Over the past decade, the number of older patients undergoing hematopoietic cell transplantation (HCT) has increased significantly in both United States and Worldwide (1,2). While the development of reduced-intensity conditioning, improved supportive care, and newer antimicrobials have all contributed to the increase (3), better recognition of biological age as one of the major determinants of transplant-related mortality also plays an important role (4). Traditionally, hematopoietic cell transplant-comorbidity index (HCT-CI) and pre-transplant Karnofsky Performance Scale (KPS) assessed by clinicians are used to determine the overall fitness of an older patient (5,6). Recently, comprehensive geriatric assessment (CGA) of a patient’s function, mobility, medication, cognition, nutrition, psychosocial status, social support, and comorbidities, has been increasingly used to identify geriatric deficits prior to transplant; and impairment in Lawton instrumental activities of daily living (IADL) has been consistently found to be one of the domains that is associated with inferior outcomes (7–9). However, the prevalence of deficits in other geriatric domains, their relationship with established HCT-CI and KPS, and their impact on transplant outcomes have remained largely unknown. We have previously reported a cohort of older patients who received official geriatric consultation prior to transplant and the high prevalence of functional impairment, polypharmacy, potentially inappropriate medication use, and malnutrition (10). In order to better utilize geriatrics resource, we have since prospectively tested a patient self-reported, electronic rapid fitness assessment tool (eRFA), originally developed for older pre-operative cancer patients (11), as a screening instrument for older transplant patients and are now reporting results from this pilot study.
In total, 48 patients aged 50 years and older have completed eRFA prior to either initial transplant (n=32) or geriatrics (n=16) clinic consultation appointment. We choose 50 years and older since this was the population of transplant patients with observed geriatric deficits (7). The median time from eRFA completion to transplant was 63.5 days (range 14–441), and the median time to complete survey was 10 minutes (range 5–96). Patients self-completed the survey 81% of time, with the remaining 19% completed by family caregivers. Most patients, 75%, were married or in a domestic partnership; 81% had at least some college education; and 85% were living with family or partner. The convenience and time spent on eRFA was generally comparable to our published experience for older surgical patients and therefore feasible for the HCT population (11).
We summarized patient’s clinical and transplant characteristics in table 1. There were 28 patients who underwent autologous HCT (ASCT) for either myeloma or lymphoma, and 20 patients who underwent allogeneic HCT (allo-HCT) for either acute myeloid leukemia, myelodysplastic syndrome, or lymphoma (Table 1). The median age of the cohort was 69.5 years (range 50.3–79.5) and was similar among ASCT and allo-HCT groups. HCT-CI was high risk (≥3) and KPS was less than 90 in about two-third of patients. Forty percent of allo-HCT and all ASCT patients received a myeloablative conditioning regimen. With a median followup of 15 months for survivors, 12 patients had died including 4 in the ASCT group (2 each for non-relapse mortality and relapse/progression of disease) and 8 in the allo-HCT group (5 for non-relapse mortality and 3 for relapse/progression of disease). Statistical analysis was not performed due to low event rate and the relatively short followup period.
Table 1.
Baseline Characteristics
| Autologous HCT (n=28) | Allogeneic HCT (n=20) | |
|---|---|---|
| Age, years (median, range) | 69.9 (50.3 – 79.5) | 68.8 (51.4 – 76.6) |
| Female gender (n, %) | 14 (50) | 9 (45) |
| Transplant diagnosis (n, %) | Multiple Myeloma, 12 (43) NHL, 16 (57) |
AML/MDS, 13 (65) HL and NHL, 7 (35) |
| Disease Status | ||
| CR/VGPR/PR | 25 (89) | 13 (65) |
| Stable/Progressive disease | 3 (11) | 7 (35) |
| HCT-CI | ||
| 0-2 | 10 (36) | 7 (35) |
| ≥3 | 18 (64) | 13 (65) |
| Clinician-assessed KPS | ||
| ≥90 | 12 (43) | 6 (30) |
| <90 | 16 (57) | 14 (70) |
| Conditioning intensity | ||
| Myeloablative | 28 (100) | 8 (40) |
| RIC/NMA | 0 (0) | 12 (60) |
Abbreviations: HCT, Hematopoietic cell transplant; AML/MDS, Acute myeloid leukemia/Myelodysplastic Syndrome; NHL, Non-Hodgkin’s Lymphoma; HL, Hodgkin’s Lymphoma; CR/VGPR/PR, Complete response/Very good partial response/Partial response; HCT-CI, Hematopoietic cell transplant-comorbidity index; KPS, Karnofsky Performance Scale; RIC/NMA, Reduced-intensity/non-myeloablative conditioning.
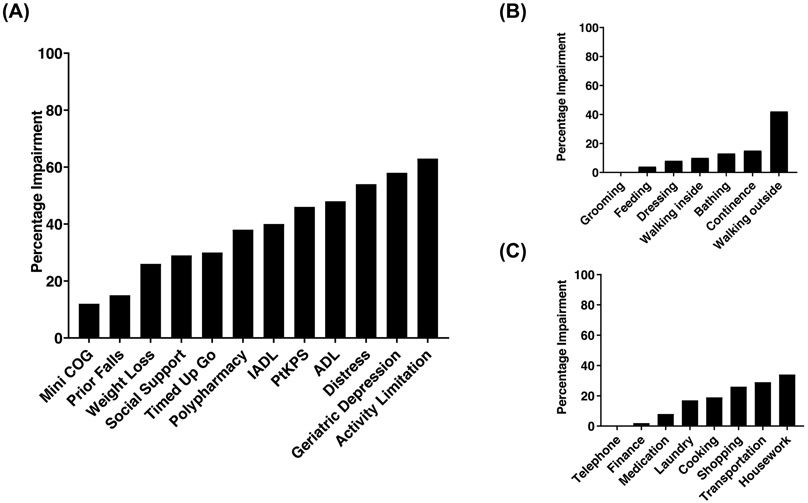
We summarized the prevalence of geriatric deficits in our cohort of patients in Figure 1A. The definition of impairment is based on following cutoff scores: patient-reported KPS <90, Katz basic activities of daily living (ADL) score <14, Lawton instrumental activities of daily living (IADL) score <16, more than 1 fall the past year, timed get-up-go (TUG) ≥10 seconds, mini-cog score <3, social support score <17, social activity limitation score ≥8, weight loss ≥10 pounds, distress level ≥4, geriatric depression score ≥1, and polypharmacy (number of medications ≥5) (11). Consistent with previous findings (7–10), we identified high prevalence of functional and physical impairments in ADL (48%), IADL (40%), patient self-rated KPS (46%), and TUG (30%). Among patients with ADL and IADL impairments, the predominant deficits involved ambulatory activities, which included walking outside (42%), housework (34%), transportation (29%), and shopping (26%) (Figure 1B and 1C). In addition, 38% patients had polypharmacy; and only 1 patient reported poor vision and no patient reported poor hearing.
Figure 1.
(A) Geriatric Impairment (percentage) in individual domains of eRFA.
(B) Impairment (percentage) in individual items of ADL.
(C) Impairment (percentage) in individual items of IADL.
Interestingly, we identified high prevalence of distress (54%), depressive symptoms (58%), and social activity limitations (63%) in these patients prior to transplant (Figure 1A). While several previous studies had documented pre-transplant depression and distress in general HCT patients (12,13), these impairments were significantly higher in our cohort of older patients. This distinct pattern of impairment highlighted the overall clinical complexity of our transplant patient population. In addition, the high social activity limitation with moderate impairment in social support system indicated a potential unmet need in older patients’ social functioning prior to transplant. We are examining these issues in depth using validated survey instruments such as Psychological Assessment of Candidates for Transplantation (PACT, 14), their impact on transplant outcomes, and effects of potential interventions.
Finally, we explored the associations between individual eRFA domains with well-established HCT-CI and clinician-assessed KPS prior to transplant. As shown in Table 2 using Fisher’s exact test, no individual eRFA domain was significantly associated with HCT-CI. In contrast, clinician-assessed KPS was significantly associated with individual eRFA functional and physical domains including limitations in ADL, IADL, TUG, and patient self-assessed KPS (Table 2). Clinician-assessed KPS was also significantly associated with patient-reported weight loss. These results suggested that eRFA provided complementary information to HCT-CI on the patient’s overall risk for HCT. In addition, while clinician-assessed KPS was associated with the patient’s functional and physical status prior to HCT, eRFA may provide additional risk stratifying information such as cognition, psychosocial status, distress, and polypharmacy.
Table 2.
Associations between Geriatric Deficits with HCT-CI and Clinician-assessed KPS Prior to Transplant
| HCT-CI (≥3 vs 0-2) | Clinician KPS (<90 vs ≥90) | |||
|---|---|---|---|---|
| Geriatric Deficit/Frailty | % impaired | p-value | % impaired | p-value |
| Pt-assessed KPS (<90) | 45 vs 47 | >0.999 | 60 vs 22 | 0.017 |
| Prior falls | 13 vs 18 | 0.692 | 14 vs 17 | >0.999 |
| Weight loss | 33 vs 13 | 0.166 | 37 vs 6 | 0.033 |
| Distress | 48 vs 65 | 0.368 | 53 vs 56 | >0.999 |
| Polypharmacy (≥5) | 42 vs 29 | 0.536 | 37 vs 39 | >0.999 |
| Timed get-up-go | 33 vs 25 | 0.735 | 41 vs 7 | 0.033 |
| ADL | 55 vs 35 | 0.237 | 63 vs 22 | 0.008 |
| IADL | 45 vs 29 | 0.363 | 53 vs 17 | 0.016 |
| Social support | 29 vs 29 | >0.999 | 33 vs 22 | 0.521 |
| Social activity limitation | 53 vs 68 | 0.361 | 70 vs 50 | 0.222 |
| Geriatric depression | 58 vs 59 | >0.999 | 70 vs 39 | 0.068 |
| Mini-cog | 11 vs 13 | >0.999 | 14 vs 7 | 0.65 |
Abbreviations: HCT-CI, Hematopoietic cell transplant-comorbidity index; KPS, Karnofsky Performance Scale; ADL, Activities of daily living; IADL, Instrumental activities of daily living.
Our study is limited by its single institution design with predominantly urban patient population including some 50–60 years old, small sample size, and short follow-up period. In addition, the optimal timing of initial and/or subsequent eRFA and action plans on how to utilize it to guide transplant decision-making for individual patient remain to be developed and refined. Despite these limitations, we have shown here prospectively that adopting a patient-reported, electronic geriatric impairment screening instrument is entirely feasible in our transplant population and may help triage patients appropriately for in depth geriatrics evaluation and management. Importantly, we have identified significant psychosocial symptoms and distress among older patients prior to HCT, suggesting that increased multidisciplinary care collaboration among transplantation, geriatrics, rehabilitation, psychiatry, and social work may be warranted. Finally, these patient-reported outcomes may provide complementary information to traditional transplant indices such as HCT-CI and KPS. Large scale multicenter prospective cohort studies such as the upcoming BMT-CTN 1704 CHARM study are likely needed to examine the impact of individual geriatric domains on HCT outcomes in addition to functional limitations (15).
Acknowledgement:
This research was supported in part by the NIH/NCI Cancer Center Support Grant P30 CA008748. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This work was presented in part at the annual Transplantation and Cellular Therapy Meetings of ASBMT & CIBMTR in Houston, Texas from February 20–24, 2019.
Footnotes
Conflict of Interest:
All authors declare no conflict of interest related to the submitted research work.
References:
- 1.D’Souza A, Fretham C. Current uses and outcomes of hematopoietic cell transplantation (HCT): CIBMTR Summary Slides, 2017.
- 2.Gratwohl A, Pasquini M, Aljurf M, Atsuta Y, Baldomero H, Foeken L et al. One million haematopoietic stem-cell transplants: a retrospective observational study. The Lancet Haematology 2015; 2: e91–100. [DOI] [PubMed] [Google Scholar]
- 3.Giralt SA. Hematopoietic cell transplantation for older adults. In: Handbook of Geriatric Oncology - Practical Guide to Caring for the Older Cancer Patient. Demos Medical, 2019, pp 241–254. [Google Scholar]
- 4.Artz A Biologic vs physiologic age in the transplant candidate. Hematology 2016; 2016: 99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sorror ML. How I assess comorbidities before hematopoietic cell transplantation. Blood 2013; 121: 2854–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McClune BL, Weisdorf DJ, Pedersen TL, da Silva G, Tallman MS, Sierra J et al. Effect of Age on Outcome of Reduced-Intensity Hematopoietic Cell Transplantation for Older Patients with Acute Myeloid Leukemia in First Complete Remission or With Myelodysplastic Syndrome. Journal of Clinical Oncology 2010; 28. doi: 10.1200/JCO.2009.25.4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muffly LS, Kocherginsky M, Stock W, Chu Q, Bishop MR, Godley LA et al. Geriatric assessment to predict survival in older allogeneic hematopoietic cell transplantation recipients. Haematologica 2014; 99: 1373–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deschler B, Ihorst G, Schnitzler S, Bertz H, Finke J. Geriatric assessment and quality of life in older patients considered for allogeneic hematopoietic cell transplantation: a prospective risk factor and serial assessment analysis. Bone marrow transplantation 2018; 53: 565–575. [DOI] [PubMed] [Google Scholar]
- 9.Nawas MT, Andreadis C, Martin TG, Wolf JL, Ai WZ, Kaplan LD et al. Limitation in Patient-Reported Function is Associated with Inferior Survival in Older Adults Undergoing Autologous Hematopoietic Cell Transplantation. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation 2019. doi: 10.1016/j.bbmt.2019.01.028. [DOI] [PubMed] [Google Scholar]
- 10.Lin RJ, Shahrokni A, Dahi PB, Jakubowski AA, Devlin S, Maloy MA et al. Pretransplant comprehensive geriatric assessment in hematopoietic cell transplantation: a single center experience. Bone marrow transplantation 2018. doi: 10.1038/s41409-018-0151-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shahrokni A, Tin A, Downey RJ, Strong V, Mahmoudzadeh S, Boparai MK et al. Electronic Rapid Fitness Assessment: A Novel Tool for Preoperative Evaluation of the Geriatric Oncology Patient. Journal of the National Comprehensive Cancer Network: JNCCN 2017; 15: 172–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trask P, Paterson A, Riba M, Brines B, Griffith K, Parker P et al. Assessment of psychological distress in prospective bone marrow transplant patients. Bone Marrow Transpl 2002; 29: 917. [DOI] [PubMed] [Google Scholar]
- 13.Pillay B, Lee SJ, Katona L, Burney S, Avery S. Psychosocial factors associated with quality of life in allogeneic stem cell transplant patients prior to transplant. Psycho Oncol 2014; 23: 642–649. [DOI] [PubMed] [Google Scholar]
- 14.Hong S, Rybicki L, Corrigan D, Dabney J, Hamilton BK, Kalaycio M et al. Psychosocial Assessment of Candidates for Transplant (PACT) as a tool for psychological and social evaluation of allogeneic hematopoietic cell transplantation recipients. Bone Marrow Transpl 2019; 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bruijnen CP, van Harten-Krouwel DG, Koldenhof JJ, Emmelot-Vonk MH, Witteveen PO. Predictive value of each geriatric assessment domain for older patients with cancer: a systematic review. J Geriatr Oncol 2019; March 26. [DOI] [PubMed] [Google Scholar]