Abstract
Purpose
Salivary gland neoplasms are rare cancers of the head and neck region. Radical treatment in tumors of large salivary glands is surgery. Adjuvant treatment depends on the presence of risk factors that worsen the prognosis, but the role of these factors in patients treated by surgery with radio- or radiochemotherapy still remains unclear. The aim of the study is assessment of treatment results and identification of the risk factors affecting the prognosis in patients with tumors of large salivary glands subjected to adjuvant radio- or radiochemotherapy.
Patients and Methods
The study included 126 patients with local stage large salivary gland cancer who were treated surgically with adjuvant radio- or radiochemotherapy. The study excluded inoperable patients, patients with distant metastases, patients in a poor general condition and patients with contraindications to adjuvant treatment. They were treated between 2006 and 2016 and evaluated in terms of OS (overall survival), CSS (cancer-specific survival), RFS (relapse-free survival) and LRFS (local relapse-free survival).
Results
During a 44-month follow-up, 5-OS, CSS, RFS and LRFS were 55%, 68%, 60% and 73%, respectively. Multivariate analysis showed that OS was influenced by the following parameters: WHO performance status, TNM stage (T and N parameters), radicality of surgery, histopathological type, applied method of radiotherapy planning and tumor volume. WHO performance status, T and N parameters of the TNM stage and large volume of elective area influenced CSS, and the T parameter of the TNM stage, the dose below 60Gy and tumor volume influenced RFS and LRFS. Chemoradiotherapy can be used in N-positive patients.
Conclusion
The analysis indicates that the TNM grade, histopathological type, patient’s condition, radicality of the procedure, technique and dose of radiotherapy are the most important prognostic factors in these patients.
Keywords: salivary gland cancer, parotid cancer, radiotherapy, radiochemotherapy, risk factors, prognosis
Introduction
Salivary gland neoplasms are rare cancers of the head and neck region. According to SEER analysis, they constitute 8.1% of tumors in this anatomical region and 0.2% of all cancers.1 Most cases are recorded in the sixth decade of life.2 The incidence rate in men and women is similar – the ratio of men to women is 1.3:1.2 These tumors develop in large salivary glands (parotid glands, submandibular gland, sublingual gland) and small salivary glands that are found in the mucosa of the upper gastrointestinal tract and in the upper respiratory tract. The whole population is dominated by tumors of large salivary glands, and the most common among them are parotid tumors, constituting 64–80% of salivary gland tumors. 7–11% of salivary gland tumors are tumors of the submandibular gland, and less than 1% are tumors of the sublingual gland.3 Neoplasms of small salivary glands constitute from 9% to 23%. 25% of salivary gland neoplasms are malignant and the most common of these are mucoepidermoid carcinoma (34%), adenoid-cystic carcinoma (22%) and adenocarcinoma (18%).4
Radical treatment in tumors of large salivary glands is based on surgery appropriate to their stage and histopathological diagnosis.5 Adjuvant treatment (radio- or radiochemotherapy) depends on the presence of risk factors that worsen the prognosis.5 Currently, there are no clearly defined risk factors indicating an increased likelihood of local recurrence or distant metastases in patients undergoing adjuvant therapy, or associated indications for intensification of treatment. The following study presents an analysis of the risk factors based on a retrospective assessment of the influence of individual factors on the prognosis in a group of patients with cancer of large salivary glands undergoing adjuvant treatment – radio- or radiochemotherapy.
Materials and Methods
A retrospective analysis of a group of 126 patients with cancer of large salivary glands treated in the Center of Oncology of the Lublin Region between 2006 and 2016 was conducted. The characteristics of the patients are presented in Table 1. The study included patients with local stage cancer of large salivary glands according to the TNM (T – tumor, N – nodes, M – metastases)6 staging system (stages I–IVb, T1–4, N0–3, M0), radically treated by surgery and adjuvant radio- or radiochemotherapy. The study excluded patients not operated on, patients with distant metastases, patients in a poor general condition (with a WHO performance status score of 4) and patients with contraindications to adjuvant radio- or radiochemotherapy. The extent of surgical treatment was dependent on the initial stage of the disease according to the TNM staging system and included tumor removal, removal of the salivary gland with the tumor, removal of the salivary gland with the tumor and selective or radical unilateral or bilateral lymphadenectomy. All patients were qualified for adjuvant radio- or radiochemotherapy that was administered using irradiation methods available at the time (2-D – two-dimensional technique, 3-D three-dimensional conformal technique, IMRT – intensity-modulated radiotherapy technique). The median of the total dose was 60Gy (Gray) (40–72Gy). The minimum dose that provided local control in the treated patients was 60Gy. 29 (22%) patients did not follow the original treatment plan for various reasons. In 18 (14%) patients, treatment was discontinued due to toxicity, and 11 (9%) patients discontinued the treatment, refusing its continuation. Concomitant chemotherapy based on Cisplatin was used in 19 patients.
Table 1.
Characteristics of the Patients
| Parameter | Number of Patients (Percent)/Median (Range) |
|---|---|
| Age | 61.5 (19–88 years) |
| - 19–49 | 33 (26%) |
| - 50–61 | 29 (23%) |
| - 62–70 | 31 (25%) |
| - 71–88 | 33 (26%) |
| Gender | |
| - Female | 66 (52%) |
| - Male | 60 (48%) |
| WHO | |
| - 0 | 53 (42%) |
| - 1 | 53 (42%) |
| - 2 | 18 (14%) |
| - 3 | 2 (2%) |
| Location | |
| - parotid gland | 73 (58%) |
| - submandibular gland | 53 (42%) |
| Time from surgery to radio- or radiochemotherapy | |
| <9 weeks | 61 (48%) |
| ≥9 weeks | 65 (52%) |
| Clinical nerve palsy | |
| - yes | 33 (26%) |
| - no | 93 (74%) |
| Radicality | |
| - R0 | 64 (51%) |
| - R1 | 51 (48%) |
| - R2 | 11 (9%) |
| Nerve palsy after surgery | |
| - yes | 37 (29%) |
| - no | 89 (71%) |
| Histopathological type | |
| - squamous cell carcinoma | 29 (23%) |
| - adenocarcinoma | 20 (16%) |
| - adenoid cystic | 22 (17%) |
| - undifferentiated carcinoma | 14 (11%) |
| - acinic cell carcinoma | 13 (10%) |
| - other (polymorphus adenocarcinoma, salivary duct carcinoma, mucoepidermoid carcinoma high grade, mucoepidermoid carcinoma intermediate and low grade, myoepitelial carcinoma, carcinoma ex pleomorfic adenoma | 4 (3%), 8 (6%), 8 (6%), 3 (2%), 3 (2%), 2 (2%) |
| Neuroinvasion | |
| - yes | 41 (33%) |
| - no | 85 (67%) |
| Angioinvasion | |
| - yes | 18 (14%) |
| - no | 108 (86%) |
| TNM Stage | |
| I | 18 (14%) |
| II | 29 (23%) |
| III | 27 (21%) |
| IVab | 52 (41%) |
| T1 | 22 (17%) |
| T2 | 39 (31%) |
| T3 | 30 (24%) |
| T4 | 35 (28%) |
| N0 | 83 (66%) |
| N 1–3 | 43 (34%) |
| Technique of radiation therapy | |
| - 2D | 26 (21%) |
| - 3D | 29 (23%) |
| - IMRT | 71 (56%) |
| Dose | 60 (40–72) Gy |
| <60Gy | 28 (22%) |
| ≥60Gy | 98 (78%) |
| Chemotherapy | |
| - yes | 19 (15%) |
| - no | 107 (85%) |
| Initial level of hemoglobin | |
| <12.5mg/dL | 10 (25%) |
| ≥12.5mg/dL | 30 (75%) |
| Tumor volume | 54.1 cm3 (3.7–197.7) cm3 |
| ≤10cm3 | 20 (24%) |
| 10.1–50cm3 | 28 (33%) |
| 50.1–100cm3 | 24 (29%) |
| >100cm3 | 12 (14%) |
| Irradiation area | |
| - only surgical bed with margin | 25 (20%) |
| - surgical bed + lnd. group I–II | 27 (21%) |
| - surgical bed + unilateral lnd. | 45 (36%) |
| - surgical bed + bilateral lnd | 28 (22%) |
| Tumor bed volume (dose ≥ 60Gy) | 151.5cm3 (43.6–392.1)cm3 |
| ≤100cm3 | 16 (19%) |
| 100.1–200cm3 | 33 (31%) |
| 200.1–300cm3 | 18 (21%) |
| >300cm3 | 17 (20%) |
| Elective area volume (dose ≥ 50Gy) | 278 cm3 (103.2–633.4) cm3 |
| ≤ 150cm3 | 42 (50%) |
| 150.1–300cm3 | 19 (23%) |
| 300.1–450cm3 | 12 (14%) |
| 450.1–600cm3 | 8 (10%) |
| >600cm3 | 3 (4%) |
Abbreviations: R0, radical surgery; R1, microscopic nonradical surgery, R2, macroscopic nonradical surgery; lnd, lymphadenectomy.
In the study group, the following curves were analyzed: local control (LRFS – local relapse-free survival), time to relapse (RFS – relapse-free survival), overall survival time (OS – overall survival) and cancer-specific survival (CSS). The Kaplan–Meier method was used for statistical analysis. Patient follow-up was reported to the date the patient was last seen in the hospital. The study identified and determined the impact of such epidemiological factors as age, gender, WHO performance status,7 cancer location and the time between surgical treatment and adjuvant radio- or radiochemotherapy. The analysis also included clinical factors: nerve palsy, radicality of the surgical procedure, histopathological type, TNM stage, neuro- or angioinvasion, hemoglobin level at the start of treatment, the dose at the surgical bed and elective area, irradiation area and the applied technique of radiotherapy planning. The influence of chemotherapy on OS, CSS, RFS and LRFS was analyzed as well. In patients who were irradiated to a dose of at least 60Gy at the surgical bed and the planning was carried out using a conformal technique (3-D or IMRT), the following volume parameters were analyzed: the tumor volume, determined on the basis of CT performed before surgery, and the volume of the surgical bed and the elective area, determined on the basis of CT for treatment planning. In order to gauge the impact of the irradiation range on prognosis, the area and volume of the surgical bed and the elective area were analyzed (as continuous variable), depending on the features T (I–IVab) and N (N-negative or N-positive) on the TNM scale.
An attempt was also made to find factors that determined the decision on concurrent adjuvant therapy and the effect of concurrent adjuvant therapy on survival in different groups. The Log-rank test was used to determine the differences in OS, CSS, RFS and LRFS between patients with the presence and absence of individual factors. The Cox proportional hazard model was used to analyze the influence of continuous independent variables on survival times. A stepwise regression of the Cox model of all the aforementioned risk factors was performed. The findings of the analysis, together with statistically significant results, are presented in the paper. Spearman’s rank-order correlation test was used to assess the presence of a correlation between the stage of advancement and the irradiated area or volume. Non-parametric Kruskal–Wallis tests were used to analyze the effect of tumor volume on the type of relapse. The significance level in all tests was p=0.05. The statistical analysis was conducted using Statistica 13.1 (StatSoft Poland). The present study was approved by the Ethics Committee of the Medical University in Lublin (Lublin, Poland) (approval no. KE-0254/340/2018). Written informed consent was obtained from all participants. Participants’ privacy is ensured by anonymizing the data included in the manuscript and database. The study was conducted in accordance with the Declaration of Helsinki.
Results
Over the entire follow-up period, which was 44 months on average (3–195 months), 60 patients (48%) died, 37 of whom died due to the cancer (30% of all patients). In the analyzed group of patients, 2-, 5- and 10-year overall survival was 68%, 55% and 32%, respectively, and cancer-specific survival was 82%, 68% and 42%, respectively.
During the whole period of follow-up, 43 patients (34%) were recurrent. More than half of them (29 patients – 67% of all relapses) had locoregional recurrences – 23 patients (18%) at the surgical site and 6 patients (5%) at local nodes. Pulmonary metastases were the most frequent in distant relapses (7 patients – 50% metastases). In the analyzed group of patients, 2-, 5- and 10-year relapse-free survival was 69%, 60% and 44%, respectively, whereas the local relapse-free survival was 81%, 73% and 53%, respectively.
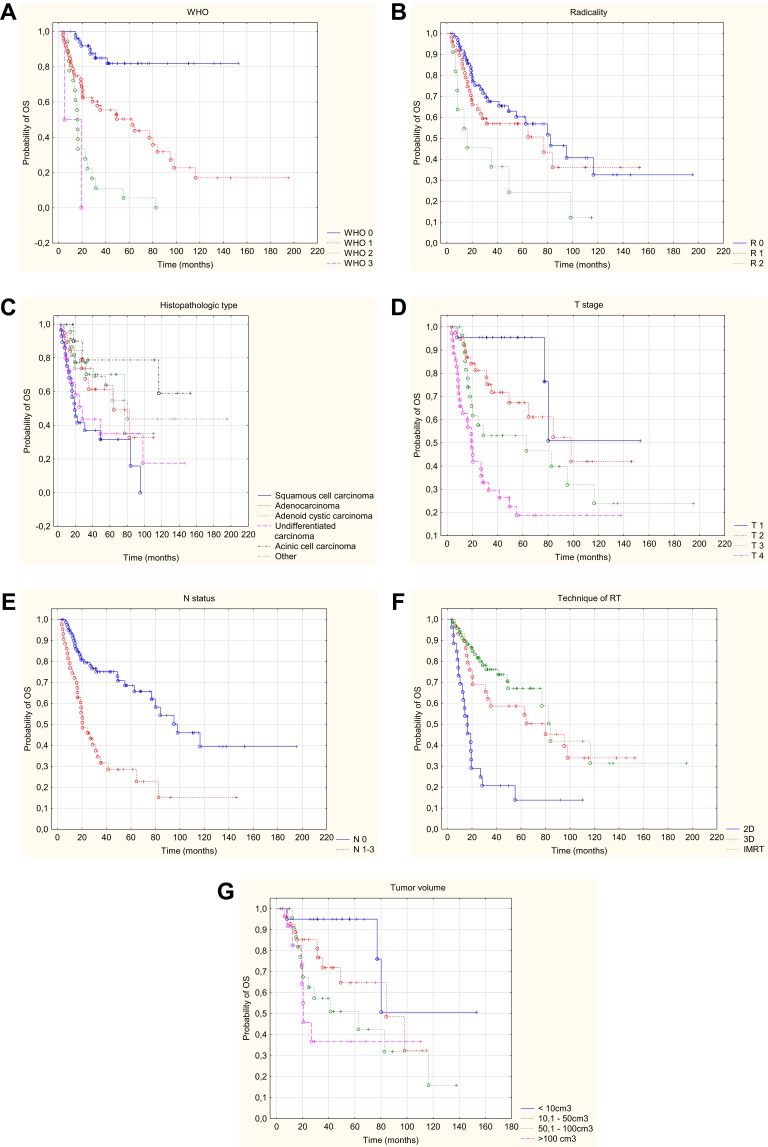
Univariate analysis allowed to conclude that the following risk factors had a statistically significant (p<0.05) impact on OS: age of patients at the time of disease (older patients lived shorter), WHO performance status (shorter OS in patients in a poorer condition), tumor location (slightly better prognosis for patients with tumors in the parotid gland), initial or postoperative cranial nerve palsy (presence of paralysis worsened the survival), radicality of surgery (the worst prognosis in patients with R2 resection), histopathological type (worse prognosis in patients with squamous cell carcinoma), worse prognosis in the presence of neuroinvasion. There was a deterioration in survival with an increase in the stage (including worsening of survival with an increase in the local stage of advancement – the T feature, and invasion of lymph nodes – presence of the N-positive parameter). Worse survival was also characteristic of patients who had hemoglobin level lower than 12.5mg/dl, patients who waited for adjuvant treatment more than 9 weeks after surgery, cases where the two-dimensional technique of radiotherapy planning was used, as well as cases where the dose at the surgical site was lower than 60Gy. Larger tumor volume, larger surgical bed volume, as well as larger volume of the elective area worsened overall survival. Data on 2-, 5- and 10-year survival with p-value are provided in Table 2. Multivariate analysis based on the Cox regression model allowed to conclude that the only independent risk factors that deteriorated the survival were: a higher WHO grade, non-radical surgery, squamous cell type, higher T-grade, positive N, the dose at the surgical site and volume of tumor (Figure 1A–G.) Statistically significant parameters are given in Table 3.
Table 2.
The Influence of the Analyzed Parameters on the 2-, 5- and 10-Year Overall Survival (OS)
| Parameter | Groups | 2-Year OS (%) | 5-Year OS (%) | 10-Year OS (%) | χ2 Test-Value | p-value |
|---|---|---|---|---|---|---|
| Age | 19–49 | 88 | 72 | 72 | 19.646 | 0.002 |
| 50–61 | 78 | 75 | 13 | |||
| 62–70 | 62 | 53 | 16 | |||
| 71–88 | 44 | 23 | 23 | |||
| Gender | Female | 66 | 51 | 24 | 0.864 | 0.387 |
| Male | 70 | 58 | 38 | |||
| WHO | 0 | 91 | 82 | 82 | 38.469 | <0.001 |
| 1 | 63 | 50 | 17 | |||
| 2 | 28 | 6 | 0 | |||
| 3 | 0 | 0 | 0 | |||
| Location | Parotid | 75 | 67 | 39 | 1.975 | 0.048 |
| Submandibular | 63 | 45 | 24 | |||
| Clinical Nerve palsy | Yes | 39 | 27 | 19 | 3.797 | <0.001 |
| No | 79 | 66 | 34 | |||
| Radicality | R0 | 75 | 60 | 33 | 8.200 | 0.017 |
| R1 | 64 | 56 | 35 | |||
| R2 | 45 | 24 | 12 | |||
| Nerve palsy after surgery | Yes | 43 | 27 | 17 | 4.114 | <0.001 |
| No | 80 | 68 | 33 | |||
| Histopathological type | Squamous | 41 | 32 | 0 | 13.646 | 0.018 |
| Adenocarcinoma | 84 | 61 | 33 | |||
| Cystic adenoid carcinoma | 77 | 70 | 35 | |||
| Undifferentiated | 67 | 32 | 19 | |||
| Acinic | 83 | 72 | 56 | |||
| Other | 74 | 64 | 45 | |||
| Neuroinvasion | Yes | 50 | 34 | 20 | 3.248 | 0.001 |
| No | 77 | 65 | 36 | |||
| Angioinvasion | Yes | 55 | 41 | 40 | 1.150 | 0.250 |
| No | 70 | 57 | 31 | |||
| Stage | I | 94 | 94 | 51 | 23.219 | <0.001 |
| II | 85 | 74 | 44 | |||
| III | 62 | 58 | 26 | |||
| IVab | 52 | 29 | 25 | |||
| T | 1 | 96 | 96 | 51 | 30.977 | <0.001 |
| 2 | 81 | 67 | 42 | |||
| 3 | 61 | 53 | 24 | |||
| 4 | 42 | 19 | 19 | |||
| N | positive | 48 | 28 | 16 | 4.230 | <0.001 |
| negative | 79 | 69 | 40 | |||
| Time | <9 weeks | 76 | 65 | 42 | 2.295 | 0.022 |
| ≥9 weeks | 60 | 43 | 19 | |||
| Technique of RT | 2D | 29 | 14 | 14 | 4.036 | <0.001 |
| 3D | 69 | 59 | 34 | |||
| IMRT | 83 | 67 | 31 | |||
| Dose | <60Gy | 35 | 26 | 9 | 26.350 | <0.001 |
| ≥60Gy | 78 | 62 | 37 | |||
| CHT | yes | 72 | 60 | 30 | 0.880 | 0.929 |
| no | 68 | 54 | 30 | |||
| Hemoglobin level | <12.5 mg/dL | 40 | 40 | N/A | 2.253 | 0.024 |
| ≥12.5 mg/dL | 83 | 72 | N/A | |||
| Tumor volume | ≤ 10 cm3 | 95 | 95 | 51 | 11.725 | 0.008 |
| 10.1–50 cm3 | 85 | 65 | N/A | |||
| 50.1–100 cm3 | 63 | 51 | 16 | |||
| >100 cm3 | 46 | 37 | N/A | |||
| Irradiation area | Only surgical bed with margin | 88 | 75 | 63 | 15.561 | 0.001 |
| Surgical bed + lnd. group I-II | 78 | 63 | 39 | |||
| Surgical bed + unilateral lnd. | 67 | 56 | 24 | |||
| Surgical bed + bilateral lnd | 43 | 23 | 8 | |||
| Tumor bed volume (dose ≥ 60Gy) | ≤100 cm3 | 94 | 94 | 50 | 10.050 | 0.019 |
| 100.1–200 cm3 | 84 | 65 | 39 | |||
| 200.1–300 cm3 | 76 | 63 | 0 | |||
| >300 cm3 | 50 | 37 | N/A | |||
| Elective area volume (dose ≥ 50Gy) | ≤150 cm3 | 90 | 83 | 43 | 10.725 | 0.029 |
| 150.1–300 cm3 | 65 | 46 | 0 | |||
| 300.1–450 cm3 | 65 | 64 | N/A | |||
| 450.1–600 cm3 | 62 | 33 | 0 | |||
| >600 cm3 | 67 | 33 | N/A |
Note: Statistically significant results in bold.
Abbreviations: p, significance level; R0, radical surgery; R1, non-radical microscopic surgery; R2, non-radical macroscopic surgery; RT, radiotherapy; CHT, chemotherapy; RT, radiotherapy; 2D, two-dimensional planning; 3D, three-dimensional planning; IMRT, planning with intensity-modulated radiation therapy
Figure 1.
Kaplan–Meier curve of OS with respect to WHO status (A), radicalism (B), histopathologic type (C), T – stage (D), N – status (E), technique of radiotherapy (F), tumor volume (G).
Table 3.
Results of Cox Multivariate Analysis
| Endpoint | Parameter | Chi-square | p-value | Hazard Ratio (HR) | 95% HR Lower | 95% HR Upper |
|---|---|---|---|---|---|---|
| OS | WHO | 19.540 | <0.001 | 2.331 | 1.601 | 3.393 |
| Radicality | 14.177 | <0.001 | 2.020 | 1.401 | 2.914 | |
| Squamous | 7.258 | 0.007 | 2.240 | 1.245 | 4.029 | |
| T | 6.639 | 0.010 | 1.513 | 1.104 | 2.073 | |
| N | 7.097 | 0.007 | 2.131 | 1.221 | 3.719 | |
| RT technique | 5.823 | 0.016 | 0.659 | 0.469 | 0.924 | |
| Tumor vol. | 9.571 | 0.002 | 1.745 | 1.226 | 2.482 | |
| CSS | WHO | 14.616 | <0.001 | 2.341 | 1.514 | 3.620 |
| T | 8.42013 | 0.004 | 1.779 | 1.206 | 2.626 | |
| N | 4.709 | 0.030 | 2.173 | 1.078 | 4.383 | |
| Elective vol. | 7.721 | 0.005 | 0.137 | 0.792 | 1.591 | |
| RFS | T | 17.396 | <0.001 | 2.052 | 1.464 | 2.877 |
| Dose | 9.881 | 0.002 | 0.353 | 0.185 | 0.676 | |
| Tumor vol. | 10.326 | 0.001 | 1.978 | 1.305 | 2.999 | |
| LRFS | T | 9.565 | 0.002 | 1.898 | 1.264 | 2.850 |
| Dose | 5.436 | 0.019 | 0.391 | 0.177 | 0.861 | |
| Tumor vol. | 4.926 | 0.026 | 1.793 | 1.071 | 3.002 |
Abbreviations: p, significance level; CI, confidence interval; RT, radiotherapy; OS, overall survival; CSS, cancer-specific survival; RFS, relapse-free survival; LRFS, local relapse-free survival; T, tumor; N, nodes; vol, volume.
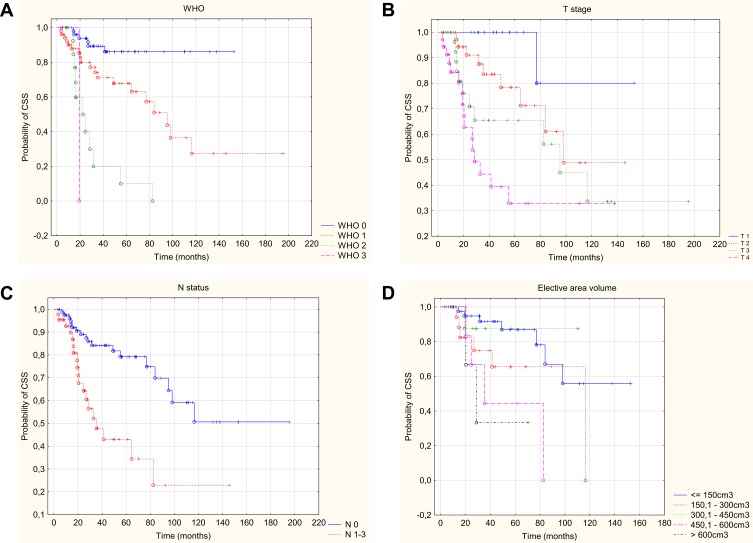
In the univariate analysis, the following factors were found to have a statistically significant effect on the deterioration of CSS: worse WHO performance status, nerve palsy, neuroinvasion, higher grade on the TNM scale (including higher T and positive N), two-dimensional planning technique, dose at the surgical site lower than 60Gy, larger tumor volume and volume of elective area. Data on 2-, 5- and 10-year cancer-specific survival with p-value are provided in Table 4. The multivariate analysis based on the Cox regression model allowed to conclude that the only independent risk factors that deteriorated cancer-specific survival were: a higher WHO grade, a higher T grade, positive N feature on the TNM scale and larger volume of elective area (Figure 2). Statistically significant parameters are given in Table 3.
Table 4.
The Influence of the Analyzed Parameters on the 2-, 5- and 10-Year Cancer-Specific Survival (CSS)
| Parameter | Groups | 2-Year CSS (%) | 5-Year CSS (%) | 10-Year CSS (%) | χ2 Test-Value | p-value |
|---|---|---|---|---|---|---|
| Age | 19–49 | 82 | 75 | 75 | 3.037 | 0.386 |
| 50–61 | 89 | 81 | 20 | |||
| 62–70 | 84 | 73 | 22 | |||
| 71–88 | 73 | 40 | 40 | |||
| Gender | Female | 80 | 64 | 35 | 0.956 | 0.339 |
| Male | 84 | 73 | 52 | |||
| WHO | 0 | 94 | 86 | 86 | 19.412 | <0.001 |
| 1 | 80 | 68 | 27 | |||
| 2 | 50 | 11 | 0 | |||
| 3 | 0 | 0 | 0 | |||
| Location | Parotid | 83 | 62 | 36 | 1.068 | 0.285 |
| Submandibular | 81 | 75 | 50 | |||
| Clinical nerve palsy | Yes | 61 | 47 | 38 | 2.292 | 0.021 |
| No | 89 | 75 | 41 | |||
| Radicality | R0 | 84 | 69 | 43 | 1.444 | 0.485 |
| R1 | 79 | 69 | 43 | |||
| R2 | 90 | 72 | 36 | |||
| Nerve palsy after surgery | Yes | 66 | 46 | 33 | 2.740 | 0.006 |
| No | 88 | 77 | 42 | |||
| Histopathological type | Squamous | 64 | 55 | 0 | 6.758 | 0.239 |
| Adenocarcinoma | 94 | 69 | 37 | |||
| Cystic adenoid carcinoma | 82 | 74 | 37 | |||
| Undifferentiated | 80 | 60 | 30 | |||
| Acinic | 100 | 87 | 65 | |||
| Other | 83 | 72 | 72 | |||
| Neuroinvasion | Yes | 73 | 54 | 36 | 2.050 | 0.040 |
| No | 86 | 75 | 45 | |||
| Angioinvasion | Yes | 68 | 50 | 50 | 1.390 | 0.164 |
| No | 85 | 71 | 43 | |||
| Stage | I | 100 | 100 | 80 | 16.118 | 0.001 |
| II | 92 | 79 | 48 | |||
| III | 74 | 74 | 38 | |||
| IVab | 74 | 43 | 37 | |||
| T | 1 | 100 | 100 | 80 | 22.324 | <0.001 |
| 2 | 91 | 78 | 49 | |||
| 3 | 76 | 66 | 34 | |||
| 4 | 62 | 33 | 33 | |||
| N | Positive | 67 | 43 | 23 | 3.511 | <0.001 |
| Negative | 89 | 79 | 51 | |||
| Time | <9 weeks | 85 | 73 | 54 | 1.444 | 0.146 |
| ≥9 weeks | 79 | 61 | 28 | |||
| Technique of RT | 2D | 53 | 25 | 25 | 3.718 | 0.004 |
| 3D | 81 | 73 | 50 | |||
| IMRT | 90 | 76 | 36 | |||
| Dose | <60Gy | 50 | 37 | 13 | 11.013 | <0.001 |
| ≥60Gy | 89 | 75 | 49 | |||
| CHT | Yes | 82 | 68 | 34 | 0.489 | 0.625 |
| No | 82 | 68 | 42 | |||
| Hemoglobin level | <12.5 mg/dL | 64 | 64 | N/A | 1.016 | 0.310 |
| ≥12.5 mg/dL | 86 | 75 | N/A | |||
| Tumor volume | ≤ 10 cm3 | 100 | 100 | 80 | 9.572 | 0.022 |
| 10.1–50 cm3 | 92 | 73 | N/A | |||
| 50.1–100 cm3 | 85 | 64 | 24 | |||
| >100 cm3 | 64 | 51 | N/A | |||
| Irradiation area | Only surgical bed with margin | 91 | 77 | 66 | 17.747 | <0.001 |
| Surgical bed + lnd. group I–II | 92 | 74 | N/A | |||
| Surgical bed + unilateral lnd. | 86 | 78 | 39 | |||
| Surgical bed + bilateral lnd | 56 | 30 | 10 | |||
| Tumor bed volume (dose ≥ 60Gy) | ≤ 100 cm3 | 100 | 100 | 80 | 6.581 | 0.086 |
| 100.1–200 cm3 | 90 | 72 | 43 | |||
| 200.1–300 cm3 | 87 | 72 | 0 | |||
| >300 cm3 | 74 | 54 | N/A | |||
| Elective area volume (dose ≥ 50Gy) | ≤ 150 cm3 | 95 | 87 | 56 | 9.978 | 0.044 |
| 150.1–300 cm3 | 82 | 66 | 0 | |||
| 300.1–450 cm3 | 87 | 87 | N/A | |||
| 450.1–600 cm3 | 83 | 44 | N/A | |||
| >600 cm3 | 66 | 33 | N/A |
Note: Statistically significant results in bold.
Abbreviations: p, significance level; R0, radical surgery; R1, non-radical microscopic surgery; R2, non-radical macroscopic surgery; RT, radiotherapy; CHT, chemotherapy; RT, radiotherapy; 2D, two-dimensional planning; 3D, three-dimensional planning; IMRT, planning with intensity-modulated radiation therapy.
Figure 2.
Kaplan–Meier curve of CSS with respect to WHO status (A), T – stage (B), N – status (C), elective area volume (D).
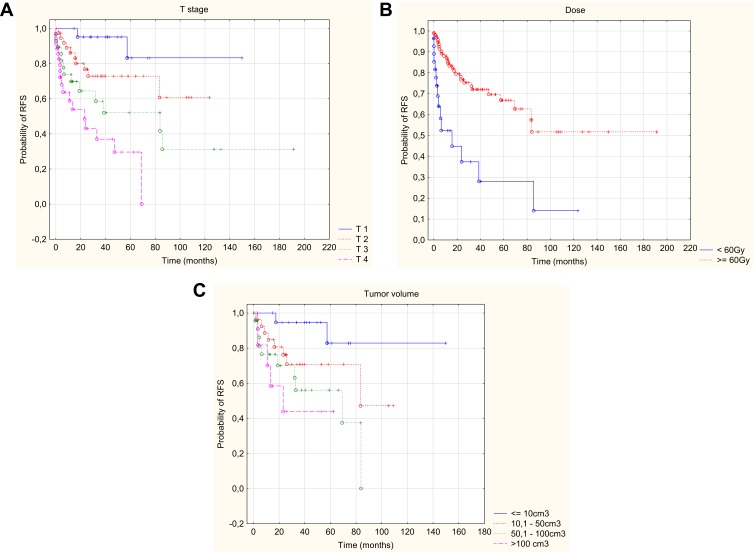
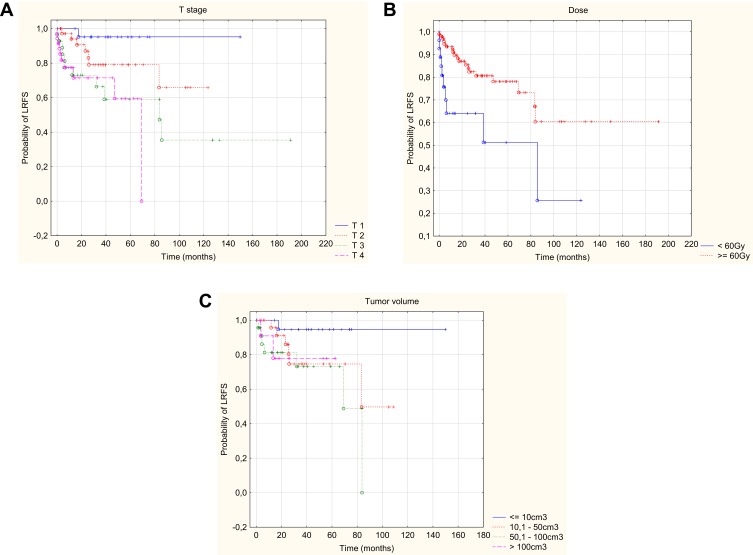
In addition, the univariate analysis showed that higher risk of relapse occurred in patients with a worse WHO performance status, nerve palsy, presence of neuroinvasion, higher TNM (including a higher T and positive N), larger tumor volume and when the dose at the surgical site was below 60Gy. Higher risk of local recurrence occurred in patients with worse WHO performance status, squamous histopathological type, a higher TNM stage (including a higher T-feature), a dose at the surgical site below 60Gy, and in patients irradiated using two-dimensional planning. Data on 2-, 5- and 10-year relapse-free and local relapse-free survival with p-value are provided in Tables 5 and 6. In addition, the multivariate analysis allowed to conclude that the only independent risk factors worsening the relapse-free survival and local relapse-free survival were: higher T parameter on the TNM stage scale, larger tumor volume and the dose at the surgical site lower than 60Gy (Figures 3 and 4). Statistically significant parameters are given in Table 3.
Table 5.
The Influence of the Analyzed Parameters on the 2-, 5- and 10-Year Relapse-Free Survival (RFS)
| Parameter | Groups | 2-Year RFS (%) | 5-Year RFS (%) | 10-Year RFS (%) | χ2 Test-Value | p-value |
|---|---|---|---|---|---|---|
| Age | 19–49 | 72 | 72 | 62 | 2.280 | 0.516 |
| 50–61 | 78 | 66 | 22 | |||
| 62–70 | 64 | 45 | 29 | |||
| 71–88 | 69 | 51 | 51 | |||
| Gender | Female | 69 | 53 | 45 | 1.105 | 0.269 |
| Male | 69 | 66 | 47 | |||
| WHO | 0 | 88 | 85 | 75 | 17.941 | <0.001 |
| 1 | 58 | 45 | 31 | |||
| 2 | 43 | 21 | 0 | |||
| 3 | 0 | 0 | 0 | |||
| Location | Parotid | 67 | 61 | 40 | 0.789 | 0.430 |
| Submandibular | 73 | 60 | 50 | |||
| Clinical nerve palsy | Yes | 53 | 50 | 23 | 2.592 | 0.009 |
| No | 75 | 64 | 52 | |||
| Radicality | R0 | 70 | 60 | 45 | 0.669 | 0.715 |
| R1 | 69 | 45 | 43 | |||
| R2 | 68 | 68 | 34 | |||
| Nerve palsy after surgery | Yes | 45 | 40 | 24 | 2.995 | 0.003 |
| No | 78 | 55 | 52 | |||
| Histopathological type | Squamous | 55 | 46 | 0 | 6.525 | 0.258 |
| Adenocarcinoma | 64 | 64 | 43 | |||
| Cystic adenoid carcinoma | 76 | 59 | 59 | |||
| Undifferentiated | 64 | 64 | 43 | |||
| Acinic | 78 | 43 | 67 | |||
| Other | 83 | 73 | 73 | |||
| Neuroinvasion | Yes | 55 | 50 | 27 | 2.268 | 0.023 |
| No | 76 | 63 | 55 | |||
| Angioinvasion | Yes | 66 | 56 | 56 | 0.701 | 0.482 |
| No | 70 | 63 | 43 | |||
| Stage | I | 100 | 88 | 88 | 17.724 | <0.001 |
| II | 80 | 75 | 56 | |||
| III | 70 | 65 | 39 | |||
| IVab | 67 | 34 | 23 | |||
| T | 1 | 95 | 83 | 83 | 20.922 | <0.001 |
| 2 | 77 | 73 | 61 | |||
| 3 | 64 | 53 | 31 | |||
| 4 | 43 | 50 | 0 | |||
| N | Positive | 46 | 36 | 24 | 3.117 | 0.002 |
| Negative | 81 | 70 | 53 | |||
| Time | <9 weeks | 73 | 62 | 56 | 0.949 | 0.343 |
| ≥9 weeks | 65 | 57 | 29 | |||
| Technique of RT | 2D | 44 | 30 | 30 | 4.928 | 0.085 |
| 3D | 70 | 70 | 46 | |||
| IMRT | 76 | 60 | 45 | |||
| Dose | <60Gy | 44 | 28 | 14 | 3.489 | <0.001 |
| ≥60Gy | 78 | 67 | 52 | |||
| CHT | Yes | 56 | 48 | 48 | 0.991 | 0.321 |
| No | 71 | 62 | 44 | |||
| Hemoglobin level | <12.5 mg/dL | 43 | 43 | N/A | 1.212 | 0.225 |
| ≥12.5 mg/dL | 68 | 56 | N/A | |||
| Tumor volume | ≤10 cm3 | 95 | 83 | 83 | 9.956 | 0.019 |
| 10.1–50 cm3 | 76 | 71 | N/A | |||
| 50.1–100 cm3 | 70 | 56 | 0 | |||
| >100 cm3 | 44 | 44 | N/A | |||
| Irradiation area | Only surgical bed with margin | 83 | 68 | 55 | 13.555 | 0.004 |
| Surgical bed + lnd. group I-II | 76 | 71 | N/A | |||
| Surgical bed + unilateral lnd. | 74 | 70 | 42 | |||
| Surgical bed + bilateral lnd | 38 | 19 | 19 | |||
| Tumor bed volume (dose ≥ 60Gy) | ≤100 cm3 | 93 | 93 | 78 | 6.204 | 0.102 |
| 100.1–200 cm3 | 80 | 72 | N/A | |||
| 200.1–300 cm3 | 67 | 59 | N/A | |||
| >300 cm3 | 50 | 50 | 0 | |||
| Elective area volume (dose ≥ 50Gy) | ≤ 150 cm3 | 88 | 85 | 56 | 7.205 | 0.125 |
| 150.1–300 cm3 | 70 | 52 | N/A | |||
| 300.1–450 cm3 | 60 | N/A | N/A | |||
| 450.1–600 cm3 | 58 | 58 | 0 | |||
| >600 cm3 | 33 | 33 | N/A |
Note: Statistically significant results in bold.
Abbreviations: p, significance level; R0, radical surgery; R1, non-radical microscopic surgery; R2, non-radical macroscopic surgery; RT, radiotherapy; CHT, chemotherapy; RT, radiotherapy; 2D, two-dimensional planning; 3D, three-dimensional planning; IMRT, planning with intensity-modulated radiation therapy.
Table 6.
The Influence of the Analyzed Parameters on 2-, 5- and 10-Year Local Relapse-Free Survival (LRFS)
| Parameter | Groups | 2-Year LRFS(%) | 5-Year LRFS(%) | 10-Year LRFS(%) | χ2 Test-Value | p-value |
|---|---|---|---|---|---|---|
| Age | 19–49 | 90 | 90 | 77 | 6.963 | 0.073 |
| 50–61 | 85 | 85 | 28 | |||
| 62–70 | 73 | 55 | 37 | |||
| 71–88 | 73 | 49 | 49 | |||
| Gender | Female | 80 | 67 | 58 | 0.870 | 0.384 |
| Male | 82 | 79 | 55 | |||
| WHO | 0 | 96 | 96 | 85 | 19.689 | 0.002 |
| 1 | 74 | 63 | 43 | |||
| 2 | 58 | 19 | 0 | |||
| 3 | 0 | 0 | 0 | |||
| Location | Parotid | 81 | 73 | 41 | 0.616 | 0.538 |
| Submandibular | 81 | 74 | 62 | |||
| Clinical nerve palsy | Yes | 74 | 67 | 33 | 1.610 | 0.107 |
| No | 84 | 75 | 61 | |||
| Radicality | R0 | 84 | 73 | 55 | 0.662 | 0.718 |
| R1 | 82 | 73 | 58 | |||
| R2 | 68 | 68 | 34 | |||
| Nerve palsy after surgery | Yes | 83 | 74 | 58 | 1.081 | 0.279 |
| No | 76 | 69 | 42 | |||
| Histopathological type | Squamous | 61 | 51 | 0 | 11.104 | 0.049 |
| Adenocarcinoma | 83 | 66 | 33 | |||
| Cystic adenoid carcinoma | 95 | 95 | 95 | |||
| Undifferentiated | 64 | 64 | 43 | |||
| Acinic | 91 | 73 | 55 | |||
| Other | 88 | 81 | 81 | |||
| Neuroinvasion | Yes | 82 | 73 | 64 | 0.924 | 0.355 |
| No | 79 | 74 | 39 | |||
| Angioinvasion | Yes | 77 | 66 | 66 | 0.756 | 0.449 |
| No | 82 | 74 | 52 | |||
| Stage | I | 100 | 100 | 100 | 10.256 | 0.017 |
| II | 87 | 77 | 58 | |||
| III | 79 | 73 | 43 | |||
| IVab | 70 | 59 | 39 | |||
| T | 1 | 95 | 95 | 95 | 12.233 | 0.007 |
| 2 | 89 | 77 | 66 | |||
| 3 | 73 | 59 | 35 | |||
| 4 | 72 | 60 | 0 | |||
| N | Positive | 69 | 61 | 40 | 1.684 | 0.092 |
| Negative | 86 | 78 | 59 | |||
| Time | <9 weeks | 83 | 78 | 71 | 1.247 | 0.213 |
| ≥9 weeks | 79 | 66 | 34 | |||
| Technique of RT | 2D | 53 | 35 | 35 | 7.141 | 0.028 |
| 3D | 89 | 89 | 59 | |||
| IMRT | 85 | 74 | 56 | |||
| Dose | <60Gy | 64 | 52 | 26 | 2.653 | 0.008 |
| ≥60Gy | 86 | 78 | 60 | |||
| CHT | Yes | 66 | 57 | 57 | 1.202 | 0.229 |
| No | 84 | 76 | 54 | |||
| Hemoglobin level | <12.5 mg/dL | 67 | 67 | N/A | 0.021 | 0.983 |
| ≥12.5 mg/dL | 75 | 59 | N/A | |||
| Tumor volume | ≤10 cm3 | 95 | 95 | 95 | 5.129 | 0.162 |
| 10.1–50 cm3 | 86 | 74 | N/A | |||
| 50.1–100 cm3 | 81 | 73 | 0 | |||
| >100 cm3 | 78 | 78 | N/A | |||
| Irradiation area | Only surgical bed with margin | 91 | 76 | 61 | 5.129 | 0.163 |
| Surgical bed + lnd.group I-II | 88 | 78 | N/A | |||
| Surgical bed + unilateral lnd. | 81 | 77 | 5 | |||
| Surgical bed + bilateral lnd | 61 | 61 | 61 | |||
| Tumor bed volume (dose ≥ 60Gy) | ≤ 100cm3 | 94 | 94 | 94 | 5.543 | 0.136 |
| 100.1–200 cm3 | 83 | 83 | N/A | |||
| 200.1–300 cm3 | 73 | 64 | N/A | |||
| >300 cm3 | 77 | 77 | 0 | |||
| Elective area volume (dose ≥ 50Gy) | ≤150 cm3 | 94 | 88 | 63 | 8.759 | 0.067 |
| 150.1–300 cm3 | 80 | 70 | N/A | |||
| 300.1–450 cm3 | 75 | N/A | N/A | |||
| 450.1–600 cm3 | 58 | 58 | 0 | |||
| >600 cm3 | 100 | 100 | N/A |
Note: Statistically significant results in bold.
Abbreviations: p, significance level; R0, radical surgery; R1, non-radical microscopic surgery; R2, non-radical macroscopic surgery; RT, radiotherapy; CHT, chemotherapy; RT, radiotherapy; 2D, two-dimensional planning; 3D, three-dimensional planning; IMRT, planning with intensity-modulated radiation therapy.
Figure 3.
Kaplan–Meier curve of RFS with respect of T – stage (A), dose (B), tumor volume (C).
Figure 4.
Kaplan–Meier curve of LRFS with respect of T – stage (A), dose (B), tumor volume (C).
The analysis showed that the T stage on the TNM scale positively correlated with the irradiation range (R=0.254, p=0.004), the volume of the surgical bed (R=0.791, p<0.001) and the volume of the elective area (R=0.573, p<0.001). Also, the status of regional lymph nodes (features N-negative and N-positive) correlated with the irradiation range (R=0.504, p<0.001), the volume of the surgical bed (R=0.379, p<0.001) and the volume of the elective area (R=0.755, p<0.001). An analysis of the influence of the irradiation range at individual T stages on the TNM scale showed that increasing the irradiation range at stage T1 (at least for a bed with lymph nodes of groups I–III) improves CSS, and increasing the volume of the elective area (over 150 cm3) extends RFS and LRFS. In addition, at T4, increasing the volume of the elective area (over 300 cm3) extends LRFS (p<0.05, Table 7). In the remaining stages (T2–T3), neither the range nor the volume of irradiation affected any of the parameters tested (p>0.05, Table 7). In the case of patients with the N-negative feature, increasing the irradiation range (at least for a bed with lymph nodes of groups I–III) extended CSS and RFS. Irradiation range did not affect prognosis in patients with the N-positive feature. Also, the volume of irradiation (volume of the surgical bed, volume of the elective area) did not affect the prognosis, neither in patients without lymph node metastases (N-negative) nor in patients with lymph node metastases (N-positive) (Table 8).
Table 7.
Influence of the Irradiation Range, Surgical Bed Volume and Volume of the Elective Area in T Stages on OS, CSS, RFS and LRFS
| Tumor Stage | T1 | T2 | T3 | T4 | |||||
|---|---|---|---|---|---|---|---|---|---|
| Test Log-Rank | χ2 Test-Value | p-value | χ2 Test-Value | p-value | χ2 Test-Value | p-value | χ2 Test-Value | p-value | |
| Irradiation area vs: | OS | −4.432 | 0.218 | −1.082 | 0.781 | −6.177 | 0.103 | −3.043 | 0.385 |
| CSS | −9.2 | 0.026 | −3.604 | 0.307 | −5.831 | 0.12 | −3.647 | 0.302 | |
| RFS | −5.373 | 0.146 | −1.918 | 0.589 | −5.479 | 0.14 | −3.102 | 0.376 | |
| LRFS | −4.697 | 0.195 | −3.306 | 0.307 | −2.444 | 0.485 | −0.361 | 0.948 | |
| Tumor bed volume vs: | OS | 0.423 | 0.672 | −0.436 | 0.803 | −0.984 | 0.611 | −0.992 | 0.609 |
| CSS | 0 | 1 | −1.219 | 0.543 | −0.448 | 0.799 | −0.073 | 0.963 | |
| RFS | 0.671 | 0.501 | −1.154 | 0.561 | −0.48 | 0.786 | −0.258 | 0.878 | |
| LRFS | 0.461 | 0.644 | −4.453 | 0.107 | −1.304 | 0.52 | −1.428 | 0.489 | |
| Elective area volume vs: | OS | 0.338 | 0.734 | −5.265 | 0.071 | −5.032 | 0.284 | −0.603 | 0.962 |
| CSS | 0 | 1 | −1.249 | 0.535 | −1.582 | 0.52 | −2.668 | 0.615 | |
| RFS | −2.12 | 0.033 | −2.157 | 0.339 | −0.218 | 0.994 | −3.866 | 0.962 | |
| LRFS | −2.287 | 0.022 | −2.987 | 0.224 | −0.397 | 0.982 | −11.434 | 0.022 | |
Note: Statistically significant results in bold.
Abbreviations: p, significance level; χ2, chi square test; OS, overall survival; CSS, cancer-specific survival; RFS, relapse-free survival; LRFS, local relapse-free survival; T, tumor.
Table 8.
Influence of the Irradiation Range, Surgical Bed Volume and Volume of the Elective Area in N Stages on OS, CSS, RFS and LRFS
| Nodal Status | N0 | N 1–3 | |||
|---|---|---|---|---|---|
| Test Log-Rank | χ2 Test-Value | p-value | χ2 Test-Value | p-value | |
| Irradiation area vs: | OS | −4.409 | 0.22 | −5.023 | 0.17 |
| CSS | −8.608 | 0.035 | −6.808 | 0.078 | |
| RFS | −8.979 | 0.029 | −4.263 | 0.234 | |
| LRFS | −6.811 | 0.078 | −0.246 | 0.969 | |
| Tumor bed volume vs: | OS | −2.474 | 0.480 | −2.896 | 0.408 |
| CSS | −3.983 | 0.263 | −1.280 | 0.734 | |
| RFS | −5.517 | 0.138 | −1.091 | 0.779 | |
| LRFS | −6.298 | 0.098 | −2.521 | 0.472 | |
| Elective area volume vs: | OS | −3.766 | 0.152 | −0.194 | 0.979 |
| CSS | −2.247 | 0.325 | −0.716 | 0.870 | |
| RFS | −2.464 | 0.292 | −0.600 | 0.897 | |
| LRFS | −3.831 | 0.147 | −3.348 | 0.341 | |
Note: Statistically significant results in bold.
Abbreviations: p, significance level; χ2, chi square test; OS, overall survival; CSS, cancer-specific survival; RFS, relapse-free survival; LRFS, local relapse-free survival; N, nodes.
The median tumor volume in the entire analyzed group was 54.1 cm3. In the group of patients without relapse, the median volume was 48.5 cm3, in the group of patients with local recurrence – 66.5 cm3, and in the group of patients with generalized relapse – 68.9 cm3. The differences were not statistically significant (Kruskal–Wallis test: H (2, N=84) = 2.629 p=0.269).
The retrospective analysis did not show any effect of chemotherapy on OS, CSS, RFS and LRFS. The results are shown in Tables 2, 4 and 5. The frequency of chemotherapy in groups of patients with selected parameters was analyzed, and then the results of treatment were compared in patients with chemoradiotherapy and patients with only radiotherapy. There were no differences in the frequency of chemotherapy in patients with a different local stage – T parameter (χ2=1.492, p=0.684), radical or non-radical surgery (χ2=0.030, p=0.862), with and without neuroinvasion (χ2=0.390, p=0.530), and with or without angioinvasion (χ2=2.640, p=0.104). However, there was a significantly more frequent use of concurrent adjuvant therapy in patients with squamous cell carcinoma (χ2=4.600, p=0.032) and metastases in regional lymph nodes (χ2=11.710, p<0.001). The influence of chemotherapy on OS, CSS, RFS and LRFS was analyzed in a group of patients with squamous cell carcinoma and in patients with lymph node metastases. There were no statistically significant differences between patients with squamous cell carcinoma who received chemoradiotherapy and those who received only radiotherapy in terms of OS (χ2=1.279, p=0.201), CSS (χ2=1.139, p=0.255), RFS (χ2=1.147, p=0.251) and LRFS (χ2=0.799, p=0.424). There were, however, statistically significant differences between patients with lymph node metastases and without lymph node metastases (in favor of patients with N-negative) in OS (χ2=3.177, p=0.001) and in CSS (χ2=2.463, p=0.014) in favor of patients with chemoradiotherapy, without statistically significant differences in RFS (χ2=1.738, p=0.082) and LRFS (χ2=0.457, p=0.648).
Discussion
The study indicates a relatively good prognosis in patients with local stage salivary gland cancer who have undergone surgery followed by adequate adjuvant treatment, although a relapse (local or distant) makes it significantly worse.8 In the studied group of patients, 5-OS, 5-CSS, 5-RFS and 5-LRFS were 55%, 68%, 60% and 73%, respectively. These results are slightly lower than in the available literature. In the study by Al-Mamgani et al,9 which involved 186 patients undergoing radiotherapy, 5-year OS, CSS, DFS and LRC were 68%, 80%, 83% and 89%, respectively. In the study by Huang et al,10 in which 85 patients were irradiated using IMRT or 3-D methods of radiotherapy planning, 5-OS, 5-DFS and 5-LRC were 82%, 77.5% and 88.4%, respectively. In older studies, these results are slightly lower. In the study by Poorten et al,11 5-OS and 5-DFS were 76% and 69%, respectively, and in the study by Kirkbride et al,12 5-OS and 5-LRC were 68% and 81%, respectively. Only in the study by Vander Poorten et al,13 involving 151 patients, 69% of whom underwent surgery with adjuvant radiotherapy, 5-OS and 5-DFS were worse – 46% and 64%, respectively. The reasons for these differences are complex and result from the selection of patients in each analysis. For example, in Huang et al,10 more than half of the patients were in stage I/II on the TNM scale, and only 27% in stage IV. In the analyzed group, only 37% of patients were in stage I/II, and 41% were in stage IV. In the study by Al-Mamgani et al,9 only 24% of patients were in stage T3–4, while in our analysis 48% of patients were in stage T3–4. In all of these studies, the prognosis in patients was significantly influenced by a variety of risk factors.
The present study discusses in detail the impact of these factors on prognosis. The Cox multivariate analysis shows that the most important risk factor for total death, cancer-specific death, total and local relapse is the stage. This is confirmed by the literature data. An extensive analysis by Spiro14 indicates that the stage, in particular the size of the tumor over 4 cm, is a stronger prognostic factor than the histopathological type. Similarly, in the study by Renchana et al,15 the T1–T2 tumor size is a significantly better prognostic factor than T3–4, and this factor is a more important parameter than the degree of malignancy or histopathological type. Regional nodes involvement (N-positive feature) in the study group is also a significant prognostic factor. In the multivariate Cox analysis, invasion of lymph nodes considerably deteriorated the OS, as well as the CSS and RFS in the univariate analysis. These results are confirmed by the data from the articles cited above10,13–15. Unfortunately, due to the number of patients (in some groups lower than 10 pt), there is a lack of statistical power to the conducted analysis stratified by the tumor T and N stage.
In the analyzed group of patients, 13 histopathological types were found, among which the most common was squamous cell carcinoma. The remaining ones included: NOS adenocarcinoma, adenoid cystic carcinoma, undifferentiated carcinoma, acinic cell carcinoma, and others, which accounted for less than 10% of all cases. The percentage of patients with individual histological types differs from their prevalence in the whole population.16 This is due to the fact that patients with particularly prognostically bad histopathological types were qualified for radiotherapy. For instance, squamous cell carcinoma accounts for only 6–14% of salivary gland cancers in the general population.16 It is also the type that had the statistically worst impact on overall survival. This is confirmed by the literature data. In a comprehensive analysis of over 2000 patients, Lee et al17 demonstrated that squamous cell carcinoma is worse than other histopathological types, although the difference is not statistically significant (OS: HR 0.97, CI (0.94–1.00), p=0.053). Median survival for squamous cell carcinoma, adenocarcinoma, adenoid cystic carcinoma and mucoepidermoid carcinoma was: 1.9y, 4.2y, 12.1y and 9.5y, respectively. Median time to recurrence for squamous cell carcinoma and adenoid-cystic carcinoma was 2.8y and 29.6y, respectively.17 In the studied group of patients, those with squamous cell carcinoma had the worst prognosis, and the differences were statistically significant. 5-year OS for squamous, adenocarcinoma and adenoid-cystic carcinoma was 32%, 61% and 70%, respectively (p=0.018).
In the analyzed group of patients, the dose at the surgical site was in the range of 40–72Gy. As mentioned above, a dose lower than 60Gy was given to 29 patients. In these patients, treatment was discontinued due to its significant toxicity.18 Patients irradiated with a dose lower than 60Gy showed worse prognosis. It had a statistically significant effect on prognosis in both the univariate analysis (for OS, CSS, RFS and LRFS) and the multivariate analysis (for RFS and LRFS). This dependence was also demonstrated by Garden et al,19 who analyzed 198 patients with adenoid-cystic carcinoma treated by surgery with adjuvant radiotherapy. The study showed a trend towards better local control with a dose increase. This was statistically significant in patients with a positive margin with a crude control rate of 40% for doses <56Gy and 88% for doses ≥56Gy (p=0.006). Similarly, in another study by Garden et al,20 the dose >60Gy was considered to improve local control in patients with positive margins or neuroinvasion. Also, the applied technique of radiotherapy planning influenced the results of treatment. However, due to the fact that patients were previously treated using a simpler two-dimensional planning technique, whereas in recent years they are treated with new planning techniques (3-D, then IMRT), the better treatment results may be associated with other elements of diagnostic and therapeutic procedures related to the progress in oncology. Other researchers also indicate a good prognosis in patients who have used new treatment planning techniques. In the analysis by Huang et al10 cited above, the IMRT, 3-D and 2-D techniques were used in 77%, 23% and 0%, respectively, yielding excellent results for 5-OS and 5-DFS (82% and 77.5%, respectively), as opposed to a 17 years older study by Vander Poorten et al,13 where 5-OS and 5-DFS were 46% and 64%, respectively.
In the examined group of patients, the following volume parameters were analyzed: tumor volume before treatment, volume of the surgical bed and nodal regions determined during radiotherapy planning. To unify the study group as much as possible, only patients treated with a dose of at least 60Gy, planned in conformal techniques, were analyzed. The impact of the volume of the tumor, surgical bed and electively irradiated lymph nodes on overall survival was demonstrated. In addition, the influence of the tumor volume on the percentage of all relapses, including local ones, was demonstrated. Similarly, many studies identify tumor volume as a determinant of prognosis.21 In a study on larynx cancer, Knegjens et al22 showed a significant effect of tumor volume on local control. The risk of local recurrence increases by 14% for each 10 cm3 of tumor volume (95% CI, 8% to 21%). Also, the larger the tumor volume, the higher the risk of locoregional relapse and distant metastases. Studer et al23 found that a 2-year nodal control was 95%, 90% and 75% for the following tumor volume ranges, respectively: 1–15 cm3, >15–70 cm3 and >70 cm3 (p=0.04), and only 4% of patients with cancer of the head and neck region with a volume of less than 70 cm3 had distant metastases, compared with 25% of patients with a tumor volume greater than 70 cm3. In our study, the median tumor volume in patients without relapse was 48.5 cm3, with local relapse – 66.5 cm3, and with distant metastases – 68.9 cm3. In salivary gland tumors, the adverse effect of larger tumor volume on prognosis was confirmed in a study by Almuhaimid et al.24 The metabolic volume of MTV tumor (determined on the basis of PET-CT) was shown to be an independent factor increasing the risk of metastasis (adjusted odds ratio 4.80, 95% confidence interval 1.09–21.20; p=0.039).
The analysis of our own group of patients in various stages indicates that an increase in the irradiation range and volume of both the surgical bed and the elective area worsens the prognosis. This conclusion is misleading, given that the range and volume of irradiation positively correlate with the stage of advancement. After dividing the entire group of patients according to stages depending on the T and N features on the TNM scale, it was found that greater range and volume of irradiation not only does not worsen survival, but in some cases improves prognosis. This relationship is not as evident as in the study by Hsieh et al25 where it was shown that, in the presence of the N-positive feature, irradiation of the area of elective lymph nodes on both sides of the neck reduces the percentage of local relapses, or as in the study by Chen et al26 in which the use of irradiation of elective lymph nodes reduced 10-year percentage of nodal recurrences from 26% to 0%. Our analysis indicates that increasing the range of irradiation and the volume of elective areas may improve treatment results, especially at the lowest and the highest stage expressed by the T feature on the TNM scale, as well as in the absence of metastases in regional lymph nodes. Irrespective of the stage, irradiation of the surgical bed alone may increase the risk of nodal relapse. The volume of the elective lymph node area should be at least 150 cm3 in stage T1 and at least 300 cm3 in stage T4.
In the analyzed group of patients, a deteriorating factor for OS, CSS as well as for RFS and LRFS is neuroinvasion. This is also confirmed by other studies.27 In the study by Garden et al19 cited above, neuroinvasion was found to be one of the risk factors that deteriorated crude failure rates from 18% to 9% (p=0.02), and in the analysis by Huang et al it affected OS (p=0.03), DFS (p=0.009) and LRC (p=0.049).
Numerous publications identify hemoglobin levels as an important determinant of prognosis.21,28–31 Correlation of lower hemoglobin level with a worse effect of radiotherapy was demonstrated in cancers of the head and neck region, lungs, cervix and bladder.28 In the case of head and neck cancers, the study was based primarily on the most common squamous cell carcinomas, in particular larynx cancer.29–31 The cut-off value was considered to be 12mg/dl, below which the prognosis was worse. Studies in the available literature did not analyze the effect of hemoglobin on head and neck tumors other than squamous cell carcinomas. In the analyzed group of patients with salivary gland cancers, the effect of hemoglobin level lower than 12.5 mg/dl on overall survival was demonstrated. The effect of low hemoglobin on the relapse rate has not been shown, which may, however, be related to the small number of patients analyzed.
A number of studies identify the radicality of the surgical procedure as a factor affecting the prognosis.27,32–34 In our study, the radicality had a significant impact only on overall survival, both in the univariate analysis and in the multivariate analysis. Also, the age of patients at the time of the disease had a negative influence on the prognosis, which is confirmed by some other publications.10 In addition, the WHO performance status determines the prognosis, affecting all endpoints analyzed in the study (in the multivariate analysis, only OS and CSS). Although there are no studies on this topic, the impact of the general condition seems to be indisputable and should play an important role in qualifying patients for treatment.
Our study did not show any benefits of using chemotherapy in any of the endpoints examined. This may be due to the selection of patients in particular groups. Patients who were assumed to have a worse prognosis underwent more aggressive treatment – chemoradiotherapy, so they cannot be easily compared with better prognosis patients treated with adjuvant radiotherapy. For this reason, two subgroups were analyzed, in which the number of patients undergoing chemoradiation was significantly different from the other patients in study. They were patients with squamous cell carcinoma and metastases to regional lymph nodes. A statistically significant positive effect of the use of chemotherapy on overall survival and cancer-specific survival was demonstrated only in the group with nodal metastases. There was no statistically significant effect on RFS and LRFS. There is no literature in the field of randomized trials comparing adjuvant radiochemotherapy and radiotherapy. In many cases, the addition of chemotherapy results from the extrapolation of research into other cancers of the head and neck region.35 This is particularly evident in patients with squamous cell carcinoma.36 Studies comparing the results of treatment of patients with chemotherapy and without chemotherapy did not show any benefits of chemotherapy,37–42 however, these are retrospective studies on a small group of patients, and the lack of differences may result from the selection of patients mentioned earlier. Only a prospective randomized trial could show any obvious benefits of using adjuvant chemoradiotherapy.
Conclusion
The severity of the disease on the TNM scale, and in particular the T parameter, is the most important independent factor that worsens the prognosis in all the analyzed endpoints. The invasion of lymph nodes also plays a significant role in prognosis, although to a lesser extent. Among the analyzed histopathological types, the most unfavorable prognosis is in the case of squamous cell carcinoma, the presence of which is an independent factor that deteriorates the overall survival. A non-radical surgery, neuroinvasion, low hemoglobin level, high volume of tumor and a poor general condition also deteriorate survival. It is recommended to use a dose over 60Gy at the surgical bed, take into account the area of elective lymph nodes, and implement new planning techniques to reduce the risk of relapse. Although the role of adjuvant chemoradiotherapy is still unclear, it may be beneficial to patients with regional lymph node metastases. It is necessary to identify risk factors whose presence should influence the modification of adjuvant therapy in this group of patients.
Disclosure
The authors declare that there are no conflicts of interest regarding the publication of this article.
References
- 1.Carvalho AL, Nishimoto IN, Califano JA, Kowalski LP. Trends in incidence and prognosis for head and neck cancer in the United States: a site-specific analysis of the SEER database. Int J Cancer. 2005;114(5):806–816. doi: 10.1002/ijc.20740 [DOI] [PubMed] [Google Scholar]
- 2.Licitra L, Grandi C, Prott FJ, Schornagel JH, Bruzzi P, Molinari R. Major and minor salivary glands tumours. Crit Rev Oncol Hematol. 2003;45(2):215–225. doi: 10.1016/S1040-8428(02)00005-7 [DOI] [PubMed] [Google Scholar]
- 3.El-Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ. WHO Classification of Head and Neck Tumours. WHO Classification of Tumours. 4th ed. Vol. 9 2017. [Google Scholar]
- 4.Ho K, Lin H, Ann DK, Chu PG, Yen Y. An overview of the rare parotid gland cancer. Head Neck Oncol. 2011;3:40. doi: 10.1186/1758-3284-3-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bell RB, Dierks EJ, Homer L, Potter BE. Management and outcome of patients with malignant salivary gland tumors. J Oral Maxillofac Surg. 2005;63:917–928. doi: 10.1016/j.joms.2005.03.006 [DOI] [PubMed] [Google Scholar]
- 6.Lydiatt WM, Mukherji SK, O’Sullivan B, et al. Salivary glands In: Amin MB, editor. AJCC Cancer Staging Manual, 8th ed. New York: Springer; 2017:95. [Google Scholar]
- 7.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the eastern cooperative oncology group. Am J Clin Oncol. 1982;5(6):649–656. doi: 10.1097/00000421-198212000-00014 [DOI] [PubMed] [Google Scholar]
- 8.Kordzińska-Cisek I, Grzybowska-Szatkowska L. The retrospective analysis of recurrent salivary gland cancer after surgery and adjuvant radio- or chemoradiotherapy. Biuletyn Polskiego Towarzystwa Onkologicznego Nowotwory. 2018;3(5–6):293–299. [Google Scholar]
- 9.Al-Mamgani A, van Rooij P, Verduijn GM, Meeuwis CA, Levendag PC. Long-term outcomes and quality of life of 186 patients with primary parotid carcinoma treated with surgery and radiotherapy at the Daniel den Hoed Cancer Center. Int J Radiat Oncol Biol Phys. 2012;84:189–195. [DOI] [PubMed] [Google Scholar]
- 10.Huang BS, Chen WY, Cheng-En H. Outcomes and prognostic factors for surgery followed by modern radiation therapy in parotid gland carcinomas. Jpn J Clin Oncol. 2016;46(9):832–838. doi: 10.1093/jjco/hyw067 [DOI] [PubMed] [Google Scholar]
- 11.Poorten VV, Hart A, Vauterin T, et al. Prognostic index for patients with parotid carcinoma: international external validation in a Belgian-German database. Cancer. 2009;115:540–550. doi: 10.1002/cncr.v115:3 [DOI] [PubMed] [Google Scholar]
- 12.Kirkbride P, Liu FF, O’Sullivan B, et al. Outcome of curative management of malignant tumours of the parotid gland. J Otolaryngol. 2001;30:271–279. doi: 10.2310/7070.2001.19527 [DOI] [PubMed] [Google Scholar]
- 13.Vander Poorten VL, Balm AJ, Hilgers FJ, et al. The development of a prognostic score for patients with parotid carcinoma. Cancer. 1999;85:2057–2067. doi: [DOI] [PubMed] [Google Scholar]
- 14.Spiro RH. Controversies in the management of salivary gland disease In: McGurk M, Renehan A, editors. Factors Affecting Survival in Salivary Gland Cancers. Oxford:: Oxford University Press; 2001:143–150. [Google Scholar]
- 15.Renehan AG, Gleave EN, Slevin NJ, McGurk M. Clinico-pathological and treatment-related factors influencing survival in parotid cancer. Br J Cancer. 1999;80:1296–1300. doi: 10.1038/sj.bjc.6990501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kordzińska-Cisek I, Grzybowska Szatkowska L. Salivary gland cancer – epidemiology. Nowotwory J Oncol. 2018;68(1):22–27. doi: 10.5603/NJO.2018.0005 [DOI] [Google Scholar]
- 17.Lee RJ, Tan AP, Tong EL, Satyadev N, Christensen RE. Epidemiology, prognostic factors, and treatment of malignant submandibular gland tumors. A population-based cohort analysis. JAMA Otolaryngol Head Neck Surg. 2015;141(10):905–912. doi: 10.1001/jamaoto.2015.1745 [DOI] [PubMed] [Google Scholar]
- 18.Kordzińska-Cisek I, Grzybowska-Szatkowska L. Complications of radio- and radiochemotherapy in patients undergoing major salivary gland cancer surgery. Otolaryngol Pol. 2019;73(3):26–31. doi: 10.5604/00306657 [DOI] [PubMed] [Google Scholar]
- 19.Garden AS, Weber RS, Morrison WH, et al. The influence of positive margins and nerve invasion in adenoid cystic carcinoma of the head and neck treated with surgery and radiation. Int J Radiat Oncol Biol Phys. 1995;32:619–626. [DOI] [PubMed] [Google Scholar]
- 20.Garden AS, el-Naggar AK, Morrison WH, Callender DL, Ang KK, Peters LJ. Postoperative radiotherapy for malignant tumors of the parotid gland. Int J Radiat Oncol Biol Phys. 1997;37(1):79–85. [DOI] [PubMed] [Google Scholar]
- 21.Rutkowski T. The role of tumor volume in radiotherapy of patients with head and neck cancer. RadiatOncol. 2014;9:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knegjens JL, Hauptmann M, Pameijer FA, et al. Tumor volume as prognostic factor in chemoradiation for advanced head and neck cancer. Head Neck. 2011;33(3):375–382. doi: 10.1002/hed.21459 [DOI] [PubMed] [Google Scholar]
- 23.Studer G, Lütolf UM, El-Bassiouni M, Rousson V. Glanzmann C volumetric staging (VS) is superior to TNM and AJCC staging in predicting outcome of head and neck cancer treated with IMRT. Acta Oncol. 2007;46(3):386–394. [DOI] [PubMed] [Google Scholar]
- 24.Almuhaimid TM, Lim WS, Roh JL, et al. Pre-treatment metabolic tumor volume predicts tumor metastasis and progression in high-grade salivary gland carcinoma. J Cancer Res Clin Oncol. 2018;144(12):2485–2493. doi: 10.1007/s00432-018-2760-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hsieh CE, Lee LY, Chou YC, et al. Nodal failure patterns and utility of elective nodal irradiation in submandibular gland carcinoma treated with postoperative radiotherapy - a multicenter experience. RadiatOncol. 2018;13(1):184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen AM, Garcia J, Lee NY, Bucci MK, Eisele DW. Patterns of nodal relapse after surgery and postoperative radiation therapy for carcinomas of the major and minor salivary glands: what is the role of elective neck irradiation? Int J Radiat Oncol Biol Phys. 2007;67(4):988–994. [DOI] [PubMed] [Google Scholar]
- 27.Speight PM, Barrett AW. Prognostic factors in malignant tumours of the salivary glands. Br J Oral Maxillofac Surg. 2009;47(8):587–593. doi: 10.1016/j.bjoms.2009.03.017 [DOI] [PubMed] [Google Scholar]
- 28.Dische S. Radiotherapy and anemia – the clinical experience. RadiotherOncol. 1991;20:35–40. [DOI] [PubMed] [Google Scholar]
- 29.Fujii M, Ohguri T, Yahara K, et al. Concurrent hyperfractionated chemoradiotherapy for head and neck squamous cell carcinoma: the prognostic impact of the overall treatment time and completion rates of chemotherapy. Springerplus. 2015;4:446. doi: 10.1186/s40064-015-1244-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCloskey SA, Jaggernauth W, Rigual NR, et al. Radiation treatment interruptions greater than one week and low hemoglobin levels (12 g/dL) are predictors of local regional failure after definitive concurrent chemotherapy and intensity-modulated radiation therapy for squamous cell carcinoma of the head and neck. Am J Clin Oncol. 2009;32:587–591. [DOI] [PubMed] [Google Scholar]
- 31.Rutkowski T, Wygoda A, Skladowski K, et al. Predictors of radiotherapy outcome in patients with T2 supraglottic carcinoma. Eur Arch Otorhinolaryngol. 2012;269:923–929. doi: 10.1007/s00405-011-1847-9 [DOI] [PubMed] [Google Scholar]
- 32.Lloyd S, Yu JB, Wilson LD, et al. Determinants and patterns of survival in adenoid cystic carcinoma of the head and neck, including an analysis of adjuvant radiation therapy. Am J Clin Oncol. 2011;34:76–81. doi: 10.1097/COC.0b013e3181d26d45 [DOI] [PubMed] [Google Scholar]
- 33.Ellington CL, Goodman M, Kono SA, et al. Adenoid cystic carcinoma of the head and neck: incidence and survival trends based on 1973–2007 surveillance, epidemiology, and end results data. Cancer. 2012;118:4444–4451. doi: 10.1002/cncr.27408 [DOI] [PubMed] [Google Scholar]
- 34.Ouyang DQ, Liang LZ, Zheng GS, et al. Risk factors and prognosis for salivary gland adenoid cystic carcinoma in southern china: a 25-year retrospective study. Medicine. 2017;96:e5964. doi: 10.1097/MD.0000000000005964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cortesina G, Airoldi M, Palonta F. Current role of chemotherapy in exclusive and integrated treatment of malignant tumours of salivary glands. Acta Otorhinolaryngol Ital. 2005;25:179–181. [PMC free article] [PubMed] [Google Scholar]
- 36.Pignon JP, le Maitre A, Maillard E, Bourhis J; Group M-NC. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiat Oncol. 2009;92:4–14. doi: 10.1016/j.radonc.2009.04.014 [DOI] [PubMed] [Google Scholar]
- 37.Pederson AW, Salama JK, Haraf DJ, et al. Adjuvant chemoradiotherapy for locoregionally advanced and high-risk salivary gland malignancies. Head Neck Oncol. 2011;3:31. doi: 10.1186/1758-3284-3-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schoenfeld JD, Sher DJ, Norris CM Jr, et al. Salivary gland tumors treated with adjuvant intensity-modulated radiotherapy with or without concurrent chemotherapy. Int J Radiat Oncol Biol Phys. 2012;82:308–314. [DOI] [PubMed] [Google Scholar]
- 39.Tanvetyanon T, Qin D, Padhya T, et al. Outcomes of postoperative concurrent chemoradiotherapy for locally advanced major salivary gland carcinoma. Arch Otolaryngol Head Neck Surg. 2009;135:687–692. doi: 10.1001/archoto.2009.70 [DOI] [PubMed] [Google Scholar]
- 40.Airoldi M, Gabriele AM, Gabriele P, et al. Concomitant chemoradiotherapy followed by adjuvant chemotherapy in parotid gland undifferentiated carcinoma. Tumori. 2001;87:14–17. doi: 10.1177/030089160108700103 [DOI] [PubMed] [Google Scholar]
- 41.Pederson AW, Haraf DJ, Blair EA, et al. Chemoreirradiation for recurrent salivary gland malignancies. RadiotherOncol. 2010;95:308–311. [DOI] [PubMed] [Google Scholar]
- 42.Bouyon A, Hans S, Durdux C, Housset M. Postoperative treatment of malignant tumors of the parotid gland: radiotherapy, concomitant chemotherapy and radiation therapy? Cancer Radiothér. 2007;11:465–475. doi: 10.1016/j.canrad.2007.07.002 [DOI] [PubMed] [Google Scholar]