Abstract
Glioblastoma is a devastating disease with a dismal prognosis. While recent advancements in cancer immunotherapy have led to improvements in treating other types of cancer, patients with glioblastoma have not benefited from these new therapies and techniques. Fortunately, neurosurgeons and oncologists at Washington University School of Medicine conducting a cutting edge clinical trial are looking to overcome these persistent challenges in treating glioblastoma through combining a personalized vaccine with new immunotherapy drugs.
Introduction
Glioblastoma multiforme (GBM) is the most common primary malignant brain tumor and accounts for approximately 17,000 new cases in the United States annually.1 GBM unfortunately has the worst prognosis of primary gliomas, with a miserable median survival of 12–15 months despite aggressive treatment.1,2 The current first line standard of care includes maximal surgical resection, adjuvant radiotherapy with concurrent temozolomide chemotherapy following resection, and six months of maintenance temozolomide after concurrent chemoradiotherapy.1 Tumors in a large majority of patients will recur, and the lack of effective standard second-line options leads to patients rapidly succumbing to the disease; the current 5 year survival rate is less than 10%.1 Due to the abysmal prognosis associated with GBM, new safe and effective therapies are desperately needed.
Cancer immunotherapy is a new and active area of cancer research, where various therapies are used to evoke an immune response against a tumor. Contemporary cancer immunotherapies include targeting immune checkpoint signaling pathways with inhibitory antibodies, checkpoint blockade immunotherapy (CBI), or priming the immune response with therapeutic vaccines. Therapeutic vaccinations aim to prime the immune response against tumor antigens, which can include shared tumor antigens and/or personalized tumor-specific antigens, called neoantigens. While other therapies, including cellular therapies such as chimeric antigen receptor (CAR) T cells have shown positive data in other cancer types, this review will focus on past applications of CBI and therapeutic vaccines. We will also present a pioneering clinical trial that combines a personalized therapeutic vaccine with CBI.
Checkpoint Blockade Immunotherapy in Glioblastoma
Landmark discoveries in checkpoint inhibition have revolutionized oncology treatment options for previously devastating diagnoses, resulting in a well-deserved Nobel Prize. Two major immunotherapy targets are the negative immune regulatory checkpoint proteins cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and Programmed cell death protein 1 (PD-1) or its ligand, PD-L1. Both CTLA-4 and PD-1/PD-L1 are coreceptor molecules on the surface of T-cells that inhibit T cell function and play key roles in guarding against autoimmunity.3 The CTLA-4 pathway regulates T-cell proliferation and priming in the lymph nodes, while the PD-1/PD-L1 pathway regulates T cell response in the tissues later in the immune response.4 CBIs targeting these pathways can improve the anti-tumor immune response.
CBI has shown efficacy in preclinical orthotopic transplantable GBM mouse models, such as GL261 and SMA-560. Mice with intracranially implanted GL261 tumors show a survival benefit when treated with either anti PD-1, anti PD-L1, or anti CLTA-4 treatment.5,6 These effects of anti PD-1 or CTLA-4 therapy in GL261 are synergistic when combined with radiotherapy.7,8 Anti CTLA-4 treatment also confers a survival benefit in mice with intracranially implanted SMA-560 GBM cell line tumors.9
Both anti CTLA-4 and anti PD-1/PD-L1 CBI antibodies have been FDA approved or have shown preliminary success in melanoma, squamous and non-squamous non-small cell lung cancer, small cell lung cancer, metastatic renal cell carcinoma, urothelial cancers, head and neck squamous cell carcinoma, and colorectal cancer.10 The immune system can apparently control tumors in many environments as shown by the success of CBI in multiple organ systems; ongoing clinical trials are investigating the efficacy of CBI in other systems.10
Due to the success of CBIs in other cancer types, many clinical trials for CBI in newly diagnosed and recurrent GBM are currently underway, but none have reported convincing positive results in large patient cohorts. The Checkmate 498 open label trial for patients with newly diagnosed GBM and an unmethylated MGMT promoter comparing nivolumab (anti PD-1) combined with radiotherapy against standard of care temozolomide with radiotherapy did not meet the primary endpoint of overall survival.11 The ongoing sister phase III Checkmate 548 (NCT02667587) trial for patients with newly diagnosed GBM and methylated MGMT will compare nivolumab versus placebo combined with standard radiotherapy plus temozolomide. While a phase II trial comparing pembrolizumab (anti PD-1) against concurrent pembrolizumab and bevacizumab appeared safe in both cohorts, it also showed minimal anti-tumor activity in the pembrolizumab only cohort, and combination therapy did not show improved outcome compared to historical bevacizumab controls.12 A phase Ib trial of pembrolizumab in 25 patients with recurrent PD-1 positive GBM reported 1 partial response and 12 patients with stable disease.13 In a trial of patients with recurrent GBM, neoadjuvant (pre-reresection) combined with adjuvant pembrolizumab conferred a survival benefit over adjuvant pembrolizumab alone.14 The results from this neoadjuvant administration study suggest that a strong priming response associated with increased tumor antigen exposure may lead to a better anti-tumor response later. A trial treating 6 recurrent GBM patients with pembrolizumab and bevacizumab reported no dose-limiting toxicities and clinical benefit in 3 of 6 patients.15,16
In addition to CBI monotherapy, ongoing clinical trials for both newly diagnosed and recurrent GBM are investigating combination CBI of anti CTLA-4 and anti PD-1. Results from these trials demonstrate that CBI is safe and tolerable in these populations, but do not show a survival benefit. Results from the Checkmate-143 phase I study comparing nivolumab (anti PD-1) alone or in combination with ipilimumab (anti CTLA-4) in patients with recurrent GBM showed no grade 3 or greater SAEs out of 10 patients treated with nivolumab monotherapy (3 mg/kg every 2 weeks).17 Only 2 of 20 patients in the Checkmate 143 trial treated with nivolumab at 3 mg/kg plus ipilimumab at 1 mg/kg every 3 weeks for 4 doses followed by maintenance nivolumab at 3 mg/kg every 2 weeks experienced grade 3 or greater SAEs.17 A lower initial 4 doses of nivolumab at 1 mg/kg with higher ipilimumab at 3 mg/kg led to a larger (7/10) proportion of SAEs without a dramatic increase in treatment response.17 Furthermore, cohort 2 of the Checkmate 143 phase III study, which randomized GBM patients to nivolumab monotherapy or bevacizumab monotherapy, did not show any difference in median overall survival, 12 month overall survival, or progression free survival.17
Although the completed trials investigating CBIs in patients with GBM have established a safe treatment protocol, they have not shown a survival benefit compared to standard therapy. The GBM microenvironment and standard of care treatment conditions cause immunosuppression, and the low mutational burden does not create an immunogenic environment present in other tumors.18,19 The challenging tumor biology along with past negative trial results suggest that combination therapies are likely necessary to see a clinical benefit.
Therapeutic Neoantigen Vaccination in Glioblastoma
Tumor antigens distinguish tumors from normal tissue and serve as potential targets for immune system neutralization. Shared tumor antigens and neoantigens are both subsets of tumor antigens. Normal tissues or limited subsets of normal tissues express low levels of shared tumor antigens, but tumors overexpress these shared antigens. Examples include MAGE in melanoma20 and HER2/neu in breast cancer.21 Tumor-specific antigens, or neoantigens, are the result of somatic mutations only present in the tumor.22 Therefore, while tumor cells may express neoantigens, normal tissue does not due to a lack of somatic mutations present. The first neoantigen described was mutated cyclin-dependent kinase 4 in melanoma.23 Other groups have since described neoantigens in multiple other types of cancer.24,25
Because they are targets for immune cells, vaccinating a patient against their own tumor antigens could stimulate an anti-tumor immune response. The obvious benefit to using neoantigens over shared tumor antigens is that they are unique to the tumor, therefore mitigating autoimmune effects. Phase II clinical trials in patients with GBM using rindopepimut, a vaccine against the neoantigen EGFRvIII, combined with standard temozolomide and radiation showed benefits in overall survival and progression free survival over historical controls.26–28 However, a phase III trial of rindopepimut with temozolomide failed to show a survival benefit.29 Additionally, EGFRvIII is only expressed in 20–30% of GBMs, so most patients would not be candidates for this therapy.30 While disappointing, these results warrant investigation into combining neoantigen vaccines with additional immunotherapy such as CBI. Within the past year, several publications show that personalized neoantigen vaccine therapies for glioblastoma are feasible, generate neoantigen specific T cells that infiltrate the tumor, and have favorable safety profiles.31–33 A predictive approach to identify immunogenic neoantigens is necessary to streamline any potential therapy that would benefit more than a small subset of patients.
The Future of Brain Tumor Immunotherapy
Given the favorable safety profile and different mechanisms of immune activation, combining CBI with neoantigen vaccines makes therapeutic sense. These two approaches could synergistically increase the anti-tumor immune response, using CBI to overcome the problem of immunosuppression and using neoantigen vaccination to counteract the poor immunogenicity of the tumor. Blocking CTLA-4 and PD-1 with CBIs potentially enhances anti-tumor neoantigen-specific T cell responses in melanoma34 and NSCLC respectively.35
An innovative clinical trial (NCT03422094) currently recruiting patients at Washington University School of Medicine (WUSM) combines a state-of-the-art personalized neoantigen vaccine (NeoVax) with different CBIs, specifically nivolumab (anti PD-1) and ipilimumab (anti CTLA-4). Eligible patients must have newly diagnosed GBM with an unmethylated MGMT promoter. Patients who enroll in the trial receive NeoVax and their designated nivolumab or ipilimumab infusions at Siteman Cancer Center in St. Louis. This trial seeks to investigate how the timing of CBI administration combined with a personalized neoantigen vaccine affects the clinical and immunological response. Because CTLA-4 has a role in early priming and PD-1 in later local tissue response, sequentially administering different CBIs which target these separate pathways could synergistically boost the anti-tumor immune response.
Preclinical data has shown a synergistic effect of combining a vaccine with either anti PD-136 or anti CTLA-437 in mice with intracranially implanted GL261 tumors. Multiple clinical trials in various cancer types have investigated concurrent tumor antigen vaccination with checkpoint blockade monotherapy without an increase in adverse events over CBI alone.38 These results indicate that combining a vaccine with CBIs should be safe for patients with GBM.
Creating a neoantigen vaccine specific for each tumor requires a robust, accurate, and cost-effective pipeline. Fortunately, investigators at the Siteman Cancer Center and the McDonnell Genome Institute at WUSM have developed an immunogenomics approach to identify patient neoantigens for a vaccine. Compared to cancer genome sequencing, identifying neoantigens with tumor versus normal tissue exome sequencing is robust, accurate, and conserves resources.39 To ensure that the tumor expresses enough of the identified mutant neoantigens, RNA sequencing will measure tumor mRNA mutant allele expression levels of neoantigens identified by tumor/normal exome sequencing. An in silico prioritization algorithm then filters the mutant neoantigens to identify the top candidates that have high HLA class I or class II allele epitope binding affinity for presentation to the patient T cells. Scientists then manufacture a vaccine against the prioritized neoantigens with a synthetic long peptide vaccine combined with a poly-ICLC adjuvant. In preclinical studies, our group successfully predicted and screened neoantigens from the GL261 and SMA-560 murine GBM models using this screening strategy.40
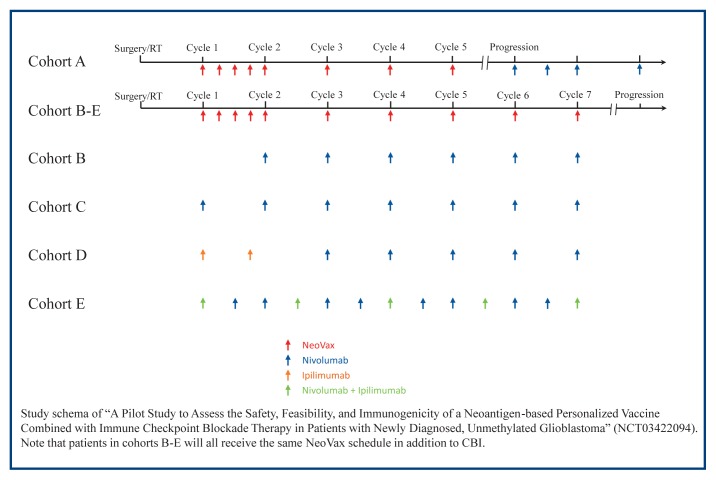
Patients who have a potential GBM and want to participate in the trial can receive surgery and treatment from experts at Barnes Jewish Hospital and Siteman Cancer Center. After surgery, patients receive the standard course radiotherapy (60 Gy over 30 treatments, Monday through Friday for 6 weeks) without temozolomide. Temozolomide is excluded due to the immunosuppressive effects and lack of efficacy in MGMT unmethylated GBM, a similar strategy adopted in Checkmate 498 to avoid the immunosuppressive effects of chemotherapy. During radiotherapy, scientists and physicians at WUSM analyze the tumor and generate a personalized neoantigen vaccine as described above. After radiotherapy, patients enter the vaccine priming phase where they receive their unique NeoVax treatment weekly for 4 weeks. After priming, patients then move to the boosting phase, where they receive NeoVax once every 4 weeks. While the NeoVax schedule is the same for all patients, the schedule for CBIs differs among the cohorts. Patients in cohort A will receive only NeoVax until progression, when they begin nivolumab infusions every other week. Patients in cohort B receive only NeoVax during the priming phase, and then receive nivolumab and NeoVax during the boosting phase. Patients in cohort C receive nivolumab and NeoVax during the priming and boosting phases. Patients in cohort D receive ipilimumab and NeoVax during the priming phase, and then switch to nivolumab and NeoVax during the boosting phase. Patients in cohort E receive all 3: nivolumab, ipilimumab, and NeoVax during both the priming and boosting phases. Figure 1 depicts the administration schedule for the NeoVax trial.
Figure 1.
To date, only 8 other trials across various cancer types are recruiting patients for clinical trials involving a therapeutic vaccine and combination CBI. Some of the ongoing trials will begin reporting results next year. The NeoVax trial at WUSM is the only enrolling trial administering a therapeutic vaccine with combination checkpoint blockade immunotherapy for patients with glioblastoma. Our group will soon open a new clinical trial (NCT04015700) investigating a therapeutic DNA vaccine with combination CBI.
Conclusion
GBM is a uniformly lethal disease. While recent achievements in immuno-oncology have improved the prognoses of many cancer diagnoses, these benefits have not yet translated to GBM. These setbacks are likely due to the unique and immunosuppressive nature of GBM, which will require ingenuity to overcome. Despite these roadblocks, trailblazing new treatments like NeoVax and combination checkpoint blockade at Washington University are giving hope to patients with GBM and shaping the future of cancer treatment.
Footnotes
Andrew T. Coxon, MS2, Department of Neurological Surgery; Tanner M. Johanns, MD, PhD, Assistant Professor of Medicine, Division of Medical Oncology; and Gavin P. Dunn, MD, PhD, (above), Associate Professor of Neurological Surgery, Director of Brain Tumor Immunology and Therapeutics, and the Andrew M. and Jane M. Bursky Center for Human Immunology and Immunotherapy Programs; all are at Washington University School of Medicine, St. Louis, Missouri.
Contact: gpdunn@wustl.edu
Disclosure
GPD is a co-founder and Chief Scientific Officer of Immunovalent Therapeutics, Inc. TMJ has served on scientific advisory boards for Geneos Therapeutics and NovoCure.
References
- 1.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Omuro A, DeAngelis LM. Glioblastoma and other malignant gliomas: A clinical review. JAMA. 2013;310(17):1842–1850. doi: 10.1001/jama.2013.280319. [DOI] [PubMed] [Google Scholar]
- 3.Korman AJ, Peggs KS, Allison JP. Checkpoint blockade in cancer immunotherapy. Adv Immunol. 2006;90:297–339. doi: 10.1016/S0065-2776(06)90008-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buchbinder EI, Desai A. CTLA-4 and PD-1 Pathways: Similarities, Differences, and Implications of Their Inhibition. Am J Clin Oncol. 2016;39(1):98–106. doi: 10.1097/COC.0000000000000239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wainwright DA, Chang AL, Dey M, et al. Durable therapeutic efficacy utilizing combinatorial blockade against IDO, CTLA-4, and PD-L1 in mice with brain tumors. Clin Cancer Res. 2014;20(20):5290–5301. doi: 10.1158/1078-0432.CCR-14-0514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reardon DA, Gokhale PC, Klein SR, et al. Glioblastoma Eradication Following Immune Checkpoint Blockade in an Orthotopic, Immunocompetent Model. Cancer Immunology Research. 2016;4(2):124–135. doi: 10.1158/2326-6066.CIR-15-0151. [DOI] [PubMed] [Google Scholar]
- 7.Zeng J, See AP, Phallen J, et al. Anti-PD-1 blockade and stereotactic radiation produce long-term survival in mice with intracranial gliomas. Int J Radiat Oncol Biol Phys. 2013;86(2):343–349. doi: 10.1016/j.ijrobp.2012.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Belcaid Z, Phallen JA, Zeng J, et al. Focal radiation therapy combined with 4-1BB activation and CTLA-4 blockade yields long-term survival and a protective antigen-specific memory response in a murine glioma model. PLoS ONE. 2014;9(7):e101764. doi: 10.1371/journal.pone.0101764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fecci PE, Ochiai H, Mitchell DA, et al. Systemic CTLA-4 blockade ameliorates glioma-induced changes to the CD4+ T cell compartment without affecting regulatory T-cell function. Clin Cancer Res. 2007;13(7):2158–2167. doi: 10.1158/1078-0432.CCR-06-2070. [DOI] [PubMed] [Google Scholar]
- 10.Bersanelli M, Buti S. From targeting the tumor to targeting the immune system: Transversal challenges in oncology with the inhibition of the PD-1/PD-L1 axis. World J Clin Oncol. 2017;8(1):37–53. doi: 10.5306/wjco.v8.i1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bristol-Myers Squibb Announces Phase 3 CheckMate −498 Study Did Not Meet Primary Endpoint of Overall Survival with Opdivo (nivolumab) Plus Radiation in Patients with Newly Diagnosed MGMT-Unmethylated Glioblastoma Multiforme | BMS Newsroom. [Accessed July 30, 2019]. https://news.bms.com/press-release/corporatefinancial-news/bristol-myers-squibb-announces-phase-3-checkmate-498-study-did.
- 12.Reardon DA, Nayak L, Peters KB, et al. Phase II study of pembrolizumab or pembrolizumab plus bevacizumab for recurrent glioblastoma (rGBM) patients. JCO. 2018;36(15_suppl):2006–2006. doi: 10.1200/JCO.2018.36.15_suppl.2006. [DOI] [Google Scholar]
- 13.Reardon DA, Kim T-M, Frenel J-S, et al. ATIM-35. RESULTS OF THE PHASE IB KEYNOTE-028 MULTI-COHORT TRIAL OF PEMBROLIZUMAB MONOTHERAPY IN PATIENTS WITH RECURRENT PD-L1-POSITIVE GLIOBLASTOMA MULTIFORME (GBM) Neuro-Oncology. 2016;18(suppl_6):vi25–vi26. doi: 10.1093/neuonc/now212.100. [DOI] [Google Scholar]
- 14.Cloughesy TF, Mochizuki AY, Orpilla JR, et al. Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nat Med. 2019;25(3):477–486. doi: 10.1038/s41591-018-0337-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kurz SC, Cabrera LP, Hastie D, et al. PD-1 inhibition has only limited clinical benefit in patients with recurrent high-grade glioma. Neurology. 2018;91(14):e1355–e1359. doi: 10.1212/WNL.0000000000006283. [DOI] [PubMed] [Google Scholar]
- 16.Mantica M, Pritchard A, Lieberman F, Drappatz J. Retrospective study of nivolumab for patients with recurrent high grade gliomas. J Neurooncol. 2018;139(3):625–631. doi: 10.1007/s11060-018-2907-4. [DOI] [PubMed] [Google Scholar]
- 17.Omuro A, Vlahovic G, Lim M, et al. Nivolumab with or without ipilimumab in patients with recurrent glioblastoma: Results from exploratory phase I cohorts of CheckMate 143. Neuro-oncology. 2018;20(5):674–686. doi: 10.1093/neuonc/nox208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGranahan T, Therkelsen KE, Ahmad S, Nagpal S. Current State of Immunotherapy for Treatment of Glioblastoma. Curr Treat Options Oncol. 2019;20(3) doi: 10.1007/s11864-019-0619-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hodges TR, Ott M, Xiu J, et al. Mutational burden, immune checkpoint expression, and mismatch repair in glioma: Implications for immune checkpoint immunotherapy. Neuro-oncology. 2017;19(8):1047–1057. doi: 10.1093/neuonc/nox026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van der Bruggen P, Traversari C, Chomez P, et al. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science. 1991;254(5038):1643–1647. doi: 10.1126/science.1840703. [DOI] [PubMed] [Google Scholar]
- 21.Peoples GE, Goedegebuure PS, Smith R, Linehan DC, Yoshino I, Eberlein TJ. Breast and ovarian cancer-specific cytotoxic T lymphocytes recognize the same HER2/neu-derived peptide. Proc Natl Acad Sci USA. 1995;92(2):432–436. doi: 10.1073/pnas.92.2.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parmiani G, De Filippo A, Novellino L, Castelli C. Unique human tumor antigens: Immunobiology and use in clinical trials. J Immunol. 2007;178(4):1975–1979. doi: 10.4049/jimmunol.178.4.1975. [DOI] [PubMed] [Google Scholar]
- 23.Wölfel T, Hauer M, Schneider J, et al. A p16INK4a-insensitive CDK4 mutant targeted by cytolytic T lymphocytes in a human melanoma. Science. 1995;269(5228):1281–1284. doi: 10.1126/science.7652577. [DOI] [PubMed] [Google Scholar]
- 24.Echchakir H, Mami-Chouaib F, Vergnon I, et al. A point mutation in the alpha-actinin-4 gene generates an antigenic peptide recognized by autologous cytolytic T lymphocytes on a human lung carcinoma. Cancer Res. 2001;61(10):4078–4083. [PubMed] [Google Scholar]
- 25.Brändle D, Brasseur F, Weynants P, Boon T, Van den Eynde B. A mutated HLA-A2 molecule recognized by autologous cytotoxic T lymphocytes on a human renal cell carcinoma. J Exp Med. 1996;183(6):2501–2508. doi: 10.1084/jem.183.6.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sampson JH, Aldape KD, Archer GE, et al. Greater chemotherapy-induced lymphopenia enhances tumor-specific immune responses that eliminate EGFRvIII-expressing tumor cells in patients with glioblastoma. Neurooncology. 2011;13(3):324–333. doi: 10.1093/neuonc/noq157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sampson JH, Heimberger AB, Archer GE, et al. Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma. J Clin Oncol. 2010;28(31):4722–4729. doi: 10.1200/JCO.2010.28.6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schuster J, Lai RK, Recht LD, et al. A phase II, multicenter trial of rindopepimut (CDX-110) in newly diagnosed glioblastoma: The ACT III study. Neuro-oncology. 2015;17(6):854–861. doi: 10.1093/neuonc/nou348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weller M, Butowski N, Tran DD, et al. Rindopepimut with temozolomide for patients with newly diagnosed, EGFRvIII-expressing glioblastoma (ACT IV): A randomised, double-blind, international phase 3 trial. Lancet Oncol. 2017;18(10):1373–1385. doi: 10.1016/S1470-2045(17)30517-X. [DOI] [PubMed] [Google Scholar]
- 30.Wong AJ, Ruppert JM, Bigner SH, et al. Structural alterations of the epidermal growth factor receptor gene in human gliomas. Proc Natl Acad Sci USA. 1992;89(7):2965–2969. doi: 10.1073/pnas.89.7.2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hilf N, Kuttruff-Coqui S, Frenzel K, et al. Actively personalized vaccination trial for newly diagnosed glioblastoma. Nature. 2019;565(7738):240–245. doi: 10.1038/s41586-018-0810-y. [DOI] [PubMed] [Google Scholar]
- 32.Keskin DB, Anandappa AJ, Sun J, et al. Neoantigen vaccine generates intratumoral T cell responses in phase Ib glioblastoma trial. Nature. 2019;565(7738):234–239. doi: 10.1038/s41586-018-0792-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johanns TM, Miller CA, Liu CJ, et al. Detection of neoantigen-specific T cells following a personalized vaccine in a patient with glioblastoma. Oncoimmunology. 2019;8(4):e1561106. doi: 10.1080/2162402X.2018.1561106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Rooij N, van Buuren MM, Philips D, et al. Tumor exome analysis reveals neoantigen-specific T-cell reactivity in an ipilimumab-responsive melanoma. J Clin Oncol. 2013;31(32):e439–442. doi: 10.1200/JCO.2012.47.7521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348(6230):124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Antonios JP, Soto H, Everson RG, et al. PD-1 blockade enhances the vaccination-induced immune response in glioma. JCI Insight. 2016;1(10) doi: 10.1172/jci.insight.87059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Agarwalla P, Barnard Z, Fecci P, Dranoff G, Curry WT. Sequential immunotherapy by vaccination with GM-CSF-expressing glioma cells and CTLA-4 blockade effectively treats established murine intracranial tumors. J Immunother. 2012;35(5):385–389. doi: 10.1097/CJI.0b013e3182562d59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morse MA, Lyerly HK. Checkpoint blockade in combination with cancer vaccines. Vaccine. 2015;33(51):7377–7385. doi: 10.1016/j.vaccine.2015.10.057. [DOI] [PubMed] [Google Scholar]
- 39.Matsushita H, Vesely MD, Koboldt DC, et al. Cancer exome analysis reveals a T-cell-dependent mechanism of cancer immunoediting. Nature. 2012;482(7385):400–404. doi: 10.1038/nature10755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johanns TM, Ward JP, Miller CA, et al. Endogenous Neoantigen-Specific CD8 T Cells Identified in Two Glioblastoma Models Using a Cancer Immunogenomics Approach. Cancer Immunol Res. 2016;4(12):1007–1015. doi: 10.1158/2326-6066.CIR-16-0156. [DOI] [PMC free article] [PubMed] [Google Scholar]