Abstract
Membrane separators are key elements of microbial fuel cells (MFCs), especially of those constructed in a dual-chamber configuration. Until now, membranes made of Nafion have been applied the most widely to set-up MFCs. However, there is a broader agreement in the literature that Nafion is expensive and in many cases, does not meet the actual (mainly mass transfer-specific) requirements demanded by the process and users. Driven by these issues, there has been notable progress in the development of alternative materials for membrane fabrication, among which those relying on the deployment of ionic liquids are emerging. In this review, the background of and recent advances in ionic liquid-containing separators, particularly supported ionic liquid membranes (SILMs), designed for MFC applications are addressed and evaluated. After an assessment of the basic criteria to be fulfilled by membranes in MFCs, experiences with SILMs will be outlined, along with important aspects of transport processes. Finally, a comparison with the literature is presented to elaborate on how MFCs installed with SILM perform relative to similar systems assembled with other, e.g., Nafion, membranes.
Keywords: ionic liquid, supported ionic liquid membrane, membrane separator, Nafion, microbial fuel cell, bioelectrochemical system
1. Introduction
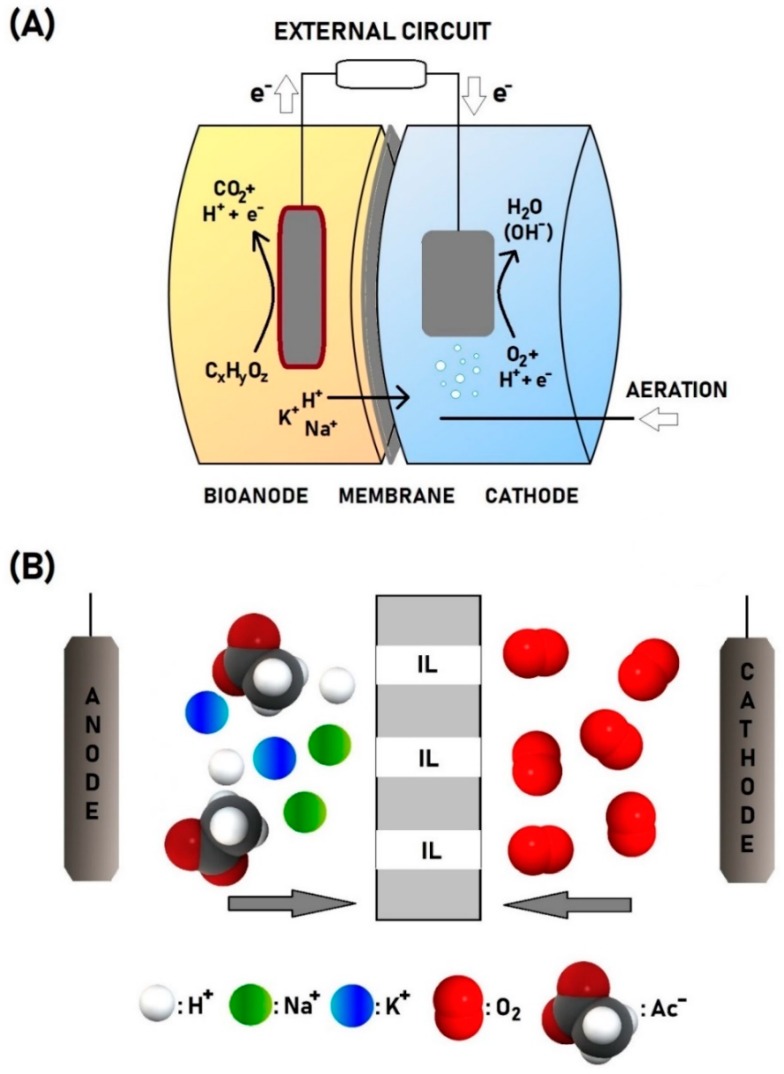
Microbial fuel cells (MFCs) represent one particular type of bioelectrochemical system, in which organic matter (e.g., environmental pollutants such as wastewaters) is removed and transformed into electricity [1,2,3]. Architecturally, MFCs (especially those designed in a dual-compartment layout) consist of three substantial elements, namely, the two electrodes (anode and cathode) and a separator—most frequently a membrane—in between them (Figure 1A) [4,5,6]. In fact, their characteristics have an imperative effect on the achievable process efficiency for several reasons, as follows. First of all, electrode properties such as those of the anode affect the development of electrochemically-active biofilms on its surface [7]. Anodes, from the point of view of exoelectrogenic strains, can be seen as terminal electron acceptors utilized under anaerobic conditions [8]. In this sense, anodes are interfaces connecting the biofilm with the (normally abiotic) cathode electrode (as a terminal electron donor, e.g., for oxygen reduction) by receiving the electrons (liberated from microbial metabolic pathways of organic matter oxidation) and conveying the charge to the external circuit containing (a resistor and) the cathode [9,10,11]. Furthermore, to increase the electrochemical efficiency of a bioelectrochemical system such as MFC in terms of the current production and power output, the total internal resistance of the MFC should be reduced [12,13]. This, from the perspective of electrodes, should result in decreased charge transfer resistances and overpotentials [14,15,16].
Figure 1.
The scheme of a double-chamber microbial fuel cell (MFC) structure and working principles (A), and the main components transported through the ionic liquid (IL)-containing membrane (B).
Besides the electrodes, the traits of the separator (typically a membrane, as mentioned) are of equal importance for the maintenance of ion transfer [6,17,18]. In essence, for an adequate MFC performance, separators need to ensure the selective passage of certain ionic species (mostly protons, instead of competing ions such as Na+ and K+ often found in remarkable quantities in the anolyte, but which is dependent on the type of membrane), while others should be held back (Figure 1B) [19]. This is advantageous for restricting the occurrence of so-called pH-splitting between electrodes, which has been proven to deteriorate the MFC performance [20,21,22]. Furthermore, separators have to prevent the mixing of reactants, in particular, substrates fed to the anode chamber and the oxygen supplied to the cathode (Figure 1B). If these criteria are not fulfilled due to an insufficient mass transfer resistance, the subsequent crossover of substances leads to significant losses and a sub-optimal MFC efficacy [6,23]. On top of that, higher (ionic) conductivity is also favored for the sake of lowered total internal resistance since separators can be considered an Ohmic resistance feature [24,25]. An additional aspect to consider about membrane separators used in MFC is the operating stability. For instance, reliable membrane separators should withstand chemical as well as biological fouling, which presents a threat, especially in the long-term [26,27]. Such impacts may notably alter the initial (physico-chemical) features of the membrane and as a result, cyclic regeneration or even replacement may be inevitable for keeping the MFC in a good condition.
Overall, the above issues have induced intense R&D in the field of separation technology, e.g., membranes to be applied in MFCs, where one of the latest, emerging directions is linked to the employment of ionic liquids (ILs) [28]. ILs are salts, comprised of (inorganic or organic) anion and (organic) cation parts that can be varied to adjust the IL properties, in agreement with the actual demands. Accordingly, ILs are taken into account as tailor-made compounds with broad recognition in various chemical- and biotechnological areas, thanks to their negligible vapor pressure (non-volatility), remarkable ionic conductivity, and wide electrochemical potential window [29].
In the next sections, progress related to the design and use of novel separators prepared with ILs for MFCs in the form of supported ionic liquid membranes (SILMs) will be reviewed to highlight the most crucial findings of this specific subject and enlighten the perspectives of these materials.
2. Research Progress with Supported Ionic Liquid Membranes for Microbial Fuel Cell Applications
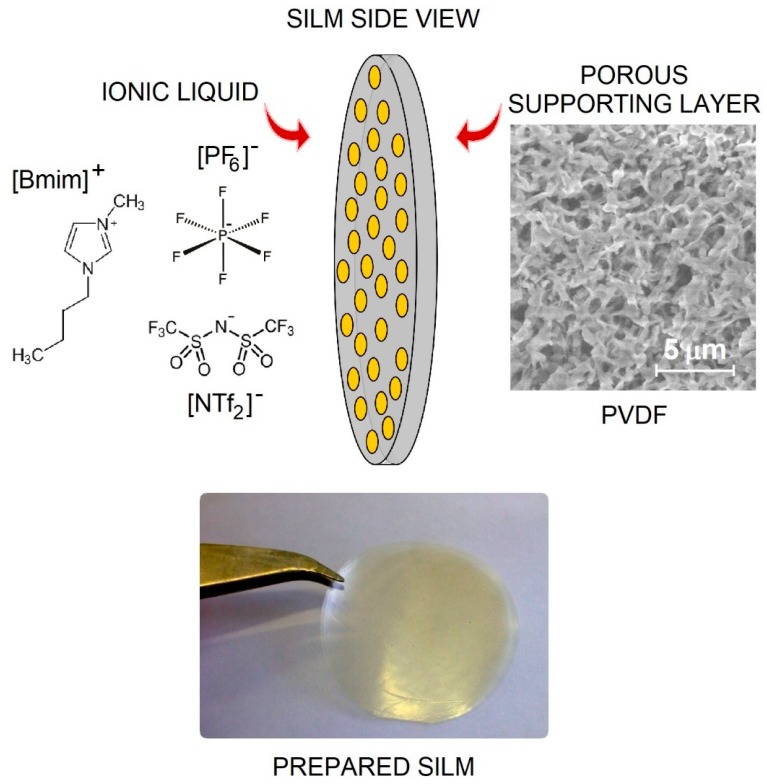
Complementing MFCs with supported liquid membrane (SLM) technology has been shown to be effective for increasing the power output of the process [30]. If the SLM is prepared using an ionic liquid, an SILM is eventually obtained. In Table 1, demonstrative examples of using SILM in MFCs are listed. In fact, for membrane fabrication, the ionic liquids comprising imidazolium-type cations have been almost exclusively tested. As for the anion of these ILs, combinations resulting in a lower water miscibility (increased hydrophobicity) are preferred (e.g., [NTf2]− and [PF6]− instead of [Cl]−) in order to act against the extraction of IL from the pores of the support material in an aqueous environment characterizing an MFC. Less variation is noticeable in terms of the porous support material made of either Nylon or PVDF (Figure 2). Since the application of SILM in MFCs is an emerging field, mostly acetate, as a simple, easily degradable substrate, has been used for fundamental studies. The compatibility of the IL with a given support matrix will significantly influence the global stability of the SILM obtained. Nevertheless, besides the purely physico-chemical factors taking place in the half-cells of a dual-chamber MFC, such as mass transport processes and mixing conditions, the interference of SILMs with the underlying microbial culture/consortia (to be seen as the biological component of the MFC) should also be taken into consideration. From this point of view, two main scenarios exist.
Table 1.
SILMs used in various MFC studies.
| Configuration | Inoculum | Substrate | SILM | Reference | |
|---|---|---|---|---|---|
| Ionic Liquid | Support Layer | ||||
| Single-Chamber | Mixed Culture | Wastewater | [mtoa][Cl] | Nylon | [32] |
| [omim][NTf2] | Nylon | ||||
| [omim][BF4] | Nylon | ||||
| [omim][PF6] | Nylon | ||||
| Dual-Chamber | Mixed Culture | Acetate | [hmim][PF6] | PVDF | [25] |
| [bmim][NTf2] | PVDF | ||||
| Dual-Chamber | Mixed Culture | Acetate | [hmim][PF6] | PVDF | [23] |
| [bmim][NTf2] | PVDF | ||||
| Dual-Chamber | Mixed Culture | Acetate | [bmim][PF6] | PVDF | [33] |
Figure 2.
The structure, scheme, and image of a supported ionic liquid membrane (SILM).
On the one hand, if the leakage of ILs from the pores occurs, it will probably affect the activity of electro-active bacteria (EAB). Many ILs have been reported to possess some kind of toxic behavior in various microorganisms. Recently, it was shown by Nemestóthy et al. [31] through a kinetic study that ILs such as [bmim][Cl] and [bmim][Ac], depending on their concentrations, caused a notable loss of metabolic activity of an anaerobic hydrogen-producing community. Furthermore, Hernández-Fernández et al. [32] pointed out that the escape of IL from SILMs to the anolyte contributed to depression of the MFC performance, probably due to perturbation of the whole-cell, electrochemically-active, living biocatalysts. Literature works addressing the stability of SILMs have explained that hydrodynamics in the liquid phase embracing the SILM play a key role and the operation of MFC under static/gently mixed conditions can be seen as a beneficial strategy for enhancing the durability of IL-containing physical separators [33,34,35]. To overcome the instability of SILMs in aqueous media/polar solvents, alternative directions in ionic liquid-containing membrane development for double-compartment microbial fuel cells have appeared. These rely on the blending of various ILs and organic polymers, resulting in a polymer-inclusion membrane, and can be regarded as a possible way forward [36,37,38].
On the other hand, just like in most (filtration) processes where a membrane is employed, chemical and biological fouling of the membrane installed in the bioelectrochemical system is a real threat [26]. Therefore, over time, the deposition of chemical agents, substances, and microbes can be expected. Obviously, the properties of the membrane will then deteriorate, followed by the decrease of the MFC’s power generation capacity. As analyzed in the review of Koók et al. [27] based on previous literature findings, proper selection of the IL to be embedded in the support layer can potentially suppress biofouling. For instance, it has been found by Jebur et al. [39] that liquid membranes prepared with imidazolium cation-based hydrophilic (with [Cl]− and [Br]− anions) and hydrophobic (with [NTf2]− anion) ILs considerably lowered the numbers of growing colonies for strains such as Staphylococcus aureus and Pseudomonas aeruginosa. Therefore, a suitable choice and deployment of ionic liquids could be seen as a potential way to design membranes with a more efficient resistance to microbiological attacks and counteract the biofouling of membrane separators in MFCs [27]. However, due to the limited information available on this aspect, additional research will be needed to assess the traits of biological foulants in light of those of SILMs and understand the possible mechanisms, cross-effects, and interdependencies.
It is worth mentioning that SILMs seem to be advantageous in special bioelectrochemical applications, such as hyper-thermophilic MFCs (>80 °C) [40]. In these systems, extreme thermophilic bacteria serves as biocatalysts; however, common proton exchange membranes (PEM), such as Nafion, fail to stay hydrated for proper functioning. To overcome the issue of the temperature sensitivity of PEMs, Mistry et al. [41] investigated the possible use of ILs in the form of supported liquid membranes. After modifying Nafion and Hyflon PEMs with [bmim][NTf2] IL (soaking at different temperatures), SILMs with a high thermal stability and promising anhydrous proton conductivity could be prepared due to successful ion exchange between the proton of –SO3H groups of the PEM and the [bmim]+ of IL and the relatively high mobility of the H+[NTf2]− ion pair [41].
3. Transport Processes in Ionic Liquid Membranes and at Water/IL Interfaces
As ILs are subject to growing interest for electrochemical applications, including membrane technology, the transport of various compounds into and in the IL phase should be elucidated. In this respect, the transport of (i) water, (ii) IL components, (iii) ionic solutes, and in some cases (iv) gaseous compounds can be addressed.
3.1. Mutual Solubility of Water and Hydrophobic Ionic Liquids
An SILM is considered stable when only minimal loss of IL from the porous support to the surrounding aqueous media and a consistent membrane operation are ensured. For this, the first thing to inspect is usually the mutual solubility of water and IL and the influencing factors. In general, ILs with hydrophobic anions are applied for bioelectrochemical applications in the form of a membrane (SILM, in most cases), in order to minimize the water uptake of the liquid membrane and the leakage of IL from the supporting layer. Although the ILs commonly used for such a purpose (prepared by using [PF6]−, [NTf2]−, or [DCA]− anions combined, for example, with 1-alkyl-3-methylimidazolium ([Cnmim]+), trioctylmethylammonium ([mtoa]+), trihexyl(tetradecyl)phosphonium ([P666,14]+), and 1-butyl-1-methylpyrrolidinium ([Pyrr14]+)) are called hydrophobic, they still show more than negligible interactions with water [42,43,44,45]. Moreover, it is known that in addition to the positive effect of a longer alkyl chain length of the cation on the hydrophobicity of IL, mainly the anion defines the hydrophobicity and the extent of miscibility with water [43,44,46,47]. Therefore, the hygroscopic character and sensitivity towards hydrolysis of [PF6]− may cause considerable water solubility and simultaneously, the dissolution of IL in the aqueous phase (the latter aspect is minor, but still, the contamination of aqueous phase by IL is best avoided) [34,45]. The [NTf2]− anion is also hygroscopic and slightly soluble in water; however, to a much lower extent when compared to [PF6]− [48]. It was shown that in SILM prepared with [Cnmim][PF6] (n = 4, 8, 10) being in touch with aqueous phase at both sides, after a given lag-time for reaching the critical water content, continuous water transport occurs [34]. It turned out that water is transferred into the IL phase and then forms clusters or so-called microenvironments, after which a steady permeation of H2O can be obtained [34]. Moreover, it can be said that the water present in the IL phase has an effect on the mobility of the cation and anion of ILs, mainly through distraction of the electrostatic interactions between them [49].
3.2. Effect of Aqueous Ions on the Mutual Solubility of Water and IL
As the solubility of the salts in aqueous media depends on the presence (and type) of ionic solutes, the effect of the common ions in MFC electrolytes on the IL dissolution to the aqueous phase may be a crucial aspect of SILM stability. MFC anolytes contain a wide spectra of ions from the—mainly wastewater-based—seed source, such as K+, Na+, Ca2+, Mg2+, and NH4+ cations and Cl−, SO42−, and CH3COO− (shortly Ac−) anions, as well as ions of the buffering species (HPO42−, H2PO4−, HCO3−, and CO32−) or cathodic product (OH−) [18].
As was shown for aqueous solutions of water-miscible ILs, the addition of so-called “kosmotropic” (order-making) salts can confine the solubility of ILs in water via the salting-out effect [50,51,52]. To adopt this concept in the field of SILM-based MFCs would be highly beneficial, since the loss of IL from the pores of the supporting layer could be further decreased by moderating the solubility of ILs in water. Freire et al. [53] studied the salting-out of hydrophobic [bmim][NTf2] IL from the aqueous phase by using various salts, and concluded that at low salt concentrations (~0.1–0.2 M), a salting-in effect can be observed, followed by salting-out at higher concentrations. Salting-in is manifested in the breaking of the water structure, leading to the stabilization of hydrophobic moieties in the solution by direct ion binding, and thus resulting in an increased solubility of the solute (in our case, IL) in water [53,54]. Since most of the ion concentrations in an MFC range within several mM, it can be said, that under these conditions, SILMs may need to face the salting-in effect—increased solubility in water—in MFCs. However, by using more concentrated buffer solutions (e.g., >200 mM) or applying elevated salt concentrations, the salting-out of IL could be promoted, which could lead to extended SILM stability due to minimized IL loss. However, considering the possible negative effect of a high salt concentration on the biological activity or the rate of membrane/electrode fouling, realistic concentration limits should be maintained.
For instance, Lefebvre et al. [55] investigated the effect of an elevated NaCl concentration on the performance of two-chambered MFCs, and it was found that increasing the amount of NaCl can be tolerated by anodophilic bacteria and boost MFC efficiency up to a given value (~340 mM). Following this point, a further raise in concentration caused significant performance losses. By taking into account the contribution of various ions to the salting-out of IL from water, it can be said that an MFC operation at elevated H2PO4−, PO43−, and SO42− concentrations (100–200 mM) may be feasible and advantageous, while higher Na+, K+, Ca2+, and Mg2+ concentrations may suppress the solubility of water in the IL phase (although, this latter aspect shows less significance) [53,56]. In addition, it can be said that high amounts of H+ and NH4+ should be avoided, as these compounds may effectively contribute to the increased mutual solubility of IL and water [53,57].
3.3. Transport of Ionic Solutes
The transport of ionic solutes is more complex and the exact mechanisms underlying this process have not been fully clarified, so intense research is currently being undertaken. Nevertheless, separate discussion seems to be needed when the transport of ionic species takes place either (i) at the water/IL interface or (ii) in the IL phase. On the one hand, at immiscible water/IL interfaces, it seems that H+ transfers into the IL via so-called void-assisted ion-paired proton transfer, which means that proton transport is facilitated by pairing with hydrophobic anions and filling the voids as a capacitive layer in the interfacial IL phase, as it was found for highly hydrophobic IL with a [P66614]+ cation and [FAP]− (tris(-pentafluoroethyl)trifluorophosphate) anion [43]. On the other hand, alkali metal cations did not follow such facilitated transfer, as their hydrated ionic radii exceeded the estimated size of the voids (being a consequence of the anisotropic nature of ILs) [43]. In the IL bulk phase, although proton transport becomes hindered by the non-polar alkyl groups of the cations, protons have a higher mobility than other diffusing cationic species. This observation highlights the possible use of ILs in MFCs for the enhanced transfer of protons in the presence of other cations, which could contribute to a better performance and pH balance between the anodic- and cathodic-side electrolytes.
The transport of alkali metal ions seems to depend on the presence of water microenvironments in IL, through which they move via diffusion. The molecular diffusion is determined by the size of the transferring ionic species, as well as the viscosity of the IL [34]. For instance, Na+ and Cl− are usually characterized by a low solubility in hydrophobic ionic liquids, and they transfer equimolarly. Therefore, it was concluded that small solute transport is not influenced by the selectivity of the IL towards them [34,58]. However, as was found in the case of different forms of thymol blue as a solute, the transfer of larger molecules may be affected by the affinity of IL in relation to them [34]. This mechanism may be of interest for MFCs in terms of anodic organic compounds (as agents interfering with the cathode-surface reactions due to crossover), including substrates. It is also important to note that the presence of various ions in aqueous media may alter the mutual solubility of water and IL [53,57]. In conclusion, it is obvious that ILs provide a special electrolyte media for ion (and related water) transfer, which could be of great interest for MFCs. Therefore, further R&D in this field can be proposed.
4. Outlook and Perspectives of SILMs in MFCs
In agreement with the previous discussion, mass transport features across the SILMs ought to be determined for MFC process characterization, especially in terms of (i) the substrate, (ii) ions, and (iii) oxygen, which is the most common reactant at the MFC cathode (Figure 1B). In the case of the oxygen mass transfer properties of membranes, for instance, it was argued that membranes enabling too highly dissolved O2 fluxes will eventually make the MFCs work in a sub-optimal way. Suggestions regarding the critical values of oxygen mass transfer coefficients (kO) have been suggested in papers such as those by Bakonyi et al. [6] and Koók et al. [23]. Inadequate electricity generation by MFC will be directly reflected in measures of electrochemical performance, among which the power density (Pd, mW m−2 anode surface) is the most common one.
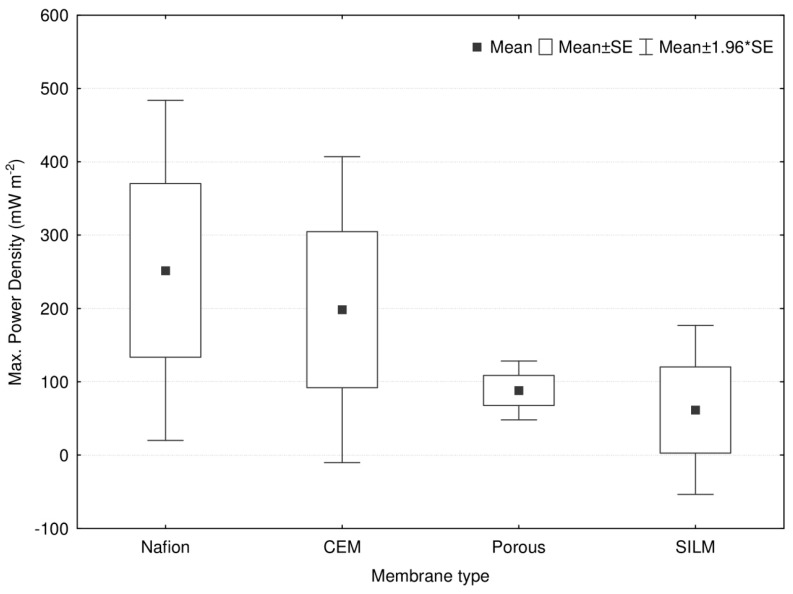
In terms of Pd by MFCs applying SILM as separator, it can be deduced that values fluctuate remarkably. For instance, the maximal Pd derived from total cell polarization tests can vary from 1.4 [25] to 179 mW m−2 [33]. It is noteworthy, however, that such improvements may not only be associated with the actual type of IL, e.g., [bmim][PF6] instead of [bmim][NTf2] on the same PVDF support membrane (Table 1), but are also likely dependent on changing other factors of the MFC architecture, as well as inoculum sources from which EABs are enriched. As the ultimate aim of manufacturing and testing SILMs for MFC is to provide promising candidates as alternatives to the Nafion PEM (proton exchange membrane), a comparative evaluation can show how far the research on and development of SILMs have reached. As a matter of fact, experiences with Nafion membranes in MFCs (acetate substrate, mixed culture as the seed source, dual-chamber configuration, and batch mode) indicate that the maximum Pd could be in a similar range—14.4 [59], 17.7 [60], 38 [61,62], 43.6 [63], 57.5 [64], 65 [65], 118 [66], 126.7 [67], and 173.3 [68]—to that attainable in MFCs with SILMs (1.4–179 mW m−2) [23,25,33]. To extend the comparative evaluation of SILMs, it is worth taking a look at the performances of MFCs operated with membrane candidates (such as cation exchange membranes (CEM) and porous (cheap) materials) proposed to compete with Nafion. In Table 2, such a compilation of the literature can be observed for systems with similar underlying working principles. The studies listed in Table 2 have been screened and selected after a careful search of the literature, following certain guidelines and filters suggested by Ge et al. [69] and Whitaker et al. [70]. The main steps were (i) the choice of database: SCOPUS; (ii) the determination of keywords: e.g., “bioelectrochemical system”, “microbial fuel cell”, “membrane”, and “separator”; and (iii) a manual check of the relevance and availability of essential data to be assessed (configuration, inoculum, substrate, operating mode, and maximum power density). In Figure 3, no real differences are detectable and thus, it seems that all MFCs, regardless of the type of membrane separator, could produce comparable maximum power densities. This conclusion is supported by the outcomes of the Tukey hones significant difference (HSD) test presented in Table 3, where no values of p < 0.05 (criteria of significant difference) could be noted for any pairs of membrane categories. However, these statistical results should be treated with care due to the lower number of data and should be revisited in the future on a bigger population of samples.
Table 2.
Comparative table for MFCs operated with various types of membranes.
| Configuration | Operating Mode | Inoculum | Substrate | Membrane Type | Maximum Power Density (mW m−2 anode) | Reference |
|---|---|---|---|---|---|---|
| Dual-chamber | batch | Mixed culture | Acetate | Nafion 117 | 107.9 | [71] |
| Dual-chamber | batch | Mixed culture | Acetate | Nafion 117 | 38.0 | [61] |
| Dual-chamber | batch | Mixed culture | Acetate | Nafion 117 | 65.0 | [65] |
| Dual-chamber | batch | Mixed culture | Acetate | Nafion 117 | 38.0 | [62] |
| Dual-chamber | batch | Mixed culture | Acetate | Nafion 117 | 1013.0 | [72] |
| Dual-chamber | batch | Mixed culture | Acetate | Nafion 117 | 118.0 | [66] |
| Dual-chamber | batch | Mixed culture | Acetate | Nafion 117 | 1225.0 | [73] |
| Dual-chamber | batch | Mixed culture | Acetate | Nafion 117 | 43.6 | [63] |
| Dual-chamber | batch | Mixed culture | Acetate | Nafion 117 | 17.7 | [60] |
| Dual-chamber | batch | Mixed culture | Acetate | Nafion 117 | 126.7 | [67] |
| Dual-chamber | batch | Mixed culture | Acetate | Nafion 117 | 57.5 | [64] |
| Dual-chamber | batch | Mixed culture | Acetate | Nafion 177 | 173.3 | [68] |
| Dual-chamber | batch | Mixed culture | Acetate | CEM | 33.0 | [62] |
| Dual-chamber | batch | Mixed culture | Acetate | CEM | 902.0 | [72] |
| Dual-chamber | batch | Mixed culture | Acetate | CEM | 112.0 | [66] |
| Dual-chamber | batch | Mixed culture | Acetate | CEM | 114.0 | [66] |
| Dual-chamber | batch | Mixed culture | Acetate | CEM | 82.0 | [66] |
| Dual-chamber | batch | Mixed culture | Acetate | CEM | 12.6 | [60] |
| Dual-chamber | batch | Mixed culture | Acetate | CEM | 320.0 | [74] |
| Dual-chamber | batch | Mixed culture | Acetate | CEM | 11.3 | [75] |
| Dual-chamber | batch | Mixed culture | Acetate | Porous | 5.0 | [62] |
| Dual-chamber | batch | Mixed culture | Acetate | Porous | 36.0 | [62] |
| Dual-chamber | batch | Mixed culture | Acetate | Porous | 36.0 | [62] |
| Dual-chamber | batch | Mixed culture | Acetate | Porous | 121.0 | [66] |
| Dual-chamber | batch | Mixed culture | Acetate | Porous | 114.0 | [66] |
| Dual-chamber | batch | Mixed culture | Acetate | Porous | 74.0 | [66] |
| Dual-chamber | batch | Mixed culture | Acetate | Porous | 117.0 | [66] |
| Dual-chamber | batch | Mixed culture | Acetate | Porous | 41.6 | [63] |
| Dual-chamber | batch | Mixed culture | Acetate | Porous | 5.4 | [75] |
| Dual-chamber | batch | Mixed culture | Acetate | Porous | 246.7 | [67] |
| Dual-chamber | batch | Mixed culture | Acetate | Porous | 163.9 | [76] |
| Dual-chamber | batch | Mixed culture | Acetate | Porous | 97.0 | [64] |
| Dual-chamber | batch | Mixed culture | Acetate | SILM | 179.0 | [33] |
| Dual-chamber | batch | Mixed culture | Acetate | SILM | 4.2 | [23] |
| Dual-chamber | batch | Mixed culture | Acetate | SILM | 1.4 | [25] |
Figure 3.
Illustrative comparison of MFCs based on the data of Table 2 with regard to the type of membrane used.
Table 3.
The p-values derived from Tukey’s HSD test for the assessment of significance.
| Membrane Type | Nafion | CEM | Porous | SILM |
|---|---|---|---|---|
| Nafion | - | 0.976 | 0.510 | 0.735 |
| CEM | 0.976 | - | 0.834 | 0.895 |
| Porous | 0.510 | 0.834 | - | 0.998 |
| SILM | 0.735 | 0.895 | 0.998 | - |
Accordingly, SILMs can be taken into account as plausible separators for microbial fuel cells. However, due to the early-stage of research on these materials (covering approximately a period of 4–5 years), further feedback will be advantageous to reveal their pros and cons during application, especially those related to their stability, biofouling resistance, and simultaneous contribution to MFC efficiency.
5. Conclusions
In this paper, the progress that has been achieved with regards to microbial fuel cells operated using membranes containing ionic liquids has been overviewed. It has been shown that the mass transport processes taking place across a membrane and how supported ionic liquid membranes may contribute to efficiently running the process have to be considered in MFCs. Ionic liquids and support materials for the fabrication of SILMs were evaluated in light of literature experiences and a comparative assessment with other membrane-assisted MFCs demonstrated the potential of SILMs as alternative separator candidates for this kind of bioelectrochemical system.
Acknowledgments
The financial support of this work by the National Research, Development and Innovation Office (NKFIH, Hungary) under grant number FK 131409 is duly acknowledged. The authors wish to thank the Project no. 2017-00015, which has been implemented with the support provided from the National Research, Development and Innovation Fund of Hungary, financed under the 2017-2.2.5-TÉT-FR funding scheme.
Funding
National Research, Development and Innovation Office (NKFIH, Hungary) (FK 131409); National Research, Development and Innovation Fund of Hungary (Project no. 2017-00015; 2017-2.2.5-TÉT-FR).
Conflicts of Interest
The authors declare they have no any conflict of interest.
References
- 1.Zhang Q., Hu J., Lee D.J. Microbial fuel cells as pollutant treatment units: Research updates. Bioresour. Technol. 2016;217:121–128. doi: 10.1016/j.biortech.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 2.Koók L., Rózsenberszki T., Nemestóthy N., Bélafi-Bakó K., Bakonyi P. Bioelectrochemical treatment of municipal waste liquor in microbial fuel cells for energy valorization. J. Clean. Prod. 2016;112:4406–4412. doi: 10.1016/j.jclepro.2015.06.116. [DOI] [Google Scholar]
- 3.Pandey P., Shinde V.N., Deopurkar R.L., Kale S.P., Patil S.A., Pant D. Recent advances in the use of different substrates in microbial fuel cells toward wastewater treatment and simultaneous energy recovery. Appl. Energy. 2016;168:706–723. doi: 10.1016/j.apenergy.2016.01.056. [DOI] [Google Scholar]
- 4.Patil S.A., Gildemyn S., Pant D., Zengler K., Logan B.E., Rabaey K. A logical data representation framework for electricity-driven bioproduction processes. Biotechnol. Adv. 2015;33:736–744. doi: 10.1016/j.biotechadv.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 5.Logan B.E., Hamelers B., Rozendal R., Schröder U., Keller J., Freguia S., Aelterman P., Verstraete W., Rabaey K. Microbial fuel cells: Methodology and technology. Environ. Sci. Technol. 2006;40:5181–5192. doi: 10.1021/es0605016. [DOI] [PubMed] [Google Scholar]
- 6.Bakonyi P., Koók L., Kumar G., Tóth G., Rózsenberszki T., Nguyen D.D., Chang S.W., Zhen G., Bélafi-Bakó K., Nemestóthy N. Architectural engineering of bioelectrochemical systems from the perspective of polymeric membrane separators: A comprehensive update on recent progress and future prospects. J. Membr. Sci. 2018;564:508–522. doi: 10.1016/j.memsci.2018.07.051. [DOI] [Google Scholar]
- 7.Wei J., Liang P., Huang X. Recent progress in electrodes for microbial fuel cells. Bioresour. Technol. 2011;102:9335–9344. doi: 10.1016/j.biortech.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 8.Zhou M., Chi M., Luo J., He H., Jin T. An overview of electrode materials in microbial fuel cells. J. Power Sources. 2011;196:4427–4435. doi: 10.1016/j.jpowsour.2011.01.012. [DOI] [Google Scholar]
- 9.Baudler A., Schmidt I., Langner M., Greiner A., Schröder U. Does it have to be carbon? Metal anodes in microbial fuel cells and related bioelectrochemical systems. Energy Environ. Sci. 2015;8:2048–2055. doi: 10.1039/C5EE00866B. [DOI] [Google Scholar]
- 10.Hindatu Y., Annuar M.S.M., Gumel A.M. Mini-review: Anode modification for improved performance of microbial fuel cell. Renew. Sustain. Energy Rev. 2017;73:236–248. doi: 10.1016/j.rser.2017.01.138. [DOI] [Google Scholar]
- 11.Santoro C., Arbizzani C., Erable B., Ieropoulos I. Microbial fuel cells: From fundamentals to applications. A review. J. Power Sources. 2017;356:225–244. doi: 10.1016/j.jpowsour.2017.03.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kashyap D., Dwivedi P.K., Pandey J.K., Kim Y.H., Kim G.M., Sharma A., Goel S. Application of electrochemical impedance spectroscopy in bio-fuel cell characterization: A review. Int. J. Hydrogen Energy. 2014;39:20159–20170. doi: 10.1016/j.ijhydene.2014.10.003. [DOI] [Google Scholar]
- 13.Miller A., Singh L., Wang L., Liu H. Linking internal resistance with design and operation decisions in microbial electrolysis cells. Environ. Int. 2019;126:611–618. doi: 10.1016/j.envint.2019.02.056. [DOI] [PubMed] [Google Scholar]
- 14.ElMekawy A., Hegab H.M., Dominguez-Benetton X., Pant D. Internal resistance of microfluidic microbial fuel cell: Challenges and potential opportunities. Bioresour. Technol. 2013;142:672–682. doi: 10.1016/j.biortech.2013.05.061. [DOI] [PubMed] [Google Scholar]
- 15.Rismani-Yazdi H., Carver S.M., Christy A.D., Tuovinen O.H. Cathodic limitations in microbial fuel cells: An overview. J. Power Sources. 2008;180:683–694. doi: 10.1016/j.jpowsour.2008.02.074. [DOI] [Google Scholar]
- 16.Cristiani P., Carvalho M.L., Guerrini E., Daghio M., Santoro C., Li B. Cathodic and anodic biofilms in Single Chamber Microbial Fuel Cells. Bioelectrochemistry. 2013;92:6–13. doi: 10.1016/j.bioelechem.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 17.Leong J.X., Daud W.R.W., Ghasemi M., Liew K.B., Ismail M. Ion exchange membranes as separators in microbial fuel cells for bioenergy conversion: A comprehensive review. Renew. Sustain. Energy Rev. 2013;28:575–587. doi: 10.1016/j.rser.2013.08.052. [DOI] [Google Scholar]
- 18.Oliot M., Galier S., Roux de Balmann H., Bergel A. Ion transport in microbial fuel cells: Key roles, theory and critical review. Appl. Energy. 2016;183:1682–1704. doi: 10.1016/j.apenergy.2016.09.043. [DOI] [Google Scholar]
- 19.Daud S.M., Kim B.H., Ghasemi M., Daud W.R.W. Separators used in microbial electrochemical technologies: Current status and future prospects. Bioresour. Technol. 2015;195:170–179. doi: 10.1016/j.biortech.2015.06.105. [DOI] [PubMed] [Google Scholar]
- 20.Li W.W., Sheng G.P., Liu X.W., Yu H.Q. Recent advances in the separators for microbial fuel cells. Bioresour. Technol. 2011;102:244–252. doi: 10.1016/j.biortech.2010.03.090. [DOI] [PubMed] [Google Scholar]
- 21.Rahimnejad M., Bakeri G., Ghasemi M., Zirepour A. A review on the role of proton exchange membrane on the performance of microbial fuel cell. Polym. Adv. Technol. 2014;25:1426–1432. doi: 10.1002/pat.3383. [DOI] [Google Scholar]
- 22.Harnisch F., Schröder U. Selectivity versus mobility: Separation of anode and cathode in microbial bioelectrochemical systems. ChemSusChem. 2009;2:921–926. doi: 10.1002/cssc.200900111. [DOI] [PubMed] [Google Scholar]
- 23.Koók L., Nemestóthy N., Bakonyi P., Göllei A., Rózsenberszki T., Takács P., Salekovics A., Kumar G., Bélafi-Bakó K. On the efficiency of dual-chamber biocatalytic electrochemical cells applying membrane separators prepared with imidazolium-type ionic liquids containing [NTf2]− and [PF6]− anions. Chem. Eng. J. 2017;324:296–302. doi: 10.1016/j.cej.2017.05.022. [DOI] [Google Scholar]
- 24.Xu J., Sheng G.P., Luo H.W., Li W.W., Wang L.F., Yu H.Q. Fouling of proton exchange membrane (PEM) deteriorates the performance of microbial fuel cell. Water Res. 2012;46:1817–1824. doi: 10.1016/j.watres.2011.12.060. [DOI] [PubMed] [Google Scholar]
- 25.Koók L., Nemestóthy N., Bakonyi P., Zhen G., Kumar G., Lu X., Su L., Saratale G.D., Kim S.H., Gubicza L. Performance evaluation of microbial electrochemical systems operated with Nafion and supported ionic liquid membranes. Chemosphere. 2017;175:350–355. doi: 10.1016/j.chemosphere.2017.02.055. [DOI] [PubMed] [Google Scholar]
- 26.Noori M.T., Ghangrekar M.M., Mukherjee C.K., Min B. Biofouling effects on the performance of microbial fuel cells and recent advances in biotechnological and chemical strategies for mitigation. Biotechnol. Adv. 2019;37:107420. doi: 10.1016/j.biotechadv.2019.107420. [DOI] [PubMed] [Google Scholar]
- 27.Koók L., Bakonyi P., Harnisch F., Kretzschmar J., Chae K.J., Zhen G., Kumar G., Rózsenberszki T., Tóth G., Nemestóthy N., et al. Biofouling of membranes in microbial electrochemical technologies: Causes, characterization methods and mitigation strategies. Bioresour. Technol. 2019;279:327–338. doi: 10.1016/j.biortech.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 28.Abejón R., Pérez-Acebo H., Garea A. A bibliometric analysis of research on supported ionic liquid membranes during the 1995–2015 period: Study of the main applications and trending topics. Membranes. 2017;7:63. doi: 10.3390/membranes7040063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yan X., Anguille S., Bendahan M., Moulin P. Ionic liquids combined with membrane separation processes: A review. Sep. Purif. Technol. 2019;222:230–253. doi: 10.1016/j.seppur.2019.03.103. [DOI] [Google Scholar]
- 30.Fradler K.R., Michie I., Dinsdale R.M., Guwy A.J., Premier G.C. Augmenting microbial fuel cell power by coupling with supported liquid membrane permeation for zinc recovery. Water Res. 2014;55:115–125. doi: 10.1016/j.watres.2014.02.026. [DOI] [PubMed] [Google Scholar]
- 31.Nemestóthy N., Bakonyi P., Rózsenberszki T., Kumar G., Koók L., Kelemen G., Kim S.H., Bélafi-Bakó K. Assessment via the modified gompertz-model reveals new insights concerning the effects of ionic liquids on biohydrogen production. Int. J. Hydrogen Energy. 2018;43:18918–18924. doi: 10.1016/j.ijhydene.2018.08.174. [DOI] [Google Scholar]
- 32.Hernández-Fernández F.J., Pérez de los Ríos A., Mateo-Ramírez F., Godínez C., Lozano-Blanco L.J., Moreno J.I., Tomás-Alonso F. New application of supported ionic liquids membranes as proton exchange membranes in microbial fuel cell for waste water treatment. Chem. Eng. J. 2015;279:115–119. doi: 10.1016/j.cej.2015.04.036. [DOI] [Google Scholar]
- 33.Koók L., Kaufer B., Bakonyi P., Rózsenberszki T., Rivera I., Buitrón G., Bélafi-Bakó K., Nemestóthy N. Supported ionic liquid membrane based on [bmim][PF6] can be a promising separator to replace Nafion in microbial fuel cells and improve energy recovery: A comparative process evaluation. J. Membr. Sci. 2019;570:215–225. doi: 10.1016/j.memsci.2018.10.063. [DOI] [Google Scholar]
- 34.Fortunato R., Afonso C.A.M., Reis M.A.M., Crespo J.G. Supported liquid membranes using ionic liquids: Study of stability and transport mechanisms. J. Membr. Sci. 2004;242:197–209. doi: 10.1016/j.memsci.2003.07.028. [DOI] [Google Scholar]
- 35.Fortunato R., Afonso C.A.M., Benavente J., Rodriguez-Castellón E., Crespo J.G. Stability of supported ionic liquid membranes as studied by X-ray photoelectron spectroscopy. J. Membr. Sci. 2005;256:216–223. doi: 10.1016/j.memsci.2005.02.023. [DOI] [Google Scholar]
- 36.Hernández-Fernández F.J., De Los Ríos A.P., Mateo-Ramírez F., Juarez M.D., Lozano-Blanco L.J., Godínez C. New application of polymer inclusion membrane based on ionic liquids as proton exchange membrane in microbial fuel cell. Sep. Purif. Technol. 2016;160:51–58. doi: 10.1016/j.seppur.2015.12.047. [DOI] [Google Scholar]
- 37.Baicha Z., Salar-García M.J., Ortiz-Martínez V.M., Hernández-Fernández F.J., de los Ríos A.P., Maqueda Marín D.P., Collado J.A., Tomás-Alonso F., El Mahi M. On the Selective Transport of Nutrients through Polymer Inclusion Membranes Based on Ionic Liquids. Processes. 2019;7:544. doi: 10.3390/pr7080544. [DOI] [Google Scholar]
- 38.Salar-García M.J., Ortiz-Martínez V.M., de los Ríos A.P., Hernández-Fernández F.J. A method based on impedance spectroscopy for predicting the behavior of novel ionic liquid-polymer inclusion membranes in microbial fuel cells. Energy. 2015;89:648–654. doi: 10.1016/j.energy.2015.05.149. [DOI] [Google Scholar]
- 39.Jebur M., Sengupta A., Chiao Y.H., Kamaz M., Qian X., Wickramasinghe R. Pi electron cloud mediated separation of aromatics using supported ionic liquid (SIL) membrane having antibacterial activity. J. Membr. Sci. 2018;556:1–11. doi: 10.1016/j.memsci.2018.03.064. [DOI] [Google Scholar]
- 40.Fu Q., Fukushima N., Maeda H., Sato K., Kobayashi H. Bioelectrochemical analysis of a hyperthermophilic microbial fuel cell generating electricity at temperatures above 80 °C. Biosci. Biotechnol. Biochem. 2015;79:1200–1206. doi: 10.1080/09168451.2015.1015952. [DOI] [PubMed] [Google Scholar]
- 41.Mistry M.K., Subianto S., Choudhury N.R., Dutta N.K. Interfacial interactions in aprotic ionic liquid based protonic membrane and its correlation with high temperature conductivity and thermal properties. Langmuir. 2009;25:9240–9251. doi: 10.1021/la901330y. [DOI] [PubMed] [Google Scholar]
- 42.Samec Z., Langmaier J., Kakiuchi T. Charge-transfer processes at the interface between hydrophobic ionic liquid and water. Pure Appl. Chem. 2009;81:1473–1488. doi: 10.1351/PAC-CON-08-08-36. [DOI] [Google Scholar]
- 43.De Eulate E.A., Silvester D.S., Arrigan D.W.M. Void-assisted ion-paired proton transfer at water-ionic liquid interfaces. Angew. Chem. Int. Ed. 2015;54:14903–14906. doi: 10.1002/anie.201507556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Malik M.A., Hashim M.A., Nabi F. Ionic liquids in supported liquid membrane technology. Chem. Eng. J. 2011;171:242–254. doi: 10.1016/j.cej.2011.03.041. [DOI] [Google Scholar]
- 45.Yao C., Pitner W.R., Anderson J.L. Ionic liquids containing the tris(pentafluoroethyl)trifluorophosphate anion: A new class of highly selective and ultra hydrophobic solvents for the extraction of polycyclic aromatic hydrocarbons using single drop microextraction. Anal. Chem. 2009;81:5054–5063. doi: 10.1021/ac900719m. [DOI] [PubMed] [Google Scholar]
- 46.Freire M.G., Santos L.M.N.B.F., Fernandes A.M., Coutinho J.A.P., Marrucho I.M. An overview of the mutual solubilities of water-imidazolium-based ionic liquids systems. Fluid Phase Equilibria. 2007;261:449–454. doi: 10.1016/j.fluid.2007.07.033. [DOI] [Google Scholar]
- 47.Zhou T., Chen L., Ye Y., Chen L., Qi Z., Freund H., Sundmacher K. An overview of mutual solubility of ionic liquids and water predicted by COSMO-RS. Ind. Eng. Chem. Res. 2012;51:6256–6264. doi: 10.1021/ie202719z. [DOI] [Google Scholar]
- 48.O’Mahony A.M., Silvester D.S., Aldous L., Hardacre C., Compton R.G. Effect of water on the electrochemical window and potential limits of room-temperature ionic liquids. J. Chem. Eng. Data. 2008;53:2884–2891. doi: 10.1021/je800678e. [DOI] [Google Scholar]
- 49.Kumar P., Prakash P., Ramya K.R., Venkatnathan A. Probing translational and rotational dynamics in hydrophilic/hydrophobic anion based imidazolium ionic liquid-water mixtures. Soft Matter. 2018;14:6109–6118. doi: 10.1039/C8SM00765A. [DOI] [PubMed] [Google Scholar]
- 50.Trindade J.R., Visak Z.P., Blesic M., Marrucho I.M., Coutinho J.A.P., Canongia Lopes J.N., Rebelo L.P.N. Salting-out effects in aqueous ionic liquid solutions: Cloud-point temperature shifts. J. Phys. Chem. B. 2007;111:4737–4741. doi: 10.1021/jp067022d. [DOI] [PubMed] [Google Scholar]
- 51.Najdanovic-Visak V., Canongia Lopes J.N., Visak Z.P., Trindade J., Rebelo L.P.N. Salting-out in aqueous solutions of ionic liquids and K3PO4: Aqueous biphasic systems and salt precipitation. Int. J. Mol. Sci. 2007;8:736–748. doi: 10.3390/i8080736. [DOI] [Google Scholar]
- 52.Zafarani-Moattar M.T., Hamzehzadeh S. Salting-out effect, preferential exclusion, and phase separation in aqueous solutions of chaotropic water-miscible ionic liquids and kosmotropic salts: Effects of temperature, anions, and cations. J. Chem. Eng. Data. 2010;55:1598–1610. doi: 10.1021/je900681b. [DOI] [Google Scholar]
- 53.Freire M.G., Carvalho P.J., Silva A.M.S., Santos L.M.N.B.F., Rebelo L.P.N., Marrucho I.M., Coutinho J.A.P. Ion specific effects on the mutual solubilities of water and hydrophobic ionic liquids. J. Phys. Chem. B. 2009;113:202–211. doi: 10.1021/jp8080035. [DOI] [PubMed] [Google Scholar]
- 54.Kalra A., Tugcu N., Cramer S.M., Garde S. Salting-in and salting-out of hydrophobic solutes in aqueous salt solutions. J. Phys. Chem. B. 2001;105:6380–6386. doi: 10.1021/jp010568+. [DOI] [Google Scholar]
- 55.Lefebvre O., Tan Z., Kharkwal S., Ng H.Y. Effect of increasing anodic NaCl concentration on microbial fuel cell performance. Bioresour. Technol. 2012;112:336–340. doi: 10.1016/j.biortech.2012.02.048. [DOI] [PubMed] [Google Scholar]
- 56.Tomé L.I.N., Varanda F.R., Freire M.G., Marrucho I.M., Coutinho J.A.P. Towards an understanding of the mutual solubilities of water and hydrophobic ionic liquids in the presence of salts: The anion effect. J. Phys. Chem. B. 2009;113:2815–2825. doi: 10.1021/jp810141d. [DOI] [PubMed] [Google Scholar]
- 57.Mazan V., Boltoeva M.Y., Tereshatov E.E., Folden C.M. Mutual solubility of water and hydrophobic ionic liquids in the presence of hydrochloric acid. RSC Adv. 2016;6:56269–56270. doi: 10.1039/C6RA06791C. [DOI] [Google Scholar]
- 58.Ghareh Bagh F.S., Mjalli F.S., Hashim M.A., Hadj-Kali M.K.O., Alnashef I.M. Solubility of sodium chloride in ionic liquids. Ind. Eng. Chem. Res. 2013;52:11488–11493. doi: 10.1021/ie401282y. [DOI] [Google Scholar]
- 59.Zinadini S., Zinatizadeh A.A., Rahimi M., Vatanpour V., Rahimi Z. High power generation and COD removal in a microbial fuel cell operated by a novel sulfonated PES/PES blend proton exchange membrane. Energy. 2017;125:427–438. doi: 10.1016/j.energy.2017.02.146. [DOI] [Google Scholar]
- 60.Di Palma L., Bavasso I., Sarasini F., Tirillò J., Puglia D., Dominici F., Torre L. Synthesis, characterization and performance evaluation of Fe3O4/PES nano composite membranes for microbial fuel cell. Eur. Polym. J. 2018;99:222–229. doi: 10.1016/j.eurpolymj.2017.12.037. [DOI] [Google Scholar]
- 61.Min B., Cheng S., Logan B.E. Electricity generation using membrane and salt bridge microbial fuel cells. Water Res. 2005;39:1675–1686. doi: 10.1016/j.watres.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 62.Jung R.K., Cheng S., Oh S.E., Logan B.E. Power generation using different cation, anion, and ultrafiltration membranes in microbial fuel cells. Environ. Sci. Technol. 2007;41:1004–1009. doi: 10.1021/es062202m. [DOI] [PubMed] [Google Scholar]
- 63.Tang X., Guo K., Li H., Du Z., Tian J. Microfiltration membrane performance in two-chamber microbial fuel cells. Biochem. Eng. J. 2010;52:194–198. doi: 10.1016/j.bej.2010.08.007. [DOI] [Google Scholar]
- 64.Choi S., Kim J.R., Cha J., Kim Y., Premier G.C., Kim C. Enhanced power production of a membrane electrode assembly microbial fuel cell (MFC) using a cost effective poly [2,5-benzimidazole] (ABPBI) impregnated non-woven fabric filter. Bioresour. Technol. 2013;128:14–21. doi: 10.1016/j.biortech.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 65.Tao H.C., Sun X.N., Xiong Y. A novel hybrid anion exchange membrane for high performance microbial fuel cells. RSC Adv. 2015;5:4659–4663. doi: 10.1039/C4RA11638K. [DOI] [Google Scholar]
- 66.Kondaveeti S., Lee J., Kakarla R., Kim H.S., Min B. Low-cost separators for enhanced power production and field application of microbial fuel cells (MFCs) Electrochim. Acta. 2014;132:434–440. doi: 10.1016/j.electacta.2014.03.046. [DOI] [Google Scholar]
- 67.Neethu B., Bhowmick G.D., Ghangrekar M.M. A novel proton exchange membrane developed from clay and activated carbon derived from coconut shell for application in microbial fuel cell. Biochem. Eng. J. 2019;148:170–177. doi: 10.1016/j.bej.2019.05.011. [DOI] [Google Scholar]
- 68.Oh S.E., Logan B.E. Proton exchange membrane and electrode surface areas as factors that affect power generation in microbial fuel cells. Appl. Microbiol. Biotechnol. 2006;70:162–169. doi: 10.1007/s00253-005-0066-y. [DOI] [PubMed] [Google Scholar]
- 69.Ge Z., Li J., Xiao L., Tong Y., He Z. Recovery of Electrical Energy in Microbial Fuel Cells. Environ. Sci. Technol. Lett. 2014;1:137–141. doi: 10.1021/ez4000324. [DOI] [Google Scholar]
- 70.Whitaker J., Ludley K.E., Rowe R., Taylor G., Howard D.C. Sources of variability in greenhouse gas and energy balances for biofuel production: A systematic review. Gcb Bioenergy. 2010;2:99–112. doi: 10.1111/j.1757-1707.2010.01047.x. [DOI] [Google Scholar]
- 71.Tiwari B.R., Noori M.T., Ghangrekar M.M. A novel low cost polyvinyl alcohol-Nafion-borosilicate membrane separator for microbial fuel cell. Mater. Chem. Phys. 2016;182:86–93. doi: 10.1016/j.matchemphys.2016.07.008. [DOI] [Google Scholar]
- 72.Leong J.X., Daud W.R.W., Ghasemi M., Ahmad A., Ismail M., Liew K. Ben Composite membrane containing graphene oxide in sulfonated polyether ether ketone in microbial fuel cell applications. Int. J. Hydrogen Energy. 2015;40:11604–11614. doi: 10.1016/j.ijhydene.2015.04.082. [DOI] [Google Scholar]
- 73.Daud S.M., Daud W.R.W., Kim B.H., Somalu M.R., Bakar M.H.A., Muchtar A., Jahim J.M., Lim S.S., Chang I.S. Comparison of performance and ionic concentration gradient of two-chamber microbial fuel cell using ceramic membrane (CM) and cation exchange membrane (CEM) as separators. Electrochim. Acta. 2018;259:365–376. doi: 10.1016/j.electacta.2017.10.118. [DOI] [Google Scholar]
- 74.Bavasso I., Di Palma L., Petrucci E. Treatment of wastewater in H-type MFC with protonic exchange membrane: Experimental study of organic carbon and ammonium reduction with electrochemical characterization. Chem. Eng. Trans. 2016;47:223–228. [Google Scholar]
- 75.Pasternak G., Yang Y., Santos B.B., Brunello F., Hanczyc M.M., Motta A. Regenerated silk fibroin membranes as separators for transparent microbial fuel cells. Bioelectrochemistry. 2019;126:146–155. doi: 10.1016/j.bioelechem.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 76.Kim K.Y., Chae K.J., Yang E., Lee M.Y., Kim I.S. Influence of pressurized anode chamber on ion transports and power generation of UF membrane microbial fuel cells (UF-MFCs) J. Power Sources. 2015;279:731–736. doi: 10.1016/j.jpowsour.2015.01.006. [DOI] [Google Scholar]