Abstract
The baroreflex is a critical physiological mechanism controlling cardiovascular function by modulating both the sympathetic and parasympathetic activities. Here, we report that electrical activation of the baroreflex attenuates joint inflammation in experimental arthritis induced by the administration of zymosan into the femorotibial cavity. Baroreflex activation combined with lumbar sympathectomy, adrenalectomy, celiac subdiaphragmatic vagotomy or splenectomy dissected the mechanisms involved in the inflammatory modulation, highlighting the role played by sympathetic inhibition in the attenuation of joint inflammation. From the immunological standpoint, baroreflex activation attenuates neutrophil migration and the synovial levels of inflammatory cytokines including TNF, IL-1β and IL-6, but does not affect the levels of the anti-inflammatory cytokine IL-10. The anti-inflammatory effects of the baroreflex system are not mediated by IL-10, the vagus nerve, adrenal glands or the spleen, but by the inhibition of the sympathetic drive to the knee. These results reveal a novel physiological neuronal network controlling peripheral local inflammation.
Keywords: Baroreflex, Inflammation, Neuroimmunomodulation, Sympathetic activity, Parasympathetic activity
1. Introduction
The nervous system senses peripheral inflammation triggering behavioral and cardiovascular physiological responses to maintain physiological homeostasis (Borovikova et al., 2000; Tracey, 2002; Maier and Watkins, 2003; Volman et al., 2005; Bassi et al., 2012). Clinical and experimental studies revealed that alterations of the autonomic nervous system associate with peripheral and chronic inflammatory disorders (Mravec, 2007; Stojanovich, 2009; Pongratz and Straub, 2013; Zubcevic et al., 2014). Recent studies from Tracey and coworkers have defined the inflammatory reflex, as a “mechanism by which the nervous system reflexively regulates the inflammatory response in real time, just as it controls heart rate and other vital functions” (Tracey, 2002; Ulloa, 2005). These studies reveal that the efferent neural-to-immune arm of this reflex system – is mediated by the activation of the vagus nerve (Tracey, 2002; Huston et al., 2006; Rosas-Ballina and Tracey, 2009). This nerve is the main component of the parasympathetic nervous system and its activation reduces cytokine production in the spleen (Rosas-Ballina and Tracey, 2009). On the other hand, the sympathetic nervous system can also regulate the innate immune system (Katafuchi et al., 1993; Kox et al., 2014; Martelli et al., 2014). Patients with chronic rheumatoid arthritis exhibit autonomic dysfunctions characterized by reduced parasympathetic and increased sympathetic tone (Pongratz and Straub, 2013), these effects associated with increased plasma levels of inflammatory cytokines such as TNF, IL-1β, and IL-6 (Tetta et al., 1990; Danis et al., 1992). Furthermore, a high sympathetic activity is linked to severe clinical signs and increased peripheral inflammation in rheumatoid arthritis (Hürlimann et al., 2002; Huston and Tracey, 2011; Pongratz and Straub, 2013), suggesting that low sympathetic activity could ameliorate inflammation in rheumatoid arthritis. Together, these studies indicate that both the parasympathetic and sympathetic nervous systems can control systemic inflammation.
Cardiovascular homeostasis is essential for the survival of humans and the maintenance of blood perfusion and oxygenation of vital organs (Abboud and Benson, 2015). The baroreflex is a critical physiological mechanism controlling cardiovascular homeostasis (Di Rienzo et al., 2001) by inhibiting the sympathetic outflow and increasing the parasympathetic drive, to control the arterial pressure and heart rate. The baroreceptors are mechanoreceptor sensory neurons located in the aortic arch, carotid sinuses, and major blood vessels that monitor the arterial pressure. These neurons can promote a neural response inhibiting the sympathetic activity, and increasing the parasympathetic tone (Krieger et al., 1982; Chapleau et al., 1988). Several studies demonstrated that hypertensive subjects have a higher inflammatory profile (Khraibi, 1991; Dzielak, 1992; Fu, 1995; Shen et al., 1995; Suzuki et al., 1995) combined with the attenuation of baroreflex function (Ferguson et al., 1984; Gronda et al., 2014). Therefore, it is conceivable that the higher inflammatory profile is linked to baroreflex dysfunction in arterial hypertension.
Taking into account that anesthesia attenuates baroreflex function (Palmisano et al., 1991; Tanaka and Nishikawa, 1999; Akine et al., 2001; Bassani et al., 2013), our laboratory developed a technique to electrically activate the aortic depressor nerve, an afference of the baroreflex, in conscious rats. We demonstrated that electrical stimulation of the aortic depressor nerve activates the baroreflex promoting bradycardia and hypotension, under physiological (De Paula et al., 1999; Durand et al., 2009) and pathophysiological conditions (Salgado et al., 2007; Durand et al., 2009, 2012), without the undesirable effects of anesthesia. Moreover, electrical stimulation of the aortic depressor nerve in conscious rats shifts the sympathovagal balance toward a parasympathetic predominance (Krieger et al., 1982; Chapleau et al., 1988).
Therefore, we investigated whether the activation of the baroreflex, by electrical stimulation of the aortic depressor nerve in conscious rats, controls peripheral inflammation in experimental arthritis. Furthermore, we also investigated the mechanisms mediating baroreflex control of peripheral inflammation by performing lumbar sympathectomy (the knee joint receives only sympathetic innervation) or celiac subdiaphragmatic vagotomy to distinguish the role played by sympathetic and parasympathetic efferences, respectively, and adrenalectomy or splenectomy to preclude a role played by these organs during the baroreflex activation.
2. Materials and methods
The experimental protocols comply with the recommendations of the SBNeC (Brazilian Society of Neuroscience and Behavior), the Ethical Principles of the Brazilian College of Animal Experimentation (COBEA Protocol 137/2013), and the US National Institutes of Health Guide for The Care and Use of Laboratory Animals.
2.1. Animal experiments
Male Wistar rats (250–300 g), obtained from the Main Animal Facility of the Medical School of Ribeirão Preto, University of São Paulo, were housed upon arrival at the animal facility in plastic cages under a 12-h light/dark cycle (lights on at 7 am) at 20 °C ± 1 °C and maintained in groups of five per cage (40 × 33 ×18 cm). The animals had unrestricted access to food and tap water. The number of animals used was the minimum required to ensure reliability of the results, and every effort was made to minimize animal discomfort.
2.2. Surgical procedures
Animals were anesthetized with a ketamine and xylazine mixture (50 mg/kg and 10 mg/kg) administered into the right posterior calf muscle. After the confirmation of a surgical plane of anesthesia by lack of response to a foot pinch, animals were maintained in supine position, and a medial laparotomy was performed, while one of the following procedures was applied:
Splenectomy (SPLENX): the spleen was removed after ligation of the splenic blood vessels.
Sympathectomy (SYMPX): the right lumbar sympathetic ganglia (L2–L3 level) were dissected near the renal artery; the L5 ganglion was identified at the level of aorta bifurcation, and all pathways connecting L2–L5 were excised.
Subdiaphragmatic vagotomy (VAGX): the posterior wall of the esophagus was visualized to show the celiac branch of the vagus nerve, the celiac branch was followed until its exit from the esophageal hiatus and then 1–2 mm length of the nerve was removed.
Bilateral adrenalectomy (ADX) was performed through a dorsal approach; to avoid body electrolyte loss, rats were provided with free access to 0.9% NaCl.
At the end of surgery, all animals received an injection of a polyvalent veterinary antibiotic (Pentabiótico, 0.2 mL, intramuscular; Fort Dodge, Campinas, SP, Brazil) and an injection of the anti-inflammatory and analgesic flunixin meglumine (Banamine, 25 mg/kg, subcutaneous; Schering-Plough, Cotia, SP, Brazil). A post-surgery period of 7–10 days elapsed until the implantation of the electrodes into the aortic depressor nerve. No significant postoperative weight loss or mortality was observed in the animals.
2.3. Electrode implantation
Animals were anesthetized with a cocktail of ketamine and xylazine (50 mg/kg and 10 mg/kg, i.p.) and subjected to a ventral neck surgery under microscope to isolate the left aortic depressor nerve below its joint to the superior laryngeal nerve. The aortic depressor nerve was implanted with a bipolar stainless steel electrode with an inter-leads distance of 2 mm. The electrodes were constructed by attaching two 40 mm-long stainless-steel wires (0.008 inches bare, 0.011 inches Teflon coated; model 791400; A-M Systems, Sequim, WA, USA) to a small plug (GF-6; Microtech, Boothwyn, PA, USA). The bared tips of the electrodes consisted of 2 mm lengths, forming hooks that were implanted around the aortic depressor nerve. First, the electrode was tunneled through the sternocleidomastoid muscle and the small plug was exteriorized in the nape of the neck. Next, the short segment of the aortic depressor nerve that was implanted with the bipolar stainless steel electrodes was carefully covered with silicone impression material (Kwik-Sil silicone elastomer; World Precision Instruments, Sarasota, FL, USA). The sham group underwent similar surgical procedures but was not subjected to electrical stimulation of the aortic depressor nerve. Under the same anesthesia, the left carotid artery was catheterized with polyethylene tubing (PE-50; Becton Dickinson, Sparks, MD) for recording the pulsatile arterial pressure (PAP). The catheter was tunneled subcutaneously, exteriorized in the nape of the neck and sutures closed the surgical incision sites. Flunixin meglumine (Banamine, 25 mg/kg, subcutaneous; Schering-Plough, Cotia, SP, Brazil) was injected immediately after the end of surgery.
2.4. Electrical stimulation of the aortic depressor nerve
Twenty-four hours after electrode implantation into the aortic depressor nerve, the awakened rats had the PAP recorded. In brief, the arterial catheter was connected to a pressure transducer (MLT844; ADInstruments, Bella Vista, Australia) and signal was amplified (ML224; ADInstruments, Bella Vista, Australia) and sampled by a IBM/PC computer (Core 2 duo, 2.2 GHz, 4 GB ram) equipped with an analog-to-digital interface (2 kHz; ML866, ADInstruments, Bella Vista, Australia). A quiet environment was maintained to avoid stress and rats had the PAP recorded at baseline conditions for 15 min. Next, the electrodes were connected to an external stimulator (1M1C, AVS Projetos, São Carlos, SP, Brazil) and rats were subjected to electrical stimulation (intensity 0.5 mA; pulse width 0.25 ms; frequency 15 Hz) of the aortic depressor nerve for 2 min. Only those rats that showed no sign of distress during electrical stimulation of the aortic depressor nerve were used in the study. PAP recordings were processed with LabChart 7.0 (ADInstruments, Bella Vista, Australia), a computer software capable of detecting inflection points and generate mean arterial pressure (MAP) and heart rate (HR) time series.
2.5. Zymosan injection and arthritis assessment
Immediately after the electrical stimulation of the aortic depressor nerve, rats were injected with zymosan (100 μg/50 μL) or saline (50 μL) into the femorotibial cavity throughout a 30G needle. After 6 h the animals were decapitated for blood collection and exposition of the synovial bursa. Joint edema was accessed through the measure of knee diameter by a paquimeter (Marberg, Brazil), and joint’s clinical performance was scored as follows: 0 = no evidence of inflammation; 1 = edema of the femorotibial cavity (slight edema); 2 = edema involving all joint capsule surrounding the knee (large edema); 3 = the same as 2 plus small hemorrhagic spots along the synovial bursa; 4 = the same as 2 plus large hemorrhagic spots or blood/pus leakage. Synovial cavities were then opened, washed with a mixture of PBS/EDTA by a micropipette, diluted (1:5) and the total number of leukocytes was determined by Neubauer chamber through an optical microscope (400×). The results are depicted as neutrophils/joint cavity.
2.6. Cytokines measured by ELISA
The collected synovial fluids were immediately frozen in liquid nitrogen and stored at −70 °C. On the day of the assay, the samples were defrosted and maintained in ice until the end of the experiment. The samples were homogenized in 500 μL of the appropriate buffer containing protease inhibitors followed by centrifugation for 10 min at 2000×g to collect the supernatant. The supernatant was used to measure the levels of TNF (catalog#DY510), IL-1β (catalog#DY501), IL-6 (catalog#DY506), and IL-10 (catalog#DY522) by enzyme-linked immunosorbent assay (ELISA) using Duo set kits from R&D Systems (Minneapolis, MN, USA). Briefly, microtiter plates were coated overnight at 4 °C with an immunoaffinity-purified polyclonal sheep antibody against TNF (4 μg/mL), IL-1β (0.8 μg/mL), IL-6 (4 μg/mL), or IL-10 (4 μg/mL). After blocking the plates, recombinant murine TNF, IL-1β, IL-6 or IL-10 were used as standards at defined concentrations, and the samples were then added in duplicate and incubated overnight at 4 °C. Rabbit biotinylated immunoaffinity purified polyclonal antibodies (pAbs) anti-TNF (225 ng/mL), anti-IL-1β (350 ng/mL), anti-IL-6 (400 ng/mL), or anti-IL-10 (300 ng/mL) was added, followed by incubation at room temperature for 1 h. Finally, 50 μL of avidin-horseradish peroxidase (1:5000 dilution; DAKO A/S, Glostrup, Denmark) were added to each well for 30 min. Then, the plates were washed, and the color reagent OPD (200 μg per well, Sigma, St. Louis, MO, USA) was added. After 15 min, the reaction was stopped with H2SO4 and the optical density (OD) measured at 490 nm. The results were expressed as pg·mL−1 of TNF, IL-1β, IL-6, and IL-10, based on standard curves.
2.7. Statistical analysis
Neutrophil migration to the synovial tissue was statistically analyzed by one-way analysis of variance (ANOVA) followed by the Tukey’s multiple comparison post hoc test. The hemodynamic parameters were analyzed with the two-way ANOVA for repeated measures followed by the Tukey’s post hoc test when indicated. The time course of joint diameter and clinical score were analyzed with the two-way ANOVA for repeated measures followed by the Bonferroni’s post hoc test when indicated. The experimental sample n refers to the number of animals. Differences were considered statistically significant when p < 0.05.
3. Results
3.1. The hemodynamic responses to electrical stimulation of the aortic depressor nerve validate baroreflex activation
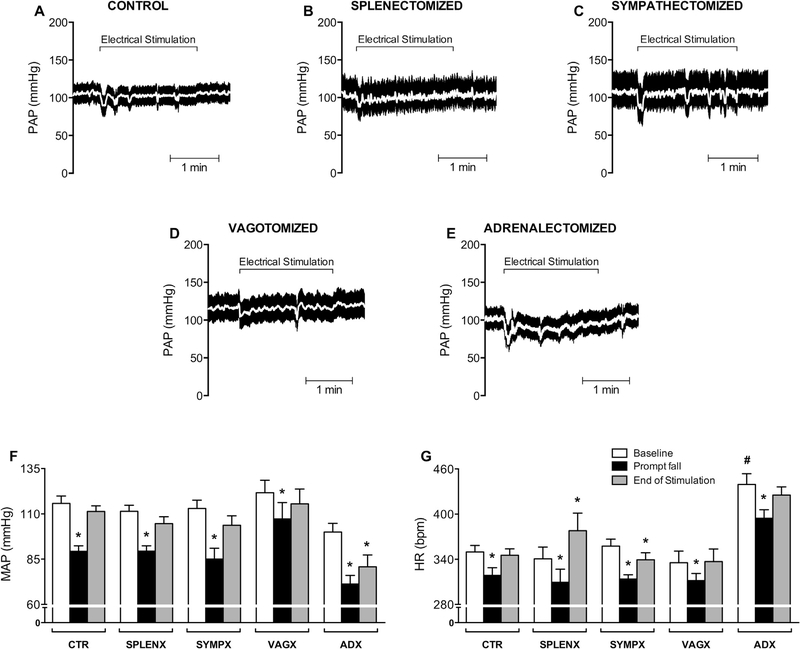
Tracings of the pulsatile arterial pressure (PAP) from animals representative of every group show a prompt hypotensive reaction at the beginning of the electrical stimulation of the aortic depressor nerve (Fig. 1A–E). There is a recovery to baseline levels at the end of the electrical aortic depressor nerve stimulation. These results are a normal physiological response that confirms the proper stimulation of the aortic depressor nerve. The analyses of the mean arterial pressure (MAP) show that all animal groups had similar baseline values for MAP (Fig. 1F: clear bars) and HR (Fig. 1G: clear bars), except the adrenolectomized (ADX) group that showed tachycardia at a baseline conditions as compared with the other groups.
Fig. 1.
Hemodynamic responses to electrical stimulation of the aortic depressor nerve. Tracing of pulsatile arterial pressure (PAP) [white line represents mean arterial pressure] during 2 min of electrical stimulation of the aortic depressor nerve. (A) Control (CTR; n = 9); (B) splenectomized (SPLENX; n = 5); (C) sympathectomized (SYMPX; n = 8); (D) vagotomized (VAGX; n = 6); (E) adrenalectomized (ADX; n = 4) animals. Bar graphs (F and G) show the mean arterial pressure (MAP) and heart rate (HR). Bars represent mean ± standard error. *p < 0.05 compared to baseline within of each group; #p < 0.05 compared to baseline in all other groups (Two-way ANOVA for repeated measures followed by the Tukey’s post hoc test).
The aortic depressor nerve stimulation for 2 min elicited a significant prompt (within the first 5 s) fall of MAP and HR (Fig. 1F, G: black bars) in all experimental groups. This hypotensive response was transitory in all the experimental groups but not in the adrenalectomized animals (Fig. 1F: ADX gray bar). Likewise, the aortic nerve stimulation causes bradycardia in all the experimental groups. This bradycardia was transitory in all the experimental groups but not in the in the sympathectomized (SYMPX) animals that showed sustained bradycardia. On the other hand, the splenectomized (SPLENX) animals showed a transitory bradycardia followed by significant tachycardia (Fig. 1G: gray bars). Together, these results indicate that the baroreflex was conspicuously activated by electrical stimulation of the aortic depressor nerve.
3.2. Effects of baroreflex activation on the knee-joint inflammatory profile
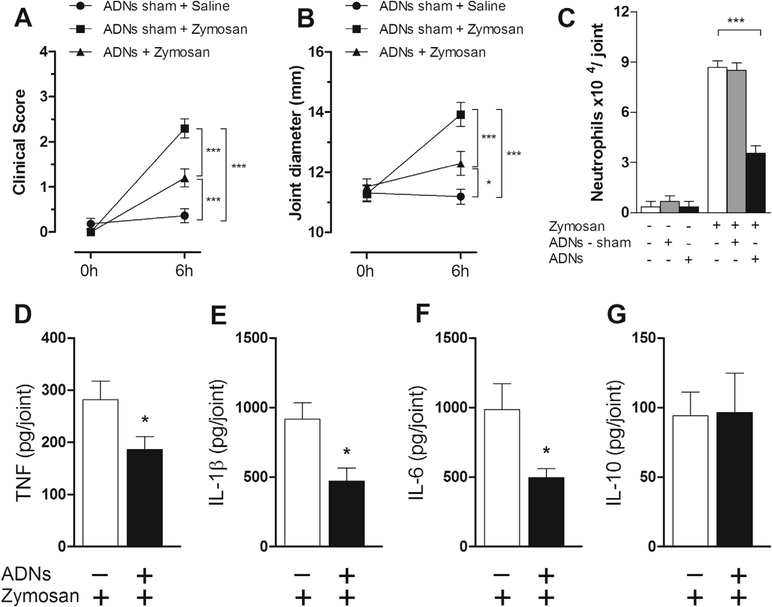
First, we analyzed whether baroreflex activation attenuates experimental arthritis. Our results indicate that baroreflex activation attenuated the clinical score of arthritis (Fig. 2A). This treatment produced a better clinical score (Fig. 2A) as compared to the sham-stimulated animals (ADNs-sham). Baroreflex activation also reduced joint diameter by more than 10% (Fig. 2B) and inhibited neutrophil migration to the synovial cavity by more than 50% (Fig. 2C). Next, we analyzed whether the baroreflex activation could reduce articular cytokine levels. Baroreflex activation significantly inhibited synovial TNF levels by over 30%; and IL-1β and IL-6 levels by over 50% (Fig. 2D–F). This effect was specific, as the baroreflex activation did not affect the synovial levels of anti-inflammatory cytokines such as IL-10 (Fig. 2G). These results suggest that baroreflex activation improves the clinical score of experimental arthritis by regulating peripheral inflammation.
Fig. 2.
Electrical activation of the baroreflex attenuated the clinical score of experimental arthritis. Effects of the aortic depressor nerve stimulation (ADNs) or sham stimulation (ADNs – sham) over the inflammatory profile of the femorotibial joint in animals challenged with intra-articular zymosan. (A) Clinical score of the knee joint; (B) articular edema, and (C) neutrophilic infiltration in the synovial tissue. (D) Synovial levels of TNF, (E) IL-1β, (F) IL-6, and (G) IL-10. Zymosan (n = 6); ADN sham (n = 5); ADNs (n = 5). Bars represent means ± standard error. *p < 0.05; **p < 0.01; ***p < 0.001 (One-way ANOVA followed by Tukey’s post hoc test.)
3.3. Anti-inflammatory effect of baroreflex activation does not depend on the spleen, vagus nerve or adrenal glands
Previous studies indicated that an inflammatory reflex involves a peripheral neural pathway including the spleen, the celiac branch of the vagus nerve or the adrenal glands (Huston et al., 2006; Torres-Rosas et al., 2014). Therefore, we examined whether splenectomy, vagotomy or adrenalectomy affect the anti-inflammatory effects of baroreflex activation. Despite the fact that splenectomy produced an unexpected inflammatory effect (Fig. 3C), baroreflex activation in splenectomized rats still improved the clinical score of the knee joint, reduced joint diameter by more than 80% and reduced neutrophil migration by more than 100% (Fig. 3A–C). Furthermore, baroreflex activation improved the clinical score of the knee joint in vagotomized and adrenalectomized animals (Fig. 3D and G), reduced knee diameter by more than 10% (Fig. 3E and H) and decrease neutrophil migration to the synovial cavity by more than 55% (Fig. 3F and I). Together, these results depict a new physiological mechanism for peripheral neuro-immune modulation independent of the spleen, the vagus nerve and the adrenal glands, but mediated by the sympathetic arm of the baroreflex.
Fig. 3.
The Anti-inflammatory effect of electrical activation of the baroreflex is independent of the spleen, vagus nerve or adrenal glands. Effects of the aortic depressor nerve stimulation (ADNs) over the inflammatory profile of the femorotibial joint after intra-articular administration of zymosan in sham ADNs (sham-ADNs), sham surgery (Shams), splenectomized (SPLENX – upper panels), vagotomized (VAGX – middle panels) or adrenalectomized (ADX – lower panels). Clinical score (A, D and G), articular edema (B, E and H), and neutrophilic infiltration (C, F and I). Bars represent means ± standard error. SPLENX group: sham (n = 6); SPLENX (n = 6); sham-s + ADNs (n = 5); SPLENX + ADNs (n = 5). VAGX group: sham (n = 6); VAGX (n = 6); Sham-s + ADNs (n = 4); VAGX + ADNs (n = 6); ADX group: sham (n = 6); ADX (n = 6); Sham-s + ADNs (n = 5); ADX + ADNs (n = 5). *p < 0.05; $ $, **p < 0.01; $ $ $, ***p < 0.001 (Two-way ANOVA for repeated measures followed by Bonferroni’s post hoc test.)
3.4. Anti-inflammatory effect of baroreflex activation depends on the integrity of the local sympathetic chain
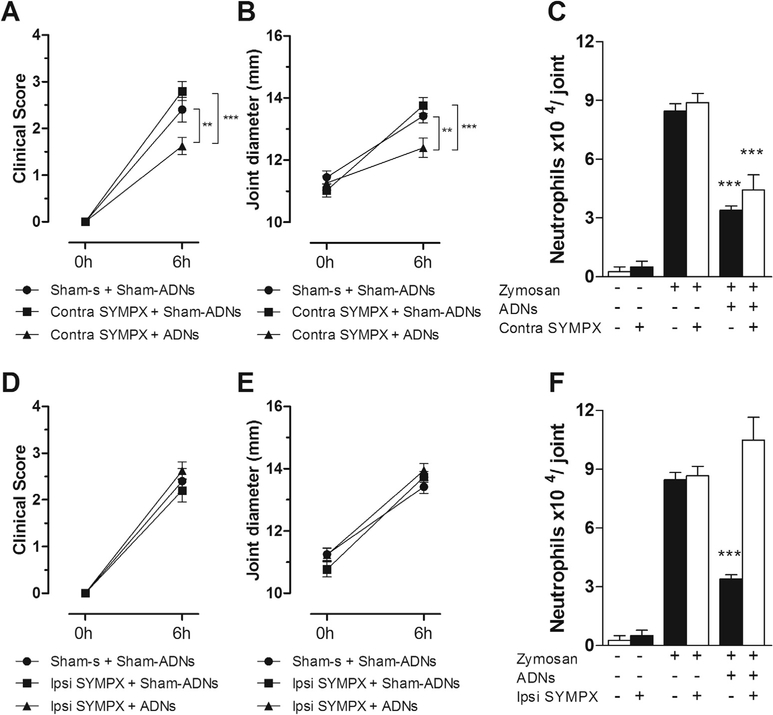
Taking into account that the baroreflex afferents send projections the aortic baroreceptors to supra-spinal structures involved in the modulation of the autonomic nervous system (Guyenet, 2006), and that the knee synovial tissue exhibits a substantial sympathetic innervation (Schaible and Straub, 2014), we examined whether the joint’s sympathetic innervation mediates the anti-inflammatory effect of baroreflex activation. To accomplish this task, the rats underwent unilateral sympathectomy (L2–L6) prior to baroreflex activation. While baroreflex activation improved the knee’s clinical score (Fig. 4A), inhibited joint diameter by more than 10% (Fig. 4B) and neutrophil migration by more than 50% (Fig. 4C) in the contralateral knee to the sympathectomy (contra to SYMPX), this effect was blocked in the ipsilateral (Fig. 4D–F) sympathectomized (ipsi to SYMPX) joint. These findings from demonstrated the dependence of the sympathetic integrity for the baroreflex anti-inflammatory effect. Collectively, these results depict a new neuro-immune pathway involving the baroreflex and the inhibition of sympathetic afferences.
Fig. 4.
The anti-inflammatory effect of electrical activation of the baroreflex depends on the integrity of local sympathetic chain. Effects of the aortic depressor nerve stimulation (ADNs) on the inflammatory profile of the femorotibial joint after intra-articular administration of zymosan in sympathectomized (SYMPX) animals. Clinical score (A and D), articular edema (B and E), and neutrophilic infiltration (C and F) in the contralateral (contra: A, B and C) or ipsilateral (ipsi: D, E and F) knee-joint to the sympathectomy (SYMPX). Zymosan (n = 5); SYMPX (n = 5); ADNs (n = 5); SYMPX + ADNs (n = 8). Bars represent means ± standard error. *p < 0.05; **p < 0.01; ***p < 0.001 (Two-way ANOVA for repeated measures followed by Bonferroni’s post hoc test.)
4. Discussion
Previous studies showed that the neuro-immune reflex requires a peripheral neural pathway, comprising the spleen, the celiac branch of the vagus nerve or the adrenal glands (Tracey, 2002; Torres-Rosas et al., 2014). Thus, the neuro-immune regulation of the inflammatory process is affected when one of these components is disturbed. Since these studies stimulated only efferent neural pathways, for instance from the vagus or the sciatic nerve, it is not clear whether the stimulation of an afferent neural pathway activates the neuro-immune reflex. Our current results indicate that stimulation of the afferences of the baroreflex induces an anti-inflammatory mechanism independent on the spleen, celiac vagus and the adrenal glands. However, the stimulation of the afferences of the baroreflex involved the inhibition of the sympathetic drive, highlighting a new pathway for the anti-inflammatory reflex.
Electrical activation of the baroreflex, as clearly demonstrated by the hemodynamic responses shown in Fig. 1, induced a peripheral anti-inflammatory effect in the knee joint, preventing neutrophil migration and reducing inflammatory cytokine levels of TNF, IL-1β and IL-6 combined with the improvement of the clinical score and edema attenuation. These findings concur with the beneficial effects of anti-TNF therapy preventing the cellular infiltration and improving the clinical score of rheumatoid arthritis (Hürlimann et al., 2002; Edrees et al., 2005). Of note, peripheral vagal denervation comprising the celiac ganglion did not completely abrogate the inhibitory effect of baroreflex activation on the femorotibial joint. This finding indicates that the efferent parasympathetic arm of the baroreflex arch is not required to control peripheral inflammation. Moreover, the anti-inflammatory baroreflex pathway was not mediated by the spleen as demonstrated in splenectomized animals. This observation contrasts with the mechanism proposed for the anti-inflammatory reflex in sepsis that modulates systemic inflammation by inhibiting the production of inflammatory cytokines in the spleen (Tracey, 2002; Huston et al., 2006; Huston and Tracey, 2011). However, this is a more systemic mechanism that was analyzed in other experimental models, e.g. sepsis. Of note, our data corroborate previous studies showing that inflammatory processes in areas away from the abdominal cavity, for instance, lungs and skin, are autonomous and independent of the spleen or the celiac branch of the vagus nerve (Levy et al., 2013; Ortiz-Pomales et al., 2013).
Next, we investigated the role of the adrenal glands in the anti-inflammatory effect of the baroreflex activation. The adrenal cortex produces corticosterone, the major glucocorticoid in rodents, and a well-known hormone that exhibits compelling immunosuppressive effects (Simpson and Waterman, 1988). An endocrine deficiency in corticosterone release is associated with chronic inflammatory diseases including arthritis (Sternberg et al., 1989). Furthermore, catecholaminergic hormones released from the adrenal medulla have anti-inflammatory properties in sepsis after neuronal activation (Torres-Rosas et al., 2014). However, activation of the baroreflex suppressed neutrophil recruitment in adrenalectomized animals, indicating that the baroreflex control of inflammation in an experimental model of arthritis is independent of the adrenal glands, and it represents a new mechanism of neuro-modulation.
An important observation in the present study is that the integrity of the lumbar sympathetic chain is required for the baroreflex modulation of inflammation in the femur–tibial joint. Our results indicate that baroreflex activation improved the clinical score and decreased the edema and neutrophil count only in the contralateral joint of the sympathectomy because the sympathetic chain was intact. By contrast, baroreflex activation did not act in the sympathectomized ipsilateral joint, indicating that this anti-inflammatory mechanism involves the integrity of the baroreflex arch, including specific local sympathetic innervation. These results show that the baroreflex anti-inflammatory network is independent of immunosuppressive factors released by the adrenal glands or the spleen.
In summary, this is the first evidence that activation of the baroreflex network elicits an anti-inflammatory neuronal network that is independent of the canonical neuroimmune pathways mediated by the vagal modulation of the spleen or the adrenal glands. The most important finding is that the integrity of the lumbar sympathetic chain was required for the inflammatory modulation triggered by baroreflex stimulation upon the femur–tibial joint. This finding corroborates previous studies where sympathetic loss leads to more severe arthritic inflammation (Donnerer et al., 1991; Weidler et al., 2005; Jänig and Green, 2014). However, other works have shown that activation of the sympathetic nervous system is the main mechanism responsible for the anti-inflammatory effects elicited by neural stimulation (Katafuchi et al., 1993; Kox et al., 2014; Martelli et al., 2014).
The activation of the baroreflex improved the clinical score, and decreased the edema and neutrophil count only in the contralateral joint of the sympathectomy, but not in the ipsilateral joint. These results highlight that this anti-inflammatory mechanism is mediated by specific local sympathetic innervation. In other words, the anti-inflammatory baroreflex mechanism requires the integrity of the whole reflex arch. These results evidence that baroreflex electrical activation elicits an anti-inflammatory mechanism mediated by sympathetic inhibition and not parasympathetic – vagal – activation, and is independent of immunosuppressive factors released by the adrenal glands or the spleen. Therefore, baroreflex electrical activation could be used as a new therapeutic approach to inhibit inflammatory factors such as a TNF in rheumatoid arthritis, in agreement with current anti-TNF therapies (Danis et al., 1992; Hürlimann et al., 2002; Edrees et al., 2005).
Acknowledgments
This work was supported by FAPESP project Grant (2011/20343-4; 2012/04237-2; 2013/08216-2; 2013/20549-7) and CNPq project Grant (870325/1997-3; 475715/2012-8; 302787/2011-9). GSB holds a PhD scholarship from CNPq (proc. No. 142068/2012-8). FB holds a scholarship for Scientific Initiation from FAPESP (proc. No. 2014/15386-4). We are grateful to Ms. Ieda Regina dos Santos for technical assistance.
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- Abboud FM, Benson CJ, 2015. ASICs and cardiovascular homeostasis. Neuropharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akine A, Suzuka H, Hayashida Y, Kato Y, 2001. Effects of ketamine and propofol on autonomic cardiovascular function in chronically instrumented rats. Auton. Neurosci. 87, 201–208. [DOI] [PubMed] [Google Scholar]
- Bassani T, Bari V, Marchi A, Wu MA, Baselli G, Citerio G, Beda A, de Abreu MG, Güldner A, Guzzetti S, Porta A, 2013. Coherence analysis overestimates the role of baroreflex in governing the interactions between heart period and systolic arterial pressure variabilities during general anesthesia. Auton. Neurosci 178, 83–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassi GS, Kanashiro A, Santin FM, de Souza GEP, Nobre MJ, Coimbra NC, 2012. Lipopolysaccharide-induced sickness behaviour evaluated in different models of anxiety and innate fear in rats. Basic Clin. Pharmacol. Toxicol 110, 359–369. [DOI] [PubMed] [Google Scholar]
- Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ, 2000. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 405, 458–462. [DOI] [PubMed] [Google Scholar]
- Chapleau MW, Hajduczock G, Abboud FM, 1988. Mechanisms of resetting of arterial baroreceptors: an overview. Am. J. Med. Sci [DOI] [PubMed] [Google Scholar]
- Danis VA, Franic GM, Rathjen DA, Laurent RM, Brooks PM, 1992. Circulating cytokine levels in patients with rheumatoid arthritis: results of a double blind trial with sulphasalazine. Ann. Rheum. Dis 51, 946–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Paula PM, Castania JA, Bonagamba LGH, Salgado HC, Machado BH, 1999. Hemodynamic responses to electrical stimulation of the aortic depressor nerve in awake rats. Am. J. Phys 277, R31–R38. [DOI] [PubMed] [Google Scholar]
- Di Rienzo M, Parati G, Castiglioni P, Tordi R, Mancia G, Pedotti A, 2001. Baroreflex effectiveness index: an additional measure of baroreflex control of heart rate in daily life. Am. J. Physiol. Regul. Integr. Comp. Physiol 280, 744–751. [DOI] [PubMed] [Google Scholar]
- Donnerer J, Amann R, Lembeck F, 1991. Neurogenic and non-neurogenic inflammation in the rat paw following chemical sympathectomy. Neuroscience 45, 761–765. [DOI] [PubMed] [Google Scholar]
- Durand MT, Fazan R Jr., Salgado MC, Salgado HC, 2009. Acute and chronic electrical activation of baroreceptor afferents in awake and anesthetized subjects. Braz. J. Med. Biol. Res 42 (1), 53–60. [DOI] [PubMed] [Google Scholar]
- Durand MT, Mota AL, Barale AR, Castania JA, Fazan R Jr., Salgado HC, 2012. Time course of the hemodynamic responses to aortic depressor nerve stimulation in conscious spontaneously hypertensive rats. Braz. J. Med. Biol. Res 45 (5), 444–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzielak DJ, 1992. The immune system and hypertension. Hypertension 19, 36–44. [DOI] [PubMed] [Google Scholar]
- Edrees AF, Misra SN, Abdou NI, 2005. Anti-tumor necrosis factor (TNF) therapy in rheumatoid arthritis: correlation of TNF-alpha serum level with clinical response and benefit from changing dose or frequency of infliximab infusions. Clin. Exp. Rheumatol. 23 (4), 469–474. [PubMed] [Google Scholar]
- Ferguson DW, Abboud FM, Mark AL, 1984. Selective impairment of baroreflexmediated vasoconstrictor responses in patients with ventricular dysfunction Circulation 69: 451–460. Eur. J. Heart Fail 16, 977–983. [DOI] [PubMed] [Google Scholar]
- Fu ML, 1995. Do immune system changes have a role in hypertension? J. Hypertens 13 (11), 1259–1265. [DOI] [PubMed] [Google Scholar]
- Gronda E, Seravalle G, Brambilla G, Costantino G, Casini A, Alsheraei A, Lovett EG, Mancia G, Grassi G, 2014. Chronic baroreflex activation effects on sympathetic nerve traffic, baroreflex function, and cardiac haemodynamics in heart failure: a proof-of-concept study. Eur. J. Heart Fail 16, 977–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG, 2006. The sympathetic control of blood pressure. Nat. Rev. Neurosci. 7, 335–346. [DOI] [PubMed] [Google Scholar]
- Hürlimann D, Forster A, Noll G, Enseleit F, Chenevard R, Distler O, Béchir M, Spieker LE, Neidhart M, Michel BA, Gay RE, Lüscher TF, Gay S, Ruschitzka F, 2002. Anti-tumor necrosis factor-a treatment improves endothelial function in patients with rheumatoid arthritis. Circulation 106, 2184–2187. [DOI] [PubMed] [Google Scholar]
- Huston JM, Tracey KJ, 2011. The pulse of inflammation: heart rate variability, the cholinergic anti-inflammatory pathway and implications for therapy. J. Intern. Med 269, 45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huston JM, Ochani M, Rosas-Ballina M, Liao H, Ochani K, Pavlov VA, Gallowitsch-Puerta M, Ashok M, Czura CJ, Foxwell B, Tracey KJ, Ulloa L, 2006. Splenectomy inactivates the cholinergic antiinflammatory pathway during lethal endotoxemia and polymicrobial sepsis. J. Exp. Med 203, 1623–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jänig W, Green PG, 2014. Acute inflammation in the joint: its control by the sympathetic nervous system and by neuroendocrine systems. Auton. Neurosci 182, 42–54. [DOI] [PubMed] [Google Scholar]
- Katafuchi T, Take S, Hori T, 1993. Roles of sympathetic nervous system in the suppression of cytotoxicity of splenic natural killer cells in the rat. J. Phys 465, 343–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khraibi AA, 1991. Association between disturbances in the immune system and hypertension. Am. J. Hypertens. 4, 635–641. [DOI] [PubMed] [Google Scholar]
- Kox M, van Eijk LT, Zwaag J, van den Wildenberg J, Sweep FCGJ, van der Hoeven JG, Pickkers P, 2014. Voluntary activation of the sympathetic nervous system and attenuation of the innate immune response in humans. Proc. Natl. Acad. Sci. 111, 7379–7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger EM, Salgado HC, Michelini LC, 1982. Resetting of the baroreceptors. Int. Rev. Physiol 26, 119–146. [PubMed] [Google Scholar]
- Levy G, Fishman JE, Dazhong X, Chandler BTJ, Feketova E, Wei D, Qin Y, Vamsi A, Ulloa L, Deitch EA, 2013. Parasympathetic stimulation via the vagus nerve prevents systemic organ dysfunction by abrogating gut injury and lymph toxicity in trauma and hemorrhagic shock. Shock 39 (1), 39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier SF, Watkins LR, 2003. Immune-to-central nervous system communication and its role in modulating pain and cognition: implications for cancer and cancer treatment. Brain Behav. Immun 17, 125–131. [DOI] [PubMed] [Google Scholar]
- Martelli D, Yao ST, McKinley MJ, McAllen RM, 2014. Reflex control of inflammation by sympathetic nerves, not the vagus. J. Phys 592, 1677–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mravec B, 2007. Letter to the editor: autonomic dysfunction in autoimmune diseases: consequence or cause? Lupus 16, 767–768. [DOI] [PubMed] [Google Scholar]
- Ortiz-Pomales YT, Krzyzaniak M, Coimbra R, Baird A, Eliceiri BP, 2013. Vagus nerve stimulation blocks vascular permeability following burn in both local and distal sites. Burns 39, 68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmisano BW, Clifford PS, Hoffmann RG, Seagard JL, Coon RL, Kampine JP, 1991. Depression of baroreflex control of heart rate by halothane in growing piglets. Anesthesiology 75, 512–519. [DOI] [PubMed] [Google Scholar]
- Pongratz G, Straub RH, 2013. Role of peripheral nerve fibres in acute and chronic inflammation in arthritis. Nat. Rev. Rheumatol 9, 117–126. [DOI] [PubMed] [Google Scholar]
- Rosas-Ballina M, Tracey KJ, 2009. Cholinergic control of inflammation. J. Intern. Med 265, 663–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salgado HC, Barale AR, Castania JA, Machado BH, Chapleau MW, Fazan R Jr., 2007. Baroreflex responses to electrical stimulation of aortic depressor nerve in conscious SHR. Am. J. Phys 292, H593–H600. [DOI] [PubMed] [Google Scholar]
- Schaible H-G, Straub RH, 2014. Function of the sympathetic supply in acute and chronic experimental joint inflammation. Auton. Neurosci 182, 55–64. [DOI] [PubMed] [Google Scholar]
- Shen K, Sung KL, Whittemore DE, DeLano FA, Zweifach BW, Schmid-Schönbein GW, 1995. Properties of circulating leukocytes in spontaneously hypertensive rats. Biochem. Cell Biol 73 (7–8), 491–500. [DOI] [PubMed] [Google Scholar]
- Simpson ER, Waterman MR, 1988. Regulation of the synthesis of steroidogenic enzymes in adrenal cortical cells by ACTH. Annu. Rev. Physiol. 50, 427–440. [DOI] [PubMed] [Google Scholar]
- Sternberg EM, Hill JM, Chrousos GP, Kamilaris T, Listwak SJ, Gold PW, Wilder RL, 1989. Inflammatory mediator-induced hypothalamic-pituitaryadrenal axis activation is defective in streptococcal cell wall arthritissusceptible Lewis rats. Proc. Natl. Acad. Sci 86, 2374–2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojanovich L, 2009. Autonomic dysfunction in autoimmune rheumatic disease. Autoimmun. Rev 8, 569–572. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Zweifach BW, Forrest MJ, Schmid-Schönbein GW, 1995. Modification of leukocyte adhesion in spontaneously hypertensive rats by adrenal corticosteroids. J. Leukoc. Biol 1, 20–26. [PubMed] [Google Scholar]
- Tanaka M, Nishikawa T, 1999. Sevoflurane speeds recovery of baroreflex control of heart rate after minor surgical procedures compared with isoflurane. Anesth. Analg 89, 284–289. [DOI] [PubMed] [Google Scholar]
- Tetta C, Camussi G, Modena V, Di Vittorio C, Baglioni C, 1990. Tumour necrosis factor in serum and synovial fluid of patients with active and severe rheumatoid arthritis. Ann. Rheum. Dis 49, 665–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Rosas R, Yehia G, Pena G, Mishra P, del Rocio Thompson-Bonilla M, Moreno-Eutimio MA, Arriaga-Pizano LA, Isibasi A, Ulloa L, 2014. Dopamine mediates vagal modulation of the immune system by electroacupuncture. Nat. Med 20, 291–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey KJ, 2002. The inflammatory reflex. Nature 420, 853–859. [DOI] [PubMed] [Google Scholar]
- Ulloa L, 2005. The vagus nerve and the nicotinic anti-inflammatory pathway. Nat. Rev. Drug Discov 4, 673–684. [DOI] [PubMed] [Google Scholar]
- Volman TJ, Hendriks T, Goris RJ, 2005. Zymosan-induced generalized inflammation: experimental studies into mechanisms leading to multiple organ dysfunction syndrome. Shock 23, 291–297. [DOI] [PubMed] [Google Scholar]
- Weidler C, Holzer C, Harbuz M, Hofbauer R, Angele P, Schölmerich J, Straub RH, 2005. Low density of sympathetic nerve fibres and increased density of brain derived neurotrophic factor positive cells in RA synovium. Ann. Rheum. Dis 64, 13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubcevic J, Jun JY, Kim S, Perez PD, Afzal A, Shan Z, Li W, Santisteban MM, Yuan W, Febo M, Mocco J, Feng Y, Scott E, Baekey DM, Raizada MK, 2014. Altered inflammatory response is associated with an impaired autonomic input to the bone marrow in the spontaneously hypertensive rat. Hypertension 63, 542–550. [DOI] [PMC free article] [PubMed] [Google Scholar]