Abstract
Vitamin A (VA) is an important nutrient for weaning piglets. It plays a significant role in the normal formation, development, and maintenance of epithelial cells. Previous studies have shown that VA supplements could improve the host’s intestinal barrier function. Therefore, we hypothesized that VA supplements can affect intestinal function in weaned piglets by regulating intestinal stem cells. Thirty-two 21-d-old weaned [(Yorkshire × Landrace) × Duroc] piglets with an average weight of 8.34 ± 0.13 kg were randomly divided into 4 treatment groups, with 1) 2 mg/kg (control), 2) 4 mg/kg, 3) 8 mg/kg, and 4) 16 mg/kg doses of VA, respectively. The experiment lasted for 14 d. Weaned piglets were given ad libitum access to food and water during the test. The ADG (linear, P = 0.020) and G:F (linear, P = 0.005) of the piglets were found to increase significantly from days 8 to 14. The Lgr5+ gene expression (P = 0.012) in the jejunum mucosa of the 16 mg/kg VA group was increased. The jejunum villus height (P = 0.027) and villi surface area (P = 0.035) were significantly increased in the 4 mg/kg VA treatment group. The crypt depth increased significantly in the 4 and 8 mg/kg VA treatment groups (quadratic, P = 0.043), and the ratios of villus height to crypt depth significantly increased in the 16 mg/kg VA group (quadratic, P = 0.015). The maltase (P = 0.032), sucrose (P = 0.041), and alkaline phosphatase activity (linear, P = 0.024) were significantly increased when further supplemented with 4 mg/kg VA. Slc2a2 mRNA abundance was significantly increased in the 2 mg/kg VA group (linear, P = 0.024). Moreover, the budding rates, buddings number per organoid, and Chromogranin A and Muc2 expression of piglet intestinal organoids were significantly reduced (P < 0.05) by VA and its metabolites (retinoic acid). Compared with the control group, the expression of Spp1 and Trop2 increased. These results indicated that VA may increase the stemness of intestinal stem cell in vitro. This study suggested that VA could affect growth performance and intestinal function by regulating intestinal stem cells in the jejunum of weaned piglets.
Keywords: enzyme activity, growth performance, intestinal development, intestinal stem cells, vitamin A, weaning piglets
Introduction
The weaning transition is a critical period for piglet growth. The piglets’ growth and development after weaning is very sensitive to a variety of stressors, which has an adverse effect on intestinal health and leads to huge economic losses in swine husbandry (van Beers-Schreurs and Bruininx, 2002). Weaning stress usually affects the intestinal stem cells in weaned piglets, resulting in negative effects such as diarrhea (Yang et al., 2016a). At the same time, this phase is accompanied by significant changes in gastrointestinal physiology, microbiology, and immunology (Hampson, 1986; Pluske et al., 1997). Hence, it is necessary for piglets to supplement some essential nutrients (such as vitamin A [VA]) to relieve weaning stress.
Vitamin A is an important nutrient for piglets. It plays a key role in vision, fetal development, reproduction, and epithelial cells (Blomhoff, 1994; Balmer and Blomhoff, 2002; Blomhoff and Blomhoff, 2006). There is evidence that VA can be involved in the synthesis of glycoproteins (Wolf et al., 1979). This is important for the normal formation, development, and maintenance of epithelial cells. VA deficiency leads to reduced synthesis of serum glycoprotein in rats (Wolf et al., 1979). β-Carotene, an antioxidant, is a form of VA that plays an important role in preventing lipid peroxidation and tumor formation and delaying aging (Sarkar et al., 1995; Bhatia, 1998). Previous studies have shown that VA supplements could improve the host’s intestinal barrier function (Lima et al., 2010). Weaning stress in piglets causes severe intestinal damage in the first 2 wk after weaning (Montagne et al., 2007). We hypothesized that dietary VA supplements can improve intestinal development, morphology, and mucosal enzyme activity in weaning piglets. This study focused on the effects of VA on growth performance, organ index, intestinal morphological structure, intestinal digestive enzyme activity, blood biochemical parameters, and intestinal stem cells in weaned piglets.
Materials and methods
The experimental design and procedures in this study were approved by the Animal Care and Use Committee of the Hunan Normal University, Changsha City, Hunan, China (Chen et al., 2019).
Animals and experimental treatments
This animal experiment was carried out in the animal house of the Institute of Subtropical Agroecology, Chinese Academy of Sciences. Thirty-two piglets [(Yorkshire × Landrace) × Duroc] at 8.34 ± 0.13 kg average BW were weaned at the age of 3 wk and randomly assigned to 1 of the 4 basic diets containing 2 (control group), 4, 8, or 16 mg/kg VA (Royal DSM NV, Shanghai, China). The VA in the diet was added in the form of retinol acetate. The basic diet (Table 1) met the nutritional requirements of the National Research Council (NRC, 2012) for piglets weighing 7 to 11 kg, and it contained 0.20 mg/kg VA. The piglets were fed for 14 d—8 replicate pens per treatment group and 1 piglet in each pen. The piglets were free to feed and water throughout the experimental period. They were regularly observed for illness, diarrhea, or other abnormal behavior. Following previously described methods (Yin et al., 2001), the piglets’ growth performance was determined by calculating the ADG, ADFI, and the ratio of ADG to ADFI (G:F) throughout the trial period.
Table 1.
Ingredient and chemical composition of weaning piglet diets, as-fed basis
| Dietary vitamin A, mg/kg | ||||
|---|---|---|---|---|
| Items | 2 | 4 | 8 | 16 |
| Ingredient, % | ||||
| Corn | 40.14 | 40.14 | 40.14 | 40.14 |
| Extruded corn1 | 20 | 20 | 20 | 20 |
| Soybean meal | 9 | 9 | 9 | 9 |
| Fish meal | 7 | 7 | 7 | 7 |
| Plasma protein powder | 5 | 5 | 5 | 5 |
| Whey powder | 9 | 9 | 9 | 9 |
| Glucose2 | 3 | 3 | 3 | 3 |
| Soybean oil | 3.8 | 3.8 | 3.8 | 3.8 |
| Stone powder | 1.05 | 1.05 | 1.05 | 1.05 |
| Choline chloride | 0.1 | 0.1 | 0.1 | 0.1 |
| Antioxidants | 0.05 | 0.05 | 0.05 | 0.05 |
| Citric acid | 0.5 | 0.5 | 0.5 | 0.5 |
| Salt | 0.1 | 0.1 | 0.1 | 0.1 |
| l-Lys HCl | 0.45 | 0.45 | 0.45 | 0.45 |
| dl-Met | 0.2 | 0.2 | 0.2 | 0.2 |
| l-Thr | 0.14 | 0.14 | 0.14 | 0.14 |
| l-Trp | 0.02 | 0.02 | 0.02 | 0.02 |
| Mineral premix3 | 0.15 | 0.15 | 0.15 | 0.15 |
| Vitamin premix4 | 0.3 | 0.3 | 0.3 | 0.3 |
| Total | 100 | 100 | 100 | 100 |
| Calculated composition | ||||
| CP, % | 18.65 | 18.65 | 18.65 | 18.65 |
| ME, kcal/kg | 3,407 | 3,407 | 3,407 | 3,407 |
| VA, mg/kg | 2.20 | 4.20 | 8.20 | 16.20 |
| Ca, % | 0.80 | 0.80 | 0.80 | 0.80 |
| Total P, % | 0.56 | 0.56 | 0.56 | 0.56 |
| Available P, % | 0.38 | 0.38 | 0.38 | 0.38 |
| l-Lys5, % | 1.37 | 1.37 | 1.37 | 1.37 |
| Thr5, % | 0.81 | 0.81 | 0.81 | 0.81 |
| Trp5, % | 0.22 | 0.22 | 0.22 | 0.22 |
| SAA5,6, % | 0.75 | 0.75 | 0.75 | 0.75 |
| DCAD7, mEq/kg DM | 2.32 | 2.32 | 2.32 | 2.32 |
1Bulk density is 0.3 to 0.5 kg/L, puffing temperature is 100 to 150 °C. The moisture content of the finished product is 8% to 10%, and the degree of gelatinization is above 90%.
2Dextrose monohydrate.
3Mineral premix per kilogram of feed: 150 mg Fe (FeSO4), 100 mg Zn (ZnSO4), 30 mg Mn (MnSO4), 25 mg Cu (CuSO4), 0.5 mg I (KIO3), 0.3 mg Co (CoSO4), and 0.3 mg Se (Na2SeO3).
4Vitamin premix supplied per kilogram of feed: 220 IU vitamin D3; 16 IU vitamin E; 0.5 mg vitamin K3; 17.5 μg vitamin B12; 3.5 mg riboflavin; 30 mg niacin; 10 mg d-pantothenic acid; 0.05 mg biotin; 0.3 mg folic acid; 1.0 mg thiamine; 7 mg pyridoxine; and, 4.0 mg ethoxyquin.
5Standardized ileal digestible.
6SAA = Met + Cys.
7DCAD = dietary cation–anion difference, which was calculated according to the formula: [(%Na/0.023 + %K/0.039)] − [(%Cl/0.0355 + %S/0.016)].
Sample collection and measurements
At 14 d, after the piglets had been made to fast overnight, approximately 10 mL of blood samples was collected from them by jugular puncture in conventional blood collection tubes. Afterwards, these piglets were euthanized using the previously recommended method on the 14th day (Yan et al., 2018). The weight of the liver, spleen, 2 kidneys, and the total length of the small intestine of each piglet was recorded. The organ indexes (ratio of total weight or total length to BW) were calculated based on the 14th-day BW of the weaned piglets. Jejunum and ileum samples (at least 2 cm)—from which intestinal contents had been removed—were immediately collected and stored in a 4% formalin solution at room temperature. The jejunum and ileum segments of the piglets were cut longitudinally along the intestine; the contents of the intestine were then rinsed with PBS solution. The upper intestinal mucosa was carefully collected with a glass slide, wrapped in tin foil, frozen in liquid nitrogen, and brought back to the laboratory for −80 °C frozen storage until it was required for analysis.
Intestinal morphology analysis
The jejunum and ileum samples soaked in the 4% formalin solution were embedded in paraffin in accordance with standard paraffin embedding procedures (Barea et al., 2011) and then cut into 4-μm-thick sections and stained with hematoxylin and eosin. The villi and crypts of the intestine were observed under a microscope in a 40× combined magnification and image processing and analysis system (Version 4.12, Leica Imaging Systems Ltd., Cambridge, UK). The villus height (VH), crypt depth (CD), and villus width (VW) of the jejunum and ileum were measured using Image-pro Plus 6.0 software. The ratio of VH to CD (VH:CD) and the villus surface area (VSA) were calculated. VSA (= π × VH × VW) was calculated according to the method recommended by Kisielinski et al. (2002). At least 20 intact villi and their associated crypts were selected from the intestinal section of each piglet. The corresponding mean value of VH:CD and VSA in each piglet was calculated and used for further analysis (Chen et al., 2019).
Analysis of intestinal enzyme activities
The piglets’ jejunum and ileum mucosa tissue samples were homogenized in liquid nitrogen, immediately collected in PBS solution, allowed to stand for 3 h, and centrifuged (3,000 × g, 4 °C, 10 min). The supernatants were taken for analysis of intestinal maltase, sucrase, lactase, and alkaline phosphatase activities using the purchased kits according to the manufacturer’s instructions (Nanjing Jiancheng Bioengineering Institute, China).
Real-time quantitative PCR
The total RNA of the jejunum and ileum samples were isolated from the liquid nitrogen frozen and ground tissues using RNAiso Plus (Code No. 9109, TaKaRa, Dalian, China) and then treated with the PrimeScript RT reagent Kit with gDNA Eraser (Code No. RR047A, TaKaRa, Dalian, China) to remove the genomic DNA (42 °C, 2 min). Reverse transcription was conducted at 37 °C for 15 min and at 85 °C for 5 s, as per the instructions in the product manual. The methods of total RNA extraction, reverse transcription, and RT-qPCR of organoid samples are the same as those of tissue samples. Primers used in this study were designed via Primer design software 6.0 for leucine-rich-repeat-containing G-protein-coupled receptor 5 (Lgr5+), solute carrier family 15 member 1 (Slc15a1/PepT1), solute carrier family 7 member 1 (Slc7a1/CAT1), solute carrier family 6 member 19 (Slc6a19/B0AT1), solute carrier family 1 member 1 (Slc1a1/EAAT3), solute carrier family 2 member 2 (Slc2a2/GLUT2), solute carrier family 27 member 4 (Slc27a4/FATP4), Chromogranin A, Muc2, Spp1, Trop2, and the housekeeping gene (β-actin) according to pig gene sequence (Table 2). The cDNA was obtained by reverse transcription and diluted 5 times with RNase-free water for real-time quantitative PCR (ABI 7900HT Fast Real-Time PCR System; Applied Biosystems, Carlsbad, CA) analysis using a final volume of 10 μL. The first step was pre-denaturation (95 °C, 30 s); the second step was PCR reaction (95 °C, 5 s and 60 °C, 34 s at 40 cycles). There were 3 repetitions per gene, with each sample corresponding to β-actin as a reference. The target gene mRNA relative expression (RE) was calculated as RE = 2−ΔΔCt(treat−control); −ΔΔCt (treat − control) = (Ct target gene − Ct β-actin)treat − (Ct target gene − Ct β-actin)control. The target mRNA and β-actin mRNA were amplified with comparable efficiencies. The double distilled water (ddH2O) was used instead of cDNA for the negative control, as described in previous research (Yang et al., 2016b,c).
Table 2.
Primers used for real-time PCR analysis
| Genes | Primers | Primers sequences, 5′ to 3′ | Size, bp | NCBI accession number |
|---|---|---|---|---|
| Lgr5 + | Forward | GCCTTTGTAGGCAACCCTTC | 121 | NM_001315762.1 |
| Reverse | AGGCACCATTCAAAGTCAGTG | |||
| Slc15a1/PepT1 | Forward | CATCGCCATACCCTTCTG | 144 | NM_214347.1 |
| Reverse | TTCCCATCCATCGTGACATT | |||
| Slc7a1/CAT1 | Forward | CAACGACCGGACCAAAACAC | 193 | NM_001012613.1 |
| Reverse | CTGGTACACCATGTTCGGCT | |||
| Slc6a19/B 0 AT1 | Forward | CCTGACGCTTATCAACGGGT | 137 | XM_003359855.4 |
| Reverse | AGTTCATGTCGCAGGTCTGG | |||
| Slc1a1/EAAT3 | Forward | GGCACCGCACTCTACGAAGCA | 177 | NM_001164649.1 |
| Reverse | GCCCACGGCACTTAGCACGA | |||
| Slc2a2/GLUT2 | Forward | GACACGTTTTGGGTGTTCCG | 149 | NM_001097417.1 |
| Reverse | GAGGCTAGCAGATGCCGTAG | |||
| Slc27a4/FATP4 | Forward | AGACACACGTTGGACCTTCC | 188 | XM_021069609.1 |
| Reverse | GCAGGTTGGTGTTGATGAGC | |||
| Chromogranin A | Forward | ACTCCGAGGAGATGAACGGA | 205 | NM_001164005.2 |
| Reverse | CTTGGAGGACGCCTCTTCTG | |||
| Muc2 | Forward | GCTCCAGAGAGAAGGCAGAACC | 171 | XM_021082584.1 |
| Reverse | CTCAGGTGCACAGCGAACTC | |||
| Spp1 | Forward | GCCTCTGCCCTTCCAGTTAAA | 210 | NM_214023.1 |
| Reverse | CTCAGGGCTTTCGTTGGACT | |||
| Trop2 | Forward | CATTACGAGCACCCCACCAT | 239 | XM_003127967.4 |
| Reverse | GTGAGGCGCTTCATGGAGAA | |||
| β-Actin | Forward | AGTTGAAGGTGGTCTCGTGG | 215 | XM_003124280.5 |
| Reverse | TGCGGGACATCAAGGAGAAG |
Three-dimensional culture of piglet intestinal organoids
We first need to prepare precool PBS, thaw Matrigel (BD Biosciences, SanJose, CA) at 4 °C, and prewarm 24-well plate (LabServ, Thermo Fisher Scientific) at 37 °C. Seven-day-old piglets were euthanized, small intestine (jejunum) was taken out and stored in PBS on ice; proximal part of small intestine was cut longitudinally, washed in PBS, scraped off villi using a cover slips slowly and lightly, and removed the fat as completed as possible. Then, the small intestine was washed 2 times in PBS, cut into small pieces (0.2 to 0.3 cm) to make resuspend easier, transferred to a 50-mL tube, and washed 3 to 4 times with ice-cold PBS. Then, the tissue was incubated with 2 mM ethylenediaminetetraacetic acid (Sigma–Aldrich) at 4 °C for 30 min for epithelial isolation. The tube was swayed for 5 min until high crypt purity was obtained and then were filtered through a 70-μm cell strainer. Crypt suspension was added with 10% (vol/vol) FCS and spin down at 300 × g for 5 min. The supernatant was discarded. The crypts were resuspended in 15-mL advanced DMEM-GF (Gibco, Grand Island, NY) and spin at 150 × g for 2 min. The supernatant was discarded. Crypts were suspended in phenol-red free Matrigel. Then, there was a 50-µl droplet of Matrigel-crypts mix in the each well center of a 24-well plate, and was incubated at 37 °C with 5% CO2 for 15 min, subsequently. Five hundred microliters of culture medium, which consisted of Wnt3a, Noggin, and R-Spondin 1-conditioned medium, B27 supplement (Invitrogen), N2 supplement (Invitrogen, Carlsbad, CA), glutamine (Sigma-Aldrich), N-acetyl cysteine (Sigma-Aldrich), recombinant murine epidermal growth factor (PeproTech, Rocky Hill, NJ), nicotinamide (Sigma-Aldrich), and SB202190 (Sigma-Aldrich), was added per well after Matrigel got solidification. The culture medium was maintained until passaging organoids. The passaging was performed every 5 d with a 1:3 split ratio. Each well contains at least 10 organoids. The concentrations of retinol and retinoic acid for application to the organoids were confirmed according to the calculated concentrations of VA in Table 1 and previous studies (Matsumoto et al., 2016). The organoids were treated with 0, 5, 10, and 15 μM retinol (Aladdin, V111674, Shanghai, China) and retinoic acid (Aladdin, R106320, Shanghai, China) for 5 d and observed under a microscope in a 20× combined magnification and image processing and analysis system (Version 4.12, Leica Imaging Systems Ltd., Cambridge, UK) on the third and fifth days, respectively. The number of budded and nonbudded organoids, as well as the number of budding on each budding organoid, was counted. We calculated the ratio of budded organoids to all organoids and the average budded numbers per budding organoid. RT-qPCR was used to detect the mRNA abundance of cell differentiation markers (Chromogranin A and Muc2) and embryonic stem cell markers (Spp1 and Trop2) in organoids.
Statistical Analysis
All data were analyzed in accordance with the mixed model procedure (PROC MIXED) of the SAS software (Version 9.4; SAS Institute, Inc., Cary, NC). Linear and quadratic effects of treatment were investigated. Before analysis, all data were tested for the normality using the univariate procedure. Data were presented as means ± SEM. Differences were considered significant at P < 0.05.
Results
Growth performance and diarrhea rate
There were no significant differences in the BW, ADFI, and diarrhea rates of piglets among the 4 VA diets throughout the trial (Table 3). However, dietary VA supplementation significantly increased both ADG (linear, P = 0.020) and G:F (linear, P = 0.005) of piglets from days 8 to 14.
Table 3.
Growth performance of weaned piglets with different VA concentrations1
| Dietary VA, mg/kg | Contrast, P < | |||||||
|---|---|---|---|---|---|---|---|---|
| Items | 2 | 4 | 8 | 16 | SEM | Model P value | Linear | Quadratic |
| BW, kg | ||||||||
| 0 d | 8.33 | 8.34 | 8.34 | 8.34 | 0.127 | 0.976 | 0.979 | 0.991 |
| 7 d | 9.18 | 9.25 | 9.33 | 9.08 | 0.171 | 0.793 | 0.901 | 0.653 |
| 14 d | 10.38 | 10.50 | 10.80 | 10.66 | 0.182 | 0.535 | 0.505 | 0.738 |
| ADFI, g/d | ||||||||
| 0 to 7 d | 237.30 | 255.45 | 252.60 | 239.06 | 15.277 | 0.666 | 0.986 | 0.624 |
| 8 to 14 d | 388.67 | 381.89 | 407.58 | 400.78 | 9.689 | 0.476 | 0.495 | 0.999 |
| 0 to 14 d | 312.98 | 318.67 | 330.09 | 319.92 | 10.995 | 0.646 | 0.757 | 0.733 |
| ADG, g/d | ||||||||
| 0 to 7 d | 120.54 | 129.46 | 141.97 | 105.36 | 14.784 | 0.666 | 0.812 | 0.464 |
| 8 to 14 d | 172.32 | 178.57 | 209.82 | 225.89 | 9.201 | 0.039 | 0.020 | 0.781 |
| 0 to 14 d | 146.43 | 154.02 | 175.89 | 165.62 | 9.525 | 0.404 | 0.372 | 0.652 |
| G:F, g/d:g/d | ||||||||
| 0 to 7 d | 0.49 | 0.50 | 0.45 | 0.51 | 0.038 | 0.779 | 0.981 | 0.805 |
| 8 to 14 d | 0.44 | 0.47 | 0.51 | 0.56 | 0.017 | 0.025 | 0.005 | 0.825 |
| 0 to 14 d | 0.46 | 0.48 | 0.51 | 0.51 | 0.016 | 0.229 | 0.203 | 0.696 |
| Diarrhea rate, % | ||||||||
| 0 to 14 d | 20.54 | 15.18 | 10.71 | 19.64 | 2.730 | 0.208 | 0.776 | 0.209 |
1Values are expressed as mean ± SEM, n = 8.
Organ index analysis and Lgr5+ mRNA abundance
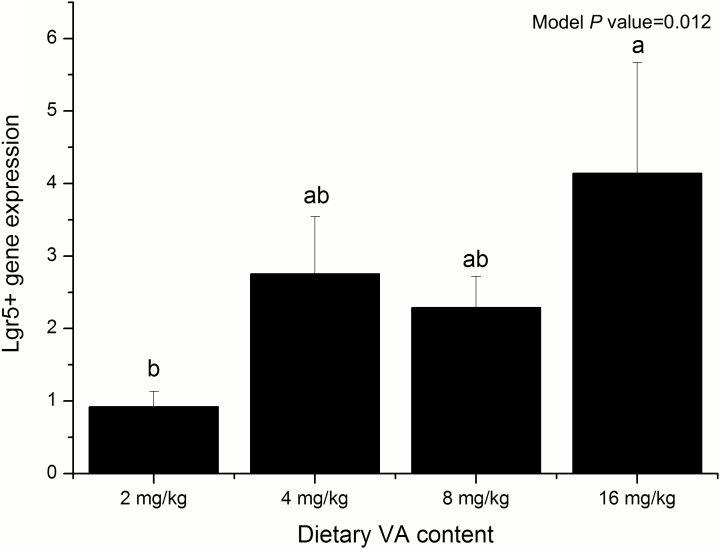
There was no significant difference in the total weight of the liver, spleen, kidneys, and small intestinal length and the relative weight based on the day 14 BW of the weaned piglets (Table 4). The mRNA abundance of Lgr5+ gene was significantly increased in the 16 mg/kg VA group compared with that in the control group (P = 0.012, Fig. 1).
Table 4.
Organ index of weaned piglets with different VA concentrations1
| Dietary VA, mg/kg | Contrast, P < | |||||||
|---|---|---|---|---|---|---|---|---|
| Items2 | 2 | 4 | 8 | 16 | SEM | Model P value | Linear | Quadratic |
| Liver | ||||||||
| Total weight, g | 211.00 | 216.44 | 224.00 | 225.81 | 3.917 | 0.609 | 0.154 | 0.821 |
| Relative weight, g/kg | 20.33 | 20.63 | 20.78 | 21.20 | 0.191 | 0.216 | 0.121 | 0.887 |
| Spleen | ||||||||
| Total weight, g | 23.88 | 26.81 | 25.81 | 23.94 | 0.792 | 0.140 | 0.910 | 0.143 |
| Relative weight, g/kg | 2.31 | 2.55 | 2.38 | 2.25 | 0.065 | 0.144 | 0.530 | 0.154 |
| Both kidneys | ||||||||
| Total weight, g | 55.94 | 58.31 | 59.00 | 56.86 | 0.958 | 0.165 | 0.702 | 0.261 |
| Relative weight, g/kg | 5.42 | 5.56 | 5.53 | 5.55 | 0.097 | 0.237 | 0.706 | 0.763 |
| Small intestine | ||||||||
| Total length, m | 9.24 | 9.23 | 9.61 | 9.68 | 0.112 | 0.177 | 0.099 | 0.846 |
| Relative length, m/kg | 0.90 | 0.88 | 0.90 | 0.91 | 0.015 | 0.539 | 0.773 | 0.645 |
1Values are expressed as mean ± SEM, n = 8.
2Relative weight = the ratio of total weight to BW of 14 d old; relative length = the ratio of total length to BW of 14 d old.
Figure 1.
The expression of Lgr5+ gene within weaning piglets’ jejunum mucosa at different VA concentrations. Different lowercase letters indicate statistical significance (P < 0.05). Values are expressed as mean ± SEM, n = 8.
Intestinal morphology histology
This study found that the jejunum VH (P = 0.027) and VSA (P = 0.035) were significantly increased in the 4 mg/kg VA treatment group (Table 5). The CD in ileum was significantly increased in the 4 and 8 mg/kg VA treatment groups (quadratic, P = 0.043), and the ileum VH:CD ratio was significantly increased in the 16 mg/kg VA group (quadratic, P = 0.015). Other indicators showed no significant difference among the 4 groups.
Table 5.
Intestinal morphology histology of weaned piglets with different VA concentrations1
| Dietary VA, mg/kg | Contrast, P < | |||||||
|---|---|---|---|---|---|---|---|---|
| Items2 | 2 | 4 | 8 | 16 | SEM | Model P value | Linear | Quadratic |
| VH, μm | ||||||||
| Jejunum | 343.83 | 357.08 | 311.39 | 327.14 | 7.262 | 0.027 | 0.133 | 0.062 |
| Ileum | 321.08 | 300.98 | 319.27 | 316.19 | 6.156 | 0.312 | 0.949 | 0.505 |
| CD, μm | ||||||||
| Jejunum | 257.05 | 262.00 | 245.63 | 240.20 | 5.664 | 0.335 | 0.202 | 0.654 |
| Ileum | 222.36 | 241.61 | 240.19 | 219.78 | 4.786 | 0.185 | 0.828 | 0.043 |
| VW, μm | ||||||||
| Jejunum | 136.74 | 140.72 | 138.49 | 138.58 | 1.928 | 0.522 | 0.855 | 0.633 |
| Ileum | 130.54 | 132.74 | 134.83 | 130.41 | 1.620 | 0.299 | 0.911 | 0.330 |
| VH:CD, μm:μm | ||||||||
| Jejunum | 1.36 | 1.38 | 1.28 | 1.38 | 0.036 | 0.366 | 0.870 | 0.586 |
| Ileum | 1.45 | 1.26 | 1.34 | 1.44 | 0.031 | 0.025 | 0.828 | 0.015 |
| VSA, μm | ||||||||
| Jejunum | 147484.54 | 157421.48 | 135795.17 | 142154.11 | 3568.284 | 0.035 | 0.232 | 0.063 |
| Ileum | 131501.77 | 125264.26 | 135438.62 | 129994.09 | 3085.549 | 0.469 | 0.844 | 0.951 |
1Values are expressed as mean ± SEM, n = 8.
Small intestinal brush border enzyme activity and nutrient transporter mRNA abundance
Changes in dietary VA content did not significantly affect the jejunal mucosal digestive enzyme activity in weaned piglets (Table 6). However, the maltase (P = 0.032), sucrose (P = 0.041), and alkaline phosphatase activity (linear, P = 0.024) in the ileum mucosa were significantly reduced when supplemented with 8 and 16 mg/kg VA. The mRNA abundance of the jejunal and ileal nutrient transporters was presented in Table 7. Slc2a2 mRNA abundance was significantly increased in the 2 mg/kg VA group (linear, P = 0.024). However, there was no significant difference in the abundance of other nutrient transporters.
Table 6.
Enzyme activity of weaned piglets with different VA concentrations in intestinal mucosa1
| Dietary VA, mg/kg | Contrast, P < | |||||||
|---|---|---|---|---|---|---|---|---|
| Items | 2 | 4 | 8 | 16 | SEM | Model P value | Linear | Quadratic |
| Jejunal mucosa | ||||||||
| Maltase | 196.36 | 244.60 | 164.73 | 212.61 | 20.640 | 0.604 | 0.870 | 0.997 |
| Sucrase | 59.69 | 98.36 | 68.37 | 49.74 | 7.631 | 0.124 | 0.365 | 0.058 |
| Lactase | 18.76 | 31.95 | 31.40 | 29.74 | 3.762 | 0.243 | 0.361 | 0.343 |
| Alkaline phosphatase | 467.34 | 417.14 | 389.76 | 424.68 | 41.149 | 0.523 | 0.686 | 0.625 |
| Ileal mucosa | ||||||||
| Maltase | 136.08 | 231.58 | 104.94 | 262.62 | 22.734 | 0.032 | 0.179 | 0.455 |
| Sucrase | 35.84 | 55.47 | 16.73 | 34.55 | 4.843 | 0.041 | 0.282 | 0.919 |
| Alkaline phosphatase | 284.43 | 293.01 | 174.86 | 123.21 | 29.394 | 0.028 | 0.024 | 0.593 |
1Values are expressed as mean ± SEM, n = 8.
Table 7.
The mRNA abundance of nutrient transporters in intestinal mucosa of weaning piglets with different VA concentrations1
| Dietary VA, mg/kg | Contrast, P < | |||||||
|---|---|---|---|---|---|---|---|---|
| Items2 | 2 | 4 | 8 | 16 | SEM | Model P value | Linear | Quadratic |
| Jejunal mucosa | ||||||||
| Slc15a1 | 1.17 | 1.03 | 1.28 | 1.17 | 0.068 | 0.468 | 0.694 | 0.919 |
| Slc7a1 | 0.90 | 0.95 | 0.94 | 1.03 | 0.056 | 0.450 | 0.470 | 0.987 |
| Slc6a19 | 1.10 | 1.02 | 1.12 | 0.91 | 0.065 | 0.382 | 0.406 | 0.630 |
| Slc1a1 | 1.38 | 0.99 | 1.15 | 1.37 | 0.122 | 0.297 | 0.896 | 0.234 |
| Slc2a2 | 1.05 | 1.04 | 1.23 | 1.08 | 0.076 | 0.452 | 0.680 | 0.662 |
| Slc27a4 | 1.00 | 1.01 | 1.00 | 1.02 | 0.027 | 0.841 | 0.850 | 0.830 |
| Ileal mucosa | ||||||||
| Slc15a1 | 1.04 | 0.70 | 0.84 | 0.81 | 0.068 | 0.363 | 0.390 | 0.260 |
| Slc7a1 | 0.88 | 0.75 | 0.84 | 0.74 | 0.081 | 0.528 | 0.658 | 0.944 |
| Slc6a19 | 1.04 | 0.77 | 0.77 | 0.86 | 0.061 | 0.352 | 0.316 | 0.144 |
| Slc1a1 | 1.24 | 0.97 | 0.94 | 1.05 | 0.066 | 0.137 | 0.340 | 0.163 |
| Slc2a2 | 1.06 | 0.65 | 0.71 | 0.69 | 0.063 | 0.024 | 0.053 | 0.118 |
| Slc27a4 | 1.18 | 1.08 | 0.85 | 1.07 | 0.072 | 0.153 | 0.406 | 0.285 |
1Values are expressed as mean ± SEM, n = 8.
2 Slc15a1, solute carrier family 15 member 1, Slc7a1, solute carrier family 7 member 1, Slc6a19, solute carrier family 6 member 19, Slc1a1, solute carrier family 1 member 1, Slc2a2, solute carrier family 2 member 2, Slc27a4, solute carrier family 27 member 4.
Growth of piglet intestinal organoids and mRNA abundance of markers
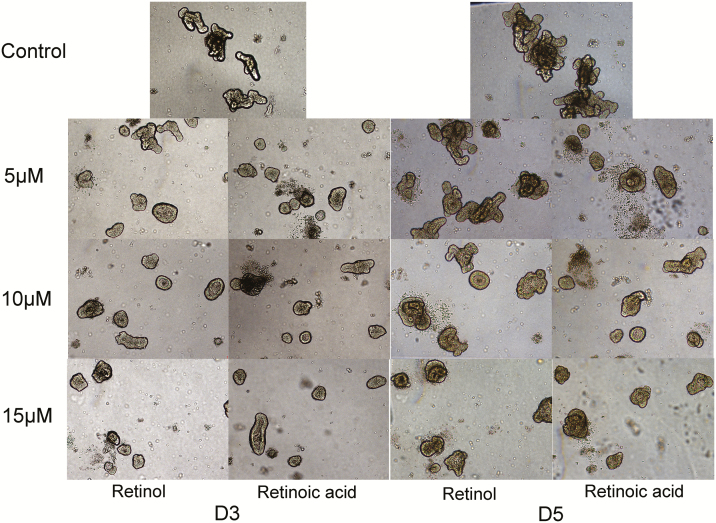
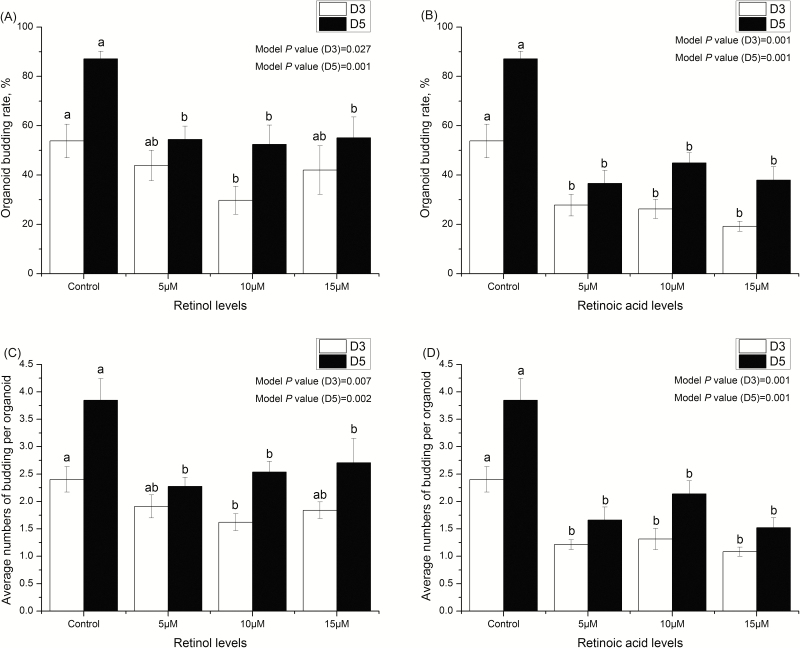
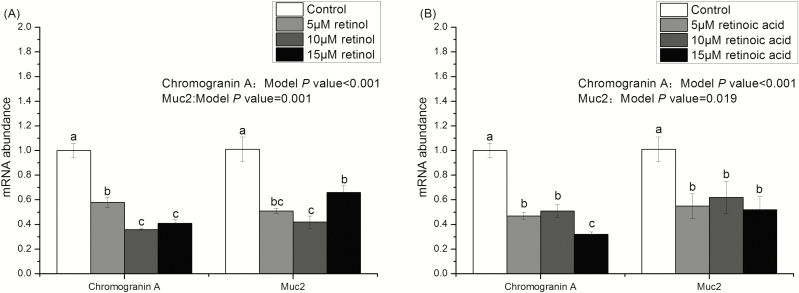
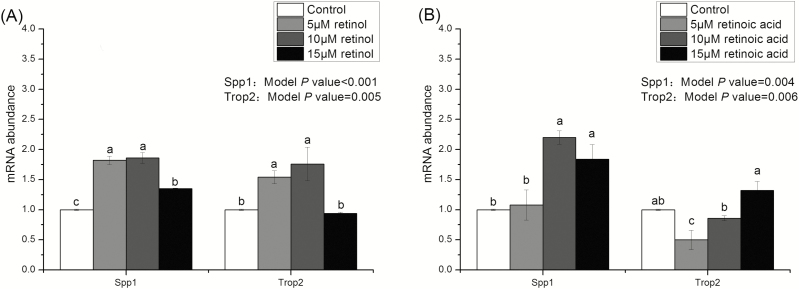
Figures 2 and 3 show that the growth of intestinal organoids of piglets treated with 0 (control), 5, 10, and 15 μM retinol and retinoic acids. Compared with the control group, budding rates and the number of buddings per organoid in retinol acid- and retinoic acid-treated organoids were significantly reduced (P < 0.05). Moreover, the effects of retinol and retinoic acid on budding rates and the number of buddings per organoid were similar on days 3 and 5. Figures 4 and 5 are the mRNA abundance of the cell differentiation markers and embryonic stem cell markers in the organoids collected on day 5, respectively. This study showed that the expression of Chromogranin A and Muc2 (cell differentiation markers) in retinol- and retinoic acid-treated organoids was suppressed (P < 0.05). Meanwhile, the mRNA abundance of Spp1 and Trop2 (embryonic stem cell markers) increased significantly (P < 0.05).
Figure 2.
The organoid images observed under a microscope in a 20× combined magnification and image processing and analysis system.
Figure 3.
The growth of intestinal organoids in piglets treated with different retinol and retinoic acid concentrations. (A) The organoid budding rates on the third and fifth days treated with 0 (control), 5, 10, and 15 μM retinol. (B) The organoid budding rates on the third and fifth days treated with 0 (control), 5, 10, and 15 μM retinoic acid. (C) The average budding numbers of per organoid on the third and fifth days treated with 0 (control), 5, 10, and 15 μM retinol. (D) The average budding numbers of per organoid on the third and fifth days treated with 0 (control), 5, 10, and 15 μM retinoic acid. Different lowercase letters indicate statistical significance (P < 0.05). Values are expressed as mean ± SEM, n = 8.
Figure 4.
The mRNA abundance of Chromogranin A and Muc2 in organoids treated with different concentrations of retinol and retinoic acid. Different lowercase letters indicate statistical significance (P < 0.05). Values are expressed as mean ± SEM, n = 8.
Figure 5.
The mRNA abundance of Spp1 and Trop2 in organoids treated with different concentrations of retinol and retinoic acid. Different lowercase letters indicate statistical significance (P < 0.05). Values are expressed as mean ± SEM, n = 8.
Discussion
The trial focused on the effects of dietary VA supplementation on the growth and intestinal function in weaned piglets. VA is an important nutrient for regulating piglet growth. Previous studies have shown that retinol can protect intestinal epithelium from the intestinal toxicity of C. difficile TxA (Maciel et al., 2007). It can be seen that VA dietary supplementation affects the intestinal epithelial structure, and thus the intestinal function, of piglets. The post-weaning diet is a major factor in regulating the growth and maturation of the small intestine in weaned piglets (Cera et al., 1988). Weaning stress causes a sharp decrease in feed intake and growth rate in piglets. It has been reported that pigs fed on VA-restricted diets had lower ADG and feed efficiency during the growing period (Ayuso et al., 2015). VA deficiency has also been associated with low growth in children (West et al., 1997). The NRC had similar reports on VA deficiency in piglets (NRC, 2012), but other authors have not found any evidence that VA deficiency has any effect on pig growth performance (Ching et al., 2002; D’Souza et al., 2003; Olivares et al., 2009a, 2009b, 2011). In our current work, the supplement of 16 mg/kg VA in the diet significantly increased the piglets’ ADG and feed remuneration in the second week after weaning. This was a little different from the outcomes in previous studies. It must be taken into account that the experimental subjects in this study were 21-d-old weaned pigs with an initial BW of about 8.3 kg. The piglets at this stage were more susceptible to weaning stress than the experimental animals used by previous researchers. Therefore, our work verifies that VA supplementation helped relieve weaning stress at this stage, which led to a significant improvement in piglet growth performance.
Disaccharidases are located on the brush border of the small intestine mucosa. Disaccharidase activity determines carbohydrate digestion and transport capacity in weaned piglets. It is an important marker for assessing intestinal development in animals (Huygelen et al., 2015; Pieper et al., 2016). In this study, the digestive enzymes activity of ileum mucosa in the 4 mg/kg VA treatment group increased significantly. This shows that the VA supplement alleviates intestinal damage. Intestinal function is closely related to intestinal morphological structure (Pluske et al., 1996). Weaning usually causes damage to the intestinal structure of piglets, which is characterized by a decrease in VH and increase in CD (Hampson, 1986). Weaned piglets absorb nutrients primarily through the villus on the inside of the intestines. The increased VSA allows the weaned piglets to absorb more nutrients into the body. As the VH decreases, the area of contact between the nutrients and intestinal villus decreases, which limits the absorption of nutrients. VH and VSA of jejunum in the 4 mg/kg VA treatment group were significantly increased. Four mg/kg VA may be the optimal dose for weaning piglets among 4 treatment groups. The VH:CD ratio in the 2 mg/kg VA group significantly increased, caused by the gradual migration of stem cells from the base of the crypt to the top of the villi. This also has a positive effect on intestinal repair.
The intestinal epithelium can achieve a process of self-renewal in adult mammals (Schepers et al., 2011). Lgr5+ (leucine-rich-repeat-containing G-protein-coupled receptor 5) is a stem cell located above the Paneth cells in the small intestine expressed only at the base of the crypt. It has been reported to be a marker of intestinal stem cells (Barker et al., 2007; Yan et al., 2012). The jejunum is the main site of digestion and absorption of nutrients. The intestinal stem cells can be detected via a RT-qPCR detection of Lgr5+ gene expression in the jejunum. In this study, we found that the Lgr5+ gene in the 16 mg/kg VA treatment group was highly expressed in the jejunum mucosa compared with the control group. The small intestinal alkaline phosphatase is a tissue-specific phosphatase (TSAP). Its expression is associated with cell differentiation and is also commonly considered a marker of cell differentiation (Barnard and Warwick, 1993; Hodin et al., 1996; Kovaříková et al., 2000; Hýžd’alová et al., 2008). It maintains the intestinal barrier’s integrity by limiting bacterial transepithelial channels and dephosphorylation of bacterial lipopolysaccharides. Additionally, alkaline phosphatase can also increase the intestinal digestion and absorption functions by catalyzing the hydrolysis of various phosphorylated compounds and lysing phosphate esters that do not readily penetrate cell membranes (Grant et al., 2015). In this study, the alkaline phosphatase activity in the ileal mucosa of the 8 and 16 mg/kg VA treatment groups was found to be significantly reduced. A high concentration of dietary VA has been found to inhibit intestinal epithelial cell differentiation.
Previous studies have shown that VA absence resulted in an uncontrolled proliferation of epithelial stem cells that fail to differentiate into the normal phenotype in many lining epithelia during development (Rexer et al., 2001). Retinol is a form of VA. Retinoic acid is the major active metabolite of VA. The anabolic process of retinoic acid is sequentially carried out in the small intestine, liver and target cells. The small intestine is the first gateway for contact with dietary VA as well as the unique organ for its absorption and metabolism. Dietary VA enters the small intestine and is metabolized to produce retinoic acid. The un-metabolized retinol (which may contain retinol acetate) forms a mixture with the metabolically produced retinoic acid, which is absorbed by the intestinal epithelial cells and absorbed by the piglets. The self-renewing epithelium of the small intestine includes crypts and villi. Cells are newly created in the crypts and are lost due to apoptosis at the tip of the villi. Most crypts can be cultured in a 3D model of the intestinal organoids, comprising several crypt domains surrounding a central lumen lined by a villus-like epithelium (“villus domain”). This is a good in vitro model for studying intestinal stem cells (Sato et al., 2009; Sato et al., 2011). Our study found that both retinol and retinoic acid inhibited the budding of organoids compared to the control group. The expression of chromogranin A and Muc2 (intestinal cell differentiation marker) in organoids treated with retinol and retinoic acid was suppressed. The expression of differentiation markers was significantly reduced. There were a large number of spherical organoids in the medium. These spherical organoids are similar to embryonic small intestine organoids that lack differentiated cells. Embryonic stem cells are highly undifferentiated cells (Mustata et al., 2013). Studies have shown that the expression of Spp1 and Trop2 (embryonic stem cell markers) in organoids treated with retinol and retinoic acid increased significantly. The number of differentiated cells decreased and the number of undifferentiated embryonic stem cells increased. The differentiation of organoids treated by VA and its metabolites was inhibited. In vivo, the results of alkaline phosphatase and Lgr5+ gene abundance in the 16 mg/kg VA group showed inhibition of cell differentiation and increased crypt stem cells. The reason may be that high-dose supplementation of VA may limit the differentiation of intestinal stem cells, leading to an increase in stem cell stemness. Thus, our finding that VA is an important regulator of stemness and differentiation in the intestinal crypt provides important insight for the cellular mechanism that maintains homeostasis.
Proteins, sugars, and fats are broken down into free AA, monosaccharides, and fatty acids after entering the small intestine, respectively. They are absorbed into the cells by animals via the corresponding transporter or other forms. Slc2a2 is a transport carrier associated with glucose. Our results only observed significant differences in the Slc2a2 gene expression, whereas other indicators did not differ significantly. When VA was added to the piglet diet, ADG was higher in weaned piglets as the concentration of VA increased. However, from the perspective of the intestinal lumen development, the addition of high concentration of VA seems to reduce the expression of GLUT2 and reduce the activity of TSAP. The sample we collect was the terminal ileum, which was connected to the large intestine. Studies have shown that high concentrations of VA have longer small intestinal lengths and may have the potential to better digest and absorb nutrients. Fewer nutrients may be available to reach the terminal ileum, and related enzyme activities and expression of nutrient transporters are reduced. In the future, I will verify this conjecture based on more experiments.
In conclusion, this study suggests that VA supplementation affects the growth in weaned piglets. Moreover, VA can affect intestinal function by regulating the intestinal stem cells within the jejunum, which provides guidance for feed formula of weaned piglets.
Glossary
Abbreviations
- CD
crypt depth
- RE
relative expression
- TSAP
tissue-specific phosphatase
- VA
vitamin A
- VH
villus height
- VSA
villus surface area
- VW
villus width
Funding
This work was supported by Key Programs of Frontier Scientific Research of the Chinese Academy of Sciences (grant no. QYZDY-SSW-SMC008), the Natural Science Foundation of Hunan Province (grant no. 2017JJ1020), and the Young Elite Scientists Sponsorship Program by CAST (grant no. YESS20160086).
Conflict of interest
The authors declare that they have no competing interests.
LITERATURE CITED
- Ayuso M., Ovilo C., Fernández A., Nuñez Y., Isabel B., Daza A., López-Bote C. J., and Rey A. I.. . 2015. Effects of dietary vitamin A supplementation or restriction and its timing on retinol and α-tocopherol accumulation and gene expression in heavy pigs. Anim. Feed Sci. Tech. 202:62–74. doi: 10.1016/j.anifeedsci.2015.01.014 [DOI] [Google Scholar]
- Balmer J. E., and Blomhoff R.. . 2002. Gene expression regulation by retinoic acid. J. Lipid Res. 43:1773–1808. doi: 10.1194/jlr.r100015-jlr200 [DOI] [PubMed] [Google Scholar]
- Barea R., Nieto R., Vitari F., Domeneghini C., and Aguilera J. F.. . 2011. Effects of pig genotype (Iberian v. Landrace × Large White) on nutrient digestibility, relative organ weight and small intestine structure at two stages of growth. Animal 5:547–557. doi: 10.1017/S1751731110002181 [DOI] [PubMed] [Google Scholar]
- Barker N., van Es J. H., Kuipers J., Kujala P., van den Born M., Cozijnsen M., Haegebarth A., Korving J., Begthel H., Peters P. J., . et al. 2007. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449:1003–1007. doi: 10.1038/nature06196 [DOI] [PubMed] [Google Scholar]
- Barnard J. A., and Warwick G.. . 1993. Butyrate rapidly induces growth inhibition and differentiation in HT-29 cells. Cell Growth Differ. 4:495–501. [PubMed] [Google Scholar]
- Bhatia A. L. 1998. The anti-aging role of vitamin A and β-carotene. Indian J. Gerontol. 12:70–79. [Google Scholar]
- Blomhoff R. 1994. Vitamin A in health and disease. Boca Raton (FL): CRC Press, Taylor & Francis Group. [Google Scholar]
- Blomhoff R., and Blomhoff H. K.. . 2006. Overview of retinoid metabolism and function. J. Neurobiol. 66:606–630. doi: 10.1002/neu.20242 [DOI] [PubMed] [Google Scholar]
- Cera K. R., Mahan D. C., Cross R. F., Reinhart G. A., and Whitmoyer R. E.. . 1988. Effect of age, weaning and postweaning diet on small intestinal growth and jejunal morphology in young swine. J. Anim. Sci. 66:574–584. doi: 10.2527/jas1988.662574x [DOI] [PubMed] [Google Scholar]
- Chen C. C., Wang Z. B., Li J. Z., Li Y. L., Huang P. F., Ding X. Q., Yin J., He S. P., Yang H. S., and Yin Y. L.. . 2019. Dietary vitamin E affect small intestinal histomorphology, digestive enzyme activity and the expression of nutrient transporters by inhibiting proliferation of intestinal epithelial cells within jejunum in weaned piglets. J. Anim. Sci. 97:1212–1221. doi: 10.1093/jas/skz023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching S., Mahan D. C., Wiseman T. G., and Fastinger N. D.. . 2002. Evaluating the antioxidant status of weanling pigs fed dietary vitamins A and E. J. Anim. Sci. 80:2396–2401. doi: 10.2527/2002.8092396x [DOI] [PubMed] [Google Scholar]
- D’Souza D. N., Pethick D. W., Dunshea F. R., Pluske J. R., and Mullan B. P.. . 2003. Nutritional manipulation increases intramuscular fat levels in the Longissimus muscle of female finisher pigs. Austral. J. Agric. Res. 54:745–749. doi: 10.1071/AR03009 [DOI] [Google Scholar]
- Grant C. N., Mojica S. G., Sala F. G., Hill J. R., Levin D. E., Speer A. L., Barthel E. R., Shimada H., Zachos N. C., and Grikscheit T. C.. . 2015. Human and mouse tissue-engineered small intestine both demonstrate digestive and absorptive function. Am. J. Physiol. Gastrointest. Liver Physiol. 308:G664–G677. doi: 10.1152/ajpgi.00111.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson D. J. 1986. Alterations in piglet small intestinal structure at weaning. Res. Vet. Sci. 40:32–40. doi: 10.1016/S0034-5288(18)30482-X [DOI] [PubMed] [Google Scholar]
- Hodin R. A., Meng S., Archer S., and Tang R.. . 1996. Cellular growth state differentially regulates enterocyte gene expression in butyrate-treated HT-29 cells. Cell Growth Differ. 7:647–653. [PubMed] [Google Scholar]
- Huygelen V., De Vos M., Prims S., Vergauwen H., Fransen E., Casteleyn C., Van Cruchten S., and Van Ginneken C.. . 2015. Birth weight has no influence on the morphology, digestive capacity and motility of the small intestine in suckling pigs. Livest Sci. 182:129–136. doi: 10.1016/j.livsci.2015.11.003 [DOI] [Google Scholar]
- Hýžd’alová M., Hofmanová J., Pacherník J., Vaculová A., and Kozubík A.. . 2008. The interaction of butyrate with TNF-α during differentiation and apoptosis of colon epithelial cells: Role of NF-κB activation. Cytokine 44:33–43. doi: 10.1016/j.cyto.2008.06.003 [DOI] [PubMed] [Google Scholar]
- Kisielinski K., Willis S., Prescher A., Klosterhalfen B., and Schumpelick V.. . 2002. A simple new method to calculate small intestine absorptive surface in the rat. Clin. Exp. Med. 2:131–135. doi: 10.1007/s102380200018 [DOI] [PubMed] [Google Scholar]
- Kovaříková M., Pacherník J., Hofmanová J., Zadák Z., and Kozubík A.. . 2000. TNF-α modulates the differentiation induced by butyrate in the HT-29 human colon adenocarcinoma cell line. Eur. J. Cancer 36:1844–1852. doi: 10.1016/S0959-8049(00)00178-7 [DOI] [PubMed] [Google Scholar]
- Lima A. A., Soares A. M., Lima N. L., Mota R. M., Maciel B. L., Kvalsund M. P., Barrett L. J., Fitzgerald R. P., Blaner W. S., and Guerrant R. L.. . 2010. Effects of vitamin A supplementation on intestinal barrier function, growth, total parasitic, and specific Giardia spp infections in Brazilian children: A prospective randomized, double-blind, placebo-controlled trial. J. Pediatr. Gastroenterol. Nutr. 50:309–315. doi: 10.1097/MPG.0b013e3181a96489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciel A. A., Oriá R. B., Braga-Neto M. B., Braga A. B., Carvalho E. B., Lucena H. B., Brito G. A., Guerrant R. L., and Lima A. A.. . 2007. Role of retinol in protecting epithelial cell damage induced by Clostridium difficile toxin A. Toxicon 50:1027–1040. doi: 10.1016/j.toxicon.2007.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto T., Mochizuki W., Nibe Y., Akiyama S., Matsumoto Y., Nozaki K., Fukuda M., Hayashi A., Mizutani T., Oshima S., . et al. 2016. Retinol promotes in vitro growth of proximal colon organoids through a retinoic acid-independent mechanism. PLoS One 11:e0162049. doi: 10.1371/journal.pone.0162049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagne L., Boudry G., Favier C., Le Huërou-Luron I., Lallès J. P., and Sève B.. . 2007. Main intestinal markers associated with the changes in gut architecture and function in piglets after weaning. Br. J. Nutr. 97:45–57. doi: 10.1017/S000711450720580X [DOI] [PubMed] [Google Scholar]
- Mustata R. C., Vasile G., Fernandez-Vallone V., Strollo S., Lefort A., Libert F., Monteyne D., Pérez-Morga D., Vassart G., and Garcia M. I.. . 2013. Identification of Lgr5-independent spheroid-generating progenitors of the mouse fetal intestinal epithelium. Cell Rep. 5:421–432. doi: 10.1016/j.celrep.2013.09.005 [DOI] [PubMed] [Google Scholar]
- NRC 2012. Nutrient requirements of swine. 11th rev. ed. Natl. Acad. Press, Washington, DC. [Google Scholar]
- Olivares A., Daza A., Rey A. I., and López-Bote C. J.. . 2009a. Dietary vitamin A concentration alters fatty acid composition in pigs. Meat Sci. 81:295–299. doi: 10.1016/j.meatsci.2008.07.029 [DOI] [PubMed] [Google Scholar]
- Olivares A., Rey A. I., Daza A., and Lopez-Bote C. J.. . 2009b. High dietary vitamin A interferes with tissue α-tocopherol concentrations in fattening pigs: A study that examines administration and withdrawal times. Animal 3:1264–1270. doi: 10.1017/S175173110900487X [DOI] [PubMed] [Google Scholar]
- Olivares A., Rey A. I., Daza A., and López-Bote C. J.. . 2011. Low levels of dietary vitamin A increase intramuscular fat content and polyunsaturated fatty acid proportion in liver from lean pigs. Livest Sci. 137:31–36. doi: 10.1016/j.livsci.2010.09.023 [DOI] [Google Scholar]
- Pieper R., Scharek-Tedin L., Zetzsche A., Rohe I., Kroger S., Vahjen W., and Zentek J.. . 2016. Bovine milk-based formula leads to early maturation-like morphological, immunological, and functional changes in the jejunum of neonatal piglets. J. Anim. Sci. 94:989–999. doi: 10.2527/jas.2015-9942 [DOI] [PubMed] [Google Scholar]
- Pluske J. R., Hampson D. J., and Williams I. H.. . 1997. Factors influencing the structure and function of the small intestine in the weaned pig: A review. Livest. Prod. Sci. 51:215–236. doi: 10.1016/S0301-6226(97)00057-2 [DOI] [Google Scholar]
- Pluske J., Williams I., and Aherne F.. . 1996. Maintenance of villous height and crypt depth in piglets by providing continuous nutrition after weaning. Anim. Sci. 62:131–144. doi: 10.1017/S1357729800014417 [DOI] [Google Scholar]
- Rexer B. N., Zheng W. L., and Ong D. E.. . 2001. Retinoic acid biosynthesis by normal human breast epithelium is via aldehyde dehydrogenase 6, absent in MCF-7 cells. Cancer Res. 61:7065–7070. [PubMed] [Google Scholar]
- Sarkar A., Bishayee A., and Chatterjee M.. . 1995. Beta-carotene prevents lipid peroxidation and red blood cell membrane protein damage in experimental hepatocarcinogenesis. Cancer Biochem. Biophys. 15:111–125. [PubMed] [Google Scholar]
- Sato T., van Es J. H., Snippert H. J., Stange D. E., Vries R. G., van den Born M., Barker N., Shroyer N. F., van de Wetering M., and Clevers H.. . 2011. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 469:415–418. doi: 10.1038/nature09637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T., Vries R. G., Snippert H. J., van de Wetering M., Barker N., Stange D. E., van Es J. H., Abo A., Kujala P., Peters P. J., . et al. 2009. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459:262–265. doi: 10.1038/nature07935 [DOI] [PubMed] [Google Scholar]
- Schepers A. G., Vries R., van den Born M., van de Wetering M., and Clevers H.. . 2011. Lgr5 intestinal stem cells have high telomerase activity and randomly segregate their chromosomes. Embo J. 30:1104–1109. doi: 10.1038/emboj.2011.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Beers-Schreurs H., and Bruininx E.. . 2002. Nutritional management to prevent disorders in post-weaning pig health. Nutrition health of the gastrointestinal tract. Wageningen Acad. Publ., Wageningen, The Netherlands: p. 135–158. [Google Scholar]
- West K. P., LeClerq S. C., Shrestha S. R., Wu L. S., Pradhan E. K., Khatry S. K., Katz J., Adhikari R., and Sommer A.. . 1997. Effects of vitamin A on growth of vitamin A-deficient children: Field studies in Nepal. J. Nutr. 127:1957–1965. doi: 10.1093/jn/127.10.1957 [DOI] [PubMed] [Google Scholar]
- Wolf G., Kiorpes T. C., Masushige S., Schreiber J. B., Smith M. J., and Anderson R. S.. . 1979. Recent evidence for the participation of vitamin A in glycoprotein synthesis. Fed. Proc. 38:2540–2543. [PubMed] [Google Scholar]
- Yan K. S., Chia L. A., Li X., Ootani A., Su J., Lee J. Y., Su N., Luo Y., Heilshorn S. C., Amieva M. R., . et al. 2012. The intestinal stem cell markers Bmi1 and Lgr5 identify two functionally distinct populations. Proc. Natl. Acad. Sci. USA 109:466–471. doi: 10.1073/pnas.1118857109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan S. L., Long L. N., Zong E. Y., Huang P. F., Li J. Z., Li Y. L., Ding X. Q., Xiong X., Yin Y. L., and Yang H. S.. . 2018. Dietary sulfur amino acids affect jejunal cell proliferation and functions by affecting antioxidant capacity, Wnt/beta-catenin, and the mechanistic target of rapamycin signaling pathways in weaning piglets. J. Anim. Sci. 96:5124–5133. doi: 10.1093/jas/sky349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H. S., Xiong X., Wang X. C., Li T. J., and Yin Y. L.. . 2016a. Effects of weaning on intestinal crypt epithelial cells in piglets. Sci. Rep. UK 6:36939. doi: 10.1038/srep36939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Xiong X., Wang X., and Yin Y.. . 2016b. Mammalian target of rapamycin signaling pathway changes with intestinal epithelial cells renewal along crypt-villus axis. Cell. Physiol. Biochem. 39:751–759. doi: 10.1159/000445665 [DOI] [PubMed] [Google Scholar]
- Yang H. S., Xiong X., and Yin Y. L.. . 2016c. Metabolomic analysis of intestinal epithelial cell maturation along the crypt–villus axis. RSC Adv. 6:27566–27574. doi: 10.1039/C5RA27722A [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y. L., Baidoo S. K., Schulze H., and Simmins P. H.. . 2001. Effects of supplementing diets containing hulless barley varieties having different levels of non-starch polysaccharides with β-glucanase and xylanase on the physiological status of the gastrointestinal tract and nutrient digestibility of weaned pigs. Livest. Prod. Sci. 71:97–107. doi: 10.1016/S0301-6226(01)00214-7 [DOI] [Google Scholar]