Introduction
Hundreds of influenza-associated pediatric deaths are reported annually, with children under the age of two at the highest risk of mortality [1]. Four medications are approved for influenza treatment but for children under the age of two, oseltamivir is the only approved drug [2]. Oseltamivir is a prodrug of its active form, oseltamivir carboxylate (OC) [3].
Since oseltamivir can only be administered enterally, a nasogastric (NG) tube is used for those unable to take medications orally. Alternatively, it can be delivered through pre-existing enteric tubes in patients with medical complexity, a significant proportion of children hospitalized for influenza [4]. Pharmacokinetic (PK) studies of oseltamivir administered through NG tubes in critically ill children have had varying results; OC exposure has been reported to be higher and lower when compared to previous studies in children who took oseltamivir orally [5, 6]. Since pediatric influenza deaths often occur within 7 days of symptom onset, adequate OC concentrations early in illness are critical [1]. No study has directly compared, in the same cohort of critically ill children, oseltamivir and OC concentrations between oral and enteric tube administration. Therefore, we designed a prospective cohort study to investigate the effects of route administration of oseltamivir on plasma concentrations in critically ill children with suspected influenza. We hypothesized patients who require oseltamivir administration through an enteric tube would have significantly different concentrations that may be subtherapeutic or potentially toxic.
Materials and Methods
Study Design
We performed a prospective cohort study in critically ill children admitted to the Pediatric Intensive Care Unit (PICU) at Cincinnati Children’s Hospital Medical Center (CCHMC). The study was approved by the CCHMC Institutional Review Board and conducted between January 2015 and March 2016 (two influenza seasons). All patients older than 2 weeks of age who were admitted to the PICU and prescribed oseltamivir for suspected or confirmed influenza were eligible for the study, regardless of reason for admission. Legal authorized representatives of patients older were approached for consent (see Text, Supplemental Digital Content 1).
PK Sample Collection
The clinical team determined oseltamivir dosing, treatment length and administration route based on patient clinical status. Clinical dosing was based off the FDA label as follows (Table 1): ages 2 weeks to 1 year: 3 mg/kg twice daily; ages 1 to 12 years: <15 kg or less: 30 mg twice daily; 15.1–23 kg: 45 mg twice daily; 23.1–40 kg: 60 mg twice daily; 40.1 kg or more: 75 mg twice daily; ages > 12 years: 75 mg twice daily. The formulations used were either suspension or capsule. Each subject used the same formulation throughout their treatment duration.
Table 1: Dosing information and diagnoses for enrolled subjects.
PO = oral administration. Tube = enteric tube administration.
NG = nasogastric; G = gastric; GJ = gastrojejunal; RSV = respiratory syncytial virus
| Patient and Dosing Characteristics | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Subject # (based on enrollment order) | Age (yrs) | Weight (kg) | Reason for PICU admission | Influenza Testing result/Other infectious diagnosis | Route (Tube [NG, G,GJ]/PO) | Formulation | Day of Oseltamivir when PK sampling done | Dosing # | Dosagea (mg) | Food within 2 hours of oseltamivir doses? |
| Subjects administered oseltamivir orally (PO) | ||||||||||
| 3 | 6 | 19.5 | Uncompensated septic shock | Influenza A | PO | Suspension | 1 | 1 | 45 | No |
| 5 | 7 | 25 | Respiratory failureb | Influenza B | PO | Capsule | 1 | 1 | 60 | No |
| 10 | 5 | 15.2 | Status asthmaticus | Influenza A | PO | Suspension | 2 | 3 | 45 | No |
| 11 | 11 | 64.8 | Septic shock, respiratory distress | Negative/Rhinovirus | PO | Capsule | 1 | 2 | 75 | Yes |
| Subjects administered oseltamivir by enteric tube | ||||||||||
| 1 | 6 | 22 | Respiratory failure | Negative/RSV | Tube (GJ) | Suspension | 1 | 1 | 45 | No |
| 2 | 2 | 9 | Respiratory failure | Negative/RSV | Tube (NG) | Suspension | 1 | 1 | 30 | No |
| 4 | 8 | 18.9 | Septic shock, hypoxia | Influenza B | Tube (G) | Suspension | 1 | 1 | 45 | No |
| 6 | 15 | 46.7 | Septic shock, respiratory failure | Influenza B/Haemophilus influenzae, Streptococcus pneumoniae | Tube (G) | Suspension | 1 | 1 | 78c | Yes |
| 4 | 7 | |||||||||
| 7 | 0.67 | 9.7 | Respiratory failure | Influenza A/RSV | Tube (NG) | Suspension | 1 | 2 | 30 | Yes |
| 4 | 8 | |||||||||
| 8 | 13 | 32 | Respiratory failure | Influenza A | Tube (G) | Suspension | 1 | 1 | 60 | Yes |
| 4 | 7 | |||||||||
| 9 | 19 | 64.5 | Septic shock, hemorrhage, respiratory failure | Influenza B/Pseudomonas aeruginosa, Stenotrophomonas, Candida | Tube (NG) | Suspension | 4 | 7 | 78 | No |
Dosage recommendations (https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/021087s062lbl.pdf):
Ages 2 weeks – 1 year: 3 mg/kg twice daily
Ages 1–12 years: <15 kg or less: 30 mg twice daily; 15.1–23 kg: 45 mg twice daily; 23.1–40 kg: 60 mg twice daily; 40.1 kg or more: 75 mg twice daily
Ages > 12 years: 75 mg twice daily
Defined as requiring positive pressure ventilation or intubation
Higher dose given due to rounding of volume of suspension
The enteric tube cohort included patients with NG tubes placed during the index hospitalization or pre-existing enteric tubes (e.g. G or GJ tubes). During the first influenza season (January-June 2015), PK samples were collected before and after the first oseltamivir dose. Sparse PK sampling was done (see Figure, Supplemental Digital Content 2). Several patients received oseltamivir prior to consent, and therefore samples from early time points were not obtained. Based on missed blood sampling opportunities, we modified the protocol for the second influenza season (January – March 2016) to obtain complete patient PK sampling. For the second season we collected PK samples before and after the first dose after consent was obtained (see Figure, Supplemental Digital Content 2).
Measurement of Drug and Metabolite Concentrations
Oseltamivir and OC concentrations were measured using a validated liquid chromatography-tandem mass spectrometry assay by BASi, Inc (West Lafayette, IN). Measured oseltamivir and OC concentrations were compared to PK model-based predictions using the oseltamivir population PK model developed with concentrations in pediatric, adult and geriatric patients by Kamal et al [7].
Clinical Data Collection
Clinical data were collected during the study, including time on supplemental oxygen and number of ventilator days (see Text, Supplemental Digital Content 1).
Pharmacokinetic Modeling to Estimate PK parameters
PK analysis was performed with MWPharm (Mediware, Prague, Czech Republic) [8] using an oseltamivir population PK model [7] and Bayesian estimation for patients with complete PK sampling. Maximum concentration (Cmax) and area under the concentration-time curve for 12 hours post-dose (AUC0–12hr) were estimated and compared for patients who took oseltamivir by mouth (PO) versus by enteric tube.
Results
During the first influenza season, six patients were enrolled (see Figure, Supplemental Digital Content 3), of whom only 3 had complete PK sampling (see Figure, Supplemental Digital Content 2). Under the revised protocol in the second season, five subjects had PK sampling obtained. For patients who remained in the PICU on day 4, PK samples were obtained around dose seven. In total, 11 subjects had PK sampling. None of the subjects received oseltamivir prior to hospitalization.
Four and seven patients were in the PO cohort and tube cohort, respectively. The median age of the entire cohort was 7 years (range 0.7–19 years) and 45% of subjects were male (see Table, Supplemental Digital Content 4). There were no group differences in baseline characteristics. Both cohorts had comparable illness severity at time of PICU admission, as demonstrated by pediatric risk of mortality (PRISM) III (6 [0–8] vs. 0 [0–4.5], tube vs. PO, p = 0.41) and pediatric logistic organ dysfunction (PELOD) scores (13.3 ± 3.5 vs. 14.3 ± 10, tube vs. PO, p = 0.82). No patient had hepatic dysfunction that would affect metabolism of oseltamivir to OC, but one patient (subject 3) did have acute kidney injury (AKI).
Concentrations of Oseltamivir and Metabolite
Dosing route and regimens were determined by clinical teams based on influenza testing results and clinical status (Table 1). For patients who tested negative for influenza, oseltamivir was appropriately discontinued. The measured oseltamivir and OC concentrations obtained on treatment days 1 and 2 were compared with published PK model-based predictions [7] (Fig. 1A). Within the PO group, 91% (10/11) of measurable oseltamivir concentrations were within the 90% prediction interval of the model. In contrast, only 47% (9/19) of oseltamivir concentrations measured in enteric tube patients were within this range, with 42% (8/19) of oseltamivir concentrations falling below the predicted 5th percentile. For the metabolite, 92% (12/13) of measured OC concentrations were within the 90% prediction interval in the PO group compared to 47% (8/17) in the enteric tube group. The one measured OC concentration above the 95th percentile is from subject 3 in the PO group who had AKI. No measured OC concentration in the PO group was below the predicted 5th percentile compared to 53% (9/17) of OC concentrations in the tube group.
Figure 1:
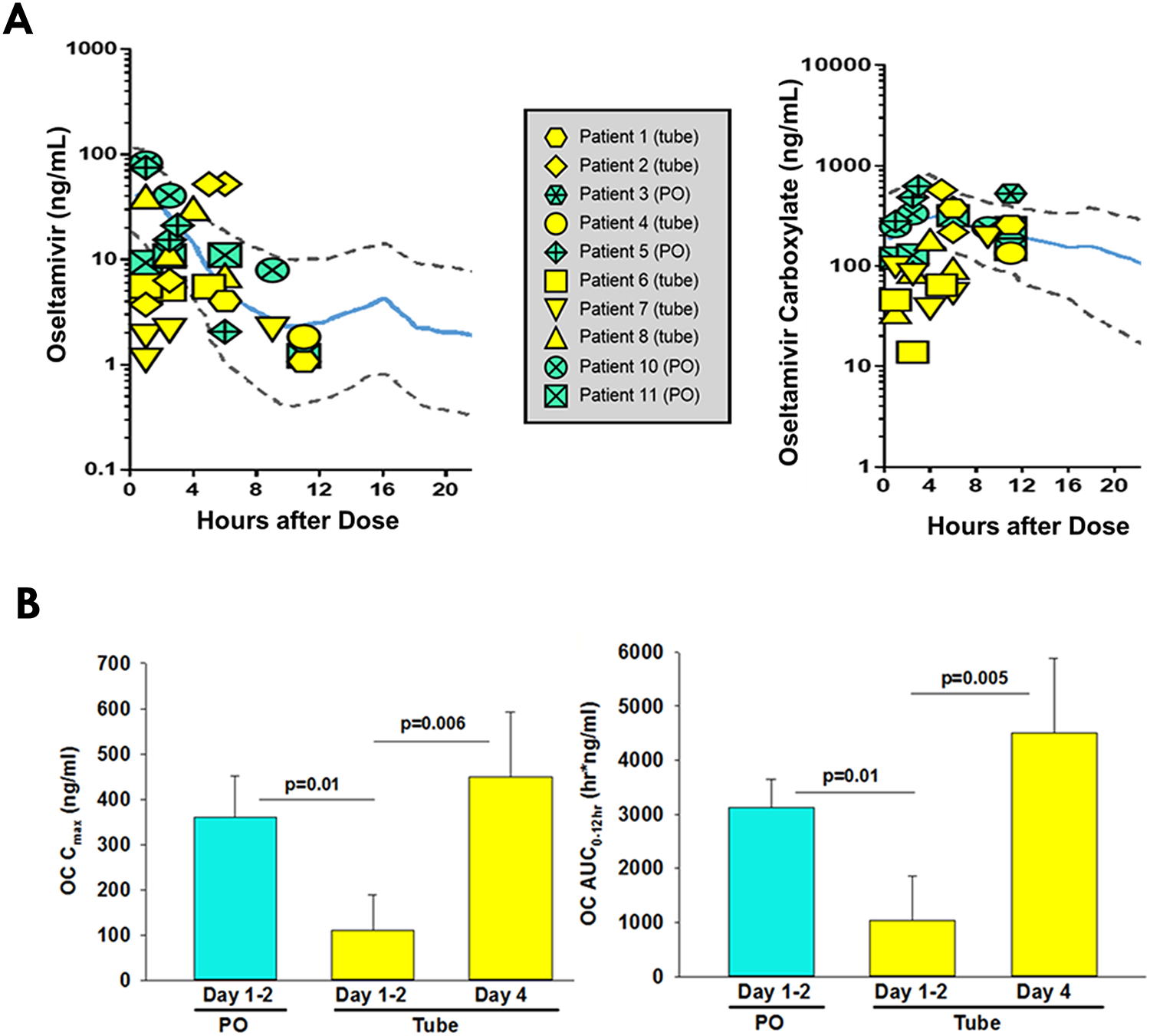
(A) Comparison of measured oseltamivir (left) and metabolite (right) concentrations to the oseltamivir PK prediction model by Kamal et al. [7]. Measured concentrations of oseltamivir and its active metabolite, oseltamivir carboxylate, for our study population were superimposed onto the median (solid blue lines) and 90% prediction interval (area between dashed lines) of an oseltamivir pharmacokinetic model based on data from pediatric, adult and geriatric patients. (Amended with permission from American Society for Microbiology) (B) Comparison of estimated mean Cmax and AUC0–12hr of OC metabolite between PO and tube groups. (Left) Cmax of OC and (right) AUC0–12hr of OC. The p values compare the means between the two groups. PO = oral administration. Tube = enteric tube administration. Cmax = estimated maximum concentration. AUC = estimated Area Under the Curve. Statistical significance defined as p≤0.05 using student’s t-test.
Pharmacokinetic Analysis
When comparing the PO and enteric tube patients who had sufficient samples for PK modeling, and thus did not include the patient with AKI, there were no significant differences in oseltamivir Cmax or AUC0–12hr despite differences in concentrations as described above. On days 1 and 2, the Cmax and AUC0–12hr estimates of OC were significantly lower in the enteric tube group (109±80 ng/mL and 1,036±824 hr*ng/ml, respectively) compared with the PO group (360±92 ng/mL and 3,122±523 hr*ng/ml, p<0.05) (Fig. 1B). The OC AUC0–12hr for the enteric tube group was below the suggested target range of 2,000–8,000 hr*ng/mL, defined by previous pediatric studies [9]. By day 4, OC Cmax and AUC0–12hr in the enteric tube group increased significantly (448±144 ng/mL and 4,501±1,393 hr*ng/ml) and were comparable with those in the PO group on days 1–2.
Clinical Outcomes
Enteric tube patients required supplemental oxygen for statistically significant longer time compared to PO patients (median 190 hr [range 92–1524] versus 33 hr [range 6.5–105]). There was a higher percentage of patients who required mechanical ventilation or non-invasive positive pressure ventilation in the enteric tube group versus PO group (86% vs 25%, p=0.09).
Discussion
This is the first study, to our knowledge, directly comparing the two routes of oseltamivir administration prospectively in critically ill pediatric patients. Within our cohort, we found that oseltamivir delivered by enteric tube resulted in lower concentrations and exposure (AUC0–12hr) to the active metabolite OC in the first two treatment days. Previously published oseltamivir PK data in critically ill children on extracorporeal membrane oxygenation (ECMO) support requiring enteric tube administration have reported lower than expected metabolite concentrations as well [6]. The authors concluded that ECMO does not appear to affect oseltamivir PK but instead NG administration and gastric dysmotility may have significant influence on OC concentrations. Among critically ill children with influenza, treatment with a neuraminidase inhibitor within 48 hours of illness onset is significantly associated with survival [10]. If patients receiving oseltamivir by enteric tube are not achieving metabolite concentrations as expected in the first 48 hours, they may be at risk of higher mortality.
There are several potential reasons for the pharmacokinetic differences between the two groups in our study. We initially hypothesized that patients who receive enteric tube administration would have higher acuity of critical illness. If patients with enteric tubes were more severely ill, they may have decreased absorption or poor motility from gastroparesis leading to delayed absorption. We found no statistically significant difference between illness severity scores in our groups in contrast to our hypothesis. Our inability to show statistical significance in severity scores may reflect the small study sample size.
We found no difference in the Cmax and AUC0–12hr of the prodrug, oseltamivir, but lower exposures to the metabolite, suggesting differences in drug metabolism. Oseltamivir is hydrolyzed by carboxylesterases into OC. It is known that proinflammatory cytokines, such as interleukin-6, reduce expression and activity of carboxylesterases [11]. Patients in the tube group may have higher inflammatory mediators leading to reduced metabolite conversion, and thus lower plasma OC concentrations. However, this was not evaluated in the current study.
The drug could have adsorbed to the enteric tubes, which were made of different materials (silicone for G/J tubes, polyurethane for NG tubes). However, with a low Poctanol/water [12], oseltamivir is not highly lipophilic and would not be expected to adsorb to enteric tubes in significant quantities.
There are several limitations of this study. First, we grouped patients with NG tubes newly placed during the hospitalization of interest and those with pre-existing G-tubes into the same cohort. Patients with G-tubes likely have chronic medical conditions that could contribute to delayed motility or altered drug absorption. In addition, different formulations of oseltamivir were used. All enteric tube patients received oseltamivir as a suspension, but half of PO patients received oseltamivir as an intact capsule. Finally, the small number of patients enrolled in this study is an additional limitation.
Understanding the mechanisms causing the differences in pharmacokinetics between the oral and enteric tube cohorts is critical. Further studies are warranted to validate these findings in a larger cohort of patients, especially to distinguish possible differences between delivery through a newly placed NG tube or through a pre-existing enteric tube. Clinicians should be aware of possible under-dosing of oseltamivir in critically ill children when an enteric tube is used.
Supplementary Material
Conflicts of Interest and Sources of support:
Dr. Kaplan reports financial support to the institution (Cincinnati Children’s Hospital Medical Center [CCHMC]) for work on a DSMB of a clinical trial (Eli Lilly) not related in any way to the work in the manuscript. Dr. Wong and the institution, CCHMC, hold patents for sepsis-related biomarkers. All remaining authors report no conflict of interest.
This work was supported by funding from the American Academy of Pediatrics Resident Research Grant; the National Center for Advancing Translational Sciences of the National Institutes of Health [Award Number 5UL1TR001425] and the Cincinnati Children’s Hospital Medical Center Division of Critical Care Medicine.
References
- 1.Shang M, Blanton L, Brammer L, Olsen SJ, Fry AM: Influenza-Associated Pediatric Deaths in the United States, 2010–2016. Pediatrics 2018, 141(4). [DOI] [PubMed] [Google Scholar]
- 2.What You Should Know About Flu Antiviral Drugs [www.cdc.gov/flu/antivirals/whatyoushould.htm]
- 3.He G, Massarella J, Ward P: Clinical pharmacokinetics of the prodrug oseltamivir and its active metabolite Ro 64–0802. Clin Pharmacokinet 1999, 37(6):471–484. [DOI] [PubMed] [Google Scholar]
- 4.Buchan SA, Chung H, Karnauchow T et al. : Characteristics and Outcomes of Young Children Hospitalized With Laboratory-confirmed Influenza or Respiratory Syncytial Virus in Ontario, Canada, 2009–2014. Pediatr Infect Dis J 2019, 38(4):362–369. [DOI] [PubMed] [Google Scholar]
- 5.Giraud C, Manceau S, Oualha M et al. : High levels and safety of oseltamivir carboxylate plasma concentrations after nasogastric administration in critically ill children in a pediatric intensive care unit. Antimicrob Agents Chemother 2011, 55(1):433–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wildschut ED, de Hoog M, Ahsman MJ, Tibboel D, Osterhaus AD, Fraaij PL: Plasma concentrations of oseltamivir and oseltamivir carboxylate in critically ill children on extracorporeal membrane oxygenation support. PLoS One 2010, 5(6):e10938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kamal MA, Van Wart SA, Rayner CR et al. : Population pharmacokinetics of oseltamivir: pediatrics through geriatrics. Antimicrob Agents Chemother 2013, 57(8):3470–3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Proost JH, Meijer DK: MW/Pharm, an integrated software package for drug dosage regimen calculation and therapeutic drug monitoring. Comput Biol Med 1992, 22(3):155–163. [DOI] [PubMed] [Google Scholar]
- 9.Oo C, Barrett J, Hill G et al. : Pharmacokinetics and dosage recommendations for an oseltamivir oral suspension for the treatment of influenza in children. Paediatr Drugs 2001, 3(3):229–236. [DOI] [PubMed] [Google Scholar]
- 10.Louie JK, Yang S, Samuel MC, Uyeki TM, Schechter R: Neuraminidase inhibitors for critically ill children with influenza. Pediatrics 2013, 132(6):e1539–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang J, Shi D, Yang D, Song X, Yan B: Interleukin-6 alters the cellular responsiveness to clopidogrel, irinotecan, and oseltamivir by suppressing the expression of carboxylesterases HCE1 and HCE2. Mol Pharmacol 2007, 72(3):686–694. [DOI] [PubMed] [Google Scholar]
- 12.Oseltamivir phosphate, MSDS No. 04 7175 5 [Online]; Roche: Basel, Switzerland, September 5, 2016. https://www.roche.com/dam/jcr:77724bca-b696-459d-9a52-ea176445b376/en/0471755.20160509.11037.pdf (accessed Dec 14, 2017). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


