Abstract
Background
The bacterium Coxiella burnetii is the etiological agent of Q fever and is mainly transmitted via inhalation of infectious aerosols. DNA of C. burnetii is frequently detected in ticks, but the role of ticks as vectors in the epidemiology of this agent is still controversial. In this study, Ixodes ricinus and Dermacentor marginatus adults as well as I. ricinus nymphs were fed on blood spiked with C. burnetii in order to study the fate of the bacterium within putative tick vectors.
Methods
Blood-feeding experiments were performed in vitro in silicone-membrane based feeding units. The uptake, fecal excretion and transstadial transmission of C. burnetii was examined by quantitative real-time PCR as well as cultivation of feces and crushed tick filtrates in L-929 mouse fibroblast cells and cell-free culture medium.
Results
Ticks successfully fed in the feeding system with engorgement rates ranging from 29% (D. marginatus) to 64% (I. ricinus adults). Coxiella burnetii DNA was detected in the feces of both tick species during and after feeding on blood containing 105 or 106 genomic equivalents per ml blood (GE/ml), but not when fed on blood containing only 104 GE/ml. Isolation and cultivation demonstrated the infectivity of C. burnetii in shed feces. In 25% of the I. ricinus nymphs feeding on inoculated blood, a transstadial transmission to the adult stage was detected. Females that molted from nymphs fed on inoculated blood excreted C. burnetii of up to 106 genomic equivalents per mg of feces.
Conclusions
These findings show that transstadial transmission of C. burnetii occurs in I. ricinus and confirm that I. ricinus is a potential vector for Q fever. Transmission from both tick species might occur by inhalation of feces containing high amounts of viable C. burnetii rather than via tick bites.
Keywords: Coxiella burnetii, Ticks, Transmission
Background
Coxiella burnetii is an obligate intracellular bacterium and distributed worldwide with the exception of New Zealand. It is known as the causal agent of Q fever and affects a wide range of hosts including humans [1, 2]. Infected ruminants, considered as the main reservoir, show no symptoms or fertility-related complications and can shed the bacteria in large amounts during parturition or defecation [3, 4]. Human Q fever infection is often presented as an acute flu-like disease with possible complications such as pneumonia or hepatitis [5, 6]. Moreover, a chronic form mainly affecting heart-valves as well as a fatigue syndrome can occur [7, 8]. The most relevant infection route of Q fever is the inhalation of contaminated dusts or aerosols. Persons with close contact to infected domestic ruminants, such as farmers or veterinarians, may be at higher risk for acquiring this disease [9, 10].
Coxiella burnetii was first isolated from an infected Dermacentor andersoni tick [11] and was then isolated from 40 different tick species, including Ixodes ricinus, the most common tick in central Europe [12–15]. The tick D. marginatus in particular is considered to be a competent vector. The spatial distribution of this tick matches the increased occurrence of Q fever cases in southwest Germany [16, 17]. In early studies, the presence of C. burnetii in tick organs was examined by staining methods [18, 19]. With the introduction of PCR methods, several studies investigating the prevalence of tick-borne pathogens in Europe showed C. burnetii DNA in over 10% of the examined arthropods [20, 21]. Contrary, in other studies, no C. burnetii-specific DNA was detected in ticks [22–24]. In addition, tick feces samples were found to contain C. burnetii or DNA of this agent [17, 25]. However, recent research questioned the role of ticks in Q fever transmission. During the large Q fever outbreak in the Netherlands 2007–2010, ticks obtained from environment, pets, wildlife and livestock were tested, but no infected tick could be found, even from infected herds [26]. Thus, the risk of acquiring Q fever by ticks is commonly assessed as being low. Furthermore, Coxiella-like bacteria were discovered as primarily non-pathogenic tick endosymbionts [27, 28]. Their close genetic relationship to C. burnetii could have led to misidentifications by PCR [29, 30]. These partially contradictory findings demonstrate the need for further research on possible transmission routes of C. burnetii between different life stages of ticks and from ticks to their hosts.
To study blood-feeding and vector competence of hematophagous arthropods under controlled laboratory conditions, several in vitro feeding systems have been established [31–35]. These methods allow detailed analyses of blood-borne pathogen transmission via insect or tick vectors. In this study, the uptake, survival and transstadial transmission of C. burnetii in ticks, as well as excretion of the bacteria via feces, was analyzed using a membrane-based in vitro feeding system.
Methods
Ticks
Adult female and male I. ricinus and D. marginatus as well as I. ricinus nymphs were obtained from a laboratory colony (Insect Services, Berlin, Germany) at the age of 14 to 37 weeks after molting. The colonies were free of Borrelia spp. and Rickettsia spp. Until feeding, I. ricinus ticks were maintained at 4–8 °C and D. marginatus at room temperature at a relative humidity (RH) of > 90%. At least 24 h before the beginning of tick feeding experiments, I. ricinus were moved to room temperature.
Membrane production
Artificial tick feeding was performed in silicone-membrane sealed glass tubes following the protocol by Kröber and Guerin [36]. Briefly, the silicone mass was mixed according to the recipe published by Krull et al. [37] and spread out on lens cleaning tissue (Whatman, Maidstone, UK) with the rounded side of a gel releaser (Bio-Rad, Hercules, USA). Membranes were left to polymerize for at least 16 h at > 95% RH. Membrane thickness was measured with the Inductive Dial Comparator 2000 (Mahr, Göttingen, Germany). Membranes with a thickness of 70–100 µm were used for the feeding of I. ricinus adults. Ixodes ricinus nymphs and adult D. marginatus were fed on membranes of 60–80 µm.
Artificial feeding system
Sealed feeding units were made by gluing the silicone membrane with Elastosil E41 (Wacker Chemie, München, Germany) to borosilicate tubes of 50 mm length, 30 mm outer diameter, 28 mm inner diameter (Neubert Glas, Geschwenda, Germany). A square piece of mosquito netting (15 × 15 mm, Draht Driller, Freiburg, Germany) was loosely glued to the membrane [37] to stimulate the fixation of adult ticks. An extract of washed sheep wool (Alana, Karlsruhe, Germany) was prepared with dichloromethane (Carl Roth, Karlsruhe, Germany) as described by Böhme [38]. Ninety µl of the extract (10 mg/ml) was added to the membrane of each feeding unit, which were subsequently allowed to dry for 2 h at room temperature before ticks were placed in the feeding unit [38].
Feeding of adult I. ricinus
Blood used for feeding experiments was taken from the jugular vein of cattle (Thüringer Landesamt für Verbraucherschutz, Bad Langensalza, Germany, registration no. 04-102/15) in heparinized blood vials (Sarstedt, Nümbrecht, Germany). Of this blood, 3.1 ml supplemented with glucose (2 g/l) were added to each well of a 6-well plate (Sarstedt). As a phagostimulant, 1 mmol/l ATP (Carl Roth) dissolved in physiological sodium chloride solution was added to the blood [39]. The plate was pre-warmed at 37 °C. Blood was changed every 10–14 h. The tick feeding units placed in the 6-well plates were incubated at 37 °C, a RH of > 70% and a day–night regime with a cycle of 15 h light and 9 h darkness. All environmental factors were continuously monitored by a data logger (MSR, Seuzach, Switzerland). As a further stimulus, the CO2 level was increased to 2.5% [40]. Each feeding unit contained 7–9 female and 5 male I. ricinus.
During the first 12 h of each feeding experiment, ticks were fed on blood (without bacteria) for attachment before starting inoculation. For the infection experiments 104 (2 units), 105 (2 units) or 106 (5 units) genomic equivalents (GE)/ml of Coxiella burnetii Nine Mile phase II RSA 439 (obtained from the strain collection at Friedrich Löffler Institute Jena) were subsequently added to the wells of the feeding unit during every blood change, except for the mock infection control (2 units). In one of the experiments, attached females feeding on Coxiella-inoculated blood were removed from the membrane after 48 h of feeding and transferred to a fresh feeding unit in order to continue feeding on Coxiella-free blood (4 units). During each blood change, the membranes and ticks were inspected and feeding units were rinsed with warm 0.9% sodium chloride solution. Samples of tick feces of every feeding unit were removed daily with fine forceps and stored at 4 °C until further processing. Single ticks were removed during feeding at different days. Detached replete ticks were removed from the membrane, transferred to a 15 ml Falcon tube and individually stored at 21–23 °C, > 90% RH.
Feeding of D. marginatus
Blood for feeding of D. marginatus adults was prepared the same way as for I. ricinus adults. The experiments were performed with addition of 106 GE/ml C. burnetii in 4 feeding units or mock inoculation in 2 feeding units. Per feeding unit, 7 female and 5 male D. marginatus were used. Feeding chambers were incubated on a 38 °C heating plate with an ambient temperature of 28–30 °C. This provides a temperature gradient, resembling the animal skin and environmental temperatures. Blood change as well as storage of feces samples and engorged ticks was carried out as in I. ricinus adult experiments.
Feeding of I. ricinus nymphs
The feeding of I. ricinus nymphs was performed in the same setting as for D. marginatus. CO2 was used at a concentration of 4% and the feeding units were covered with a moist piece of cotton wool to prevent desiccation of ticks. In each feeding unit, 50–60 nymphs were placed. One feeding unit remained as mock infected, whereas the other were constantly supplemented with 106 GE/ml throughout feeding. Blood change was performed similar to the adult feeding and engorged nymphs were removed and transferred to 1.5 ml tubes and maintained at 21–23 °C and a RH of over 90%. Shortly after engorgement, 5 mock infected nymphs and 6 nymphs, which were exposed to C. burnetii were removed and tested by qPCR.
Cultivation of Coxiella burnetii
All experiments with C. burnetii Nine Mile Phase II RSA 439 were carried out under biosafety laboratory 2 conditions. Coxiella burnetii was cultivated in L-929 mouse fibroblasts, maintained in Dulbecco’s Modified Eagle Medium (DMEM; Life Technologies, Carlsbad, USA) supplemented with 5% fetal calf serum (FCS; Life Technologies). Medium was changed weekly, and the cells were harvested after an average of two weeks when Coxiella-containing vacuoles were visible. Purification of bacteria from host cells was performed via needle purification using 22 G cannulas as smallest size according to [41], and genomic equivalent measurement was performed by quantitative real-time PCR as described below. Coxiella burnetii was stored in a 45 mM phosphate-buffered 0.25 M sucrose solution with 10% glycerol in aliquots at − 80 °C.
DNA isolation and quantitative real-time polymerase chain reaction (PCR)
Female ticks from feeding experiments were stored in 70% ethanol at 4 °C. Prior to DNA-extraction, ticks were washed in PBS and dried. Every tick was weighed individually using a micro balance. In addition, feces per day and group were weighed in order to normalize the PCR results to the mass of feces. Lysis of ticks and feces was performed in a Tris-EDTA-lysozyme-buffer [42], containing 0.25 M Tris-HCl (pH 7.5), 10 mM EDTA and 0.4% (w/v) lysozyme, with micropestles (Eppendorf, Wesseling-Berzdorf, Germany) and in the case of ticks, an additional two rounds of centrifugation (14000×g, 5 min, 20 °C) in 1.5 ml tubes. After 4 h of lysis with 0.2 % (w/v) proteinase K at 56 °C, DNA extraction was performed according to the High Pure PCR Template Preparation Kit (Roche, Basel, Switzerland). In addition, DNA was extracted from 200 µl blood that was used to feed previously infected ticks with the same DNA extraction kit according the whole blood protocol. Quantitative real-time PCR was performed with a Lightcycler 480 II (Roche) targeting the C. burnetii single-copy gene icd [43]. For each reaction 2 µl of template DNA or standard was used [44]. The program included 45 cycles of 15 s at 95 °C and 30 s at 60 °C. A cut-off for positivity was set to a Cq-value of 35, which was equal to 10 copies per reaction. For confirmation of DNA extraction in I. ricinus, a quantitative real-time PCR targeting the I. ricinus housekeeping genes actin and elongation factor 2 was conducted using the primers described in [45].
Isolation of Coxiella burnetii from feces
Tick feces samples originating from one group fed on blood inoculated with 106 GE/ml C. burnetii and from one mock infected group were weighed and used for cultivation in L-929 cells. Briefly, ninety-two mg feces from mock infected and 102 mg feces from infected ticks were dissolved in 10 ml DMEM containing 5% FCS, filtered through 0.45 µm syringe filters (TPP, Trasadingen, Switzerland). From the filtrate, 4.5 ml were transferred to a 25 cm2 flask (Greiner bio-one, Kremsmünster, Austria) of confluent murine L-929 cells. The volume was then extended to 6 ml with DMEM containing 5% FCS. Medium was changed weekly, and cells were visually monitored at 400× magnification. For PCR-based affirmation of the visible increase of vacuoles, every three days a square of approximately 1.5 cm2 of the cell layer was removed with PBS (pH 7.4) using a cell scraper. Cells were centrifuged (15000×g for 5 min at 4 °C) and the pellet resuspended in lysis buffer. DNA was extracted and C. burnetii DNA was quantified using the isocitrate dehydrogenase (icd) qPCR. For normalization the QuantiTect Probe RT-PCR Kit (Qiagen, Hilden, Germany) with the QuantiTect Primer for Ribosomal Protein L22 (rpl22) (Qiagen) targeting a mouse housekeeping gene was used, and the icd results were normalized to the average Cq values of rpl22.
Isolation of C. burnetii from eclosed adults
Of the females molted from infected nymphs, two were bisected and qPCR was performed on one half whereas the other half was used for the cultivation of bacteria. For this purpose, halved ticks were suspended in 3 ml DMEM overnight before filtration through 0.45 µm syringe filters. One ml of this filtrate was used for inoculation of L-929 cells whereas the remaining filtrate was centrifuged at 15000×g for 5 min at 4 °C. Acidified citrate cysteine medium-2 (ACCM-2) was prepared as specified by the manufacturer (Sunrise Science, San Diego, USA). The pellet was resuspended in 2 ml ACCM-2 and placed into a 12-well plate. As a control, 2 ml ACCM-2 was inoculated with 105 GE/ml C. burnetii RSA 439. The cultures were incubated at 37 °C with 5% CO2 and 2.5% O2 [46]. At day 1, 4 and 7, 100 µl were removed and used for qPCR quantification of C. burnetii. Inoculated L-929 cells were visually examined biweekly for signs of Coxiella containing vacuoles.
Reinfection experiment
In order to examine transstadial transmission, females, which were fed as nymphs on Coxiella-containing blood, were fed with blood without C. burnetii. Therefore, two groups of 4 females and 3 males each were used. An additional uninfected group of the same composition was used as a negative control. Small feeding units (20 × 2.5 × 25 mm) were used in a 12-well-plate with 1 ml blood supplemented with 2 g/l glucose and of 1 mM ATP. During each blood change, 200 µl of blood was collected for qPCR analysis. Feces were removed every second day and also tested by qPCR and cultivation in L-929 cells.
Statistical analysis
Data were analyzed using Excel to calculate the mean and standard deviation. SPSS Statistics V22.0 was used for performing unpaired two-tailed t-tests and Mann–Whitney U-tests, depending on data distribution. Pearson’s Chi-square test was performed for analysis of the engorgement rates. P-values < 0.05 were considered statistically significant.
Results
Behavior of I. ricinus and D. marginatus in the in vitro feeding assay
In five independent experiments a total number of 141 female I. ricinus were fed. Within the first 24 h of feeding, 58 ± 12% (mean ± standard deviation) of female I. ricinus ticks attached to the membrane. The attachment rate varied in different experiments between 84% and 100% at day 4. An engorgement rate of 49 ± 15% was achieved after 14.5 ± 3.1 days, as shown in Table 1. Adults feeding on inoculated blood took longer as for those taking an uninfected blood meal, but the difference was not statistically significant (Mann-Whitney U-test, U = 41.5, Z = − 1.453, P = 0.157).
Table 1.
In vitro feeding of ticks
| Species | Life stage | Engorgement rate (%) | Engorgement weight (mg) | Duration of feeding (days) | Time until oviposition | Molting rate (%) |
|---|---|---|---|---|---|---|
| Ixodes ricinus | Adults | 49 ± 15 | 186.2 ± 61.8 | 14.5 ± 3.1 | 14.7 ± 4.9 daysa | – |
| Nymphs | ~ 50 | 3.0 ± 1.0 | 7.9 ± 1.8 | 10 ± 5 weeks | 92 | |
| Dermacentor margiatus | Adults | 29 | 414.6 ± 136.7 | 14.8 ± 3.4 | 4 daysb | – |
aDays until oviposition of I. ricinus were calculated from 15 egg-laying ticks
bIn the D. marginatus experiment, only 1 tick laid eggs after 4 days
Notes: Feeding parameters of in vitro experiments with I. ricinus adults and nymphs and D. marginatus adults (mean values ± standard deviation)
Visible feces production of ticks started at day 2 post-attachment. For DNA extraction, up to 40 mg of daily pooled feces per feeding group was used. During the rapid engorgement phase, which occurred after approximately 10 days of in vitro feeding, feces production decreased and the ticks engorged to an average weight of 186.2 ± 61.8 mg (average initial weight 2 mg).
The in vitro feeding assay was also established for D. marginatus. In two independent experiments fixation rates of 25% and 33% at day 4 and an engorgement rate of 29% (8 of 28 female D. marginatus) in both experiments were observed. This engorgement rate is lower than the one of I. ricinus adults, but the difference was not statistically significant (χ2 = 3.798, df = 1, P = 0.051). The engorgement weight was 414.6 ± 136.7 mg (average initial weight 8 mg) and engorged females had fed for an average of 14.8 ± 3.4 days. The engorgement weight of D. marginatus was significantly higher than the weight of engorged I. ricinus (t(43) = −5 .356, P < 0.001). It was also noticed that females often detached and reattached during feeding.
Feeding was also performed with a total number of 200 I. ricinus nymphs. Approximately 50% of the nymphs engorged to an average weight of 3.0 ± 1.0 mg (average weight of unfed nymphs: 0.48 ± 0.31 mg) (Table 1). The remaining nymphs did not attach to the membrane or did not fully engorge. Nymphs fully engorged within 7.9 ± 1.8 days. After removal of 11 nymphs for PCR testing, within 10 ± 5 weeks 92% (82/89) of the engorged nymphs molted into adults, of which 59% were females.
Infected I. ricinus can carry C. burnetii for at least seven weeks
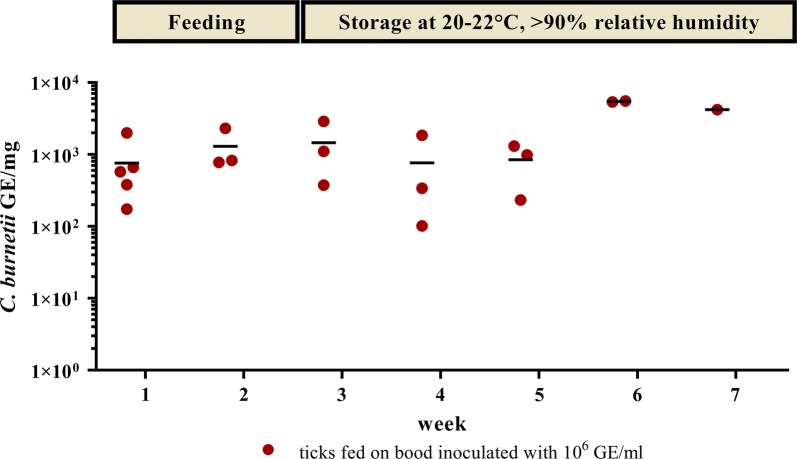
To analyze the uptake of C. burnetii by ticks in the feeding system, 2–4 I. ricinus females exposed to 106 GE/ml containing blood and one female tick exposed to negative blood were tested weekly for the presence of Coxiella-DNA, starting one week after beginning of feeding.
A slight but statistically not significant (t(6) = − 0.969, P = 0.370) increase in C. burnetii DNA load was detected during feeding, i.e. in the first two weeks (Fig. 1). In all the female I. ricinus that fed on inoculated blood DNA of C. burnetii was found (ranging from 102 to 103 GE/mg) up to 5 weeks after feeding. In contrast, ticks feeding on negative blood remained C. burnetii negative (data not shown).
Fig. 1.
Coxiella burnetii DNA in ticks during and after feeding. Each red dot represents one tick, which was used for DNA extraction after feeding on blood inoculated with 106 GE/ml of C. burnetii Nine Mile phase II. The means are indicated by a horizontal line. During the first two time points, the ticks were still feeding. Ticks were kept separated from each other after detaching from the membrane. Mock-infected ticks (one per time point, not shown) had no Cq-value. The data derive from two independent experiments and were normalized for the two Ixodes ricinus housekeeping genes actin and elongation factor 2
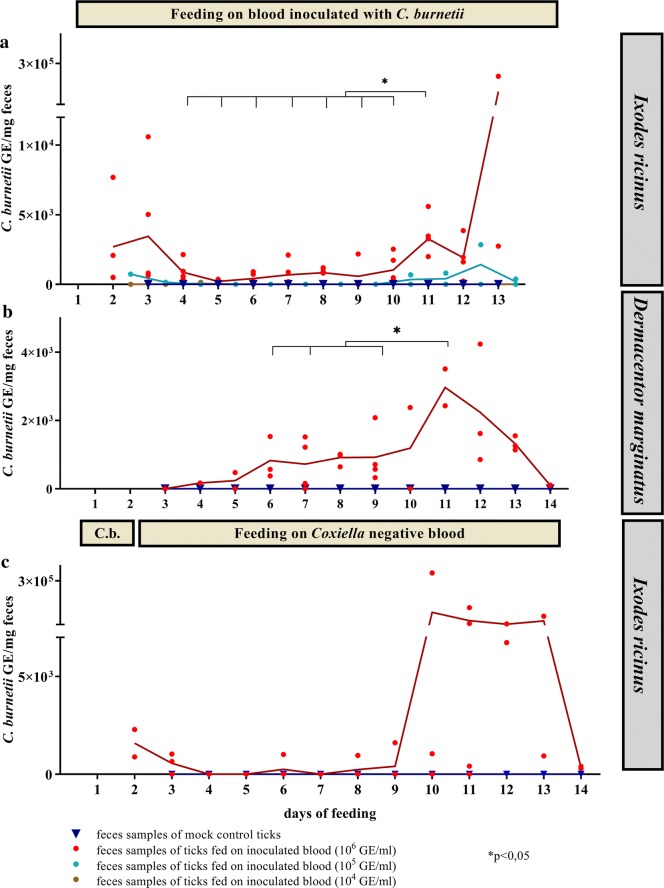
Coxiella burnetii is excreted via feces by adult I. ricinus and D. marginatus
Coxiella burnetii DNA could readily be detected in feces of groups feeding on blood inoculated with 106 GE/ml, but a decrease of the DNA content was observed after 3 days. However, after 9 days of feeding, the amount of C. burnetii DNA increased again. The DNA load at day 11 differed significantly from the DNA loads at the days 4–10 (e.g. t(8) = − 4.631, P = 0.009 at day 5; t(8) = − 2.768, P = 0.024 at day 10). Feces of ticks fed on 105 GE/ml Coxiella-containing blood displayed a similar time-dependent excretion of bacteria (Fig. 2a), whereas a dose of 104 GE/ml was not sufficient to obtain Coxiella DNA-containing feces. In D. marginatus, feces excretion started between day 3 and 6. In comparison to I. ricinus, larger amounts of feces were deposited, and also male D. marginatus attached and defecated for longer time periods. Similar to I. ricinus, in the feces samples examined by PCR the bacterial DNA load was highest between day 11 and 12 with up to 2 × 103 GE per milligram feces (Fig. 2b) and was significantly different to the days 6 (t(3) = 3.508, P = 0.039), 7 (t(4) = − 3.413, P = 0.027) and 9 (t(4) = − 3.024, P = 0.039).
Fig. 2.
Coxiella DNA in feces. In (a) and (b) ticks were fed during the whole feeding experiment with blood inoculated with C. burnetii in different concentrations. The means of the replicates of each data set are connected with a line. The data for I. ricinus (a) represent three independent experiments with five different feeding units inoculated with 106 GE/ml blood (n = 5), 104 GE/ml (n = 2), 105 GE/ml (n = 2) and negative controls (n = 2). For D. marginatus (b), the data represent two independent experiments with feeding units on blood inoculated with 106 GE/ml (n = 4) and negative controls (n = 2). c Ticks were fed on 106 GE/ml inoculated blood for 36 h (indicated by the horizontal bar labelled with ‘C.b.’) (n = 4). Attached ticks were subsequently removed and placed in a Coxiella-free feeding unit. Ticks constantly feeding on Coxiella-negative blood served as negative controls (n = 2). Statistical significance of DNA loads in comparison to day 11 is indicated by an asterisk
To test whether shorter feeding periods would be sufficient for the excretion of C. burnetii via feces, the experimental setup was modified: I. ricinus were allowed to feed on C. burnetii-spiked blood for 36 h before being transferred to another unit where they continued feeding on bacteria-free blood. Here, a similar increase of C. burnetii DNA after approximately 11 days was observed in the feces (Fig. 2c), although the differences were not statistically significant.
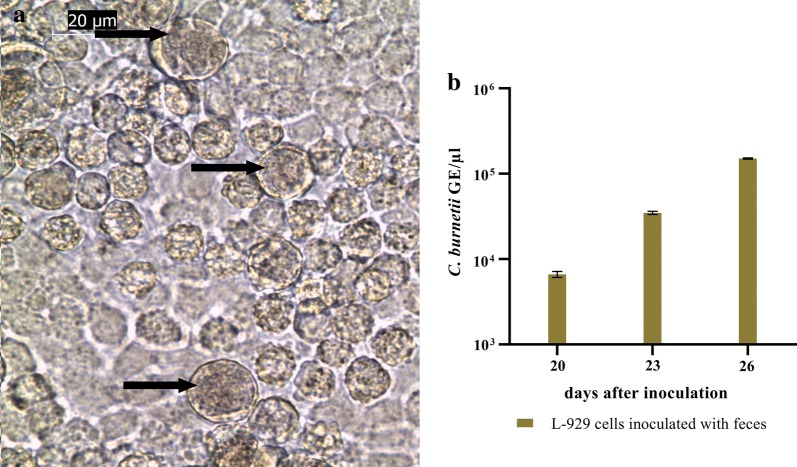
Next, we investigated whether the C. burnetii DNA in the tick feces derived from viable bacteria. Feces samples taken at day 10 from ticks fed on blood containing 106 GE/ml (transferred to non-infected blood after 36 h, see above) were inoculated to L-929 cells. Samples from Coxiella negative blood-feeding were used as controls. In the cells incubated with feces from C. burnetii exposed ticks, first microscopically visible vacuoles appeared at day 18 after inoculation (Fig. 3a) and an increase in vacuoles was visible within the following ten days. At day 20, 23 and 26 after inoculation cells were harvested and analyzed by real-time PCR. An increase of Coxiella DNA in relation to the DNA of mouse fibroblasts (L-929) could be detected (Fig. 3b), whereas the flask inoculated with feces of negative controls remained free of Coxiella DNA. These data show that C. burnetii excreted in the feces from ticks feeding on infected blood are viable.
Fig. 3.
Growth of Coxiella burnetii from tick feces in L-929 cells. a Coxiella-containing vacuoles (indicated by arrows) 19 days after inoculation of L-929 cells with feces of ticks, fed for 36 h on Coxiella-containing blood. The picture was taken at 400× magnification. b Cells were removed from the flask at three different time points and genomic equivalents were determined by quantitative PCR. Samples were measured in duplicate, the mean values and standard deviation are indicated. The data were normalized for the mouse housekeeping gene rpl22
Coxiella burnetii is transstadially transmitted from I. ricinus nymphs to adults
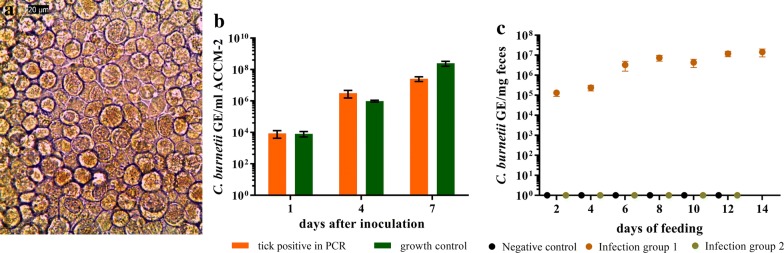
In order to examine transstadial transmission of C. burnetii, I. ricinus nymphs were infected with 106 GE/ml using the in vitro feeding system. Via qPCR, uptake of Coxiella DNA was confirmed by testing 6 previously infected nymphs, whereas 5 mock infected nymphs remained negative. Two weeks after molting, 40 adults, which were fed on C. burnetii inoculated blood as nymphs, and 26 adults fed on negative blood were tested by real-time PCR. Of the nymphs which were fed on inoculated blood 25% were positive for C. burnetii DNA. One female tick was bisected and one half was analyzed for C. burnetii DNA by PCR. The Cq-value was 22.69. After inoculation of axenic medium and L-929 cells with a filtrate of the other half of the tick, growth of C. burnetii in both settings was observed (Fig. 4a, b).
Fig. 4.
Transstadial transmission of C. burnetii from nymphs to adult I. ricinus. Nymphs were fed on blood containing 106 GE/ml and were allowed to transform into adults. a Of the PCR C. burnetii-positive adult ticks, one half of a bisected tick was used for cultivation with L-929 cells. Vacuoles typical for Coxiella infections appeared 24 days after inoculation. b Material from the same tick was used for cultivation in cell-free ACCM-2 medium, and C. burnetii DNA was detected by PCR. Negative controls tested in parallel showed no Cq-value in the PCR (not shown). c Eight females molted from previously infected nymphs were divided into two infection groups, each consisting of 4 females and 3 Coxiella-negative males, and fed again on sterile blood. Feces were removed every two days and tested by quantitative PCR. Infection group 1 excreted C. burnetii DNA with their feces, whereas in feces of infection group 2 and in the negative control group no C. burnetii DNA was detected (here shown as 100). Samples in b and c were analyzed in three independent measurements in duplicates, the mean values and standard deviations are indicated
Coxiella burnetii can be excreted via feces by adult ticks infected as nymphs
Eight female ticks which were fed with C. burnetii as nymphs were divided into two groups and fed again on sterile blood. In this reinfection experiment, all negative control ticks and 3 out of 4 ticks of each infection group were attached. In one of the infection groups, C. burnetii DNA was detected in feces at every time point, revealing an increasing DNA concentration up to 6 × 106 GE/mg feces (Fig. 4c). The bacteria contained in the feces were infective as verified by culture in L-929 cells and PCR (Cq = 25.39) at day 21 post-inoculation. These results suggest that C. burnetii is transmitted transstadially from I. ricinus nymphs to adults and that infected adults excrete the bacteria with their feces during feeding on a non-infected host. Samples of blood on which these ticks had fed were tested at 7 different time points during the first 7 days of feeding, but Coxiella DNA was not detected.
Discussion
The significance of ticks as potential vectors for C. burnetii is still under debate. Several laboratory-based studies have revealed that many tick species get infected upon feeding on C. burnetii positive animals and that they carry the bacterium into their next developmental life stage. In addition, transmission of C. burnetii to non-infected laboratory animals by ticks has been demonstrated [47]. Besides these principle findings, many questions remain unresolved, including the most significant mechanism of infection (e.g. via feces or saliva) or the effectiveness of transstadial transmission. It is important to investigate these aspects of C. burnetii in ticks, in order to fully understand the role these arthropods play in the epidemiology of Q fever.
In this study, we have investigated uptake of C. burnetii by I. ricinus and D. marginatus via blood-feeding. By using an artificial membrane feeding system it could be demonstrated that I. ricinus becomes infected by feeding on bacteria-containing blood and that the bacteria are detectable within the ticks for at least seven weeks. The feces, which are constantly being deposited during blood-feeding, contain viable C. burnetii. This excretion of C. burnetii was shown to be time dependent, as a peak of bacteria in feces was detectable at approximately 11 days upon start of feeding. The amount of C. burnetii found in the ticks and in their feces also correlated with the concentration of the bacteria in the blood, and was highest when feeding with 106 GE/ml blood, the highest concentration used in this study. Titers of 106 are commonly found in blood of humans with an acute Q fever infection [48]. Lower titers, 105 GE/ml blood, created a similar bacteria excretion profile, whereas in the 104 GE/ml-group C. burnetii DNA could not be detected, indicating the need of a highly bacteremic host for excretion via feces.
The appearance of C burnetii in tick feces 9 days after start of feeding on infected blood suggests a replication of C. burnetii in the cells of the midgut, confirming earlier studies [19, 25]. At the same time, we cannot exclude that this increase was in part or completely caused by the secretion of more concentrated feces during this period.
Tick feces are known as a potential source for infection via inhalation of infected dusts [49]. Our data suggest that, at least for I. ricinus and D. marginatus, tick feces are not always infectious but that the amount of active C. burnetii in feces strongly increases towards the end of the feeding period, provided that the tick was not already infected with C. burnetii before the blood meal (see below).
The use of an in vitro feeding system allows for well-controlled experimental conditions, especially for analyzing the effects of bacterial concentration and duration of feeding. In addition, no infection experiments with mammalian hosts are required. By using guinea pigs as hosts, Siroky et al. [50] demonstrated the principle vector competence of Hyalomma aegyptium for C. burnetii. However, the exact mechanisms of transmission could not be analyzed, which is possible with in vitro systems such as the one used in our study.
Our results demonstrate that adult ticks which became infected with C. burnetii as nymphs, contain viable bacteria and excrete them via feces during feeding on non-infected blood. This excretion of bacteria followed a different time course and contained higher concentrations as compared to non-infected ticks feeding on infected blood. DNA of C. burnetii was present in high concentrations from the start of feeding and did constantly increase during the blood meal. At the same time no bacterial DNA could be detected in the blood used for feeding of these adults. Although we cannot exclude that minimal amounts of Coxiella were secreted into the blood, given the detection limit of 10 GE per reaction [41], this finding might indicate that the primary route of transmission of C. burnetii by ticks is via feces. In the field, this transmission could occur in several ways. Dried feces can form infectious dusts which are inhaled by one or more potential hosts. Additionally, the feces can contaminate the bite wound caused by the tick, similar to transmission of the parasite Trypanosoma cruzi by reduviid bugs [51]. Likewise, Rickettsia prowazekii, the etiological agent of louse-borne epidemic typhus is only excreted through feces and not via saliva. The infection consequently takes place when the itching sting is scratched and the pathogen rubbed into the skin [52]. Also, the amount of feces might have influence on transmission efficiency. Dermacentor marginatus deposits larger amounts than I. ricinus, as observed by us (data not shown) and others [38], thereby secreting more bacteria.
From the I. ricinus nymphs which were fed on C. burnetii-infected blood, only about 25% were positive for the bacteria after molting into adults. This is in accordance to Siroky et al. [50], where 29% of adults which as larvae fed on C. burnetii-positive guinea pigs were infected. The result is also similar to data obtained with in vitro feeding systems and the tick-borne pathogens Rickettsia monacensis and Anaplasma phagocytophilum [35]. It indicates that transstadial transmission is not 100% efficient, but that roughly one out of four nymphs feeding on an infected host is able to transmit C. burnetii as an adult, under the selected experimental conditions. Accordingly, in our study, bacterial DNA in the feces was found only in one out of two experimental groups during the feeding of adults which were infected as nymphs. These results might contribute to the observation that ticks do not play a major role in transmitting Q fever in the field, although in principle they are competent vectors [47].
Our experiments were conducted with the Phase II Nine Mile strain of C. burnetii. This strain is non-pathogenic to humans due to a different LPS-structure when compared to the virulent Phase I strain [14]. The Phase II bacteria were chosen due to safety issues, as performing the in vitro assays requires extensive direct handling of infected ticks and their feces. However, we cannot exclude that the differences in surface structures could influence the behavior of the bacteria in the tick or in this in vitro feeding system. Therefore, key findings of our study should be repeated using virulent C. burnetii strains.
Conclusions
This study provides evidence that the tick species I. ricinus and D. marginatus secrete viable C. burnetii via feces when feeding on infected blood. Moreover, approximately 25% of I. ricinus that became infected in the nymph stage still contained C. burnetii as adults and shed bacteria with their feces upon feeding on non-infected blood. As no C. burnetii was detected in the blood these adults fed on, our data support the idea that secretion via feces is a possible mechanism by which ticks can distribute and transmit C. burnetii.
Acknowledgements
We thank Steffen Jakob and Ulrike Ehlert for the excellent technical assistance.
Abbreviations
- ACCM
acidified citrate cysteine medium
- GE
genomic equivalents
- icd
isocitrate dehydrogenase
- PBS
phosphate-buffered saline
- PCR
polymerase chain reaction
- RH
relative humidity
- rpl22
ribosomal protein L22
Authors’ contributions
SK performed the experiments. AMN and AS contributed to the design of the study. SU, GRM, KMS, SK and KH designed the study and interpreted the results. SU and SK wrote the paper. All authors read and approved the final manuscript.
Funding
This study was supported by the German Federal Ministry for Education and Research (BMBF) via the Research Network of Zoonotic Infectious Diseases, project Q-GAPS (Q fever - German Interdisciplinary Program for Research), grant number 0IKI1726G and 01KI1726C. AMN received financial support from the BMBF as part of the Junior Research Group ‘Tick-borne Zoonoses’ with grant number 01KI1720.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Ethics approval and consent to participate
Usage of bovine blood was approved by the Thüringer Landesamt für Verbraucherschutz, Germany, registration No. 04-102/15.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Sophia Körner, Email: Sophia.koerner@fli.de.
Gustavo R. Makert, Email: Gustavo.makert@izi.fraunhofer.de
Katja Mertens-Scholz, Email: katja.mertens-scholz@fli.de.
Klaus Henning, Email: klaus.henning@fli.de.
Martin Pfeffer, Email: pfeffer@vetmed.uni-leipzig.de.
Alexander Starke, Email: alexander.starke@vetmed.uni-leipzig.de.
Ard M. Nijhof, Email: ard.nijhof@fu-berlin.de
Sebastian Ulbert, Email: Sebastian.ulbert@izi.fraunhofer.de.
References
- 1.Jones RM, Nicas M, Hubbard AE, Reingold AL. The infectious dose of Coxiella burnetii (Q fever) Appl Biosaf. 2006;11:32–41. doi: 10.1177/153567600601100106. [DOI] [Google Scholar]
- 2.van Schaik EJ, Chen C, Mertens K, Weber MM, Samuel JE. Molecular pathogenesis of the obligate intracellular bacterium Coxiella burnetii. Nat Rev Microbiol. 2013;11:561–573. doi: 10.1038/nrmicro3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roest HJ, van Gelderen B, Dinkla A, Frangoulidis D, van Zijderveld F, Rebel J, et al. Q fever in pregnant goats: pathogenesis and excretion of Coxiella burnetii. PLoS ONE. 2012;7:48949. doi: 10.1371/journal.pone.0048949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gache K, Rousset E, Perrin JB, De Cremoux R, Hosteing S, Jourdain E, et al. Estimation of the frequency of Q fever in sheep, goat and cattle herds in France: results of a 3-year study of the seroprevalence of Q fever and excretion level of Coxiella burnetii in abortive episodes. Epidemiol Infect. 2017;145:3131–3142. doi: 10.1017/S0950268817002308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marrie TJ. Coxiella burnetii pneumonia. Eur Respir J. 2003;21:713–719. doi: 10.1183/09031936.03.00099703. [DOI] [PubMed] [Google Scholar]
- 6.Jang YR, Shin Y, Jin CE, Koo B, Park SY, Kim MC, et al. Molecular detection of Coxiella burnetii from the formalin-fixed tissues of Q fever patients with acute hepatitis. plos ONE. 2017;12:0180237. doi: 10.1371/journal.pone.0180237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maurin M, Raoult D. Q fever. Clin Microbiol Rev. 1999;12:518–553. doi: 10.1128/CMR.12.4.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morroy G, Keijmel SP, Delsing CE, Bleijenberg G, Langendam M, Timen A, et al. Fatigue following acute Q fever: a systematic literature review. PLoS ONE. 2016;11:0155884. doi: 10.1371/journal.pone.0155884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fenga C, Gangemi S, De Luca A, Calimeri S, Lo Giudice D, Pugliese M, et al. Seroprevalence and occupational risk survey for Coxiella burnetii among exposed workers in Sicily, southern Italy. Int J Occup Med Environ Health. 2015;28:901–907. doi: 10.13075/ijomeh.1896.00448. [DOI] [PubMed] [Google Scholar]
- 10.Meadows SL, Jones-Bitton A, McEwen SA, Jansen J, Patel SN, Filejski C, et al. Prevalence and risk factors for Coxiella burnetii seropositivity in small ruminant veterinarians and veterinary students in Ontario, Canada. Can Vet J. 2017;58:397–399. [PMC free article] [PubMed] [Google Scholar]
- 11.Davis GE, Cox HR. A filter-passing infectious agent isolated from ticks. Isolation from Dermacentor andersonii, reactions with animals, and filtration experiments. Publ Health Rep. 1938;53:2259–2276. doi: 10.2307/4582746. [DOI] [Google Scholar]
- 12.Kaaserer B, Rehacek J, Urvögly J, Kovacova E, Lukacova M, Kocianova E. Erst-isolation des Q-Fieber Erregers Coxiella burnietii aus Ixodes ricinus-Zecken in Tirol (Österreich) Verein Innsbruck. 1994;81:223–227. [Google Scholar]
- 13.Spitalská E, Kocianová E. Detection of Coxiella burnetii in ticks collected in Slovakia and Hungary. Eur J Epidemiol. 2003;18:263–266. doi: 10.1023/A:1023330222657. [DOI] [PubMed] [Google Scholar]
- 14.Angelakis E, Raoult D. Q fever. Vet Microbiol. 2010;140:297–309. doi: 10.1016/j.vetmic.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 15.Rizzoli A, Silaghi C, Obiegala A, Rudolf I, Hubálek Z, Földvári G, et al. Ixodes ricinus and its transmitted pathogens in urban and peri-urban areas in Europe: new hazards and relevance for public health. Front Public Health. 2014;2:251. doi: 10.3389/fpubh.2014.00251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liebisch A. Das Q-Fieber als Naturherdinfektion in Süddeutschland. Bundesgesundheitsblatt. 1977;20:185–191. [Google Scholar]
- 17.Sting R, Breitling N, Oehme R, Kimmig P. Untersuchungen zu Vorkommen von Coxiella burnetii bei Schafen und Zecken der Gattung Dermacentor in Baden-Würtemberg. Dtsch Tierarztl Wochenschr. 2004;111:390–394. [PubMed] [Google Scholar]
- 18.Kordova N, Rehacek J. Experimental infection of ticks in vivo and their organs in vitro with filterable particles of Coxiella burneti. Acta Virol. 1959;3:201–209. [PubMed] [Google Scholar]
- 19.Zupancicovå-Majerska M, Rehåcek J, Kovåcovå E. Localization of Coxiella burnetii and Rickettsiae of the Rocky Mountain spotted fever group in ticks. Acta Virologica. 1972;16:63–70. [PubMed] [Google Scholar]
- 20.Bonnet S, de la Fuente J, Nicollet P, Liu X, Madani N, Blanchard B, et al. Prevalence of tick-borne pathogens in adult Dermacentor spp. ticks from nine collection sites in France. Vector Borne Zoonotic Dis. 2013;13:226–236. doi: 10.1089/vbz.2011.0933. [DOI] [PubMed] [Google Scholar]
- 21.Szymańska-Czerwińska M, Galińska EM, Niemczuk K, Zasępa M. Prevalence of Coxiella burnetii infection in foresters and ticks. Ann Agric Environ Med. 2013;20:699–704. [PubMed] [Google Scholar]
- 22.Reye AL, Hübschen JM, Sausy A, Muller CP. Prevalence and seasonality of tick-borne pathogens in questing Ixodes ricinus ticks from Luxembourg. Appl Environ Microbiol. 2010;76:2923–2931. doi: 10.1128/AEM.03061-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schötta AM, Wijnveld M, Stockinger H, Stanek G. Approaches for reverse line blot-based detection of microbial pathogens in Ixodes ricinus ticks collected in Austria and impact of the chosen method. Appl Environ Microbiol. 2017;83:00489-17. doi: 10.1128/AEM.00489-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pilloux L, Baumgartner A, Jaton K, Lienhard R, Ackermann-Gäumann R, Beuret C, et al. Prevalence of Anaplasma phagocytophilum and Coxiella burnetii in Ixodes ricinus ticks in Switzerland: an underestimated epidemiologic risk. N Microb N Infect. 2018;27:22–26. doi: 10.1016/j.nmni.2018.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith DJW. Studies in the epidemiology of Q fever. Aust J Exp Biol Med Sci. 1941;19:133–136. doi: 10.1038/icb.1941.21. [DOI] [Google Scholar]
- 26.Sprong H, Tijsse-Klasen E, Langelaar M, De Bruin A, Fonville M, Gassner F, et al. Prevalence of Coxiella burnetii in ticks after a large outbreak of Q fever. Zoonoses Public Health. 2012;59:69–75. doi: 10.1111/j.1863-2378.2011.01421.x. [DOI] [PubMed] [Google Scholar]
- 27.Duron O. The IS1111 insertion sequence used for detection of Coxiella burnetii is widespread in Coxiella-like endosymbionts of ticks. FEMS Microbiol Lett. 2015;362:132. doi: 10.1093/femsle/fnv132. [DOI] [PubMed] [Google Scholar]
- 28.Machado-Ferreira E, Vizzoni VF, Balsemão-Pires E, Moerbeck L, Gazeta GS, Piesman J, et al. Coxiella symbionts are widespread into hard ticks. Parasitol Res. 2016;115:4691–4699. doi: 10.1007/s00436-016-5230-z. [DOI] [PubMed] [Google Scholar]
- 29.Duron O, Noël V, McCoy KD, Bonazzi M, Sidi-Boumedine K, Morel O, et al. The recent evolution of a maternally-inherited endosymbiont of ticks led to the emergence of the Q fever pathogen, Coxiella burnetii. PLoS Pathog. 2015;11:1004892. doi: 10.1371/journal.ppat.1004892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elsa J, Duron O, Séverine B, González-Acuña D, Sidi-Boumedine K. Molecular methods routinely used to detect Coxiella burnetii in ticks cross-react with Coxiella-like bacteria. Infect Ecol Epidemiol. 2015;5:29230. doi: 10.3402/iee.v5.29230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bauer B, Wetzel H. A new membrane for feeding Glossina morsitans Westw (Diptera, Glossinidae) Bull Entomol Res. 1976;64:563–565. doi: 10.1017/S0007485300006246. [DOI] [Google Scholar]
- 32.Waladde SM, Young AS, Morzaria SP. Artificial feeding of ixodid ticks. Parasitol Today. 1996;12:272–278. doi: 10.1016/0169-4758(96)10027-2. [DOI] [PubMed] [Google Scholar]
- 33.Makert GR, Vorbrüggen S, Krautwald-Junghanns ME, Voss M, Sohn K, Buschmann T, et al. A method to identify protein antigens of Dermanyssus gallinae for the protection of birds from poultry mites. Parasitol Res. 2016;115:2705–2713. doi: 10.1007/s00436-016-5017-2. [DOI] [PubMed] [Google Scholar]
- 34.Fourie JJ, Stanneck D, Luus HG, Beugnet F, Wijnveld M, Jongejan F. Transmission of Ehrlichia canis by Rhipicephalus sanguineus ticks feeding on dogs and on artificial membranes. Vet Parasitol. 2013;197:595–603. doi: 10.1016/j.vetpar.2013.07.026. [DOI] [PubMed] [Google Scholar]
- 35.Oliver JD, Lynn GE, Burkhardt NY, Price LD, Nelson CM, Kurtti TJ, et al. Infection of immature Ixodes scapularis (Acari: Ixodidae) by membrane feeding. J Med Entomol. 2016;53:409–415. doi: 10.1093/jme/tjv241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kröber T, Guerin PM. In vitro feeding assays for hard ticks. Trends Parasitol. 2007;23:445–449. doi: 10.1016/j.pt.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 37.Krull C, Böhme B, Clausen PH, Nijhof AM. Optimization of an artificial tick feeding assay for Dermacentor reticulatus. Parasit Vectors. 2017;10:60. doi: 10.1186/s13071-017-2000-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Böhme B. In-vitro-Fütterung von Dermacentor reticulatus und Ixodes ricinus und Entwicklung eines teilautomatisierten Fütterungssystems für Schildzecken. Doctoral disseration. Freie Universität, Berlin; 2016.
- 39.Soares SF, Louly CC, Marion-Poll F, Ribeiro MF, Borges LM. Study on cheliceral sensilla of the brown dog tick Rhipicephalus sanguineus (Latreille, 1806) (Acari: Ixodidae) involved in taste perception of phagostimulants. Acta Trop. 2013;126:75–83. doi: 10.1016/j.actatropica.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 40.Voigt WP, Young AS, Mwaura SN, Nyaga SG, Njihia GM, Mwakima FN, et al. In vitro feeding of instars of the ixodid tick Amblyomma variegatum on skin membranes and its application to the transmission of Theileria mutans and Cowdria ruminatium. Parasitology. 1993;107:257–263. doi: 10.1017/S0031182000079233. [DOI] [PubMed] [Google Scholar]
- 41.Samuel JE, Hendrix LR. Laboratory maintenance of Coxiella burnetii. Curr Protoc Microbiol. 2009;Chapter 6:Unit 6C.1. doi: 10.1002/9780471729259.mc06c01s15. [DOI] [PubMed] [Google Scholar]
- 42.Brennan RE, Samuel JE. Evaluation of Coxiella burnetii antibiotic susceptibilities by real-time PCR assay. J Clin Microbiol. 2003;41:1869–1874. doi: 10.1128/JCM.41.5.1869-1874.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klee SR, Tyczka J, Ellerbrok H, Franz T, Linke S, Baljer G, et al. Highly sensitive real-time PCR for specific detection and quantification of Coxiella burnetii. BMC Microbiol. 2006;6:2. doi: 10.1186/1471-2180-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dresler J, Klimentova J, Pajer P, Salovska B, Fucikova AM, Chmel M, et al. Quantitative proteome profiling of Coxiella burnetii reveals major metabolic and stress differences under axenic and cell culture cultivation. Front Microbiol. 2019;10:2022. doi: 10.3389/fmicb.2019.02022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Honig Mondekova H, Sima R, Urbanova V, Kovar V, Rego ROM, Grubhoffer L, et al. Characterization of Ixodes ricinus fibrinogen-related proteins (Ixoderins) discloses their function in the tick innate immunity. Front Cell Infect Microbiol. 2017;7:509. doi: 10.3389/fcimb.2017.00509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Omsland A, Cockrell DC, Howe D, Fischer ER, Virtaneva K, Sturdevant DE, et al. Host cell-free growth of the Q fever bacterium Coxiella burnetii. Proc Natl Acad Sci USA. 2009;106:4430–4434. doi: 10.1073/pnas.0812074106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Duron O, Sidi-Boumedine K, Rousset E, Moutailler S, Jourdain E. The importance of ticks in Q fever transmission: what has (and has not) been demonstrated? Trends Parasitol. 2015;31:536–552. doi: 10.1016/j.pt.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 48.Panning M, Kilwinski J, Greiner-Fischer S, Peters M, Kramme S, Frangoulidis D, et al. High throughput detection of Coxiella burnetii by real-time PCR with internal control system and automated DNA preparation. BMC Microbiol. 2008;8:77. doi: 10.1186/1471-2180-8-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schliesser T. Epidemiology and importance of Q fever in German livestock. Wien Tieraerztl Monat. 1991;78:7–12. [Google Scholar]
- 50.Siroký P, Kubelová M, Modrý D, Erhart J, Literák I, Spitalská E, et al. Tortoise tick Hyalomma aegyptium as long term carrier of Q fever agent Coxiella burnetii-evidence from experimental infection. Parasitol Res. 2010;107:1515–1520. doi: 10.1007/s00436-010-2037-1. [DOI] [PubMed] [Google Scholar]
- 51.Tyler KM, Engman DM. The life cycle of Trypanosoma cruzi revisited. Int J Parasitol. 2001;31:472–481. doi: 10.1016/S0020-7519(01)00153-9. [DOI] [PubMed] [Google Scholar]
- 52.Bechah Y, Capo C, Mege JL, Raoult D. Epidemic typhus. Lancet Infect Dis. 2008;8:417–426. doi: 10.1016/S1473-3099(08)70150-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article.