Abstract
Maximal safe resection can improve patient outcomes for a variety of brain tumor types including low- and high-grade gliomas, pituitary adenomas, and other pathologies. Numerous intraoperative adjuncts exist to guide surgeons with maximizing extent of resection. Three distinct strategies exist including: 1) surgical navigation; 2) intraoperative imaging; and 3) tumor fluorescence. Surgical navigation involves registration of high-resolution three-dimensional imaging to the patient’s cranial surface anatomy, allowing real-time localization of tumor and brain structures. Intraoperative imaging devices like intraoperative magnetic resonance imaging (iMRI), intraoperative computed tomography (iCT), 3-D fluoroscopy, and intraoperative ultrasonography (iUS) allow near real time visualization to assess the extent of resection. Intraoperative fluorescence via intravenous fluorescein or oral 5-aminolevulinic acid (5-ALA) causes brain tumors to “light up”, which can be viewed through surgical optics using selective filters and specific wavelength light sources. A general overview, as well as implementation and utilization of some of these image guidance strategies at Washington University and by Siteman Cancer Center neurosurgeons at Barnes Jewish Hospital, is discussed in this review.
Introduction
Maximal safe resection of brain tumors is the “holy grail” of neurosurgical neurooncology. This applies to low- and high-grade gliomas, pituitary adenomas, brain metastases, and a variety of other pathologies. Maximizing the extent of resection has been demonstrated to improve progression free survival and overall survival for many different tumor types.1–5 A variety of modalities have been implemented in neurosurgery to improve neurosurgeon’s ability to assess intraoperatively the extent of resection in an effort maximize tumor removal and to preserve critical neurological functions. This review will discuss advances in neurosurgical guidance techniques including surgical navigation; intraoperative imaging modalities including iMRI, iCT, iUS; and tumor fluorescence. In particular, neurosurgeons of Washington University and Siteman Cancer Center practicing at Barnes Jewish and St. Louis Children’s Hospitals have significant expertise with intraoperative imaging.5–11
Surgical Navigation
Resection of brain tumors is complicated by poor visualization, complex three-dimensional anatomy, and the critical importance of anatomic structures. Intraoperative localization during surgical resection is critical to optimize maximal safe extent of resection. Neurosurgeons commonly utilize surgical navigation to improve localization. In the most general case, surgical navigation requires registration of the patient’s surface cranial anatomy with high-resolution three-dimensional images. Neurosurgeons use optical and electromagnetic devices to create three dimensional maps of the patient’s cranial surface, which can be registered with high accuracy to high-resolution pre-operative CT and MRI scans loaded on the intraoperative computer (Figure 1). Registration of the patient’s cranial surface in real and imaging space allows the surgeon to place a probe anywhere within the intracranial space and see this location on the uploaded imaging. Surgical navigation techniques give the neurosurgeon a kind of “x-ray vision”, allowing gross estimation of tumor borders, extent of resection, and proximity to important brain structures.
Figure 1. Surgical navigation.
Surgical navigation (Stealth™ Medtronic, Inc, Louisville, CO) enables the neurosurgeon to continuously review the anatomical location during surgery using preoperatively acquired MRI studies register to the patient’s surface cranial anatomy. This technique improves accurate selection of safe surgical corridors and maximizes safe tumor removal. In this example, pre-operative post-contrast T1-weighted MRI identifies a ring-enhancing glioblastoma. Pre-operative MRI DTI tractography are merged to demonstrate the relationship of the tumor to the corticospinal motor fibers (left, green: red, right) and the left arcuate fasciculus language fibers (orange).
Advanced MRI modalities can be incorporated to improve surgical navigation. Diffusion tensor imaging (DTI) can identify critical white matter tracts involving motor (corticospinal fibers), language (arcuate fasciculus), vision (optic radiations), and other vital subcortical tracts. Functional imaging such as task-based functional MRI (fMRI) and resting state MRI (rsMRI, non-task based) can also be incorporating into the surgical navigation system. The fMRI identifies areas of cortical function by measuring changes in the BOLD signal during simple tasks, while rsMRI measures interactions between cortical regions to identify underlying functional networks.12 These modalities can be registered to structural imaging to identify relationships between areas of critical function and tumor. Integration of these modalities into the surgical navigation assists with maximization of resection while preserving function.
Intraoperative Imaging
Surgical navigation techniques are highly accurate immediately after registration; however, tissue shifts caused by the operative approach, tumor resection, cerebrospinal fluid drainage, and other factors impair this accuracy. Images used for navigation can be updated and improved intraoperatively with iMRI, iCT, and iUS as will be discussed below.
Intraoperative ultrasound (iUS) has been applied in neurosurgery for over 30 years.13,14 It is readily available and relatively inexpensive. Intraoperative ultrasound permits real time visualization enabling the surgeon the ability to estimate the margins of a lesion and the extent of resection. There are several significant advantages of iUS compared with other intraoperative imaging modalities. Of the available imaging techniques, iUS is the only true real time modality, allowing immediate assessment of tumor tissue and brain shift during surgery. It can be deployed quickly, is commonly available in the operating room, and is lower cost. Additionally, iUS (and iCT) does not impact pacemakers and other implanted devices, which is a limitation of iMRI.14 The principle disadvantages of iUS are poor resolution relative to iCT and iMRI, as well as relatively steep learning curve. The utility of iUS has improved with advanced 3D techniques, integration into surgical navigation platforms, and implementation of color Doppler techniques to visualize vasculature such as the internal carotid artery and its branches during brain tumor resection.14
Diagnostic CT imaging has become ubiquitous in neurosurgery over the last 30 years given the low cost, versatility, acquisition speed, and spatial resolution (particularly for osseous structures). Intraoperative CT is used during brain tumor resection at some centers; can be particularly helpful, and in fact is generally superior tool, for assessing osseous anatomy for tumors involving the skull, skull base, and spine.15 (Figure 2). Intraoperative CT and 3D fluoroscopy are typically faster and relatively less expensive than intraoperative MRI modalities. However, iCT does have significant limitations in soft tissue resolution compared to iMRI. 3-D fluoroscopy, can produce “CT-like” images, and has been shown to be quite useful for spinal surgery and some cranial procedures15 (Figure 3).
Figure 2. Intraoperative CT.
Sagittal post-contrast T1 weighted MRI (a) showing patient with cervical chordoma involving C2 and the prevertebral tissues (arrow) in the posterior oropharynx. intraoperative 3-D fluoroscopy (b, sagittal; e axial) during a transoral approach for tumor resection demonstrating some residual C2 body (arrows) involved with tumor that lead to additional resection prior to closure. Postoperative CT (c, sagittal; f, axial) demonstrating more complete resection (arrows). intraoperative MRI (d, sagittal) demonstrating resection of the chordoma. Postoperative lateral C-spine x-ray image after posterior instrumented occipital-cervical fusion for stabilization (g).
Figure 3. Intraoperative CT.
3-D fluoroscopy (O-Arm, Medtronic, Minneapolis, Minnesota) can produce “CT like” images, and has been shown to be quite useful for spinal surgery and some cranial procedures. Intraoperative CT and 3D fluoroscopy can be particularly helpful, and in fact, superior in terms of the ability to assess osseous anatomy for tumors involving the bone of the skull, skull base, and spine (see example in Figure 2).
For more than 20 years, iMRI has been advancing as a modality in neurosurgical operating rooms.16,17 Of the available intraoperative imaging modalities, iMRI offers the highest soft tissue spatial resolution. Numerous centers worldwide have implemented iMRI strategies using either high or low magnetic field devices. At Barnes Jewish Hospital, Washington University physicians have been using a movable six-ton 1.5 tesla high field intraoperative MRI device mounted on ceiling rails.6 This device is situated in a middle storage room in between two neurosurgical operating rooms, and therefore can be used intermittently by surgeons in two different adjacent rooms during overlapping surgeries. Using this strategy, the neurosurgeon performs brain tumor resection until perceived maximal safe resection, at which point preparations are made for iMRI. Specifically designed imaging coils are placed around the patient’s head, additional sterile drapes are applied, and a safety checklist is completed prior to moving the iMRI device into the operating field (Figure 4). Acquired images can then be integrated into the surgical navigation system, enabling accurate navigation to resect identified residual tumor when accessible and safe. In recent years, an iMRI modality termed continuous MR thermography has facilitated performance of laser interstitial thermal therapy (LITT), a minimally invasive technique by which a laser probe is placed within brain lesions using surgical navigation for laser ablation18 (see related article by Kim and colleagues in this issue).
Figure 4. Intraoperative MRI.
iMRI suite at Barnes-Jewish Hospital in St. Louis with a movable iMRI device (IMRIS Inc, Minnetonka, Minnesota). After completion of standard brain tumor resection, prior to closure of the craniotomy, additional sterile drapes are applied and safety checks are completed (upper left), external 8-channel coils are applied (upper right), the doors of the magnet storage bay located in between 2 operating rooms are opened (lower left), and the movable 6-ton ceiling mounted iMRI device is moved into position (approximately 90 seconds) to enable acquisition of 1.5 Tesla high field high resolution iMRI images to assess the extent of tumor resection (lower right).
Washington University neurosurgeons have employed the iMRI strategy in more than 2,000 brain tumor surgeries, one of the largest experiences with this technique in North America. Clinical outcome studies by Washington University neurosurgeons as well as others worldwide have demonstrated that iMRI can improve the safe extent of resection increasing progression free and overall survival for patients with low and high-grade gliomas, pituitary adenomas and a variety of other pediatric and adult brain tumors. Pediatric neurosurgeons at St. Louis Children’s Hospital have successfully treated children with various brain tumors using the iMRI strategy employed at Barnes Jewish Hospital.9,11 Washington University neurosurgeons are part of a multi-center collaboration, the IMRIS Multi-Center Intraoperative MRI Neurosurgery Database, (I-MiND) which is studying the impact of iMRI and other treatment modalities on outcomes for patients with brain tumors and other complex neurological disorders across multiple centers in North America.
Tumor Fluorescence
Fluorescent agents including fluorescein and 5-ALA are being used in US centers to visualize brain tumors during surgeries. These agents help identify brain-tumor interfaces and hard to visualize residual tumor. The principle advantage of this technique is that information is immediate and continuously available to the surgeon.
Fluorescein has been utilized in medicine and other non-medical industries for decades.19 Ophthalmology and other areas of medicine utilize intravenous or topical application of fluorescein allows better visualization of certain pathologies. In neurosurgery, intravenous administration of fluorescein can aid in visualization of blood vessels during vascular neurosurgical procedures such as clipping of cerebral aneurysms or resection or arteriovenous malformations. Modification of the technique of has been proven to enhance the neurosurgeons ability to visualize brain tumors.19 Intraoperative imaging with fluorescein relies on the breakdown of blood brain barrier that is inherent with many brain tumors, particularly malignant gliomas.20 Intravenous fluorescein is administered early in brain tumor surgeries. The neurosurgeon uses a microscope with selective fluorescent filters that enable enhanced visualization of the brain tumor (Figure 5). Advantages include high available of fluorescein, low cost, proven safety, and ease of use. The major disadvantage is limited to specificity, which could result in retained residual tumor, or worse, injury to critical brain structures. Extreme care is needed on the part of the neurosurgeon to understand the limits of this technology.
Figure 5. Fluorescein for brain tumors.
Upper row: Patient with left frontal glioblastoma as seen on T1 weighted axial post-contrast MRI (preoperative upper left, postoperative upper right). Middle row: Interhemispheric intraoperative microsurgical view during resection of the tumor under standard white light (left) and under 560 nm yellow fluorescence filter after intravenous injection of fluorescein (right) using the Zeiss Pentero microscope (Carl Zeiss Meditec, Inc., Dublin, California). Note the demarcation between the green fluorescence of the tumor and the absence of fluorescence of the adjacent brain. Lower row: En bloc gross specimens with standard white light (left) and fluorescence filter (right) demonstrating the fluorescence of the tumor after the intravenous injection of fluorescein.
5-aminolevulinic acid (5-ALA) also improves visualization of select brain tumors. Mechanistically, 5-ALA is preferentially metabolized by certain tumors to protoporphyrin IX,21 which can be excited with a blue light and visualized using a selective blue filter through surgical optics as a fluorescent pink hue (Figure 6). The use of this fluorescent agent can facilitate detection of tumor that may not be readily apparent using surgical microscopes or other techniques. 5-ALA is more specific than fluorescein as it is metabolized preferentially by the tumor. Patients can have a mild photosensitivity for 24–48 hours after administration of this agent, but the worldwide experience with thousands of patients have demonstrated this as a very well tolerated strategy.22 Brain tumor resection using 5-ALA has been extensively studied in Europe and other continents. A randomized prospective study demonstrated that 5-ALA improved extent of resection and progression free survival for malignant gliomas.23 The Food and Drug Administration (FDA) in the United States approved 5-ALA in the commercial product of Gleolan (NXDC, Inc) in 2018. The neurosurgeons of Washington University and the Siteman Cancer Center at Barnes Jewish Hospital are among the first in the United States to employ this recently FDA approved agent.
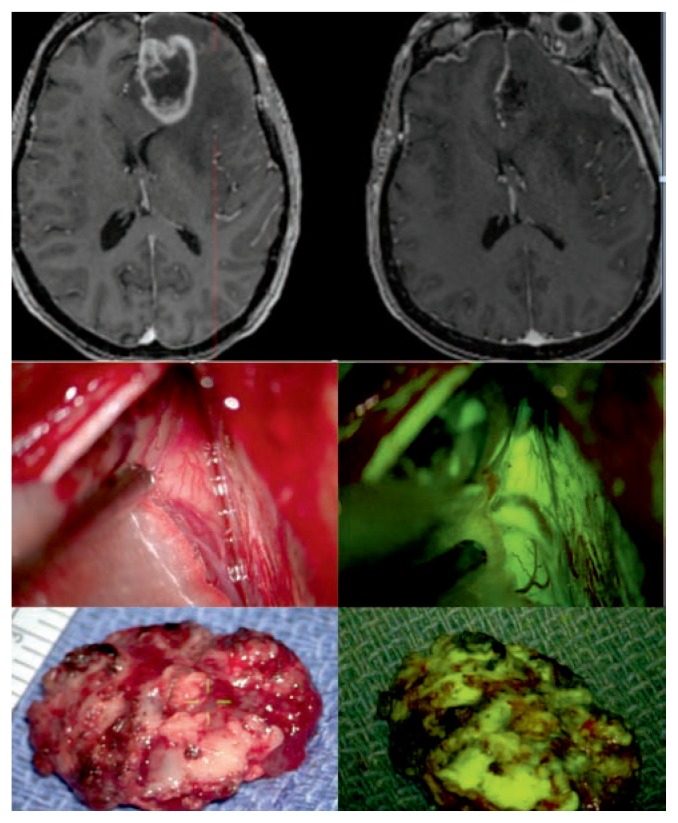
Figure 6. 5-ALA for brain tumors.
Patient with recurrent left frontal-parietal glioblastoma as seen on preoperative T1 weighted axial post contrast MRI, (upper left 3 images), and on intraoperative MRI after 5-ALA guide resection (lower left 3 images). Upper right: intraoperative microsurgical view of the view during craniotomy for resection of the tumor under standard white light using the Zeiss Pentero microscope (Carl Zeiss Meditec, Inc., Dublin, California). Lower right: 400 nm blue fluorescence filter after oral administration of 5-ALA (Gleolan™) several hours preoperatively (right). The pink fluorescence signifies the presence of protoporphyrin IX, the metabolite visualized in the tumor which preferentially metabolizes 5-ALA.
Summary
Maximizing the extent of safe resection is an important goal in the removal of a variety of brain tumor types. Surgical navigation, intraoperative imaging, and tumor fluorescence are powerful modalities that advance this goal by improving brain tumor localization and visualization. These strategies can be used independently, in combination with each other, or in combination with traditional neurosurgical physiologic monitoring techniques including cortical/subcortical motor stimulation, awake craniotomy to identify and preserve areas of language, somatosensory and motor evoke potentials, and monitoring of cranial nerve electromyography. Additionally, advanced MRI techniques such as DTI tractography, fMRI, and rsMRI offer visualization of critical cortical / subcortical areas, improving safe tumor resection. Structural and functional imaging can be performed using iMRI, and surgical navigation scans can be updated with these scans to account for brain shift during surgery. Integration of intraoperative imaging modalities, navigations tools and techniques for identification and preservation of critical function are leading to more precise and safe maximal brain tumor resection by the neurosurgeons of Washington University and the Siteman Cancer Center practicing at Barnes Jewish and St. Louis Children’s Hospitals.
Acknowledgment
The authors would like to thank Jeanene Crain and Rhonda Quint for their assistance in preparation and submission of this manuscript.
Footnotes
Michael R. Chicoine, MD, (above), is the August A. Busch, Jr. Professor of Neurological Surgery; Peter Sylvester, MD, Neurosurgery Resident PGY6; Alexander T. Yahanda, BS; and Amar Shah, MD, are all in the Department of Neurological Surgery, Washington University School of Medicine, St. Louis, Missouri.
Contact: chicoinem@wustl.edu
Disclosure
MC received funding from 1) IMRIS Inc. for an unrestricted educational grant to support an iMRI database and outcomes analysis project, the IMRIS Muliticenter intraoperative MRI Neurosurgery Database (I-MiND); 2) The Head for the Cure Foundation; and 3) Mrs. Carol Rossfeld and The Alex & Alice Aboussie Family Charitable Foundation.
References
- 1.Lacroix M, Abi-Said D, Fourney DR, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: Prognosis, extent of resection, and survival. J Neurosurg. 2001;95(2):190–198. doi: 10.3171/jns.2001.95.2.0190. [DOI] [PubMed] [Google Scholar]
- 2.McGirt MJ, Chaichana KL, Gathinji M, et al. Independent association of extent of resection with sur vival in patients with malignant brain astrocytoma. J Neurosurg. 2009;110(1):156–162. doi: 10.3171/2008.4.17536. [DOI] [PubMed] [Google Scholar]
- 3.McGirt MJ, Chaichana KL, Attenello FJ, et al. Extent of surgical resection is independently associated with sur vival in patients with hemispheric infiltrating low-grade gliomas. Neurosurgery. 2008;63(4):700–707. doi: 10.1227/01.NEU.0000325729.41085.73. author reply 707–708. [DOI] [PubMed] [Google Scholar]
- 4.Sanai N, Polley M-Y, McDermott MW, Parsa AT, Berger MS. An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg. 2011;115(1):3–8. doi: 10.3171/2011.7.JNS10998. [DOI] [PubMed] [Google Scholar]
- 5.Sylvester PT, Evans JA, Zipfel GJ, et al. Combined high-field intraoperative magnetic resonance imaging and endoscopy increase extent of resection and progression-free sur vival for pituitary adenomas. Pituitary. 2015;18(1):72–85. doi: 10.1007/s11102-014-0560-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chicoine MR, Lim CCH, Evans JA, et al. Implementation and preliminary clinical experience with the use of ceiling mounted mobile high field intraoperative magnetic resonance imaging between two operating rooms. Acta Neurochir Suppl. 2011;109:97–102. doi: 10.1007/978-3-211-99651-5_15. [DOI] [PubMed] [Google Scholar]
- 7.Akbari SHA, Sylvester PT, Kulwin C, et al. Initial Experience Using Intraoperative Magnetic Resonance Imaging During a Trans-Sulcal Tubular Retractor Approach for the Resection of Deep-Seated Brain Tumors: A Case Series. Oper Neurosurg Hagerstown Md. 2019;16(3):292–301. doi: 10.1093/ons/opy108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haydon DH, Chicoine MR, Dacey RG. The impact of high-field-strength intraoperative magnetic resonance imaging on brain tumor management. Neurosurgery. 2013;60(Suppl 1):92–97. doi: 10.1227/01.neu.0000430321.39870.be. [DOI] [PubMed] [Google Scholar]
- 9.Karsy M, Akbari SH, Limbrick D, et al. Evaluation of pediatric glioma outcomes using intraoperative MRI: A multicenter cohort study. J Neurooncol. 2019;143(2):271–280. doi: 10.1007/s11060-019-03154-7. [DOI] [PubMed] [Google Scholar]
- 10.Leuthardt EC, Lim CCH, Shah MN, et al. Use of movable high-field-strength intraoperative magnetic resonance imaging with awake craniotomies for resection of gliomas: Preliminary experience. Neurosurgery. 2011;69(1):194–205. doi: 10.1227/NEU.0b013e31821d0e4c. discussion 205–206. [DOI] [PubMed] [Google Scholar]
- 11.Shah MN, Leonard JR, Inder G, et al. Intraoperative magnetic resonance imaging to reduce the rate of early reoperation for lesion resection in pediatric neurosurgery. J Neurosurg Pediatr. 2012;9(3):259–264. doi: 10.3171/2011.12.PEDS11227. [DOI] [PubMed] [Google Scholar]
- 12.Zhang D, Johnston JM, Fox MD, et al. Preoperative sensorimotor mapping in brain tumor patients using spontaneous fluctuations in neuronal activity imaged with functional magnetic resonance imaging: Initial experience. Neurosurgery. 2009;65(6 Suppl):226–236. doi: 10.1227/01.NEU.0000350868.95634.CA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Unsgaard G, Ommedal S, Muller T, Gronningsaeter A, Nagelhus Hernes TA. Neuronavigation by intraoperative three-dimensional ultrasound: Initial experience during brain tumor resection. Neurosurgery. 2002;50(4):804–812. doi: 10.1097/00006123-200204000-00022. discussion 812. [DOI] [PubMed] [Google Scholar]
- 14.Sastry R, Bi WL, Pieper S, et al. Applications of Ultrasound in the Resection of Brain Tumors. J Neuroimaging Off J Am Soc Neuroimaging. 2017;27(1):5–15. doi: 10.1111/jon.12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schichor C, Terpolilli N, Thorsteinsdottir J, Tonn JC. Intraoperative Computed Tomography in Cranial Neurosurgery. Neurosurg Clin N Am. 2017;28(4):595–602. doi: 10.1016/j.nec.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 16.Sutherland GR, Kaibara T, Louw D, Hoult DI, Tomanek B, Saunders J. A mobile high-field magnetic resonance system for neurosurgery. J Neurosurg. 1999;91(5):804–813. doi: 10.3171/jns.1999.91.5.0804. [DOI] [PubMed] [Google Scholar]
- 17.Black PM, Moriarty T, Alexander E, et al. Development and implementation of intraoperative magnetic resonance imaging and its neurosurgical applications. Neurosurgery. 1997;41(4):831–842. doi: 10.1097/00006123-199710000-00013. discussion 842–845. [DOI] [PubMed] [Google Scholar]
- 18.Hawasli AH, Bagade S, Shimony JS, Miller-Thomas M, Leuthardt EC. Magnetic resonance imaging-guided focused laser interstitial thermal therapy for intracranial lesions: Single-institution series. Neurosurgery. 2013;73(6):1007–1017. doi: 10.1227/NEU.0000000000000144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stummer W, Suero Molina E. Fluorescence Imaging/Agents in Tumor Resection. Neurosurg Clin N Am. 2017;28(4):569–583. doi: 10.1016/j.nec.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 20.Acerbi F, Cavallo C, Broggi M, et al. Fluorescein-guided surgery for malignant gliomas: A review. Neurosurg Rev. 2014;37(4):547–557. doi: 10.1007/s10143-014-0546-6. [DOI] [PubMed] [Google Scholar]
- 21.Ferraro N, Barbarite E, Albert TR, et al. The role of 5-aminolevulinic acid in brain tumor surgery: A systematic review. Neurosurg Rev. 2016;39(4):545–555. doi: 10.1007/s10143-015-0695-2. [DOI] [PubMed] [Google Scholar]
- 22.Teixidor P, Arráez MÁ, Villalba G, et al. Safety and Efficacy of 5-Aminolevulinic Acid for High Grade Glioma in Usual Clinical Practice: A Prospective Cohort Study. PloS One. 2016;11(2):e0149244. doi: 10.1371/journal.pone.0149244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stummer W, Pichlmeier U, Meinel T, et al. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: A randomised controlled multicentre phase III trial. Lancet Oncol. 2006;7(5)(06):392–401. 70665–9. doi: 10.1016/S1470-2045. [DOI] [PubMed] [Google Scholar]