Abstract
Today, fat grafting has wide applicability across plastic surgery disciplines, including both aesthetic and reconstructive procedures. However, much controversy has surrounded adipose tissue transfer throughout the 20th century, necessitating extensive research to improve the fat grafting process and to better understand its associated complications and benefits. Initial concerns included the technical difficulties of properly handling and processing adipose to ensure adequate outcomes. As these issues were addressed, more modern concerns were raised by the U.S Food and Drug Administration and the general scientific community regarding the oncological potential of adipose tissue and its potential interference with breast cancer screenings. Today, many formalized clinical studies have evidenced the safety of fat grafting, allowing the procedure to gain widespread popularity and opening avenues for future applications.
Keywords: fat grafting, autologous adipose tissue transfer, safety, regulations
The use of fat grafting can be first dated to 1893, when German surgeon Gustav Adolf Neuber performed an autologous adipose tissue transfer from the arm to the orbit to improve the cosmesis of a postinfectious scar. 1 However, over the next two decades, paraffin injection would overshadow adipose transfer as the primary technique to correct a variety of aesthetic concerns due to its apparent superiority stemming from its high melting point, ability to remain inert, and relative ease of delivery. 2 With increased paraffin use, though, several complications arose, most notably tissue penetration, leading to paraffinomas that facilitated infection and were linked to pulmonary emboli. 3 These complications led to a shift away from paraffin use and a reexploration of fat grafting in the first half of the 20th century. At this time, autologous fat transfer began to be used in both aesthetic and reconstructive settings in the face, breast, abdomen, and hands, especially to improve the appearance of traumatic injuries sustained during the world wars. 4 5 6 7
However, more widespread usage of adipose tissue transfer highlighted its shortcomings, namely unpredictable reabsorption rates and the formation of fibroses and oily cysts. In 1987, a position paper released by the American Society of Plastic and Reconstructive Surgeons (ASPRS) Ad-Hoc Committee on New Procedures unequivocally condemned the use of autologous fat injection in breast augmentation, as the committee was apprehensive that graft degeneration and necrosis could cause otherwise detectable breast lesions to go undiscovered. 8 Once again, fat grafting fell out of favor. Then, in the 1990s, surgeons Chajchir and Coleman developed standardized techniques to stabilize the adipocyte, such as rinsing and purifying the lipoaspirate during fat extraction and processing, improving wound bed vascularization, and minimizing trauma during graft injection. 9 10 11 12 Such methods consequently reduced complication rates and renewed interest in fat grafting, allowing the procedure to regain popularity and once again be implemented in multiple areas across plastic surgery.
As the technique of fat grafting became more refined, further research was conducted to better understand the cellular basis of adipose in an effort to characterize its optimal use. In 2002, Zuk et al published a seminal article identifying adipose tissue as a dense source of mesenchymal stem cells, explaining its possible role in regenerative medicine and opening new doors for adipose research and use in stem cell therapy. 13 Further insight into adipose-derived stem cells (ADSCs) found that the majority of stem cells were found in the stromal vascular fraction (SVF) of a traditional lipoaspirate, distinguishing standard autologous fat grafting from “stem-cell enhanced” fat grafting. 14 15 While exciting, the discovery of this association between fat grafting and stem-cell mediated cell proliferation also prompted hesitation in the plastic surgery community. Concerns were raised surrounding the oncological potential of adipose, not to mention the still-present worry that fat grafting could interfere with breast cancer screenings. It became evident that without further understanding of the basic science and clinical principles behind adipose transfer, plastic surgery organizations such as the American Society of Plastic Surgeons (ASPS) could not establish formal guidelines and protocols for its applications.
Over the next decade, significant research was performed to appreciate the clinical relevance of fat grafting in an effort to best identify appropriate target patient populations, optimize surgical techniques, and obtain accurate measures of outcomes and complication rates to better inform patients. These efforts culminated in a seminal publication by the ASPS Fat Graft Task Force in 2009 stating that the available literature at the time supported that there was no association between fat grafting and higher rates of malignancy and that the risk of interference with breast cancer detection was nonsignificant. The ASPS publication also acknowledged, however, that there was still a paucity of studies in this field and demanded further clinical investigations to validate their preliminary findings. 16 Fig. 1 summarizes important historical fat grafting milestones leading to current practice.
Fig. 1.
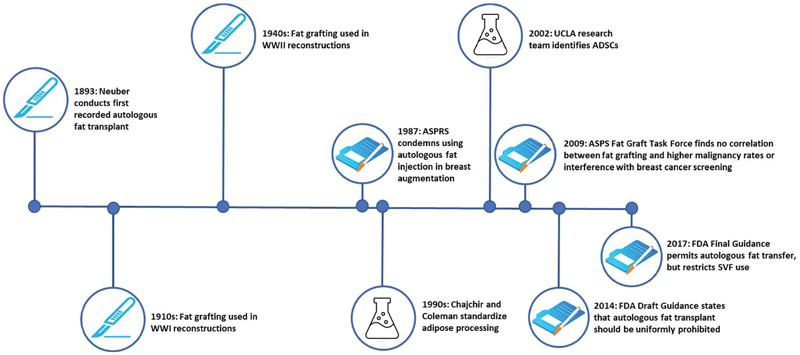
History and timeline of key fat grafting applications and regulations. ADSCs, adipose-derived stem cells; ASPRS, American Society of Plastic and Reconstructive Surgeons; ASPS, American Society of Plastic Surgeons; FDA, U.S. Food and Drug Administration; SVF, stromal vascular fraction; UCLA, University of California at Los Angeles; WWI, World War I; WWII, World War II.
The ASPS statement on fat grafting provided the foundation for this procedure to incorporate itself into standard plastic surgery practice. While the statement did not explicitly recommend adipose transfer, it popularized the concept that problems were more likely to stem from the surgeon's technique and experience rather than from poorly understood biological mechanisms. Today, as fat grafts have been shown to have the ability to be harvested from several readily available autologous donor sites and as technological advances have allowed for grafting to be performed in outpatient settings, the procedure has integrated itself as a mainstay practice in plastic surgery.
Main Concerns
Table 1 summarizes the main fat grafting concerns and the key publications that addressed them.
Table 1. Summary of main fat grafting concerns and important contributing publications.
| Major concerns | Important papers | Publishing journal | Year Published |
|---|---|---|---|
| Processing, delivery, and storage of fat grafts | Chajchir and Benzaquen 9 | Plastic and Reconstructive Surgery | 1989 |
|
Coleman
11
Butterwick et al 22 |
Aesthetic Plastic Surgery
Dermatologic Surgery |
1995 2006 |
|
| Khouri and Khouri 21 | Plastic and Reconstructive Surgery | 2017 | |
| Krastev et al 26 | JAMA Facial Plastic Surgery | 2018 | |
| Fat transfer in facial surgery | Krastev et al 26 | JAMA Facial Plastic Surgery | 2018 |
| Oncological potential of ADSCs | ASPRS Ad-Hoc Committee on New Procedures 8 | Plastic Surgical Nursing | 1987 |
| Zuk et al 13 | Molecular Biology of the Cell | 2002 | |
| Gutowski et al 16 | Plastic and Reconstructive Surgery | 2009 | |
| Rigotti et al 33 | Aesthetic Plastic Surgery | 2010 | |
| Kronowitz et al 34 | Plastic and Reconstructive Surgery | 2016 | |
| Interference with cancer screenings | ASPRS Ad-Hoc Committee on New Procedures 8 | Plastic Surgical Nursing | 1987 |
| Kneeshaw et al 38 | Breast | 2006 | |
| Gutowski et al 16 | Plastic and Reconstructive Surgery | 2009 | |
| FDA perspective | Zuk et al 13 | Molecular Biology of the Cell | 2002 |
| U.S. Department of Health and Human Services; U.S. Food and Drug Administration 41 | – | 2014 | |
| Johnson et al 42 | Aesthetic Plastic Surgery | 2017 | |
| U.S. Department of Health and Human Services; U.S. Food and Drug Administration 43 44 | – | 2017 |
Abbreviations: ADSCs, adipose-derived stem cells; ASPRS, American Society of Plastic and Reconstructive Surgeons; FDA, U.S. Food and Drug Administration; JAMA, Journal of the American Medical Association.
Processing, Delivery, and Storage of Fat Grafts
Many of the original complications associated with fat grafting, such as unpredictable reabsorption and oil cyst formation, were addressed with the advent of standardized adipose extraction, purification, and injection methods. 9 10 11 12 Nowadays, reports of poor aesthetic results stemming from graft volume loss are relatively uncommon, and experienced surgeons can decrease the risk of such complications with intraoperative overcorrection. 17 18 Additionally, various surgical complications have been reported in the literature, including postoperative infection, formation of seroma or hematoma, and potentially fatal fat embolism. 19 20 21 However, these reports are rare and express that the severity and rate of complications associated with fat grafting are more correlated with the proficiency and technique of the surgeon rather than the procedure itself. 16
The efficacy of fresh adipose as opposed to previously stored or frozen adipose has also been explored. Butterwick et al found that using frozen lipoaspirate resulted in similar, if not better, aesthetic and longevity outcomes in 10 patients who underwent fat grafting for hand rejuvenation. 22 In addition, experimental studies focusing on cell viability have shown that frozen adipose must be stored using a controlled freezing approach and a cryoprotective agent to optimize graft outcomes. 23 24 25
Application of Fat Transfer in Facial Surgery
Particular attention has been paid to the use of autologous fat transfer in facial reconstructive surgery given fragmented evidence outlining its effectiveness and outcomes. Many plastic surgeons had been hesitant to rely on fat grafting as the corrective technique of choice for facial deformity due to the fear that the high visibility and aesthetic importance of the face would magnify any imperfections from the fat grafting process, in particular loss of graft volume over time. In an effort to simplify interpretation of the existing body of literature on fat grafting in facial reconstructive surgery, Krastev et al in 2018 conducted a meta-analysis examining 51 studies comprising 1,533 patients. Patient satisfaction rates were reported to be 91.1%, and surgeon satisfaction rates were reported to be 88.6%, indicating that the modern use of fat grafting in facial reconstruction yields largely successful results for all involved. 26
Oncological Potential of Adipose-Derived Stem Cells
In the recent past, the main concern regarding fat grafting has revolved around its use in postmastectomy patients with a history of breast cancer. Adipose tissue has been shown to consist of large concentrations of multipotent ADSCs, which, in the presence of cytokines and growth factors, implicates adipose tissue in regenerative and angiogenic roles. 27 28 Though it is adipose tissue's regenerative qualities that make it valuable in reconstructive procedures—especially in irradiated tissues with poor vascular beds—neoplastic processes also depend on these regenerative mechanisms. 21 29 As such, there exists a concern that adipose tissue may provide residual malignant cells a favorable environment for reproliferation, potentially leading to an increased risk of cancer recurrence in breast cancer patients.
Basic science studies have experimentally noted that ADSCs increase the migration capacity and the growth of breast cancer cells. Charvet et al discovered that 10 times as many breast cancer cells grown in an ADSC coculture underwent significant cell migration as opposed to the cancer cells grown in a cancer cell culture only. 30 Similarly, Orbay et al found that in mice studies, the injection of breast cancer cells with ADSCs and/or fat graft increased migration and growth rate of neoplastic cells significantly. 31
With this concern in mind, many surgeons remain hesitant to perform autologous fat grafting, citing the lack of evidence and/or the increased perceived oncological risk of the procedure. 32 Though this concern was justified in animal models, several clinical studies have established a lack of associated oncological risk with fat grafting in humans. Epitomizing these studies is Rigotti et al's single-center case–control study of 137 patients with a long-term follow-up of 7.6 years. 33 After comparative analysis and relapse-free survival probability estimations, the authors concluded that fat grafting in breast reconstruction had no significant effect on the recurrence of breast cancer in postmastectomy patients. Kronowitz et al further validated the safety of fat grafting, evidencing no increase in cancer recurrence across local or systemic levels in a single-center matched controlled study of 1,024 breasts. 34
Interference with Breast Cancer Screening
The degeneration of transferred fat tissue and subsequent scarring and calcification also posed a barrier for fat grafting to become widely accepted. The 1987 ASPRS position statement deploring autologous fat transfer for breast augmentation stunted scientific discussion into the field, and, for a time, adipose tissue transfer to the breast was considered a taboo procedure worthy of a malpractice suit. 35 It is important to note that although the original ASPRS statement did not cite significantly relevant studies at the time, the basis of their concern was rooted in the biological mechanisms of adipose degeneration. It has been well-described across the literature that fat necrosis can occur in autologous fat transfer, resulting in benign inflammatory processes that lead to microcalcification of adipose that has the potential to be either mistaken for or mask breast cancer. 36 This concern, in part, is reflective of the radiological technology available in the late 20th century; today, advances have been made such that radiologists can consistently discriminate between necrotic and neoplastic calcifications. 37 38 39 40
The Perspective of the U.S. Food and Drug Administration
With the discovery that adipose tissue contained a dense population of mesenchymal stem cells, a largely unregulated marketplace erupted, offering unsubstantiated stem cell therapies. Because existing guidelines did not differentiate traditional fat grafting from cell-assisted lipotransfer procedures, this marketplace was allowed to exist under the umbrella of safety provided by established autologous fat grafting. In an attempt to regulate this inappropriate use of fat grafting, the U.S Food and Drug Administration (FDA) published a Draft Guidance in 2014 stating their position that the separation and reinjection of fractionated adipose tissue, especially to the breast, should be prohibited within the larger scope of regulating human cells, tissue, and cellular- and tissue-based products. 41 Though the development for evidence-based protocols regarding fat grafting is necessary, the FDA's overly broad assertion on its nonuse led to many logistical challenges for both physicians and patients by delaying treatment, increasing already high clinical costs, and creating hindersome legal navigation in coupling established adipose cell injection with the novelty and stigma of stem cell treatment. 42 Over the next 2 years, the ASPS contended with the FDA's position, citing it to be prohibitive for patients looking for an inexpensive and proven treatment modality that for years had been shown to be successful in terms of safety and outcomes.
After significant dispute, the FDA released its final guidance statement in November 2017, the positions of which are maintained to this day. To the satisfaction of plastic surgeons, the FDA shifted their stance on autologous fat grafts, reclassifying them as products allowed to be used without premarket approval. However, SVFs used in cell-assisted lipotransfer techniques became subject to greater scrutiny, noting that the malignancy risk associated with the higher concentration of stem cells in SVF warrants heightened regulation of their use. 14 43 44
Conclusion
The viability of fat grafting has been under almost constant scrutiny since its first use by Neuber over a century ago. While the concept itself has made great strides, there has been continued stigma around fat grafting stemming largely from unsubstantiated opinions and improper levels of examination. In an effort to bridge the knowledge gap around fat grafting, a substantial body of research has been conducted, demonstrating that fat grafting does not lead to a significantly different oncological potential in patients who receive the treatment, nor are these patients subject to missed or incorrect breast cancer diagnoses. Autologous fat transfer has now achieved widespread acceptance among plastic surgeons and, more importantly, has been shown to have successful and safe patient outcomes.
Funding Statement
Funding None.
Conflicts of Interest None declared.
Products/Devices/Drugs
None.
References
- 1.Neuber G. Ueber die Wiederanheilung vollständig vom Körper getrennter, die ganze Fettschicht enthaltender Hautstücke. Zbl F Chirurgie. 1893;30:16. [Google Scholar]
- 2.Mazzola R F, Mazzola I C. The fascinating history of fat grafting. J Craniofac Surg. 2013;24(04):1069–1071. doi: 10.1097/SCS.0b013e318292c447. [DOI] [PubMed] [Google Scholar]
- 3.Glicenstein J. Les premiers “fillers”, vaseline et paraffine. Du miracle à la catastrophe. Ann Chir Plast Esthet. 2007;52(02):157–161. doi: 10.1016/j.anplas.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 4.Lexer E. Free transplantation. Ann Surg. 1914;60(02):166–194. doi: 10.1097/00000658-191408000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller C C. Chicago, IL: Oak Press; 1926. Cannula implants and review of implantation technics in esthetic surgery. [Google Scholar]
- 6.Al-Hayder S, Gramkow C, Trojahn Kølle S-F. Use of autologous fat grafting for the correction of burn scar contracture in the hand: a case report. Case Reports Plast Surg Hand Surg. 2017;4(01):81–83. doi: 10.1080/23320885.2017.1369883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klinger M, Lisa A, Klinger Fet al. Regenerative approach to scars, ulcers and related problems with fat grafting Clin Plast Surg 20154203345–352., viii [DOI] [PubMed] [Google Scholar]
- 8.No Authors.Report on autologous fat transplantation. ASPRS Ad-Hoc Committee on New Procedures, September 30, 1987 Plast Surg Nurs 1987704140–141. [PubMed] [Google Scholar]
- 9.Chajchir A, Benzaquen I.Fat-grafting injection for soft-tissue augmentation Plast Reconstr Surg 19898406921–934., discussion 935 [PubMed] [Google Scholar]
- 10.Coleman S R. The technique of periorbital lipoinfiltration. Oper Tech Plast Reconstr Surg. 1994;1(03):120–126. [Google Scholar]
- 11.Coleman S R. Long-term survival of fat transplants: controlled demonstrations. Aesthetic Plast Surg. 1995;19(05):421–425. doi: 10.1007/BF00453875. [DOI] [PubMed] [Google Scholar]
- 12.Coleman S R. Facial recontouring with lipostructure. Clin Plast Surg. 1997;24(02):347–367. [PubMed] [Google Scholar]
- 13.Zuk P A, Zhu M, Ashjian P et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13(12):4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rohrich R J, Wan D. Making sense of stem cells and fat grafting in plastic surgery: the hype, evidence, and evolving U.S. Food and Drug Administration regulations. Plast Reconstr Surg. 2019;143(02):417e–424e. doi: 10.1097/PRS.0000000000005207. [DOI] [PubMed] [Google Scholar]
- 15.Yoshimura K, Shigeura T, Matsumoto D et al. Characterization of freshly isolated and cultured cells derived from the fatty and fluid portions of liposuction aspirates. J Cell Physiol. 2006;208(01):64–76. doi: 10.1002/jcp.20636. [DOI] [PubMed] [Google Scholar]
- 16.Gutowski K A, Baker S B, Coleman S R et al. Current applications and safety of autologous fat grafts: a report of the ASPS Fat Graft Task Force. Plast Reconstr Surg. 2009;124(01):272–280. doi: 10.1097/PRS.0b013e3181a09506. [DOI] [PubMed] [Google Scholar]
- 17.Pierrefeu-Lagrange A C, Delay E, Guerin N, Chekaroua K, Delaporte T. Radiological evaluation of breasts reconstructed with lipomodeling [in French] Ann Chir Plast Esthet. 2006;51(01):18–28. doi: 10.1016/j.anplas.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 18.Pulagam S R, Poulton T, Mamounas E P. Long-term clinical and radiologic results with autologous fat transplantation for breast augmentation: case reports and review of the literature. Breast J. 2006;12(01):63–65. doi: 10.1111/j.1075-122X.2006.00188.x. [DOI] [PubMed] [Google Scholar]
- 19.Cohen O, Lam G, Karp N, Choi M. Determining the oncologic safety of autologous fat grafting as a reconstructive modality: an institutional review of breast cancer recurrence rates and surgical outcomes. Plast Reconstr Surg. 2017;140(03):382e–392e. doi: 10.1097/PRS.0000000000003576. [DOI] [PubMed] [Google Scholar]
- 20.Hang-Fu L, Marmolya G, Feiglin D H. Liposuction fat-fillant implant for breast augmentation and reconstruction. Aesthetic Plast Surg. 1995;19(05):427–437. doi: 10.1007/BF00453876. [DOI] [PubMed] [Google Scholar]
- 21.Khouri R K, Jr, Khouri R K. Current clinical applications of fat grafting. Plast Reconstr Surg. 2017;140(03):466e–486e. doi: 10.1097/PRS.0000000000003648. [DOI] [PubMed] [Google Scholar]
- 22.Butterwick K J, Bevin A A, Iyer S. Fat transplantation using fresh versus frozen fat: a side-by-side two-hand comparison pilot study. Dermatologic Surg. 2006;32(05):640–644. doi: 10.1111/j.1524-4725.2006.32135.x. [DOI] [PubMed] [Google Scholar]
- 23.Gir P, Brown S A, Oni G, Kashefi N, Mojallal A, Rohrich R J. Fat grafting: evidence-based review on autologous fat harvesting, processing, reinjection, and storage. Plast Reconstr Surg. 2012;130(01):249–258. doi: 10.1097/PRS.0b013e318254b4d3. [DOI] [PubMed] [Google Scholar]
- 24.Son D, Oh J, Choi T et al. Viability of fat cells over time after syringe suction lipectomy: the effects of cryopreservation. Ann Plast Surg. 2010;65(03):354–360. doi: 10.1097/SAP.0b013e3181bb49b8. [DOI] [PubMed] [Google Scholar]
- 25.Wolter T P, von Heimburg D, Stoffels I, Groeger A, Pallua N. Cryopreservation of mature human adipocytes: in vitro measurement of viability. Ann Plast Surg. 2005;55(04):408–413. doi: 10.1097/01.sap.0000181345.56084.7d. [DOI] [PubMed] [Google Scholar]
- 26.Krastev T K, Beugels J, Hommes J, Piatkowski A, Mathijssen I, van der Hulst R. Efficacy and safety of autologous fat transfer in facial reconstructive surgery: a systematic review and meta-analysis. JAMA Facial Plast Surg. 2018;20(05):351–360. doi: 10.1001/jamafacial.2018.0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosen E D, MacDougald O A. Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol. 2006;7(12):885–896. doi: 10.1038/nrm2066. [DOI] [PubMed] [Google Scholar]
- 28.Yoshimura K, Sato K, Matsumoto D. Berlin, Heidelberg: Springer-Verlag; 2010. Cell-assisted lipotransfer for breast augmentation: grafting of progenitor-enriched fat tissue. [Google Scholar]
- 29.Mojallal A, Saint-Cyr M, Garrido I. Autologous fat transfer: controversies and current indications for breast surgery. J Plast Reconstr Aesthetic Surg. 2009;62(05):708–710. doi: 10.1016/j.bjps.2009.01.037. [DOI] [PubMed] [Google Scholar]
- 30.Charvet H J, Orbay H, Harrison L, Devi K, Sahar D E. In vitro effects of adipose-derived stem cells on breast cancer cells harvested from the same patient. Ann Plast Surg. 2016;76 03:S241–S245. doi: 10.1097/SAP.0000000000000802. [DOI] [PubMed] [Google Scholar]
- 31.Orbay H, Hinchcliff K M, Charvet H J, Sahar D E. Fat graft safety after oncologic surgery: addressing the contradiction between in vitro and clinical studies. Plast Reconstr Surg. 2018;142(06):1489–1499. doi: 10.1097/PRS.0000000000004992. [DOI] [PubMed] [Google Scholar]
- 32.Kling R E, Mehrara B J, Pusic A L et al. Trends in autologous fat grafting to the breast: a national survey of the American Society of Plastic Surgeons. Plast Reconstr Surg. 2013;132(01):35–46. doi: 10.1097/PRS.0b013e318290fad1. [DOI] [PubMed] [Google Scholar]
- 33.Rigotti G, Marchi A, Stringhini P et al. Determining the oncological risk of autologous lipoaspirate grafting for post-mastectomy breast reconstruction. Aesthetic Plast Surg. 2010;34(04):475–480. doi: 10.1007/s00266-010-9481-2. [DOI] [PubMed] [Google Scholar]
- 34.Kronowitz S J, Mandujano C C, Liu J et al. Lipofilling of the breast does not increase the risk of recurrence of breast cancer: a matched controlled study. Plast Reconstr Surg. 2016;137(02):385–393. doi: 10.1097/01.prs.0000475741.32563.50. [DOI] [PubMed] [Google Scholar]
- 35.Coleman S R, Saboeiro A P.Fat grafting to the breast revisited: safety and efficacy Plast Reconstr Surg 200711903775–785., discussion 786–787 [DOI] [PubMed] [Google Scholar]
- 36.Hogge J P, Robinson R E, Magnant C M, Zuurbier R A. The mammographic spectrum of fat necrosis of the breast. Radiographics. 1995;15(06):1347–1356. doi: 10.1148/radiographics.15.6.8577961. [DOI] [PubMed] [Google Scholar]
- 37.Leibman A J, Styblo T M, Bostwick J., III Mammography of the postreconstruction breast. Plast Reconstr Surg. 1997;99(03):698–704. doi: 10.1097/00006534-199703000-00015. [DOI] [PubMed] [Google Scholar]
- 38.Kneeshaw P J, Lowry M, Manton D, Hubbard A, Drew P J, Turnbull L W. Differentiation of benign from malignant breast disease associated with screening detected microcalcifications using dynamic contrast enhanced magnetic resonance imaging. Breast. 2006;15(01):29–38. doi: 10.1016/j.breast.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 39.Yunus M, Ahmed N, Masroor I, Yaqoob J. Mammographic criteria for determining the diagnostic value of microcalcifications in the detection of early breast cancer. J Pak Med Assoc. 2004;54(01):24–29. [PubMed] [Google Scholar]
- 40.Chala L F, de Barros N, de Camargo Moraes P et al. Fat necrosis of the breast: mammographic, sonographic, computed tomography, and magnetic resonance imaging findings. Curr Probl Diagn Radiol. 2004;33(03):106–126. doi: 10.1067/j.cpradiol.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 41.U.S. Department of Health and Human Services; U.S. Food and Drug Administration.Human Cells, Tissues, and Cellular and Tissue-Based Products (HCT/Ps) from Adipose Tissue: Regulatory Considerations. Draft Guidance for IndustryAvailable at:https://www.ifats.org/assets/docs/01%20-%20Guidance%20-Draft%20Adipose%20Tissue.pdf. Accessed August 20, 2019
- 42.Johnson M L, Johnson L, Mahabir R C, Bernard R. Perspectives on the FDA draft guidances for use of adipose tissue. Aesthetic Surg J. 2017;37(05):622–625. doi: 10.1093/asj/sjx049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.U.S. Food and Drug Administration.Regulatory Considerations for Human Cells, Tissues, and Cellular and Tissue-Based Products: Minimal Manipulation and Homologous UseAvailable at:https://www.fda.gov/regulatory-information/search-fda-guidance-documents/regulatory-considerations-human-cells-tissues-and-cellular-and-tissue-based-products-minimal. Accessed August 20, 2019
- 44.U.S. Department of Health and Human Services; U.S. Food and Drug Administration.Same Surgical Procedure Exception under 21 CFR 1271.15(b): Questions and Answers Regarding the Scope of the ExceptionGuidance for Industry. Available at:https://www.fda.gov/media/89920/download. Accessed August 20, 2019


