Abstract
The efficiency of Au/TiO2 based catalysts in 1-phenylethanol oxidation was investigated. The role of support modifiers (La2O3 or CeO2), influence of gold loading (0.5% or 4%) and redox pretreatment atmosphere, catalyst recyclability, effect of oxidant: tert-butyl hydroperoxide (TBHP) or O2, as well as the optimization of experimental parameters of the reaction conditions in the oxidation of this alcohol were studied and compared with previous studies on 1-octanol oxidation. Samples were characterized by temperature-programmed oxygen desorption (O2-TPD) method. X-ray photoelectron spectroscopy (XPS) measurements were carried out for used catalysts to find out the reason for deactivation in 1-phenylethanol oxidation. The best catalytic characteristics were shown by catalysts modified with La2O3, regardless of the alcohol and the type of oxidant. When O2 was used, the catalysts with 0.5% Au, after oxidative pretreatment, showed the highest activity in both reactions. The most active catalysts in 1-phenylethanol oxidation with TBHP were those with 4% Au and the H2 treatment, while under the same reaction conditions, 0.5% Au and O2 treatment were beneficial in 1-octanol oxidation. Despite the different chemical nature of the substrates, it seems likely that Au+(Auδ+) act as the active sites in both oxidative reactions. Density functional theory (DFT) simulations confirmed that the gold cationic sites play an essential role in 1-phenylethanol adsorption.
Keywords: gold, heterogeneous catalysis, alcohol oxidation, 1-phenylethanol, TBHP, DFT
1. Introduction
Alcohol oxidation is one of the most important transformations in industrial organic chemistry and a challenging process in terms of green chemistry [1,2]. Traditional methods involve the use of toxic, expensive, stoichiometric metal oxidants and harmful organic solvents, and often require harsh reaction conditions [3,4]. Catalysis research is working towards favourable solutions to these problems through the development of effective heterogeneous catalysts for environment friendly applications.
One example is the development and study of catalytic systems for the oxidation of 1-phenylethanol, which is an aromatic alcohol with high reactivity. The main oxidation product of its oxidation is acetophenone (methylphenylketone, C6H5COCH3). Acetophenone is used in perfumes, soaps and creams, as well as a flavouring substance in food, soft drinks and tobacco. It is also used as a solvent and has a sleeping effect, important in the manufacture of medicines.
There are many studies reported for 1-phenylethanol oxidation, using both homogeneous and heterogeneous catalysis (Table 1). From the summarized results, it can be concluded that the catalytic oxidation of this alcohol, a representative of cyclic alcohols, can be carried out under mild conditions: using green oxidizing agents such as oxygen, air and peroxides, both with a solvent and in its absence, in moderate temperature ranges: from room temperature to 150 °C and, in most cases, at atmospheric pressure. Also, when using peroxides, a new trend is the reaction under the influence of microwave heating, which, in comparison with the traditional process, allows obtaining high yields in a short time.
Table 1.
Catalytic oxidation of 1-phenylethanol using homogeneous and heterogeneous catalysts.
| Catalyst | Oxidant | P, Atm | Solvent | T, °C | Reaction Time, h | c R | dYac, % | Ref. |
|---|---|---|---|---|---|---|---|---|
| [Cu2(R)(CH3O)(NO3)]2(CH3O)2 | TBHP | 1 | - | 80 | b 1MW | 71 | 54 | [5] |
|
g [Cu(κONN’HL)(NO3) (N,N-dimethylformamide(DMF)](NO3)∙H2 |
TBHP | 1 | - | 80 | 2 | 1000 | 12 | [6] |
|
g [Cu(κONN’HL)(NO3)(DMF)] (NO3)∙H2+K2CO3 |
TBHP | 1 | - | 80 | 2 | 1000 | 62 | [6] |
| h [Cu(1κNOO’,2κO’,3κO”L)]n | TBHP | 1 | - | 120 | b 1MW | 250 | 66 | [7] |
|
h [Cu(1κNOO’,2κO’,3κO”L)]n +2,2,6,6-Tetramethylpiperidin-1-yl)oxyl(TEMPO) |
TBHP | 1 | - | 120 | b 1MW | 250 | 81 | [7] |
| h [Cu(κNOO’HL)Cl(CH3OH)] | TBHP | 1 | - | 120 | b 1MW | 250 | 82 | [7] |
|
h [Cu(κNOO’HL)Cl(CH3OH)] +TEMPO |
TBHP | 1 | - | 120 | b 1MW | 250 | 92 | [7] |
| Carbon nanotubes (CNTs) | TBHP | 1 | - | 80 | b 1MW | 50 | 8 | [8] |
| Graphene oxide (GO) | TBHP | 1 | - | 80 | b 1MW | 50 | 14 | [8] |
| CoCl2 | TBHP | 1 | - | 80 | b 1MW | 50 | 28 | [8] |
| CoCl2–5%CNTs | TBHP | 1 | - | 80 | b 1MW | 50 | 85 | [8] |
| CoCl2–5% GO | TBHP | 1 | - | 80 | b 1MW | 50 | 72 | [8] |
| CuO | TBHP | 1 | - | 80 | b 1MW | 50 | 16 | [8] |
| CuO–1%CNTs | TBHP | 1 | - | 80 | b 1MW | 50 | 59 | [8] |
| Fe2O3 | TBHP | 1 | - | 80 | b 1MW | 50 | 10 | [8] |
| Fe2O3–1%CNTs | TBHP | 1 | - | 80 | b 1MW | 50 | 32 | [8] |
| Fe2O3–CoCl2–5%CNTs | TBHP | 1 | - | 80 | b 1MW | 50 | 73 | [8] |
| V2O5 | TBHP | 1 | - | 80 | b 1MW | 50 | 45 | [8] |
| CoCl2–V2O5–5%CNTs | TBHP | 1 | - | 80 | b 1MW | 50 | 54 | [8] |
| [FeCl2(L)(2,20bipy)] | TBHP | 1 | - | 150 | b 1MW | 333 | 99 | [9] |
| [FeCl2(L)(2,20bipy)] | TBHP | 1 | - | 150 | 46 | 333 | 99 | [9] |
| [Fe(bipy)3](CF3SO3)2 | H2O2 | 1 | CH3CN | 100 | 0.5 | 100 | 62 | [10] |
| [Fe(bipy)3](CF3SO3)2)+2-pyridinecarboxylic acid | H2O2 | 1 | CH3CN | 100 | 0.5 | 100 | 93 | [10] |
| VOPO4+TEMPO | O2 | 4 | H2O | 80 | 6 | 20 | a 38.5(89) | [11] |
| NiO/SiO2 | O2 | 1 | p-xylen | 100 | 6 | 12 | 51 | [12] |
| MnO2 commercial | TBHP | 1 | e ACN:tol | RT | 7 | 1 | 84 | [13] |
| MnO2 commercial | TBHP | 1 | e ACN:tol | 40 | 7 | 20 | 34 | [13] |
| MnO2 commercial | H2O2 | 1 | e ACN:tol | 40 | 5 | 20 | 0 | [13] |
| MnO2 commercial | TBHP | 1 | e ACN:tol | 80 | 7 | 10 | 67 | [13] |
| MnO2 commercial | - | 1 | e ACN:tol | 80 | 24 | 1 | 30 | [13] |
| NbP–C | H2O2 | 1 | CH3CN | 90 | 24 | 11 | a 72 | [14] |
| CeCrO3 | TBHP | 1 | DMSO | 90 | 6 | 10 | a 100 | [15] |
| 15wt.% Ag- Octahedral molecular sieve-2 | TBHP | 1 | CH3CN | 75 | 4 | 625 | a 71.5 | [16] |
| 0.9wt.% Pd/Aerosil380 | O2 | 10 | H2O | 100 | 6 | 262 | 44.9 | [17] |
| 0.9wt.% Pd/Aerosil380 | O2 | 10 | H2O | 100 | 12 | 262 | 75.1 | [17] |
| 1.0wt.% Pd/60wt.% Polyketone (PK)–SiO2 | O2 | 10 | H2O | 100 | 6 | 262 | 62.2 | [17] |
| 1.0wt.% Pd/60wt.% PK–SiO2 | O2 | 10 | H2O | 100 | 12 | 262 | 100 | [17] |
| 1.0wt.% Pd/76wt.% PK–SiO2 | O2 | 10 | H2O | 100 | 6 | 262 | 58.3 | [17] |
| 1.0wt.% Pd/76wt.% PK–SiO2 | O2 | 10 | H2O | 100 | 12 | 262 | 94.8 | [17] |
| 3wt.%Pd/O-Diamonds(Dia) | O2 | 1 | o-xylen | 100 | 4 | 1428 | a 27.9 | [18] |
| 3wt.%Pd/CeO2/O-Dia | O2 | 1 | o-xylen | 100 | 4 | 1428 | a 72.5 | [18] |
| 1.57wt.% Pd/CeO2 | O2 | 1 | - | 120 | 2 | 649 | a 91 | [19] |
| 1.44wt.% Pd/apatite | O2 | 1 | - | 120 | 2 | 649 | a 90 | [19] |
| 10.10wt.% Ru/Mg–LaO | O2 | 1 | toluene | 80 | 4 | 10 | 96 | [20] |
| 10.10wt.% Ru/SiO2 | O2 | 1 | toluene | 80 | 4 | 10 | 45 | [20] |
| 10.10wt.% Ru/Al2O3 | O2 | 1 | toluene | 80 | 4 | 10 | 40 | [20] |
| 10.10wt.% Ru/MgO | O2 | 1 | toluene | 80 | 4 | 10 | 36 | [20] |
| 10.10wt.% Ru/TiO2 | O2 | 1 | toluene | 80 | 4 | 10 | 36 | [20] |
| 1.54wt.% Au/CeO2 | O2 | 1 | - | 120 | 2 | 649 | 95 | [19] |
| 1wt.% gold nanoparticles (Au NPs)/Ionic liquid(IL)/N-hydroxyphthalimide(NHPI) | O2 | 4 | - | 100 | 24 | 1356 | a 60(47) | [21] |
| 1wt.% Au NPs/IL/NHPI | O2 | 4 | - | 160 | 24 | 6780 | a 77 (58) | [21] |
| 1wt.%Au NPs– supported ionic liquid-like phases | H2O2 | 1 | H2O | 150 | 0.25 | 8 | >90 | [22] |
| 1wt.% Au/Active carbon | TBHP | 1 | - | 150 | b 2 MW | 500 | 55 | [23] |
| 1wt.% Au/carbon xerogel | TBHP | 1 | - | 150 | b 2 MW | 500 | 90 | [23] |
| 1wt.% Au/Graphite | TBHP | 1 | - | 150 | b 2 MW | 500 | 63 | [23] |
| 1wt.% Au/Microdiamonds | TBHP | 1 | - | 150 | b 2 MW | 500 | 100 | [23] |
| 1wt.% Au/Nanodiamonds for liquid dispersion | TBHP | 1 | - | 150 | b 2 MW | 500 | 83 | [23] |
| 1wt.%Au/Silicone carbide | TBHP | 1 | - | 150 | b 2 MW | 500 | 73 | [23] |
| 0.89wt.% Au/Hydrotalcite (Ht) | Air | 1 | toluene | 80 | 0.33 | 222 | 99 | [24] |
| 0.89wt.% Au/Ht | Air | 1 | toluene | 40 | 3 | 222 | 99 | [24] |
| 0.89wt.% Au/Ht | Air | 1 | toluene | 27 | 6 | 222 | 99 | [24] |
| 0.89wt.% Au/Al2O3 | Air | 1 | toluene | 27 | 3 | 222 | 71 | [24] |
| 0.89wt.% Au/MgO | Air | 1 | toluene | 27 | 3 | 222 | 71 | [24] |
| 0.89wt.% Au/TiO2 | Air | 1 | toluene | 27 | 3 | 222 | 14 | [24] |
| 0.89wt.% Au/TiO2+Na2CO3 | Air | 1 | toluene | 27 | 3 | 222 | 65 | [24] |
| 0.89wt.% Au/SiO2 | Air | 1 | toluene | 27 | 3 | 222 | <1 | [24] |
| 1.8wt.% Au/Layered double hydroxide | O2 | 1 | toluene | 80 | 2 | 200 | 99 | [25] |
| 1.0wt.% Au/CuaMgbAlcOx | O2 | 1 | f mes. | 90 | 1 | 1181 | 85.1 | [26] |
| 5wt.% Au/TiO2 | O2 | 1 | - | 120 | 6 | 500 | 99 | [27] |
| 5wt.% Au/Carbon black | O2 | 1 | - | 120 | 4 | 500 | 65 | [27] |
| 5wt.% Au/Single wall carbon nanotubes | O2 | 1 | - | 120 | 3 | 500 | 99 | [27] |
| 5wt.% Au/MnO2-R | O2 | 4 | - | 120 | 8 | 40,000 | 81 | [28] |
| Au–Pd (2 wt.%, 1:1)/Sodium titanate nanotubes | Air | 1 | - | 120 | 10 | 10,000 | a 84(86) | [29] |
| 7.8wt.% Au/TiO2 | O2 | 10 | H2O | 100 | 8 | 100 | 100 | [30] |
| 7.8wt.% Au/TiO2+ K2CO3 | O2 | 10 | H2O | 100 | 2 | 100 | 93 | [30] |
| 10.83wt.% Au-dendrimers/ Mesoporous SiO2SBA-15+ 3 eq. K3PO4 |
O2 | 1 | CH2Cl2/H2O | RT | 24 | 33 | 99.1 | [31] |
| 0.5 wt.%(Au0–Pd0)/high surface area-BaAl2O4 | O2 | 20 | - | 140 | 0.83 | 50,000 | a 97 | [32] |
| 4wt.% Au/La2O3/TiO2 | TBHP | 1 | - | 80 | 1 | 5000 | 98 | This |
| 4wt.% Au/La2O3/TiO2 | O2 | 1 | f mes. | 80 | 1 | 100 | 99 | work |
a Instead of yield, data on conversion of alcohol are given, and acetophenone selectivity in brackets; b MW—microwave irradiation; c R—Alcohol/Active metal ratio (mol/mol); d Yac—yield of acetophenone; e ACN:tol—CH3CN:toluene (3:1); f mes.—mesitylene; g [Cu(κONN’HL)(NO3) (DMF)](NO3)∙H2—copper complex with Schiff base ligand (HL) formed from salicylic aldehyde and aminoethylpiperazine (details on structure in [6]); h [Cu(1κNOO’,2κO’,3κO”-L)] (1) and [Cu(κNOO’-HL)Cl(CH3OH)] (2)—two aroylhydrazone Cu(II) complexes in two different tautomeric forms ((1) enol and (2) keto), H2L = 2-hydroxy(2-hydroxybenzylidene)benzohydrazide (details in [7]).
Some disadvantages exist for homogeneous catalysts, namely the inability to reuse them, and the need for addition of bases and radicals, which researchers often apply to increase the yield of acetophenone. However, an exception is the work where some of us were able to synthesize catalysts based on iron complexes with low solubility, which were efficiently reused [9].
When heterogeneous base metals catalysts were used, selective oxidation of 1-phenylethanol at room temperature was possible, however, a large catalyst load and a long reaction time up to 24 h were required [13]. Catalysts based on palladium, silver, and ruthenium, exceeded the activity of previous catalyst systems, as expected, and were not deactivated during the recycling tests [16,17]. However, the disadvantages in the case of silver and palladium catalysts would include the formation of by-products [16,18,19], and a high ratio of ruthenium to alcohol needed when using ruthenium catalysts [20].
Gold-containing systems have been extensively investigated in this process [19,21,22,23,24,25,26,27,28,29,30,31,32]. The main feature of these systems is their high activity and selectivity; however, there is a tendency to their gradual deactivation due to an increase in the size of gold nanoparticles (Au NPs) during reaction and recycling tests. Also, in most cases, the authors suggest that highly dispersed gold in the metallic state is responsible for the excellent activity in the oxidation of 1-phenylethanol [24,26,27,29,31]. However, there are also supporters of the cationic nature of gold active centres in this process [23,28,33] and Liang et al. [25] considered negative charged gold as an active site.
Unfortunately, there are very few works in which the mechanism of both aerobic and especially peroxidative oxidation of 1-phenylethanol is proposed. Furthermore, when using TBHP as the oxidizing agent, the role of the catalyst is attributed to the decomposition of this oxidant into radicals responsible for the direct oxidation of alcohol [34,35]. In general, it can be concluded that supported gold-containing systems are effective catalysts in the oxidation of 1-phenylethanol; however, even in the oxidation of such a reactive alcohol, the study of the mechanism of formation of the active surface of gold-containing systems responsible for excellent catalytic performance is still the subject of numerous discussions.
Previously, in a series of our works [36,37,38,39], it was shown that 1-octanol, a representative of the less reactive alcohols, whose physical properties impose constraints to implementation of green chemistry approaches, could be effectively oxidized under mild conditions and using Au/(MexOy)/TiO2 catalysts. It was found that the formation of an active surface responsible for the catalytic properties is strictly dependent on the modifying additives used for improving metal-support interaction, a way to transform and stabilize positively charged gold active sites. It would be expected that such catalysts would exhibit higher catalytic activity in the oxidation of more reactive alcohols, such a 1-phenylethanol, which lead us to carry out the present study. Moreover, this work should also allow comparing the catalytic behaviours towards different types of primary alcohols, one inactivated (alkyl) and another activated (bearing an aromatic ring), represented by 1-octanol and 1-phenylethanol, respectively.
Therefore, in this work, we continued to investigate Au/TiO2 based catalysts, unmodified and modified with lanthana and ceria, aiming to assess their performance in 1-phenylethanol oxidation under mild conditions. We also wanted to compare the results with those previously obtained for 1-octanol oxidation, using the same catalysts, to find out whether the nature of the active sites of catalysts in 1-phenylethanol oxidation is the same as in 1-octanol, depending on a number of factors: the influence of the nature of the oxidizing agent, support nature, the pretreatment atmosphere and gold content. Additionally, to find a theoretical conclusion about the effect of gold cationic sites on alcohol activation, DFT simulations were performed.
2. Materials and Methods
Titania P25 (nonporous, 70% anatase and 30% rutile, particle size: 21 nm, purity: 99.5%, Evonik Degussa GmbH, (Essen, Germany) was used as the starting support and was modified by impregnation with aqueous solutions of La(NO3)3·6H2O or Ce(NO3)3·6H2O (Merck, Darmstadt, Germany) in a molar ratio Ti/Me (La, Ce) = 40. Au was loaded on the supports with a nominal loading of 0.5 and 4 wt.% using HAuCl4·3H2O (Merck, Darmstadt, Germany) as precursor, by deposition-precipitation with urea (Merck, Darmstadt, Germany) in the absence of light following the previously reported procedure [40,41]. Briefly, the gold precursor (4.2 × 10−3 M) and urea (0.42 M) were dissolved in distilled water, and thereafter the support was added to the solution. The resulting mixture was heated to 80 °C and kept at constant temperature for 16 h, with stirring. The initial pH was 2.4. The pH was adjusted to 7.5 by the end of gold deposition. After the deposition–precipitation procedure, the samples were centrifuged (11,000 rpm, 15 min), washed with water then centrifuged four times, and dried under vacuum 2 h at 80 °C. After drying, the samples were stored at room temperature in a desiccator under vacuum, away from light, in order to prevent any alteration.
Catalysts were previously characterized by adsorption of N2 at −196 °C on a Micromeritics Tristar 3000 Apparatus, Micromeritics Instrument Corporation (Norcross, GA, USA), X-ray diffraction (XRD) on a Philips XPert PRO diffractometer (Amsterdam, Netherlands), X-ray photoelectron spectroscopy (XPS), on a ESCALAB 200A, VG Scientific (Waltham, MA, USA), energy-dispersive X-ray spectroscopy (EDX), transmission electron microscopy (TEM), as well as scanning transmission electron microscopy-high angle annular dark field (STEM-HAADF) using one single microscope (JEOL JEM-2100F, JEOL Ltd., Tokyo, Japan), temperature programmed reduction (H2-TPR) on a ChemiSorb 2750, (Micromeritics Instrument Corporation, Norcross, GA, USA) [38,39,42], and diffuse reflectance Fourier transform infrared (DRIFT) spectra of adsorbed CO [43] on a Bruker EQUINOX 55/S FTIR spectrometer (Billerica, MA, USA).
In this work, temperature-programmed oxygen desorption (O2-TPD) was used to assess the nature of the interaction of oxygen with the surface of the catalyst and the support. All experiments were performed on a “Chemosorb” chemisorption analyzer (Neosib, Novosibirsk, Russia) equipped with a thermal conductivity detector (TCD), which was calibrated with O2 prior analysis. A sample (0.2 g) pretreated at 300 °C in helium flow (60 mL/min) was saturated with oxygen at 40 °C for 1 h. Oxygen desorption was carried out in a helium stream from 40 to 650 °C with a heating rate of 20 °C/min.
The used (i.e., after the reaction test) catalysts were also characterized by X-ray photoelectron spectroscopy. Therefore, the surface composition and the chemical state of gold were determined by XPS analysis, performed on an ESCALAB 200A spectrometer (VG Scientific, Waltham, MA, USA) using Al Kα radiation (1486.6 eV). A pass-energy of 40 eV and a step size of 0.1 eV/step were selected. The charge effect was corrected using the C1s peak as a reference (binding energy of 285 eV). The CASA XPS software (version 2.3.15, CASA Software Ltd, Teignmouth, UK, http://www.casaxps.com/) was used for data analysis.
The catalytic properties for 1-phenylethanol oxidation were studied using catalyst samples either without treatment or after pre-treatment at 300 °C in H2 or O2 atmosphere. Therefore, catalysts samples will be denoted hereinafter as n% Au/(MexOy)/TiO2_X, where n is the gold content in wt.%, Me is the modifier metal (La or Ce) and X indicates the applied pretreatment atmosphere (O2 or H2). The catalytic tests were carried out in a batch reactor operated under atmospheric conditions at the temperature 80 °C for 6 h. The stirring reaction mixture was as follows: 1.27 µmol of Au were introduced in 1-phenylethanol (6 mmol) as the substrate, TBHP (70% v/v aqueous solution, 14.6 mmol) as the oxidizing agent, in a base- and solvent-free medium.
After the reaction test, the mixture was allowed to cool down to room temperature. Typically, to conduct the product analysis, 10 µL of benzaldehyde (internal standard) and 1 mL of MeCN were added to 100 µL of such reaction mixture. The resulting sample was centrifuged for 15 min and analyzed by gas chromatography (GC) using the internal standard method. Blank tests indicated that only traces (4%) of ketone were generated. Chromatographic analyses were undertaken using a GC 8000 series gas chromatograph (Fisons Instruments, Loughborough, UK) equipped with a BP-20 (WAX) capillary column (SGE Analytical Science Europe Ltd, Milton Keynes, UK) and a flame ionization detector (FID) detector (Fisons Instruments, Loughborough, UK). Molar yield (%) was defined based on substrate, i.e., moles of product per 100 mol of substrate, determined by GC. Attribution of peaks was made by comparison with chromatograms of genuine samples and, in some cases, by gas chromatography mass-spectrometry (GC–MS) analyses with He as the carrier gas using a Clarus 600C instrument (Perkin Elmer, Waltham, MA, USA), equipped with a 30 m × 0.22 mm × 25 µm BPX5 (SGE Analytical Science Europe Ltd, Milton Keynes, UK) capillary column. According to the GC-MS acetophenone was the only product in both aerobic and peroxidative oxidation of 1-phenylethanol.
To adequately compare the catalytic results of aerobic oxidation of 1-phenylethanol with those of 1-octanol, the conditions selected for the oxidation of 1-phenylethanol with molecular oxygen were as follows: alcohol/gold ratio (R) = 100 mol/mol, 25 mL of a 0.1 M solution of 1-phenylethanol in mesitylene, O2 flow = 30 mL/min, stirring 800 rpm. And to compare the catalytic results of the peroxidative oxidation of 1-octanol with of those of 1-phenylethanol, the conditions selected for the peroxidative oxidation of 1-octanol were as follows: R = 5000 mol/mol, T = 80 °C, TBHP/alcohol = 2.43 mmol/mmol, stirring 800 rpm, without solvent or base added. In the absence of support/catalyst in the reaction mixture no activity was observed in both aerobic and peroxidative oxidation of 1-octanol.
To perform the recycling experiments, the used sample was separated from the reaction mixture by centrifugation (5000 rpm, 15 min) and decantation, washed 4 times with 5 mL of acetonitrile and dried at 50 °C to constant weight. It was then reused for the oxidation test as described above.
The adsorption of 1-phenylethanol on gold nanoparticles was simulated in a scalar-relativistic approach using the density functional (DFT) method PBE [44]. The tetrahedral Au20 and Au20+ clusters are considered as models of gold nanoparticles. The Au20 cluster has been obtained experimentally [45] and is a popular model for studying structural effects in catalysis [46,47]. Because the cluster has atoms located at the top, on the facet and on the edge and having different coordination number, we can effectively apply this model to study the structural effects in the adsorption of phenylethanol. A cationic gold cluster was obtained by removing one electron from Au20 with subsequent optimization of the structure. The effect of the support was ignored as a first approximation.
The interaction of 1-phenylethanol or 2-phenylethanol with Au20 cluster was simulated:
| Au20 + CH3–CH(OH)–Ph → (CH3–CH(OH)–Ph)Au20 | (1) |
| Au20 + OH–CH2–CH2–Ph → (OH–CH2–CH2–Ph)Au20 | (2) |
Different coordination modes of alcohol on Au20 by the OH group or the phenyl fragment were considered. The structures of (OH–CH2–CH2–Ph)Au20 isomers were optimized, and the total energies of the reagents and products were calculated taking into account the energy of zero vibrations. The change in total energy and standard enthalpies of the reaction (1) were determined according to the formula:
| ΔE = E(phenylethanol–Au20) − E(Au20) − E(phenylethanol) | (3) |
The calculations were performed in the PRIRODA program (version 17, Russia) [48] using a Lomonosov supercomputer [49].
3. Results and Discussion
3.1. Catalytic Results
3.1.1. Peroxidative Oxidation of 1-Phenylethanol
Since we had the goal of finding out the same active sites in both processes, the reaction conditions should be the same. Therefore, as in the case of 1-octanol oxidation, all experiments on both peroxidative and aerobic oxidation of 1-phenylethanol were carried out at a temperature of 80 °C (conventional heating) (Scheme 1).
Scheme 1.
Oxidation of 1-phenylethanol over Au/(MexOy)/TiO2 catalysts.
We started the catalytic experiments with TBHP as oxidizing agent with a very low catalyst loading equal to the ratio of 1-phenylethanol/Au of 5000 and in no-solvent conditions. The results are presented in Table 2. As expected, both unmodified and modified catalysts proved to be much more active in the oxidation of 1-phenylethanol than in that of 1-octanol. Taking into account the high alcohol/gold ratio, the catalysts, even in the as-prepared state, reached more than 50% of acetophenone yield after 6 h of reaction, despite the low activity in the first hours (Scheme 1). As in the oxidation of 1-octanol [38,39], the sample modified with lanthanum oxide demonstrated the best catalytic performance (Table 2): 98% yield of acetophenone was achieved already in 1 h when 4% Au/La2O3/TiO2 pretreated in H2 was used. It should also be noted that the activity of the studied catalysts increased with increasing gold content.
Table 2.
Catalytic results of peroxidative oxidation of 1-phenylethanol 1.
| Entry | Sample | Yield of Acetophenone (mol%) at Time (h) | ||||||
|---|---|---|---|---|---|---|---|---|
| 0.25 | 0.5 | 1 | 2 | 3 | 4 | 6 | ||
| 1 | - | 0 | 0 | 1 | 2 | 3 | 3 | 4 |
| 2 | TiO2 | 2 | 2 | 2 | 2 | 2 | 4 | 6 |
| 3 | CeO2/TiO2 | 3 | 3 | 3 | 4 | 6 | 8 | 14 |
| 4 | La2O3/TiO2 | 3 | 3 | 3 | 6 | 8 | 10 | 17 |
| 5 | 0.5% Au/TiO2_H2 | 25 | 40 | 55 | 80 | 100 | 100 | 100 |
| 6 | 0.5% Au/TiO2_O₂ | 18 | 30 | 43 | 69 | 81 | 98 | 100 |
| 7 | 0.5% Au/TiO2_as | 1 | 1 | 3 | 9 | 16 | 28 | 51 |
| 8 | 4% Au/TiO2_H₂ | 33 | 46 | 75 | 100 | 100 | 100 | 100 |
| 9 | 4% Au/TiO2_O₂ | 30 | 49 | 65 | 99 | 100 | 100 | 100 |
| 10 | 4% Au/TiO2_as | 1 | 2 | 6 | 19 | 28 | 45 | 82 |
| 11 | 0.5% Au/CeO2/TiO2_H₂ | 27 | 37 | 60 | 85 | 100 | 100 | 100 |
| 12 | 0.5% Au/CeO2/TiO2_O₂ | 28 | 36 | 72 | 90 | 100 | 100 | 100 |
| 13 | 0.5% Au/CeO2/TiO2_as | 2 | 3 | 3 | 7 | 14 | 28 | 61 |
| 14 | 4% Au/CeO2/TiO2_H₂ | 36 | 49 | 85 | 100 | 100 | 100 | 100 |
| 15 | 4% Au/CeO2/TiO2_O₂ | 22 | 41 | 75 | 100 | 100 | 100 | 100 |
| 16 | 4% Au/CeO2/TiO2_as | 3 | 4 | 10 | 25 | 46 | 67 | 100 |
| 17 | 0.5% Au/La2O3/TiO2_H₂ | 31 | 45 | 55 | 88 | 100 | 100 | 100 |
| 18 | 0.5% Au/La2O3/TiO2_O₂ | 38 | 49 | 61 | 96 | 100 | 100 | 100 |
| 19 | 0.5% Au/La2O3/TiO2_as | 1 | 1 | 2 | 8 | 18 | 42 | 84 |
| 20 | 4% Au/La2O3/TiO2_H₂ | 41 | 55 | 98 | 100 | 100 | 100 | 100 |
| 21 | 4% Au/La2O3/TiO2_O₂ | 22 | 40 | 67 | 100 | 100 | 100 | 100 |
| 22 | 4% Au/La2O3/TiO2_as | 1 | 2 | 6 | 29 | 54 | 100 | 100 |
1 Reaction conditions: TBHP: 1-phenylethanol = 2.43; T = 80 °C, stirring, R= 5000 (1.27 µmol of Au).
However, it should be emphasized that the supports also showed some activity under these conditions, as the yield of acetophenone increased with time and depended on the support nature (Entries 2–4, Table 2). In the absence of a catalyst or support, the formation of a small amount of acetophenone was also observed (Entry 1, Table 2). For the oxidation of 1-phenylethanol with molecular oxygen, no activity was observed when using supports only, and neither in the absence of a support/catalyst, as will be seen below. This shows the very good activity of TBHP, which, when decomposed by heating or by reaction with a metal (see Introduction), forms radicals (t-BuO·, t-BuOO·), responsible for the direct oxidation of alcohols [34]. Therefore, in the referred work, 97% acetophenone was obtained using 6 equivalents of TBHP at 100 °C in 24 h, without adding any catalysts or bases [35].
When using the La-modified sample, which showed a medium activity in 1-phenylethanol peroxidative oxidation, direct dependence of the yield of acetophenone on catalyst loading was observed (Table 3). Thus, 100% acetophenone was reached already after 15 min of reaction when the total gold amount was increased from 1.27 to 10 µmol. It should be noted that experiments using hydrogen peroxide (30% aqueous solution) as oxidant were not effective.
Table 3.
Effect of the total gold amount on the peroxidative oxidation of 1-phenylethanol 1 using 4% Au/La2O3/TiO2_O2.
| Au Amount (µmol) | Yield of Acetophenone (mol%) at Time (min) | |||||
|---|---|---|---|---|---|---|
| 5 | 15 | 30 | 60 | 120 | 180 | |
| 1.27 | 15 | 22 | 40 | 67 | 100 | 100 |
| 5 | 33 | 40 | 50 | 81 | 100 | 100 |
| 10 | 80 | 100 | 100 | 100 | 100 | 100 |
| 20 | 100 | 100 | 100 | 100 | 100 | 100 |
1 1-phenylethanol (6 mmol), TBHP (70% aqueous solution, 14.6 mmol), T = 80 °C.
In the case of the 4% Au/La2O3/TiO2_H2 catalyst, only 11% of the product yield was achieved after 6 h of reaction, under the same conditions used with TBHP (T = 80 °C, 6 mmol 1-phenylethanol, 14.6 mmol H2O2, 1.27 µmol of Au). With the same catalyst, a 98% yield of acetophenone was obtained after 1 h using TBHP (Entry 20, Table 2). Thus, conditions close to the principles of green chemistry were selected to compare with the above-mentioned oxidation process of 1-octanol: a solvent-free oxidation of 1-phenylethanol with Au/(MexOy)/TiO2 catalysts, for 6 h, at T = 80 °C, using a minimum gold loading (1.27 µmol), and TBHP as a green oxidant, without any additives.
3.1.2. Aerobic Oxidation of 1-Phenylethanol
After replacing TBHP with molecular oxygen, keeping the same alcohol/gold ratio (R = 5000), we did not observe any conversion, even after 6 h of reaction (Table 4).
Table 4.
Effect of alcohol/Au ratio (R) on aerobic oxidation of 1-phenylethanol 1 with 4% Au/La2O3/TiO2_H2 catalyst.
| Catalyst | R | Yield of Acetophenone, % |
|---|---|---|
| 4% Au/La2O3/TiO2_H₂ | 5000 | 0 2 |
| 500 | 50 2 | |
| 100 | 98 3 |
1 Reaction conditions: 0.1 M 1-phenylethanol in mesitylene, T = 80 °C, 30 mL/min O2. 2 After 6 h. 3 After 0.5 h.
The next step was to investigate the effect of the alcohol/gold ratio. With a ten-fold increase in catalyst loading (R = 500), 50% conversion was observed after 6 h of reaction. A complete conversion of 1-phenylethanol, using this catalyst, could be achieved only at R = 100, with 98% acetophenone yield being reached after just 30 min of reaction.
Such a different behaviour in the catalytic activity probably lies in the different oxidative capacity of oxygen and TBHP. Thus, the catalytic activity in the aerobic oxidation of 1-phenylethanol of the remaining catalysts was studied with R = 100. As it can be seen in Table 5, catalysts with a low gold content with an oxidative pretreatment were the most active, as in the case of 1-octanol (Figure 1b,e,f) [39].
Table 5.
Catalytic results of aerobic oxidation of 1-phenylethanol 1.
| Entry | Catalyst | Yield of Acetophenone (mol%) at Time (h) | ||||||
|---|---|---|---|---|---|---|---|---|
| 0.25 | 0.5 | 1 | 2 | 3 | 4 | 6 | ||
| 1 | 0.5% Au/TiO2_H₂ | 21 | 25 | 31 | 40 | 45 | 47 | 50 |
| 2 | 0.5% Au/TiO2_O₂ | 26 | 32 | 38 | 44 | 49 | 52 | 58 |
| 3 | 4% Au/TiO2_H₂ | 32 | 36 | 35 | 41 | 42 | 43 | 44 |
| 4 | 4% Au/TiO2_O₂ | 19 | 21 | 25 | 30 | 35 | 38 | 40 |
| 5 | 0.5% Au/CeO2/TiO2_H₂ | 45 | 50 | 60 | 65 | 68 | 70 | 72 |
| 6 | 0.5% Au/CeO2/TiO2_O₂ | 70 | 86 | 96 | 99 | 100 | 100 | 100 |
| 7 | 4% Au/CeO2/TiO2_H₂ | 53 | 59 | 65 | 69 | 71 | 73 | 78 |
| 8 | 4% Au/CeO2/TiO2_O₂ | 40 | 44 | 50 | 55 | 59 | 65 | 70 |
| 9 | 0.5% Au/La2O3/TiO2_H₂ | 90 | 95 | 97 | 100 | 100 | 100 | 100 |
| 10 | 0.5% Au/La2O3/TiO2_O₂ | 95 | 98 | 100 | 100 | 100 | 100 | 100 |
| 11 | 4% Au/La2O3/TiO2_H₂ | 88 | 96 | 99 | 100 | 100 | 100 | 100 |
| 12 | 4% Au/La2O3/TiO2_O₂ | 79 | 88 | 93 | 97 | 98 | 99 | 100 |
1 Reaction conditions: R = 100, 0.1 M 1-phenylethanol in mesitylene; T = 80 °C, 30 mL/min O2, stirring = 800 rpm.
Figure 1.
Time evolution of catalytic peroxidative (a,c,e) and aerobic (b,d,f) oxidation of 1-octanol. Reaction conditions of solvent-free peroxidative oxidation: R = 5000, TBHP: 1-octanol = 2.43; T = 80 °C, stirring = 800 rpm. Reaction conditions of aerobic oxidation: R = 100, 0.1 M 1-octanol in n-heptane; T = 80 °C, 30 mL/min O2, stirring = 800 rpm.
3.1.3. Peroxidative and Aerobic Oxidation of 1-Octanol
Since this work also aimed at making a comparison with previous results obtained for the oxidation of 1-octanol, we also studied the effects of the oxidizing agent in the oxidation of this substrate. The trends of catalytic behaviour of the catalysts in the peroxidative and aerobic oxidation of 1-octanol were similar in terms of activity (Figure 1). The most active catalysts were those with low Au loading, after an oxidative pretreatment. Regarding selectivity, it is important to note that when using molecular oxygen as an oxidizing agent, a different behaviour is observed in the distribution of oxidation products, depending on the pretreatment, the nature of the support and the gold content [39]. However, when TBHP was used as oxidizing agent, in the absence of a solvent, the main product, in all cases, was acid with a small amount of ester (up to 20%). This is probably due to the peroxide used as oxidizing agent which decomposed producing water [34], that is needed for octanoic acid formation, according to the Scheme 2 (route A) from [36,39].
Scheme 2.
Possible reaction pathways for the oxidation of 1-octanol on supported gold catalysts (Adapted from [36,39]).
Therefore, according to all catalytic results, under comparable reaction conditions, 1-phenylethanol can be much more efficiently and selectively oxidized over Au/MexOy/TiO2 catalysts than 1-octanol. Moreover, the best results in the oxidation of both alcohols were achieved using TBHP.
In all cases, the best catalytic characteristics were shown by catalysts modified with lanthanum oxide, regardless of the alcohol and the type of oxidizing agent. Also, when a solvent was used and molecular oxygen was present as an oxidizing agent, the catalysts with the lowest gold content after oxidative pretreatment showed the highest activity in both 1-phenylethanol and 1-octanoloxidation.
The only difference was that under no-solvent peroxidative conditions, the most active catalysts in 1-phenylethanol oxidation are those with a high load of gold and the hydrogen treatment, while under the same reaction conditions, low gold loading and oxygen treatment were beneficial in 1-octanol oxidation.
3.2. Catalyst Characterization
Catalysts were previously characterized by several techniques [38,39,42,43]. The N2 adsorption and EDX analysis (Table 6) showed that the textural properties and the content of gold cannot be the reason for the different catalytic behaviour observed for the catalysts. In addition, TEM and STEM-HAADF measurements showed no direct correlations between the average Au particle size and catalytic properties. A number of nanoparticles in the range 1–7 nm with different distributions were observed in all catalysts (Table 6).
Table 6.
Textural properties of supports and catalysts, analytical content and particle size of Au. Adapted from [38,39].
| Sample | SBET, m2/g | EDX Au Content, wt.% | Au Average Particle Size, nm |
|---|---|---|---|
| TiO2 | 55 | - | - |
| La2O3/TiO2 | 48 | - | - |
| CeO2/TiO2 | 48 | - | - |
| 0.5% Au/TiO2_H2 | 54 | 0.4 | 4.4 |
| 0.5% Au/TiO2_O2 | 54 | 0.4 | 4.2 |
| 4% Au/TiO2_H2 | 50 | 4.0 | 2.9 |
| 4% Au/TiO2_O2 | 50 | 4.0 | 3.3 |
| 0.5% Au/CeO2/TiO2_H2 | 47 | 0.3 | 3.4 |
| 0.5% Au/CeO2/TiO2_O2 | 47 | 0.3 | 3.8 |
| 4% Au/CeO2/TiO2_H2 | 46 | 4.1 | 2.8 |
| 4% Au/CeO2/TiO2_O2 | 46 | 4.1 | 2.4 |
| 0.5% Au/La2O3/TiO2_H2 | 47 | 0.5 | 2.8 |
| 0.5% Au/La2O3/TiO2_O2 | 47 | 0.5 | 2.4 |
| 4% Au/La2O3/TiO2_H2 | 43 | 3.3 | 2.6 |
| 4% Au/La2O3/TiO2_O2 | 43 | 3.3 | 2.7 |
XPS measurements of catalysts showed that gold formed different electronic states on the support surface—ions Au+ and Au3+, and neutral gold nanoparticles. The ratio among these states depends strongly on the support and the modifier nature (Table 7). XPS results for ceria modified samples are presented in Figure A1.
Table 7.
Effect of gold content (0.5 or 4 wt.%) and redox treatment (H2 or O2) on contribution of different electron states of Au calculated according to XPS for Au/TiO2, Au/CeO2/TiO2, Au/La2O3/TiO2 catalysts.
| Catalyst | Au(0, 1+ or 3+) Relative Content, % | ||
|---|---|---|---|
| Au0 | Au1+ | Au3+ | |
| 1 0.5% Au/TiO2_H2 | 91 | 9 | 0 |
| 1 0.5% Au/TiO2_O2 | 84 | 16 | 0 |
| 1 4% Au/TiO2_H2 | 73 | 14 | 11 |
| 1 4% Au/TiO2_O2 | 89 | 11 | 0 |
| 3 0.5% Au/CeO2/TiO2_H2 | 91 | 9 | 0 |
| 3 0.5% Au/CeO2/TiO2_O2 | 85 | 15 | 0 |
| 2 4% Au/CeO2/TiO2_H2 | 68 | 20 | 12 |
| 3 4% Au/CeO2/TiO2_O2 | 90 | 10 | 0 |
| 1 0.5% Au/La2O3/TiO2_H2 | 80 | 20 | 0 |
| 1 0.5% Au/La2O3/TiO2_O2 | 65 | 35 | 0 |
| 1 4% Au/La2O3/TiO2_H2 | 81 | 19 | 0 |
| 1 4% Au/La2O3/TiO2_O2 | 83 | 17 | 0 |
According to the H2-TPR and CO2-TPD results, it was concluded that modification by La oxide favored the formation of very stable ionic species Auδ+ (0 < δ < 1) by their localization on the strong basic Lewis sites, formed by two-electron orbitals of the oxygen atom on the support surface (data not shown, obtained in [39]). The highest portion of such gold states was observed in the Au/La2O3/TiO2 sample, while they were practically absent in unmodified and modified with ceria catalysts. Also, according to DRIFT CO (Figure A2, Reproduced from Ref. [43] with permission from the Royal Society of Chemistry) and XPS data, the contribution of singly charged ions and their stability were maximum for the most active catalysts (4% Au/La2O3/TiO2_H2 and 0.5% Au/La2O3/TiO2_O2). Thus, although the amount of monovalent ions (determined by XPS) for some materials was comparable to the values found for lanthanum-modified catalysts, strong and stable gold ions were only found in lanthanum-modified samples, according to the applied DRIFT CO method [43].
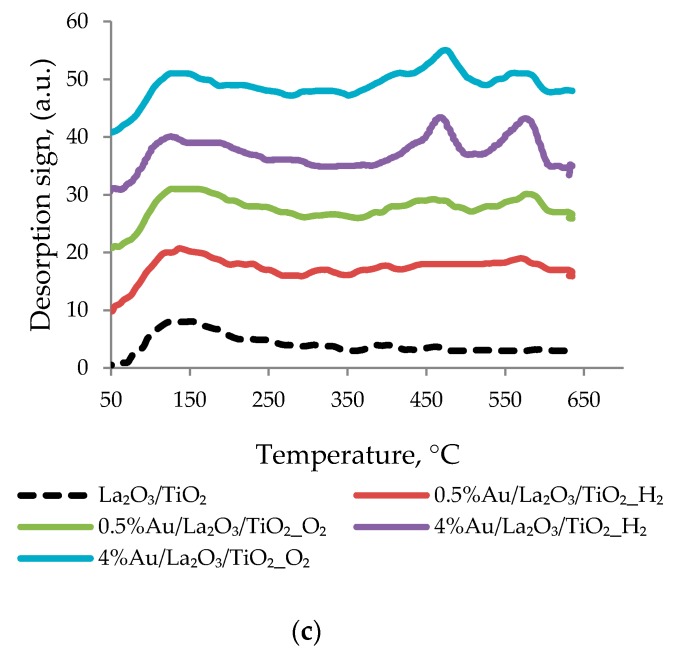
O2-TPD was used to assess the nature of the interaction of oxygen with the surface of the catalyst and the support. The O2-TPD profiles obtained for the catalysts and their corresponding supports (Figure 2) show the presence of several peaks of oxygen desorption corresponding to different forms of adsorbed oxygen.
Figure 2.
O2-TPD profiles for Au nanoparticles on TiO2 (a)CeO2/TiO2 (b)and La2O3/TiO2 (c)with different pretreatments.
Moreover, all the catalysts showed a wide peak of oxygen desorption in the range of 50–350 °C, which may be due to the adsorption of O2−on TiO2, according to [50]. It should be noted that pure titania showed three overlapping peaks (at 95, 205 and 292 °C) in this temperature range. After gold deposition on TiO2, a slight change in the shape of these peaks was observed. After titania was modified with ceria and lanthana, the shape and position of the wide peak in the low-temperature region changed, overlapping maxima were observed at 100, 190 and 265 °C for CeO2/TiO2 and at 110, 230 and 310 °C for La2O3/TiO2.
The deposition of gold on the ceria-modified support did not cause a significant change in the O2-TPD profiles. However, after the deposition of gold on the surface of lanthana-modified titania, high-temperature peaks appeared in the range of 400–600 °C, and catalysts with 4% Au had a noticeably high intensity, with peaks at 460 and 560 °C. These desorption peaks are likely to occur at higher temperatures and correspond to adsorbed O− on the surface of TiO2, as described in [50,51]. Addition of lanthana and gold effectively stimulate the dissociation of O2 to O−, which has a higher activity than the super-oxo form O2− in the oxidation reaction [50,52,53]. Thus, these results support the previously established favorable features of doping titania with lanthana (formation and stabilization of single charged gold ions through their localization on strong basic sites of La2O3/TiO2). Besides they revealed another promoting role of modifying additives of lanthana: providing the most active type of oxygen for effective oxidation of alcohols.
When comparing the XPS and the catalytic results of the catalysts in the peroxidative and aerobic oxidation of 1-phenylethanol (Table 8), there is no apparent correlation. Nevertheless, the most active catalysts (4% Au/La2O3/TiO2_H2 and 0.5% Au/La2O3/TiO2_O2) show the largest contribution of monovalent gold ions (Entries 1,2, Table 8).
Table 8.
Catalytic results of the most active catalysts in the peroxidative and aerobic oxidation of 1-phenylethanol and contribution of various electronic states of gold in these catalysts, calculated by XPS.
| Entry | Sample | Yield of Acetophenone in 2 h, mol % | Relative Au Content, % | |
|---|---|---|---|---|
| Au0 | Au+ | |||
| 1 | 4% Au/La2O3/TiO2_H₂ 1,a | 100 | 81 | 19 |
| 2 | 0.5% Au/La2O3/TiO2_O₂ 2,a | 100 | 65 | 35 |
| 3 | 4% Au/La2O3/TiO2_H₂_1c 1,b | 90 | 83 | 17 |
| 4 | 4% Au/La2O3/TiO2_H₂_6c 1,c | 59 | 89 | 11 |
1R = 5000, TBHP: 1-phenylethanol; T = 80 °C, stirring = 800 rpm. 2 R = 100, 0.1 M 1-phenylethanol in mesitylene; T = 80 °C, O2 = 30 mL/min, stirring = 800 rpm. a XPS performed for the catalyst before reaction. b XPS performed for the used catalyst after 1 cycle of reaction. c XPS performed for the used catalyst after 6 cycles of reaction.
3.3. Catalyst Recycling Tests
The catalyst recyclability in the peroxidative oxidation of 1-phenylethanolwas investigated up to six consecutive cycles, as described in the Experimental part, with the best performing catalyst, i.e., 4% Au/La2O3/TiO2_H2. As can be seen in Figure 3, there was a gradual catalyst deactivation during the recycling tests. Particularly, in the second cycle, the catalyst maintained 90% of activity, whereas in the third cycle a loss of 23% of its initial activity was observed. Consecutive decreasing of activity stopped at the sixth cycle where the yield of acetophenone kept the same level as in the fifth cycle (58–59%). Nevertheless, high selectivity to acetophenone was preserved in each cycle.
Figure 3.
Effect of the catalyst recycling on the yield of acetophenone from peroxidative oxidation of 1-phenylethanol under catalyzed by 4% Au/La2O3/TiO2_H2 (1.27 µmol Au, TBHP: 1-phenylethanol = 2.43,2 h, T = 80 °C).
To find out the cause of the observed catalyst deactivation, XPS analysis of the catalyst after the first and last (6th) cycle was performed (Figure 4) and compared with the XPS results for the catalyst before reaction. As in the “fresh” catalyst, before reaction (Entry 1, Table 8), two electronic states of gold were found in the used catalysts: metallic (Au0) with binding energy (BE) (Au4f7/2) 84.2 and single charged ions (Au+) with BE (Au4f7/2) 85.2 eV in catalyst after the 1st cycle and Au0 with BE (Au4f7/2) 84.1 eV and (Au+) with BE (Au4f7/2) 85 eV [54,55,56,57] in catalyst after 6th cycles were detected. However, according to XPS measurements, the surface concentration of gold is different among the studied samples (Entries 1,3,4, Table 8). It can be seen from Table 8 that, with each cycle, loss of catalytic activity decreased proportionally to the contribution of monovalent gold. That can probably be the reason for deactivation, as also in the case of 1-octanol (see detailed information in Table A1 as well as discussion of previous results from [39]), since gold monovalent ions were the proposed active species for the reaction.
Figure 4.
XPS of used 4%Au/La2O3/TiO2_H2 catalyst after the 1st (a) and 6th (b) cycles of 1-phenylethanol peroxidative oxidation (reaction conditions as in Figure 1).
3.4. Quantum Chemical Simulation of the Alcohol Adsorption on a Gold Cluster
The purpose of this study was to understand the following issues relating to the nature of the active sites of the gold nanoparticles in the activation of phenylethanol at the atomic level:
-
(i)
Which phenylethanol coordination on a gold cluster is preferred (by OH– or C6H5– groups)?
-
(ii)
How do the structural features of the catalyst surface, including availability of low coordinated gold atoms, affect the adsorption of the alcohol?
-
(iii)
What is the effect of gold cationic sites on alcohol activation?
The optimized structures of phenylethanol-Au20 complexes, in which the alcohol is coordinated to a gold atom by the OH group, are shown in Figure 5. The energy changes during the formation of the complexes and the corresponding standard enthalpies are collected in Table 9. According to the calculated data, the most favorable coordination during the interaction of phenylethanol with Au20 is carried out at the top of the cluster. Both 1-phenylethanol and 2-phenylethanol bind to the top gold atom with similar values of adsorption energy (57–60 kJ/mol). The binding energies of alcohol on the edge and facet gold atoms are significantly lower (considering 1-phenylethanol as an example).
Figure 5.

Optimized structures of Au20 clusters and phenylethanol-Au20 complexes (coordination by OH group).
Table 9.
The calculated values of the energy change (ΔE, kJ/mol) and standard enthalpy (ΔH, kJ/mol) in the reactions of 1-phenylethanol (1) or of 2-phenylethanol (2) with the Au20z cluster (z = 0, +1).
| z | Complex | Isomer | Type of Coordination | ΔE | ΔH | |
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | OH– | top | −57 | −54 |
| 0 | 2 | 1 | OH– | top | −60 | −57 |
| 0 | 3 | 2 | OH– | edge | −33 | −29 |
| 0 | 4 | 2 | OH– | facet | −30 | −31 |
| 0 | 5 | 2 | C6H5– | top | −49 | −45 |
| 0 | 6 | 2 | C6H5– | edge | −23 | −21 |
| +1 | 1 | 2 | OH– | top | −97 | −92 |
| +1 | 2 | 1 | OH– | top | −112 | −108 |
| +1 | 3 | 2 | OH– | edge | −75 | −70 |
| +1 | 4 | 2 | OH– | facet | −56 | −54 |
| +1 | 5 | 2 | C6H5– | top | −102 | −100 |
| +1 | 6 | 2 | C6H5– | edge | −75 | −72 |
When alcohol is coordinated on the cluster through a benzene fragment (Figure 6), the adsorption energies decrease at all sites of Au20, compared to OH– group coordination. Among two different ways of alcohol coordination (OH– or C6H5–), coordination with the OH– group is advantageous (Complex 2, Figure 5). In this case, low-coordinated gold atoms are the most active in the activation of alcohol.
Figure 6.
Optimized structures of phenylethanol-Au20 complexes (coordination by C6H5 fragment).
Then we examined how the gold cationic sites affect alcohol adsorption. The interaction of 1-phenylethanol or 2-phenylethanol with an Au20+ cluster was simulated at different coordinations (by the OH group or the phenyl fragment):
| Au20+ + CH3–CH(OH)–Ph → (CH3–CH(OH)–Ph)Au20+ | (4) |
| Au20+ + OH–CH2–CH2–Ph → (OH–CH2–CH2–Ph)Au20+ | (5) |
The optimized structures of phenylethanol-Au20+complexes have similar features with neutral phenylethanol-Au20. In contrast, the calculated energy changes in reaction 4 (Table 9) are larger than in reaction 1. The binding energy of phenylethanol to low-coordinated cationic gold atoms through the OH– group is significantly higher than on the neutral cluster. When 1-phenylethanol is coordinated by an aromatic fragment on Au20+, the adsorption energy increases and becomes almost the same as the coordination of alcohol with the OH group. Based on the study, it can be concluded that the cationic sites play an important role in phenylethanol adsorption.
4. Conclusions
From the above results, it could be concluded that Au/(MexOy)/TiO2 systems were highly effective in the oxidation of 1-phenylethanol, and the catalysts modified with lanthana were the most active, as in the oxidation of 1-octanol. Furthermore, comparing our results on 1-phenylethanol oxidation with other gold supported catalysts (Table 1), it could be concluded that the Au/(MexOy)/TiO2 systems are one of the most effective in peroxidative selective oxidation of 1-phenylethanol with TBHP, given the high alcohol/gold ratio (R = 5000), and low temperature (T = 80 °C) used without bases and solvents.
In the absence of a solvent and using TBHP as an oxidizing agent, the most active catalysts are those with a high load of gold and the hydrogen treatment was beneficial. In the case of using solvent and oxygen as an oxidizing agent, as in the oxidation of 1-octanol, the catalysts with the lowest gold content after oxidative pretreatment showed the highest activity. The reason of such a difference most likely is the reduction of some part of unstable gold ions to metallic state in a 0.5% Au/La2O3/TiO2_O2 catalyst by electron transfer under the influence of TBHP (even catalysts in «as prepared state» could provide high activity in 1-phenylethanol oxidation with TBHP after 6 h). Moreover, it was previously shown that there are the same contribution of stable gold ions in these two catalysts (0.5% Au/La2O3/TiO2_O2 and 4% Au/La2O3/TiO2_H2); furthermore, the difference in catalytic activity of 1-phenylethanol oxidation on these catalysts was not so noticeable.
The most active catalysts had a high concentration of stable monovalent gold ions, and deactivation of catalysts in 1-phenylethanol oxidation is parallel to the reduction of Au+ (Auδ+) states, that confirms the cationic nature of the active sites. The promoting role of lanthanum additives consists not only in the formation of the most stable Au+ (Auδ+) species on the surface of La-modified TiO2 due to their localization at the strong basic Lewis sites, as proved earlier, but also in the presence of the most active type of oxygen, contributing to a more efficient oxidation of alcohols.
Thus, a general conclusion can be drawn that, despite the different nature of the studied alcohols, the nature of the active sites of Au/(MexOy)/TiO2 catalysts in both the aerobic and peroxidative oxidation of 1-phenylethanol and 1-octanol is the same and monovalent gold ions are the active sites in these processes. Herein, the concentration, strength and stability of these sites are determined by the gold content, the nature of the support and modifier, and the pretreatment atmosphere.
The adsorption of 1-phenylethanol on Au clusters was simulated using DFT calculations. Among two different ways of alcohol coordination, that with the OH– group is the most advantageous one. In this case, low-coordinated gold atoms are the most active in the activation of alcohol. The binding energy of 1-phenylethanol with low-coordinated cationic gold atoms through the OH– group is significantly higher than that on the neutral cluster. Therefore, based on the quantum chemical calculations, it was concluded that the cationic sites play an important role in 1-phenylethanol adsorption, what confirms our suggestions on the gold cationic nature based on experimental results. The theoretical results were similar for 2-phenylethanol.
Acknowledgments
Authors thank Carlos Sá (CEMUP) for the assistance with XPS analyses.
Appendix A
Figure A1.
Au4f XP spectra of Au/CeO2/TiO2 samples with different gold content (0.5 or 4 wt.%) pretreated in H2 or O2 flow at 300 °C for 1 h: 0.5%Au/CeO2/TiO2_O2 (a), 0.5%Au/CeO2/TiO2_H2 (b) and 4%Au/CeO2/TiO2_O2 (c).
Appendix B
Figure A2.
DRIFT spectra of CO adsorbed on the most active catalysts in peroxidative and aerobic oxidation of 1-phenylethanol. Reproduced from Ref. [43] with permission from the Royal Society of Chemistry, 2019.
Appendix C
Table A1.
Catalytic results of the most active catalyst in the aerobic oxidation of 1-octanol and contribution of various electronic states of gold in this catalyst, calculated by XPS. Adapted from [39].
| Entry | Sample | Conversion of 1-Octanol After 6 h, ca.% |
Relative Au Content, % | |
|---|---|---|---|---|
| Au0 | Au+ | |||
| 1 | 0.5% Au/La2O3/TiO2_O₂ 1 | 63 | 65 | 35 |
| 2 | 0.5% Au/La2O3/TiO2_O₂ 2,a | 31 | 87 | 13 |
| 3 | 0.5% Au/La2O3/TiO2_H₂ 1,b | 34 | 87 | 13 |
1 XPS performed for the catalyst before reaction. 2 XPS performed for the catalyst after reaction. a XPS performed for the used catalyst after 6 h of reaction. b XPS performed for the catalyst pretreated in H2 at 500 °C for 1 h.
As we can see in Table A1, there was also significant a loss of 50% of its initial activity already in the second cycle of 1-octanol oxidation with O2 (Entry 2, Table A1). At the same time, a proportional decrease of monovalent ions in this sample after the first catalytic cycle is also observed. However, on the basis of H2-TPR analysis it was shown that such deactivation is related to the reduction of unstable monovalent gold ions (20–23%). Moreover, in order to check the stability of the mentioned gold ions and also confirm that they are the active sites, 0.5% Au/La2O3/TiO2_O2 sample was treated in a hydrogen atmosphere at higher temperature (500 °C), for reduction of unstable gold ions. According to the XPS analysis of this sample (Entry 3, Table A1), it was found a decrease of 13% in the surface concentration of Auδ+ ions. This reduced sample was tested in a 1-octanol oxidation reaction. The initial and final conversion of 1-octanol on this sample was reduced almost two times, as the activity of the sample after the recycling test, where it was also observed 13% of gold monovalent ions.
Author Contributions
E.P. carried out the catalysts preparation and pretreatments, performed all catalytic tests, interpreted XPS data and wrote the first draft of the paper; E.K. participated on the conceptualization and methodology of most characterization methods, compared 1-octanol and 1-phenylethanol catalytic results and participated on the writing; A.P.C.R. and L.M.D.R.S.M. were responsible for methodology of catalytic tests in 1-phenylethanol oxidation and supervised those experiments; D.G. was responsible for TPD analyses; S.A.C.C. was responsible for the XPS analyses, D.P. and C.J. performed the DFT calculations and respective interpretation; S.A.C.C., A.J.L.P., A.P., V.C.C. and N.B. provided the means for the realization of this work and contributed to the supervision and paper revision. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partially supported by the Fundação para a Ciência e Tecnologia (FCT), project UIDB/00100/2020 of Centro de Química Estrutural. Tomsk Polytechnic University Competitiveness Enhancement Program, project VIU-RSCBMT-65/2019 and Russian Foundation of Basic Research, project 18-29-24037, and Spanish MINECO project CTQ2017-86170-Rare acknowledged as well.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Barati B., Moghadam M., Rahmati A., Mirkhani V., Tangestaninejad S., Mohammadpoor-Baltork I. Direct oxidation of alcohols to carboxylic acids over ruthenium hydride catalyst with diphenylsulfoxide oxidant. Inorg. Chem. Commun. 2013;29:114–117. doi: 10.1016/j.inoche.2012.12.014. [DOI] [Google Scholar]
- 2.Corberán V.C., González-Pérez M.E., Martínez-González S., Gómez-Avilés A. Green oxidation of fatty alcohols: Challenges and opportunities. Appl. Catal. A Gen. 2014;474:211–223. doi: 10.1016/j.apcata.2013.09.040. [DOI] [Google Scholar]
- 3.Tojo G., Fernández M. Oxidation of Primary Alcohols to Carboxylic Acids: A Guide to Current Common Practice. Springer; Berlin, Germany: 2006. [Google Scholar]
- 4.Zhao M., Li J., Song Z., Desmond R., Tschaen D.M., Grabowski E.J.J., Reider P.J. A novel chromium trioxide catalyzed oxidation of primary alcohols to the carboxylic acids. Tetrahedron Lett. 1998;39:5323–5326. doi: 10.1016/S0040-4039(98)00987-3. [DOI] [Google Scholar]
- 5.Anbu S., Alegria E.C.B.A., Pombeiro A.J.L. Catalytic activity of a benzoyl hydrazone based dimericdicopper(II) complex in catechol and alcohol oxidation reactions. Inorg. Chim. Acta. 2015;431:139–144. doi: 10.1016/j.ica.2014.11.038. [DOI] [Google Scholar]
- 6.Nesterova O.V., Nesterov D.S., Krogul-Sobczak A., Guedes da Silva M.C., Pombeiro A.J.L. Synthesis, crystal structures and catalytic activity of Cu(II) and Mn(III)Schiff base complexes: Influence of additives on the oxidation catalysis of cyclohexane and 1-phenylehanol. J. Mol. Catal. A Chem. 2017;426:506–515. doi: 10.1016/j.molcata.2016.09.005. [DOI] [Google Scholar]
- 7.Sutradhar M., Alegria E.C.B.A., Roy Barman T., Scorcelletti F., Guedes da Silva M.C., Pombeiro A.J.L. Microwave-assisted peroxidative oxidation of toluene and 1-phenylethanol with monomeric keto and polymeric enolaroylhydrazone Cu(II) complexes. Mol. Catal. 2017;439:224–232. doi: 10.1016/j.mcat.2017.07.006. [DOI] [Google Scholar]
- 8.Ribeiro A.P.C., Fontolan E., Alegria E.C.B.A., Kopylovich M.N., Bertani R., Pombeiro A.J.L. The influence of multiwalled carbon nanotubes and graphene oxide additives on the catalytic activity of 3d metal catalysts towards 1-phenylethanol oxidation. J. Mol. Catal. A Chem. 2017;426:557–563. doi: 10.1016/j.molcata.2016.07.015. [DOI] [Google Scholar]
- 9.Karmakar A., Martins L.M.D.R.S., Guedes da Silva M.F.C., Hazra S., Pombeiro A.J.L. Solvent-free microwave-assisted peroxidative oxidation of alcohols catalyzed by iron(III)-TEMPO catalytic systems. Catal. Lett. 2015;145:2066–2076. doi: 10.1007/s10562-015-1616-2. [DOI] [Google Scholar]
- 10.Cozzi I.S., Crotti C., Farnetti E. Microwave-assisted green oxidation of alcohols with hydrogen peroxide catalyzed by iron complexes with nitrogen ligands. J. Organomet. Chem. 2018;878:38–47. doi: 10.1016/j.jorganchem.2018.10.003. [DOI] [Google Scholar]
- 11.Du Z., Ma J., Ma H., Gao J., Xu J. Synergistic effect of vanadium–phosphorus promoted oxidation of benzylic alcohols with molecular oxygen in water. Green Chem. 2010;12:590–592. doi: 10.1039/b924602a. [DOI] [Google Scholar]
- 12.Sasaki T., Ichikuni N., Hara T., Shimazu S. Study on the promoting effect of nickel silicate for 1-phenylethanol oxidation on supported NiO nanocluster catalysts. Catal. Today. 2018;307:29–34. doi: 10.1016/j.cattod.2017.05.076. [DOI] [Google Scholar]
- 13.Bhaumik C., Stein D., Vincendeau S., Poli R., Manoury E. Oxidation of alcohols by TBHP in the presence of sub-stoichiometric amounts of MnO2. C. R. Chim. 2016;19:566–570. doi: 10.1016/j.crci.2016.02.012. [DOI] [Google Scholar]
- 14.Reis M.C., Barros S.D.T., Lachter E.R., San Gil R.A.S., Floresc J.H., Pais da Silva M.I., Onfroyd T. Synthesis, characterization and catalytic activity of meso-niobium phosphate in the oxidation of benzyl alcohols. Catal. Today. 2012;192:117–122. doi: 10.1016/j.cattod.2012.05.025. [DOI] [Google Scholar]
- 15.Burange A.S., Jayaram R.V., Shukla R., Tyagi A.K. Oxidation of benzylic alcohols to carbonyls using tert-butyl hydroperoxide over pure phase nanocrystalline CeCrO3. Catal. Commun. 2013;40:27–31. doi: 10.1016/j.catcom.2013.05.019. [DOI] [Google Scholar]
- 16.Yadav G.D., Yadav A.R. Selective liquid phase oxidation of secondary alcohols into ketones by tert-butyl hydroperoxide on nano-fibrous Ag-OMS-2 catalyst. J. Mol. Catal. A Chem. 2013;M380:70–77. doi: 10.1016/j.molcata.2013.09.018. [DOI] [Google Scholar]
- 17.Antonetti C., Toniolo L., Cavinato G., Forte C., Ghignoli C., Ishake R., Cavani F., Raspolli Galletti A.M. A hybrid polyketone–SiO2 support for palladium catalysts and their applications in cinnamaldehyde hydrogenation and in 1-phenylethanol oxidation. Appl. Catal. A Gen. 2015;496:40–50. doi: 10.1016/j.apcata.2015.01.045. [DOI] [Google Scholar]
- 18.Yasueda T., Seike R., Ikenaga N., Miyake T., Suzuki T. Palladium-loaded oxidized diamond catalysis for the selective oxidation of alcohols. J. Mol. Catal. A Chem. 2009;306:136–142. doi: 10.1016/j.molcata.2009.02.039. [DOI] [Google Scholar]
- 19.Abad A., Almela C., Corma A., Garcıa H. Efficient chemoselective alcohol oxidation using oxygen as oxidant. Superior performance of gold over palladium catalysts. Tetrahedron. 2006;62:6666–6672. doi: 10.1016/j.tet.2006.01.118. [DOI] [Google Scholar]
- 20.Kantam M.L., Reddy R.S., Pal U., Sudhakar M., Venugopal A., Jeeva Ratnam K., Figueras F., Reddy Chintareddy V., Nishinad Y. Ruthenium/magnesium–lanthanum mixed oxide: An efficient reusable catalyst for oxidation of alcohols by using molecular oxygen. J. Mol. Catal. A Chem. 2012;359:1–7. doi: 10.1016/j.molcata.2012.03.013. [DOI] [Google Scholar]
- 21.Hosseini-Monfared H., Meyer H., Janiak C. Dioxygen oxidation of 1-phenylethanol with gold nanoparticles and N-hydroxyphthalimide in ionic liquid. J. Mol. Catal. A Chem. 2013;372:72–78. doi: 10.1016/j.molcata.2013.02.007. [DOI] [Google Scholar]
- 22.Restrepo J., Lozano P., Burguete M.I., García-Verdugo E., Luis S.V. Gold nanoparticles immobilized onto supported ionic liquid-like phases for microwave phenylethanol oxidation in water. Catal. Today. 2015;255:97–101. doi: 10.1016/j.cattod.2014.12.023. [DOI] [Google Scholar]
- 23.Carabineiro S.A.C., Ribeiro A.P.C., Buijnsters J.G., Avalos-Borja M., Pombeiro A.J.L., Figueiredo J.L., Martins L.M.D.R.S. Solvent-free oxidation of 1-phenylethanol catalysed by gold nanoparticles supported on carbon powder materials. Catal. Today. 2020 doi: 10.1016/j.cattod.2019.06.041. in press. [DOI] [Google Scholar]
- 24.Mitsudome T., Noujima A., Mizugaki T., Jitsukawa K., Kaneda K. Efficient aerobic oxidation of alcohols using a hydrotalcite-supported gold nanoparticle catalyst. Adv. Synth. Catal. 2009;351:1890–1896. doi: 10.1002/adsc.200900239. [DOI] [Google Scholar]
- 25.Liang W., Xiangju M., Fengshou X. Au nanoparticles supported on a layered double hydroxide with excellent catalytic properties for the aerobic oxidation of alcohols. Chin. J. Catal. 2010;31:943–947. [Google Scholar]
- 26.Haider P., Grunwaldt J.D., Baiker A. Gold supported on Mg, Al and Cu containing mixed oxides: Relation between surface properties and behavior in catalytic aerobic oxidation of 1-phenylethanol. Catal. Today. 2009;141:349–354. doi: 10.1016/j.cattod.2008.06.003. [DOI] [Google Scholar]
- 27.Shanahan A.E., McNamara J.A., Sullivan H.J., Byrne M. An insight into the superior performance of a gold nanocatalyst on single wall carbon nanotubes to that on titanium dioxide and amorphous carbon for the green aerobic oxidation of aromatic alcohols. New Carbon Mater. 2017;32:242–251. doi: 10.1016/S1872-5805(17)60121-5. [DOI] [Google Scholar]
- 28.Wang L., He L., Liu Q., Liu Y., Chen M., Cao Y., He H., Fan K. Solvent-free selective oxidation of alcohols by molecular oxygen over gold nanoparticles supported on β-MnO2 nanorods. Appl. Catal. A Gen. 2008;344:150–157. doi: 10.1016/j.apcata.2008.04.013. [DOI] [Google Scholar]
- 29.Nepak D., Darbha S. Selective aerobic oxidation of alcohols over Au–Pd/sodium titanate nanotubes. Catal. Commun. 2015;58:149–153. doi: 10.1016/j.catcom.2014.09.018. [DOI] [Google Scholar]
- 30.Yang X., Wang X., Liang C., Su W., Wang C., Feng Z., Li C., Qiu J. Aerobic oxidation of alcohols over Au/TiO2: An insight on the promotion effect of water on the catalytic activity of Au/TiO2. Catal. Commun. 2008;9:2278–2281. doi: 10.1016/j.catcom.2008.05.021. [DOI] [Google Scholar]
- 31.Li H., Zheng Z., Cao M., Cao R. Stable gold nanoparticle encapsulated in silica-dendrimers organic–inorganic hybrid composite as recyclable catalyst for oxidation of alcohol. Microporous Mesoporous Mater. 2010;136:42–49. doi: 10.1016/j.micromeso.2010.07.017. [DOI] [Google Scholar]
- 32.Mertens P.G.N., Corthals S.L.F., Yeb X., Poelman H., Jacobs P.A., Sels B.F., Vankelecom I.F.J., De Vos D.E. Selective alcohol oxidation to aldehydes and ketones over base-promoted gold–palladium clusters as recyclable quasi homogeneous and heterogeneous metal catalysts. J. Mol. Catal. A Chem. 2009;313:14–21. doi: 10.1016/j.molcata.2009.07.017. [DOI] [Google Scholar]
- 33.Abad A., Concepcion P., Corma A., Garcia H. A collaborative effect between gold and a support induces the selective oxidation of alcohols. Angew. Chem. Int. Ed. 2005;44:4066–4069. doi: 10.1002/anie.200500382. [DOI] [PubMed] [Google Scholar]
- 34.Daliran S., Santiago-Portillo A., Navalón S., Oveisi A., Álvaro M., Ghorbani-Vaghei R., Azarifar D., García H. Cu(II)-Schiff base covalently anchored to MIL-125(Ti)-NH2 as heterogeneous catalyst for oxidation reactions. J. Coll. Interface Sci. 2018;532:700–710. doi: 10.1016/j.jcis.2018.07.140. [DOI] [PubMed] [Google Scholar]
- 35.Wu J., Liu Y., Ma X., Liu P., Gu C., Dai B. Metal-free oxidation of secondary benzylic alcohols using aqueous TBHP. Synth. Commun. 2006;46:1747–1758. doi: 10.1080/00397911.2016.1223307. [DOI] [Google Scholar]
- 36.Kotolevich Y., Kolobova E., Khramov E., Farıas M.H., Zubavichus Y., Tiznado H., González-Pérez M.E., Corberán V.C., Mota-Morales J.D., Pestryakov A.N., et al. n-octanol oxidation on Au/TiO2 catalysts promoted with La and Ce oxides. J. Mol. Catal. 2017;427:1–10. doi: 10.1016/j.molcata.2016.09.003. [DOI] [Google Scholar]
- 37.Kotolevich Y., Kolobova E., Mamontov G., Khramov E., Cabrera Ortega J.E., Tiznado H., Farias M.H., Bogdanchikova N., Zubavichus Y., Mota-Morales J.D., et al. Au/TiO2 catalysts promoted with Fe and Mg for n-octanol oxidation under mild conditions. Catal. Today. 2016;278:104–112. doi: 10.1016/j.cattod.2016.05.002. [DOI] [Google Scholar]
- 38.Kolobova E., Pakrieva E., Pascual L., Cortés Corberán V., Bogdanchikova N., Farias M., Pestryakov M. Selective oxidation of n-octanol on unmodified and La-modified nanogold catalysts: Effect of metal content. Catal. Today. 2019;333:127–132. doi: 10.1016/j.cattod.2018.04.046. [DOI] [Google Scholar]
- 39.Pakrieva E., Kolobova E., Mamontov G., Bogdanchikova N., Farias M.H., Pascual L., Cortés Corberán V., Martinez Gonzalez S., Carabineiro S.A.C., Pestryakov A. Green oxidation of n-octanol on supported nanogold catalysts: Formation of gold active sites under combined effect of gold content, additive nature and redox pretreatment. ChemCatChem. 2019;11:1615–1624. doi: 10.1002/cctc.201801566. [DOI] [Google Scholar]
- 40.Zanella R., Giorgio S., Henry C.R., Louis C. Alternative methods for the preparation of gold nanoparticles supported on TiO2. J. Phys. Chem. B. 2002;106:7634–7642. doi: 10.1021/jp0144810. [DOI] [Google Scholar]
- 41.Zanella R., Louis C. Influence of the conditions of thermal treatments and of storage on the size of the gold particles in Au/TiO2 samples. Catal. Today. 2005;107:768–777. doi: 10.1016/j.cattod.2005.07.008. [DOI] [Google Scholar]
- 42.Kolobova E., Maki-Arvela P., Pestryakov A., Pakrieva E., Pascual L., Smeds A., Rahkila J., Sandberg T., Peltonen J., Murzin D.Y. Reductive amination of ketones with benzylamine over gold supported on different oxides. Catal. Lett. 2019;149:3432–3446. doi: 10.1007/s10562-019-02917-1. [DOI] [Google Scholar]
- 43.Kolobova E.N., Pakrieva E.G., Carabineiro S., Bogdanchikova N., Kharlanov A., Kazantsev S.O., Hemming J., Mäki-Arvela P., Pestryakov A.N., Murzin D. Oxidation of a wood extractive betulin to biologically active oxo-derivatives using supported gold catalysts. Green Chem. 2019;21:3370–3382. doi: 10.1039/C9GC00949C. [DOI] [Google Scholar]
- 44.Perdew J.P., Burke K., Ernzerhof M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996;77:3865–3868. doi: 10.1103/PhysRevLett.77.3865. [DOI] [PubMed] [Google Scholar]
- 45.Xiao L., Tollberg B., Hu X., Wang L. Structural study of gold clusters. J. Chem. Phys. 2006;124:114309. doi: 10.1063/1.2179419. [DOI] [PubMed] [Google Scholar]
- 46.Mukhamedzyanova D.F., Ratmanova N.K., Pichugina D.A., Kuzmenko E.N. A structural and stability evaluation of Au12 from an isolated cluster to the deposited material. J. Phys. Chem. C. 2012;116:11507–11518. doi: 10.1021/jp212367z. [DOI] [Google Scholar]
- 47.Beletskaya A.V., Pichugina D.A., Shestakov A.F., Kuz’menko N.E. Formation of H2O2 on Au20 and Au19Pd Clusters: Understanding the Structure Effect on the Atomic Level. J. Phys. Chem. A. 2013;117:6817–6826. doi: 10.1021/jp4040437. [DOI] [PubMed] [Google Scholar]
- 48.Laikov D.N., Ustynyuk Y.A. PRIRODA-04: A Quantum-ChemicalProgram Suite. New Possibilities in the Study of Molecular Systems with the Application of Parallel Computing. Russ. Chem. Bull. 2005;54:820–826. doi: 10.1007/s11172-005-0329-x. [DOI] [Google Scholar]
- 49.Sadovnichy V., Tikhonravov A., Voevodin V., Opanasenko V. Contemporary High Performance Computing: From Petascale toward Exascale. CRC Press; Boca Raton, FL, USA: 2013. [Google Scholar]
- 50.Yu J., Wu G., Mao D. Effect of La2O3 on catalytic performance of Au/TiO2 for CO oxidation. Acta Phys.-Chim. Sin. 2008;24:1751–1755. doi: 10.1016/S1872-1508(08)60071-6. [DOI] [Google Scholar]
- 51.Lee K.J., Kumar P.A., Maqbool M.S., Rao K.N., Song K.H., Ha H.P. Ceria added Sb-V2O5/TiO2 catalysts for low temperature NH3 SCR: Physico-chemical properties and catalytic activity. Appl. Catal. B. 2013;142:705–717. doi: 10.1016/j.apcatb.2013.05.071. [DOI] [Google Scholar]
- 52.Carja G., Kameshima Y., Okada K., Madhusoodana C.D. Mn–Ce/ZSM5 as a new superior catalyst for NO reduction with NH3. Appl. Catal. B. 2007;73:60–64. doi: 10.1016/j.apcatb.2006.06.003. [DOI] [Google Scholar]
- 53.Kang M., Park E.D., Kim J.M., Yie J.E. Manganese oxide catalysts for NOx reduction with NH3 at low temperatures. Appl. Catal. A. 2007;327:261–269. doi: 10.1016/j.apcata.2007.05.024. [DOI] [Google Scholar]
- 54.Casaletto M.P., Longo A., Martorana A., Prestianni A., Venezia A.M. XPS study of supported gold catalysts: The role of Au0 and Au+δ species as active sites. Surf. Interface Anal. 2006;38:215–218. doi: 10.1002/sia.2180. [DOI] [Google Scholar]
- 55.Costa V.V., Estrada M., Demidova Y., Prosvirin I., Kriventsov V., Cotta R.F., Fuentes S., Simakov A., Gusevskaya E. Gold nanoparticles supported on magnesium oxide as catalysts for the aerobic oxidation of alcohols under alkali-free conditions. J. Catal. 2012;292:148–156. doi: 10.1016/j.jcat.2012.05.009. [DOI] [Google Scholar]
- 56.Feng R., Li M., Liu J. Synthesis of core–shell Au@Pt nanoparticles supported on Vulcan XC-72 carbon and their electrocatalytic activities for methanol oxidation. Colloids Surf. A. 2012;406:6–12. doi: 10.1016/j.colsurfa.2012.04.030. [DOI] [Google Scholar]
- 57.Pestryakov A.N., Lunin V.V., Bogdanchikova N., Temkin O.N., Smolentseva E. Active states of gold in small and big metal particles in CO and methanol selective oxidation. Fuel. 2013;110:48–53. doi: 10.1016/j.fuel.2012.10.012. [DOI] [Google Scholar]