Abstract
Context-specific signaling is a prevalent theme in cancer biology wherein individual molecules and pathways can have multiple or even opposite effects depending on the tumor type. TAK1 represents a particularly notable example of such signaling diversity in cancer progression. Originally discovered as a TGF-β-activated kinase, over the years it has been shown to respond to numerous other stimuli to phosphorylate a wide range of downstream targets and elicit distinct cellular responses across cell and tissue types. Here we present a comprehensive review of TAK1 signaling and provide important therapeutic perspectives related to its function in different cancers.
Keywords: TAK1 signal transduction, cancer biology
INTRODUCTION
Transforming growth factor-β activated kinase 1 (TAK1) belongs to a family of mitogen-activated protein three kinases (MAP3Ks) and is also referred to as MAP3K7 (1). But aside from mediating MAPK signaling, it can also phosphorylate numerous other downstream targets including IKKβ in NFκB activation (2–4), αTAT1 in microtubule acetylation (5), p62 in the maintenance of cellular redox homeostasis (6), and AMPK in metabolic regulation (7). Moreover, TAK1 modulates canonical forms of cytokine signal transduction by interacting with SMAD and STAT proteins (8, 9), and plays a central role in development by modulating Wnt signaling (10). Hence, TAK1 proves to have an integral role in signal transduction across multiple pathways and cellular processes.
There are also numerous activators of TAK1 including cytokines, pathogens, DNA-damage, hypoxia and osmotic stress. These varying conditions and stimuli signal through TAK1 to modulate the cell cycle, cell differentiation, immune responses, cell migration and redox homeostasis. Dysregulation of these pathways is often associated with malignancies and therefore TAK1 is considered a key therapeutic target in multiple tumor settings (4, 11–14).
A key challenge in the field is fully integrating how crucial changes in major signaling networks drive carcinogenesis and tumor progression. One recurring theme is that the same molecule can mediate opposing roles in tumor suppression and progression, depending on the cellular and environmental context, as well as stage of pathology. Instances here include TGFβ (15), NFκB (16), and MAPK (17) signaling, across different tumor types and pathological stages. Indeed, TAK1 provides a powerful model system to investigate such contextual signaling due to the large number of pathways and processes that it regulates, but also highlights the challenges faced in systems biology and cancer therapeutics. Here we review the current knowledge of TAK1 function and consider how it mediates cellular homeostasis and drives tumor progression.
REGULATION OF TAK1 FUNCTION
Composition of the TAK1 complex and activation
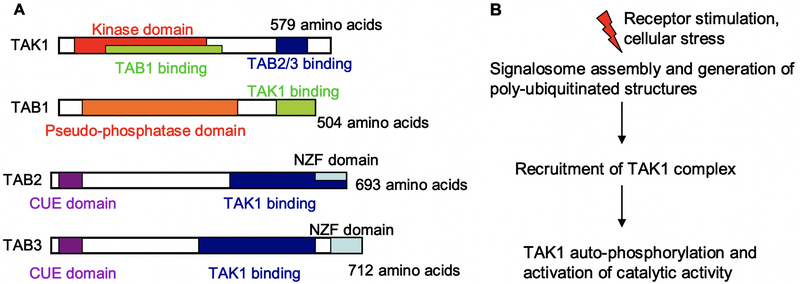
TAK1 activation requires TAK1-binding proteins (TAB1–3), where it forms an oligomeric complex with TAB1 and either TAB2 or TAB3 (18–22). To date, it remains to be clarified whether differential complex stoichiometries specify divergent functional responses. The TAK1-TAB1 interaction occurs in the kinase domain of TAK1, while TAB2 and TAB3 bind to a region near the carboxy terminus (Fig 1A).
Fig 1. TAK1 architecture and activation.
(A) Schematic representations of the different domains of the constituent molecules of the TAK1 complex, with amino acid length in humans. The kinase activity of TAK1 is regulated by binding interactions with TAB1 and TAB2/3. The latter molecules contain NZF and CUE domains, which bind poly-ubiquitinated (pUb) structures. (B) General outline of TAK1 activation. Receptor stiumuli and cell stress activate signaling pathways that generate pUb structures. The TAK1 complex is recruited to these structures through TAB2/3, resulting in the activation of TAK1 catalytic activity.
A defining feature of TAB2 and TAB3 are the presence of Npl4 zinc finger (NZF) binding domains, which bind lysine-63 (K63)-linked poly-ubiquitinylated (pUb) structures. Cellular stimuli elicit the generation of pUb chains, and binding of the TAK complex to these structures through TAB2/3 (Fig 1B). This binding event activates TAK1 catalytic activity, resulting in auto-phosphorylation and full enzymatic activation (23–25).
Also, TAB2 and TAB3 contain CUE domains (coupling of ubiquitin conjugation to endoplasmic reticulum degradation) which allow for binding pUb chains albeit with much lower affinities than NZF domains. To date, the structural details of the TAB-TAK interaction have been derived from both biochemical and crystal data, although much of the dynamic mechanisms underlying pUb-dependent TAK1 catalytic activation remain undefined. Similarly, another molecular aspect of interest in the field is to understand the structural and functional consequences of tandem TAB pUb-binding domains.
Of note however, is that TAK1 function is regulated by mechanisms in addition to ubiquitination. For instance, the O-linked GlcNAcylation of TAB1 regulates TAK1 function in osmotic shock and IL1 signaling (26), and the calcium-calmodulin dependent kinase CaMKII phosphorylates TAK1 in Wnt signaling (27). Moreover, constituents of the TAK1 complex are modified by a large number of post-translational modifications (PTMs), with intricate consequences on TAK1 activity (3). Hence an important line of investigation will be to understand how the multiple signaling networks that impinge upon TAK1 are integrated to elicit the myriad roles that the enzyme orchestrates.
TAK1 signal transduction
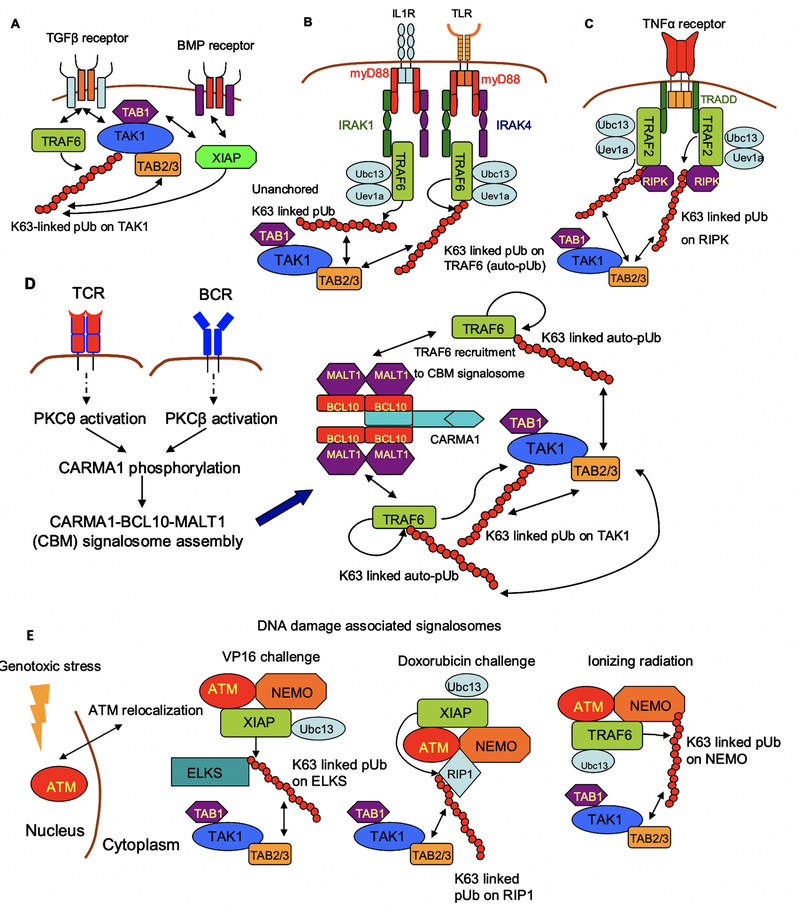
A striking feature of TAK1 activation pertains to the variety of signaling network architectures that regulate enzymatic function (Fig 2). For instance, TAK1 directly interacts with ALK5 TGFβ receptor, and receptor stimulation activates the ubiquitin conjugating system by recruiting the E3 ligase TRAF6 (28, 29) (Fig 2A). A similar mechanism is found in BMP (bone morphogenetic protein) signaling, where receptor-ligand interactions recruit the E3 ligase XIAP to the receptor, culminating in the generation of K63-linked pUb and TAK1 activation (30) (Fig 2A). Hence TAK1 activation upon TGFβ and BMP stimulation occurs through membrane proximal mechanisms.
Fig 2. Mechanisms of TAK1 activation in response to different forms of stimuli.
(A) Stimulation of TGFβ and BMP receptors activate TAK1 through membrane-proximal mechanisms, whereby the receptor directly interacts with the E3 ligases TRAF6 and XIAP, respectively. (B and C) Stimulation of IL1, TLR and TNFα receptors assemble signaling complexes through adaptor proteins. The adaptors in IL1 and TLR signaling are myD88 and IRAKs 1 and 4, while TRADD is the adaptor in TNFα signaling. These adaptors recruit the pUb-conjuagting system, comprised of TRAF6 or TRAF2, Ubc13 and Uev1A. (D) Stimulation of T and B cell receptors activate TAK1 through PKC activation, and the assembly of the CBM Signalosome. This Signalosome provides the scaffold for TRAF6 mediated pUb, and TAK1 activation. (E) TAK1 activation in response to genotoxic stress relies on the cytoplasmic relocalization of the nuclear kinase ATM. A cytoplasmic ATM-NEMO complex assembles the pUb-conjugating system comprised of either TRAF6 or XIAP, depending on the type of cellular stress.
An added layer of complexity mediates TAK1 activation in IL1, TLR and TNFα signaling (20, 25, 31). In these instances, receptor-stimulation recruits adaptor molecules, which in turn recruit the ubiquitin conjugating system. Signaling through TLRs and the IL1 receptor recruits myD88, which provides a platform for the recruitment of IRAK1 and IRAK4 (Fig 2B). The ubiquitin conjugation system is assembled on the nucleated receptor complex, critically relying on IRAK binding surfaces. The E3 ligase in both instances is TRAF6, which mediates the generation of K63-linked pUb. In the case of TLR signaling, TRAF6 is auto-ubiquitinated while in IL1 signaling TRAF6 ubiquitinates TAK1.
A similar design architecture is found in TNFα signal transduction, wherein receptor stimulation recruits the adaptor TRADD (Fig 2C). The ubiquitin conjugating system is assembled on the ligated receptor-TRADD scaffold. The E3 ubiquitin ligase varies according to the specific system, but TRAF2, TRAF5 and cIAP1/2 have been reported (14). The E3 ligase acts in conjunction with the ubiquitin conjugating molecules Ubc13 and Uev1A, to generate the K63-linked pUb scaffold.
A more elaborate signaling pathway elicits TAK1 activation upon T cell and B cell receptor stimulation (TCR and BCR, respectively) (Fig 2D). A crucial intermediary in both receptor signaling systems is the activation of protein kinase C (PKC) - PKCθ in T cells and PKCβ in B cells (32, 33). The activated kinase phosphorylates CARMA1 (also known as CARD11), thus exposing its CARD domain. Phosphorylated CARMA1 provides a platform for the formation of the CBM signalosome, comprised of CARMA1, BCL10 and MALT11. The ubiquitin conjugating system is assembled on the CBM signalosome, and the E3 ligase TRAF6 mediates the formation of the pUb scaffold.
A complementary signaling architecture arises in TAK1 activation upon genotoxic stress (Fig 2E). This pathway relies on the cytoplasmic relocalization of the nuclear kinase ATM (ataxia-telangiectasia mutated), and the formation of a cytoplasmic ATM-NEMO complex (34– 36). The ATM-NEMO complex assembles the ubiquitin conjugating system, as well as signalosomes that vary in stoichiometries and constituent molecules in a stimulus-specific manner.
In response to the DNA damaging agent VP16, the ATM-NEMO complex assembles the E3 ligase XIAP, alongside Ubc13. The pUb scaffold is generated on ELKS (glutamate, leucine, lysine, and serine-rich protein), leading to TAK1 activation (35). In response to ionizing radiation, the ATM-NEMO complex recruits TRAF6 with Ubc13, and the pUb chain is generated on NEMO. Finally, in response to doxorubicin and etoposide challenge, the ATM-NEMO complex recruits RIP1, and RIP is ubiquitinated by a XIAP-Ubc13 complex (36).
Hence TAK1 activation can occur at all layers of cellular signal transduction, spanning from the membrane proximal layer of receptor-ligand interactions, to cytoplasmic signalosome assembly. Enzymatic activation occurs in response to a variety of ligands, including cytokines and antigen receptor stimulation. Cellular stress in the form of DNA damage, hypoxia (37, 38), and osmotic shock (39, 40) also activate TAK1, and the molecular mechanisms in these latter instances are active areas of investigation. Of further intrigue is the observation that the TAK1 complex is expressed in both the nucleus and cytoplasm of neutrophils, and the nuclear fraction mediates NFκB activation (41).
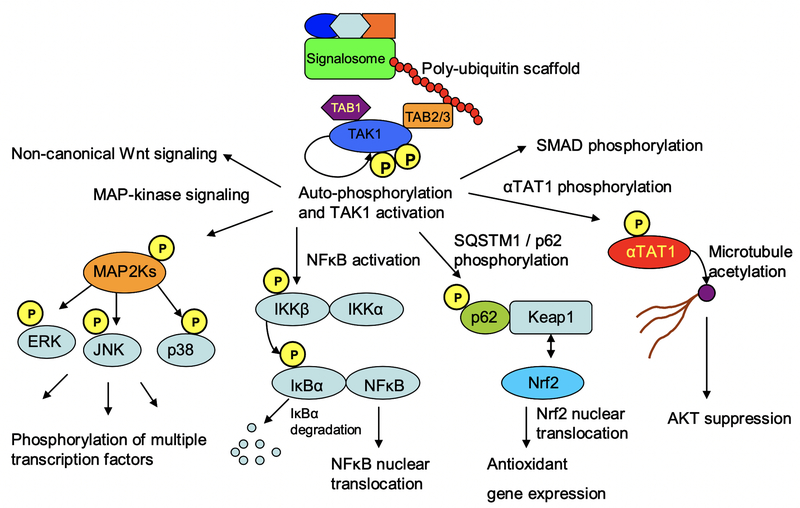
The large variety of stimuli that activate TAK1 is complemented by an equally diverse array of molecules that serve as TAK1 substrates (Fig 3). These include MAP2-Kinases such as MKK3, MKK4, MKK6 and MKK7, which phosphorylate MAP-Kinases such as ERK, p38 and JNK. In parallel, TAK1 mediates NFκB activation by phosphorylating IKKβ, eliciting IκBα phosphorylation and degradation (1–4). In addition, TAK1 regulates diverse processes ranging from microtubule (MT) acetylation to cellular redox homeostasis and metabolic regulation. It does so by phosphorylating molecules such as the MT acetyl-transferase αTAT1 (5), the redox regulatory molecule p62 (6), as well as the metabolic regulator AMPK (7). Moreover, TAK1 modulates canonical TGFβ signaling by phosphorylating SMAD molecules, and mediates non-canonical Wnt signaling (8–10). Hence TAK1 integrates diverse forms of stimuli to regulate a wide variety of cellular processes.
Fig 3. TAK1 signal transduction.
Active TAK1 orchestrates signal transduction through multiple pathways. These include MAP-kinase signaling, NFκB activation, regulation of cellular redox homeostasis through p62 phosphorylation, modulation of AKT activity through αTAT1 phosphorylation and microtubule acetylation, as well as the modulation of canonical forms of TGFβ-superfamily signaling through SMAD phosphorylation. Moreover, TAK1 mediates non-canonical Wnt signaling pathways in development and morphogenesis.
These processes include cell growth, proliferation and survival; cell motility and migratory capacity; inflammation, immunity and immune regulation; as well as cellular redox and metabolic homeostasis. The dysregulation of all these processes underlie the hallmark traits of cancer, and TAK1 function and dysfunction mediate central roles across multiple tumors. We next consider how TAK1 function mediates tumor suppression, carcinogenesis, and tumor progression.
TAK1 IN CANCER BIOLOGY
Cell cycle dysregulation is a hallmark feature of cancer (42–44). Tumor cells exhibit sustained proliferation, often evading growth suppressors and resisting programmed cell death. Malignant proliferation is also accompanied by metastatic dissemination of tumor cells to distant sites through epithelial-to-mesenchymal (EMT) transition, facilitated by the accumulation of reactive oxygen and nitrogen species (ROS and RNS respectively) that promote degradation of the extracellular matrix (ECM) components and create an inflammatory tumor microenvironment. However, tumor cells can often evade immune surveillance, and hence a paradoxical scene emerges wherein an inflammatory tumor microenvironment is complemented by cancer cell immune evasion. TAK1 is intricately involved in these hallmark traits while simultaneously maintaining homeostasis and tumor suppression (Fig 4). We consider these dual roles in turn.
Fig 4. Context dependent TAK1 signaling in cancer biology.
Signal transduction through multiple signal transduction systems regulate TAK1 activity. These include receptor signaling, as well as cell stress pathways such as DNA damage. TAK1 integrates these signals and mediates downstream signaling through multiple pathways. These include MAPK and NFκB signaling, microtubule acetylation, and cellular redox homeostasis. Perturbations to TAK1 activity result in diverse phenotypes and are found across multiple types of cancer.
TAK1 in tumor suppression
Conditional knockout studies have shown that TAK1 acts as a tumor suppressor in certain cases. For instance, hepatocyte-specific TAK1 deletion in mice have demonstrated cell death, inflammation, and compensatory cell proliferation, culminating in hepatic carcinogenesis (45–48). Hepatocytes in these mice undergo apoptosis as well as necrosis, resulting in liver dysplasia and biliary ductopenia. Within a month after birth, liver cell death is accompanied by compensatory proliferation, followed by tumor onset at four months.
The molecular mechanism attributed to this phenotype involves the cell survival role that TAK1 mediates through NFκB signaling. NFκB activates the transcription of multiple pro-survival genes, such as the IAP family proteins and c-FLIP, which inhibit caspase activation and suppress cellular apoptosis. Deletion of TAK1 abrogates NFκB activation, and hence the expression of cell survival molecules, leading to cell death.
Dying cells release inflammatory mediators known as alarmins, which in turn activate liver resident Kupffer macrophage cells to release the pro-inflammatory TNFα. Whereas in normal mice TNFα activates JNK and NFκB signaling to promote inflammation, in the absence of TAK1, TNFα renders cells susceptible to the apoptotic pathway, eliciting cell death, compensatory proliferation, and hepatic carcinogenesis (45, 47).
Intriguingly, in response to LPS stimulation, TAK1 deficient liver cells exhibit enhanced JNK, FoxO3a and TAO2 phosphorylation relative to wild type cells. Hyperactivation of these molecules is reversed in cells that lack the IKK subunit, NEMO, as well as TAK1 (46). These double knockout mice do not develop liver cancer as spontaneously as the single mutants. The molecular mechanisms that underlie the coordinate effects of NEMO and TAK1 require further investigation. One distinct possibility is that deletion of NEMO results in enhanced nuclear localization of NFκB, thus rescuing cell survival.
A further homeostatic aspect of TAK1 function relates to cellular metabolism. TAK1 has been shown to phosphorylate and activate AMPK (7), resulting in enhanced lipid catabolism in the forms of fatty acid oxidation and autophagy (49, 50). These effects occur through inhibition of mTORC1, and the induction of oxidative transcriptional programs mediated by the AMPK-activated transcription factor PPARα. Predictably, hepatocyte-specific TAK1 deletion results in reduced AMPK activation, with consequential suppression of autophagy and lipid oxidation that lead to hepatic carcinogenesis, thus demonstrating a link between TAK1 and AMPK function in liver metabolism and homoeostasis (48). Given the widespread role of TAK1 and AMPK, it is likely that TAK1 regulates cellular metabolism through AMPK signaling in a variety of cellular contexts, in addition to the liver.
Instances of TAK1 deletions in cancer patients have been reported across a variety of tumor types (51–54). In particular, the chromosomal deletion of an 800-kilobase locus on 6q15, which contains the TAK1 gene, is often linked to prostate cancer, pediatric T-cell acute lymphoblastic leukemia (T-All), and acute myeloid leukemia (AML). The loss of TAK1 in these cancers suggests a certain propensity of the TAK1 locus to be deleted across cellular divisions. Although the underlying selective pressures need to be clarified, it is noteworthy that this region lies close to the centromere. These chromosomal deletions not only confer a selective advantage during malignant transformation and tumor progression but also raise crucial questions as to why cells that harbor TAK1 deletions in prostate and blood cancer are not eliminated by apoptosis and instead enter programs of uncontrolled proliferation.
One possible link between the loss of TAK1 function and carcinogenesis is that TAK1 suppresses tumor onset by regulating cellular redox homeostasis (6, 55–60). Indeed, cellular ROS accumulation has been shown to stimulate the nuclear localization of the transcription factor Nrf2, which mediates the expression of multiple anti-oxidant genes (55). Notably, Nrf2 nuclear translocation is known to be inhibited by p62, a cytoplasmic retention process that is reversed when TAK1 phosphorylates p62 to disrupt the Nrf2-p62 interaction (6). Thus, in this context TAK1 positively regulates the anti-oxidant transcriptional response through p62 phosphorylation- which would be dysregulated upon loss of TAK1 resulting in genomic instabilities that underlie cancer biology and tumor progression.
A link between TAK1 deficiency, redox imbalance and tumor progression is particularly evident in metastatic skin squamous cell carcinoma (56, 61). Deficiency of TAK1 results in elevated cellular ROS, earlier EMT onset, and enhanced cellular invasive capacity. The disruption of redox homeostasis results in enhanced signaling of the Rac1 and RhoA GTPases, leading to greater cell motility. TAK1 deficiency also results in higher expression of EMT-associated transcription factors (TFs). The molecular mechanisms that underlie these effects require further research. However, it is clear that TAK1 lies at the nexus of an intricate signaling network, involving anti-oxidant gene expression, inhibition of EMT-associated TFs, and small GTPase activity. Loss of TAK1 thus simultaneously results in many of the hallmark traits of cancer, in the form of excess ROS and ECM degradation, enhanced metastatic capacity, and uncontrolled proliferation.
In parallel, TAK1 also modulates canonical TGFβ signaling by phosphorylating SMAD effectors and enhancing expression of the inhibitory SMAD7 (61). Hence TAK1 is posited to set an upper limit to TGFβ signaling. Exposure of cells to TGFβ in the tumor microenvironment mediates a central role in metastasis (15), while TAK1 deficiency in the above settings augment metastatic invasion. Thus, there seems to be a positive feedback mechanism where TGFβ in the ECM is complemented by cellular loss of TAK1. Cellular responses mediated by TGFβ are restrained by TAK1 under homeostatic settings. However, in the instance of metastatic skin squamous cell carcinoma, the loss of TAK1 results in the potentiation of TGFβ signaling, ultimately eliciting an aggressive metastatic phenotype.
TAK1 in tumor progression
In contrast to the studies mentioned above, TAK1 function is strongly associated with tumor progression and metastatic invasion in many other cases. Multiple correlation-based studies have implicated elevated TAK1 expression and activity across a range of cancers including esophageal (62), osteosarcoma (63), thyroid (64), gastric (65) and ovarian (66). Inhibition of TAK1 activity in these instances resulted in reduced cell proliferation and invasion, as well as the induction of apoptosis.
The central mechanism implicated in these studies involves TAK1 signaling through NFκB. Inhibition of TAK1 reduces nuclear localization of the NFκB subunit p65, resulting in decreased Cyclin D1 gene expression. Cyclin D1 regulates cell cycle progression and cell division (67, 68), and hence reduced expression levels inhibit tumor progression. Another outcome of TAK1 inhibition is increased levels of caspase 3 cleavage and cytosolic cytochrome c, as well as lower amounts of the pro-survival factor Bcl2. Hence the emergent scenario is one where TAK1 inhibition abrogates NFκB activation, which results in the downregulation of pro-survival factors, and the stimulation of cellular apoptotic pathways. In parallel, TAK1 function also modulates cell cycle progression and proliferation, hence setting a delicate balance between homeostasis and pathogenesis (Fig 4).
Another theme that emerges from these studies is that TAK1 is critically involved in metastasis. A prominent example is its role in breast cancer metastasis (69–75). Metastatic invasion requires ECM degradation and the upregulation of molecules that mediate cell migration including matrix metalloproteinases (MMPs) and chemokine receptors (CCRs). TAK1 is directly linked to enhanced expression of both these classes of molecules in breast cancer, in particular molecules such as MMP9, COX2 and CCR7, thus suggesting TAK1 mediated upregulation of entire sets of molecules in breast cancer metastasis. In other malignancies such as thyroid (64) and gastric (65) cancer, inhibition of TAK1 have resulted in reduced invasive capacity due to reduced expression levels of MMP9. Hence TAK1 activity results in the transcriptional upregulation of multiple molecules that mediate cell migration and metastatic invasion.
An intriguing feature of TAK1 function in cancer biology relates to the diverse feedback loops that promote pathological progression. A notable example is related to TAK1 activation by DNA damage and genotoxic stress (34–36). Under homeostatic conditions, TAK1 activity elicits cellular processes that promote DNA repair and inhibit cell cycle progression. However, in cancer context which often involves genomic instabilities, this same homeostatic mechanism drives TAK1 hyperactivation and elicits signal transduction through the pathways that TAK1 regulates, resulting in tumor progression.
A further instance of such pathological feedback mechanisms pertains to the environmental factors in which the cytokine and TAK1 function. Although cytokine receptors such as IL1 and TNFα are known activators of TAK1 during inflammation and immunity, these inflammatory cytokines can also mediate epithelial-to-mesenchymal transition (EMT) (43, 76), as well as decisions of survival, death and differentiation. Therefore, the combination of cytokine environment and TAK1 activity appear to be critical in the delicate balance between homeostasis and pathology especially in certain cases such as liver cancers as discussed previously (45–48) wherein TAK1 loss contributes to an initial wave of cell death, then compensatory cell proliferation followed by liver carcinogenesis which occur in the presence of TNFα and TGFβ. In contrast, the persistent presence of TNFα, IL1 and TGFβ in the tumor microenvironment (43, 76) results in TAK1 hyperactivation, increased receptor expression for these inflammatory cytokines (31), and a positive feedback effect that culminates in the induction of multiple molecules that mediate metastatic invasion.
A similar interplay is evident between TGFβ and TAK1 function. Under homeostasis, TAK1 modulates canonical TGFβ signaling through direct interactions with SMAD molecules (8, 9), as well as transcriptional induction of the inhibitory SMAD7 (61). In the instance of head and neck cancer discussed previously (61), the concomitant loss of TAK1 and cellular exposure to TGFβ elicits an aggressive metastatic phenotype. In a contrasting circumstance of breast cancer metastasis (73), TGFβ stimulates TAK1 to upregulate the expression of molecules that mediate metastatic invasion. Hence the same cytokines that mediate host immunity and homeostasis through TAK1 function, are co-opted in cancer biology to establish multiple self-reinforcing mechanisms that aid tumor progression. Moreover, the combinatorial effects of the particular cytokines present, and TAK1 activity levels, exert contrasting effects in homeostasis and cancer.
Yet another link between TAK1 and tumor progression relates to the regulation of metabolic pathways. AMPK, a key sensor enzyme in cellular energetics, is regulated by a number of post-translational modifications including TAK1-induced phosphorylation that catalytically activates AMPK (7, 49, 50). In parallel, AMPK is also allosterically activated by ADP and AMP binding. This is important in times of nutrient deprivation, where increased ratios of ADP and AMP relative to ATP activate AMPK. AMPK activation in turn coordinates cellular metabolic pathways to elicit two over-arching effects. The first pertains to lipid metabolism as we previously discussed in the context of liver cancer. AMPK activation inhibits fatty acid synthesis, while activating lipid catabolism and fatty acid uptake. This mediates an important homeostatic role, since disruption of TAK1 signaling and AMPK activation results in hepatic carcinogenesis (48).
The second effect relates to glucose metabolism, in that AMPK activity stimulates glucose uptake and glycolysis (49, 50). This occurs through multiple mechanisms. The first relates to the enhanced localization of glucose transporters, such as GLUT1 and GLUT4, to the cell membrane (77, 78). AMPK activity also increases the expression of glycolytic enzymes such as hexokinase 2, as well as that of glucose transporters such as GLUT4 (79, 80). Lastly, AMPK stimulates glycolytic flux by phosphorylating glycolytic enzymes such as 6-phosphofructo2-kinase (81, 82). Hence TAK1-mediated activation of AMPK stimulates glycolytic metabolism at multiple levels.
The combination of enhanced lipid catabolism and glycolytic flux represent two important factors of tumor cell metabolism that fuel uncontrolled cell proliferation (42). TAK1 signaling through AMPK likely regulates both these features of cancer cell metabolism. Moreover, TAK1 is also activated by polyunsaturated fatty acids such as arachidonic acid, leading to p38 MAPK activation and enhanced adhesive and invasive cellular phenotypes that mediate tumor growth and metastasis (71). It remains to be resolved whether the lipid environment of the ECM activates TAK1 and AMPK signaling to elicit features of tumor cell metabolism, and whether the extracellular lipid environment, combined with intracellular TAK1 and AMPK signaling, further potentiate this self-reinforcing mechanism to enhance pathological progression.
The tumor microenvironment often facilitates metastatic invasion through overt inflammation and tissue damage. Paradoxically, cancer cells evade immune destruction within this inflammatory microenvironment. The combination of inflammation and cancer cell immune evasion is due to the cytokines present within the tumor microenvironment, which can recruit immune cells and promote an inflammatory state, while others restrain overt inflammation. Instances of the former include TNFα and IL1, while an important example of the latter type is TGFβ. Signaling induced by all these cytokines rely on TAK1 activity, and hence TAK1 likely orchestrates a central role in inflammation and cancer. Consequently, an important direction will be to investigate the cell-type specific actions of TAK1, in immune and cancer cells, under the contextual effects of the tumor microenvironment.
TAK1 in immune function and cancer biology
Research over the past decades has revealed a complex interplay between immune and cancer cells (43, 76). Overt inflammation often leads to carcinogenesis, while tumor cells promote an inflammatory microenvironment. Instances of such positive feedback are observed in inflammatory bowel disease and colorectal cancer, chronic asthma and pancreatitis in bronchial and pancreatic cancer respectively, and infection with human papillomavirus in cervical cancer. Inflammatory cytokines promote carcinogenesis and tumor progression, while cancer cells stimulate inflammatory immune responses and further malignant transformation. TAK1 plays a fundamental role in the inflammatory immune response across all immune cell types, as well as orchestrating defining features of cancer cell biology. The cell type specific actions of TAK1 underlie its contextual roles in cancer biology, and we review specific instances below.
Macrophages are central mediators of the innate immune response and tumor progression (76). Tumor associated macrophages (TAMs) secrete inflammatory cytokines such as IL1 and TNFα. The expression and secretion of these cytokines rely on TAK1 signaling, and cytokine receptor stimulation activates TAK1. Such positive feedback between TAK1 activity and cytokine secretion is restrained by coordinated degradation of TAK1 in response to NFκB activation. This relies on the de-ubiquitination of K63-linked pUb mediated by Cyld, and the creation of K48-linked TAK1 pUb chains mediated by the E3 ligase Itch (83). This transition between pUb structures downregulates TAK1 activity, and stimulates its proteasomal degradation. Mice deficient in Itch and Cyld exhibit enhanced macrophage mediated inflammation, and tumor progression in lung carcinoma.
The critically important role of TAK1 in macrophage activation and inflammation suggests that TAK1 function underlies many aspects of the inflammatory tumor microenvironment. Alongside broad expression across immune cells, TAK1 is also expressed in cancer cells, and is activated by the cytokines produced by inflammatory immune cells. Hence the emergent scenario is one where TAK1 signaling in immune cells elicits an inflammatory microenvironment, triggers cancer cell responses culminating in tumor progression, and leads to further inflammation. Thus, TAK1 signaling lies at the nexus of the multiple positive feedback loops that elicit the inflammatory tumor microenvironment.
But interestingly, in contrast to macrophages and other immune cells, TAK1 reportedly mediates different modes of signal transduction in neutrophils. Specific deletion of TAK1 in neutrophils results in augmented cell numbers through development (84). In response to LPS stimulation, these cells exhibit greater levels of IKK, p38 and JNK phosphorylation. This culminates in higher secretion levels of the inflammatory cytokines IL1, TNFα and IL6. Concurrent deletion of p38 with TAK1 restores cellular phenotypes to wild type levels. The molecular mechanisms attributed to these effects involve enhanced binding of TAB1 to p38 in the absence of TAK1, leading to p38 activation. The absence of TAK1 also results in greater levels of ROS, leading to enhanced phosphorylation of IKK and p38. Hence genetic knockout of TAK1 elicits inflammatory neutrophil phenotypes.
Additionally, TAK1 localizes to both the cytoplasmic as well as nuclear compartments in neutrophils (41). It does so alongside its binding partners, TAB1, TAB2 and TAB4, as well as the IKK complex and NFκB. Consequently, TAK1 signaling occurs in both the cytoplasm and nucleus of neutrophils. In contrast to the genetic ablation study mentioned above, pharmacological inhibition of TAK1 with 5z-7-oxozeaenol impairs neutrophil TNFα secretion. These discrepancies between the pharmacological and genetic perturbations to TAK1 signaling in neutrophils remain undetermined, although one distinct possibility is that 5z-7-oxozeaenol has off-target effects including inhibition of other kinases such as ERK and MEK. In addition, genetic TAK1 ablation may trigger compensatory adjustments in signaling networks during maturation that will require more in-depth studies including cell type-specific conditional knock-out models at specific developmental stages.
The proinflammatory effects of TAK1 illustrate the seemingly paradoxical example where immune cells normally recognize and eliminate tumor cells in homeostatic settings, but tumor progression relies on immune evasion by cancer cells. Here, an important contributing factor likely involves the secretion of TGFβ by stromal cells in the tumor microenvironment, and consequential immuno-suppression (15, 85, 86). Exposure of T cells to TGFβ elicits a regulatory phenotype, which restrains tissue inflammation. Hence cancer cells in certain contexts may be better protected from the immune system by tolerogenic cytokines such as TGFβ, even while tumor progression is aided by an inflammatory microenvironment.
This dual immuno-modulatory effect of TGFβ has been well documented in cancer biology. Indeed, in the early stages of carcinogenesis, the cytokine exerts a tumor suppressive role, by inhibiting cell proliferation and promoting apoptosis. But in more advanced pathological stages, TGFβ promotes the invasive capacity of metastatic cancer. As a major effector of TGFβ signaling in the tumor microenvironment, targeting TAK1 represents a powerful strategy in cancer therapy.
TAK1 in tumor angiogenesis
A further hallmark of cancer biology pertains to the ability of tumors to stimulate angiogenesis and neo-vascularization (42). Tissue vascularization is regulated by the balance of angiogenic inducers and suppressors. Instances of inducers include vascular endothelial growth factor-A (VEGFA) and fibroblast growth factor (FGF), while thrombospondin-1 (TSP-1) is one suppressor. These molecules act upon vascular endothelial cells to regulate the sprouting and splitting of new blood vessels. Neo-vascularization becomes quiescent in adulthood, mostly being transiently activated during pathophysiologic states such as wound healing and tumor growth. TAK1 mediates key aspects of angiogenesis during development and tumor progression.
Vascularization during embryonic development requires TAK1 function (87). Endothelial cell specific deletion of TAK1 results in impaired vascularization, due to endothelial cell death and abrogation of migratory capacity. The molecular mechanisms that underlie this are similar to TAK1 function in the previously discussed settings. The exposure of endothelial cells to cytokines such as TNFα stimulates cell death in the absence of TAK1, and endothelial cell death results in vascular collapse. In parallel to cell survival, TAK1 also mediates cell migration. In the context of embryonic vascularization, new blood vessels are formed through the migration of tip cells towards guidance cues such as cytokines and growth factors. The absence of TAK1 results in impaired migratory capacity, and hence aberrant vascular formation.
The maintenance of vascular integrity in adulthood also requires TAK1 (88). As in embryonic development, conditional deletion of endothelial TAK1 also results in vascular defects due to endothelial cell death in response to TNFα. This is particularly pronounced in the liver and intestine where immune cells constitutively release TNFα in response to the commensal microbiota.
The integral role that TAK1 mediates in angiogenesis and vascular homeostasis suggests that TAK1 function is involved in tumor vascularization. This has been shown in a subcutaneous tumor model, where deletion of TAK1 from tumor endothelial cells results in regression of tumor vascular networks, and reduction in tumor size (88). Similar to the previously mentioned contexts, this is due to endothelial cell death in response to inflammation, particularly TNFα. Hence inhibiting TAK1 function could provide a means to abrogate tumor vascularization, as well as targeting many of the other hallmark traits of cancer as described next.
Targeting TAK1 in cancer therapy
A central challenge in all cancer therapy is the minimization of undesirable side effects. This is particularly relevant for therapeutic strategies targeting TAK1 given its pleiotropic roles in different tissues. However, innovative strategies that inhibit TAK1 function exclusively in cancer cells holds immense clinical promise.
Because TAK1 has a key role in cell survival and the inhibition of apoptosis, inhibiting this kinase could provide a means to induce cancer cell death. This has been demonstrated in multiple types including colon (89, 90), lung (91), and certain blood cancers (92). Additionally, the more recent development of highly specific inhibitors such as Takinib, which induces tumor remission in the presence of TNFα, have shown promising efficacy and specificity (93). In some cases, such as pancreatic cancer, TAK1 inhibitors abrogate chemo-resistance to first-line therapies (94).
Mechanistically, most TAK1-related cancer therapies appear to induce cell death. In colon cancer, combined MEK and TAK1 inhibition elicits apoptosis in malignant cells harboring KRAS mutations. In lung cancer, the combined blockade of BMP and TGFβ receptors inhibits TAK1 expression and induces cell death. Similarly, TAK1 inhibition in conjunction with TRAIL stimulation, a member of the TNFα superfamily, induces cell death in a cell culture model of lung cancer (95). Hence an important direction of further research will be to understand the specific molecular alterations that underlie different types of cancers, and how TAK1 inhibition can be deployed in combination with other therapeutic agents to optimize treatment regimes. The fact that TAK1 deletion induces cell death in the presence of cytokines such as TNFα and TGFβ (45, 47), alongside the effectiveness of Takinib in the presence of TNFα (93), suggest that the loss of TAK1 activity in the presence of the stimulators of TAK1 signaling is an effective means to induce cancer cell death. Hence the simultaneous application of TAK1 inhibitors with cytokines such as TNFα and TGFβ, is predicted to induce cancer cell death. In a similar manner, TAK1 inhibition in the presence of chemotherapy, which activates TAK1 signaling due to genotoxic insults, induces cell death and abrogates chemoresistance (94). On the other hand, cancers that exhibit TAK1 deletions, can be effectively targeted using activators of TAK1 signaling. Hence chemotherapy, and the administration of TNFα or TGFβ, are predicted to be effective therapies in the previously mentioned instances of pancreatic and blood cancers (51–53). An important challenge in this regard pertains to the selective targeting of cancer cells. Nanoparticles and ultrasound guided drug delivery systems (96, 97) represent promising strategies for the localized administration of therapies targeting TAK1 function in cancer.
Given that TAK1 also orchestrates metastatic invasion, inhibiting this kinase may prove effective in countering cancer metastasis. This is particularly relevant in documented cases such as in thyroid, gastric, ovarian and breast cancers (62–64, 67, 73). In addition to countering metastatic invasive capacity, TAK1 inhibition could also be effective at inducing cell death, since activators of TAK1 signaling, such as IL1, TNFα and TGFβ, facilitate metastatic invasion.
PERSPECTIVES
An ongoing goal in cancer research is to develop a comprehensive understanding of the signaling networks that culminate in disease progression. These networks span multiple spatial and temporal scales, from single cells to microenvironments and organism levels. Characterizing these networks will increasingly rely on mass spectrometry/proteomics, RNA-seq and other systems-based technologies that quantify cellular and physiological states. The application of these methods towards understanding TAK1 function could provide a powerful model system in cancer research, as well as systems biology. Such approaches will ultimately yield important insights towards novel approaches in cancer therapy.
Footnotes
Conflict of Interest: The authors declare no potential conflicts of interest
References
- 1.Yamaguchi K, Shirakabe K, Shibuya H, Irie K, Oishi I, Ueno N et al. Identification of a member of the MAPKKK family as a potential mediator of TGF-β signal transduction. Science 1995; 270: 2008–2011. [DOI] [PubMed] [Google Scholar]
- 2.Dai L, Aye Thu C, Liu XY, Xi J, Cheung PC. TAK1, more than just innate immunity. IUBMB Life 2012; 64: 825–834. [DOI] [PubMed] [Google Scholar]
- 3.Hirata Y, Takahashi M, Morishita T, Noguchi T, Matsuzawa A. Post-translational modifications of the the TAK1-TAB complex. Int J Mol Sci 2017; 18: 205, 10.3390/ijms18010205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sakurai H Targeting of TAK1 in inflammatory disorders and cancer. Trends Pharmacol Sci 2012; 33: 522–530. [DOI] [PubMed] [Google Scholar]
- 5.Shah N, Kumar S, Zaman N, Pan C, Bloodworth J, Lei W et al. TAK1 activation of alpha-TAT1 and microtubule hyperacetylation control AKT signaling and cell growth. Nat Commun 2018; 9: 1696, DOI: 10.1038/s41467-018-04121-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hashimoto K, Simmons AN, Kajino-Sakamoto R, Tsuji Y, Ninomiya-Tsuji J. TAK1 regulates the Nrf2 antioxidant system through modulating p62/SQSTM1. Antioxid Redox Signal 2016; 25: 17, DOI: 10.1089/ars.2016.6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xie M, Zhang D, Dyck JR, Li Y, Zhang H, Morishima M et al. A pivotal role for endogenous TGF-beta-activated kinase-1 in the LKB1/AMP- activated protein kinase energy-sensor pathway. Proc Natl Acad Sci USA 2006; 103: 17378–17383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ohkawara B, Shirakabe K, Hyodo-Miura J, Matsuo R, Ueno N, Matsumoto K, et al. Role of the TAK1-NLK-STAT3 pathway in TGF-β-mediated mesoderm induction. Gene Dev 2004; 18: 381–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoffmann A, Preobrazhenska O, Wodarczyk C, Medler Y, Winkel A, Shahab S et al. Transforming growth factor-β-activated kinase-1 (TAK1), a MAP3K, interacts with SMAD proteins and interferes with osteogenesis in murine mesenchymal progenitors. J Biol Chem 2005; 280: 27271–27283. [DOI] [PubMed] [Google Scholar]
- 10.Smit L, Baas A, Kuipers J, Korswagen H, van de Wetering M, Clevers H. Wnt activates the Tak1/Nemo-like kinase pathway. J Biol Chem 2004; 279: 17232–17240. [DOI] [PubMed] [Google Scholar]
- 11.Santoroa R, Carbonea C, Piroa G, Chiaob PJ, Melisia D. TAK-ing aim at chemoresistance: the emerging role of MAP3K7 as a target for cancer therapy. Drug Resist Updat 2017; 33–35: 36–42. [DOI] [PubMed] [Google Scholar]
- 12.Roh YS, Song J, Seki E. TAK1 regulates hepatic cell survival and carcinogenesis. J Gastroenterol 2014; 49: 185–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim SI, Choi ME. TGF-β-activated kinase-1: New insights into the mechanism of TGF-β signaling and kidney disease. Kidney Res Clin Pract 2012; 31: 94–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mihaly SR, Ninomiya-Tsuji J, Morioka S. TAK1 control of cell death. Cell Death Differ 2014; 21: 1667–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neuzillet C, Tijeras-Raballand A, Cohen R, Cros J, Faivre S, Raymond E, et al. Targeting the TGFβ pathway for cancer therapy. Pharmacol Therapeut 2015; 147: 22–31. [DOI] [PubMed] [Google Scholar]
- 16.Park MH, Hong JT. Roles of NFκB in cancer and inflammatory diseases and their therapeutic approaches. Cells 2016; 5: 15, DOI: 10.3390/cells5020015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burotto M, Chiou VL, Jung-Min L, Kohn E. The MAPK pathway across different malignancies: a new perspective. Cancer 2014; 120: 3446–3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shibuya H, Yamaguchi K, Shirakabe K, Tonegawa A, Gotoh Y, Ueno N et al. TAB1: An activator of the TAK1 MAPKKK in TGF-β signal transduction. Science 1996; 272: 1179–1182. [DOI] [PubMed] [Google Scholar]
- 19.Besse A, Lamothe B, Campos AD, Webster WK, Maddineni U, Lin SC et al. TAK-1 dependent signaling requires functional interaction with TAB2/TAB3. J Biol Chem 2007; 282: 3918–3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takaesu G, Kishida S, Hiyama A, Yamaguchi K, Shibuya H, Irie K et al. TAB2, a novel adaptor protein, mediates activation of TAK1 MAPKKK by linking TAK1 to TRAF6 in the IL-1 signal transduction pathway. Mol Cell 2000; 5: 649–658. [DOI] [PubMed] [Google Scholar]
- 21.Cheung PC, Nebreda AR, Cohen P. TAB3, a new binding partner of the protein kinase TAK1. Biochem J 2004; 378: 27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sakurai H, Miyoshi H, Mizukami J, Sugita T. Phosphorylation-dependent activation of TAK1 mitogen-activated protein kinase kinase kinase by TAB1. FEBS Lett 2000; 474: 141–145. [DOI] [PubMed] [Google Scholar]
- 23.Yu Y, Ge N, Xie M, Sun W, Burlingame S, Pass AK et al. Phosphorylation of Thr-178 and Thr-184 in the TAK1 T-loop is required for interleukin (IL)-1-mediated optimal NF-κB and AP-1 activation as well as IL-6 gene expression. J Biol Chem 2008, 283: 24497–24505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singhirunnusorn P, Suzuki S, Kawasaki N, Saiki I, Sakurai H. Critical roles of threonine 187 phosphorylation in cellular stress induced rapid and transient activation of transforming growth factor-β activated kinase 1 (TAK1) in a signaling complex containing TAK1-binding protein TAB1 and TAB2. J Biol Chem 2005; 280: 7359–7368. [DOI] [PubMed] [Google Scholar]
- 25.Hamidi A, von Bulow V, Hamidi R, Winssinger N, Barluenga S, Heldin CH, et al. Polyubiquitination of transforming growth factor β (TGF-β)-associated kinase 1 mediates nuclear factor-κB activation in response to different inflammatory stimuli. J Biol Chem 2012; 287: 123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang D, Xu Z, Tao T, Liu X, Sun X, Ji Y et al. Modification of TAK1 by O-linked N-acetylglucosamine facilitates TAK1 activation and promotes M1 macrophage polarization. Cell Signal 2016; 28: 1742–1752. [DOI] [PubMed] [Google Scholar]
- 27.Ishitani T, Kishida S, Hyodo-Miura J, Ueno N, Yasuda J, Waterman M et al. The TAK1-NLK Mitogen-Activated Protein Kinase cascade functions in the Wnt-5a/Ca2+ pathway to antagonize Wnt/β-Catenin signaling. Mol Cell Biol 2003; 23: 131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sorrentino A, Thakur N, Grimsby S, Marcusson A, von Bulow V, Schuster N et al. The type I TGF-β receptor engages TRAF6 to activate TAK1 in a receptor kinase-independent manner. Nat Cell Biol 2008; 10: 1199–1207. [DOI] [PubMed] [Google Scholar]
- 29.Yamashita M, Fatyol K, Jin C, Wang X, Liu Z, Zhang YE. TRAF6 mediates Smad-independent activation of JNK and p38 by TGF-β. Mol Cell 2008; 31: 918–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamaguchi K, Nagai S, Ninomiya-Tsuji J, Nishita M, Tamai K, Irie K, et al. XIAP, a cellular member of the inhibitor of apoptosis protein family, links the receptors to TAB1–TAK1 in the BMP signaling pathway. EMBO J 1999; 18: 179–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sato S, Sanjo H, Takeda K, Ninomiya-Tsuji J, Yamamoto M, Kawai T, et al. Essential function for the kinase TAK1 in innate and adaptive immune responses. Nat Immunol 2005; 6: 1087–1095. [DOI] [PubMed] [Google Scholar]
- 32.Sun L, Deng L, Ea CK, Xia ZP, Chen ZJ. The TRAF6 ubiquitin ligase and TAK1 kinase mediate IKK activation by BCL10 and MALT1 in T lymphocytes. Mol Cell 2004; 14: 289–301. [DOI] [PubMed] [Google Scholar]
- 33.Shinohara H, Yasuda T, Aiba Y, Sanjo H, Hamadate M, Watarai H et al. PKC β regulates BCR-mediated IKK activation by facilitating the interaction between TAK1 and CARMA1. J Exp Med 2005; 202: 1423–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hinz M, Stilmann M, Arslan SC, Khanna KK, Dittmar G, Scheidereit C. A cytoplasmic ATM-TRAF6-cIAP1 module links nuclear DNA damage signaling to ubiquitin-mediated NFκB activation. Mol Cell 2010; 40: 63–74. [DOI] [PubMed] [Google Scholar]
- 35.Wu ZH, Wong ET, Shi Y, Niu J, Chen Z, Miyamoto S, et al. ATM- and NEMO-dependent ELKS ubiquitination coordinates TAK1-mediated IKK activation in response to genotoxic stress. Mol Cell 2010; 40: 75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang Y, Xia F, Hermance N, Mabb A, Simonson S, Morrissey S, et al. A cytosolic ATM/NEMO/RIP1 complex recruits TAK1 to mediate the NF-κB and p38 mitogen-activated protein kinase (MAPK)/MAPK-activated protein 2 responses to DNA damage Mol Cell Biol 2011; 31: 2774–2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blanco S, Santos C, Lazo PA. Vaccinia-related kinase 2 modulates the stress response to hypoxia mediated by TAK1. Mol Cell Biol 2007; 27: 7273–7283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Melvin A, Mudie S, Rocha S. Further insights into the mechanism of hypoxia-induced NF-κB. Cell Cycle 2011; 10: 879–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Inagaki M, Omori E, Kim JY, Komatsu Y, Scott G, Ray MK, et al. TAK1-binding protein 1, TAB1, mediates osmotic stress-induced TAK1 activation but is dispensable for TAK1-mediated cytokine signaling. J Biol Chem 2008; 283: 33080–33086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huangfu WC, Omori E, Akira S, Matsumoto K, Ninomiya-Tsuji J. Osmotic stress activates the TAK1–JNK pathway while blocking TAK1-mediated NF-κB activation: TAO2 regulates TAK1 pathways. J Biol Chem 2006; 281: 28802–28810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ear T, Fortin CF, Simard FA, McDonald PP. Constitutive association of TGF-β-activated kinase 1 with the IκB kinase complex in the nucleus and cytoplasm of human neutrophils and its impact on downstream processes. J Immunol 2010; 184: 3897–3906. [DOI] [PubMed] [Google Scholar]
- 42.Hanahan D and Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144: 646–674. [DOI] [PubMed] [Google Scholar]
- 43.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010; 140: 883–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med 2010; 49: 1603–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Inokuchi S, Aoyama T, Miura K, Osterreicher CH, Kodama Y, Miyai K, et al. Disruption of TAK1 in hepatocytes causes hepatic injury, inflammation, fibrosis, and carcinogenesis. Proc Natl Acad Sci USA 2010; 107: 844–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bettermann K, Vucur M, Haybaeck J, Koppe C, Janssen J, Heymann F, et al. TAK1 suppresses a NEMO-dependent but NF-κB-independent pathway to liver cancer. Cancer Cell 2010; 17: 481–496. [DOI] [PubMed] [Google Scholar]
- 47.Yang L, Inokuchi S, Roh YS, Song J, Loomba R, Park EJ, et al. Transforming growth factor-beta signaling in hepatocytes promotes hepatic fibrosis and carcinogensis in mice with hepatocyte-specific deletion of TAK1. Gastroenterology 2013; 144: 1042–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Inokuchi-Shimizu S, Park EJ, Roh YS, Yang L, Zhang B, Song J, et al. TAK1-mediated autophagy and fatty acid oxidation prevent hepatosteatosis and tumorigenesis. J Clin Invest 2014; 124: 3566–3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jeon SM. Regulation and function of AMPK in physiology and diseases. Exp Mol Med 2016; 48: e245, doi: 10.1038/emm.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dasgupta B, Chhipa RR. Evolving lessons on the complex role of AMPK in normal physiology and cancer. Trends Pharmacol Sci 2016; 37: 192–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu W, Chang BL, Cramer S, Koty PP, Li T, Sun J, et al. Deletion of a small consensus region at 6q15, including the MAP3K7 gene, is significantly associated with high-grade prostate cancers. Clin Cancer Res 2007; 13: 5028–5033. [DOI] [PubMed] [Google Scholar]
- 52.Wu M, Shi L, Cimic A, Romero L, Sui G, Lees CJ, et al. Suppression of Tak1 promotes prostate tumorigenesis. Cancer Res 2012; 72: 2833–2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cordas Dos Santos DM, Eiler J, Sosa Vizaino A, Orlova EZimmermann M, Stanulla M, Schrappe M, et al. MAP3K7 is recurrently deleted in pediatric T-lymphobastic leukemia and affects cell proliferation independently of NF-κB. BMC Cancer 2018; 18: 663, 10.1186/s12885-018-4525-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lamothe B, Lai YJ, Hur L, Orozco NM, Wang J, Campos AD, et al. Deletion of TAK1 in the myeloid lineage results in the spontaneous development of myelomonocytic leukemia in mice. PLos One 2012; 7: e51228, 10.1371/journal.pone.0051228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kansanen E, Kuosmanen SM, Leinonen H, Levonen AL. The Keap1-Nrf2 pathway: mechanisms of activation and dysregulation in cancer. Redox Biol 2013; 1: 45–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lam CR, Tan C, Teo Z, Tay CY, Phua T, Wu YL, et al. Loss of TAK1 increases cell traction force in a ROS-dependent manner to drive epithelial-mesenchymal transition of cancer cells. Cell Death Dis 2013; 4: e848, 10.1038/cddis.2013.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Omori E, Morioka S, Matsumoto K, Ninomiya-Tsuji J. TAK1 regulates reactive oxygen species and cell death in keratinocytes, which is essential for skin integrity. J Biol Chem 2008; 283: 26161–26168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lam CRI, Tan MJ, Tan SH, Tang MBY, Cheung PCF, Tan NS. TAK1 regulates SCF expression to modulate PKBα activity that protects keratinocytes from ROS-induced apoptosis. Cell Death Differ 2011; 18: 1120–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kajino-Sakamoto R, Omori E, Nighot PK, Blislager AT, Matsumoto K, Ninomiya-Tsuji J. TGF-β-activated kinase 1 signaling maintains intestinal integrity by preventing accumulation of reactive oxygen species in the intestinal epithelium. J Immunol 2010; 185: 4729–4737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ogura Y, Hindi SM, Sato S, Xiong G, Akira S, Kumar A. TAK1 modulates satellite stem cell homeostasis and skeletal muscle repair. Nat Commun 2015; 6: 10123, DOI: 10.1038/ncomms10123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Freudlsperger C, Bian Y, Wise SC, Burnett J, Coupar J, Yang X, et al. TGF-β and NF-κB signal pathway cross-talk is mediated through TAK1 and SMAD7 in a subset of head and neck cancers. Oncogene 2013; 32: 1549–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wen J, Hu Y, Luo KJ, Yang H, Zhang SS, Fu JH. Positive transforming growth factor-β activated kinase-1 expression has an unfavorable impact on survival in T3N1–3M0 esophageal squamous cell carcinomas. Ann Thorac Surg 2013; 95: 285–291. [DOI] [PubMed] [Google Scholar]
- 63.Wei Y, Zhou R, Wang Q, Beibei F, Jing W, Wang H. Expression and function of TAK1 in osteosarcoma tissue. Int J Clin Exp Med 2016; 9: 10891–10898. [Google Scholar]
- 64.Lin P, Niu W, Peng C, Zhang Z, Niu J. The role of TAK1 expression in thyroid cancer. Int J Clin Exp Pathol 2015; 8: 14449–14456. [PMC free article] [PubMed] [Google Scholar]
- 65.Yang Y, Qiu Y, Tang M, Wu Z, Hu W, Chen C. Expression and function of transforming growth factor-β-activated protein kinase 1 in gastric cancer. Mol Med Rep 2017; 16: 3103–3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cai PCH, Shi L, Liu VWS, Tang HWM, Liu IJ, Leung THY, et al. Elevated TAK1 augments tumor growth and metastatic capacities of ovarian cancer cells through activation of NF-κB signaling. Oncotarget 2014; 5: 7549–7562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Terada Y, Inoshita S, Nakashima O, Kuwahara M, Sasaki S, Marumo F. Regulation of cyclin D1 expression and cell cycle progression by mitogen-activated protein kinase cascade. Kidney Int 1999; 56: 1258–1261. [DOI] [PubMed] [Google Scholar]
- 68.Guttridge DC, Albanese C, Reuther JY, Pestell RG, Baldwin AS. NF-κB controls cell growth and differentiation through transcriptional regulation of cyclin D1. Mol Cell Biol 1999; 19: 5785–5799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Iriondo O, Liu Y, Lee G, Elhodaky M, Jimenez C, Lin L, et al. TAK1 mediates microenvironment-triggered autocrine signals and promotes triple-negative breast cancer lung metastasis. Nat Commun 2018; 9: 1994, DOI: 10.1038/s41467-018-04460-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Safina A, Sotomayor P, Limoge M, Morrison C, Bakin AV. TAK1-TAB2 signaling contributes to bone destruction by breast carcinoma cells. Mol Cancer Res 2011; 9: 71042–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ray DM, Myers PH, Painter JT, Hoenerhoff MJ, Olden K, Roberts JD. Inhibition of transforming growth factor-β activated kinase-1 blocks cancer cell adhesion, invasion, and metastasis. Br J Cancer 2012; 107: 129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jiang L, Yu L, Zhang X, Lei F, Wang L, Liu X et al. miR-892b silencing activates NF-κB and promotes aggressiveness in breast cancer. Cancer Res 2016; 76: 1101–1111. [DOI] [PubMed] [Google Scholar]
- 73.Safina A, Ren M-Q, Vandette E, Bakin AV. TAK1 is required for TGFβ1-mediated regulation of matrix metalloproteinase-9 and metastasis. Oncogene 2008; 27: 1198–1207. [DOI] [PubMed] [Google Scholar]
- 74.Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, et al. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol 2000; 2: 737–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huang HL, Chiang CH, Hung WC, Hou MF. Targeting of TGF-β-activated protein kinase 1 inhibits chemokine (C-C motif) receptor 7 expression, tumor growth and metastasis in breast cancer. Oncotarget 2014; 6: 995–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gonzalez H, Hagerling C, Werb Z. Roles of the immune system in cancer: from tumor initation to metastatic progression. Genes Dev 2018; 32: 1267–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Taylor EB, An D, Kramer HF, Yu H, Fujii NL, Roeckl KS, et al. Discovery of TBC1D1 as an insulin-, AICAR-, and contraction-stimulated signaling nexus in mouse skeletal muscle. J Biol Chem 2008; 283: 9787–9796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wu N, Zheng B, Shaywitz A, Dagon Y, Tower C, Bellinger G et al. AMPK-dependent degradation of TXNIP upon energy stress leads to enhanced glucose uptake via GLUT1. Mol Cell 2013; 49: 1167–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zheng D, MacLean PS, Pohnert SC, Knight JB, Olson AL, Winder WW et al. Regulation of muscle GLUT-4 transcription by AMP-activated protein kinase. J Appl Physiol 2001; 91: 1073–1083. [DOI] [PubMed] [Google Scholar]
- 80.Stoppani J, Hildebrandt AL, Sakamato K, Cameron-Smith D, Goodyear LJ, Neufer PD. AMP-activated protein kinase activates transcription of the UCP3 and HKII genes in rat skeletal muscle. Am J Physiol Endocrinol Metab 2002; 283: E1239–1248. [DOI] [PubMed] [Google Scholar]
- 81.Marsin AS, Bertrand L, Rider MH, Deprez J, Beauloye C, Vincent MF et al. Phosphorylation and activation of heart PFK-2 by AMPK has a role in the stimulation of glycolysis during ischaemia. Curr Biol 2000; 10: 1247–1255. [DOI] [PubMed] [Google Scholar]
- 82.Marsin AS, Bouzin C, Bertrand L, Hue L. The stimulation of glycolysis by hypoxia in activated monocytes is mediated by AMP-activated protein kinase and inducible 6-phosphofructo-2-kinase. J Biol Chem 2002; 277: 30778–30783. [DOI] [PubMed] [Google Scholar]
- 83.Ahmed N, Zeng M, Sinha I, Polin L, We WZ, Rathinam C et al. The E3 ligase Itch and deubiquitinase Cyld act together to regulate Tak1 and inflammation. Nat Immunol 2011; 12: 1176–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ajibade AA, Wang Q, Cui J, Zou J, Xia X, Wang M et al. TAK1 negatively regulates NF-κB and p38 MAP kinase activation in Gr-1+CD11b+ neutrophils. Immunity 2012; 36: 43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Massague J TGFβ in cancer. Cell 2008; 134: 215–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Syed V TGFβ signaling in cancer. J Cell Biochem 2016; 117: 1279–1287. [DOI] [PubMed] [Google Scholar]
- 87.Morioka S, Inagaki M, Komatasu Y, Mishina Y, Matsumoto K, Ninomiya-Tsuji J. TAK1 kinase signaling regulates embryonic angiogenesis by modulating endothelial cell survival and migration. Blood 2012; 120: 3846–3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Naito H, Iba T, Wakabayashi T, Tai-Nagara I, Suehiro JI, Jia W et al. TAK1 prevents endothelial apoptosis and maintains vascular integrity. Dev Cell 2019; 48: 151–166. [DOI] [PubMed] [Google Scholar]
- 89.Singh A, Sweeney MF, Yu M, Burger A, Greninger P, Benes C et al. TAK1 inhibition promotes apoptosis in KRAS-dependent colon cancers. Cell 2012; 148: 639–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.McNew KL, Whipple WJ, Mehta AK, Grant TJ, Ray L, Kenny C, et al. MEK and TAK1 regulate apoptosis in colon cancer cells with KRAS-dependent activation of proinflammatory signaling. Mol Cancer Res 2016; 14: 1204–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Augeri DJ, Langenfeld E, Castle M, Gilleran JA, Langenfeld J. Inhibition of BMP and TGFβ receptors downregulates expression of XIAP and TAK1 leading to lung cancer cell death. Mol Cancer 2016; 15: 27, DOI 10.1186/s12943-016-0511-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Buglio D, Palakurthi S, Byth K, Vega F, Toader D, Saeh J et al. Essential role of TAK1 in regulating mantle cell lymphoma survival. Blood 2012; 120: 347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Totzke J, Gurbani D, Raphemot R, Hughes P, Bodoor K, Carlson DA et al. Takinib, a selective TAK1 inhibitor, broadens the therapeutic efficacy of TNF-α inhibition for cancer and autoimmune disease. Cell Chem Biol 2017; 24: 1029–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Melisi D, Xia Q, Paradiso G, Ling J, Moccia T, Carbone C et al. Modulation of pancreatic cancer chemoresistance by inhibition of TAK1. J Natl Cancer Inst 2011; 103: 1190–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Choo MK, Kawasaki N, Singhirunnusorn P, Koizumi K, Sato S, Akira S et al. Blockade of transforming growth factor-β-activated kinase 1 activity enhances TRAIL-induced apoptosis through activation of a caspase cascade. Mol Cancer Ther 2006; 5: 2970–2976. [DOI] [PubMed] [Google Scholar]
- 96.Vallet-Regi M, Colilla M, Izquierdo-Barba I, Manzano M. Mesoporous silica nanoparticles for drug delivery: current insights. Molecules 2018; 23: 47, 10.3390/molecules23010047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chowdhury SM, Lee T, Willmann JK. Ultrasound-guided drug delivery in cancer. Ultrasonography 2017; 36: 171–184. [DOI] [PMC free article] [PubMed] [Google Scholar]