Abstract
Band 3 protein (B3p) exchanging Cl− and HCO3− through erythrocyte membranes is responsible for acid balance, ion distribution and gas exchange, thus accounting for homeostasis of both erythrocytes and entire organisms. Moreover, since B3p cross links with the cytoskeleton and the proteins underlying the erythrocyte membrane, its function also impacts cell shape and deformability, essential to adaptation of erythrocyte size to capillaries for pulmonary circulation. As growing attention has been directed toward this protein in recent years, the present review was conceived to report the most recent knowledge regarding B3p, with specific regard to its anion exchange capability under in vitro oxidative conditions. Most importantly, the role of natural antioxidants, i.e., curcumin, melatonin and Mg2+, in preventing detrimental oxidant effects on B3p is considered.
Keywords: erythrocytes, band 3 protein, anion exchange, SO4=, oxidative stress, antioxidants
1. Introduction
Oxidative stress is due to an imbalance between reactive oxygen species (ROS) formation and the antioxidant defense systems, initiating pro-oxidant processes. Both radical and non-radical oxygen-based molecules, such as hydroxyl radical (OH•), hydrogen peroxide (H2O2), singlet oxygen (O2), and superoxide (O2•) are considered to be ROS [1]. Metabolism basically implies ROS production; however, the organism has an antioxidant defense system, importantly and constantly contributing to keep redox balance at an acceptable level. Among the main endogenous antioxidants, enzymes, such as superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx), and non-enzymatic antioxidants, like reduced glutathione (GSH), are able to rapidly neutralize ROS [2]. Antioxidants assumed with the diet, such as vitamin C, vitamin E, carotenoids, minerals (Zn, Mn, Cu, Se, Mg) and polyphenols (flavonoids, phenolic acids), impact the activity of endogenous antioxidants, thus contributing to maintaining redox homeostasis [3]. One of the most critical features in human physiology is redox homeostasis, playing a pivotal role in cellular physiology, as well as in the development of several diseases. Under oxidative stress, the excessive ROS production influences several cell signaling pathways, being a common patho-physiological mechanism which underlies many chronic diseases [4,5,6]. ROS are defined as small highly reactive chemical species, with one or more unpaired electrons, able to oxidize other compounds such as proteins, membrane lipids, nucleic acids and polysaccharides, finally resulting in cell damage.
Early investigations on the human erythrocyte suggested that this cell does not respond to external stimuli and that G proteins, protein kinases, phospholipases and phosphatases contained inside erythrocytes represent non-functional vestiges of signaling pathways active in erythroid precursor cells. However, more recent evidence has demonstrated that human red blood cells are highly responsive to the external environment and that the cell’s machinery related to signaling proteins likely comprises components critically involved in the erythrocyte’s communication with the extracellular medium [7].
It has been shown that oxidative damage reduces survival and rheological properties of circulating red blood cells, affecting their shape, which strictly correlates with Band 3 protein function (B3p) [8,9]. Band 3 protein is importantly involved in maintaining erythrocyte deformability as well as ion balance, and is essential to gas exchange capability, and may account for homeostasis of both erythrocytes and the entire organism under healthy and oxidative stress conditions.
2. Role of Band 3 Protein in Erythrocytes
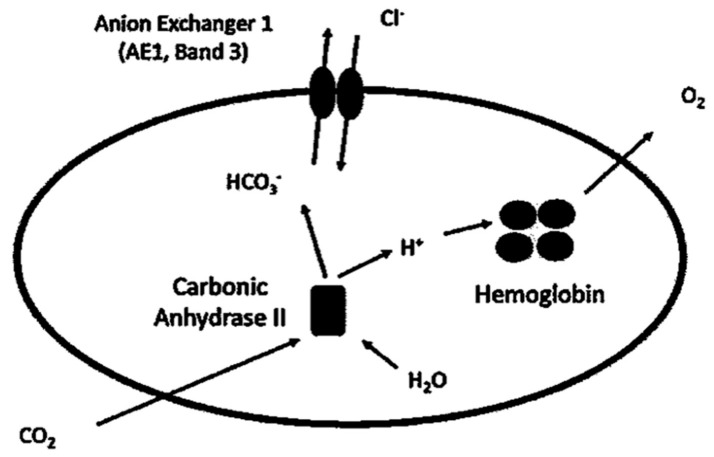
Band 3 protein, present in millions of copies on the erythrocyte membrane [10], is the most abundant integral protein, the crystal structure of which was determined in 2015 [11]. The erythrocyte membrane is made of a phospholipid bilayer with integral proteins linked to the cytoskeleton through a protein network underneath the cytoplasmatic side of membrane [12]. Band 3 protein exchanges chloride and bicarbonate (Cl−/HCO3−) anions across the plasma membrane (Figure 1), which is necessary to guarantee efficient respiration [13].
Figure 1.
Anion exchange through Band 3 protein [13]. At tissue level, CO2 diffuses through erythrocytes membrane and is converted with H2O to HCO3− and H+ by the enzyme carbonic anhydrase II. HCO3− is extruded in exchange for Cl−, while H+ is buffered by hemoglobin. At pulmonary level, the direction of ion exchange through Band 3 protein is reversed (not shown), with HCO3− entering the cell in exchange for Cl− and with CO2 finally expired by lungs.
2.1. Role in CO2 Transport
At tissue level, carbon dioxide (CO2) diffusing into erythrocytes is hydrated by intracellular carbonic anhydrase II (CAII), resulting in bicarbonate (HCO3−) production. This anion is then extruded out of the cell in electroneutral exchange for chloride (Cl−) [13,14]. At pulmonary capillary level, the system is reversed: HCO3− entering erythrocytes via B3p in exchange for Cl− is converted by CAII to CO2, which leaves the cell by permeating through the plasma membrane, to be finally expired by the lungs [15]. Human respiration requires a fast conversion between CO2 and HCO3−, with, on the one hand, CA II facilitating this reversible reaction inside erythrocytes and, on the other hand, B3p providing passage for HCO3− across the plasma membrane. These two proteins represent crucial actors of the CO2 metabolism. As intracellular H2O is needed for CO2/HCO3− conversion, aquaporin-1 (AQP1), abundantly present in erythrocytes, is considered a part of B3p complexes or involved in CO2 transport. With regard to the latter point, the Fluorescence Resonance Energy Transfer (FRET) technique has been useful for identifying the interaction between AQP1 and B3p at 8 nm distance, which falls within the range useful for dipole-dipole interaction. Importantly, interaction between B3p and AQP1 was adaptable to changes in membrane tonicity, which suggests that AQP1 involvement in response to tonicity could be associated with its function in B3p-mediated exchange. For this reason, AQP1 seems to be critically implicated in blood CO2 transport and, in turn, respiration [16]. Their primary function is to allow tissue oxygenation and CO2 elimination, but erythrocytes, flowing in the blood stream, make tissue oxygenation effective due to their biconcave shape, which corresponds to a greater area available for gas exchange and, notably, lets them adapt to the narrow capillaries.
2.2. Role in Erythrocyte Rigidity
Biconcave shape maintaining and erythrocytes size adaptation through capillaries is due to a peculiar plasma membrane arrangement, including structural proteins and cytoskeletal proteins, providing erythrocytes with unique structure.
On the other hand, membrane deformability lets erythrocytes restore their original size when flowing through larger vessels. Therefore, alterations in deformability of erythrocytes may result in changes in microcirculatory blood flow and delivery of oxygen to the tissues. In addition, other factors, including Na+/K+-ATPase, are also responsible for maintenance of erythrocytes deformability. In particular, Na+/K+-ATPase, involved in intracellular ionic homeostasis maintenance, consequently affecting cell volume regulation and, in turn, erythrocyte deformability. Reduced deformability of erythrocytes has been shown to have an index of adverse outcomes in the form of cardiovascular diseases, impact on the rheological properties of blood and possibly representing a cardiovascular risk factor [17]. Hence, erythrocyte rigidity represents an important factor affecting oxygen delivery to the tissues, since oxygen-carrying capacity decreases when erythrocytes become more rigid. In this way, increased red blood cell rigidity results in an impaired peripheral perfusion and, finally, tissue oxygenation [18]. As an integral membrane protein, B3p, in addition to gas exchange across the cell membrane, is also involved in erythrocyte mechanical and osmotic properties, such as docking of glycolytic enzymes and maintenance of cell shape [19]. Such functions are mediated by both C-terminal membrane domain for anion exchange and a N-terminal cytosolic domain, which is mainly involved in protein–protein interactions, by coupling plasma membrane to the underlying cytoskeleton, through cysteine –SH groups [20,21,22]. The link between erythrocyte membrane, embedded proteins, namely B3p, and cytoskeletal components is quite complex. According to the model proposed by Kodippili and co-authors [23], a part of B3p is linked to glycophorin A or B, Rh-associated glycoproteins, and other polypeptides bound to cytoskeleton through ankyrin bridge. Another part of B3p is linked to glycophorin C, Rh-associated glycoproteins, GLUT1, actin, protein 4.1 and spectrin, while the remaining part freely diffuses in the lipid bilayer, although interacting with spectrin. Based on this evidence, Reithmeier and co-authors [13] contributed to shedding light on the B3p structure, its arrangement in the lipid bilayer, anion binding and transport. In this regard, B3p functions, in addition to anion exchange and spectrin-actin cytoskeleton anchoring, deal with regulation of glycolytic enzymes, control of erythrocyte lifespan docking for peripheral membrane proteins, such as protein 4.1, protein 4.2, and several phosphatases and kinases. Defects or deficiencies in B3p may lead to a reduced cohesion between cytoskeleton and lipid bilayer, with a consequent loss of membrane surface area typical of hereditary spherocytosis.
Band 3 protein functions, including anion exchange capability, critically depends on a complex net of interactions between lipid bilayer, embedded proteins, hemoglobin and cytoskeleton. With regard to hemoglobin, its link to the erythrocyte membrane contributes to targeting and, in turn, removal of senescent erythrocytes by macrophages. A model has been proposed to explain this process: the link of modified forms of hemoglobin (hemichromes) to plasma membrane affects cytoskeleton integrity, resulting in clustering of B3p. Hemichrome formation, as recently reported by Welbourn and co-authors [24], seems to be involved in storage lesions of blood used for transfusions. Damage deriving from storage may possibly depend on hemichrome binding to the plasma membrane, reduction in the B3p monomer, and increase in B3p degradation products along with a loss of vesicles bearing aggregated hemoglobin, thus resulting in a decreased cell flexibility and a compromised blood flux efficiency.
The exchange of the physiological substrates HCO3−/Cl− has already been proven by a rate of exchange across the plasma membrane that is so fast (5 × 104 ions/s at 37 °C) that it cannot be easily determined [15]. Nonetheless, the efficiency of B3p can be effectively monitored by measuring the rate constant for the uptake of another anion, SO4=, slower and, hence, more easily detectable than Cl− or HCO3− transport [25]. SO4= uptake determination has been recognized as a good tool for monitoring erythrocyte homeostasis [26]. In recent years, B3p anion exchange monitoring has been widely used to assay the effect of different experimental conditions, performed by applying oxidants and toxins in vitro [8,27], or in diseases associated with membrane protein degradation [28,29,30,31]. Consequently, this review, for the first time, collects the most recent knowledge about B3p function, taking into account that red blood cells represent a suitable cell model to evaluate cell response to oxidative conditions, due to their sensitivity to oxidation and simple metabolism, with specific regard to its anion exchange capability. The purpose is to provide a broader and more complete view of the activity of the anion transport mediated by the B3p in human erythrocytes and, more importantly, to report about the beneficial effect of antioxidants on B3p exposed to oxidant conditions in vitro.
3. Anion Exchange through Band 3 Protein in Different Oxidative Experimental Conditions and the Role of Antioxidants
Oxidant molecules, transferred by the blood stream, exert their action on the cell membrane with possible effects on transport systems and, in turn, on erythrocyte homeostasis. For this reason, increasing attention has been addressed to ion transport system function and underlying pathways, with the aim of considering them as parameters to verify at which level oxidants act and, possibly, to define novel targets for drug development.
3.1. Curcumin
It has been demonstrated that acidification of the external medium (pH 6.5, range not hemolytic) can lead to a significant decrease in anion exchange capability, which is reversed by treatment with Curcumin (Table 1). Curcumin, indian saffron turmeric yellow, is a hydrophobic pigment deriving from the rhizome (turmeric) of Curcuma longa herb. It has long been used in medicine and as a food-coloring agent, and its several beneficial effects have already been shown [32,33]. Erythrocytes, when exposed to external medium at different pH values, may exhibit alteration of cytoskeletal and integral membrane proteins, including B3p, resulting in membrane destabilization and ionic imbalance, possibly provoked by oxidative damage (Figure 2). In this latter regard, a significant reduction in anion exchange efficiency has been actually detected, probably due to the acidic medium oxidizing hemoglobin, which produces ROS, with consequent damage to the cell membrane. According to this model, it is clear that perturbations of the external medium may reflect on B3p inefficiency mediated by alterations at level of cytoplasmic proteins [34]. Curcumin protects erythrocyte membranes against oxidative stress by scavenging free radicals, thus acting as an antioxidant molecule [35], similarly to other natural antioxidant compounds. The choice of verifying the efficiency of a specific ion exchanger, such as B3p, was carried out to add more knowledge about the impact of oxidative stress on cell membrane and ion transport system in an anucleate cell.
Table 1.
Rate constant (min−1) for SO4= uptake.
| Condition | Rate Constant (min−1) | Time (min) | n |
|---|---|---|---|
| Human erythrocytes (ctr) [8,34,37,40,41] | 0.066 ± 0.001 | 18 | 18 |
| Medium pH 6.5 [34] | 0.035 ± 0.001 | 29 | 6 |
| 300 μM H2O2 [37] | 0.032 ± 0.001 | 31 | 6 |
| 600 μM H2O2 [37] | 0.030 ± 0.001 | 33 | 3 |
| 1 mM Diamide [28] | 0.029 ± 0.002 | 32 | 5 |
| 0.1 mM Orthovanadate [28] | 0.031 ± 0.001 | 34 | 5 |
| 0.5 mM NEM [41] | 0.030 ± 0.001 | 33 | 5 |
| 1 mM NEM [41] | 0.033 ± 0.003 | 30 | 5 |
| 2 mM NEM [41] | 0.023 ± 0.002 | 43 | 7 |
| 10 μM Curcumin in Medium pH 6.5 [34] | 0.048 ± 0.001 | 20 | 6 |
| 10 mM Mg2+ 300 μM H2O2 [41] | 0.058 ± 0.005 | 17 | 5 |
| 10 mM Mg2+ 600 μM H2O2 [41] | 0.057 ± 0.001 | 17 | 5 |
| 10 mM Mg2+ 0.5 mM NEM [41] | 0.060 ± 0.002 | 16 | 6 |
| 10 mM Mg2+ 1 mM NEM [41] | 0.056 ± 0.002 | 18 | 6 |
| 10 mM Mg2+ 2 mM NEM [41] | 0.055 ± 0.002 | 18 | 6 |
| 100 μM Melatonin + 300 μM H2O2 [40] | 0.078 ± 0.001 | 13.5 | 10 |
| 10 μM H2O2 (Preconditioning) + 300 μM H2O2 [37] | 0.051 ± 0.001 | 19 | 4 |
Figure 2.
Light microscope observations of untreated human red blood cells [8,34,37,40,41] (A,D,G), or exposed to either pH 6.5 SO4= medium (B) or to pH 6.5 SO4= medium plus 10 μM curcumin [34] (C); human erythrocytes exposed to either 300 μM H2O2 (E), or exposed to 10–300 μM H2O2 (pre-conditioning) [37] (F) in SO4= medium incubation; human erythrocytes exposed to either 300 µM H2O2 (H), or exposed to 100 Melatonin plus 300 µM H2O2 [40] (I) in SO4= medium incubation. 400× magnification. Arrows indicate erythrocytes morphological alterations. Red blood cells shape is irregular due to spines, if compared to the untreated erythrocytes. Pictures are modified from references cited above.
3.2. Hydrogen Peroxide-Induced Oxidative Conditions and the Beneficial Effect of Antioxidants
In addition to the evidence that oxidants provide detrimental effects on membrane transport systems, the damage at the level of B3p may also result from altered interactions with cytoplasmic proteins. To better focus on this aspect, B3p efficiency has been studied when erythrocytes are exposed to hydrogen peroxide (H2O2). Hydrogen peroxide, commonly used in vitro to model oxidant conditions, is a scarcely reactive non-radical compound easily permeating plasma membranes. It promotes HO• radical formation after binding with transition metals, usually present inside the cell, leading to lipid peroxidation [36]. Therefore, red blood cells, frequently exposed to oxidative conditions [10], may be considered as a good model to study oxidative stress effects despite having potent endogenous antioxidant machineries [8]. It has been shown that H2O2 induces oxidative stress at not hemolytic concentrations and reduces B3p efficiency, not associated with a reduced cell size [37]. Morphological alterations detected under H2O2 seem not to be linked to eryptosis, which actually develops at much higher H2O2 concentrations [38]. In this latter regard, oxidative stress at membrane level may elicit phosphatidylserine (PS) exposure in erythrocytes [39], which implies loss of PS asymmetry, activation of blood coagulation and recognition of red blood cells by macrophages, to remove damaged erythrocytes from circulation.
The reduced efficiency of SO4= uptake through B3p, observed under H2O2-induced oxidative conditions, can be prevented or attenuated by a short-time pre-incubation of red blood cells with low H2O2 concentrations followed by a stronger oxidative stress (Table 1). Pre-incubation allows red blood cells to adapt to a mild oxidative stress, owing to their higher resistance to oxidants, similarly to what demonstrated on other cell types (Figure 2) [42]. In this case, no antioxidant has been provided from the outside to mitigate the impact of oxidative stress, but the endogenous antioxidant system of erythrocytes, including SOD, CAT, GPx, and Peroxyredoxin 2, is essential to cell survival when oxidative conditions are being applied. Specifically, CAT contributes to ROS neutralization in erythrocytes following oxidative stress induced by H2O2 [37]. This peculiar response of erythrocytes to oxidative stress is a sort of defense against oxidants and is called preconditioning [37]. This strategy is based on an augmented efficiency of antioxidant enzymes, sustained by low-concentrated oxidants, without the intervention of antioxidant molecules, which represents an impressive homeostatic response of erythrocytes.
Nonetheless, the effect of antioxidants on a validated in vitro model of oxidative stress-induced alterations of B3p has been studied.
3.3. Magnesium
In particular, the possible antioxidant effect of Magnesium (Mg2+) on erythrocytes following oxidative conditions induced by H2O2-NEM- Orthovanadate and Diamide has been proved (Table 1) [28,41]. These treatments with oxidants lead to alterations in red blood cells redox state and, moreover, in KCl co-transport activation. Such effects result in the blockage of phosphatase (PTP) activity on B3p tyrosine phosphorylation [43] induced by cell shrinkage. Magnesium, the second most abundant intracellular ion after K+, with concentrations ranging between 5 and 30 mM, modulates cell volume regulation, enzymes activity and erythrocytes membrane physical properties [44]. Previous studies on the effect of Mg2+ deficiency on membrane function and red blood cell metabolism have been performed in animal models [43,45] and in oxidative stress-related diseases, such as preeclampsia, with hypoxia due to preterm labor [46]. It has already been reported that, in hamsters, magnesium deficiency increases red blood cells’ susceptibility to injury due to free radicals and, in rats, provokes a significant reduction in red blood cells GSH, restored by administration of d-propanolol or vitamin E [47,48]. Hence, Mg2+ has been chosen as a good candidate to study its possible beneficial effect in protecting B3p anion exchange capability in case of oxidative damage.
Among the most interesting outcomes, magnesium pre-exposure has been shown to prevent the reduced efficiency of SO4= uptake through B3p, in both normal and G6PDH-deficient red blood cells, characterized by reduced GSH production and greater sulfhydryl-group (–SH groups) oxidation in in vitro models [28,41]. Specifically, the evidence that oxidative stress decreases the efficiency of anion transport could be due to either structural changes of B3p by SH-groups oxidation or to cell shrinkage after increased K+ efflux [28]. In this regard, Mg2+ exerts its effect on KCC-mediated cell shrinkage observed under oxidative stress. To better focus on the possible antioxidant effect of Mg2+, phosphorylation of Tyrosine and Syk kinases expression levels have been measured in normal erythrocytes following oxidative stress induced by H2O2 and NEM [41]. It is already known that human erythrocytes provide a metabolic response to oxidative conditions in order to maximize the production of NADPH, to restore GSH content and Thioredoxin, and to activate Syk kinases, responsible for phosphorylation of Tyrosines associated with B3p oxidation [43]. The hyperphosphorylation of B3p has been described in several prooxidant hemolytic disorders [49], and it has been shown that oxidized B3p is selectively phosphorylated [50] by Syk kinase, which is responsible for Tyr 8 and Tyr 21 phosphorylation [43,51,52]. Magnesium beneficial effect is not mediated by phosphorylative pathways, but the metal would prevent alterations and protects erythrocyte homeostasis via membrane organization, cross linking between B3p and cytoplasmic proteins, thus impairing Syk docking. Certainly, the rate constant restoration due to pre-exposure to Mg2+ also depends on GSH and –SH group protection, suggesting a role of endogenous antioxidant system stabilized by Mg2+ [28,41,49].
3.4. Melatonin
Melatonin (Mel) is an antioxidant that has been used both in vitro and in vivo on animal models or in clinics to counteract oxidative stress-associated pathologies. Melatonin, a neurohormone derived from tryptophan and predominantly produced by the pineal gland of vertebrates [53], is a multifunctional and evolutionarily conserved molecule acting as a powerful antioxidant fighting against oxidative stress [54,55,56]. In vivo studies have already demonstrated that Mel displays antioxidant properties even greater than those of vitamin E, vitamin C, and α-carotene [54,55]. Additionally, Mel can promote the function of various enzymatic antioxidants, i.e., GPx SOD, GR, and CAT, as well as GSH synthesis, increasing intracellular GSH content [55,57]. In a recent study, a correlation between Mel effect, oxidative stress, and B3p anion exchange capability was shown for the first time. In particular, the authors reported that pre-treatment of red blood cells with Mel impairs the reduction in rate constant for SO4= uptake (Table 1) and the reduction in B3p expression levels due to H2O2. Since H2O2-induced oxidative stress on red blood cells involves neither lipid peroxidation nor oxidation of membrane –SH groups nor methaemoglobin (MetHb) formation [37], the beneficial effect of Mel in protecting B3p should not depend on these mechanisms. Hence, to shed more light on the effect of this molecule, Mel antioxidant power has been assayed in the absence of CAT activity. In such experimental conditions, Mel’s efficacy in impairing the reduction of the rate constant for SO4= uptake due to oxidative conditions and in protecting B3p expression levels was demonstrated [40]. Melatonin also exerts its effect by delaying membrane protein degradation and precipitation of hemin on red blood cells membrane. This could probably explain the preventative effect of Mel against the reduction in B3p expression levels observed after H2O2, thus corroborating the hypothesis that the beneficial action of Mel is exerted at the membrane level (Figure 2) [58,59].
4. Conclusions
Erythrocytes are reasonably employed as models for studies on cellular homeostasis, being continuously threatened by oxidative events associated with high ROS levels. The present review offers more knowledge about the effect of oxidative stress on exchange through B3p under different in vitro experimental conditions, with the double purpose of not only considering B3p efficiency as a good tool for monitoring erythrocyte homeostasis, but also of making it eligible as a target for antioxidants to protect erythrocytes homeostasis. In this context, what has been performed in vitro to study the impact of oxidative stress along with the beneficial effect of antioxidants on B3p could contribute to propose an effective use of antioxidant compounds, possibly introduced by diet, to prevent detrimental effects of oxidative conditions, namely at erythrocytes level. Hence, antioxidant supplementation can be reasonably considered as a tool for improving the endogenous antioxidant defense and to fight against free radicals.
Acknowledgments
The authors are grateful to L. Romano for encouraging the authors to start studying Band 3 protein and for reviewing the paper.
Author Contributions
All authors contributed to performing the literature search and writing the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
Alessia Remigante is supported by the Programma Operativo Nazionale (PON) RI 2014.2020 fellowship.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Halliwell B., Rafter J., Jenner A. Health promotion by flavonoids, tocopherols, tocotrienols, and other phenols: Direct or indirect effects? Antioxidant or not? Am. J. Clin. Nutr. 2005;81:268S–276S. doi: 10.1093/ajcn/81.1.268S. [DOI] [PubMed] [Google Scholar]
- 2.Neha K., Haider M.R., Pathak A., Yar M.S. Medicinal prospects of antioxidants: A review. Eur. J. Med. Chem. 2019;178:687–704. doi: 10.1016/j.ejmech.2019.06.010. [DOI] [PubMed] [Google Scholar]
- 3.Zujko M.E., Witkowska A.M., Waskiewicz A., Sygnowska E. Estimation of dietary intake and patterns of polyphenol consumption in Polish adult population. Adv. Med. Sci. 2012;57:375–384. doi: 10.2478/v10039-012-0026-6. [DOI] [PubMed] [Google Scholar]
- 4.Uttara B., Singh A.V., Zamboni P., Mahajan R.T. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009;7:65–74. doi: 10.2174/157015909787602823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khansari N., Shakiba Y., Mahmoudi M. Chronic inflammation and oxidative stress as a major cause of age-related diseases and cancer. Recent Pat. Inflamm. Allergy Drug Discov. 2009;3:73–80. doi: 10.2174/187221309787158371. [DOI] [PubMed] [Google Scholar]
- 6.Cervantes Gracia K., Llanas-Cornejo D., Husi H. CVD and Oxidative Stress. J. Clin. Med. 2017;6 doi: 10.3390/jcm6020022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferru E., Giger K., Pantaleo A., Campanella E., Grey J., Ritchie K., Vono R., Turrini F., Low P.S. Regulation of membrane-cytoskeletal interactions by tyrosine phosphorylation of erythrocyte band 3. Blood. 2011;117:5998–6006. doi: 10.1182/blood-2010-11-317024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morabito R., Romano O., La Spada G., Marino A. H2O2-Induced Oxidative Stress Affects SO4= Transport in Human Erythrocytes. PLoS ONE. 2016;11:e0146485. doi: 10.1371/journal.pone.0146485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Zwieten R., Verhoeven A.J., Roos D. Inborn defects in the antioxidant systems of human red blood cells. Free Radic. Biol. Med. 2014;67:377–386. doi: 10.1016/j.freeradbiomed.2013.11.022. [DOI] [PubMed] [Google Scholar]
- 10.Steck T.L. The organization of proteins in the human red blood cell membrane. A review. J. Cell Biol. 1974;62:1–19. doi: 10.1083/jcb.62.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arakawa T., Kobayashi-Yurugi T., Alguel Y., Iwanari H., Hatae H., Iwata M., Abe Y., Hino T., Ikeda-Suno C., Kuma H., et al. Crystal structure of the anion exchanger domain of human erythrocyte band 3. Science. 2015;350:680–684. doi: 10.1126/science.aaa4335. [DOI] [PubMed] [Google Scholar]
- 12.Bennett V., Baines A.J. Spectrin and ankyrin-based pathways: Metazoan inventions for integrating cells into tissues. Physiol. Rev. 2001;81:1353–1392. doi: 10.1152/physrev.2001.81.3.1353. [DOI] [PubMed] [Google Scholar]
- 13.Reithmeier R.A., Casey J.R., Kalli A.C., Sansom M.S., Alguel Y., Iwata S. Band 3, the human red cell chloride/bicarbonate anion exchanger (AE1, SLC4A1), in a structural context. Biochim. Biophys. Acta. 2016;1858:1507–1532. doi: 10.1016/j.bbamem.2016.03.030. [DOI] [PubMed] [Google Scholar]
- 14.Sterling D., Reithmeier R.A., Casey J.R. A transport metabolon. Functional interaction of carbonic anhydrase II and chloride/bicarbonate exchangers. J. Biol. Chem. 2001;276:47886–47894. doi: 10.1074/jbc.M105959200. [DOI] [PubMed] [Google Scholar]
- 15.Frumence E., Genetet S., Ripoche P., Iolascon A., Andolfo I., Le Van Kim C., Colin Y., Mouro-Chanteloup I., Lopez C. Rapid Cl−/HCO3− exchange kinetics of AE1 in HEK293 cells and hereditary stomatocytosis red blood cells. Am. J. Physiol. Cell Physiol. 2013;305:C654–C662. doi: 10.1152/ajpcell.00142.2013. [DOI] [PubMed] [Google Scholar]
- 16.Hsu K., Lee T.Y., Periasamy A., Kao F.J., Li L.T., Lin C.Y., Lin H.J., Lin M. Adaptable interaction between aquaporin-1 and band 3 reveals a potential role of water channel in blood CO2 transport. FASEB J. 2017;31:4256–4264. doi: 10.1096/fj.201601282R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Banerjee S., Banerjee M., Sarkar R.N. Diabetes mellitus and aging. J. Indian Med. Assoc. 1998;96:147–148. [PubMed] [Google Scholar]
- 18.Saldanha C. Human Erythrocyte Acetylcholinesterase in Health and Disease. Molecules. 2017;22 doi: 10.3390/molecules22091499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anong W.A., Franco T., Chu H., Weis T.L., Devlin E.E., Bodine D.M., An X., Mohandas N., Low P.S. Adducin forms a bridge between the erythrocyte membrane and its cytoskeleton and regulates membrane cohesion. Blood. 2009;114:1904–1912. doi: 10.1182/blood-2009-02-203216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steck T.L., Ramos B., Strapazon E. Proteolytic dissection of band 3, the predominant transmembrane polypeptide of the human erythrocyte membrane. Biochemistry. 1976;15:1153–1161. doi: 10.1021/bi00650a030. [DOI] [PubMed] [Google Scholar]
- 21.Tanner M.J., Martin P.G., High S. The complete amino acid sequence of the human erythrocyte membrane anion-transport protein deduced from the cDNA sequence. Biochem. J. 1988;256:703–712. doi: 10.1042/bj2560703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lux S.E., John K.M., Kopito R.R., Lodish H.F. Cloning and characterization of band 3, the human erythrocyte anion-exchange protein (AE1) Proc. Natl. Acad. Sci. USA. 1989;86:9089–9093. doi: 10.1073/pnas.86.23.9089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kodippili G.C., Spector J., Hale J., Giger K., Hughes M.R., McNagny K.M., Birkenmeier C., Peters L., Ritchie K., Low P.S. Analysis of the mobilities of band 3 populations associated with ankyrin protein and junctional complexes in intact murine erythrocytes. J. Biol. Chem. 2012;287:4129–4138. doi: 10.1074/jbc.M111.294439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Welbourn E.M., Wilson M.T., Yusof A., Metodiev M.V., Cooper C.E. The mechanism of formation, structure and physiological relevance of covalent hemoglobin attachment to the erythrocyte membrane. Free Radic. Biol. Med. 2017;103:95–106. doi: 10.1016/j.freeradbiomed.2016.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jennings M.L. Proton fluxes associated with erythrocyte membrane anion exchange. J. Membr. Biol. 1976;28:187–205. doi: 10.1007/BF01869697. [DOI] [PubMed] [Google Scholar]
- 26.Romano L., Passow H. Characterization of anion transport system in trout red blood cell. Am. J. Physiol. 1984;246:C330–C338. doi: 10.1152/ajpcell.1984.246.3.C330. [DOI] [PubMed] [Google Scholar]
- 27.Morabito R., Marino A., Romano P., Rigano C., La Spada G. Sulphate and chloride-dependent potassium transport in human erythrocytes are affected by crude venom from nematocysts of the jellyfish Pelagia noctiluca. Cell. Physiol. Biochem. 2013;32:86–95. doi: 10.1159/000356630. [DOI] [PubMed] [Google Scholar]
- 28.Teti D., Crupi M., Busa M., Valenti A., Loddo S., Mondello M., Romano L. Chemical and pathological oxidative influences on band 3 protein anion-exchanger. Cell. Physiol. Biochem. 2005;16:77–86. doi: 10.1159/000087734. [DOI] [PubMed] [Google Scholar]
- 29.Morabito R.R.A., Bagnato G., Neal R.W., Sciortino D., D’Angelo T., Iannelli F., Iannelli F., Cordova F., Cirillo M., La Spada G. Band 3 Protein Function and Oxidative Stress in Erythrocytes from Systemic Sclerosis Patients with Interstitial Lung Disease. Eur. J. Clin. Biomed. Sci. 2017;3:80–84. doi: 10.11648/j.ejcbs.20170304.12. [DOI] [Google Scholar]
- 30.Morabito R., Remigante A., Cavallaro M., Taormina A., La Spada G., Marino A. Anion exchange through band 3 protein in canine leishmaniasis at different stages of disease. Pflugers Arch. 2017;469:713–724. doi: 10.1007/s00424-017-1974-2. [DOI] [PubMed] [Google Scholar]
- 31.Morabito R.R.A., Loddo S., Trichilo V., Dossena S., Marino A. Hyperglycemia affects anion exchange through Band 3 protein: An In Vitro and In Vivo study on human erythrocytes; Proceedings of the SIBS Società Italiana di Biologia Sperimentale Ancona; Ancona, Italy. 9–10 November 2018. [Google Scholar]
- 32.Gupta S.C., Patchva S., Koh W., Aggarwal B.B. Discovery of curcumin, a component of golden spice, and its miraculous biological activities. Clin. Exp. Pharmacol. Physiol. 2012;39:283–299. doi: 10.1111/j.1440-1681.2011.05648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kelkel M., Jacob C., Dicato M., Diederich M. Potential of the dietary antioxidants resveratrol and curcumin in prevention and treatment of hematologic malignancies. Molecules. 2010;15:7035–7074. doi: 10.3390/molecules15107035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morabito R., Falliti G., Geraci A., Spada G.L., Marino A. Curcumin Protects-SH Groups and Sulphate Transport after Oxidative Damage in Human Erythrocytes. Cell. Physiol. Biochem. 2015;36:345–357. doi: 10.1159/000430256. [DOI] [PubMed] [Google Scholar]
- 35.Singh N., Kamath V., Narasimhamurthy K., Rajini P.S. Protective effect of potato peel extract against carbon tetrachloride-induced liver injury in rats. Environ. Toxicol. Pharmacol. 2008;26:241–246. doi: 10.1016/j.etap.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 36.Heo S.J., Park E.J., Lee K.W., Jeon Y.J. Antioxidant activities of enzymatic extracts from brown seaweeds. Bioresour. Technol. 2005;96:1613–1623. doi: 10.1016/j.biortech.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 37.Morabito R., Remigante A., Di Pietro M.L., Giannetto A., La Spada G., Marino A. SO4= uptake and catalase role in preconditioning after H2O2-induced oxidative stress in human erythrocytes. Pflugers Arch. 2017;469:235–250. doi: 10.1007/s00424-016-1927-1. [DOI] [PubMed] [Google Scholar]
- 38.Shan F., Yang R., Ji T., Jiao F. Vitamin C Inhibits Aggravated Eryptosis by Hydrogen Peroxide in Glucose-6-Phosphated Dehydrogenase Deficiency. Cell. Physiol. Biochem. 2016;39:1453–1462. doi: 10.1159/000447848. [DOI] [PubMed] [Google Scholar]
- 39.Minetti M., Mallozzi C., Di Stasi A.M. Peroxynitrite activates kinases of the src family and upregulates tyrosine phosphorylation signaling. Free Radic. Biol. Med. 2002;33:744–754. doi: 10.1016/S0891-5849(02)00891-2. [DOI] [PubMed] [Google Scholar]
- 40.Morabito R., Remigante A., Marino A. Melatonin Protects Band 3 Protein in Human Erythrocytes against H2O2-Induced Oxidative Stress. Molecules. 2019;24 doi: 10.3390/molecules24152741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morabito R., Remigante A., Marino A. Protective Role of Magnesium against Oxidative Stress on SO4= Uptake through Band 3 Protein in Human Erythrocytes. Cell. Physiol. Biochem. 2019;52:1292–1308. doi: 10.33594/000000091. [DOI] [PubMed] [Google Scholar]
- 42.Zhu J., Kang L., Ye Q., Fan G., Liang Y., Yan C., Orgah J. Effects of Shenfu injection and its main components on the contraction of isolated rat thoracic aortic rings. PLoS ONE. 2013;8:e78026. doi: 10.1371/journal.pone.0078026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pantaleo A., Ferru E., Pau M.C., Khadjavi A., Mandili G., Matte A., Spano A., De Franceschi L., Pippia P., Turrini F. Band 3 Erythrocyte Membrane Protein Acts as Redox Stress Sensor Leading to Its Phosphorylation by p (72) Syk. Oxid. Med. Cell. Longev. 2016;2016:6051093. doi: 10.1155/2016/6051093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bede O.N.D., Surányi A., Horváth I., Szlávik M., Gyurkovits K. Effects of magnesium supplementation on the glutathione redox system in atopic asthmatic children. Inflamm. Res. 2008;57:279–286. doi: 10.1007/s00011-007-7077-3. [DOI] [PubMed] [Google Scholar]
- 45.De Franceschi L., Brugnara C., Beuzard Y. Dietary magnesium supplementation ameliorates anemia in a mouse model of β-Thalassemia. Blood. 2016;90:1283–1290. doi: 10.1182/blood.V90.3.1283. [DOI] [PubMed] [Google Scholar]
- 46.Chernyshova E.S., Zaikina Y.S., Tsvetovskaya G.A., Strokotov D.I., Yurkin M.A., Serebrennikova E.S., Volkov L., Maltsev V.P., Chernyshev A.V. Influence of magnesium sulfate on HCO3/Cl transmembrane exchange rate in human erythrocytes. J. Theor. Biol. 2016;393:194–202. doi: 10.1016/j.jtbi.2015.12.023. [DOI] [PubMed] [Google Scholar]
- 47.De Franceschi L.V.M.E., Fumagalli L., Brugnara C., Turrini F., Motta R., Veghini E., Corato C., Alper S.L., Berton G. K-Cl cotransport modulation by intracellular Mg in erythrocytes from mice bred for low and high Mg levels. Am. J. Physiol. Cell Physiol. 2001;281:C1385–C1395. doi: 10.1152/ajpcell.2001.281.4.C1385. [DOI] [PubMed] [Google Scholar]
- 48.Freedman A.M.T., Stafford R.E., Dickens B.F., Cassidy M.M., Muesing R.A., Weglicki W.B. Erythrocytes from mag-nesium-deficient hamsters display an enhanced susceptibility to oxidative stress. Am. J. Physiol. Cell Physiol. 1992;262:C1371–C1375. doi: 10.1152/ajpcell.1992.262.6.C1371. [DOI] [PubMed] [Google Scholar]
- 49.Crupi M., Romano L., Romano P., Venza M., Venza I., Teti D. Erythrocytes anion transport and oxidative change in beta-thalassaemias. Cell Biol. Int. 2010;34:655–662. doi: 10.1042/CBI20090472. [DOI] [PubMed] [Google Scholar]
- 50.Pantaleo A., Ferru E., Giribaldi G., Mannu F., Carta F., Matte A., de Franceschi L., Turrini F. Oxidized and poorly glycosylated band 3 is selectively phosphorylated by Syk kinase to form large membrane clusters in normal and G6PD-deficient red blood cells. Biochem. J. 2009;418:359–367. doi: 10.1042/BJ20081557. [DOI] [PubMed] [Google Scholar]
- 51.Bordin L., Fiore C., Bragadin M., Brunati A.M., Clari G. Regulation of membrane band 3 Tyr-phosphorylation by proteolysis of p72(Syk) and possible involvement in senescence process. Acta Biochim. Biophys. Sin. 2009;41:846–851. doi: 10.1093/abbs/gmp071. [DOI] [PubMed] [Google Scholar]
- 52.Bordin L., Ion-Popa F., Brunati A.M., Clari G., Low P.S. Effector-induced Syk-mediated phosphorylation in human erythrocytes. Biochim. Biophys. Acta. 2005;1745:20–28. doi: 10.1016/j.bbamcr.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 53.Rahman K. Studies on free radicals, antioxidants, and co-factors. Clin. Interv. Aging. 2007;2:219–236. [PMC free article] [PubMed] [Google Scholar]
- 54.Korkmaz A., Reiter R.J., Topal T., Manchester L.C., Oter S., Tan D.X. Melatonin: An established antioxidant worthy of use in clinical trials. Mol. Med. 2009;15:43–50. doi: 10.2119/molmed.2008.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reiter R.J., Tan D.X., Mayo J.C., Sainz R.M., Leon J., Czarnocki Z. Melatonin as an antioxidant: Biochemical mechanisms and pathophysiological implications in humans. Acta Biochim. Pol. 2003;50:1129–1146. doi: 10.18388/abp.2003_3637. [DOI] [PubMed] [Google Scholar]
- 56.Radogna F., Diederich M., Ghibelli L. Melatonin: A pleiotropic molecule regulating inflammation. Biochem. Pharmacol. 2010;80:1844–1852. doi: 10.1016/j.bcp.2010.07.041. [DOI] [PubMed] [Google Scholar]
- 57.Anisimov V.N., Popovich I.G., Zabezhinski M.A., Anisimov S.V., Vesnushkin G.M., Vinogradova I.A. Melatonin as antioxidant, geroprotector and anticarcinogen. Biochim. Biophys. Acta. 2006;1757:573–589. doi: 10.1016/j.bbabio.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 58.Tesoriere L., D’Arpa D., Conti S., Giaccone V., Pintaudi A.M., Livrea M.A. Melatonin protects human red blood cells from oxidative hemolysis: New insights into the radical-scavenging activity. J. Pineal Res. 1999;27:95–105. doi: 10.1111/j.1600-079X.1999.tb00602.x. [DOI] [PubMed] [Google Scholar]
- 59.Srour M.A., Bilto Y.Y., Juma M. Evaluation of different methods used to measure malonyldialdehyde in human erythrocytes. Clin. Hemorheol. Microcirc. 2000;23:23–30. [PubMed] [Google Scholar]