Abstract
Cardiopulmonary bypass (CPB) causes a systemic inflammatory response reaction that may contribute to post-operative complications. One cause relates to the air/blood interface from the extracorporeal circuit. The modulatory effects of blending nitric oxide (NO) gas into the ventilation/sweep gas of the membrane lung was studied in a porcine model of air-induced inflammation in which NO gas was added and compared to controls with or without an air/blood interface. Healthy swine were supported on partial bypass under 4 different test conditions. Group 1: no air exposure, Group 2: air alone, Group 3: air plus 50 ppm NO, and Group 4: air plus 500 ppm NO. of the NO gas blended into the ventilation/sweep site of the membrane lung. The platelets and leucocytes were activated by air alone. Addition of NO to the sweep gas attenuated the inflammatory response created by the air/blood interface in this model.
Keywords: Cardiopulmonary Bypass, Nitric Oxide gas, Inflamation, CD11b, air, cardiotomy suction
INTRODUCTION
Every year, hundreds of thousands of cardiac procedures are performed using standard cardiopulmonary bypass (CPB). (1) The duration of CPB is associated with the frequency and severity of respiratory, renal, neurologic, and cardiac events. (2–7) These adverse events are thought to result in part from a systemic inflammatory reaction or stress response induced by CPB. (8,9) Foreign surface exposure and air/blood interface are felt to be the primary causes.(10–16)
Nitric oxide (NO) is a gas produced also by endothelial cells of the vascular system and has been shown to increase vasodilation and prevent leukocyte and platelet activation and aggregation in small animal models (17) and in a small number of randomized clinical CPB trials. (18,19) Nitric oxide has been demonstrated clinically to improve myocardial protection, fluid balance and post-operative complications attenuating the inflammatory stress produced during extracorporeal support.(20–22) A porcine extracorporeal circulation (ECC) model was developed to create a controlled model of air-induced leucocyte and platelet activation. In these experiments the effect of blending NO gas into the sweep gas of the artificial lung in conjunction with a controlled air/blood interface was evaluated.
METHODS
Porcine Surgical Model
Under a protocol approved by the Institutional Animal Care and Use Committee (IACUC), twenty-two healthy adult swine weighing 46.5±7.6 Kg were anesthetized, placed in a supine position, intubated and ventilated. The animals were anesthetized and ventilated with a tidal volume of 6–7 cc/Kg and a rate of 12–15 breaths/min. The rate was titrated as necessary to maintain PaCO2 40–50 mmHg with a normal pH. The FiO2 was maintained between 40–60% and adjusted to maintain the PaO2 100–250 mmHg throughout the experimental course. Once placed on ECC, the ventilation rate was reduced further to adjust the PaCO2 to normal values.
The right cervical vessels were exposed for cannulation. The pigs were fully anticoagulated using a bolus dose of unfractionated heparin (300 units/Kg) and maintained at activated clotting time >400 seconds. A 19–23 Fr Biomedicus venous drainage cannula (Medtronic Cardiopulmonary, Minneapolis, MN) was advanced into the superior vena cava (SVC), with a 12–14 Fr DLP model 77012 arterial reinfusion cannula (Medtronic Cardiopulmonary, Minneapolis, MN) placed in the right carotid artery.
Extracorporeal Circuit
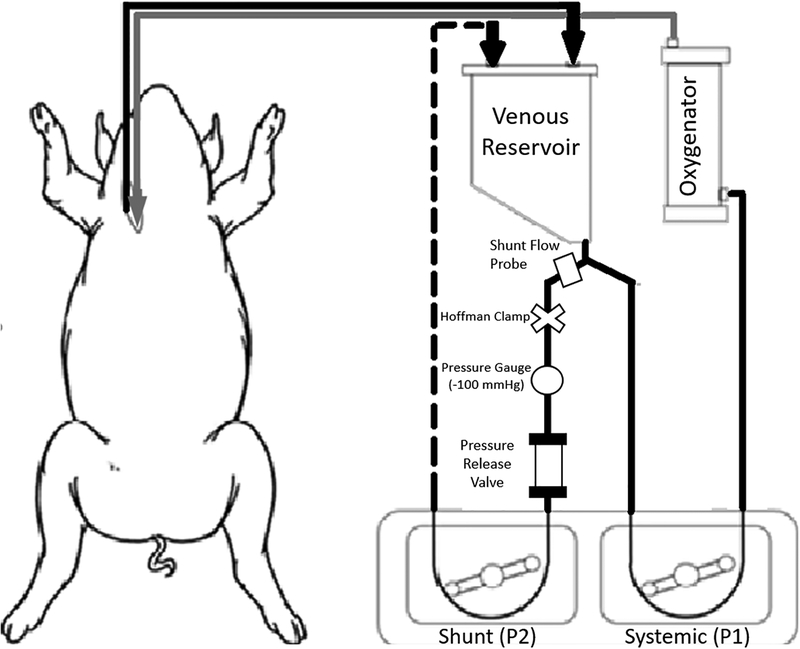
Cannulae were connected to an ECC circuit that consisted of 3/8” PVC tubing, polycarbonate connectors, a Terumo 15FX polypropylene membrane lung with an open venous reservoir that contained both an integrated cardiotomy filter and 120μ defoamer (Terumo Cardiovascular, Ann Arbor, MI), Sarns dual water heater/cooler (Terumo Cardiovascular, Ann Arbor, MI), and Cobe roller pump (LivaNova, Arvada, CO) (Figure 1). In the groups in which an air/blood interface was applied, the circuit also included a loop to simulate cardiotomy suction and/or ventricular venting. This shunt included an 8-foot segment of 1/4” PVC tubing which, via a second roller pump diverted blood from the venous reservoir outlet, past a blood flowmeter and a VRV-11 one-way relief valve (Quest Medical, Dallas, TX) back to the venous reservoir. The negative 100mmHg pressure required to open the pressure relief valve to air was achieved by tightening a Hoffmann clamp positioned proximal to the relief valve. Subtracting the measured flow rate from the roller pump flow rate estimates the volume of entrained air. This portion of the extracorporeal flow was exposed to room air at a rate of 3–3.5 L/min at −100 mmHg pressure before being returned to the venous reservoir.
Figure 1: Extracorporeal bypass circuit.
Blood drained (by gravity) into the open venous reservoir. Blood exiting the reservoir was pumped either through a polypropylene membrane oxygenator by the systemic roller pump (P1) back into the animal or diverted by a second roller pump (P2) to create an air: blood interface shunt that was recirculated back to the venous reservoir. The air- blood interface was controlled by a Hoffmann clamp to expose the blood to a negative pressure of 100 mmHg. A pressure relief valve opened to introduce air into the shunt at −100 mmHg.
Blood was drained by gravity from the venous cannula in the right atrium into the defoamer/filter in the venous reservoir. Blood from the reservoir was perfused through the membrane lung and returned to the arterial circulation through the arterial infusion cannula at a rate of 1.6–2.0 L/min (35–43 mL/min/Kg) for 120 minutes at normothermia. The ventilation/sweep gas (with or without NO) to the membrane oxygenator was 100% oxygen at a 1:1 gas: blood ratio. At conclusion of ECC, the cannulae were removed and replaced with both venous and arterial access lines. Local anesthesia was infiltrated, and the incision was closed. Pigs were then recovered from anesthesia and placed in housing to be followed for 96 hours. In all animals, pain management was obtained with opioid agonist (Fentanyl Patch 50 mcg/hour for the first 72hr) and Buprenorphine (0.3 mg) if needed thereafter. This pain protocol was used to minimize the potential interaction of an anti-inflammatory agent with our CPB strategy.
Experimental Groups
Four experimental groups were designated. The first group was a Control (n=5) in which ECC was established without any air/blood mixing. No blood was exposed to a large air/blood interface except for the open venous reservoir.
The remaining groups were exposed to a controlled air/blood interface through the shunt diagrammed in Figure 1. The second group (Air) (n=5) consisted of blood diverted through the shunt with only 100% O2 blended into the sweep gas of the membrane lung. The third (Air-50 ppm) and fourth (Air-500 ppm) groups were like the second group with the addition of NO gas blended into the 100% O2 sweep gas of the membrane lung at a concentration of either 50 ppm (n=6) or 500 ppm (n=6). In Groups 2–4, the air: blood shunt rate was maintained between 700–800 mL/min (15–17.5 mL/Kg/min.
Nitric Oxide addition to the ventilation/sweep gas to the membrane lung
Nitric oxide (NO) was continuously administered and blended into the ventilation/sweep gas of the membrane lung at a dose of 50 ppm (n=6) and 500 ppm (n=6) in the third and fourth groups during the 120 minutes of ECC. NO gas was generated by an electrochemical method that has been described previously.(23) 250 mL of nitrite electrolyte (7 mM CuSO4, 7.1 mM 1,4,7-trimethyl-1,4,7-triazacyclonane (Me3TACN), 1 M NaNO2 in HEPES 0.5 M pH 7.3 buffer) was circulated through vitreous carbon open cell foam (24 pore/cm, Goodfellow) working and counter electrodes (12.8 mL each) separated by a 1 mm thick perforated insulator, then through the shell of a 2.1 m2 surface area hollow silicone fiber (Permselect, PDMSXA-2.1) gas separator membrane module. NO was generated by Cu(II)Me3TACN mediated nitrite reduction on the polarized working electrode. The generated NO gas was separated from the electrolyte into 0.1 L/min N2 recipient gas stream in the lumen. The generated NO gas was blended into the ventilation gas after the separation of condensed humidity from the gas separator membrane module. The NO enriched ventilation gas was sampled from the inlet port of the oxygenator through a 0.060” × 24” Nafion moisture exchanger (Permapure) with 150 SCCM flow rate, and the NO and NO2 concentration of the sample gas were measured using calibrated commercial amperometric NO and NO2 sensors (Alphasense Ltd, Essex, United Kingdom). The current for electrochemical generation of NO was feedback-controlled by a microcontroller (Ruggeduino) based on the measured NO level in the ventilation gas in order to maintain steady 50 ppm or 500 ppm NO levels continuously throughout the 120-minute ECC period.
After recovery from anesthesia, pigs were placed in animal housing in accordance with IACUC standards and observed for 96 hours. Food and water were supplied ad libitum, and animal health was monitored with daily postoperative checks and blood draws. Blood samples were drawn to assess complete blood count (CBC), platelet and leucocyte activation by flow cytometry for CD11b and P-selectin (markers for leucocyte and platelet activation) at baseline, 120 min (off ECC), and 6, 24, 48, 72, and 96 hours post ECC. CD11b and P-selectin levels are markers for. After 96 hours, pigs were re-anesthetized and dynamic pulmonary compliance was assessed after 5 minutes on standardized ventilator settings: 100% FiO2, positive end-expiratory pressure 5 cmH2O, respiratory rate 10 breaths/minute, tidal volume 10 mL/Kg. Pigs were then euthanized while under anesthesia. Tissue samples were collected from the left ventricular anterior wall, left lingular pulmonary lobe, left liver lobe edge, and the full thickness of one kidney and sent for pathologic examination by a veterinary pathologist.
Statistical Analysis
Differences were analyzed and compared within each test group and between the test groups. The extracorporeal flow data were analyzed and compared using unpaired one-way ANOVA with a p-value <0.05 considered as significant. Baseline measurements displayed a non-parametric distribution, thus non-parametric Wilcoxon rank-sum testing was used to determine statistical significance, with p-value <0.05 considered significant. Statistical analysis was performed using STATA version 15 (STATA Corporation, TX, USA).
RESULTS:
Platelet Counts
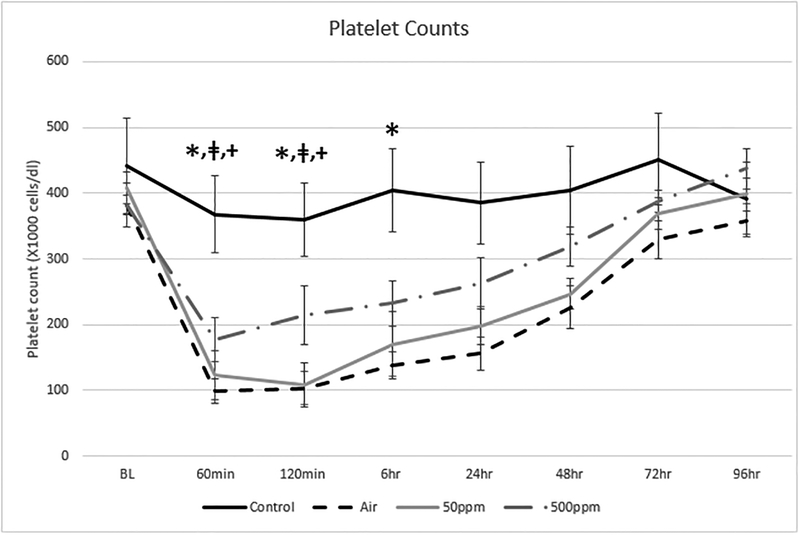
Figure 2 shows the change in platelet counts in the four test groups. There was a reduction in platelet counts in all air/blood interface groups that was not observed in the Control group. Platelet counts returned to normal in all air/blood interface groups.
Figure 2: Changes in Platelet Counts in the 4 test groups.
The values are expressed as the mean and standard deviation. There was a significant decline in platelets between the Control and Air groups*: p= 0.0122, Control and 50 ppm groupsǂ: p=0.0225 and Control and 500 ppm groups+: p=0.0358 after 120 minutes of ECC. There was only a significant difference between the Control and Air groups* at 6 hours; p=0.0122.
Leucocyte Counts
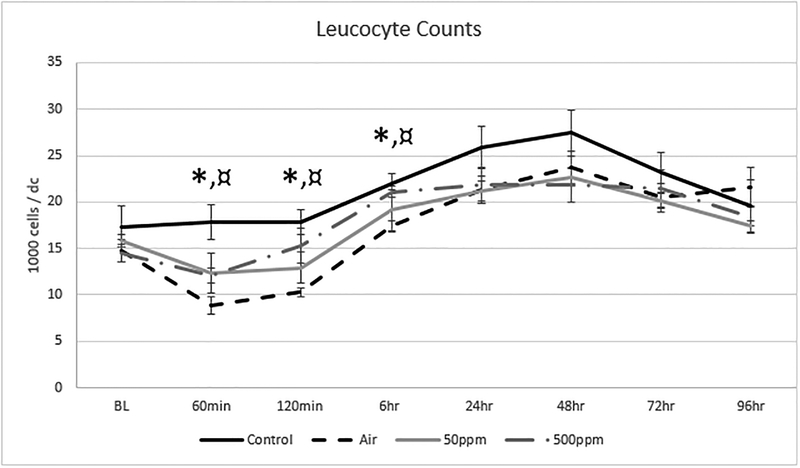
Figure 3 shows the change in leucocyte counts in the four test groups. There was a wide baseline spread in leucocyte counts that was not statistically significant between groups. The leucocyte counts were maintained in the Control group compared to a decline in counts in all air/blood interface groups during the ECC period. There was a significant difference between the Control and Air groups at 120 minutes and 6 hours, but not between the Control and any NO groups. There was a rebound in leucocyte counts after 6 hours in all groups that ultimately returned towards baseline levels in all groups. The addition of 500 ppm of NO attenuated the reduction in white blood counts compared to the Air group without NO at 120 minutes and 6 hours (p=0.011 and 0.001, respectively).
Figure 3: Changes in Leucocyte Counts in the 4 test groups.
The values are expressed as the mean and standard deviation. There was a decline and rebound in all groups except for the Control group in which there was an elevation in counts after 2 hours of ECC. Leucocyte counts returned to baseline levels by 96 hours in all groups. There was a significant difference in leucocyte counts between Control and Air groups*: p=0.0122 at 120 min and p=0.0122 at 6 hours, respectively. There was also a significant difference in leucocyte counts between Air and 500ppm groups¤: p=0.0137 at 120 min and p=0.0081 at 6 hours, respectively. There was no difference between the Control and 500ppm groups.
Flow Cytometry: Granulocytes: CD11b
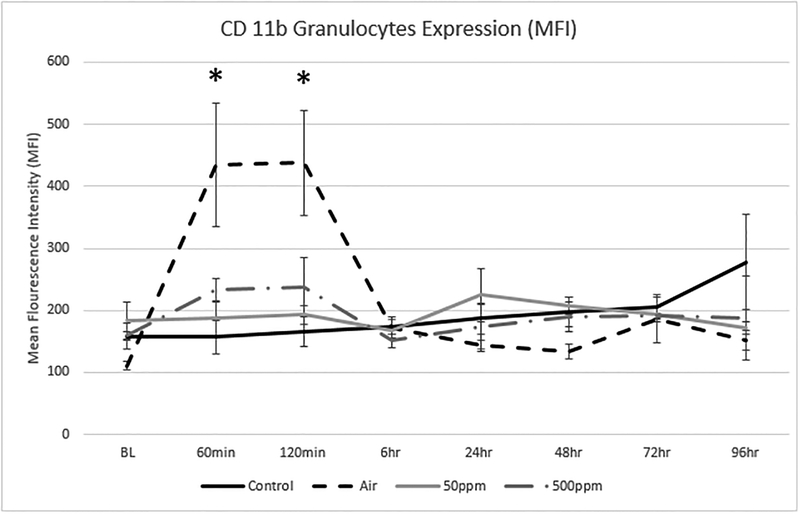
Figure 4 shows the change in expression for CD11b on granulocytes. CD11b is a marker for leucocyte activation. The values are expressed as a mean fluorescence index. There was a significant increase in CD11b expression in the Air group as compared to the Control group at 60 and 120 minutes (p=0.05 and 0.03, respectively) indicating granulocyte activation associated with air exposure. This activation was prevented in groups exposed to either dose of NO.
Figure 4: Changes in Granulocyte CD11b expression in the 4 test groups.
The values are expressed as the mean and standard deviation. Granulocyte CD 11b expression rose swiftly in the Air group and was statically significant to the Control group* at 60 min (p=0.0465) and 120 min (p=0.0278). Elevation of CD11b was minimal in the remaining groups, suggesting NO has a protective effect in the activation of granulocyte CD11b. There were no statistical differences between the Control and NO groups.
Flow Cytometry: Monocytes: CD11b
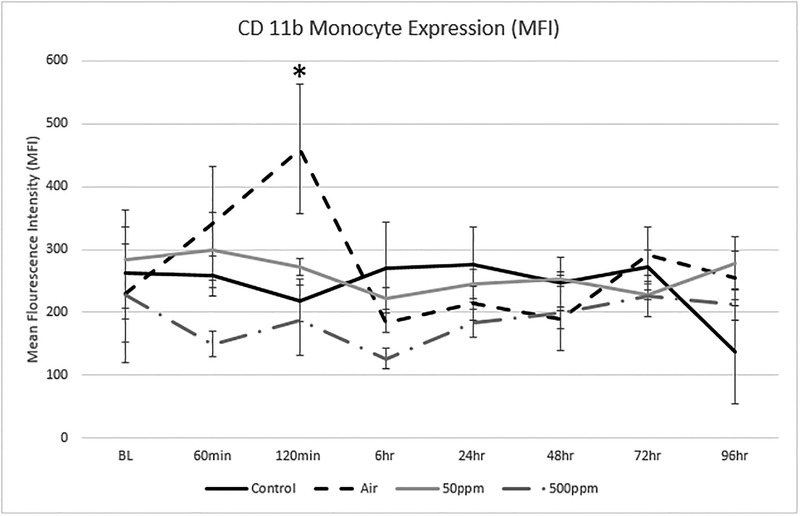
Figure 5 shows the change in expression for CD11b on monocytes. There was a significant increase in monocyte CD11b expression at 120 minutes in the Air group which was not seen in the Control or the groups exposed to either dose of NO.
Figure 5: Changes in Monocyte CD11b expression in the 4 test groups.
The values are expressed as the mean and standard deviation. Monocyte CD11b expression was elevated in the Air group and was statically significant to the Control group* at 120 min (p=0.0367). There were no other statistical differences between groups. Changes in Monocyte CD11b were maintained closer to baseline values, suggesting NO had a protective effect in the activation of Monocyte CD11b.
Flow Cytometry: P- Selectin
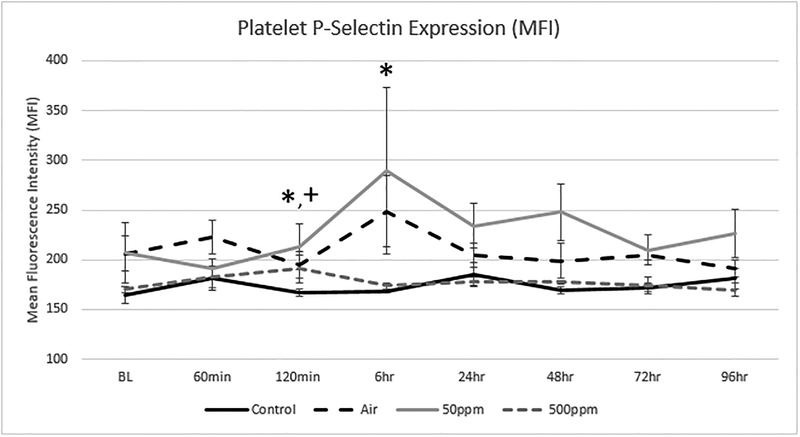
Figure 6 shows the changes in P- selectin expression in the four groups. P-selectin is a marker for platelet activation. P-selectin increased at 6 hours in the Air group as well as in the 50 ppm NO group. This was not seen in the Control group nor in the 500 ppm group (p= 0.008 and 0.33, respectively).
Figure 6: Changes in Platelet P-Selectin expression in the 4 test groups.
There was a wide spread in measured expression. The values are expressed as the mean and standard deviation. P-Selectin expression was slightly elevated in the Air* (p=0.0367) and 500 ppm NO+ (p=0.020) groups compared to Control. There was also a statistical difference between the Control and Air* (p=0.0212) groups at 6 hours.
Pathology
There were some multifocal areas of mildly hypereosinophilic myofibers seen in the cardiac tissue of all animals. The pulmonary tissue in all groups had minimal small infiltrates of lymphocytes and macrophages. The liver showed similar scattered inflammatory cell infiltrates within portal areas. Renal samples also contained minimal interstitial infiltrates of lymphocytes and macrophages. There were no appreciable differences in the pathologic findings between groups.
DISCUSSION:
It is widely known that during cardiac surgery, CPB induces a generalized inflammatory response. (24) The inflammatory response to CPB can be measured by detection of activation and aggregation of leucocytes and platelets that circulate in the blood. (14–16) Foreign surface exposure and an air/blood interface are likely to contribute to this phenomenon.
Sources of an air/blood interface occur in an open venous reservoir as well as cardiotomy suction and ventricular venting. By comparison, the air/blood interface has been eliminated during Extracorporeal Membrane Oxygenation (ECMO) which allows use for many days or weeks without this inflammatory response. Previous studies conducted in this laboratory implicate both the magnitude and duration of the air/blood interface and negative pressure in this phenomenon. (25, 26)
Nitric oxide has a role in the regulation of systemic inflammation (26) and its use has been the focus of multiple scientific and clinical investigations. It is known that NO regulates apoptosis (27), inhibits platelet adhesion and aggregation (28), monocyte adherence and migration (29) and attenuates generation of oxygen free radicals. (30) NO is inhibited during CPB and administration of the NO precursor l-arginine during surgery may improve the endogenous NO levels. (31) The ischemia-reperfusion related injury can be reduced within the kidney, liver, and lung with inhaled NO, although the optimal dose in unknown. (32) Inhaled exogenous NO (80 ppm) administered during experimental ischemia and reperfusion reduced markers of myocardial injury in an experimental setting. (33, 34)
In this experimental model, the addition of an air/blood interface caused an inflammatory and coagulation effect distinct from extracorporeal circulation without the air/blood interface. The addition of NO to the ventilation/sweep gas impacted certain features of this experimental phenomenon including a reduction in the platelet and granulocyte activation. There was also preservation of the leucocyte count in the NO groups with lower CD11b expression, suggesting modulation of the immune response.
This study was designed to evaluate the effect of air as a single variable in a partial ECC model. The effects on platelets and granulocytes were demonstrated, but there was no organ injury. This may relate to the “dose” of the air/blood interface and/or the duration of ECC. CPB typically involves a sternotomy or thoracotomy, higher extracorporeal flow rates, hypothermia and myocardial arrest and preservation in addition to the blood/air interface associated with cardiotomy suction and ventricular venting. The lack of organ injury could be related to the absence of these other important pro-inflammatory insults.
In clinical use of cardiopulmonary bypass with cardiac surgery, any intervention or novel therapy that would modulate the detrimental or untoward side effects of associated systemic inflammation with CPB would have a profound impact. Pro-inflammation has higher incidence of risks including acute kidney injury, which is associated with higher costs, morbidity and death. (35–37)
The physiologic effects of NO have been investigated in a number of studies in which NO gas has been administered into the ventilation port of a membrane lung. In one in-vitro study, 3 different concentrations of NO gas (15, 40, 75 ppm) were ventilated for 24 hours through the gas port of a polymethylpentene membrane lung and ECMO circuit primed with fresh human blood. Although no dose response relationship was established, platelet counts were better preserved in circuits that were exposed to NO in the sweep gas, consistent with our findings. (35)
A clinical randomized study evaluated the impact of gaseous NO delivered into the sweep gas of a pediatric CPB circuit during cardiac surgery. Sixteen children were randomly assigned to receive 20 ppm NO or placebo. The patients receiving NO had significantly lower troponin levels at 12, 24, and 48 hours (p<0.05) and lower B-type natriuretic peptide levels at 12 and 24 hours (p<0.05). The study patients also had higher hemoglobin level at 48 hours without associated differences in bleeding or transfusions. (20) In another clinical trial, 198 children undergoing cardiac surgery were randomly assigned to receive 20 ppm NO or placebo into the sweep gas on CPB. Those receiving NO had a lower incidence of post-operative low cardiac output syndrome, with the greatest effect occurring in younger children. (21) Nitric oxide has also been evaluated within the sweep gas of patients on ECMO. In a 60-patient controlled randomized study, data was collected on 60 coronary artery bypass patients in which 40 ppm NO was delivered into the gas intake of the membrane lung. Nitric oxide gas delivered into the CPB circuit had a cardioprotective effect where the level of cardiac troponin cTnI, Creatinine Kinase-MB value and the vasoactive drug and inotrophic support (VIS) value was significantly lower to those patients without NO treatment. (36) In a series of 30 consecutive children supported on ECMO, 20 ppm NO delivered into the sweep gas was not associated with any adverse effects. (22)
Conclusions:
Nitric oxide was applied to the sweep gas of the membrane lung in a series of animals supported on ECC with an air/blood interface analogous to CPB with cardiotomy suction. The addition of NO attenuated the inflammatory response with a reduction in platelet loss and expression of CD11b on granulocytes. Additional studies will include addition of a large thoracotomy to better replicate clinical CPB, more precise dosing studies of NO, and application in different animal models.
Sources of Funding & Conflict of Interest:
This study was supported by NIH grants 1R21HL125961–01 and 1R21HD08707–01. The authors report no conflict of interest for this manuscript
REFERENCES:
- 1.D’Agostino RS, Jacobs JP, Badhwar V, et al. The Society of Thoracic Surgeons Adult Cardiac Surgery Database: 2018 Update on Outcomes and Quality. Ann Thorac Surg 2018;105:15–23. [DOI] [PubMed] [Google Scholar]
- 2.Holmes JHt, Connolly NC, Paull DL, et al. Magnitude of the inflammatory response to cardiopulmonary bypass and its relation to adverse clinical outcomes. Inflamm Res 2002;51:579–586. [DOI] [PubMed] [Google Scholar]
- 3.Chang CH, Chen SW, Fan PC, et al. Sequential organ failure assessment score predicts mortality after coronary artery bypass grafting. BMC Surg 2017;17:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laffey JG, Boylan JF, Cheng DC. The systemic inflammatory response to cardiac surgery: implications for the anesthesiologist. Anesthesiology 2002;97:215–252. [DOI] [PubMed] [Google Scholar]
- 5.Salis S, Mazzanti VV, Merli G, et al. Cardiopulmonary bypass duration is an independent predictor of morbidity and mortality after cardiac surgery. J Cardiothorac Vasc Anesth 2008;22:814–822. [DOI] [PubMed] [Google Scholar]
- 6.Zheng J, Xiao Y, Chong M, et al. The effect of cardiopulmonary bypass duration on renal injury after congenital heart surgery in infants and young children. Adv Clin Exp Med 2013;22:693–698. [PubMed] [Google Scholar]
- 7.Mamikonian L, Mamo L, Smith P, Koo J, Lodge A, Turi J. Cardiopulmonary bypass is associated with hemolysis and acute kidney injury in neonates, infants, and children. Pediatr Crit Care Med 2014;15: 11–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Warren OJ; Smith AJ; Alexiou C; Rogers PL; Jawad N; Vincent C; Darzi AW; Athanasiou T. The inflammatory response to cardiopulmonary bypass: part 1--mechanisms of pathogenesis. Journal of Cardiothoracic & Vascular Anesthesia. 23(2):223–31, 2009. April. [DOI] [PubMed] [Google Scholar]
- 9.Warren OJ; Watret AL; de Wit KL; Alexiou C; Vincent C; Darzi AW; Athanasiou T. The inflammatory response to cardiopulmonary bypass: part 2--anti-inflammatory therapeutic strategies. Journal of Cardiothoracic & Vascular Anesthesia. 23(3):384–93, 2009. June. [DOI] [PubMed] [Google Scholar]
- 10.Partrick DA, Moore EE, Fullerton DA, Barnett CC Jr., Meldrum DR, Silliman CC. Cardiopulmonary bypass renders patients at risk for multiple organ failure via early neutrophil priming and late neutrophil disability. J Surg Res 1999;86:42–49. [DOI] [PubMed] [Google Scholar]
- 11.El-Sabbagh AM, Toomasian CJ, Toomasian JM, Ulysse G, Major T, Bartlett RH. Effect of air exposure and suction on blood cell activation and hemolysis in an in vitro cardiotomy suction model. ASAIO J 2013;59:474–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pohlmann JR, Toomasian JM, Hampton CE, Cook KE, Annich GM, Bartlett RH. The relationships between air exposure, negative pressure, and hemolysis. ASAIO J 2009;55:469–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skrabal CA, Khosravi A, Choi YH, et al. Pericardial suction blood separation attenuates inflammatory response and hemolysis after cardiopulmonary bypass. Scand Cardiovasc J 2006;40:219–223. [DOI] [PubMed] [Google Scholar]
- 14.Warren OJ, Smith AJ, Alexiou C, et al. The inflammatory response to cardiopulmonary bypass: part 1--mechanisms of pathogenesis. J Cardiothorac Vasc Anesth 2009;23:223–231. [DOI] [PubMed] [Google Scholar]
- 15.Westerberg M, Gabel J, Bengtsson A, Sellgren J, Eidem O, Jeppsson A. Hemodynamic effects of cardiotomy suction blood. J Thorac Cardiovasc Surg 2006;131:1352–1357. [DOI] [PubMed] [Google Scholar]
- 16.Gabel J, Westerberg M, Bengtsson A, Jeppsson A. Cell salvage of cardiotomy suction blood improves the balance between pro- and anti-inflammatory cytokines after cardiac surgery. Eur J Cardiothorac Surg 2013;44:506–511. [DOI] [PubMed] [Google Scholar]
- 17.Major TC, Brant DO, Reynolds MM, et al. The attenuation of platelet and monocyte activation in a rabbit model of extracorporeal circulation by a nitric oxide releasing polymer. Biomaterials 2010;31:2736–2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bennett M, Thuys C, Augustin S, Schultz B, Bottrell S, Horton A, Bednarz A, Horton S. The Safe Addition of Nitric Oxide into the Sweep Gas of the Extracorporeal Circuit during Cardiopulmonary Bypass and Extracorporeal Life Support. J Extra Corpor Technol. 2018. December;50(4):260–264. [PMC free article] [PubMed] [Google Scholar]
- 19.Jacobson J Nitric oxide: platelet protectant properties during cardiopulmonary bypass/ECMO. J Extra Corpor Technol. 2002. June;34(2):144–7. [PubMed] [Google Scholar]
- 20.Checchia PA, Bronicki RA, Muenzer JT, et al. Nitric oxide delivery during cardiopulmonary bypass reduces postoperative morbidity in children--a randomized trial. J Thorac Cardiovasc Surg 2013;146:530–536. [DOI] [PubMed] [Google Scholar]
- 21.James C, Millar J, Horton S, Brizard C, Molesworth C, Butt W. Nitric oxide administration during paediatric cardiopulmonary bypass: a randomised controlled trial. Intensive Care Med 2016;42:1744–1752. [DOI] [PubMed] [Google Scholar]
- 22.Chiletti R, Horton S, Bednarz A, Bartlett R, Butt W. Safety of nitric oxide added to the ECMO circuit: a pilot study in children. Perfusion. 2018. January;33(1):74–76. [DOI] [PubMed] [Google Scholar]
- 23.Qin Y, Zajda J, Brisbois E, Ren H, Toomasian J, Major T, Rojas-Pena A, Carr B, Johnson T, Haft J, Bartlett R, Hunt A, Lehnert N, Meyerhoff M. Portable nitric oxide (NO) generator based on electrochemical reduction of nitrite for potential applications in inhaled NO therapy and cardiopulmonary bypass surgery. Mol Pharm. 2017; 14(11):3762–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paparella D, Yau TM, Young E (2002) Cardiopulmonary bypass induced inflammation: pathophysiology and treatment. An update. Eur J Cardiothorac Surg 21:232–244. [DOI] [PubMed] [Google Scholar]
- 25.Carr B, Johnson TJ, Gomez-Rexrode A, Mohammed A, Coughlin MA, Toomasian JM, Bartlett RH, Haft JW. Inflammatory Effects of Blood-Air Interface in a Porcine Cardiopulmonary Bypass Model. ASAIO J 2019, [In Press] doi: 10.1097/MAT.000000000000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guzik TJ, Korbut R, Adamek-Guzik T (2003) Nitric oxide and superoxide in inflammation and immune regulation. J Physiol Pharmacol 54(4):469–487. [PubMed] [Google Scholar]
- 27.Uchiyama T, Otani H, Okada T, Ninomiya H, Kido M, Imamura H (2002) Nitric oxide induces caspase-dependent apoptosis and necrosis in neonatal rat cardiomyocytes. J Mol Cell Cadiol 34:1049–1061. [DOI] [PubMed] [Google Scholar]
- 28.Sawicki G, Salas E, Murat J, Miszta-Lane H, Radomski MW (1997) Release of gelatinase A during platelet activation mediates aggregation. Nature 386:616–619. [DOI] [PubMed] [Google Scholar]
- 29.Comini L, Bachetti T, Agnoletti L, Gaia G, Curello S, Milanesi B (1999) Induction of functional inducible nitric oxide synthetase in monocytes of patients with congestive heart failure: link with tumour necrosis factoralpha. Eur Heart J 20:1503–1513. [DOI] [PubMed] [Google Scholar]
- 30.Van Dervort AL, Yan L, Madara PJ, Cobb JP, Wesley RA, Corriveau CC (1994) Nitric oxide regulates endotoxin-induced TNF-alpha production by human neutrophils. J Immunol 152:4102–4109. [PubMed] [Google Scholar]
- 31.Zakkar M, Guida G, Suleiman MS, Angelini GD (2015) Cardiopulmonary bypass and oxidative stress. Oxid Med Cell Longevity 2015:189863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Phillips L, Toledo AH, Lopez-Neblina F, Anaya-Prado R, Toledo-Pereyra LH (2009) Nitric oxide mechanism of protection in ischaemia and reperfusion injury. J Invest Surg 22(1):46–55. [DOI] [PubMed] [Google Scholar]
- 33.Lin X, Huang Y, Pokreisz P, Vermeersch P, Marsboom G, Swinnen M, Verbeken E, Santos J, Pelles M, Gillijns H, Van de Wert F, Bloch K, Janssens S (2007) Nitric oxide inhalation improves microvascular flow and decreases infarction size after myocardial ischaemia and reperfusion. J Am Coll Cardiol 50(8):808–817. [DOI] [PubMed] [Google Scholar]
- 34.Nagasaka Y, Fernandez BO, Garcia-Saura MF, Petersen B, Ichinose F, Bloch KD, Feelisch M, Zapol WM (2008) Brief periods of nitric oxide inhalation protect against myocardial ischaemia-reperfusion injury. Anesthesiology 109(4):675–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brown JR, Kramer RS, Coca SG, Parikh CR. Duration of Acute Kidney Injury Impacts Long-Term Survival After Cardiac Surgery. Ann Thorac Surg 2010;90:1142–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dasta JF, Kane-Gill SL, Durtschi AJ, Pathak DS, Kellum JA. Costs and outcomes of acute kidney injury (AKI) following cardiac surgery. Nephrol Dial Transplant (2008) 23: 1970–4. [DOI] [PubMed] [Google Scholar]
- 37.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics−−2012 update: A report from the American Heart Association. Circulation. 2012. January 3;125(1):e2–e220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mellgren K, Friberg LG, Mellgren G, Hedner T, Wennmalm A, Wadenvik H. Nitric oxide in the oxygenator sweep gas reduces platelet activation during experimental perfusion. Ann Thorac Surg 1996;61:1194–1198. [DOI] [PubMed] [Google Scholar]
- 39.Kamenshchikov NO, Mandel IA, Podoksenov YK, Svirko YS, Lomivorotov VV, Mikheev SL, Kozlov BN, Shipulin VM, Nenakhova AA, Anfinogenova YJ. Nitric oxide provides myocardial protection when added to the cardiopulmonary bypass circuit: Randomized trial. J Thorac Cardiovasc Surg 2018; in press. [DOI] [PubMed] [Google Scholar]