Abstract
BACKGROUND
Inflammatory bowel diseases are managed by different methods, which may not be well tolerated because of their side effects. Recently, pro-prebiotics are considered as a supplementary treatment in gastrointestinal diseases. In this study, the effect of Lactocare® (ZistTakhmir Company) was investigated on the disease severity in mild to moderate ulcerative colitis.
METHODS
In this randomized, double-blind clinical trial (Iranian Registry of Clinical Trials number: IRCT201407271264N5), 60 patients with mild to moderate ulcerative colitis were included. An 8-week trial was carried out comparing Lactocare® as a supplement with standard therapy against placebo. Simple Clinical Colitis Activity Index (SCCAI) was measured at baseline and after 8 weeks. Statistical analysis was performed using paired ttest to assess the temporal changes (before and after the treatment) in the mean of SCCAI in each group. Chi-square test was used to compare the response rates. Odds ratios (OR) and the 95% confidence intervals (95%CI) were also calculated. p values of less than 0.05 were considered significant.
RESULTS
A significant decreased mean SCCAI was seen in the intervention group (4.56 ± 2.56) vs. placebo group (6.54 ± 2.47) (p < 0.05). Response to treatment was seen in 64.3% of the treatment group vs. 47% in the placebo group (p = 0.18). Response to treatment was observed in 90.9% of patients with ulcerative colitis for more than 5 years compared with 44.4% of the control group (p = 0.01).
CONCLUSION
Regarding the effectiveness of pre-probiotics in mitigating symptoms in patients with ulcerative colitis, it could be suggested to try pre-probiotics in the standard treatment particularly in those with more than five years ofthe disease.
Keywords: Ulcerative colitis, Pre-probiotics, Simple Clinical Colitis Activity Index (SCCAI)
INTRODUCTION
Ulcerative colitis (UC) and Crohn’s disease (CD) are two main forms of inflammatory bowel disease (IBD), a chronic relapsing inflammatory disorder of the gastrointestinal tract. Although a multi-factorial etiology in IBD is widely acknowledged, the exact etiology remains unclear.1,2 An exaggerated mucosal immune response to commensal gut bacteria has been proposed to drive the inflammatory process in genetically susceptible individuals.1,3 Currently, induction of remission and maintaining in this phase are the main treatment strategies.2
To achieve this goal in patients with UC, topical or systemic 5-aminosalicylic acid (5-ASA) is the first choice.4 Immunomodulators, including azathioprine and 6-mercaptopurine, are used in persistent cases or when adverse events to 5-ASA occurred. Intravenous corticosteroids, cyclosporine, and anti-TNFa agents are prescribed when UC is severe and refractory. But aside from the expenses of the above-mentioned therapies, significant disadvantages are reported such as increased risk of infections seen with immunosuppressive therapies and anti-TNFa agents. In addition, surgical intervention will be eventually needed in one-third of the patients despite these therapeutic options.2,5,6
Manipulation of the microbial composition has been a topic of research in recent decades, which showed promising results considering the role of intestinal microbiota in the pathogenesis of IBD. Pre- and probiotics are among the most interesting components act on the microbial composition and showed benefits in controlling IBD.5,7
Probiotics are live exogenous non-pathogenic microorganisms prescribed to function via several potential mechanisms including adjustment of the microbial composition, function, growth, enhancement of local immune responses, and improvement of the integrity of the gut barrier.2,5,6 Various strains of bacteria (mostly VSL # 3 and E. Coli Nissle 1917) have been studied as probiotic complements and compared with mesalazine as the standard treatment. Some of them showed equal effectiveness in maintaining the remission phase or preventing recurrent flare-ups.2,3,5
On the contrary, prebiotics, non-digestible dietary components stimulate the growth and metabolism of beneficial bacteria to modulate the endogenous luminal microflora already present in the colon. There is fewer evidence for prebiotic use than that for probiotics in UC.2,3 So far, the use of prebiotics in UC is not accepted in clinical practice.
It seems that a combination of probiotics and prebiotics would enhance the healing power of probiotics by increasing the number of needed microbiota in the lumina by prebiotics.3
Up to now, there is no clear concise conclusion to suggest the widespread use of probiotics due to the several differences of the clinical outcome measures (primary and secondary endpoints), various inclusion and exclusion criteria, and a low number of cases remain in the study up to the endpoint and most importantly choosing a proper control group that is the Achill’s tendon of the clinical trials.
This clinical trial was designed to evaluate the effect of a brand of pre-probiotics named Lactocare® (ZistTakhmir) on the disease severity of patients with UC compared with placebo in Gorgan, Northeast of Iran.
MATERIALS AND METHODS
Ethical consideration:
This double-blind, semi-randomized, placebo-controlled parallel study was first registered in the Iranian Registry of Clinical Trials (No: IRCT201407271264N5).
The study project was approved by the local Ethics Committee of Golestan University of Medical Sciences. Informed consent was obtained from all participants after explaining the goals of the study to the patients. There was no obligation for entering into the study and routine medications were not stopped in anyone.
All authors confirmed to have access to the study data and reviewed and approved the final manuscript.
Study population:
This study was conducted on patients with mild to moderate UC who were referred to Golestan Research Center of Gastroenterology and Hepatology (GRCGH) during November 2014-December 2014. Mild to moderate UC was defined according to the Simple Clinical Colitis Activity Index (SCCAI).8 This clinical index is determined using six factors including 1- bowel frequency during a day, 2- bowel frequency during the night, 3- the urgency of defecation, 4- seeing blood in the stool, 5-general well-being and 6- extra-intestinal features (each extra-intestinal presentation calculated as one score). The scoring system of SCCAI is shown in table 1.
Table 1. Clinical scoring system for the Simple Clinical Colitis Activity Index (SCCAI)7 .
| Symptoms | Score |
|
Bowel frequency (day)
1–3 4–6 7–9 > 9 |
0 1 2 3 |
|
Bowel frequency (night)
1–3 4–6 |
1 2 |
|
Urgency of defecation
Hurry Immediately Incontinence |
1 2 3 |
|
Blood in stool
Trace Occasionally frank Usually frank |
1 2 3 |
|
General well being
Very well Slightly below par Poor Very poor Terrible |
0 1 2 3 4 |
| Extracolonic features | 1 per manifestation |
Sample Size:
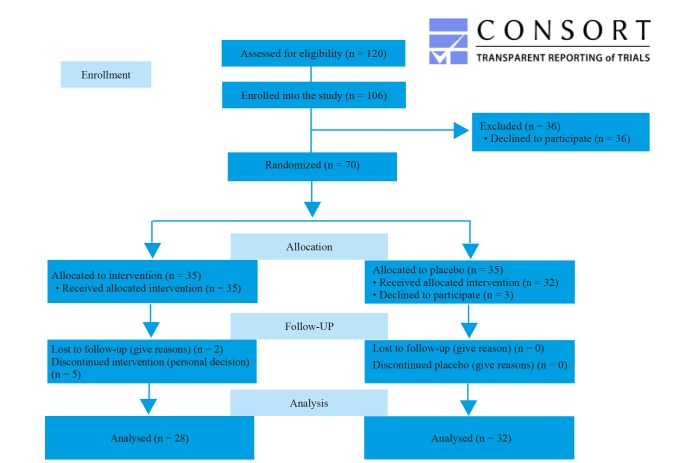
In this clinical trial, 120 patients were recruited and assessed for the inclusion criteria. Among them, 106 were evaluated as eligible to participate in the study. When starting the random allocation into intervention or placebo groups, 36 declined to participate in the study because of their own decision to not receive any additional medication. 70 patients were then allocated randomly into 35 in the intervention group and 35 in the placebo group. The sample size was calculated based on a power of 80% and a statistical significance (α) 95% (p = 0.05). We assumed that a response to treatment after 8 weeks in patients who received probiotics is 71% and in the patients who received the placebo is 44%.
After 8 weeks of follow-up, there were 28 patients (two were lost to follow up and five decided not to continue) in the intervention group and 32 in the placebo group (three discontinued intervention as a personal decision). Figure 1.
Fig.1.
CONSORT 2010 Flow Diagram
Study protocol:
All patients were enrolled in the clinical trial according to the same protocol. Demographic characteristics and medical history were recorded for each patient. The severity of UC was determined according to simple clinical colitis activity, assessed by questionnaires. Eligible patients aged between 18-60 years were randomly divided into two groups including the treatment group and the placebo group. The treatment group received Lactocare® (ZistTakhmir, Iran) capsules containing seven strains (including Lactobacillus casei , Lactobacillus acidophilus, Lactobacillus rhamnosus , Lactobacillus bulgaricus, Bifidobacterium breve, Bifidobacterium longum , Streptococcus thermophiles with prebiotic froctooligosaccharide) twice daily for 8 weeks in addition to their standard drug regimen. Lactocare® was provided in capsules containing 1 × 109 CFU kept in the refrigerator below 4°C. Placebo was also provided in similar packages with no identification sign, which were prepared by the same company of the probiotic (ZistTakhmir) containing starch and kept in the same situation. The packages were just labeled as A or B and neither physicians nor patients knew the containing materials. Our pharmacist was the only one who knew what A or B stands for and she was not involved in randomization of patients or analyzing data.
Inclusion criteria:
1- Diagnosis of UC established by previous colonoscopy with consistent histology and clinical course; 2- UC involving at least rectosigmoid region, whose activity was confirmed by colonoscopy prior to the study; 3- Mild-to-moderate relapsing UC, defined as simple clinical colitis activity; 4- Relapsing episodes for less than 4 weeks before the study entry; 5- aminosalicylic acid, at least 4 weeks before the study at a stable dose (mesalazine at least 1.6 g/day) or 6-mercaptopurine (at least 1 mg/kg/day) at least 3 months before the study entry at a stable dose.
Exclusion criteria:
1- Crohn’s disease or pouchitis; 2- Severe UC according to simple clinical colitis activity index; 3- Use of oral steroids within the last 4 weeks before study entry; 4- Use of antibiotics within the last 2 weeks before study entry; 5- Change in the dose of oral 5-aminosalicylic acid within the last 4 weeks before the study entry and within the 8-week study period or a change in the dose of oral 6- mercaptopurine and azathioprine drugs within the last 3 months before the study and 6- Use of rectal 5- aminosalicylic acid, or steroids within 1 week before the study.
Primary endpoint:
The primary endpoint was the improvement of the gastrointestinal symptoms related to UC. This evaluation was done by measuring SCCAI before and after the 8-week treatment with Lactocare® or placebo. As all included patients had mild to moderate UC, mean differences of the baseline and after intervention SCCAI was considered as the response.
Statistical assessment:
Statistical analysis was performed using appropriate tests. Paired ttest was used to assess the temporal changes (before and after the treatment) in the mean of SCCAI in each of the two groups. Chi-square test was used to compare the response rates between the two groups. We also calculated and reported odds ratios (OR) and 95% confidence intervals (95%CI). Pvalues of less than 0.05 were considered significant.
RESULTS
A total of 60 patients including 28 in the treatment group and 32 in the placebo group were enrolled. The baseline characteristics of the patients have been presented in table 2.
Table 2. Demographic characteristics of UC patients entered into the trial .
| Characteristics | Lactocare ® | Placebo |
|
Age
< 40 yrs ≥ 40 yrs |
22 (56.4) 8 (38.1) |
17 (43.6) 13 (61.9) |
|
Sex
Male, N (%) Female, N (%) |
12 (42.9) 19 (52.8) |
16 (57.1) 17 (47.2) |
|
Duration
< 5 yrs ≥ 5 yrs |
18 (56.3) 13 (40.6) |
14 (43.8) 19 (59.4) |
The assessment of patients with UC in the treatment group (after 8-week treatment with Lactocare® twice a day) showed that the mean (SD) of SCCAI has been significantly decreased from 6.54 (2.74) to 4.65 (2.65) (p = 0.017). While the difference between mean (SD) SCCAI values was not significant in the placebo group before and after the treatment (5.7 [3] to 5.21 [2.2]; p = 0.17).
Response to Lactocare® was 64.3% vs. 47% in the placebo group (p = 0.18). In multivariate regression analysis,the duration of disease was considered into account. Results showed that treatment with Lactocare® in those with UC for a duration of five years or more yielded a 90.9% response (OR = 12.5, 95%CI = 1.309-119.321; p = 0.012) but in those diagnosed less than five years it was 47.1% (OR = 0.89, 95%CI = 0.216-3.662; p = 0.870). (Table 3).
Table 3. Response to treatment with Lactocare® or placebo in UC patients regards to the duration of disease .
| Variable | Response (N) | Response (%) | OR | 95%CI | P-value | ||
| Duration | < 5yrs | Lactocare® | 8 | 47.1 | 0.89 | 0.216-3.662 | 0.870 |
| Placebo | 7 | 50 | - | ||||
| ≥ 5 yrs | Lactocare® | 10 | 90.9 | 12.5 | 1.309-119.321 | 0.012 | |
| Placebo | 8 | 44.4 | - |
DISCUSSION
In this double-blindrandomized clinical trial performed on mild-to-moderate UC, a decrease in the clinical index of disease activity was studied using 8 weeks of Lactocare® or placebo. Results showed no significant priority of Lactocare® to placebo overall (64.3% vs. 47%), although it was clinically notable.Taking the duration of disease into account showed interesting results. In those who were diagnosed for 5 years or more,a decrease of the disease severity was seen in 90.9% but in newer cases of UC (less than 5 years), disease activity was decreased in 47.1% of the patients.
Lactocare® is a pre-probiotics (synbiotic) used in this clinical trial. There are few studies about the combination of probiotics and prebiotics as a complementary treatment in patients with IBD.1 Indeed, there is one similar study that used synbiotics in UC; although the outcome measures were different from ours. Fujimori and colleagues in a clinical trial in 2009 allocated 120 patients with UC into three groups to compare the effects of prebiotics, probiotics, or synbiotics on their quality of life. In the probiotics group, the emotional function was significantly improved (p = 0.03). In the prebiotics group, bowel function (p = 0.04); and in the synbiotic group, systemic and social functions were improved (p = 0.008 and = 0.02, respectively). They suggested adding synbiotics to the routine treatments of UC patients. The significant effects failed to continue until the end of the study in all groups.3 Despite the differences between the tools of measuring outcomes in Fujimori’s study and the present study, an improved outcome was seen after using synbiotics.
In an animal model, Ivanovska and co-workers showed the significant beneficial effect of synbiotic microparticles administration once a day. Effect of synbiotic was more significant in the promotion of the lactobacilli growth in colitic rats than probiotic.9
Sood and others in a multicenter, randomized, double-blind, placebo-controlled trial in India (2009) included 147 patients with mild-to-moderate UC and allocated them into those receiving VSL # 3, a high potency probiotic, or placebo twice daily for 12 weeks. They were assessed at weeks 6 and 12 for three points or more decrease in the disease activity index. At week 6, 50% of patients in the group given VSL # 3 (25, 32.5%) and 10% of group given placebo (p = 0.001) reported a decrease in this index. This significant priority of probiotic continued at 12th week, 42.9% in VSL # 3 compared with 15.7% in the placebo group (p = 0.001).1
The exact mechanism of pre-probiotics’ actions is still unclear but it has been assumed that they have a protective role by keeping away harmful pathogens from intestinal mucosa surface probably by modulating the membrane permeability and the mucosal immune system. They would hold the homeostasis of bowel mucosal system, intestinal barrier function, and modulate the immune response that needs a continuous balance between pro- and anti-inflammatory components.7,10-11 Pre-probiotic agents are likely to become an integral component of treating IBD in combination with traditional anti-inflammatory and immunosuppressive agents to re-boost the host immune system.12
CONCLUSIONS
One important result achieved in the present study was the role of disease duration in response to complementary pre-probiotics treatment. As mentioned in the results section (table 3), the overall response was not significantly different between the two groups but when duration was added to the multivariate regression analysis, the results changed. Those with longer duration responded significantly better to the intervention (Lactocare®) compared with those with a shorter one (less than 5 years).
In those with longer duration of disease, a significant change in the gut microbiota would be occurred due to the use of different antibiotics and medications related to their intestinal and extra-intestinal complaints. So, the better response in this group of patients would be better explained by the local anti-inflammatory role assumed for pre-probiotics through modulating the gut flora as explored in the previous studies.13
This concept would be better examined in a larger population of patients with IBD with longer follow-ups to be able to claim with more confidence about the effect of pre-probiotics on the disease activity.
Limitations:
In the present study, we had just two points of measuring the disease activity index; first at the baseline before the intervention and then at the end of study (after 8 weeks). But in most studies especially when the duration of the study was longer (12 weeks or more) there were more points for measurements.
Routine medications were almost equal in all patients (sulfasalazine) with some exceptions on those who were on the treatment with mesalazine or Asacol® that could be ignored due to the small numbers.
Financial supports:
This research project was funded by the Research Deputy of Golestan University of Medical Sciences.
Authors’ contribution:
TA, AAT, and NR contributed to the design and conception of the research project, critical revision of the article, and final approval of the version to be published. HR, MF and SB contributed in collecting and interpretation of data, drafting the article and revising it critically for important intellectual content. GR contributed to the acquisition and data analysis and interpretation, drafting the article, and critical revision of the article.
Also all authors agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Acknowledgments
This paper was extracted from a thesis dedicated to achieve MD degree from Golestan University of Medical Sciences. Authors tend to acknowledge all colleagues and patients helped us in Golestan Research Center of Gastroenterology and Hepatology (GRCGH).
Please cite this paper as:
Amiriani T, Rajabli N, Faghani M, Besharat S, Roshandel GR, AkhavanTabib A, Joshaghani HR. Effect of Lactocare® Synbiotic on Disease Severity in Ulcerative Colitis: A Randomized Placebo-Controlled Double-Blind Clinical Trial. Middle East J Dig Dis 2020;12: 27-33. doi: 10.15171/mejdd.2020.160.
Footnotes
ETHICAL APPROVAL There is nothing to be declared.
CONFLICT OF INTEREST The authors declare no conflict of interest related to this work.
References
- 1.Sood A, Midha V, Makharia GK, Ahuja V, Singal D, Goswami P. et al. The probiotic preparation, VSL# 3 induces remission in patients with mild-to-moderately active ulcerative colitis. Clin Gastroenterol Hepatol. 2009;7:1202–9 e1. doi: 10.1016/j.cgh.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 2.Derikx LA, Dieleman LA, Hoentjen F. Probiotics and prebiotics in ulcerative colitis. Best Pract Res Clin Gastroenterol. 2016;30:55–71. doi: 10.1016/j.bpg.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 3.Fujimori S, Gudis K, Mitsui K, Seo T, Yonezawa M, Tanaka S. et al. A randomized controlled trial on the efficacy of synbiotic versus probiotic or prebiotic treatment to improve the quality of life in patients with ulcerative colitis. Nutrition. 2009;25:520–5. doi: 10.1016/j.nut.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 4.Kruis W, Frič P, Pokrotnieks J, Lukáš M, Fixa B, Kaščák M. et al. Maintaining remission of ulcerative colitis with the probiotic Escherichia coli Nissle 1917 is as effective as with standard mesalazine. Gut. 2004;53:1617–23. doi: 10.1136/gut.2003.037747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miele E, Pascarella F, Giannetti E, Quaglietta L, Baldassano RN, Staiano A. Effect of a probiotic preparation (VSL# 3) on induction and maintenance of remission in children with ulcerative colitis. Am J Gastroenterol. 2009;104:437–43. doi: 10.1038/ajg.2008.118. [DOI] [PubMed] [Google Scholar]
- 6.Sang LX, Chang B, Zhang WL, Wu XM, Li XH, Jiang M. Remission induction and maintenance effect of probiotics on ulcerative colitis: a meta-analysis. World J Gastroenterol. 2010;16:1908–15. doi: 10.3748/wjg.v16.i15.1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scaldaferri F, Gerardi V, Lopetuso LR, Del Zompo F, Mangiola F, Boškoski I. et al. Gut microbial flora, prebiotics, and probiotics in IBD: their current usage and utility. Biomed Res Int. 2013;2013:435268. doi: 10.1155/2013/435268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walmsley R, Ayres R, Pounder R, Allan R. A simple clinical colitis activity index. Gut. 1998;43:29–32. doi: 10.1136/gut.43.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ivanovska TP, Mladenovska K, Zhivikj Z, Pavlova MJ, Gjurovski I, Ristoski T. et al. Synbiotic loaded chitosan-Ca-alginate microparticles reduces inflammation in the TNBS model of rat colitis. Int J Pharm. 2017;527:126–134. doi: 10.1016/j.ijpharm.2017.05.049. [DOI] [PubMed] [Google Scholar]
- 10.Reiff C, Kelly D. Inflammatory bowel disease, gut bacteria and probiotic therapy. Int J Med Microbiol. 2010;300:25–33. doi: 10.1016/j.ijmm.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 11. Plaza-Díaz J, Ruiz-Ojeda FJ, Vilchez-Padial LM, Gil A. Evidence of the Anti-Inflammatory Effects of Probiotics and Synbiotics in Intestinal Chronic Diseases. Nutrients 2017;9. pii: E555. 10.3390/nu9060555. [DOI] [PMC free article] [PubMed]
- 12.Sartor RB. Therapeutic manipulation of the enteric microflora in inflammatory bowel diseases: antibiotics, probiotics, and prebiotics. Gastroenterology. 2004;126:1620–33. doi: 10.1053/j.gastro.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 13.Ghosh S, van Heel d, Playford RJ. Probiotics in inflammatory bowel disease: is it all gut flora modulation? Gut. 2004;53:620–2. doi: 10.1136/gut.2003.034249. [DOI] [PMC free article] [PubMed] [Google Scholar]