Abstract
In this study, we identified cadmium (Cd) as a potential endocrine disruptor that impairs laying performance, egg quality, and eggshell deposition and induces oxidative stress and inflammation in the eggshell glands of laying hens. A total of 480 38-wk-old laying hens were randomly assigned into 5 groups that were fed a basal diet (control) or a basal diet supplemented with Cd (provided as CdCl2·2.5 H2O) at 7.5, 15, 30, and 60 mg Cd per kg feed for 9 wk. The results showed that, when compared with the control group, a low dose of dietary Cd (7.5 mg/kg) had positive effects on egg quality by improving albumen height, Haugh unit, yolk color, and shell thickness at the third or ninth week. However, with the increase in the dose and duration of Cd exposure, the laying performance, egg quality, and activities of eggshell gland antioxidant enzymes (catalase [CAT], glutathione peroxide [GSH-Px]), and ATPase (Na+/K+-ATPase, Ca2+-ATPase, and Mg2+-ATPase) deteriorated, and the activity of total nitric oxide synthase (T-NOS) and the level of malondialdehyde (MDA) increased significantly (P < 0.05). The histopathology and real-time quantitative PCR results showed that Cd induced endometrial epithelial cell proliferation accompanied by upregulation of the mRNA levels of progesterone receptor (PgR) and epidermal growth factor receptor (EGFR), downregulation of the mRNA levels of estrogen receptor α (ERα) and interleukin 6 (IL6), and inflammation of the eggshell gland accompanied by significantly increased expression of complement C3 and pro-inflammatory cytokine tumor necrosis factor α (TNFα) (P < 0.05). In addition, the ultrastructure of the eggshell showed that dietary supplementation with 7.5 mg/kg Cd increased the palisade layer and total thickness of the shell, but with the increase in dietary Cd supplementation (30 and 60 mg/kg) the thickness of the palisade layer and mammillary layer decreased significantly (P < 0.05), and the outer surface of the eggshell became rougher. Correspondingly, the expression of calbindin 1 (CALB1), ovocalyxin-32 (OCX-32), ovocalyxin-36 (OCX-36), osteopontin (SPP1), and ovocledidin-17 (OC-17) decreased significantly (P < 0.05) with increasing dietary Cd supplementation. Conclusively, the present study demonstrates that dietary supplementation with Cd negatively affects laying performance, egg quality, and eggshell deposition by disturbing the metabolism of eggshell glands in laying hens but has a positive effect on egg quality at low doses.
Keywords: cadmium, egg quality, eggshell biomineralization, eggshell gland, laying hens, oxidative stress
Introduction
Cadmium (Cd) is a heavy metal that occurs in nature at low concentrations. It is one of the major chemical pollutants found in the daily environment of developed countries, mainly in association with other heavy metals such as the sulfide ores of zinc, copper, and lead (Godt et al., 2006). Human activities contribute to the widespread occurrence of Cd, including mining residues, the combustion of fossil fuels, and the electroplating and manufacture of nickel-Cd batteries (Martelli et al., 2006). For these reasons, Cd is found in almost everything we eat, drink, and breathe (Thompson and Bannigan, 2008). Animals are exposed to Cd from various sources through these contaminated media, and Cd accumulates in various tissues and organs (Wang et al., 2017). Cd has a long biological half-life of 10 to 30 yr and is difficult to discharge (Duc Phuc et al., 2016). The bioaccumulation of Cd eventually leads to serious consequences for animal production, economic benefits, and even human health. Cd pollution in animal production has been a problem for several countries, and in some situations, the concentration of Cd in manure and animal feed can even reach 130 mg/kg (Sant’Ana et al., 2005; Li et al., 2010). In vivo, Cd can interfere with the transport and metabolism of calcium ions (Choong et al., 2014), mimic and displace the essential metals in various biomolecules and enzymes. For example, Cd can occupy the active sites of zinc-metalloenzymes, including carbonic anhydrase (CA), to alter their activities and behaviors (Aravind and Prasad, 2004). Many studies have reported that the exposure of laying hens to Cd results in decreased egg production (Olgun and Bahtiyarca, 2015) and eggshell thickness (48 mg Cd/kg diets) (Leach et al., 1979). However, the results of these studies also showed a positive effect on the laying performance or egg quality of hens supplemented with low levels of Cd (Leach et al., 1979; Olgun and Bahtiyarca, 2015).
In addition, Cd is a typical environmental xenoestrogens and is considered a potential endocrine disruptor that mimics the function of steroid hormones (Byrne et al., 2009), which can induce hormone-dependent MCF-7 cell proliferation (Brama et al., 2007), increase the expression of estrogen-regulated genes (Garcia-Morales et al., 1994), and activate the estrogen receptor (ER) (Brama et al., 2007). There is reasonable evidence suggesting that Cd may be associated with uterine disease in women (Jackson et al., 2008). The ability to activate ERα is central to the induction of cell proliferation by estrogen and estrogen mimics in many cancers and other disease processes (Osborne and Schiff, 2005).
The eggshell of birds plays an important role in determining the physical and antimicrobial defense of the egg and regulates the exchange of metabolic gases and water (Hincke et al., 2012). Eggshell quality is a very important factor not only for hatchability (Dauwea et al., 2004) but also for food safety and egg marketing (Park et al., 2018). In laying hens, the process of eggshell formation involves calcium carbonate (CaCO3) synthesis and calcite crystal deposition which are facilitated by matrix proteins in the eggshell gland (uterus) (Nys et al., 2004). Various studies found that calbindin 1 (CALB1), osteopontin (SPP1), and CA were closely related to mammillary layer thickness, fracture toughness, and egg shape, respectively (Brionne et al., 2014; Chen et al., 2015).
Therefore, the eggshell gland is not only a biological organ responsible for eggshell formation but also a target organ of steroid hormones, such as estrogen and progesterone, or environmental estrogens (Lundholm, 1991). The response of the eggshell gland to Cd levels may be the main mechanism through which Cd affects eggshell secretion. However, the toxic effects of Cd exposure on eggshell glands and on the expression of genes involved in eggshell formation in laying hens are not clear. The purpose of this study was to determine the effect of different levels of dietary Cd supplementation on laying performance, egg quality, eggshell gland antioxidant and related enzyme activities, eggshell gland histopathology, the expression of pro-inflammatory cytokines, and the protein involved in eggshell formation in laying hens.
Materials and methods
Birds, diets, and management
All experimental protocols involving animals were approved by the Animal Care and Welfare Committee of Animal Science College and the Scientific Ethical Committee of Zhejiang University (No. ZJU2013105002, Hangzhou, China). Hens were housed in 1-layer ladder cages (density of approximately 3,000 cm2 per hen). Throughout the study, birds were kept in a naturally ventilated windowed poultry house with a temperature between 23 and 26 °C, relative humidity between 65% and 75%, and 16 h/d of illumination. All animals were treated humanely according to the criteria outlined in the “Guide for the Care and Use of Laboratory Animals” prepared by the National Academy of Science and published by the National Institute of Health. Ethical regulations in accordance with national and institutional guidelines for the protection of animal welfare were adhered to during experiments (Public Health Service [PHS], 2002).
For the experiment, a total of 480 Hy-Line Brown laying hens, 38 wk of age, were obtained from a commercial poultry layer farm in Jiande, China. After 3 d of acclimation, the hens were randomly assigned to 5 treatments, each of which included 6 replicates of 16 laying hens. The treatment protocol was as follows: 1) basal diet (control), 2) basal diet + 7.5 mg/kg Cd (provided as CdCl2·2.5 H2O), 3) basal diet + 15 mg/kg Cd, 4) basal diet + 30 mg/kg Cd, and 5) basal diet + 60 mg/kg Cd. The 30 and 60 mg/kg levels were selected as slightly toxic levels while the lower levels were considered to be representative of the amounts of Cd that would occur in feedstuffs due to environmental contamination (Czarnecki and Baker, 1982; Li et al., 2010). Diets and water were provided ad libitum throughout the experimental period. The whole experimental period lasted 10 wk, including a 1-wk adaptation period and a 9-wk experimental stage. The compositions of the basal diet are presented in Table 1. The actual concentrations of dietary Cd were detected by graphite furnace atomic absorption spectrometry (GFAAS) as described by NHFPC (2015). Briefly, for Cd analysis, 1.5 g of feed sample (12 samples per treatment) was weighed. The sample was transferred to a microwave digestion vessel and digested with a mixed solution of 5 mL of HNO3 and 1 mL of H2O2 in a microwave oven (MDS-2000, CEM Corp., Matthews, NC, USA). The acid was evaporated to dryness. The sample was rinsed 3 times with 1 mL of 1% HNO3, transferred to a 10 mL volumetric flask and brought to volume with 1% HNO3. Twenty microliters of samples and configured Cd standard solutions were introduced into the graphite tube using a sample dispenser, and the absorbance was measured. In this experimental method, the limit of detection and limit of quantification were 0.001 and 0.003 mg/kg, respectively. The results for diets 1 to 5 were 0.47 ± 0.01, 7.58 ± 0.09, 15.56 ± 0.18, 30.55 ± 0.11, and 60.67 ± 0.15 mg Cd/kg diet, respectively. CdCl2·2.5 H2O was purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China; purity ≥ 99%).
Table 1.
Ingredient compositions and nutrient levels of basal diet for hens (as-fed basis)
| Basal ingredients | Composition | Nutrient level2 | Value |
|---|---|---|---|
| Corn, % | 65.00 | Metabolizable energy, MJ/kg | 2.65 |
| Soybean meal (42.0% Crude Protein), % | 21.00 | Crude protein, % | 15.73 |
| Feather meal, % | 1.00 | Ether extract, % | 6.32 |
| Fish meal, % | 1.00 | Lysine, % | 0.78 |
| Calcium carbonate, % | 7.00 | Methionine, % | 0.34 |
| Premix1, % | 5.00 | Cysteine, % | 0.32 |
| Total phosphorus, % | 0.61 | ||
| Total, % | 100.00 | Calcium, % | 3.45 |
1The premix provided following per kilogram of diet: vitamin A, 7,000 IU; vitamin D3, 2,500 IU; vitamin E, 49.5 mg; vitamin K3, 1 mg; vitamin B1, 1.5 mg; vitamin B2, 4 mg; vitamin B6, 2 mg; vitamin B12, 0.02 mg; niacin, 30 mg; folic acid, 0.55 mg; pantothenic acid, 10 mg; biotin, 0.16 mg; choline, 500 mg; Sodium chloride, 2,500mg; Cu, 20 mg; Fe, 70 mg; Mn, 100 mg; Zn, 70 mg; I, 0.4 mg; Se, 0.5 mg.
2Estimated from Chinese feed database provided with tables of feed composition and nutritive values in China (Xiong et al., 2015).
Collection of samples and measurement
Feed consumption, egg production rate (EPR), and egg weight (EW) for each replicate were measured daily. Average EW (AEW), average daily feed intake (ADFI), and feed conversion ratio (FCR) were calculated. Egg quality assessment was carried out at the third and ninth weeks. A total of 30 eggs per treatment (5 eggs per replicate) were randomly collected for egg quality measurement. The EW, albumen height, yolk color, Haugh unit, and eggshell strength were measured using a digital egg tester (DET-6000, Nabel Co. Ltd., Kyoto, Japan). Eggshell thickness was measured using a dial gauge micrometer (547-350, Mitutoyo, Kawasaki, Japan). Then, the albumen, yolk, and eggshell of the eggs (2 eggs per replicate) collected in the ninth week were separated and weighed for the Cd accumulation analysis using GFAAS according to the method used for the detection of Cd residues in the feed. At the end of the experimental period, 12 birds per treatment (2 birds per replicate) were randomly selected during the rapid growth phase of calcification (18 ± 1 h post ovulation). The birds were euthanized by cervical dislocation, and then eggs were carefully removed from the shell gland. Two portions of the eggshell gland were snap frozen in liquid nitrogen and stored at −80 °C for antioxidant, molecular, and Cd residue analyses. Another portion of the eggshell gland was collected and fixed in 4% paraformaldehyde for histopathological analysis.
Microstructural analysis of eggshell
Two eggs from each replicate were sampled and processed to examine the ultrastructure of the eggshell. The interior contents of selected eggs were removed, the shells were cleaned with tap water, and the shell membranes were carefully peeled with a tweezer. After drying thoroughly at room temperature, eggshell samples (1 cm2) were prepared by cutting from the equator of the shell, mounted on aluminum stubs, coated with gold using an ion coater, and viewed with scanning electron microscopy at 3.0 kV (JEOL JSMT330A, Hitachi Ltd., Tokyo, Japan). According to the method of Parsons (1982), the palisade layer thickness and mammillary layer thickness were measured from the cross section of the shell. Three scanned images were obtained for each treatment group.
Eggshell gland oxidative stress variables and ATPase assays
Eggshell gland tissue samples were prepared as 10% tissue homogenate. The homogenate was centrifuged at 3,000 rpm for 10 min at 4 °C. The supernatant was collected for the following analysis. The activities of catalase (CAT), glutathione peroxidase (GSH-Px), total superoxide dismutase (T-SOD), total nitric oxide synthase (T-NOS), adenosine triphosphate (ATPase) (Na+/K+-ATPase, Ca2+-ATPase, and Mg2+-ATPase), and the content of malondialdehyde (MDA) were assayed using commercially available assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the instructions of the manufacturer.
Histopathological analysis
The eggshell gland tissues fixed with 4% paraformaldehyde were trimmed and embedded in paraffin wax. The paraffin-embedded sections were cut into 5 to 6 µm thick sections using a microtome (Leica Microsystems, RM2016) and then stained with hematoxylin and eosin (H & E) for histopathological observation by optical microscopy at a final magnification of 400× (Nikon Eclipse 80i, Nikon, Tokyo, Japan).
Total RNA extraction and quantitative real-time PCR
Total RNA was extracted and used for the reverse transcription of complementary DNA (cDNA) as previously described (Chen et al., 2015). Quantitative real-time PCR was performed in triplicate in the CFX96 Touch Real-Time PCR detection system (Bio-Rad Laboratories, Hercules, California, USA) using TB Green Premix Ex Taq (Takara RR420A) according to the manufacturer’s instructions, and the expression of target genes was normalized to that of β-actin. The primer sequences for qRT-PCR are presented in Table 2. The relative expression of each gene was calculated by the ΔΔCt method as described previously (Livak and Schmittgen, 2001).
Table 2.
Primer used for quantitative real-time PCR
| Target gene | Primer | Primer sequence, 5′–3′ | Accession no. |
|---|---|---|---|
| β-Actin | Forward | TCCCTGGAGAAGAGCTATGAA | NM_205518.1 |
| Reverse | CAGGACTCCATACCCAAGAAAG | ||
| CA | Forward | GGGAATGCCAAACCTGAAATAC | NM_205317.1 |
| Reverse | GCAGAGCGAAAGAGAGAATGA | ||
| OPN | Forward | GACACTGACGAGTCTGATGAAG | NM_204535.4 |
| Reverse | CGTTCTCTACGCTCTGATGTT | ||
| OCX-32 | Forward | CCAAGAAGAGGACCACAGATT | NM_204534.4 |
| Reverse | CAACAGCATTGTCCTTCCTTATC | ||
| OCX-36 | Forward | CAAGCTGATCTCTGGCTTACTG | XM_025142254.1 |
| Reverse | GGAAGGTGTATGGCTGGATATG | ||
| CALB1 | Forward | GGTGCCAGCAGCTAAAGTCA | XM_027452451.1 |
| Reverse | TGGCCAGTTCAGTAAGCTCC | ||
| OC-17 | Forward | CAATGCCTTCGTCTGCAAAG | KF835610.1 |
| Reverse | GTGGGTCCGTTTATTGCAGTG | ||
| C3 | Forward | CATACGGGTGAAGAAGGACAA | NM_205405.3 |
| Reverse | CTTGCTGATGAGGTAGGAGAAG | ||
| IL1β | Forward | CTCACAGTCCTTCGACATCTTC | XM_015297469.1 |
| Reverse | CGGTACATACGAGATGGAAACC | ||
| IL6 | Forward | GATCCGGCAGATGGTGATAAA | XM_015281283.2 |
| Reverse | GAGGATTGTGCCCGAACTAAA | ||
| TNFα | Forward | GACAGCCTATGCCAACAAGTA | AY765397.1 |
| Reverse | TCCACATCTTTCAGAGCATCAA | ||
| ERα | Forward | TATTGATGATCGGCTTAGTCTGGC | NM_205183.2 |
| Reverse | CGAGCAGCAGTAGCCAGTAGCA | ||
| PgR | Forward | TCCTTGCTGACCAGTCTAAATC | NM_205262.1 |
| Reverse | GTTAGCCACCACACCTTTCT | ||
| EGFR | Forward | CACTCCCACACTAGCTCATTAC | XM_015281328.2 |
| Reverse | TGCCTGCCTACACACTTATTC | ||
| PCNA | Forward | GAGCAGAAGACAATGCGGATA | XM_003212379.3 |
| Reverse | TGCACAGGAGATGACAACAG |
Statistical analysis
For statistical evaluation, the normality of data distribution was tested with the Kolmogorov–Smirnov test. The time- and dose-dependent effects of Cd on the laying performance and egg quality of laying hens were tested by 2-way analysis of variance (2-way ANOVA) followed by the least significant difference (LSD) post hoc tests using SPSS 20.0 (SPSS Inc., Chicago, IL, USA). Linear regression analysis (SPSS 20.0, SPSS Inc., Chicago, IL, USA) was used to assess the dose–response relationship of Cd exposure with laying performance and egg quality. Other data of the experiment were statistically analyzed by 1-way ANOVA, and Tukey’s post hoc test was used to compare the significant differences between means. All data are presented as the mean ± SE, and the significance level was set at P < 0.05 for all measurements.
Results and discussion
Laying performance
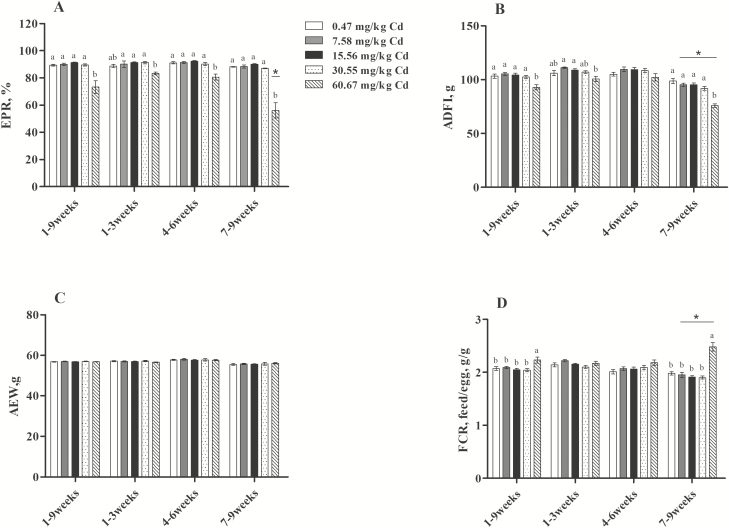
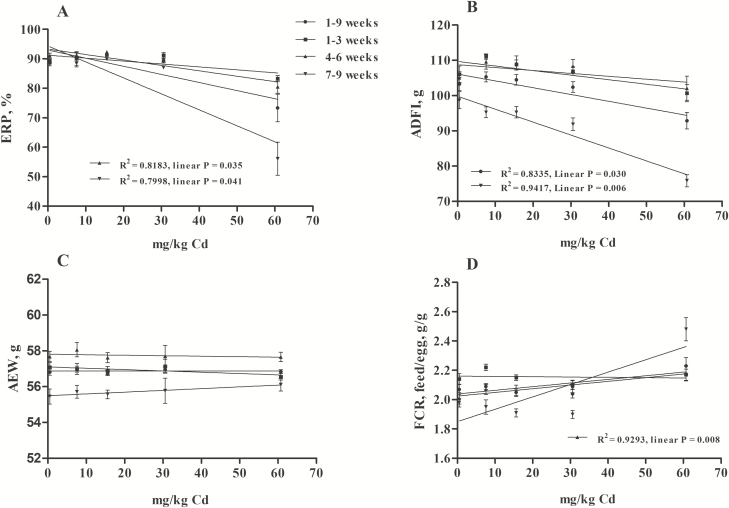
There is a dose-dependent and time-dependent relationship between environmental Cd exposure and accumulation (Yu et al., 2008). The effects of dietary Cd treatments on laying performance are summarized in Figure 1. Throughout the experimental period, the EPR decreased significantly (P < 0.05) in the group supplemented with 60.67 mg/kg Cd compared with the other groups and was the lowest during wk 7 to 9 (FEPR [8,75] = 11.245, P < 0.05; Figure 1A). There was no significant difference in AEW among the groups (P > 0.05; Figure 1C). From 7 to 9 wk, the diet supplemented with 60.67 mg/kg Cd resulted in a marked decrease in the ADFI compared with the control group, as well as with other stages (FADFI [8,75] = 3.927, P < 0.05; Figure 1B). In contrast, the FCR was negatively affected by dietary 60.67 mg/kg Cd (P < 0.05), beginning at 7 to 9 wk (FFCR [8,75] = 5.651, P < 0.05; Figure 1D). In addition, the linear regression equation constructed for the relationship between the dietary inclusion level of Cd and laying performance revealed that the EPR was negatively and significantly correlated with the dose of Cd in diets at 4 to 6 wk (Y = −0.1841x + 93.308, R2 = 0.8183, P < 0.05) and 7 to 9 wk (Y = −0.5425x + 94.38, R2 = 0.7998, P < 0.05; Figure 2A). The ADFI was negatively and significantly correlated with the dose of Cd in diets at 7 to 9 wk (Y = −0.3668x + 99.813, R2 = 0.9417, P < 0.05) and 1 to 9 wk (Y = −0.193x + 106.1, R2 = 0.8335, P < 0.05; Figure 2B). In contrast, the FCR was positively and significantly correlated with the dose of Cd in diets at 4 to 6 wk (Y = 0.0025x + 2.0243, R2 = 0.9293, P < 0.05; Figure 2D). The results obtained in the present study confirmed that the toxic range of Cd was connected with the dose and duration of exposure. Similarly, many studies have shown that exposure to Cd resulted in decreased egg production (Leach et al., 1979; Rahman et al., 2007) and ADFI and increased FCR (Czarnecki et al., 1982; Olgun and Bahtiyarca, 2015). However, we found that the diets supplemented with 7.58, 15.56, and 30.55 mg/kg Cd had no effect on these factors, which disagreed with the report by Olgun and Bahtiyarca (2015) suggesting that diet supplemented with 15 mg/kg Cd (12 wk) significantly decreased the EPR and ADFI. This contradictory finding may be caused by the different tolerances of different breeds and ages of laying hens to Cd.
Figure 1.
Effects of Cd on laying performance of laying hens. (A) EPR, egg production rate; (B) AEW, average egg weight; (C) ADFI, average daily feed intake; (D) FCR, feed conversion ratio. Values are represented as the mean ± SE (n = 6 replicates per treatment and n = 96 hens per treatment). a–cDifferent letters within the same time points indicate significant differences among different doses of Cd, *P < 0.05 vs. groups of 1 to 3 and 4 to 6 wk (2-way ANOVA, LSD tests, P < 0.05).
Figure 2.
The dose–response relationship between Cd exposure and laying performance (n = 6 replicates per treatment and n = 96 hens per treatment, mean ± SE). (A) EPR, egg production rate, Y4–6 wk = −0.1841x + 93.308, Y7–9 wk = −0.5425x + 94.38; (B) ADFI, average daily feed intake, Y7–9 wk = −0.3668x + 99.813, Y1–9 wk = −0.193x + 106.1; (C) AEW, average egg weight; (D) FCR, feed conversion ratio, Y4–6 wk = 0.0025x + 2.0243.
Egg quality and Cd residues in tissues and eggs
Many researchers have found that the administration of Cd resulted in negative effects on egg quality, but it may be useful when low levels are added to diets (Leach et al., 1979; Rahman et al., 2007; Olgun and Bahtiyarca, 2015). In the current study, similar results were obtained: the albumen height and Haugh unit were improved in the groups administered low levels of dietary Cd (7.58 mg/kg) after 3 wk of treatment (P < 0.05) compared with the control group (0.47 mg/kg), and the positive of Cd impact on yolk color and eggshell thickness continued even with long-term Cd treatment (9 wk; Table 3). The reason may be associated with the function of Cd to mimic the effects of estrogen, which could maintain yolk deposition (Nakada et al., 1994) and enhance the synthesis of yolk precursors (Li et al., 2014). There is no explanation for the positive impact on albumen quality and eggshell thickness. However, as a disruptor of the endocrine system (Johnson et al., 2003), with the increase in dose and duration of exposure, the negative effects of Cd on egg quality were significant. Our results showed that the quality of the eggs, including albumen height, Haugh unit, and eggshell strength, was inferior at the ninth week compared with the third week. Compared with the control group, the groups supplemented with 30.55 and 60.67 mg/kg dietary Cd had significantly decreased albumen height, Haugh unit, and eggshell thickness (P < 0.05; Table 3). Linear regression analysis revealed that the relationships between the dose of dietary Cd and eggshell strength and thickness were negative and significant at both the third week (Yeggshell strength = −0.0114x + 3.9529, R2 = 0.8027, P < 0.05; Yeggshell thickness = −0.0006x + 0.3424, R2 = 0.8318, P < 0.05) and ninth week (Yeggshell strength = −0.0192x + 4.035, R2 = 0.9563, P < 0.05; Yeggshell thickness = −0.0008x + 0.3455, R2 = 0.8981, P < 0.05). The relationship between the dose of dietary Cd and the Haugh unit was negative and significant at only the ninth week (Y = −0.1937x + 81.187, R2 = 0.8439, P < 0.05; Table 3). However, the effects of Cd on albumen height and Haugh unit both presented dose- and time-dependent patterns (FAlbumen height [4,140] = 5.254, P < 0.05; FHaugh unit [4, 140] = 4.607, P < 0.05). These results demonstrated that Cd exposure mainly affects the quality of the egg albumen and eggshell in a time- or dose-dependent manner.
Table 3.
Effect of Cd on egg quality and Cd residues in eggshell glands and eggs of laying hens1
| Time | Dietary Cd dosage, mg/kg | ||||||
|---|---|---|---|---|---|---|---|
| Items | (wk) | 0.47 | 7.58 | 15.56 | 30.55 | 60.67 | Linear P-value |
| Egg quality2 | |||||||
| Albumen height, mm | 3rd | 6.70 ± 0.11b | 7.30 ± 0.17a | 6.96 ± 0.16a,b | 6.60 ± 0.20b | 6.44 ± 0.11b | 0.212 |
| 9th | 6.20 ± 0.13a | 6.13 ± 0.13a,* | 6.59 ± 0.07a | 5.17 ± 0.15b,* | 5.00 ± 0.13b,* | 0.083 | |
| Yolk color | 3rd | 6.04 ± 0.10a,b | 6.36 ± 0.11a | 6.11 ± 0.11a,b | 6.04 ± 0.10a,b | 5.71 ± 0.10b | 0.098 |
| 9th | 5.00 ± 0.11b | 5.47 ± 0.09a | 5.03 ± 0.09b | 5.17 ± 0.08a,b | 4.97 ± 0.12b | 0.538 | |
| Haugh unit | 3rd | 82.38 ± 0.66b,c | 86.41 ± 0.94a | 84.83 ± 1.00a,b | 79.84 ± 1.31c | 79.91 ± 0.82c | 0.199 |
| 9th | 80.64 ± 0.85a | 80.41 ± 0.79a,* | 80.11 ± 0.45a,* | 72.06 ± 0.94b,* | 70.47 ± 0.95b,* | 0.0285 | |
| Eggshell strength, kgf/m3 | 3rd | 3.89 ± 0.11a | 3.77 ± 0.10a | 3.82 ± 0.11a | 3.82 ± 0.10a | 3.15 ± 0.08b | 0.0316 |
| 9th | 3.89 ± 0.10a,b | 4.00 ± 0.07a | 3.74 ± 0.14a,b | 3.52 ± 0.10b | 2.82 ± 0.13c,* | 0.0147 | |
| Eggshell thickness, mm | 3rd | 0.34 ± 0.003a | 0.33 ± 0.003a | 0.34 ± 0.003a | 0.33 ± 0.003a | 0.30 ± 0.003b | 0.0408 |
| 9th | 0.34 ± 0.003b | 0.35 ± 0.003a | 0.33 ± 0.003b,c | 0.32 ± 0.002c | 0.30 ± 0.004d | 0.0049 | |
| Cadmium residues3, μg/kg | |||||||
| Eggshell | 9th | 197.5 ± 14.93c | 290.0 ± 25.50b,c | 432.5 ± 38.16b | 632.5 ± 58.51a | 785.5 ± 41.13a | 0.007 |
| Albumen | 9th | 4.96 ± 0.07e | 9.00 ± 0.08d | 12.09 ± 0.12c | 15.21 ± 0.37b | 32.52 ± 0.39a | 0.002 |
| Yolk | 9th | 8.10 ± 0.27e | 11.90 ± 0.32d | 13.36 ± 0.13c | 16.92 ± 0.42b | 34.38 ± 0.27a | 0.003 |
| Total egg4 | 9th | 29.04 ± 1.80d | 43.62 ± 3.12c,d | 62.94 ± 4.64c | 89.81 ± 7.24b | 123.39 ± 4.84a | 0.002 |
| Eggshell gland | 9th | 11.75 ± 0.48e | 70.25 ± 1.49d | 97.00 ± 3.49c | 123.75 ± 4.39b | 207.50 ± 3.48a | 0.005 |
1Values are represented as the mean ± SE.
2 n = 6 replicates per treatment and n = 30 eggs per treatment.
3 n = 6 replicates per treatment and n = 12 eggs or eggshell glands per treatment.
4Cd residue in total egg (μg/kg) = (Cd residue in eggshell (μg/kg) × Eggshell weight (g) + Cd residue in albumen (μg/kg) × Albumen weight (g) + Cd residue in yolk (μg/kg) × Yolk weight (g)) / Total egg weight (g).
5 Y = −0.1937x + 81.187, R2 = 0.8439.
6 Y = −0.0114x + 3.9529, R2 = 0.8027.
7 Y = −0.0192x + 4.0351, R2 = 0.9563.
8 Y = −0.0006x + 0.3424, R2 = 0.8318.
9 Y = −0.0008x + 0.3455, R2 = 0.8981.
a–eMeans within a row with different superscripts are significantly different (P < 0.05).
*P < 0.05 vs. groups of third week. Two-way ANOVA, LSD tests for egg quality, 1-way ANOVA, Tukey post hoc test for Cd residues. Linear regression analysis was used to assess the dose–response relationship between Cd exposure and egg quality and Cd residues. kgf/m2, kilogram-force/m2.
Egg laying is recognized as a way for adult females to excrete environmental contaminants (e.g., metals) (Kim and Oh, 2014). Therefore, the eggs of birds can be used as an effective bioindicator for monitoring environmental Cd contamination (Shahbaz et al., 2013). In this study, as shown in Table 3, the concentration of Cd in eggs was closely related to that in the diet; with the increase in dietary Cd (from 0.47 to 60.67 mg/kg), the accumulation of Cd in the eggshell, albumen, yolk, and total egg increased significantly (P < 0.05). Interestingly, the main accumulation site of Cd was the eggshell, while the accumulation of Cd was low in the albumen and yolk. Similarly, Sato et al. (1997) found that the Cd concentration in yolk and albumen of eggs was not significantly increased despite the high Cd accumulation in the liver and the follicle walls of the ovary. This phenomenon may be mainly attributed to the follicle walls, which play a role in protecting the follicle yolks against Cd toxicity (Sato et al., 1997). During the experimental period, the Cd content in the total egg exceeded the maximum permitted levels of Cd in fresh eggs (Cd < 0.05 mg/kg, GB 2762-2017) (CFDA, 2017) when the diet was supplemented with 15.56 mg/kg Cd. Nevertheless, diets contaminated with 7.58 mg/kg Cd or less still meet the requirements of food safety for Cd residues in fresh eggs. In addition, the Cd concentration in the eggshell gland followed a linear trend, which increased significantly with increasing dietary Cd (P < 0.05). The eggshell gland is the main site of eggshell formation. The toxic effects of Cd on calcium homeostasis and various proteins related to the eggshell formation may be the key factors affecting eggshell quality, which will be discussed in the following experiments.
Ultrastructure of the eggshell
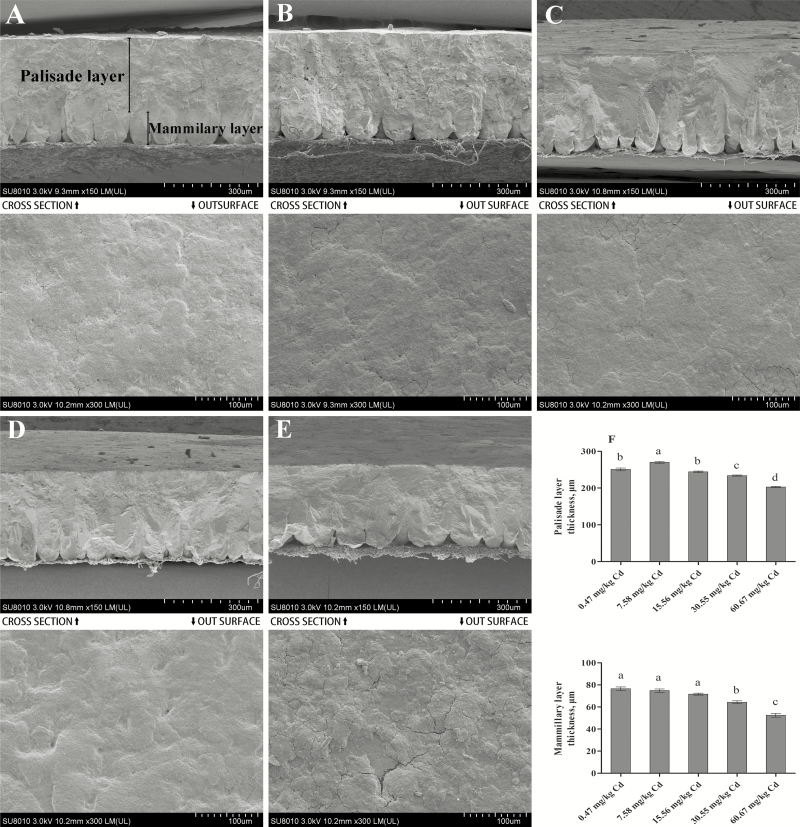
Ultrastructural studies have shown that the eggshell is comprised of morphologically different calcified layers, including the cuticle layer, mammillary layer, palisade layer, vertical crystal layer, and shell membrane (Ruiz et al., 2000). Among them, the palisade column, which comprises approximately two-thirds of the total thickness of the eggshell, is considered to be a major determinant of shell stiffness and strength (Parsons, 1982; Ruiz et al., 2000). In the current study, the palisade layer thickness increased significantly in the low dose Cd-supplemented group (7.58 mg/kg) compared with the control group (P < 0.05) and then decreased with the increase in dietary Cd dose; significant decreases (P < 0.05) were observed in the 30.55 and 60.67 mg/kg Cd groups compared with the control group (Figure 3). The changes in the thickness of the palisade layer were consistent with those of the eggshell thickness and strength. Many studies have found that the effective thickness and densities of the mammillary layer are also meaningful indicators that reflect the mechanical properties of eggshells (Bunk and Balloun, 1978; Gongruttananun, 2017). Furthermore, eggs with a thicker mammillary layer have a higher ability to hatch successfully (Bunk and Balloun, 1978). This layer provides approximately 80% of the total calcium requirement of the chick before hatching (Dieckert et al., 1989). Our results showed that the thickness of the mammillary layer presented a decreasing trend with a significant (P < 0.05) decrease in the high-dose Cd-supplemented groups (30.55 and 60.67 mg/kg) compared with the control group (Figure 3). In addition, the results revealed that the outer surface of eggshell became rougher in the groups administrated 30.55 and 60.67 mg/kg Cd (Figure 3A–E). This may be attributed to the effect of Cd on the expression of ovocalyxin-32 (OCX-32), which mainly becomes incorporated into the outer layers of eggshell and the cuticle and regulates the termination of eggshell calcification (Fulton et al., 2012). The effect of Cd on the expression of OCX-32 will be discussed in the following experiment. These results indicated that Cd could affect eggshell quality by affecting the thickness of the palisade and mammillary layers, as well as shell outer surface deposition. In addition, we speculated that the high accumulation of Cd in eggshell might affect the embryo development through the mammillary layer.
Figure 3.
Ultrastructure of the eggshell of laying hens using scanning electron microscopy after Cd exposure. Scanning electron micrographs of eggshell in laying hens fed 0.47, 7.58, 15.56, 30.55, or 60.67 mg/kg Cd. (A–E) Cross section of eggshells (magnified ×150, scale bar = 300 μm), and out surface of eggshell (magnified ×300, scale bar = 100 μm). Eight eggs with each micrograph. (F) Thickness of the palisade layer and mammillary layer. Each value is the mean ± SE (n = 6 replicates per treatment and n = 12 eggs per treatment). a–dColumn with different superscript letters were significantly different (P < 0.05).
Oxidative stress variables and ATPase activities in the eggshell gland
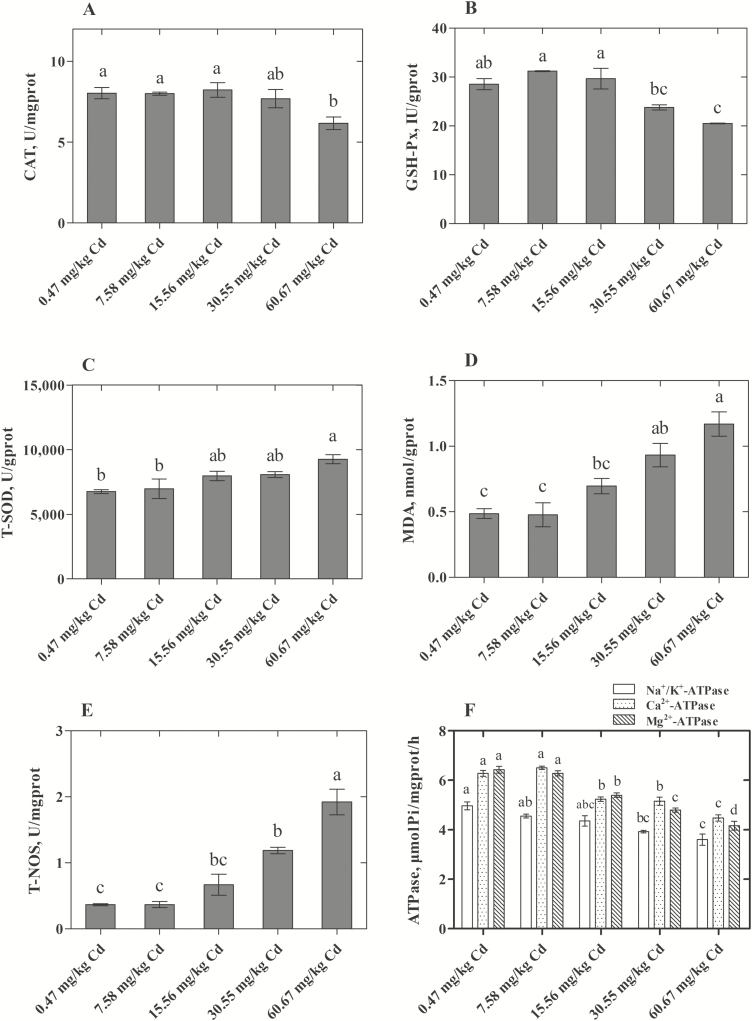
The role of Cd-induced oxidative stress in various cells and organs of the body by disrupting the balance of the oxidant/antioxidant system is well documented (Valko et al., 2005). Various studies have demonstrated that the uterus is one of the target tissues affected by Cd (Nasiadek et al., 2014). In the current study, we evaluated the related enzyme activities of oxidative stress in the eggshell gland and found that the activities of T-NOS increased significantly with increasing doses of dietary Cd (30.55 and 60.67 mg/kg; Figure 4E). Zhong et al. (2015) found that Cd could induce ROS generation by modulating the activity of NOS. Another key player in the ROS metabolism is hydrogen peroxide (H2O2) (Dröge, 2002), which can be formed by SOD catalyzing the dismutation of superoxide radicals (O2·−) and be detoxified by the enzyme CAT. Therefore, changes in the activities of SOD and CAT can be indicators of oxidative stress induced by Cd. Our results showed that the activity of CAT decreased in the uterus in the group administered the highest Cd dose (60.67 mg/kg Cd; Figure 4A). However, the activity of T-SOD increased with increasing of dietary Cd supplementation and was significantly increased in the 60.67 mg/kg Cd group (Figure 4C). But higher antioxidative enzyme activities under oxidative stress are not a general rule, which may depend on the strength of the stressor or the duration of exposure (Cheung et al., 2001). In addition, we found that Cd could cause the lipid peroxidation (LPO) in the eggshell gland, and the concentration of MDA increased significantly in the 30.55 and 60.67 mg/kg Cd groups compared with the control group (P < 0.05; Figure 4D). Because MDA is one of the soluble degradation products of lipids formed during the process of LPO, the concentration of MDA can be used to reflect the extent of LPO (Janero et al., 1990). In addition to CAT, the selenium-dependent enzyme GSH-Px plays an important role in both converting H2O2 to water and removing the lipid hydroperoxides (Maiorino et al., 1991; Reiter et al., 1995). The activity of GSH-Px (Figure 4B) in the eggshell gland decreased significantly in the groups administered 30.55 and 60.67 mg/kg Cd. These results demonstrated that oxidative stress was one of the mechanisms of the toxic effects of Cd on the eggshell gland of hens.
Figure 4.
Effect of Cd on oxidative stress variables and ATPase activities in the eggshell gland of laying hens. (A) CAT, catalase; (B) GSH-Px, glutathione peroxide; (C) T-SOD, total superoxide dismutase; (D) MDA, malondialdehyde; (E) T-NOS, total nitric oxide synthase; (F) ATPase (Na+/K+-ATPase, Ca2+-ATPase, and Mg2+-ATPase). Each value is the mean ± SE (n = 6 replicates per treatment and n = 12 hens per treatment). a–dColumn with different superscript letters were significantly different (P < 0.05).
In addition, we found that the activities of ATPases, including Na+/K+ ATPase, Ca2+-ATPase, and Mg2+-ATPase, decreased significantly (P < 0.05) in the groups supplemented with 30.55 and 60.67 mg/kg dietary Cd (Figure 4F). Na+/K+ ATPase (sodium pump), as a heterodimer protein, mainly regulates membrane potential and cation transport across the sarcolemmal membrane to maintain the balance of cell homeostasis (Ostadal et al., 2003). Mg2+-ATPase is also a significant ATPase in ionic transport and oxidative phosphorylation and is responsible for the transepithelial regulation of Mg2+ ions (Atli and Canli, 2013). Ca2+-ATPase plays an important role in mediating calcium transport in the uterus of oviparous amniotes during the process of eggshell formation (Parker et al., 2008). Because of the similar size and charge between Cd2+ and Ca2+, Cd2+ can compete with Ca2+ in a number of biological processes, thereby disrupting intracellular Ca2+ homeostasis (Sutoo et al., 1990). The ATP-dependent Ca “pump” was inhibited by Cd, which may result in the inhibition of Ca translocation toward the lumen of the shell gland and ultimately affect eggshell quality. Therefore, our results showed that Cd could exert toxicity by disrupting cell ion metabolism and cell homeostasis in the eggshell gland of hens.
Histopathological variations and relative gene expression of the eggshell gland
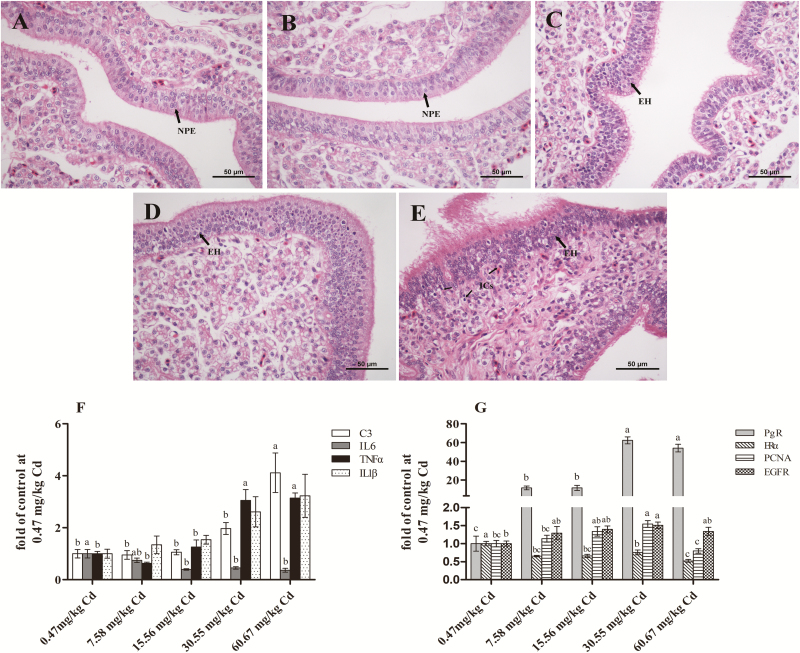
Various studies found that Cd could disrupt the endocrine system of females, due to its wide spectrum of toxic effects on the uterus and mammary gland of rats and the developing human reproductive tract (Johnson et al., 2003, Kippler et al., 2012). Johnson et al. (2003) reported that the exposure of ovariectomized rats to Cd increased the proliferation of the endometrium in the uterus. Similarly, our histological results revealed that the endometrium of the eggshell gland presented obvious hyperplasia with increasing dietary Cd supplementation (Figure 5C–E). In addition, the eggshell gland tissue in the group treated with a high dose of dietary Cd (60.67 mg/kg) exhibited significant pathological damage (Figure 5E). Pathological damage to the eggshell gland has been confirmed to negatively affect its normal secretory function and egg quality (Li et al., 2017). Thus, we examined the gene expression of inflammation-related factors in the eggshell gland (Figure 5F) and showed that the expression of complement C3 and the pro-inflammatory cytokine TNFα increased significantly (P < 0.05) with a high dose of dietary Cd (60.67 mg/kg). Complement is mainly synthesized in the liver. However, the local complement, especially C3, can regulate inflammation in a variety of organs (Giacomassi et al., 2017). Intriguingly, the pro-inflammatory cytokine IL6 decreased significantly in the 15.56, 30.55, and 60.67 mg/kg groups compared with the other 2 groups (P < 0.05). This finding is inconsistent with many previous studies showing that Cd could increase IL6 production in a variety of cells (Souza et al., 2004; Låg et al., 2010). However, this finding is similar to the study by Yoshioka et al (1999), who found that IL6 could significantly inhibit stromal cell proliferation in endometrial tissue from the secretory phase. Furthermore, treatment with estradiol and progesterone induced an inhibitory response to IL6 in endometrial cells from the proliferative phase. These results showed that Cd could induce the proliferation of the endometrium and inflammation of the eggshell gland and that the toxic effects of Cd on the eggshell gland might be closely related to its environmental xenoestrogen properties.
Figure 5.
Eggshell gland histomorphology and histopathology and relative gene expression from various groups of experimental laying hens. The eggshell gland sections were stained with hematoxylin & eosin (×400 magnification, 6 animals with each pathology section). (A) Control (Cadmium 0.47 mg/kg), (B) Cadmium 7.58 mg/kg, (C) Cadmium 15.56 mg/kg, (D) Cadmium 30.55 mg/kg, (E) Cadmium 60.67 mg/kg. NPE, normal proliferative endometrium; EH, endometrial hyperplasia; ICs, Inflammatory cells. Bar scale = 50μm. (F, G) Method of 2-ΔΔCt was applied for calculation of relative gene expression with β-actin as the endogenous control and the average ΔCt value of 0.47μM Cd group as the calibrator to normalize the signal. C3, component C3; IL6, interleukin 6; TNFα, tumor necrosis factor alpha; IL1β, interleukin 1 beta; PgR, progesterone receptor; ERα, estrogen receptor α; PCNA, proliferating cell nuclear antigen; EGFR, epidermal growth factor receptor. Each value is the mean ± SE (n = 6). a–dColumn with different superscript letters were significantly different (P < 0.05).
To confirm the environmental xenoestrogen properties of Cd and preliminarily explore the mechanism of Cd-induced endometrial epithelial cell proliferation in the eggshell gland of laying hens, we examined the expression of ERα, progesterone receptor (PgR), proliferating cell nuclear antigen (PCNA), and epidermal growth factor receptor (EGFR;Figure 5G) and found that Cd induced a significant increase (P < 0.05) in PgR mRNA levels and a decrease (P < 0.05) in ERα mRNA levels in the groups treated with 7.58, 15.56, 30.55, and 60.67 mg/kg Cd compared with the control group. Similar results were also observed in Mcf-7 breast cancer cells by Garcia-Morales et al. (1994), who indicated that Cd might induce Mcf-7 breast cancer cell proliferation and PgR expression by modulating the ER. The increase in the expression of PCNA further confirmed the result that Cd induced endometrial proliferation of the eggshell gland. However, it is controversial whether Cd induces cell proliferation by activating the ER. The results of Gao et al. (2015) showed that low concentrations of Cd stimulated cell proliferation in estrogen-responsive uterine cells by the nongenomic activation of MAPK rather than by classical ER-mediated pathways. Similarly, Wei et al. (2015) reported that EGFR played a critical role in Cd promoting cell proliferation. Our results showing that the expression of EGFR genes increased with the increase in dietary Cd dose and that the difference between the 30.55 mg/kg Cd group and the control group was significant (P < 0.05), seemed to support the above views, so the mechanism through which Cd promotes the proliferation of the eggshell gland endometrium of laying hens needs further study.
Gene expression of proteins involved in eggshell formation in the eggshell gland
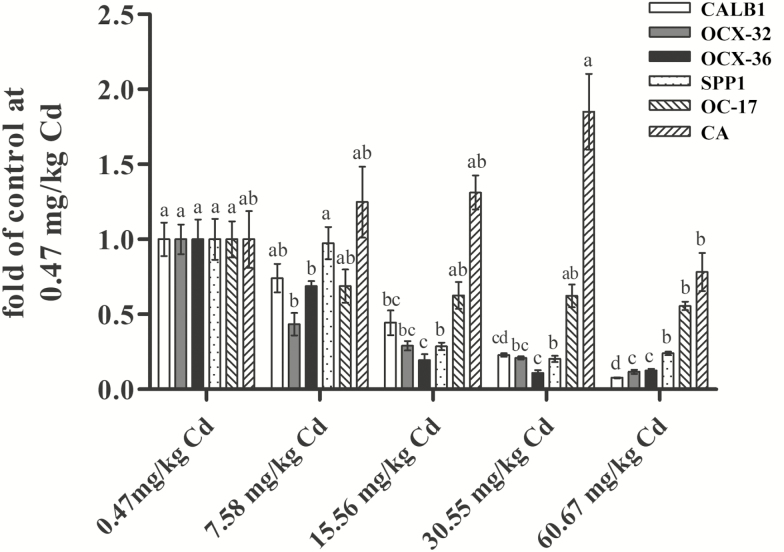
To investigate the effect of Cd on the mechanism of eggshell formation, we measured the gene expression of proteins involved in eggshell formation in the eggshell gland (Figure 6). Eggshell is rich in calcium and various glycoproteins. Different layers of eggshell are formed by the deposition of the organic matter and inorganic matter secreted by the eggshell gland. Proteins such as CA, ovocledidin-17 (OC-17), ovocalyxin-36 (OCX-36), OCX-32, CALB1, and SPP1 play an important role in eggshell calcification (Hincke et al., 1995; Gautron et al., 2007; Brionne et al., 2014).
Figure 6.
Effects of Cd on expression of calbindin 1 (CALB1), ovocalyxin-32 (OCX-32), ovocalyxin-36 (OCX-36), osteopontin (SPP1), ovocledidin-17 (OC-17), and carbonic anhydrase (CA) in the eggshell gland of hens treated with cadmium chloride. Method of 2−ΔΔCt was applied for the calculation of relative gene expression with β-actin as the endogenous control and the average ΔΔCt value of 0 μM Cd group as the calibrator to normalize the signal. Each value is the mean ± SE (n = 6). a–dColumn with different superscript letters were significantly different (P < 0.05).
In the ultrastructure study, we speculated that the disorder of the outer shell surface deposition may be related to the expression of OCX-32. The results showed that the gene expression of OCX-32 in the eggshell gland decreased significantly (P < 0.05) with increasing dietary Cd dose (7.58 to 60.67 mg/kg) compared with the control group. Chen et al. (2015) reported that calcium deficiency could reduce OCX-32 expression in the uterus of ducks, which could be reversed by repletion of adequate dietary calcium. Therefore, the effect of Cd on calcium homeostasis may be the major reason for these adverse results. OCX-36, which is only expressed in the eggshell gland in the oviduct of laying hens, is present predominately in the inner part of the shell and shell membrane (Gautron et al., 2007). It may protect against bacterial invasion in both embryonated eggs (relevant to avian reproductive success) and unfertilized table eggs (relevant to food safety) (Cordeiro et al., 2013). The suppression of OCX-36 expression (7.58 to 60.67 mg/kg Cd) indicated that Cd treatment, even at a low dose, might weaken the resistance of eggs to pathogens.
It is known that the shell stiffness and strength are mainly determined by the palisade and mammillary layers. SPP1 is localized specifically in the base of the mammillary layers (mammillary cores) and in the outermost part of the palisade layer of the shell (Fernandez et al., 2003). Another protein OC-17 was found to be distributed throughout the shell matrix but concentrated in the mammillary layers (Hincke et al., 1995). In the current study, the decrease in palisade and mammillary layer thickness induced by Cd supplementation may be associated with the suppression of SPP1 and OC-17 expression in the eggshell gland. This is because these proteins are associated with the initiation of eggshell calcification (Sharp and Silyn-Roberts, 1984) and calcite growth (Freeman et al., 2010). In addition, CaCO3 is a major component of avian eggshell. CALB1 is an important protein responsible for Ca transport in the eggshell gland of laying hens (Bar et al., 1996). CA plays a critical role in catalyzing the change in metabolic CO2 to bicarbonate ions (Hulikova et al., 2014), which drives the deposition of CaCO3 in eggshell mainly dependent on dietary calcium (Chen et al., 2015). Our results showed that the mRNA expression of CALB1 decreased significantly with increasing dietary Cd supplementation (15.56, 30.55, and 60.67 mg/kg Cd), but interestingly, the expression of CA increased with increasing dietary Cd supplementation and then decreased at a dose of 60.67 mg/kg. Similarly, Caricato et al. (2010) found that a low dose of Cd could enhance the CA protein expression in the digestive gland in mussels. However, diets contaminated with 7.58 mg/kg Cd had no significant effects on the expression of SPP1, OC-17, CALB1, and CA, which was not consistent with the changes affected by Cd on the eggshell strength and thickness. Therefore, the mechanism by which low-dose Cd improves eggshell quality still needs further study. However, a high dietary dose of Cd impaired the eggshell microstructure, probably by suppressing the process of eggshell formation, including outer or inner shell membrane formation, Ca transport, and calcite crystal calcification, thus negatively affecting eggshell quality.
In conclusion, dietary supplementation with Cd at 60.67 mg/kg decreased laying performance and egg quality, exacerbated oxidative damage and inflammatory response, and disrupted the endocrine system in the eggshell gland of laying hens. No negative effects on performance and egg quality were found with the addition of 7.58 and 15.56 mg/kg Cd to diets. Diets supplemented with 7.58 mg/kg Cd even exerted positive effects on egg quality. The study suggested that diets contaminated with Cd at a concentration of 7.58 mg/kg or less in the short term have no negative effect on hen health, laying performance, egg quality, and egg safety. Cd-contaminated diets should be replaced with a normal diet in time. In addition, the current study for the first time discussed the toxic effects of Cd on the expression of proteins related to eggshell deposition in the eggshell gland and preliminarily explored the possible mechanism of Cd affecting the eggshell biomineralization and the proliferation of endometrial epithelium of eggshell gland in laying hens.
Acknowledgment
This study was supported by the Modern Agro-industry Technology Research System of China (CARS-40-K10).
Glossary
Abbreviations
- AEW
average egg weight
- CA
carbonic anhydrase
- CALB
calbindin
- CAT
catalase
- EGFR
epidermal growth factor receptor
- EPR
egg production rate
- ERα
estrogen receptor
- EW
egg weight
- FCR
feed conversion ratio
- GFAAS
graphite furnace atomic absorption spectrometry
- GSH-Px
glutathione peroxide
- LPO
lipid peroxidation
- MDA
malondialdehyde
- PCNA
proliferating cell nuclear antigen
- PgR
progesterone receptor
- TNFα
tumor necrosis factor α
- T-NOS
total nitric oxide synthase
- T-SOD
total superoxide dismutase
- SPP
osteopontin
Conflict of interest statement
The authors declare that they have no conflict of interest.
Literature cited
- Aravind P., and Prasad M. N. V.. 2004. Carbonic anhydrase impairment in cadmium-treated Ceratophyllum demersum L. (free floating freshwater macrophyte): toxicity reversal by zinc. J. Anal. At. Spectrom. 19:52–57. doi: 10.1039/b307282g [DOI] [Google Scholar]
- Atli G., and Canli M... 2013. Metals (Ag+, Cd2+, Cr6+) affect ATPase activity in the gill, kidney, and muscle of freshwater fish Oreochromis niloticus following acute and chronic exposures. Environ. Toxicol. 28:707–717. doi: 10.1002/tox.20766 [DOI] [PubMed] [Google Scholar]
- Bar A., Vax E., Hunziker W., Halevy O., and Striem S... 1996. The role of gonadal hormones in gene expression of calbindin (Mr 28,000) in the laying hen. Gen. Comp. Endocrinol. 103:115–122. doi: 10.1006/gcen.1996.0100 [DOI] [PubMed] [Google Scholar]
- Brama M., Gnessi L., Basciani S., Cerulli N., Politi L., Spera G., Mariani S., Cherubini S., Scotto d’Abusco A., Scandurra R.,. et al. 2007. Cadmium induces mitogenic signaling in breast cancer cell by an ERalpha-dependent mechanism. Mol. Cell. Endocrinol. 264:102–108. doi: 10.1016/j.mce.2006.10.013 [DOI] [PubMed] [Google Scholar]
- Brionne A., Nys Y., Hennequet-Antier C., and Gautron J... 2014. Hen uterine gene expression profiling during eggshell formation reveals putative proteins involved in the supply of minerals or in the shell mineralization process. BMC Genomics 15:220. doi: 10.1186/1471-2164-15-220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunk M. J., and Balloun S. L... 1978. Ultrastructure of the mammillary region of low puncture strength avian eggshells. Poult Sci. 57:639–647. doi: 10.3382/ps.0570639 [DOI] [Google Scholar]
- Byrne C., Divekar S. D., Storchan G. B., Parodi D. A., and Martin M. B... 2009. Cadmium–a metallohormone? Toxicol. Appl. Pharmacol. 238:266–271. doi: 10.1016/j.taap.2009.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caricato R., Roberto C., Lionetto M. G., Giulia L. M., Dondero F., Francesco D., Viarengo A., Aldo V., Schettino T., and Trifone S... 2010. Carbonic anhydrase activity in Mytilus galloprovincialis digestive gland: sensitivity to heavy metal exposure. Comp. Biochem. Physiol. C. Toxicol. Pharmacol. 152:241–247. doi: 10.1016/j.cbpc.2010.04.011 [DOI] [PubMed] [Google Scholar]
- Chen W., Zhao F., Tian Z. M., Zhang H. X., Ruan D., Li Y., Wang S., Zheng C. T., and Lin Y. C... 2015. Dietary calcium deficiency in laying ducks impairs eggshell quality by suppressing shell biomineralization. J. Exp. Biol. 218(Pt 20):3336–3343. doi: 10.1242/jeb.124347 [DOI] [PubMed] [Google Scholar]
- Cheung C. C., Zheng G. J., Li A. M., Richardson B. J., and Lam P. K... 2001. Relationships between tissue concentrations of polycyclic aromatic hydrocarbons and antioxidative responses of marine mussels, Perna viridis. Aquat. Toxicol. 52:189–203. doi: 10.1016/s0166-445x(00)00145-4 [DOI] [PubMed] [Google Scholar]
- China food and drug administration (CFDA). 2017. Maximum levels of contaminants in foods. GB 2762-2017. Beijing: China Standard Press. [Google Scholar]
- Choong G., Liu Y., and Templeton D. M... 2014. Interplay of calcium and cadmium in mediating cadmium toxicity. Chem. Biol. Interact. 211:54–65. doi: 10.1016/j.cbi.2014.01.007 [DOI] [PubMed] [Google Scholar]
- Cordeiro C. M., Esmaili H., Ansah G., and Hincke M. T... 2013. Ovocalyxin-36 is a pattern recognition protein in chicken eggshell membranes. Plos One 8:e84112. doi: 10.1371/journal.pone.0084112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czarnecki G. L., and Baker D. H... 1982. Tolerance of the chick to excess dietary cadmium as influenced by dietary cysteine and by experimental infection with Eimeria acervulina. J. Anim. Sci. 54:983–988. doi: 10.2527/jas1982.545983x [DOI] [PubMed] [Google Scholar]
- Dauwea T., Janssensa E., Kempenaersb B., and Eens M... 2004. The effect of heavy metal exposure on egg size, eggshell thickness and the number of spermatozoa in blue tit Parus caeruleus eggs. Environ. Pollut. 129:125–129. doi: 10.1016/j.envpol.2003.09.028 [DOI] [PubMed] [Google Scholar]
- Dieckert J. W., Dieckert M. C., and Creger C. R... 1989. Calcium reserve assembly: a basic structural unit of the calcium reserve system of the hen egg shell. Poult. Sci. 68:1569–1584. doi: 10.3382/ps.0681569 [DOI] [PubMed] [Google Scholar]
- Dröge W. 2002. Free radicals in the physiological control of cell function. Physiol. Rev. 82:47–95. doi: 10.1152/physrev.00018.2001 [DOI] [PubMed] [Google Scholar]
- Duc Phuc H., Kido T., Dung Manh H., Thai Anh L., Phuong Oanh N. T., Okamoto R., Ichimori A., Nogawa K., Suwazono Y., and Nakagawa H... 2016. A 28-year observational study of urinary cadmium and β2-microglobulin concentrations in inhabitants in cadmium-polluted areas in Japan. J. Appl. Toxicol. 36:1622–1628. doi: 10.1002/jat.3327 [DOI] [PubMed] [Google Scholar]
- Fernandez M. S., Escobar C., Lavelin I., Pines M., and Arias J. L... 2003. Localization of osteopontin in oviduct tissue and eggshell during different stages of the avian egg laying cycle. J. Struct. Biol. 143:171–180. doi: 10.1016/j.jsb.2003.08.007 [DOI] [PubMed] [Google Scholar]
- Freeman C. L., Harding J. H., Quigley D., and Rodger P. M... 2010. Structural control of crystal nuclei by an eggshell protein. Angew. Chem. Int. Ed. Engl. 49:5135–5137. doi: 10.1002/anie.201000679 [DOI] [PubMed] [Google Scholar]
- Fulton J. E., Soller M., Lund A. R., Arango J., and Lipkin E... 2012. Variation in the ovocalyxin-32 gene in commercial egg-laying chickens and its relationship with egg production and egg quality traits. Anim. Genet. 43(Suppl 1):102–113. doi: 10.1111/j.1365-2052.2012.02384.x [DOI] [PubMed] [Google Scholar]
- Gao X. H., Yu L. D., Moore A. B., Kissling G. E., Waalkes M. P., and Dixon D... 2015. Cadmium and proliferation in human uterine leiomyoma cells: evidence of a role for EGFR/MAPK pathways but not classical estrogen receptor pathways. Environ Health Persp. 123(4):331–336. doi: 10.1289/ehp.1408234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Morales P., Saceda M., Kenney N., Kim N., Salomon D. S., Gottardis M. M., Solomon H. B., Sholler P. F., Jordan V. C., and Martin M. B... 1994. Effect of cadmium on estrogen receptor levels and estrogen-induced responses in human breast cancer cells. J. Biol. Chem. 269:16896–16901. [PubMed] [Google Scholar]
- Gautron J., Murayama E., Vignal A., Morisson M., McKee M. D., Réhault S., Labas V., Belghazi M., Vidal M. L., Nys Y.,. et al. 2007. Cloning of ovocalyxin-36, a novel chicken eggshell protein related to lipopolysaccharide-binding proteins, bactericidal permeability-increasing proteins, and plunc family proteins. J. Biol. Chem. 282:5273–5286. doi: 10.1074/jbc.M610294200 [DOI] [PubMed] [Google Scholar]
- Giacomassi C., Buang N., Ling G. S., Crawford G., Cook H. T., Scott D., Dazzi F., Strid J., and Botto M... 2017. Complement C3 exacerbates imiquimod-induced skin inflammation and psoriasiform dermatitis. J. Invest. Dermatol. 137:760–763. doi: 10.1016/j.jid.2016.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godt J., Scheidig F., Grosse-Siestrup C., Esche V., Brandenburg P., Reich A., and Groneberg D. A... 2006. The toxicity of cadmium and resulting hazards for human health. J. Occup. Med. Toxicol. 1:22. doi: 10.1186/1745-6673-1-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gongruttananun N. 2017. Induced molt using cassava meal. 2. Effects on eggshell quality, ultrastructure, and pore density in late-phase laying hens. Poult. Sci. 97:1050–1058. doi: 10.3382/ps/pex365. [DOI] [PubMed] [Google Scholar]
- Hincke M. T., Nys Y., Gautron J., Mann K., Rodriguez-Navarro A. B., and McKee M. D... 2012. The eggshell: structure, composition and mineralization. Front. Biosci. (Landmark Ed). 17:1266–1280. doi: 10.2741/3985 [DOI] [PubMed] [Google Scholar]
- Hincke M. T., Tsang C. P., Courtney M., Hill V., and Narbaitz R... 1995. Purification and immunochemistry of a soluble matrix protein of the chicken eggshell (ovocleidin 17). Calcif. Tissue Int. 56:578–583. doi: 10.1007/bf00298593 [DOI] [PubMed] [Google Scholar]
- Hulikova A., Aveyard N., Harris A. L., Vaughan-Jones R. D., and Swietach P... 2014. Intracellular carbonic anhydrase activity sensitizes cancer cell pH signaling to dynamic changes in CO2 partial pressure. J. Biol. Chem. 289:25418–25430. doi: 10.1074/jbc.M114.547844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson L. W., Zullo M. D., and Goldberg J. M... 2008. The association between heavy metals, endometriosis and uterine myomas among premenopausal women: National Health and Nutrition Examination Survey 1999–2002. Hum. Reprod. 23:679–687. doi: 10.1093/humrep/dem394 [DOI] [PubMed] [Google Scholar]
- Janero D. R. 1990. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic. Biol. Med. 9:515–540. doi: 10.1016/0891-5849(90)90131-2 [DOI] [PubMed] [Google Scholar]
- Johnson M. D., Kenney N., Stoica A., Hilakivi-Clarke L., Singh B., Chepko G., Clarke R., Sholler P. F., Lirio A. A., Foss C.,. et al. 2003. Cadmium mimics the in vivo effects of estrogen in the uterus and mammary gland. Nat. Med. 9:1081–1084. doi: 10.1038/nm902 [DOI] [PubMed] [Google Scholar]
- Kim J., and Oh J. M... 2014. Trace element concentrations in eggshells and egg contents of black-tailed gull (Larus crassirostris) from Korea. Ecotoxicology 23:1147–1152. doi: 10.1007/s10646-014-1256-0 [DOI] [PubMed] [Google Scholar]
- Kippler M., Tofail F., Gardner R., Rahman A., Hamadani J. D., Bottai M., and Vahter M... 2012. Maternal cadmium exposure during pregnancy and size at birth: a prospective cohort study. Environ. Health Perspect. 120:284–289. doi: 10.1289/ehp.1103711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Låg M., Rodionov D., Ovrevik J., Bakke O., Schwarze P. E., and Refsnes M... 2010. Cadmium-induced inflammatory responses in cells relevant for lung toxicity: expression and release of cytokines in fibroblasts, epithelial cells and macrophages. Toxicol. Lett. 193:252–260. doi: 10.1016/j.toxlet.2010.01.015 [DOI] [PubMed] [Google Scholar]
- Leach R. M. Jr, Wang K. W., and Baker D. E... 1979. Cadmium and the food chain: the effect of dietary cadmium on tissue composition in chicks and laying hens. J. Nutr. 109:437–443. doi: 10.1093/jn/109.3.437 [DOI] [PubMed] [Google Scholar]
- Li J., Leghari I. H., He B., Zeng W., Mi Y., and Zhang C... 2014. Estrogen stimulates expression of chicken hepatic vitellogenin II and very low-density apolipoprotein II through ER-α. Theriogenology 82:517–524. doi: 10.1016/j.theriogenology.2014.05.003 [DOI] [PubMed] [Google Scholar]
- Li R. Q., Qi X. F., Han X. Y., Liu C. H., Wang J., Wang R. C., Wang J. Y., and Huang J. Y... 2017. Deterioration of eggshell quality is related to calbindin in laying hens infected with velogenic genotype VIId Newcastle disease virus. Theriogenology 91:62–68. doi: 10.1016/j.theriogenology.2016.12.030 [DOI] [PubMed] [Google Scholar]
- Li Y. X., Xiong X., Lin C. Y., Zhang F. S., Wei L., and Wei H... 2010. Cadmium in animal production and its potential hazard on Beijing and Fuxin farmlands. J. Hazard. Mater. 177:475–480. doi: 10.1016/j.jhazmat.2009.12.057 [DOI] [PubMed] [Google Scholar]
- Livak K. J., and Schmittgen T. D... 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T) method. Method. 25:402–408. doi: 10.1006/meth.2001 [DOI] [PubMed] [Google Scholar]
- Lundholm C. E. 1991. Influence of chlorinated hydrocarbons, Hg2+ and methyl-Hg+ on steroid hormone receptors from eggshell gland mucosa of domestic fowls and ducks. Arch. Toxicol. 65:220–227. doi: 10.1007/bf02307312 [DOI] [PubMed] [Google Scholar]
- Maiorino M., Chu F. F., Ursini F., Davies K. J., Doroshow J. H., and Esworthy R. S... 1991. Phospholipid hydroperoxide glutathione peroxidase is the 18-kDa selenoprotein expressed in human tumor cell lines. J. Biol. Chem. 266:7728–7732. [PubMed] [Google Scholar]
- Martelli A., Rousselet E., Dycke C., Bouron A., and Moulis J. M... 2006. Cadmium toxicity in animal cells by interference with essential metals. Biochimie 88:1807–1814. doi: 10.1016/j.biochi.2006.05.013 [DOI] [PubMed] [Google Scholar]
- Nakada T., Asato L., Oikawa T., Koja Z., and Tanaka K... 1994. Effect of estrogen on yolk deposition and atresia of ovarian follicles in hypophysectomized hens. Japanese Poult. Sci. 31:162–167. doi: 10.2141/jpsa.31.162. [DOI] [Google Scholar]
- Nasiadek M., Skrzypińska-Gawrysiak M., Daragó A., Zwierzyńska E., and Kilanowicz A... 2014. Involvement of oxidative stress in the mechanism of cadmium-induced toxicity on rat uterus. Environ. Toxicol. Pharmacol. 38:364–373. doi: 10.1016/j.etap.2014.07.007. [DOI] [PubMed] [Google Scholar]
- National Health and Family Planning Commission of China (NHFPC). 2015. National food cadmium standard: determination of cadmium in foods. GB/T 5009.15-2015. Beijing: China Standards Press. [Google Scholar]
- Nys Y., Gautron J., Garcia-Ruiz J. M., and Hincke M. T... 2004. Avian eggshell mineralization: biochemical and functional characterization of matrix proteins. CR. Palevol. 3(6–7):549–562. doi: 10.1016/j.crpv.2004.08.002 [DOI] [Google Scholar]
- Olgun O., and Bahtiyarca Y... 2015. Effects of dietary cadmium and boron supplementation on performance, eggshell quality and mineral concentrations of bone in laying hens. Biol. Trace Elem. Res. 167:56–62. doi: 10.1007/s12011-015-0291-x [DOI] [PubMed] [Google Scholar]
- Osborne C. K., and Schiff R... 2005. Estrogen-receptor biology: continuing progress and therapeutic implications. J. Clin. Oncol. 23:1616–1622. doi: 10.1200/JCO.2005.10.036 [DOI] [PubMed] [Google Scholar]
- Ostadal P., Elmoselhi A. B., Zdobnicka I., Lukas A., Chapman D., and Dhalla N. S... 2003. Ischemia-reperfusion alters gene expression of Na+-K+ ATPase isoforms in rat heart. Biochem. Biophys. Res. Commun. 306:457–462. doi: 10.1016/s0006-291x(03)00986-0 [DOI] [PubMed] [Google Scholar]
- Park J. A., and Sohn S. H... 2018. The influence of hen aging on eggshell ultrastructure and shell mineral components. Korean J. Food Sci. Anim. Resour. 38:1080–1091. doi: 10.5851/kosfa.2018.e41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker S. L., Lindsay L. A., Herbert J. F., Murphy C. R., and Thompson M. B... 2008. Expression and localization of Ca2+-ATPase in the uterus during the reproductive cycle of king quail (Coturnix chinensis) and zebra finch (Poephila guttata). Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 149:30–35. doi: 10.1016/j.cbpa.2007.09.014 [DOI] [PubMed] [Google Scholar]
- Parsons A. H. 1982. Structure of the eggshell. Poult Sci. 61:2013–2021. doi: 10.3382/ps.0612013 [DOI] [Google Scholar]
- Public Health Service (PHS) 2002. Policy on Humane Care and Use of Laboratory Animals. Washington (DC): US Department of Health and Human Services. [Google Scholar]
- Rahman M. S., Sasanami T., and Mori M... 2007. Effects of cadmium administration on reproductive performance of Japanese quail (Coturnix japonica). J Poult Sci. 44:92–97. doi: 10.2141/jpsa.44.92 [DOI] [Google Scholar]
- Reiter R. J., Melchiorri D., Sewerynek E., Poeggeler B., Barlow-Walden L., Chuang J., Ortiz G. G., and Acuña-Castroviejo D... 1995. A review of the evidence supporting melatonin’s role as an antioxidant. J. Pineal Res. 18:1–11. doi: 10.1111/j.1600-079x.1995.tb00133.x [DOI] [PubMed] [Google Scholar]
- Ruiz J., and Lunam C. A... 2000. Ultrastructural analysis of the eggshell: contribution of the individual calcified layers and the cuticle to hatchability and egg viability in broiler breeders. Br. Poult. Sci. 41:584–592. doi: 10.1080/713654975 [DOI] [PubMed] [Google Scholar]
- Sant’Ana M. G., Moraes R., and Bernardi M. M... 2005. Toxicity of cadmium in Japanese quail: evaluation of body weight, hepatic and renal function, and cellular immune response. Environ. Res. 99:273–277. doi: 10.1016/j.envres.2005.06.003 [DOI] [PubMed] [Google Scholar]
- Sato S., Okabe M., Emoto T., Kurasaki M., and Kojima Y... 1997. Restriction of cadmium transfer to eggs from laying hens exposed to cadmium. J. Toxicol. Environ. Health 51:15–22. doi: 10.1080/00984109708984008 [DOI] [PubMed] [Google Scholar]
- Shahbaz M., Hashmi M. Z., Malik R. N., and Yasmin A... 2013. Relationship between heavy metals concentrations in egret species, their environment and food chain differences from two Headworks of Pakistan. Chemosphere 93:274–282. doi: 10.1016/j.chemosphere.2013.04.078 [DOI] [PubMed] [Google Scholar]
- Sharp R. M., and Silyn-Roberts H... 1984. Development of preferred orientation in the eggshell of the domestic fowl. Biophys. J. 46:175–179. doi: 10.1016/S0006-3495(84)84010-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza V., Escobar Md Mdel M. C., Gómez-Quiroz L., Bucio L., Hernández E., Cossio E. C., and Gutiérrez-Ruiz M. C... 2004. Acute cadmium exposure enhances AP-1 DNA binding and induces cytokines expression and heat shock protein 70 in HepG2 cells. Toxicology 197:213–228. doi: 10.1016/j.tox.2004.01.006 [DOI] [PubMed] [Google Scholar]
- Sutoo D., Akiyama K., and Imamiya S... 1990. A mechanism of cadmium poisoning: the cross effect of calcium and cadmium in the calmodulin-dependent system. Arch. Toxicol. 64:161–164. doi: 10.1007/bf01974404 [DOI] [PubMed] [Google Scholar]
- Thompson J., and Bannigan J... 2008. Cadmium: toxic effects on the reproductive system and the embryo. Reprod. Toxicol. 25:304–315. doi: 10.1016/j.reprotox.2008.02.001 [DOI] [PubMed] [Google Scholar]
- Valko M., Morris H., and Cronin M. T... 2005. Metals, toxicity and oxidative stress. Curr. Med. Chem. 12:1161–1208. doi: 10.2174/0929867053764635 [DOI] [PubMed] [Google Scholar]
- Wang L. X., Zhang S. P., Wang Z., Xu M., Yuan L., Cui J. S., and Liu S. J... 2017. A protective role of Heme-regulated eIF2a kinase in cadmium-induced liver and kidney injuries. Chemosphere 185:284–289. doi: 10.1016/j.chemosphere.2017.07.018 [DOI] [PubMed] [Google Scholar]
- Wei Z., Song X., and Shaikh Z. A... 2015. Cadmium promotes the proliferation of triple-negative breast cancer cells through EGFR-mediated cell cycle regulation. Toxicol. Appl. Pharmacol. 289:98–108. doi: 10.1016/j.taap.2015.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong B. H., Luo Q. Y., Zhao F., and Pang Z. H.. 2015. Tables of feed composition and nutritive values in China. China Feed. 21-22:23–39. doi: 10.15906/j.cnki.cn11-2975/s.20152108 [DOI] [Google Scholar]
- Yoshioka H., Harada T., Iwabe T., Nagano Y., Taniguchi F., Tanikawa M., and Terakawa N... 1999. Menstrual cycle-specific inhibition of the proliferation of endometrial stromal cells by interleukin 6 and its soluble receptor. Am. J. Obstet. Gynecol. 180:1088–1094. doi: 10.1016/s0002-9378(99)70599-5 [DOI] [PubMed] [Google Scholar]
- Yu X., Hong S., and Faustman E. M... 2008. Cadmium-induced activation of stress signaling pathways, disruption of ubiquitin-dependent protein degradation and apoptosis in primary rat Sertoli cell-gonocyte cocultures. Toxicol. Sci. 104:385–396. doi: 10.1093/toxsci/kfn087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong L. Y., Wang L. M., Xu L. R., Liu Q. L., Jiang L. L., Zhi Y. E., Lu W., and Zhou P... 2015. The role of nitric oxide synthase in an early phase Cd-induced acute cytotoxicity in MCF-7 cells. Biol. Trace Elem. Res. 164:130–138. doi: 10.1007/s12011-014-0187-1 [DOI] [PubMed] [Google Scholar]