Abstract
Endometriosis is a hormone-responsive gynecologic disease, signifying its connotations across a woman’s life span. Previous studies suggested that endocrine disrupting chemicals were risk factors for endometriosis. Nevertheless, little is known on exposure to organophosphate, pyrethroid and phenoxy acid pesticides on endometriosis diagnosis. In this study, we determined the concentrations of 11 pesticides, metabolites of organophosphate and pyrethroid insecticides, and phenoxy herbicides, in urine collected from 619 reproductive-age women in Utah and California, using liquid chromatography-tandem mass spectrometry. The association of urinary concentrations of pesticides with an increase in the odds of endometriosis diagnosis was examined in 594 women who underwent laparoscopy/laparotomy (operative cohort: n = 471) or pelvic magnetic resonance imaging (population cohort: n = 123), during 2007–2009. 2-Isopropyl-4-methyl-6-hydroxypyrimidine (IMPY), malathion dicarboxylic acid (MDA), para-nitrophenol (PNP), 3,5,6-trichloro-2-pyridinol (TCPY), 3-phenoxybenzoic acid (3-PBA), and 2,4-dichlorophenoxyacetic acid (2,4-D) were detected in ≥95% of the urine samples analyzed. Urinary concentrations of IMPY, MDA, PNP, 3-PBA and 2,4-D tended to be higher in younger, non-Hispanic black, nulliparous and less affluent women. IMPY was the most dominant compound in urine followed by PNP and TCPY. When women in the 4th quartile of IMPY and the 2nd quartile of TCPY concentrations (μg/g creatinine) were compared with women in the 1st quartile, the odds ratios (ORs) for diagnosis of endometriosis increased significantly in unadjusted models (IMPY OR = 1.89, 95% confidence interval (CI) = 1.12–3.20; TCPY OR = 1.65, 95% CI = 1.02–2.69) for the operative (n = 471) and entire data set (n = 594), respectively. Our results suggest that exposure to elevated concentrations of diazinon (the parent compound of IMPY) and chlorpyrifos and chlorpyrifos-methyl (parent compounds of TCPY) may be associated with endometriosis.
Keywords: Endometriosis, organophosphate, pyrethroid, phenoxyacid, pesticide, urine
Graphical Abstract
1. Introduction
Endometriosis is an estrogen-dependent gynecologic disease, affecting around 176 million women worldwide (Kvaskoff et al., 2015). At least 11% of menstruating women in the U.S. are reported to have asymptomatic endometriosis (Buck Louis et al., 2011). Health implications of endometriosis include infertility and a higher risk of gravid, cancer and cardiovascular diseases (Kvaskoff et al., 2015; Shafrir et al., 2018; Smarr et al., 2016). Although several factors such as genetic predisposition, immune dysfunction and hormonal imbalances are likely involved in the pathogenesis of endometriosis, its etiology remains elusive (Kunisue et al., 2012). It is reported that 145,000 cases of endometriosis annually result from exposure to endocrine disrupting chemicals (EDCs) across European Union (Hunt et al., 2016). However, studies that describe the link between endometriosis and EDCs, such as dioxins (Eskenazi et al., 2002; Pauwels et al., 2001), organochlorine pesticides (Buck Louis et al., 2012a; Porpora et al., 2009; Upson et al., 2013), polybrominated diphenyl ethers (Buck Louis et al., 2012a), polychlorinated biphenyls (Buck Louis et al., 2012a; Pauwels et al., 2001; Reddy et al., 2006; Trabert et al., 2010), per- and poly-fluoroalkyl substances (Buck Louis et al., 2012b; Campbell et al., 2016; Wang et al., 2017), benzophenone-type UV filters (Kunisue et al., 2012), bisphenol A (Buck Louis et al., 2013) and phthalates (Buck Louis et al., 2013; Reddy et al., 2006; Upson et al., 2013; Weuve et al., 2010), have yielded mixed results (Smarr et al., 2016). Little is known on the association between exposure to pesticides, especially organophosphates/pyrethroids, and endometriosis (Table S1) serving as the impetus for study (Dewailly et al., 2014; Li and Kannan, 2018; Smarr et al., 2016; Ye et al., 2017).
Organophosphorus (OP) and pyrethroid (PYR) insecticides as well as phenoxy acid (PA) herbicides are the most widely used pesticides in agriculture, homes, and gardens globally (Oulhote and Bouchard, 2013). The general population can be exposed to these pesticides via diet, inhalation, and dermal absorption (Gracia-Lor et al., 2017; McKelvey et al., 2013). Since 2000, biomonitoring studies in the U.S. and several European countries have reported exposure to pesticides and their metabolites in urine (Becker et al., 2006; Dewailly et al., 2014; Oulhote and Bouchard, 2013; Swan et al., 2003). Our recent study showed ubiquitous exposure to OP and PYR metabolites as well as PAs in populations in eight countries, with higher concentrations measured in females than in males (Li and Kannan, 2018). Several epidemiologic studies have reported association between exposure to these pesticides and reproductive anomalies (e.g., poor semen quality) (Coker et al., 2018; Furlong et al., 2014; Saillenfait et al., 2015; Swan et al., 2003). Thus, it is plausible that exposure to OP, PYR and PA pesticides may affect hormone-dependent diseases such as endometriosis.
Epidemiologic studies linking environmental exposure with endometriosis should consider methodologic challenges. First, choice of sampling framework e.g., clinical- versus population-based cohort is of interest (Buck Louis et al., 2013). Second, diagnostic criteria for endometriosis is a key factor in describing its etiology. Whereas surgical visualization is the clinical gold standard for the diagnosis of endometriosis, self-reported data may underestimate this phenotype in asymptomatic women (Smarr et al., 2016; Weuve et al., 2010). Third, chemicals measured should reflect timing of exposure during disease onset and development (Buck Louis et al., 2012a; Upson et al., 2013; Wang et al., 2017). Fourth, choice of biological media (e.g., fat, blood or urine) should be compatible with the accumulation characteristics of EDCs (Smarr et al., 2016). Lastly, potential confounders of endometriosis should be taken into consideration in the association between disease development and EDCs exposure (Mumford et al., 2017; Itoh et al., 2009).
In this study, the association between exposure to 11 pesticides and their metabolites (also referred to as ‘Universal Pesticides’ as per CDC’s NHANES report) and endometriosis in reproductive-age U.S. women was examined. Using a matched cohort design, an operative cohort comprising women aged 18–44 years (n = 492) was recruited from 14 participating surgical centers in Utah and California during 2007–2009 and matched to a cohort (n = 127) of similarly aged women recruited from the general population surrounding participating clinical sites.
2. Materials and methods
2.1. Study cohorts
Details of the study design and demographic characteristics of the study population have been previously described (Buck Louis et al., 2011; 2012a; Kunisue et al., 2012). Urine samples (n = 626) collected from menstruating women for the Endometriosis, Natural history, Diagnosis, and Outcomes (ENDO) Study in 2007–2009 were used in this study. In brief, women were eligible if they met the following four criteria: no history of surgically visualized endometriosis, no hormone injection treatment over the past two years, no breastfeeding for over 6 months, and no history of cancer. Due to limited urine volume, seven samples were excluded from chemical analysis in this study relative to that reported in Kunisue et al. (2012). The operative cohort comprised 495 currently menstruating women, aged 18–44 years, scheduled for a laparoscopy or laparotomy at 14 participating surgical centers in Salt Lake City, Utah, or San Francisco, California. Among the 492 women in the operative cohort, 471 (96%) underwent laparoscopy or laparotomy, of whom 188 (40%) were diagnosed with endometriosis. The clinical gold standard of surgical visualization together with histologic confirmation was used to define endometriosis diagnoses. The endometriosis cases were categorized into four stages (I - minimal [n = 106; 58%], II - mild [n = 27; 15%], III - moderate [n = 22; 12%] and IV - severe [n = 28; 15%]) according to the Revised American Society for Reproductive Medicine’s classification (ASRM, 1997). The population cohort (n = 131) was matched to the operative cohort by age and residence within the 50-mile catchment area of the participating surgical centers. Women in the population cohort underwent standardized pelvic magnetic resonance imaging (MRI) for the assessment of endometriosis in light of having no indication for surgery and recognizing that MRI is more sensitive for diagnosing stages 3 and 4 (Buck Louis et al., 2012a; 2012b; 2013). Among the 127 women in the population cohort, 123 (97%) underwent pelvic MRI, of whom 14 (11%) were diagnosed with endometriosis.
2.2. Data and urine collection
A baseline personal interview was completed and information pertaining to age, standardized anthropometric assessment, race/ethnicity, education, marital status, gravidity, parity, household income, smoking and drinking habits were collected approximately 2 months prior to laparoscopy or laparotomy or MRI. Demographic information was missing for <1.5% of the women. Institutional Review Board approvals were obtained from all participating study sites, and all women provided full consent before any data collection. Upon enrollment, women from both cohorts provided non-fasting urine specimens (~120 mL). All urine samples were stored in a freezer at −20 °C until chemical analysis. The residual urine samples previously analyzed for phytoestrogens (Mumford et al., 2017) were used for pesticide analysis, in this study.
2.3. Sample preparation and analysis
Urine samples were analyzed for 11 pesticides and their metabolites: 2-isopropyl-4-methyl-6-hydroxypyrimidine (IMPY), malathion dicarboxylic acid (MDA), para-nitrophenol (PNP), 3,5,6-trichloro-2-pyridinol (TCPY), 3-phenoxybenzoic acid (3-PBA), 4-fluoro-3-phenoxybenzoic acid (4F-3PBA), 2,4-dichlorophenoxyacetic acid (2,4-D), 2,4,5-trichlorophenoxyacetic acid (2,4,5-T), trans/cis-3-(2,2-dichlorovinyl)-2,2-dimethyl-cyclopropane-1-carboxylic acid (trans/cis-DCCA) and cis-3-(2,2-dibromovinyl)-2,2-dimethyl-cyclopropane-l-carboxylic acid (cis-DBCA), The analytical method has been described in detail previously (Li and Kannan, 2018). In brief, urine samples were extracted using solid phase extraction with 13C4-IMPY, 13C4-MDA, D4-PNP, 13C3-TCPY, 13C6-3-PBA, 13C6-4F-3PBA, 13C6-2,4-D, 13C6-2,4,5-T, 13C2-trans-DCCA, 13C2-cis-DCCA and l3C2-cis-DBCA spiked as internal standards.
Analyte separation and quantification were achieved using an ultrahigh performance liquid chromatography system (ACQUITY Class I, Waters, Milford, MA, USA) coupled with tandem mass spectrometry (ABSCIEX 5500, Applied Biosystems, Foster City, CA, USA). A 3-μL aliquot of the sample was injected onto a Betasil C18 column (100 × 2.1 mm, 5 μm; Thermo Electron Corp., Waltham, MA, USA) serially connected to a Javelin Betasil C18 guard column (20 × 2.1 mm, 5 μm; Thermo Electron Corp., Waltham, MA, USA). The instrument was operated in the electrospray ionization negative (for MDA, PNP, TCPY, 3-PBA, 4F-3PBA, 2,4-D, 2,4,5-T, trans/cis-DCCA and cis-DBCA) or positive mode (for IMPY) with multiple reaction monitoring.
The target analytes were quantified using an internal standard method (i.e., isotope dilution) with a 12 to 14-point calibration curve prepared at concentrations ranging from 0.01 to 200 ng/mL. The correlation coefficient (r) was ≥0.999 for all the compounds. Several procedural blanks were analyzed. Trace concentrations (ng/mL) of target compounds (0.16 of IMPY, 0.02 of MDA, 0.24 of PNP, 0.12 of TCPY, 0.02 of 2,4-D, 0.05 of 2,4,5-T, 0.002 of 3-PBA, and 0.008 of trans-DCCA) were detected in procedural blanks, and these concentrations were subtracted from those measured for samples. The accuracy (% mean recovery) and precision tests were conducted by replicate analysis of a synthetic urine sample (Sigma-Aldrich, Round Rock, TX, USA) fortified at low (1 ng/mL), medium (10 ng/mL) and high concentrations (100 ng/mL) of target chemicals. The recoveries of all target analytes were in the range of 83–108%, with a relative standard deviation (RSD) of <15%. A total of 35 urine samples were analyzed in duplicate for the evaluation of method precision. The limit of detection (LOD), defined as a signal-to-noise ratio of 3, ranged from 0.001 to 0.043 ng/mL (Table S2).
2.4. Statistical analysis
Descriptive statistic and comparison of basic characteristics of women with and without endometriosis for each cohort were evaluated using the Student’s-t test and Mann-Whitney U test. Medians were calculated and stratified by endometriosis stage and cohorts. All instrument-derived concentrations were retained without any substitution for values below the LOD, to avoid introducing bias (Buck Louis et al., 2012a; Kunisue et al., 2012; Mumford et al., 2017; Pollack et al., 2015). The concentrations were log-transformed (χ + 1) to normalize their distributions. Urinary concentrations of pesticides and their metabolites were also normalized for creatinine content. Data were analyzed using SPSS 19.0 (SPSS Inc., Chicago, IL, USA).
The logarithm transformed concentrations of each urinary pesticides were categorized into four quartiles. Binary or multivariate unconditional logistic regression was used to estimate crude and adjusted odds ratio (OR) and corresponding 95% confidence interval (Cl) for an endometriosis diagnosis by comparing the 2nd, 3rd, and 4th quartile concentrations of each analyte to that of the 1st quartile concentration (the lowest as the reference). Adjusted logistic regression models included potential confounders such as site, age (years), race/ethnicity, parity, household income, smoking, drinking and urinary creatinine (μg/g). Potential dose-response relationship was examined across quartiles by coding the median log (χ + 1) quartile concentration of each analyte as a continuous variable in the regression models. Sensitivity analysis was conducted for the operative cohort by restricting endometriosis to stages III and IV and by inclusion of parity conditional on gravidity (never pregnant, pregnant without births, and pregnant with births) given parity’s uncertain pathway with the studied pesticides and endometriosis (Buck Louis et al., 2006; 2012a; 2013; Kunisue et al., 2012). Homogeneity was tested on pesticide concentrations at log-transformed (χ + 1) from the operative and population cohorts.
3. Results and discussion
3.1. Urinary concentrations of pesticides and their metabolites
A total of 619 urine samples collected during 2007–2009 from the operative and population cohorts of endometriosis were analyzed. The detection frequency and volume-based and creatinine-adjusted concentrations of pesticides and their metabolites in urine are presented in Table 1. IMPY, MDA, PNP, TCPY, 2,4-D and 3-PBA were the most frequently detected pesticides/metabolites with detection frequencies in the range of 95–100%. The metabolites of pyrethroid insecticides, including 4F-3PBA and trans/cis-DCCA (metabolites of cyfluthrin, flumethrin, cypermethrin and permethrin) and cis-DBCA (a specific metabolite of deltamethrin) were detected in 47–80% of the samples analyzed. The detection rate for 2,4,5-T was only 0.8% reflecting its limited use relative to other pesticides. The detection frequencies of pesticides and their metabolites found in this study were similar to those reported previously from other populations (Li and Kannan, 2018; Li et al., 2019). The detection rates of urinary trans/cis-DCCA were lower in our ENDO specimens (67–80%) than those reported previously for other populations (90–100%) (Li and Kannan, 2018; Li et al., 2019). This could be partly due to the population characteristics of this ENDO study. The median (range) concentrations (ng/mL) in all samples (n = 619) were: 2.7 (0.11–120) for IMPY, 0.64 (<LOD–21) for PNP, 0.60 (<LOD–8.9) for TCPY, 0.25 (<LOD–7.9) for 2,4-D, 0.22 (<LOD–23) for MDA, 0.17 (<LOD–24) for 3-PBA, 0.09 (<LOD–34) for cis-DCCA, 0.06 (<LOD–37) for trans-DCCA, 0.01 (<LOD–4.9) for 4F-3PBA, and <LOD (<LOD–7.3) for cis-DBCA. Further analyses of the data were restricted to those analytes that had a detection frequency of >80% in all samples to exclude possible bias.
Table 1.
Descriptive statistics of concentrations (ng/mL; μg/g creatinine) of urinary pesticides and their metabolites in the ENDO study (n = 619a).
| Analyte | Unit | DFb (%) | Percentile |
||||
|---|---|---|---|---|---|---|---|
| 25th | 50th | 75th | 95th | 100th | |||
| IMPY | ng/mL | 100 | 1.64 | 2.70 | 4.77 | 12.3 | 120 |
| μg/g creatinine | 1.78 | 3.16 | 5.80 | 18.3 | 222 | ||
| MDA | ng/mL | 97.6 | 0.092 | 0.217 | 0.520 | 1.70 | 23.0 |
| μg/g creatinine | 0.121 | 0.243 | 0.482 | 1.79 | 24.3 | ||
| PNP | ng/mL | 99.2 | 0.328 | 0.637 | 1.18 | 3.24 | 21.1 |
| μg/g creatinine | 0.422 | 0.707 | 1.16 | 2.83 | 23.1 | ||
| TCPY | ng/mL | 95.0 | 0.278 | 0.601 | 1.14 | 2.76 | 8.91 |
| μg/g creatinine | 0.353 | 0.703 | 1.15 | 2.59 | 10.2 | ||
| 2,4-D | ng/mL | 99.7 | 0.144 | 0.249 | 0.457 | 1.08 | 7.85 |
| μg/g creatinine | 0.169 | 0.271 | 0.465 | 1.36 | 5.13 | ||
| 2,4,5-T | ng/mL | 0.80 | <LODc | <LOD | <LOD | <LOD | 0.046 |
| μg/g creatinine | -d | - | - | - | 0.294 | ||
| 3-PBA | ng/mL | 97.9 | 0.069 | 0.166 | 0.426 | 1.90 | 24.0 |
| μg/g creatinine | 0.094 | 0.199 | 0.402 | 1.98 | 34.3 | ||
| 4F-3PBA | ng/mL | 76.3 | 0.001 | 0.008 | 0.022 | 0.089 | 4.91 |
| μg/g creatinine | 0.001 | 0.009 | 0.020 | 0.082 | 2.68 | ||
| trans-DCCA | ng/mL | 66.9 | <LOD | 0.055 | 0.273 | 1.85 | 37.2 |
| μg/g creatinine | - | 0.065 | 0.240 | 2.00 | 50.0 | ||
| cis-DCCA | ng/mL | 79.5 | 0.017 | 0.091 | 0.270 | 1.43 | 34.3 |
| μg/g creatinine | 0.025 | 0.106 | 0.271 | 1.39 | 19.9 | ||
| cis-DBCA | ng/mL | 47.0 | <LOD | <LOD | 0.058 | 0.313 | 7.31 |
| μg/g creatinine | - | - | 0.060 | 0.256 | 6.67 | ||
| Σ11 pesticidese | ng/mL | 100 | 3.88 | 6.45 | 10.5 | 22.8 | 131 |
| μg/g creatinine | 4.62 | 7.03 | 11.5 | 28.9 | 241 | ||
Out of n = 626, 7 have non-detectable urine volume (3 in operative and 4 in population cohort).
Detection frequency.
Limit of detection.
Not available.
Sum concentration of the 11 pesticides and their metabolites.
Spearman correlation analysis showed significant positive correlations between unadjusted and creatinine-adjusted concentrations of pesticides and their metabolites in the operative or population or the combined cohort (p < 0.01; Table S3). A significant positive correlation was also found between unadjusted and creatinine-adjusted concentrations for sum concentrations of six pesticides (p < 0.01). These findings suggest that urine excretion volume at sampling (i.e., dilution factor) did not affect the measured concentrations of pesticides (Li and Kannan, 2018). Further discussion on urinary pesticide concentrations was based on unadjusted values, unless specified otherwise.
3.2. Relationship with demographic characteristics
Urinary concentrations of IMPY, MDA, PNP, TCPY, 3-PBA and 2,4-D and demographic characteristics of the cohorts are summarized in Table 2. The median concentrations (ng/mL) in all samples were on the order of: IMPY (2.7) > PNP (0.64) > TCPY (0.60) > 2,4-D (0.25) > MDA (0.22) > 3-PBA (0.17). The pattern of higher OP concentrations than those of PYRs in urine has been reported previously (Calafat et al., 2017; Garí et al., 2018; Li and Kannan, 2018; Li et al., 2019). Earlier studies on urinary pesticide concentrations have shown that PNP (metabolite of parathion and methyl parathion) and TCPY (metabolite of chlorpyrifos and chlorpyrifos-methyl) were the dominant OPs (Calafat et al., 2017; Li and Kannan, 2018; Li et al., 2019; Swan et al., 2003). In the current study, IMPY (metabolite of diazinon) was the dominant urinary pesticide metabolite followed by PNP and TCPY, suggesting the prevalence of exposure to diazinon, parathion, methyl parathion, chlorpyrifos and chlorpyrifos-methyl. IMPY accounted for 10–20% of the ‘Universal Pesticides’ concentration in urine from Japan, Korea and Saudi Arabia; diazinon is commonly used in indoor pest control (Li and Kannan, 2018). Although the use of OPs for indoor and garden pest control has been banned in the U.S. since 2000 (Barr et al., 2010), they are still widely used in agriculture, accounting for 33% (20 million lbs) of all insecticides used in the U.S. in 2012 (EPA, 2017).
Table 2.
Urinary concentrations of pesticides and their metabolites by demographic characteristics.
| Characteristics | Median (ng/mL) |
||||||
|---|---|---|---|---|---|---|---|
| IMPY | MDA | PNP | TCPY | 3-PBA | 2,4-D | Σ6 pesticidesa | |
| Total (n = 619b) | 2.7 | 0.22 | 0.64 | 0.60 | 0.17 | 0.25 | 5.9 |
| Sampling site | ** | * | ** | ** | |||
| Utah (n = 520) | 3.1 | 0.22 | 0.64 | 0.62 | 0.16 | 0.25 | 6.1 |
| California (n = 99) | 1.8 | 0.15 | 0.67 | 0.52 | 0.23 | 0.24 | 4.5 |
| Age (years) | * | ** | * | ** | |||
| 20–29 (n = 219) | 3.1 | 0.32 | 0.68 | 0.66 | 0.18 | 0.26 | 6.6 |
| 30–39 (n = 261) | 2.5 | 0.18 | 0.53 | 0.56 | 0.17 | 0.22 | 5.5 |
| ≥40 (n= 138) | 2.6 | 0.14 | 0.62 | 0.60 | 0.16 | 0.27 | 5.7 |
| Body mass index (kg/m2) | |||||||
| <25.0 (n = 268) | 2.5 | 0.21 | 0.61 | 0.62 | 0.17 | 0.26 | 6.2 |
| 25.0–29.9 (n = 151) | 2.7 | 0.21 | 0.62 | 0.55 | 0.17 | 0.21 | 5.5 |
| >30.0 (n = 195) | 3.0 | 0.22 | 0.67 | 0.61 | 0.17 | 0.25 | 6.1 |
| Race/ethnicity | * | * | ** | ||||
| Hispanic (n = 81) | 2.4 | 0.27 | 0.71 | 0.58 | 0.22 | 0.24 | 6.3 |
| Non-Hispanic white (n = 470) | 2.7 | 0.23 | 0.62 | 0.59 | 0.16 | 0.25 | 5.9 |
| Non-Hispanic black (n = 10) | 2.5 | 0.13 | 1.4 | 0.97 | 0.52 | 0.41 | 7.0 |
| Asian/Hawaiian/Pacific Islander/Alaska | 2.7 | 0.17 | 0.53 | 0.72 | 0.17 | 0.28 | 5.8 |
| Native/American Indian (n = 34) | |||||||
| Others (n = 24) | 2.9 | 0.11 | 0.55 | 0.61 | 0.31 | 0.19 | 6.1 |
| Education | |||||||
| <High school graduate (n = 28) | 3.1 | 0.16 | 0.74 | 0.46 | 0.23 | 0.24 | 5.7 |
| High school graduate (n = 82) | 2.7 | 0.27 | 0.65 | 0.59 | 0.16 | 0.21 | 5.4 |
| Technical School graduate (n = 252) | 2.6 | 0.23 | 0.63 | 0.66 | 0.16 | 0.25 | 5.7 |
| ≥College graduate (n = 253) | 2.7 | 0.20 | 0.62 | 0.59 | 0.18 | 0.26 | 6.4 |
| Marital status | ** | ||||||
| Married/living as married (n = 445) | 2.8 | 0.21 | 0.62 | 0.61 | 0.14 | 0.24 | 6.0 |
| Others (n = 169) | 2.6 | 0.23 | 0.65 | 0.59 | 0.22 | 0.29 | 5.9 |
| Gravidity | ** | ||||||
| Nulligravida (n = 212) | 3.1 | 0.26 | 0.67 | 0.60 | 0.20 | 0.28 | 6.5 |
| Gravid (n = 405) | 2.6 | 0.19 | 0.61 | 0.60 | 0.16 | 0.24 | 5.5 |
| Parity | ** | ** | * | * | |||
| Nulliparous (n = 271) | 2.7 | 0.24 | 0.69 | 0.60 | 0.20 | 0.29 | 6.3 |
| Parous (n = 348) | 2.7 | 0.19 | 0.58 | 0.60 | 0.14 | 0.23 | 5.5 |
| Household incomec | * | ** | ** | ** | * | ||
| Below poverty (n = 69) | 3.1 | 0.33 | 0.80 | 0.73 | 0.29 | 0.33 | 7.0 |
| Within 180% poverty (n = 73) | 3.5 | 0.31 | 0.80 | 0.71 | 0.17 | 0.27 | 6.5 |
| Above 180% poverty (n = 468) | 2.5 | 0.19 | 0.57 | 0.58 | 0.14 | 0.23 | 5.7 |
| Smoking | * | ||||||
| No (n = 541) | 2.6 | 0.23 | 0.62 | 0.62 | 0.16 | 0.26 | 6.0 |
| Yes (n = 78) | 3.0 | 0.18 | 0.67 | 0.51 | 0.21 | 0.21 | 5.7 |
| Drinking | ** | ** | ** | * | ** | ||
| No (n = 479) | 2.9 | 0.25 | 0.65 | 0.65 | 0.17 | 0.27 | 6.2 |
| Yes (n = 140) | 2.2 | 0.15 | 0.60 | 0.47 | 0.14 | 0.21 | 5.1 |
Sum concentration of the six pesticides and their metabolites.
Out of n = 626, 7 have non-detectable urine volume (3 in operative and 4 in population cohort).
On the basis of the 2007 HHS Poverty guidelines accounting for the numbers of persons in the household for the 48 contiguous states and District of Columbia.
p < 0.05,
p < 0.01.
We found significantly higher concentrations of IMPY, MDA and sum of the six pesticides in urine samples from women who resided in Utah than those who resided in California (Table 2). However, the median concentration of 3-PBA measured in urine samples from California (0.23 ng/mL) was significantly higher than that from Utah (0.16 ng/mL). Urinary concentrations of benzophenone-type UV filters also presented a significant regional difference in this ENDO cohort (Kunisue et al., 2012). In addition, urinary concentrations of IMPY, MDA, PNP, 3-PBA and 2,4-D tended to be higher for younger (age), non-Hispanic black (race), nulliparous (parity) and less affluent (household income) women (Table 2). These findings suggest that the U.S. women with such characteristics might have higher exposure to those pesticides than do the others. The general population is commonly exposed to pesticides through the ingestion of food and water (McKelvey et al., 2013; Oulhote and Bouchard, 2013). Body mass index and education level had no effect on urinary pesticide concentrations in the studied women.
Urinary concentrations of 2,4-D were significantly lower in smokers than in nonsmokers (Table 2). A similar pattern was found in the urinary concentrations of two UV filters in this ENDO cohort (Kunisue et al., 2012). This can be explained by greater activities of cytochrome P450 (CYP) drug-metabolizing enzymes, induced by tobacco smoking, which can enhance metabolic transformation of endogenous and xenobiotic chemicals (Hukkanen et al., 2011). Additionally, urinary concentrations of IMPY, MDA, TCPY, 2,4-D and sum of the six pesticides were significantly lower in drinkers than in nondrinkers. It has been reported that induction of CYP450 enzymes was positively correlated with alcohol consumption (Zanger and Schwab, 2013). Thus, pesticides might be efficiently metabolized and eliminated in active smokers and alcoholics relative to nonsmokers and teetotalers.
3.3. Relationship of pesticides with endometriosis
The overall distribution of pesticide concentrations in urine was similar between the operative and population cohorts (Fig. 1). There was no significant difference in sum concentrations of the six pesticides between women with and without endometriosis in both cohorts (p > 0.05). IMPY was the most dominant compound followed by PNP and TCPY in either operative or population cohort. When the concentrations measured in the operative and population cohorts were examined separately, significantly higher concentrations of PNP and TCPY were found in women with (p < 0.05) and without (p < 0.05) endometriosis in the operative cohort than those in the population cohort. In addition, women without endometriosis had significantly higher concentrations of 3-PBA (p < 0.05) but lower concentrations of IMPY (p < 0.05) in the operative cohort than the population cohort. It is worth to note that prior to this study, no earlier study had examined urinary concentrations of ‘Universal Pesticides’ in association with endometriosis.
Fig. 1.
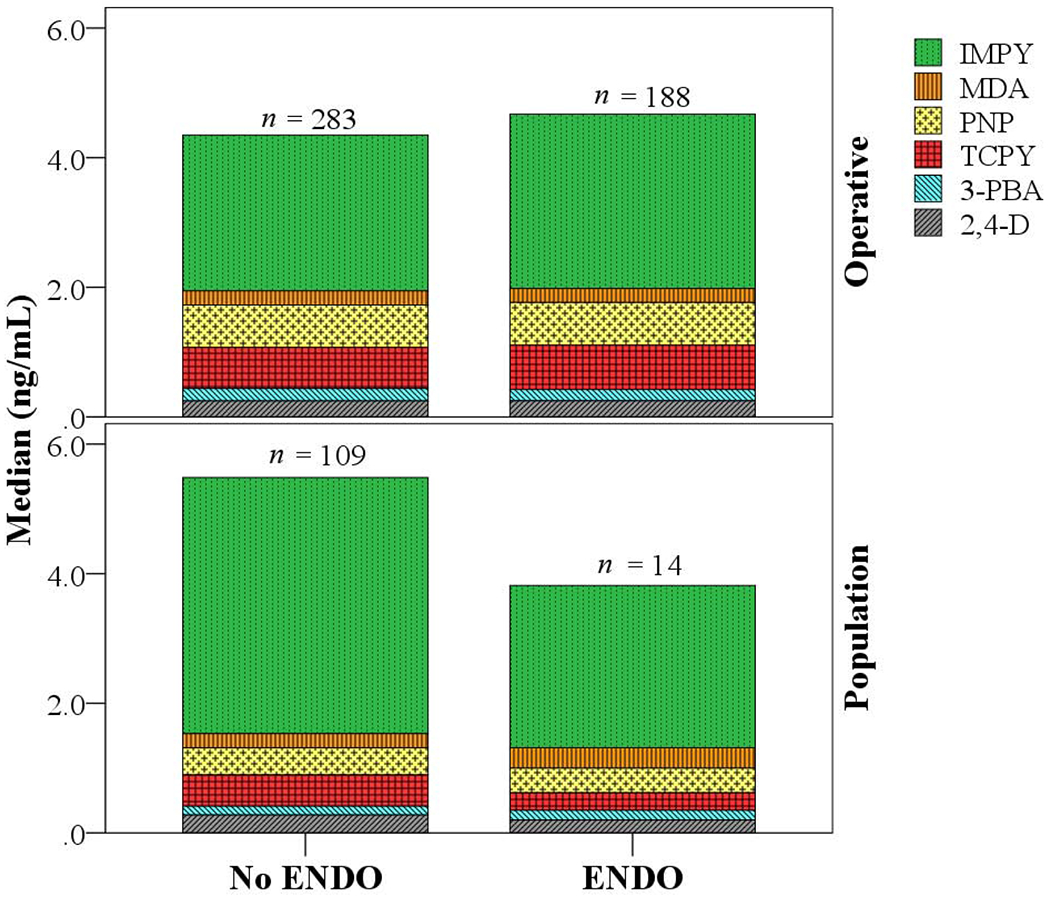
Urinary concentrations of pesticides and their metabolites in the operative and population cohorts of the ENDO study.
3.4. Correlation analysis between studied pesticides
Significant correlations (r = 0.11–0.35; p < 0.05) were found among all six analytes except between 3-PBA and MDA (r = 0.08; p > 0.05) (Table 3). Significant positive correlations were also found between creatinine-adjusted concentrations of pesticides, with the exception of between 3-PBA and IMPY, MDA, TCPY and 2,4-D (r = 0.01–0.04; p > 0.05). These results were similar to those reported for correlations of urinary concentrations of ‘Universal Pesticides’ in general populations across eight countries, in our earlier study (Li and Kannan, 2018). The positive correlations among the six ‘Universal Pesticides’ in urine indicate that women were exposed to OP pesticides (i.e., diazinon, malathion, methyl parathion, parathion, chlorpyrifos and chlorpyrifos-methyl), 2,4-D and PYR insecticides (i.e., permethrin, cypermethrin and cyfluthrin) simultaneously.
Table 3.
Pearson correlation coefficients (r) of urinary (log (χ + 1); ng/mL) and creatinine-adjusted concentrations (log (χ + 1); μg/g creatinine; in Italic) of pesticides and their metabolites in the ENDO study (n = 619a).
| IMPY | MDA | PNP | TCPY | 3-PBA | 2,4-D | |
|---|---|---|---|---|---|---|
| IMPY | 1.0 | |||||
| 1.0 | ||||||
| MDA | 0.19** | 1.0 | ||||
| 0.17** | 1.0 | |||||
| PNP | 0.26** | 0.30** | 1.0 | |||
| 0.18** | 0.12** | 1.0 | ||||
| TCPY | 0.14** | 0.24** | 0.35** | 1.0 | ||
| 0.10* | 0.13** | 0.19** | 1.0 | |||
| 3-PBA | 0.11** | 0.08 | 0.31** | 0.14** | 1.0 | |
| 0.04 | 0.01 | 0.14** | 0.02 | 1.0 | ||
| 2,4-D | 0.14** | 0.24** | 0.30** | 0.23** | 0.21** | 1.0 |
| 0.16** | 0.20** | 0.08* | 0.09* | 0.04 | 1.0 |
Out of n = 626, 7 have non-detectable urine volume (3 inoperative and 4 in population cohort).
p < 0.05,
p <0.01, significant value of correlation (2-tailed).
3.5. Odds ratio for endometriosis diagnosis
The (un)adjusted ORs and corresponding 95% CIs for each pesticide and sum of the six pesticides (in quartiles) are presented for both cohorts in Table S4. In comparison to the 1st quartile urinary concentrations, the ORs for endometriosis diagnosis in other quartiles denoted no association in either operative or population or the combined cohort because the confidence intervals for these analytes included the value of one. Of note is the consistency in the direction of ORs for all the studied compounds in sensitivity analyses that restricted the choice of affected individuals with endometriosis to stages III and IV (Table S5). However, when the ORs for endometriosis diagnosis were calculated based on urinary creatinine concentrations (μg/g creatinine), the unadjusted ORs were significantly elevated for IMPY (OR = 1.89, 95% CI = 1.12–3.20) in the 4th quartile operative cohort, and TCPY (OR = 1.65, 95% CI = 1.02–2.69) in the 2nd quartile combined cohort, relative to the 1st quartile, respectively (Table 4). This denotes an approximate 65–89% increase in the odds of an endometriosis diagnosis in women with the higher concentrations of IMPY (5.85–100 μg/g creatinine) or TCPY (0.36–0.70 μg/g creatinine) compared to women with the 1st quartile concentrations of these compounds, respectively. A similar result of significantly elevated OR for IMPY (OR = 1.71, 95% CI = 1.01–2.91) in the 4th quartile was observed when we restricted for the operative cohort by inclusion of parity conditional on gravidity (Table S5). Linear trends for all analytes across the quartiles were significant in adjusted models in the operative cohort and the entire data set (Tables 4, S4 and S5).
Table 4.
Odds of an endometriosis diagnosis by urinary concentrations (log (χ + 1); μg/g creatinine) of pesticides and their metabolites in the operative (188 women with and 283 without endometriosis) and population (14 women with and 109 without endometriosis) cohorts from the ENDO study.
| Analyte (μg/g creatinine) | Operative cohort (n = 471) |
Population cohort (n = 123) |
All (n = 594) |
|||
|---|---|---|---|---|---|---|
| Crude ORa (95% CI)b | Adjusted ORc (95% CI) | Crude OR (95% CI) | Adjusted OR (95% CI) | Crude OR (95% CI) | Adjusted OR (95% CI) | |
| IMPY | ||||||
| 1st quartile (0.04–1.78) | reference | reference | reference | reference | reference | reference |
| 2nd quartile (1.79–3.20) | 1.03 (0.60, 1.76) | 1.07 (0.61, 1.88) | 0.35 (0.06, 1.94) | 0.32 (0.04, 2.25) | 0.99 (0.61, 1.62) | 1.02 (0.61, 1.69) |
| 3rd quartile (3.21–5.84) | 1.35 (0.79, 2.29) | 1.42 (0.81, 2.47) | 0.96 (0.25, 3.73) | 0.74 (0.15, 3.67) | 1.41 (0.88, 2.28) | 1.47 (0.89, 2.43) |
| 4th quartile (5.85–100) | 1.89e (1.12, 3.20) | 1.72 (0.98, 3.00) | 0.35 (0.06, 1.94) | 0.24 (0.04, 1.61) | 1.20 (0.74, 1.95) | 1.07 (0.64, 1.77) |
| p-trendd | 0.007 | <0.001 | 0.406 | 0.744 | 0.295 | <0.001 |
| MDA | ||||||
| 1st quartile (<0.004–0.12) | reference | reference | reference | reference | reference | reference |
| 2nd quartile (0.13–0.24) | 0.93 (0.55, 1.57) | 0.94 (0.54, 1.63) | 0.62 (0.10, 4.01) | 0.62 (0.09, 4.34) | 0.86 (0.53, 1.40) | 0.80 (0.48, 1.34) |
| 3rd quartile (0.25–0.49) | 1.00 (0.59, 1.69) | 0.91 (0.52, 1.58) | 1.67 (0.36, 7.68) | 1.62 (0.29, 9.18) | 0.98 (0.61, 1.59) | 0.91 (0.55, 1.50) |
| 4th quartile (0.50–24.3) | 1.25 (0.75, 2.11) | 1.19 (0.69, 2.06) | 1.39 (0.28, 6.80) | 1.23 (0.22, 6.79) | 1.23 (0.76, 1.97) | 1.11 (0.68, 1.84) |
| p-trend | 0.293 | <0.001 | 0.539 | 0.899 | 0.223 | <0.001 |
| PNP | ||||||
| 1st quartile (<0.001–0.43) | reference | reference | reference | reference | reference | reference |
| 2nd quartile (0.44–0.71) | 0.90 (0.53, 1.52) | 0.79 (0.45, 1.36) | 1.04 (0.19, 5.59) | 1.12 (0.19, 6.68) | 1.09 (0.67, 1.77) | 1.04 (0.63, 1.73) |
| 3rd quartile (0.72–1.17) | 1.41 (0.84, 2.37) | 1.34 (0.78, 2.31) | 2.15 (0.49, 9.51) | 1.77 (0.38, 8.32) | 1.41 (0.88, 2.28) | 1.44 (0.88, 2.38) |
| 4th quartile (1.18–23.1) | 0.91 (0.54, 1.54) | 0.83 (0.47, 1.45) | 0.67 (0.10, 4.30) | 0.47 (0.06, 3.67) | 1.10 (0.67, 1.79) | 1.07 (0.64, 1.79) |
| p-trend | 0.983 | <0.001 | 0.887 | 0.894 | 0.653 | <0.001 |
| TCPY | ||||||
| 1st quartile (<0.02–0.35) | reference | reference | reference | reference | reference | reference |
| 2nd quartile (0.36–0.70) | 1.24 (0.73, 2.08) | 1.13 (0.66, 1.95) | 0.93 (0.21, 4.10) | 1.06 (0.22, 5.19) | 1.65 (1.02, 2.69) | 1.52 (0.92, 2.51) |
| 3rd quartile (0.71–1.15) | 1.11 (0.66, 1.88) | 1.07 (0.62, 1.85) | 1.00 (0.23, 4.43) | 1.16 (0.24, 5.68) | 1.39 (0.85, 2.28) | 1.29 (0.78, 2.14) |
| 4th quartile (1.16–8.44) | 0.98 (0.58, 1.66) | 0.90 (0.52, 1.57) | 0.45 (0.08, 2.65) | 0.60 (0.09, 3.87) | 1.21 (0.74, 1.99) | 1.12 (0.67, 1.87) |
| p-trend | 0.788 | <0.001 | 0.398 | 0.873 | 0.676 | <0.001 |
| 3-PBA | ||||||
| 1st quartile (<0.001–0.09) | reference | reference | reference | reference | reference | reference |
| 2nd quartile (<0.10–0.20) | 0.80 (0.48, 1.34) | 0.84 (0.49, 1.44) | 1.56 (0.24, 10.0) | 1.94 (0.28, 13.5) | 0.99 (0.61, 1.60) | 1.06 (0.65, 1.74) |
| 3rd quartile (<0.21–0.40) | 0.73 (0.44, 1.24) | 0.77 (0.44, 1.34) | 3.48 (0.64, 18.8) | 3.31 (0.58, 18.9) | 1.07 (0.66, 1.73) | 1.14 (0.69, 1.87) |
| 4th quartile (<0.41–34.3) | 0.81 (0.48, 1.36) | 0.88 (0.50, 1.53) | 1.61 (0.25, 10.4) | 1.48 (0.21, 10.3) | 0.98 (0.60, 1.59) | 1.14 (0.68, 1.89) |
| p-trend | 0.614 | <0.001 | 0.659 | 0.895 | 0.937 | <0.001 |
| 2,4-D | ||||||
| 1st quartile (<0.006–0.17) | reference | reference | reference | reference | reference | reference |
| 2nd quartile (0.18–0.27) | 1.28 (0.76, 2.16) | 1.07 (0.62, 1.85) | 2.16 (0.49, 9.57) | 2.15 (0.44, 10.5) | 1.36 (0.85, 2.19) | 1.18 (0.72, 1.93) |
| 3rd quartile (0.28–0.47) | 1.26 (0.74, 2.12) | 1.17 (0.68, 2.01) | 0.30 (0.03, 3.06) | 0.24 (0.02, 2.57) | 1.00 (0.62, 1.62) | 0.94 (0.57, 1.54) |
| 4th quartile (0.48–5.13) | 0.96 (0.57, 1.64) | 0.85 (0.49, 1.48) | 1.33 (0.27, 6.53) | 1.14 (0.22, 6.05) | 0.85 (0.52, 1.38) | 0.76 (0.46, 1.27) |
| p-trend | 0.628 | <0.001 | 0.894 | 0.899 | 0.212 | <0.001 |
| Σ6 pesticidesf | ||||||
| 1st quartile (1.22–4.30) | reference | reference | reference | reference | reference | reference |
| 2nd quartile (4.31–6.55) | 0.96 (0.57, 1.64) | 0.91 (0.52, 1.58) | 1.38 (0.28, 6.76) | 1.38 (0.25, 7.70) | 1.04 (0.64, 1.69) | 1.08 (0.65, 1.79) |
| 3rd quartile (6.56–10.7) | 1.33 (0.79, 2.24) | 1.25 (0.72, 2.17) | 1.80 (0.39, 8.27) | 1.42 (0.28, 7.18) | 1.34 (0.83, 2.16) | 1.34 (0.82, 2.20) |
| 4th quartile (10.8–109) | 1.40 (0.83, 2.35) | 1.31 (0.75, 2.28) | 0.67 (0.10, 4.30) | 0.41 (0.06, 3.01) | 0.98 (0.60, 1.60) | 0.89 (0.53, 1.48) |
| p-trend | 0.124 | <0.001 | 0.687 | 0.843 | 0.947 | <0.001 |
Odds ratios from unadjusted logistic regressions.
95% confidence interval of odds ratio.
Odds ratios from multivariable logistic regressions adjusting for demographic characteristics of site, age, race/ethnicity, parity, household income, smoking and drinking.
Tests for linear trend were performed using the median urinary concentration of analyte in each quartile as a continuous variable in the model.
Values with ORs >1 are highlighted in bold.
Sum concentration of the six pesticides and their metabolites.
Our findings suggest an increase in the odds of an endometriosis diagnosis for IMPY and TCPY, which suggests that these pesticide metabolites are associated with the diagnosis of endometriosis. To our knowledge, no earlier study examined the association between IMPY/TCPY and endometriosis. However, TCPY was found to be one of the dominant OPs in urine across eight countries and the U.S. general population (Li and Kannan, 2018; Li et al., 2019), similar to that found in this study. It is worth noting that IMPY was the most dominant urinary pesticide found in this ENDO study. In vitro studies have reported estrogenic potencies of chlorpyrifos and diazinon (parent compounds of TCPY and IMPY, respectively), which suggests that these pesticides may be potent xenoestrogens (Andersen et al., 2002; Kojima et al., 2004; Serra et al., 2019). EDCs can interact with nuclear receptors to exert their effect (Katz et al., 2016) and nuclear receptors have been reported to play an important role in endometriosis progression (Cho et al., 2018). Chlorpyrifos can also induce oxidative stress and apoptosis in peripheral blood lymphocytes of rats (Ojha and Gupta, 2017). These observations emphasize the need for further studies that examine endometriosis following exposure to chlorpyrifos and diazinon.
4. Conclusions
To our knowledge, this is the first epidemiologic study to investigate the occurrence of urinary metabolites of OP and PYR insecticides, and phenoxy herbicides in U.S. women and their association with incident endometriosis. Our preliminary evidence suggests that exposure to diazinon (the parent compound of IMPY) as well as chlorpyrifos and chlorpyrifos-methyl (parent compounds of TCPY) may be associated with increased odds of an incident endometriosis diagnosis. These exploratory findings await corroboration in further studies. Furthermore, efforts are needed in delineating etiologic mechanisms of these non-persistent pesticides in related with endometriosis development.
Despite the fact that this study adds novel information that links universal pesticides exposure to incident endometriosis, there are some limitations that should be taken into consideration when drawing conclusions. First, a single spot urine sample was analyzed from both cohorts, and the suitability of spot urine to represent exposure over time is questionable (Li et al., 2019). Second, the small sample size in the population cohort limits the power of the study. Third, MRI diagnosis for endometriosis in the population cohort was not able to specifically identify stages I and II of the disease. Our findings should be considered as exploratory and warrants further corroboration.
Supplementary Material
Highlights.
Eleven pesticides and their metabolites were analyzed in urine of endometriosis cohorts.
Six pesticides/metabolites had a detection frequency ≥95% in urine.
Odds ratios for endometriosis diagnosis were significant for IMPY and TCPY.
Exposure to diazinon and chlorpyrifos may be associated with endometriosis.
Acknowledgments
This study was supported by the Intramural Research Program, Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), National Institutes of Health (contracts NO1-DK-6-3428, NO1-DK-6-3427, 10001406-02).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no competing financial interest.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Andersen HR, Vinggaard AM, Rasmussen TH, Gjermandsen IM, Bonefeld-Jørgensen EC, 2002. Effects of currently used pesticides in assays for estrogenicity, androgenicity, and aromatase activity in vitro. Toxicol Appl Pharmacol 179, 1–12. [DOI] [PubMed] [Google Scholar]
- ASRM, 1997. American Society for Reproduction Medicine (ASRM), Revised American Society for Reproduction Medicine classification of endometriosis: 1996. Fertil Steril 67, 817–821. [DOI] [PubMed] [Google Scholar]
- Barr DB, Olsson AO, Wong LY, Eidunka S, Baker SE, Whitehead RD, Magsumbol MS, Williams BL, Needham LL, 2010. Urinary concentrations of metabolites of pyrethroid insecticides in the general U.S. population: National Health and Nutrition Examination Survey 1999–2002. Environ Health Perspect 118, 742–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker K, Seiwert M, Angerer J, Kolossa-Gehring M, Hoppe HW, Ball M, Schulz C, Thumulla J, Seifert B, 2006. GerES IV pilot study: assessment of the exposure of German children to organophosphorus and pyrethroid pesticides. Int J Hyg Environ Health 209, 221–233. [DOI] [PubMed] [Google Scholar]
- Buck Louis G, Dukic V, Heagerty PJ, Louis TA, Lynch CD, Ryan LM, Schisterman EF, Trumble A, the Pregnancy Modeling Working Group, 2006. Analysis of repeated pregnancy outcomes. Stat Methods Med Res 15, 103–126. [DOI] [PubMed] [Google Scholar]
- Buck Louis GM, Chen Z, Peterson CM, Hediger ML, Croughan MS, Sundaram R, Stanford JB, Varner MW, Fujimoto VY, Giudice LC, Trumble A, Parsons PJ, Kannan K, 2012a. Persistent lipophilic environmental chemicals and endometriosis: the ENDO study. Environ Health Perspect 120, 811–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck Louis GM, Hediger ML, Peterson CM, Croughan M, Sundaram R, Stanford J, Chen Z, Fujimoto VY, Varner MW, Trumble A, Giudice LC, on behalf of the ENDO Study Working Group, 2011. Incidence of endometriosis by study population and diagnostic method: the ENDO study. Fertil Steril 96, 360–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck Louis GM, Peterson CM, Chen Z, Croughan M, Sundaram R, Stanford J, Varner MW, Kennedy A, Giudice L, Fujimoto VY, Sun L, Wang L, Guo Y, Kannan K, 2013. Bisphenol A and phthalates and endometriosis: the Endometriosis: Natural History, Diagnosis and Outcomes Study. Fertil Steril 100, 162–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck Louis GM, Peterson CM, Chen Z, Hediger ML, Croughan MS, Sundaram R, Stanford JB, Fujimoto VY, Varner MW, Giudice LC, Kennedy A, Sun L, Wu Q, Kannan K, 2012b. Perfluorochemicals and endometriosis the ENDO study. Epidemiology 23, 799–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, Ye X, Valentin-Blasini L, Li Z, Mortensen ME, Wong LY, 2017. Coexposure to non-persistent organic chemicals among American pre-school aged children: a pilot study. Int J Hyg Environ Health 220, 55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell S, Raza M, Pollack AZ, 2016. Perfluoroalkyl substances and endometriosis in US women in NHANES 2003–2006. Reprod Toxicol 65, 230–235. [DOI] [PubMed] [Google Scholar]
- Cho YJ, Lee SH, Park JW, Han M, Park MJ, Han SJ, 2018. Dysfunctional signaling underlying endometriosis: current stage of knowledge. J Mol Endocrinol 60, doi: 10.1530/JME-17-0227. [DOI] [PubMed] [Google Scholar]
- Coker E, Chevrier J, Rauch S, Bradman A, Obida M, Crause M, Bornman R, Eskenazi B, 2018. Association between prenatal exposure to multiple insecticides and child body weight and body composition in the VHEMBE South African birth cohort. Environ Int 113, 122–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewailly E, Forde M, Robertson L, Kaddar N, Laouan Sidi EA, Cote S, Gaudreau E, Drescher O, Ayotte P, 2014. Evaluation of pyrethroid exposures in pregnant women from 10 Caribbean countries. Environ Int 63, 201–206. [DOI] [PubMed] [Google Scholar]
- EPA, 2017. United States Environmental Protection Agency (EPA). Pesticides industry sales and usage 2008–2012 market estimates, https://www.epa.gov/sites/production/files/2017-01/documents/pesticides-industry-sales-usage-2016_0.pdf, Accessed date: 1 June 2018. [Google Scholar]
- Eskenazi B, Mocarelli P, Warner M, Samuels S, Vercellini P, Olive D, Needham LL, Patterson DG, Brambilla P, Gavoni N, Casalini S, Panazza S, Turner W, Gerthoux PM, 2002. Serum dioxin concentrations and endometriosis: a cohort study in Seveso, Italy. Environ Health Perspect 110, 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furlong MA, Engel SM, Barr DB, Wolff MS, 2014. Prenatal exposure to organophosphate pesticides and reciprocal social behavior in childhood. Environ Int 70, 125–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garí M, González-Quinteiro Y, Bravo N, Grimalt JO, 2018. Analysis of metabolites of organophosphate and pyrethroid pesticides in human urine from urban and agricultural populations (Catalonia and Galicia). Sci Total Environ 622–623, 526–533. [DOI] [PubMed] [Google Scholar]
- Gracia-Lor E, Castiglioni S, Bade R, Been F, Castrignano E, Covaci A, Gonzalez-Marino I, Hapeshi E, Kasprzyk-Hordern B, Kinyua J, Lai FY, Letzel T, Lopardo L, Meyer MR, O’Brien J, Ramin P, Rousis NI, Rydevik A, Ryu Y, Santos MM, Senta I, Thomaidis NS, Veloutsou S, Yang Z, Zuccato E, Bijlsma L, 2017. Measuring biomarkers in wastewater as a new source of epidemiological information: current state and future perspectives. Environ Int 99, 131–150. [DOI] [PubMed] [Google Scholar]
- Hukkanen J, Jacob III P, Peng M, Dempsey D, Benowitz NL, 2011. Effect of nicotine on cytochrome P450 1A2 activity. Br J Clin Pharmacol 72, 836–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt PA, Sathyanarayana S, Fowler PA, Trasande L, 2016. Female reproductive disorders, diseases, and costs of exposure to endocrine disrupting chemicals in the European Union. J Clin Endocrinol Metab 101, 1562–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh H, Iwasaki M, Hanaoka T, Sasaki H, Tanaka T, Tsugane S, 2009. Urinary phthalate monoesters and endometriosis in infertile Japanese women. Sci Total Environ 408, 37–42. [DOI] [PubMed] [Google Scholar]
- Katz TA, Yang Q, Trevino LS, Walker CL, Al-Hendy A, 2016. Endocrine-disrupting chemicals and uterine fibroids. Fertil Steril 106, 967–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima H, Katsura E, Takeuchi S, Niiyama K, Kobayashi K, 2004. Screening for estrogen and androgen receptor activities in 200 pesticides by in vitro reporter gene assays using Chinese hamster ovary cells. Environ Health Perspect 112, 524–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunisue T, Chen Z, Buck Louis GM, Sundaram R, Hediger ML, Sun L, Kannan K, 2012. Urinary concentrations of benzophenone-type UV filters in U.S. women and their association with endometriosis. Environ Sci Technol 46, 4624–4632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvaskoff M, Mu F, Terry KL, Harris HR, Poole EM, Farland L, Missmer SA, 2017. Endometriosis: a high-risk population for major chronic diseases? Hum Reprod Update 21, 500–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li AJ, Martinez-Moral MP, Kannan K, 2019. Temporal variability in urinary pesticide concentrations in repeated-spot and first-morning-void samples and its association with oxidative stress in healthy individuals. Environ Int 130, 104904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li AJ, Kannan K, 2018. Urinary concentrations and profiles of organophosphate and pyrethroid pesticide metabolites and phenoxyacid herbicides in populations in eight countries. Environ Int 121, 1148–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKelvey W, Jacobson JB, Kass D, Barr DB, Davis M, Calafat AM, Aldous KM, 2013. Population-based biomonitoring of exposure to organophosphate and pyrethroid pesticides in New York City. Environ Health Perspect 121, 1349–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumford SL, Week J, Kannan K, Buck Louis GM, 2017. Urinary phytoestrogen concentrations are not associated with incident endometriosis in premenopausal women. J Nutr 147, 227–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojha A, Gupta YK, 2017. Study of commonly used organophosphate pesticides that induced oxidative stress and apoptosis in peripheral blood lymphocytes of rats. Hum Exp Toxicol 36, 1158–1168. [DOI] [PubMed] [Google Scholar]
- Oulhote Y, Bouchard MF, 2013. Urinary metabolites of organophosphate and pyrethroid pesticides and behavioral problems in Canadian children. Environ Health Perspect 121, 1378–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauwels A, Schepens PJC, D’Hooghe T, Delbeke L, Dhont M, Brouwer A, Weyler J, 2001. The risk of endometriosis and exposure to dioxins and polychlorinated biphenyls: a case-control study of infertile women. Hum Reprod 16, 2050–2055. [DOI] [PubMed] [Google Scholar]
- Pollack AZ, Buck Louis GM, Chen Z, Sun L, Trabert B, Guo Y, Kannan K, 2015. Bisphenol A, benzophenone-type ultraviolet filters, and phthalates in relation to uterine leiomyoma. Environ Res 137, 101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porpora MG, Medda E, Abballe A, Bolli S, De Angelis I, di Domenico A, Ferro A, Ingelido AM, Maggi A, Panici PB, De Felip E, 2009. Endometriosis and organochlorinated environmental pollutants: a case-control study on Italian women of reproductive age. Environ Health Perspect 117, 1070–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy BS, Rozati R, Reddy S, Kodampur S, Reddy P, Reddy R, 2006. High plasma concentrations of polychlorinated biphenyls and phthalate esters in women with endometriosis: a prospective case control study. Fertil Steril 85, 775–779. [DOI] [PubMed] [Google Scholar]
- Saillenfait AM, Ndiaye D, Sabate JP, 2015. Pyrethroids: exposure and health effects - an update. Int J Hyg Environ Health 218, 281–292. [DOI] [PubMed] [Google Scholar]
- Serra H, Scholze M, Altenburger R, Busch W, Budzinski H, Brion F, Aït-Aïssa S, 2019. Combined effects of environmental xeno-estrogens within multi-component mixtures: comparision of in vitro human- and zebrafish-based estrogenicity bioassays. Chemosphere 227, 334–344. [DOI] [PubMed] [Google Scholar]
- Shafrir AL, Farland LV, Shah DK, Harris HR, Kvaskoff M, Zondervan K, Missmer SA, 2018. Risk for and consequences of endometriosis: a critical epidemiologic review. Best Pract Res Clin Obstet Gynaecol 51, 1–15. [DOI] [PubMed] [Google Scholar]
- Smarr MM, Kannan K, Buck Louis GM, 2016. Endocrine disrupting chemicals and endometriosis. Fertil Steril 106, 959–966. [DOI] [PubMed] [Google Scholar]
- Swan SH, Kruse RL, Liu F, Barr DB, Drobnis EZ, Redmon JB, Wang C, Brazil C, Overstreet JW, the Study for Future Families Research Group, 2003. Semen quality in relation to biomarkers of pesticide exposure. Environ Health Perspect 111, 1478–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trabert B, De Roos AJ, Schwartz SM, Peters U, Scholes D, Barr DB, Holt VL, 2010. Non-dioxin-like polychlorinated biphenyls and risk of endometriosis. Environ Health Perspect 118, 1280–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upson K, De Roos AJ, Thompson ML, Sathyanarayana S, Scholes D, Barr DB, Holt VL, 2013. Organochlorine pesticides and risk of endometriosis: findings from a population-based case-control study. Environ Health Perspect 121, 1319–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upson K, Sathyanarayana S, De Roos AJ, Thompson ML, Scholes D, Dills R, Holt VL, 2013. Phthalates and risk of endometriosis. Environ Res 126, 91–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Zhang R, Jin F, Lou H, Mao Y, Zhu W, Zhou W, Zhang P, Zhang J, 2017. Perfluoroalkyl substances and endometriosis-related infertility in Chinese women. Environ Int 102, 207–212. [DOI] [PubMed] [Google Scholar]
- Weuve J, Hauser R, Calafat AM, Missmer SA, Wise LA, 2010. Association of exposure to phthalates with endometriosis and uterine leiomyomata: findings from NHANES, 1999–2004. Environ Health Perspect 118, 825–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Pan W, Zhao Y, Zhao S, Zhu Y, Liu W, Liu J, 2017. Association of pyrethroids exposure with onset of puberty in Chinese girls. Environ Pollut 227, 606–612. [DOI] [PubMed] [Google Scholar]
- Zanger UM, Schwab M, 2013. Cytochrome P450 enzymes in drug metabolism: regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol Ther 138, 103–141. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


