1. Introduction
Co-use of tobacco and cannabis within the past 30 days is highly prevalent (1–17), with 70% of cannabis-using adults in the U.S. reporting tobacco use in the past 30 days compared to 25% of non-using adults (12). Young adults have the highest prevalence of both cannabis and tobacco use of any age group (6, 18) and are particularly likely to co-use. In 2014–2015, 21% of 18–24 year-olds reported co-use in the past 30 days compared to 4.5% reporting cannabis-only use (6). Cannabis use has also been rising among young adults (19), and prevalence of daily or almost daily cannabis use among 18–25 year-olds, which occurs primarily among co-users (15), has increased from 4.3% in 2002 to 6.4% in 2014 (19).
Beyond the well-known harms of tobacco use, co-use has been tied to higher risk of substance use problems relative to single product use. These include greater risk of cannabis use disorder (CUD) (2, 3, 20–22) and greater difficulty with cannabis cessation (10, 23) relative to cannabis-only use, as well as faster progression to nicotine dependence or higher tobacco use compared to tobacco-only use (8, 24, 25). However, the findings for smoking cessation are mixed. Some studies show better or no worse success in tobacco cessation (26, 27) for co-users compared to tobacco-only users, while others show lower odds of quit attempts or smoking cessation (25, 28–32) among co-users.
There is concern that increasing normalization of cannabis use could lead to greater co-use. Many studies have called for prevention and control programs to address co-use in order to reduce the public health impacts from both products (9, 16, 22, 26), but there is a lack of understanding of the relationship between cannabis and tobacco use among co-users that is required to inform such programs (16).
We define co-use as use of both cannabis and tobacco within the same 30-day period. Co-use can take many forms. Individuals can use the products separately or they can use them concurrently, defined here as use of one product while feeling the effects of another. Concurrent use can include coadministration, the practice of consuming tobacco and cannabis simultaneously through a blunt or a spliff. Blunts consist of cannabis rolled inside of a hollowed-out cigar or cigarillo wrapper. Spliffs are joints that contain both tobacco and cannabis. Co-administration through vaporizing cannabis or cannabis extracts containing tetrahydrocannabinol (THC), the psychoactive chemical in cannabis, along with nicotine is possible but not widely reported. Concurrent use can also take the form of “chasing”, the practice of consuming tobacco immediately after cannabis; however, concurrent use does not necessarily involve either co-administration or chasing.
There are several theoretical explanations for co-use, including shared predisposition to substance use (6, 33) mediated by genetic (34–36) and cultural/environmental (3, 34, 36) factors, as well as the “gateway” hypothesis (3, 10, 37, 38). However, these explanations do not specify the interrelatedness of products or why they might be used concurrently, defined here as using one product while still feeling the effects of the other. Theories that seek to explain the relationship between tobacco and cannabis use yield conflicting hypotheses about concurrent use. The theory of synergistic (5, 12) and/or compensatory effects (39, 40) suggest that the products are used concurrently to amplify positive, and/or counteract negative, effects. However, the theory that co-users substitute one product for another (5, 41) suggests separate use. Additionally, environmental factors could cause use of both products to be used at the same time (e.g. when socializing) or different times (e.g. e-cigarette use in public settings, cannabis use in private settings) (5, 42, 43) without a causal link.
There is also limited empirical research on the relatedness of cannabis and tobacco use among cousers. Akbar et al. (2019) found that 72% of adult co-users agree that they use tobacco and cannabis for different reasons, and 54% view their use of the two products as “completely separate.” Yet, 40% of cousers report using cannabis and tobacco simultaneously though blunts (cannabis smoked through cigar wrappers) and spliffs (joints rolled with both cannabis and tobacco), 86% report “chasing” (smoking tobacco immediately after cannabis), and 78% report higher use of tobacco when under the influence of cannabis at least “occasionally” (16). The high prevalence of blunt use among co-users (16, 44–47) is a consistent finding suggestive of interrelated use of the products, but it is unclear if co-administration, , explains concurrent use entirely, particularly given limited research on “chasing” (16, 48) and other forms of concurrent use. The inconsistent predictions of existing models and limited past empirical research suggest that further study of co-use behaviors is needed (49).
Furthermore, most prior observational research used cross-sectional and/or cohort designs (9, 48, 50, 51) or qualitative methods (5), which rely on conscious recall and causal reconstruction of events. Ecological momentary assessment (EMA) has the distinct feature of collecting data in, or near real-time from participants in their natural settings (39, 42, 52–56). Some studies have used EMA to capture cannabis and tobacco use patterns among young adults. In one study, participants had higher working memory skills when tobacco was used concurrently with cannabis compared to when they used cannabis alone (39). Using a sample of 31 young adults, Berg et al. (2019) found that cannabis predicted tobacco use during the next four-hour window (53). To our knowledge, no EMA studies have specifically assessed the relatedness of tobacco and cannabis use during the same interval of time among co-users.
The aims of this study were to: 1) quantify the association between tobacco and cannabis in a sample of young adult co-users during four epochs per day over 28 days, and 2) explore and characterize variability in this association as a function of baseline cannabis use and dependence, tobacco use and dependence, and demographic characteristics of co-users.
2. Methods
2.1. Participants and recruitment
Participants were recruited through flyers, local newspapers, and social media advertisements in two large urban cities in the Northeastern United States. Participants were screened online with telephone confirmation. Eligibility criteria were: 1) aged 18–24 years at screening, 2) currently use cannabis at least 2 days per week, and 3) currently use tobacco products “every day” or “some days” (including e-cigarettes). Participants were excluded if they 1) reported symptoms of severe withdrawal from alcohol, 2) reported illicit drug use (other than cannabis) in past 30 days, 3) reported symptoms of major psychiatric disorder, 4) were currently pregnant/lactating or planning to become pregnant, or 5) had potentially lethal alcohol consumption (as evidenced by BAC >0.20 based on reported drinks, gender, and weight) in the past 3 months.
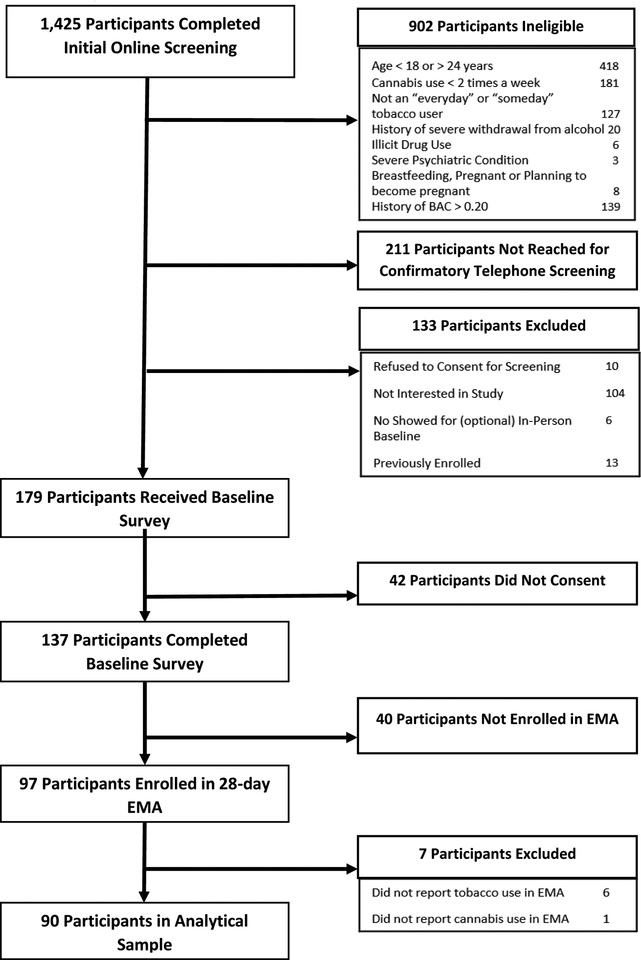
Fig. 1. summarizes recruitment of participants. Of 1,425 who completed screening, 902 were found ineligible; the most common reasons for ineligibility were age not in range (N=418), use of cannabis less than twice per week (N=181), heavy alcohol use (N=139), and not some days/every day tobacco users (N=127). Following online screening, 211 individuals could not be reached for follow-up telephone screening and an additional 133 participants were excluded for the following reasons: 10 did not consent to telephone screening, 104 were not interested in participating in the study, 6 did not show for an in-person baseline visit (which was made optional after ~10 participants were enrolled) and could not be reached rescheduled, and 13 were found to have been previously enrolled. After completing electronic informed consent, participants (N = 137) completed an online baseline survey that collected information on tobacco, cannabis, and alcohol use patterns, attitudes about tobacco and cannabis use, mental health symptoms, and personality characteristics. Participants (N=97) received a training on automated interactive voice response (IVR) procedures and were enrolled in a 28-day EMA study. Of the 40 not enrolled in IVR, some were affected by the early termination of enrollment in IVR following loss of study personnel. Ethical approval was provided by the Institutional Review Boards of Chesapeake Research Review, LLC and the Battelle Memorial Institute.
Fig. 1.
Participant enrollment flowchart.
2.2. Procedures
Participants received three IVR calls to their mobile phones per day for 28-days. Calls were scheduled to occur at random times within 4-hour periods dictated by each participant’s sleep cycle but constrained to be at least 2 hours apart. Each missed call was re-attempted twice, with follow-up calls made at 10-minute intervals. To reduce missing data, participants were able to make inbound calls to the IVR system to complete missed or dropped calls for up to 30 minutes after the end of the 4-hour window for that call, provided the next call of the day had not occurred. Disconnected calls resumed on the last incomplete item. Participants received $25 for completing the baseline survey, $20 per week in which they completed any IVR calls, and a bonus of $1.00 per call. Participants recieved $2.00 per week for completing at least one call on 6 days or $5.00 per week for completing at least one call on 7 days in a week. The maximum incentive for IVR was $184 over four weeks.
Each participant reported on four epochs per day. During morning calls, participants reported on two epochs: 1) “after you took last night’s survey until you went to bed”, and 2) “since you woke up this morning”. During midday calls, participants were asked to report on use behavior that had occurred since their last survey, or, if the survey was not completed in the morning, since they woke up. During evening calls, participants reported on behaviors since the prior survey. In order to quantify the overlap of tobacco and cannabis use among participants, we excluded 7 participants who reported no tobacco use (N=6) and one that reported no cannabis use.
2.3. Measures
During screening and baseline surveys, age, gender, race/ethnicity, education level and other demographic information were collected. Cannabis-related measures collected at baseline included frequency of use (days per week) and history (age of first use, years of regular use), and the Cannabis Use Disorders Identification Test-Revised (CUDIT-R) (57). Tobacco-related measures included current tobacco use frequency (some days/every day), age of first tobacco use, and time to first tobacco use per day.
During IVR calls, participants reported use of tobacco, alcohol, and cannabis. Information on modes of cannabis use (blunt, spliff, joint/bowl/bong/one-hitter/pipe, edibles, vaporizer, concentrates, hookah to smoke cannabis, e-cigarette to vaporize pot/cannabis/THC liquid/THC e-juice) was collected if cannabis use was reported. If tobacco use was reported, modes of tobacco use (cigarette, cigar/little cigar, hookah/shisha/waterpipe tobacco, e-cigarette) were assessed. Participants could select multiple modes of cannabis or tobacco use. Detailed analysis of modes of cannabis use is outside of the scope of this article. Any epochs with spliff and/or blunt use were classified as “co-administration”. We did not correct for reporting co-administration as non-tobacco; results of a corrected analysis are reported in the supplemental material. The order of cannabis and tobacco use (tobacco before, after, before & after) was collected if both were reported during the same epoch.
2.4. Statistical analyses
We assessed the correlates of EMA compliance among the 90 participants using mixed effects logistic regression with predicted marginals. We modeled compliance as a function of follow-up time using linear, quadratic, and cubic terms (i.e. days, days2, and days3), day of week, epoch timing, participant age (18–20, 21–24, missing), race/ethnicity (African American, White, Hispanic/Asian/Mixed/Other), gender, education level (no high school diploma/GED, diploma/GED, some college or higher), and baseline-reported frequency of tobacco and cannabis use. Due to the small sample size, we aggregated Hispanic ethnicity with Asian, multiple races, and other races into one category.
To visualize the overlap between tobacco and cannabis use (aim 1), we plotted the difference in the probability of cannabis use during epochs with tobacco compared to epochs without tobacco for each participant. Mixed effects logistic regression with random intercepts was used to model cannabis use as a function of tobacco use while adjusting for time-varying confounders (time of day, day of week, and a dummy variable for alcohol use). As random intercepts controlled directly for individuals’ propensity to use cannabis without tobacco, we did not include fixed baseline characteristics in the models.
Tobacco use was decomposed into two variables in order to distinguish between within-person and between-person effects. The prevalence of tobacco use during EMA for each participant was included to capture between-person effects (i.e. association between frequency of tobacco use and cannabis use among individuals). A binary variable for tobacco use, centered at the participant’s prevalence of tobacco use, was used to estimate within-person effects (i.e. the association between tobacco use and cannabis use within individuals). The within-person association is of interest in this study. The odds ratio (OR) for the within-person association was interpreted as a measure of the degree of overlap of tobacco and cannabis use, with OR>1 indicating more concurrent use, and OR<1 indicating more separate use, and OR=1 indicating that products are used independently. To test whether the overlap of the products was due exclusively to co-administration, we repeated the analysis excluding epochs with any blunt or spliff use.
To explore heterogeneity in the association between tobacco and cannabis use (aim 2), we interacted tobacco use variables with baseline participant characteristics that may have moderated the association. These included age, race/ethnicity, gender, cannabis use variables (CUDIT-R scores, age of first cannabis, years of regular cannabis use), and tobacco use variables (tobacco use frequency, tobacco use with 30 minutes of waking, age at first tobacco use). Due to the exploratory nature of aim #2, we did not adjust for multiple testing.
We assessed the impact of compliance through sensitivity analysis. We repeated the primary analysis with interactions for study follow-up time (weeks 1 & 2 vs. 3 & 4) and for high (>median) and low (<=median) compliance participants. Mixed effect regression models were fit assuming compound symmetry, with a Huber-White Sandwich estimator for robustness to misspecification of the within-person correlation model. All analyses were performed using Stata version 15 (58).
3. Results
3.1. Demographics & baseline cannabis and tobacco use
Table 1 describes demographics and baseline tobacco and cannabis use for the 90 participants included the analysis. Participants used cannabis an average of 6.0 (1.6) days per week and tobacco use was reported “every day” by 74% and “some days” by 26% of included participants. Participants excluded from the analysis did not differ considerably from those included in the analysis, except in their frequency of tobacco use.
Table 1.
Baseline Descriptive Statistics of Participants
| Included in Analysis (N=90) | ||
|---|---|---|
| N | % | |
| Age | ||
| 18–20 | 33 | 37% |
| 21–24 | 55 | 61% |
| Missing | 2 | 2% |
| Gender | ||
| Male | 53 | 59% |
| Female | 37 | 41% |
| Race/Ethnicity | ||
| African American | 20 | 22% |
| Caucasian | 46 | 51% |
| Hispanic | 9 | 10% |
| Asian | 2 | 2% |
| Other/More than one race | 13 | 13% |
| Employment Status | ||
| Full-time student | 29 | 32% |
| Employed Full-time or Part-time | 43 | 48% |
| Unemployed or Disabled | 18 | 20% |
| Educational Attainment | ||
| < High School or GED | 5 | 6% |
| High school/GED | 21 | 23% |
| Some College or Higher | 64 | 71% |
| Tobacco Use Frequency | ||
| Every day | 68 | 76% |
| Some days | 22 | 24% |
| Time to First Use of Tobacco Upon Waking | ||
| <=5 mins | 15 | 17% |
| 6–30 mins | 22 | 24% |
| 31–60 mins | 14 | 14% |
| >60 mins | 38 | 42% |
| Not reported | 1 | 1% |
| Mean | SD | |
| Days of Cannabis Use per Week | 6.0 | 1.6 |
| First Age of Cannabis Use | 15.3 | 2.4 |
| Years of Regular Cannabis Use | 4.3 | 2.6 |
| CUDIT-R1 | 13.4 | 5.0 |
| First Age of Tobacco Use | 15.1 | 2.7 |
CUDIT-R: Cannabis Use Disorder Identification Test – Revised
3.2. Compliance with IVR
Among the 90 participants, 5,550 out of 10,080 possible IVR surveys were completed (55.1% compliance). Predicted marginals based on logistic regression models (S1 Table) showed that adjusted compliance declined significantly over the 28 days of EMA from 78.8% on day 1 to 36.6% on day 28. Compliance was also significantly lower on weekend days (51.7%, 52.4%) than on weekdays (53.1 – 56.3%). There were no significant differences by call timing, participant age, gender, race, education, or, importantly, by baseline frequency of tobacco or cannabis use.
3.3. Temporal association between cannabis and tobacco use (Aim #1)
During the 28 days of EMA, participants reported using neither product in 41.0% of epochs, concurrent cannabis and tobacco in 22.2% of epochs, tobacco-only in 19.3% of epochs, and cannabis-only in 17.6% of epochs. When both were used, tobacco was used after cannabis 15.9% of the time, before cannabis 5.5% of the time, and both before and after cannabis 79% of the time.
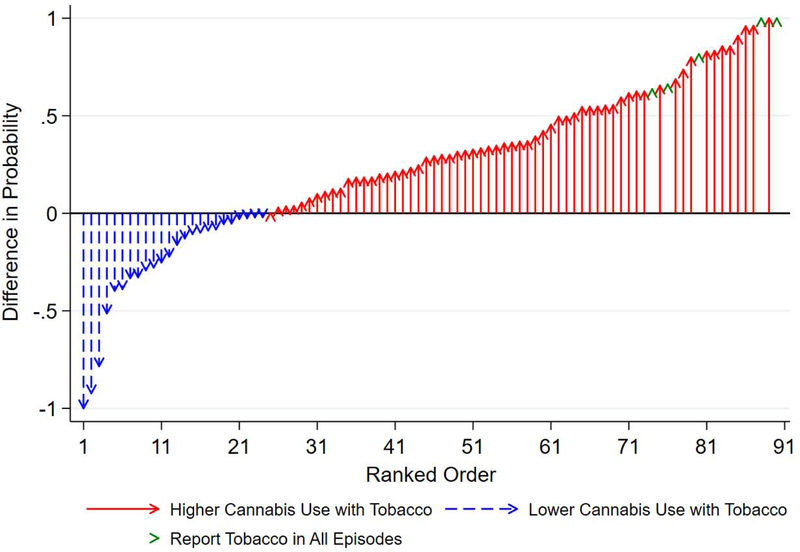
In Fig. 2, we plotted the difference in the observed probability of cannabis use as a function of tobacco use for each participant. Arrows show the extent to which the probability of cannabis use was higher (or lower) in epochs with tobacco use than in epochs without tobacco use. For most participants, the probability of cannabis use was higher with tobacco than without tobacco. Assuming the 5 participants who reported tobacco in every epoch have zero cannabis use without tobacco, the average probability of cannabis use was 25.5 (standard deviation=42.7) percentage points higher with tobacco than without tobacco.
Fig. 2.
Difference in observed probability of cannabis use as a function of tobacco use by participant Caption: Arrows represent the difference in probability of cannabis use with tobacco vs. probability of cannabis without tobacco. N=61 have higher probabilities of cannabis use with tobacco (red arrows), N=24 participants have equal or lower cannabis use when using tobacco (blue arrows), and N=5 participants did not report any epochs without tobacco (green arrowheads). Participants’ data was sorted in ascending order.
Table 2 presents the results of mixed effects logistic regression models for cannabis use. Adjusted odds of cannabis use were 5.36 (95% C.I.: 3.12 – 9.18, p<0.001) times higher if tobacco was reported in the same epoch than if it was not. For a participant with the average prevalence of tobacco use during EMA (24.2%), the predicted probability of cannabis use without tobacco was 29.5% compared to 58.9% with tobacco. Cannabis use was also associated with alcohol use (OR=1.58, 1.17 −2.13, p=0.002). There were no significant between-person effects.
Table 2.
Random intercept logistic regression models of cannabis use as a function of tobacco use and confounders
| Cannabis Use | Cannabis Use | ||||
|---|---|---|---|---|---|
| All Epochs | Excluding Epochs with Co-Administration1 | ||||
| OR (95% C.I.) | p-value | OR (95% C.I.) | p-value | ||
| Tobacco Use in Epoch (Within-Person Effect) | 5.36 (3.12, 9.18) | <0.001 | 3.43 (1.90, 6.19) | <0.001 | |
| Prevalence of Tobacco Use during EMA (Between-Person Effect) | 1.04 (0.21, 5.28) | 0.962 | 0.88 (0.16, 4.23) | 0.888 | |
| Alcohol Use in Epoch | Yes | 1.58 (1.17, 2.13) | 0.002 | 1.64 (1.17, 2.29) | 0.004 |
| Epoch Timing | Morning | Ref | |||
| Midday | 1.88 (1.42, 2.48) | <0.001 | 1.76 (1.24, 2.48) | 0.002 | |
| Evening | 3.04 (2.33, 3.96) | <0.001 | 3.11 (2.23, 4.33) | <0.001 | |
| Late Night | 3.93 (2.81, 5.50) | <0.001 | 3.76 (2.43, 5.81) | <0.001 | |
| Day of Week | Sunday | 0.99 (0.82, 1.19) | 0.877 | 1.03 (0.81, 1.31) | 0.807 |
| Monday | Ref | Ref | |||
| Tuesday | 0.87 (0.71, 1.08) | 0.209 | 0.89 (0.67, 1.19) | 0.426 | |
| Wednesday | 0.79 (0.61, 1.01) | 0.055 | 0.95 (0.68, 1.33) | 0.766 | |
| Thursday | 0.90 (0.73, 1.12) | 0.351 | 0.97 (0.74, 1.29) | 0.864 | |
| Friday | 1.00 (0.80, 1.26) | 0.966 | 1.05 (0.79, 1.40) | 0.741 | |
| Saturday | 0.95 (0.76, 1.20) | 0.665 | 0.94 (0.70, 1.25) | 0.656 | |
| Constant | 0.21 (0.14, 0.34) | <0.001 | 0.08 (0.05, 0.15) | <0.001 | |
| ICC2 | 0.295 | 0.462 | |||
| Participants | N | 90 | 89 | ||
| Epochs | N | 5,499 | 4,541 | ||
Co-administration refers to use of blunts or spliffs
ICC: intra-class correlation coefficient
Co-administration through blunts and spliffs was reported in half (50.0%) of epochs with concurrent use. Excluding these, the association remained large and significant (OR=3.43; 1.90 – 6.19, p<0.001). In sensitivity analyses (not shown), there were no significant differences in the association between the first two weeks of EMA (62.6% average compliance) and last two weeks (47.5%) or between participants with above-median (80.2%) and below-median (31.1%) compliance.
3.4. Variability in the association between cannabis and tobacco use (Aim #2)
Table 3 presents the odds ratios for interaction models and predicted marginal effects for significant interactions. Baseline frequency of cannabis use significantly modified the association between products. For each additional day of cannabis use per week reported at baseline, the odds ratio for the within-person association between cannabis and tobacco use_increases by a factor of 1.37 (1.021.82; p=0.034). Later age of first cannabis use was associated with a smaller overlap between products (OR = 0.83/year, 0.70–0.99, p=0.049). There were no other significant interactions.
Table 3.
Results from interaction models exploring heterogeneity in the association between cannabis and tobacco use.
| Interaction Variable | Levels | Interaction OR1 | p-value | Predicted Marginal Effects of Interaction |
|---|---|---|---|---|
| Age | 21–24 years | 1.10 | 0.845 | |
| (Ref=18–20, NR) | ||||
| Race/Ethnicity | White | Ref | ||
| (Ref=White, non-Hispanic) | African American | 1.84 | 0.380 | |
| Hispanic, Other | 1.16 | 0.814 | ||
| Gender | Male | 0.54 | 0.203 | |
| (Ref=Female) | ||||
| Education | Ref=Less than High School Diploma/GED | Ref | ||
| HSD/GED | 2.05 | 0.514 | ||
| Some College/Higher | 2.07 | 0.376 | ||
| Cannabis Use Frequency | Days/Week | 1.37 | 0.034 | 5.2 percentage points/day |
| Age of First Cannabis Use | Years | 0.83 | 0.049 | −3.0 percentage points/year |
| Years of Regular Cannabis Use | Years | 1.10 | 0.249 | |
| CUDIT-R2 | Points | 1.10 | 0.140 | |
| Tobacco Use Frequency | Ref=Every day | Ref | ||
| Somedays | 0.59 | 0.350 | ||
| Use of Tobacco Within 30 Minutes of Waking | Yes (Ref=No) | 0.69 | 0.547 | |
| Age of First Tobacco | Years | 0.96 | 0.618 |
Each variable was modelled separately. All interaction models control for main effects of average tobacco use, tobacco use during epoch (centered), alcohol use, epoch timing, day of week, and the interaction variable. Models include interactions for prevalence of tobacco use (between-person) and tobacco use during epoch (within-person). Reported interactions are for within-person effects only.
CUDIT-R: Cannabis Use Disorder Identification Test – Revised
3. Discussion
This study measured the degree of overlap in cannabis and tobacco use during four epochs in a day, and explored its variability, among co-using young adults. On average, cannabis and tobacco use were significantly more likely to occur together than would be expected if their use were independent. Half of epochs with concurrent use involved co-administration through blunts or spliffs; however, cannabis and tobacco were associated even when co-administration was not reported. There was significant variability in the association among participants. Heavier cannabis users and those who began using cannabis at younger ages were more likely to use the products concurrently than lighter users and later initiators. The overlap between cannabis and tobacco suggests that use of the products was related. Furthermore, the relatedness seems to be higher for more frequent users and earlier initiators, which could be early indicators of problematic cannabis use.
The findings of this EMA study build upon those of a recent cross-sectional study of adult co-users (16). Though our results broadly agree with the findings of Akbar et al. (2019), baseline tobacco use variables did not explain the variability in our young adult sample, while some variables related to cannabis use (frequency of use, age of initiation) did. This contrast could be due to methodological differences between the studies. There could also be differences between younger and older adult cousers, which should be explored in future research.
Research is also needed to investigate the mechanisms that link cannabis and tobacco use among co-users. Though tobacco was used both before and after cannabis in 79% of epochs with concurrent use, and simultaneous use was very common, it was nearly three times more common for tobacco to follow cannabis (15.9%) than to precede it (5.5%). Cannabis may stimulate cravings for tobacco or reduce reluctance to use tobacco, though a clinical trial did not identify such an effect in non-dependent individuals (41), or participants may be intentionally “chasing” with tobacco to enhance and/or prolong their “high” or offset the sedative effects of cannabis. Identification of the mechanisms that relate cannabis and tobacco use could provide targets for treatment and prevention among co-users.
Most existing treatments for dual dependence in co-users (59–64) tend to treat the two addictions through separate approaches. Though this population was not treatment-seeking, this study suggests that integrated treatment strategies that target the link between cannabis and tobacco use may be more effective than those approaching the two substances in isolation. Similar strategies have been proposed for treatment of nicotine dependence and alcohol use disorder (65, 66). However, opportunities for integrated treatment will be limited if treatment-seeking co-users prefer to quit only one substance or only one at a time (43). Prevention strategies directed at young adult co-users should target the widespread practice of co-administration, which provides an opportunity for occasional tobacco use to become habitual through its association with cannabis use and may increase risks of cannabis dependence.
4.1. Limitations
This study has several limitations. First, to be conservative, we did not correct for misreporting of blunts and spliffs as non-tobacco. The results of an analysis with correction are presented in the supplemental materials. After recoding, the degree of overlap increases substantially.
Secondly, temporal associations between cannabis and tobacco use may be confounded by unobserved factors. Though we controlled for time of day and alcohol use, we did not capture the location or the activities of participants. Some of the observed heterogeneity may be due to environments wherein one substance (e.g. an e-cigarette) could be used while another (e.g. smoked cannabis) could not.
Third, while our sample draws from a diverse population, participants were recruited from two large Northeastern metropolitan areas, one of which legalized cannabis possession for adults 21 and older. We also excluded co-users with recent heavy alcohol use or illicit drug use. Findings may not be generalizable to co-users who abuse other substances or who are located in other settings. Participation bias may limit the generalizability of this study’s findings more than for studies using less-intensive methods.
Fourth, the 3 to 6-hour periods of time between surveys that define epochs may be too coarse, as tobacco and cannabis use separated by a few hours may be unrelated. Future EMA studies should include passive devices, such as electronic lighters or e-cigarettes that continuously log usage data, to collect data with higher frequency.
Lastly, compliance is an important metric in EMA studies, as missing data can bias findings. However, the mixed effects models used in the analysis are robust to missing data under the missing at random assumption. Also, while 55.1% compliance is low by the standards of EMA studies, which often report compliance exceeding 80–90% (39, 67), this study excluded a smaller proportion of EMA participants from the analysis than two recent EMA studies of substance use (7% vs. 19% and 29%) (53, 54). Participants in this study were also asked to complete more calls (84 vs. 12) (67) over longer period of time (28 vs. 7 days) (39) than in other studies. Sensitivity analysis revealed no significant differences in the association between high and low compliance periods or participants; therefore, we find no evidence of bias due to low compliance. Future EMA studies of young adults should consider using behavioral reinforcement strategies and/or and passive data collection devices to overcome imperfect compliance.
4.2. Conclusions
This study found a significant temporal association between cannabis and tobacco use among young adult co-users that was due only in part to the practice of co-administration through blunts and spliffs. Frequent cannabis use and earlier age of initiation were associated with more concurrent use of cannabis and tobacco. Given the overlap, cannabis and tobacco use are likely to be related among cousers, particularly those with more frequent cannabis use. Treatment of co-users may benefit from considering the relationship between the products in integrative approaches.
Supplementary Material
Highlights.
The link between cannabis and tobacco use by young adults is not well understood.
28-day ecological momentary assessment (EMA) was used to track co-users.
Cannabis and tobacco use were significantly temporally associated among co-users.
There was considerable variability in the temporal association across participants.
Heavier cannabis users were more likely to use cannabis and tobacco concurrently.
Acknowledgements
We thank Andrea Villanti for providing input during study conceptualization and grant application. Bonnie King provided financial management assistance to the project on which this study is based and proofread the final draft. We would like to thank the research participants for their time.
Funding Statement
Research reported in this publication was supported by the National Institute on Drug Abuse of the National Institutes of Health under Award Number R21DA041548. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funder had no role in this publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interests
E.N.P. is employed by Canopy Growth Corp., a licensed producer of cannabis. All work on this study, other than approval of the final manuscript, occurred prior to E.N.P.’s employment by Canopy. R.V. has received consulting fees or honoraria for work related to cannabis from Zynerba Pharmaceuticals, Canopy Health Innovations, and Brain Solutions Inc., companies that produce, market, or support commercial production and/or distribution of cannabis products. All other authors declare that they have no competing interests.
References
- 1.Agrawal A, Budney AJ, Lynskey MT. The co-occurring use and misuse of cannabis and tobacco: a review. Addiction. 2012;107(7):1221–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agrawal A, Lynskey MT. Tobacco and cannabis co-occurrence: does route of administration matter? Drug Alcohol Depend. 2009;99(1–3):240–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agrawal A, Lynskey MT, Madden PA, Pergadia ML, Bucholz KK, Heath AC. Simultaneous cannabis and tobacco use and cannabis-related outcomes in young women. Drug Alcohol Depend. 2009;101(1–2):8–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agrawal A, Silberg JL, Lynskey MT, Maes HH, Eaves LJ. Mechanisms underlying the lifetime co-occurrence of tobacco and cannabis use in adolescent and young adult twins. Drug Alcohol Depend. 2010;108(1–2):49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berg CJ, Payne J, Henriksen L, Cavazos-Rehg P, Getachew B, Schauer GL, et al. Reasons for Marijuana and Tobacco Co-use Among Young Adults: A Mixed Methods Scale Development Study. Subst Use Misuse. 2018;53(3):357–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohn AM, Abudayyeh H, Perreras L, Peters EN. Patterns and correlates of the co-use of marijuana with any tobacco and individual tobacco products in young adults from Wave 2 of the PATH Study. Addict Behav. 2019;92:122–7. [DOI] [PubMed] [Google Scholar]
- 7.Cohn A, Villanti A, Richardson A, Rath JM, Williams V, Stanton C, et al. The association between alcohol, marijuana use, and new and emerging tobacco products in a young adult population. Addict Behav. 2015;48:79–88. [DOI] [PubMed] [Google Scholar]
- 8.Dai H, Hao J. Electronic cigarette and marijuana use among youth in the United States. Addict Behav. 2017;66:48–54. [DOI] [PubMed] [Google Scholar]
- 9.Hindocha C, Freeman TP, Ferris JA, Lynskey MT, Winstock AR. No Smoke without Tobacco: A Global Overview of Cannabis and Tobacco Routes of Administration and Their Association with Intention to Quit. Front Psychiatry. 2016;7:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peters EN, Budney AJ, Carroll KM. Clinical correlates of co-occurring cannabis and tobacco use: a systematic review. Addiction. 2012;107(8):1404–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rabin RA, George TP. A review of co-morbid tobacco and cannabis use disorders: Possible mechanisms to explain high rates of co-use. The American journal on addictions. 2015;24(2):105–16. [DOI] [PubMed] [Google Scholar]
- 12.Schauer GL, Berg CJ, Kegler MC, Donovan DM, Windle M. Differences in Tobacco Product Use Among Past Month Adult Marijuana Users and Nonusers: Findings From the 2003–2012 National Survey on Drug Use and Health. Nicotine & Tobacco Research. 2015:ntv093. [DOI] [PubMed] [Google Scholar]
- 13.Schauer GL, Peters EN. Correlates and trends in youth co-use of marijuana and tobacco in the United States, 2005–2014. Drug Alcohol Depend. 2018;185:238–44. [DOI] [PubMed] [Google Scholar]
- 14.Webster L, Chaiton M, Kirst M. The co-use of tobacco and cannabis among adolescents over a 30-year period. J Sch Health. 2014;84(3):151–9. [DOI] [PubMed] [Google Scholar]
- 15.Goodwin RD, Pacek LR, Copeland J, Moeller SJ, Dierker L, Weinberger A, et al. Trends in Daily Cannabis Use Among Cigarette Smokers: United States, 2002–2014. Am J Public Health. 2018;108(1):137–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akbar SA, Tomko RL, Salazar CA, Squeglia LM, McClure EA. Tobacco and cannabis co-use and interrelatedness among adults. Addict Behav. 2019;90:354–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seaman EL, Green KM, Wang MQ, Quinn SC, Fryer CS. Examining prevalence and correlates of cigarette and marijuana co-use among young adults using ten years of NHANES data. Addict Behav. 2019;96:140–7. [DOI] [PubMed] [Google Scholar]
- 18.Schauer GL, Berg CJ, Kegler MC, Donovan DM, Windle M. Assessing the overlap between tobacco and marijuana: Trends in patterns of co-use of tobacco and marijuana in adults from 2003–2012. Addict Behav. 2015;49:26–32. [DOI] [PubMed] [Google Scholar]
- 19.Azofeifa A, Mattson ME, Schauer G, McAfee T, Grant A, Lyerla R. National Estimates of Marijuana Use and Related Indicators - National Survey on Drug Use and Health, United States, 2002–2014. MMWR Surveill Summ. 2016;65(11):1–28. [DOI] [PubMed] [Google Scholar]
- 20.Weinberger AH, Pacek LR, Wall MM, Zvolensky MJ, Copeland J, Galea S, et al. Trends in cannabis use disorder by cigarette smoking status in the United States, 2002–2016. Drug Alcohol Depend. 2018;191:45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Swift W, Coffey C, Carlin JB, Degenhardt L, Calabria B, Patton GC. Are adolescents who moderate their cannabis use at lower risk of later regular and dependent cannabis use? Addiction. 2009;104(5):806–14. [DOI] [PubMed] [Google Scholar]
- 22.Ream GL, Benoit E, Johnson BD, Dunlap E. Smoking tobacco along with marijuana increases symptoms of cannabis dependence. Drug and alcohol dependence. 2008;95(3):199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gray KM, Riggs PD, Min S-J, Mikulich-Gilbertson SK, Bandyopadhyay D, Winhusen T. Cigarette and cannabis use trajectories among adolescents in treatment for attention-deficit/hyperactivity disorder and substance use disorders. Drug and alcohol dependence. 2011;117(2–3):242–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rubinstein ML, Rait MA, Prochaska JJ. Frequent marijuana use is associated with greater nicotine addiction in adolescent smokers. Drug Alcohol Depend. 2014;141:159–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weinberger AH, Platt J, Copeland J, Goodwin RD. Is Cannabis Use Associated With Increased Risk of Cigarette Smoking Initiation, Persistence, and Relapse? Longitudinal Data From a Representative Sample of US Adults. J Clin Psychiatry. 2018;79(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rabin RA, Ashare RL, Schnoll RA, Cinciripini PM, Hawk LW Jr., Lerman C, et al. Does cannabis use moderate smoking cessation outcomes in treatment-seeking tobacco smokers? Analysis from a large multi-center trial. The American journal on addictions. 2016;25(4):291–6. [DOI] [PubMed] [Google Scholar]
- 27.Humfleet G, Munoz R, Sees K, Reus V, Hall S. History of alcohol or drug problems, current use of alcohol or marijuana, and success in quitting smoking. Addict Behav. 1999;24(1):149–54. [DOI] [PubMed] [Google Scholar]
- 28.Vogel EA, Rubinstein ML, Prochaska JJ, Ramo DE. Associations between marijuana use and tobacco cessation outcomes in young adults. J Subst Abuse Treat. 2018;94:69–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weinberger AH, Pacek LR, Wall MM, Gbedemah M, Lee J, Goodwin RD. Cigarette smoking quit ratios among adults in the USA with cannabis use and cannabis use disorders, 2002–2016. Tob Control. 2019. [DOI] [PubMed] [Google Scholar]
- 30.Strong DR, Myers MG, Pulvers K, Noble M, Brikmanis K, Doran N. Marijuana use among US tobacco users: Findings from wave 1 of the population assessment of tobacco health (PATH) study. Drug Alcohol Depend. 2018;186:16–22. [DOI] [PubMed] [Google Scholar]
- 31.Ford DE, Vu HT, Anthony JC. Marijuana use and cessation of tobacco smoking in adults from a community sample. Drug Alcohol Depend. 2002;67(3):243–8. [DOI] [PubMed] [Google Scholar]
- 32.Stapleton JA, Keaney F, Sutherland G. Illicit drug use as a predictor of smoking cessation treatment outcome. Nicotine Tob Res. 2009;11(6):685–9. [DOI] [PubMed] [Google Scholar]
- 33.Vanyukov MM, Tarter RE, Kirisci L, Kirillova GP, Maher BS, Clark DB. Liability to substance use disorders: 1. Common mechanisms and manifestations. Neurosci Biobehav Rev. 2003;27(6):507–15. [DOI] [PubMed] [Google Scholar]
- 34.Agrawal A, Grant JD, Lynskey MT, Madden PA, Heath AC, Bucholz KK, et al. The genetic relationship between cannabis and tobacco cigarette use in European- and African-American female twins and siblings. Drug Alcohol Depend. 2016;163:165–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Agrawal A, Lynskey MT, Kapoor M, Bucholz KK, Edenberg HJ, Schuckit M, et al. Are genetic variants for tobacco smoking associated with cannabis involvement? Drug Alcohol Depend. 2015;150:183–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Agrawal A, Madden PA, Bucholz KK, Heath AC, Lynskey MT. Initial reactions to tobacco and cannabis smoking: a twin study. Addiction. 2014;109(4):663–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Badiani A, Boden JM, De Pirro S, Fergusson DM, Horwood LJ, Harold GT. Tobacco smoking and cannabis use in a longitudinal birth cohort: evidence of reciprocal causal relationships. Drug and alcohol dependence. 2015;150:69–76. [DOI] [PubMed] [Google Scholar]
- 38.Patton GC, Coffey C, Carlin JB, Sawyer SM, Lynskey M. Reverse gateways? Frequent cannabis use as a predictor of tobacco initiation and nicotine dependence. Addiction. 2005;100(10):1518–25. [DOI] [PubMed] [Google Scholar]
- 39.Schuster RM, Mermelstein RJ, Hedeker D. Ecological momentary assessment of working memory under conditions of simultaneous marijuana and tobacco use. Addiction. 2016;111(8):1466–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hindocha C, Freeman TP, Xia JX, Shaban NDC, Curran HV. Acute memory and psychotomimetic effects of cannabis and tobacco both ‘joint’ and individually: a placebo-controlled trial. Psychol Med. 2017;47(15):2708–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hindocha C, Lawn W, Freeman TP, Curran HV. Individual and combined effects of cannabis and tobacco on drug reward processing in non-dependent users. Psychopharmacology (Berl). 2017;234(21):3153–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Phillips KT, Phillips MM, Lalonde TL, Prince MA. Does social context matter? An ecological momentary assessment study of marijuana use among college students. Addict Behav. 2018;83:154–9. [DOI] [PubMed] [Google Scholar]
- 43.McClure EA, Tomko RL, Salazar CA, Akbar SA, Squeglia LM, Herrmann E, et al. Tobacco and cannabis co-use: Drug substitution, quit interest, and cessation preferences. Exp Clin Psychopharmacol. 2019;27(3):265–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schauer GL, King BA, Bunnell RE, Promoff G, McAfee TA. Toking, Vaping, and Eating for Health or Fun: Marijuana Use Patterns in Adults, U.S., 2014. Am J Prev Med. 2016;50(1):1–8. [DOI] [PubMed] [Google Scholar]
- 45.Schauer GL, Berg CJ, Kegler MC, Donovan DM, Windle M. Differences in Tobacco Product Use Among Past Month Adult Marijuana Users and Nonusers: Findings From the 2003–2012 National Survey on Drug Use and Health. Nicotine Tob Res. 2016;18(3):281–8. [DOI] [PubMed] [Google Scholar]
- 46.Schauer GL, Peters EN. Correlates and trends in youth co-use of marijuana and tobacco in the United States, 2005–2014. Drug and Alcohol Dependence. 2018;185:238–44. [DOI] [PubMed] [Google Scholar]
- 47.Cohn A, Johnson A, Ehlke S, Villanti AC. Characterizing substance use and mental health profiles of cigar, blunt, and non-blunt marijuana users from the National Survey of Drug Use and Health. Drug Alcohol Depend. 2016;160:105–11. [DOI] [PubMed] [Google Scholar]
- 48.Ream GL, Benoit E, Johnson BD, Dunlap E. Smoking tobacco along with marijuana increases symptoms of cannabis dependence. Drug Alcohol Depend. 2008;95(3):199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Walsh H, Hindocha C, Duaso M. Commentary on Popova et al. (2017): Co-used and co-administered tobacco and cannabis (marijuana) require further investigation. Addiction. 2017;112(10):1830–1. [DOI] [PubMed] [Google Scholar]
- 50.Doran N, Myers MG, Correa J, Strong DR, Tully L, Pulvers K. Marijuana use among young adult non-daily cigarette smokers over time. Addict Behav. 2019;95:91–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fairman BJ. Cannabis problem experiences among users of the tobacco-cannabis combination known as blunts. Drug and alcohol dependence. 2015;150:77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shiffman S Ecological momentary assessment (EMA) in studies of substance use. Psychological assessment. 2009;21(4):486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Berg CJ, Haardorfer R, Payne JB, Getachew B, Vu M, Guttentag A, et al. Ecological momentary assessment of various tobacco product use among young adults. Addict Behav. 2019;92:38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mead EL, Chen JC, Kirchner TR, Butler J 3rd, Feldman RH. An Ecological Momentary Assessment of Cigarette and Cigar Dual Use Among African American Young Adults. Nicotine Tob Res. 2018;20(suppl_1):S12–S21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen-Sankey JC, Choi K, Kirchner TR, Feldman RH, Butler J 3rd, Mead EL. Flavored cigar smoking among African American young adult dual users: An ecological momentary assessment. Drug Alcohol Depend. 2019;196:79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shiffman S, Stone AA, Hufford MR. Ecological momentary assessment. Annu Rev Clin Psychol. 2008;4:1–32. [DOI] [PubMed] [Google Scholar]
- 57.Adamson SJ, Kay-Lambkin FJ, Baker AL, Lewin TJ, Thornton L, Kelly BJ, et al. An improved brief measure of cannabis misuse: the Cannabis Use Disorders Identification Test-Revised (CUDIT-R). Drug Alcohol Depend. 2010;110(1–2):137–43. [DOI] [PubMed] [Google Scholar]
- 58.StataCorp. Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC; 2017. [Google Scholar]
- 59.Lee DC, Budney AJ, Brunette MF, Hughes JR, Etter JF, Stanger C. Treatment models for targeting tobacco use during treatment for cannabis use disorder: case series. Addict Behav. 2014;39(8):1224–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hill KP, Toto LH, Lukas SE, Weiss RD, Trksak GH, Rodolico JM, et al. Cognitive behavioral therapy and the nicotine transdermal patch for dual nicotine and cannabis dependence: a pilot study. The American journal on addictions. 2013;22(3):233–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Becker J, Hungerbuehler I, Berg O, Szamrovicz M, Haubensack A, Kormann A, et al. Development of an integrative cessation program for co-smokers of cigarettes and cannabis: demand analysis, program description, and acceptability. Subst Abuse Treat Prev Policy. 2013;8:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Becker J, Haug S, Sullivan R, Schaub MP. Effectiveness of different Web-based interventions to prepare co-smokers of cigarettes and cannabis for double cessation: a three-arm randomized controlled trial. J Med Internet Res. 2014;16(12):e273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Beckham JC, Adkisson KA, Hertzberg J, Kimbrel NA, Budney AJ, Stephens RS, et al. Mobile contingency management as an adjunctive treatment for co-morbid cannabis use disorder and cigarette smoking. Addict Behav. 2018;79:86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee DC, Walker DD, Hughes JR, Brunette MF, Scherer E, Stanger C, et al. Sequential and simultaneous treatment approaches to cannabis use disorder and tobacco use. J Subst Abuse Treat. 2019;98:39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kalman D, Kim S, DiGirolamo G, Smelson D, Ziedonis D. Addressing tobacco use disorder in smokers in early remission from alcohol dependence: the case for integrating smoking cessation services in substance use disorder treatment programs. Clin Psychol Rev. 2010;30(1):12–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Giorgi I, Fiabane E, Vittadini G, Anastasi S, Benvenuto A, Malovini A, et al. Outcome Evaluation of an Integrated Treatment for Comorbid Alcohol and Nicotine Addiction: An Exploratory Study. Archives of Psychiatric Nursing. 2017;31(4):429–30. [DOI] [PubMed] [Google Scholar]
- 67.Lipperman-Kreda S, Gruenewald PJ, Grube JW, Bersamin M. Adolescents, alcohol, and marijuana: Context characteristics and problems associated with simultaneous use. Drug Alcohol Depend. 2017;179:55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.