Abstract
The melanocortin (MC) system consists of neuropeptides that are cleaved from the polypeptide precursor pro-opiomelanocortin (POMC). In the brain, MC neuropeptides signal primarily through the MC-3 and MC-4 receptors, which are widely expressed throughout the brain. While the MC system has been largely studied for its role in food intake and body weight regulation, converging evidence has emerged over approximately the last 20-years showing that alcohol (ethanol), and other drugs of abuse influence the central MC system, and that manipulating MC receptor signalling modulates ethanol intake. Although there is divergent evidence, the wealth of data appears to suggest that activating MC signalling, primarily through the MC-4 receptor, is protective against excessive ethanol consumption. In the present review, we first describe the MC system and then detail how ethanol exposure and consumption alters central MC and MC-receptor expression and levels. This is followed by a review of the data, from pharmacological and genetic studies, which show that manipulations of MC receptor activity alter ethanol intake. We then briefly highlight studies implicating a role for the MC system in modulating neurobiological responses and intake of other drugs of abuse, including amphetamine, cocaine and opioids. Finally, we introduce relatively new observations that the drug, bupropion (BUP), a drug that activates central MC activity, significantly reduces ethanol intake in rodent models when administered alone and in combination with the non-selective opioid receptor antagonist, naltrexone. Phase II clinical trials are currently underway to assess the efficacy of BUP as a treatment for alcohol use disorders.
Keywords: Melanocortin, agouti-related protein, alcohol, MC-4 receptor, bupropion, naltrexone
1. Introduction
Alcohol use, and specifically high-risk drinking, is considered one of the most serious public health problems worldwide, being the fifth leading risk factor for the global disease (Lim et al., 2012). According to World Health Organization, harmful use of alcohol is associated with 3.3 million of deaths every year and 5.1% of disability-adjusted life years (DALYs) (World Health Organization, 2018). The estimated annual cost of alcohol misuse, including alcohol use disorder (AUD), in the United States is $249 billion (Sacks et al., 2015). Given this, in 2018 the World Health Assembly approved a resolution to urge countries to strengthen their national responses to public health problems caused by the harmful use of alcohol (World Health Organization, 2018).
Further, in 2017, 70% of the USA population reported drinking alcohol in the past 12 months (Grant et al., 2017) and two in five of alcohol drinkers aged 18 and older reported to have heavy episodic drinking (Manthey et al., 2019). Heavy episodic or “binge” drinking is commonly defined as consuming enough alcohol to achieve blood concentration in excess of 80 mg/dl in a short period of time (National Institute on Alcohol Abuse and Alcoholism, 2004). This typically corresponds to consumption of five standard drinks or more for men (four or more for women) in about 2 hours. People who exhibit binge drinking have a higher risk of many serious health conditions, including heart disease, hypertension and type 2 diabetes (Courtney and Polich, 2009). It has also been demonstrated that binge drinking increased the risk of developing AUDs, especially when binge drinking begins in adolescents (Manthey et al., 2019).
Despite the high prevalence of harmful use of alcohol and the associated risks, pharmacotherapy options to help AUD patients achieve abstinence or reduce their alcohol consumption are rather limited. In fact, there are only three medications for treating AUDs approved by the U.S. Food and Drug Administration (FDA); that is, acamprosate, disulfiram and naltrexone. Recently, nalmefene, and opioid antagonist, was approved by the European Medicines Agency as a treatment for alcohol dependence (Goh and Morgan, 2017). However, the efficacy of these pharmacological treatments remains modest (Franck and Jayaram-Lindström, 2013). Additionally, they present some limitations such as occurrence of side effects and high dropout rates. With this in mind, the development of more efficacious and safer therapies to treat aspects of AUDs are needed. Over approximately the last 20 years, pre-clinical studies have suggested that neuropeptides may represent a new target for the therapeutic treatment of AUDs. In this review, we focus on one of these promising targets, neuropeptides of the melanocortin system.
2. Overview of the melanocortin system
The melanocortin (MC) system is composed of peptides that are cleaved from proopiomelanocortin (POMC), a polypeptide precursor synthesized in the anterior and intermediate lobes of the pituitary and in neurons of the arcuate nucleus of hypothalamus (Arc) and the nucleus of the solitary tract (NTS) (Cawley et al., 2016). After POMC protein cleavage by two prohormone convertases (PC1/3 and PC2), several melanocortin peptides are produced, including α-, β-, and γ-melanocyte stimulating hormones (MSH), as well as β-endorphin and adrenocorticotrophic hormone (ACTH) (Haskell-Luevano et al., 1999). The actions of the MC peptides are mediated by five G protein-coupled receptors (GPCRs) subtypes, namely MC1–5R, coupled to Gαs that stimulate the adenylyl cyclase activity and cAMP levels (Dores et al., 2016). Recently, in vitro studies demonstrated that MC3-R and MC4-R could also couple to ERK1/2-MAPK signal activation indicating that both receptors can couple to Gαi in addition to Gαs (Mountjoy, 2015)
Each MC ligand exhibits differential affinity for the MC receptors (MCR) which highlights the unique nature of each receptor. While all the MC peptides are approximately equipotent at the MC3-R (Roselli-Rehfuss et al., 1993), only ACTH can bind to MC2-R (Fridmanis et al., 2017). Another aspect that differentiates the MCRs is the tissue distribution profiles. All five MCR subtypes are expressed throughout the peripheral tissues, but only MC3-R and MC4-R have been detected in the brain (Mountjoy, 2010). The distribution of MC4-R is particularly enriched in cortex, hippocampus, amygdala, brainstem and hypothalamus (Gelez et al., 2010). In relation with the MC3-R, the greatest expression is found in the hypothalamus and the limbic system (Lindblom et al., 1998; Roselli-Rehfuss et al., 1993). Interestingly, unique to the MC system is the presence of endogenous antagonists, agouti-signalling protein (ASP) and agouti-related protein (AgRP) that exert their actions via antagonizing MCR (Chai et al., 2003). In addition, it has been demonstrated that AgRP entails inverse agonist activity at MC4-R; that is, directly decreasing cAMP levels within the cell (Haskell-Luevano and Monck, 2001; Nijenhuis et al., 2001). AgRP is highly expressed in the arcuate nucleus of hypothalamus (Arc) (Ollmann et al., 1997; Shutter et al., 1997) and is co-localized with neurons producing neuropeptide Y (NPY) and GABA (Krashes et al., 2013). By contrast, POMC neurons exhibit heterogeneous phenotypes that are not only GABAergic but also glutamatergic and cholinergic cells (Meister et al., 2006; Wittmann et al., 2013). A growing body of evidence suggest that these transmitters (α-MSH and AgRP) display opposing actions on MC3-Rs and MC4-Rs, affecting neuronal circuits and further hypothalamic-dependent physiological functions.
The MC peptides regulate multiple physiological functions from food intake and neuroendocrine function to sexual responses (Cone, 2006). The role of MC system in the regulation of food intake and energy homeostasis has been the best documented. Previous pharmacological studies demonstrated that central administration of a full agonist of MC3-and/or MC4-R potently inhibit food intake. Thus, the POMC-derived peptide α-MSH inhibits food intake and stimulates energy expenditure via activation of MC3-R-and MC4-R– expressing neurons, whereas AgRP antagonizes the effect of α-MSH on MC receptors, causing an increase in food intake and a decrease in energy expenditure (Atasoy et al., 2012; Yang and Harmon, 2003). Given the importance of MC4-R in appetite and energy regulation, it has been proposed as a drug target for obesity (Fani et al., 2014).
3. Role of MC on alcohol-related behaviours
Human evidence linking the MC system to human AUD is sparse, though one study identified linkage of a rare variants of POMC exons associated with overall substance use disorders, and alcohol, cocaine, and marijuana dependence in African American subjects (Wang et al., 2012). On the other hand, substantial pre-clinical evidence has emerged over approximately the last 20 years that MC neuropeptides also have potent and modulating effects on ethanol consumption (Navarro, 2017; Olney et al., 2014a). Several studies suggest a role for the MC system in high alcohol preference and consumption in mice and rats. For example, higher expression of POMC mRNA are exhibited in the Arc in selectively bred Sardinian alcohol-preferring (sP) rats (Zhou et al., 2013) and High Alcohol preferring (HAP) rats (Kinoshita et al., 2004) relative to their non-ethanol preferring counterparts. Other rats selectively bred to prefer alcohol, such as Alko Alcohol (AA) rats, have significantly higher ratio of POMC/AgRP expression in the Arc compared with Alko-non Alcohol (ANA) rats (Lindblom et al., 2002a). Interestingly, AA rats also exhibited low level of MC3-R in the nucleus accumbens (NAc) and high levels in the paraventricular nucleus (PVN), ventral medial (VMH) and Arc regions of the hypothalamus, when compared to ANA rats. Regarding MC4-R, AA rats showed higher levels in the VMH compared with the ANA rats (Lindblom et al., 2002a). Studies carried out on inbred strains of mice are in accordance with these data. Thus, high drinking C57BL/6 mice have a higher basal POMC mRNA levels in the hypothalamus than alcohol avoiding DBA/2 mice obtained from Charles River (St. Constant, Quebec, Canada) (Jamensky and Gianoulakis, 1999). Taking together, these data suggest a potential contribution of the MC system to the innate tendency of these animals to show high alcohol drinking and/or preference. Thus, the potential value of MC system as a biomarker for identifying at-risk individuals for developing AUDs is high.
Further, evidence of alterations of the MC system by alcohol exposure is extensive. For example, it has been demonstrated that chronic exposure to an ethanol liquid-diet can reduce levels of POMC and convertase PC1/3 (Navarro et al., 2013), as well as α-MSH levels in the Arc, regions of the extended amygdala, and the thalamus in Sprague-Dawley rats (Navarro et al., 2008; Rainero et al., 1990). However, an increase in α-MSH (Kokare et al., 2006; Müschen et al., 2019) or POMC levels (Rasmussen et al., 2002) have been reported during withdrawal after chronic ethanol exposure. In the same way, acute exposure to ethanol also leads to changes in the MC system. Interestingly, acute intraperitoneal (i.p.) administration of alcohol increase AgRP level in C57BL/6J mice but not in low ethanol drinking 129/SvJ mice (Cubero et al., 2010). Conversely, ethanol failed to alter POMC mRNA expression or α-MSH immunoreactivity in the Arc (Cubero et al., 2010; Kinoshita et al., 2000). Contradictory with these data, acute i.p. injection of ethanol significantly reduces α-MSH immunoreactivity in the Arc (Kokare et al., 2008). In spite of the differences observed between studies presented above, likely related to factors such as route of administration, duration of treatment, species studied, or method used to detect POMC and MC peptides, studies that entail voluntary ethanol consumption consistently show that repeated bouts of ethanol drinking induces changes in the MC system. For example, voluntary ethanol consumption increased hypothalamic POMC levels in C57BL/6J mice (De Waele and Gianoulakis, 1994) and POMC mRNA levels in the NAc shell and hypothalamus of sP rats (Zhou et al., 2013). In the same direction, self-administration of alcohol in an operant conditioning paradigm increases α-MSH IR in Arc, NAc and bed nucleus of the stria terminalis (Shelkar et al., 2015). When binge-like ethanol exposure occurs, a reduction in α-MSH IR in several hypothalamic regions and increase in AgRP IR in the PVN was observed (Lerma-Cabrera, Carvajal et al., 2013a; Sprow et al., 2016). These data suggest that ethanol’s effect on the MC system may be part of a mechanism contributing to increase ethanol drinking during the early stages of AUDs prior to the transition to dependence. Specifically, blunted α-MSH signalling and increased AgRP activity observed after binge drinking may compromise the protective effect exerted by the MC system and lead to continued binge-like drinking, ultimately leading to ethanol dependence.
The studies above provide compelling evidence that ethanol exposure, consumption, self-administration, and withdrawal after chronic ethanol exposure produce alterations in the central MC system and to the endogenous MC antagonist, AgRP. Pharmacological and genetic studies show that in addition to ethanol’s influence on the MC system, MCR signalling also modulates ethanol intake and neurobiological responses to ethanol. While most studies have focused on how the MC system modulates ethanol intake, it is interesting to note recent evidence showing a role for MC receptor signalling in the modulation of pain stemming from ethanol withdrawal. Interestingly, withdrawal from ethanol during dependence produces hyperalgesia, and intranasal, intraventricular, and CeA administration of a MC4-R antagonist blocked withdrawal-induced hyperalgesia (Roitsch Hellard et al., 2017; Avegno et al., 2018).
A summary of pharmacological and genetic studies implicating a role for the MC system in modulating ethanol intake can be found in Table 1. Perhaps the first study to provide evidence that MCR signalling modulates ethanol consumption showed that central infusion of the non-selective MCR agonist, melanotan-II (MTII), significantly blunted voluntary ethanol intake in selectively-bred AA rats (Ploj et al., 2002). Shortly thereafter, it was shown that both peripheral and central administration of MTII reduced voluntary ethanol intake in C57BL/6J mice, and that the ability of MTII to reduce ethanol intake was blocked be pre-treatment with the MCR antagonist, AgRP-(83–132), showing that the actions of MTII on ethanol intake were MCR-mediated (Navarro et al., 2003). Furthermore, the MC4-R appears to be the primary receptor that modulates the effect of MCR agonist on ethanol intake. For example, central infusion of MTII reduced ethanol intake similarly in mutant mice lacking the MC3-R and wild-type mice, while central infusion of a selective MC4-R agonist significantly blunted ethanol drinking (Navarro et al., 2005). Additionally, while peripheral administration of MTII significantly reduced ethanol intake in both mutant mice lacking MC4-R and wild-type control mice, MTII-induced reduction of ethanol intake was significantly blunted when given centrally to MC4-R mice, further evidence that the central action of MCR agonist on ethanol intake are mediated by the MC4-R (Navarro et al., 2011). However, it does appear that that MC3-R plays a significant role, as central infusion of MTII more robustly blunts ethanol intake in mutant mice lacking the MC3-R. This latter observation suggests that the MC3-R opposes the actions of MC4-R signalling in the modulation of ethanol intake (Olney et al., 2014b). There is also direct evidence for a role of AgRP in modulating ethanol intake, as central infusion of AgRP-(83–132) significantly increased voluntary ethanol intake in C57BL/6J mice (Navarro et al., 2005), and genetic deletion of AgRP was associated with a significant reduction of both operant self-administration of ethanol and binge-like ethanol intake in male and female mice. Interestingly, female, but not male, mutant mice lacking AgRP showed blunted voluntary consumption of ethanol, suggesting sex differences in the role of AgRP in modulating voluntary intake (Navarro et al., 2009).
Table 1.
Summary of effect of MCR agonists and antagonists, and gene knockouts, on alcohol-related behaviours
| Treatment | Mode of administration | Species | Effect on ethanol intake | Reference |
|---|---|---|---|---|
| MTII | i.c.v. | C57 mice | Decreased ethanol intake | Navarro et al., (2005, 2003) |
| AA rats | Decreased ethanol intake | Ploj et al., (2002) | ||
| msP rats | Decreased ethanol intake | Polidori et al., (2006) | ||
| MC4R −/− | No effect | Navarro et al., (2011) | ||
| MC3−/− | Decreased ethanol intake | Navarro et al., (2005) | ||
| MC3 −/− | Enhanced reduction of binge-like ethanol drinking | Olney et al., (2014b) | ||
| Intra-LH | C57 mice | Blunted binge-like ethanol consumption | Sprow et al., (2016) | |
| Intra-amygdala | P rats | Decreased voluntary consumption | York et al., (2011) | |
| α-MSH | Intra-VTA | Sprague-Dawley rats | Increased self-administration of ethanol | Shelkar et al., (2015) |
| MC4R selective | i.c.v | C57 mice | Decreased ethanol intake | Navarro et al., (2005) |
| agonist | Intra-VTA and - NAc | Sprague-Dawley rats | Decreased ethanol intake | Carvajal et al., (2017); Lerma-Cabrera et al., (2012) |
| Intra-NAc | Sprague-Dawley rats | Decreased ethanol palatability | Lerma-Cabrera et al., (2013b) | |
| MC4R antagonist | Intra-VTA | Sprague-Dawley rats | Blunt ethanol self-administration | (Shelkar et al., 2015) |
| AgRP | i.c.v. | C57 mice | Increased ethanol intake | Navarro et al., (2005) |
| Blocked MTII-induced reduced ethanol intake | Navarro et al., (2003) | |||
| AA rats | No effect | Ploj et al., (2002) | ||
| msP rats | No effect | Polidori et al., (2006) | ||
| Intra-LH | C57 mice | Increased binge-like ethanol consumption | Sprow et al., (2016) | |
| None | AgRP −/− | Decrease ethanol intake Decrease ethanol self-administration | Navarro et al., (2009) |
The brain regions in which MCR signalling modulates ethanol intake have also been studies. It has been demonstrated that administration of selective a MC4-R agonist into the VTA or NAc-shell blunted ethanol drinking in rats (Carvajal, Lerma-Cabrera et al., 2017; Lerma-Cabrera et al., 2012), indicating that MC system interacts directly with the mesolimbic dopaminergic reward pathway. Similarly, with the use of taste-reactivity procedures in rats to directly assess the hedonic evaluation of intraorally infused taste stimuli, it was found the NAcshell, but not LH, infusion of a selective MC4-R agonist reduced appetitive responding, and increased aversive responding, for intraorally infused ethanol (Lerma-Cabrera et al., 2013b). Regardless, when delivered into the LH, a brain area critical for modulation of the reinforcing properties of rewards (Marchant et al., 2012), infusion of MTII significantly blunted, and a selective MC4-R antagonist increased, binge-like ethanol consumption in C57BL/6J mice (Sprow et al., 2016). Finally, site-directed infusion of MTII into the amygdala blunted ethanol intake in selectively bred P rats (York et al., 2011).
While there is a large body of evidence that stimulating MCR signalling blunts ethanol intake, it must also be noted that there are inconsistencies in the literature. In a study involving Marchigan-Sardinian (msP) rats, a line selectively bred for high ethanol intake, central infusion of MTII only transiently reduced ethanol intake and AgRP had no effect. On the other hand, MTII robustly reduced feeding while AgRP increased food intake in msP rats. The authors concluded that MCR signalling does not modulate the pharmacodynamic effect of ethanol in msP rats, but may influence ethanol intake through regulation of caloric balance (Polidori et al., 2006). On the other hand, another study showed that MTII regulates intake of caloric and non-caloric reinforcing substances, which would indicate that MC signalling regulates the reinforcing properties of substances independent of calories (Navarro et al., 2011). Additionally, VTA infusion of α-MSH increased operant self-administration of ethanol in rats, while VTA infusion of a selective MC4-R antagonist blunt ethanol self-administration (Shelkar et al., 2015). Thus, while the majority of evidence points to increase MCR signalling as protective against ethanol intake, there are inconsistencies which may be explained by the rodent model employed (mice versus rats and selectively-bred models), the specific consumption paradigm used (voluntary consumption, binge-like drinking procedures, or ethanol self-administration) and route and site of administration, to name a few obvious differences between studies. Such inconsistencies highlight the need for caution when developing potential MC-based pharmacotherapies to treat AUDs. Attention to the factors that may contribute to individual differences in outcome success, such as population differences and differences in ethanol use patterns, will be necessary.
4. The MC system and other drugs of abuse
The peptide POMC has been implicated in the modulation of neurobiological responses to several drugs of abuse. In the last decades, several molecular and behavioural studies have shown that the MC system is involved in the regulation of neurochemical brain systems underlying the pharmacodynamic actions of drugs of abuse, in particular the dopamine system. For example, there is an established interaction between MC signalling and the dopaminergic system (Hao et al., 2015; Roseberry et al., 2015) and anatomical studies have shown that MC receptors are expressed very broadly in dopaminergic enriched brain regions, such as VTA and NAc (Alvaro et al., 1996; Mountjoy, 2015). Further, pharmacological studies have shown that acute central administration of AgRP increased dopamine in the medial prefrontal cortex (mPFC), but not in the NAc (Davis et al., 2011). Additionally, chronic central administration of MTII alters dopamine (DA) receptor binding in the NAc (Lindblom et al., 2002b) and direct administration of α-MSH into the VTA or LH stimulates dopamine release in the NAc (Legrand et al., 2015; Lindblom et al., 2001; Sánchez et al., 2001). DA increase in NAc was blocked by pre-treatment with a selective antagonist for MC4-R (Lindblom et al., 2001). Additionally, chronic administration of MTII alters dopamine 1 (D1) and dopamine 2 (D2) receptor expression in the striatum, VTA, and brainstem (Lindblom et al., 2002b) and chronic administration of haloperidol, the non-selective D1/D2 antagonist resulted in a significant upregulation of MC4-R mRNA in striatum (Alvaro, 2003), supporting the bidirectional interaction between the MC and DA systems. In addition, it has been demonstrated that application of α-MSH modifies excitatory synapses in D1 dopamine receptor-expressing medium spiny neurons in the NAc (Lim et al., 2012), suggesting that bidirectional interaction presented by the MC and DA systems could be mediated by MC4-R and D1 Receptors.
Given the evidence that the MC system regulates dopamine activity, it is not surprising that MCR signalling has also been linked to the behavioural and neurobiological responses to psychostimulant drugs such as cocaine and amphetamine. Thus, chronic administration of cocaine reduces the expression of POMC mRNA in the striatum and NAc (Valenza et al., 2016) and increases the expression of MC4-R mRNA in the striatum, hippocampus and NAc (Alvaro, 2003; Hsu et al., 2005). Further, acute administration of amphetamine increased POMC and MC3-R expression in the hypothalamus (Yu et al., 2018) Consistently, manipulating MCR signalling modulates the rewarding properties of amphetamine. Acute, central administration of MTII enhances the reinforcing properties of amphetamine, reflected by a potentiation of amphetamine’s ability to lower the threshold of electrical lateral hypothalamus selfstimulation (LHSS) in rats (Cabeza de Vaca et al., 2002). However, chronic administration of MTII fails to influence the effects of repeated amphetamine administration on LHSS, interpreted as potential tolerance to amphetamine with chronic exposure (Cabeza de Vaca et al., 2005). In this same line, Hsu and collaborators (2005) showed that site-directed administration into the NAc of the a non-selective MCR antagonist blocked self-administration of cocaine as well as the conditioning of place preference induced by cocaine, suggesting that blockade of MCR signalling in the NAc attenuates the reinforcing properties of cocaine (Cui and Lutter, 2013). Finally, there is also evidence that nicotine administration causes an upregulation of central MC-3R and MC4-R (Tapinc et al., 2017) and that MC4-R blockade blunts stress-induced reinstatement of nicotine-seeking in rats (Qi et al., 2015).
Both chronic administration and withdrawal of morphine produces a decrease in the expression of POMC mRNA in the hypothalamus (Bronstein et al., 1990; Le Merrer et al., 2009; Pintér-Kübler et al., 2013) and MC4-R mRNA in the NAc, periaqueductal gray (PAG) and striated nucleus (Alvaro et al., 1996), regions that modulate the reinforcing properties of opioids, opioid tolerance and opioid-induced psychomotor stimulation (Kalivas and Stewart, 1991). On the other hand, acute administration of morphine increases the expression of the mRNA of MC4-R in the amygdala (Starowicz et al., 2003). Behavioural studies also point to the role of the MC system in modulating neurobiological responses to opioids. Thus, for example, central administration of γ-MSH decreases the acquisition of heroin self-administration in rats (van Ree, 1983). Similarly, central administration of selective MC4-R antagonist, HS014, blocks the development of morphine tolerance (Niu et al., 2012). On the other hand, administration of a non-selective MCR antagonist into the CeA (Starowicz et al., 2003) or a selective MC4-R antagonist into the spinal cord (Starowicz et al., 2005) causes the restoration of the morphine tolerance response. Similarly, central administration of a selective MC4-R antagonist increases the antinocioceptive effects of morphine (Ercil et al., 2005). Thus, in addition to modulating neurobiological responses to ethanol, there is a wealth of evidence that the MC system also modulates neurobiological responses to stimulant drugs and opioids.
5. Bupropion, an activator of the MC system and potential therapeutic target for treating AUDs
The pre-clinical data above provide consistent evidence that ethanol and other drugs of abuse promote alteration in the central MC and AgRP systems, and conversely, manipulating MCR modulates drug and alcohol intake, with the wealth of evidence suggesting that activation of MCR is protective. This is suggestive evidence that targeting MCR may be an effective therapeutic target for treating drug and AUDs. Unfortunately, there are currently no clinically available pharmaceutical drugs that are designed to target MCRs, with the exception of a non-selective MC receptor agonist, bremelanotide (Vyleesi ®), a recently FDA-approved medication used to treat hypoactive sexual desire disorder in premenopausal women (Kingsberg et al., 2019). Interestingly, bupropion (BUP), a norepinephrine and DA re-uptake inhibitor (Foley et al., 2006; Nomikos et al., 1992), may be an effective strategy for treating AUDs as an activator of central MC signalling. Previous work to identify treatments for obesity found that simultaneous administration of BUP and the non-selective opioid receptor antagonist, naltrexone (NAL), synergistically reduced body weight which exceed the effects on monotherapy with either drug (Greenway et al., 2009a; Greenway et al., 2010), evidence which ultimately helped generate the FDA approved weigh loss drug Contrave®. Further, in vitro evidence showed that BUP stimulates murine POMC neurons, and to a greater degree when BUP and NAL are simultaneously applied (Greenway et al., 2009b). In turn, stimulation of POMC neurons would promote increased activity of the MC system. Greenway and colleagues hypothesized that the increased effectiveness of BUP when combined with NAL was the result of blockade of auto-inhibitory opioid receptor on POMC neurons in the hypothalamus allowing BUP to stimulate POMC, and by extension, MC activity, without inhibitory opposition (Greenway et al., 2009b). Interestingly, we have previously shown that combined MTII and NAL synergistically blunted binge-like ethanol intake in mice to a greater degree than either drug alone (Navarro et al., 2015), a striking parallel to the work by Greenway and colleagues that assessed the effects of combined BUP + NAL (Greenway et al., 2009a). We hypothesized that combined MTII + NAL therapy reduced ethanol intake via a similar mechanism by which BUP + NAL reduced food intake and obesity. This led us to the question, will BUP, alone and in combination with NAL, blunt ethanol intake in pre-clinical mouse models? We recently showed that peripheral administration of BUP significantly blunted binge-like ethanol intake and ethanol drinking following chronic intermittent access to ethanol in male C57BL/6J mice, and that peripheral administration of subthreshold doses of BUP and NAL that did not significantly blunt binge-like ethanol intake alone did so when combined in a cocktail. On the other hand, BUP alone or in combination with NAL failed to alter sucrose intake, and natural reward that entails calories (Navarro et al., 2019). BUP + NAL has also recently been found to reduce ethanol intake in male, but not female, mice (Zhou et al., 2019) and in rats selectively bred for high alcohol intake (Nicholson et al., 2018).
We recently replicated the combined BUP + NAL study based on Navarro et al., 2019 in male C57BL/6J mice. Data from this study are presented in Figure 1. Using the “drinking in the dark” procedure to model binge-like ethanol drinking (Rhodes et al., 2005; Thiele and Navarro, 2014), mice were given i.p. injection of a 3 mg/kg dose of NAL followed by an i.p. injection of a 20 mg/kg dose of BUP approximately 30 minutes before access to a 20% (v/v) ethanol solution. Other groups received two injections of vehicle, or an injection of vehicle and an injection of BUP or NAL. Consistent with Navarro et al., 2019, we again found that while BUP and NAL in subthreshold doses failed to significantly blunt binge-like ethanol intake, combined BUP + NAL was effective for up to an hour after treatment. As noted by Navarro et al., 2019, the short half-life of non-extended release BUP likely accounts for the transient effects of BUP, which were not evident by the second hour of testing. In light of our pre-clinical data and the clinical availability of therapeutic BUP and NAL, we recently performed an open-label pilot phase II clinical trial study and found suggestive evidence that combined BUP + NAL may be an effective approach to treated problematic binge drinking in humans (Walters et al., in press). We are currently conducting a more thorough phase II clinical trial experiment to determine the efficacy of BUP to curb binge drinking.
Figure 1:
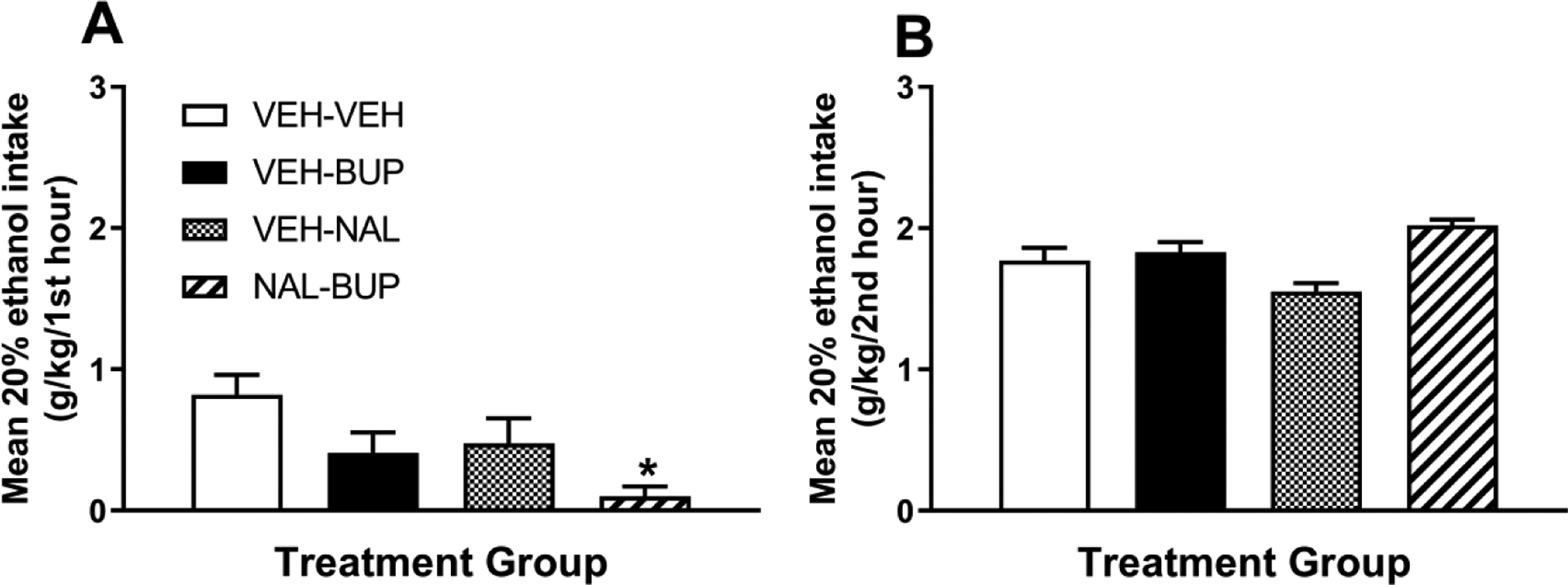
Binge-like ethanol consumption of a 20% (v/v) ethanol solution in male C57BL/6J mice beginning approximately 30 minutes after intraperitoneal (i.p.) injection of two injections of vehicle (VEH-VEH, n = 12), an i.p. injection of vehicle followed by an injection of a 20 mg/kg dose of BUP (VEH-BUP, n = 13), an i.p. injection of vehicle followed by an injection of a 3 mg/kg NAL (VEH-NAL, n = 12), or an i.p. injection of a 3 mg/kg dose of NAL followed by an injection of a 20 mg/kg dose of BUP (NAL-BUP, n = 12). There was a significant effect of drug treatment during the first hour of testing [F(3, 45) = 4.43, p = 0.008), and Bonferroni corrected t-tests indicated that relative to the VEH-VEH group, the NAL-BUP group drank significantly less ethanol (panel A). However, as seen in panel B, there were no group differences in ethanol intake during the second hour of testing [F(3, 45) = 0.67, p > 0.05]. Assessment of blood ethanol concentrations (VEH-VEH = 93.9 + 18.61 mg/dl; VEH-BUP = 88.93 + 15.49 mg/dl; VEHNAL = 44.51 + 9.07 mg/dl; NAL-BUP = 46.55 + 7.33 mg/dl) was statistically significant [F(3, 45) = 3.86, p = 0.015] though Bonferroni corrected t-tests failed to find individual group differences. All data are presented as mean + SEM. *p < 0.05 relative to VEH-VEH group.
6. Conclusions
In the present review, we have summarized previous pre-clinical studies that have shown the ethanol and drugs of abuse interact with the MC and AgRP systems, and that manipulating MCR significantly modulates ethanol intake, with the majority of evidence suggesting that activation of MC4-R is protective against excessive intake. MCR signalling may modulate drug and ethanol intake via modulation of central DA activity. While there are currently no clinically available drugs for targeting the MC system (with the exception of the recently FDA-approved drug, bremelanotide), evidence suggests that BUP stimulates central MC activity, making it an attractive target for treating AUDs. It should be noted that since BUP also functions as a DA and NE re-uptake inhibitor, this drug may influence ethanol intake via a variety of central actions. BUP has been approved by the FDA since 1985 primarily as an antidepressant medication (Ascher et al., 1995), but it is also used off-label to treating nicotine addiction (Foley et al., 2006) and for weight-loss therapy (Anderson et al., 2002). Our pre-clinical data support a potential role for BUP, alone and in combination with NAL (a drug that is already FDA-approved for treating AUDs), as a therapeutic target for treating excessive alcohol consumption. Finally, while we have initiated phase II clinical trial studies to assess the efficacy of BUP for treating AUDs, future large-scale phase III trials will be necessary. In summary, the present review provides converging evidence the targeting the MC system should continue to be considered a therapeutic target for treating drug and AUDs.
Highlights.
Alcohol and other drugs of abuse influence the central melanocortin system
Activation of melanocortin receptor signalling significantly reduces alcohol intake
Bupropion, which stimulates central melanocortin activity, reduces alcohol intake
Clinical trials are assessing the efficacy of bupropion for treating AUD
Acknowledgements
This work was supported by the NIH [AA013573, AA022048]; UNC School of Medicine/TraCS Translational Team Science Award [TTSA018P1]; and Program “José Castillejo” mobility stays abroad for young doctors [CAS18/00198].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alvaro JD, 2003. Molecular and Behavioral Interactions Between Central Melanocortins and Cocaine. J. Pharmacol. Exp. Ther 304, 391–399. 10.1124/jpet.102.040311 [DOI] [PubMed] [Google Scholar]
- Alvaro JD, Tatro JB, Quillan JM, Fogliano M, Eisenhard M, Lerner MR, Nestler EJ, Duman RS, 1996. Morphine down-regulates melanocortin-4 receptor expression in brain regions that mediate opiate addiction. Mol. Pharmacol 50. [PubMed] [Google Scholar]
- Anderson JW, Greenway FL, Fujioka K, Gadde KM, McKenney J, O’Neil PM, 2002. Bupropion SR Enhances Weight Loss: A 48-Week Double-Blind, Placebo-Controlled Trial. Obes. Res 10, 633–641. 10.1038/oby.2002.86 [DOI] [PubMed] [Google Scholar]
- Ascher JA, Cole JO, Colin JN, Feighner JP, Ferris RM, Fibiger HC, Golden RN, Martin P, Potter WZ, Richelson E, 1995. Bupropion: a review of its mechanism of antidepressant activity. J. Clin. Psychiatry 56, 395–401. [PubMed] [Google Scholar]
- Atasoy D, Betley JN, Su HH, Sternson SM, 2012. Deconstruction of a neural circuit for hunger. Nature 488, 172–177. 10.1038/nature11270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avegno EM, Lobell TD, Itoga CA, Baynes BB, Whitaker AM, Weera MM, Edwards S, Middleton JW, & Gilpin NW, 2018. Central amygdala circuits mediate hyperalgesia in alcohol-dependent rats. J. Neurosci, 38, 7761–7773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronstein DM, Przewlocki R, Akil H, 1990. Effects of morphine treatment on pro-opiomelanocortin systems in rat brain. Brain Res. 519, 102–111. 10.1016/0006-8993(90)90066-K [DOI] [PubMed] [Google Scholar]
- Cabeza de Vaca S, Hao J, Afroz T, Krahne LL, Carr KD, 2005. Feeding, body weight, and sensitivity to non-ingestive reward stimuli during and after 12-day continuous central infusions of melanocortin receptor ligands. Peptides 26, 2314–2321. 10.1016/j.peptides.2005.03.041 [DOI] [PubMed] [Google Scholar]
- Cabeza de Vaca S, Kim GY, Carr K, 2002. The melanocortin receptor agonist MTII augments the rewarding effect of amphetamine in ad-libitum-fed and food-restricted rats. Psychopharmacology (Berl). 161, 77–85. 10.1007/s00213-002-0998-1 [DOI] [PubMed] [Google Scholar]
- Carvajal F, Lerma-Cabrera JM, Alcaraz-Iborra M, Navarro M, Thiele TE, Cubero I, 2017. Nucleus Accumbens MC4-R Stimulation Reduces Food and Ethanol Intake in Adult Rats Regardless of Binge-Like Ethanol Exposure during Adolescence. Front. Behav. Neurosci 11, 1–10. 10.3389/fnbeh.2017.00167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawley NX, Li Z, Loh YP, 2016. 60 YEARS OF POMC: Biosynthesis, trafficking, and secretion of pro-opiomelanocortin-derived peptides. J. Mol. Endocrinol 56, T77–97. 10.1530/JME-15-0323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai B-X, Neubig RR, Millhauser GL, Thompson DA, Jackson PJ, Barsh GS, Dickinson CJ, Li J-Y, Lai Y-M, Gantz I, 2003. Inverse agonist activity of agouti and agouti-related protein. Peptides 24, 603–9. [DOI] [PubMed] [Google Scholar]
- Cone RD, 2006. Studies on the Physiological Functions of the Melanocortin System. Endocr. Rev 27, 736–749. 10.1210/er.2006-0034 [DOI] [PubMed] [Google Scholar]
- Courtney KE, Polich J, 2009. Binge drinking in young adults: Data, definitions, and determinants. Psychol. Bull 135, 142–56. 10.1037/a0014414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubero I, Navarro M, Carvajal F, Lerma-Cabrera JM, Thiele TE, 2010. Ethanol-Induced Increase of Agouti-Related Protein (AgRP) Immunoreactivity in the Arcuate Nucleus of the Hypothalamus of C57BL/6J, but not 129/SvJ, Inbred Mice. Alcohol. Clin. Exp. Res 34, 693–701. 10.1111/j.1530-0277.2009.01138.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui H, Lutter M, 2013. The expression of MC4Rs in D1R neurons regulates food intake and locomotor sensitization to cocaine. Genes, Brain Behav. 12, 658–665. 10.1111/gbb.12057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JF, Choi DL, Shurdak JD, Krause EG, Fitzgerald MF, Lipton JW, Sakai RR, Benoit SC, 2011. Central melanocortins modulate mesocorticolimbic activity and food seeking behavior in the rat. Physiol. Behav 102, 491–495. 10.1016/j.physbeh.2010.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Waele JP, Gianoulakis C, 1994. Enhanced activity of the brain β-endorphin system by free-choice ethanol drinking in C57BL/6 but not DBA/2 mice. Eur. J. Pharmacol 258, 119–129. 10.1016/0014-2999(94)90064-7 [DOI] [PubMed] [Google Scholar]
- Dores RM, Liang L, Davis P, Thomas AL, Petko B, 2016. 60 YEARS OF POMC: Melanocortin receptors: evolution of ligand selectivity for melanocortin peptides. J. Mol. Endocrinol 56, T119–T133. 10.1530/JME-15-0292 [DOI] [PubMed] [Google Scholar]
- Ercil NE, Galici R, Kesterson RA, 2005. HS014, a selective melanocortin-4 (MC4) receptor antagonist, modulates the behavioral effects of morphine in mice. Psychopharmacology (Berl). 180, 279–285. 10.1007/s00213-005-2166-x [DOI] [PubMed] [Google Scholar]
- Fani L, Bak S, Delhanty P, van Rossum EFC, van den Akker ELT, 2014. The melanocortin-4 receptor as target for obesity treatment: a systematic review of emerging pharmacological therapeutic options. Int. J. Obes. (Lond) 38, 163–9. 10.1038/ijo.2013.80 [DOI] [PubMed] [Google Scholar]
- Foley KF, DeSanty KP, Kast RE, 2006. Bupropion: pharmacology and therapeutic applications. Expert Rev. Neurother 6, 1249–1265. 10.1586/14737175.6.9.1249 [DOI] [PubMed] [Google Scholar]
- Franck J, Jayaram-Lindström N, 2013. Pharmacotherapy for alcohol dependence: status of current treatments. Curr. Opin. Neurobiol 23, 692–699. 10.1016/j.conb.2013.05.005 [DOI] [PubMed] [Google Scholar]
- Fridmanis D, Roga A, Klovins J, 2017. ACTH Receptor (MC2R) Specificity: What Do We Know About Underlying Molecular Mechanisms? Front. Endocrinol. (Lausanne) 8, 13 10.3389/fendo.2017.00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelez H, Poirier S, Facchinetti P, Allers KA, Wayman C, Bernabé J, Alexandre L, Giuliano F, 2010. Neuroanatomical distribution of the melanocortin-4 receptors in male and female rodent brain. J. Chem. Neuroanat 40, 310–324. 10.1016/j.jchemneu.2010.09.002 [DOI] [PubMed] [Google Scholar]
- Goh ET, Morgan MY, 2017. Review article: pharmacotherapy for alcohol dependence - the why, the what and the wherefore. Aliment. Pharmacol. Ther 45, 865–882. 10.1111/apt.13965 [DOI] [PubMed] [Google Scholar]
- Grant BF, Chou SP, Saha TD, Pickering RP, Kerridge BT, Ruan WJ, Huang B, Jung J, Zhang H, Fan A, Hasin DS, 2017. Prevalence of 12-Month Alcohol Use, High-Risk Drinking, and DSM-IV Alcohol Use Disorder in the United States, 2001–2002 to 2012–2013: Results From the National Epidemiologic Survey on Alcohol and Related Conditions. JAMA psychiatry 74, 911–923. 10.1001/jamapsychiatry.2017.2161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenway FL, Dunayevich E, Tollefson G, Erickson J, Guttadauria M, Fujioka K, Cowley MA, NB-201 Study Group, 2009a. Comparison of combined bupropion and naltrexone therapy for obesity with monotherapy and placebo. J. Clin. Endocrinol. Metab 94, 4898–906. 10.1210/jc.2009-1350 [DOI] [PubMed] [Google Scholar]
- Greenway FL, Fujioka K, Plodkowski RA, Mudaliar S, Guttadauria M, Erickson J, Kim DD, Dunayevich E, COR-I Study Group, 2010. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 376, 595–605. 10.1016/S0140-6736(10)60888-4 [DOI] [PubMed] [Google Scholar]
- Greenway FL, Whitehouse MJ, Guttadauria M, Anderson JW, Atkinson RL, Fujioka K, Gadde KM, Gupta AK, O’Neil P, Schumacher D, Smith D, Dunayevich E, Tollefson GD, Weber E, Cowley MA, 2009b. Rational Design of a Combination Medication for the Treatment of Obesity. Obesity 17, 30–39. 10.1038/oby.2008.461 [DOI] [PubMed] [Google Scholar]
- Hao Y, Tian X-B, Liu T-T, Liu C, Xiang H-B, Zhang J-G, 2015. Original Article MC4R expression in pedunculopontine nucleus involved in the modulation of midbrain dopamine system, Int J Clin Exp Pathol [PMC free article] [PubMed] [Google Scholar]
- Haskell-Luevano C, Chen P, Li C, Chang K, Smith MS, Cameron JL, Cone RD, 1999. Characterization of the neuroanatomical distribution of agouti-related protein immunoreactivity in the rhesus monkey and the rat. Endocrinology 140, 1408–15. 10.1210/endo.140.3.6544 [DOI] [PubMed] [Google Scholar]
- Haskell-Luevano C, Monck EK, 2001. Agouti-related protein functions as an inverse agonist at a constitutively active brain melanocortin-4 receptor. Regul. Pept 99, 1–7. [DOI] [PubMed] [Google Scholar]
- Hsu R, Taylor JR, Newton SS, Alvaro JD, Haile C, Han G, Hruby VJ, Nestler EJ, Duman RS, 2005. Blockade of melanocortin transmission inhibits cocaine reward. Eur. J. Neurosci 21, 2233–2242. 10.1111/j.1460-9568.2005.04038.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamensky NT, Gianoulakis C, 1999. Comparison of the proopiomelanocortin and proenkephalin opioid peptide systems in brain regions of the alcohol-preferring C57BL/6 and alcohol-avoiding DBA/2 mice. Alcohol 18, 177–87. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Stewart J, 1991. Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res. Rev 16, 223–244. 10.1016/0165-0173(91)90007-U [DOI] [PubMed] [Google Scholar]
- Kingsberg SA, Clayton AH, Portman D, Williams LA, Krop J, Jordan R, Lucas J, & Simon JA, 2019. Bremelanotide for the treatment of hypotactive sexual desire disorder: Two randomized phase 3 trials. Obstet. Gynecol, 134, 899–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita H, Harbuz MS, Nishiguchi M, Ouchi H, Minami T, Utsumi T, Motomura H, Hishida S, 2004. High Alcohol Preferring (HAP) And Low Alcohol Preferring (LAP) Rats Show Altered Proopiomelanocortin (POMC) Messenger Rna Expression In The Arcuate Nucleus. Alcohol Alcohol. 39, 406–409. 10.1093/alcalc/agh076 [DOI] [PubMed] [Google Scholar]
- Kinoshita H, Jessop DS, Finn DP, Coventry TL, Roberts DJ, Ameno K, Ijiri I, Harbuz MS, 2000. Acute ethanol decreases NPY mRNA but not POMC mRNA in the arcuate nucleus. Neuroreport 11, 3517–9. [DOI] [PubMed] [Google Scholar]
- Kokare DM, Chopde CT, Subhedar NK, 2006. Participation of α-melanocyte stimulating hormone in ethanol-induced anxiolysis and withdrawal anxiety in rats. Neuropharmacology 51, 536–545. 10.1016/j.neuropharm.2006.04.011 [DOI] [PubMed] [Google Scholar]
- Kokare DM, Singru PS, Dandekar MP, Chopde CT, Subhedar NK, 2008. Involvement of alpha-melanocyte stimulating hormone (α-MSH) in differential ethanol exposure and withdrawal related depression in rat: Neuroanatomical-behavioral correlates. Brain Res. 1216, 53–67. 10.1016/j.brainres.2008.03.064 [DOI] [PubMed] [Google Scholar]
- Krashes MJ, Shah BP, Koda S, Lowell BB, 2013. Rapid versus Delayed Stimulation of Feeding by the Endogenously Released AgRP Neuron Mediators GABA, NPY, and AgRP. Cell Metab. 18, 588–595. 10.1016/j.cmet.2013.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Merrer J, Becker JAJ, Befort K, Kieffer BL, 2009. Reward Processing by the Opioid System in the Brain. Physiol. Rev 89, 1379–1412. 10.1152/physrev.00005.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legrand R, Lucas N, Breton J, Déchelotte P, Fetissov SO, 2015. Dopamine release in the lateral hypothalamus is stimulated by α-MSH in both the anticipatory and consummatory phases of feeding. Psychoneuroendocrinology 56, 79–87. 10.1016/j.psyneuen.2015.02.020 [DOI] [PubMed] [Google Scholar]
- Lerma-Cabrera JM, Carvajal F, Alcaraz-Iborra M, De La Fuente L, Navarro M, Thiele TE, Cubero I, 2013b. Adolescent binge-like ethanol exposure reduces basal ??-MSH expression in the hypothalamus and the amygdala of adult rats. Pharmacol. Biochem. Behav 110, 66–74. 10.1016/j.pbb.2013.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerma-Cabrera JM, Carvajal F, Chotro G, Gaztañaga M, Navarro M, Thiele TE, Cubero I, 2013a. MC4-R signaling within the nucleus accumbens shell, but not the lateral hypothalamus, modulates ethanol palatability in rats. Behav. Brain Res 239, 51–54. 10.1016/j.bbr.2012.10.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerma-Cabrera JM, Carvajal F, De la Torre L, De la Fuente L, Navarro M, Thiele TE, Cubero I, 2012. Control of food intake by MC4-R signaling in the lateral hypothalamus, nucleus accumbens shell and ventral tegmental area: Interactions with ethanol. Behav. Brain Res 234, 51–60. 10.1016/j.bbr.2012.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, AlMazroa MA, Amann M, Anderson HR, Andrews KG, Aryee M, Atkinson C, Bacchus LJ, Bahalim AN, Balakrishnan K, Balmes J, Barker-Collo S, Baxter A, Bell ML, Blore JD, Blyth F, Bonner C, Borges G, Bourne R, Boussinesq M, Brauer M, Brooks P, Bruce NG, Brunekreef B, Bryan-Hancock C, Bucello C, Buchbinder R, Bull F, Burnett RT, Byers TE, Calabria B, Carapetis J, Carnahan E, Chafe Z, Charlson F, Chen H, Chen JS, Cheng AT-A, Child JC, Cohen A, Colson KE, Cowie BC, Darby S, Darling S, Davis A, Degenhardt L, Dentener F, Des Jarlais DC, Devries K, Dherani M, Ding EL, Dorsey ER, Driscoll T, Edmond K, Ali SE, Engell RE, Erwin PJ, Fahimi S, Falder G, Farzadfar F, Ferrari A, Finucane MM, Flaxman S, Fowkes FGR, Freedman G, Freeman MK, Gakidou E, Ghosh S, Giovannucci E, Gmel G, Graham K, Grainger R, Grant B, Gunnell D, Gutierrez HR, Hall W, Hoek HW, Hogan A, Hosgood HD, Hoy D, Hu H, Hubbell BJ, Hutchings SJ, Ibeanusi SE, Jacklyn GL, Jasrasaria R, Jonas JB, Kan H, Kanis JA, Kassebaum N, Kawakami N, Khang Y-H, Khatibzadeh S, Khoo J-P, Kok C, Laden F, Lalloo R, Lan Q, Lathlean T, Leasher JL, Leigh J, Li Y, Lin JK, Lipshultz SE, London S, Lozano R, Lu Y, Mak J, Malekzadeh R, Mallinger L, Marcenes W, March L, Marks R, Martin R, McGale P, McGrath J, Mehta S, Memish ZA, Mensah GA, Merriman TR, Micha R, Michaud C, Mishra V, Hanafiah KM, Mokdad AA, Morawska L, Mozaffarian D, Murphy T, Naghavi M, Neal B, Nelson PK, Nolla JM, Norman R, Olives C, Omer SB, Orchard J, Osborne R, Ostro B, Page A, Pandey KD, Parry CD, Passmore E, Patra J, Pearce N, Pelizzari PM, Petzold M, Phillips MR, Pope D, Pope CA, Powles J, Rao M, Razavi H, Rehfuess EA, Rehm JT, Ritz B, Rivara FP, Roberts T, Robinson C, Rodriguez-Portales JA, Romieu I, Room R, Rosenfeld LC, Roy A, Rushton L, Salomon JA, Sampson U, Sanchez-Riera L, Sanman E, Sapkota A, Seedat S, Shi P, Shield K, Shivakoti R, Singh GM, Sleet DA, Smith E, Smith KR, Stapelberg NJ, Steenland K, Stöckl H, Stovner LJ, Straif K, Straney L, Thurston GD, Tran JH, Van Dingenen R, van Donkelaar A, Veerman JL, Vijayakumar L, Weintraub R, Weissman MM, White RA, Whiteford H, Wiersma ST, Wilkinson JD, Williams HC, Williams W, Wilson N, Woolf AD, Yip P, Zielinski JM, Lopez AD, Murray CJ, Ezzati M, 2012. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380, 2224–2260. 10.1016/S0140-6736(12)61766-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindblom J, Kask A, Hägg E, Härmark L, Bergström L, Wikberg J, 2002b. Chronic infusion of a melanocortin receptor agonist modulates dopamine receptor binding in the rat brain. Pharmacol. Res 45, 119–124. 10.1006/phrs.2001.0913 [DOI] [PubMed] [Google Scholar]
- Lindblom J, Opmane B, Mutulis F, Mutule I, Petrovska R, Klusa V, Bergström L, Wikberg JE, 2001. The MC4 receptor mediates alpha-MSH induced release of nucleus accumbens dopamine. Neuroreport 12, 2155–8. [DOI] [PubMed] [Google Scholar]
- Lindblom J, Schiöth HB, Larsson A, Wikberg JE, Bergström L, 1998. Autoradiographic discrimination of melanocortin receptors indicates that the MC3 subtype dominates in the medial rat brain. Brain Res. 810, 161–171. 10.1016/S0006-8993(98)00918-4 [DOI] [PubMed] [Google Scholar]
- Lindblom J, Wikberg JE, Bergström L, 2002a. Alcohol-preferring AA rats show a derangement in their central melanocortin signalling system. Pharmacol. Biochem. Behav 72, 491–496. 10.1016/S0091-3057(02)00719-0 [DOI] [PubMed] [Google Scholar]
- Manthey J, Shield KD, Rylett M, Hasan OSM, Probst C, Rehm J, 2019. Global alcohol exposure between 1990 and 2017 and forecasts until 2030: a modelling study. Lancet S0140–6736, 32744–2. 10.1016/S0140-6736(18)32744-2 [DOI] [PubMed] [Google Scholar]
- Marchant NJ, Millan EZ, McNally GP, 2012. The hypothalamus and the neurobiology of drug seeking. Cell. Mol. Life Sci 69, 581–597. 10.1007/s00018-011-0817-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister B, Gömüç B, Suarez E, Ishii Y, Dürr K, Gillberg L, 2006. Hypothalamic proopiomelanocortin (POMC) neurons have a cholinergic phenotype. Eur. J. Neurosci 24, 2731–2740. 10.1111/j.1460-9568.2006.05157.x [DOI] [PubMed] [Google Scholar]
- Mountjoy KG, 2015. Pro-Opiomelanocortin (POMC) Neurones, POMC-Derived Peptides, Melanocortin Receptors and Obesity: How Understanding of this System has Changed Over the Last Decade. J. Neuroendocrinol 27, 406–418. 10.1111/jne.12285 [DOI] [PubMed] [Google Scholar]
- Mountjoy KG, 2010. Distribution and Function of Melanocortin Receptors within the Brain. Springer, New York, NY, pp. 29–48. 10.1007/978-1-4419-6354-3_3 [DOI] [PubMed] [Google Scholar]
- Müschen LH, Rhein M, Hoppe V, John N, Schwabe K, Frieling H, Bleich S, Muschler MAN, 2019. Alcohol Withdrawal and Proopiomelanocortin Neuropeptides in an Animal Model of Alcohol Dependence. Neuropsychobiology 1–10. 10.1159/000499844 [DOI] [PubMed] [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism, 2004. NIAAA Council Approves Definition of Binge Drinking. NIAAA Newsl. 3, 3:3. [Google Scholar]
- Navarro M, 2017. The Role of the Melanocortin System in Drug and Alcohol Abuse, International Review of Neurobiology. 10.1016/bs.irn.2017.06.009 [DOI] [PubMed] [Google Scholar]
- Navarro M, Carvajal F, Lerma-Cabrera JM, Cubero I, Picker MJ, Thiele TE, 2015. Evidence that Melanocortin Receptor Agonist Melanotan-II Synergistically Augments the Ability of Naltrexone to Blunt Binge-Like Ethanol Intake in Male C57BL/6J Mice. Alcohol. Clin. Exp. Res 39, 1425–1433. 10.1111/acer.12774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro M, Cubero I, Chen AS, Chen HY, Knapp DJ, Breese GR, Marsh DJ, Thiele TE, 2005. Effects of melanocortin receptor activation and blockade on ethanol intake: a possible role for the melanocortin-4 receptor. Alcohol Clin Exp Res 29, 949–957. https://doi.org/00000374-200506000-00004 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro M, Cubero I, Knapp DJ, Breese GR, Thiele TE, 2008. Decreased Immunoreactivity of the Melanocortin Neuropeptide α-Melanocyte-Stimulating Hormone (α-MSH) After Chronic Ethanol Exposure in Sprague-Dawley Rats. Alcohol. Clin. Exp. Res 32, 266–276. 10.1111/j.1530-0277.2007.00578.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro M, Cubero I, Knapp DJ, Thiele TE, 2003. MTII-induced reduction of voluntary ethanol drinking is blocked by pretreatment with AgRP-(83–132). Neuropeptides 37, 338–344. 10.1016/j.npep.2003.10.003 [DOI] [PubMed] [Google Scholar]
- Navarro M, Cubero I, Ko L, Thiele TE, 2009. Deletion of agouti-related protein blunts ethanol self-administration and binge-like drinking in mice. Genes, Brain Behav. 8, 450–458. 10.1111/j.1601-183X.2009.00493.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro M, Cubero I, Thiele TE, 2013. Decreased Immunoreactivity of the Polypeptide Precursor Pro-Opiomelanocortin (POMC) and the Prohormone Convertase PC1/3 After Chronic Ethanol Exposure in Sprague-Dawley Rats. Alcohol. Clin. Exp. Res 37, 399–406. 10.1111/j.1530-0277.2012.01951.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro M, Lerma-Cabrera JM, Carvajal F, Lowery EG, Cubero I, Thiele TE, 2011. Assessment of voluntary ethanol consumption and the effects of a melanocortin (MC) receptor agonist on ethanol intake in mutant C57BL/6J mice lacking the MC-4 receptor. Alcohol. Clin. Exp. Res 35, 1058–1066. 10.1111/j.1530-0277.2011.01438.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro M, Luhn KL, Kampov-Polevoy AB, Garbutt JC, Thiele TE, 2019. Bupropion, Alone and in Combination with Naltrexone, Blunts Binge-Like Ethanol Drinking and Intake Following Chronic Intermittent Access to Ethanol in Male C57BL/6J Mice. Alcohol. Clin. Exp. Res 43, 783–790. 10.1111/acer.13992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson ER, Dilley JE, Froehlich JC, 2018. Co-Administration of Low-Dose Naltrexone and Bupropion Reduces Alcohol Drinking in Alcohol-Preferring (P) Rats. Alcohol. Clin. Exp. Res 42, 571–577. 10.1111/acer.13577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijenhuis WAJ, Oosterom J, Adan RAH, 2001. AgRP(83–132) Acts as an Inverse Agonist on the Human-Melanocortin-4 Receptor. Mol. Endocrinol 15, 164–171. 10.1210/mend.15.1.0578 [DOI] [PubMed] [Google Scholar]
- Niu Z, Ma J, Chu H, Zhao Y, Feng W, Cheng Y, 2012. Melanocortin 4 receptor antagonists attenuates morphine antinociceptive tolerance, astroglial activation and cytokines expression in the spinal cord of rat. Neurosci. Lett 529, 112–117. 10.1016/j.neulet.2012.09.034 [DOI] [PubMed] [Google Scholar]
- Nomikos GG, Damsma G, Wenkstern D, Fibiger HC, 1992. Effects of chronic bupropion on interstitial concentrations of dopamine in rat nucleus accumbens and striatum. Neuropsychopharmacology 7, 7–14. [PubMed] [Google Scholar]
- Ollmann MM, Wilson BD, Yang YK, Kerns JA, Chen Y, Gantz I, Barsh GS, 1997. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science 278, 135–8. 10.1126/science.278.5335.135 [DOI] [PubMed] [Google Scholar]
- Olney JJ, Navarro M, Thiele TE, 2014. Targeting central melanocortin receptors: A promising novel approach for treating alcohol abuse disorders. Front. Neurosci 8, 1–9. 10.3389/fnins.2014.00128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olney JJ, Sprow GM, Navarro M, Thiele TE, 2014. The protective effects of the melanocortin receptor (MCR) agonist, melanotan-II (MTII), against binge-like ethanol drinking are facilitated by deletion of the MC3 receptor in mice. Neuropeptides 48, 47–51. 10.1016/j.npep.2013.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pintér-Kübler B, Ferenczi S, Núnez C, Zelei E, Polyák A, Milanés MV, Kovács KJ, 2013. Differential Changes in Expression of Stress- and Metabolic-Related Neuropeptides in the Rat Hypothalamus during Morphine Dependence and Withdrawal. PLoS One 8, e67027 10.1371/journal.pone.0067027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploj K, Roman E, Kask A, Hyytiä P, Schiöth HB, Wikberg JES, Nylander I, 2002. Effects of melanocortin receptor ligands on ethanol intake and opioid peptide levels in alcohol-preferring AA rats. Brain Res. Bull 59, 97–104. 10.1016/s0361-9230(02)00844-4 [DOI] [PubMed] [Google Scholar]
- Polidori C, Geary N, Massi M, 2006. Effect of the melanocortin receptor stimulation or inhibition on ethanol intake in alcohol-preferring rats. Peptides 27, 144–149. 10.1016/j.peptides.2005.07.008 [DOI] [PubMed] [Google Scholar]
- Qi X, Yamada H, Corrie LW, Ji Y, Bauzo RM, Alexander JC, & Bruijnzeel AW, 2015. A critical role for the melanocortin 4 receptor in stress-induced relapse to nicotine seeking in rats. Addict. Biol 20, 324–335. [DOI] [PubMed] [Google Scholar]
- Rainero I, De Gennaro T, Visentin G, Brunetti E, Cerrato P, Torre E, Portaleone P, Pinessi L, 1990. Effects of chronic ethanol treatment on α-MSH concentrations in rat brain and pituitary. Neuropeptides 15, 139–141. 10.1016/0143-4179(90)90145-O [DOI] [PubMed] [Google Scholar]
- Rasmussen DD, Boldt BM, Wilkinson CW, Mitton DR, 2002. Chronic daily ethanol and withdrawal: 3. Forebrain pro-opiomelanocortin gene expression and implications for dependence, relapse, and deprivation effect. Alcohol. Clin. Exp. Res 26, 535–546. 10.1111/j.1530-0277.2002.tb02572.x [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC, 2005. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol. Behav 84, 53–63. 10.1016/j.physbeh.2004.10.007 [DOI] [PubMed] [Google Scholar]
- Roitsch Hellard EA, Impastato RA, & Gilpin NW, 2017. Intra-cerebral and intra-nasal melanocortin-4 receptor antagonist blocks withdrawal hyperalgesia in alcohol dependent rats. Addict. Biol, 22, 692–701. [DOI] [PubMed] [Google Scholar]
- Roseberry AG, Stuhrman K, Dunigan AI, 2015. Regulation of the mesocorticolimbic and mesostriatal dopamine systems by α-melanocyte stimulating hormone and agouti-related protein. Neurosci. Biobehav. Rev 56, 15–25. 10.1016/j.neubiorev.2015.06.020 [DOI] [PubMed] [Google Scholar]
- Roselli-Rehfuss L, Mountjoy KG, Robbins LS, Mortrud MT, Low MJ, Tatro JB, Entwistle ML, Simerly RB, Cone RD, 1993. Identification of a receptor for gamma melanotropin and other proopiomelanocortin peptides in the hypothalamus and limbic system. Proc. Natl. Acad. Sci. U. S. A 90, 8856–60. 10.1073/pnas.90.19.8856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks JJ, Gonzales KR, Bouchery EE, Tomedi LE, Brewer RD, 2015. 2010 National and State Costs of Excessive Alcohol Consumption. Am. J. Prev. Med 49, e73–e79. 10.1016/j.amepre.2015.05.031 [DOI] [PubMed] [Google Scholar]
- Sánchez MS, Barontini M, Armando I, Celis ME, 2001. Correlation of Increased Grooming Behavior and Motor Activity with Alterations in Nigrostriatal and Mesolimbic Catecholamines After α-Melanotropin and Neuropeptide Glutamine–Isoleucine Injection in the Rat Ventral Tegmental Area. Cell. Mol. Neurobiol 21, 523–533. 10.1023/A:1013871407464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelkar GP, Kale AD, Singh U, Singru PS, Subhedar NK, Kokare DM, 2015. Alpha-melanocyte stimulating hormone modulates ethanol self-administration in posterior ventral tegmental area through melanocortin-4 receptors. Addict. Biol 20, 302–315. 10.1111/adb.12126 [DOI] [PubMed] [Google Scholar]
- Shutter JR, Graham M, Kinsey AC, Scully S, Lüthy R, Stark KL, 1997. Hypothalamic expression of ART, a novel gene related to agouti, is up-regulated in obese and diabetic mutant mice. Genes Dev. 11, 593–602. 10.1101/gad.11.5.593 [DOI] [PubMed] [Google Scholar]
- Sprow GM, Rinker JA, Lowery-Gointa EG, Sparrow AM, Navarro M, Thiele TE, 2016. Lateral hypothalamic melanocortin receptor signaling modulates binge-like ethanol drinking in C57BL/6J mice. Addict. Biol 21, 835–46. 10.1111/adb.12264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starowicz K, Obara I, Przewłocki R, Przewlocka B, 2005. Inhibition of morphine tolerance by spinal melanocortin receptor blockade. Pain 117, 401–11. 10.1016/j.pain.2005.07.003 [DOI] [PubMed] [Google Scholar]
- Starowicz K, Sieja A, Bilecki W, Obara I, Przewlocka B, 2003. The effect of morphine on MC4 and CRF receptor mRNAs in the rat amygdala and attenuation of tolerance after their blockade. Brain Res. 990, 113–119. 10.1016/S0006-8993(03)03444-9 [DOI] [PubMed] [Google Scholar]
- Tapinc DE, IIgin R, Kaya E, Gozen O, Ugur M, Koylu EO, Kanit L, Keser A, & Balkan B, 2017. Gene expression of pro-opiomelanocortin and melanocortin receptors is regulated in the hypothalamus and mesocorticolimbic system following nicotine adminisration. Neurosci. Lett 10, 75–79. [DOI] [PubMed] [Google Scholar]
- Thiele TE, Navarro M, 2014. “Drinking in the dark” (DID) procedures: A model of binge-like ethanol drinking in non-dependent mice. Alcohol 48, 235–241. 10.1016/j.alcohol.2013.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenza M, Picetti R, Yuferov V, Butelman ER, Kreek MJ, 2016. Strain and cocaine-induced differential opioid gene expression may predispose Lewis but not Fischer rats to escalate cocaine self-administration. Neuropharmacology 105, 639–650. 10.1016/j.neuropharm.2016.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ree JM, 1983. The influence of neuropeptides related to pro-opiomelanocortin on acquisition of heroin self-administration of rats. Life Sci. 33, 2283–2289. 10.1016/0024-3205(83)90261-8 [DOI] [PubMed] [Google Scholar]
- Walter TJ, Navarro M, Thiele TE, Perdersen C, Kampov-Polevoy A, & Garbutt JC, in press. An open-label study of naltrexone and bupropion combination therapy for treating binge drinking in human subjects. Alcohol Alcoholism. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Gelernter J, Kranzler HR, & Zhang H, 2012. Identification of POMC exonic variants associated with substance dependence and body mass index. PLoS One, 7, e45300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann G, Hrabovszky E, Lechan RM, 2013. Distinct glutamatergic and GABAergic subsets of hypothalamic pro-opiomelanocortin neurons revealed by in situ hybridization in male rats and mice. J. Comp. Neurol 521, 3287–3302. 10.1002/cne.23350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization, 2018. Global Status Report on Alcohol and Health 2018, Global Status Report on Alcohol and Health 2018. 10.1017/CBO9781107415324.004 [DOI]
- Yang YK, Harmon CM, 2003. Recent developments in our understanding of melanocortin system in the regulation of food intake. Obes. Rev 4, 239–48. [DOI] [PubMed] [Google Scholar]
- York DA, Boghossian S, Park-York M, 2011. Melanocortin activity in the amygdala influences alcohol intake. Pharmacol. Biochem. Behav 98, 112–119. 10.1016/j.pbb.2010.12.010 [DOI] [PubMed] [Google Scholar]
- Yu CH, Hsieh YS, Chen PN, Chen JR, Kuo DY, 2018. Knockdown of the transcript of ERK in the brain modulates hypothalamic neuropeptide-mediated appetite control in amphetamine-treated rats. Br. J. Pharmacol 175, 726–739. 10.1111/bph.14120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Colombo G, Niikura K, Carai MAM, Femenía T, García-Gutiérrez MS, Manzanares J, Ho A, Gessa GL, Kreek MJ, 2013. Voluntary alcohol drinking enhances proopiomelanocortin gene expression in nucleus accumbens shell and hypothalamus of Sardinian alcohol-preferring rats. Alcohol. Clin. Exp. Res 37 Suppl 1, E131–40. 10.1111/j.1530-0277.2012.01867.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Leri F, Low MJ, Kreek MJ, 2019. Sex differences in the effect of bupropion and naltrexone combination on alcohol drinking in mice. Pharmacol. Biochem. Behav 181, 28–36. 10.1016/j.pbb.2019.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]


