Abstract
Purpose:
Elevated synovial fluid concentrations of pro-inflammatory cytokines, degradative enzymes, and cartilage breakdown markers at the time of anterior cruciate ligament (ACL) reconstruction are associated with worse postoperative patient-reported outcomes and cartilage quality. However, it remains unclear if this is due to a more robust or dysregulated inflammatory response or is a function of a more severe injury. The objective of this study was to evaluate the association of the molecular composition of the synovial fluid, patient demographics and injury characteristics to cartilage degradation after acute ACL injury.
Methods:
We performed a cluster analysis of synovial fluid (SF) concentrations of pro- and anti-inflammatory cytokines, and biomarkers of cartilage degradation, bony remodeling, and hemarthrosis. We evaluated the association of biomarker clusters with patient demographics, days between injury, VAS pain, SF aspirate volumes and bone bruise volumes measured on MRI.
Results:
Two clusters were identified from the 35 patients included in this analysis; Dysregulated Inflammation and Low Inflammation. The Dysregulated Inflammation cluster consisted of 10 patients and demonstrated significantly greater concentrations of biomarkers of cartilage degradation (p<0.05) as well as a lower ratio of anti-inflammatory to pro-inflammatory cytokines (p=0.053) when compared to the Low Inflammation cluster. Patient demographics, bone bruise volumes, SF aspirate volumes, pain, and concomitant injuries did not differ between clusters.
Conclusion:
A subset of patients exhibited dysregulation of the inflammatory response following acute ACL injury which may increase the risk of posttraumatic osteoarthritis. This response does not appear to be a function of injury severity.
Keywords: knee, inflammation, cytokine, cartilage, biomarker
Introduction
Following traumatic injury, increased cytokine burden and a dysregulated inflammatory response are associated with severe complications such as multiple organ dysfunction, wound complications, and longer intensive care and hospital stays.(1–3) As a consequence, some in the orthopaedic trauma research community have investigated individual patient’s immunologic response, damage-associated molecular patterns (DAMPs) and injury characteristics. Identifying “Precision Injury Signatures” may not only determine if there is an association with short-term complications for polytraumatized patients (ClinicalTrials.gov Identifier: ), but also if these injury signatures may influence diagnosis and treatment in the future.(3, 4)
While life-threatening complications are extremely rare after acute anterior cruciate ligament (ACL) injury, approximately 50% of patients develop posttraumatic osteoarthritis (PTOA) within 15 years after injury.(5–7) ACL injury initiates an innate immunologic response resulting in a cascade of cytokine and catabolic enzyme activity that is associated with increased biomarkers of cartilage turnover.(8–12) Specifically, after acute ACL injury significant increases in synovial fluid concentrations of pro-inflammatory cytokines interleukin-1α (IL-1α) and IL-1β, catabolic enzyme matrix metalloproteinase-3 (MMP-3), and biomarkers of cartilage breakdown (C-terminal crosslinked telopeptide of type II collagen (CTXII), Cartilage oligomeric matrix protein (COMP)), and bone turnover (C-terminal crosslinked telopeptide of type I collagen (CTXI), N-terminal crosslinked telopeptide of type I collagen (NTXI)) have been reported.(9, 11, 13) While inflammatory and chondrodegenerative biomarkers are elevated after ACL injury and reconstruction,(10, 11, 14) it remains unknown why roughly half of ACL-injured patients demonstrate an early onset of PTOA whereas the other half do not.
Elevated pro-inflammatory cytokine, degradative enzyme, and cartilage breakdown marker concentrations in the synovial fluid at the time of ACL reconstruction have been associated both with worse patient-reported outcomes and cartilage quality 2 to 3 years after surgery.(15, 16) Increased synovial fluid concentrations of IL-1α and MMP-9 at the time of ACL reconstruction have been associated with worse patient reported outcomes at a minimum of two years after surgery.(16) In addition, increased synovial fluid concentrations of a sulfated glycosaminoglycan (sGAG), a biomarker of cartilage degradation, at the time of surgery have been associated with inferior cartilage quality (i.e. increased T1 and T2 wavelengths on MRI) three years after surgery.(15) However, it remains unclear if elevated cytokine expression and increased synovial fluid concentrations of cartilage breakdown markers on the day of surgery in a subset of patients are related to a greater, more persistent inflammatory response or whether these concentrations are the result of a more severe initial injury. For example, results from a recent animal study suggest that the magnitude and duration of bone marrow edema following ACL injury may be related to the forces borne by the joint at the time of injury.(17) These knowledge gaps provide the strong rationale for assessing synovial fluid profiles early after injury as well as factors associated with the severity of injury.
We hypothesized that information from more acute analyses that incorporate features of the immunologic response and injury characteristics will differentiate patient subsets and provide information that could identify patients that may require anti-inflammatory treatment to augment the standard of care. Therefore, the purposes of this exploratory study were to determine if 1) different inflammatory phenotypes can be determined from the molecular composition of the synovial fluid within 8 days of ACL injury, and 2) patient demographic or injury characteristics differ between inflammatory phenotypes.
Methods
This study is a secondary analysis of 41 ACL-injured subjects enrolled at one site as part of a multicenter randomized trial (ClinicalTrials.gov ID: ).(10, 11) All patients provided informed consent prior to participating in this IRB-approved trial. The original study was powered to assess the effect of an intra-articular corticosteroid injection versus saline prior to ACL reconstruction. The current analyses involve only baseline data collected prior to any treatment being administered. As such, an a priori power analysis was not performed for this exploratory secondary analysis.
Patients
The study included skeletally mature patients between the ages of 14 and 32 that sustained an acute ACL rupture during sports participation. Patients were excluded if they had previous ligamentous or meniscus injury to contralateral knee, a history of previous traumatic ipsilateral knee injury and/or surgery, or clinical evidence of posterior cruciate ligament injury or more than grade 1 medial or lateral collateral ligament injury. Patients were also excluded if the injury occurred more than 8 days prior to enrollment. Because the original study assessed the effect of a corticosteroid injection, patients were also excluded if they had a known allergy to triamcinolone acetonide, intra-articular cortisone injection into either knee within 3 months of injury, or a history of any inflammatory disease or immunocompromise.(11) Patients were not excluded on the basis of biological sex, race, or concomitant meniscus or articular cartilage injury. Patient demographics were recorded at the baseline visit and pain was assessed on the day of enrollment using as 10 cm Visual Analogue Scale (VAS).
Severity of Injury
The severity of injury was assessed in three ways: 1) concomitant injuries to the menisci or articular cartilage, 2) the volume of synovial fluid aspirated from the knee at the baseline visit, and 3) the volume of the bone bruises on each patient’s preoperative MRI scan. Concomitant injuries to the menisci and articular cartilage were confirmed arthroscopically at the time of surgery. Knee aspiration was performed aseptically within 8 days of injury, and intraarticular placement was confirmed using the “squish” test.(18) The knee was aspirated to dryness and the volume of synovial fluid that was aspirated was recorded. The volume of preoperative bone bruises was calculated from each patient’s preoperative magnetic resonance imaging (MRI) scan.(19) Only patients with MRIs within slice thicknesses < 5 mm were included provided T2 or proton density (PD) sequences in the coronal, axial, and sagittal plane were available. Bone bruise volumes were measured from the T2 or PD weighted coronal images using a modified version of Roemer and Bohndorf’s technique.(19, 20) Bone bruise volumes from each of the four bony regions (medial tibial plateau, medial femoral condyle, lateral tibial plateau, and lateral femoral condyle) were then summed and expressed as the total bone bruise volume (mm3).(19)
Biomarker Analyses
Synovial fluid samples were spun for 10 minutes at 3500 RPM and the supernatant aliquoted and stored at −80° C until analyzed. At the conclusion of the study, samples were shipped to the Duke University Biomarker Shared Resource laboratory for analysis (VBK/JLH).(11) Synovial fluid concentrations of cytokines and biomarkers of cartilage degradation, bone turnover, and hemarthrosis were assessed using previously described methods (Table 1).(10, 11, 15)
Table 1.
| Biomarker* | Abbreviation | Volume (Dilution) | CV† | LLOD‡ | Number (%) below LLOD |
|---|---|---|---|---|---|
| Pro-inflammatory | |||||
| Interleukin-1α (pg/mL) Catalog # K151RBD, Meso Scale Discovery, Rockville, MD |
IL-1α | 25 μl (1:2) |
9.9% | 0.238 pg/ml | 24 (69%) |
| Interleukin-1β (pg/mL) Catalog #K151QPD, Meso Scale Discovery, Rockville, MD |
IL-1β | 25 μl (1:2) |
5.7% | 0.033 pg/ml | 16 (46%) |
| Anti-inflammatory | |||||
| Interleukin-1 receptor antagonist (pg/mL) Catalog # DRA00B, R&D Systems, Minneapolis, MN |
IL-1RA | 100 μl (None) |
4.4% | 6.3 pg/ml | 0 (0%) |
| Cartilage degradation | |||||
| Sulfated glycosaminoglycan (μg/mL) Catalog # BP-004, Kamiya Biomedical Company, Seattle, WA |
sGAG | 25 μl (None) |
1.8% | N/A | 0 (0%) |
| C-terminal crosslinked telopeptide of type II collagen (ng/mL) Cartilaps®, Catalog # AC-10F1, Immunodiagnostic Systems, Inc, Fountain Hills, AZ |
CTX-II | 40 μl (None) |
4.3% | 0.20 μg/L | 0 (0%) |
| Cartilage oligomeric matrix protein (μg/mL) Catalog #RD194080200, BioVendor, Asheville, NC |
COMP | 5 μl (1:2500) |
1.9% | 0.4 ng/ml | 0 (0%) |
| Matrix metalloproteinase 3 plex (MMP-1, MMP-3, MMP-9) (pg/mL) Catalog # K15043C, Meso Scale Discovery, Rockville, MD |
MMP-1 | 5 μl (1:20) |
3.5% | 1.74 pg/ml | 0 (0%) |
| MMP-3 | 4.5% | 4.34 pg/ml | 0 (0%) | ||
| MMP-9 | 3.5% | 14.3 pg/ml | 0 (0%) | ||
| Bone Turnover | |||||
| N-terminal crosslinked telopeptide of type I collagen (nM BCE) Catalog # 9021, Osteomark ® NTx; Alere, Scarborough, ME |
NTX-I | 25 μl (1:5) |
2.3% | N/A | 0 (0%) |
| Hemarthrosis | |||||
| Bilirubin + biliverdin (μmol/L) In-house ELISA assay developed by Kraus Laboratory |
B+B | 30 μl (None) |
1.3% | 1.0 μmol/L | 0 (0%) |
CV = Mean inter-assay coefficient of variation
LLOD = Lower limits of detection
Cytokines
Proteomic analysis has demonstrated that the cytokine-cytokine receptor pathway is significantly upregulated following acute ACL injury,(13) and pro-inflammatory cytokines IL-1α and IL-1β and anti-inflammatory IL-1 receptor antagonist (IL-1RA) are pivotal in the pathological processes leading to joint tissue breakdown.(21, 22) After ACL injury, patients with elevated synovial fluid IL-1 levels have also demonstrated more severe chondral damage.(23) IL-1α has previously been demonstrated to be predictive of patient-reported outcomes after ACL reconstruction,(16) and increased expression of IL-1β has been associated with more rapid cartilage degradation amongst osteoarthritis patients.(24) On the contrary, concentrations of the chondroprotective IL-1RA significantly decrease after ACL injury resulting in relatively unopposed activity of IL-1.(25) As such, lower IL-1RA has been associated with more severe chondral damage.(23)
For IL-1α and IL-1β values that were below the lower limit of detection (LLOD), 0.5 LLOD imputed values were used for statistical analyses. We also calculated a ratio of IL-1RA to IL-1β to assess the balance of pro- versus anti-inflammatory cytokines. Lower ratios are indicative of a greater imbalance between anti-inflammatory IL-1RA relative to concentration of pro-inflammatory cytokines.
Degradative Enzymes
We have demonstrated that pro-inflammatory stimulation of meniscus cells increases matrix metalloproteinase (MMP) and cytokine activity,(26) and the combination of pro-inflammatory cytokines and compressive loading like what may be seen during sporting and high demand activities further results in degradative enzyme activity and increased production of pro-inflammatory mediators.(27) Similarly after ACL injury, Amano et al. reported significant correlations between MMP-1 and MMP-3 with pro-inflammatory cytokine concentrations in the synovial fluid.(15) In addition, we have previously reported that synovial fluid MMP-9 on the day of ACL reconstruction was significantly greater for those with inferior patient-reported outcomes at minimum 2 year follow-up.(16)
Biomarkers of Cartilage Degradation
Three biomarkers of cartilage degradation were included in this study: sulfated glycosaminoglycan (sGAG), C-terminal crosslinked telopeptide type II collagen (CTXII), and cartilage oligomeric matrix protein (COMP). Due to both the mechanical insult of injury and enzymatic degradation, Glycosaminoglycans (GAGs) content increases immediately after injury.(28) In addition, increased synovial fluid concentrations of sulfated GAG (sGAG) at the time of ACL reconstruction were predictive of worse matrix composition on MRI at 3 years.(15) Following cartilage degradation, CTXII is released into the synovial fluid. CTXII has been identified as a biomarker for the diagnosis, staging, and evaluating the prognosis of hip and knee OA,(29, 30) and has also been demonstrated to be responsive over short testing periods (3 months).(31) We have reported that CTXII correlates with the degree of joint destruction and increases significantly within 1 month after ACL injury.(10, 11) COMP is also a marker of cartilage breakdown. Increased COMP has been predictive of joint space narrowing and osteophyte formation in those with idiopathic knee OA.(32) In addition, ACL-injured patients have also demonstrated significantly increased COMP when compared to healthy volunteers.(33)
Biomarker of Bone Turnover
NTXI is a marker of bone remodeling, and has been shown to be predictive of cartilage thinning for those with idiopathic OA.(34) In addition, NTXI has been demonstrated to increase following ACL injury.(9)
Marker of Hemarthrosis
Bilirubin/biliverdin concentration has been used as a biomarker of hemarthrosis and has been shown to be significantly elevated following lower extremity trauma.(35) Bilirubin/biliverdin has also been previously used to quantify hemarthrosis after ACL injury.(15)
Statistical Analyses
The statistical methods utilized in the current study replicate those previously used by Amano et al.(15) Biomarker data were not normally distributed, and consistent with Amano et al., were transformed using Box-Cox transformation.(15) A 2-cluster analysis was selected to both be consistent with Amano et al. but also because of the hypothesis that there may be 2 underlying clusters of patients based on the 50% prevalence of PTOA within 15 years after ACL injury.(5–7, 15)
After performing the cluster analysis, patients were then grouped by cluster assignment. Patient age, BMI, days between injury and baseline VAS pain, synovial fluid aspirate volume and bone bruise volumes were compared between clusters using two-tailed independent t-tests. Sex and the prevalence of concomitant meniscal and/or articular cartilage injuries were compared between clusters using chi-square or Fisher Exact tests as appropriate. Biomarker concentrations were compared between cluster using two-tailed independent t-tests. All analyses were performed using SPSS Statistics 24 (IBM, Armonk, NY) and statistical significance was determined with p ≤ 0.05.
Results
Of the 41 patients originally enrolled at our site, complete biomarker, imaging, and surgical data were available for 35 patients (85.4%); the MRIs of 6 patients did not meet our standards for analysis. Two clusters were identified (Table 2). The variables that contributed the most to cluster assignment were MMP-1 (ANOVA F = 121.4, p < 0.001), MMP-3 (F = 13.6, p = 0.001), IL-1RA (F = 11.0, p = 0.002), sGAG (F = 5.1, p = 0.03), and IL-1β (F = 4.7, p = 0.04).(Figure 1)
Table 2.
Comparison of synovial fluid biomarker concentrations between the 2 clusters. Values are presented as mean ± standard deviation or number (%)*
| Dysregulated Inflammation Cluster | Low Inflammation Cluster | p† | |
|---|---|---|---|
| Cytokine Concentrations | |||
| IL-1α (pg/mL) | 0.92 ± 2.11 | 0.32 ± 0.47 | 0.39 |
| # < LLOD | 5/10 (50%) | 19/25 (76%) | 0.23 |
| IL-1β (pg/mL) | 1.30 ± 2.63 | 0.17 ± 0.25 | 0.21 |
| # < LLOD | 2/10 (20%) | 13/25 (52%) | 0.13 |
| IL-1RA (pg/mL) | 15,988 ± 14,012 | 4,653 ± 6,420 | 0.03 |
| IL-1RA:IL-1β ratio | 49,814 ± 40,816 | 126,632 ± 178,787 | 0.053 |
| Cartilage Degradation | |||
| CTX-II (ng/mL) | 0.74 ± 0.38 | 0.84 ± 0.44 | 0.54 |
| COMP (μg/mL) | 52.0 ± 11.6 | 52.0 ± 12.3 | > 0.99 |
| sGAG (μg/mL) | 441.3 ± 149.5 | 323.1 ± 135.9 | 0.03 |
| MMP-1 (pg/mL) | 1,057,992 ± 285,749 | 178,556 ± 178,658 | < 0.001 |
| MMP-3 (pg/mL) | 2,551,651 ± 2,154,629 | 927,773 ± 410,839 | 0.04 |
| MMP-9 (pg/mL) | 13,185 ± 7,792 | 40,095 ± 46,222 | 0.009 |
| Bone Turnover | |||
| NTX-I (μg/mL) | 19.7 ± 9.8 | 21.2 ± 6.3 | 0.68 |
| Hemarthrosis | |||
| Bilirubin+Biliverdin (μmol/L) | 32.1 ± 14.1 | 31.0 ± 26.1 | 0.91 |
Bold, italics denotes statistically significant differences between patient clusters
Raw non-transformed data presented in this table; however, transformed data were used for statistical analyses
Figure 1.
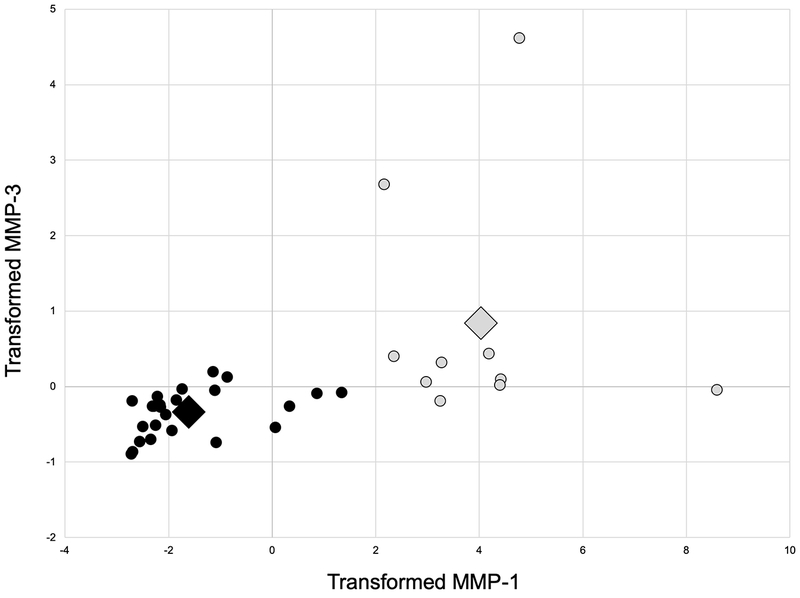
Scatterplot of transformed matrix metalloproteinase-1 (MMP-1) and MMP-3 of the two clusters (Grey = Dysregulated Inflammation Cluster, Black = Low Inflammation Cluster; Large diamond represents the centroid for each cluster).
One cluster consisting of 10 patients demonstrated significantly greater MMP-1, MMP-3, sGAG, and anti-inflammatory IL-1RA, and significantly lower MMP-9. While IL-1RA was increased for Cluster 1, the ratio of IL-1RA to IL-1β tended to be lower when compared to the other cluster of 25 patients (p = 0.053, Table 2). Because of the imbalance of pro- versus anti-inflammatory cytokines, we termed this the Dysregulated Inflammation cluster to be consistent with previous studies of polytrauma patients that used similar terminology (Immune Dysregulation).(2, 3) Patient demographics, bone bruise volumes, synovial fluid aspirate volumes, pain, and concomitant injuries did not differ between the Dysregulated Inflammation and Low Inflammation clusters (Table 3).
Table 3.
Comparison of patient and injury characteristics between the 2 clusters.*
| Dysregulated Inflammation Cluster | Low Inflammation Cluster | p | |
|---|---|---|---|
| Demographics | |||
| Age (y) | 21.4 ± 5.1 | 18.7 ± 3.9 | 0.15 |
| Sex (F/M; %F) | 5/5 (50%) | 10/15 (40%) | 0.71 |
| BMI (kg/m2) | 25.3 ± 4.1 | 23.4 ± 2.6 | 0.18 |
| Injury Characteristics | |||
| Days after injury | 5.6 ± 1.3 | 3.8 ± 2.2 | 0.02 |
| Aspirate volume (cc) | 34.2 ± 25.8 | 22.0 ± 16.4 | 0.10 |
| Bone bruise volume (mm3) | 14.6 ± 19.6 | 8.3 ± 7.7 | 0.18 |
| VAS Pain | 3.4 ± 3.1 | 5.5 ± 2.9 | 0.08 |
| Medial meniscus tear | 5/10 (50%) | 20/25 (80%) | 0.11 |
| Lateral meniscus tear | 7/10 (70%) | 16/25 (64%) | > 0.99 |
| Articular cartilage lesion | 1/10 (10%) | 0/25 (0%) | 0.29 |
Bold, italics denotes statistically significant differences between patient clusters
Discussion
The purposes of this study were to determine if 1) inflammatory phenotypes can be determined from the molecular composition of the synovial fluid within 8 days of ACL injury, and 2) if patient demographic or injury characteristics differ between inflammatory phenotypes. Our hypothesis of different patient subsets based on synovial fluid profiling was supported by these results. The most important finding of this study suggests that a subset of patients inherently exhibit a dysregulated inflammatory response after acute injury that is not a function of injury severity. The current results are similar to a cluster analyses by Namas et al. performed during the first 5 days following blunt polytrauma that identified 2 patient clusters differing significantly on the basis of multiple inflammatory mediators.(2) Just as in our study, the Namas et al. clusters did not differ in terms of patient demographics or injury severity. Moreover, the proportion of patients within their early immune dysregulation cluster (9/33 patients, 27.3%)(2) was very similar to the proportion of patients in the Dysregulated Inflammation cluster in the current study (10/35, 28.5%). Collectively, these studies suggest there may be a subset of patients predisposed to a dysregulated inflammatory response after acute injury.
The current results differ somewhat from a previous cluster analysis performed with synovial fluid samples collected on the day of ACL reconstruction (about 6 weeks after injury).(15) In this study, they too identified a similar proportion of patients (10/26, 38.5%) that fit within an inflammatory cluster with elevated pro-inflammatory cytokine concentrations, MMP-1, and MMP-3.(15) However, the inflammatory cluster demonstrated significantly lower sGAG concentrations at the time of surgery unlike the Dysregulated Inflammation cluster in our study. Our Dysregulated Inflammation cluster demonstrated significantly increased sGAG approximately 4 days post injury. The reason for this difference is unclear but may be related to the time between injury and sample collection (mean 4 versus 64 days after injury); the two studies assessed different periods in the response to injury. Patient age also differed between studies (mean 19.5 versus 34.0 years). Patterns of cytokines and chemokines differ greatly between elderly trauma patients over the age of 65 when compared to patients under 30 years of age.(36) Patient age differed by only 15 years between the current study and Amano et al.; it is not known the degree to which this amount of age difference may have impacted the synovial fluid immune profiles.
While the results of the current study differ slightly from the previous cluster analysis by Amano et al.,(15) it is becoming increasingly clear that there is a subset of patients with dysregulated inflammatory responses after acute injury. Not only is there a more robust pro-inflammatory response, the corresponding innate anti-inflammatory response is muted in this subset of patients. More than 20 years ago, Dr. Scott Dye described a theory of the knee being “a biological [car] transmission with an envelope of function.”(37) He suggested that the knee is an organ that is not only effected by the treatment provided but also by the complex interplay between anatomic, physiologic, and biomechanical factors. Specific to the current topic, he theorized that there may be phenotypical variations in the response to injury and perhaps genetic predisposition to dysregulation of molecular and/or cellular homeostasis.(37) The response to acute injury, whether polytrauma, ACL injury, or meniscus injury, involves a complex dynamic interaction of multiple pathways including the NF-κB, cytokine-cytokine receptor interaction, and osteoclast differentiation pathways, among others.(13, 38–40) Similar pathways are also implicated in the development and progression of knee osteoarthritis.(41–43) Because of the complex nature of this individualized response, a better understanding of the dynamic interplay between multiple pathways may be necessary in order to improve both short- and long-outcomes in the future.(2, 3)
It was intriguing to find that the proportion of patients demonstrating dysregulated inflammatory responses after trauma or ACL injury ranged from 27% to 38%. This suggests that the biologic response to injury does not fully explain the early onset of PTOA after ACL injury as 50% of patients develop PTOA within 15 years of injury. This leaves a notable proportion of patients that do not exhibit dysregulated inflammatory responses but still progress to PTOA. There are several additional factors that may contribute to progression to PTOA. Mechanical loading may influence cartilage degradation, and OA patients with a greater cartilage turnover in response to light exercise also demonstrate significantly greater cartilage thinning over the ensuing two years.(44) Altered joint mechanics secondary to concomitant injuries treated with chondroplasty or partial meniscectomy can also accelerate the development of OA.(45) The chronic pro-inflammatory effects of altered chondral and meniscal forces were not assessed in this study. In addition, the combination of increased body mass and decreased activity seen in the years after ACL reconstruction(46) may contribute to the progression to PTOA even in those that do not exhibit inflammatory dysregulation immediately after injury. There is also the possibility of reinjury that could account, at least in part, for the “missing” additional incident 25% PTOA. The progression to PTOA is undoubtedly multifactorial, further justifying the need to assess personalized signatures after injury in order to alter the path of this complex condition.
This study was not without limitation. First, it represents pilot work to assess the synovial fluid profiles of 35 patients immediately following ACL injury; the results should be considered discovery based for future hypothesis generation. There may the potential for additional clusters or patient phenotypes that could be identified with a larger sample size. Second, our study was focused on IL-1 whereas others have suggested assessing a wider spectrum of both pro- and anti-inflammatory cytokines.(2, 4, 15) Additionally, even at a mean of 4 days post injury, a large number of samples had IL-1α and IL-1β values below the lower limits of detection, especially in the Low Inflammation cluster. Finally, we employed very narrow inclusion/exclusion criteria, and additional studies will be necessary to determine if these results are generalizable to older age groups and those with multiple ligament injuries or other acute knee injuries.
In conclusion, increased cartilage breakdown and degradative enzyme activity after ACL injury may be related to a dysregulated inflammatory response demonstrated by a subset of patients and do not appear to be a function of injury severity. We identified a cluster of patients with a dysfunctional inflammatory response that also demonstrated significantly greater concentrations of biomarkers previously associated with PTOA progression, namely sGAG, MMP-1, and MMP-3.(15, 47) Future studies are necessary to determine if 1) early anti-inflammatory treatment may alter the cycle of persistent inflammation and cartilage degradation in this subset of patients, and 2) the timing of surgery should be earlier to avoid the “second-hit” of inflammation associated with surgery or delayed until after the initial inflammatory response has subsided.
Acknowledgements
This study received funding from The Arthritis Foundation of America. Dr. Lattermann was supported by a K-23 career development award from the NIH-NIAMS (5K23AR060275). Data collection and study administration was supported by the University of Kentucky CTSA award (UL1TR000117). This study was conducted at the University of Kentucky and all analyses of biological specimens were performed at Duke University. Results of the present study do not constitute endorsement by the American College of Sports Medicine, and the authors declare no conflicts of interest. The results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation.
References
- 1.Levy RM, Mollen KP, Prince JM, Kaczorowski DJ, Vallabhaneni R, Liu S, et al. Systemic inflammation and remote organ injury following trauma require HMGB1. Am J Physiol Regul Integr Comp Physiol. 2007;293(4):R1538–R44. [DOI] [PubMed] [Google Scholar]
- 2.Namas RA, Almahmoud K, Mi Q, Ghuma A, Namas R, Zaaqoq A, et al. Individual-specific principal component analysis of circulating inflammatory mediators predicts early organ dysfunction in trauma patients. J Crit Care. 2016;36:146–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McKinley TO, Gaski GE, Vodovotz Y, Corona BT, Billiar TR. Diagnosis and management of polytraumatized patients with severe extremity trauma. J Orthop Trauma. 2018;32(Suppl 3):S1–S6. [DOI] [PubMed] [Google Scholar]
- 4.Lamparello AJ, Namas RA, Constantine G, McKinley TO, Elster E, Vodovotz Y, et al. A conceptual time window-based model for early stratification of trauma patients. J Intern Med. 2019;286(1):2–15. [DOI] [PubMed] [Google Scholar]
- 5.Lohmander LS, Roos H. Knee ligament injury, surgery, and osteoarthritis. Truth or consequences? Acta Orthop. 1994;65(6):605–9. [DOI] [PubMed] [Google Scholar]
- 6.Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35(10):1756–69. [DOI] [PubMed] [Google Scholar]
- 7.Oiestad BE, Engebretsen L, Storheim K, Risberg MA. Knee osteoarthritis after anterior cruciate ligament injury: a systematic review. Am J Sports Med. 2009;37(7):1434–43. [DOI] [PubMed] [Google Scholar]
- 8.Cameron M, Buchgraber A, Passler H, Vogt M, Thonar E, Fu FH, et al. The natural history of the anterior cruciate ligament-deficient knee. Am J Sports Med. 1997;25(6):751–4. [DOI] [PubMed] [Google Scholar]
- 9.Catterall JB, Stabler TV, Flannery CR, Kraus VB. Changes in serum and synovial fluid biomarkers after acute injury (NCT00332254). Arthritis Res Ther. 2010;12(6):R229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lattermann C, Jacobs CA, Proffitt Bunnell M, Jochimsen KN, Abt JP, Reinke EK, et al. Logistical challenges and design considerations for studies using acute anterior cruciate ligament injury as a potential model for early posttraumatic arthritis. J Orthop Res. 2017;35(5):641–50. [DOI] [PubMed] [Google Scholar]
- 11.Lattermann C, Jacobs CA, Proffitt Bunnell M, Huston LJ, Gammon LG, Johnson DL, et al. A multicenter study of early anti-inflammatory treatment in patients with acute ACL tear. Am J Sports Med. 2017;45(2):325–33. [DOI] [PubMed] [Google Scholar]
- 12.Svoboda SJ, Harvey TM, Owens BD, Brechue WF, Tarwater PM, Cameron KL. Changes in serum biomarkers of cartilage turnover after anterior cruciate ligament injury. Am J Sports Med. 2013;41(9):2108–16. [DOI] [PubMed] [Google Scholar]
- 13.King JD, Rowland G, Villasante Tezanos AG, Warwick J, Kraus VB, Lattermann C, et al. Joint fluid proteome after anterior cruciate ligament rupture reflects an acute inflammatory and chondrodegenerative state. Cartilage. 2018;Epub ahead of print(DOI: 10.1177/1947603518790009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larsson S, Struglics A, Lohmander LS, Frobell R. Surgical reconstruction of ruptured anterior cruciate ligament prolongs trauma-induced increase of inflammatory cytokines in synovial fluid: an exploratory analysis in the KANON trial. Osteoarthritis Cartilage. 2017;25(9):1443–51. [DOI] [PubMed] [Google Scholar]
- 15.Amano K, Huebner JL, Stabler TV, Tanaka M, McCulloch CE, Lobach I, et al. Synovial fluid profile at the time of anterior cruciate ligament reconstruction and its association with cartilage matrix composition 3 years after surgery. Am J Sports Med. 2018;46(4):890–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lattermann C, Conley CE- W, Johnson DJ, Reinke EK, Huston LJ, Huebner JL, et al. Select biomarkers on the day of anterior cruciate ligament reconstruction predict poor patient-reported outcomes at 2-year follow-up: a pilot study. BioMed Research International. 2018;Article ID 9387809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pedersen DR, El-Khoury GY, Thedens DR, Saad-Eldine M, Phisitkul P, Amendola A. Bone contusion progression from traumatic knee injury: association of rate of contusion resolution with injury severity. Open Access J Sports Med. 2017;8:9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glattes RC, Spindler KP, Blanchard GM, Rohmiller MT, McCarty EC, Block J. A simple, accurate method to confirm placement of intra-articular knee injection. Am J Sports Med. 2004;32(4):1029–31. [DOI] [PubMed] [Google Scholar]
- 19.Lattermann C, Jacobs CA, Reinke EK, Scaramuzza EA, Huston LJ, Dunn WR, et al. Are bone bruise characteristics and articular cartilage pathology associated with inferior outcomes two and six years after anterior cruciate ligament reconstruction? Cartilage. 2017;8(2):139–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roemer FW, Bohndorf K. Long-term osseous sequelae after acute trauma of the knee joint evaluated by MRI. Skeletal Radiol. 2002;31:615–23. [DOI] [PubMed] [Google Scholar]
- 21.Jacques C, Gosset M, Berenbaum F, Gabay C. The role of IL-1 and IL-1Ra in joint inflammation and cartilage degradation. Vitam Horm. 2006;74:371–403. [DOI] [PubMed] [Google Scholar]
- 22.Lotz M Cytokines in cartilage injury and repair. Clin Orthop Relat Res. 2001(391 Suppl):S108–15. [DOI] [PubMed] [Google Scholar]
- 23.Marks PH, Donaldson ML. Inflammatory cytokine profiles associated with chondral damage in the anterior cruciate ligament-deficient knee. Arthroscopy. 2005;21(11):1342–7. [DOI] [PubMed] [Google Scholar]
- 24.Attur M, Belitskaya-Levy I, Oh C, Krasnokutsky S, Greenberg J, Samuels J, et al. Increased interleukin-1b gene expressin in peripheral blood leukocytes is associated with increased pain and predicts risk of progression of symptomatic knee osteoarthritis. Arthritis Rheum. 2011;63(7):1908–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cameron M, Buchgraber A, Passler H, Vogt M, Thonar E, Fu F, et al. The natural history of the anterior cruciate ligament-deficient knee. Changes in synovial fluid cytokine and keratan sulfate concentrations. American Journal of Sports Medicine. 1997;25(6):751–4. [DOI] [PubMed] [Google Scholar]
- 26.Stone AV, Loeser RF, Vanderman KS, Long DL, Clark SC, Ferguson CM. Pro-inflammatory stimulation of meniscus cells increases production of matrix metalloproteinases and additional catabolic factors involved in osteoarthritis pathogenesis. Osteoarthritis Cartilage. 2014;22(2):264–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cook AE, Stoker AM, Leary EV, Pfeiffer FM, Cook JL. Metabolic responses of meniscal explants to injury and inflammation ex vivo. J Orthop Res. 2018;36:2657–63. [DOI] [PubMed] [Google Scholar]
- 28.Waters NP, Stoker AM, Carson WL, Pfeiffer FM, Cook JL. Biomarkers affected by impact velocity and maximum strain of cartilage during injury. J Biomech. 2014;47(3185–3195). [DOI] [PubMed] [Google Scholar]
- 29.Nepple JJ, Thomason KM, An TW, Harris-Hayes M, Clohisy JC. What is the utility of biomarkers for assessing the pathophysiology of hip osteoarthrits? A systematic review. Clin Orthop Related Res. 2015;473:1683–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kraus VB, Collins JE, Hargrove D, Losina E, Nevitt M, Katz JN, et al. Predictive validity of biochemical biomarkers in knee osteoarthritis: data from the FNIH OA Biomarkers Consortium. Ann Rheum Dis. 2016;ePub ahead of print(doi: 10.1136/annrheumdis-2016-209252). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hunt MA, Pollock CL, Kraus VB, Saxne T, Peters S, Huebner JL, et al. Relationships amongst osteoarthritis biomarkers, dynamic knee joint load, and exercise: results from a randomized controlled pilot study. BMC Musculoskelet Disord. 2013;14:115. doi: 10.1186/1471-2474-14-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Golightly YM, Marshall SW, Kraus VB, Renner JB, Villaveces A, Casteel C, et al. Biomarkers of incident radiographic knee osteoarthritis: Do they vary by chronic knee symptoms? Arthritis Rheum. 2011;63(2276-2283). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palmieri-Smith RM, Wojtys EM, Potter HG. Early cartilage changes after anterior cruciate ligament injury: evaluation with imaging and serum biomarkers - a pilot study. Arthroscopy. 2016;32(1309-1318). [DOI] [PubMed] [Google Scholar]
- 34.Kraus VB, Collins JE, Hargrove D, Losina E, Nevitt M, Katz JN, et al. Predictive validity of biochemical biomarkers in knee osteoarthritis: data from the FNIH OA Biomarkers Consortium. Ann Rheum Dis. 2017;76(1):186–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adams SB, Setton LA, Bell RD, Easley ME, Huebner JL, Stabler T, et al. Inflammatory cytokines and matrix metalloproteinases in the synovial fluid after intra-articular ankle fracture. Foot Ankle Int. 2015;36(11):1264–71. [DOI] [PubMed] [Google Scholar]
- 36.Lamparello AJ, Namas RA, Abdul-Malak O, Vodovotz Y, Billiar TR. Young and aged blunt trauma patients display major differences in circulating inflammatory mediator profiles after severe injury. J Am Coll Surg. 2019;228(2):148–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dye SF. The knee as a biologic transmission with an envelope of function. A theory. Clin Orthop Relat Res. 1996;323:10–8. [DOI] [PubMed] [Google Scholar]
- 38.Pan W, Zhang AQ, Gu W, Gao JW, Du DY, Zhang LY, et al. Identification of haplotype tag single nucleotide polymorphisms within the nuclear factor-kB family genes and their clinical relevance in pateints with major trauma. Critical Care. 2015;19(95):DOI 10.1186/s13054-015-0836-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abdul-Malak O, Vodovotz Y, Zaaqoq A, Guardado J, Almahmoud K, Yin J, et al. Elevated admission base deficity is associated with a complex dynamic network of systemic inflammation which drives clinical trajectories in blunt trauma patients. Mediat Inflamm. 2016;Article ID 7950374: 10.1155/2016/7950374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brophy RH, Sandell LJ, Rai MF. Traumatic and degenerative meniscus tears have different gene expression signatures. Am J Sports Med. 2017;45(1):114–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saito T, Tanaka S. Molecular mechanisms underlying osteoarthritis devlepment: Notch and NF-kB. Arthritis Res Ther. 2017;19(94):DOI 10.1186/s13075-017-1296-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li B, Bai L, Shen P, Sun Y, Chen Z, Wen Y. Identification of differentially expressed microRNAs in knee anterior cruciate ligament tissues surgically removed from patients with osteoarthritis. Int J Mol Med. 2017;40(4):1105–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marcu KB, Otero M, Olivotto E, Borzi RM, Goldring MB. NF-kB signaling: multiple angles to target OA. Curr Drug Targets. 2010;11(5):599–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chu CR, Sheth S, Erhart-Hledik JC, Do B, Titchenal MR, Andriacchi TP. Mechanically stimulated biomarkers signal cartilage changes over 5 years consistent with disease progression in medial knee osteoarthritis patients. J Orthop Res. 2018;36:891–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jones MH, Spindler KP. Risk factors for radiographic joint space narrowing and patient reported outcomes of post-traumatic osteoarthritis after ACL reconstruction: data from the MOON cohort. J Orthop Res. 2017;35(7):1366–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spindler KP, Huston LJ, Chagin KM, KAttan MW, Reinke EK, Amendola A, et al. Ten-year outcomes and risk factors after anterior cruciate ligament reconstruction: a MOON longitudinal prospective cohort study. Am J Sports Med. 2018;46(4):815–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lohmander LS, Hoerrner LA, Lark MW. Metalloproteinases, tissue inihibitor, and proteoglycan fragments in knee synovial fluid in human osteoarthritis. Arthritis Rheum. 1993;36:181–9. [DOI] [PubMed] [Google Scholar]


