Abstract
Suicidal ideation increases in adolescence, especially for anxious youth, and is a frequent precursor to suicide. This study examined whether neural processing of social rejection interacted with negative social experiences to predict suicidal ideation. Thus, to our knowledge this is the first study to examine how brain function may interact with the environment to contribute to suicidal ideation in youth, consistent with a developmental psychopathology perspective. 36 anxious youth (ages 11 to 16) completed diagnostic interviews and questionnaires, an ecological momentary assessment (EMA) protocol, and a functional magnetic resonance imaging (fMRI) paradigm. Results showed that youth experienced greater severity of suicidal ideation when they exhibited heightened activation to social rejection in the right anterior insula and also experienced high levels of peer victimization or EMA-measured daily negative social experiences. Findings provide preliminary evidence that alterations in neural processing of social rejection interacts with exposure to negative social experiences to contribute to suicidal ideation.
Keywords: brain function, social rejection processing, negative social experiences, suicidal ideation
Suicide rates have increased over the past 20 years, and suicide is the second leading cause of death for adolescent youth in the United States1. Suicidal ideation (SI), which is defined as thoughts of or desire to end one’s own life, is a frequent precursor to suicide attempts and death by suicide2. Lifetime prevalence rates of SI begin to rise in early adolescence, and increase until about age 173. Thus, it is important to better understand and predict the development of SI among youth during the adolescent developmental period to help identify early risk and modifiable targets for improved prevention of suicide.
Multiple theories of suicide posit that SI is motivated by the intense emotional distress or pain that occurs in the context of social disconnection or rejection4–6. For example, Schneidman (1993) posited that suicide is a solution for ending unbearable emotional pain that arises in part from disruptions in relationships or social isolation (i.e. “psychache”). Consistent with these theories, interpersonal difficulties are among the strongest proximal predictors of thoughts of suicide7, 8. Thus, neural function associated with heightened affective responses to social rejection may be associated with SI. Moreover, altered neural function to social rejection may interact with negative social experiences (e.g., rejection, separation, conflict, or other problems with peers, family, or other close relationships) to contribute to SI. Specifically, youth who exhibit maladaptive neural processing of social rejection may be at elevated risk for SI when they encounter a number of negative social experiences, because such experiences are likely to bring about particularly intense and unpleasant affective responses for these youth. On the other hand, youth with this same neurobehavioral vulnerability may still be at relatively low risk for SI under conditions of low levels of negative social experiences, because they are less likely to encounter events that bring about intense negative affect.
Investigating the interaction between neural function and social experiences is in line with a developmental psychopathology framework, which emphasizes interplay among biological and environmental factors in the etiology of psychiatric problems9. Understanding how social rejection processing and social experiences are linked to suicide risk is especially important during the adolescent period, when youth experience increases in the significance of social relationships and social stressors10, 11.
In the current study, we examined the extent to which altered neural processing of social rejection in two key brain regions, insula (AI) and dorsal anterior cingulate cortex (dACC)12, interacted with negative social experiences to predict SI. Specifically, we examined two forms of negative social experiences: peer victimization, and daily negative social experiences as measured by ecological momentary assessment (EMA) methods. Therefore, this is the first study to the best of our knowledge to examine how brain function may interact with environmental factors to contribute to suicide risk in youth. We tested this model in a sample of anxious youth given that this population is at high risk for SI13, 14 and exhibit aberrant emotion processing of social-evaluative threat and peer rejection15.
Neural Processing of Social Rejection and Association with Suicidal Ideation
Given that social separation is a threat to survival, social rejection processing is theorized to involve neural components important for the detection and avoidance of danger12. These components include sensory processing regions such as the primary and secondary somatosensory cortices (S1, S2) and posterior insula (PI), and affective processing regions such as the AI and ACC12, 16. Neural models of social rejection processing posit that the AI and dACC in particular play a crucial role in the negatively affective responses to social disconnection or rejection12, 16. These regions are repeatedly activated during neural paradigms eliciting social rejection and grief/loss across both youth and adults, and heightened activation in these regions are associated with greater subjective distress after exclusion12, 17–20. Therefore, given that activation of the AI and dACC are linked to unpleasant emotional responses to social rejection, greater activation of these regions may also be associated with desire for suicide.
The few existing functional neuroimaging studies focusing on youth suicidal thoughts or behaviors have generally showed alterations in neural regions implicated in emotion processing, such as limbic and prefrontal areas, as well as the insula and ACC21–24. Two recent studies examined associations between neural processing of social rejection and nonsuicidal self-injury (NSSI), which is highly correlated with SI and suicidal behavior25, 26. These studies suggest that altered neural processing of social rejection in affective regions, including the mPFC and ACC, is linked to suicide risk. One other study in adults showed that neural functional alterations in the insula was linked to a history of suicide attempts in females27. However, none of these studies examined associations between neural processing of social rejection and SI16.
Negative Social Experiences as a Moderator of Association between Neural Processing of Social Rejection and Suicidal Ideation
Multiple suicide theories emphasize the role of social disconnection, separation, or loss in contributing to the desire for suicide6. Empirical evidence further shows that interpersonal stressors, social rejection experiences, and feeling rejected by others, are strongly linked to SI28, and are frequent proximal predictors7, 8. Among adolescent youth, social factors such as peer victimization, social isolation, and parent-adolescent conflict, are associated with SI29–31. Thus, SI appears to be especially likely to occur in the presence of negative social experiences, and altered neural processing of social rejection may interact with negative social experiences to contribute to SI. Specifically, youth who exhibit heightened activation to social rejection in the AI and dACC may be especially vulnerable to SI when they encounter high levels of real-world negative social experiences given that these experiences might be especially likely to elicit intense negative affect for these youth.
In the current study, we investigated two forms of negative social experiences as moderators of the association between neural activation to social rejection in the dACC and AI, and SI. First, we assessed peer victimization because evidence suggests this is a particularly deleterious negative social experience in adolescence linked to SI32. Second, we used EMA to assess for daily negative social experiences, which is a more ecologically valid measure of day to day negative social experiences as they naturally occur33, 34.
Present Study
The current study examined the interaction between neural activation to social rejection and negative social experiences (peer victimization and EMA daily negative social experiences) and association with SI severity in a sample of anxious youth during the adolescent developmental period. We hypothesized that youth with greater blood oxygenation level dependent (BOLD) response in bilateral AI and dACC to social rejection and exposure to high levels of peer victimization would experience higher levels of SI compared to other youth. Similarly, we hypothesized that youth with both greater BOLD response in these regions in response to social rejection and high levels of EMA daily negative social experiences would experience higher levels of SI. We used the Chatroom Interact Task to assess for neural activation to social rejection, in which adolescent participants interact online with virtual peers19, 35. This task was developed to be a more ecologically valid interactive paradigm that more closely simulates day-to-day peer social rejection experiences in adolescence compared to existing social rejection neuroimaging paradigms that were originally designed for adult populations36.
Method
Participants
The current study included participants from a larger study examining the development of depression among anxious youth following anxiety treatment37. All youth met Diagnostic and Statistical Manual for Mental Disorders (DSM 4th ed.; American Psychiatric Association 1994) criteria for an anxiety disorder (separation anxiety disorder, generalized anxiety disorder, and/or social phobia), and completed a randomized treatment for anxiety (16 sessions of either cognitive-behavioral therapy or child-centered supportive therapy) two years prior to the current study. The present study included 36 youth (53% girls) who completed an fMRI scan and all relevant concurrent measures. Age of youth at the time of the current study ranged from 11 to 16 (M = 13.56, SD = 1.50). The sample was 93% Caucasian, 6% African-American, and 1% Biracial. Average family income for the sample was approximately $65,000. Trained interviewers administered the Schedule for Affective Disorders and Schizophrenia for School-Aged Children (K-SADS; Kaufman et al. 1997) to youth participants, and to their parents about their child, to assess for the presence of a DSM-IV diagnosis of an anxiety disorder following treatment. At the time of the current study, approximately 28% of participants met full criteria for one of the following anxiety disorders: separation anxiety disorder (N=1), social phobia (N =1), generalized anxiety disorder (N=5), or comorbid diagnoses of social phobia and generalized anxiety disorder (N = 3). Additionally, child-reported scores on the Screen for Anxiety Disorders38 indicated average levels of anxiety symptoms (M = 19.58, SD = 11.5) were above the recommended cutoff for remission (raw score = 12;39).
Youth were excluded from the larger treatment study if they were taking psychotropic medications, were acutely suicidal or homicidal, had a developmental disorder, or had an IQ below 70 as assessed by the Wechsler Abbreviated Scale of Intelligence40. Youth were also excluded if they were not suited for fMRI procedures for reasons such as pregnancy or ferromagnetic objects in the body. In addition, youth were excluded if they had a primary diagnosis of any of the following disorders: major depressive disorder, obsessive compulsive disorder, post-traumatic stress disorder, conduct disorder, substance abuse or dependence, ADHD (predominantly hyperactive-impulsive type or combined type), or a lifetime diagnosis of schizophrenia, schizoaffective disorder, bipolar disorder, or depression with psychosis. 9 participants met criteria for a secondary, comorbid diagnosis of Specific Phobia (N = 5), ADHD (N = 2), Tourette Syndrome (N = 1), or Mood Disorder NOS (N = 1).
Procedure
This study’s procedures were approved by the university’s Institutional Review Board. Parents provided parental consent and youth assented to participation. Youth completed questionnaires via secure web-based surveys, and diagnostic interviews during a laboratory visit that occurred approximately 2 years after treatment completion (Day 1 of current study). Within one week following the laboratory visit, participants began the EMA protocol. Youth returned to participate in the fMRI Chatroom Interact Task (Day 2) following completion of EMA (average amount of time elapsed between the EMA and fMRI task was 28 days). All fMRI scans took place at the Magnetic Resonance Research Center (MRRC) at the University of Pittsburgh. Youth were trained to minimize head motion and had the opportunity to practice the fMRI task in a simulator prior to the scan. Participants received compensation for completing assessments.
Measures
Suicidal ideation (SI).
The 4 item SI Composite of the Mood and Feelings Questionnaire was used to assess severity of SI (MFQ-SI41). The MFQ-SI assesses severity of suicidal thoughts, and includes items such as “thought life was not worth living” and “thought about killing self”. Each item was coded as either 0 (“not true”), 1 (“sometimes”), or 2 (“true”). Higher scores indicated greater SI severity. The MFQ-SI is a validated scale for assessing SI, showing strong reliability and concurrent and predictive validity in previous research41.
Depressive symptoms.
The Children’s Depression Rating Scale – Revised (CDRS-R42) was used to assess severity of depressive symptoms. The CDRS-R is a validated semi-structured clinician-rated instrument consisting of 17 items assessing depression. The CDRS-R integrates information from the parent, child, and clinical observations to determine severity of youth depressive symptoms and has demonstrated high internal consistency (α=0.85) and good test-retest reliability (r=0.92). CDRS scores ≥ 40 typically define clinical levels of depression43.
Peer victimization.
Peer victimization was measured via the Victim subscale of the Peer Relations Questionnaire (PRQ)44. This subscale is made up of 5 items assessing victimization (e.g., “I get picked on by others”), where participants respond on a four-point Likert-type scale ranging from 1 = “never” to 4 = “very often”. High scores reflect higher rates of self-reported victimization. Authors have reported adequate validity and reliability coefficients which range between α = .71 to α = .8644.
Ecological Momentary Assessment (EMA) Protocol.
Daily negative social experiences were assessed as part of the EMA protocol, similar to methods used in previous studies 33, 45. Staff called youth participants on study cell phones for the EMA protocol. The protocol consisted of two blocks (5 consecutive days of calls per block) over a 2 week period (1 block per week). Each block consisted of 14 calls over two weekend days and three weekdays (i.e., 4 PM on Thursday to 9:30 PM on Monday, 28 total calls). Phone calls were made throughout the day; however, weekday calls were limited to after-school hours. The number of completed calls was high (M = 85%). Youth were prompted to describe a time when they experienced the most negative affect in response to a self-nominated event that occurred within the past hour. Trained coders classified negative events into various categories (e.g., peer, family, health, school, etc.). Interrater agreement was adequate for coding event category (Cohen’s Kappa = 0.70). Proportions were calculated for negative social experiences endorsed, which included calls with all negative events coded as related to peers, family, or romantic partners (e.g., disagreements, unable to spend time with parents/friends), grief/loss (e.g., teacher passed away), or being alone, excluded or separated from others (e.g., others went to do something and left youth alone). All other events were considered to be non-social, such as school or health related events.
fMRI Chatroom Interact Task.
The Chatroom Interact Task was designed to investigate neural response to social acceptance and rejection19. The task takes the form of a structured online interaction to give the impression that participants and peers are interacting in real time while maintaining sufficient standardization. Participants were told that they would interact with other peers in a “chat game” over the internet during the upcoming fMRI visit, although in reality the Chatroom Interact task consisted of predetermined, computer-generated responses from fictional peers.
On Day 1, participants selected photographs and fictitious biographical profiles for potential peers with E-prime 1.0 computer software46. First, participants selected the top 5 colored pictures of same-sex youth that they would be most interested in interacting with at their next visit. Selections were made from within sets of 20 photographs for each age (9–11, 12–14 or 15–17) and gender grouping. The pictures of peers were of child actors who consented to be photographed for the task. Next, participants were presented with 5 names and profiles that were ostensibly associated with each of the peers in the pictures previously selected. The participant was asked to rank these peers based on their profiles in order from 1 (most excited to chat with) to 5 (least excited to chat with). Participants also completed their own biographical profile using a standardized template, and were told the information would be shared with the other peers in the chat task. Research staff also took the participant’s photograph. On Day 2, participants returned to the laboratory and were told that they had been matched with two peers selected from the first visit for the ‘chat game’. The top 2 pictures and profiles of peers selected by the participant from Day 1 were always used for the fMRI task.
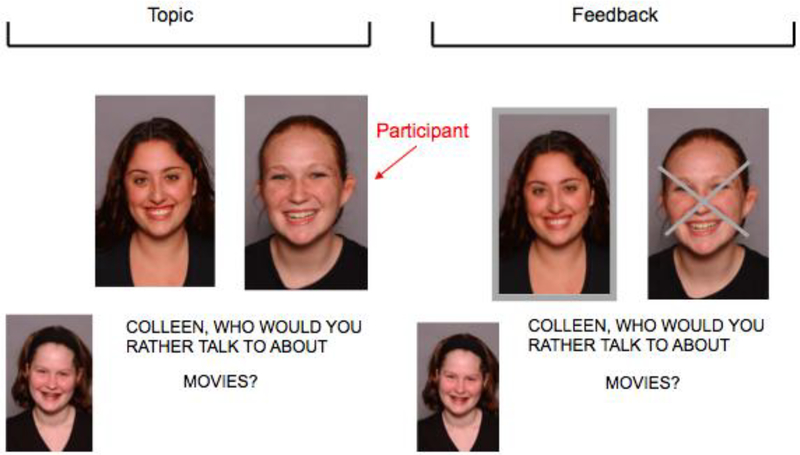
During the scanning session, stimuli were presented using E-prime 1.0. The profiles and pictures for the participant and selected peers were presented at the start of the task for the participant to review. The task used a slow-event related design and proceeded in 4 blocks, each containing 15 trials. Participants were chosen (accepted) or not chosen (rejected) to discuss a series of topics (e.g., music, movies) in blocks 2 and 3. The picture of the peer making the choice was shown at the bottom left corner of the screen, and the pictures of the participant and second peer were shown next to each other in the middle of the screen. At the beginning of each trial, the question ‘Who would you rather talk to about …’ with the selected topic for that trial (i.e. … ‘music?’) appeared on the screen for 3.34 s. Feedback was then provided about whether the participant was accepted or rejected for 10.02 s. The picture of the rejected person was superimposed with an ‘X’ and the picture of the accepted person was highlighted around the border (See 1). The participant indicated whether the person on the left or the right was chosen in all trials with a response glove on the right hand. Topics were presented randomly and repeated in each block, but with a different person making the selection for each block. Participants experienced one ‘accept’ block in which they were chosen two-thirds of the time, and one ‘reject’ block in which they were rejected two-thirds of the time (15 accept and 15 reject trials in total). The order of accept and reject blocks and trials were randomized across each participant. The participant made choices between the two virtual peers in Block 1, although data from this block was not used in analyses. In block 4 (control block), the participant viewed a picture of themselves and a peer, and pressed a button using the response glove to indicate if a dot appeared on the face on the left or right. Each trial was 13.36 seconds long, resulting in 200.4 seconds per block (total run time of task = 808.28 seconds). Participants were debriefed at the conclusion of the task and informed that in reality they had been playing with a preset computer program.
BOLD Functional MRI Acquisition and Preprocessing
Image Acquisition.
Images were acquired on a Siemens 3T Trio scanner (Erlangen, Germany). Thirty-two 3.2 mm slices were acquired parallel to the AC–PC line using a posterior-to-anterior echo planar (EPI) pulse sequence (T2*-weighted imaged depicting BOLD signal; TR = 1670 ms, TE = 29 ms, FOV = 205 mm, flip angle = 75°). There were 484 volumes in total. High-resolution T1-weighted MPRAGE images (1 mm, axial) were also collected for use in cross-registration.
Preprocessing.
MRI images were preprocessed using SPM1247. Volumes were manually reoriented to the AC-PC line, and slice time corrected. Images were then realigned to correct for head motion, segmented, and coregistered to the participant’s mean functional image. Realigned images were spatially normalized to a standard MNI template (Montreal Neurological Institute template) using a 12-parameter affine model and voxels were resampled to be 2 mm3. Normalized images were spatially smoothed using a 6 mm full-width at half-maximum Gaussian filter. High-pass temporal filtering (0.008Hz /128sec in SPM) was applied to remove low-frequency drift in the time series. Volumes with motion greater than 5mm/5° and global intensities more than 3 SD from the mean were detected using the ARTDetect toolbox48. Volumes were repaired using interpolation methods in the ArtRepair toolbox for SPM49 if no more than 25% of volumes per session were detected as outliers. Repaired volumes were used for first-level analyses.
Data Analytic Approach
ROI Moderation Analyses.
First level analyses were conducted by modeling the fMRI response based on hemodynamic response function (HRF) convolved with the vectors of two feedback conditions (rejection and acceptance) and one control condition. Three regressors were modeled for rejection, acceptance, and control conditions. We also entered six movement parameters into our model as regressors of no interest to control for movement-related signal change. Statistical images were then created for the rejection > acceptance contrast19.
We then examined group level neural activation within a priori anatomically defined bilateral AI and dACC regions of interest (ROI) using Automated Anatomical Labeling (AAL) atlas50. Mean parameter estimates for the rejection > acceptance contrast for each participant were extracted from the ROIs using MARSBAR toolbox51. The extracted ROI parameter estimates were then used for regression analyses.
Three independent regressions were conducted using the SPSS macro PROCESS52 to examine the moderating effect of peer victimization on the association between neural activation to social rejection and SI (i.e. neural activation to social rejection x peer victimization) for each ROI (left AI, right AI, and dACC). Three more independent regressions were conducted to test the moderating effect of EMA daily negative social experiences on SI (i.e. neural activation to social rejection EMA daily negative social experiences). For all analyses, ROI parameter estimates and social experience variables were entered as main effects in each separate regression model, and depressive symptom scores were also included as a covariate, given the strong association between depression and SI3. The interaction effect for each of the six regressions was entered last. All predictors were centered to reduce multicollinearity. Type I error was controlled using a Bonferroni correction (p < .008). Significant interactions were probed using Johnson-Neyman procedures via PROCESS to identify regions of significance52.
Results
Descriptive Statistics
Means, standard deviations and bivariate correlations for primary continuous variables are presented in Table 1. Age was not correlated with any of the primary variables. Independent sample t-tests showed that girls exhibited higher levels of peer victimization (M = 7.58, SD = 2.39) than boys (M = 6.12, SD = 1.54); t(34) = 2.21, p < .05. Girls also exhibited higher levels of depressive symptoms (M = 24.05, SD = 3.62) than boys (M = 20.03, SD = 2.78); t(34) = 3.71, p < .01. No gender differences were observed for any of the other variables. T-tests also showed that type of treatment youth received for anxiety (child-centered or cognitive behavioral therapy) prior to the current study was not associated with any variables.
Table 1.
Means, standard deviations, and bivariate correlations for primary continuous variables.
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| 1. R AI | - | .90*** | .70*** | .25 | .02 | −.08 | .12 | −.08 |
| 2. L AI | .90*** | - | .80*** | .02 | −.09 | −.17 | .02 | −.09 |
| 3. d ACC | .70*** | .80*** | - | −.09 | −.13 | −.26 | −.13 | −.05 |
| 4. SI | .25 | .02 | −.09 | - | .30 | .22 | .20 | .25 |
| 5. CDRS-R | .02 | −.09 | −.13 | .30 | - | .22 | .29 | .07 |
| 6. PRQ-victim | .12 | −.17 | −.26 | .22 | .22 | - | .09 | −.22 |
| 7. EMA neg. social. | .12 | .02 | −.13 | .20 | .29 | .09 | - | .14 |
| 8. Age | −.08 | −.09 | −.05 | .25 | .07 | −.22 | .14 | - |
| Mean (SD) | −.04 (.22) | −.02 (.22) | −.05(.15) | .29 (.71) | 22.13 (3.85) | 6.86 (2.16) | .14 (.15) | 13.62(1.50) |
Note.
p <.001.
R AI = right anterior insula; L AI = left anterior insula; CDRS-R = The Children’s Depression Rating Scale – Revised; PRQ-victim = Peer Relations Questionnaire – Victim Scale; EMA neg. social exper. = proportion of negative social experiences endorsed during ecological momentary assessment.
ROI Moderation Analyses
Neural activation to social rejection x peer victimization.
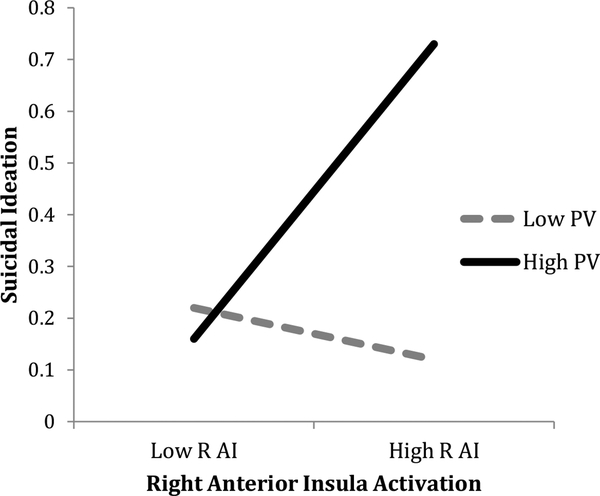
As shown in Table 2, results of regression analyses showed that peer victimization moderated the association between right AI activation in response to rejection vs. acceptance and SI after controlling for youth depressive symptoms. The Johsnon-Neyman post-hoc analyses showed that there was a significant positive association between right AI activation and SI only when peer victimization levels were above the 58th percentile (PRQ-V scores above 6.97). Figure 2 depicts the interaction effect by graphing the simple slopes between AI activation and SI at high (75th percentile) and low (25th percentile) levels of peer victimization.
Table 2.
Right Anterior Insula x Peer Victimization Predicting SI
| Predictors | ΔR2 | b | SE | t |
|---|---|---|---|---|
| Main Effects | .17 | |||
| R AI | .85 | .44 | 1.92 | |
| PRQ-Victim | .07 | .05 | 1.44 | |
| CDRS-R | .04 | .03 | 1.55 | |
| Interaction Effect | .19 | |||
| CDRS-R × R AI | .67 | .22 | 2.99** | |
| Model R2 = 36, F(4, 31) = 4.35, p < .01 | ||||
Note.
p < .01.
R AI = right anterior insula; CDRS-R = The Children’s Depression Rating Scale – Revised; PRQ-victim = Peer Relations Questionnaire – Victim Scale.
Figure 2. Mean Level of Activation in Right Anterior Insula x Peer Victimization Predicting.
Note. R AI = right anterior insula; PV = peer victimization.
Peer victimization did not moderate the association between left AI and SI, b = .17, SE = .29, p = .56. Peer victimization also did not moderate the association between dACC and SI, b = .03, SE = .39, p = .94.
Neural activation to social rejection x EMA daily negative social experiences.
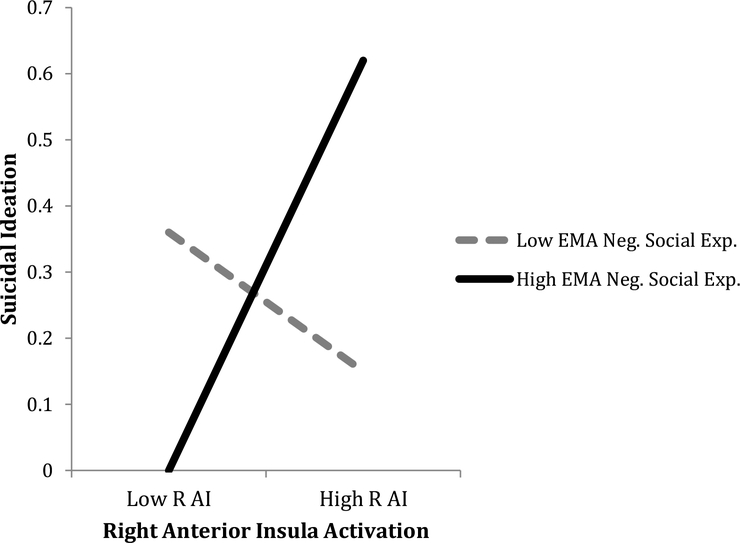
Table 3 shows that EMA measured daily negative social experiences also significantly moderated the association between right AI activation and SI. Johsnon-Neyman post-hoc results showed that that there was a significant positive association between right AI activation and SI only when the proportion of negative social experiences were above the 66th percentile (or above .14). Figure 3 depicts the interaction effect.
Table 3.
Right Anterior Insula x Daily Negative Social Experiences Predicting SI
| Predictor | ΔR2 | b | SE | t |
|---|---|---|---|---|
| Main Effects | .15 | |||
| R AI | .88 | .42 | 2.09* | |
| EMA neg. social exp. | −.17 | .70 | −.24 | |
| CDRS-R | .04 | .03 | 1.37 | |
| Interaction Effect | .28 | |||
| EMA neg. social exp. × R AI | 12.01 | 3.09 | 3.88*** | |
| Model R2 = 43 F(4, 31) = 5.86, p < .01 | ||||
Note.
p <.001.
R AI = right anterior insula.
Figure 3. Mean level of Activation in Right Anterior Insula x Daily Negative Social Experiences Predicting Suicidal Ideation.
Note. R AI = right anterior insula; EMA Neg. Social Exp. = EMA negative social experiences.
EMA measured daily negative social experiences did not moderate the association between left AI and SI, b = 2.54, SE = 5.50, p = .65, nor did it moderate the association between dACC and SI, b = 2.09, SE = 7.43, p = .78.
Exploratory Whole Brain Analyses
We conducted supplemental, exploratory whole brain regression analyses to further investigate regions in which brain activation during social rejection might be associated with SI. We regressed SI scores onto neural activation during rejection > acceptance (p < .005, uncorrected), controlling for depressive symptoms. Results showed activation in the right AI and inferior frontal gyrus, left inferior frontal gyrus, and right occipital gyrus (Table 4).
Table 4.
Regions of activation during rejection > acceptance condition showing correlations with suicidal ideation (uncorrected p <.005)
| Region | BA | Cluster size (# of voxels) | x | y | z | t |
|---|---|---|---|---|---|---|
| Right anterior insula/inferior frontal gyrus | 13/47 | 65 | 40 | 14 | −18 | 4.18 |
| Right middle occipital gyrus | 19 | 37 | 48 | −76 | 16 | 3.39 |
| Left inferior frontal gyrus | 44 | 16 | −56 | 14 | 14 | 3.09 |
Discussion
We investigated the extent to which the interaction between neural processing of social rejection and negative social experiences were associated with SI severity in a clinical sample of anxious adolescents, a population at elevated risk for SI. Results showed that youth reported higher levels of SI if they exhibited heightened activation to social rejection in the right AI, and also experienced peer victimization or EMA measured daily negative social experiences.
This study integrates and extends separate bodies of work in the areas of affective neuroscience and suicide to make a novel contribution to the understanding of the development of suicide risk in adolescent youth. Specifically, findings are consistent with prior theory and research suggesting that heightened AI activation to social rejection is associated with more intense negative emotional responses12. The AI appears to be loci for integration of interoception (i.e. stimuli within the body) with cognitive and emotional awareness, and, and is thought to also be critical in the affective component of physical pain (i.e. the “unpleasantness” of pain) during physical pain processing53, 54. Therefore, findings are consistent with studies suggesting that responses to social rejection and physical pain partially share neural components12. In fact, given the strong evidence for overlap between neural processing of social rejection and pain, some researchers have referred to affective responses to social rejection as “social pain”12, 16, 55. Furthermore, results are consistent with suicide theories and research suggesting that suicidal thoughts are motivated by the desire to escape intense emotional pain (e.g., “psychache”)5, 56, particularly in the context of negative social experiences5, 6. Taken together, findings support the hypothesis that youth with heightened AI activation to social rejection are more susceptible to increased negative affective responses to social rejection (or “social pain”) and desire for suicide when these youth encounter negative social experiences.
Our findings, together with two other recent studies examining associations between neural processing of social rejection and other indices of suicide risk (i.e. NSSI)25, 26, suggest that alterations in neural function during social rejection processing may be especially relevant for increased vulnerability for suicide in adolescents. Adolescents may be particularly vulnerable to alterations in AI activation to social rejection, given age-related changes in insula function during processing of both interoceptive and social information11, 57. While developmental shifts in AI function are thought to facilitate flexible, affective responding to changing social contexts11, 57, these neural changes may also contribute to the rise in SI during the critical adolescent period.
Previous studies within the suicide risk literature have focused either on brain function22, 24, or social factors32, 58. Our findings emphasize the importance of considering how brain function may interact with social experiences to contribute to suicide risk, consistent with a developmental psychopathology perspective. Moreover, although a good deal of research attention has been given to the association between more severe negative social experiences, such as bullying/peer victimization and suicidality29, 32, our findings suggest that the frequent occurrence of a broad range of negative social experiences, ranging from harsher (e.g., peer victimization) to more typical day to day experiences (e.g., disagreements, being excluded from outings with family or friends), may all have the potential to increase SI among youth exhibiting altered neural function during social rejection processing.
Finally, given that this is the first study to examine the association between brain function during peer social rejection processing and SI among youth, we conducted supplemental, exploratory whole brain regression analyses to further investigate potential neural correlates of SI. Results supported that SI is associated with neural activation to social rejection in the right AI. In addition, activation in regions implicated in facial and emotion processing (occipital gyrus and inferior frontal gyrus) was associated with SI, consistent with prior studies examining neural function and suicidal thoughts and behaviors23, 24.
Interestingly, ROI moderation and whole brain analyses implicated activation in the right AI specifically. There is some evidence for lateralization of function within the AI that is consistent with these results. The right AI in particular may be more likely to provide an interface between the mapping of interoceptive information and the representation of internal bodily states as subjective feelings, and underlie negative affect and physical pain53, 59. We also did not find support for our hypothesis that activation in the dACC is linked to SI, either alone, or in interaction with negative social experiences. Future studies are needed to examine whether heightened dACC activation is less relevant for SI in anxious youth and other youth populations.
Finally, findings also have implications for understanding elevated rates of SI and potential interventions among anxious youth populations. Previous research with anxious samples implicates the AI in increased attention to interoceptive sensations, a core feature of anxiety disorders53, 60. We speculate that AI activation may underlie heightened perceptions of internal bodily changes in response to social rejection in anxious youth, contributing to a more intense negative emotional experience and desire for suicide. Therefore, heightened AI activation to social rejection may at least partially explain elevated risk for SI in anxious youth, and be an effective treatment target. If findings are replicated, current neuromodulatatory interventions used to treat physical pain that regulate AI activation, such as real-time fMRI neurofeedback61, might have success in preventing or reducing SI for anxious youth, particularly those youth who encounter a higher number of negative social experiences.
Future research is needed to address limitations of this study. The sample size in this study was moderate, and so findings must be interpreted with caution. Study findings may not generalize to other populations of youth, such as non-anxious youth, or other racial/ethnic groups given that the sample was primarily comprised of Caucasian and African American participants. Constructs were also assessed concurrently at a single timepoint, which further limits interpretations about how brain function and social experiences may prospectively contribute to the development of SI. In addition, future research is needed to further investigate how functioning and connectivity among other brain regions associated with social rejection processing (e.g. somatosensory cortex), and emotion processing (e.g., amygdala, prefrontral regions), are linked to suicide risk. Finally, although the Chatroom Interact Task is one of the few available tasks specifically designed to assess neural activation to peer social rejection in adolescents, the task still may be limited in its ability to capture neural features of affective responses to real-world social rejection during typical face-to-face interactions in schools or other social environments.
Summary
SI increases in adolescence and is a frequent precursor to suicidal behavior and suicide. This study examined whether neural activation to social rejection interacted with negative social experiences to predict SI, and thus is the first study to our knowledge to examine how brain function may interact with aspects of the environments to predict SI. Social rejection processing and social experiences may be especially relevant to understanding suicide risk during the adolescent period, when youth experience increases in the significance of social relationships and social stressors. Results showed that youth experienced greater severity of SI when they exhibited heightened neural activation to social rejection in the right anterior insula and also experienced high levels of peer victimization or EMA-measured daily negative social experiences. Findings are consistent with prior theory and research suggesting that heightened AI activation to social rejection is associated with more intense negative emotional responses, or “social pain”16. Furthermore, results are consistent with suicide theories and research suggesting that suicidal thoughts are motivated by the desire to escape intense emotional pain (e.g., “psychache”;5, 56, particularly in the context of negative social experiences. Taken together, findings support the hypothesis that youth with heightened AI activation to social rejection are more susceptible to increased negative affective responses to social rejection and desire for suicide when these youth encounter negative social experiences.
Figure 1. Example of fMRI Chatroom Interact Task Trial.
Footnotes
This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Centers for Disease Control and Prevention NCfIPaC. Web-based Injury Statistics Query and Reporting System 2013. Available from: http://webappa.cdc.gov.
- 2.Nock MK 2016. Recent and needed advances in the understanding, prediction, and prevention of suicidal behavior. Depression and anxiety 33:460–3. [DOI] [PubMed] [Google Scholar]
- 3.Nock MK, Green JG, Hwang I, McLaughlin KA, Sampson NA, Zaslavsky AM, et al. 2013. Prevalence, correlates, and treatment of lifetime suicidal behavior among adolescents: results from the National Comorbidity Survey Replication Adolescent Supplement. JAMA psychiatry 70:300–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baumeister RF 1990. Suicide as escape from self. Psychological review 97:90. [DOI] [PubMed] [Google Scholar]
- 5.Shneidman ES 1993. Commentary: Suicide as psychache. The Journal of nervous and mental disease 181:145–7. [DOI] [PubMed] [Google Scholar]
- 6.Van Orden KA, Witte TK, Cukrowicz KC, Braithwaite SR, Selby EA, Joiner TE Jr 2010. The interpersonal theory of suicide. Psychological review 117:575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nock MK, Prinstein MJ, Sterba SK 2009. Revealing the form and function of self-injurious thoughts and behaviors: A real-time ecological assessment study among adolescents and young adults. Journal of abnormal psychology 118:816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Victor SE, Scott LN, Stepp SD, Goldstein TR 2018. I Want You to Want Me: Interpersonal Stress and Affective Experiences as Within- Person Predictors of Nonsuicidal Self- Injury and Suicide Urges in Daily Life. Suicide and Life- Threatening Behavior. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cicchetti D, Rogosch FA 2002. A developmental psychopathology perspective on adolescence. Journal of consulting and clinical psychology 70:6. [DOI] [PubMed] [Google Scholar]
- 10.Hankin BL, Mermelstein R, Roesch L 2007. Sex differences in adolescent depression: Stress exposure and reactivity models. Child development 78:279–95. [DOI] [PubMed] [Google Scholar]
- 11.Guyer AE, Silk JS, Nelson EE 2016. The neurobiology of the emotional adolescent: From the inside out. Neuroscience & Biobehavioral Reviews 70:74–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eisenberger NI 2012. The pain of social disconnection: examining the shared neural underpinnings of physical and social pain. Nature Reviews Neuroscience 13:421–34. [DOI] [PubMed] [Google Scholar]
- 13.O’Neil KA, Puleo CM, Benjamin CL, Podell JL, Kendall PC 2012. Suicidal ideation in anxiety- disordered youth. Suicide and Life- Threatening Behavior 42:305–17. [DOI] [PubMed] [Google Scholar]
- 14.Gallagher M, Prinstein MJ, Simon V, Spirito A 2014. Social anxiety symptoms and suicidal ideation in a clinical sample of early adolescents: Examining loneliness and social support as longitudinal mediators. Journal of abnormal child psychology 42:871–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silk JS, Davis S, McMakin DL, Dahl RE, Forbes EE 2012. Why do anxious children become depressed teenagers? The role of social evaluative threat and reward processing. Psychological medicine 42:2095–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eisenberger NI 2012. The neural bases of social pain: evidence for shared representations with physical pain. Psychosomatic medicine 74:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumar P, Waiter GD, Dubois M, Milders M, Reid I, Steele JD 2017. Increased neural response to social rejection in major depression. Depression and anxiety 34:1049–56. [DOI] [PubMed] [Google Scholar]
- 18.Giletta M, Hastings PD, Rudolph KD, Bauer DJ, Nock MK, Prinstein MJ 2017. Suicide ideation among high-risk adolescent females: Examining the interplay between parasympathetic regulation and friendship support. Development and psychopathology 29:1161–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silk JS, Siegle GJ, Lee KH, Nelson EE, Stroud LR, Dahl RE 2013. Increased neural response to peer rejection associated with adolescent depression and pubertal development. Social cognitive and affective neuroscience:nst 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsu D, Yttredahl A, Sankar A 2018. 66. Neuroimaging Evidence for Targeting Abnormal Responses to the Social Environment in Major Depressive Disorder. Biological Psychiatry 83:S27. [Google Scholar]
- 21.Johnston JA, Wang F, Liu J, Blond BN, Wallace A, Liu J, et al. 2017. Multimodal neuroimaging of frontolimbic structure and function associated with suicide attempts in adolescents and young adults with bipolar disorder. American journal of psychiatry 174:667–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller AB, McLaughlin KA, Busso DS, Brueck S, Peverill M, Sheridan MA 2018. Neural Correlates of Emotion Regulation and Adolescent Suicidal Ideation. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging 3:125–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pan L, Hassel S, Segreti A, Nau S, Brent D, Phillips M 2013. Differential patterns of activity and functional connectivity in emotion processing neural circuitry to angry and happy faces in adolescents with and without suicide attempt. Psychological medicine 43:2129–42. [DOI] [PubMed] [Google Scholar]
- 24.Quevedo K, Ng R, Scott H, Martin J, Smyda G, Keener M, et al. 2016. The neurobiology of self-face recognition in depressed adolescents with low or high suicidality. Journal of abnormal psychology 125:1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Groschwitz RC, Plener PL, Groen G, Bonenberger M, Abler B 2016. Differential neural processing of social exclusion in adolescents with non-suicidal self-injury: An fMRI study. Psychiatry Research: Neuroimaging 255:43–9. [DOI] [PubMed] [Google Scholar]
- 26.Brown RC, Plener PL, Groen G, Neff D, Bonenberger M, Abler B 2017. Differential neural processing of social exclusion and inclusion in adolescents with non-suicidal self-injury and young adults with borderline personality disorder. Frontiers in psychiatry 8:267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olié E, Jollant F, Deverdun J, de Champfleur NM, Cyprien F, Le Bars E, et al. 2017. The experience of social exclusion in women with a history of suicidal acts: a neuroimaging study. Scientific reports 7:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu RT, Miller I 2014. Life events and suicidal ideation and behavior: a systematic review. Clinical psychology review 34:181–92. [DOI] [PubMed] [Google Scholar]
- 29.Heilbron N, Prinstein MJ 2010. Adolescent peer victimization, peer status, suicidal ideation, and nonsuicidal self-injury: Examining concurrent and longitudinal associations. Merrill-Palmer Quarterly 56:388–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giletta M, Calhoun CD, Hastings PD, Rudolph KD, Nock MK, Prinstein MJ 2014. Multilevel risk factors for suicidal ideation among at-risk adolescent females: the role of hypothalamic-pituitary-adrenal axis responses to stress. Journal of abnormal child psychology: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.King CA, Merchant CR 2008. Social and interpersonal factors relating to adolescent suicidality: A review of the literature. Archives of Suicide Research 12:181–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holt MK, Kaufman Kantor G, Finkelhor D 2008. Parent/child concordance about bullying involvement and family characteristics related to bullying and peer victimization. Journal of School Violence 8:42–63. [Google Scholar]
- 33.Oppenheimer CW, Ladouceur CD, Waller JM, Ryan ND, Allen KB, Sheeber L, et al. 2016. Emotion socialization in anxious youth: Parenting buffers emotional reactivity to peer negative events. Journal of abnormal child psychology 44:1267–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smyth JM, Stone AA 2003. Ecological momentary assessment research in behavioral medicine. Journal of Happiness studies 4:35–52. [Google Scholar]
- 35.Olino TM, Silk JS, Osterritter C, Forbes EE 2015. Social reward in youth at risk for depression: a preliminary investigation of subjective and neural differences. Journal of child and adolescent psychopharmacology 25:711–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Williams KD, Jarvis B 2006. Cyberball: A program for use in research on interpersonal ostracism and acceptance. Behavior research methods 38:174–80. [DOI] [PubMed] [Google Scholar]
- 37.Silk JS, Tan PZ, Ladouceur CD, Meller S, Siegle GJ, McMakin DL, et al. 2018. A randomized clinical trial comparing individual cognitive behavioral therapy and child-centered therapy for child anxiety disorders. Journal of Clinical Child & Adolescent Psychology 47:542–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Birmaher B, Brent DA, Chiappetta L, Bridge J, Monga S, Baugher M 1999. Psychometric properties of the Screen for Child Anxiety Related Emotional Disorders (SCARED): a replication study. Journal of the American Academy of Child & Adolescent Psychiatry 38:1230–6. [DOI] [PubMed] [Google Scholar]
- 39.Caporino NE, Sakolsky D, Brodman DM, McGuire JF, Piacentini J, Peris TS, et al. 2017. Establishing clinical cutoffs for response and remission on the Screen for Child Anxiety Related Emotional Disorders (SCARED). Journal of the American Academy of Child & Adolescent Psychiatry 56:696–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wechsler D 1999. Manual for the Wechsler abbreviated intelligence scale (WASI). San Antonio, TX: The Psychological Corporation. [Google Scholar]
- 41.Hammerton G, Zammit S, Potter R, Thapar A, Collishaw S 2014. Validation of a composite of suicide items from the Mood and Feelings Questionnaire (MFQ) in offspring of recurrently depressed parents. Psychiatry research 216:82–8. [DOI] [PubMed] [Google Scholar]
- 42.Poznanski EO, Mokros HB. Children’s depression rating scale, revised (CDRS-R): Western Psychological Services Los Angeles; 1996.
- 43.Mayes TL, Bernstein IH, Haley CL, Kennard BD, Emslie GJ 2010. Psychometric properties of the Children’s Depression Rating Scale-Revised in adolescents. Journal of child and adolescent psychopharmacology 20:513–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rigby K, Slee PT 1993. Dimensions of interpersonal relation among Australian children and implications for psychological well-being. The Journal of social psychology 133:33–42. [DOI] [PubMed] [Google Scholar]
- 45.Silk JS, Forbes EE, Whalen DJ, Jakubcak JL, Thompson WK, Ryan ND, et al. 2011. Daily emotional dynamics in depressed youth: A cell phone ecological momentary assessment study. Journal of experimental child psychology 110:241–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schneider W, Eschman A, Zuccolotto A. E-Prime: User’s guide: Psychology Software Incorporated; 2002.
- 47.Ashburner J, Barnes G, Chen C, Daunizeau J, Flandin G, Friston K, et al. 2014. SPM12 manual. Wellcome Trust Centre for Neuroimaging, London, UK. [Google Scholar]
- 48.Whitfield-Gabrieli S, Nieto-Castanon A, Ghosh S 2011. Artifact detection tools (ART). Camb, Ma Release Version 7:11. [Google Scholar]
- 49.Mazaika PK, Hoeft F, Glover GH, Reiss AL 2009. Methods and software for fMRI analysis of clinical subjects. Neuroimage 47:S58. [Google Scholar]
- 50.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. 2002. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15:273–89. [DOI] [PubMed] [Google Scholar]
- 51.Brett M, Anton J-L, Valabregue R, Poline J-B, editors. Region of interest analysis using an SPM toolbox. 8th international conference on functional mapping of the human brain; 2002: Sendai. [Google Scholar]
- 52.Bolin JH 2014 Hayes, Andrew F. (2013). Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression- Based Approach. New York, NY: The Guilford Press. Journal of Educational Measurement 51:335–7. [Google Scholar]
- 53.Critchley HD, Wiens S, Rotshtein P, Öhman A, Dolan RJ 2004. Neural systems supporting interoceptive awareness. Nature neuroscience 7:189. [DOI] [PubMed] [Google Scholar]
- 54.Wiech K, Lin C-s, Brodersen KH, Bingel U, Ploner M, Tracey I 2010. Anterior insula integrates information about salience into perceptual decisions about pain. Journal of Neuroscience 30:16324–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.MacDonald G, Leary MR 2005. Why does social exclusion hurt? The relationship between social and physical pain. Psychological bulletin 131:202. [DOI] [PubMed] [Google Scholar]
- 56.Hooley JM, Franklin JC, Nock MK 2014. Chronic pain and suicide: understanding the association. Current pain and headache reports 18:1–6. [DOI] [PubMed] [Google Scholar]
- 57.Li D, Zucker NL, Kragel PA, Covington VE, LaBar KS 2017. Adolescent development of insula- dependent interoceptive regulation. Developmental science 20:e12438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Giletta M, Prinstein MJ, Abela JR, Gibb BE, Barrocas AL, Hankin BL 2015. Trajectories of suicide ideation and nonsuicidal self-injury among adolescents in mainland China: Peer predictors, joint development, and risk for suicide attempts. Journal of consulting and clinical psychology 83:265. [DOI] [PubMed] [Google Scholar]
- 59.Craig AD, Craig A 2009. How do you feel—now? The anterior insula and human awareness. Nature reviews neuroscience 10. [DOI] [PubMed] [Google Scholar]
- 60.Paulus MP, Stein MB 2006. An insular view of anxiety. Biological psychiatry 60:383–7. [DOI] [PubMed] [Google Scholar]
- 61.Emmert K, Breimhorst M, Bauermann T, Birklein F, Van De Ville D, Haller S 2014. Comparison of anterior cingulate vs. insular cortex as targets for real-time fMRI regulation during pain stimulation. Frontiers in behavioral neuroscience 8:350. [DOI] [PMC free article] [PubMed] [Google Scholar]