Abstract
The Gamma Knife Center of St. Louis has established itself as a key facility offering stereotactic radiosurgery (SRS) for a variety of neuro-oncologic disorders. Since the Gamma Knife unit was first brought to Washington University in 1997, we have treated 5,696 patients. In this review, we discuss the effective role of Gamma Knife SRS in the treatment strategies for patients with neuro-oncologic disorders including brain metastases, meningiomas, pituitary adenomas, and acoustic neuromas. While there is active ongoing research evaluating the most effective treatment for patients with these disorders, it is clear that best management practices may be tailored for individual patients utilizing SRS either alone or in conjunction alternative treatment strategies including open neurosurgical procedures, laser thermos-ablative surgery, and even new medical oncological treatment strategies.
Introduction
The concept and development of stereotactic radiosurgery (SRS) was initially proposed by Dr. Lars Leksell incorporating his Leksell stereotactic frame to guide radiation to precisely irradiate selected targets within the brain.1 Radiosurgery accomplishes this by delivering multiple convergent beams of gamma radiation emitted from cobalt-60 directed toward a central point to provide the conformal delivery of high dose radiation to a specific tumor volume within the brain and to avoid delivering high-dose irradiation to normal brain tissue. The prototype Gamma Knife unit utilizing gamma radiation was initially developed in 1968 at the Karolinska Institute in Stockholm, Sweden.2,3
The widespread use of Gamma Knife stereotactic radiosurgery was made possible by the development of CT and MR stereotactic imaging techniques and corresponding development of computer technology and software that allow for creation of treatment plans unique for each patient that are extremely accurate and efficient, allowing most patients to be treated as outpatients. During frame-based SRS, the patient is initially placed in a Leksell stereotactic frame. The patient undergoes neuro-imaging with MRI or CT studies that permit generation and definition of image-guided Leksell stereotactic coordinates. The Gamma Knife software allows the creation of three-dimensional treatment plans to simulate radiation isodose lines and allow the neurosurgeon and radiation oncologist to define a prescription dose of radiation at the tumor target. Ultimately, the treatment plan simulates the delivery of high radiation in a conformal manner with a single treatment fraction. The radiation dose falls rapidly outside the volume of the tumor target and avoids delivering significant radiation doses to normal brain. (Table 1).
Table 1.
Quantification of all SRS completed at a single institution, Washington University in St. Louis
| Lesion | Sample size (n=5201) | |
|---|---|---|
|
| ||
| Astrocytoma | 112 | |
|
| ||
| Acoustic/trigeminal schwannoma | 439 | |
|
| ||
| Functional | ||
| Trigeminal neuralgia | 739 | |
| Glossopharyngeal neuralgia | 5 | |
| Tremor | 12 | |
|
| ||
| Vascular | ||
| Arteriovenous fistula | 10 | |
| Arteriovenous malformation | 347 | |
| Cavernoma | 4 | |
|
| ||
| Meningioma | 525 | |
|
| ||
| Metastases | 2502 | |
| Melanoma | 277 | |
| Breast | 369 | |
| Lung | 1387 | |
| Renal | 142 | |
| Colon | 55 | |
| Other | 272 | |
|
| ||
| Sellar Lesions | ||
| Pituitary adenoma | 290 | |
| Craniopharyngioma | 7 | |
|
| ||
| Other | 734 | |
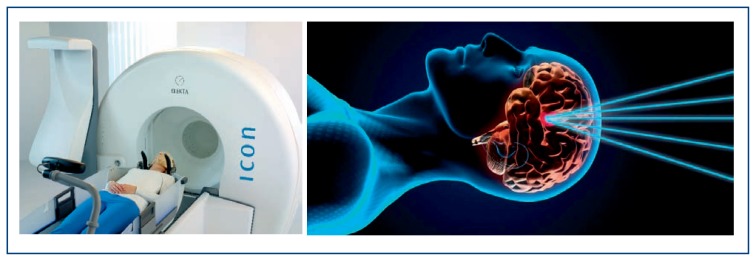
Current radiosurgery units may utilize frameless radiosurgery techniques that allow repeated treatment fractions, or hypo-fractionated radiosurgery. While earlier Gamma Knife radiosurgery units have solely utilized single fraction treatments, the most recent models with an attached CT scanner (ICON Gamma Knife unit) allows frameless SRS techniques and hypo-fractionated therapy. Figure 1 shows the Gamma Knife ICON unit (a) with a schematic representation of the central core allowing collimation of multiple beams of gamma radiation delivered to stereotactic target (b).
Figure 1. Stereotactic Radiosurgery.
Left, example of a patient within the Gamma Knife Icon system, that is able to deliver SRS with either frame-based or frameless techniques. Right, a schematic representation of a highly focused point by collimating beams of radiation from its cobalt sources to a lesion as selected by a treatment team comprised of neurosurgeons, radiation oncologists, and physicists.
Gamma Knife Stereotactic Radiosurgery in Treatment of Metastatic Brain Disease
Metastatic disease represents a prevalent form of brain tumors, comprising 9–17% all intracranial neoplasms.4 Most commonly, intracranial metastases represent secondary lesions from lung cancer, breast cancer, or melanoma cumulatively representing approximately 75% of brain metastases.5 Although untreated brain metastases are associated with a median survival as low as one month, standard of care treatment significantly improves outcomes.6 Positive prognostic factors include high Karnofsky Performance Status (KPS), age < 65 years, control of systemic disease, response to steroid treatment, and lack of neurocognitive impairment. In retrospective analyses examining patients with a variety of systemic cancer, up to 41% were found to have greater than three intracranial lesions.7
Current guidelines for treatment of metastatic brain disease include a multi-faceted treatment approach variably incorporating stereotactic radiosurgery, whole brain radiation therapy (WBRT), and surgery. Dexamethasone at a dose up to 4 mg 4x/day is frequently prescribed for symptomatic treatment of cerebral edema, with a majority of patients demonstrating neurological improvement within 72 hours of treatment. Antiepileptic medications are used both prophylactically and therapeutically to reduce the incidence of seizures. Systemic management of disease generally includes a medical oncology regimen tailored to the primary malignancy. Traditionally, intracranial metastases are often poorly responsive to standard chemotherapy. Treatment responsiveness is thought to be limited by blood-brain barrier penetration. In some intracranial tumors, radiation treatment may function synergistically to increase chemotherapy efficacy and improve progression-free survival. Selective utilization of targeted chemotherapy or immunotherapy regimens improve responsiveness of systemic disease to primary malignancies and may have a potential role in mitigating growth of metastatic lesions in the CNS. The roles of these new therapies (targeted and immune) are still being evaluated for possible efficacy within the CNS in controlling metastatic brain disease.
Traditionally, WBRT represented the goldstandard treatment for treatment of metastatic disease to the brain.8 During the 20th century a wide number of clinical trials optimized WBRT treatment durations and regimens with current clinical practice utilizing a prescribed dose of 30 Gy over 10 fractions. Experience has demonstrated that WBRT, initially thought to represent a low-risk treatment option, is associated with significant adverse effects including delayed toxicity, most notably significant for adverse neurocognitive effects that may be both profound and permanent. The associated neurocognitive decline has a characteristic biphasic pattern, with an initial transient decline at four months post-treatments and a secondary severe decline that starts months to years after treatment, more recently quantified in a meta-analysis to represent a cognitive decline of 31–57% at 3 months and 48–89% at one year.9 Active research includes leveraging WBRT with hippocampal sparing in an attempt to provide the treatment efficacy of WBRT while attempting to avoid its neurocognitive effects on memory, with long-term outcomes ongoing. The significant neurocognitive effects of WBRT have spawned the advance of stereotactic radiosurgery options that provide high-dose radiation to the metastatic tumors specific and avoidance of both vital structures and normal brain tissue.
Surgery has been and continues to have a critical role in treatment of metastatic disease. A well-designed clinical trial published in the New England Journal of Medicine established the utility of surgical resection of single metastatic tumors demonstrating improved survival and decreased local recurrence rates compared with treatment with WBRT alone.10 In addition, surgical resection provides both immediate relief of mass effect and treatment for lesions that might be too large for SRS therapy. Gamma Knife SRS provides an increasingly important alternative modality for treatment of metastatic brain disease in patients. With the advent of more focused radiotherapeutic options, WBRT and neurosurgical resection are often not the primary modality for treatment of patients with metastatic brain disease, but may be utilized in selected patients with extensive intracranial disease burden or with lesions characterized by large tumor volumes and significant mass effect.
In multiple studies, cumulatively with more than 6,000 patients, Gamma Knife SRS for patients with brain metastases showed an average of 84–97% local control rate. SRS can also decrease peri-tumoral edema as characterized by a decreased steroid dependence at six months post treatment. Delayed radiation effects typically develop 9–12 months following SRS in a minority of patients. Post-surgical adjuvant SRS is a necessary component of treatment for surgically resected metastases as surgical resection alone demonstrates a local recurrence rate of 46%.11 Finally, SRS may be utilized as a primary treatment option for patients who are deemed poor surgical candidates for either medical comorbidities or location of lesions either in deep or eloquent brain locations, or for lesions less than 3 cm in maximal diameter.
Historically, SRS has also been employed in the treatment for patients with up to three brain metastases, while in the past WBRT was more often considered to treat four or more lesions. A Lancet Oncology observational study of nearly 1,200 patients demonstrated similar median overall survival for patients treated with SRS with two to four tumors versus those with five to ten metastases, without significant difference in neurocognitive function, therefore suggesting the possibility that SRS may be effectively utilized as a treatment option with patients with greater numbers of intracranial metastases.12 Treatment of intracranial metastatic disease with multiple tumors with Gamma Knife SRS remains a controversial topic, and its utilization as an alternative treatment option to WBRT in these situations vary with institutional preference. There is a lack of definitive evidence to support level one guidelines for WBRT versus SRS in treatment of metastatic diseases with larger numbers of lesions. Figure 2 demonstrates the effectiveness of Gamma Knife SRS in treatment of metastatic disease.
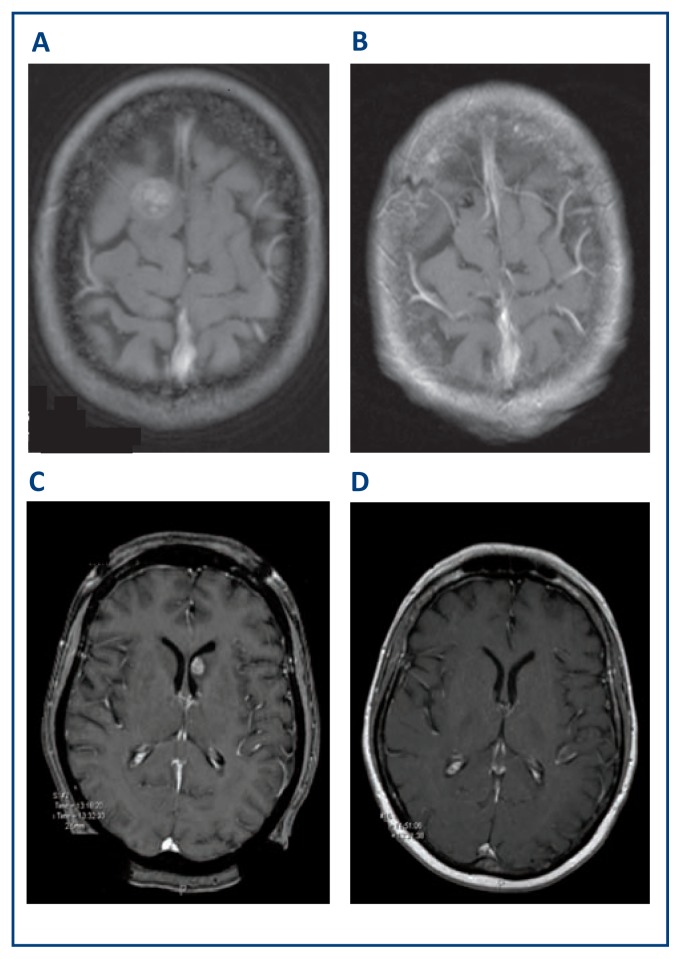
Figure 2. Stereotactic Radiosurgery Treatment of Metastatic Tumors.
Metastatic melanoma pre- and post-Gamma Knife SRS (A) and (B) respectively six months after treatment. Metastatic lung carcinoma pre- and post-Gamma Knife SRS (C) and (D) respectively three months after treatment.
Stereotactic Radiosurgery for Treatment of Meningiomas
Meningiomas are most often benign neoplasms arising from arachnoidal cap cells along the inner surface of the dura, and represent approximately 25% of primary intracranial tumors in the United States. The large majority (~97%) of these meningothelial neoplasms are WHO grade 1 (typical) or WHO grade 2 (atypical), while the remainder may be considered anaplastic or malignant WHO grade 3. Meningiomas occur more frequently in the elderly and female patients, and usually arise from intracranial or intraspinal regions with much less common intraventricular or extradural (intra-osseous) locations. Meningiomas generally grow at a slow rate with doubling times varying from 415 days to eight years.13,14 Symptoms generally are reflected in their size and location. Large tumors may result in headaches, secondary to increased intracranial pressure, and focal neurologic deficits such as seizures (cortical), weakness (paracentral), ataxia (cerebellar), and cranial nerve dysfunction (medial sphenoidal or petro-clival) secondary to mass effect on the adjacent brain parenchyma. Meningiomas may also produce symptoms from peritumoral edema. MR imaging of meningiomas typically show isointense lesions on non-contrast studies, and are best visualized with MR T1 contrast-enhancing studies.
Clinical management of meningiomas includes an assessment of their symptomology, growth rate, and age of patient. Indeed, many patients with meningiomas may be observed with surveillance neuro-imaging studies. This is common management strategy with incidental meningiomas in the elderly and poor operative candidates. Classic treatment of meningiomas includes surgical resection, stereotactic radiosurgery, or a combination of both modalities. Surgery has maintained a continued key role in management of meningiomas, offering effective rapid relief of mass effect, while complete or near total surgical resection result in improved long-term tumor control. The role of radiosurgery in now well-established as a key treatment modality in the management of selected patients with meningiomas. A retrospective study of 5,300 patients with meningiomas undergoing SRS from 15 centers showed a five-year and 10-year progression-free survival of 95.2% and 88.6% respectively, with morbidity of 6.6%. A similar long-term study of 290 consecutive patients showed progression-free control rates of 88.7 and 87.2% at 10 years and 20 years at follow up, respectively, with adverse radiation effects in 3.1% of patients.15
Meningiomas located along the skull base affecting critical structures offer a difficult management problem. Such tumors often involve the orbit, cavernous sinus, or petro-clival regions. Aggressive surgical resection of these complicated meningiomas in the past were associated with a high incidence of injury to cranial nerves, eloquent regions of the brain, or essential vascular structures. With the advent of SRS, surgical goals may be modified with plans for less aggressive surgical resections with the knowledge that radiosurgery offers on excellent option for long-term management of incompletely resected meningiomas. This is particularly exemplified for large meningiomas in surgically inaccessible locations. Such lesions may require pre-surgical planning for subtotal surgical resections, with planned adjuvant Gamma Knife radiosurgery to treat residual tumor, knowing its capability to provide excellent long-term control of tumor growth while minimizing injury associated with gross total resection of the tumor. Such a multi-modality approach offers the more efficient and safer treatment paradigm in these patients.
Stereotactic Radiosurgery in Treatment of Pituitary Adenomas
Pituitary adenomas account for approximately 10–20% of intracranial neoplasms.16 Pituitary adenomas are classified in two main biological categories, either secretory or non-functioning adenomas, and two morphological categories, microadenomas or macroadenomas, based on size. Larger tumors are more likely to result in compression of the optic chiasm, resulting in bitemporal visual field deficit, and/ or symptoms of panhypopituitarism. Secretory pituitary adenomas are associated with clinical syndromes; for example, prolactin-secreting adenomas may cause Forbes-Albright syndrome with amenorrhea-infertility in women and erectile dysfunction-infertility in men, while growth hormone-secreting adenomas cause acromegaly and ACTH-secreting adenomas cause Cushing’s disease.
The initial treatment of pituitary adenomas varies depending on size and biochemical function. Conservative management may be indicated in incidental and non-functional microadenomas with observation and surveillance MR imaging. Prolactinomas are typically treated medically with dopamine agonists. Other secretory adenomas are commonly surgically treated with endoscopic, transphenoidal biopsy and surgical resection to achieve a biochemical “cure”. Similarly, endoscopic resection may be employed to treat macroadenomas that cause mass effect on the optic structures, and allow rapid decompression by removing the suprasellar component of the tumor, though frequently complete surgical resections may be limited by invasion of surrounding structures like the cavernous sinus. Incompletely resected or recurrent tumors can be treated with radiation to enhance long-term control of tumor growth. Radiosurgery may have a primary or a complementary role for patients with small tumors, in patients who are poor surgical candidates, or in patients treated with partial surgical debulking to prevent tumor recurrence. In addition, SRS may be utilized to treat acromegaly or Cushing’s disease in patients who have persistent secretory disease post-operatively and/or who have failed medical management.
Outcomes of Gamma Knife radiosurgery following treatment of nonfunctioning pituitary adenomas in 125 patients over 22 years showed tumor control rates of 99%, 94% and 76% at one, five, and 10 years respectively though with less effective control associated with larger tumors greater than 4.5 cm.3,17 Similarly, secretory tumors demonstrate slower treatment responses; Pollock reported 46 patients with acromegaly treated with Gamma Knife radiosurgery, with endocrinologic normalization rate of 60% at five years.18 Gamma Knife radiosurgery may work in a delayed fashion with remission rates occurring variably over time. Kobayashi reported in a group of patients with Cushing’s Disease a normalization of ACTH levels in 35% and significant improvement in an additional 25% with a mean follow up of 64 months.19 The role of Gamma Knife radiosurgery in patients with secretory pituitary adenomas provides an additional therapeutic modality often combined with other treatment options including surgical or medical therapy.
Stereotactic Radiosurgery for Treatment of Vestibular Schwannomas
Intracranial schwannomas are characterized as capsulated benign tumors arising from Schwann cells along cranial nerves. The most common intracranial schwannoma, acoustic, or vestibular schwannoma, accounts for approximately 10% of intracranial neoplasms and occurs in the cerebello-pontine angle typically arising from the vestibular component of the eighth cranial nerve and extending into the internal auditory canal. Less frequent intracranial schwannomas may arise from the fifth, seventh, or twelfth cranial nerve, and rarely on other cranial nerves.20
Prior to Gamma Knife stereotactic radiosurgery, surgical resection was essentially the only treatment option for patients with vestibular schwannomas with high risk of morbidity given the tumor’s deep location near the brainstem nuclei and cranial nerves. Progress in microsurgical techniques have decreased surgical risks with improved post-operative morbidity and less frequent injury to the facial nerve resulting in facial palsy. Gamma Knife stereotactic radiosurgery is now established as a non-invasive alternative to microsurgical resection, and typically provides successful long-term control. In a retrospective study, 829 patients with a vestibular schwannoma treated with SRS demonstrated a tumor control rate without need for open surgery of 97% over a 15-year period, with facial neuropathy in less than 1% of patients.21 Furthermore, Gamma Knife radiosurgery can be combined with partial surgical resection for treatment of previously inoperable large vestibular schwannomas. A group of 40 patients with large vestibular schwannomas (median volume of 3.3 cm3) were underwent incomplete surgical tumor resection followed by a median dose of 12 Gy SRS at the fiftieth prescription isodose line. The patients showed an 86% tumor control rate over a 10-year follow-up.22 Furthermore, these patients demonstrated high rate of facial nerve preservation as demonstrated by 95% of patients remaining grade 1–2 in the House-Brackmann facial nerve scale. While there is significant overlap in the efficacy of Gamma Knife radiosurgery and open microsurgical resection, judicious use of each modality in selected patients provide better treatment options in individual patients with vestibular schwannomas. Figure 3 demonstrates the highly conformal prescription isodose line in Gamma Knife SRS in treatment of vestibular schwannoma.
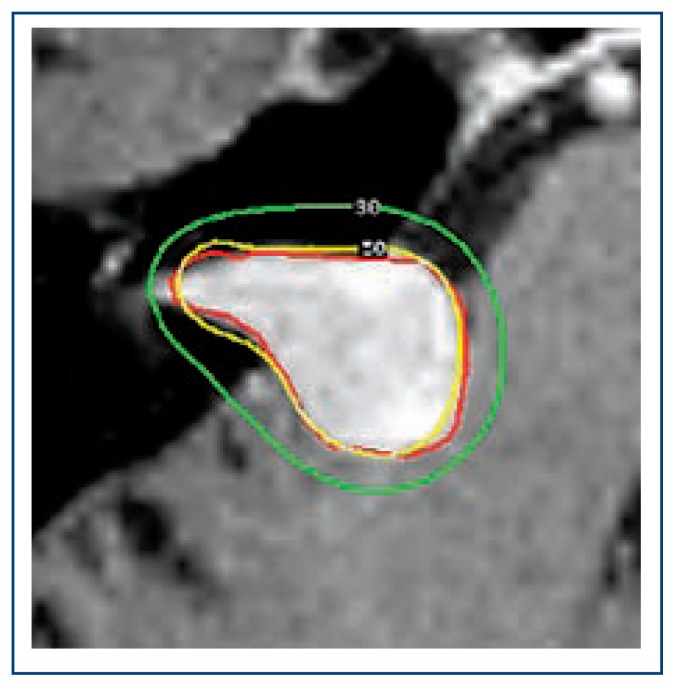
Figure 3. Radiosurgery plan for treatment of vestibular schwanomma.
Red outline represents contoured tumor target, while the yellow line represents the 50% prescription isodose of radiation (12Gy), and the green line represents the 30% isodose (7.2 Gy). Radiosurgery is able to prescribe a high radiation dose inside the yellow prescription isodose line conformal with the edge of the shwannoma with rapid radiation fall off.
Conclusion
In the past few decades, stereotactic radiosurgery techniques have changed the clinical treatment paradigms in managing neuro-oncology patients. SRS offers improved treatment options for patients with brain metastatic disease, introducing a major shift away from the use of whole brain radiation in many patients. Radiosurgery has also demonstrated significant promise as an alternative minimally invasive therapy in managing patients with benign brain tumors such as meningiomas, pituitary adenomas, and vestibular schwannomas.
Footnotes
Keith M. Rich, MD, (above), MSMA member since 1986, is Professor of Neurosurgery, and Rupen Desai, MD, is Neurosurgery Resident PGY4, Department of Neurosurgery, Washington University School of Medicine, St. Louis, Missouri.
Contact: richk@wustl.edu
Disclosure
None reported.
References
- 1.Leksell L. The stereotactic method and radiosurgery of the brain. Acta Chir Stand. 1951;102:316–319. [PubMed] [Google Scholar]
- 2.Leksell L. Stereotactic radiosurgery. J Neurol Neurosurg Psych. 1983;46:797–803. doi: 10.1136/jnnp.46.9.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sheehan JP, Niranjan S, Sheehan JM, Jane JA, Laws ER, et al. Stereotactic radiosurgery for pituitary adenomas: an intermediate review of its safety, efficacy and role in neurosurgeical treatment armamentarium. J Neurosug. 2005;102:678–691. doi: 10.3171/jns.2005.102.4.0678. [DOI] [PubMed] [Google Scholar]
- 4.Nayak L, Lee EQ, Wen PY. Epidemiology of Brain Metastases. Current Oncology Reports. 2012;14:48–58. doi: 10.1007/s11912-011-0203-y. [DOI] [PubMed] [Google Scholar]
- 5.Frank Lagerwaard FJ, Levendag Peter C, Nowak Peter JCM, Eijkenboom Wilhelmina MH, Hanssens Patrick EJ, Schmitz Paul IM. Identification of prognostic factors in patients with brain metastases: a review of 1292 patients. International J Radiation Oncology Biol Physics. 1999;43(98):795–803. 00442–8. doi: 10.1016/S0360-3016. [DOI] [PubMed] [Google Scholar]
- 6.Langer C, Mehta MP. Current management of brain metastases, with a focus on systemic options. J Clin Oncol. 2005 Sep 1;23(25):6207–19. doi: 10.1200/JCO.2005.03.145. [DOI] [PubMed] [Google Scholar]
- 7.Fabi Alessandra, Felici Alessandra, Metro Giulio, Mirri Alessandra, et al. Brain metastases from solid tumors:disease outcome according to type of treatment and therapeutic recources of the treating center. J Exp Clin Cancer Res. 2011:10. doi: 10.1186/1756-9966-30-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Felice F1, Musio D2, Cassese R2, Tombolini V3. Radiotherpeutic treatment approaches for brain metastases. Anticancer Res. 2014 Dec;34(12):6913–8. [PubMed] [Google Scholar]
- 9.Tallet AV1, Azria D, Barlesi F, Spano JP, Carpentier AF, Gonçalves A, Metellus P. Neurocognitive function impairment after whole brain radiotherapy for brain metastes:actual assement. Radiat Oncol. 2012;7:77. doi: 10.1186/1748-717X-7-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patchell RA, Tibbs PA, Walsh JW, Dempsey RJ, Maruyama Y, et al. A randomized trial of surgery in the treatment of single metastases to the brain”. N Engl J Med. 1990;322:494–500. doi: 10.1056/NEJM199002223220802. [DOI] [PubMed] [Google Scholar]
- 11.Patchell RA1, Tibbs PA, Regine WF, Dempsey RJ, et al. Postoperative radiotherapy in the treatment of single metastases to the brain:a randomized trial. JAMA. 1998;280:1485–9. doi: 10.1001/jama.280.17.1485. [DOI] [PubMed] [Google Scholar]
- 12.Alngi F, Fiorentino A, Navarria P, Bello Lorenzo. Stereotacti radiosurgery for patienswith brain metastases. Lancet Oncology. 2014;15:246–247. doi: 10.1016/S1470-2045(14)70151-2. [DOI] [PubMed] [Google Scholar]
- 13.Jaaskelainen J, Haltia M, Laasonen E, Wahlstrom T, Valtonen S. The growth rate of intracranial meningiomas and its relation to histology. An analysis of 43 patients. Surg Neurol. 1985;24(2):165–72. doi: 10.1016/0090-3019(85)90180-6. [DOI] [PubMed] [Google Scholar]
- 14.Jung HW, Yoo H, Paek SH, Choi KS. Long-term outcome and growth rate of subtotally resected petroclival meningiomas: experience with 38 cases. Neurosurgery. 2000;46(3):567–74. doi: 10.1097/00006123-200003000-00008. discussion 74–5. [DOI] [PubMed] [Google Scholar]
- 15.Santacroce A, Walier M, Regis J, et al. Long-term tumor control of benign intracranial meningiomas after radiosurgery in a series of 4565 patients. Neurosurgery. 2012;70(1):32–9. doi: 10.1227/NEU.0b013e31822d408a. discussion 9. [DOI] [PubMed] [Google Scholar]
- 16.Laws ER, Jr, Vance ML. Radiosurgery for pituitary tumors and craniopharyngiomas. Neurosurg Clin N Am. 1999;10:327–336. [PubMed] [Google Scholar]
- 17.Park KJ, Kano H, Parry PV, et al. Long-term outcomes after gamma knife stereotactic radiosurgery for nonfunctional pituitary adenomas. Neurosurgery. 2011;69(6):1188–99. doi: 10.227/NEU.0b013e318222afed. [DOI] [PubMed] [Google Scholar]
- 18.Pollock BE, Jacob JT, Brown PD, Nippoldt TB. Radiosurgery of growth hormone-producing pituitary adenomas: factors associated with biochemical remission. J Neurosurg. 2007;106(5):833–8. doi: 10.3171/jns.2007.106.5.833. [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi T, Kida Y, Mori Y. Gamma knife radiosurgery in the treatment of Cushing disease: long-term results. J Neurosurg. 2002;97(5 Suppl):422–8. doi: 10.3171/jns.2002.97.supplement. [DOI] [PubMed] [Google Scholar]
- 20.Russell DS, Rubinstein LJ. Pathology of tumors of the nervous system. Edward Arnold; London: 1989. [Google Scholar]
- 21.Lunsford LD, Niranjan A, Flickinger JC, Maitz A, Kondziolka D. Radiosurgery of vestibular schwannomas: summary of experience in 829 cases. J Neurosurg. 2005;102:195–9. [PubMed] [Google Scholar]
- 22.Iwai Y, Ishibashi K, Watanabe Y, Uemura G, Yamanaka K. Functional Preservation After Planned Partial Resection Followed by Gamma Knife Radiosurgery for Large Vestibular Schwannomas. World Neurosurg. 2015;16(15):00247–8. doi: 10.1016/j.wneu.2015.03.012. [DOI] [PubMed] [Google Scholar]