Abstract
Stress is known to influence smoking relapse. Experimental studies indicate acute stress increases nicotine-seeking behavior; yet neurobiological mechanisms remain poorly understood. Herein, we investigated disrupted excitatory neural activity in the dorsolateral prefrontal cortex (dlPFC) as a mechanism of stress-induced nicotine-seeking behavior. Non-treatment-seeking cigarette smokers were screened for psychiatric, medical, and neuroimaging contraindications. Using a double-blind, placebo-controlled, randomized crossover design, participants (N=21) completed two oral-dosing sessions: stress (yohimbine 54mg + hydrocortisone 10mg) vs. placebo (lactose 54mg + lactose 10mg). During each experimental session, working memory proficiency, dlPFC excitatory neural activity, nicotine-seeking behavior, and subjective effects were measured. dlPFC excitatory neural activity was quantified via glutamate modulation during working memory performance using functional proton magnetic resonance spectroscopy. Nicotine-seeking behavior was assayed using a cigarette puffs vs. money choice progressive ratio task. Results indicated yohimbine + hydrocortisone evoked a sustained physiological stress response (elevated heart rate, blood pressure, saliva cortisol, and saliva α-amylase levels; ps<.05). Relative to placebo levels, acute stress increased nicotine-seeking behavior (ps<.05), disrupted dlPFC glutamate modulation (p=.025), and impaired dlPFC function (working memory proficiency; ps<.05). The stress-induced increase in nicotine-seeking behavior was linearly related to the stress-induced disruption of dlPFC glutamate modulation (R2=0.24–0.37; ps<.05). These findings suggest disrupted dlPFC excitatory neural activity is a neurobiological correlate of acute stress-induced nicotine-seeking behavior. These findings further emphasize the central role of the dlPFC in regulating drug-seeking behavior. Future studies are needed to evaluate interventions to improve dlPFC resilience to acute stress effects, including neurostimulation, working memory training, and ‘anti-stress’ medications.
Keywords: stress, drug seeking, nicotine use disorder, prefrontal cortex, functional proton magnetic resonance spectroscopy, glutamate
INTRODUCTION
Tobacco use remains the leading cause of preventable death, accounting for more than 7 million deaths each year worldwide (WHO, 2017). Nearly 70% of adult cigarette smokers want to stop smoking and more than half attempted to quit in the past year (Wang et al., 2018). Food and Drug Administration (FDA)-indicated pharmacotherapies for smoking cessation attenuate craving and withdrawal symptoms, and yet, relapse rates remain unacceptable high (Eisenberg et al., 2008). Factors that precipitate relapse are complex and extend beyond craving and withdrawal. There is strong evidence that stress contributes to cigarette smoking relapse (McKee et al., 2011). Individuals who relapsed to smoking often cited ‘stress’ as a precipitating factor (al’Absi, 2006; Hughes, 2009). Preclinical studies indicate experimental stress reliably increases drug-seeking behavior across drugs of abuse, including nicotine (reviewed (Mantsch et al., 2016)). Yet, it is not clear how acute stress motivates nicotine-seeking behavior – how does stress alter cognitive function and/or neural network communication such that nicotine-seeking behavior increases?
A neurocognitive framework for approaching this question posits that two neural systems, ‘top-down’ (reflective) and ‘bottom-up’ (impulsive), dynamically interact to regulate behavior (Bechara, 2005). The top-down system, anchored by the prefrontal cortex (PFC), is responsible for goal-directed behavior, decision-making, and inhibitory control. Conversely, the bottom-up system, which includes the amygdala and striatum, is associated with incentive salience, sensation-seeking, and impulsive actions. The relative influence of these neural systems on behavior is not static; rather, their respective influence changes dynamically as environmental cues, interoceptive signals, and affective states are integrated. Stress has been hypothesized to influence both systems (Koob, 2008). Stress may enhance bottom-up signals, including nicotine craving (McKee et al., 2011), and/or impair top-down inhibitory control (McKee et al., 2015). From this neurocognitive framework, we focused on the dorsolateral PFC (dlPFC) as an index of the top-down neural system, and investigated the question; does acute stress increase nicotine-seeking behavior by disrupting dlPFC function?
Stress can be defined as a challenge to organism homeostasis. Stressful events activate the hypothalamic-pituitary-adrenal (HPA)-axis and autonomic nervous system (ANS) and increase circulating levels of noradrenaline and cortisol. Noradrenaline and cortisol mediate many of the physiological effects of stress, including elevated heart rate and blood pressure. The noradrenergic system, in particular, has been repeatedly shown to play a central role in stress-induced drug-seeking behavior (reviewed (Mantsch et al., 2016)). Yohimbine (YOH) is a presynaptic noradrenergic α2-autoreceptor antagonist that disinhibits noradrenaline release. YOH is widely used in preclinical research to evoke an acute physiological stress response (via increased plasma noradrenaline levels) and has been shown to reliably increase drug-seeking behavior (Mantsch et al., 2016). In the brain, noradrenaline levels exhibit an inverted ‘U’-shaped relationship with dlPFC function, i.e., too little, and too much, noradrenergic stimulation impairs dlPFC function. In non-human primates, elevated noradrenergic stimulation impaired spatial working memory proficiency and suppressed dlPFC neural spiking frequency (reviewed (Arnsten, 2009)). Noradrenergic stimulation also impaired working memory proficiency in humans (reviewed (Chamberlain et al., 2006)). Based on preclinical research indicating that stress impairs dlPFC function (reviewed (Arnsten, 2009)), we chose to focus on the top-down neural system, specifically the dlPFC, in our investigation of acute stress effects on nicotine-seeking behavior. Simplistically, stress may impair dlPFC cognitive processes (e.g., inhibitory control, decision-making) and thus, disinhibit nicotine-seeking/self-administration behavior.
Here we quantified dlPFC function and excitatory neural activity using a verbal 2-back task. Verbal 2-back is a well-validated working memory task reliably associated with dlPFC activation (Owen et al., 2005) and is neutral with respect to nicotine-dependence (i.e., not confounded by nicotine craving and/or withdrawal) and thus, facilitates isolation of acute stress effects. Participants performed the verbal 2-back during proton functional magnetic resonance spectroscopy (1H fMRS) acquisition in the dlPFC. 1H fMRS is a novel neuroimaging technique that facilitates quantitative in vivo measurement of dynamic changes in glutamate levels during task performance. Prior studies indicate 1H fMRS-measured increases in glutamate levels reflect phasic increases in excitatory neurotransmission and metabolic activity (Sonnay et al., 2016; Stanley and Raz, 2018). We recently piloted this approach among healthy subjects and found 2-back performance significantly increased left dlPFC glutamate levels, as hypothesized (Woodcock et al., 2018b). These findings validated our 1H fMRS 2-back approach. Moreover, 1H fMRS is not confounded by neurovascular coupling. Elevated blood pressure and heart rate are hallmarks of a stress response, which may influence other imaging metrics, e.g., blood oxygen-level dependent (BOLD) fMRI; thus, we chose to use 1H fMRS to investigate acute stress effects on dlPFC neural activity.
In this study, we hypothesized that acute stress would: 1) disrupt dlPFC excitatory neural activity (glutamate modulation), 2) impair dlPFC function (2-back response accuracy), and 3) increase nicotine-seeking behavior, relative to placebo levels. Finally, we hypothesized the magnitude of stress-induced nicotine-seeking would be linearly related to stress effects on dlPFC glutamate modulation. If supported, our findings would provide preliminary evidence of a putative neurobiological mechanism, i.e., disrupted dlPFC excitatory neural activity, through which acute stress may act to increase nicotine-seeking behavior. We hypothesize that acute stress, especially noradrenergic stimulation, disinhibits nicotine-seeking behavior by disrupting dlPFC inhibitory control, similar to prior research (McKee et al., 2015).
METHODS
Participants
The local Institutional Review Board approved all study procedures (conducted in accordance with the Declaration of Helsinki). Interested candidates (n=105) were invited for in-person screening. Sobriety was verified (expired breath alcohol <.02%) before informed consent procedures. Assessments included: self-report questionnaires (e.g., Fagerstrom Test for Nicotine Dependence: FTND) (Heatherton et al., 1991), brief computerized psychiatric interview (MINI-6) (Sheehan and Lecrubier, 2010), expired breath carbon monoxide (CO; biomarker of recent smoking), urine sample (tested for substance use and pregnancy), electrocardiogram (ECG), resting vital signs (blood pressure [BP] and heart rate [HR]), and MRI contraindications (self-report). Among those invited for an in-person screening, 53 did not show up (and were dropped) and 26 were found to be ineligible. Participants who satisfied all inclusion/exclusion criteria (Supplemental Table S1) and provided written informed consent were invited to participate in the study (n=27). Experimental sessions were completed on non-consecutive weekdays (Monday-Friday; 76% completed both sessions within 7 days). Participants who completed both sessions were included in analyses (N=21). Female participants were scheduled during the luteal phase of the menstrual cycle (self-report) to minimize stress response variability (Kirschbaum et al., 1999). Participants were compensated up to $200 for their time.
Experimental Procedures
Participants completed experimental sessions on separate days (placebo vs. stress; randomized crossover design) under double-blind and placebo-controlled conditions (blind maintained by MKG). Participants smoked cigarettes ad libitum before/after each session. Experimental procedures are detailed in Figure 1. Upon arrival (11:00am), sobriety was verified (expired breath alcohol <.02%). Physiological and subjective effects were measured periodically throughout each session. Saliva samples were collected via oral swab three times during each session (Salimetrics®, State College, PA) and assayed for cortisol and α-amylase levels. Seated and resting vital signs (blood pressure (BP) and heart rate (HR)) were measured at four time points throughout procedures. A periodic self-report battery was also collected at four time points and consisted of: Minnesota Nicotine Withdrawal Scale (MNWS) (Hughes and Hatsukami, 1986), Brief Questionnaire of Smoking Urges (QSU) (Cox et al., 2001), State-Trait Anxiety Inventory (STAI; state version) (Spielberger, 1983), and Positive and Negative Affect Scale (PANAS) (Watson et al., 1988).
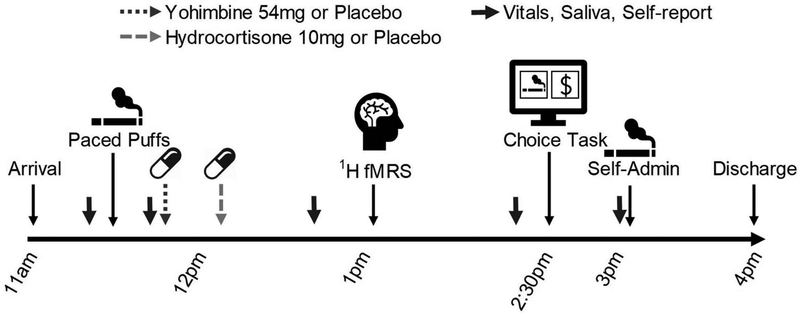
Figure 1:
Experimental Timeline. At 11am, the participant arrived at the laboratory and were screened for sobriety (expired breath alcohol < .02%). At 11:20am, a saliva sample was collected. At 11:30am, the participant smoked in Room A (Paced Puffs procedure). At 11:40am, vital signs and self-report measures were collected. At 11:45am, the participant orally self-administered (swallowed) the first compound: either 54mg yohimbine (YOH) or 54mg lactose (placebo). At 12:15pm, the participant orally self-administered the second compound: either 10mg hydrocortisone (HYD) or 10mg lactose (placebo). At 12:40pm, vital signs, saliva, and self-report data were collected. At 12:50pm, the participant was escorted to the MRI center for a 1:00pm scan (1H fMRS started around 1:15pm). At 2:00pm, the participant was escorted back to the laboratory. At 2:20pm, vital signs and self-report data were collected. At 2:30pm, the participant completed the Puffs vs. Money Choice Task (nicotine-seeking task; Room B). At 3:00pm, vital signs, saliva, and self-report data were collected. At 3:05pm, the participant self-administered (smoked) earned cigarette puffs in Room A. At 4pm, the participant was debriefed, paid, and discharged.
At 11:30am, participants completed the ‘paced puff’ procedure. Participants smoked one cigarette puff (1–2s inhale; preferred brand; experimenter provided) every minute for 5min (6 total puffs; video-verified) as an experimental control for recent nicotine exposure. Participants smoked in only one experimental room (Room A; externally-ventilated). At 11:45am, the first capsule (non-descript opaque capsule) was self-administered (swallowed; 54mg yohimbine [‘stress’] or 54mg lactose [‘placebo’]). At 12:15pm, participants self-administered the second capsule (10mg hydrocortisone [HYD; ‘stress’] or 10mg lactose [‘placebo’]). At 12:50am, participants were escorted to the MRI scan which was completed from 1–2pm (see Neuroimaging below). Following the scan, participants were escorted back to the laboratory and completed the Choice Task (described below) in Room B from 2:30–3pm. At 3:05pm, participants moved to the experimental smoking room (Room A) and self-administered earned cigarette puffs (1–2s inhale; video-verified; preferred brand; experimenter provided). Separate rooms for nicotine-seeking task (Room A) and nicotine self-administration (Room B) minimized the influence of environmental cues on choice behavior. Participants were monitored until discharge at 4pm.
Pharmacology/Biomarkers
YOH is a presynaptic α2-autoreceptor antagonist that blocks negative feedback and disinhibits noradrenaline release (Goldberg and Robertson, 1983). Biomarkers of YOH dosing include BP and saliva α-amylase levels (indirect biomarker of β-adrenoceptor stimulation) (Greenwald et al., 2013). HYD is metabolized to cortisol and binds as an agonist to glucocorticoid and mineralocorticoid receptors (Meikle and Tyler, 1977). Saliva cortisol is a well-validated correlate of plasma cortisol levels (Kahn et al., 1988). In combination, YOH+HYD increase the neurochemical constituents, noradrenaline and cortisol, of a ‘natural’ stressful event.
Neuroimaging
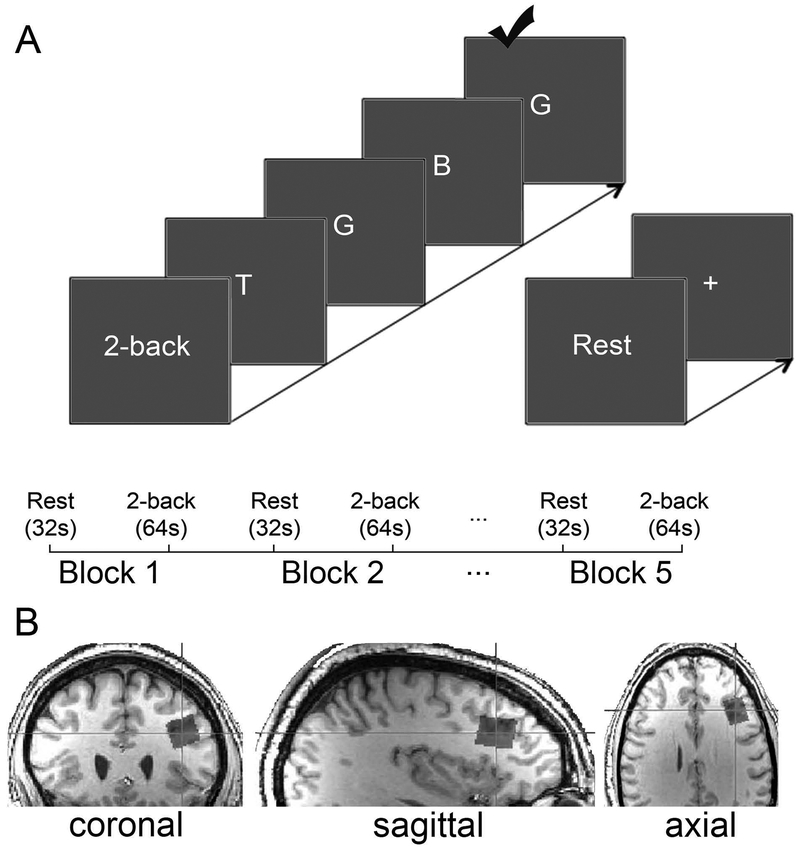
dlPFC function and excitatory neural activity were interrogated via verbal 2-back task performance (Owen et al., 2005) during 1H fMRS spectra acquisition (Woodcock et al., 2018b). Before each MRI scan, participants practiced the 2-back task outside of the scanner until deemed proficient by the experimenter (Figure 2A). Inside the scanner, participants completed 5 blocks of passive visual fixation (‘rest’; 32s) and 5 blocks of verbal 2-back (64s) during continuous 1H MRS spectra acquisition. During each 2-back block, a 4s task prompt (‘2-back’) was followed by 20 uppercase letters presented serially (3s/letter; 500ms letter presented; 2500ms blank screen; 6 target letters). Response latency, response accuracy, i.e., percentage of correct responses, and d-prime (d’ = ZHit - ZFalseAlarm) (Macmillan and Creelman, 1990) were outcome measures of interest (feedback not provided to participants). Neuroimaging was conducted on a 3T Siemens Verio system (32-channel receive-only volume head coil) and included a structural scan (T1-weighted; MPRAGE), B0-field shim (left dlPFC; 25×25×25mm; FASTESTMAP (Gruetter and Tkáč, 2000)), automated voxel placement procedure (Woodcock et al., 2018a) (left dlPFC; Figure 2B),1H MRS spectra acquisition (15 spectra; 480s; PRESS with OVS and VAPOR; voxel dimensions 15×20×15mm; TE=23ms; TR=4.0s; 8 averages/spectrum; bandwidth = 2kHz; 2048 data points) and water-unsuppressed 1H MRS spectrum acquisition (2 averages; TE=23ms; TR=10s). A relatively short TE minimized diffusion and J-evolution, while the relatively long TR reduced T1-weighting.
Figure 2:
Verbal 2-back task and voxel location. A) The verbal 2-back task consisted of five repetitions of alternating periods of passive visual fixation ‘Rest’ (32s; 2s prompt “Rest”; 30s static centered fixation cross) and 2-back (64s; 4s prompt “2-back”; 20 capitalized centered letters presented serially [3s/letter; 500ms on-screen; 2500ms blank screen]; 6 targets/block). B) The voxel location is depicted in orthonormal slices. The voxel (15x20x15mm; 4.5cm3) was in the left dorsolateral prefrontal cortex (Brodmann Areas 45/46).
Cigarette Puffs vs. Money Choice Task
After the 11:30am paced puff procedure, the Choice Task was the only other opportunity for participants to smoke during each experimental session. During this 30min task, participants could earn one cigarette puff or $0.25 (Tidey et al., 1999) via computer ‘mouse’ clicking on 11 independent choice trials. In each trial, the participant selected either ‘puff’ or ‘money’ on the computer. To earn one unit of that selection, the participant had to satisfy the response requirement (progressive ratio schedule of ‘mouse’ clicks [identical schedules for puffs and money]: 5, 12, 33, 100, 180, 340, 540, 835, 1220, 1660, and 2275). At 3:05pm (after the Choice Task), participants moved to experimental Room A, were provided one cigarette (preferred brand), and instructed to smoke the exact number of puffs earned (1–2s inhale; video-verified) at their chosen pace.
Nicotine-Seeking Behavior
Nicotine-seeking behavior was quantified two ways. First, the percentage of ‘mouse’ clicks to earn cigarette puffs relative to the total number of ‘mouse’ clicks for both puffs and money was calculated. This is a continuous measure of nicotine-seeking behavior scaled to total behavioral output; referred to as ‘normalized nicotine-seeking behavior’ for the remainder of the manuscript. Second, the total number of cigarette puffs earned was quantified. This is a ‘real-world’ measure of nicotine-seeking behavior as subjects smoked the exact number of cigarette puffs earned during the task.
Analysis Strategy
1H MRS spectra were analyzed by a blinded experimenter using LCModel v6.3 (post-processing and quantification were 100% automated) (Provencher, 2008). Consecutive spectra were corrected for eddy current, zero- and first-order phase, and B0-shift, and averaged to improve signal-to-noise ratio (SNR) which resulted in 32s temporal resolution (8 averages/spectra). T1-weighted images were B1-field corrected and segmented into partial volume maps of cerebrospinal fluid, gray matter, and white matter using FreeSurfer and FSL tools (Dale et al., 1999; Smith et al., 2004). Finally, voxel tissue composition and appropriate correction factors [e.g., T1 and T2 relaxation; (Posse et al., 2007)] were used to quantify absolute glutamate concentration (mmol/kg wet weight) (Gasparovic et al., 2006). We used a classical block design: alternating periods of 2-back (64s) and visual fixation rest (32s). 2-back task blocks were longer than rest blocks (64s vs. 32s) and thus, more spectra were acquired during 2-back than rest (16 vs. 8 spectra). Accordingly, we cleaved each 2-back block into ‘early’ 2-back (first 32s) and ‘late’ 2-back (final 32s) to match the temporal resolution and SNR of the rest measurements (32s) and avoid SNR and Cramer-Rao Lower-Bound (CRLB%) bias, an approach we used previously (Woodcock et al., 2018b). Glutamate modulation was calculated as percentage change from rest to early and late 2-back (separately): [(2-back – rest)/(rest)] * 100. Voxel placement accuracy was analyzed by co-registering each subject’s voxel to template space and calculating 3D geometric voxel overlap with the template voxel (Woodcock et al., 2018a).
All data were evaluated for missing and extreme values, and normality (Shapiro-Wilk test of normality; skewness/kurtosis statistics). Variable distributions were on a case-by-case basis and normalized with either statistical transformations (log10) or winsorization (extreme values; ≥1 SD from nearest value) prior to outcome analyses. Power analyses, calculated for the Choice Task using G*Power (v3.0.10), indicated that 21 participants was sufficient to detect a moderate within-subject main effect (Cohen’s f≥0.25) at the recommended power (0.80) and α=.05 for correlated measures (r=.70) (Cohen, 1992; Erdfelder et al., 1996).
Within-subject two-way repeated measures analyses of variance (rmANOVAs) were used to evaluate a priori hypotheses. Main effects of experimental session (placebo vs. stress) were considered for glutamate modulation, 2-back behavioral data, and nicotine-seeking behavior. For subjective and physiological responses, which were collected multiple times throughout each experimental session, omnibus experimental session by time point interactions were considered. Bivariate correlations examined our central hypothesis: stress effects on nicotine-seeking behavior were linearly related to stress effects on dlPFC excitatory neural activity. Four Pearson correlations evaluated the relationship between dlPFC excitatory neural activity (early and late 2-back glutamate modulation) and nicotine-seeking behavior (normalized nicotine-seeking behavior and cigarette puffs earned/smoked). FTND score, i.e., nicotine dependence level, was hypothesized to be related to nicotine-seeking behavior during placebo (test of ecological validity). If found, FTND will be covaried to isolate acute stress effects on nicotine-seeking behavior. FTND was evaluated as a covariate for all measures (only included when significant). Descriptive statistics are presented as mean ± one standard deviation (M ± 1 SD), and in figures, error bars depict ± one standard error of the mean (SEM), unless otherwise noted. Significance threshold was p≤.05 for all analyses.
RESULTS
Sample Characteristics
Participants (N=21) were 28.0±3.9 years old (range: 21–34), mostly male (85.7%), and African-American (71.4%). Participants reported smoking 17.2±5.9 cigarettes/day and were moderately nicotine dependent (FTND=6.1±2.0; Table 1).
Table 1:
Sample characteristics (N = 21)
| Characteristics | M ± 1 SD or % |
|---|---|
| Smoking (cigarettes/day) | 17.2 ± 5.9 |
| Age at first cigarette | 16.5 ± 3.5 |
| Menthol cigarette (%) | 85.7% |
| FTND score | 6.1 ± 2.0 |
| Age (yrs) | 28.0 ± 3.9 |
| Gender (% Male) | 85.7% |
| Race (% African-American) | 71.4% |
| Education (yrs) | 13.1 ± 2.4 |
| Alcohol use (days/month) | 3.8 ± 4.9 |
| Used alcohol in past month (%) | 71.4% |
| Marijuana use (days/month) | 4.3 ± 5.4 |
| Used marijuana in past month (%) | 47.6% |
Note: M = mean; SD = standard deviation; FTND = Fagerstrom Test for Nicotine Dependence.
dlPFC Function and Excitatory Neural Activity
Throughout the Results section, rmANOVA effect sizes are provided (partial η2, but denoted as η2) and interpreted as small (≤.09), moderate (0.10–0.24), and large (≥.25). Results are presented in order of importance to our hypotheses.
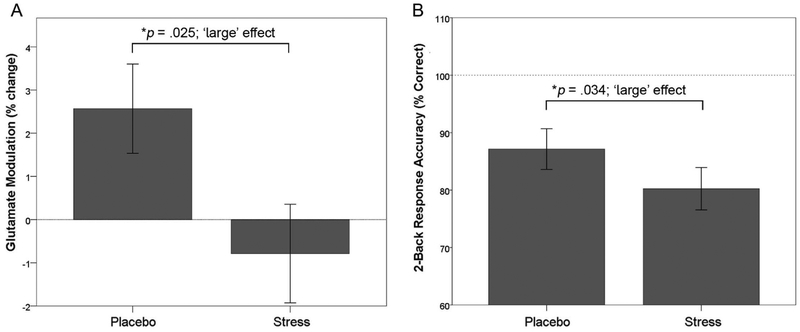
Glutamate modulation, i.e., the percentage change in dlPFC glutamate levels from rest to 2-back performance, was evaluated using a two-way rmANOVA. Results indicated YOH+HYD significantly impaired early 2-back glutamate modulation relative to placebo modulation levels (F(1,72)=6.02; p=.025; η2=0.25; Figure 3A). Follow-up analyses indicated glutamate levels were 2.7% higher during 2-back performance than during rest in the placebo session (F(1,83)=8.12; p<.01; η2=0.09; not shown) but glutamate levels were unchanged during the stress session (p=.58). Behavioral data indicate YOH+HYD significantly impaired 2-back response accuracy relative to placebo levels (F(1,40)=6.01, p=.034, η2=0.38; 87.1±13.3% correct vs. 78.2±15.1% correct; Figure 3B). Further, YOH+HYD significantly impaired d’ across task blocks, relative to placebo (F(1,44)=3.57, p=.013, η2=0.25; not shown). These findings indicate YOH+HYD disrupted dlPFC excitatory neural activity and impaired working memory proficiency relative to placebo levels. YOH+HYD did not alter 2-back response latency relative to placebo levels (ps≥.40). During placebo, late 2-back glutamate levels were not higher than rest (i.e., no glutamate modulation; p>.40) and thus, stress effects on late 2-back glutamate modulation were not evaluated. FTND was not related to glutamate modulation or 2-back response metrics (ps>.25), and thus, not covaried in analyses. Voxel placement accuracy was reliable across participants and experimental sessions (85.6±16.8% overlap with the template voxel in template brain space).
Figure 3:
Stress disrupted dlPFC glutamate modulation and impaired working memory proficiency. A) During placebo, glutamate levels during early 2-back were significantly higher by 2.7% than rest levels (F(1,83)=8.12; p<.01; η2=0.09) denoted as ‘glutamate modulation’ or % change relative to rest levels. Also, stress significantly attenuated early 2-back glutamate modulation relative to placebo modulation levels (F(1,72)=6.02; p=.025; η2=0.25, large effect). B) Stress significantly impaired 2-back response accuracy relative to placebo levels (F(1,40)=6.01, p=.034, η2=0.38, large effect). Group means ± 1 SEM are depicted. Note: SEM = standard error of the mean.
Nicotine-Seeking Behavior
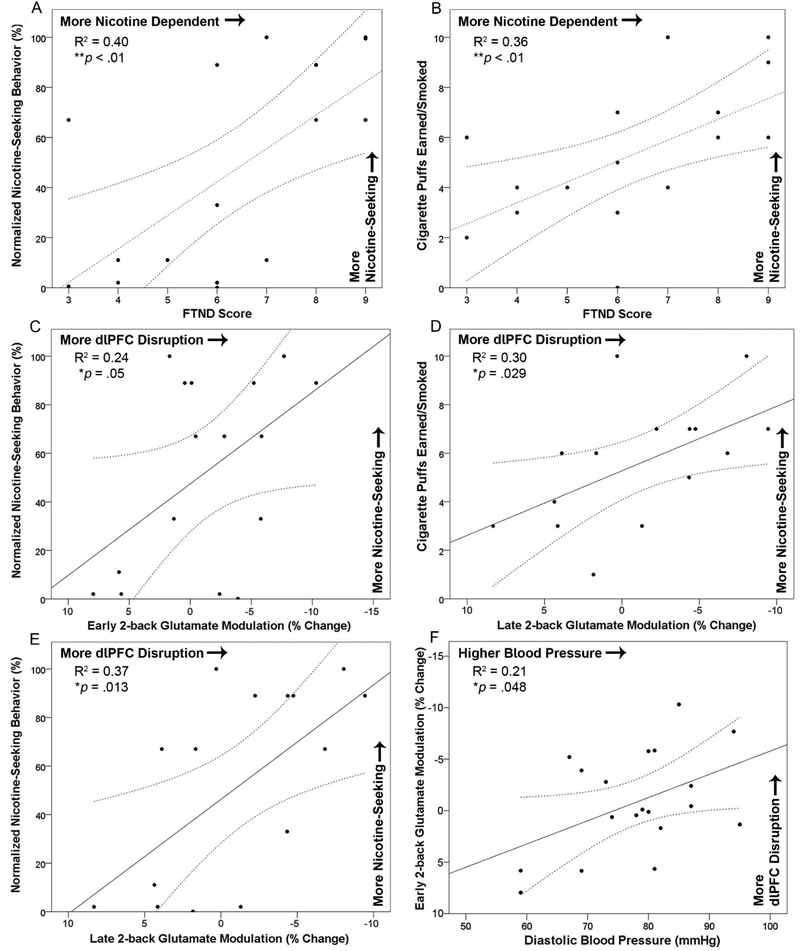
During placebo, nicotine dependence level (FTND score) was positively correlated with percentage of total ‘mouse’ clicks allocated to earn cigarette puffs (i.e., ‘normalized nicotine-seeking behavior’; R2=0.40, p<.01, Figure 4A) and with the number of cigarette puffs earned/smoked (R2=0.36, p<.01; Figure 4B). FTND score explained 36–40% of the variance in nicotine-seeking behavior during placebo indicating nicotine-seeking behavior measured in the laboratory scaled with cigarette smoking behavior outside of the laboratory, i.e., our paradigm has ecological validity. To isolate acute stress effects on nicotine-seeking behavior (independent of nicotine dependence level), FTND score was covaried in subsequent analyses. Controlling for FTND score, rmANCOVA indicated YOH+HYD significantly increased normalized nicotine-seeking behavior (F(1,16)=5.55, p=.032, η2=0.26; 51.7±40.0%, Range=10–100%) and number of cigarette puffs earned/smoked (F(1,16)=4.93, p=.041, η2=0.24; 5.6±2.4, Range=1–10), relative to placebo levels (normalized nicotine-seeking: 46.7±41.6%, Range=0–100%; puffs: 5.3±2.7, Range=0–10). Exploratory analyses revealed a significant FTND score by experimental session interaction on cigarette puffs earned/smoked (F(1,16)=4.88, p=.042, η2=0.23) and normalized nicotine-seeking (F(1,16)=6.16, p=.025, η2=0.28 which indicated YOH+HYD increased nicotine-seeking behavior more among less nicotine-dependent individuals compared to more nicotine-dependent individuals. Stated differently, more nicotine-dependent individuals exhibited less malleable nicotine-seeking behavior in response to YOH+HYD than less dependent individuals.
Figure 4:
Upper Panel. During placebo, FTND score was significantly positively correlated with A) normalized nicotine-seeking behavior (R2=0.40, p<.01) and B) cigarette puffs earned and smoked (R2=0.36, p<.01). A linear trend was fitted to the data with 95% confidence intervals displayed as curved dashed lines. These correlations indicate that participants who reported higher nicotine dependence levels exhibited greater nicotine-seeking behavior during the placebo session, suggesting the puffs vs. money choice task paradigm has ecological validity. Lower Panels. The effects of stress on dlPFC glutamate modulation were linearly related to the effects of stress on nicotine-seeking behavior. During the stress session, greater dlPFC disruption was correlated with more nicotine-seeking across metrics; C) early 2-back glutamate modulation and normalized nicotine-seeking behavior (R2=0.24, p=.05), D) late 2-back glutamate modulation and cigarette puffs earned/smoked (R2=0.30, p=.029), and E) late 2-back glutamate modulation and normalized nicotine-seeking behavior (R2=0.37, p=.013). F) During the stress session, diastolic blood pressure was significantly correlated with early 2-back glutamate modulation indicating higher blood pressure was related to greater dlPFC disruption (R2=0.21, p=.048). Note: FTND = Fagerstrom Test for Nicotine Dependence; SEM = standard error of the mean.
Bivariate Correlations
Four planned bivariate correlations examined relationships between dlPFC glutamate modulation and nicotine-seeking behavior during the stress session. Pearson correlations indicated early 2-back glutamate modulation was significantly related to normalized nicotine-seeking behavior (R2=0.24, p=.05; Figure 4C), but not cigarette puffs earned/smoked (R2=0.13, p=.17; not shown). Late 2-back glutamate modulation was significantly related to cigarette puffs earned/smoked (R2=0.30, p=.029; Figure 4D) and normalized nicotine-seeking behavior (R2=0.37, p=.013; Figure 4E). These correlations suggest the extent to which stress disrupted dlPFC excitatory activity was linearly related to the extent to which stress increases nicotine-seeking behavior, accounting for 24–37% of the variance in nicotine-seeking behavior.
Exploratory Pearson correlations examined relationships between physiological stress biomarkers and dlPFC glutamate modulation. During the stress session, diastolic blood pressure (after the 1H fMRS scan) was significantly related to early 2-back glutamate modulation (R2=0.21, p=.048; Figure 4F) and ‘trend’-level related to late 2-back glutamate modulation (R2=0.20, p=.058; not shown). The extent to which stress increased diastolic blood pressure was linearly related to the extent to which stress disrupted dlPFC excitatory activity, accounting for 21% of the variance. Systolic blood pressure and saliva markers were not significantly related to dlPFC glutamate modulation (ps>.05).
Subjective Effects
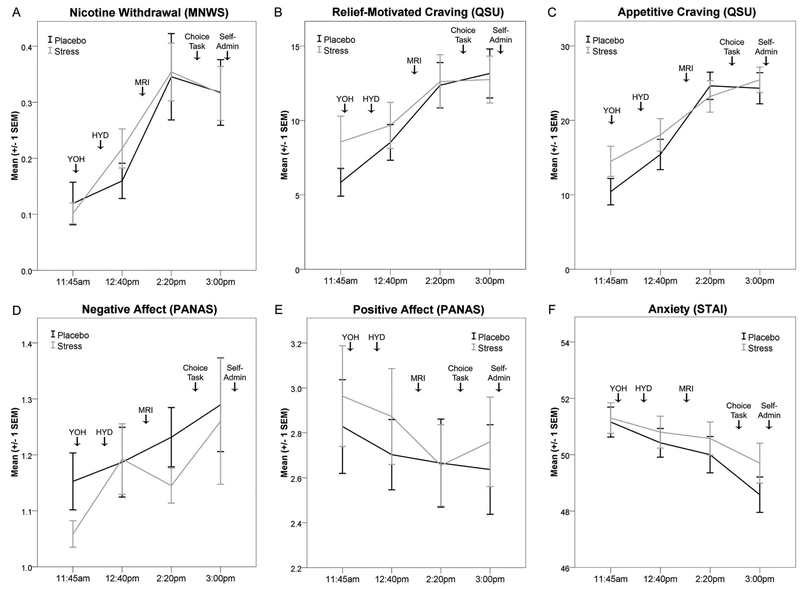
No self-report measures exhibited significant experimental session by time point interactions (ps>.10; Figure 5). Exploratory analyses indicate nicotine withdrawal symptoms, relief-motivated craving, appetitive craving, and negative affect each significantly increased throughout each experimental session (ps<.05) but were not significantly affected by YOH+HYD. Peak nicotine withdrawal severity was between ‘slight’ and ‘mild’, while appetitive and relief-motivated craving peaked between ‘moderate’ and ‘substantial.’ Anxiety levels significantly decreased throughout each session (p<.05) but were not affected by YOH+HYD. Experimental session order was significantly related to positive affect (p<.01) and thus, was included as a covariate. Controlling for session order, positive affect increased throughout both experimental sessions (p=.036) but was not affected by YOH+HYD (p>.20). FTND was not related to any subjective effect’s measures (ps>.05), and thus, was not covaried in the above analyses.
Figure 5:
Stress did not alter subjective effects measures. Subjective effects (group mean ± one SEM) are depicted for each experimental session (light gray = stress; dark gray = placebo). A) Nicotine withdrawal severity (MNWS). B) Relief-motivated craving (QSU). C) Appetitive craving (QSU). D) Negative affect (PANAS). E) Positive affect (PANAS). F) Anxiety levels (STAI; state version). Note: SEM = standard error of the mean; MNWS = Minnesota Nicotine Withdrawal Scale; QSU = Brief Questionnaire of Smoking Urges; PANAS = Positive and Negative Affect Scale; STAI = State-Trait Anxiety Index; YOH = yohimbine or placebo dose; HYD = hydrocortisone or placebo dose; MRI = magnetic resonance imaging; Self-admin = self-administration of cigarette puffs earned during the Choice Task.
Physiological Effects
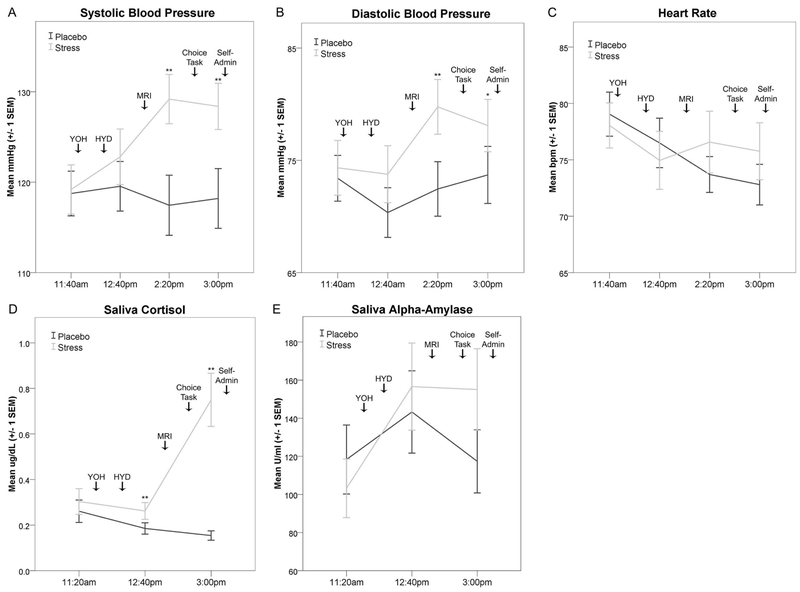
Two-way rmANOVAs indicated systolic and diastolic BP (mmHg), HR (bpm), and saliva cortisol (ug/dL; log10-transformed) exhibited significant omnibus experimental session by time point interactions, as expected. YOH+HYD significantly increased systolic and diastolic BP relative to placebo levels (systolic: F(3,57)=8.33, p<.001, η2=0.31, Figure 6A; diastolic: F(3,57)=2.98, p=.039, η2=0.14, Figure 6B). YOH+HYD significantly increased HR and saliva cortisol relative to placebo levels (HR: F(3,57)=3.38, p=.024, η2=0.15, Figure 6C; cortisol: F(2,38)=25.13, p<.001, η2=0.57, Figure 6D). Relative to baseline, YOH+HYD significantly increased saliva α-amylase levels (U/mL) during the stress session (F(2,38)=5.02, p=.012, η2=0.21, Figure 6E), but the hypothesized experimental session by time point interaction was not significant (p=.13). FTND was not related to any physiological biomarkers (ps>.20) and thus, was not included as a covariate for the above analyses. These data indicate YOH+HYD evoked a significant and sustained physiological stress response associated with moderate-to-large effect sizes.
Figure 6:
Yohimbine (54mg; oral) + Hydrocortisone (10mg; oral) evoked a sustained physiological stress response. Physiological effects (group mean ± one SEM) are depicted for each experimental session (light gray = stress; dark gray = placebo). Drop down arrows indicate the timing of experimental procedures. A) Systolic blood pressure (mmHg). B) Diastolic blood pressure (mmHg). C) Heart rate (beats per minute; bpm). D) Saliva cortisol levels (µg/dl). E) Saliva α-amylase levels (U/ml). Significant pairwise session differences at each time point are noted: *p<.05; **p<.01. Note: mmHg = millimeters of mercury; SEM = standard error of the mean; µg/dl = micrograms per deciliter; U/ml = units per milliliter; YOH = yohimbine dose; HYD = hydrocortisone dose; MRI = magnetic resonance imaging; Self-admin = self-administration of cigarette puffs earned during the Choice Task.
Session Order Effects
Experimental session order (stress or placebo first) was not significantly related to nicotine-seeking behavior (ps>.25); choice task behavioral output (total number of ‘mouse’ clicks; ps>.40); glutamate modulation (ps>.60); working memory response accuracy, latency, or d’ (ps>.25); or physiological biomarkers (ps>.20). Session order was not related to any self-report measures (ps>.08) other than positive affect. Finally, subjects did not correctly identify the stress session more accurately than the placebo condition (62% vs. 67%); only marginally better than random chance (50%), suggesting the experimental ‘blinding’ was effective.
DISCUSSION
This study investigated acute stress effects among non-treatment-seeking cigarette smokers. Principal findings were five-fold. Relative to placebo levels, acute stress: 1) disrupted dlPFC excitatory neural activity (glutamate modulation), 2) impaired dlPFC function (2-back response accuracy), and 3) increased normalized nicotine-seeking behavior and cigarette puffs earned/smoked. Fourth, bivariate correlations indicated greater stress-induced nicotine-seeking behavior was linearly related to greater disruption of dlPFC glutamate modulation during 2-back performance. These findings suggest disrupted dlPFC excitatory neural activity is a neurobiological correlate of acute stress-induced nicotine-seeking behavior. Fifth, higher diastolic blood pressure during stress was linearly related to lower dlPFC 2-back glutamate modulation suggesting noradrenergic stimulation is central to disrupting dlPFC excitatory neural activity.
Nicotine-seeking behavior was assayed using a behavioral economic approach: money vs. cigarette puffs choice progressive ratio task (e.g., (Greenwald et al., 2013)). This approach yielded direct measures of appetitive nicotine motivation relative to a reinforcing alternative choice: money. As hypothesized, nicotine-dependence level (FTND score) was positively correlated with nicotine-seeking behavior during placebo indicating our approach has ecological validity and scales with cigarette smoking behavior outside of the laboratory. To isolate acute stress effects, we covaried nicotine-dependence level in analyses and found that acute stress increased normalized nicotine-seeking behavior and cigarette puffs earned/smoked, relative to placebo levels. Acute stress accounted for 24–26% of the variance in the change in nicotine-seeking behavior from the placebo to the stress session. Follow-up analyses indicated stress increased nicotine-seeking behavior more among less nicotine-dependent individuals suggesting they exhibit more malleable cigarette smoking behavior. ‘Normalized nicotine-seeking behavior’ was an important metric in this study because preclinical studies have reported that YOH increased non-contingent motor responding (Le et al., 2011; Mantsch et al., 2016). This metric accounted for total behavioral output and indicated that stress shifted choice preference to nicotine-seeking behavior. Moreover, we found no evidence that YOH increased motor output (total ‘mouse’ clicks or mean click rate). Our findings are consistent with prior human research that found stress decreased latency to smoking using a lapse paradigm (McKee et al., 2015; McKee et al., 2011) and preclinical studies that found stress increased drug-seeking behavior (Mantsch et al., 2016).
dlPFC function and excitatory neural activity were investigated using verbal 2-back (Owen et al., 2005) during 1H fMRS acquisition. This approach was previously validated in healthy controls (Woodcock et al., 2018b). Our findings indicate dlPFC glutamate levels during 2-back task performance were significantly higher than during rest in the placebo session. Based on extensive preclinical research, elevated glutamate levels measured with 1H fMRS reflect increased excitatory neurotransmission and metabolic activity. 13C MRS research indicates a nearly 1:1 ratio between glutamate-glutamine cycling rate (excitatory neurotransmission) and cerebral metabolic rate of glucose [CMRGLC; oxidative metabolism; reviewed (Rothman et al., 2011)]. Thus, phasic increases in excitatory neurotransmission correspond with increased metabolic activity – both of which are reflected in glutamate modulation measured via 1H fMRS (Sonnay et al., 2016). Non-human primate research indicates that excitatory feed-forward microcircuits in the dlPFC maintain working memory traces (reviewed (Arnsten, 2009)). We speculate that our glutamate modulation findings reveal these neurobiological processes at the macroscopic scale. Specifically, working memory traces, i.e., the neural maintenance of letters during 2-back task performance, drive a phasic increase in excitatory neural activity that we measured as glutamate modulation in the dlPFC (Stanley and Raz, 2018; Woodcock et al., 2018b).
Acute stress significantly impaired dlPFC function (2-back response accuracy and d’) and disrupted dlPFC excitatory neural activity (glutamate modulation), relative to placebo levels. Stress-induction has previously been shown to impair dlPFC function and working memory proficiency across species: human (Chamberlain et al., 2006; Qin et al., 2009), non-human primate (Arnsten, 2009), and rodent (Roozendaal et al., 2004). In particular, elevated noradrenaline levels have been shown to attenuate dlPFC neural spiking frequency and impair spatial working memory in non-human primates (reviewed (Arnsten, 2009)). Noradrenergic stimulation may disrupt dlPFC excitatory microcircuits that maintain working memory traces and thus, impair proficiency (Arnsten, 2009). Indeed, we found that higher diastolic blood pressure during stress, a marker influenced by noradrenergic stimulation, was related to disrupted dlPFC glutamate modulation.
The central hypothesis of this study is that disrupted dlPFC activity is a neurobiological correlate of stress-induced nicotine-seeking behavior. Our findings support this hypothesis. Specifically, the extent to which stress disrupted dlPFC glutamate modulation was linearly related to the extent to which stress increased nicotine-seeking behavior and accounted for 24–37% of the variance. These data are consistent with prior clinical research indicating working memory proficiency and neural activity predicted smoking behavior outside of the laboratory (Loughead et al., 2015; Patterson et al., 2010). Our findings highlight the role of the dlPFC in nicotine-seeking behavior and suggest disrupted dlPFC activity may be a mechanism of stress-induced nicotine-seeking behavior. The dlPFC has a well-established role in cognitive processes that regulate substance use behavior (e.g., decision-making, delayed gratification, and self-control) (Bechara, 2005; Hare et al., 2009; Hare et al., 2014; Nestor et al., 2011). Simplistically, our findings may indicate that stress disrupted subjects’ ability to delay gratification or exhibit inhibitory control, and thus, appetitive nicotine motivation was disinhibited. Alternatively, from a neuroeconomic perspective, stress may have enhanced the expected value of cigarette smoking (or decreased the expected value of money) and shifted choice preference to cigarette puffs (Rangel et al., 2008). Finally, stress may have altered interoceptive signals. Interoceptive signals are processed in the insula and translated into subjective feelings/states that can influence decision-making (Noël et al., 2013). Thus, stress may have altered interoceptive signals or insula activity and shifted subjects’ decision-making preference to nicotine-seeking (e.g., (Janes et al., 2010)). Stress may influence other neural systems, in addition to the dlPFC, that contribute to drug-seeking behavior. However, our findings highlight the importance of the dlPFC and provide preliminary evidence of a mechanistic relationship between stress, dlPFC excitatory neural activity, and nicotine-seeking behavior.
Stress has also been hypothesized to motivate substance use by increasing drug craving and/or withdrawal. Herein, we found that stress did not alter self-reported cigarette craving or nicotine withdrawal symptom severity. Our findings suggest a dissociation between acute stress effects on craving/withdrawal symptoms and nicotine-seeking behavior. There may be some degree of automaticity between stress and nicotine-seeking behavior, independent of the subjective perception of craving and/or withdrawal symptoms. For example, McKee and colleagues found that stressful imagery amplified cigarette craving, decreased latency to smoke, and increased number of cigarettes smoked, relative to neutral imagery (McKee et al., 2015). Guanfacine, an α2A-noradrenergic agonist, blunted the effects of stress on cigarette smoking behavior, but not stress-induced craving (McKee et al., 2015). Thus, stress-induced craving and nicotine self-administration can be disentangled, and noradrenergic stimulation and the dlPFC may play central roles in their dissociation.
In this study, a pharmacological stress-induction approach was used rather than a psychosocial approach because of important methodological advantages, including neurochemical specificity, sustained duration of action, dose-response control, and experimental blinding. Relative to placebo, oral pretreatment with YOH (54mg) and HYD (10mg) elicited a significant and sustained physiological stress response throughout experimental procedures as indicated by biomarkers: systolic and diastolic BP, HR, saliva cortisol, and saliva α-amylase. The magnitude of stress response elicited herein was similar to a robust psychosocial stress-induced approach based on qualitative comparison of effect sizes from the published literature (Woodcock et al., 2019). Importantly, subjects did not correctly identify the stress session more than the placebo session, indicating the experimental ‘blinding’ was effective.
As stated above, we found no evidence that acute stress altered subjective cigarette craving, nicotine withdrawal, anxiety, or affect, relative to placebo levels. Our findings are contrary to prior studies that found elevated craving or withdrawal following stress-induction (e.g., (Greenwald et al., 2013; McKee et al., 2015; McKee et al., 2011; Sinha, 2009)). Our unexpected null findings may reflect poor measurement sensitivity or inconsistent participant responding. However, we believe these explanations are unlikely. We used psychometrically-validated ‘gold-standard’ self-report measures. Also, as hypothesized, craving and withdrawal symptoms significantly increased as a function of experimental nicotine abstinence during each experimental sessions, suggesting consistent participant responding. We speculate the YOH+HYD doses used herein evoked a subtle subjective stress response which was not detected with self-report measures.
It is important to note study limitations. First, the pharmacological stress-induction approach used herein may differ from ‘real life’ stress and subjective stress effects were not measured in this study. Psychosocial stress-induction approaches, especially script-driven mental imagery (e.g., (Sinha, 2009)), more closely approximate ‘naturalistic’ stress. However, pharmacological stress-induction had important methodological advantages for our purposes. Second, this was a small and demographically-homogenous sample: especially for brain-behavior correlation analyses (n=16). Third, laboratory nicotine-seeking behavior may not generalize to ‘real world’ smoking. However, we observed strong relationships between nicotine-dependence level and placebo nicotine-seeking behavior suggesting our paradigm has ecological validity. Fourth, we were not able to disentangle noradrenaline vs. cortisol effects.
Conclusions
In summary, our findings indicate acute stress increases nicotine-seeking behavior, disrupts dlPFC excitatory neural activity, and impairs dlPFC function. Further, greater stress-induced disruptions of dlPFC activity were linearly related to greater stress-induced nicotine-seeking behavior. These data provide preliminary evidence that disrupted dlPFC excitatory neural activity may be a neurobiological correlate of stress-induced nicotine-seeking behavior. Future studies are needed to evaluate interventions to improve dlPFC resilience to acute stress effects, including neurostimulation, working memory training, and ‘anti-stress’ medications.
Supplementary Material
Acknowledgments
The authors thank Caroline Zajac-Benitez, Dr. Muzamil Arshad, Chaitali Anand, Jonathan Lynn, Andrew Neff, Lisa Sulkowski, and Dr. Paul Burghardt for their assistance.
Role of funding source
Research reported in this publication was generously supported by the National Institute on Drug Abuse of the National Institutes of Health under F31 DA040369 (awarded to EAW), T32 DA022975 (awarded to EAW), K99 DA048125 (awarded to EAW), and 2 R01 DA015462 (awarded to MKG). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Funding also generously provided by the Young Investigator Grant (Wayne State University; awarded to EAW), State of Michigan (Joe Young Sr./Helene Lycaki funds), and the Detroit Wayne Mental Health Authority. Funding sources were not involved in the design, execution, analysis, or interpretation of data described in this manuscript. This study is registered as a clinical trial ( NCT03670212).
Footnotes
Conflict of Interest
All authors declare no ethical or financial conflict of interest with respect to the content of this work.
References
- al’Absi M (2006) Hypothalamic-pituitary-adrenocortical responses to psychological stress and risk for smoking relapse. International journal of psychophysiology : official journal of the International Organization of Psychophysiology 59:218–227. [DOI] [PubMed] [Google Scholar]
- Arnsten AF (2009) Stress signalling pathways that impair prefrontal cortex structure and function. Nature Reviews Neuroscience 10:410–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A (2005) Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nature neuroscience 8:1458–1463. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Muller U, Blackwell AD, Robbins TW, Sahakian BJ (2006) Noradrenergic modulation of working memory and emotional memory in humans. Psychopharmacology 188:397–407. [DOI] [PubMed] [Google Scholar]
- Cohen J (1992) A power primer. Psychological bulletin 112:155. [DOI] [PubMed] [Google Scholar]
- Cox LS, Tiffany ST, Christen AG (2001) Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine & Tobacco Research 3:7–16. [DOI] [PubMed] [Google Scholar]
- Dale A, Fischl B, Sereno M (1999) Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage 9:179–194. [DOI] [PubMed] [Google Scholar]
- Eisenberg MJ, Filion KB, Yavin D, Bélisle P, Mottillo S, Joseph L, Gervais A, O’Loughlin J, Paradis G, Rinfret S (2008) Pharmacotherapies for smoking cessation: a meta-analysis of randomized controlled trials. Canadian Medical Association Journal 179:135–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdfelder E, Faul F, Buchner A (1996) GPOWER: A general power analysis program. Behavior research methods, instruments, & computers 28:1–11. [Google Scholar]
- Gasparovic C, Song T, Devier D, Bockholt HJ, Caprihan A, Mullins PG, Posse S, Jung RE, Morrison LA (2006) Use of tissue water as a concentration reference for proton spectroscopic imaging. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine 55:1219–1226. [DOI] [PubMed] [Google Scholar]
- Goldberg M, Robertson D (1983) Yohimbine: a pharmacological probe for study of the alpha 2-adrenoreceptor. Pharmacological Reviews 35:143–180. [PubMed] [Google Scholar]
- Greenwald MK, Lundahl LH, Steinmiller CL (2013) Yohimbine increases opioid-seeking behavior in heroin-dependent, buprenorphine-maintained individuals. Psychopharmacology 225:811–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruetter R, Tkáč I (2000) Field mapping without reference scan using asymmetric echo‐planar techniques. Magnetic resonance in medicine 43:319–323. [DOI] [PubMed] [Google Scholar]
- Hare TA, Camerer CF, Rangel A (2009) Self-control in decision-making involves modulation of the vmPFC valuation system. Science 324:646–648. [DOI] [PubMed] [Google Scholar]
- Hare TA, Hakimi S, Rangel A (2014) Activity in dlPFC and its effective connectivity to vmPFC are associated with temporal discounting. Frontiers in neuroscience 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, FAGERSTROM KO (1991) The Fagerström test for nicotine dependence: a revision of the Fagerstrom Tolerance Questionnaire. British journal of addiction 86:1119–1127. [DOI] [PubMed] [Google Scholar]
- Hughes JR (2009) Smokers’ beliefs about the inability to stop smoking. Addictive behaviors 34:1005–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami D (1986) Signs and symptoms of tobacco withdrawal. Archives of general psychiatry 43:289–294. [DOI] [PubMed] [Google Scholar]
- Janes AC, Pizzagalli DA, Richardt S, Chuzi S, Pachas G, Culhane MA, Holmes AJ, Fava M, Evins AE, Kaufman MJ (2010) Brain reactivity to smoking cues prior to smoking cessation predicts ability to maintain tobacco abstinence. Biological psychiatry 67:722–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn J-P, Rubinow DR, Davis CL, Kling M, Post RM (1988) Salivary cortisol: a practical method for evaluation of adrenal function. Biological Psychiatry 23:335–349. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH (1999) Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosomatic medicine 61:154–162. [DOI] [PubMed] [Google Scholar]
- Koob GF (2008) A role for brain stress systems in addiction. Neuron 59:11–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le A, Funk D, Juzytsch W, Coen K, Navarre BM, Cifani C, Shaham Y (2011) Effect of prazosin and guanfacine on stress-induced reinstatement of alcohol and food seeking in rats. Psychopharmacology 218:89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loughead J, Wileyto EP, Ruparel K, Falcone M, Hopson R, Gur R, Lerman C (2015) Working memory-related neural activity predicts future smoking relapse. Neuropsychopharmacology 40:1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macmillan NA, Creelman CD (1990) Response bias: Characteristics of detection theory, threshold theory, and” nonparametric” indexes. Psychological Bulletin 107:401. [Google Scholar]
- Mantsch JR, Baker DA, Funk D, Lê AD, Shaham Y (2016) Stress-induced reinstatement of drug seeking: 20 years of progress. Neuropsychopharmacology 41:335–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Potenza MN, Kober H, Sofuoglu M, Arnsten AF, Picciotto MR, Weinberger AH, Ashare R, Sinha R (2015) A translational investigation targeting stress-reactivity and prefrontal cognitive control with guanfacine for smoking cessation. Journal of psychopharmacology 29:300–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Sinha R, Weinberger AH, Sofuoglu M, Harrison EL, Lavery M, Wanzer J (2011) Stress decreases the ability to resist smoking and potentiates smoking intensity and reward. Journal of psychopharmacology 25:490–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meikle AW, Tyler FH (1977) Potency and duration of action of glucocorticoids: effects of hydrocortisone, prednisone and dexamethasone on human pituitary-adrenal function. The American journal of medicine 63:200–207. [DOI] [PubMed] [Google Scholar]
- Nestor L, McCabe E, Jones J, Clancy L, Garavan H (2011) Differences in “bottom-up” and “top-down” neural activity in current and former cigarette smokers: evidence for neural substrates which may promote nicotine abstinence through increased cognitive control. Neuroimage 56:2258–2275. [DOI] [PubMed] [Google Scholar]
- Noël X, Brevers D, Bechara A (2013) A neurocognitive approach to understanding the neurobiology of addiction. Current opinion in neurobiology 23:632–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen AM, McMillan KM, Laird AR, Bullmore E (2005) N‐back working memory paradigm: A meta‐analysis of normative functional neuroimaging studies. Human brain mapping 25:46–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson F, Jepson C, Loughead J, Perkins K, Strasser AA, Siegel S, Frey J, Gur R, Lerman C (2010) Working memory deficits predict short-term smoking resumption following brief abstinence. Drug and alcohol dependence 106:61–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posse S, Otazo R, Caprihan A, Bustillo J, Chen H, Henry PG, Marjanska M, Gasparovic C, Zuo C, Magnotta V (2007) Proton echo‐planar spectroscopic imaging of J‐coupled resonances in human brain at 3 and 4 Tesla. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine 58:236–244. [DOI] [PubMed] [Google Scholar]
- Provencher S (2008) LCModel. Version. [Google Scholar]
- Qin S, Hermans EJ, van Marle HJ, Luo J, Fernández G (2009) Acute psychological stress reduces working memory-related activity in the dorsolateral prefrontal cortex. Biological psychiatry 66:25–32. [DOI] [PubMed] [Google Scholar]
- Rangel A, Camerer C, Montague PR (2008) A framework for studying the neurobiology of value-based decision making. Nature Reviews Neuroscience 9:545–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, McReynolds JR, McGaugh JL (2004) The basolateral amygdala interacts with the medial prefrontal cortex in regulating glucocorticoid effects on working memory impairment. The Journal of neuroscience 24:1385–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman DL, De Feyter HM, Graaf RA, Mason GF, Behar KL (2011) 13C MRS studies of neuroenergetics and neurotransmitter cycling in humans. NMR in biomedicine 24:943–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan D, Lecrubier Y (2010) The Mini International Neuropsychiatric Interview Version 6.0 (MINI 6.0). Medical Outcomes System Inc: Jacksonville, FL. [Google Scholar]
- Sinha R (2009) Modeling stress and drug craving in the laboratory: implications for addiction treatment development. Addiction biology 14:84–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S, Jenkinson M, Woolrich M, Beckmann C, Behrens T, Johansen-Berg H, Bannister P, De Luca M, Drobnjak I, Flitney D, Niazy R, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady J, Matthews P (2004) Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23 Suppl 1:S208–219. [DOI] [PubMed] [Google Scholar]
- Sonnay S, Duarte JM, Just N, Gruetter R (2016) Compartmentalised energy metabolism supporting glutamatergic neurotransmission in response to increased activity in the rat cerebral cortex: A 13C MRS study in vivo at 14.1 T. Journal of Cerebral Blood Flow & Metabolism 36:928–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD (1983) Manual for the State-Trait Anxiety Inventory STAI (form Y)(“self-evaluation questionnaire”).
- Stanley JA, Raz N (2018) Functional Magnetic Resonance Spectroscopy: The “New” MRS for Cognitive Neuroscience and Psychiatry Research. Frontiers in Psychiatry 9:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidey JW, Higgins ST, Bickel WK, Steingard S (1999) Effects of response requirement and the availability of an alternative reinforcer on cigarette smoking by schizophrenics. Psychopharmacology 145:52–60. [DOI] [PubMed] [Google Scholar]
- Wang TW, Asman K, Gentzke AS, Cullen KA, Holder-Hayes E, Reyes-Guzman C, Jamal A, Neff L, King BA (2018) Tobacco product use among adults—United States, 2017. Morbidity and Mortality Weekly Report 67:1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A (1988) Development and validation of brief measures of positive and negative affect: the PANAS scales. Journal of personality and social psychology 54:1063. [DOI] [PubMed] [Google Scholar]
- WHO (2017) WHO report on the global tobacco epidemic, 2017: monitoring tobacco use and prevention policies. World Health Organization. [Google Scholar]
- Woodcock E, Arshad M, Khatib D, Stanley J (2018a) Automated Voxel Placement: A Linux-Based Suite of Tools for Accurate and Reliable Single Voxel Coregistration. J Neuroimaging Psychiatry Neurol 3:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock EA, Anand C, Khatib D, Diwadkar VA, Stanley JA (2018b) Working Memory Modulates Glutamate Levels in the Dorsolateral Prefrontal Cortex during 1H fMRS. Frontiers in psychiatry 9:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock EA, Greenwald MK, Khatib D, Diwadkar VA, Stanley JA (2019) Pharmacological stress impairs working memory performance and attenuates dorsolateral prefrontal cortex glutamate modulation. NeuroImage 186:437–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.