Abstract
Glycosyltransferases are key enzymes involved in the assembly of repeating units of exopolysaccharides (EPS). A glycosyltransferase generally consists of the N-terminal and the C-terminal domain, however, the functional role of these domains in EPS biosynthesis remains largely unknown. In this study, homologous overexpression was employed to investigate the effects of EpsFN, a truncated form of rhamnosyltransferase EpsF with only the N-terminal domain, on EPS biosynthesis in Streptococcus thermophilus 05-34. Reverse transcription qPCR and Western blotting analysis confirmed the successful expression of epsFN in 05-34 at the transcription and translation level, respectively. Further analysis showed that the monosaccharide composition and yield of EPS were not affected by the overexpression of epsFN, whereas the molecular mass decreased by 5-fold. Accordingly, the transcription levels of genes involved in EPS biosynthesis, including chain-length determination gene epsC, were down-regulated by 5- to 6-fold. These results indicated that the N-terminal domain of EpsF alone could influence the molecular mass of EPS, probably via lowering the concentration of sugar precursors, which may lead to decreased expression of genes responsible for chain-length determination.
Keywords: Streptococcus thermophilus, Chain length, N-terminal domain of rhamnosyltransferase, Exopolysaccharides
Introduction
Microbial exopolysaccharides (EPS) are a wide group of secreted polymers that can be assembled as capsular polysaccharides (CPS) tightly associated with cell surface, or released as extracellular slime in the surrounding of the cell (Kleerebezem et al., 1999; Nwodo, Green & Okoh, 2012). Among microbial EPS, those produced by lactic acid bacteria (LAB) have received increasing attention for conferring textural and rheological properties to fermented products (Lebeer et al., 2009; Caggianiello, Kleerebezem & Spano, 2016). Many of the EPS produced by LAB strains are heteropolysaccharides, which are built up from repeating units consisting of two or more types of monosaccharides, such as galactose, glucose and rhamnose (Remus et al., 2012; Caggianiello, Kleerebezem & Spano, 2016). Heteropolysaccharides are commonly synthesized by the Wzx/Wzy-dependent pathway reported for EPS and O-antigen biosynthesis in Gram-negative bacteria (Whitfield, 1995). In this pathway, first, a priming glycosyltransferase catalyzes the transfer of a sugar-1-phosphate from a nucleotide diphospho-sugar to the undecaprenyl (C55) phosphate lipid moiety. Subsequently, a series of glycosyltransferases catalyze the addition of other sugar residues until the repeat unit is completed. Then, the lipid-linked repeat unit is translocated across the membrane by the flippase Wzx, and subsequently polymerized by the polymerase Wzy (Islam & Lam, 2014). In Gram-positive bacteria, a membrane protein complex Wzd/Wze (designated EpsC/EpsD in LAB) is postulated to be responsible for chain length determination and secretion of the mature EPS (Bentley et al., 2006; Lebeer et al., 2009).
Glycosyltransferases play important roles in the biosynthesis of repeating units of EPS via catalyzing the formation of glycosidic bonds between sugar residues. Donor sugars for these reactions are usually activated in the form of nucleoside diphosphate sugars, such as UDP-Glucose, UDP-Galactose and dTDP-Rhamnose. Transfer of the sugar residue results in either inversion or retention of the anomeric stereochemistry of the donor sugar, as such, glycosyltransferases are classified as inverting or retaining glycosyltransferases, respectively (Lairson et al., 2008). Also, glycosyltransferases are currently classified into 107 families in the CAZy database (www.cazy.org) based on amino acid sequence similarities (Campbell et al., 1997; Coutinho et al., 2003). Moreover, two structural folds, GT-A and GT-B, have been identified for the nucleotide sugar-dependent glycosyltransferases (Breton et al., 2006; Lairson et al., 2008). Both the GT-A and GT-B folds contain two Rossmann-like domains (β/α/β), which are proposed to be involved in substrate binding (Lesk, 1995). Several structural studies of glycosyltransferases showed that domains responsible for donor binding are located on the N-terminal of GT-A glycosyltransferases and the C-terminal of GT-B type, respectively (Martinez-Fleites et al., 2006; Steiner et al., 2010). Additionally, positively charged residues and α-helixes on the N-terminal region of several GT-B glycosyltransferases can also participate in stabilizing the phosphate group of donor sugars (Albesa-Jové et al., 2014). Upon binding of the donor, glycosyltransferases may undergo a conformational change, facilitating the binding of acceptor molecules and the subsequent sugar transfer (Qasba, Ramakrishnan & Boeggeman, 2005).
Construction of N- or C-terminal truncated forms of enzymes has been employed to investigate the structure–function relationships of glucosyltransferase and fucosyltransferase in homopolysaccharides biosynthesis (Bastida, Fernández-Mayoralas & García-Junceda, 2002; Del Moral et al., 2008). It has been revealed that deletion of the C-terminal 65 amino acids of a α-1,6-fucosyltransferase from Rhizobium sp. resulted in its increased affinity to GDP-Fucose donor (Bastida, Fernández-Mayoralas & García-Junceda, 2002). In addition, a α-1,2-fucosyltransferase lacking its N-terminal domain was reported to lose glycosyltransferase activity in Helicobacter pylori (Wang et al., 1999). More recently, it has been demonstrated that truncation of domain V of the glucosyltransferase GTF180 resulted in increased yield, yet decreased molecular-mass of oligosaccharides produced by Lactobacillus reuteri (Meng et al., 2015). These studies suggest possible roles of specific domains of glycosyltransferases in determining enzyme activity, substrate specificity, and properties of EPS produced.
The in situ production of EPS by ropy Streptococcus thermophilus strains has been strongly associated with the improved viscosity, water retention and the mouthfeel of yogurts (Broadbent et al., 2003; Purwandari, Shah & Vasiljevic, 2007). Our previous studies have shown that yogurt fermented with an EPS producing strain S. thermophilus 05-34 exhibited enhanced physical and sensory properties (Qin et al., 2011). Recently, a typical region encoding genes involved in EPS biosynthesis has been detected in the draft genome sequence of S. thermophilus 05-34 (accession number: QFLC00000000). Furthermore, a gene epsFN (DIS31_02545), encoding the N-terminal region of a rhamnosyltransferase EpsF, was found within this eps cluster. Similarly, the truncated version of EpsF has also been found in several other S. thermophilus strains (Li et al., 2018; Wu et al., 2015). In this study, the effects of this N-terminal region of rhamnosyltransferase on the biosynthesis of EPS in 05-34 were investigated.
Materials and Methods
Bacterial strains, plasmids and growth conditions
The bacterial strains and plasmids used in this study are listed in Table 1. S. thermophilus was routinely maintained in de Man–Rogosa–Sharpe (MRS) broth at 37 °C. For EPS isolation, S. thermophilus was cultured in 10% (w/v) sterilized reconstituted skim milk (RSM). Lactococcus lactis NZ9000, used as a host in cloning experiments, was cultured at 30 °C in M17 broth supplemented with 0.5% (w/v) D-glucose (GM17 medium). When required, chloramphenicol (Cm) was added at 5 μg/mL for both L. lactis and S. thermophilus.
Table 1. Bacterial strains and plasmids used in this study.
| Strain or plasmid | Relevant characteristics | Source of reference |
|---|---|---|
| Strains | ||
| S. thermophilus 05-34 | EPS-producing strain isolated from Tibetan kefir grains | CGMCC16303 Qin et al. (2011) |
| S. thermophilus 05CK | S. thermophilus 05-34 carrying pSlpA-8148 | This study |
| S. thermophilus 05epsF | S. thermophilus 05-34 carrying pSlpA-epsF | This study |
| S. thermophilus 05Fh6 | S. thermophilus 05-34 carrying pSlpA-epsFh6 | This study |
| L. lactis NZ9000 | Plasmid-free derivative of L. lactis MG1363 pepN::nisRK | Kuipers et al. (1998) |
| Plasmids | ||
| pNZ8148 | Cmr, inducible expression vector carrying the nisA promoter PnisA | Mierau & Kleerebezem (2005) |
| pSlpA-8148 | Cmr, pNZ8148 derivative carrying constitutive promoter PslpA instead of PnisA | This study |
| pSlpA-epsF | Cmr, pSlpA-8148 derivative carrying the epsFN gene | This study |
| pSlpA-epsFh6 | Cmr, pSlpA-8148 derivative carrying the epsFN gene with a 3′-sequence encoding a C-terminal His-tag | This study |
Construction of EpsFN overexpression strain and Western blot analysis
In order to realize constitutive expression, a 318-bp DNA fragment was synthesized as Fig. S1, which consists of the ribosomal-binding site (RBS) and the constitutive promoter PslpA from the gene encoding the S-layer surface protein of Lactobacillus acidophilus (Boot et al., 1996). This DNA fragment was synthesized by Sangon (Beijing, China) and then inserted into the vector pNZ8148 between BglII and NcoI, resulting in the expression vector pSlpA-8148. Subsequently, the epsFN gene (DIS31_02545) was amplified by PCR from the genomic DNA of S. thermophilus 05-34 with primers F-epsF (5′-CATGCCATGGGAACAAAAACAGTTTATATCG-3′) and R-epsF (5′-CGCGAGCTCCTATGAGCTTGTAGGACTATTC-3′). Restriction sites used for subsequent cloning are underlined: NcoI and SacI for F-epsF and R-epsF, respectively. The amplicon obtained was digested by NcoI and SacI, and then inserted into pSlpA-8148. The ligation mixture was introduced into L. lactis NZ9000 by electroporation (Holo & Nes, 1989), and transformants were selected on GM17 agar supplemented with Cm. Plasmids were isolated from L. lactis transformants using QIAGEN Miniprep Spin Kit (Qiagen Inc, Germany), and further verified by restriction analysis and sequencing using the primer R-8148 (5′-CAATCAAAGCAACACGTG-3′). The resulting recombinant plasmid pSlpA-espF was transformed into S. thermophilus 05-34 by electroporation in a 0.2 cm cuvette at 1.5 kV, 25 μF and 200 Ω (Marciset & Mollet, 1994), and the recombinant strain was designated as S. thermophilus 05epsF.
To confirm the overexpression of EpsFN in S. thermophilus 05-34, the epsFN gene was also amplified by PCR using primers F-epsF and R-epsFh6 (5′-CGCGAGCTCTAATGATGATGATGATGATGTGAGCTTGTAGGACTATTC-3′), thereby introducing a 6× His tag coding sequence to the 3′-end of epsFN for Western blot analysis. This amplicon was cloned into pSlpA-8148 as described above, and then transformed into S. thermophilus 05-34, yielding the recombinant strain S. thermophilus 05Fh6. Meanwhile, the plasmid pSlpA-8148 was also introduced into S. thermophilus 05-34, resulting in the control strain S. thermophilus 05CK. Overnight cultures of S. thermophilus 05CK and S. thermophilus 05Fh6 were inoculated into 20 mL of fresh MRS medium containing 5 μg/mL of Cm. Intracellular proteins were extracted as previously described (Wang et al., 2015). Aliquots of 50 μg proteins from S. thermophilus 05CK and S. thermophilus 05Fh6 were separated by a 15.5% Tricine-SDS-PAGE gel, and then transferred to a nitrocellulose membrane using Bio-Rad Mini Trans-Blot® system. The membrane was blocked with TBS + 5% (w/v) skim milk overnight at 4 °C, and then incubated with a 1:5,000 dilution of anti His-Tag mouse monoclonal antibody (CWBiotech, Beijing, China) followed by a 1:5,000 dilution of HRP-conjugated goat Anti-Mouse IgG (CWBiotech, Beijing, China). Protein bands were visualized using a cECL Western Blot Kit (CWBiotech, Beijing, China) according to the manufacturer’s instructions.
RNA isolation and reverse transcription qPCR (RT-qPCR)
S. thermophilus 05epsF and S. thermophilus 05CK were grown in 10% RSM medium (initial pH 7.0) at 37 °C for 30 h. Each 5 mL of fermentation culture was mixed with 5 mL 2% (w/v) sodium citrate, 10 mg Pronase E and 20 μl β-mercaptoethanol, and incubated in water bath at 50 °C for 3 h. The mixture was centrifuged at 6,000g for 5 min at 4 °C and the supernatant was discarded. The total RNA was isolated using RNAprep pure cell/bacteria kit following the manufacture’s instruction (Tiangen, Beijing, China). Subsequently, reverse transcription was carried out with PrimeScript II 1st strand cDNA synthesis Kit (Takara, Beijing, China), with 750 ng of total RNA as the template. Specific primers were designed using PRIMER V5 software to amplify the 100- to 150-bp regions of interest genes including epsA, epsC, eps2C and epsG (Table S1), and their specificity was checked before quantitative analyses. Semi-quantitative PCR was performed using a 1:20 dilution of cDNA as template, and the PCR products were visualized on 2% agarose gels stained with ethidium bromide. Real-Time PCR (qPCR) was performed using SYBR Green assay kit (Tiangen, Beijing, China) in a Roche LightCycler® 96 Real-Time PCR System (Roche Applied Science, Rotkreuz, Switzerland). Gene expressions were calculated by the 2ΔΔCT method (Schmittgen & Livak, 2008) by using the 16S rRNA as the reference gene.
Isolation and purification of EPS
S. thermophilus 05epsF and S. thermophilus 05CK were propagated in MRS broth at 37 °C for 24 h, followed by two more precultures inoculated in MRS broth. For EPS isolation, cell cultures were inoculated (2%, v/v) into 10% RSM broth (initial pH 7.0) and grown for 30 h at 37 °C. The extraction of EPS was performed as previously described with some modifications (Cerning et al., 1994). Briefly, cultures were heated at 100 °C for 15 min and centrifuged at 6,000g for 15 min to remove the cells. In order to precipitate proteins, trichloroacetic acid (TCA) was added in the supernatant to a final concentration of 4% (v/v), stored at 4 °C for 2 h, and then centrifuged at 6,000g for 15 min. Crude EPS was precipitated by adding cold ethanol to the supernatant at a ratio of 3:1 (v/v), mixed thoroughly and kept for 24 h at 4 °C. The pellet containing EPS was obtained by centrifugation, dialyzed and freeze-dried. The crude EPS was purified as previously described (Li et al., 2016). The yield of EPS was quantified using the phenol-sulfuric method using glucose as a standard (DuBois et al., 1956). Concentration of protein and nucleic acid of purified fraction was determined spectroscopically at 280 and 260 nm, respectively.
Molecular mass and monosaccharide composition of EPS
In order to determine the average molecular mass of the EPS sample, an Agilent1100 series HPLC system (Agilent, Santa Clara, CA, USA) was conducted with an RI detector. EPS samples were eluted with deionized water at a flow rate of 0.8 mL/min. Based on the linear regression equation drawn through dextran standards (Sigma–Aldrich, St. Louis, MO, USA), the molecular mass of EPS was analyzed through GPC data processing software. The monosaccharide composition was determined by gas chromatography coupled with mass spectrograph (GC-MS). Some pretreatments were performed as previously described (Li et al., 2016). The treated samples were used for GC-MS with the conditions as follows: initial column temperature was set at 140 °C with a rate of 1.5 °C/min to reach 200 °C and with a rate of 10 °C/min to 250 °C, then the highest temperature was held for 5 min, and samples were injected into the column with N2 as the carrier gas at a flow rate of one mL/min. The monosaccharide composition of EPS was determined by comparison with the retention time of monosaccharide standards.
Statistical analysis
All the experiments were independently conducted in triplicates. Results were presented as the mean value ± standard error. When two groups were compared, an unpaired student t test with Welch’s correction was used to calculate the p values.
Results
Genetic organization of the eps cluster in S. thermophilus 05-34
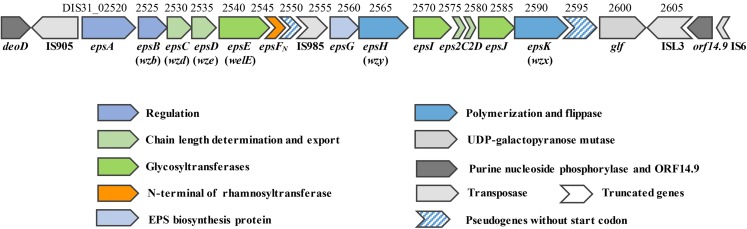
The draft genome of 05-34 has been sequenced by our team recently (accession number: QFLC00000000). A 16.3 kb region encoding enzymes required for EPS biosynthesis was identified on the chromosome (Fig. 1). Based on amino acid sequence and protein structural similarity, putative functions were assigned to 18 genes (DIS31_02520~DIS31_02605) within this region (Table S2). Among them, four genes (DIS31_02520~DIS31_02535) at the 5′-end are highly conserved in S. thermophilus species, and were assigned for regulation (epsAB) and chain-length control (epsCD) functions in EPS biosynthesis. Two truncated genes eps2C and eps2D (DIS31_02575 and DIS31_02580) encode proteins that show 94% identity to the C-terminus of Lp_1197 and N-terminus of Lp_1198, respectively, which were annotated as EPS chain-length controlling proteins in Lactobacillus plantarum WCFS1. Genes epsH (DIS31_02565) and epsK (DIS31_02590) encode polymerase Wzy and flippase Wzx of the typical Wzx/Wzy-dependent pathway for EPS biosynthesis, respectively (Table S2). In addition, a pseudogene DIS31_02595 was also assigned as flippase, however, no translational start codon was detected at its 5′-end. Thus, DIS31_02595 was considered to be an inactivated gene. The central region of the eps cluster encodes an EPS biosynthesis protein EpsG (DIS31_02560) with unknown function, and several glycosyltransferases. Among them, EpsE (DIS31_02540) was 100% identical to the priming glycosyltransferase of S. thermophilus NCFB 2393, which was shown to transfer glucosyl 1-phosphate to the undecaprenyl phosphate (Almirón-Roig et al., 2000). In addition, proteins EpsI (DIS31_02570) and EpsJ (DIS31_02585) showed 100% identity to β-1,3-glucosyltransferase (QBR99840.1) and 98% identity to GT-2 family glycosyltransferase (QBR99843.1) from S. thermophilus, respectively. Notably, the protein encoded by gene epsFN (DIS31_02545) was composed of only 87 amino acids, which displayed 99% identity to the N-terminal section of rhamnosyltransferase EpsF with 390 amino acids in S. thermophilus EU20 and S. thermophilus NCFB2393. Homologous overexpression of epsFN was carried out to explore its role in EPS biosynthesis in 05-34.
Figure 1. Genetic organization of the eps cluster and flanking regions in S. thermophilus 05-34.
Gene_locus tags and gene names are marked above and below the genes, respectively. Gene names in brackets are adopted from the bacterial polysaccharide gene nomenclature (BPGN) system (Reeves et al., 1996).
EpsFN was successfully overexpressed in S. thermophilus 05-34
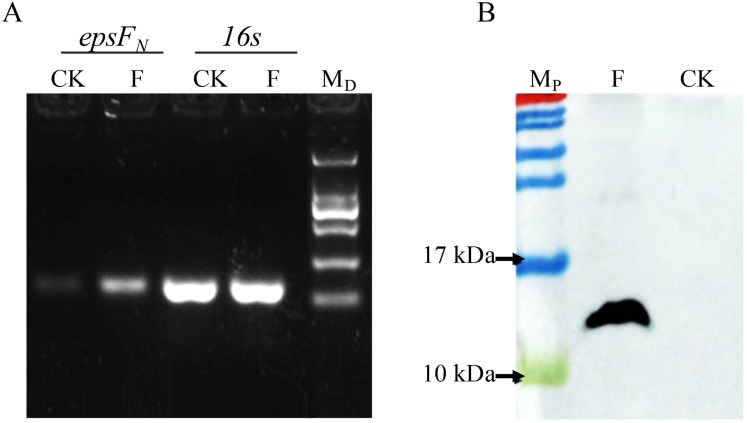
The epsFN gene was amplified by PCR and cloned into plasmid pSlpA-8148. DNA sequencing verified that the length of the amplicon was 264 bp, which showed 100% identity with the epsFN gene of S. thermophilus 05-34. Semi-quantitative PCR revealed the elevated transcription level of epsFN under the control of promoter pSlpA in strain S. thermophilus 05epsF compared to the control strain S. thermophilus 05CK (Fig. 2A). Then RT-qPCR assay showed that the mRNA level of epsFN was 370-fold higher in strain S. thermophilus 05epsF than that in S. thermophilus 05CK. Western blotting assay further revealed the production of a 12-kDa protein in S. thermophilus 05Fh6, which corresponded to the expected size of EpsFN-His6, while no corresponding bands were observed in the control strain S. thermophilus 05CK (Fig. 2B). These results indicated that the gene epsFN was successfully over-expressed in S. thermophilus 05-34.
Figure 2. Detection of the over-expression of epsFN in S. thermophilus by Semi-quantitative PCR (A) and Western blot (B).
(A) Agarose electrophoresis of PCR products of epsFN and 16s rRNA, CK: S. thermophilus 05CK; F: S. thermophilus 05epsF; MD: DNA maker DL2000 (Tiangen). (B) Western blot analysis, CK: S. thermophius 05CK; F: S. thermophilus 05Fh6; MP: PageRuler Prestained Protein Ladder (Thermo Scientic, Waltham, MA, USA).
The overexpression of EpsFN did not affect the yield of EPS in S. thermophilus
S. thermophilus 05epsF was cultivated in 10% RSM broth (initial pH 7.0) at 37 °C for 30 h, and then EPS from RSM samples was isolated and purified. According to the elution profile of EPS from S. thermophilus 05epsF, there was only one single and relatively symmetrical peak at OD490 in fraction No. 11, while the peak of the elution profile in the control group appeared in fraction No. 10. These results suggested that overexpression of epsFN in S. thermophilus 05-34 decreased the average molecular mass of EPS. The purified EPS fraction showed no absorption at 260 nm or 280 nm by ultraviolet detection, suggesting that there was no nucleic acids or protein contamination in EPS samples. Based on the glucose standard, the yield of EPS produced by S. thermophilus 05epsF was 124 ± 4 mg/L, showing no significant difference with that of the control group (131 ± 2 mg/L, p = 0.18).
S. thermophilus 05epsF produced EPS with unchanged composition but reduced molecular mass
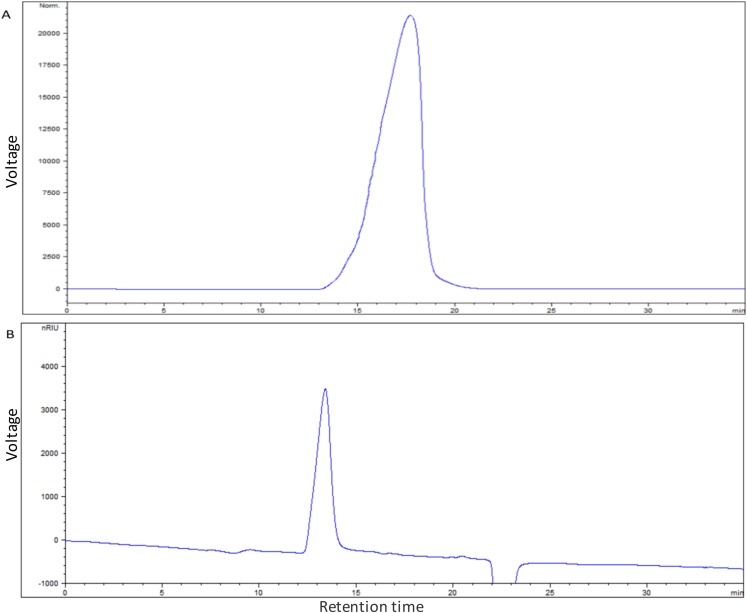
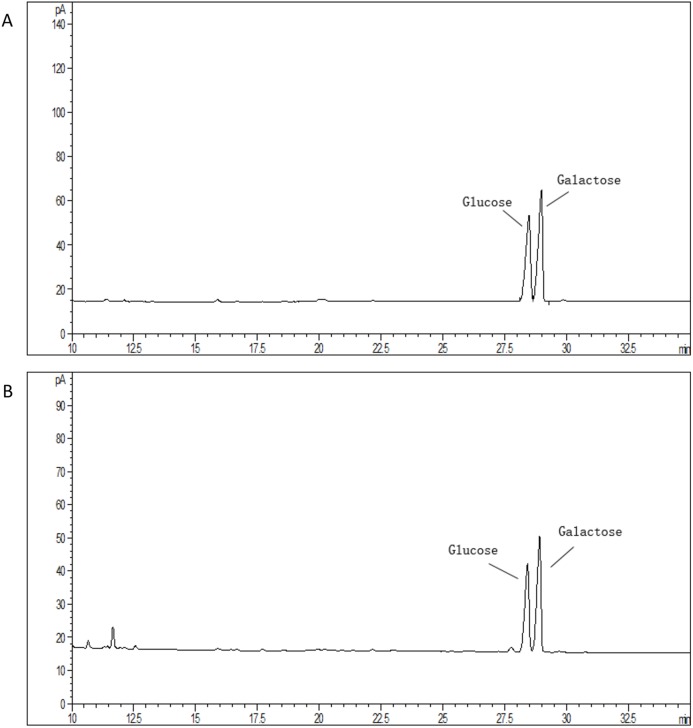
The average molecular mass of EPS produced by S. thermophilus 05epsF was determined using GPC software to be 8.8 × 104 Da (Fig. 3A), while the molecular mass of EPS produced by S. thermophilus 05CK was 4.6 × 105 Da (Fig. 3B). Based on the retention time of different monosaccharide standards, the monomer analysis by GC-MS indicated that the EPS produced by S. thermophilus 05epsF and S. thermophilus 05CK were both composed of galactose and glucose in an approximate ratio of 1.0:0.8 with a trace amount of mannose, which was probably derived from cell wall polysaccharides or MRS medium composition (Figs. 4A and 4B). Characteristics of EPS produced by S. thermophilus 05CK and 05epsF were summarized in Table 2.
Figure 3. Molecular mass of EPS produced by S. thermophilus 05epsF (A) and S. thermophilus 05CK (B).
According to the linear regression equation drawn through dextran standards, the molecular mass of EPS samples was determined based on their retention time (X-axis) using gel permeation chromatography (GPC).
Figure 4. Monosaccharide composition profile of hydrolyzed EPS from S. thermophilus 05epsF (A) and S. thermophilus 05CK (B).
Table 2. EPS characteristics produced by S. thermophilus 05CK and 05epsF.
| S. thermophilus 05CK | S. thermophilus 05epsF | |
|---|---|---|
| Yield (mg/L) | 131 ± 2 | 124 ± 4 |
| Monosaccharide composition | galactose and glucose | galactose and glucose |
| Molar ratio (Gal/Glc) | 1.25 | 1.25 |
| Molecular mass (Da) | 4.6 × 105 | 8.8 × 104 |
| Elution time (fraction No.) | 10 | 11 |
The overexpression of EpsFN caused a down-regulation of eps genes in S. thermophilus
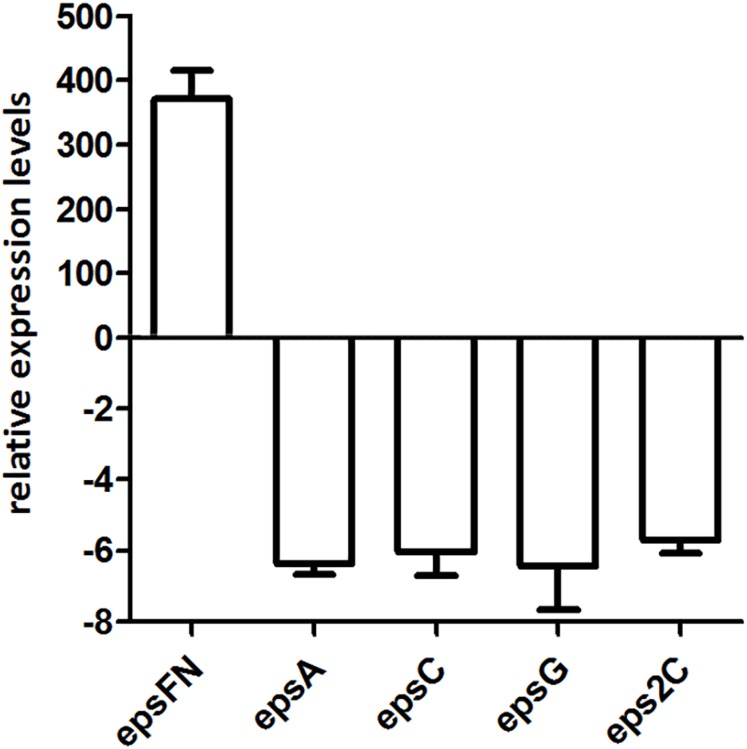
Since the molecular mass of EPS produced by S. thermophilus 05epsF decreased 5-fold than that of the control strain, we analyzed the transcription level of chain-length determining genes epsC and eps2C, regulatory gene epsA, and gene epsG encoding the EPS biosynthesis protein. RT-qPCR results revealed that the transcription levels of these genes in strain S. thermophilus 05epsF were 5- to 6-fold lower than that of the control strain (Fig. 5), indicating that the over-expression of EpsFN led to a down-regulation of genes within the eps cluster in S. thermophilus 05-34. Therefore, the reduced molecular mass of EPS may result from the decreased level of these genes, especially the down-regulation of epsC in S. thermophilus 05epsF.
Figure 5. RT-qPCR analysis of the expression levels of epsFN, epsA, epsC, epsG and eps2C in S. thermophilus 05epsF.
The fold changes calculated are relative to the transcript levels in S. thermophilus 05epsF compared to those in S. thermophilus 05CK. Error bars represent the standard errors of the results obtained by three independent experiments (p < 0.05).
Discussion
Nucleotide sugar-dependent glycosyltransferases play important roles in the assembly of repeating units of EPS in bacteria. In this study, the functional role the 87-amino acid fragment of rhamnosyltransferase EpsF in EPS biosynthesis was investigated. Notably, the overexpression of this N-terminal region (EpsFN) did not result in the incorporation of rhamnose residues into the repeating units of EPS produced by 05-34, despite this strain harbors the four enzymes RmlABCD (gene locus DIS31_09015 ~ 09025, DIS31_00470) required for the biosynthesis of dTDP-Rhamnose, the substrate of EpsF (Zeidan et al., 2017). Thus, it is proposed that EpsFN lost the catalytic function as a rhamnosyltransferase due to rearrangements leading to loss of C-terminal domain.
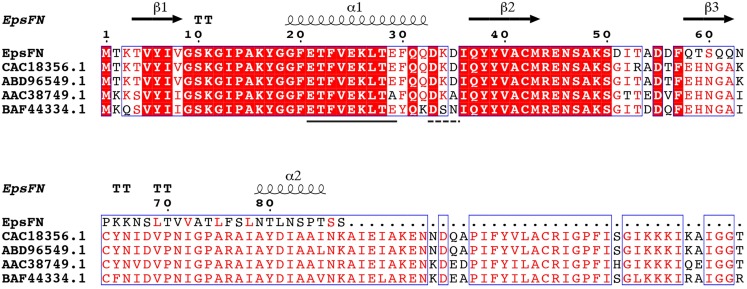
Protein sequence analysis of the full length version EpsF (GenBank Accession No: ABD96549.1, EpsF from S. thermophilus EU20) showed that it belongs to the glycosyltransferase family 4 (GT-4) and displays a GT-B type fold. Secondary structure analysis showed that EpsFN contains two α-helices and three β-strands, which form the conserved N-terminal segment of Rossmann folds that responsible for nucleotides binding (Rao & Rossmann, 1973). Further sequence alignment analysis of EpsFN with homologous rhamnosyltransferases from other Streptococcus species revealed the presence of an EX7E motif (residues 21–29) and a DxD motif (residues 33–35) (Fig. 6), which were proposed to be involved in the stabilizing of donor substrates and the interaction with phosphate groups of nucleotides, respectively (Coutinho et al., 2003; Breton et al., 2006). Taken together, it is speculated that EpsFN may still have the ability to interact with nucleotide sugar molecules, and thus to affect the EPS biosynthesis in 05-34.
Figure 6. Secondary structure prediction and sequence alignment of EpsFN from S. thermophilus 05-34 with homologous rhamnosyltransferases from Streptococcus spp.
Sequence alignment was performed with EpsF homologs from S. thermophilus NCFB2393 (CAC18356.1), S. thermophilus EU20 (ABD96549.1), Streptococcus pneumoniae (AAC38749.1) and Streptococcus oralis 10557 (BAF44334.1) using I-TASSER (Zhang, 2009) and ESPript softwares (Robert & Gouet, 2014). Secondary structure elements of EpsFN are shown above the protein sequence. Conserved residues are shown in a red background. The conserved EX7E and DxD motifs are indicated by solid and dashed lines, respectively.
In the EpsFN overexpression strain, the molecular mass of EPS decreased from 4.6 × 105 Da to 8.8 × 104 Da, and the transcription level of genes within the eps cluster including epsC, eps2C, epsA and epsG also showed a remarkable down-regulation. A previous study by our team revealed that when the molecular mass of EPS increased from 2.5 × 104 Da to 4.7 × 105 Da in 05-34, the corresponding transcription level of epsC showed a 2.7-fold up-regulation (Li et al., 2016). Protein structural homologs analysis revealed a high similarity (99.9%; E-value = 3.90E−25) of EpsC to the chain-length regulation protein WzzE of Escherichia coli (Table S2). The impact of the expression level of WzzE homologs on lipopolysaccharide O-antigen chain length distribution has been reported in Shigella (Carter et al., 2007) and Pseudomonas (Huszczynski et al., 2019). In addition, Bender, Cartee & Yother (2003) showed that inactivation of cps2C or cps2D led to the production of only short-chain polymers in S. pneumoniae. Therefore, it is assumed that there is a correlation between the transcription level of chain-length determining genes and the molecular mass of EPS in S. thermophilus 05-34. Furthermore, the concentration of nucleotide sugars has also been shown to modulate the chain length of CPS in S. pneumoniae (Forsee, Cartee & Yother, 2009). Sequence alignment revealed that EpsFN has conserved motifs for nucleotide binding, suggesting an ability to interact with sugar precursors. Thus, we assume that lacking of the major parts of rhamnosyltransferase might lead to nonspecific interactions of EpsFN with sugar precursors, and further a decrease in available precursor concentrations. It is proposed that the synthesis of repeat units is coordinated with the polymerization process during EPS biosynthesis (Bouazzaoui & LaPointe, 2006). The reduced cellular level of sugar precursors may lead to a decrease in the amount of repeat units available for polymerization, resulting in the release of premature polysaccharides with shorter chain length in S. thermophilus 05epsF.
Also, it is possible that the decrease in molecular mass was caused by disturbed interaction between EpsFN and other proteins involved in EPS assembly. In the model of Wzx/Wzy-dependent pathway, EpsC (Wzz), Wzx, Wzy, the priming glycosyltransferase, and CpsA in the case of CPS biosynthesis, were proposed to form the assembly machinery (Grangeasse, 2016). The overexpressed EpsFN might impact the stoichiometry or stability of the assembly machinery, in some way, leading to the premature termination of shorter-length EPS in S. thermophilus 05epsF.
Notably, the overexpression of EpsFN did not change the yield of EPS produced by S. thermophilus 05-34. The production of EPS in thermophilic LAB strains is reported to be growth-associated, since the energy used for EPS biosynthesis is provided by the central carbon metabolism of the producing cell (De Vuyst & Degeest, 1999). Although EpsFN caused a decrease in EPS chain length, the EPS biosynthetic capacity could remain the same in S. thermophilus 05epsF, since its growth was not affected by EpsFN. In this situation, once the shorter-chain length polysaccharide was released, the assembly of a new chain would begin immediately. Thus, we speculated that S. thermophilus 05epsF may produce increased number of shorter-chain length EPS, resulting in a total yield similar to that of the control strain.
Conclusions
The current study describes the effect of EpsFN, an 87-amino acid N-terminal domain of EpsF, on EPS biosynthesis. Our results showed that overexpression of this N-terminal domain did not change the yield or monosaccharide composition of EPS produced by S. thermophilus 05-34, whereas the molecular mass decreased from 4.6 × 105 Da to 8.8 × 104 Da. Accordingly, the transcription level of genes within the eps cluster showed a remarkable down-regulation in the EpsFN overexpression strain, suggesting that EpsFN has an impact on the transcription level of eps cluster, potentially via nonspecific interactions with sugar precursors and lowering their intracellular concentration.
Supplemental Information
The transcriptional start site (+1), −35 and −10 regions are indicated in bold type. The Ribosomal-binding site (RBS) is indicated in box. Restriction sites are underlined.
A1–A3: Agarose electrophoresis of PCR products of epsFN and 16s rRNA from three independent experiments. M: DNA maker DL2000; 1, 2: PCR products of epsFN using the genome of S. thermophilus05CK and 05epsF as template, respectively; 3, 4: PCR products of 16s rRNA using the genome of S. thermophilus05CK and 05epsF as template, respectively. B1–B3: Western blot analysis of three independent experiments CK:S. thermophius05CK; F: S. thermophilus 05Fh6; MP: PageRuler Prestained Protein Ladder (Thermo Scientic, Waltham, MA, USA).
Acknowledgments
We thank Professor Willem M. de Vos (Wageningen University) for the gift of plasmid pNZ8148.
Funding Statement
This work was supported by the National Natural Science Foundation of China (No. 21476250). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
Guohong Wang conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Jiaxi Li performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Shuxin Xie performed the experiments, prepared figures and/or tables, and approved the final draft.
Zhengyuan Zhai analyzed the data, prepared figures and/or tables, and approved the final draft.
Yanling Hao conceived and designed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Data Availability
The following information was supplied regarding data availability:
The draft genome sequence of Streptococcus thermophilus 05-34 is available at GenBank: QFLC00000000.
References
- Albesa-Jové et al. (2014).Albesa-Jové D, Giganti D, Jackson M, Alzari PM, Guerin ME. Structure–function relationships of membrane-associated GT-B glycosyltransferases. Glycobiology. 2014;24(2):108–124. doi: 10.1093/glycob/cwt101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almirón-Roig et al. (2000).Almirón-Roig E, Gasson MJ, Mulholland F, Griffin AM. The complete cps gene cluster from Streptococcus thermophilus NCFB, 2393 involved in the biosynthesis of a new exopolysaccharide. Microbiology. 2000;146(11):2793–2802. doi: 10.1099/00221287-146-11-2793. [DOI] [PubMed] [Google Scholar]
- Bastida, Fernández-Mayoralas & García-Junceda (2002).Bastida A, Fernández-Mayoralas A, García-Junceda E. C-Terminal truncation of α 1,6-fucosyltransferase from Rhizobium sp. does not annul the transferase activity of the enzyme. Bioorganic & Medicinal Chemistry. 2002;10(3):737–742. doi: 10.1016/S0968-0896(01)00327-3. [DOI] [PubMed] [Google Scholar]
- Bender, Cartee & Yother (2003).Bender MH, Cartee RT, Yother J. Positive correlation between tyrosine phosphorylation of CpsD and capsular polysaccharide production in Streptococcus pneumoniae. Journal of Bacteriology. 2003;185(20):6057–6066. doi: 10.1128/JB.185.20.6057-6066.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley et al. (2006).Bentley SD, Aanensen DM, Mavroidi A, Saunders D, Rabbinowitsch E, Collins M, Donohoe K, Harris D, Murphy L, Quail MA, Samuel G, Skovsted IC, Kaltoft MS, Barrell B, Reeves PR, Parkhill J, Spratt BG. Genetic analysis of the capsular biosynthetic locus from all 90 Pneumococcal serotypes. PLOS Genetics. 2006;2(3):e31. doi: 10.1371/journal.pgen.0020031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boot et al. (1996).Boot HJ, Kolen CP, Andreadaki FJ, Leer RJ, Pouwels PH. The Lactobacillus acidophilus S-layer protein gene expression site comprises two consensus promoter sequences, one of which directs transcription of stable mRNA. Journal of Bacteriology. 1996;178(18):5388–5394. doi: 10.1128/JB.178.18.5388-5394.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouazzaoui & LaPointe (2006).Bouazzaoui K, LaPointe G. Use of antisense RNA to modulate glycosyltransferase gene expression and exopolysaccharide molecular mass in Lactobacillus rhamnosus. Journal of Microbiological Methods. 2006;65(2):216–225. doi: 10.1016/j.mimet.2005.07.011. [DOI] [PubMed] [Google Scholar]
- Breton et al. (2006).Breton C, Šnajdrová L, Jeanneau C, Koča J, Imberty A. Structures and mechanisms of glycosyltransferases. Glycobiology. 2006;16(2):29R–37R. doi: 10.1093/glycob/cwj016. [DOI] [PubMed] [Google Scholar]
- Broadbent et al. (2003).Broadbent JR, McMahon DJ, Welker DL, Oberg CJ, Moineau S. Biochemistry, genetics, and applications of exopolysaccharide production in Streptococcus thermophilus: a review. Journal of Dairy Science. 2003;86(2):407–423. doi: 10.3168/jds.S0022-0302(03)73619-4. [DOI] [PubMed] [Google Scholar]
- Caggianiello, Kleerebezem & Spano (2016).Caggianiello G, Kleerebezem M, Spano G. Exopolysaccharides produced by lactic acid bacteria: from health-promoting benefits to stress tolerance mechanisms. Applied Microbiology and Biotechnology. 2016;100(9):3877–3886. doi: 10.1007/s00253-016-7471-2. [DOI] [PubMed] [Google Scholar]
- Campbell et al. (1997).Campbell JA, Davies GJ, Bulone V, Henrissat B. A classification of nucleotide-diphospho-sugar glycosyltransferases based on amino acid sequence similarities. Biochemical Journal. 1997;326(3):929–939. doi: 10.1042/bj3260929u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter et al. (2007).Carter JA, Blondel CJ, Zaldivar M, Alvarez SA, Marolda CL, Valvano MA, Contreras I. O-antigen modal chain length in Shigella flexneri 2a is growth-regulated through RfaH-mediated transcriptional control of the wzy gene. Microbiology. 2007;153(10):3499–3507. doi: 10.1099/mic.0.2007/010066-0. [DOI] [PubMed] [Google Scholar]
- Cerning et al. (1994).Cerning J, Renard CMGC, Thibault JF, Bouillanne C, Landon M, Desmazeaud MJ, Topisirovic L. Carbon source requirements for exopolysaccharide production by Lactobacillus casei CG11 and partial structure analysis of the polymer. Applied and Environmental Microbiology. 1994;60(11):3914–3919. doi: 10.1128/AEM.60.11.3914-3919.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutinho et al. (2003).Coutinho PM, Deleury E, Davies GJ, Henrissat B. An evolving hierarchical family classification for glycosyltransferases. Journal of Molecular Biology. 2003;328(2):307–317. doi: 10.1016/S0022-2836(03)00307-3. [DOI] [PubMed] [Google Scholar]
- De Vuyst & Degeest (1999).De Vuyst L, Degeest B. Heteropolysaccharides from lactic acid bacteria. FEMS Microbiology Reviews. 1999;23(2):153–177. doi: 10.1111/j.1574-6976.1999.tb00395.x. [DOI] [PubMed] [Google Scholar]
- Del Moral et al. (2008).Del Moral S, Olvera C, Rodriguez M, Munguia A. Functional role of the additional domains in inulosucrase (IslA) from Leuconostoc citreum CW28. BMC Biochemistry. 2008;9(1):6. doi: 10.1186/1471-2091-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuBois et al. (1956).DuBois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Analytical Chemistry. 1956;28(3):350–356. doi: 10.1021/ac60111a017. [DOI] [Google Scholar]
- Forsee, Cartee & Yother (2009).Forsee WT, Cartee RT, Yother J. A kinetic model for chain length modulation of Streptococcus pneumoniae cellubiuronan capsular polysaccharide by nucleotide sugar donor concentrations. Journal of Biological Chemistry. 2009;284(18):11836–11844. doi: 10.1074/jbc.M900379200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grangeasse (2016).Grangeasse C. Rewiring the pneumococcal cell cycle with serine/threonine- and tyrosine-kinases. Trends in Microbiology. 2016;24(9):713–724. doi: 10.1016/j.tim.2016.04.004. [DOI] [PubMed] [Google Scholar]
- Holo & Nes (1989).Holo H, Nes IF. High-frequency transformation, by electroporation, of Lactococcus lactis subsp. cremoris grown with glycine in osmotically stabilized media. Applied and Environmental Microbiology. 1989;55(12):3119–3123. doi: 10.1128/AEM.55.12.3119-3123.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huszczynski et al. (2019).Huszczynski SM, Coumoundouros C, Pham P, Lam JS, Khursigara CM. Unique regions of the polysaccharide copolymerase Wzz2 from Pseudomonas aeruginosa are essential for O-specific antigen chain length control. Journal of Bacteriology. 2019;201(15):e00165-19. doi: 10.1128/JB.00165-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam & Lam (2014).Islam ST, Lam JS. Synthesis of bacterial polysaccharides via the Wzx/Wzy-dependent pathway. Canadian Journal of Microbiology. 2014;60(11):697–716. doi: 10.1139/cjm-2014-0595. [DOI] [PubMed] [Google Scholar]
- Kleerebezem et al. (1999).Kleerebezem M, Van Kranenburg R, Tuinier R, Boels IC, Zoon P, Looijesteijn E, Hugenholtz J, De Vos WM. Exopolysaccharides produced by Lactococcus lactis: from genetic engineering to improved rheological properties? In: Konings WN, Kuipers OP, Veld JHJH, editors. Lactic Acid Bacteria: Genetics, Metabolism and Applications. Dordrecht: Springer; 1999. pp. 357–365. [PubMed] [Google Scholar]
- Kuipers et al. (1998).Kuipers OP, De Ruyter PGGA, Kleerebezem M, De Vos WM. Quorum sensing-controlled gene expression in lactic acid bacteria. Journal of Biotechnology. 1998;64(1):15–21. doi: 10.1016/S0168-1656(98)00100-X. [DOI] [Google Scholar]
- Lairson et al. (2008).Lairson LL, Henrissat B, Davies GJ, Withers SG. Glycosyltransferases: structures, functions, and mechanisms. Annual Review of Biochemistry. 2008;77(1):521–555. doi: 10.1146/annurev.biochem.76.061005.092322. [DOI] [PubMed] [Google Scholar]
- Lebeer et al. (2009).Lebeer S, Verhoeven TLA, Francius G, Schoofs G, Lambrichts I, Dufrene Y, Vanderleyden J, De Keersmaecker SCJ. Identification of a gene cluster for the biosynthesis of a long, galactose-rich exopolysaccharide in Lactobacillus rhamnosus GG and functional analysis of the priming glycosyltransferase. Applied and Environmental Microbiology. 2009;75(11):3554–3563. doi: 10.1128/AEM.02919-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesk (1995).Lesk AM. NAD-binding domains of dehydrogenases. Current Opinion in Structural Biology. 1995;5(6):775–783. doi: 10.1016/0959-440X(95)80010-7. [DOI] [PubMed] [Google Scholar]
- Li et al. (2018).Li B, Ding X, Evivie SE, Jin D, Meng Y, Huo G, Liu F. Short communication: genomic and phenotypic analyses of exopolysaccharides produced by Streptococcus thermophilus KLDS SM. Journal of Dairy Science. 2018;101(1):106–112. doi: 10.3168/jds.2017-13534. [DOI] [PubMed] [Google Scholar]
- Li et al. (2016).Li D, Li J, Zhao F, Wang G, Qin Q, Hao Y. The influence of fermentation condition on production and molecular mass of EPS produced by Streptococcus thermophilus 05-34 in milk-based medium. Food Chemistry. 2016;197:367–372. doi: 10.1016/j.foodchem.2015.10.129. [DOI] [PubMed] [Google Scholar]
- Marciset & Mollet (1994).Marciset O, Mollet B. Multifactorial experimental design for optimizing transformation: electroporation of Streptococcus thermophilus. Biotechnology and Bioengineering. 1994;43(6):490–496. doi: 10.1002/bit.260430609. [DOI] [PubMed] [Google Scholar]
- Martinez-Fleites et al. (2006).Martinez-Fleites C, Proctor M, Roberts S, Bolam DN, Gilbert HJ, Davies GJ. Insights into the synthesis of lipopolysaccharide and antibiotics through the structures of two retaining glycosyltransferases from family GT4. Chemistry & Biology. 2006;13(11):1143–1152. doi: 10.1016/j.chembiol.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Meng et al. (2015).Meng X, Dobruchowska JM, Pijning T, Gerwig GJ, Kamerling JP, Dijkhuizen L. Truncation of domain V of the multidomain glucansucrase GTF180 of Lactobacillus reuteri 180 heavily impairs its polysaccharide-synthesizing ability. Applied Microbiology and Biotechnology. 2015;99(14):5885–5894. doi: 10.1007/s00253-014-6361-8. [DOI] [PubMed] [Google Scholar]
- Mierau & Kleerebezem (2005).Mierau I, Kleerebezem M. 10 years of the nisin-controlled gene expression system (NICE) in Lactococcus lactis. Applied Microbiology and Biotechnology. 2005;68(6):705–717. doi: 10.1007/s00253-005-0107-6. [DOI] [PubMed] [Google Scholar]
- Nwodo, Green & Okoh (2012).Nwodo U, Green E, Okoh A. Bacterial exopolysaccharides: functionality and prospects. International Journal of Molecular Sciences. 2012;13(12):14002–14015. doi: 10.3390/ijms131114002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purwandari, Shah & Vasiljevic (2007).Purwandari U, Shah NP, Vasiljevic T. Effects of exopolysaccharide-producing strains of Streptococcus thermophilus on technological and rheological properties of set-type yoghurt. International Dairy Journal. 2007;17(11):1344–1352. doi: 10.1016/j.idairyj.2007.01.018. [DOI] [Google Scholar]
- Qasba, Ramakrishnan & Boeggeman (2005).Qasba PK, Ramakrishnan B, Boeggeman E. Substrate-induced conformational changes in glycosyltransferases. Trends in Biochemical Sciences. 2005;30(1):53–62. doi: 10.1016/j.tibs.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Qin et al. (2011).Qin QQ, Xia BS, Xiong Y, Zhang SX, Luo YB, Hao YL. Structural characterization of the exopolysaccharide produced by Streptococcus thermophilus 05-34 and its in situ application in yogurt. Journal of Food Science. 2011;76(9):C1226–C1230. doi: 10.1111/j.1750-3841.2011.02397.x. [DOI] [PubMed] [Google Scholar]
- Rao & Rossmann (1973).Rao ST, Rossmann MG. Comparison of super-secondary structures in proteins. Journal of Molecular Biology. 1973;76(2):241–256. doi: 10.1016/0022-2836(73)90388-4. [DOI] [PubMed] [Google Scholar]
- Reeves et al. (1996).Reeves PR, Hobbs M, Valvano MA, Skurnik M, Whitfield C, Coplin D, Kido N, Klena J, Maskell D, Raetz CRH, Rick PD. Bacterial polysaccharide synthesis and gene nomenclature. Trends in Microbiology. 1996;4(12):495–503. doi: 10.1016/S0966-842X(97)82912-5. [DOI] [PubMed] [Google Scholar]
- Remus et al. (2012).Remus DM, Van Kranenburg R, Van Swam II, Taverne N, Bongers RS, Wels M, Wells JM, Bron PA, Kleerebezem M. Impact of 4 Lactobacillus plantarum capsular polysaccharide clusters on surface glycan composition and host cell signaling. Microbial Cell Factories. 2012;11(1):149. doi: 10.1186/1475-2859-11-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert & Gouet (2014).Robert X, Gouet P. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Research. 2014;42(W1):W320–W324. doi: 10.1093/nar/gku316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmittgen & Livak (2008).Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nature Protocols. 2008;3(6):1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Steiner et al. (2010).Steiner K, Hagelueken G, Messner P, Schäffer C, Naismith JH. Structural basis of substrate binding in WsaF, a rhamnosyltransferase from Geobacillus stearothermophilus. Journal of Molecular Biology. 2010;397(2):436–447. doi: 10.1016/j.jmb.2010.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al. (1999).Wang G, Boulton PG, Chan NWC, Palcic MM, Taylor DE. Novel Helicobacter pylori α1,2-fucosyltransferase, a key enzyme in the synthesis of Lewis antigens. Microbiology. 1999;145(11):3245–3253. doi: 10.1099/00221287-145-11-3245. [DOI] [PubMed] [Google Scholar]
- Wang et al. (2015).Wang G, Li D, Ma X, An H, Zhai Z, Ren F, Hao Y. Functional role of oppA encoding an oligopeptide-binding protein from Lactobacillus salivarius Ren in bile tolerance. Journal of Industrial Microbiology & Biotechnology. 2015;42(8):1167–1174. doi: 10.1007/s10295-015-1634-5. [DOI] [PubMed] [Google Scholar]
- Whitfield (1995).Whitfield C. Biosynthesis of lipopolysaccharide O antigens. Trends in Microbiology. 1995;3(5):178–185. doi: 10.1016/S0966-842X(00)88917-9. [DOI] [PubMed] [Google Scholar]
- Wu et al. (2015).Wu Q, Tun HM, Leung FC-C, Shah NP. Genomic insights into high exopolysaccharide-producing dairy starter bacterium Streptococcus thermophilus ASCC 1275. Scientific Reports. 2015;4(1):133. doi: 10.1038/srep04974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidan et al. (2017).Zeidan AA, Poulsen VK, Janzen T, Buldo P, Derkx PMF, Øregaard G, Neves AR. Polysaccharide production by lactic acid bacteria: from genes to industrial applications. FEMS Microbiology Reviews. 2017;41(Suppl. 1):S168–S200. doi: 10.1093/femsre/fux017. [DOI] [PubMed] [Google Scholar]
- Zhang (2009).Zhang Y. I-TASSER: Fully automated protein structure prediction in CASP8. Proteins-structure Function and Bioinformatics. 2009;77(S9):100–113. doi: 10.1002/prot.22588. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The transcriptional start site (+1), −35 and −10 regions are indicated in bold type. The Ribosomal-binding site (RBS) is indicated in box. Restriction sites are underlined.
A1–A3: Agarose electrophoresis of PCR products of epsFN and 16s rRNA from three independent experiments. M: DNA maker DL2000; 1, 2: PCR products of epsFN using the genome of S. thermophilus05CK and 05epsF as template, respectively; 3, 4: PCR products of 16s rRNA using the genome of S. thermophilus05CK and 05epsF as template, respectively. B1–B3: Western blot analysis of three independent experiments CK:S. thermophius05CK; F: S. thermophilus 05Fh6; MP: PageRuler Prestained Protein Ladder (Thermo Scientic, Waltham, MA, USA).
Data Availability Statement
The following information was supplied regarding data availability:
The draft genome sequence of Streptococcus thermophilus 05-34 is available at GenBank: QFLC00000000.