Abstract
Neutrophils are peripheral immune cells that represent the first recruited innate immune defense against infections and tissue injury. However, these cells can also induce overzealous responses and cause tissue damage. Although the role of neutrophils activating the immune system is well established, only recently their critical implications in neuro-immune interactions are becoming more relevant. Here, we review several aspects of neutrophils in the bidirectional regulation between the nervous and immune systems. First, the role of neutrophils as a diffuse source of acetylcholine and catecholamines is controversial as well as the effects of these neurotransmitters in neutrophil’s functions. Second, neutrophils contribute for the activation and sensitization of sensory neurons, and thereby, in events of nociception and pain. In addition, nociceptor activation promotes an axon reflex triggering a local release of neural mediators and provoking neutrophil activation. Third, the recruitment of neutrophils in inflammatory responses in the nervous system suggests these immune cells as innovative targets in the treatment of central infectious, neurological and neurodegenerative disorders. Multidisciplinary studies involving immunologists and neuroscientists are required to define the role of the neurons-neutrophils communication in the pathophysiology of infectious, inflammatory, and neurological disorders.
Keywords: Neutrophil, Neuroimmunomodulation, Nicotinic receptors, Adrenoceptors, Neuroinflammation, Neuroimmunology
1. Introduction
1.1. Neutrophils: an overview
Neutrophils are short-lived polymorphonuclear leukocytes that are continuously generated from myeloid precursors in the bone marrow. Neutrophils are activated by bacterial and tissue damaged products, such as cytokines, damage-associated molecular patterns (DAMPs), and growth factors. These factors increase the neutrophil lifespan and ensure their migration and infiltration into the inflammatory focus through a concentration gradient of chemotactic stimulus [1]. Neutrophils are a critical component of the innate immune system essential to fight microorganisms and clear cellular debris in both septic and aseptic processes. Neutrophils can kill pathogens through different mechanisms: phagocytosis, degranulation of proteinases, and the release of reactive oxygen/nitrogen species (ROS and RNS), and neutrophil extracellular traps (NETs). ROS are products of the “cellular respiratory burst”, which is initiated by reducing oxygen to superoxide anions through the NADPH oxidase NOX2, an enzyme assembled in the phagosome membrane. From the formation of superoxide, hydrogen peroxide (H2O2) is produced and released into the phagosome space [213]. Neutrophils also release myeloperoxidase (MPO) into the phagosome by degranulated lysosomes. As a consequence, chloride ions are oxidized by H2O2 to generate hypochlorous acid (HOCl), a strong cell membrane oxidant [214]. The nitric oxide (NO) is produced by inducible NO synthase isoform (iNOS). iNOS produces high levels of NO in response to inflammatory mediators and/or to pathogen-associated molecular patterns (PAMPs). iNOS is regulated at transcriptional level and its activity is calcium-independent [2]. In addition to inflammation-induced iNOS expression, this enzyme has a constitutive expression in both murine and human resting neutrophils [3]. The iNOS-derived NO is a microbicidal and host cell-cytotoxic mediator by itself, but it can react with superoxide resulting in peroxynitrite, which is a stronger cytotoxic factor [4].
NETs were first described as a stick web of DNA conjugated with antimicrobial enzymes, such as elastase and MPO, that capture and kill bacteria in the extracellular milieu [5]. This process is not specific for bacteria and many other pathogens including fungi, parasites, and viruses, can also activate neutrophils to produce NETs. Depending of the stimulus, NETosis can occur through different pathways. For example, incubation of neutrophils with phorbol-12-myristate-13-acetate (PMA) dissociates azyrophilic granules containing elastase and MPO via the oxidative burst. These enzymes are then translocated into the nucleus, where they activate the protein-arginine deiminase 4 (PAD4), which is responsible for the deamination of arginine into citrulline. This process results in chromatin decondensation, followed by cell membrane lyse, and NETs release. This pathway is known as a ‘suicidal NETosis’, because it induces cell death [6]. By contrast, ‘vital NETosis’ does not induce cell suicide. Vital NETosis occurs in response to bacteria and fungi, and results in the release of NETs via vesicles, allowing neutrophils to still perform phagocytosis and chemotaxis [7,8]. Although NETs release may help to control infection, it can also cause organ damage. In animal models of autoimmune diseases, such as systemic lupus erythematosus, rheumatoid arthritis, and psoriasis, NETs are spontaneously induced causing tissue damage [9]. As described later in this review, NETs production has also implications in CNS disorders including multiple sclerosis (MS) [10,11], Alzheimer’ disease [12] and stroke [13,14].
Neutrophils deficiency to kill microorganisms can cause immunosuppression and increases the risk of opportunistic infections. For example, individuals with chronic granulomatous disease, a hereditary condition impairing NADPH oxidase, are more susceptible to microbial infection and sepsis [15]. However, neutrophils’ mediators are unspecific as they affect both microbial and host cells, leading to tissue and organ damage as found in auto-immune, infectious, and traumatic disorders [16]. Therefore, neutrophils are key players of the immune response being either a friend or foe for the host according to the inflammatory context.
1.2. Neuro-immune interaction: neutrophils in a neuro-immune context
Emerging evidences show a complex and bidirectional communication between the nervous and the immune systems [17–21]. The nervous system encompasses both central (brain and spinal cord) and the peripheral (autonomic and enteric) systems. The autonomic nervous system controls organ functions through the balance between the sympathetic and parasympathetic systems. In the sympathetic network, preganglionic neurons originated along the thoracolumbar segments of the spinal cord synapse with ganglionic neurons in the pre- or paravertebral ganglia. These ganglionic neurons release norepinephrine on peripheral tissues and activate local adrenergic receptors. In the parasympathetic network, preganglionic neurons originated in the brainstem nuclei and along the sacral spinal cord synapse with ganglionic neurons located near the target organ. These ganglionic neurons release acetylcholine that subsequently activates local cholinergic receptors. The vagus nerve is the principal nerve of the parasympathetic system and plays a pivotal role connecting the brain with the most important organs including the heart, lungs, liver, and the adrenal glands. The adrenal medulla acts as a sympathetic ganglion releasing catecholamines directly into the bloodstream and inducing a systemic effect rather than modulating specific organs. Several studies demonstrated the regulation of the immune system by autonomic nervous networks. Most of these neuro-immune interactions has been described in monocytes/macrophages and lymphocytes [22–24]. However, the role of neutrophils in the neuro-immune panorama in (patho)-physiological conditions is poorly understood.
Previous neuro-immune studies reported neutrophil recruitment as a response to pathological conditions, as determined by blood cytokine levels as inflammatory markers. We have used neutrophil recruitment as a biological signal of local/acute inflammation. We investigated neuromodulation of inflammation in experimental arthritis [25–28], using neutrophil migration as the main hallmark for local inflammation. Despite the key role of neutrophils in tissue damage, few studies investigated their role in the neural circuits, probably because of their short lifespan [29,30]. The half-life of neutrophils is approximately 10−19 h in mice and humans, and treatment with adrenergic or cholinergic drugs cannot be performed for long periods of time after their isolation from the blood. Moreover, mature neutrophils are found almost exclusively in the bloodstream and in inflamed tissue, but not in secondary lymphoid organs such as the lymph nodes or the spleen. The presence of mature neutrophils in the blood represents the first line of defense and, their quick migration into the injured site is essential to fight infections [31]. In contrast to neutrophils, direct interactions between the nervous and the immune systems are mediated through neuro-immune synapses between peripheral nerves and lymphocytes/macrophages. Lymphocytes are distributed in primary (thymus and bone marrow) and secondary (spleen and lymph nodes) lymphoid organs, which are innervated by post-ganglionic sympathetic nerves that interact with resident lymphocytes through synapsis-like structures [24,32,33]. In the thymus and spleen, these sympathetic innervations are responsible for the maturation of T and B lymphocytes [34,35], respectively. On the other hand, macrophages are present in many nonlymphoid organs, where they are regulated through direct sympathetic innervations as described in the liver, and intestine [22,36]. The barrier tissues are the major sites where immune cells traffic and reside; in particular, the intestinal mucosa alone harbors more lymphocytes than all the lymphoid organs combined. Therefore, the interference of such neural inputs in tissue-resident lymphoid populations cannot be excluded. Moreover, lymphoid structures rich in lymphocytes, such as thymus, are innervated by parasympathetic vagal fibers [37]. Moreover, considering the importance of chronic low-grade inflammation as a key factor in the development of cardiovascular diseases and metabolic syndrome [38–41], it is also essential to mention the implications of neuro-immune interface for many pathological states, such as obesity and insulin resistance, and their related diseases including hypertension, atherosclerosis, diabetes, and stroke [42–47].
From a clinical perspective, the study of neuro-immune interactions is allowing the design of new therapeutic strategies for infectious and inflammatory disorders. For example, electrical stimulation of the vagus nerve activates the splenic nerve to release norepinephrine, which in turn activates splenic lymphocytes to produce acetylcholine. Acetylcholine activates the alpha7 subunit of nicotinic acetylcholine receptors (α7nAChR) on macrophages and inhibits the production of inflammatory factors [17,24]. This neural circuits (“inflammatory reflex”) inspired the design of bioelectronic devices for the treatment of autoimmune conditions such as rheumatoid arthritis and Crohn’ disease [48–50]. Furthermore, the vagal signals to the spleen decrease the activation of circulating neutrophils by modulating the expression of CD11b [51]. These results evidence that vagal stimulation can be exploited to modulate neutrophil recruitment in infectious and inflammatory disorders.
In this review, the first section has focused on how neutrophils contribute to the neuronal regulation of the immune system in response to the catecholaminergic/ cholinergic neurotransmitters produced by specific neuronal networks. In return, neutrophils can also produce both neurotransmitters, to feedback the neuronal network, and cytokines to modulate the immune system. These atypical neural mechanisms are behind those classical anti- and pro-inflammatory mediators already described as chemokines and cytokines (Fig. 1A). The second section will discuss the bidirectional crosstalk between neutrophils and sensory neurons and their contribution to pain, and neurogenic inflammation. Pain, one of the cardinal points of inflammation, has relevant clinical importance that, together with fever, shows some neuroimmune peculiarities (Fig. 1B). Finally, the third section of this article discusses the role of neutrophils in neurologic and neurodegenerative disorders affecting the central nervous system (CNS) (Fig. 1C).
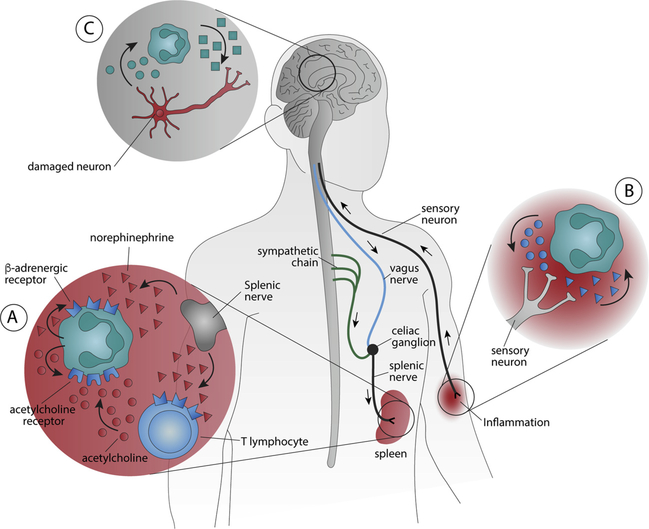
Fig. 1. The neuro-immune interactions of neutrophils.
(A) Neutrophils contribute to the neuronal regulation of the immune system. Neurotransmitters produced by either neuronal pathways (such as norepinephrine produced by the splenic nerve) or immune cells (acetylcholine produced by T lymphocytes) modulate neutrophil activity and the production of secondary messengers that, in turn, regulate both neurons and immune cells (including neutrophils by autocrine mechanism). (B) Neutrophils also modulate neuronal sensory signals by producing neurotransmitters to regulate the activation, sensitization, and maintenance of sensory neurons as observed in pain. Sensory neurons can also modulate neutrophils inhibiting their microbicidal functions by releasing neuropeptides. (C) Neutrophils can also cross the blood-brain-barrier (BBB) and release cytotoxic and inflammatory factors that induce neuronal damage in the central nervous system and contribute to neurological and neurodegenerative disorders.
2. Neutrophils as an immunological and diffuse source of neurotransmitters
Recent studies show that immune cells are an important non-neuronal source of neurotransmitters that allow the bidirectional crosstalk between the nervous and the immune system. When activated, neutrophils produce acetylcholine and catecholamines that can feedback the original neuronal network and also to transfer the neuronal signal to other immune cells, including neutrophils themselves. In neurons, tyrosine hydroxylase (TH) initiates the synthesis of catecholamines converting the amino acid L-tyrosine to L-DOPA, the precursor for dopamine synthesis. Next, the vesicular monoamine transporter (VMAT) translocates this neurotransmitter into vesicles, where dopamine is hydroxylated in the β position by dopamine-β-hydroxylase to generate norepinephrine, which is converted into epinephrine by the phenylethanolamine N-methyltransferase. Likewise, neutrophils also have all the enzymatic machinery necessary for the synthesis, metabolism, storage, and uptake of catecholamines [52,53]. It has been detected and quantified the amounts of dopamine, norepinephrine, epinephrine, and their metabolites, such as DL-3,4-dihydroxyphenylglycol (DHPG; a norepinephrine metabolite) and metanephrine (MET; epinephrine metabolite), in human neutrophils isolated from peripheral blood by high performance liquid chromatography (HPLC) [53]. These levels are similar to those reported in rat neutrophils by using enzyme-linked immunosorbent assay (ELISA) [52]. Furthermore, rat neutrophils also produce mRNA for both TH and dopamine-β-hydroxylase [52]. In fact, the intracellular levels of dopamine, norepinephrine, and DHPG are reduced in neutrophils treated with α-metil-ρ-tyrosine, a classical inhibitor of TH [53]. Likewise, treatment of human neutrophils with reserpine, an VMAT inhibitor, reduces the intracellular concentrations of norepinephrine and dopamine [53]. Together, these results indicate that human, rat, and murine neutrophils produce catecholamines through a mechanism similar to that reported in neurons. After depolarization, catecholamines are released by the vesicles into the extracellular milieu to exert their effects until they are reuptake or processed into inactive metabolites. Incubation of human neutrophils with desipramine (inhibits monoamine reuptake) markedly decreases their intracellular levels of norepinephrine [53].
Neutrophils can also produce acetylcholine. Peripheral human granulocytes are a non-neural source to produce and storage acetylcholine, but they do not synthetize significant amounts of acetylcholine as compared with lymphocytes [54]. Norepinephrine can stimulate splenic modulatory T lymphocytes expressing choline acetyltransferase to produce acetylcholine, which inhibits the production of inflammatory cytokines from splenic resident macrophages and prevent systemic inflammation in experimental sepsis [24]. Future studies are needed to determine the condition by which neutrophil-derived acetylcholine can induce (i) pro-inflammatory effects, as described for catecholamines [52], or (ii) an anti-inflammatory mechanism, as described for T lymphocytes in the spleen [24].
2.1. Catecholamines and their effects in neutrophils
Catecholamines have a critical role mediating the crosstalk between the nervous and the immune systems. Epinephrine and norepinephrine bind to either α (α1A, α1B, α1D, and α2A, α2B, α2C) or β (β1, β2, and β3) adrenoceptors (ARs), two families of adrenoceptors with distinct structural and pharmacological properties [55]. ARs are coupled to G proteins as their principal second messengers. α1ARs activate Gq/11, a subfamily of heterotrimeric G proteins that activates the phospholipase C (PLC)-calcium-diacyl glycerol (DAG)- protein kinase C (PKC) pathway in vascular (α1A, α1B, α1DARs) [56] and non-vascular systems (α1AARs). α2ARs activates Gi proteins to decrease the outflow of catecholamines (α2A and α2CARs) and modulate cognitive and behavioral disorders (α2A, α2B and α2CARs). βARs are coupled to Gs proteins, which activate the adenylyl cyclase and increase the intracellular cAMP levels. Thus, βARs can activate protein kinase A (PKA) to increase cardiac contractility (mainly β1ARs) and relax bronchial smooth muscles (mainly β2ARs) [57]. Due to the vast array of biological functions regulated by the adrenoceptors, it is easy to understand the importance of these receptors as potential therapeutic targets in multiple pathologies.
Recent studies showed that neutrophils express both αARs and βARs including α1A, α2C, α1D, β1, β2, and β3ARs but not α2BARs mRNA [52,58–63]. The expression of αARs in neutrophils has been confirmed in multiple studies related to diverse physiological conditions [52,61]. For instance, α1 and α2ARs exert opposite effects in neutrophils as they increase and decrease CD11b expression, respectively [62]. On the other hand, catecholamines can be produced by neutrophils increasing the extension of tissue damage through a autocrine mechanism via α2ARs activation [52].
βARs are predominantly anti-inflammatory. in vitro studies showed that βARs modulate neutrophil oxidative burst, chemotaxis, NET formation, and the expression of adhesion molecules, leukotriene B4 (LTB4), and chemokines/cytokines [62–65]. The intracellular βAR signaling, especially those associated with cAMP and PKA activation, modulate most to the neutrophil functions [66,67]. For example, isoproterenol, a β-adrenergic agonist, inhibits neutrophil oxidative burst induced by N-Formyl-methionyl-leucyl-phenylalanine (fMLP), a chemotactic peptide, or calcium ionophores by increasing intracellular calcium and cAMP levels [65]. However, a recent study also showed that βARs inhibit superoxide production in human neutrophils via a cAMP-independent mechanism [68]. Finally, adrenergic agents selectively inhibit the oxidative burst of human neutrophils without affecting elastase release [64].
Treatment of neutrophils with adrenergic agonists can attenuate cellular responses by inducing the desensitization and internalization of the adrenoceptors [69]. From a clinical perspective, neutrophils from patients treated with adrenergic agonists or presenting elevated levels of endogenous catecholamines are less capable to migrate or generate microbicidal agents. Furthermore, incubation of neutrophils with norepinephrine reduces cell chemotaxis by impairing the cytoskeleton remodeling [70]. Neutrophil phagocytosis and chemotaxis is also impaired in animals that underwent experimental stroke [70], a wellknown condition that induces a sustained sympathetic activation [71]. Thus, the use of β-blockers could prevent the immunosuppression and subsequent infection that usually follows stroke conditions [47], as isoproterenol reduced the expression of βARs in neutrophils [72].
Several sympathetic dysfunctions are mediated by end-target βARs and therefore neutrophils could be a useful model to study autonomic alterations. For example, reduction in βARs expression and receptor responsiveness in peripheral neutrophils have been reported in neonates and elderly, respectively. These results indicate that adrenergic signaling can change during development, depending on the physiological homeostasis or sympathetic dysfunctions [73,74]. Other studies also reported that β2ARs are reduced in neutrophils from diabetic children and hypertensive subjects, but increased in patients with post-traumatic stress disorder [60,75,76]. The potential role of β2ARs in other conditions such as psoriasis and atopic dermatitis appears to be controversial. Although there are some indications suggesting an irregularity in the function or expression of neutrophil β2ARs in psoriasis [77], posterior studies reported no change in the density and affinity of these receptor in neutrophils isolated of patients with psoriasis [78] or atopic dermatitis [59]. Moreover, neutrophil count in peripheral blood is also modulated by circadian oscillations by down-regulating CXCL-12 in the bone marrow, which can allow neutrophil release. This down-regulation is mediated by sunlight stimulation of β3ARs [79].
Another catecholamine that showed a great anti-inflammatory potential is dopamine [80,81]. There are two families of dopaminergic receptors: D1-type (including D1 and D5 subtypes) and D2-type (D2, D3, and D4 subtypes) [82]. D3 and D5 receptors are commonly expressed in human neutrophils, whereas D2 and D4 expression showed significant variability [83]. Dopaminergic receptors seem to be functional and interfere in neutrophil activity (e.g. phagocytosis) as observed in sympathectomized mice [84]. The uptake, synthesis, storage, and release of dopamine in neutrophils support the hypothesis of dopaminergic regulation of human neutrophil functions [53]. Dopaminergic agonists decreases nitric oxide production [85], and dopamine decreases ROS production in stimulated neutrophils [86–88], but only high concentrations of dopamine impairs neutrophil phagocytosis of bacterial pathogens [88].
2.2. Cholinergic and nicotinic receptors in neutrophils
Acetylcholine (ACh) is a neurotransmitter found in central and peripheral synapses that binds to muscarinic (mAChRs) and nicotinic (nAChRs) cholinergic receptors [89]. nAChRs are cationic channels composed of five homologous subunits, which are encoded by a large multi-gene family [18]. Although nicotine, a component of tobacco and the canonical nAChR agonist, displays potent anti-inflammatory effects when signaling through α7nAChRs in macrophages [90]. These receptors are considered a central component of the “inflammatory reflex” [90]. α7nAChRs are found in neurons and non-neuronal cells (e.g. microglia, astroglia, oligodendrocytes, endothelial and leukocytes) [89] and appear to be the main functional cholinergic receptors modulating the immune system. The expression of nAChRs in neutrophil’ surface has been studied by high affinity binding assays [91,92]. In humans, it has already been described that neutrophils isolated from blood also express α7nAChRs and α3β4 nAChRs [91]. More recently, the expression of mRNA for nAChR subunits, mostly α2–9 and β2–4, were detected in stimulated murine neutrophils [92]. The expression of these nAChRs strongly suggests the regulation of neutrophils by nicotinic agonists. Treatment of neutrophils with nicotine diminishes the expression of integrin adhesion molecules (CD11b and L-selectin) and endothelial intercellular adhesion molecule 1 (ICAM-1) [93], and inhibits their microbicidal properties, such as phagocytosis and chemotaxis, without affecting superoxide production [94]. Treatment with nicotine enhances elastase degranulation and generation of eicosanoids, such as prostaglandin E2 and LTB4, in these cells [94], but other studies showed opposite effects [95–97]. For example, treatment with nicotine interferes with the ability of neutrophils to kill periodontal pathogens, without affecting bacteria uptake [95]. Nicotine can also halt the degranulation and the production ROS, including superoxide, in stimulated neutrophils [95–97]. By contrast, cholinergic antagonists decreases neutrophil phagocytic migration activity without affecting cell oxidative burst [54] and, although nicotine did not affect the oxidative burst, it reacts with neutrophil-derived HOCl to generate nicotinechloramine, a membrane pore-forming compound [98]. Noteworthy, all these studies were performed under viable nicotine concentrations that failed to induce cellular cytotoxicity in neutrophils [94,95,97]. The different results in these studies can be due to the incubation times and concentration of nicotine, source of neutrophil, medium composition, and specificity and particularities of the assays utilized. In summary, despite the controversy, most studies show that nicotine substantially impairs neutrophil functions that may contribute to the higher susceptibility to infections reported in tobacco smokers.
Recent studies showed that the cholinergic regulation of neutrophils is not exclusively mediated by extracellular receptors of the cell membrane. For example, nicotine still modulates neutrophil activity even in the presence of first generation hydrophilic muscarinic or nicotinic antagonists [96]. In fact, acetylcholine and acetylcholine-like structures have lipophilic characteristics, allowing their translocation into the cytoplasm where α7nAchRs are also found (e.g. mitochondria) [99–101]. Thus, extracellular ligands can also target intracellular nicotinic receptors exerting particular biological properties in neutrophils, where each specific subtype of nicotinic receptor could exert distinct and opposite functions.
The immune properties of nicotine on neutrophils have clinical implication for infectious disorders. For example, nicotine increases survival in endotoxemia and experimental sepsis by inhibiting the production and release of systemic inflammatory mediators, such as tumor necrosis factor (TNF) and high mobility group box protein 1 (HMGB-1) [102]. Other studies showed that nicotine also inhibits neutrophil infiltration into vital organs, reducing organ damage and failure [103,104]. On the other hand, multiple studies showed that decreasing neutrophil recruitment to the infected area impairs bacterial clearance in experimental peritonitis induced by E. coli inoculation [105] and pneumonia [106]. In fact, α7nAchR-deficient mice show accentuated neutrophil migration toward the infectious focus and improved bacterial clearance [107]. These contradictory effects appear to depend on exposure range to α7nAchR agonists at different time points after the infection. Activation of nicotinic receptors in early phases of the infection prevent neutrophil recruitment into the infectious site allowing the spread of microorganisms; whereas nicotinic activation at later time points, can inhibit massive neutrophil recruitment into vital organs preserving tissue integrity [20]. Other studies reported that the disparity between the pro- and anti-inflammatory effects of nicotine is due to the differences in the period of exposition. The pre-treatment (before the arthritis induction; mimicking long-term exposition) exacerbated arthritis severity, whereas the post-treatment (therapeutic use) improved the inflammatory and clinical signs in arthritic rats [108]. Future studies will be needed to determine whether how the dosage and method of administration (bolus injection vs. continuous infusion) affect these results [109].
The chronic exposure to nicotine, as observed in smokers, exacerbates neutrophil activity as observed in patients with rheumatoid arthritis, and periodontal and lung diseases. Chronic stimulation of nAChRs in neutrophil increases IL-8 production by peroxynitrite generation, nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) activation and inhibitor of kB (IκB) degradation [110]. Indeed, smokers have higher plasma levels of IL-8 associated to blood neutropenia. Nicotine expands the neutrophil lifespan by inhibiting the diphospho-inositol pentakisphosphate (InsP7) and Akt pathway [111,112], contributing to cell accumulation and tissue damage in emphysema and bronchitis [113]. Chronic activation of α7nAchRs also increases NETs release via Akt and PAD4 activation [114]. Treatment with nicotine worsens the clinical score in murine collagen-induced arthritis, by increasing plasma levels of MPO-DNA complex, a known marker for NETs [109]. The authors also reported higher plasmatic levels of MPO-DNA complex in smoker subjects, and that nicotine acts as a potent inducer of NETosis via α7AChR activation. These results are consistent with the epidemiological studies showing that cigarette smoking predispose to rheumatoid arthritis development [114].
3. Bidirectional regulation of sensory neuron-neutrophil functions
The tissue injury caused by physical trauma or infection generates a local synthesis of mediators and DAMPs by neuronal and non-neuronal cells. In an initial stage, the generation of arachidonic acid metabolites enhances the production of prostanoids, a subclass of eicosanoids (e.g., prostaglandin E2), which increase vascular tissue permeability and activate afferent neurons (Fig. 1A–C). These events are associated with the appearance of critical cardinal signs of inflammation, including redness, heat, swelling, and pain. Physiologically, pain protects the inflamed tissue by sending a warning (nociceptive) signal to the brain [115,116]. The activation and/or sensitization of the peripheral endings of nsensory neurons (mostly nociceptors) by inflammatory mediators alerts the organism about an infection or a tissue injury, shunning it from further damage for proper healing [117]. Thereafter, leukocytes are attracted to the site of the injury (i) to fight against infection, to repair tissue damage, and (ii) to mediate a complex neuro-immune interaction with the sensory neurons [118]. From a clinical perspective, dysregulation in the resolution of acute inflammation may result in persistent and exacerbated inflammatory disorders, as observed in rheumatoid arthritis and neuropathic pain [119,120].
Neutrophil migration is an early event in the cellular phase of inflammation and it is responsible for the elimination of infectious agents and cellular debris. Within a few hours after the tissue damage, neutrophils are the most predominant leukocytes that migrate to the injured site, fighting the infection, and orchestrating the wound healing. Furthermore, they are also an important source of chemical mediators that affect the sensitivity of primary afferents neurons, such as cytokines and chemokines [121,122] (Fig. 1D–E). Cytokine-stimulated neutrophils induce, in turn, the release of additional mediators and trigger a complement alternative pathway amplifying nociception [123,124]. The pronociceptive action of neutrophils was first reported by our group showing that neutrophil recruitment toward the joints of dogs enhanced articular nociception promoted by the administration of lipopolysaccharides. Subsequent studies showed that intra-plantar administration of LTB4 and the complement component 5a (C5a) in rat paw elicited mechanical hypernociception by a mechanism dependent on neutrophil migration [125,126]. Furthermore, the administration of an allosteric C5aR antagonist inhibited C5a-induced neutrophil migration reducing mechanical hyperalgesia in experimental models of inflammatory and neuropathic pain [127]. Our studies were confirmed by other investigators showing that neutrophils participate in the genesis of different pathological types of pain [128–132]. Regarding inflammatory pain models, treatment with fucoidin reduces neutrophil recruitment and mechanical allodynia during carrageenan-induced inflammation [133], and the depletion of neutrophils with vinblastine sulfate or anti-neutrophil antibody decreases mechanical hyperalgesia induced by paw incision in mice [134]. In humans, there is a strong relationship between the hyperalgesic effects of LTB4 and the kinetic of neutrophil migration [135,136]. Other studies have observed that a neutrophil infiltration into the joint of patients suffering from arthritis precedes the clinical signs of inflammation and, therefore, this cellular event could be considered a predictive signal of pain development [137,138]. Antibody-induced neutropenia inhibits edema formation, but not the mechanical and thermal thresholds on complete Freund’s adjuvant (CFA)- and zymosan-induced pain [139,140]. Although these studies used different methods to induce neutropenia, these data suggest that neutrophils may not be the only leukocytes modulating nociception in these inflammatory conditions, although future studies would be required to confirm this hypothesis.
Other studies suggest that neutrophils can also contribute to neuropathic pain. Neutrophils are almost absent in an intact nerve, but a significant infiltration of neutrophils and macrophages have been observed at the site of the nerve lesion in experimental models of neuropathy [141]. A substantial endoneurial neutrophil invasion was reported at the site of a partial transection of the sciatic nerve, and the depletion of circulating neutrophils reduced the development of thermal hyperalgesia [131]. Further studies indicated that genetic ablation of mediators or receptors mediating neutrophils adhesion and migration improves mechanical hyperalgesia in experimental neuropathic pain [142,143]. Another interesting study showed that chronic constriction injury of peripheral nerve induces neutrophil infiltration into the dorsal root ganglia (DRG), ipsilateral to the nerve lesion, and it correlates with an increase in MCP-1 expression [144]. Moreover, we also reported that fucoidin or depletion of neutrophils with antibody anti-Ly6G inhibited the expression of TNF in DRGs and reduced the mechanical threshold after infection with Herpes Simplex Virus Type-1 in a murine model of acute herpetic neuralgia [145]. Finally, although the functional implications of neutrophils on neuronal axon and soma remain unknown, some studies suggest that neutrophil-derived elastase present in DRG and nerves is an important mediator for an induction of pain hypersensitivity in experimental models of neuropathy [146,147]. Taken together, these data support a direct role of neutrophils and its mediators in etiology and maintenance of neuropathic pain.
At the lesion site, neutrophils release inflammatory mediators, such as cytokines, that activate other cells, inducing edema, hyperalgesia, and migration of other leukocytes. However, the hypernociceptive potential of neutrophils is not directly associated with the release of nociceptive cytokines [148,149]. On the contrary, this effect depends on the potential of neutrophils to release direct-acting mediators through a coordinated cascade of factors. In fact, an inflammatory stimulus in the rodent paw induces an initial formation of bradykinin that triggers resident cells and neutrophils to release inflammatory factors, PGE2, and sympathetic amines that will have a direct action on neurons and nociception [130,150–153]. We reported that in vitro neutrophils stimulated with IL-1β produced PGE2 through a mechanism that is inhibited by fucoidin [133]. These results evidence that infiltrated neutrophils contribute to mechanical hypernociception, at least by releasing direct-acting mediators, such as PGE2 and sympathetic amines. Neutrophils also modulate other peripheral mechanisms of inflammatory pain including the production of ROS, metalloproteases, and hydrogen protons [154–157]. Furthermore, neutrophils may generate endothelins, that synergize with other hyperalgesic mediators and increases the nociceptors excitability and, consequently, pain sensitivity [158]..
Finally, nociceptors activation promotes an axon reflex and generates action potentials that propagate antidromically, triggering a rapid and local release of neural mediators, such as substance P (SP), neuropeptides calcitonin gene related peptide (CGRP), vasoactive intestinal peptide (VIP), and gastrin releasing peptide (GRP). These neuronal mediators produce an independent inflammatory response similar to the innate immune system. This process is known as “neurogenic inflammation” [159–162] (Fig. 2C). Neuropeptides released by sensory neurons, such as GRP and VIP, induce neutrophil chemotaxis and can also act as anti-microbicidal components [163–165]. On the other hand, recent studies showed that CGRP released from sensory neurons during host-pathogen interactions reduces neutrophil recruitment as well as their microbicidal activities and thereby promoting immunosuppression [166,167]. Although most of the studies suggest an indirect contribution of neutrophils to hyperalgesia, the development and use of drugs to mudulate neutrophil migration to the focus of lesions should be considered as a potential strategy against inflammatory persistent pain.
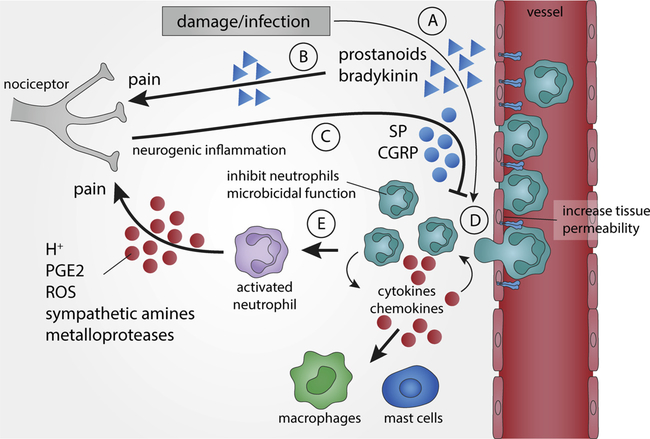
Fig. 2. Bidirectional regulation of sensory neuron-neutrophil functions.
Infection or tissue damage induces the production of inflammatory factors such as arachidonic acid metabolites and prostanoids that (A) increase tissue permeability to circulating neutrophils, and (B) can induce nociception and pain by activating sensory neuronal pathways. (C) The activation of nociceptive terminals triggers an axonal reflex that generates neurogenic substance P (SP) and calcitonin gene-related peptide (CGRP) increasing tissue permeability and inhibiting neutrophil microbicidal functions (neurogenic inflammation). (D) Neutrophils migrate into tissue to produce cytokines and chemokines that attract other leukocytes such as mast cells and circulating neutrophils. (E) Infiltrated neutrophils are activated and can produce multiple factors such as prostaglandin E2 (PGE2), sympathetic amines, reactive oxygen species (ROS), hydrogen protons (H+), and metalloproteases that modulate nociceptors and inflammatory pain.
4. Neutrophil in the central nervous system
Early studies suggested that the CNS is an “immune privileged site” due to the impenetrable blood-brain barrier (BBB), and the lack of antigen-presenting cells and lymphatic vasculature [168]. Nonetheless, this concept has been changed over the past few years [169], and recent studies show the presence of lymphatic vessels in the brain, and alterations of the BBB during CNS disorders allows a bidirectional communication between peripheral leukocytes and CNS [170,171]. Neutrophils are immune cells with a short lifespan that can contribute to the brain damage during the acute stage of cerebral injury. Under physiological conditions, neutrophils are hardly found in brain parenchyma due the BBB [172]. However, a small number of neutrophils are present in the meninges, pia membrane, and the cerebrospinal fluid. During CNS disorders, including during central infections, immune and non-immune resident cells of the cerebral tissue generate chemoattractant mediators that induce neutrophil infiltration into the cerebral tissue [168] (Fig. 3A–C). In fact, avoiding neutrophil infiltration into the CNS after brain injuries diminishes neuronal damage [173]. On the other hand, inhibition of neutrophils in patients may increase the risk of opportunistic infections [174]. Therefore, future studies will require determining the role of neutrophils in specific neurological disorders. This could lead to the design of specific therapies against the detrimental effects of the neutrophils, without affecting their beneficial protective roles. In this review, we discussed the participation of CNS-infiltrating neutrophils addressing the main aspects of inflammatory mediators and cellular types involved as well as the damaged structures in autoimmune (e.g. multiple sclerosis), neurodegenerative (e.g. Alzheimer’ disease) and infectious (e.g. sepsis, fungal and parasite infections) diseases and ischemic stroke.
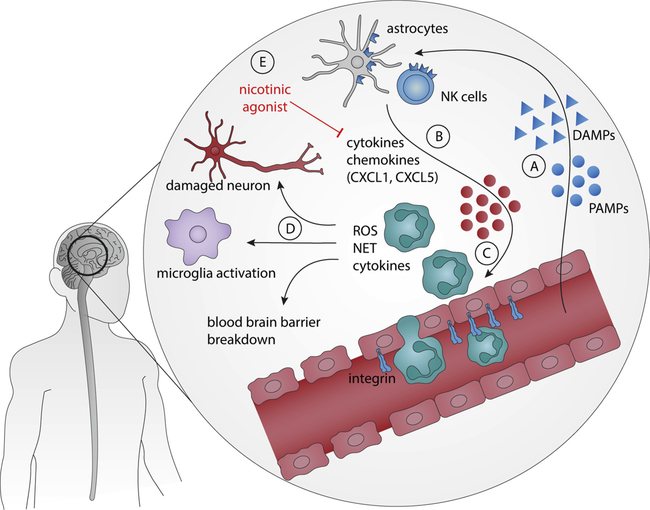
Fig. 3. Role of neutrophils in the central nervous system.
A) During the presence of neurodegenerative (e.g. Alzheimer), inflammatory (e.g. stroke) or infectious (e.g. sepsis, viral infection) conditions in the central nervous system (CNS). (B) Resident cells, such as astrocytes and NK cells, release different chemotactic substances, mainly chemokines. (C) These chemical mediators allow neutrophils to cross the blood-brain barrier (BBB) and produce high quantities of harmful mediators to neurons, such as reactive oxygen and nitrogen species (ROS and RNS), neutrophil extracellular traps (NETs) and cytokines. (D) In addition, these mediators induce microglial activation, ruptures on BBB, and demyelination and axonal loss. (E) The pharmacological activation of nicotinic receptors by acetylcholine or nicotinic agonists decreases neutrophil infiltration to the CNS by reducing the levels of chemokines.
Currently, little is known about the involvement of neutrophils in the pathogenesis of multiple sclerosis (MS). Neutrophils are recruited to the CNS through the release of chemokines, such as CXCL1 and CXCL5, which are produced different cells, including the astrocytes - via Th17-derived IL-17 - and mast cells from the meninges [175,176]. IL-17−/− mice show reduced neutrophil infiltration into the brain, but not in the spinal cord. In this case, astrocytes express CXCL2 and CXCL5 mediated by IFN-γ. Curiously, IFN-γ did not induce chemokine production by astrocytes in vitro, suggesting that it might act indirectly. Clinical studies reported that MS patients have increased levels of neutrophils and systemic NETs [10,11], and the plasma levels of CXCL1, CXCL5, and elastase correlated with clinical disability [177]. Interestingly, neutrophil recruitment into the CNS differ between IL-17 (to the brain) or interferon (IFN)-γ (to the spinal cord) production in experimental autoimmune encephalomyelitis (EAE), a multiple sclerosis model [178]. When in the CNS, neutrophils increase the permeability of the BBB and disrupt myelination [179]. Thus, neutrophil infiltration into the CNS leads to demyelination and axonal loss (Fig. 3D).
Neutrophil infiltration into the CNS also appears to contribute to the development of neurodegenerative disorders, such as Alzheimer’ disease. Neutrophil infiltration occurs in the regions with amyloid-β deposits by a LFA-1 integrin mechanism, and further brain injury appears following NETs and IL-17 release [12]. Different studies demonstrated that Alzheimer’s patients present high number of neutrophils in the peripheral blood [180]. TNF levels modulation using isoindolin-1,3 dithione increased the presence of “healthy” neutrophils in the CNS, and improved the clinical score of 3xTgAD mice [181]. Of note, an increasing number of investigators propose that targeting α7AChR in neutrophils may modulate neuro-inflammatory and cognitive dysfunctions in Alzheimer’s disease [182]. Multiple studies showed that activation of α7- and α9nAChR reduces the levels of CXCL12 and CCL2 in the CNS, and improves the clinical outcomes by preventing neutrophil infiltration [182,183] (Fig. 3E).
During infections by microorganism that invade the CNS, neutrophils can perform protective or deleterious roles depending on the nature of the pathogen and host defense. Infection susceptibility is increased in immunosuppressed individuals, as usually observed in neutropenia or infected HIV patients. For example, fungal CNS infection by Candida albicans promotes a protective recruitment of CXCR2-expressing neutrophils through microglia-induced IL-1β and CXCL1 [184,185]. Toxoplasma gondii can lead to cerebral toxoplasmosis, and the infiltration of neutrophil promotes host defense contributing to IFN-γ production and parasite control during the early stages of infection [186]. On the other hand, during sepsis, a systemic infectious condition, multiple vital organs are damaged by neutrophil infiltration, including the brain. Sepsis can also induce cognitive dysfunction and neurological disorders that appears to be associated with neutrophil filtration. Neutrophil adheres to the cerebrovascular endothelium via β2-integrins [187]. Recent studies showed that natural killer (NK) cells have an essential role in neutrophil recruitment into the brain during sepsis via chemokine production and microglial interaction [188]. Recent studies found neutrophils in the CNS, even fourteen days after the septic challenge, that are associated wih high levels of TNF and CXCL1 along with behavioral alterations [189].
In animal models of ischemic stroke, injury induces damaged cells to release DAMPs, which activate resident cells to produce chemokines, such as CXCL2 and CXCL8, resulting in neutrophil recruitment into the CNS. Neutrophils activation starts in the peripheral blood by systemic HMGB1, and then they infiltrate into the CNS due to an increased expression of very late antigen-4 (VLA-4), allowing their migration and adhesion to the brain blood vessels [173,190]. In the brain, neutrophils are activated releasing NETs, and also interacting with microglia, which results in tissue damage and the disruption of the BBB [13,14] (Fig. 3D). Of note, patients who suffered hemorrhagic stroke showed low levels of oxidative respiratory in the isolated neutrophils, which inversely correlated with the plasma levels of norepinephrine [191]. This suppression could explain the higher susceptibility to infections in post-stroke patients. The susceptibility to nosocomial infections due to reduced neutrophil infiltration after stroke was improved by α7nAChR pharmacologic blockage or genetic deletion [192]. Hypoperfusion enhances the interaction of neutrophils with the vasculature and promotes their adhesion by inducing the expression of selectins in the surface of the endothelial cells [193]. Neutrophils first accumulated within hours in the leptomeninges and perivascular spaces before they infiltrate into the parenchyma [194]. Neutropenic animals display reduced blood flow in the injured hemisphere after traumatic brain injury [195]. Hypoperfusion and neutrophils adherence promote ischemia and even early coagulopathy [196]. Likewise, neutrophils also contribute to vascular dysfunction during and after the injury in a hypoxia-ischemia model [197]. The interaction between activated neutrophils and endothelium is normally associated with secondary injury after traumatic brain injury [171].
Other neurological disorders are also associated by neutrophil infiltration into the CNS, including viral infection [198], febrile seizure [199], and Entero‐Behçet’s disease [200]. However, the molecular mechanisms mediating the role of neutrophils in the pathogenesis of CNS disorders are not well-known, and future research is needed to determine the specific contribution of neutrophils to distinct neurological disorders.
5. Conclusion and perspectives
Although neutrophils are classical innate immune cells, they are a significant non-neural source of neurotransmitters (e.g. catecholamines and acetylcholine) exerting both paracrine and autocrine, self-regulatory modulation in inflammatory conditions. From a clinical perspective, neutrophils can interact with the central and peripheral nervous systems being responsible for the genesis of central inflammatory/neurodegenerative conditions and pain, respectively. Thus, inhibition of neutrophils could be a promising strategy against inflammatory and neurogenic pain or brain damage. The main findings in the current literature that demonstrate the role of neutrophils in a neuro-immune context are shown in the Table 1.
Table 1.
The main findings showing the role of neutrophils in a neuro-immune context.
| Experimental Condition | Stimulus | Neutrophil Target | Neutrophil Release Factors | Effect | Ref. |
|---|---|---|---|---|---|
| Arthritis | Vagus Nerve Stimulation or β-adrenoceptor Agonists | β-Adrenoceptors | – | Vagal stimulation reduced neutrophil migration and arthritic joint inflammation by activating specific sympatho-excitatory brain nuclei | [26,27,28] |
| Acute lung injury | LPS | TLR4 | Norepinephrine and Epinephrine | Neutrophil-derived catecholamine mediate lung injury | [52] |
| Stroke | Norephnephrine | Adrenoceptors | – | Activation of sympathetic nervous system weakens neutrophil migration, activation, and phagocytosis, inhibiting their critical host defense functions | [70] |
| Sepsis | Nicotine | α7-Nicotinic Receptors | Nicotine reduces neutrophil recruitment to the infected area during sepsis development | [106] | |
| Pain | IL-1β | – | PGE2, ROS, Elastase | Neutrophils participate in the cascade of events leading to inflammatory hypernociception | [133,144,146] |
| Multiple Esclerosis | IL-17 | – | – | Neutrophils are required for tissue damage in the brain and development of experimental encephalomyelitis | [175,176] |
| Alzheimer’s disease | – | – | IL-17, NETs | Neutrophils contribute to Alzheimer’s disease pathogenesis and cognitive impairment | [12] |
| Experimental models of inflammation (air-pouch and peritonitis) | α2-Adrenoceptor Agonist | α2-Adrenoceptors | – | α2-Adrenoceptor agonists prevent the accumulation of neutrophils in inflammatory focus in vivo | [61] |
| In vitro assay | Ephnephrine, Epinephrine and Isoprenaline (β-Agonist) | Adrenoceptors | – | Adrenergic agents reduced neutrophil migration mainly through β-adrenoceptor activation and inhibit neutrophil extracellular traps | [62,63] |
Neutrophil represents a heterogeneous population of cells with different characteristics. They can be subdivided into at least two distinct subpopulations [201,202] named N1 and N2 neutrophils and represent their immune-stimulating or suppressive potential, respectively. These two subpopulations of neutrophils have significant clinical implications in multiple infectious and inflammatory disorders, including autoimmunity and cancer. Unfortunately, there are no specific molecular markers yet to purify and study these subpopulations. Also, the specific brain micro-environmental cues that regulate their activities remain to be elucidated. Further understanding of these cues will allow the use of neutrophils as specific CNS targets to control inflammation in neurological and neurodegenerative disorders.
Importantly, it is not clear for how long the neutrophils survive in the brain. Recent studies indicate that their lifespan may significantly vary in distinct disease conditions [203,204]. Neutrophil survival time in the brain can depend on specific factors, such as adenosine, ATP, glutamate, and hypoxia [205–207]. Neutrophils can also modulate other CNS cells such as astrocytes and induce a complex cellular process modulating neuro-inflammation in neurological and neurodegenerative disorders [208]. Future studies are also needed to determine whether neutrophil recruitment into the brain is similar to that in peripheral tissues. Recent studies suggest that the rolling and migration of neutrophils in the brain venue are coordinated by different mechanisms. However, the specific molecules involved in the adhesion and transmigration of neutrophils in these vessels are still not known and require future explorations [1].
It is also unknown whether neutrophils that enter the brain could return to the bloodstream, and consequently to peripheral tissues. Interestingly, brain cells and neurotransmitters can affect neutrophil migration and prolong their survival time [209,210]. Peripheral neutrophils can be reprogrammed into a phenotype with specific enhanced function [201]. Recent studies have described a phenomenon called ‘reverse migration’ (when neutrophils migrate from the organ that they infiltrated back to the blood vessels) [211]. It is unknown whether this phenomenon also happens in the CNS. Interestingly, lung tumor induces osteoblastic cells in bone marrow to release primed neutrophils, which promote tumor development [212]. Future studies will explore whether pathological brain tissue may prime neutrophils and change their function in peripheral organs.
Finally, experimental and clinical studies will be needed to determine the clinical implications of neutrophils in chronic neurological and neurodegenerative disorders. Further studies are needed to describe whether neutrophil infiltration is a process involving specific regions or a global event in the brain as observed in EAE model [178]. These studies could enable the development of new therapies to target the recruitment of neutrophil in neurodegenerative or infectious diseases. This approach could avoid the systemic immunosuppression induced by a general depletion of neutrophils. Moreover, the modulatory role of neurotransmitters on neutrophils is still largely controversial and may be explored. Therefore, multidisciplinary studies involving immunologists and neuroscientists will be required to define the role of the neurons/neutrophils communication in the pathophysiology of infectious, inflammatory, and neurological disorders.
Acknowledgments
The research leading to these results has received funding from the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, grants 11/20343-4, 11/19670-0, 12/04237-2, 13/08216-2, 15/25364-0, and 16/08385-7). AK is supported by National Council for Scientific and Technological Development (CNPq) and Coordination for the Improvement of Higher Education Personnel (CAPES). AB is supported by Instituto Serrapilheira/Serra (Grant 1708-15285).
Abbreviations
- ACh
acetylcholine
- ARs
adrenoceptors
- βARs
beta-adrenoceptors
- BBB
blood-brain barrier
- C5a
complement component 5a
- CFA
complete Freund’s adjuvant
- CGD
chronic granulomatous disease
- CGRP
calcitonin gene-related peptide
- CNS
central nervous system
- DAMPs
damage-associated molecular patterns
- DHPG
DL-3,4-dihydroxyphenylglycol
- DRG
dorsal root ganglia
- ELISA
enzyme-linked immunosorbent assay
- fMLP
N-formyl-methionyl-leucyl-phenylalanine
- GRP
gastrin releasing peptide
- H+
hydrogen protons
- H2O2
hydrogen peroxide
- HMGB-1
high mobility group box protein 1
- HOCl
hypochlorous acid
- HPLC
high performance liquid chromatography
- ICAM-1
intercellular adhesion molecule 1
- IFN
interferon
- IL
interleukin
- iNOS
inducible NO synthase
- InsP7
diphospho-inositol pentakisphosphate
- IκB
inhibitor of kB
- LTB4
leukotriene B4
- mAChRs
muscarinic receptors
- MET
methanephrine hydrochloride
- MPO
myeloperoxidase
- MS
multiple sclerosis
- nAChRs
nicotinic receptors
- NETs
neutrophil extracellular traps
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- NK
natural killer
- NO
nitric oxide
- PAMPs
pathogen-associated molecular patterns
- PGE2
prostaglandin E2
- PMA
phorbol-12-myristate-13-acetate
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- SP
substance P
- TH
tyrosine hydroxylase
- TNF-α
tumor necrosis factor α
- VIP
vasoactive intestinal peptide
- VLA-4
very late antigen-4
- VMAT
vesicular monoamine transporter
- α7nAChR
alpha7 subunit of nicotinic acetylcholine receptors
Footnotes
Declaration of Competing Interest
None.
References
- [1].Kolaczkowska E, Kubes P, Neutrophil recruitment and function in health and inflammation, Nat. Rev. Immunol 13 (2013) 159–175, 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- [2].Vannini F, Kashfi K, Nath N, The dual role of iNOS in cancer, Redox Biol. 6 (2015) 334–343, 10.1016/j.redox.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Jyoti A, Singh AK, Dubey M, Kumar S, Saluja R, Keshari RS, Verma A, Chandra T, Kumar A, Bajpai VK, Barthwal MK, Dikshit M, Interaction of inducible nitric oxide synthase with rac2 regulates reactive oxygen and nitrogen species generation in the human neutrophil phagosomes: implication in microbial killing, Antioxid. Redox Signal 20 (2014) 417–431, 10.1089/ars.2012.4970. [DOI] [PubMed] [Google Scholar]
- [4].Carreras MC, Pargament GA, Catz SD, Poderoso JJ, Boveris A, Kinetics of nitric oxide and hydrogen peroxide production and formation of peroxynitrite during the respiratory burst of human neutrophils, FEBS Lett. 341 (1994) 65–68, 10.1016/0014-5793(94)80241-6. [DOI] [PubMed] [Google Scholar]
- [5].Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A, Neutrophil extracellular traps kill bacteria, Science 303 (2004) 1532–1535, 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- [6].Papayannopoulos V, Neutrophil extracellular traps in immunity and disease, Nat. Rev. Immunol 18 (2018) 134–147, 10.1038/nri.2017.105. [DOI] [PubMed] [Google Scholar]
- [7].Yipp BG, Petri B, Salina D, Jenne CN, Scott BNV, Zbytnuik LD, Pittman K, Asaduzzaman M, Wu K, Meijndert HC, Malawista SE, de Boisfleury Chevance A, Zhang K, Conly J, Kubes P, Infection-induced NETosis is a dynamic process involving neutrophil multitasking in vivo, Nat. Med 18 (2012) 1386–1393, 10.1038/nm.2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Byrd AS, O’Brien XM, Johnson CM, Lavigne LM, Reichner JS, An extracellular matrix-based mechanism of rapid neutrophil extracellular trap formation in response to Candida albicans, J. Immunol 190 (2013) 4136–4148, 10.4049/jimmunol.1202671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Jorch SK, Kubes P, An emerging role for neutrophil extracellular traps in non-infectious disease, Nat. Med 23 (2017) 279–287, 10.1038/nm.4294. [DOI] [PubMed] [Google Scholar]
- [10].Bisgaard AK, Pihl-Jensen G, Frederiksen JL, The neutrophil-to-lymphocyte ratio as disease actvity marker in multiple sclerosis and optic neuritis, Mult. Scler. Relat. Disord 18 (2017) 213–217, 10.1016/j.msard.2017.10.009. [DOI] [PubMed] [Google Scholar]
- [11].Tillack K, Naegele M, Haueis C, Schippling S, Wandinger K-P, Martin R, Sospedra M, Gender differences in circulating levels of neutrophil extracellular traps in serum of multiple sclerosis patients, J. Neuroimmunol 261 (2013) 108–119, 10.1016/j.jneuroim.2013.05.004. [DOI] [PubMed] [Google Scholar]
- [12].Zenaro E, Pietronigro E, Della Bianca V, Piacentino G, Marongiu L, Budui S, Turano E, Rossi B, Angiari S, Dusi S, Montresor A, Carlucci T, Nanì S, Tosadori G, Calciano L, Catalucci D, Berton G, Bonetti B, Constantin G, Neutrophils promote Alzheimer’s disease-like pathology and cognitive decline via LFA-1 integrin, Nat. Med 21 (2015) 880–886, 10.1038/nm.3913. [DOI] [PubMed] [Google Scholar]
- [13].Perez-de-Puig I, Miró-Mur F, Ferrer-Ferrer M, Gelpi E, Pedragosa J, Justicia C, Urra X, Chamorro A, Planas AM, Neutrophil recruitment to the brain in mouse and human ischemic stroke, Acta Neuropathol. 129 (2015) 239–257, 10.1007/s00401-014-1381-0. [DOI] [PubMed] [Google Scholar]
- [14].Kim S-W, Lee H, Lee H-K, Kim I-D, Lee J-K, Neutrophil extracellular trap induced by HMGB1 exacerbates damages in the ischemic brain, Acta Neuropathol. Commun 7 (2019) 94, 10.1186/s40478-019-0747-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Baehner RL, Nathan DG, Leukocyte oxidase: defective activity in chronic granulomatous disease, Science (New York, N. Y.) 155 (1967) 835–836 (Accessed November 19, 2018), http://www.ncbi.nlm.nih.gov/pubmed/6018195. [DOI] [PubMed] [Google Scholar]
- [16].Epstein FH, Weiss SJ, Tissue destruction by neutrophils, N. Engl. J. Med 320 (1989) 365–376, 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- [17].Pavlov VA, Chavan SS, Tracey KJ, Molecular and functional neuroscience in immunity, Annu. Rev. Immunol 36 (2018) 783–812, 10.1146/annurev-immunol-042617-053158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ulloa L, The vagus nerve and the nicotinic anti-inflammatory pathway, Nat. Rev. Drug Discov 4 (2005) 673–684, 10.1038/nrd1797. [DOI] [PubMed] [Google Scholar]
- [19].Inoue T, Tanaka S, Okusa MD, Neuroimmune interactions in inflammation and acute kidney injury, Front. Immunol 8 (2017) 945, 10.3389/fimmu.2017.00945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kanashiro A, Sônego F, Ferreira RG, Castanheira FVS, Leite CA, Borges VF, Nascimento DC, Cólon DF, Alves-Filho JC, Ulloa L, Cunha FQ, Therapeutic potential and limitations of cholinergic anti-inflammatory pathway in sepsis, Pharmacol. Res 117 (2017), 10.1016/j.phrs.2016.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Levine YA, Faltys M, Chernoff D, Harnessing the inflammatory reflex for the treatment of inflammation-mediated diseases, Cold Spring Harb. Perspect. Med (2019) a034330, 10.1101/cshperspect.a034330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Phillips RJ, Powley TL, Macrophages associated with the intrinsic and extrinsic autonomic innervation of the rat gastrointestinal tract, Auton. Neurosci 169 (2012) 12–27, 10.1016/j.autneu.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Vida G, Peña G, Kanashiro A, Thompson-Bonilla MDR, Palange D, Deitch EA, Ulloa L, β2-adrenoreceptors of regulatory lymphocytes are essential for vagal neuromodulation of the innate immune system, Faseb J. 25 (2011), 10.1096/fj.11-191007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Rosas-Ballina M, Olofsson PS, Ochani M, Valdes-Ferrer SI, Levine YA, Reardon C, Tusche MW, Pavlov VA, Andersson U, Chavan S, Mak TW, Tracey KJ, Acetylcholine-synthesizing t cells relay neural signals in a vagus nerve circuit, Science 334 (2011) 98–101, 10.1126/science.1209985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Bassi GS, Brognara F, Castania JA, Talbot J, Cunha TM, Cunha FQ, Ulloa L, Kanashiro A, Dias DPM, Salgado HC, Baroreflex activation in conscious rats modulates the joint inflammatory response via sympathetic function, Brain Behav. Immun. 49 (2015) 140–147, 10.1016/j.bbi.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Bassi GS, Dias DPM, Franchin M, Talbot J, Reis DG, Menezes GB, Castania JA, Garcia-Cairasco N, Resstel LBM, Salgado HC, Cunha FQ, Cunha TM, Ulloa L, Kanashiro A, Modulation of experimental arthritis by vagal sensory and central brain stimulation, Brain Behav. Immun (2017), 10.1016/j.bbi.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kanashiro A, Franchin M, Bassi GSGS, Reis Santana DADA, Cunha TMTM, Cunha FQFQ, Ulloa L, Rodrigues GJGJ, Inhibition of spinal p38 MAPK prevents articular neutrophil infiltration in experimental arthritis via sympathetic activation, Fundam. Clin. Pharmacol 32 (2018) 155–162, 10.1111/fcp.12338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kanashiro A, de Lucena TO, Schneider AH, Alves HR, Bassi GS, Dutra SGV, de Queiróz Cunha F, Ulloa L, do Carmo Malvar D, Regulation of murine arthritis by systemic, spinal, and intra-articular adrenoceptors, Pharmacol. Rep (2019), 10.1016/j.pharep.2019.06.010. [DOI] [PubMed] [Google Scholar]
- [29].Basu S, Hodgson G, Katz M, Dunn AR, Evaluation of role of G-CSF in the production, survival, and release of neutrophils from bone marrow into circulation, Blood 100 (2002) 854–861 (Accessed September 24, 2018), http://www.ncbi.nlm.nih.gov/pubmed/12130495. [DOI] [PubMed] [Google Scholar]
- [30].Lahoz-Beneytez J, Elemans M, Zhang Y, Ahmed R, Salam A, Block M, Niederalt C, Asquith B, Macallan D, Human neutrophil kinetics: modeling of stable isotope labeling data supports short blood neutrophil half-lives, Blood 127 (2016) 3431–3438, 10.1182/blood-2016-03-700336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Sônego F, Castanheira FVS, Ferreira RG, Kanashiro A, Leite CAVG, Nascimento DC, Colón DF, Borges VF, Alves-Filho JC, Cunha FQ, Paradoxical roles of the neutrophil in sepsis: protective and deleterious, Front. Immunol 7 (2016), 10.3389/fimmu.2016.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Rosas-Ballina M, Ochani M, Parrish WR, Ochani K, Harris YT, Huston JM, Chavan S, Tracey KJ, Splenic nerve is required for cholinergic antiinflammatory pathway control of TNF in endotoxemia, Proc. Natl. Acad. Sci. U. S. A 105 (2008) 11008–11013, 10.1073/pnas.0803237105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Bulloch K, Pomerantz W, Autonomic nervous system innervation of thymic-related lymphoid tissue in wildtype and nude mice, J. Comp. Neurol 228 (1984) 57–68, 10.1002/cne.902280107. [DOI] [PubMed] [Google Scholar]
- [34].Kohm AP, Sanders VM, Norepinephrine and beta 2-adrenergic receptor stimulation regulate CD4+ T and B lymphocyte function in vitro and in vivo, Pharmacol. Rev 53 (2001) 487–525 (Accessed January 10, 2019), http://www.ncbi.nlm.nih.gov/pubmed/11734616. [PubMed] [Google Scholar]
- [35].Leposavić G, Pilipović I, Perišić M, Age-associated remodeling of neural and nonneural thymic catecholaminergic network affects thymopoietic productivity, Neuroimmunomodulation 18 (2011) 290–308, 10.1159/000329499. [DOI] [PubMed] [Google Scholar]
- [36].Neuhuber WL, Tiegs G, Innervation of immune cells: evidence for neuroimmunomodulation in the liver, Anat. Rec 280A (2004) 884–892, 10.1002/ar.a.20093. [DOI] [PubMed] [Google Scholar]
- [37].Antonica A, Ayroldi E, Magni F, Paolocci N, Lymphocyte traffic changes induced by monolateral vagal denervation in mouse thymus and peripheral lymphoid organs, J. Neuroimmunol 64 (1996) 115–122 (Accessed July 18, 2018), http://www.ncbi.nlm.nih.gov/pubmed/8632053. [DOI] [PubMed] [Google Scholar]
- [38].Grandl G, Wolfrum C, Hemostasis, endothelial stress, inflammation, and the metabolic syndrome, Semin. Immunopathol 40 (2018) 215–224, 10.1007/s00281-017-0666-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].McMaster WG, Kirabo A, Madhur MS, Harrison DG, Inflammation, Immunity, and Hypertensive End-Organ Damage, Circ. Res 116 (2015) 1022–1033, 10.1161/CIRCRESAHA.116.303697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Nosalski R, McGinnigle E, Siedlinski M, Guzik TJ, Novel immune mechanisms in hypertension and cardiovascular risk, Curr. Cardiovasc. Risk Rep 11 (2017) 12, 10.1007/s12170-017-0537-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Saltiel AR, Olefsky JM, Inflammatory mechanisms linking obesity and metabolic disease, J. Clin. Invest 127 (2017) 1–4, 10.1172/JCI92035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Carnevale D, Pallante F, Fardella V, Fardella S, Iacobucci R, Federici M, Cifelli G, De Lucia M, Lembo G, The angiogenic factor PlGF mediates a neuroimmune interaction in the spleen to allow the onset of hypertension, Immunity 41 (2014) 737–752, 10.1016/j.immuni.2014.11.002. [DOI] [PubMed] [Google Scholar]
- [43].Carnevale D, Perrotta M, Pallante F, Fardella V, Iacobucci R, Fardella S, Carnevale L, Carnevale R, De Lucia M, Cifelli G, Lembo G, A cholinergic-sympathetic pathway primes immunity in hypertension and mediates brain-to-spleen communication, Nat. Commun 7 (2016) 13035, 10.1038/ncomms13035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Chang EH, Chavan SS, Pavlov VA , Cholinergic control of inflammation, metabolic dysfunction, and cognitive impairment in obesity-associated disorders: mechanisms and novel therapeutic opportunities, Front. Neurosci 13 (2019), 10.3389/fnins.2019.00263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].van Gils JM, Derby MC, Fernandes LR, Ramkhelawon B, Ray TD, Rayner KJ, Parathath S, Distel E, Feig JL, Alvarez-Leite JI, Rayner AJ, McDonald TO, O’Brien KD, Stuart LM, Fisher EA, Lacy-Hulbert A, Moore KJ, The neuroimmune guidance cue netrin-1 promotes atherosclerosis by inhibiting the emigration of macrophages from plaques, Nat. Immunol 13 (2012) 136–143, 10.1038/ni.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Vasamsetti SB, Florentin J, Coppin E, Stiekema LCA, Zheng KH, Nisar MU, Sembrat J, Levinthal DJ, Rojas M, Stroes ESG, Kim K, Dutta P, Sympathetic neuronal activation triggers myeloid progenitor proliferation and differentiation, Immunity 49 (2018), [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Wong CHY, Jenne CN, Lee W-Y, Léger C, Kubes P, Functional innervation of hepatic iNKT cells is immunosuppressive following stroke, Science (New York, N. Y.) 334 (2011) 101–105, 10.1126/science.1210301. [DOI] [PubMed] [Google Scholar]
- [48].Kanashiro A, Bassi GS, de Queiróz Cunha F, Ulloa L , From neuroimunomodulation to bioelectronic treatment of rheumatoid arthritis, Bioelectron. Med 1 (2018) 151–165, 10.2217/bem-2018-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Bonaz B, Sinniger V, Hoffmann D, Clarençon D, Mathieu N, Dantzer C, Vercueil L, Picq C, Trocmé C, Faure P, Cracowski J-L, Pellissier S, Chronic vagus nerve stimulation in Crohn’s disease: a 6-month follow-up pilot study, Neurogastroenterol. Motil 28 (2016) 948–953, 10.1111/nmo.12792. [DOI] [PubMed] [Google Scholar]
- [50].Koopman FA, Chavan SS, Miljko S, Grazio S, Sokolovic S, Schuurman PR, Mehta AD, Levine YA, Faltys M, Zitnik R, Tracey KJ, Tak PP, Vagus nerve stimulation inhibits cytokine production and attenuates disease severity in rheumatoid arthritis, Proc. Natl. Acad. Sci 113 (2016) 8284–8289, 10.1073/pnas.1605635113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Huston JM, Rosas-Ballina M, Xue X, Dowling O, Ochani K, Ochani M, Yeboah MM, Chatterjee PK, Tracey KJ, Metz CN, Cholinergic neural signals to the spleen down-regulate leukocyte trafficking via CD11b, J. Immunol. (Baltimore, Md.: 1950) 183 (2009) 552–559, 10.4049/jimmunol.0802684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Flierl MA, Rittirsch D, Nadeau BA, Chen AJ, Sarma JV, Zetoune FS, McGuire SR, List RP, Day DE, Hoesel LM, Gao H, Van Rooijen N, Huber-Lang MS, Neubig RR, Ward PA, Phagocyte-derived catecholamines enhance acute inflammatory injury, Nature 449 (2007) 721–725, 10.1038/nature06185. [DOI] [PubMed] [Google Scholar]
- [53].Cosentino M, Marino F, Bombelli R, Ferrari M, Lecchini S, Frigo G, Endogenous catecholamine synthesis, metabolism, storage and uptake in human neutrophils, Life Sci. 64 (1999) 975–981 (Accessed September 21, 2018), http://www.ncbi.nlm.nih.gov/pubmed/10201646. [DOI] [PubMed] [Google Scholar]
- [54].Neumann S, Razen M, Habermehl P, Meyer CU, Zepp F, Kirkpatrick CJ, Wessler I, The non-neuronal cholinergic system in peripheral blood cells: effects of nicotinic and muscarinic receptor antagonists on phagocytosis, respiratory burst and migration, Life Sci. 80 (2007) 2361–2364, 10.1016/j.lfs.2007.01.010. [DOI] [PubMed] [Google Scholar]
- [55].Cotecchia S, Stanasila L, Diviani D, Protein-protein interactions at the adrenergic receptors, Curr. Drug Targets 13 (2012) 15–27 (Accessed January 11, 2019), http://www.ncbi.nlm.nih.gov/pubmed/21777184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Akinaga J, García-Sáinz JA, Pupo AS, Updates in the function and regulation of α1 -adrenoceptors, Br. J. Pharmacol 176 (2019) 2343–2357, 10.1111/bph.14617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Alexander SP, Davenport AP, Kelly E, Marrion N, Peters JA, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Southan C, Davies JA, CGTP Collaborators, The concise guide to PHARMACOLOGY 2015/16: g protein-coupled receptors, Br. J. Pharmacol 172 (2015) 5744–5869, 10.1111/bph.13348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Boreus LO, Hjemdahl P, Lagercrantz H, Martinsson A, Yao AC, Beta-adrenoceptor function in white blood cells from newborn infants: no relation to plasma catecholamine levels, Pediatr. Res 20 (1986) 1152–1155, 10.1203/00006450-198611000-00022. [DOI] [PubMed] [Google Scholar]
- [59].Pohl A, Otto J, Urbanek R, Beta-2-adrenoceptors of polymorphonuclear leukocytes in children with atopic dermatitis. Their number and affinity to the radioligand [125I]-cyanopindolol, Int. Arch. Allergy Appl. Immunol. 95 (1991) 261–265 (Accessed September 22, 2018), http://www.ncbi.nlm.nih.gov/pubmed/1682276. [DOI] [PubMed] [Google Scholar]
- [60].Gurguis GN, Andrews R, Antai-Otong D, Vo SP, Blakeley JE, Orsulak PJ, Rush AJ, Neutrophil beta2-adrenergic receptor coupling efficiency to Gs protein in subjects with post-traumatic stress disorder and normal controls, Psychopharmacology 143 (1999) 131–140 (Accessed September 22, 2018), http://www.ncbi.nlm.nih.gov/pubmed/10326775. [DOI] [PubMed] [Google Scholar]
- [61].Herrera-García AM, Domínguez-Luis MJ, Arce-Franco M, Armas-González E, Álvarez D de La Rosa, J.D. Machado, M.K. Pec, M. Feria, O. Barreiro, F. Sánchez-Madrid, F. Díaz-González, Prevention of neutrophil extravasation by α2-adrenoceptor-mediated endothelial stabilization, J. Immunol. (Baltimore, Md.: 1950) 193 (2014) 3023–3035, 10.4049/jimmunol.1400255. [DOI] [PubMed] [Google Scholar]
- [62].Scanzano A, Schembri L, Rasini E, Luini A, Dallatorre J, Legnaro M, Bombelli R, Congiu T, Cosentino M, Marino F, Adrenergic modulation of migration, CD11b and CD18 expression, ROS and interleukin-8 production by human polymorphonuclear leukocytes, Inflamm. Res 64 (2015) 127–135, 10.1007/s00011-014-0791-8. [DOI] [PubMed] [Google Scholar]
- [63].Marino F, Scanzano A, Pulze L, Pinoli M, Rasini E, Luini A, Bombelli R, Legnaro M, de Eguileor M, Cosentino M, Β 2 -Adrenoceptors inhibit neutrophil extracellular traps in human polymorphonuclear leukocytes, J. Leukoc. Biol (2018), 10.1002/JLB.3A1017-398RR. [DOI] [PubMed] [Google Scholar]
- [64].Barnett CC, Moore EE, Partrick DA, Silliman CC, Β-adrenergic stimulation down-regulates neutrophil priming for superoxide generation, but not elastase release, J. Surg. Res 70 (1997) 166–170, 10.1006/jsre.1997.5118. [DOI] [PubMed] [Google Scholar]
- [65].Nielson CP, Beta-adrenergic modulation of the polymorphonuclear leukocyte respiratory burst is dependent upon the mechanism of cell activation, J. Immunol. (Baltimore, Md.: 1950) 139 (1987) 2392–2397 (Accessed September 22, 2018), http://www.ncbi.nlm.nih.gov/pubmed/2821113. [PubMed] [Google Scholar]
- [66].Gibson-Berry KL, Whitin JC, Cohen HJ, Modulation of the respiratory burst in human neutrophils by isoproterenol and dibutyryl cyclic AMP, J. Neuroimmunol 43 (1993) 59–68 (Accessed September 19, 2018), http://www.ncbi.nlm.nih.gov/pubmed/8384637. [DOI] [PubMed] [Google Scholar]
- [67].Bazzoni G, Dejana E, Del Maschio A, Adrenergic modulation of human polymorphonuclear leukocyte activation. Potentiating effect of adenosine, Blood 77 (1991) 2042–2048 (Accessed September 19, 2018), http://www.ncbi.nlm.nih.gov/pubmed/1850310. [PubMed] [Google Scholar]
- [68].Brunskole Hummel I, Reinartz MT, Kälble S, Burhenne H, Schwede F, Buschauer A, Seifert R, Dissociations in the effects of β2-Adrenergic receptor agonists on cAMP formation and superoxide production in human neutrophils: support for the concept of functional selectivity, PLoS One 8 (2013) e64556, 10.1371/journal.pone.0064556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Galant SP, Duriseti L, Underwood S, Insel PA, Decreased beta-adrenergic receptors on polymorphonuclear leukocytes after adrenergic therapy, N. Engl. J. Med 299 (1978) 933–936, 10.1056/NEJM197810262991707. [DOI] [PubMed] [Google Scholar]
- [70].Nicholls AJ, Wen SW, Hall P, Hickey MJ, Wong CHY, Activation of the sympathetic nervous system modulates neutrophil function, J. Leukoc. Biol 103 (2018) 295–309, 10.1002/JLB.3MA0517-194RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Myers MG, Norris JW, Hachniski VC, Sole MJ, Plasma norepinephrine in stroke, Stroke 12 (2000) 200–204 (Accessed September 26, 2018), http://www.ncbi.nlm.nih.gov/pubmed/7233464. [DOI] [PubMed] [Google Scholar]
- [72].Davies AO, Lefkowitz RJ, In vitro desensitization of beta adrenergic receptors in human neutrophils. Attenuation by corticosteroids, J. Clin. Invest. 71 (1983) 565–571 (Accessed August 20, 2018), http://www.ncbi.nlm.nih.gov/pubmed/6298279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Cotter TG, O’Malley K, Decreased neutrophil cyclic AMP response to isoprenaline stimulation in the elderly, Clin. Sci. (London, England: 1979) 65 (1983) 155–157 (Accessed September 22, 2018), http://www.ncbi.nlm.nih.gov/pubmed/6305549. [DOI] [PubMed] [Google Scholar]
- [74].Roan Y, Galant SP, Decreased neutrophil Beta Adrenergic receptors in the neonate, Pediatr. Res 16 (1982) 591–593, 10.1203/00006450-198208000-00001. [DOI] [PubMed] [Google Scholar]
- [75].Corradi L, Negri F, Parini A, Partesana N, Finardi G, Decreased beta-adrenoceptors in polymorphonucleates in essential hypertension, Boll. Soc. Ital. Biol. Sper 57 (1981) 1766–1770 (Accessed September 22, 2018), http://www.ncbi.nlm.nih.gov/pubmed/6272827. [PubMed] [Google Scholar]
- [76].Schwab KO, Bartels H, Martin C, Leichtenschlag EM, Decreased beta 2-adrenoceptor density and decreased isoproterenol induced c-AMP increase in juvenile type I diabetes mellitus: an additional cause of severe hypoglycaemia in childhood diabetes? Eur. J. Pediatr 152 (1993) 797–801 (Accessed September 22, 2018), http://www.ncbi.nlm.nih.gov/pubmed/8223779. [DOI] [PubMed] [Google Scholar]
- [77].Iizuka H, Adachi K, Halprin KM, Levine V, Cyclic AMP accumulation in psoriatic skin: differential responses to histamine, AMP, and einephrine by the uninvolved and involved epidermis, J. Invest. Dermatol 70 (1978) 250–253 (Accessed September 18, 2018), http://www.ncbi.nlm.nih.gov/pubmed/205616. [DOI] [PubMed] [Google Scholar]
- [78].Fräki JE, Jakoi L, Davies AO, Lefkowitz RJ, Snyderman R, Lazarus GS, Polymorphonuclear leukocyte function in psoriasis: chemotaxis, chemokinesis, beta-adrenergic receptors, and proteolytic enzymes of polymorphonuclear leukocytes in the peripheral blood from psoriatic patients, J. Invest. Dermatol 81 (1983) 254–257 (Accessed August 20, 2018), http://www.ncbi.nlm.nih.gov/pubmed/6309987. [DOI] [PubMed] [Google Scholar]
- [79].Méndez-Ferrer S, Lucas D, Battista M, Frenette PS, Haematopoietic stem cell release is regulated by circadian oscillations, Nature 452 (2008) 442–447, 10.1038/nature06685. [DOI] [PubMed] [Google Scholar]
- [80].Haskó G, Szabó C, Merkel K, Bencsics A, Zingarelli B, Kvetan V, Vizi ES, Modulation of lipopolysaccharide-induced tumor necrosis factor-alpha and nitric oxide production by dopamine receptor agonists and antagonists in mice, Immunol. Lett 49 (1996) 143–147 (Accessed July 17, 2018), http://www.ncbi.nlm.nih.gov/pubmed/8739308. [DOI] [PubMed] [Google Scholar]
- [81].Torres-Rosas R, Yehia G, Pena G, Mishra P, del Rocio Thompson-Bonilla M, Moreno-Eutimio MA, Arriaga-Pizano LA, Isibasi A, Ulloa L, Dopamine mediates vagal modulation of the immune system by electroacupuncture, Nat. Med 20 (2014) 291–295, 10.1038/nm.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Lachowicz JE, Sibley DR, Molecular characteristics of mammalian dopamine receptors, Pharmacol. Toxicol 81 (1997) 105–113 (Accessed July 17, 2018), http://www.ncbi.nlm.nih.gov/pubmed/9335067. [DOI] [PubMed] [Google Scholar]
- [83].McKenna F, McLaughlin PJ, Lewis BJ, Sibbring GC, Cummerson JA, Bowen-Jones D, Moots RJ, Dopamine receptor expression on human T- and B-lymphocytes, monocytes, neutrophils, eosinophils and NK cells: a flow cytometric study, J. Neuroimmunol 132 (2002) 34–40 (Accessed July 17, 2018), http://www.ncbi.nlm.nih.gov/pubmed/12417431. [DOI] [PubMed] [Google Scholar]
- [84].Derevenco P, Marina C, Pavel T, Olteanu A, Junie M, Baciu I, Phagocytic response in rats following chemical sympathectomy with 6-hydroxydopamine, Rev. Roum. Physiol. (1990) 29 (1992) 57–62 (Accessed July 17, 2018), http://www.ncbi.nlm.nih.gov/pubmed/1306083. [PubMed] [Google Scholar]
- [85].Meli R, Raso GM, Gualillo O, Pacilio M, Di Carlo R, Prolactin modulation of nitric oxide and TNF-alpha production by peripheral neutrophils in rats, Life Sci. 61 (1997) 1395–1403 (Accessed July 18, 2018), http://www.ncbi.nlm.nih.gov/pubmed/9335229. [DOI] [PubMed] [Google Scholar]
- [86].Yamazaki M, Matsuoka T, Yasui K, Komiyama A, Akabane T, Dopamine inhibition of superoxide anion production by polymorphonuclear leukocytes, J. Allergy Clin. Immunol. 83 (1989) 967–972 (Accessed August 20, 2018), http://www.ncbi.nlm.nih.gov/pubmed/2541191. [DOI] [PubMed] [Google Scholar]
- [87].Matsuoka T, A sedative effect of dopamine on the respiratory burst in neonatal polymorphonuclear leukocytes, Pediatr. Res 28 (1990) 24–27, 10.1203/00006450-199007000-00006. [DOI] [PubMed] [Google Scholar]
- [88].Wenisch C, Parschalk B, Weiss A, Zedwitz-Liebenstein K, Hahsler B, Wenisch H, Georgopoulos A, Graninger W, High-dose catecholamine treatment decreases polymorphonuclear leukocyte phagocytic capacity and reactive oxygen production, Clin. Diagn. Lab. Immunol 3 (1996) 423–428 (Accessed July 18, 2018), http://www.ncbi.nlm.nih.gov/pubmed/8807207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Fujii T, Mashimo M, Moriwaki Y, Misawa H, Ono S, Horiguchi K, Kawashima K , Expression and function of the cholinergic system in immune cells, Front. Immunol 8 (2017) 1085, 10.3389/fimmu.2017.01085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, Li JH, Wang H, Yang H, Ulloa L, Al-Abed Y, Czura CJ, Tracey KJ, Nicotinic acetylcholine receptor α7 subunit is an essential regulator of inflammation, Nature 421 (2002) 384–388, 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- [91].Benhammou K, Lee M, Strook M, Sullivan B, Logel J, Raschen K, Gotti C, Leonard S, [(3)H]Nicotine binding in peripheral blood cells of smokers is correlated with the number of cigarettes smoked per day, Neuropharmacology 39 (2000) 2818–2829 (Accessed July 17, 2018), http://www.ncbi.nlm.nih.gov/pubmed/11044752. [DOI] [PubMed] [Google Scholar]
- [92].Safronova VG, Vulfius CA, Shelukhina IV, Mal’tseva VN, Berezhnov AV, Fedotova EI, Miftahova RG, Kryukova EV, Grinevich AA, Tsetlin VI, Nicotinic receptor involvement in regulation of functions of mouse neutrophils from inflammatory site, Immunobiology 221 (2016) 761–772, 10.1016/j.imbio.2016.01.016. [DOI] [PubMed] [Google Scholar]
- [93].Speer P, Zhang Y, Gu Y, Lucas MJ, Wang Y, Effects of nicotine on intercellular adhesion molecule expression in endothelial cells and integrin expression in neutrophils in vitro, Am. J. Obstet. Gynecol 186 (2002) 551–556 (Accessed July 17, 2018), http://www.ncbi.nlm.nih.gov/pubmed/11904622. [DOI] [PubMed] [Google Scholar]
- [94].Seow WK, Thong YH, Nelson RD, MacFarlane GD, Herzberg MC, Nicotine-induced release of elastase and eicosanoids by human neutrophils, Inflammation 18 (1994) 119–127 (Accessed July 31, 2018), http://www.ncbi.nlm.nih.gov/pubmed/8070897. [DOI] [PubMed] [Google Scholar]
- [95].Pabst MJ, Pabst KM, Collier JA, Coleman TC, Lemons-Prince ML, Godat MS, Waring MB, Babu JP, Inhibition of neutrophil and monocyte defensive functions by nicotine, J. Periodontol 66 (1995) 1047–1055, 10.1902/jop.1995.66.12.1047. [DOI] [PubMed] [Google Scholar]
- [96].Sasagawa S, Suzuki K, Sakatani T, Fujikura T, Effects of nicotine on the functions of human polymorphonuclear leukocytes in vitro, J. Leukoc. Biol 37 (1985) 493–502 (Accessed July 17, 2018), http://www.ncbi.nlm.nih.gov/pubmed/2984301. [DOI] [PubMed] [Google Scholar]
- [97].Sasagawa S, Kameda H, Sudo J, Tanabe T, Inhibitory effect of nicotine on chemiluminescence response of human polymorphonuclear leukocytes stimulated by opsonized zymosan in vitro, J. Toxicol. Sci 9 (1984) 1–9 (Accessed August 3, 2018), http://www.ncbi.nlm.nih.gov/pubmed/6471124. [DOI] [PubMed] [Google Scholar]
- [98].Salama SA, Arab HH, Omar HA, Maghrabi IA, Snapka RM, Nicotine mediates hypochlorous acid-induced nuclear protein damage in mammalian cells, Inflammation 37 (2014) 785–792, 10.1007/s10753-013-9797-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Lu B, Kwan K, Levine YA, Olofsson PS, Yang H, Li J, Joshi S, Wang H, Andersson U, Chavan SS, Tracey KJ, α7 nicotinic acetylcholine receptor signaling inhibits inflammasome activation by preventing mitochondrial DNA release, Mol. Med 20 (2014) 1, 10.2119/molmed.2013.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Gergalova G, Lykhmus O, Kalashnyk O, Koval L, Chernyshov V, Kryukova E, Tsetlin V, Komisarenko S, Skok M, Mitochondria express α7 nicotinic acetylcholine receptors to regulate Ca2+ accumulation and cytochrome c release: study on isolated mitochondria, PLoS One 7 (2012) e31361, 10.1371/journal.pone.0031361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Kalashnyk OM, Gergalova GL, Komisarenko SV, Skok MV, Intracellular localization of nicotinic acetylcholine receptors in human cell lines, Life Sci. 91 (2012) 1033–1037, 10.1016/j.lfs.2012.02.005. [DOI] [PubMed] [Google Scholar]
- [102].Wang H, Liao H, Ochani M, Justiniani M, Lin X, Yang L, Al-Abed Y, Wang H, Metz C, Miller EJ, Tracey KJ, Ulloa L, Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis, Nat. Med 10 (2004) 1216–1221, 10.1038/nm1124. [DOI] [PubMed] [Google Scholar]
- [103].Ni YF, Tian F, Lu ZF, Yang GD, Fu HY, Wang J, Yan XL, Zhao YC, Wang J, Jiang T, Protective effect of nicotine on lipopolysaccharide-induced acute lung injury in mice, Respiration 81 (2011) 39–46, 10.1159/000319151. [DOI] [PubMed] [Google Scholar]
- [104].Mabley J, Gordon S, Pacher P, Nicotine exerts an anti-inflammatory effect in a murine model of acute lung injury, Inflammation 34 (2011) 231–237, 10.1007/s10753-010-9228-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].van Westerloo DJ, Giebelen IAJ, Florquin S, Daalhuisen J, Bruno MJ, de Vos AF, Tracey KJ, van der Poll T, The cholinergic anti-inflammatory pathway regulates the host response during septic peritonitis, J. Infect. Dis 191 (2005) 2138–2148, 10.1086/430323. [DOI] [PubMed] [Google Scholar]
- [106].Giebelen IAJ, Leendertse M, Florquin S, van der Poll T, Stimulation of acetylcholine receptors impairs host defence during pneumococcal pneumonia, Eur. Respir. J 33 (2009) 375–381, 10.1183/09031936.00103408. [DOI] [PubMed] [Google Scholar]
- [107].Giebelen IAJ, Moine AL, van den Pangaart PS, Sadis C, Goldman M, Florquin S, van der Poll T, Deficiency of α7 cholinergic receptors facilitates bacterial clearance in Escherichia coli peritonitis, J. Infect. Dis 198 (2008) 750–757, 10.1086/590432. [DOI] [PubMed] [Google Scholar]
- [108].Yu H, Yang Y-H, Rajaiah R, Moudgil KD, Nicotine-induced differential modulation of autoimmune arthritis in the Lewis rat involves changes in interleukin-17 and anti-cyclic citrullinated peptide antibodies, Arthritis Rheum. 63 (2011) 981–991, 10.1002/art.30219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Lee J, Luria A, Rhodes C, Raghu H, Lingampalli N, Sharpe O, Rada B, Sohn DH, Robinson WH, Sokolove J, Nicotine drives neutrophil extracellular traps formation and accelerates collagen-induced arthritis, Rheumatology 56 (2016) kew449, 10.1093/rheumatology/kew449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Iho S, Tanaka Y, Takauji R, Kobayashi C, Muramatsu I, Iwasaki H, Nakamura K, Sasaki Y, Nakao K, Takahashi T, Nicotine induces human neutrophils to produce IL-8 through the generation of peroxynitrite and subsequent activation of NF-kappaB, J. Leukoc. Biol 74 (2003) 942–951, 10.1189/jlb.1202626. [DOI] [PubMed] [Google Scholar]
- [111].Zhu D, Hattori H, Jo H, Jia Y, Subramanian KK, Loison F, You J, Le Y, Honczarenko M, Silberstein L, Luo HR, Deactivation of phosphatidylinositol 3,4,5-trisphosphate/Akt signaling mediates neutrophil spontaneous death, Proc. Natl. Acad. Sci. U. S. A 103 (2006) 14836–14841, 10.1073/pnas.0605722103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Xu Y, Li H, Bajrami B, Kwak H, Cao S, Liu P, Zhou J, Zhou Y, Zhu H, Ye K, Luo HR, Cigarette smoke (CS) and nicotine delay neutrophil spontaneous death via suppressing production of diphosphoinositol pentakisphosphate, Proc. Natl. Acad. Sci. U. S. A 110 (2013) 7726–7731, 10.1073/pnas.1302906110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Aoshiba K, Nagai A, Yasui S, Konno K, Nicotine prolongs neutrophil survival by suppressing apoptosis, J. Lab. Clin. Med 127 (1996) 186–194 (Accessed July 17, 2018), http://www.ncbi.nlm.nih.gov/pubmed/8636647. [DOI] [PubMed] [Google Scholar]
- [114].Hosseinzadeh A, Thompson PR, Segal BH, Urban CF, Nicotine induces neutrophil extracellular traps, J. Leukoc. Biol 100 (2016) 1105–1112, 10.1189/jlb.3AB0815-379RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Bang SK, Ryu Y, Chang S, Im CK, Bae JH, Gwak YS, Yang CH, Kim HY, Attenuation of hypertension by C-fiber stimulation of the human median nerve and the concept-based novel device, Sci. Rep 8 (2018) 14967, 10.1038/s41598-018-33402-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Ji R-R, Chamessian A, Zhang Y-Q, Pain regulation by non-neuronal cells and inflammation, Science 354 (2016) 572–577, 10.1126/science.aaf8924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Woolf CJ, Ma Q, Nociceptors—noxious stimulus detectors, Neuron 55 (2007) 353–364, 10.1016/j.neuron.2007.07.016. [DOI] [PubMed] [Google Scholar]
- [118].Amann R, Neutrophils in inflammatory pain, Encyclopedia of Pain, Springer-Verlag, Berlin Heidelberg, 2007, 10.1007/978-3-540-29805-2_271. [DOI] [Google Scholar]
- [119].Krishnamoorthy S, Recchiuti A, Chiang N, Yacoubian S, Lee C-H, Yang R, Petasis NA, Serhan CN, Resolvin D1 binds human phagocytes with evidence for proresolving receptors, Proc. Natl. Acad. Sci. U. S. A 107 (2010) 1660–1665, 10.1073/pnas.0907342107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Ohira T, Arita M, Omori K, Recchiuti A, Van Dyke TE, Serhan CN, Resolvin E1 receptor activation signals phosphorylation and phagocytosis, J. Biol. Chem 285 (2010) 3451–3461, 10.1074/jbc.M109.044131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Ghasemlou N, Chiu IM, Julien J-P, Woolf CJ, CD11b + Ly6G − myeloid cells mediate mechanical inflammatory pain hypersensitivity, Proc. Natl. Acad. Sci 112 (2015) E6808–E6817, 10.1073/pnas.1501372112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Guerrero ATG, Verri WA, Cunha TM, Silva TA, Schivo IRS, Dal-Secco D, Canetti C, Rocha FAC, Parada CA, Cunha FQ, Ferreira SH, Involvement of LTB4 in zymosan-induced joint nociception in mice: participation of neutrophils and PGE2, J. Leukoc. Biol 83 (2008) 122–130, 10.1189/jlb.0207123. [DOI] [PubMed] [Google Scholar]
- [123].Camous L, Roumenina L, Bigot S, Brachemi S, Frémeaux-Bacchi V, Lesavre P, Halbwachs-Mecarelli L, Complement alternative pathway acts as a positive feedback amplification of neutrophil activation, Blood 117 (2011) 1340–1349, 10.1182/blood-2010-05-283564. [DOI] [PubMed] [Google Scholar]
- [124].Grace PM, Hutchinson MR, Maier SF, Watkins LR, Pathological pain and the neuroimmune interface, Nat. Rev. Immunol 14 (2014) 217–231, 10.1038/nri3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Levine JD, Lau W, Kwiat G, Goetzl EJ, Leukotriene B4 produces hyperalgesia that is dependent on polymorphonuclear leukocytes, Science (New York, N. Y.) 225 (1984) 743–745 (Accessed October 17, 2018), http://www.ncbi.nlm.nih.gov/pubmed/6087456. [DOI] [PubMed] [Google Scholar]
- [126].Levine JD, Gooding J, Donatoni P, Borden L, Goetzl EJ, The role of the polymorphonuclear leukocyte in hyperalgesia, J. Neurosci. 5 (1985) 3025–3029 (Accessed October 17, 2018), http://www.ncbi.nlm.nih.gov/pubmed/2997412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Moriconi A, Cunha TM, Souza GR, Lopes AH, Cunha FQ, Carneiro VL, Pinto LG, Brandolini L, Aramini A, Bizzarri C, Bianchini G, Beccari AR, Fanton M, Bruno A, Costantino G, Bertini R, Galliera E, Locati M, Ferreira SH, Teixeira MM, Allegretti M, Targeting the minor pocket of C5aR for the rational design of an oral allosteric inhibitor for inflammatory and neuropathic pain relief, Proc. Natl. Acad. Sci 111 (2014) 16937–16942, 10.1073/pnas.1417365111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Cunha TM, Dal-Secco D, Verri WA, Guerrero AT, Souza GR, Vieira SM, Lotufo CM, Neto AF, Ferreira SH, Cunha FQ, Dual role of hydrogen sulfide in mechanical inflammatory hypernociception, Eur. J. Pharmacol 590 (2008) 127–135, 10.1016/j.ejphar.2008.05.048. [DOI] [PubMed] [Google Scholar]
- [129].Cunha TM, Barsante MM, Guerrero AT, Verri WA, Ferreira SH, Coelho FM, Bertini R, Di Giacinto C, Allegretti M, Cunha FQ, Teixeira MM, Treatment with DF 2162, a non-competitive allosteric inhibitor of CXCR1/2, diminishes neutrophil influx and inflammatory hypernociception in mice, Br. J. Pharmacol 154 (2009) 460–470, 10.1038/bjp.2008.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Cunha FQ, Poole S, Lorenzetti BB, Ferreira SH, The pivotal role of tumour necrosis factor alpha in the development of inflammatory hyperalgesia, Br. J. Pharmacol 107 (1992) 660–664 (Accessed October 17, 2018), http://www.ncbi.nlm.nih.gov/pubmed/1472964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Perkins NM, Tracey DJ, Hyperalgesia due to nerve injury: role of neutrophils, Neuroscience 101 (2000) 745–757 (Accessed October 17, 2018), http://www.ncbi.nlm.nih.gov/pubmed/11113323. [DOI] [PubMed] [Google Scholar]
- [132].Verri WA Jr, Cunha TM, Magro DA, Guerrero ATG, Vieira SM, Carregaro V, Souza GR, das G M.M.O. Henriques, S.H. Ferreira, F.Q. Cunha, Targeting endothelin ETA and ETB receptors inhibits antigen-induced neutrophil migration and mechanical hypernociception in mice, Naunyn Schmiedebergs Arch. Pharmacol 379 (2009) 271–279, 10.1007/s00210-008-0360-1. [DOI] [PubMed] [Google Scholar]
- [133].Cunha TM, Verri WA, Schivo IR, Napimoga MH, Parada CA, Poole S, Teixeira MM, Ferreira SH, Cunha FQ, Crucial role of neutrophils in the development of mechanical inflammatory hypernociception, J. Leukoc. Biol 83 (2008) 824–832, 10.1189/jlb.0907654. [DOI] [PubMed] [Google Scholar]
- [134].Carreira EU, Carregaro V, Teixeira MM, Moriconi A, Aramini A, Verri WA, Ferreira SH, Cunha FQ, Cunha TM, Neutrophils recruited by CXCR1/2 signalling mediate post-incisional pain, Eur. J. Pain 17 (2013) 654–663, 10.1002/j.1532-2149.2012.00240.x. [DOI] [PubMed] [Google Scholar]
- [135].Bisgaard H, Kristensen JK, Leukotriene B4 produces hyperalgesia in humans, Prostaglandins 30 (1985) 791–797 (Accessed October 17, 2018), http://www.ncbi.nlm.nih.gov/pubmed/3001832. [DOI] [PubMed] [Google Scholar]
- [136].Jones AK, al-Janabi MA, Solanki K, Sobnack R, Greenwood A, Doyle DV, Britton KE, Huskisson EC, In vivo leukocyte migration in arthritis, Arthritis Rheum. 34 (1991) 270–275 (Accessed October 17, 2018), http://www.ncbi.nlm.nih.gov/pubmed/2003853. [DOI] [PubMed] [Google Scholar]
- [137].Bayley R, Kite KA, McGettrick HM, Smith JP, Kitas GD, Buckley CD, Young SP, The autoimmune-associated genetic variant PTPN22 R620W enhances neutrophil activation and function in patients with rheumatoid arthritis and healthy individuals, Ann. Rheum. Dis 74 (2015) 1588–1595, 10.1136/annrheumdis-2013-204796. [DOI] [PubMed] [Google Scholar]
- [138].Chandrashekara S, Rajendran A, Bai Jaganath A, Krishnamurthy R, Neutrophillymphocyte ratio, pain perception, and disease activity score may serve as important predictive markers for sustained remission in rheumatoid arthritis, Reumatismo 67 (2016) 109, 10.4081/reumatismo.2015.838. [DOI] [PubMed] [Google Scholar]
- [139].Rittner HL, Mousa SA, Labuz D, Beschmann K, Schäfer M, Stein C, Brack A, Selective local PMN recruitment by CXCL1 or CXCL2/3 injection does not cause inflammatory pain, J. Leukoc. Biol 79 (2006) 1022–1032, 10.1189/jlb.0805452. [DOI] [PubMed] [Google Scholar]
- [140].Suo J, Linke B, Meyer dos Santos S, Pierre S, Stegner D, Zhang DD, Denis CV, Geisslinger G, Nieswandt B, Scholich K, Neutrophils mediate edema formation but not mechanical allodynia during zymosan-induced inflammation, J. Leukoc. Biol 96 (2014) 133–142, 10.1189/jlb.3A1213-628R. [DOI] [PubMed] [Google Scholar]
- [141].Clatworthy AL, Illich PA, Castro GA, Walters ET, Role of peri-axonal inflammation in the development of thermal hyperalgesia and guarding behavior in a rat model of neuropathic pain, Neurosci. Lett 184 (1995) 5–8 (Accessed October 17, 2018), http://www.ncbi.nlm.nih.gov/pubmed/7739805. [DOI] [PubMed] [Google Scholar]
- [142].Cao L, Malon JT, Anti-nociceptive role of CXCL1 in a murine model of peripheral nerve injury-induced neuropathic pain, Neuroscience 372 (2018) 225–236, 10.1016/j.neuroscience.2017.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Liou J-T, Lee C-M, Lin Y-C, Chen C-Y, Liao C-C, Lee H-C, Day Y-J, P-selectin is required for neutrophils and macrophage infiltration into injured site and contributes to generation of behavioral hypersensitivity following peripheral nerve injury in mice, Pain 154 (2013) 2150–2159, 10.1016/j.pain.2013.06.042. [DOI] [PubMed] [Google Scholar]
- [144].Morin N, Owolabi SA, Harty MW, Papa EF, Tracy TF, Shaw SK, Kim M, Saab CY, Neutrophils invade lumbar dorsal root ganglia after chronic constriction injury of the sciatic nerve, J. Neuroimmunol 184 (2007) 164–171, 10.1016/j.jneuroim.2006.12.009. [DOI] [PubMed] [Google Scholar]
- [145].Silva JR, Lopes AH, Talbot J, Cecilio NT, Rossato MF, Silva RL, Souza GR, Silva CR, Lucas G, Fonseca BA, Arruda E, Alves-Filho JC, Cunha FQ, Cunha TM, Neuroimmune–glia interactions in the sensory ganglia account for the development of acute herpetic neuralgia, J. Neurosci 37 (2017) 6408–6422, 10.1523/JNEUROSCI.2233-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Bali KK, Kuner R, Therapeutic potential for leukocyte elastase in chronic pain states harboring a neuropathic component, Pain 158 (2017) 2243–2258, 10.1097/j.pain.0000000000001032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Vicuña L, Strochlic DE, Latremoliere A, Bali KK, Simonetti M, Husainie D, Prokosch S, Riva P, Griffin RS, Njoo C, Gehrig S, Mall MA, Arnold B, Devor M, Woolf CJ, Liberles SD, Costigan M, Kuner R, The serine protease inhibitor SerpinA3N attenuates neuropathic pain by inhibiting T cell–derived leukocyte elastase, Nat. Med 21 (2015) 518–523, 10.1038/nm.3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Green KL, Mechanism of the pro-inflammatory activity of sympathomimetic amines in thermic oedema of the rat paw, Br. J. Pharmacol 50 (1974) 243–251 (Accessed October 17, 2018), http://www.ncbi.nlm.nih.gov/pubmed/4371900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Omori K, Kida T, Hori M, Ozaki H, Murata T, Multiple roles of the PGE2 -EP receptor signal in vascular permeability, Br. J. Pharmacol 171 (2014) 4879–4889, 10.1111/bph.12815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Cunha FQ, Lorenzetti BB, Poole S, Ferreira SH, Interleukin-8 as a mediator of sympathetic pain, Br. J. Pharmacol 104 (1991) 765–767 (Accessed October 17, 2018), http://www.ncbi.nlm.nih.gov/pubmed/1797337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Cunha TM, Verri WA, Silva JS, Poole S, Cunha FQ, Ferreira SH, A cascade of cytokines mediates mechanical inflammatory hypernociception in mice, Proc. Natl. Acad. Sci 102 (2005) 1755–1760, 10.1073/pnas.0409225102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Verri WA, Cunha TM, Parada CA, Poole S, Cunha FQ, Ferreira SH, Hypernociceptive role of cytokines and chemokines: targets for analgesic drug development? Pharmacol. Ther 112 (2006) 116–138, 10.1016/j.pharmthera.2006.04.001. [DOI] [PubMed] [Google Scholar]
- [153].Lorenzetti BB, Veiga FH, Canetti CA, Poole S, Cunha FQ, Ferreira SH, Cytokine-induced neutrophil chemoattractant1 (CINC-1) mediates the sympathetic component of inflammatory mechanical hypersensitivity in rats, Eur. Cytokine Netw. 13 (2002) 456–461 (Accessed October 17, 2018), http://www.ncbi.nlm.nih.gov/pubmed/12517731. [PubMed] [Google Scholar]
- [154].Steen KH, Reeh PW, Anton F, Handwerker HO, Protons selectively induce lasting excitation and sensitization to mechanical stimulation of nociceptors in rat skin, in vitro, J. Neurosci 12 (1992) 86–95 (Accessed October 17, 2018), http://www.ncbi.nlm.nih.gov/pubmed/1309578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Twining CM, Sloane EM, Milligan ED, Chacur M, Martin D, Poole S, Marsh H, Maier SF, Watkins LR, Peri-sciatic proinflammatory cytokines, reactive oxygen species, and complement induce mirror-image neuropathic pain in rats, Pain 110 (2004) 299–309, 10.1016/j.pain.2004.04.008. [DOI] [PubMed] [Google Scholar]
- [156].Wang Z-Q, Porreca F, Cuzzocrea S, Galen K, Lightfoot R, Masini E, Muscoli C, Mollace V, Ndengele M, Ischiropoulos H, Salvemini D, A newly identified role for superoxide in inflammatory pain, J. Pharmacol. Exp. Ther 309 (2004) 869–878, 10.1124/jpet.103.064154. [DOI] [PubMed] [Google Scholar]
- [157].Woo YC, Park SS, Subieta AR, Brennan TJ, Changes in tissue pH and temperature after incision indicate acidosis may contribute to postoperative pain, Anesthesiology 101 (2004) 468–475 (Accessed October 17, 2018), http://www.ncbi.nlm.nih.gov/pubmed/15277931. [DOI] [PubMed] [Google Scholar]
- [158].Mujenda FH, Duarte AM, Reilly EK, Strichartz GR, Cutaneous endothelin-A receptors elevate post-incisional pain, Pain 133 (2007) 161–173, 10.1016/j.pain.2007.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Chiu IM, von Hehn CA, Woolf CJ, Neurogenic inflammation and the peripheral nervous system in host defense and immunopathology, Nat. Neurosci 15 (2012) 1063–1067, 10.1038/nn.3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Sauer SK, Reeh PW, Bove GM, Noxious heat-induced CGRP release from rat sciatic nerve axons in vitro, Eur. J. Neurosci 14 (2001) 1203–1208 (Accessed November 19, 2018), http://www.ncbi.nlm.nih.gov/pubmed/11703449. [DOI] [PubMed] [Google Scholar]
- [161].Edvinsson L, Ekman R, Jansen I, McCulloch J, Uddman R, Calcitonin gene-related peptide and cerebral blood vessels: distribution and vasomotor effects, J. Cereb. Blood Flow Metab 7 (1987) 720–728, 10.1038/jcbfm.1987.126. [DOI] [PubMed] [Google Scholar]
- [162].Brain SD, Williams TJ, Interactions between the tachykinins and calcitonin gene-related peptide lead to the modulation of oedema formation and blood flow in rat skin, Br. J. Pharmacol. 97 (1989) 77–82 (Accessed November 19, 2018), http://www.ncbi.nlm.nih.gov/pubmed/2470460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Brogden KA, Guthmiller JM, Salzet M, Zasloff M, The nervous system and innate immunity: the neuropeptide connection, Nat. Immunol. 6 (2005) 558–564, 10.1038/ni1209. [DOI] [PubMed] [Google Scholar]
- [164].Smith CH, Barker JN, Morris RW, MacDonald DM, Lee TH, Neuropeptides induce rapid expression of endothelial cell adhesion molecules and elicit granulocytic infiltration in human skin, J. Immunol. (Baltimore, Md.: 1950) 151 (1993) 3274–3282 (Accessed July 19, 2019), http://www.ncbi.nlm.nih.gov/pubmed/7690800. [PubMed] [Google Scholar]
- [165].Czepielewski RS, Porto BN, Rizzo LB, Roesler R, Abujamra AL, Pinto LG, Schwartsmann G, de Q Cunha F., Bonorino C, Gastrin-releasing peptide receptor (GRPR) mediates chemotaxis in neutrophils, Proc. Natl. Acad. Sci. U. S. A 109 (2012) 547–552, 10.1073/pnas.1110996109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Pinho-Ribeiro FA, Baddal B, Haarsma R, O’Seaghdha M, Yang NJ, Blake KJ, Portley M, Verri WA, Dale JB, Wessels MR, Chiu IM, Blocking neuronal signaling to immune cells treats streptococcal invasive infection, Cell 173 (2018), [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Baral P, Umans BD, Li L, Wallrapp A, Bist M, Kirschbaum T, Wei Y, Zhou Y, Kuchroo VK, Burkett PR, Yipp BG, Liberles SD, Chiu IM, Nociceptor sensory neurons suppress neutrophil and γδ T cell responses in bacterial lung infections and lethal pneumonia, Nat. Med 24 (2018) 417–426, 10.1038/nm.4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Wilson EH, Weninger W, Hunter CA, Trafficking of immune cells in the central nervous system, J. Clin. Invest 120 (2010) 1368–1379, 10.1172/JCI41911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Galea I, Bechmann I, Perry VH, What is immune privilege (not)? Trends Immunol. 28 (2007) 12–18, 10.1016/j.it.2006.11.004. [DOI] [PubMed] [Google Scholar]
- [170].Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS, Harris TH, Kipnis J, Structural and functional features of central nervous system lymphatic vessels, Nature 523 (2015) 337–341, 10.1038/nature14432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Werner JK, Stevens RD, Traumatic brain injury, Curr. Opin. Neurol 28 (2015) 565–573, 10.1097/WCO.0000000000000265. [DOI] [PubMed] [Google Scholar]
- [172].Mantovani A, Cassatella MA, Costantini C, Jaillon S, Neutrophils in the activation and regulation of innate and adaptive immunity, Nat. Rev. Immunol 11 (2011) 519–531, 10.1038/nri3024. [DOI] [PubMed] [Google Scholar]
- [173].Semple BD, Kossmann T, Morganti-Kossmann MC, Role of chemokines in CNS health and pathology: a focus on the CCL2/CCR2 and CXCL8/CXCR2 networks, J. Cereb. Blood Flow Metab 30 (2010) 459–473, 10.1038/jcbfm.2009.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Navarini AA, Lang KS, Verschoor A, Recher M, Zinkernagel AS, Nizet V, Odermatt B, Hengartner H, Zinkernagel RM, Innate immune-induced depletion of bone marrow neutrophils aggravates systemic bacterial infections, Proc. Natl. Acad. Sci. U. S. A 106 (2009) 7107–7112, 10.1073/pnas.0901162106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Christy AL, Walker ME, Hessner MJ, Brown MA, Mast cell activation and neutrophil recruitment promotes early and robust inflammation in the meninges in EAE, J. Autoimmun 42 (2013) 50–61, 10.1016/j.jaut.2012.11.003. [DOI] [PubMed] [Google Scholar]
- [176].Simmons SB, Liggitt D, Goverman JM, Cytokine-regulated neutrophil recruitment is required for brain but not spinal cord inflammation during experimental autoimmune encephalomyelitis, J. Immunol. (Baltimore, Md.: 1950) 193 (2014) 555–563, 10.4049/jimmunol.1400807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Rumble JM, Huber AK, Krishnamoorthy G, Srinivasan A, Giles DA, Zhang X, Wang L, Segal BM, Neutrophil-related factors as biomarkers in EAE and MS, J. Exp. Med 212 (2015) 23–35, 10.1084/jem.20141015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Pierson ER, Wagner CA, Goverman JM, The contribution of neutrophils to CNS autoimmunity, Clin. Immunol. (Orlando, Fla.) 189 (2018) 23–28, 10.1016/j.clim.2016.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Aubé B, Lévesque SA, Paré A, Chamma É, Kébir H, Gorina R, Lécuyer M-A, Alvarez JI, De Koninck Y, Engelhardt B, Prat A, Côté D, Lacroix S, Neutrophils mediate blood-spinal cord barrier disruption in demyelinating neuroinflammatory diseases, J. Immunol. (Baltimore, Md.: 1950) 193 (2014) 2438–2454, 10.4049/jimmunol.1400401. [DOI] [PubMed] [Google Scholar]
- [180].Le Page A, Lamoureux J, Bourgade K, Frost EH, Pawelec G, Witkowski JM, Larbi A, Dupuis G, Fülöp T, Polymorphonuclear neutrophil functions are differentially altered in amnestic mild cognitive impairment and mild alzheimer’s disease patients, J. Alzheimers Dis. 60 (2017) 23–42, 10.3233/JAD-170124. [DOI] [PubMed] [Google Scholar]
- [181].Gabbita SP, Johnson MF, Kobritz N, Eslami P, Poteshkina A, Varadarajan S, Turman J, Zemlan F, Harris-White ME, Oral TNFα modulation alters neutrophil infiltration, improves cognition and diminishes tau and amyloid pathology in the 3xTgAD mouse model, PLoS One 10 (2015) e0137305, 10.1371/journal.pone.0137305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Echeverria V, Yarkov A, Aliev G, Positive modulators of the α7 nicotinic receptor against neuroinflammation and cognitive impairment in Alzheimer’s disease, Prog. Neurobiol 144 (2016) 142–157, [DOI] [PubMed] [Google Scholar]
- [183].Jiang W, St-Pierre S, Roy P, Morley BJ, Hao J, Simard AR, Infiltration of CCR2+Ly6Chigh proinflammatory monocytes and neutrophils into the central nervous system is modulated by nicotinic acetylcholine receptors in a model of multiple sclerosis, J. Immunol. (Baltimore, Md.: 1950) 196 (2016) 2095–2108, 10.4049/jimmunol.1501613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Drummond RA, Collar AL, Swamydas M, Rodriguez CA, Lim JK, Mendez LM, Fink DL, Hsu AP, Zhai B, Karauzum H, Mikelis CM, Rose SR, Ferre EMN, Yockey L, Lemberg K, Kuehn HS, Rosenzweig SD, Lin X, Chittiboina P, Datta SK, Belhorn TH, Weimer ET, Hernandez ML, Hohl TM, Kuhns DB, Lionakis MS, CARD9-dependent neutrophil recruitment protects against fungal invasion of the central nervous system, PLoS Pathog. 11 (2015), 10.1371/journal.ppat.1005293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Drummond RA, Swamydas M, Oikonomou V, Zhai B, Dambuza IM, Schaefer BC, Bohrer AC, Mayer-Barber KD, Lira SA, Iwakura Y, Filler SG, Brown GD, Hube B, Naglik JR, Hohl TM, Lionakis MS, CARD9+ microglia promote antifungal immunity via IL-1β- and CXCL1-mediated neutrophil recruitment, Nat. Immunol 20 (2019) 559–570, 10.1038/s41590-019-0377-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Biswas A, French T, Düsedau HP, Mueller N, Riek-Burchardt M, Dudeck A, Bank U, Schüler T, Dunay IR, Behavior of neutrophil granulocytes during Toxoplasma gondii infection in the central nervous system, Front. Cell. Infect. Microbiol 7 (2017), 10.3389/fcimb.2017.00259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Blom C, Deller BL, Fraser DD, Patterson EK, Martin CM, Young B, Liaw PC, Yazdan-Ashoori P, Ortiz A, Webb B, Kilmer G, Carter DE, Cepinskas G, Human severe sepsis cytokine mixture increases β2-integrin-dependent polymorphonuclear leukocyte adhesion to cerebral microvascular endothelial cells in vitro, Criti. Care (London, England) 19 (2015) 149, 10.1186/s13054-015-0883-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].He H, Geng T, Chen P, Wang M, Hu J, Kang L, Song W, Tang H, NK cells promote neutrophil recruitment in the brain during sepsis-induced neuroinflammation, Sci. Rep 6 (2016) 27711, 10.1038/srep27711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Singer BH, Newstead MW, Zeng X, Cooke CL, Thompson RC, Singer K, Ghantasala R, Parent JM, Murphy GG, Iwashyna TJ, Standiford TJ, Cecal ligation and puncture results in long-term central nervous system myeloid inflammation, PLoS One 11 (2016) e0149136, 10.1371/journal.pone.0149136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Neumann J, Riek-Burchardt M, Herz J, Doeppner TR, König R, Hütten H, Etemire E, Männ L, Klingberg A, Fischer T, Görtler MW, Heinze H-J, Reichardt P, Schraven B, Hermann DM, Reymann KG, Gunzer M, Very-late-antigen-4 (VLA-4)-mediated brain invasion by neutrophils leads to interactions with microglia, increased ischemic injury and impaired behavior in experimental stroke, Acta Neuropathol. 129 (2015) 259–277, 10.1007/s00401-014-1355-2. [DOI] [PubMed] [Google Scholar]
- [191].Seki Y, Sahara Y, Itoh E, Kawamura T, Suppressed neutrophil respiratory burst in patients with haemorrhagic stroke, J. Clin. Neurosci 17 (2010) 187–190, 10.1016/j.jocn.2009.04.020. [DOI] [PubMed] [Google Scholar]
- [192].Lafargue M, Xu L, Carlès M, Serve E, Anjum N, Iles KE, Xiong X, Giffard R, Pittet J-F, Stroke-induced activation of the α7 nicotinic receptor increases Pseudomonas aeruginosa lung injury, FASEB J. 26 (2012) 2919–2929, 10.1096/fj.11-197384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Yang L, Froio RM, Sciuto TE, Dvorak AM, Alon R, Luscinskas FW, ICAM-1 regulates neutrophil adhesion and transcellular migration of TNF-alpha-activated vascular endothelium under flow, Blood 106 (2005) 584–592, 10.1182/blood-2004-12-4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Otxoa-de-Amezaga A, Gallizioli M, Pedragosa J, Justicia C, Miró-Mur F, Salas-Perdomo A, Díaz-Marugan L, Gunzer M, Planas AM, Location of neutrophils in different compartments of the damaged mouse brain after severe Ischemia/Reperfusion, Stroke 50 (2019) 1548–1557, 10.1161/STROKEAHA.118.023837. [DOI] [PubMed] [Google Scholar]
- [195].Uhl MW, Biagas KV, Grundl PD, Barmada MA, Schiding JK, Nemoto EM, Kochanek PM, Effects of neutropenia on edema, histology, and cerebral blood flow after traumatic brain injury in rats, J. Neurotrauma 11 (1994) 303–315, 10.1089/neu.1994.11.303. [DOI] [PubMed] [Google Scholar]
- [196].Emerich DF, Dean RL, Bartus RT, The role of leukocytes following cerebral ischemia: pathogenic variable or bystander reaction to emerging infarct? Exp. Neurol 173 (2002) 168–181, 10.1006/exnr.2001.7835. [DOI] [PubMed] [Google Scholar]
- [197].Palmer C, Roberts RL, Young PI, Timing of neutrophil depletion influences long-term neuroprotection in neonatal rat hypoxic-ischemic brain injury, Pediatr. Res 55 (2004) 549–556, 10.1203/01.PDR.0000113546.03897.FC. [DOI] [PubMed] [Google Scholar]
- [198].Marro BS, Grist JJ, Lane TE, Inducible expression of CXCL1 within the central nervous system amplifies viral-induced demyelination, J. Immunol. (Baltimore, Md.: 1950) 196 (2016) 1855–1864, 10.4049/jimmunol.1501802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Goksugur SB, Kabakus N, Bekdas M, Demircioglu F, Neutrophil-to-lymphocyte ratio and red blood cell distribution width is a practical predictor for differentiation of febrile seizure types, Eur. Rev. Med. Pharmacol. Sci 18 (2014) 3380–3385 (Accessed August 20, 2018), http://www.ncbi.nlm.nih.gov/pubmed/25491611. [PubMed] [Google Scholar]
- [200].Ohtsuka T, Entero-Behçet’s disease coexisting with long-term epilepsy and schizophrenia-like symptoms, J. Dermatol 41 (2014) 424–426, 10.1111/1346-8138.12470. [DOI] [PubMed] [Google Scholar]
- [201].Cuartero MI, Ballesteros I, Moraga A, Nombela F, Vivancos J, Hamilton JA, Corbí ÁL, Lizasoain I, Moro MA, N2 neutrophils, novel players in brain inflammation after stroke, Stroke 44 (2013) 3498–3508, 10.1161/STROKEAHA.113.002470. [DOI] [PubMed] [Google Scholar]
- [202].Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, Worthen GS, Albelda SM, Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN, Cancer Cell 16 (2009) 183–194, 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Pillay J, den Braber I, Vrisekoop N, Kwast LM, de Boer RJ, Borghans JAM, Tesselaar K, Koenderman L, In vivo labeling with 2H2O reveals a human neutrophil lifespan of 5.4 days, Blood 116 (2010) 625–627, 10.1182/blood-2010-01-259028. [DOI] [PubMed] [Google Scholar]
- [204].Tak T, Tesselaar K, Pillay J, Borghans JAM, Koenderman L, What’s your age again? Determination of human neutrophil half-lives revisited, J. Leukoc. Biol 94 (2013) 595–601, 10.1189/jlb.1112571. [DOI] [PubMed] [Google Scholar]
- [205].Vaughan KR, Stokes L, Prince LR, Marriott HM, Meis S, Kassack MU, Bingle CD, Sabroe I, Surprenant A, Whyte MKB, Inhibition of neutrophil apoptosis by ATP is mediated by the P2Y11 receptor, J. Immunol. (Baltimore, Md.: 1950) 179 (2007) 8544–8553 (Accessed January 10, 2019), http://www.ncbi.nlm.nih.gov/pubmed/18056402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Pithon-Curi TC, Schumacher RI, Freitas JJS, Lagranha C, Newsholme P, Palanch AC, Doi SQ, Curi R, Glutamine delays spontaneous apoptosis in neutrophils, Am. J. Physiol., Cell Physiol 284 (2003), 10.1152/ajpcell.00224.2002 C1355–1361. [DOI] [PubMed] [Google Scholar]
- [207].Shaftel SS, Carlson TJ, Olschowka JA, Kyrkanides S, Matousek SB, O’Banion MK, Chronic interleukin-1beta expression in mouse brain leads to leukocyte infiltration and neutrophil-independent blood brain barrier permeability without overt neurodegeneration, J. Neurosci 27 (2007) 9301–9309, 10.1523/JNEUROSCI.1418-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Pillay J, Kamp VM, van Hoffen E, Visser T, Tak T, Lammers J-W, Ulfman LH, Leenen LP, Pickkers P, Koenderman L, A subset of neutrophils in human systemic inflammation inhibits T cell responses through Mac-1, J. Clin. Invest 122 (2012) 327–336, 10.1172/JCI57990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Gupta R, Palchaudhuri S, Chattopadhyay D, Glutamate induces neutrophil cell migration by activating class I metabotropic glutamate receptors, Amino Acids 44 (2013) 757–767, 10.1007/s00726-012-1400-1. [DOI] [PubMed] [Google Scholar]
- [210].Xie L, Poteet EC, Li W, Scott AE, Liu R, Wen Y, Ghorpade A, Simpkins JW, Yang S-H, Modulation of polymorphonuclear neutrophil functions by astrocytes, J. Neuroinflamm 7 (2010) 53, 10.1186/1742-2094-7-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Colom B, Bodkin JV, Beyrau M, Woodfin A, Ody C, Rourke C, Chavakis T, Brohi K, Imhof BA, Nourshargh S, Leukotriene B4-Neutrophil elastase axis drives neutrophil reverse transendothelial cell migration in vivo, Immunity 42 (2015) 1075–1086, 10.1016/j.immuni.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Engblom C, Pfirschke C, Zilionis R, Da Silva Martins J, Bos SA, Courties G, Rickelt S, Severe N, Baryawno N, Faget J, Savova V, Zemmour D, Kline J, Siwicki M, Garris C, Pucci F, Liao H-W, Lin Y-J, Newton A, Yaghi OK, Iwamoto Y, Tricot B, Wojtkiewicz GR, Nahrendorf M, Cortez-Retamozo V, Meylan E, Hynes RO, Demay M, Klein A, Bredella MA, Scadden DT, Weissleder R, Pittet MJ, Osteoblasts remotely supply lung tumors with cancer-promoting SiglecFhigh neutrophils, Science (New York, N. Y.) 358 (2017) eaal5081, 10.1126/science.aal5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Hampton MB, Kettle AJ, Winterbourn CC, Inside the neutrophil phagosome: oxidants, myeloperoxidase, and bacterial killing, Blood 92 (9) (1998) 3007–3017. [PubMed] [Google Scholar]
- [214].Hurst JK, What really happens in the neutrophil phagosome? Free Radic. Biol. Med 53 (3) (2012) 508–520, 10.1016/j.freeradbiomed.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]