Abstract
Diminished cognitive and mood function are among the most conspicuous symptoms of Gulf War Illness (GWI). Our previous studies in a rat model of GWI have demonstrated that persistent cognitive and mood impairments are associated with substantially declined neurogenesis, chronic low-grade inflammation, increased oxidative stress and mitochondrial dysfunction in the hippocampus. We tested the efficacy of curcumin (CUR) to maintain better cognitive and mood function in a rat model of GWI because of its neurogenic, antiinflammatory, antioxidant, and memory and mood enhancing properties. Male rats were exposed daily to low doses of GWI-related chemicals, pyridostigmine bromide, N,N-diethyl-mtoluamide (DEET) and permethrin, and 5-minutes of restraint stress for 28 days. Animals were next randomly assigned to two groups, which received daily CUR or vehicle treatment for 30 days. Animals also received 5′-bromodeoxyuridine during the last seven days of treatment for analysis of neurogenesis. Behavioral studies through object location, novel object recognition and novelty suppressed feeding tests performed sixty days after treatment revealed better cognitive and mood function in CUR treated GWI rats. These rats also displayed enhanced neurogenesis and diminished inflammation typified by reduced astrocyte hypertrophy and activated microglia in the hippocampus. Additional studies showed that CUR treatment to GWI rats enhanced the expression of antioxidant genes and normalized the expression of multiple genes related to mitochondrial respiration. Thus, CUR therapy is efficacious for maintaining better memory and mood function in a model of GWI. Enhanced neurogenesis, restrained inflammation and oxidative stress with normalized mitochondrial respiration may underlie better memory and mood function mediated by CUR treatment.
Keywords: Curcumin, Gulf War Illness, Hippocampal neurogenesis, Memory impairment, Mitochondria, Mood dysfunction, Neuroinflammation, Oxidative stress, Reactive oxygen species
1. Introduction
Gulf War Illness (GWI), a chronic multisymptom condition, affects 25–32% of nearly 700,000 veterans who served in Operation Desert Storm/Desert Shield in 1991, also referred to as Gulf War (Golomb, 2008; Institute of Medicine, 2014; White et al., 2016). Cognitive and mood dysfunction, reduced concentration ability, sleep disturbances and chronic pain are the most evident central nervous system (CNS) related symptoms of GWI (Odegard et al., 2013; Janulewicz et al., 2017). These signs are typically accompanied by chronic musculoskeletal issues such as fatigue, and altered structure and function of the hippocampus (Menon et al., 2004, Chao et al., 2010; Li et al., 2011, Rayhan et al., 2013; Hubbard et al., 2014; VanRiper et al., 2017). While the precise etiology of GWI is unknown, epidemiological investigations imply that GWI in a majority of veterans is an outcome of exposure to several acetylcholinesterase inhibitors such as the nerve gas prophylactic medicine, pyridostigmine bromide (PB), and pesticides during the war (Steele et al., 2012). These studies also implied that the overall prevalence of GWI is greater in veterans who consumed a larger quantity of PB pills or veterans who used higher amounts of pesticides during the Gulf War (Sullivan et al., 2003). Combined exposures to PB and different pesticides thought to have ensued because of the following circumstances. First, veterans located in the battlefield zones consumed PB pills during the war as a prophylactic measure against a possible nerve gas attack by the enemy (Binns et al., 2008; Golomb, 1999). Second, pesticides and insect repellants were employed abundantly to counteract infectious diseases transmitted by insects and ticks in the desert. The pesticides were applied around tents, and insect repellents were sprayed on the skin and the uniform. The pesticides included the insecticide permethrin (PM) and the insect repellant N, N-diethyl-mtoluamide (DEET) (Haley and Kurt, 1997; Fricker et al., 2000; Winkenwerder, 2003). The multiple chemical exposure hypothesis is also supported by reports from the VA Research Advisory Committee on Gulf War Veterans’ Illnesses (RAC-GWVI) (Binns et al., 2008; White et al., 2016).
In congruence with the above observations, studies in a rat model have shown chronic brain dysfunction following combined exposure to low doses of chemicals including PB (oral), PM (dermal) and DEET (dermal) with or without mild stress for 28 days. Brain dysfunction is typified by impaired cognitive and mood function in association with persistently diminished new neuron production, enduring low-level inflammation, chronically elevated oxidative stress and mitochondrial dysfunction in the hippocampus (Parihar et al., 2013; Hattiangady et al., 2014; Shetty et al., 2017). Comparable changes have also been observed in other animal models of GWI, which employed exposures to various doses and/or combinations of diverse GWI related chemicals (Abdullah et al., 2011; O’Callaghan et al., 2015; Zakirova et al., 2016; Phillips and Deshpande, 2016; Locker et al., 2017).
Inflammation and increased oxidative stress associated with mitochondrial dysfunction can adversely affect cognitive and mood function either directly or indirectly via reduced hippocampal neurogenesis (Kohman and Rhodes, 2013; Jenrow et al., 2013; Parihar et al., 2013). Therefore, it is likely that chronic inflammation and oxidative stress are among the leading causes of brain dysfunction in GWI. Accordingly, compounds having potent antiinflammatory, antioxidant, pro-neurogenic properties appear to be suitable for maintaining better brain function in GWI. Curcumin (CUR), a natural phenolic component of yellow curry spice turmeric (Curcuma Longa), is one of the promising candidates for sustaining better brain function in GWI. Suitability of CUR stems from its proven antioxidant, antiinflammatory, neuroprotective, neurogenic, and cognitive and mood enhancing properties (Kim et al., 2008; Tiwari et al., 2014; Samarghandian et al., 2017). Therefore, we investigated the efficacy of CUR to maintain better cognitive and mood function in a rat model of GWI. Animals were exposed to low doses of GWI-related chemicals and mild stress daily for 28 days (Parihar et al., 2013) followed by either CUR or vehicle (VEH) treatment daily for 30 days. Animals were subjected to a battery of behavioral tests 60 days post-treatment to measure cognitive and mood function and then analyzed for the extent of neurogenesis, astrocyte hypertrophy, and microglial activation. Moreover, to ascertain the effects of CUR on increased oxidative stress and hyperactive mitochondria in the hippocampus, the expression of genes related to oxidative stress, antioxidant activity, and mitochondrial electron transport chain were measured in additional animals afflicted with chronic GWI.
2. Materials and methods
2.1. Animals
Nine-week old male Sprague Dawley rats (n = 43) acquired from Harlan (Indianapolis, IN) were housed with ad libitum access to rat chow and water. A combined institutional animal care and use committee of the Olin E. Teague Veterans’ Medical Center and the Texas A&M College of Medicine approved all experiments performed in this study. Animals were next randomly assigned to either the naïve control group (n = 11) or the GWI group (n = 32).
2.2. Study design
The timeline of various experiments is illustrated in Fig. 1. The details comprise the duration of exposure to Gulf War Illness Related Chemicals (GWIR-Cs) and stress (28 days), the duration of CUR or VEH treatment (30 days), the timeline employed for neurogenesis quantification using 5′-bromodeoxyuridine (BrdU) injections (last seven days of CUR or VEH treatment). The waiting period between treatment and commencement of behavioral tests (60 days) and the time-point of analyses of hippocampal tissues for neurogenesis, astrocyte hypertrophy and microglial activation (120 days after exposure to GWIR-Cs and stress) is also shown.
Fig. 1.
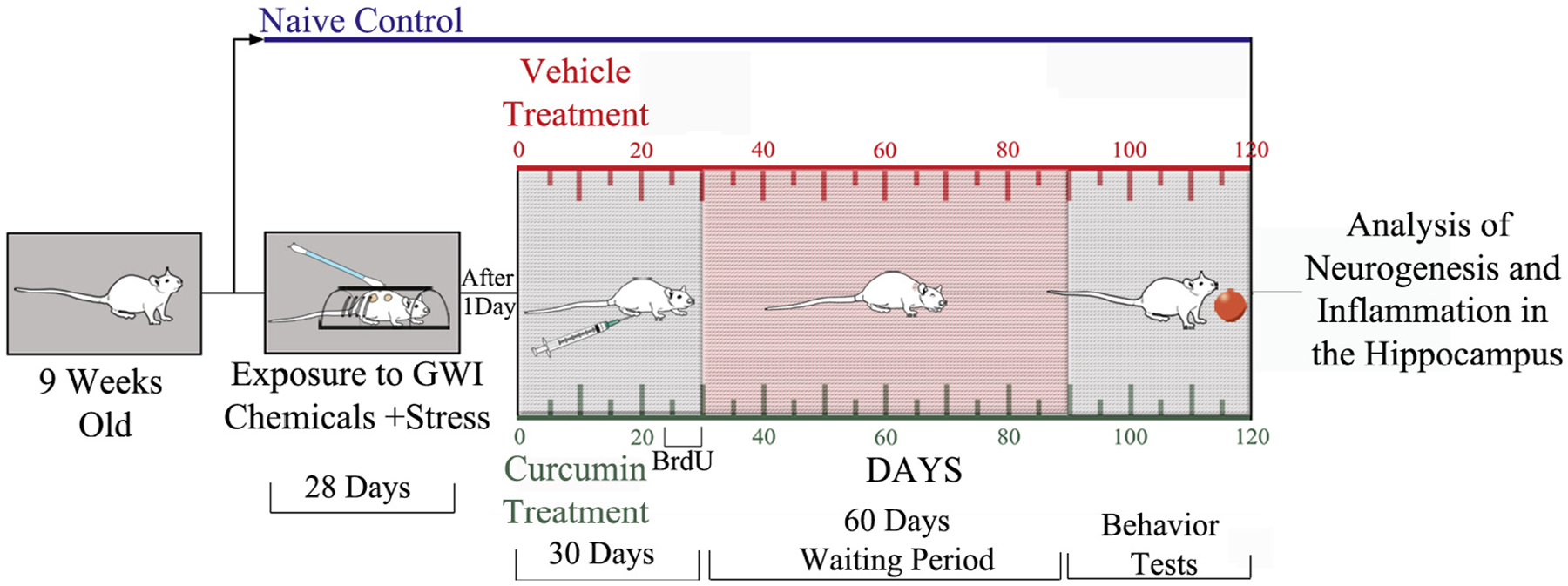
Timeline of various studies. Nine-week old Sprague-Dawley rats (n = 32) were exposed daily to Gulf War Illness related chemicals (GWIR-C’s) and 5-minutes of restraint stress for 28 days. A day later, rats exposed to GWIR-Cs and stress were randomly assigned to two groups. One group received daily curcumin (CUR, 30 mg/Kg i.p., n = 16) and the other group received vehicle (VEH, n = 16) for 30 days. Sixty days later, animals were subjected to a series of behavioral tests to assess cognitive and mood function. A group of age-matched naïve control rats (n = 11) were also included in behavioral studies. Animals in all groups were perfused with 4% paraformaldehyde following the conclusion of behavioral tests, and hippocampal tissues were processed for analyses of neurogenesis and inflammation.
2.3. Exposure of animals to GWIR-Cs and stress
The chemicals DEET and PM were purchased from Chemical Service Incorporated (West Chester, PA) whereas the drug pyridostigmine bromide (PB) was purchased from Sigma (St. Louis, MO). Animals were exposed daily to DEET, PM, and PB followed by 5 min of restraint stress for 28 days (Abdel-Rahman et al., 2002; Parihar et al., 2013; Hattiangady et al., 2014; Megahed et al., 2015; Shetty et al., 2017). The chemicals DEET (200 μl, 40 mg/Kg) and PM (200 μl, 0.13 mg/Kg) were applied dermally over shaved skin areas located on the back of the neck and the upper thoracic region between scapulae. The drug PB was administered orally via oral gavage (500 μl, 1.3 mg/Kg). A rat restrainer was used for the induction of 5 min of restraint stress, as detailed in our previous study (Parihar et al., 2011).
2.4. Administration of Curcumin
A day after exposure to GWIR-Cs and stress, GWI-rats were randomly assigned to two groups: a group receiving CUR (GWI + CUR group, n = 16) and a group receiving VEH (GWI + VEH group, n = 16). Animals in GWI + CUR group received daily CUR (Sigma, St. Louis, MO) at a dose of 30 mg/Kg in 0.1 ml of 33% DMSO (intraperitoneal) whereas, animals in GWI + VEH group received 0.1 ml of 33% DMSO. The selection of CUR dose in this study is based on results observed in several previous studies, which showed that relatively lower doses of CUR are efficacious for enhancing hippocampal neurogenesis or mood function in different animal prototypes. These include increased hippocampal neurogenesis in chronically stressed rats with 20 mg/Kg dose (Xu et al., 2007), enhanced neurogenesis and cognition in aged animals with 30 mg/Kg treatment (Dong et al., 2012) and antidepressant activity with only 10 mg/Kg dose (Kulkarni et al., 2008). The lower dose of CUR also suited the chronic nature of treatment (daily for 30 days) and the intraperitoneal route employed in this study.
The commencement of CUR administration employed in this study is based on findings in previous studies that several adverse effects that are linked to cognitive and mood dysfunction occur in the brain immediately after 28 days of exposure to GWIR-Cs and stress. These include blood-brain-barrier disruption, astrocyte hypertrophy (a sign of inflammation), decreased acetylcholinesterase activity and reduced hippocampal neurogenesis (Abdel-Rahman et al., 2002; Parihar et al., 2013). Moreover, both astrocyte hypertrophy and decreased neurogenesis persist in the hippocampus for prolonged periods in this GWI prototype (Parihar et al., 2013).
2.5. Behavioral tests for analyses of cognitive and mood function
Sixty days after CUR or VEH treatment, animals in both groups (along with age-matched naïve control animals, n = 11–16/group) were subjected to a series of behavioral tests to discern their cognitive and mood function. An object location test (OLT, a hippocampus-dependent cognitive test) measured the ability of animals to discern minor changes in the environment. A novel object recognition test (NORT) linked to the integrity of the perirhinal cortex, and the hippocampus evaluated recognition memory. A novelty suppressed feeding test (NSFT) investigated the extent of motivation and anxiety-like behavior. Animals that did not explore objects in OLT and NORT were excluded.
2.5.1. OLT
This test involved three successive trials (Hattiangady et al., 2014; Long et al., 2017). The first trial (trial-1 or habituation trial) comprised placing the rat in the center of an empty open field apparatus (100 × 100 × 60 cm) and allowing the rat to freely explore the box for 5 min. The second trial (trial-2 or sample trial) commenced 60 min after the first trial and involved placing the rat in the center of the same open field apparatus having two identical objects placed on opposite sides of the box and allowing the rat to freely explore the objects for 5 min. After an inter-trial interval of 90 min, the third trial (trial-3 or test trial) was performed for 5 min by placing the rat in the center of the same open field box with one of the objects remaining in the same location as in trial-2 and the second object moved to a new location in the open field box. The movement of the rat in trial-3 was continuously video-tracked using Noldus Ethovision XT program. The apparatus was cleaned with 70% alcohol and air-dried prior to the commencement of each trial for every rat. When the rat’s nose came within 2 cm of the object, the tracking software recorded this behavior as object exploration by the rat. Data such as the time spent in exploring the object moved to a new place or the object remaining in the familiar place, and the total time spent in object exploration were collected. Then, percentages of object exploration times spent with the object moved to a novel place vis-à-vis the object remaining in the familiar place were compared within each group. Additionally, the total distance moved, and the velocity of movement in trial-3 were collected and compared between the three groups to ascertain whether depression (or lack of motivation) interfered with the testing of cognitive function in OLT.
2.5.2. NORT
This test also comprised three sequential trials (Hattiangady and Shetty, 2012; Hattiangady et al. 2014). The first two trials comprised exploration of an empty open field apparatus (trial-1, 5 min) and exploration of two identical objects placed on opposite sides of the open field apparatus (trial-2, 5 min) with an inter-trial interval of 60 min. The trial 3 (test trial, 5 min) commenced 90 min after trial-2 and involved placing the rat in the middle of the open field apparatus with one of the objects from trial-2 remaining in the same location but the second object replaced with a novel object. The behavior of the rat was video-tracked as described for OLT above. The results such as the amount of novel object and familiar object exploration times and the total object exploration period were obtained. Then, proportions of object exploration times occupied with the novel and familiar objects were evaluated within each group. Furthermore, the total distance traveled and the speed of movement in trial-3 were compared across groups to determine whether object recognition memory testing in NORT was influenced by depression or lack of motivation in GWI groups.
2.5.3. NSFT
Each animal was first subjected to fasting for 24 h (by withdrawing food pellets from the cage), but drinking water was provided ad libitum during the fasting period. Next, a single trial lasting 5 min was conducted in an open field apparatus to measure the extent of motivation or anxiety-like behavior. A shallow plastic dish containing food pellets was placed in the center of an open field apparatus, and the rat was released from one of the corners. The movement of the rat was video tracked using Noldus Ethovision XT program to measure the latency to the first bite of food. The open field box was cleaned with 70% alcohol after every trial, and fresh food pellets were used for each rat to eliminate any odor related cues. Latency (in seconds) to the first bite of food was used as an index of motivation or anxiety-like behavior in each rat. A higher latency value implied increased anxiety-like behavior in this test.
2.6. Tissue processing and immunohistochemical methods
All animals that underwent behavioral tests were deeply anesthetized with isoflurane vapor exposure and then perfused through the heart using 4% paraformaldehyde. Animal perfusion and tissue processing protocols such as post-fixation and cryoprotection are detailed in our previous reports (Hattiangady et al., 2011, Rao et al., 2006a,b, Kodali et al., 2015). Thirty-micrometer thick coronal sections were cut through the entire hippocampus in each brain using a cryocut. The sections were collected serially in 24-well plates containing phosphate buffer and stored. Following this, in every group, animals were randomly chosen for analyses of hippocampal neurogenesis and hippocampal inflammation (6–9 animals/group). In each chosen animal, serial sections through the entire hippocampus were processed for immunohistochemistry.
The immunohistochemical studies comprised detection of 5bromodeoxyuridine (BrdU) positive newly born cells in every 15th section, doublecortin (DCX) positive newly born neurons in every 15th section, glial fibrillary acidic protein (GFAP) positive astrocytes in every 20th section and ED-1 positive activated microglia in every 20th section. The detailed methods are described in our previous reports (Hattiangady et al., 2011; Kuruba et al., 2011; Rao and Shetty, 2004; Rao et al., 2008, Kodali et al., 2015, 2016). In brief, sections were first treated with phosphate buffered saline (PBS) solution containing 20% methanol and 3% hydrogen peroxide for 20 min and then rinsed thrice in PBS. Next, the sections were treated for 30 min in PBS containing 0.1% Triton-X 100 and an appropriate serum (10%) selected based on the species in which the chosen secondary antibody was raised. Sections were then incubated 18–48 h in primary antibody solutions prepared in PBS. The primary antibodies comprised goat polyclonal anti-DCX (1:250; Santa Cruz Biotechnology, Santa Cruz, CA), mouse anti-BrdU (1:200, BD, San Jose, CA), rabbit anti-GFAP (1:1000, Dako, Santa Clara, CA), and mouse anti ED-1 (1:1000, Serotech). Sections were next washed thrice in PBS, incubated in an appropriate biotinylated secondary antibody (anti-goat, anti-mouse or anti-rabbit IgG, Vector Laboratories, Burlingame, CA) solution for 60 min, washed thrice in PBS and treated with the avidin-biotin complex reagent (ABC, Vector) for 60 min. The peroxidase reaction was then developed using vector gray or diaminobenzidine (Vector). The sections were mounted on gelatin-coated slides, dehydrated, cleared, and coverslipped.
2.7. Measurement of hippocampal neurogenesis
The extent of production of new cells in the last week of CUR or VEH treatment was measured via stereological counting of BrdU+ cells in the subgranular zone-granule cell layer (SGZ-GCL) using serial sections through the entire hippocampus (7–9 animals/group). The extent of neuronal differentiation of newly born cells and net neurogenesis was measured through BrdU and neuron-specific nuclear antigen (NeuN) dual immunofluorescence and Z-section analysis in a Nikon confocal microscope. Furthermore, the status of hippocampal neurogenesis at the time of euthanasia (~120 days after exposure to GWIR-Cs and stress in CUR and VEH groups) was measured through stereological quantification of doublecortin (DCX) positive newly born neurons (6–8 animals/group).
2.8. Analysis of hippocampal inflammation
The effect of CUR on astrocyte hypertrophy was evaluated by measuring area fractions occupied by glial-fibrillary acidic protein-positive (GFAP+) structures in different regions of the hippocampus using Image J (6–7 animals/group). The effect of CUR on microglial activity was examined through stereological counting the number of ED-1+ (CD68+) activated microglia through the entire hippocampus using serial sections in GWI + CUR and GWI + VEH groups (6–7 animals/group; Mishra et al., 2015). Furthermore, the effect of CUR for reducing the occurrence of activated microglia was ascertained through IBA-1 and ED-1 dual immunofluorescence with Z-section analyses in a confocal microscope. The percentages of activated microglia (ED-1+) among all microglia (IBA-1+) were quantified and compared between GWI + VEH and GWI + CUR groups.
2.9. Stereological cell counts
For all cell counts, the optical fractionator method in the StereoInvestigator system (Microbrightfield Inc., Williston, VT) interfaced with a Nikon E600 microscope through a color digital video camera (Optronics Inc., Muskogee, OK) was employed. This included quantification of numbers of: (i) newly generated BrdU+ cells in the SGZ-GCL; (ii) newly born DCX+ neurons in the SGZ-GCL; (iii) ED-1+ activated microglia in the dentate gyrus (DG), the CA1 subfield and the CA3 subfield. Detailed protocols of stereological cell counting are available in our earlier reports (Rao and Shetty, 2004; Hattiangady et al., 2008).
2.10. Dual immunofluorescence methods
Dual immunofluorescence studies comprised detection of: (i) neuron-specific nuclear antigen (NeuN) positive neurons among newly added cells (BrdU+ cells) in the subgranular zone-granule cell layer; and (ii) ED-1+ activated microglia among all microglia (IBA-1 + cells) in different regions of the hippocampus. For BrdU-NeuN dual immunofluorescence, representative sections from each animal were first processed for various BrdU pre-incubation treatments (Rao and Shetty, 2004; Shetty et al., 2012). Sections were washed in Tris-buffered saline (TBS), blocked in normal donkey serum, incubated overnight in a cocktail solution containing mouse anti-NeuN (1:1000, EMD Millipore, Temecula, CA) and rat anti-BrdU (1:250, Serotech) and washed in TBS. The sections were then treated with a mixture of donkey anti-mouse IgG tagged with Alexa Flour 488 (1:200, Invitrogen, Grand Island, NY), and donkey anti-rat IgG tagged with Alexa Flour 594 (1:200, Invitrogen), rinsed in TBS, and cover slipped with slow fade/antifade mounting medium (Invitrogen). Cells that exhibited BrdU (red) and NeuN (Green) co-expression were identified using a Nikon confocal microscope. Fractions of BrdU+ cells that expressed NeuN were then quantified by examination of individual BrdU+ cells in one-micrometer thick optical Z-sections.
For IBA-1 and ED-1 dual immunofluorescence representative sections from each animal were washed in PBS, blocked in normal donkey serum, incubated overnight in a primary antibody mixture containing rabbit anti-IBA-1 (1:1000, Millipore, MA) and mouse ED-1 (1:1000, Serotech), and washed in PBS. The sections were then treated with a mixture of donkey anti-rabbit IgG tagged with Alexa Fluor 594 (1:200, Invitrogen CA) and donkey anti-mouse IgG tagged with Alexa Flour 488 (1:200, Invitrogen, CA), washed in PBS, and cover slipped with slow fade/antifade mounting medium (Invitrogen CA). Cells that exhibited IBA-1 (red) and ED-1 (green) co-expression were identified using a Nikon confocal microscope. Fractions of IBA-1+ cells that expressed ED-1 were then quantified by examination of individual IBA-1+ cells in one-micrometer thick optical Z-sections.
2.11. Quantification of astrocyte hypertrophy
The area occupied by GFAP+ elements (cell body as well as processes of astrocytes) in the DG and CA1 and CA3 subfields were quantified using Image J, as detailed in our published reports (Parihar et al., 2013; Kodali et al., 2015). Briefly, images from distinct regions of the hippocampus were digitized using a 20X objective lens in a Nikon E600 microscope equipped with a digital video camera connected to a computer. Every image saved in gray scale as a bitmap file was opened in Image J software, and a binary image was generated by choosing a threshold value that preserved all GFAP+ structures but no background. The area occupied by the GFAP+ elements (i.e., the area fraction) in the binary image was then quantified by selecting “Analyze” command in the program. The area fraction of GFAP+ elements was calculated separately for every hippocampal region in each animal by using data from all chosen serial sections before the mean and SEM were determined for the total number of animals included per group.
2.12. Analyses of genes related to antioxidants and mitochondrial respiratory chain
The effects of CUR on the expression of genes related to increased oxidative stress and hyperactive mitochondria was measured in rats at an extended time-point (5 months) after exposure to GWI-chemicals and stress. Animals were treated with CUR for 30 days before hippocampal tissues were harvested. The hippocampal tissues from naïve control animals exposed to vehicle alone (VEH), GWI-Cs and stress (GWI), and GWI-Cs and stress followed by CUR treatment (GWI + CUR) were processed for quantitative real-time polymerase chain reaction (qRT-PCR). The expression of genes related to oxidative stress, antioxidant activity, and mitochondrial electron transport chain (ETC) were measured (4–5 animals/group).
For the collection of hippocampal tissues, animals were deeply anesthetized with an overdose of isoflurane and decapitated. The brain was quickly removed, snap frozen in dry ice, and stored at 80 C. For quantitative real-time polymerase chain reaction (qRT-PCR) studies, after quick thawing, the entire hippocampus from both sides was micro-dissected from each brain. For extraction of total RNA from hippocampal samples and preparation of cDNA, we used methods detailed in our recent report (Shetty et al., 2013; Shetty et al., 2017). In brief, total RNA was first extracted using RNeasy kit (Qiagen, Valencia, CA; Cat#: 74104) and the concentration and quality of RNA were determined by a Nanodrop spectrophotometer (ThermoFisher Scientific, Wilmington, DE). Total RNA (1 μg) was transcribed to cDNA using the RT2 First Strand Kit (Qiagen, Valencia, CA; Cat# 330404) and stored at −20 °C until further analysis. For measurement of genes related to oxidative stress, we used “The Rat Oxidative Stress PCR Array” (SABiosciences, Qiagen, Valencia, CA; Cat#: PARN065Z), which detected the expression of 84 key genes related to oxidative stress response, reactive oxygen species (ROS) metabolism, oxygen transport, and antioxidant activity in the hippocampus. For measuring genes linked to mitochondrial respiration, we employed “The Rat Mitochondrial Energy Metabolism PCR Array” (SABiosciences, Qiagen, Valencia, CA; Cat#PARN008Z), which profiled the expression of 84 key genes in the hippocampus, which encoded proteins that are relevant for mitochondrial respiration, such as genes encoding components of the electron transport chain and oxidative phosphorylation complexes. The methods are detailed in our recent report (Shetty et al., 2017). In brief, template cDNA from each sample was mixed with the 2X RT2 qPCR master mix (SABiosciences, Qiagen, Valencia, CA) and dH2O. This solution was then transferred to wells in rat oxidative stress RT2 Profiler™ PCR array plates or rat mitochondrial energy metabolism RT2 Profiler™ PCR array plates (25 μl/well) containing pre-dispensed gene-specific primer sets. The reactions were performed according to the manufacturer’s protocol using a CFX96 Real-Time system (Bio-Rad, Hercules, CA). A melt curve analysis was conducted to evaluate the specificity of the reaction. The Ct (threshold cycle) values of all wells were exported to an Excel spreadsheet and evaluated through web-based SABiosciences PCR array data analysis software. The 2^delta Ct values for each gene were compared between different groups.
2.13. Statistical analyses
We employed two-tailed, unpaired, Student’s t-test for analyses of data within groups, and one-way ANOVA with Newman-Keuls multiple comparison post-tests for comparison of data across three groups.
3. Results
3.1. Curcumin treated GWI rats displayed better cognitive function
Cognitive ability to perceive subtle variations in the environment was measured through an OLT. Maintenance of this function depends on the normal functioning of the hippocampal trisynaptic pathway (Warburton et al., 2013). This test is based on the role of the hippocampus in providing a cognitive map of the external world or a memory system that stores information about spatial relationships between objects and places in the organism’s environment (Manns and Eichenbaum, 2009). Accordingly, the propensity to survey the object relocated to a different locality in OLT signifies the capability of the animal to recognize minor changes in the location of objects in its immediate environment (Fig. 2 [A1]). Animals in GWI + VEH group displayed impaired spatial memory by comparable exploration of objects located in familiar and novel locations (t = 0.65, p > 0.05, Fig. 2[A3]), in contrast to naïve control animals showing a greater affinity for the object moved to a novel location (t = 4.9, p < 0.0001, Fig. 2 [A2]). On the other hand, animals in GWI + CUR group behaved akin to naïve control animals by spending a significantly greater amount of object exploration time with the object moved to a new place (t = 3.0, p < 0.01, Fig. 2 [A4]). Data for the total object exploration time, the total distance moved, and the velocity of movement in the testing phase (trial 3) were comparable between the three groups (F = 0.76–1.12, p > 0.05, Fig. 2 [A5–A7]). These findings implied that neither variable levels of object exploration nor motor deficits in one or more groups influenced the test results. Thus, CUR treated GWI rats exhibited better ability to discern minor changes in the environment.
Fig. 2.
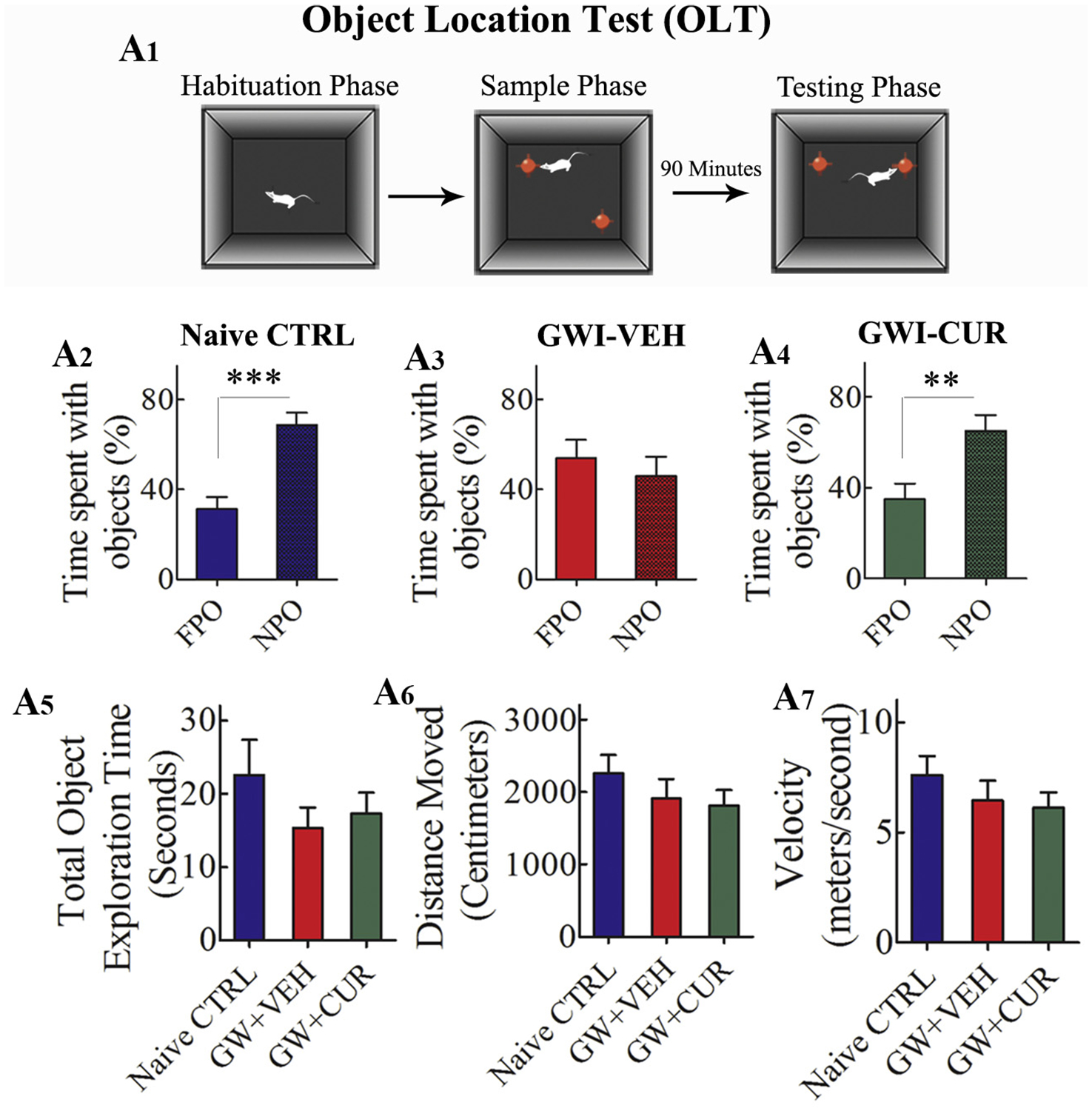
Curcumin (CUR) treated Gulf War Illness (GWI) rats displayed better ability to discern minor changes in the environment. Fig. A1 shows the various phases involved in an object location test (OLT). The bar charts in A2–A4 illustrate the performance of animals belonging to naïve control (naïve CTRL, A2), GWI animals receiving vehicle (GWI + VEH, A3), and GWI animals receiving CUR (GWI + CUR, A4) groups. Animals in GWI + VEH group showed impairment. This was evidenced by the lack of preference for the novel place object (NPO) in OLT over the familiar place object (FPO; A3). In contrast, animals in naïve control and GWI + CUR groups showed better cognitive function. This was apparent from their preference for NPO in OLT (A4). The bar charts in A5–A7 demonstrate that cognitive test results were not influenced by variability in object exploration time (A5), the total distance moved (A6) or the velocity of movement (A7) between groups. *p < 0.05, **p < 0.01, and ***p < 0.001.
3.2. Curcumin treated GWI rats exhibited better recognition memory
The object recognition memory was appraised through a NORT. This function depends primarily on the normal function of the perirhinal cortex and partially on the hippocampus (Langston and Wood, 2010). Predisposition to explore a novel object over a familiar object denotes the competence of the animal for recognition memory (Fig. 3 [A1]). Naïve control animals displayed a greater attraction for the novel object (t = 8.5, p < 0.0001, Fig. 3 [A2]) whereas animals in GWI + VEH group exhibited impaired recognition memory by spending comparable times with both familiar and novel objects (t = 0.52, p > 0.05, Fig. 3 [A3]). Animals in GWI + CUR group spent a significantly greater amount of object exploration time with the novel object (t = 2.7, p < 0.05, Fig. 3 [A4]), which matched the object exploration behavior seen in naïve control animals. Moreover, the overall object exploration time, the distance traveled, and the speed of movement in the testing phase (trial-3) were comparable across groups (F = 0.5–2.4, p > 0.05, Fig. 3 [A5–A7]). These observations confirmed that the test results were not influenced by variable levels of object exploration or motor deficits in one or more groups. Thus, CUR treatment maintained better memory function in GWI rats.
Fig. 3.
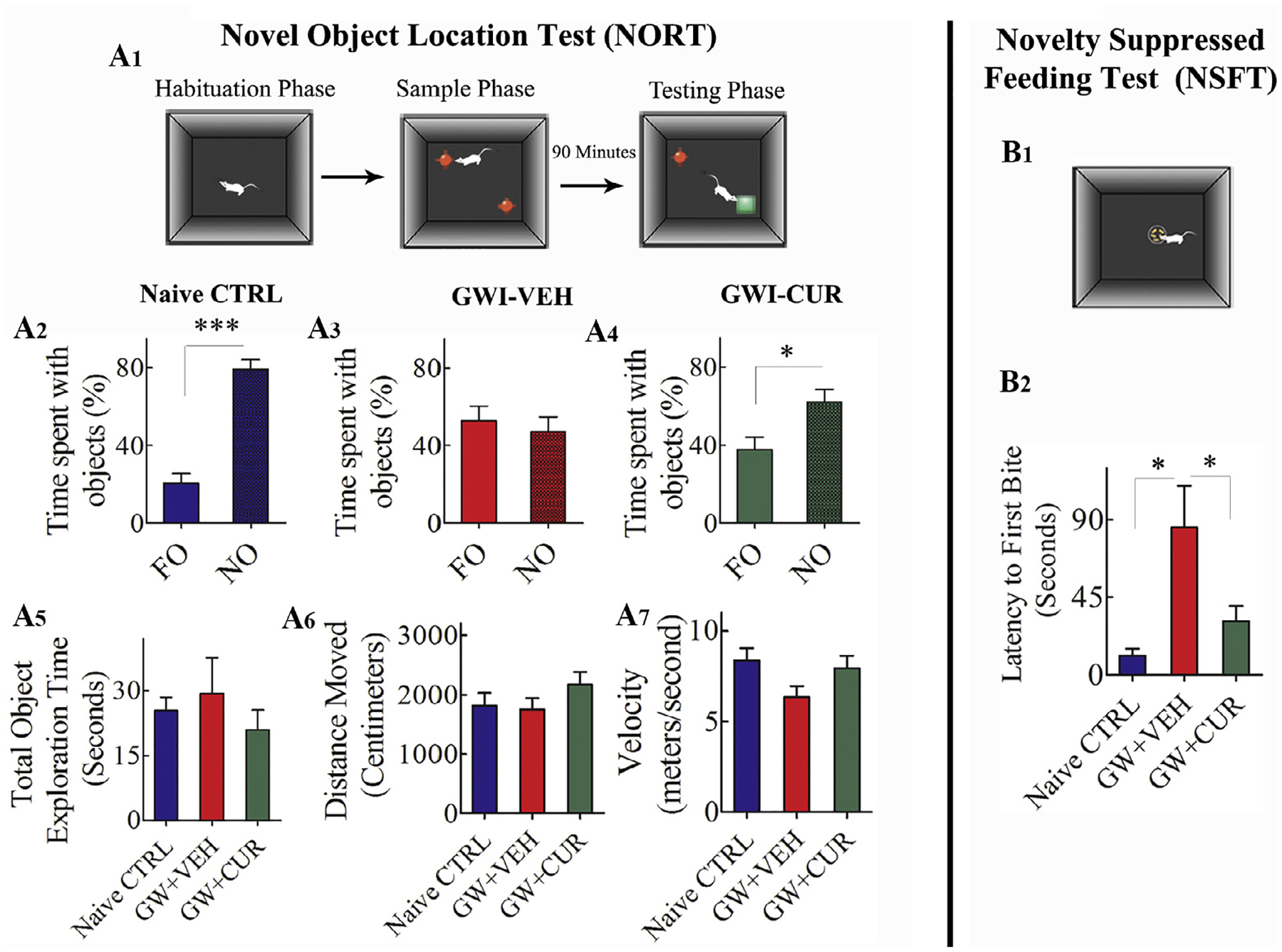
Curcumin (CUR) treated Gulf War Illness (GWI) rats exhibited better novel object recognition memory and mood function. The Fig. A1 shows the various phases involved in a novel object recognition test (NORT). The bar charts in A2–A4 illustrate the performance of animals belonging to naïve control (naïve CTRL, A2), GWI animals receiving vehicle (GWI + VEH, A3), and GWI animals receiving CUR (GWI + CUR, A4) groups. Animals in GWI + VEH group showed novel object recognition memory impairment. This was evidenced by the lack of preference for the novel object (NO) in NORT (A3). In contrast, animals in naïve control and GWI + CUR groups showed normal novel object recognition memory. This was apparent from their preference for NO in NORT (A4). The bar charts in A5–A7 demonstrate that cognitive test results were not influenced by variability in object exploration time (A5), the total distance moved (A6) or the velocity of movement (A7) between groups. The Fig. B1 shows the test apparatus used for a novelty suppressed feeding test (NSFT). The bar chart in B2 compares latency values to the first bite of food between different groups. Note that, animals in GWI + VEH group showed longer latencies than naïve control and GWI + CUR groups, implying a decreased motivation and an increased anxiety-like behavior in this group. In contrast, animals in GWI + CUR group displayed similar latencies as naïve control animals. *p < 0.05, **p < 0.01, and ***p < 0.001.
3.3. CUR treated GWI rats demonstrated better mood function
The extent of anxiety-like behavior was measured using an NSFT, which comprised measurement of the latency to approach and eat a familiar food in a novel environment (Fig. 3 [B1]). This task evaluates the capability of the animal to reconcile a struggle between a circumstance that produces enhanced anxiety and motivation to advance towards an appetitive stimulus (Merali et al., 2003). Comparison of latencies to the first bite using one-way ANOVA revealed differences in anxiety levels between groups (F = 4.2, p < 0.05, Fig. 3 [B2]). Naïve control animals displayed minimal anxiety-like behavior vis-à-vis animals in GWI + VEH group (p < 0.05, Fig. 3 [B2]), whereas animals in GWI + CUR group exhibited a behavior closer to naïve control animals (p > 0.05, Fig. 3 [B2]). Thus, CUR treatment maintained better motivation and reduced anxiety-like behavior in GWI rats.
3.4. CUR treatment enhanced hippocampal neurogenesis in GWI rats
Examination of BrdU+ cells in the SGZ-GCL suggested a reduced density of newly born cells in animals belonging to the GWI + VEH group vis-à-vis the naïve control group but similar density between naïve and GWI + CUR groups (Fig. 4 [A1–B3]). Stereological quantification confirmed that the overall number of new cells added to the SGZ-GCL varied between groups (F = 5.28, p < 0.05, Fig. 4[I]). In comparison to the naïve control group, numbers were reduced in the GWI + VEH group (p < 0.01) but comparable in the GWI + CUR group (p > 0.05; Fig. 4 [I]). Measurement of fractions of newly born cells that differentiated into NeuN-expressing neurons in the SGZ-GCL (Fig. 4 [C1–E3]) suggested no difference in the neuronal fate-choice decision by newly born cells between the three groups (F = 0.63, p > 0.05, Fig. 4 [J]). Quantification of net hippocampal neurogenesis revealed that the extent of neurogenesis varied between groups (F = 5.87, p < 0.01, Fig. 4[K]). When compared with the naïve control group, net neurogenesis was reduced in the GWI + VEH group (p < 0.05) but similar in the GWI + CUR group (p > 0.05, Fig. 4 [K]). Thus, CUR treatment increased hippocampal neurogenesis in GWI rats.
Fig. 4.
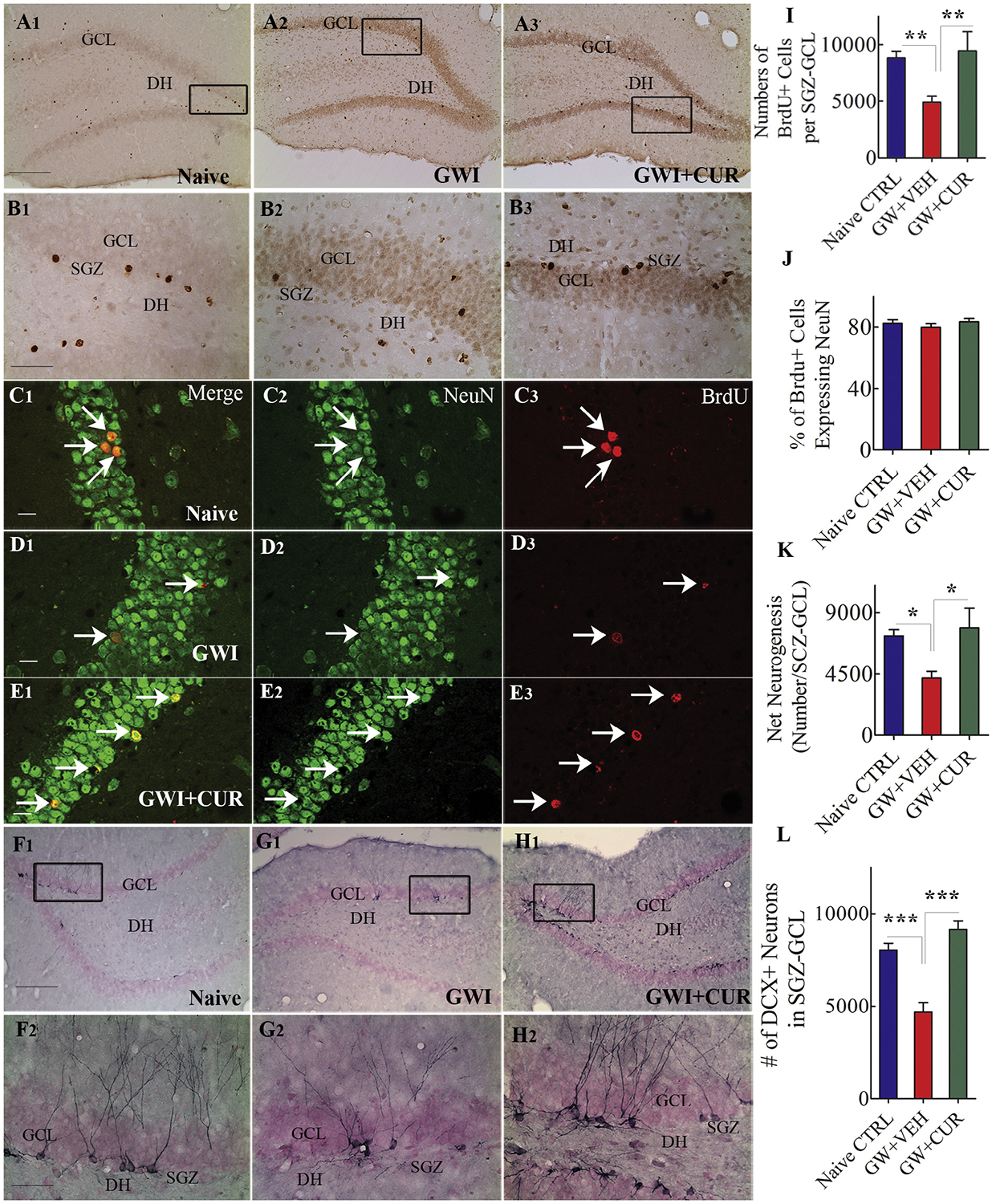
Curcumin (CUR) treatment enhanced neurogenesis in animals with Gulf War Illness (GWI). A1–B3 show distribution of BrdU+ cells in naïve control animals (A1, B1), GWI animals receiving vehicle (GWI-VEH; A2, B2), and GWI animals receiving CUR (GWI-CUR; A3, B3). The bar chart in I compares the number of BrdU+ cells in the subgranular zone-granule cell layer (SGZ-GCL) across the three groups. C1–E3 illustrate examples of BrdU+ cells that differentiated into neurons in naïve control (C1–C3), GWI-VEH (D1–D3) and GWI-CUR (E1–E3) groups. The bar charts in J and K compare percentages of BrdU+ cells expressing NeuN (J) and net neurogenesis (K) across the three groups. F1–H2 show the distribution of doublecortin+ (DCX+) neurons from animals belonging to naïve control (F1, F2), GWI-VEH (G1, G2), and GWI-CUR (H1, H2) groups. The bar chart in L compares the number of DCX+ neurons across the three groups. *p < 0.05, **p < 0.01, and ***p < 0.001. Scale bar, A1, A2, A3, F1, G1, and H1 = 200 μm; B1, B2, B3, F2, G2 and H2 = 50 μm; C1–E3 = 25 μm.
Measurement of the status of hippocampal neurogenesis 120 days after exposure to GWIR-Cs and stress (equivalent to 90 days after CUR or VEH treatment) was performed via stereological quantification of DCX+ neurons (Fig. 4 [F1–H2, and L]). This analysis also showed differences between groups (F = 24.67, p < 0.0001). In comparison to naïve control group, the numbers of DCX+ neurons were diminished in the GWI + VEH group (p < 0.001) but equivalent in the GWI + CUR group (p > 0.05; Fig. 4 [L]). Since DCX expression in rat lasts for ~14 days (Rao and Shetty 2004), DCX+ cells represent new neurons that are generated ~75 days after the termination of CUR or VEH treatment. These results imply that 30 days of CUR treatment is adequate to trigger a sustained enhanced activity of neural stem/progenitor cells to maintain higher levels of neurogenesis in the hippocampus of GWI rats.
3.5. CUR treatment reduced inflammation in the hippocampus of GWI rats
The extent of chronic inflammation in the hippocampus was measured through quantification of astrocyte hypertrophy and microglial activation (Fig. 5). Immunohistochemical staining for GFAP was used for visualizing the occurrence of astrocyte hypertrophy in different animal groups (Fig. 5 [A1–C3]). Quantification of the area fraction of GFAP+ elements in various hippocampal regions revealed differences in density between groups (F = 6.7–22.6, p < 0.01–0.0001). In comparison to the naïve control group, animals in GWI + VEH group displayed increased density of hypertrophied astrocytes in the DG, CA1 and CA3 subfields as well as the entire hippocampus (p < 0.05–0.001, Fig. 5 [D–G]). However, the extent of astrocyte elements in all measured regions in the GWI + CUR group was comparable to levels observed in the naïve control group (p > 0.05, Fig. 5 [D–G]).
Fig. 5.
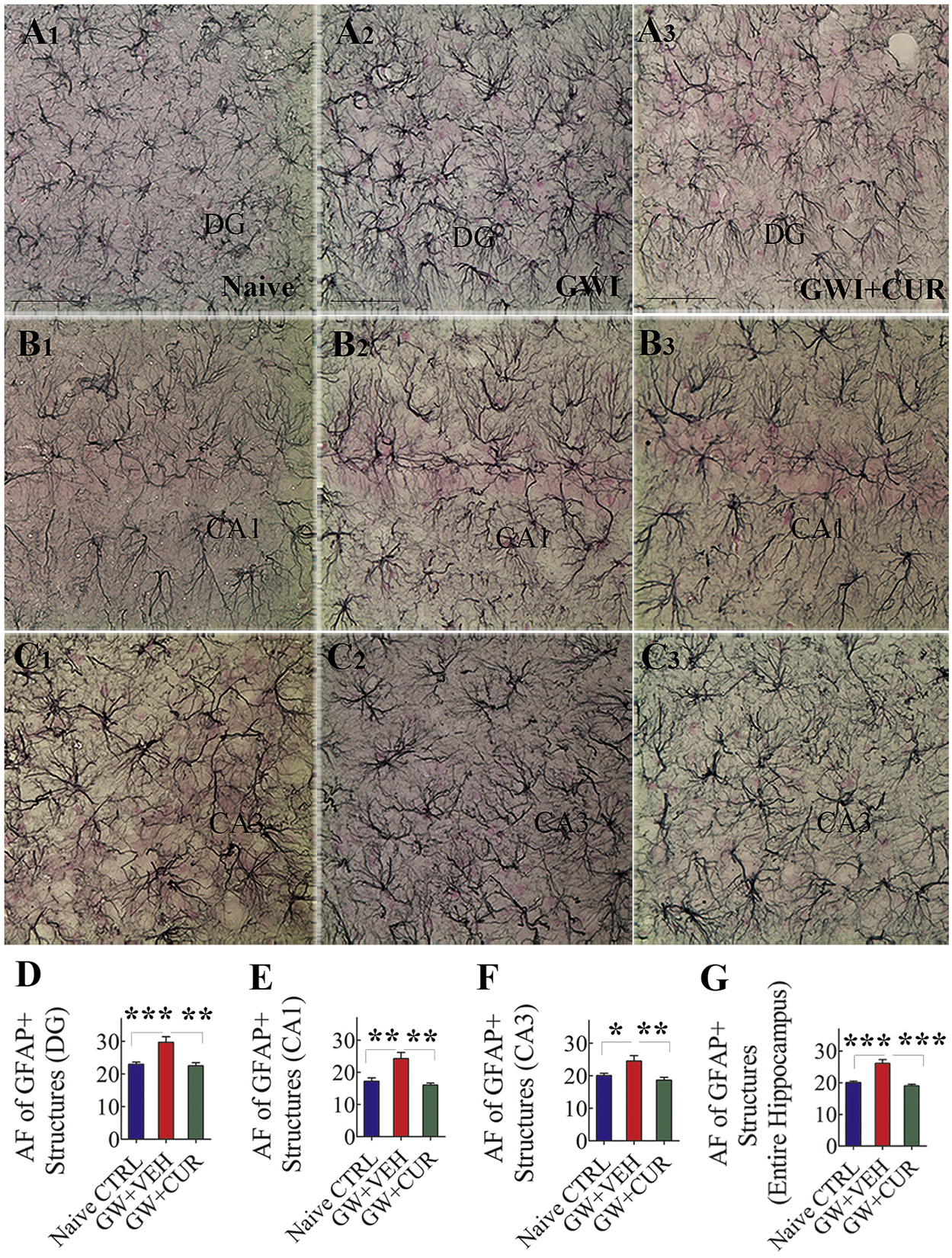
Curcumin (CUR) treatment reduced astrocyte hypertrophy in animals with Gulf War Illness (GWI). A1–C3 show the distribution and morphology of GFAP+ astrocytes in the dentate gyrus (DG; A1–A3), the CA1 subfield (B1–B3) and the CA3 subfield (C1–C3) from a naïve control animal (A1, B1, C1), a GWI animal receiving vehicle (GWI + VEH, A2, B2, C2) and a GWI animal receiving CUR (GWI-CUR; A3, B3, C3). The bar charts in D-G compare the area fraction (AF) of GFAP+ structures in the DG (D), the CA1 subfield (E), the CA3 subfield (F), and the entire hippocampus (G) between different groups. *p < 0.05, **p < 0.01, and ***p < 0.001. Scale bar, A1–C3 = 50 μm.
The presence of activated microglia (i.e., ED-1+ cells displaying less ramified processes or amoeboid morphology) among all microglia (IBA-1+ cells) was also analyzed in the hippocampus. ED-1+ activated microglia demonstrating the presence of CD68+ phagosomes, and amoeboid morphology was seen in both GWI + VEH and GWI + CUR groups but not in the naïve control group. The density of such cells appeared to be greater in the GWI + VEH group than GWI + CUR group (Fig. 6 [A1–B3; G1–H3]). Stereological quantification of ED-1+ cells revealed greater numbers of such cells in the DG and the entire hippocampus of GWI-VEH (t = 2.3–3.4, p < 0.05–0.01, Fig. 6 [C, F]). Quantification of the percentage of ED-1+ cells among IBA-1+ cells confirmed the presence of greater numbers of activated microglia in the GWI + VEH group (t = 3.2, p < 0.01, Fig. 6 [I]). Thus, the reduced occurrence of hypertrophied astrocytes and activated microglia in the hippocampus of CUR treated GWI rats demonstrated the ability of CUR to suppress the low-level neuroinflammation seen in GWI.
Fig. 6.
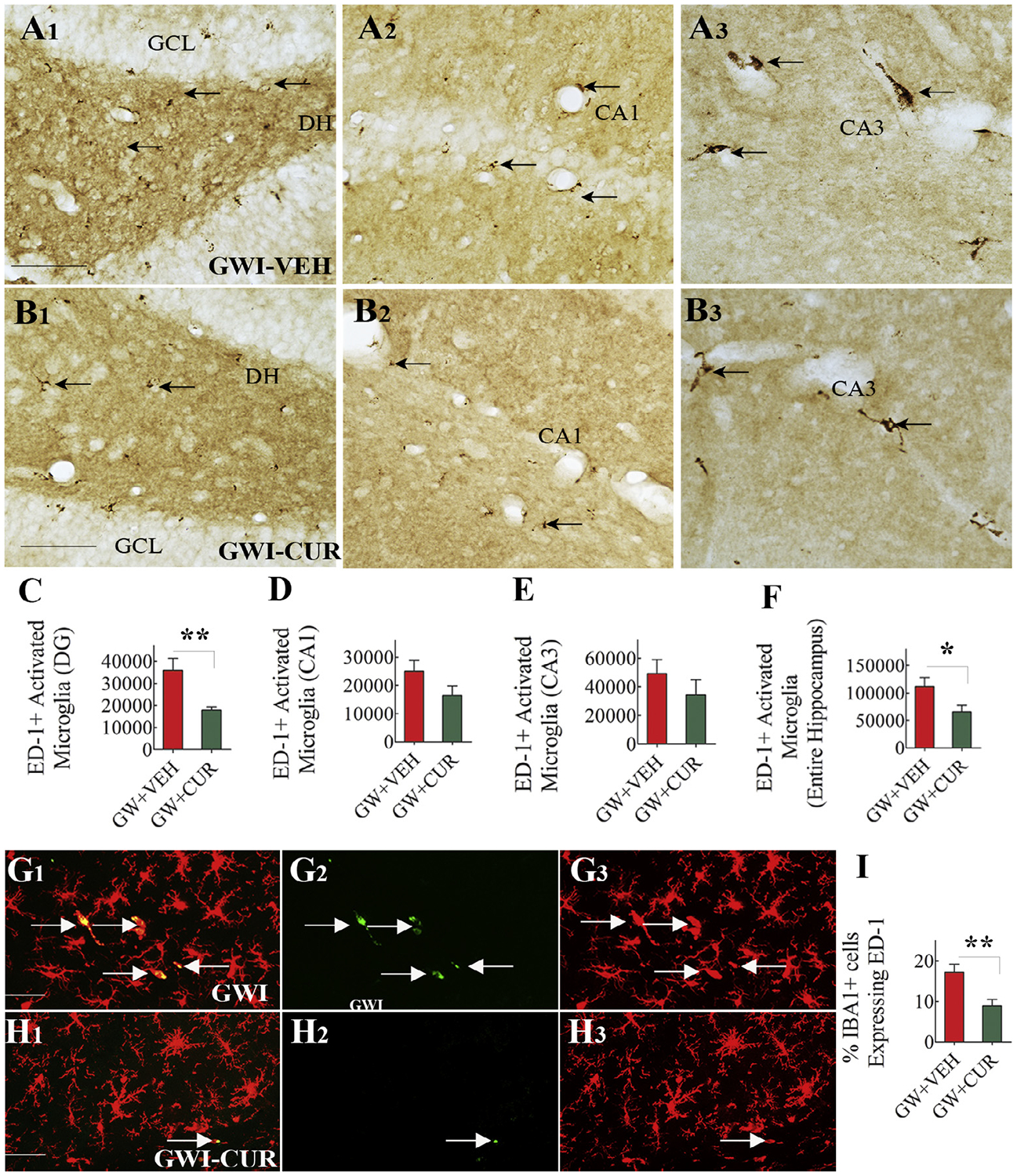
Curcumin (CUR) treatment reduced the occurrence of activated microglia. A1–B3 show the distribution of ED-1+ structures in the dentate gyrus (DG; A1,B1), the CA1 subfield (A2,B2) and the CA3 subfield (A3,B3) from a GWI animal receiving vehicle (GWI + VEH; A1–A3) and GWI animal receiving CUR (GWI-CUR; B1–B3). The bar charts in CF compare the numbers of ED-1+ structures in the DG (C), the CA1 subfield (D), the CA3 subfield (E) and the entire Hippocampus (F). G1–H3 show the distribution of IBA-1 positive microglia expressing ED-1 in animals belonging to GWI-VEH (G1–G3) and GWI-CUR (H1–H3) groups. The bar chart in I compares percentages of IBA1+ microglia expressing ED-1 across the three groups. *p < 0.05, and **p < 0.01. Scale bar, A1–B3 = 100 μm; G1–H3 = 25 μm.
3.6. CUR treatment enhanced antioxidant gene expression in GWI rats
Multiple genes related to oxidative stress displayed varied expression between naïve control, GWI and GWI + CUR groups (F = 13.8–83.8, p < 0.001–0.0001, Fig. 7 [A–B17]). In comparison to the naïve control rats, the hippocampus from GWI rats displayed increased expression of genes encoding oxidative stress markers and antioxidant proteins, implying the presence of persistent oxidative stress (Shetty et al., 2017). Interestingly, thirty days of CUR treatment in GWI rats further enhanced the expression of 17 genes encoding proteins that mediate antioxidant activity (Fig. 7). The genes include Apc, Cat, Ccs, Cygb, Gclc, Gpx1, Gpx3, Gpx4, Gpx7, Gsr, Prdx1, Prdx2, Prdx6, Sepp1, Slc38a1, Sqstm1, Txnrd2 (Fig. 7 [A–B17]). The proteins encoded by these genes and their function are detailed in Table 1. Thus, CUR treatment appeared to be modulating oxidative stress in the hippocampus of GWI rats through enhancement of multiple genes encoding proteins mediating antioxidant activity.
Fig. 7.
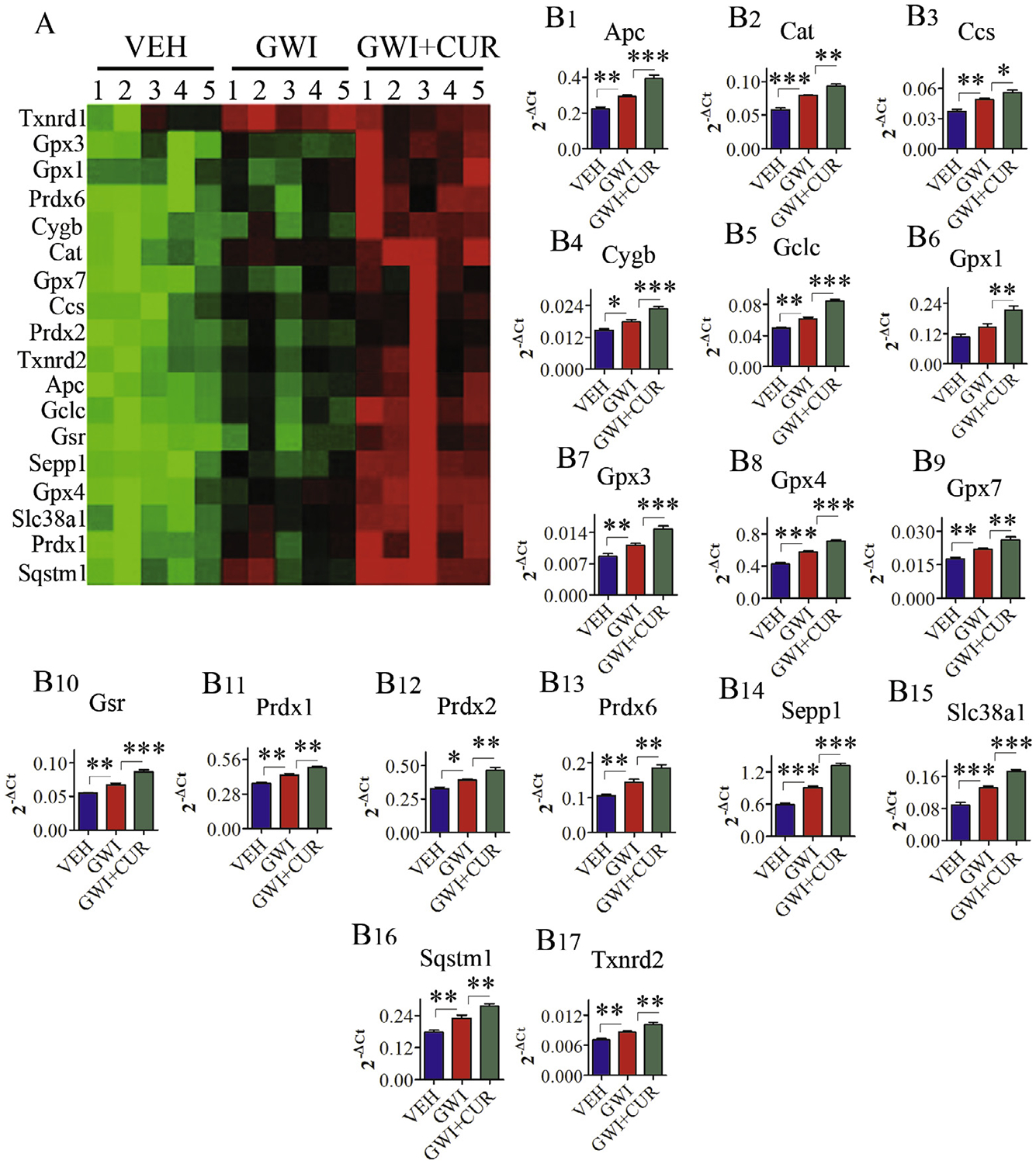
Curcumin (CUR) treatment increased the expression of genes encoding multiple antioxidant proteins in Gulf War Illness (GWI) animals. Figure A is a cluster diagram comparing the relative expression of antioxidant genes between naive control animals receiving vehicle (VEH group), animals receiving exposure to GWI chemicals and stress (GWI group), and animals receiving GWI-related chemicals and stress followed by CUR treatment (GWI + CUR group). The bar charts (B1–B17) illustrate the increased expression of 18 genes encoding different antioxidant proteins in GWI + CUR group, in comparison to VEH and GWI groups. p < 0.05, **p < 0.01, and ***p < 0.001.
Table 1.
Elevated expression of antioxidant genes.
| Gene | Protein encoded | Function |
|---|---|---|
| Apc (Adenomatous Polyposis Coli) | Encodes tumor suppressor protein | Antagonist to Wnt signaling pathway; cell migration and adhesion; chromosome segregation; and transcriptional activation |
| Cat (Catalase) | Encodes protein catalase | Converts the reactive oxygen species hydrogen peroxide to water and oxygen and thereby mitigates the toxic effects of hydrogen peroxide |
| Ccs (Copper Chaperone For Superoxide Dismutase) | Encodes Copper chaperone | Delivers copper to copper zinc superoxide dismutase 1 |
| Cygb (Cytoglobin) | Encodes hexacoordinate hemoglobin | Oxygen transport, and protection during oxidative stress. |
| Gclc (Glutamate-Cysteine Ligase Catalytic Subunit) | Encoded catalytic subunit of gamma-glutamylcysteine synthetase | First rate-limiting enzyme of glutathione synthesis |
| Gpx1 (Glutathione Peroxidase 1) | Encodes isozyme glutathione peroxidase 1 a member of glutathione peroxidase family | Protects cells against oxidative damage; protects the hemoglobin in erythrocytes from oxidative breakdown |
| Gpx3 (Glutathione Peroxidase 3) | Encodes isozyme glutathione peroxidase 3 a member of glutathione peroxidase family | Catalyzes the reduction of hydrogen peroxide, lipid peroxides and organic hydroperoxide |
| Gpx4 (Glutathione Peroxidase 4) | Encodes isozyme glutathione peroxidase 4 a member of glutathione peroxidase family | Hydrolyzes lipid hydroperoxides and protects cells against membrane lipid peroxidation |
| Gpx7 (Glutathione Peroxidase 7) | Encodes isozyme glutathione peroxidase 7 belongs glutathione peroxidase family | Protects cells from hydrogen peroxide-induced oxidative stress; suppresses acidic bile acid-induced reactive oxygen species; and protects against oxidative DNA damage and double-strand breaks |
| Gsr (Glutathione-Disulfide Reductase) | Encodes homodimeric flavoprotein a member of pyridine nucleotide-disulfide oxidoreductase family | Reduces oxidized glutathione disulfide (GSSG) to the sulfhydryl form of GSH, which is an important cellular antioxidant |
| Prdx1 (Peroxiredoxin 1) | Encodes isozyme peroxiredoxin 1 a member of the peroxiredoxin family | reduces peroxides with reducing equivalents provided through the thioredoxin system |
| Prdx2 (Peroxiredoxin 2) | Encodes isozyme peroxiredoxin 2 a member of the peroxiredoxin family | |
| Prdx6 (Peroxiredoxin 6) | Encodes isozyme peroxiredoxin 6 a member of the peroxiredoxin family | Reduces hydrogen peroxide and short chain fatty acids, phospholipid hydroperoxides and helps in redox regulation of cell |
| Sepp1 (Selenoprotein P) | Encodes a selenoprotein predominantly expressed in the liver and secreted into the plasma | Extracellular antioxidant defense through the transport of selenium |
| Slc38a1 (Solute Carrier Family 38 Member 1) | Encodes amino acid transporters | Facilitates sodium-dependent amino acid transport; mediates cotransport of glutamine and sodium ions; supplies glutamatergic and GABAergic neurons with glutamine; modulates intracellular ROS |
| Sqstm1 (Sequestosome 1) | Encodes a multifunctional protein that binds ubiquitin and regulates activation of the NF-kB signaling | Required for the formation and autophagic degradation of polyubiquitin-containing bodies; regulates the activation of NFKB1 by TNF-α, nerve growth factor and interleukin-1 |
| Txnrd2 (Thioredoxin Reductase 2) | Encodes selenocysteine-containing flavoenzyme member of the class I pyridine nucleotide-disulfide oxidoreductase family | Redox-regulated cell signaling, and defense against oxidative stress |
3.7. CUR treatment normalized the expression of genes related to mitochondrial electron transport chain in GWI rats
Many genes relevant to mitochondrial electron transport chain exhibited varied expression between naïve control, GWI and GWI + CUR groups (F = 4.4–35.7, p < 0.05–0.0001). When compared to naïve control rats, the hippocampus of GWI rats exhibited enhanced expression of multiple genes encoding mitochondrial ETC, suggesting the presence of hyperactive mitochondria or mitochondrial dysfunction in GWI (Fig. 8 [A1–A3] and Shetty et al., 2017). Thirty days of CUR treatment normalized the expression of 24 genes related to mitochondrial ETC (Fig. 8 [B1–H5]). These comprise genes that encode proteins related to all complexes of the mitochondrial respiratory chain. The normalized genes include Ndufa11, Ndufb3, Ndufs3, Ndufs6, Ndufs7 and Ndufv1 (genes encoding Complex I proteins, Fig. 8 [B1–B6]), Sdha and Sdhb (genes encoding Complex II proteins, Fig. 8 [C1–C2]), Bcs1l and Cyc1 (genes encoding Complex III proteins, Fig. 8 [D1–D2]), Cox4i2, Cox6a1, Cox7a2l, Cox15 and Cox17 (genes encoding Complex IV proteins, Fig. 8 [E1–E5]), Atp5b, Atp6ap1, Atp6v0a2 (genes encoding Complex V proteins, Fig. 8 [F1–F3]). Also, several other genes related to mitochondrial respiration exhibited normalized expression with CUR treatment. These include Ucp2, Slc25a10, Slc25a15, Slc25a20, Ppa1, Lhpp (Fig. 8 [G1–G6]). The proteins expressed by these genes and their function are detailed in Table 2. Thus, CUR treatment appeared to reverse mitochondrial dysfunction through normalization of genes related mitochondrial ETC.
Fig. 8.
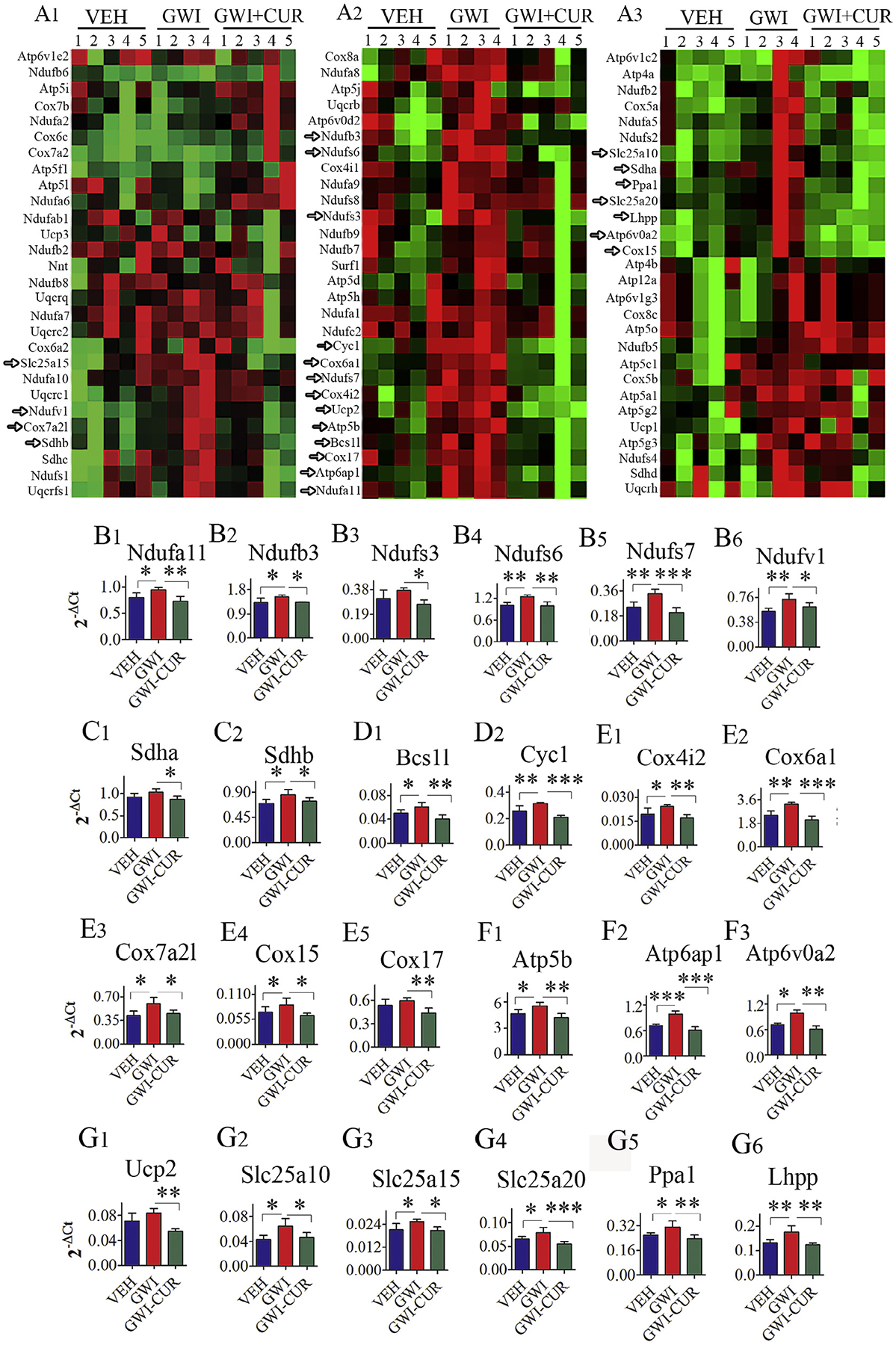
Curcumin (CUR) treatment normalized the expression of genes encoding multiple proteins relevant to the mitochondrial electron transport chain (ETC) in Gulf War Illness (GWI) rats. The figure A is a cluster diagram comparing the relative expression of genes related to the mitochondrial ETC between naive control animals receiving vehicle (VEH group), animals receiving exposure to GWI chemicals and stress (GWI group), and animals receiving GWI-related chemicals and stress followed by CUR treatment (GWI + CUR group). Arrows in A point to genes that showed increased expression in the GWI group but displayed normalized expression in the GWI + CUR group. The bar charts (B1–H5) illustrate the normalized expression of 24 genes related to the mitochondrial ETC in GWI + CUR group, in comparison to their increased expression in the GWI group. These include genes related to mitochondrial complexes I (B1–B6), II (C1–C2), III (D1–D2), IV (E1–E5) and V (F1–F2) and some other genes related to mitochondrial respiration (G1–G6). p < 0.05, **p < 0.01, and ***p < 0.001.
Table 2.
Genes exhibiting elevated expression in mitochondrial respiratory chain.
| Gene | Protein encoded | Function |
|---|---|---|
| Complex 1 (NADH:ubiquinone oxidoreductase, NADH-CoQ reductase, or NADH dehydrogenase | ||
| Ndufa11 (NADH:Ubiquinone Oxidoreductase Subunit A11) | Encodes subunit of the membrane-bound mitochondrial complex I | Transfer of electrons from NADH to the respiratory chain |
| Ndufb3 (NADH:Ubiquinone Oxidoreductase Subunit B3) | Encodes accessory subunit of NADH dehydrogenase | |
| Ndufs6 (NADH:Ubiquinone Oxidoreductase Subunit S6) | Encodes one of seven subunits in the iron-sulfur protein fraction | |
| Ndufs7 (NADH:Ubiquinone Oxidoreductase Core Subunit S7) | Encodes one of the core subunit of NADH dehydrogenase | |
| Ndufv1 (NADH:Ubiquinone Oxidoreductase Core Subunit V1) | Encodes one of the Core subunit of NADH dehydrogenase | |
| Complex II (succinate dehydrogenase or succinate-CoQ reductase) | ||
| Sdha (Succinate Dehydrogenase Complex Flavoprotein Subunit A) | Encodes flavoprotein subunit of succinate dehydrogenase | Transfers electrons from succinate to ubiquinone |
| Sdhb (Succinate Dehydrogenase Complex Iron Sulfur Subunit B) | Encodes iron-sulfur protein subunit of succinate dehydrogenase | |
| Complex III (Cytochrome bc1 complex or CoQH2-cytochrome c reductase) | ||
| Bcs1l (BCS1 Homolog, Ubiquinol-Cytochrome C Reductase Complex Chaperone) | Encodes bcs1 protein providing a chaperone like function | Assembly of complex III, maintenance of mitochondrial tubular networks, respiratory chain assembly |
| Cyc1 (Cytochrome C1) | Encodes subunit of the cytochrome bc1 complex | Transfers electrons from the Rieske iron-sulfur protein to cytochrome c |
| Complex IV (cytochrome c oxidase) | ||
| Cox4i2 (Cytochrome C Oxidase Subunit 4I2) | Encodes isoform 2 of subunit of Cytochrome c oxidase (COX) | Regulation of COX |
| Cox6a1 (Cytochrome C Oxidase Subunit 6A1) | Encodes nuclear gene encodes polypeptide 1 (liver isoform) of subunit VI a of COX | Regulation and assembly of the heteromeric complex |
| Cox7a2l (Cytochrome C Oxidase Subunit 7A2 Like) | Encodes nuclear polypeptide chains of COX | Regulatory subunit of COX and mediates the higher level of energy production in target cells by estrogen |
| Cox15 (COX15, Cytochrome C Oxidase Assembly Homolog) | Encodes nuclear gene encodes a protein which is not a structural subunit | Biogenesis of COX formation and biosynthesis of heme |
| Cox17 (COX17, Cytochrome C Oxidase Copper Chaperone) | Encodes nuclear gene encodes Copper chaperone for cytochrome c oxidase (COX) | binds two copper ions and deliver them to the Cu(A) site of COX |
| Complex V (ATP Synthase) | ||
| Atp5b (ATP Synthase, H+ Transporting, Mitochondrial F1 Complex, Beta Polypeptide) | Encodes β-subunit of catalytic core of mitochondrial ATP synthase | Facilitates synthesis of mitochondrial ATP synthase |
| Atp6ap1 (ATPase H+ Transporting Accessory Protein 1) | Encodes accessory subunit of the proton-transporting vacuolar-ATPase | Assist in the V-ATPase-mediated acidification of neuroendocrine secretory granules |
| Atp6v0a2 (ATPase H+ Transporting V0 Subunit A2) | Encodes V(0) subunit of the vacuolar ATPase | Assist acidification of intracellular compartments |
| Other Genes of Mitochondrial Respiratory Chain | ||
| Ucp2 (Uncoupling Protein 2) | Encodes member of mitochondrial anion carrier proteins | Create proton leaks across the inner mitochondrial membrane, thus uncoupling oxidative phosphorylation from ATP synthesis |
| Slc25a10 (Solute Carrier Family 25 Member 10) | Encode mitochondrial-membrane carrier proteins | Exchanges dicarboxylates, phosphates, sulfates and other small molecules, thereby providing substrates for Krebs cycle and fatty acid synthesis |
| Slc25a15 (Solute Carrier Family 25 Member 15) | Transports ornithine across the inner mitochondrial membrane from the cytosol to matrix | |
| Slc25a20 (Solute Carrier Family 25 Member 20) | Mediates the transport of acylcarnitines from the cytosol to the matrix across inner mitochondrial membrane for fatty acid oxidation. | |
| Ppa1 (Pyrophosphatase (Inorganic) 1) | Encodes proteins in inorganic pyrophosphatase family | Pyrophosphatases catalyze the hydrolysis of pyrophosphate to inorganic phosphate |
| Lhpp (Phospholysine Phosphohistidine Inorganic Pyrophosphate Phosphatase) | Encodes phosphatase protein | Hydrolyzes imidodiphosphate, 3-phosphohistidine and 6-phospholysine |
4. Discussion
Our study provides new evidence that CUR treatment is efficacious for maintaining better hippocampal function in an animal model of GWI. These functional effects comprised better memory function, and reduced anxiety-like behavior. Several cellular and molecular changes in the hippocampus mediated by CUR treatment appeared to underlie these functional benefits. These include increased neurogenesis, reduced astrocyte hypertrophy, diminished numbers of activated microglia, increased expression of genes encoding proteins mediating antioxidant activity and normalized expression of multiple genes encoding proteins related to mitochondrial respiration.
Impaired cognitive and mood function has been seen consistently in Gulf War veterans afflicted with GWI (Odegard et al., 2013; Janulewicz et al., 2017). Animal models of GWI have also demonstrated these impairments (Abdullah et al., 2011; Parihar et al., 2013; Hattiangady et al., 2014; Zakirova et al., 2016). A multitude of changes in the hippocampus and other brain regions can cause cognitive and mood dysfunction. We focused on the ability of CUR to positively modulate a few major adverse alterations in the hippocampus of GWI rats, to understand mechanisms underlying CUR mediated better hippocampal function. We first examined the effect of CUR on hippocampal neurogenesis, which is a process of new neuron addition to the hippocampal circuitry. Such neurogenesis occurs throughout life and has been found to be important for making new memories, maintaining normal mood, and pattern separation (Deng et al. 2010; Snyder et al. 2011; Fotuhi et al., 2012; Kheirbek et al. 2012; Franca et al., 2017). Persistently decreased neurogenesis in association with cognitive and mood impairments in animal models of GWI suggests a linkage between these processes (Parihar et al., 2013). Remarkably, increased hippocampal neurogenesis mediated by CUR treatment was evident in the last week of treatment as well as several months after treatment, implying the ability of CUR to trigger a sustained activity of NSCs to produce new neurons. The ability of CUR to enhance neurogenesis has been observed in several animal models earlier (Tiwari et al., 2014). CUR can enhance neurogenesis through multiple mechanisms including activation of canonical Wnt/α-catenin signaling pathway (Kim et al., 2008; Tiwari et al., 2014). CUR can also increase the expression of genes involved in cell proliferation such as reelin, nestin, and Pax6 (Tiwari et al., 2014), and activate extracellular signal-regulated kinases and p38 kinases (Kim et al., 2008). Thus, it is likely that better cognitive and mood function with CUR treatment observed in GWI rats is at least partly related to enhanced hippocampal neurogenesis.
Next, we investigated the effect of CUR on low-level chronic inflammation characterized by astrocyte hypertrophy and the increased presence of activated microglia in the hippocampus of GWI rats. This is very relevant because such inflammation has been consistently observed in animal models of GWI (Parihar et al., 2013; O’Callaghan et al., 2015; Locker et al., 2017; Shetty et al., 2017) and appears to be present in veterans suffering from GWI (O’Donovan et al., 2015; Johnson et al., 2016; Abou-Donia et al., 2017). Hippocampal inflammation can directly impair cognitive and mood function or indirectly through reducing neurogenesis (Kaltschmidt and Kaltschmidt, 2015; Ryan and Nolan, 2016; McAvoy and Sahay, 2017; Drew and Huckleberry, 2017). Interestingly, better cognitive and mood function mediated by CUR treatment in this study was associated with normalization in the morphology of astrocytes and reduced occurrence of activated microglia in different regions of the hippocampus. The ability of CUR to suppress neuroinflammation has been observed in several models of neurodegenerative and neuroinflammatory diseases (Bassani et al., 2017; Ullah et al., 2017). Additional studies include attenuation of acute inflammation after an experimental traumatic brain injury and inhibition of glial activation and inflammation in a chronic epilepsy model (Zhu et al., 2014; Kaur et al. 2015). Furthermore, CUR has been shown to suppress proinflammatory cytokine expression through multiple means. These include modulation of transcription factors such as STAT, NF-κB, AP-1, inhibition of TLR4-MAPK/NF-κB pathway, and activation of ERK1/2 and p38 signaling pathway (Shi et al., 2015; Rahimifard et al., 2017; Ullah et al., 2017). The above observations support that CUR mediated suppression of neuroinflammation is also one of the factors maintaining better cognitive and mood function in GWI rats.
We also examined the ability of CUR to normalize oxidative stress and mitochondrial function in the hippocampus of GWI rats, as both increased oxidative stress and mitochondrial dysfunction has been observed in GWI rats (Shetty et al., 2017) and veterans affected with GWI (Baraniuk et al., 2013; Koslik et al., 2014). Elevated expression of multiple genes related to mitochondrial ETC likely reflects the presence of hyperactive ETC to increased oxidative stress. Increased expression of genes encoding proteins involved in ETC may also suggest an innate compensatory response to help balance the irregular ETC (Manczak et al., 2005; Shetty et al., 2017). On the other hand, upregulation of genes encoding proteins involved in ATP synthesis likely implies an altered energy requirement due to increased protein degradation (Pecorelli et al., 2013). Both increased oxidative stress and mitochondrial dysfunction can adversely influence cognitive and mood function as well as neurogenesis (Czarny et al., 2017; Raefsky and Mattson, 2017).
Furthermore, hyperactive mitochondria can produce a greater amount of reactive oxygen species (ROS) and contribute greatly to the ongoing oxidative stress (Huttemann et al., 2012). Importantly, better cognitive and mood function mediated by CUR was associated with increased expression of multiple genes encoding antioxidant proteins which likely combated increased levels of ROS. Furthermore, normalization of multiple genes encoding proteins related to mitochondrial complexes I-V implied a reversal of mitochondrial dysfunction by CUR treatment. Normalized mitochondrial respiration would reduce the generation of ROS. The ability of CUR to normalize oxidative stress and mitochondrial function has been observed in several models of diseases previously. For instance, a recent study has shown that CUR treatment can attenuate oxidative stress and lipid peroxidation in brain tissues via increased antioxidant defense activity (Samarghandian et al., 2017). Moreover, the upregulation of Prdx genes noted in this study is consistent with earlier findings that CUR treatment can enhance Prdx genes and proteins to normalize ROS expression and lipid peroxidation (Chhunchha et al., 2011). Thus, CUR mediated increased antioxidant activity, and normalized mitochondrial function in the hippocampus have likely also played roles in sustaining better cognitive and mood function in GWI rats.
4.1. Conclusions, limitations and future directions
In this study, CUR treated GWI rats displayed better cognitive and mood function. Additional investigations suggested that CUR treatment mediated increased neurogenesis, suppression of inflammation, increased antioxidant activity and normalization of mitochondrial activity may underlie these beneficial functional effects. The functional effects of CUR are consistent with the results of a randomized clinical trial in healthy elderly population, where better attention and working memory was seen with acute CUR administration, and enhanced mood and reduced fatigue was observed with a chronic treatment (Cox et al., 2015). The findings of this study suggest that CUR may have promise for treating GWI patients. However, there are a few issues which need to be addressed in future studies. First, the current study examined the efficacy of intraperitoneal administration of CUR. As oral intake is the most commonly used route of administration in patients, it will be necessary to establish whether oral CUR treatment would have similar beneficial effects. Because of differences in the bioavailability of CUR following intraperitoneal vis-à-vis oral routes of administration, an efficacious dose of CUR for oral administration needs to be determined. Achieving efficacy with oral CUR therapy may need relatively higher doses as well as co-treatment with compounds or drugs (e.g., piperine) that enhance the bioavailability of CUR. Second, the current study examined the effects of CUR treatment immediately after 28-days of exposure to GWIR-Cs and stress. While several GWI-related pathological changes such as decreased hippocampal neurogenesis, blood brain barrier disruption, astrocyte hypertrophy, and decreased AChE activity are evident in the brain at this time-point (Abdel-Rahman et al., 2002; Parihar et al., 2013), it is unknown whether the actual GWI symptoms appear early after exposure. Therefore, it will be important to know whether delayed administration of CUR after exposure to GWIR-Cs and stress would ease cognitive and mood dysfunction. Such studies are highly relevant for considering CUR therapy for GWI patients, as >25 years have passed since the Gulf War and veterans afflicted with GWI display chronic illness.
Acknowledgments
This work was supported by grants from the Department of Veterans Affairs (VA Merit Award I01BX000883 to A.K.S., and BLR&D Research Career Scientist award 1IK6BX003612 to A.K.S.), Department of Defense (GWIRP grant, W81XWH-14-1-0572 to A. K.S.), and State of Texas (Emerging Technology Funds to A.K.S.).
Footnotes
Publisher's Disclaimer: Department of Veterans Affairs, Department of Defense, United States Government Disclaimer
Publisher's Disclaimer: The contents of this article suggest the views of authors and do not represent the views of the U.S. Department of Veterans Affairs, Department of Defense or the United States Government.
References
- Abou-Donia MB, Conboy LA, Kokkotou E, El-Masry EM, Jacobson E, Sullivan K, 2017. Screening for novel central nervous system biomarkers in Veterans with Gulf War Illness. Neurotoxicol Teratol. 61, 36–46. [DOI] [PubMed] [Google Scholar]
- Abdel-Rahman A, Shetty AK, Abou-Donia MB, 2002. Disruption of the bloodbrain barrier and neuronal cell death in cingulate cortex, dentate gyrus, thalamus, and hypothalamus in a rat model of Gulf-War syndrome. Neurobiol. Dis 10, 306–326. [DOI] [PubMed] [Google Scholar]
- Abdullah L, Crynen G, Reed J, Bishop A, Phillips J, Ferguson S, Mouzon B, Mullan M, Mathura V, Mullan M, Ait-Ghezala G, Crawford F, 2011. Proteomic CNS profile of delayed cognitive impairment in mice exposed to Gulf War agents. Neuromolecular Med. 13, 275–288. [DOI] [PubMed] [Google Scholar]
- Baraniuk JN, El-Amin S, Corey R, Rayhan R, Timbol C, 2013. Carnosine treatment for Gulf War Illness: a randomized controlled trial. Glob. J. Health. Sci 5, 69–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassani TB, Turnes JM, Moura ELR, Bonato JM, Coppola-Segovia V, Zanata SM, Oliveira RMMW, Vital MABF, 2017. Effects of curcumin on short-term spatial and recognition memory, adult neurogenesis and neuroinflammation in a streptozotocin-induced rat model of dementia of Alzheimer’s type. Behav. Brain Res 335, 41–54. [DOI] [PubMed] [Google Scholar]
- Binns JH, Barlow C, Bloom FE, Clauw DJ, Golomb BA, Graves JC et al. 2008. Gulf War Illness and the health of Gulf war veterans: scientific findings and recommendations, Research Advisory Committee Report on Gulf War Illness and Health of Gulf War Veterans. Dept of Veterans Affairs. US Government Printing Office: Washington, DC, pp 1–465 [Google Scholar]
- Chao LL, Rothlind JC, Cardenas VA, Meyerhoff DJ, Weiner MW, 2010. Effects of low-level exposure to sarin and cyclosarin during the 1991 Gulf War on brain function and brain structure in US veterans. Neurotoxicology. 31, 493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhunchha B, Fatma N, Bhargavan B, Kubo E, Kumar A, Singh DP, 2011. Specificity protein, Sp1-mediated increased expression of Prdx6 as a curcumininduced antioxidant defense in lens epithelial cells against oxidative stress. Cell. Death Dis 2, e234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox KH, Pipingas A, Scholey AB, 2015. Investigation of the effects of solid lipid curcumin on cognition and mood in a healthy older population. J. Psychopharmacol 29, 642–651. [DOI] [PubMed] [Google Scholar]
- Czarny P, Wigner P, Galecki P, Sliwinski T, 2017. The interplay between inflammation, oxidative stress, DNA damage, DNA repair and mitochondrial dysfunction in depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 80, 309–321. [DOI] [PubMed] [Google Scholar]
- Deng W, Aimone JB, Gage FH, 2010. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat. Rev. Neurosci 11, 339–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong S, Zeng Q, Mitchell ES, Xiu J, Duan Y, Li C, Tiwari JK, Hu Y, Cao X, Zhao Z, 2012. Curcumin enhances neurogenesis and cognition in aged rats: implications for transcriptional interactions related to growth and synaptic plasticity. PLoS One. 7 (2), e31211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew MR, Huckleberry KA, 2017. Modulation of Aversive Memory by Adult Hippocampal Neurogenesis. Neurotherapeutics. 14, 646–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotuhi M, Do D, Jack C, 2012. Modifiable factors that alter the size of the hippocampus with ageing. Nat. Rev. Neurol 8, 189–202. [DOI] [PubMed] [Google Scholar]
- Franca TFA, Bitencourt AM, Maximilla NR, Barros DM, Monserrat JM, 2017. Hippocampal neurogenesis and pattern separation: A meta-analysis of behavioral data. Hippocampus. 27, 937–950. [DOI] [PubMed] [Google Scholar]
- Fricker RD, Reardon E, Spektor DM, Cotton SK, Hawes-Dawson J, Pace JE, Hosek SD, 2000. Pesticide use during the Gulf War: A survey of Gulf War Veterans. RAND Corporation, Santa Monica, CA. [Google Scholar]
- Golomb BA, 1999. A review of the scientific literature as it pertains to Gulf War Illnesses:Vol 2: Pyridostigmine Bromide. Santa Monica, CA: RAND Corporation. [Google Scholar]
- Golomb BA, 2008. Acetylcholinesterase inhibitors and Gulf War Illnesses. Proc. Natl. Acad. Sci. U. S. A 105, 4295–4300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley RW, Kurt TL, 1997. Self-reported exposure to neurotoxic chemical combinations in the Gulf War. A cross-sectional epidemiologic study. JAMA 277, 231–237. [PubMed] [Google Scholar]
- Hattiangady B, Mishra V, Kodali M, Shuai B, Rao X, Shetty AK, 2014. Object location and object recognition memory impairments, motivation deficits and depression in a model of Gulf War Illness. Front. Behav. Neurosci 8, 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattiangady B, Shetty AK, 2012. Neural stem cell grafting counteracts hippocampal injury-mediated impairments in mood, memory, and neurogenesis. Stem Cells Transl. Med 1, 696–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattiangady B, Kuruba R, Shetty AK, 2011. Acute seizures in old age leads to a greater loss of CA1 pyramidal neurons, an increased propensity for developing chronic TLE and a severe cognitive dysfunction. Aging Dis. 2, 1–17. [PMC free article] [PubMed] [Google Scholar]
- Hattiangady B, Rao MS, Shetty AK, 2008. Grafting of striatal precursor cells into hippocampus shortly after status epilepticus restrains chronic temporal lobe epilepsy. Exp. Neurol 212, 468–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard NA, Hutchison JL, Motes MA, Shokri-Kojori E, Bennett IJ, Brigante RM, Haley RW, Rypma B, 2014. Central Executive Dysfunction and Deferred Prefrontal Processing in Veterans with Gulf War Illness. Clin. Psychol. Sci 2, 319–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttemann M, Helling S, Sanderson TH, Sinkler C, Samavati L, Mahapatra G, Varughese A, Lu G, Liu J, Ramzan R, Vogt S, Grossman LI, Doan JW, Marcus K, Lee I, 2012. Regulation of mitochondrial respiration and apoptosis through cell signaling: cytochrome c oxidase and cytochrome c in ischemia/reperfusion injury and inflammation. Biochim. Biophys. Acta 1817, 598–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine (IOM), 2014. Chronic Multisymptom Illness in Gulf War Veterans: Case Definitions Reexamined. National Academies Press, Washington, DC. [PubMed] [Google Scholar]
- Janulewicz PA, Krengel MH, Maule A, White RF, Cirillo J, Sisson E, Heeren T, Sullivan K, 2017. Neuropsychological characteristics of Gulf War Illness: A meta-analysis. PLoS One 12, e0177121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenrow KA, Brown SL, Lapanowski K, Naei H, Kolozsvary A, Kim JH, 2013. Selective inhibition of microglia-mediated neuroinflammation mitigates radiation-induced cognitive impairment. Radiat. Res 179, 549–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson GJ, Slater BC, Leis LA, Rector TS, Bach RR, 2016. Blood biomarkers of chronic inflammation in Gulf War Illness. PLoS One 11, e0157855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltschmidt B, Kaltschmidt C, 2015. NF-KappaB in long-term memory and structural plasticity in the adult mammalian brain. Front. Mol. Neurosci 8, 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur H, Patro I, Tikoo K, Sandhir R, 2015. Curcumin attenuates inflammatory response and cognitive deficits in experimental model of chronic epilepsy. Neurochem. Int 89, 40–50. [DOI] [PubMed] [Google Scholar]
- Kheirbek MA, Klemenhagen KC, Sahay A, Hen R, 2012. Neurogenesis and generalization: a new approach to stratify and treat anxiety disorders. Nat. Neurosci 15, 1613–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Son TG, Park HR, Park M, Kim MS, Kim HS, Chung HY, Mattson MP, Lee J., m et al. 2008. Curcumin stimulates proliferation of embryonic neural progenitor cells and neurogenesis in the adult hippocampus. J Biol. Chem 283, 14497–14505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodali M, Megahed T, Mishra V, Shuai B, Hattiangady B, Shetty AK, 2016. Voluntary running exercise-mediated enhanced neurogenesis does not obliterate retrograde spatial memory. J. Neurosci 36, 8112–8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodali M, Parihar VK, Hattiangady B, Mishra V, Shuai B, Shetty AK, 2015. Resveratrol prevents age-related memory and mood dysfunction with increased hippocampal neurogenesis and microvasculature, and reduced glial activation. Sci. Rep 5, 8075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohman RA, Rhodes JS, 2013. Neurogenesis, inflammation and behavior. Brain Behav. Immun 27, 22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koslik HJ, Hamilton G, Golomb BA, 2014. Mitochondrial dysfunction in Gulf War Illness revealed by 31Phosphorus Magnetic Resonance Spectroscopy: a case-control study. PLoS One. 9, e92887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni SK, Bhutani MK, Bishnoi M, 2008. Antidepressant activity of curcumin: involvement of serotonin and dopamine system. Psychopharmacology (Berl.) 201, 435–442. [DOI] [PubMed] [Google Scholar]
- Kuruba R, Hattiangady B, Parihar VK, Shuai B, Shetty AK, 2011. Differential susceptibility of interneurons expressing neuropeptide Y or parvalbumin in the aged hippocampus to acute seizure activity. PLoS One. 6, e24493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langston RF, Wood ER, 2010. Associative recognition and the hippocampus: differential effects of hippocampal lesions on object-place, object-context and object-place-context memory. Hippocampus. 20, 1139–1153. [DOI] [PubMed] [Google Scholar]
- Li X, Spence JS, Buhner DM, Hart J Jr, Cullum CM, Biggs MM, Hester AL, Odegard TN, Carmack PS, Briggs RW, Haley RW, 2011. Hippocampal dysfunction in Gulf War veterans: investigation with ASL perfusion MR imaging and physostigmine challenge. Radiology. 261, 218–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locker AR, Michalovicz LT, Kelly KA, Miller JV, Miller DB, O’Callaghan JP, 2017. Corticosterone primes the neuroinflammatory response to Gulf War Illness-relevant organophosphates independently of acetylcholinesterase inhibition. J. Neurochem 142, 444–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long Q, Upadhya D, Hattiangady B, Kim DK, An SY, Shuai B, Prockop DJ, Shetty AK, 2017. Intranasal MSC-derived A1-exosomes ease inflammation, and prevent abnormal neurogenesis and memory dysfunction after status epilepticus. Proc. Natl. Acad. Sci. U. S. A 114, E3536–E3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manczak M, Jung Y, Park BS, Partovi D, Reddy PH, 2005. Time-course of mitochondrial gene expressions in mice brains: implications for mitochondrial dysfunction, oxidative damage, and cytochrome c in aging. J. Neurochem 92, 494–504. [DOI] [PubMed] [Google Scholar]
- Manns JR, Eichenbaum H, 2009. A cognitive map for object memory in the hippocampus. Learn. Mem 16, 616–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAvoy KM, Sahay A, 2017. Targeting adult neurogenesis to optimize hippocampal circuits in aging. Neurotherapeutics. 14, 630–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megahed T, Hattiangady B, Shuai B, Shetty AK, 2015. Parvalbumin and neuropeptide Y expressing hippocampal GABA-ergic inhibitory interneuron numbers decline in a model of Gulf War Illness. Front. Cell. Neurosci 8, 447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon PM, Nasrallah HA, Reeves RR, Ali JA, 2004. Hippocampal dysfunction in Gulf War Syndrome. A proton MR spectroscopy study. Brain Res. 1009, 189–194. [DOI] [PubMed] [Google Scholar]
- Merali Z, Levac C, Anisman H, 2003. Validation of a simple, ethologically relevant paradigm for assessing anxiety in mice. Biol. Psychiatry 54, 552–565. [DOI] [PubMed] [Google Scholar]
- Mishra V, Shuai B, Kodali M, Shetty GA, Hattiangady B, Rao X, Shetty AK, 2015. Resveratrol treatment after status epilepticus restrains neurodegeneration and abnormal neurogenesis with suppression of oxidative stress and inflammation. Sci. Rep 5, 17807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Callaghan JP, Kelly KA, Locker AR, Miller DB, Lasley SM, 2015. Corticosterone primes the neuroinflammatory response to DFP in mice: potential animal model of Gulf War Illness. J. Neurochem 133, 708–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odegard TN, Cooper CM, Farris EA, Arduengo J, Bartlett J, Haley R, 2013. Memory impairment exhibited by veterans with Gulf War Illness. Neurocase. 19, 316–327. [DOI] [PubMed] [Google Scholar]
- O’Donovan A, Chao LL, Paulson J, Samuelson KW, Shigenaga JK, Grunfeld C, Weiner MW, Neylan TC, 2015. Altered inflammatory activity associated with reduced hippocampal volume and more severe posttraumatic stress symptoms in Gulf War veterans. Psychoneuroendocrinology. 51, 557–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parihar VK, Hattiangady B, Shuai B, Shetty AK, 2013. Mood and memory deficits in a model of Gulf War Illness are linked with reduced neurogenesis, partial neuron loss, and mild inflammation in the hippocampus. Neuropsychopharmacology. 38, 2348–2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parihar VK, Hattiangady B, Kuruba R, Shuai B, Shetty AK, 2011. Predictable chronic mild stress improves mood, hippocampal neurogenesis and memory. Mol. Psychiatry 16, 171–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecorelli A, Leoni G, Cervellati F, Canali R, Signorini C, Leoncini S, Cortelazzo A, De Felice C, Ciccoli L, Hayek J, Valacchi G, 2013. Genes related to mitochondrial functions, protein degradation, and chromatin folding are differentially expressed in lymphomonocytes of Rett syndrome patients. Mediators Inflamm. 2013, 137629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips KF, Deshpande LS, 2016. Repeated low-dose organophosphate DFP exposure leads to the development of depression and cognitive impairment in a rat model of Gulf War Illness. Neurotoxicology. 52, 127–133. [DOI] [PubMed] [Google Scholar]
- Raefsky SM, Mattson MP, 2017. Adaptive responses of neuronal mitochondria to bioenergetic challenges: Roles in neuroplasticity and disease resistance. Free Radic. Biol. Med 102, 203–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimifard M, Maqbool F, Moeini-Nodeh S, Niaz K, Abdollahi M, Braidy N, Nabavi SM, Nabavi SF, 2017. Targeting the TLR4 signaling pathway by polyphenols: A novel therapeutic strategy for neuroinflammation. Ageing Res. Rev 36, 11–19. [DOI] [PubMed] [Google Scholar]
- Rao MS, Hattiangady B, Shetty AK, 2006a. The window and mechanisms of major age-related decline in the production of new neurons within the dentate gyrus of the hippocampus. Aging Cell 5, 545–558. [DOI] [PubMed] [Google Scholar]
- Rao MS, Hattiangady B, Reddy DS, Shetty AK, 2006b. Hippocampal neurodegeneration, spontaneous seizures, and mossy fiber sprouting in the F344 rat model of temporal lobe epilepsy. J. Neurosci. Res 83, 1088–1105. [DOI] [PubMed] [Google Scholar]
- Rao MS, Hattiangady B, Shetty AK, 2008. Status epilepticus during old age is not associated with enhanced hippocampal neurogenesis. Hippocampus. 18, 931–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao MS, Shetty AK, 2004. Efficacy of doublecortin as a marker to analyse the absolute number and dendritic growth of newly generated neurons in the adult dentate gyrus. Eur. J. Neurosci 19, 234–246. [DOI] [PubMed] [Google Scholar]
- Rayhan RU, Ravindran MK, Baraniuk JN, 2013. Migraine in Gulf War Illness and chronic fatigue syndrome: prevalence, potential mechanisms, and evaluation. Front. Physiol 4, 181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan SM, Nolan YM, 2016. Neuroinflammation negatively affects adult hippocampal neurogenesis and cognition: can exercise compensate? Neurosci. Biobehav. Rev 61, 121–131. [DOI] [PubMed] [Google Scholar]
- Samarghandian S, Azimi-Nezhad M, Farkhondeh T, Samini F, 2017. Antioxidative effects of curcumin on immobilization-induced oxidative stress in rat brain, liver and kidney. Biomed. Pharmacother 87, 223–229. [DOI] [PubMed] [Google Scholar]
- Shetty AK, Hattiangady B, Rao MS, Shuai B, 2012. Neurogenesis response of middle-aged hippocampus to acute seizure activity. PLoS One 7, e43286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty GA, Hattiangady B, Shetty AK, 2013. Neural stem cell- and neurogenesisrelated gene expression profiles in the young and aged dentate gyrus. Age (Dordr). 35, 2165–2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty GA, Hattiangady B, Upadhya D, Bates A, Attaluri S, Shuai B, Kodali M, Shetty AK, 2017. Chronic oxidative stress, mitochondrial dysfunction, Nrf2 activation and inflammation in the hippocampus accompany heightened systemic inflammation and oxidative stress in an animal model of Gulf War Illness. Front. Mol. Neurosci 10, 182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X, Zheng Z, Li J, Xiao Z, Qi W, Zhang A, Wu Q, Fang Y, 2015. Curcumin inhibits Abeta-induced microglial inflammatory responses in vitro: Involvement of ERK1/2 and p38 signaling pathways. Neurosci. Lett 594, 105–110. [DOI] [PubMed] [Google Scholar]
- Snyder JS, Soumier A, Brewer M, Pickel J, Cameron HA, 2011. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature. 476, 458–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele L, Sastre A, Gerkovich MM, Cook MR, 2012. Complex factors in the etiology of Gulf War Illness: wartime exposures and risk factors in veteran subgroups. Environ. Health Perspect 120, 112–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan K, Krengel M, Proctor PS, Devine S, Heeren T, White RF, 2003. Cognitive functioning in treatment-seeking Gulf War Veterans: Pyridostigmine Bromide use and PTSD. J. Psychopath. Beh. Assess 25, 95–103. [Google Scholar]
- Tiwari SK, Agarwal S, Seth B, Yadav A, Nair S, Bhatnagar P, Karmakar M, Kumari M, Chauhan LK, Patel DK, Srivastava V, Singh D, Gupta SK, Tripathi A, Chaturvedi RK, Gupta KC, 2014. Curcumin-loaded nanoparticles potently induce adult neurogenesis and reverse cognitive deficits in Alzheimer’s disease model via canonical Wnt/beta-catenin pathway. ACS Nano. 8, 76–103. [DOI] [PubMed] [Google Scholar]
- Ullah F, Liang A, Rangel A, Gyengesi E, Niedermayer G, Munch G, 2017. High bioavailability curcumin: an anti-inflammatory and neurosupportive bioactive nutrient for neurodegenerative diseases characterized by chronic neuroinflammation. Arch. Toxicol 91, 1623–1634. [DOI] [PubMed] [Google Scholar]
- VanRiper SM, Alexander AL, Koltyn KF, Stegner AJ, Ellingson LD, Destiche DJ, Dougherty RJ, Lindheimer JB, Cook DB, 2017. Cerebral white matter structure is disrupted in Gulf War Veterans with chronic musculoskeletal pain. Pain 158, 2364–2375. [DOI] [PubMed] [Google Scholar]
- Warburton EC, Barker GR, Brown MW, 2013. Investigations into the involvement of NMDA mechanisms in recognition memory. Neuropharmacology. 74, 41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White RF, Steele L, O’Callaghan JP, Sullivan K, Binns JH, Golomb BA, Bloom FE, Bunker JA, Crawford F, Graves JC, Hardie A, Klimas N, Knox M, Meggs WJ, Melling J, Philbert MA, Grashow R, 2016. Recent research on Gulf War Illness and other health problems in veterans of the 1991 Gulf War: Effects of toxicant exposures during deployment. Cortex. 74, 449–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkenwerder W, 2003. Environmental Exposure Report: Pesticides Final Report. U.S. Department of Defense, Office of the Special Assistant to the Undersecretary of Defense (Personnel and Readiness) for Gulf War Illnesses Medical Readiness and Military Deployments, Washington, D.C. [Google Scholar]
- Xu Y, Ku B, Cui L, Li X, Barish PA, Foster TC, Ogle WO, 2007. Curcumin reverses impaired hippocampal neurogenesis and increases serotonin receptor 1A mRNA and brain-derived neurotrophic factor expression in chronically stressed rats. Brain Res. 1162, 9–18. [DOI] [PubMed] [Google Scholar]
- Zakirova Z, Crynen G, Hassan S, Abdullah L, Horne L, Mathura V, Crawford F, Ait-Ghezala G, 2016. A chronic longitudinal characterization of neurobehavioral and neuropathological cognitive impairment in a mouse model of Gulf War agent exposure. Front. Integr. Neurosci 9, 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu HT, Bian C, Yuan JC, Chu WH, Xiang X, Chen F, Wang CS, Feng H, Lin JK, 2014. Curcumin attenuates acute inflammatory injury by inhibiting the TLR4/MyD88/NF-kappaB signaling pathway in experimental traumatic brain injury. J. Neuroinflammation 11. 59-2094-11-59. [DOI] [PMC free article] [PubMed] [Google Scholar]


