Abstract
Autologous fat grafting is an aesthetic and reconstructive procedure in which an individual's own fat is harvested and injected into the soft tissues to correct contour and other abnormalities. Fat graft is considered the ideal soft tissue filler for its biocompatibility, lack of immunogenicity, and availability. The entire procedure of harvesting, processing, and transfer of fat graft affects fat graft take and effectiveness of fat grafting. This article will focus on the most common methods of fat graft processing, including centrifugation, cotton gauze rolling, sedimentation, and filtration/washing. The fragility of the harvested adipocytes makes the technique of fat graft processing of utmost importance, as blood and other unnecessary cellular fragments are removed. Each fat graft processing method has its own merits and shortcomings; however, due to a lack of well-defined prospective studies, there is no evidence to support one processing method as superior to another.
Keywords: autologous fat grafting, adipose-derived stem cells, ASCs, fat graft processing, lipoaspirate processing, liposuction, fat graft filtration, centrifugation
The concept of autologous fat grafting (AFG) has been in existence for more than a century. In 1893, Neuber was among the first to apply fat grafting to correct deformities and wounds after oncological surgery was performed. 1 The applications and techniques of natural, autologous fillers have increased since its initial use. 2 Over the past 20 years in particular, the popularity of fat grafting has significantly increased in both aesthetic and reconstructive surgeries. The applications of AFG include but are not limited to contour deformities, posttraumatic defects, burn injuries, scar contractures, breast and buttock augmentation, facial atrophy and rejuvenation, and hand rejuvenation. 3 4
While other types of soft tissue fillers have been used in some of the aforementioned applications, AFG has many qualities that make it the preferred option. The ideal filler should be easily obtainable or formulated, have reproducible delivery techniques with predictable and persistent correction, be cost-efficient, noncarcinogenic, nonteratogenic, noninflammatory, and nonmigratory, and carry minimal risk of infectious disease transmission. Adipose tissue has many of these qualities, as it offers lack of immunogenicity, relative ease of implantation, low cost, ease of obtainability, and repeatability. 5 While actual fat graft take may vary as much as 20 to 80%, retained fat has the capacity for greater longevity compared with other filler options. In general, AFG is a low-risk procedure with known potential complications including fat necrosis, cyst formation, donor or recipient site cellulitis, palpable abnormality, and contour irregularity. Since some resorption is expected, a primary goal of the procedure is to maximize graft take at the recipient site, which can be affected at any step in the fat grafting process.
Modern-day AFG is both patient- and surgeon-specific and involves identifying the optimal donor site with excess adipose tissue, harvesting fat through liposuction, processing that lipoaspirate into an injectable form, and delivering the graft in small aliquots into the recipient site. 6 Since Neuber's first observations with fat grafting—“grafts larger than an almond would not give good results”—fat grafting has become more sophisticated. 1 One particular area of emphasis has been the processing step, which is performed to reduce contaminants within the lipoaspirate while preserving adipocytes and other progenitor cells present in the graft that are fragile and highly sensitive to trauma and ischemia. 4 While donor site, cannula type, liposuction method, and injection technique may all contribute to the viability of grafted fat, these are beyond the scope of this article and will not be expounded upon.
The Scientific Basis of Fat Grafting
During fat processing, the harvested fat undergoes a process of eliminating fluid, blood, cell fragments, and oil. 7 By eliminating these agents, the remaining fat is optimized for injection and subsequent fat graft take. Studies have shown that isolated fat graft, also referred to as stromal–vascular fraction, contains a milieu of cells including endothelial cells, endothelial progenitor cells, preadipocytes, fibroblasts, vascular smooth muscle cells, and, most importantly, adipose-derived stem/stromal cells (ASCs). 8 ASCs are a subset of mesenchymal stromal/stem cells, which are multipotent cells that have the ability to differentiate into multiple lineages including adipocytes, osteoblasts, chondrocytes, and myocytes among others. 8
ASCs are believed to improve fat graft take by improving revascularization of the implanted fat. Once transferred, a fat graft can be separated into three different areas: (1) surviving zone of peripheral adipocytes, (2) regenerating zone of ASCs, which replace dying adipocytes, and (3) necrotic zone of dead adipocytes and ASCs, which are replaced by connective tissue or scar. 9 Thus, once grafted, adipose tissue demonstrates a dynamic, remodeling process, which is optimized by ASCs. Fat graft processing after harvest should aim to maximize the integrity of the adipocytes and ASCs. Currently, the various techniques of fat processing after fat harvesting include gravity separation (also referred to as decanting or sedimentation), centrifugation, cotton gauze rolling, and washing and filtration systems. 10
Fat Graft Processing Techniques
Centrifugation
In 1987, Coleman introduced a new technique in the liposuction procedure to handle fat processing for reinjection. Still commonly used today, this technique entails the separation of lipoaspirate components through centrifugation at high speeds ( Fig. 1 ). The general steps for the Coleman technique are considered the gold standard for centrifugation fat processing, though more recent literature has recommended alterations to the specific centrifugation settings. 11 Historically, the Coleman technique consists of loading 10-mL syringes with lipoaspirate, centrifugation at 3,000 rpm for 3 minutes, draining the blood and tumescent fraction from the bottom layer, and decanting and wicking the oil with a cotton pad for 3 minutes. 12 13 Coleman was against fat washing and exposing fat to air. 13
Fig. 1.
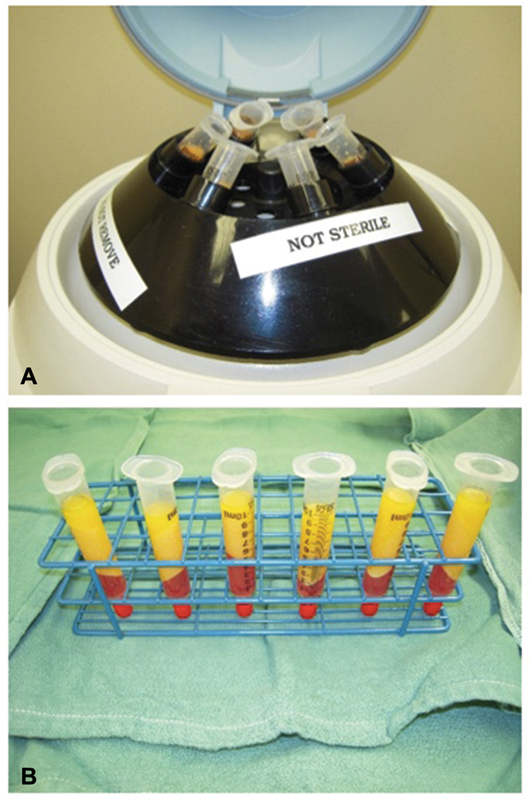
( A ) Centrifugation system with syringes set up. ( B ) After centrifugation, the resulting lipoaspirate has separate layers, with an oil layer on top that is wicked out, a blood and cell pellet layer at the bottom that is discarded, and variable density fat in the middle that is used for grafting.
The advantage of centrifugation is an increased concentration of progenitor cells in the processed fat. 12 Allen et al showed that greater percentages of highest density fractions of lipoaspirate persist over time compared with the lowest density fractions. 14 These concentrated lipoaspirates are believed to result in increased fat survival and slower reabsorption rates through a vasculogenic mechanism with increased progenitor cells and vasculogenic mediators. 14 15 Despite the efficaciousness of centrifugation in concentrating cells, there is a concern for potential damage to the adipocytes and ASCs from centrifugal forces, diminishing the concentration of each cell type and theoretically leading to poorer outcomes of fat graft. 16 17
There are multiple studies that report different centrifugation settings ( Table 1 ). 13 18 19 20 21 22 23 Some studies propose that higher centrifugal forces result in damage to fat cells with low cell viability, while very low centrifugal forces show an effect no different than simple fat decanting. 18 21 23 Interestingly, one study showed that despite the increase in peripheral damage, the number of viable cells were the same in the 500- and 1,300-rpm groups in vitro; the in vivo results favored 1,300 rpm, showing no evidence of reabsorption at 12 months. 18 Other studies have similarly shown no effect of centrifugation on adipocyte viability; Pulsfort et al demonstrated no significant histological alterations in the viability of differently centrifuged adipocytes and no apoptotic changes. 6 19 Comparing such studies is difficult due to the lack of standardization of the centrifugal force and duration of speed. Furthermore, some settings were reported in revolutions per minute, whereas other settings were reported as g-force; it was not always possible to convert between these two units, as different centrifugation machines could have different radius lengths, which would change the result of the calculation.
Table 1. Various centrifugation settings.
| Author | Rpm or g | Duration | Outcome |
|---|---|---|---|
| Coleman 13 | 3,000 rpm | 3 min | Lipoaspirate clinically viable |
| Rigotti et al 22 | 2,700 rpm | 15 min | Intact adipocytes very rare |
| Kurita et al 23 |
400
g
700 g 1,200 g 3,000 g 4,200 g |
3 min | Centrifugation at more than 3,000 g significantly damaged ASCs |
| Ferraro et al 18 | Decantation 500 rpm 1,300 rpm 3,000 rpm |
10 min 10 min 5 min 3 min |
Decantation and 500 rpm similar; greater peripheral destruction of adipocytes at 1,300 and 3,000 rpm |
| Pulsfort et al 19 | 1,000 rpm 1,500 rpm 3,000 rpm 5,000 rpm 7,500 rpm 10,000 rpm 15,000 rpm |
5 min | Centrifugation acceleration has no effect on adipocyte viability |
| Hoareau et al 21 |
100
g
400 g 900 g (3,000 rpm) 1,800 g (6,000 rpm) |
1 s, 1 min 1 min 1 min, 3 min 10 min |
Strong centrifugation (900 g , 1800 g ) is deleterious for adipose tissue compared with low centrifugation (100 g , 400 g ) |
| Asilian et al 20 | 3,400 rpm | 1 min | No difference in clinical outcome when compared with filtration/washing |
Abbreviations: ASCs, adipose-derived stem/stromal cells; g , g-force; rpm, revolutions per minute.
Cotton Gauze Rolling
Cotton gauze rolling is another commonly used method of isolating harvested fat graft, with Telfa (Medtronic) being the most popular choice of cotton gauze. Alternatives to Telfa include blue surgical towels or 4 × 4 gauze pads to absorb the undesired oil and aqueous components of the lipoaspirate. In this technique, the harvested fat is placed on top of the gauze. The back of a forceps, scalpel, or tongue depressor is used to roll the fat back and forth over the gauze. The excess tumescent and oil is absorbed in the gauze, leaving the cellular components of the fat graft behind. The harvested fat becomes more “gold” in color as the blood and other components of lipoaspirate are removed ( Fig. 2 ).
Fig. 2.
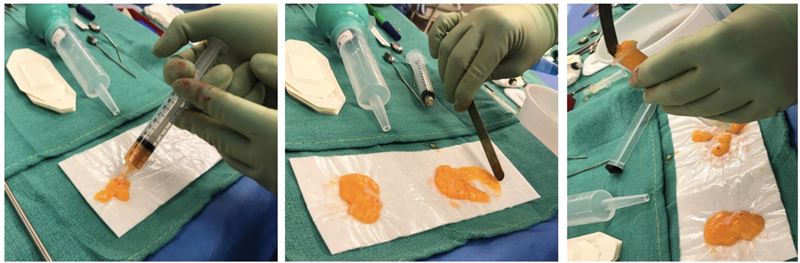
( Left ) Placing the harvested fat onto Telfa. ( Middle ) Removing debris by rolling the harvested fat onto cotton gauze. ( Right ) Placing the processed fat into a syringe for fat injection.
This method has been reported to take approximately 2 to 4 minutes, which is comparable to the time required for centrifugation. 12 Benefits of cotton gauze rolling include low cost and little trauma to the fat particles. Though previously cited as being comparable in efficiency with the time it takes to centrifuge fat, cotton gauze rolling would presumably be more time-consuming for larger quantities of fat. Similarly, cotton gauze rolling is better suited for situations in which the surgeon has an assistant who can reliably prepare the fat using cotton gauze rolling. This allows maximum efficiency as the surgeon harvests fat while his assistant prepares the harvested lipoaspirate.
Gravity Separation, Decantation, and Sedimentation
Gravity separation, decantation, and sedimentation refer to the process of allowing the lipoaspirate to settle into its three phases (oil, fat, and aqueous) with time. 24 The oil and aqueous layers are then discarded while the fat layer is extracted for injection. Commercial devices such as AquaVage (MD Resource Corp.) and Red Head (Miami Fat Supply Inc.) ( Fig. 3 ) allow for the collection and gravity separation of lipoaspirate within a sterile, closed system ( Table 2 ). There is a tube at the bottom of the container that can remove the bottommost aqueous phase of the lipoaspirate. Once the aqueous phase is completely removed, harvested fat remains with oil as the top layer. The fat layer can then be extracted with syringes, stopping when the oil layer is reached. Critics of this method state that the harvested fat may still be very “wet” if the proper amount of time has not passed for sufficient separation to occur. This results in falsely higher injection volumes of fat that is diluted by the undesired components of lipoaspirate. Benefits of these commercial devices include ease of use and single-use containers, which streamline the harvesting, processing, injecting, and cleaning process.
Fig. 3.
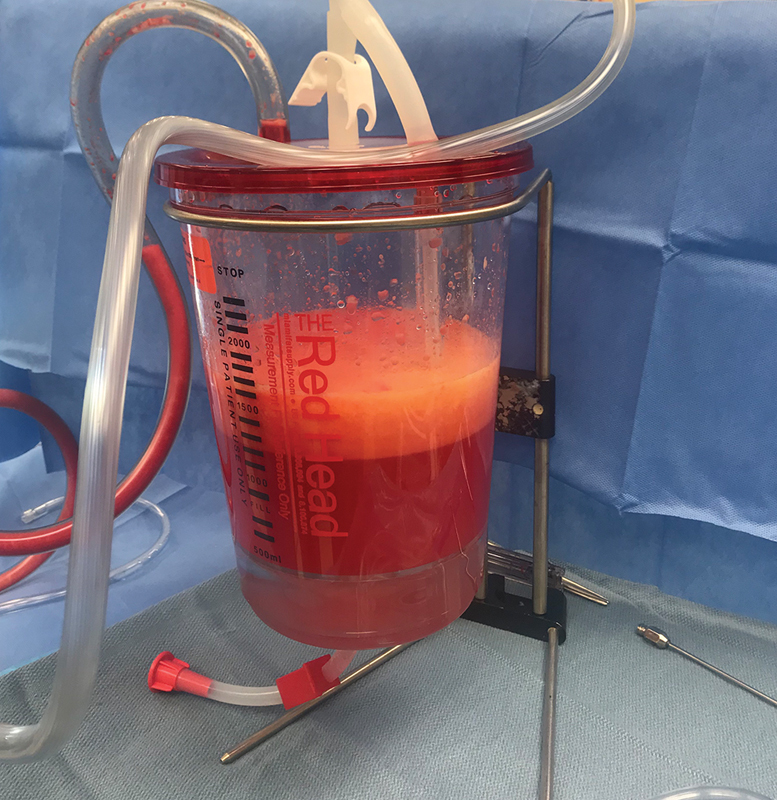
Harvesting fat graft using the Red Head system. The top layer contains variable density fat, whereas the bottom aqueous layer (containing tumescent solution and blood) is drained.
Table 2. Commercial fat graft processing products.
| Product | Company | Mechanism |
|---|---|---|
| Puregraft | Cytori Therapeutics Inc., San Diego, CA | Passive filtration combined with washing and/or centrifugation |
| REVOLVE | LifeCell Corp., Branchburg, NJ | Active filtration combined with washing and/or centrifugation |
| Tissu-Trans Filtron | Tulip Medical Products, San Diego, CA | An inline lipoaspirate filtration canister with 500- or 800-μm pores |
| LipiVage | Genesis Biosystems Inc., Lewisville, TX | Syringes allow harvest, processing, thorough filtration, and injecting |
| Red Head | Miami Fat Supply Inc., Groveland, FL | Large-volume closed-system harvesting canister; fat processed with washing and gravity |
| AquaVage | MD Resource Corp., Hayward, CA | Large-volume closed-system harvesting canister; fat processed with washing and gravity |
Washing and Filtration
Lipoaspirate can be prepared through washing and/or filtration, which is usually performed through a closed system. Washing and filtration are not mutually exclusive and can be performed together or individually. Washing is generally performed multiple times with normal saline or lactated Ringer's solution (LR), whereas filtration occurs across membranes of various pore sizes, depending on the product used. As with the previously described techniques, the goal of washing and/or filtration is to eliminate contaminants such as oil, debris, and nonviable components while obtaining the highest concentration of viable ASCs and adipocytes possible. Some hypothesize that filtration is less traumatic compared with centrifugation and better able to remove free lipid and undesired cellular content from the fat graft. 4 One study by Condé-Green et al showed that washing preserved a greater number of ASCs when compared with decanting and centrifugation. 25 Washing and filtration may be particularly effective for larger volumes of fat for which centrifugation would not be as practical. 4
Puregraft (Cytori Therapeutics Inc.) and REVOLVE (LifeCell Corp.) are two commercially available, closed-system processors that combine filtration and washing for fat graft processing ( Fig. 4 ). The Puregraft system comes in a rectangular dual filtration bag (50, 250, and 850 mL) with multiple afferent and efferent ports. 26 The manufacturer's recommended LR wash volume as well as drain time increase as harvested lipoaspirate volume increases. The fat is harvested directly into the bag and washed with LR. The bag is then inverted to allow all corners of the bag to be infiltrated; drain fluid is then egressed through a pinch clamp. 26 Approximately 250 mL of lipoaspirate can be harvested and processed within 15 minutes.
Fig. 4.
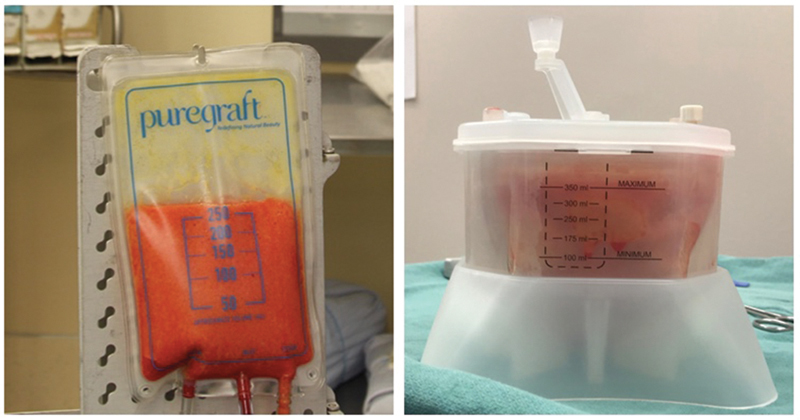
Fat graft washing and filtration using the Puregraft ( left ) and REVOLVE ( right ) systems.
Similarly, REVOLVE is an inline fat-processing system in which lipoaspirate is harvested directly into a canister device rather than a drain bag. 27 The REVOLVE device consists of an outer canister and an inner filter basket (200-μm pores) that allows fat to be separated from the tumescent fluid immediately. When 300 mL of fat is collected in the inner filter, 400 mL of LR at 37°C is added, and the fat is washed for 15 ± 5 seconds. The REVOLVE device contains rotating paddles within the filter basket, which gently sifts the tissue and ensures that the fat is thoroughly washed. The fluid is then vacuum-aspirated for 60 ± 5 seconds. The wash is then repeated as necessary. Total processing time for the REVOLVE system is approximately 10 minutes. 24 While the studies by Ansorge et al identified a significantly lower free oil and hematocrit levels in the REVOLVE specimens, there was no difference in fat retention rates clinically. 24 28
Tissu-Trans Filtron (Tulip Medical Products) is an inline lipoaspirate canister filtration-only system with 500- or 800-μm pores. This system has shown to result in a lower volume of retained graft compared with cotton gauze rolling. 28 29 For smaller volume fat harvesting and filtration processing, products such as LipiVage (Genesis Biosystems) exist, which contain a filtration system within the harvesting syringe, and the same syringe is used for harvesting, processing, and lipofilling.
Lastly, a less commonly used technique mentioned in the literature is filtering the fat with a sterile metal sieve. 20 30 This method involves allowing the aspirated fat to be left on the sieve for several minutes with gentle shaking of the sieve. Compared with cotton gauze rolling, there were significantly higher levels of inflammation in the metal sieve group; there was also a larger amount of oil left over that was not adequately separated from the desired lipoaspirate. 30 The authors concluded that the inflammation would likely lead to decreased viability of the grafted fat. 30 A separate study suggested that the results of facial fat grafting using fat processed through centrifugation and metal sieve filtration with normal saline washing were comparable, as assessed by patients and surgeons. 20
Conclusion
At this time, there is no one technique that shows superior fat graft take compared with the others. 2 Decanting appears to consistently result in a greater number of viable adipocytes as well as undesired cell components, which lead to less graft take compared with centrifugation and washing. 28 Other studies report mixed results; some claim that Telfa rolling yields the greatest amount of ASCs, whereas others support centrifugation as the optimal technique. 28 31 32 Commercial filtration–washing systems such as REVOLVE and Puregraft are the most appropriate choices for large volume fat grafting. 33 A large fraction of fat graft processing research consists of in vitro or nonhuman in vivo studies. There is a lack of standardization across all processing techniques, which makes comparison difficult and imprecise. Furthermore, even when the fat processing method is the same, the harvesting and injecting techniques and locations affect fat graft take and may vary. Additional randomized human studies are needed to determine the optimal lipoaspirate processing technique.
Funding Statement
Funding The authors have no financial interest in any of the products mentioned and have received no external support related to this study.
Footnotes
Conflicts of Interest None declared.
References
- 1.Billings E, Jr, May J W., Jr Historical review and present status of free fat graft autotransplantation in plastic and reconstructive surgery. Plast Reconstr Surg. 1989;83(02):368–381. doi: 10.1097/00006534-198902000-00033. [DOI] [PubMed] [Google Scholar]
- 2.Gir P, Brown S A, Oni G, Kashefi N, Mojallal A, Rohrich R J. Fat grafting: evidence-based review on autologous fat harvesting, processing, reinjection, and storage. Plast Reconstr Surg. 2012;130(01):249–258. doi: 10.1097/PRS.0b013e318254b4d3. [DOI] [PubMed] [Google Scholar]
- 3.Oranges C M, Striebel J, Tremp M, Madduri S, Kalbermatten D F, Schaefer D J. The impact of recipient site external expansion in fat grafting surgical outcomes. Plast Reconstr Surg Glob Open. 2018;6(02):e1649. doi: 10.1097/GOX.0000000000001649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bellini E, Grieco M P, Raposio E. The science behind autologous fat grafting. Ann Med Surg (Lond) 2017;24:65–73. doi: 10.1016/j.amsu.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rose J G, Jr, Lucarelli M J, Lemke B N et al. Histologic comparison of autologous fat processing methods. Ophthal Plast Reconstr Surg. 2006;22(03):195–200. doi: 10.1097/01.iop.0000217710.09941.10. [DOI] [PubMed] [Google Scholar]
- 6.Rohrich R J, Sorokin E S, Brown S A.In search of improved fat transfer viability: a quantitative analysis of the role of centrifugation and harvest site Plast Reconstr Surg 200411301391–395., discussion 396–397 [DOI] [PubMed] [Google Scholar]
- 7.Gutowski K A; ASPS Fat Graft Task Force.Current applications and safety of autologous fat grafts: a report of the ASPS fat graft task force Plast Reconstr Surg 200912401272–280. [DOI] [PubMed] [Google Scholar]
- 8.Moustaki M, Papadopoulos O, Verikokos C et al. Application of adipose-derived stromal cells in fat grafting: basic science and literature review. Exp Ther Med. 2017;14(03):2415–2423. doi: 10.3892/etm.2017.4811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eto H, Kato H, Suga H et al. The fate of adipocytes after nonvascularized fat grafting: evidence of early death and replacement of adipocytes. Plast Reconstr Surg. 2012;129(05):1081–1092. doi: 10.1097/PRS.0b013e31824a2b19. [DOI] [PubMed] [Google Scholar]
- 10.Gabriel A, Champaneria M C, Maxwell G P. Fat grafting and breast reconstruction: tips for ensuring predictability. Gland Surg. 2015;4(03):232–243. doi: 10.3978/j.issn.2227-684X.2015.04.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simonacci F, Bertozzi N, Grieco M P, Grignaffini E, Raposio E. Procedure, applications, and outcomes of autologous fat grafting. Ann Med Surg (Lond) 2017;20:49–60. doi: 10.1016/j.amsu.2017.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Canizares O, Jr, Thomson J E, Allen R J, Jr et al. The effect of processing technique on fat graft survival. Plast Reconstr Surg. 2017;140(05):933–943. doi: 10.1097/PRS.0000000000003812. [DOI] [PubMed] [Google Scholar]
- 13.Coleman S R.Structural fat grafting Aesthet Surg J 19981805386–388., 388 [DOI] [PubMed] [Google Scholar]
- 14.Allen R J, Jr, Canizares O, Jr, Scharf C et al. Grading lipoaspirate: is there an optimal density for fat grafting? Plast Reconstr Surg. 2013;131(01):38–45. doi: 10.1097/PRS.0b013e3182729cc6. [DOI] [PubMed] [Google Scholar]
- 15.Sarfati I, van la Parra R FD, Terem-Rapoport C A, Benyahi D, Nos C, Clough K B. A prospective randomized study comparing centrifugation and sedimentation for fat grafting in breast reconstruction. J Plast Reconstr Aesthet Surg. 2017;70(09):1218–1228. doi: 10.1016/j.bjps.2017.06.010. [DOI] [PubMed] [Google Scholar]
- 16.Ross R J, Shayan R, Mutimer K L, Ashton M W. Autologous fat grafting: current state of the art and critical review. Ann Plast Surg. 2014;73(03):352–357. doi: 10.1097/SAP.0b013e31827aeb51. [DOI] [PubMed] [Google Scholar]
- 17.Zielins E R, Brett E A, Longaker M T, Wan D C. Autologous fat grafting: the science behind the surgery. Aesthet Surg J. 2016;36(04):488–496. doi: 10.1093/asj/sjw004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferraro G A, De Francesco F, Tirino V et al. Effects of a new centrifugation method on adipose cell viability for autologous fat grafting. Aesthetic Plast Surg. 2011;35(03):341–348. doi: 10.1007/s00266-010-9613-8. [DOI] [PubMed] [Google Scholar]
- 19.Pulsfort A K, Wolter T P, Pallua N. The effect of centrifugal forces on viability of adipocytes in centrifuged lipoaspirates. Ann Plast Surg. 2011;66(03):292–295. doi: 10.1097/SAP.0b013e3181c7140e. [DOI] [PubMed] [Google Scholar]
- 20.Asilian A, Siadat A H, Iraji R. Comparison of fat maintenance in the face with centrifuge versus filtered and washed fat. J Res Med Sci. 2014;19(06):556–561. [PMC free article] [PubMed] [Google Scholar]
- 21.Hoareau L, Bencharif K, Girard A C et al. Effect of centrifugation and washing on adipose graft viability: a new method to improve graft efficiency. J Plast Reconstr Aesthet Surg. 2013;66(05):712–719. doi: 10.1016/j.bjps.2012.12.033. [DOI] [PubMed] [Google Scholar]
- 22.Rigotti G, Marchi A, Galiè Met al. Clinical treatment of radiotherapy tissue damage by lipoaspirate transplant: a healing process mediated by adipose-derived adult stem cells Plast Reconstr Surg 2007119051409–1422., discussion 1423–1424 [DOI] [PubMed] [Google Scholar]
- 23.Kurita M, Matsumoto D, Shigeura Tet al. Influences of centrifugation on cells and tissues in liposuction aspirates: optimized centrifugation for lipotransfer and cell isolation Plast Reconstr Surg 2008121031033–1041., discussion 1042–1043 [DOI] [PubMed] [Google Scholar]
- 24.Ansorge H, Garza J R, McCormack M C et al. Autologous fat processing via the Revolve system: quality and quantity of fat retention evaluated in an animal model. Aesthet Surg J. 2014;34(03):438–447. doi: 10.1177/1090820X14524416. [DOI] [PubMed] [Google Scholar]
- 25.Condé-Green A, de Amorim N F, Pitanguy I. Influence of decantation, washing and centrifugation on adipocyte and mesenchymal stem cell content of aspirated adipose tissue: a comparative study. J Plast Reconstr Aesthet Surg. 2010;63(08):1375–1381. doi: 10.1016/j.bjps.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 26.Puregraft LLC.Instructions for Use: Puregraft 850 SystemAvailable at:http://www.puregraft.com/wp-content/uploads/600-014-01-B-IFU-Puregraft-850-US.pdf. Accessed September 2, 2019
- 27.LifeCell. REVOLVE Advanced Adipose System. Available at:http://www.healthcare21.eu/wp-content/uploads/2016/11/MLC4668-R1-EU_REVOLVE-Picture-Guide_rebranded.pdf. Accessed October 13, 2019
- 28.Cleveland E C, Albano N J, Hazen A. Roll, spin, wash, or filter? Processing of lipoaspirate for autologous fat grafting: an updated, evidence-based review of the literature. Plast Reconstr Surg. 2015;136(04):706–713. doi: 10.1097/PRS.0000000000001581. [DOI] [PubMed] [Google Scholar]
- 29.Fisher C, Grahovac T L, Schafer M E, Shippert R D, Marra K G, Rubin J P. Comparison of harvest and processing techniques for fat grafting and adipose stem cell isolation. Plast Reconstr Surg. 2013;132(02):351–361. doi: 10.1097/PRS.0b013e3182958796. [DOI] [PubMed] [Google Scholar]
- 30.Minn K W, Min K H, Chang H, Kim S, Heo E J. Effects of fat preparation methods on the viabilities of autologous fat grafts. Aesthetic Plast Surg. 2010;34(05):626–631. doi: 10.1007/s00266-010-9525-7. [DOI] [PubMed] [Google Scholar]
- 31.Ibatici A, Caviggioli F, Valeriano V et al. Comparison of cell number, viability, phenotypic profile, clonogenic, and proliferative potential of adipose-derived stem cell populations between centrifuged and noncentrifuged fat. Aesthetic Plast Surg. 2014;38(05):985–993. doi: 10.1007/s00266-014-0372-9. [DOI] [PubMed] [Google Scholar]
- 32.Pfaff M, Wu W, Zellner E, Steinbacher D M. Processing technique for lipofilling influences adipose-derived stem cell concentration and cell viability in lipoaspirate. Aesthetic Plast Surg. 2014;38(01):224–229. doi: 10.1007/s00266-013-0261-7. [DOI] [PubMed] [Google Scholar]
- 33.Hanson S E, Garvey P B, Chang E I, Reece G, Liu J, Butler C E. A prospective pilot study comparing rate of processing techniques in autologous fat grafting. Aesthet Surg J. 2019;39(03):331–337. doi: 10.1093/asj/sjy154. [DOI] [PMC free article] [PubMed] [Google Scholar]


