean size values of 118–169 nm. The TiO2 additive created ROS and inhibited long-term colony formation in human intestinal epithelial Caco-2 cells at concentrations >125 µg/mL. The additive slightly induced apoptosis at a very high content of 1000 µg/mL upon exposure for 24 h [341]. This result raises a safety concern about the toxic impact of TiO2 food additives on human health [342]. Heringa et al. reported the presence of TiO2 NPs in 15 post-mortem human livers and spleens of Caucasians. Those human subjects followed a West European diet and used toothpaste, so this may have resulted from oral intake of TiO2 NPs [343].
Most in vitro cell cultivation and in vivo animal models tests clearly indicate that TiO2 NPs are toxic to mammalian cells, since they induce DNA damage, ROS generation, and apoptosis. In particular, Ag-doped TiO2 NPs act as a double-edged sword, having beneficial and adverse effects. The plasmonic effect of surface electrons of AgNP-decorated TiO2 NPs promotes light-harvesting in the visible region, as mentioned previously. This inhibits bacterial growth of E. coli and S. aureus effectively when compared to pure TiO2 NPs, as shown in Figure 20. Therefore, there exists a synergistic bactericidal effect of TiO2 and AgNPs through the photocatalytic reaction and Ag0/Ag+ species released from the AgNPs. However, metallic Ag0 and ionic Ag+ released from AgNPs can elicit a toxic effect on HepG2 cells through ROS generation (Figure 34). Furthermore, AgNPs have been found to induce a toxic effect on mammalian cells in a dose-, size- and time-dependent manner. In vivo animal studies reveal that AgNPs locate preferentially in murine target organs including the liver, spleen, kidney and brain after intratracheal instillation, intravenous or intraperitoneal injection [15].
Standalone and detached TNTs exhibit poor compatibility to human dermal fibroblasts due to ROS generation, leading to DNA damage and chromosomal aberration [308]. Ultrasonication was employed to detach TNTs from the Ti substrate. Standalone TNTs were able to pierce and penetrate through the membrane of fibroblasts, resulting in cytotoxicity. Without ultrasonication, intact TNTs adhered firmly on the Ti substrate, acting as the adhesion and growth sites for murine osteoblasts (MC3T3-E1) and human fibroblasts [226,261]. As such, anodic TNTs exhibited higher osteoblastic viability than pure Ti (Figure 35). After sputtered coating TNTs with silver for 60, 120 and 180 s (designated as ANS 60, ANS 120 and ANS 180), the ANS 60 sample still showed better biocompatibity than pure Ti. However, the cell viability of ANS 120 and ANS 180 samples decreased markedly because a longer sputtering time favored more AgNP formation on the nanotubes. Thus the ANS 180 sample exhibited the lowest cell proliferation. From Figure 21, ANS 60 showed a similar antibacterial actitvity to ANS 120 and ANS 180 samples. Therefore, a balance between bactericidal activity and cellular viability can be reached by monitoring the Ag content in ANS 60. In general, Ti-based alloy implants have inadequate antibacterial activity, so much effort has been made to improve their compatibility and antibacterial properties for clinical applications. Ti-based alloy implants with enhanced antibacterial activity and biocompatibility can be achieved by forming an Ag-doped TNTs surface layer with an optimal Ag content through anodization and sputtering. The modified TNTs formed on Ti-based implants can reduce bacterial colonization on their surfaces accordingly. Alternatively, antibacterial CuNPs can be used to replace AgNPs for doping TNTs with improved biocompatibility [98,99]. CuNPs are less toxic than AgNPs to human and bovine mammary epithelial cells [344].
Figure 35.
The viability of MC3T3-E1 murine osteoblasts obtained from the MTT assay. Error bars indicate the standard deviation (n = 5); # p < 0.05 compared with commercial pure Ti (cp-Ti). Reproduced with permission from [261]. Copyright Wiley, 2014.
However, it remains a big challenge for chemists and materials scientists to design novel nanomaterials for technological and medical applications utilizing TiO2 NPs and doped TiO2 NPs. With the increasing need for antibacterial scaffolds and wound dressings in tissue engineering, biodegradable polymers are ideal materials to immobilize TiO2 NPs to form polymer nanocomposites with improved biocompatibility and photocatalytic activity. It is of primary importance to select a proper polymeric material, suitable for those applications. As previously mentioned, mixing chitosan with TiO2 NPs can yield a biodegradable plastic film with visible light photocatalytic bactericidal activity. The film functions effectively for preserving fresh fruits and vegetables, and for prolonging shelf-life [289]. Such a plastic film can also be used as a food packaging material for killing pathogenic bacteria that causes food poisoning or food spoilage. In addition, biodegradable CS-TiO2 NPs film also finds potential applications as antibacterial scaffolds and wound dressings in orthopedics. By incorporating Cu dopant into TiO2 NPs, the resulting CS/Cu-doped TiO2 NPs shows higher photocatalytic bactericidal activity, as expected [288].
Finally, antibacterial and self-cleaning fabrics have received considerable attention in hospitals and clinics due to the increased risk of healthcare-associated infections. Those fabrics are particularly useful against nosocomial bacteria to protect patients from harmful microorganisms [298,345]. The fabrics made from cotton/Mn-doped TiO2 NPs have been reported to exhibit full inactivation of S. aureus and K. pneumoniae within 120 min under sunlight (Figure 32a,b) [298]. However, one can see that such fabrics need at least 25 wt% Mn-doped TiO2 NPs for achieving antibacterial properties. Those fillers can be removed from the fabrics upon several washing cycles, and then discharged into rivers and lakes. The recent literature data indicate the toxicity of rGO/TiO2 NPs in inducing teratogenic effects on zebrafish, although at doses considerably higher than those in aquatic environments [337]. The release of large amounts of Mn-doped TiO2 NPs would inevitably pollute the aquatic environment, and harm aquatic life in the ecosystem [346]. Moreover, Mn is a heavy metal that is toxic to mammalian cells. Manganese can induce toxicity in human bronchial epithelial cells, leading to caspase-9-mediated cell death [347,348]. Therefore, non-noble metal doped TiO2 NPs such as the N-doped TiO2 NPs photocatalyst is considere a potential replacement for Mn-doped TiO2 NPs for reducing environmental toxicity.
Thanks to the efforts of researchers, visible-light active TiO2 NPs and their nanocomposites have been found to exhibit a bactericidal effect against drug-resistant bacteria including salmonella abony, S. aureus, K. pneumoniae, P. aeruginosa and anthrax. However, it still requires substantial time and effort to develop visible-light active TiO2 NPs photocatalysts with both antibacterial activity and good cytocompatibility. Long-term, in vivo animal studies relating to the biocompatibility and cytotoxicity of those photocatalysts are required. More research studies are needed to design the toxic free, green synthesis of visible-light active TiO2 NPs with bactericidal activity for industrial, environmental and medical applications.
8. Conclusions
This review gives a summary of the synthesis, photocatalytic bacterial inactivation, biocompatibility and cytotoxic effects of visible-light active TiO2 NPs and their nanocomposites, especially over the past five years. The photocatalytic bactericidal effect of TiO2 NPs with a wide bandgap depends on the creation of an electron–hole pair under UV irradiation to generate ROS. The created radicals then interact with microorganisms, causing damage to the structure of the cell membrane and the subsequent leakage of intracellular components. However, UV-responsive TiO2 NPs exhibit poor photocatalytic efficiency under visible light. It is of practical importance to employ visible light to induce the photocatalytic bactericidal activity of TiO2 NPs. Considerable progress has recently been made in the development of TiO2 nanostuctures with good photocatalytic bactericidal activity under visible light irradiation. Visible-light active TiO2 nanomaterials can be synthetized through several techniques including sol-gel, hydrothermal/solvothermal, electrochemical anodization, and electrospinning. Among these, sol-gel is commonly used for preparing TiO2 NPs, while electrochemical anodization is an effective method for fabricating TNTs.
Doping TiO2 NPs with metal and non-metal elements can lead to a red shift in the optical absorption edge into the visible region, resulting in a reduction of the bandgap accordingly. Transition metal elements, such as Mn, Fe, V and Cu, can create a localized d-electron state in the bandgap of TiO2 NPs, thereby promoting the separation of photogenerated electron–hole pair and suppressing the recombination of charge carriers. However, defect states in the bandgap of titania would also serve as charge recombination centers under experimental conditions of high dopant concentrations. Noble metal dopants such as AgNPs exhibit plasmonic oscillation of surface electrons under visible light irradiation. As such, hot-electron transfer from excited AgNPs into the conduction band of TiO2 NPs creates ROS for bactericidal activity. In addition to photocatalytic bactericidal activity, AgNPs of Ag-doped TiO2 NPs can inactivate bacteria through the release of Ag+ ions. These cations strongly interact with the thiol groups of microorganisms and inhibit DNA replication, resulting in apoptosis. Furthermore, AgNPs also induce a toxic effect on human cells. In this respect, we must be cautious when using AgNPs as a dopant for TiO2 NPs. Taking account of the adverse effects of metal dopants, visible-light active, anion-doped TiO2 NPs using non-metal elements have attracted increasing attention as agents against pathogenic bacteria.
Given the important uses of visible-light active TiO2 NPs for various applications, their impact on human health poses a serious concern worldwide. In vitro cell cultivation and in vivo animal models studies have reported the cytotoxic effects of TiO2 NPs and Ag-doped TiO2 NPs in mammalian cells by inducing an inflammatory response, DNA damage, ROS generation, and apoptosis. For long-term safety, more research studies are needed to properly design a toxic free, green synthesis of visible-light active TiO2 NPs with bactericidal activity.
Author Contributions
S.C.T. conceived and directed the research. C.L., Y.L. and S.C.T. contributed to the writing of this review article. All authors have read and agree to the published version of the manuscript.
Funding
National Youth Science Foundation, China (Grant Number: 21703096), and Natural Science Foundation of Shandong Province, China (Grant Number: ZR2019MB053) provided the funding of this research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Economou V., Gousia P. Agriculture and food animals as a source of antimicrobial-resistant bacteria. Infect. Drug Resist. 2015;8:49–61. doi: 10.2147/IDR.S55778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rousham E.K., Unicomb L., Aminul M. Human, animal and environmental contributors to antibiotic resistance in low-resource settings: Integrating behavioural, epidemiological and one health approaches. Proc. R. Soc. B Biol. Sci. 2018;285:20180332. doi: 10.1098/rspb.2018.0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li B., Webster T.J. Bacteria antibiotic resistance: New challenges and opportunities for implant-associated orthopedic infections. J. Orthop. Res. 2018;36:22–32. doi: 10.1002/jor.23656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kavanagh N., Ryan E.J., Widaa A., Sexton G., Fennell J., O’Rourke S., Cahill K.C., Kearney C.J., O’Brien F.J., Kerrigan S.W. Staphylococcal osteomyelitis: Disease progression, treatment challenges, and future directions. Clin. Microbiol. Rev. 2018;31:e00084-17. doi: 10.1128/CMR.00084-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collignon P.J., McEwen S.A. One health—Its importance in helping to better control antimicrobial resistance. Trop. Med. Infect. Dis. 2019;4:22. doi: 10.3390/tropicalmed4010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huh A.J., Kwon Y.J. Nanoantibiotics: A new paradigm for treating infectious diseases using nanomaterials in the antibiotics resistant era. J. Control. Release. 2011;156:128–145. doi: 10.1016/j.jconrel.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Shaikh S., Nazam N., Rizvi S.M., Ahmad K., Baig M.H., Lee E.J., Choi I. Mechanistic insights into the antimicrobial actions of metallic nanoparticles and their implications for multidrug resistance. Int. J. Mol. Sci. 2019;20:2468. doi: 10.3390/ijms20102468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jesline A., John N.P., Narayanan P.M., Vani C., Murugan S. Antimicrobial activity of zinc and titanium dioxide nanoparticles against biofilm-producing methicillin-resistant Staphylococcus aureus. Appl. Nanosci. 2015;5:157–162. doi: 10.1007/s13204-014-0301-x. [DOI] [Google Scholar]
- 9.Chen C., Li S., Thomas A., Kotov N.A., Haag R. Functional graphene nanomaterials based architectures: Biointeractions, fabrications, and emerging biological applications. Chem. Rev. 2017;117:1826–1914. doi: 10.1021/acs.chemrev.6b00520. [DOI] [PubMed] [Google Scholar]
- 10.Tjong S.C., Chen H. Nanocrystalline materials and coatings. Mater. Sci. Eng. R Rep. 2004;45:1–88. doi: 10.1016/j.mser.2004.07.001. [DOI] [Google Scholar]
- 11.Tjong S.C. Nanocrystalline Materials: Their Synthesis-Structure-Property Relationships and Applications. 2nd ed. Elsevier; London, UK: 2013. [Google Scholar]
- 12.He L.X., Tjong S.C. Nanostructured transparent conductive films: Fabrication, characterization and applications. Mater. Sci. Eng. R Rep. 2016;109:1–101. doi: 10.1016/j.mser.2016.08.002. [DOI] [Google Scholar]
- 13.He L.X., Tjong S.C. Aqueous graphene oxide-dispersed carbon nanotubes as inks for the scalable production of all-carbon transparent conductive films. J. Mater. Chem. C. 2016;4:7043–7051. doi: 10.1039/C6TC01224H. [DOI] [Google Scholar]
- 14.Liao C., Li Y., Tjong S.C. Graphene nanomaterials: Synthesis, biocompatibility, and cytotoxicity. Int. J. Mol. Sci. 2018;19:3564. doi: 10.3390/ijms19113564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liao C., Li Y., Tjong S.C. Bactericidal and cytotoxic properties of silver nanoparticles. Int. J. Mol. Sci. 2019;20:449. doi: 10.3390/ijms20020449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He L., Liao C., Tjong S.C. Scalable fabrication of high-performance transparent conductors using graphene oxide-stabilized single-walled carbon nanotube inks. Nanomaterials. 2018;8:224. doi: 10.3390/nano8040224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Graves J.L., Jr., Thomas M., Ewunkem J.A. Antimicrobial nanomaterials: Why evolution matters. Nanomaterials. 2017;7:283. doi: 10.3390/nano7100283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta A., Mumtaz S., Li C.H., Hussain I., Rotello V.M. Combatting antibiotic-resistant bacteria using nanomaterials. Chem. Soc. Rev. 2019;48:415–427. doi: 10.1039/C7CS00748E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Küünal S., Visnapuu M., Volubujeva O., Soares Rosario M., Rauwel P., Rauwel E. Optimisation of plant mediated synthesis of silver nanoparticles by common weed Plantago major and their antimicrobial properties. IOP Conf. Ser. Mater. Sci. Eng. 2019;613:012003. doi: 10.1088/1757-899X/613/1/012003. [DOI] [Google Scholar]
- 20.Küünal S., Rauwel P., Rauwel E. Plant extract mediated synthesis of nanoparticles. In: Barhoum A., Makhlouf A.S., editors. Emerging Applications of Nanoparticles and Architectural Nanostructures: Current Prospects and Future Trends. Elsevier; Amsterdam, The Netherlands: 2018. pp. 411–416. Chapter 14. [DOI] [Google Scholar]
- 21.Collin F. Chemical basis of reactive oxygen species reactivity and involvement in neurodegenerative diseases. Int. J. Mol. Sci. 2019;20:2407. doi: 10.3390/ijms20102407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Regmi C., Joshi B., Ray S.K., Gyawali G., Pandey R.P. Understanding mechanism of photocatalytic microbial decontamination of environmental wastewater. Front. Chem. 2018;6:33. doi: 10.3389/fchem.2018.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ballottin D., Fulaz S., Cabrini F., Tsukamoto J., Duran N., Alves O.L., Tasic L. Antimicrobial textiles: Biogenic silver nanoparticles against Candida and Xanthomonas. Mater. Sci. Eng. C. 2017;75:582–589. doi: 10.1016/j.msec.2017.02.110. [DOI] [PubMed] [Google Scholar]
- 24.Chernousova S., Epple M. Silver as antibacterial agent: Ion, nanoparticle and metal. Angew. Chem. Int. Ed. 2013;52:1636–1653. doi: 10.1002/anie.201205923. [DOI] [PubMed] [Google Scholar]
- 25.Kedziora A., Speruda M., Krzyzewska E., Rybka J., LukowiK A., Bugla-Płoskonska G. Similarities and differences between silver ions and silver in nanoforms as antibacterial agents. Int. J. Mol. Sci. 2018;19:444. doi: 10.3390/ijms19020444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu C., Shen J., Yeung K.W.K., Tjong S.C. Development and antibacterial performance of novel polylactic acid-graphene oxide-silver nanoparticle hybrid nanocomposite mats prepared by electrospinning. ACS Biomater. Sci. Eng. 2017;3:471–486. doi: 10.1021/acsbiomaterials.6b00766. [DOI] [PubMed] [Google Scholar]
- 27.Liu C., Shen J., Liao C.Z., Yeung K.W., Tjong S.C. Novel electrospun polyvinylidene fluoride-graphene oxide-silver nanocomposite membranes with protein and bacterial antifouling characteristics. Express Polym. Lett. 2018;12:365–382. doi: 10.3144/expresspolymlett.2018.31. [DOI] [Google Scholar]
- 28.Hanaor D.A., Sorrell C.C. Review of the anatase to rutile phase transformation. J. Mater. Sci. 2011;46:855–874. doi: 10.1007/s10853-010-5113-0. [DOI] [Google Scholar]
- 29.Sarkar A., Khan G.G. The formation and detection techniques of oxygen vacancies in titanium oxide-based nanostructures. Nanoscale. 2019;11:3414–3444. doi: 10.1039/C8NR09666J. [DOI] [PubMed] [Google Scholar]
- 30.Pesci F.M., Wang G., Klug D.R., Li Y., Cowan A.J. Efficient suppression of electron-hole recombination in oxygen-deficient hydrogen-treated TiO2 nanowires for photoelectrochemical water splitting. J. Phys. Chem. C. 2013;117:25837–25844. doi: 10.1021/jp4099914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao H., Pan F., Li Y. A review on the effects of TiO2 surface point defects on CO2 photoreduction with H2O. J. Mater. 2017;3:17–32. doi: 10.1016/j.jmat.2016.12.001. [DOI] [Google Scholar]
- 32.Shwetharani R., Sakar M., Fernando C.A., Binas V., Balakrishna R.G. Recent advances and strategies to tailor the energy levels, active sites and electron mobility in titania and its doped/composite analogues for hydrogen evolution in sunlight. Catal. Sci. Technol. 2019;9:12–46. doi: 10.1039/C8CY01395K. [DOI] [Google Scholar]
- 33.Rauwel E., Galeckas A., Rauwel P. Photoluminescent cubic and monoclinic HfO2 nanoparticles: Effects of temperature and ambient. Mater. Res. Express. 2014;1:015035. doi: 10.1088/2053-1591/1/1/015035. [DOI] [Google Scholar]
- 34.Wang B., Huang W., Chi L., Al-Hashimi M., Marks T.J., Facchetti A. High-k gate dielectrics for emerging flexible and stretchable electronics. Chem. Rev. 2018;118:5690–5754. doi: 10.1021/acs.chemrev.8b00045. [DOI] [PubMed] [Google Scholar]
- 35.Markov S.L., Vidaković A.M. Testing methods for antimicrobial activity of TiO2 photocatalyst. Acta Period Technol. 2014;45:141–152. doi: 10.2298/APT1445141M. [DOI] [Google Scholar]
- 36.Rajh T., Dimitrijevic N.M., Bissonnette M., Koritarov T., Konda V. Titanium dioxide in the service of the biomedical revolution. Chem. Rev. 2014;114:10177–10216. doi: 10.1021/cr500029g. [DOI] [PubMed] [Google Scholar]
- 37.Matsunaga T.R., Tomoda Y., Nakajima T., Wake H. Photoelectrochemical sterilization of microbial cells by semiconductor powders. FEMS Microbiol. Lett. 1985;29:211–214. doi: 10.1111/j.1574-6968.1985.tb00864.x. [DOI] [Google Scholar]
- 38.Bogdan J., Zarzynska J., Plawinska-Czarnak J. Comparison of infectious agents susceptibility to photocatalytic effects of nanosized titanium and zinc oxides: A practical approach. Nanoscale Res. Lett. 2015;10:309. doi: 10.1186/s11671-015-1023-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Y., Wu T., Zhou Y., Meng C., Zhu W., Liu L. TiO2-based nanoheterostructures for promoting gas sensitivity performance: Designs, developments, and prospects. Sensors. 2017;17:1971. doi: 10.3390/s17091971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abbas M., Iftikhar H., Malik M.H., Nazir A. Surface coatings of TiO2 nanoparticles onto the designed fabrics for enhanced self-cleaning properties. Coatings. 2018;8:35. doi: 10.3390/coatings8010035. [DOI] [Google Scholar]
- 41.Huang Y., Mei L., Chen X., Wang Q. Recent developments in food packaging based on nanomaterials. Nanomaterials. 2018;8:830. doi: 10.3390/nano8100830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haggerty J.E.S., Schelhas L.T., Kitchaev D.A., Mangum J.S., Garten L.M., Sun W., Stone K.H., Perkins J.D., Toney M.F., Ceder G., et al. High-fraction brookite films from amorphous precursors. Sci. Rep. 2017;7:15232. doi: 10.1038/s41598-017-15364-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Diebold U. The surface sicence of titanium dioxide. Surf. Sci. Rep. 2003;48:53–229. doi: 10.1016/S0167-5729(02)00100-0. [DOI] [Google Scholar]
- 44.Lin X., Li J., Ma S., Liu G., Yang K., Tong M., Lin D. Toxicity of TiO2 nanoparticles to Escherichia coli: Effects of particle size, crystal phase and water chemistry. PLoS ONE. 2014;9:e110247. doi: 10.1371/journal.pone.0110247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maziarz W., Kursior A., Trenczek-Zajac A. Nanostructured TiO2-based gas sensors with enhanced sensitivity to reducing gases. Beilstein J. Nanotechnol. 2016;7:1718–1726. doi: 10.3762/bjnano.7.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Raghu A.V., Karuppanan K.K., Nampoothin J., Pullithadathil B. Wearable, flexible ethanol gas sensor based on TiO2 nanoparticles-grafted 2D-titanium carbide nanosheets. ACS Appl. Nano Mater. 2019;2:1152–1163. doi: 10.1021/acsanm.8b01975. [DOI] [Google Scholar]
- 47.Liu G., Yang H.G., Pan J., Yang Y.Q., Lu G.Q., Cheng H.M. Titanium dioxide crystals with tailored facets. Chem. Rev. 2014;114:9559–9612. doi: 10.1021/cr400621z. [DOI] [PubMed] [Google Scholar]
- 48.Al-Attafi K., Nattestad A., Wu Q., Ide Y., Yamauchi Y., Dou S.X., Kim J.H. The effect of amorphous TiO2 in P25 on dye-sensitized solar cell performance. Chem. Commun. 2018;54:381–384. doi: 10.1039/C7CC07559F. [DOI] [PubMed] [Google Scholar]
- 49.Truppi A., Petronella F., Placido T., Striccoli M. Visible-light-active TiO2-based hybrid nanocatalysts for environmental applications. Catalysis. 2018;7:100. doi: 10.3390/catal7040100. [DOI] [Google Scholar]
- 50.Tobaldi D.M., Piccirillo C., Pullar R.C., Gualtieri A.F., Seabra M.P., Castro P.M., Labrincha J.A. Silver-modified nano-titania as an antibacterial agent and photocatalyst. J. Phys. Chem. C. 2014;118:4751–4766. doi: 10.1021/jp411997k. [DOI] [Google Scholar]
- 51.Ali T., Ahmed A., Alam U., Uddin I., Tripathi P., Muneer M. Enhanced photocatalytic and antibacterial activities of Ag-doped TiO2 nanoparticles under visible light. Mater. Chem. Phys. 2018;212:325–335. doi: 10.1016/j.matchemphys.2018.03.052. [DOI] [Google Scholar]
- 52.Moongraksathum B., Chen Y.W. Anatase TiO2 co-doped with silver and ceria for antibacterial application. Catal. Today. 2018;310:68–74. doi: 10.1016/j.cattod.2017.05.087. [DOI] [Google Scholar]
- 53.Viet P.V., Phan B.T., Mott D., Maenosono S., Sang T.T., Thi C.M., Hieu L.V. Silver nanoparticle loaded TiO2 nanotubes with high photocatalytic and antibacterial activity synthesized by photoreduction method. J. Photochem. Photobiol. A. 2018;352:106–112. doi: 10.1016/j.jphotochem.2017.10.051. [DOI] [Google Scholar]
- 54.Ahmad R., Mohsin M., Ahmad D., Sardar M. Alpha amylase assisted synthesis of TiO2 nanoparticles: Structural characterization and application as antibacterial agents. J. Hazard. Mater. 2015;283:171–177. doi: 10.1016/j.jhazmat.2014.08.073. [DOI] [PubMed] [Google Scholar]
- 55.Lorenzetti M., Gongadze E., Kulkarni M., Junkar I., Iglic A. Electrokinetic properties of TiO2 nanotubular surfaces. Nanoscale Res. Lett. 2016;11:378. doi: 10.1186/s11671-016-1594-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jiang X., Lv B., Wang Y., Shen Q., Wang X. Bactericidal mechanisms and effector targets of TiO2 and Ag-TiO2 against Staphylococcus aureus. J. Med. Microbiol. 2017;66:440–446. doi: 10.1099/jmm.0.000457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pagnout C., Jomini S., Dadhwal M., Caillet C., Thomas F., Bauda P. Role of electrostatic interactions in the toxicity of titanium dioxide nanoparticles toward Escherichia coli. Colloids Surf. B Biointerfaces. 2012;92:315–321. doi: 10.1016/j.colsurfb.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 58.Liou J.W., Chang H.H. Bactericidal effects and mechanisms of visible light responsive titanium dioxide photocatalysts on pathogenic bacteria. Arch. Immunol. Ther. Exp. 2012;6:267–275. doi: 10.1007/s00005-012-0178-x. [DOI] [PubMed] [Google Scholar]
- 59.Charpentier P.A., Burgess K., Wang L., Chowdhury R.R., Lotus A.F., Moula G. Nano-TiO2/polyurethane composites for antibacterial and self-cleaning coatings. Nanotechnology. 2012;23:425606. doi: 10.1088/0957-4484/23/42/425606. [DOI] [PubMed] [Google Scholar]
- 60.Santhosh S.M., Kandasamy N. Antibiofilm activity of epoxy/Ag-TiO2 polymer nanocomposite coatings against Staphylococcus aureus and Escherichia coli. Coatings. 2015;5:95–114. doi: 10.3390/coatings5020095. [DOI] [Google Scholar]
- 61.Khorshidi B., Biswas I., Ghosh T., Thundat T., Sadrzadeh M. Polyamide-TiO2 nanocomposite membranes with enhanced thermal stability and anti-biofouling propensity. Sci. Rep. 2018;8:784. doi: 10.1038/s41598-017-18724-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang S., Liang X., Gadd G.M., Zhao Q. Advanced titanium dioxide-polytetrafluorethylene (TiO2-PTFE) nanocomposite coatings on stainless steel surfaces with antibacterial and anti-corrosion properties. Appl. Surf. Sci. 2019;490:231–241. doi: 10.1016/j.apsusc.2019.06.070. [DOI] [Google Scholar]
- 63.Ahmadi R., Tanomand A., Kazeminawa F., Kamounah F.S., Ayaseh A., Ganbarov K., Yousefi M., Katourani A., Yousefi B., Kafil H.S. Fabrication and characterization of a titanium dioxide (TiO2) reinforced with bio-nanocomposite containing Miswak (Salvadora percica L.) extract–the antimicrobial, thermophysical, and barrier properties. Int J. Nanomedic. 2019;14:3439–3454. doi: 10.2147/IJN.S201626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hegedus P., Szabó-Bárdos E., Horvath O., Szabo P., Horvath K. Investigation of a TiO2 photocatalyst immobilized with poly(vinyl alcohol) Catal. Today. 2017;284:179–186. doi: 10.1016/j.cattod.2016.11.050. [DOI] [Google Scholar]
- 65.Bui V.K., Park D., Lee Y.C. Chitosan combined with ZnO, TiO2 and Ag nanoparticles for antimicrobial wound healing applications: A mini review of the research trends. Polymers. 2017;9:21. doi: 10.3390/polym9010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Meng Y.Z., Tjong S.C. Rheology and morphology of compatibilized polyamide 6 blends containing liquid crystalline copolyesters. Polymer. 1998;39:99–107. doi: 10.1016/S0032-3861(97)00218-8. [DOI] [Google Scholar]
- 67.Meng Y.Z., Tjong S.C., Hay A.S., Wang S.J. Synthesis and proton conductivities of phosphonic acid containing poly-(arylene ether)s. J. Polym. Sci. A Polym. Chem. 2001;39:3218–3226. doi: 10.1002/pola.1304. [DOI] [Google Scholar]
- 68.Liu C., Chan K.W., Shen J., Liao C., Yeung K.W.K., Tjong S.C. Polyetheretherketone hybrid composites with bioactive nanohydroxyapatite and multiwalled carbon nanotube fillers. Polymers. 2016;8:425. doi: 10.3390/polym8120425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chan K.W., Liao C., Wong H.M., Yeung K.W.K., Tjong S.C. Preparation of polyetheretherketone composites with nanohydroxyapatite rods and carbon nanofibers having high strength, good biocompatibility and excellent thermal stability. RSC Adv. 2016;6:19417–19429. doi: 10.1039/C5RA22134J. [DOI] [Google Scholar]
- 70.Liao C., Li K., Wong H.M., Tong W.Y., Yeung K.W.K., Tjong S.C. Novel polypropylene biocomposites reinforced with carbon nanotubes and hydroxyapatite nanorods for bone replacements. Mater. Sci. Eng. C. 2013;13:1380–1388. doi: 10.1016/j.msec.2012.12.039. [DOI] [PubMed] [Google Scholar]
- 71.Liao C., Wong H.M., Yeung K.W.K., Tjong S.C. The development, fabrication and material characterization of polypropylene composites reinforced with carbon nanofiber and hydroxyapatite nanorod hybrid fillers. Int. J. Nanomed. 2014;9:1299–1310. doi: 10.2147/IJN.S58332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu C., Wong H.M., Yeung K.W., Tjong S.C. Novel electrospun polylactic acid nanocomposite fiber mats with hybrid graphene oxide and nanohydroxyapatite reinforcements having enhanced biocompatibility. Polymers. 2016;8:287. doi: 10.3390/polym8080287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Horn M., Schwerdtfeger C.F., Meagher E.P. Refinement of the structure of anatase at several temperatures. Z. Krist. 1972;136:273–281. doi: 10.1524/zkri.1972.136.3-4.273. [DOI] [Google Scholar]
- 74.Baur W.H., Khan A.A. Rutile-type compounds. IV. SiO2, GeO2 and a comparison with other rutile-type structures. Acta Crystallogr. B. 1971;27:2133–2139. doi: 10.1107/S0567740871005466. [DOI] [Google Scholar]
- 75.Fagan R., Synnott D.W., McCormack D.E., Pillai S.C. An effective method for the preparation of high temperature stable anatase TiO2 photocatalysts. Appl. Surf. Sci. 2016;371:447–452. doi: 10.1016/j.apsusc.2016.02.235. [DOI] [Google Scholar]
- 76.Higashimoto S. Titanium-dioxide-based visible-light-sensitive photocatalysis: Mechanistic insight and applications. Catalysts. 2019;9:201. doi: 10.3390/catal9020201. [DOI] [Google Scholar]
- 77.Lu Y., Zang Y., Zhang H., Zhang Y., Wang G., Zhao H. Meaningful comparison of photocatalytic properties of {001} and {101} faceted anatase TiO2 nanocrystals. Sci. Bull. 2016;61:1003–1012. doi: 10.1007/s11434-016-1109-8. [DOI] [Google Scholar]
- 78.Sajan C.P., Wageh S., Al-Ghamdhi A.A., Yu J., Cao S. TiO2 nanosheets with exposed {001} facets for photocatalytic application. Nano Res. 2016;9:3–27. doi: 10.1007/s12274-015-0919-3. [DOI] [Google Scholar]
- 79.Liu X., Du G., Li M. True photoreactivity origin of Ti3+-doped anatase TiO2 crystals with respectively dominated exposed {001}, {101}, and {100} facets. ACS Omega. 2019;4:14902–14912. doi: 10.1021/acsomega.9b01648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li M., Yin J.J., Wamer W.G., Lo Y.M. Mechanistic characterization of titanium dioxide nanoparticle-induced toxicity using electron spin resonance. J. Food Drug Anal. 2014;22:76–85. doi: 10.1016/j.jfda.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xue C., Wu J., Lan F., Liu W., Yang X., Zeng F., Xu H. Nano titanium dioxide induces the generation of ROS and potential damage in HaCaT cells under UVA irradiation. J. Nanosci. Nanotechnol. 2010;10:8500–8507. doi: 10.1166/jnn.2010.2682. [DOI] [PubMed] [Google Scholar]
- 82.Bartlet K., Movafaghi S., Dasi L.P., Kota A.K., Popat K.C. Antibacterial activity on superhydrophobic titania nanotube arrays. Colloids Surf. B Biointerfaces. 2018;166:179–186. doi: 10.1016/j.colsurfb.2018.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dong H., Zeng G., Tang L., Fan C., Zhang C., He X. An overview on limitations of TiO2-based particles for photocatalytic degradation of organic pollutants and the corresponding countermeasures. Water Res. 2015;79:128–146. doi: 10.1016/j.watres.2015.04.038. [DOI] [PubMed] [Google Scholar]
- 84.Pelaez M., Nolan N., Pillai S., Seery M., Falaras P., Kontos A.G., Dunlop P.S., Hamilton J.W., Byrne J.A., O’Shea K., et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B Environ. 2012;125:331–349. doi: 10.1016/j.apcatb.2012.05.036. [DOI] [Google Scholar]
- 85.Schneider J., Matsuoka M., Takeuchi M., Zhang J., Horiuchi Y., Anpo M., Bahnemann D.W. Understanding TiO2 photocatalysis: Mechanisms and materials. Chem. Rev. 2014;114:9919–9986. doi: 10.1021/cr5001892. [DOI] [PubMed] [Google Scholar]
- 86.Moma J., Baloyi J. Modified Titanium Dioxide for Photocatalytic Applications. In: Khan S.B., Akhtar K., editors. Photocatalysts—Applications and Attributes. Intech Open; London, UK: 2019. pp. 37–56. Chapter 3. [DOI] [Google Scholar]
- 87.Kang X., Liu S., Dai Z., He Y., Song X., Tang Z. Titanium dioxide: From engineering to applications. Catalysis. 2019;9:191. doi: 10.3390/catal9020191. [DOI] [Google Scholar]
- 88.Lin W.C., Lin Y.J. Effect of vanadium (IV)-doping on the visible light-induced catalytic activity of titanium dioxide catalysts for methylene blue degradation. Environ. Eng. Sci. 2012;29:447–452. doi: 10.1089/ees.2010.0350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yu J.H., Nam S.H., Lee J.W., Kim D.I., Boo J.H. Oxidation state and structural studies of vanadium-doped titania particles for the visible light-driven photocatalytic activity. Appl. Surf. Sci. 2019;472:46–53. doi: 10.1016/j.apsusc.2018.04.125. [DOI] [Google Scholar]
- 90.Lv T., Zhao J., Chen M., Shen K., Zhang D., Zhang J., Zhang G., Liu Q. Boosted visible-light photodegradation of methylene blue by V and Co co-doped TiO2. Materials. 2018;11:1946. doi: 10.3390/ma11101946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Khatun N., Rini E.G., Shirage P., Rajput P., Jha S.N., Sen S. Effect of lattice distortion on bandgap decrement due to vanadium substitution in TiO2 nanoparticles. Mater. Sci. Semicond. Process. 2016;50:7–13. doi: 10.1016/j.mssp.2016.04.002. [DOI] [Google Scholar]
- 92.Binas V., Venieri D., Kotzias D., Kiriakidis D. Modified TiO2 based photocatalysts for improved air and health quality. J. Mater. 2017;3:3–16. doi: 10.1016/j.jmat.2016.11.002. [DOI] [Google Scholar]
- 93.Moradi H., Eshaghi A., Hosseini S.R., Ghani K. Fabrication of Fe-doped TiO2 nanoparticles and investigation of photocatalytic decolorization of reactive red 198 under visible light irradiation. Ultrason. Sonochem. 2016;32:314–319. doi: 10.1016/j.ultsonch.2016.03.025. [DOI] [PubMed] [Google Scholar]
- 94.Crisan M., Mardare D., Ianculescu A., Dragan N., Nitoi I., Crisan D., Voicescu M., Todan L., Oancea P., Adomnitei C., et al. Iron doped TiO2 films and their photoactivity in nitrobenzene removal from water. Appl. Surf. Sci. 2018;455:201–215. doi: 10.1016/j.apsusc.2018.05.124. [DOI] [Google Scholar]
- 95.Daghrir R., Drogui P., Robert D. Modified TiO2 for environmental photocatalytic applications: A review. Ind. Eng. Chem. Res. 2013;52:3581–3599. doi: 10.1021/ie303468t. [DOI] [Google Scholar]
- 96.Li W. Influence of electronic structures of doped TiO2 on their photocatalysis. Phys. Status Solidi Rapid Res. Lett. 2014;9:10–27. doi: 10.1002/pssr.201409365. [DOI] [Google Scholar]
- 97.Ivanov S., Barylyak A., Besaha K., Bund A., Wojnarowska-Nowak R., Yaremchuk I., Kus-Liśkiewicz M. Synthesis, characterization, and photocatalytic properties of sulfur- and carbon-codoped TiO2 nanoparticles. Nanoscale Res. Lett. 2016;11:140. doi: 10.1186/s11671-016-1353-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Koklic T., Pintaric S., Zdovc I., Golob M., Umek P., Mehle A., Dobeic M., Strancar J. Photocatalytic disinfection of surfaces with copper doped TiO2 nanotube coatings illuminated by ceiling mounted fluorescent light. PLoS ONE. 2018;13:e0197308. doi: 10.1371/journal.pone.0197308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Syrek K., Grudzień J., Sennik-Kubiec A., Brudzisz A., Sulka G.D. Anodic titanium oxide layers modified with gold, silver, and copper nanoparticles. J. Nanomater. 2019;2019:9208734. doi: 10.1155/2019/9208734. [DOI] [Google Scholar]
- 100.Janczarek M., Wei Z., Endo M., Ohtani B., Kowalska E. Silver- and copper modified decahedral anatase titania particles as visible light-responsive plasmonic photocatalyst. J. Photon. Energy. 2016;7:012008. doi: 10.1117/1.JPE.7.012008. [DOI] [Google Scholar]
- 101.Wanag A., Rokicka P., Kusiak-Nejman E., Kapica-Kozar J., Wrobel R.J., Markowska-Szczupak A., Morawski A.W. Antibacterial properties of TiO2 modified with reduced graphene oxide. Ecotoxicol. Environ. Saf. 2018;147:788–793. doi: 10.1016/j.ecoenv.2017.09.039. [DOI] [PubMed] [Google Scholar]
- 102.Raja A., Selvakumar K., Rajasekaran P., Arunpandian M., Ashokkumar S., Kaviyarasu K., Asath Bahadur S., Swaminathan M. Visible active reduced graphene oxide loaded titania for photodecomposition of ciprofloxacin and its antibacterial activity. Colloids Surf. A. 2019;564:23–30. doi: 10.1016/j.colsurfa.2018.12.024. [DOI] [Google Scholar]
- 103.Tayel A., Ramadan A.R., El Seoud O.A. Titanium dioxide/graphene and titanium dioxide/graphene oxide nanocomposites: Synthesis, characterization and photocatalytic applications for water decontamination. Catalysts. 2018;8:491. doi: 10.3390/catal8110491. [DOI] [Google Scholar]
- 104.Rauwel P., Galeckas A., Salumaa M., Ducroquet F., Rauwel E. Photocurrent generation in carbon nanotube/cubic-phase HfO2 nanoparticle hybrid nanocomposites. Beilstein J. Nanotechnol. 2016;7:1075–1085. doi: 10.3762/bjnano.7.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Choi W., Termin A., Hoffmann M.R. The role of metal ion dopants in quantum-sized TiO2: Correlation between photoreactivity and charge carrier recombination dynamics. J. Phys. Chem. 1994;98:13669–13679. doi: 10.1021/j100102a038. [DOI] [Google Scholar]
- 106.Fang W.Z., Xing M.Y., Zhang J.L. A new approach to prepare Ti3+ self-doped TiO2 via NaBH4 reduction and hydrochloric acid treatment. Appl. Catal. B Environ. 2014;160–161:240–246. doi: 10.1016/j.apcatb.2014.05.031. [DOI] [Google Scholar]
- 107.Wan Z., Huang G.F., Huang W.Q., Jiao C., Yan X.G., Yang Z.M., Zhang Q.L. The enhanced photocatalytic activity of Ti3+ self-doped TiO2 by a reduction method. Mater. Lett. 2014;122:33–36. doi: 10.1016/j.matlet.2014.01.181. [DOI] [Google Scholar]
- 108.Jayashree S., Ashokkumar M. Switchable intrinsic defect chemistry of titania for catalytic applications. Catalysts. 2018;8:601. doi: 10.3390/catal8120601. [DOI] [Google Scholar]
- 109.Mathew S., Ganguly P., Rhatigan S., Kumaravel V., Byrne C., Hinder S.J., Bartlett J., Nolan M., Pillai S.C. Cu-doped TiO2: Visible light assisted photocatalytic antimicrobial activity. Appl. Sci. 2018;8:2067. doi: 10.3390/app8112067. [DOI] [Google Scholar]
- 110.Manzoor M., Rafiq A., Ikram M., Nafees M., Ali S. Structural, optical, and magnetic study of Ni-doped TiO2 nanoparticles synthesized by sol–gel method. Int. Nano Lett. 2018;8:1–8. doi: 10.1007/s40089-018-0225-7. [DOI] [Google Scholar]
- 111.Huang J.G., Guo X.T., Wang B., Li L.Y., Zhao M.X., Dong L.L., Liu X.J., Huang Y.T. Synthesis and photocatalytic activity of Mo-doped TiO2 nanoparticles. J. Spectrosc. 2015;2015:681850. doi: 10.1155/2015/681850. [DOI] [Google Scholar]
- 112.Avilés-García O., Espino-Valencia J., Romero R., Rico-Cerda J.L., Arroyo-Albiter M., Natividad R. W and Mo doped TiO2: Synthesis, characterization and photocatalytic activity. Fuel. 2017;198:31–41. doi: 10.1016/j.fuel.2016.10.005. [DOI] [Google Scholar]
- 113.Shi Z., Lai H., Yao S., Wang S. Photocatalytic activity of Fe and Ce co-doped mesoporous TiO2 catalyst under UV and visible light. J. Chin. Chem. Soc. 2012;59:614–620. doi: 10.1002/jccs.201100509. [DOI] [Google Scholar]
- 114.Aviles-Garcia O., Espino-Valencia J., Romero-Romero R., Rico-Cerda J.L., Arroyo-Albiter M., Solis-Casados D.A., Navitidad-Rangel R. Enhanced photocatalytic activity of titania by co-doping with Mo and W. Catalysts. 2018;8:631. doi: 10.3390/catal8120631. [DOI] [Google Scholar]
- 115.El Mragui A., Logvina Y., Pinto da Silva L., Zegaoui O., Estevesda Silva J.C.G. Synthesis of Fe- and Co-doped TiO2 with improved photocatalytic activity under visible irradiation toward carbamazepine degradation. Materials. 2019;12:3874. doi: 10.3390/ma12233874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nadolna J., Grzyb G., Sobczak J.W., Lisowski W. Visible light activity of rare earth metal doped (Er3+, Yb3+ or Er3+/Yb3+) titania photocatalysts. Appl. Catal. B Envorin. 2015;163:40–49. doi: 10.1016/j.apcatb.2014.07.010. [DOI] [Google Scholar]
- 117.Rozman N., Tobaldi D.M., Cvelbar U., Puliyalil H., Labrincha J.A., Legat A., Skapin A.S. Hydrothermal synthesis of rare-earth modified titania: Influence on phase composition, optical properties, and photocatalytic activity. Materials. 2019;12:713. doi: 10.3390/ma12050713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mazierski P., Lisowski W., Grzyb T., Winiarski M.J., Klimczuk T., Mikołajczyk A., Flisikowski J., Hirsch A., Kolakowska A., Puzyn T., et al. Enhanced photocatalytic properties of lanthanide-TiO2 nanotubes: An experimental and theoretical study. Appl. Catal. B Environ. 2017;205:376–385. doi: 10.1016/j.apcatb.2016.12.044. [DOI] [Google Scholar]
- 119.Xie K., Jia Q., Wang Y., Zhang W., Xu J. The electronic structure and optical properties of anatase TiO2 with rare earth metal dopants from first-principles calculations. Materials. 2018;11:179. doi: 10.3390/ma11020179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Makdee A., Unwiset P., Chanapattharapol K.C., Kidkhunthod P. Effects of Ce addition on the properties and photocatalytic activity of TiO2, investigated by X-ray absorption spectroscopy. Mater. Chem. Phys. 2018;213:431–443. doi: 10.1016/j.matchemphys.2018.04.016. [DOI] [Google Scholar]
- 121.Kasinathan K., Kennedy J., Elayaperumal M., Henini M., Malik M. Photodegradation of organic pollutants RhB dye using UV simulated sunlight on ceria based TiO2 nanomaterials for antibacterial applications. Sci. Rep. 2016;6:38064. doi: 10.1038/srep38064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Choudhury B., Borah B., Choudhury A. Extending photocatalytic activity of TiO2 nanoparticles to visible region of illumination by doping of cerium. Photochem. Photobiol. 2012;88:257–264. doi: 10.1111/j.1751-1097.2011.01064.x. [DOI] [PubMed] [Google Scholar]
- 123.Li J., Zhou H., Qian S., Liu Z., Feng J., Jin P., Liu X. Plasmonic gold nanoparticles modified titania nanotubes for antibacterial application. Appl. Phys. Lett. 2014;104:261110. doi: 10.1063/1.4885401. [DOI] [Google Scholar]
- 124.Endo M., Wei Z., Wang K., Karabiyik B., Yoshiiri K., Rokickka P., Ohtani B., Markowska-Szczupak A., Kowalska E. Noble metal-modified titania with visible-light activity for the decomposition of microorganisms. Beilstein J. Nanotechnol. 2018;9:829–841. doi: 10.3762/bjnano.9.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wysocka I., Kowalska E., Ryl J., Nowaczyk G., Zielińska-Jurek A. Morphology, photocatalytic and antimicrobial properties of TiO2 modified with mono- and bimetallic copper, platinum and silver nanoparticles. Nanomaterials. 2019;9:1129. doi: 10.3390/nano9081129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Petica A., Florea A., Gaidau C., Balan D., Anicai L. Synthesis and characterization of silver-titania nanocomposites prepared by electrochemical method with enhanced photocatalytic characteristics, antifungal and antimicrobial activity. J. Mater. Res. Technol. 2019;8:41–53. doi: 10.1016/j.jmrt.2017.09.009. [DOI] [Google Scholar]
- 127.Krajczewski J., Kolataj K., Kudelski A. Plasmonic nanoparticles in chemical analysis. RSC Adv. 2017;7:17559–17576. doi: 10.1039/C7RA01034F. [DOI] [Google Scholar]
- 128.Kim M., Lin M., Son J., Xu H., Nam J.M. Hot-electron-mediated photochemical reactions: Principles, recent advances, and challenges. Adv. Opt. Mater. 2017;5:1700004. doi: 10.1002/adom.201700004. [DOI] [Google Scholar]
- 129.Furube A., Hashimoto S. Insight into plasmonic hot-electron transfer and plasmon molecular drive: New dimensions in energy conversion and nanofabrication. NPG Asia Mater. 2017;9:e454. doi: 10.1038/am.2017.191. [DOI] [Google Scholar]
- 130.Zhang Z., Zhang C., Zheng H., Xu H. Plasmon-driven catalysis on molecules and nanomaterials. Acc. Chem. Res. 2019;52:2506–2515. doi: 10.1021/acs.accounts.9b00224. [DOI] [PubMed] [Google Scholar]
- 131.Hartland G.V., Besteiro L.V., Johns P., Govorov A.O. What’s so hot about electrons in metal nanoparticles? ACS Energy Lett. 2017;2:1641–1653. doi: 10.1021/acsenergylett.7b00333. [DOI] [Google Scholar]
- 132.Kim J., Son H.Y., Nam Y.S. Multilayered plasmonic heterostructure of gold and titania nanoparticles for solar fuel production. Sci. Rep. 2018;8:10464. doi: 10.1038/s41598-018-28789-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wu N. Plasmonic metal–semiconductor photocatalysts and photoelectrochemical cells: A review. Nanoscale. 2018;10:2679–2696. doi: 10.1039/C7NR08487K. [DOI] [PubMed] [Google Scholar]
- 134.Clavero C. Plasmon-induced hot-electron generation at nanoparticle/metal-oxide interfaces for photovoltaic and photocatalytic devices. Nat. Photonics. 2014;8:95–103. doi: 10.1038/nphoton.2013.238. [DOI] [Google Scholar]
- 135.Khan M.R., Chowdhurya M.N., Chuan T.W., Cheng C.K. Schottky barrier and surface plasmonic resonance phenomena towards the photocatalytic reaction: Study of their mechanisms to enhance the photocatalytic activity. Catal. Sci. Technol. 2015;5:2522–2531. doi: 10.1039/C4CY01545B. [DOI] [Google Scholar]
- 136.Yao G.Y., Liu Q.L., Zhao Z.Y. Studied localized surface plasmon resonance effects of Au nanoparticles on TiO2 by FDTD simulations. Catalysts. 2018;8:236. doi: 10.3390/catal8060236. [DOI] [Google Scholar]
- 137.Michalas L., Khiat A., Stathopoulos S., Prodromakis T. Electrical characteristics of interfacial barriers at metal—TiO2 contacts. J. Phys. D Appl. Phys. 2018;51:425101. doi: 10.1088/1361-6463/aadbd2. [DOI] [Google Scholar]
- 138.Hankodo C.T., Moustakas N.G., Peppel T., Springer A., Oropeza F.E., Huda A., Bustan M.D., Yudono B., Gulo F., Strunk J. Characterization and effect of Ag(0) vs. Ag(I) species and their localized plasmon resonance on photochemically inactive TiO2. Catalysts. 2019;9:323. doi: 10.3390/catal9040323. [DOI] [Google Scholar]
- 139.Ma Y., Zhi L. Graphene-based transparent conductive films: Material systems, preparation and applications. Small Methods. 2019;3:1800199. doi: 10.1002/smtd.201800199. [DOI] [Google Scholar]
- 140.Nair R.R., Blake P., Grigorenko A.N., Novoselov K.S., Booth T.J., Stauber T., Peres N.M., Geim A.K. Fines structure constant defines visual transparency of graphene. Science. 2008;320:1308. doi: 10.1126/science.1156965. [DOI] [PubMed] [Google Scholar]
- 141.Kim C.H. Nanostructured graphene: An active component in optoelectronic devices. Nanomaterials. 2018;8:328. doi: 10.3390/nano8050328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kumar P., Huo P., Zhang R., Liu B. Antibacterial properties of graphene-based nanomaterials. Nanomaterials. 2019;9:737. doi: 10.3390/nano9050737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Karahan H.E., Wiraja C., Xu C., Wei J., Wang Y., Wang L., Liu F., Chen Y. Graphene materials in antimicrobial nanomedicine: Current status and future perspectives. Adv. Healthc. Mater. 2018;7:1701406. doi: 10.1002/adhm.201701406. [DOI] [PubMed] [Google Scholar]
- 144.Gillespie N.O., Martsinovich N. Origin of charge trapping in TiO2/reduced graphene oxide photocatalytic composites: Insights from theory. ACS Appl. Mater. Interf. 2019;11:31909–31922. doi: 10.1021/acsami.9b09235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tang B., Chen H., Peng H., Wang Z., Huang W. Graphene modified TiO2 composite photocatalysts: Mechanism, progress and perspective. Nanomaterials. 2018;8:105. doi: 10.3390/nano8020105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tan L.L., Ong W.J., Chai S.P., Mohamed A.R. Reduced graphene oxide-TiO2 nanocomposite as a promising visible-light-active photocatalyst for the conversion of carbon dioxide. Nanoscale Res. Lett. 2013;8:465. doi: 10.1186/1556-276X-8-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Polat E.O., Balci O., Kakenov N., Uzlu H.B., Kocabas C., Dahiya R. Synthesis of large area graphene for high performance in flexible optoelectronic devices. Sci. Rep. 2015;5:16744. doi: 10.1038/srep16744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Guerrero-Contreras J., Caballero-Briones F. Graphene oxide powders with different oxidation degree, prepared by synthesis variations of the Hummers method. Mater. Chem. Phys. 2015;153:209–220. doi: 10.1016/j.matchemphys.2015.01.005. [DOI] [Google Scholar]
- 149.Dreyer D.R., Park S., Bielawski C.W., Ruoff R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010;39:228–240. doi: 10.1039/B917103G. [DOI] [PubMed] [Google Scholar]
- 150.Park S., An J., Potts J.R., Velamakanni A., Murali S., Ruoff R.S. Hydrazine-reduction of graphite- and graphene oxide. Carbon. 2011;49:3019–3023. doi: 10.1016/j.carbon.2011.02.071. [DOI] [Google Scholar]
- 151.Xu C., Shi X., Ji A., Shi L., Zhou C., Cui Y. Fabrication and characteristics of reduced graphene oxide produced with different green reductants. PLoS ONE. 2015;10:e0144842. doi: 10.1371/journal.pone.0144842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yeh T.F., Teng T.C., Chen L.C., Teng H. Graphene oxide-based nanomaterials for efficient photoenergy conversion. J. Mater. Chem. A. 2016;4:2014–2048. doi: 10.1039/C5TA07780J. [DOI] [Google Scholar]
- 153.Giovannetti R., Rommozzi E., Zannotti M., D’Amato C.A. Recent advances in graphene based TiO2 nanocomposites (GTiO2Ns) for photocatalytic degradation of synthetic dyes. Catalysts. 2017;7:305. doi: 10.3390/catal7100305. [DOI] [Google Scholar]
- 154.Rauwel P., Galeckas A., Ducroquet F., Rauwel E. Selective photocurrent generation in HfO2 and carbon nanotube hybrid nanocomposites under Ultra-Violet and visible photoexcitations. Mater. Lett. 2019;246:45–48. doi: 10.1016/j.matlet.2019.03.030. [DOI] [Google Scholar]
- 155.Akhavan O., Azimirad R., Safa S., Larijani M.M. Visible light photo-induced antibacterial activity of CNT–doped TiO2 thin films with various CNT contents. J. Mater. Chem. 2010;20:7386–7392. doi: 10.1039/c0jm00543f. [DOI] [Google Scholar]
- 156.Koli V.B., Dhodamani A.G., Raut A.V., Thorat N.D., Pawar S.H., Delekar S.D. Visible light photo-induced antibacterial activity of TiO2-MWCNTs nanocomposites with varying the contents of MWCNTs. J. Photochem. Photobiol. A. 2016;328:50–58. doi: 10.1016/j.jphotochem.2016.05.016. [DOI] [Google Scholar]
- 157.Koli V.B., Delekar S.D., Pawar S.H. Photoinactivation of bacteria by using Fe-doped TiO2-MWCNTs nanocomposites. J. Mater. Sci. Mater. Med. 2016;27:177. doi: 10.1007/s10856-016-5788-0. [DOI] [PubMed] [Google Scholar]
- 158.Choi J., Park H., Hoffmann M.R. Effects of single metal-ion doping on the visible-light photoreactivity of TiO2. J. Phys. Chem. C. 2010;114:783–792. doi: 10.1021/jp908088x. [DOI] [Google Scholar]
- 159.Nagpure S., Kim D.Y., Rankin S.E. Synthesis and catalytic applications of non-metal doped mesoporous titania. Inorganics. 2017;5:15. doi: 10.3390/inorganics5010015. [DOI] [Google Scholar]
- 160.Valentin C.D., Pacchioni G. Trends in non-metal doping of anatase TiO2: B, C, N and F. Catal. Today. 2013;206:12–18. doi: 10.1016/j.cattod.2011.11.030. [DOI] [Google Scholar]
- 161.Ananpattarachai J., Boonto Y., Kajitvichyanukul P. Visible light photocatalytic antibacterial activity of Ni-doped and N-doped TiO2 on Staphylococcus aureus and Escherichia coli bacteria. Environ. Sci. Pollut. Res. 2016;23:4111–4119. doi: 10.1007/s11356-015-4775-1. [DOI] [PubMed] [Google Scholar]
- 162.Zener B., Matoh L., Carraro G., Miljevic B., Korosec R.C. Sulfur-, nitrogen- and platinum-doped titania thin films with high catalytic efficiency under visible-light illumination. Beilstein J. Nanotechnol. 2018;9:1629–1640. doi: 10.3762/bjnano.9.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Cravanzola S., Cesano F., Gaziano F., Scarano D. Sulfur-doped TiO2: Structure and surface properties. Catalysts. 2017;7:214. doi: 10.3390/catal7070214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Banerjee S., Pillai S.C., Falaras P., O’Shea K.E., Byrne J.A., Dionysiou D.D. New insights into the mechanism of visible light photocatalysis. J. Phys. Chem. Lett. 2014;5:2543–2554. doi: 10.1021/jz501030x. [DOI] [PubMed] [Google Scholar]
- 165.Yu J.C., Yu J., Ho W., Jiang Z., Zhang L. Effects of F- doping on the photocatalytic activity and microstructures of nanocrystalline TiO2 powders. Chem. Mater. 2002;14:3808–3816. doi: 10.1021/cm020027c. [DOI] [Google Scholar]
- 166.Asahi R., Morikawa T., Ohwaki T., Aoki K., Taga Y. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science. 2001;13:269–271. doi: 10.1126/science.1061051. [DOI] [PubMed] [Google Scholar]
- 167.Varley J.B., Janotti A., van de Walle C.G. Mechanism of visible-light photocatalysis in nitrogen-doped TiO2. Adv. Mater. 2011;23:2343–2347. doi: 10.1002/adma.201003603. [DOI] [PubMed] [Google Scholar]
- 168.Ansari S.A., Khan M.M., Ansari M.O., Cho M.H. Nitrogen-doped titanium dioxide (N-doped TiO2) for visible light photocatalysis. New J. Chem. 2016;40:3000–3009. doi: 10.1039/C5NJ03478G. [DOI] [Google Scholar]
- 169.Yang K., Dai Y., Huang B., Whangbo M.H. Density functional characterization of the visible-light absorption in substitutional C-anion- and C-cation-doped TiO2. J. Phys. Chem. C. 2009;113:2624–2629. doi: 10.1021/jp808483a. [DOI] [Google Scholar]
- 170.Di Valentin C., Pacchioni G., Selloni A. Theory of carbon doping of titanium dioxide. Chem. Mater. 2005;17:6656–6665. doi: 10.1021/cm051921h. [DOI] [Google Scholar]
- 171.Geng H., Yin S., Yang X., Shuai Z., Liu B. Geometric and electronic structures of the boron-doped photocatalyst TiO2. J. Phys. Condens. Matter. 2006;18:87–96. doi: 10.1088/0953-8984/18/1/006. [DOI] [Google Scholar]
- 172.Patel N., Dashora A., Jaiswal R., Fernandes R., Yadav M., Kothari D.C. Experimental and theoretical investigations on the activity and stability of substitutional and interstitial boron in TiO2 photocatalyst. J. Chem. Phys. 2015;119:18581–18590. doi: 10.1021/acs.jpcc.5b05290. [DOI] [Google Scholar]
- 173.Sotelo-Vazquez C., Noor N., Kafizas A., Quesada-Cabrera R., Scanlon D.O., Taylor A., Durrant J.R., Parkin I.P. Multifunctional P-doped TiO2 films: A new approach to self-cleaning, transparent conducting oxide materials. Chem. Mater. 2015;27:3234–3242. doi: 10.1021/cm504734a. [DOI] [Google Scholar]
- 174.Gopal N.O., Lo H.H., Ke T.F., Chou C.C., Wu J.D., Sheu S.C., Ke S.C. Visible light active phosphorus-doped TiO2 nanoparticles: An EPR evidence for the enhanced charge separation. J. Phys. Chem. C. 2012;116:16191–16197. doi: 10.1021/jp212346f. [DOI] [Google Scholar]
- 175.Li D., Haneda H., Hishita S., Labhsetwar N.K. Fluorine-doped TiO2 powders prepared by spray pyrolysis and their improved photocatalytic activity for decomposition of gas-phase acetaldehyde. J. Fluor. Chem. 2005;126:69–77. doi: 10.1016/j.jfluchem.2004.10.044. [DOI] [Google Scholar]
- 176.Yang G., Wang T., Yang B., Yan Z., Ding S., Xiao T. Enhanced visible-light activity of F-N co-doped TiO2 nanocrystals via nonmetal impurity, Ti3+ ions and oxygen vacancies. Appl. Surf. Sci. 2013;287:135–142. doi: 10.1016/j.apsusc.2013.09.094. [DOI] [Google Scholar]
- 177.Janczarek M., Endo M., Zhang D., Wang K., Kowalska E. Enhanced photocatalytic and antimicrobial performance of cuprous oxide/titania: The effect of titania matrix. Materials. 2018;11:2069. doi: 10.3390/ma11112069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Haque F., Daeneke T., Kalantar Zadeh K., Ou J. Two-dimensional transition metal oxide and chalcogenide-based photocatalysts. Nano-Micro Lett. 2018;10:23. doi: 10.1007/s40820-017-0176-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kiwi J., Rtimi S. Mechanisms of the antibacterial effects of TiO2–FeOx under solar or visible light: Schottky barriers versus surface plasmon resonance. Coatings. 2018;8:391. doi: 10.3390/coatings8110391. [DOI] [Google Scholar]
- 180.Bera S., Won D.I., Rawal S.B., Kang H.J., Lee W.I. Design of visible-light photocatalysts by coupling of inorganic semiconductors. Catal. Today. 2019;335:3–19. doi: 10.1016/j.cattod.2018.11.001. [DOI] [Google Scholar]
- 181.Phung H.N., Tran V.N., Nguyen L.T., Phan L.K., Duong P.A., Le H.V. Investigating visible-photocatalytic activity of MoS2/TiO2 heterostructure thin films at various MoS2 deposition times. J. Nanomater. 2017;2017:3197540. doi: 10.1155/2017/3197540. [DOI] [Google Scholar]
- 182.Liao Y., Deng P., Wang X., Zhang D., Li F., Yang Q., Zhang H., Zhong Z. A Facile method for preparation of Cu2O-TiO2 NTA heterojunction with visible-photocatalytic activity. Nanoscale Res. Lett. 2018;13:221. doi: 10.1186/s11671-018-2637-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Li G., Huang J., Chen J., Deng Z., Huang Q., Liu Z., Guo W., Cao R. Highly active photocatalyst of Cu2O/TiO2 octahedron for hydrogen generation. ACS Omega. 2019;4:3392–3397. doi: 10.1021/acsomega.8b03404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Zhu X., Gu P., Wu H., Yang D., Sun H., Wangyang P., Li J., Tian H. Influence of substrate on structural, morphological and optical properties of TiO2 thin films deposited by reaction magnetron sputtering. AIP Adv. 2017;7:125326. doi: 10.1063/1.5017242. [DOI] [Google Scholar]
- 185.Orlianges J.C., Crunteanu A., Pothier A., Merie-Mejean T., Blondy P., Champeaus C. Titanium dioxide thin films deposited by pulsed laser deposition and integration in radio frequency devices: Study of structure, optical and dielectric properties. Appl. Surf. Sci. 2012;263:111–114. doi: 10.1016/j.apsusc.2012.09.010. [DOI] [Google Scholar]
- 186.Vahl A., Veziroglu S., Henkel B., Strunskus T., Polonskyi O., Aktas O.C., Fuapel F. Pathways to tailor photocatalytic performance of TiO2 thin films deposited by reactive magnetron sputtering. Materials. 2019;12:2840. doi: 10.3390/ma12172840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Johari N.D., Rosli J.M., Juoi J.M., Yazid S.A. Comparison on the TiO2 crystalline phases deposited via dip and spin coating using green sol–gel route. J. Mater. Res. Technol. 2019;8:2350–2358. doi: 10.1016/j.jmrt.2019.04.018. [DOI] [Google Scholar]
- 188.Manoj P.K., Koshy P., Vaidyan V.K. Transparent anatase titania films: A critical study on optical properties. Prog. Nat. Sci. Mater. Int. 2012;22:79–85. doi: 10.1016/j.pnsc.2012.03.011. [DOI] [Google Scholar]
- 189.Bai Y., Mora-Sero I., De Angelis F., Bisquert J., Wang P. Titanium dioxide nanomaterials for photovoltaic applications. Chem. Rev. 2014;114:10095–10130. doi: 10.1021/cr400606n. [DOI] [PubMed] [Google Scholar]
- 190.Rauwel E., Willinger M.G., Ducroquet F., Rauwel P., Matko I., Kiselev D., Pinna N. Carboxylic acids as oxygen sources for the atomic layer deposition of high-κ metal oxides. J. Phys. Chem. C. 2008;112:12754–12759. doi: 10.1021/jp8037363. [DOI] [Google Scholar]
- 191.Correa G.C., Bao B., Strandwitz N.C. Chemical stability of titania and alumina thin films formed by atomic layer deposition. ACS Appl. Mater. Interfaces. 2015;7:14816–14821. doi: 10.1021/acsami.5b03278. [DOI] [PubMed] [Google Scholar]
- 192.Zhang Y., Utke I., Michler J., Ilari G., Rossell M.D., Erni R. Growth and characterization of CNT–TiO2 heterostructures. Beilstein J. Nanotechnol. 2014;5:946–955. doi: 10.3762/bjnano.5.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Kumar S.G., Koteswara Rao K.S. Polymorphic phase transition among the titania crystal structures in solution based approach: From precursor chemistry to nucleation process. Nanoscale. 2014;6:11574–11632. doi: 10.1039/C4NR01657B. [DOI] [PubMed] [Google Scholar]
- 194.Padmanabhan S.C., Pillai S.C., Colreavy J., Balakrishnan S., McCormack D.E., Perova T.S., Gunko Y., Hinder S.J., Kelly J.M. A simple sol gel processing for the development of high-temperature stable photoactive anatase titania. Chem. Mater. 2007;19:4474–4481. doi: 10.1021/cm070980n. [DOI] [Google Scholar]
- 195.Behnajady M.A., Eskandarloo H. Preparation of TiO2 nanoparticles by the sol–gel method under different pH conditions and modeling of photocatalytic activity by artificial neural network. Res. Chem. Intermed. 2015;41:2001–2017. doi: 10.1007/s11164-013-1327-5. [DOI] [Google Scholar]
- 196.Lusvard G., Barani C., Giubertoni F., Paganelli G. Synthesis and characterization of TiO2 nanoparticles for the reduction of water pollutants. Materials. 2017;10:1208. doi: 10.3390/ma10101208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Sharma M., Pathak M., Kapoor P.N. The sol-gel method: Pathway to ultrapure and homogeneous mixed metal oxide nanoparticles. Asian J. Chem. 2018;30:1405–1412. doi: 10.14233/ajchem.2018.20845. [DOI] [Google Scholar]
- 198.Marami M.B., Farahmandjoum M., Khoshnevisan B. Sol–gel synthesis of Fe-doped TiO2 nanocrystals. J. Electron. Mater. 2018;47:3741–3748. doi: 10.1007/s11664-018-6234-5. [DOI] [Google Scholar]
- 199.Mogal S.I., Gandhi V.G., Mishra M., Tripathi S., Joshi P.A., Shah D.O. Single-step synthesis of silver-doped titanium dioxide: Influence of silver on structural, textural, and photocatalytic properties. Ind. Eng. Chem. Res. 2014;53:5749–5758. doi: 10.1021/ie404230q. [DOI] [Google Scholar]
- 200.Katoueizadeh E., Zebarjad S.M., Janghorban K. Synthesis and enhanced visible-light activity of N-doped TiO2 nano-additives applied over cotton textiles. J. Mater. Res. Technol. 2018;7:204–211. doi: 10.1016/j.jmrt.2017.05.011. [DOI] [Google Scholar]
- 201.Nolan T., Synnot D., Seery M., Hider S., Van Wassenhaven A., Pillai S. Effect of N-doping on the photocatalytic activity of sol-gel TiO2. J. Hazard. Mater. 2012;211–212:88–94. doi: 10.1016/j.jhazmat.2011.08.074. [DOI] [PubMed] [Google Scholar]
- 202.Zane A., Zuo R., Villamena F.A., Rockenbauer A., Foushee A.M., Flores K., Dutta P.K., Nagy A. Biocompatibility and antibacterial activity of nitrogen-doped titanium dioxide nanoparticles for use in dental resin formulations. Int. J. Nanomed. 2016;11:6459–6470. doi: 10.2147/IJN.S117584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Qin H.L., Gu G.B., Liu S. Preparation of nitrogen-doped titania with visible-light activity and its application. C. R. Chim. 2008;11:95–100. doi: 10.1016/j.crci.2007.06.004. [DOI] [Google Scholar]
- 204.Livraghi S., Chierotti M.R., Giamello E., Magnacca G., Paganini M.C., Cappelletti G., Bianchi C.L. Nitrogen-doped titanium dioxide active in photocatalytic reactions with visible light: A multi-technique characterization of differently prepared materials. J. Phys. Chem. C. 2008;112:17244–17252. doi: 10.1021/jp803806s. [DOI] [Google Scholar]
- 205.Yanagisawa K., Ovenstone J. Crystallization of anatase from amorphous titania using the hydrothermal technique: Effects of starting material and temperature. J. Phys. Chem. B. 1999;103:7781–7787. doi: 10.1021/jp990521c. [DOI] [Google Scholar]
- 206.Stride J.A., Tuong N.T. Controlled Synthesis of Titanium Dioxide Nanostructures. In: Nowotny M.K., Nowotny J., editors. Solid State Phenomena: Solid State Chemistry and Photocatalysis of Titanium Dioxide. Vol. 162. Trans Tech Publications Ltd.; Zurich, Switzerland: 2010. pp. 261–294. [Google Scholar]
- 207.Morais A., Longo C., Araujo J., Barroso M. Nanocrystalline anatase TiO2/reduced graphene oxide composite films as photoanodes for photoelectrochemical water splitting studies: The role of the reduced graphene oxide. Phys. Chem. Chem. Phys. 2015;18:2608. doi: 10.1039/C5CP06707C. [DOI] [PubMed] [Google Scholar]
- 208.De Marco L., Manca M., Giannuzzi R., Malara F., Melcarne G., Ciccarella G., Zama I., Cingolani R., Gigli G. Novel preparation method of TiO2-nanorod-based photoelectrodes for dye-sensitized solar cells with improved light-harvesting efficiency. J. Phys. Chem. C. 2010;114:4228–4236. doi: 10.1021/jp910346d. [DOI] [Google Scholar]
- 209.Falentin-Daudré C., Baumann J.S., Migonney V., Spadavecchia J. Highly crystalline sphere and rod-shaped TiO2 nanoparticles: A facile route to bio-polymer grafting. Front. Lab. Med. 2017;1:217–223. doi: 10.1016/j.flm.2017.12.003. [DOI] [Google Scholar]
- 210.Roca R.A., Leite E.R. Size and shape tailoring of titania nanoparticles synthesized by solvothermal route in different solvents. J. Am. Ceram. Soc. 2013;96:96–102. doi: 10.1111/jace.12078. [DOI] [Google Scholar]
- 211.Zhang S., Li Y., Li M. Facile Synthesis of anatase TiO2 nanospheres as anode materials for sodium-ion batteries. JOM. 2018;70:1411–1415. doi: 10.1007/s11837-018-2943-8. [DOI] [Google Scholar]
- 212.Dubey R.S., Krishnamurthy K.V., Singh S. Experimental studies of TiO2 nanoparticles synthesized by sol-gel and solvothermal routes for DSSCs application. Results Phys. 2019;14:102390. doi: 10.1016/j.rinp.2019.102390. [DOI] [Google Scholar]
- 213.Tjong S.C., Hoffman R.W., Yeager E.B. Electron and ion spectroscopic iron-chromium alloys. J. Electrochem. Soc. 1982;129:1662–1668. doi: 10.1149/1.2124232. [DOI] [Google Scholar]
- 214.Tjong S.C., Yeager E. ESCA and SIMS studies of the passive film on iron. J. Electrochem. Soc. 1981;128:2251–2254. doi: 10.1149/1.2127229. [DOI] [Google Scholar]
- 215.Louarn G., Salou L., Hoonaert A., Layrolle P. Nanostuctured surface coatings for titanium alloy implants. J. Mater. Res. 2019;34:1892–1899. doi: 10.1557/jmr.2019.39. [DOI] [Google Scholar]
- 216.Kulkarni M., Mazare A., Gongadze E., Perutkova S., Kralj-Iglič V., Milosev I., Schmuki P., Iglic A., Mozetic M. Titanium nanostructures for biomedical applications. Nanotechnology. 2015;26:062002. doi: 10.1088/0957-4484/26/6/062002. [DOI] [PubMed] [Google Scholar]
- 217.Minagar S., Wang J., Berndt C.C., Ivanova E.P., Wen C. Cell response of anodized nanotubes on titanium and titanium alloys. J. Biomed. Mater. Res. Part A. 2013;101:2726–2739. doi: 10.1002/jbm.a.34575. [DOI] [PubMed] [Google Scholar]
- 218.Su E., Justin D.F., Pratt C.R., Sarin V.K., Nguyen V.S., Oh S., Jin S. Effects of titanium nanotubes on the osseointegration, cell differentiation, mineralisation and antibacterial properties of orthopaedic implant surfaces. Bone Jt. J. 2018;100(Suppl. S1A):9–16. doi: 10.1302/0301-620X.100B1.BJJ-2017-0551.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Fu Y., Mo A. A Review on the electrochemically self-organized titania nanotube arrays: Synthesis, modifications, and biomedical applications. Nanoscale Res. Lett. 2018;13:187. doi: 10.1186/s11671-018-2597-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Liu N., Chen X., Zhang J., Schwank J.W. A review on TiO2-based nanotubes synthesized via hydrothermal method: Formation mechanism, structure modification, and photocatalytic applications. Catal. Today. 2014;225:34–51. doi: 10.1016/j.cattod.2013.10.090. [DOI] [Google Scholar]
- 221.Ahmad A., Haq E.U., Akhtar W., Arshad M., Ahmad Z. Synthesis and characterization of titania nanotubes by anodizing of titanium in fluoride containing electrolytes. Appl. Nanosci. 2017;7:701–710. doi: 10.1007/s13204-017-0608-5. [DOI] [Google Scholar]
- 222.Macak J.M., Tsuchiya H., Ghicov A., Yasuda K., Hahn R., Bauer S., Schmuki P. TiO2 nanotubes: Self-organized electrochemical formation, properties and applications. Curr. Opin. Solid State Mater. Sci. 2007;11:3–18. doi: 10.1016/j.cossms.2007.08.004. [DOI] [Google Scholar]
- 223.Do T.C., Nguyen T.Q., Nguyen K.T., Le P.H. TiO2 and Au-TiO2 nanomaterials for rapid photocatalytic degradation of antibiotic residues in aquaculture wastewater. Materials. 2019;12:2434. doi: 10.3390/ma12152434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Lai C.W. Surface morphology and growth of anodic titania nanotubes films: Photoelectrochemical water splitting studies. J. Nanomater. 2015;2015:820764. doi: 10.1155/2015/820764. [DOI] [Google Scholar]
- 225.Nguyen T.L., Ung T.D., Nguyen Q.L. Non-chapped, vertically well aligned titanium dioxide nanotubes fabricated by electrochemical etching. Adv. Nat. Sci. Nanosci. Nanotechnol. 2014;5:025016. doi: 10.1088/2043-6262/5/2/025016. [DOI] [Google Scholar]
- 226.Lan M.Y., Liu C.P., Huang H.H., Lee S.W. Both enhanced biocompatibility and antibacterial activity in Ag-decorated TiO2 nanotubes. PLoS ONE. 2013;8:e75364. doi: 10.1371/journal.pone.0075364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Li Y., Liao C., Tjong S.C. Electrospun polyvinylidene fluoride-based fibrous scaffolds with piezoelectric characteristics for bone and neural tissue engineering. Nanomaterials. 2019;9:952. doi: 10.3390/nano9070952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Al-Enizi A.M., Zagho M.M., Elzatahry A.A. Polymer-based electrospun nanofibers for biomedical applications. Nanomaterials. 2018;8:259. doi: 10.3390/nano8040259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Feng S., Zhang F., Ahmed S., Liu Y. Physico-mechanical and antibacterial properties of PLA/TiO2 composite materials synthesized via electrospinning and solution casting processes. Coatings. 2019;9:525. doi: 10.3390/coatings9080525. [DOI] [Google Scholar]
- 230.Xue J., Wu T., Dai Y., Xia Y. Electrospinning and electrospun nanofibers: Methods, materials, and applications. Chem. Rev. 2019;19:5298–5415. doi: 10.1021/acs.chemrev.8b00593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Tekmen C., Susio A., Cocen U. Titania nanofibers prepared by electrospinning. Mater. Lett. 2008;62:4470–4472. doi: 10.1016/j.matlet.2008.08.002. [DOI] [Google Scholar]
- 232.Mondal K. Recent advances in the synthesis of metal oxide nanofibers and their environmental remediation applications. Inventions. 2017;2:9. doi: 10.3390/inventions2020009. [DOI] [Google Scholar]
- 233.Albetran H., O’Connor B.H., Low I.M. Effect of calcination on band gaps for electrospun titania nanofibers heated in air–argon mixtures. Mater. Des. 2016;92:480–485. doi: 10.1016/j.matdes.2015.12.044. [DOI] [Google Scholar]
- 234.Chapman B.S., Mishra S.R., Tracy J.B. Direct electrospinning of titania nanofibers with ethanol. Dalton Trans. 2019;48:12822–12827. doi: 10.1039/C9DT01872G. [DOI] [PubMed] [Google Scholar]
- 235.Pan X., Yang M.Q., Fu X., Zhang N. Defective TiO2 with oxygen vacancies: Synthesis, properties and photocatalytic applications. Nanoscale. 2013;5:3601. doi: 10.1039/c3nr00476g. [DOI] [PubMed] [Google Scholar]
- 236.Nasr M., Balme S., Eid C., Habchi R., Miele P., Bechelany M. Enhanced visible-light photocatalytic performance of electrospun rGO/TiO2 composite nanofibers. J. Phys. Chem. C. 2017;121:261–269. doi: 10.1021/acs.jpcc.6b08840. [DOI] [Google Scholar]
- 237.Kiwi J., Rtimi S., Sanjines R., Pulgarin C. TiO2 and TiO2-doped films able to kill bacteria by contact: New evidence for the dynamics of bacterial inactivation in the dark and under light irradiation. Int. J. Photoenergy. 2014;2014:785037. doi: 10.1155/2014/785037. [DOI] [Google Scholar]
- 238.Li Y., Zhang W., Niu J., Chen Y. Mechanism of photogenerated reactive oxygen species and correlation with the antibacterial properties of engineered metal-oxide nanoparticles. ACS Nano. 2012;6:5164–5173. doi: 10.1021/nn300934k. [DOI] [PubMed] [Google Scholar]
- 239.Tsai T.M., Chang H.H., Chang K.C., Liu Y.L., Tseng C.C. A comparative study of the bactericidal effect of photocatalytic oxidation by TiO2 on antibiotic-resistant and antibiotic-sensitive bacteria. J. Chem. Technol. Biotechnol. 2010;85:1642–1653. doi: 10.1002/jctb.2476. [DOI] [Google Scholar]
- 240.Kubacka A., Diez M.S., Rojo D., Bargiela R., Ciordia S., Zapico I., Albar J.P., Barbas C., dos Santos V.A., Fernández-García M., et al. Understanding the antimicrobial mechanism of TiO2-based nanocomposite films in a pathogenic bacterium. Sci. Rep. 2015;4:4134. doi: 10.1038/srep04134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Vatansever F., de Melo W.C., Avci P., Vecchio D., Sadasivam M., Gupta A., Chandran R., Karimi M., Parizotto N.A., Yin R., et al. Antimicrobial strategies centered around reactive oxygen species—Bactericidal antibiotics, photodynamic therapy and beyond. FEMS Microbiol. Rev. 2013;37:955–989. doi: 10.1111/1574-6976.12026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Sheng H., Nakamura K., Kanno T., Sasaki K., Niwano Y. Bactericidal effect of photolysis of H2O2 in combination with sonolysis of water via hydroxyl radical generation. PLoS ONE. 2015;10:e0132445. doi: 10.1371/journal.pone.0132445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Michels H.T., Keevil C.W., Salgado C.D., Schmidt M.G. From laboratory research to a clinical trial: Copper alloy surfaces kill bacteria and reduce hospital-acquired infections. Herd. 2015;9:64–79. doi: 10.1177/1937586715592650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Rtimi S., Pulgarin C., Kiwi J. Recent developments in accelerated antibacterial inactivation on 2D Cu-titania surfaces under indoor visible light. Coatings. 2017;7:20. doi: 10.3390/coatings7020020. [DOI] [Google Scholar]
- 245.Moongraksathum B., Shang J.Y., Chen Y.W. Photocatalytic antibacterial effectiveness of Cu-doped TiO2 thin film prepared via the peroxo sol-gel method. Catalysts. 2018;8:352. doi: 10.3390/catal8090352. [DOI] [Google Scholar]
- 246.Leyland N.S., Podporska-Carroll J., Browne J., Hinder S.J., Quilty B., Pillai S.C. Highly efficient F, Cu doped TiO2 anti-bacterial visible light active photocatalytic coatings to combat hospital-acquired infections. Sci. Rep. 2016;6:24770. doi: 10.1038/srep24770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Yadav H.M., Odari S.V., Koli V.B., Mali S.S., Hong C.K., Pawar S.H., Delekar S.D. Preparation and characterization of copper-doped anatase TiO2 nanoparticles with visible light photocatalytic antibacterial activity. J. Photochem. Photobiol. A. 2014;280:32–38. doi: 10.1016/j.jphotochem.2014.02.006. [DOI] [Google Scholar]
- 248.Yadav H.M., Odari S.V., Bohara R.A., Mali S.S., Pawar S.H., Delekar S.D. Synthesis and visible light photocatalytic antibacterial activity of nickel-doped TiO2 nanoparticles against Gram-positive and Gram-negative bacteria. J. Photochem. Photobiol. A. 2014;294:130–136. doi: 10.1016/j.jphotochem.2014.07.024. [DOI] [Google Scholar]
- 249.Vollmer W., Blanot D., De Pedro M.A. Peptidoglycan structure and architecture. FEMS Microbiol. Rev. 2008;32:149–167. doi: 10.1111/j.1574-6976.2007.00094.x. [DOI] [PubMed] [Google Scholar]
- 250.Bertani B., Ruiz N. Function and biogenesis of lipopolysaccharides. EcoSal Plus. 2018 doi: 10.1128/ecosalplus.ESP-0001-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Botos I., Noinaj N., Buchanan S.K. Insertion of proteins and lipopolysaccharide into the bacterial outer membrane. Philos. Trans. R. Soc. B. 2017;372:20160224. doi: 10.1098/rstb.2016.0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Polissi A., Sperandeo P. The lipopolysaccharide export pathway in Escherichia coli: Structure, organization and regulated assembly of the Lpt machinery. Mar. Drugs. 2014;12:1023–1042. doi: 10.3390/md12021023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Dakal T.C., Kumar A., Majumdar R.S., Yadav V. Mechanistic basis of antimicrobial actions of silver nanoparticles. Front. Microbiol. 2016;7:1831. doi: 10.3389/fmicb.2016.01831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Hsueh Y.H., Lin K.S., Ke W.J., Hsieh C.T., Chiang C.L., Tzou D.Y., Liu S.T. The antimicrobial properties of silver nanoparticles in Bacillus subtilis are mediated by released Ag+ ions. PLoS ONE. 2015;10:e0144306. doi: 10.1371/journal.pone.0144306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Riaz Ahmed K.B., Nagy A.M., Brown R.P., Zhang Q., Malghan S.G., Goering P.L. Silver nanoparticles: Significance of physicochemical properties and assay interference on the interpretation of in vitro cytotoxicity studies. Toxicol. In Vitro. 2017;38:179–192. doi: 10.1016/j.tiv.2016.10.012. [DOI] [PubMed] [Google Scholar]
- 256.Gupta K., Singh R.P., Pandey A., Pandey A. Photocatalytic antibacterial performance of TiO2 and Ag-doped TiO2 against S. aureus, P. aeruginosa and E. coli. Beilstein J. Nanotechnol. 2013;4:345–351. doi: 10.3762/bjnano.4.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Garvey M., Panaitescu E., Menon L., Byrne C., Dervin S., Hinder S.J., Pillai S.C. Titania nanotube photocatalysts for effectively treating waterborne microbial pathogens. J. Catal. 2016;344:631–639. doi: 10.1016/j.jcat.2016.11.004. [DOI] [Google Scholar]
- 258.Nguyen N.T., Ozkan S., Tomanec O., Zboril A., Schmuki P. Spaced titania nanotube arrays allow the construction of an efficient N-doped hierarchical structure for visible light harvesting. ChemistryOpen. 2018;7:131–135. doi: 10.1002/open.201700199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Podporska-Carroll J., Panaitescu E., Quilty B., Wang L., Menon L., Pillai S.C. Antimicrobial properties of highly efficient photocatalytic TiO2 nanotubes. Appl. Catal. B Environ. 2015;176:70–75. doi: 10.1016/j.apcatb.2015.03.029. [DOI] [Google Scholar]
- 260.Hajjaji A., Elabidi M., Trabelsi K., Assadi A.A., Bessais B., Rtimi S. Bacterial adhesion and inactivation on Ag decorated TiO2-nanotubes under visible light: Effect of the nanotubes geometry on the photocatalytic activity. Colloids Surf. B. 2018;70:92–98. doi: 10.1016/j.colsurfb.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 261.Uhm S.H., Song D.H., Kwon J.S., Lee S.B., Han J.G., Kim K.N. Tailoring of antibacterial Ag nanostructures on TiO2 nanotube layers by magnetron sputtering. J. Biomed. Mater. Res. Part B. 2014;102:592–603. doi: 10.1002/jbm.b.33038. [DOI] [PubMed] [Google Scholar]
- 262.Dunnill C.W., Aiken Z.A., Kafizas A., Pratten J., Wilson M., Morgan D.J., Parkin I.P. White light induced photocatalytic activity of sulfur-doped TiO2 thin films and their potential for antibacterial application. J. Mater. Chem. 2009;19:8747–8754. doi: 10.1039/b913793a. [DOI] [Google Scholar]
- 263.Xue X., Wang Y., Yang H. Preparation and characterization of boron-doped titania nano-materials with antibacterial activity. Appl. Surf. Sci. 2013;264:94–99. doi: 10.1016/j.apsusc.2012.09.128. [DOI] [Google Scholar]
- 264.Sun D.S., Kau J.H., Huang H.H., Tseng Y.H., Wu W.S., Chang H.H. Antibacterial properties of visible-light-responsive carbon-containing titanium dioxide photocatalytic nanoparticles against anthrax. Nanomaterials. 2016;6:237. doi: 10.3390/nano6120237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.He P., Tao J., Huang X., Xue J. Preparation and photocatalytic antibacterial property of nitrogen doped TiO2 nanoparticles. J. Sol-Gel Sci. Technol. 2013;68:213–218. doi: 10.1007/s10971-013-3154-y. [DOI] [Google Scholar]
- 266.Fagan R., McCormack D.E., Hinder S., Pillai S.C. Improved high temperature stability of anatase TiO2 photocatalysts by N, F, P co-doping. Mater. Des. 2016;96:44–53. doi: 10.1016/j.matdes.2016.01.142. [DOI] [Google Scholar]
- 267.Li C., Sun Z., Ma R., Xue Y., Zheng S. Fluorine doped anatase TiO2 with exposed reactive (001) facets supported on porous diatomite for enhanced visible-light photocatalytic activity. Microporous Mesoporous Mater. 2017;243:281–290. doi: 10.1016/j.micromeso.2017.02.053. [DOI] [Google Scholar]
- 268.Hamilton J.W.J., Byrne J.A., Dunlop P.S.M., Dionysiou D.D., Pelaez M., O’Shea K., Synnott D., Pillai S.C. Evaluating the mechanism of visible light activity for N,F-TiO2 using photoelectrochemistry. J. Phys. Chem. C. 2014;118:12206–12215. doi: 10.1021/jp4120964. [DOI] [Google Scholar]
- 269.Abdullah A.M., Gracia-Pinilla M.A., Pillai S.C., O’Shea K. UV and visible light-driven production of hydroxyl radicals by reduced forms of N, F, and P codoped titanium dioxide. Molecules. 2019;24:2147. doi: 10.3390/molecules24112147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Milosevic I., Jayaprakash A., Greenwood B., van Driel B., Rtimi S., Bowen P. Synergistic effect of fluorinated and N doped TiO2 nanoparticles leading to different microstructure and enhanced photocatalytic bacterial inactivation. Nanomaterials. 2017;7:391. doi: 10.3390/nano7110391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Milosevic I., Rtimi S., Jayaprakash A., van Driel B., Greenwood B., Aimable A., Senna M., Bowen P. Synthesis and characterization of fluorinated anatase nanoparticles and subsequent N-doping for efficient visible light activated photocatalysis. Colloids Surf. B. 2018;171:445–450. doi: 10.1016/j.colsurfb.2018.07.035. [DOI] [PubMed] [Google Scholar]
- 272.Akhavan O., Ghaderi E. Toxicity of graphene and graphene oxide nanowalls against bacteria. ACS Nano. 2010;4:5731–5736. doi: 10.1021/nn101390x. [DOI] [PubMed] [Google Scholar]
- 273.Akhavan O., Ghaderi E., Esfandiar A. Wrapping bacteria by graphene nanosheets for isolation from environment, reactivation by sonication and inactivation by near-infrared irradiation. J. Phys. Chem. B. 2011;115:6279–6288. doi: 10.1021/jp200686k. [DOI] [PubMed] [Google Scholar]
- 274.Lu X., Feng X., Werber J.R., Chu C., Zucker I., Kim J.H., Osuji J.O., Elimelech M. Enhanced antibacterial activity through the controlled alignment of graphene oxide nanosheets. Proc. Natl. Acad. Sci. USA. 2017;114:E9793–E9801. doi: 10.1073/pnas.1710996114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Linklater D.P., Baulin V.A., Juodkazis S., Ivanova E.P. Mechano-bactericidal mechanism of graphene nanomaterials. Interface Focus. 2018;8:20170060. doi: 10.1098/rsfs.2017.0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Akhavan O., Ghaderi E. Photocatalytic reduction of graphene oxide nanosheets on TiO2 thin film for photoinactivation of bacteria in solar light irradiation. J. Phys. Chem. C. 2009;113:20214–20220. doi: 10.1021/jp906325q. [DOI] [Google Scholar]
- 277.Nica I.C., Stan M.S., Popa M., Chifiriuc M.C., Pircalabioru G.G., Lazar V., Dumitrescu I., Diamandescu L., Feder M., Baibarac M., et al. Development and biocompatibility evaluation of photocatalytic TiO2/reduced graphene oxide-based nanoparticles designed for self-cleaning purposes. Nanomaterials. 2017;7:279. doi: 10.3390/nano7090279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Andrews J.M. Determination of minimum inhibitory concentration. J. Antimicrob. Chemother. 2002;49:1049. doi: 10.1093/jac/dkf083. [DOI] [PubMed] [Google Scholar]
- 279.Macia M.D., Rojo-Molinero E., Oliver A. Antimicrobial susceptibility testing in biofilm-growing bacteria. Clin. Microbiol. Infect. 2014;20:981–990. doi: 10.1111/1469-0691.12651. [DOI] [PubMed] [Google Scholar]
- 280.Tjong S.C. Structural and mechanical properties of polymer nanocomposites. Mater. Sci. Eng. R Rep. 2006;53:73–197. doi: 10.1016/j.mser.2006.06.001. [DOI] [Google Scholar]
- 281.Jamróz E., Kulawik P., Kopel P. The effect of nanofillers on the functional properties of biopolymer-based films: A review. Polymers. 2019;11:675. doi: 10.3390/polym11040675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Gao T., Jiang M., Liu X., You G., Wang W., Sun Z., Ma A., Chen J. Patterned polyvinyl alcohol hydrogel dressings with stem cells seeded for wound healing. Polymers. 2019;11:171. doi: 10.3390/polym11010171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Mochane M.J., Motsoeneng T.S., Sadiku E.R., Mokhena T.C., Sefadi J.S. Morphology and properties of electrospun PCL and its composites for medical applications: A mini review. Appl. Sci. 2019;9:2205. doi: 10.3390/app9112205. [DOI] [Google Scholar]
- 284.Liao C., Li Y., Tjong S.C. Antibacterial activities of aliphatic polyester nanocomposites with silver nanoparticles and/or graphene oxide sheets. Nanomaterials. 2019;9:1102. doi: 10.3390/nano9081102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Rescek A., Scetar M., Hrnjak-Murgić Z., Dimitrov N., Galic K. Polyethylene/polycaprolactone nanocomposite films for food Packaging modified with magnetite and casein: Oxygen barrier, mechanical, and thermal properties. Polym. Plast. Technol. 2016;55:1450–1459. doi: 10.1080/03602559.2016.1163606. [DOI] [Google Scholar]
- 286.Xing Y., Li X., Zhang L., Xu Q., Che Z., Li W., Bai Y., Li K. Effect of TiO2 nanoparticles on the antibacterial and physical properties of polyethylene-based film. Prog. Org. Coat. 2012;73:219–224. doi: 10.1016/j.porgcoat.2011.11.005. [DOI] [Google Scholar]
- 287.Munoz-Bonilla A., Cerrada M.L., Fernández-Garcia M., Kubacla A., Ferrer M., Fernandez-Garcia M. Biodegradable polycaprolactone-titania nanocomposites: Preparation, characterization and antimicrobial properties. Int. J. Mol. Sci. 2013;14:9249–9266. doi: 10.3390/ijms14059249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Raut A.V., Yadav H.M., Gnanamani A., Pushpavanam S., Pawar S.H. Synthesis and characterization of chitosan-TiO2: Cu nanocomposite and their enhanced antimicrobial activity with visible light. Colloids Surf. B. 2011;148:566–575. doi: 10.1016/j.colsurfb.2016.09.028. [DOI] [PubMed] [Google Scholar]
- 289.Zhang X., Xiao G., Wang Y., Zhao Y., Su H., Tan T. Preparation of chitosan-TiO2 composite film with efficient antimicrobial activities under visible light for food packaging applications. Carbohydr. Polym. 2017;169:101–107. doi: 10.1016/j.carbpol.2017.03.073. [DOI] [PubMed] [Google Scholar]
- 290.Li J., Xie B., Xia K., Li Y., Han J., Zhao C. Enhanced antibacterial activity of silver doped titanium dioxide-chitosan composites under visible light. Materials. 2018;11:1403. doi: 10.3390/ma11081403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Jbeli A., Hamden Z., Bouattour S., Ferraria A.M., Conceicao D.S., Vieira Ferreira L.F., Chehimi M.M., do Rego A.M., Rei Vilar M., Boufi S. Chitosan-Ag-TiO2 films: An effective photocatalyst under visible light. Carbohydr. Polym. 2018;199:31–40. doi: 10.1016/j.carbpol.2018.06.122. [DOI] [PubMed] [Google Scholar]
- 292.Saravanan R., Aviles J., Gracia F., Mosquera E., Gupta V.K. Crystallinity and lowering band gap induced visible light photocatalytic activity of TiO2/CS (chitosan) nanocomposites. Int. J. Biol. Macromol. 2018;109:1239–1245. doi: 10.1016/j.ijbiomac.2017.11.125. [DOI] [PubMed] [Google Scholar]
- 293.Zhao Y., Tao C., Xiao G., Xu H. Controlled synthesis and wastewater treatment of Ag2O/TiO2 modified chitosan-based photocatalytic film. RSC Adv. 2017;7:11211–11221. doi: 10.1039/C6RA27295A. [DOI] [Google Scholar]
- 294.Hamden Z., Bouattour S., Ferraria A.M., Ferreira D.P., Vieira Ferreira L.F., Botelho do Rego A.M., Boufi S. In situ generation of TiO2 nanoparticles using chitosan as a template and their photocatalytic activity. J. Photochem. Photobiol. A. 2016;321:211–222. doi: 10.1016/j.jphotochem.2016.02.008. [DOI] [Google Scholar]
- 295.Kaewklin P., Siripatrawan U., Suwanagul A., Lee Y.S. Active packaging from chitosan-titanium dioxide nanocomposite film for prolonging storage life of tomato fruit. Int. J. Biol. Macromol. 2018;112:523–529. doi: 10.1016/j.ijbiomac.2018.01.124. [DOI] [PubMed] [Google Scholar]
- 296.Lavengood S.L., Zhang M. Chitosan-based scaffolds for bone tissue engineering. J. Mater. Chem. B. 2014;7:3161–3184. doi: 10.1039/c4tb00027g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Callewaert C., De Maeseneire E., Kerckhof F.M., Verliefde A., Van de Wiele T., Boon N. Microbial odor profile of polyester and cotton clothes after a fitness session. Appl. Environ. Microbiol. 2014;80:6611–6619. doi: 10.1128/AEM.01422-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Zahid M., Papadopoulou E.L., Suarato G., Binas V.D., Kiriakidis G., Gounaki I., Moira O., Venieri D., Bayer I.S., Athanassiou A. Fabrication of visible light-induced antibacterial and self-cleaning cotton fabrics using manganese doped TiO2 nanoparticles. ACS Appl. Bio Mater. 2018;1:1154–1164. doi: 10.1021/acsabm.8b00357. [DOI] [PubMed] [Google Scholar]
- 299.Pfang P.G., García-Cañete J., García-Lasheras J., Blanco A., Aunon A., Parron-Cambero R., Macías-Valcayo A., Esteban J. Orthopedic implant-associated infection by multidrug resistant Enterobacteriaceae. J. Clin. Med. 2019;8:220. doi: 10.3390/jcm8020220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Li Y., Yang Y., Li R., Tang X., Guo D., Qing Y., Qin Y. Enhanced antibacterial properties of orthopedic implants by titanium nanotube surface modification: A review of current techniques. Int. J. Nanomed. 2019;14:7217–7236. doi: 10.2147/IJN.S216175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Gollwitzer H., Haenie M., Mittelmeier W., Heidenau F., Harrasser N. A biocompatible sol-gel derived titania coating for medical implants with antibacterial modification by copper integration. AMB Express. 2018;8:24. doi: 10.1186/s13568-018-0554-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Tsou H.K., Hsieh P.Y., Chi M.H., Chung C.J., He J.L. Improved osteoblast compatibility of medical-grade polyetheretherketone using arc ionplated rutile/anatase titanium dioxide films for spinal implants. J. Biomed. Mater. Res. Part A. 2012;100:2787–2792. doi: 10.1002/jbm.a.34215. [DOI] [PubMed] [Google Scholar]
- 303.Radtke A., Topolski A., Jędrzejewski T., Kozak W., Sadowska B., Więckowska-Szakiel M., Szubka M., Talik E., Nielsen L.P., Piszczek P. The bioactivity and photocatalytic properties of titania nanotube coatings produced with the use of the low-potential anodization of Ti6Al4V alloy surface. Nanomaterials. 2017;7:197. doi: 10.3390/nano7080197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Xu L., Lv K., Yu W.Q. Effect of TiO2 nanotube layers thickness on periodontal ligament cells. Dentistry. 2016;6:369. doi: 10.4172/2161-1122.1000369. [DOI] [Google Scholar]
- 305.Piszczek P., Lewandowska Z., Radtke A., Jędrzejewski T., Kozak W., Sadowska B., Szubka M., Talik E., Flori F. Biocompatibility of titania nanotube coatings enriched with silver nanograins by chemical vapor deposition. Nanomaterials. 2017;7:274. doi: 10.3390/nano7090274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Laux P., Tentschert J., Riebeling C., Braeuning A., Creutzenberg O., Epp A., Fessard V., Haas K.H., Haase A., Hund-Rinke K., et al. Nanomaterials: Certain aspects of application, risk assessment and risk communication. Arch. Toxicol. 2018;92:121–141. doi: 10.1007/s00204-017-2144-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Wadhwa S., Rea C., O’Hare P., Mathur A., Roy S.S., Dunlop P.S.M., Byrne J.A., Burke G., Meenan B., McLaughlin J.A. Comparative in vitro cytotoxicity study of carbon nanotubes and titania nanostructures on human lung epithelial cells. J. Hazard. Mater. 2011;191:56–61. doi: 10.1016/j.jhazmat.2011.04.035. [DOI] [PubMed] [Google Scholar]
- 308.Mohamed M.A., Torabi A., Paulose M., Sakthi Kumar D., Varghese O.K. Anodically grown titania nanotube induced cytotoxicity has genotoxic origins. Sci. Rep. 2017;7:41844. doi: 10.1038/srep41844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Allegri M., Bianchi M.G., Chiu M., Varet J., Costa A.L., Ortelli S., Blosi M., Bussolati O., Poland C.A., Bergamaschi E. Shape-related toxicity of titanium dioxide nanofibres. PLoS ONE. 2016;11:e0151365. doi: 10.1371/journal.pone.0151365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Holden P.A., Gardea-Torresdey J.L., Klaessig F., Turco R., Mortimer M., Hund-Rinke K., Avery D., Barcelo D., Behra R., Cohen Y., et al. Considerations of environmentally relevant test conditions for improved evaluation of ecological hazards of engineered nanomaterials. Environ. Sci. Technol. 2016;50:6124–6145. doi: 10.1021/acs.est.6b00608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Gupta R., Xie H. Nanoparticles in daily life: Applications, toxicity and regulations. J. Environ. Pathol. Toxicol. Oncol. 2018;37:209–230. doi: 10.1615/JEnvironPatholToxicolOncol.2018026009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Khezri S.M., Shariat S.M., Tabibian S. Evaluation of extracting titanium dioxide from water-based paint sludge in auto-manufacturing industries and its application in paint production. Toxicol. Ind. Health. 2013;29:697–703. doi: 10.1177/0748233711430977. [DOI] [PubMed] [Google Scholar]
- 313.Al-Kattan A., Wichser A., Vonbank R., Brunner S., Ulrich A., Zuin S., Nowack B. Release of TiO2 from paints containing pigment-TiO2 or nano-TiO2 by weathering. Environ. Sci. Process. Impacts. 2013;15:2186–2193. doi: 10.1039/c3em00331k. [DOI] [PubMed] [Google Scholar]
- 314.Kim Y. Nanowastes treatment in environmental media. Environ. Health Toxicol. 2014;29:e2014015. doi: 10.5620/eht.e2014015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Shi X., Li Z., Chen W., Qiang L., Xia J., Chen M., Zhu L., Alvarez P.J. Fate of TiO2 nanoparticles entering sewage treatment plants and bioaccumulation in fish in the receiving streams. NanoImpact. 2016;3–4:96–103. doi: 10.1016/j.impact.2016.09.002. [DOI] [Google Scholar]
- 316.Clemente Z., Castro V.L., Moura M.A., Jonsson C.M., Fraceto L.F. Toxicity assessment of TiO2 nanoparticles in zebrafish embryos under different exposure conditions. Aquat. Toxicol. 2014;147:129–139. doi: 10.1016/j.aquatox.2013.12.024. [DOI] [PubMed] [Google Scholar]
- 317.De Matteis V. Exposure to inorganic nanoparticles: Routes of entry, immune response, biodistribution and in vitro/in vivo toxicity evaluation. Toxics. 2017;5:29. doi: 10.3390/toxics5040029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Ahamed M., Khan M.A.M., Akhtar M.J., Alhadlaq H.A., Alshamshan A. Ag-doping regulates the cytotoxicity of TiO2 nanoparticles via oxidative stress in human cancer cells. Sci. Rep. 2017;7:17662. doi: 10.1038/s41598-017-17559-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Kuku G., Culha M. Investigating the origins of toxic response in TiO2 nanoparticle-treated cells. Nanomaterials. 2017;7:83. doi: 10.3390/nano7040083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Batt J., Milward M., Chapple I., Grant M., Roberts H., Addison O. TiO2 nanoparticles can selectively bind CXCL8 impacting on neutrophil chemotaxis. Eur. Cells Mater. 2018;35:13–24. doi: 10.22203/eCM.v035a02. [DOI] [PubMed] [Google Scholar]
- 321.Ribeiro A., Gemini-Piperni S., Travassos R., Lemgruber L., Silva R.C., Rossi A.L., Farina M., Anselme K., Shokuhfar T., Shahbazian-Yassar R., et al. Trojan-like internalization of anatase titanium dioxide nanoparticles by human osteoblast cells. Sci. Rep. 2016;6:23615. doi: 10.1038/srep23615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Valentini X., Absil L., Laurent G., Robbe A., Laurent S., Muller R., Legrand A., Nonclercq D. Toxicity of TiO2 nanoparticles on the NRK52E renal cell line. Mol. Cell. Toxicol. 2017;13:419–431. doi: 10.1007/s13273-017-0046-1. [DOI] [Google Scholar]
- 323.Wang Y., Cui H., Zhou J., Li F., Wang J., Chen M., Liu Q. Cytotoxicity, DNA damage, and apoptosis induced by titanium dioxide nanoparticles in human non-small cell lung cancer A549 cells. Environ. Sci. Pollut. Res. 2014;22:5519–5530. doi: 10.1007/s11356-014-3717-7. [DOI] [PubMed] [Google Scholar]
- 324.Shi H., Magaye R., Castranova V., Zhao J. Titanium dioxide nanoparticles: A review of current toxicological data. Part. Fibre Toxicol. 2013;10:15. doi: 10.1186/1743-8977-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Mottola F., Iovine C., Santonastaso M., Romeo M.L., Pacifico S., Cobellis L., Rocco L. NPs-TiO2 and lincomycin coexposure induces DNA damage in cultured human amniotic cells. Nanomaterials. 2019;9:1511. doi: 10.3390/nano9111511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Golbamak N., Rasulev B., Cassano A., Marchese Robinson R.L., Benfenati E., Leszczynski J., Cronin M.T. Genotoxicity of metal oxide nanomaterials: Review of recent data and discussion of possible mechanisms. Nanoscale. 2015;7:2154–2198. doi: 10.1039/C4NR06670G. [DOI] [PubMed] [Google Scholar]
- 327.Huerta-García E., Zepeda-Quiroz I., Sánchez-Barrera H., Colín-Val Z., Alfaro-Moreno E., Ramos-Godinez M.D.P., López-Marure R. Internalization of titanium dioxide nanoparticles is cytotoxic for H9c2 rat cardiomyoblasts. Molecules. 2018;6:1955. doi: 10.3390/molecules23081955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Yin J.J., Liu J., Ehrenshaft M., Roberts J.E., Fu P.P., Mason R.P., Zhao B. Phototoxicity of nanotitanium dioxides in HaCaT keratinocytes—Generation of reactive oxygen species and cell damage. Toxicol. Appl. Pharmacol. 2012;263:81–88. doi: 10.1016/j.taap.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Ren Y., Liu X., Geng R., Lu Q., Rao R., Tan X., Yang X., Liu W. Increased level of α2,6-sialylated glycans on HaCaT cells induced by titanium dioxide nanoparticles under UV radiation. Nanomaterials. 2018;8:253. doi: 10.3390/nano8040253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Grassian V.H., O’Shaughnessy P.T., Adamcakova-Dodd A., Pettibone J.M., Thorne P.S. Inhalation exposure study of titanium dioxide nanoparticles with a primary particle size of 2 to 5 nm. Environ. Health Perspect. 2007;115:397–402. doi: 10.1289/ehp.9469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Liu R., Yin L., Pu Y., Lian G., Zhang J., Su Y., Xia Z., Ye B. Pulmonary toxicity induced by three forms of titanium dioxide nanoparticles via intratracheal instillation in rats. Prog. Nat. Sci. 2009;19:573–579. doi: 10.1016/j.pnsc.2008.06.020. [DOI] [Google Scholar]
- 332.Wu J., Liu W., Xue C., Zhou S., Lan F., Bi L., Xu H., Yang X., Zeng F. Toxicity and penetration of TiO2 nanoparticles in hairless mice and porcine skin after subchronic dermal exposure. Toxicol. Lett. 2009;191:1–8. doi: 10.1016/j.toxlet.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 333.Disdier C., Devoy J., Cosnefroy A., Chalansonnet M., Herlin-Boime N., Brun E., Lund A., Mabondzo A. Tissue biodistribution of intravenously administrated titanium dioxide nanoparticles revealed blood-brain barrier clearance and brain inflammation in rat. Part. Fibre Toxicol. 2015;12:27. doi: 10.1186/s12989-015-0102-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Hong J., Zhang Y.Q. Murine liver damage caused by exposure to nano-titanium dioxide. Nanotechnology. 2016;27:112001. doi: 10.1088/0957-4484/27/11/112001. [DOI] [PubMed] [Google Scholar]
- 335.Jia X., Wang S., Zhou L., Sun L. The potential liver, brain, and embryo toxicity of titanium dioxide nanoparticles on mice. Nanoscale Res. Lett. 2017;12:478. doi: 10.1186/s11671-017-2242-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Jin C., Wang F., Tang Y., Zhang X., Wang J., Yang Y. Distribution of graphene oxide and TiO2-graphene oxide composite in A549 cells. Biol. Trace Elem. Res. 2014;159:393–398. doi: 10.1007/s12011-014-0027-3. [DOI] [PubMed] [Google Scholar]
- 337.Prakash J., Venkatesan M., Praksash J.S., Bharath G., Anwer S., Veluswamy P., Prema D., Venkataprasanna K.S., Venkatasubbu G.D. Investigations on the in-vivo toxicity analysis of reduced graphene oxide/TiO2 nanocomposite in zebrafish embryo and larvae (Danio rerio) Appl. Surf. Sci. 2019;481:1360–1369. doi: 10.1016/j.apsusc.2019.03.287. [DOI] [Google Scholar]
- 338.Code of Federal Regulations (Annual Edition) [(accessed on 8 January 2020)]; Available online: https://www.govinfo.gov/app/collection/cfr.
- 339.European Food Safety Authority (EFSA) Re-evaluation of titanium dioxide (E 171) as a food additive. EFSA J. 2016;14:4545. doi: 10.2903/j.efsa.2016.4545. [DOI] [Google Scholar]
- 340.Rompelberg C., Heringa M.B., van Donkersgoed G., Drijvers J., Roos A., Westenbrink S., Peters R., van Bemmel G., brand W., Oomen A.G. Oral intake of added titanium dioxide and its nanofraction from food products, food supplements and toothpaste by the Dutch population. Nanotoxicology. 2016;10:1404–1414. doi: 10.1080/17435390.2016.1222457. [DOI] [PubMed] [Google Scholar]
- 341.Hwang J.S., Yu J., Kim H.M., Oh J.M., Choi S.J. Food additive titanium dioxide and its fate in commercial foods. Nanomaterials. 2019;9:1175. doi: 10.3390/nano9081175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Skocaj M., Filipic M., Petkovic J., Novak S. Titanium dioxide in our everyday life; is it safe? Radiol. Oncol. 2011;45:227–247. doi: 10.2478/v10019-011-0037-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Heringa M.B., Peters R.J.B., Bleys R.L.A., van der Lee M.K., Tromp P.C., van Kesteren P.C., van Eijkeren J.C., Undas A.K., Oomen A.G., Bouwmeester H. Detection of titanium particles in human liver and spleen and possible health implications. Part. Fibre Toxicol. 2018;15:15. doi: 10.1186/s12989-018-0251-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Kalinska A., Jaworski S., Wierzbicki M., Golebiewski M. Silver and copper nanoparticles—An alternative in future mastitis treatment and prevention? Int. J. Mol. Sci. 2019;20:1672. doi: 10.3390/ijms20071672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Li S.H., Zhu T.X., Huang J.Y., Guo Q.Q., Chen G.Q., Lai Y.K. Durable antibacterial and UV-protective Ag/TiO2@fabrics for sustainable biomedical application. Int. J. Nanomed. 2017;12:2593–2606. doi: 10.2147/IJN.S132035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Lammel T., Sturve J. Assessment of titanium dioxide nanoparticle toxicity in the rainbow trout (Onchorynchus mykiss) liver and gill cell lines RTL-W1 and RTgill-W1 under particular consideration of nanoparticle stability and interference with fluorometric assays. NanoImpact. 2018;11:1–19. doi: 10.1016/j.impact.2018.01.001. [DOI] [Google Scholar]
- 347.Smith M.R., Fernandes J., Go Y. Redox dynamics of manganese as a mitochondrial life-death switch. Biochem. Biophys. Res. Commun. 2017;482:388–398. doi: 10.1016/j.bbrc.2016.10.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Zhang L., Sang H., Liu Y., Li J. Manganese activates caspase-9-dependent apoptosis in human bronchial epithelial cells. Hum. Exp. Toxicol. 2013;32:1155–1163. doi: 10.1177/0960327112470272. [DOI] [PubMed] [Google Scholar]