Abstract
In mammals, many classes of noncoding RNAs (ncRNAs) are expressed at a much higher level in the brain than in other organs. Recent studies have identified a new class of ncRNAs called circular RNAs (circRNAs), which are produced by back-splicing and fusion of either exons, introns, or both exon-intron into covalently closed loops. The circRNAs are also highly enriched in the brain and increase continuously from the embryonic to the adult stage. Although the functional significance and mechanism of action of circRNAs are still being actively explored, they are thought to regulate the transcription of their host genes and sequestration of miRNAs and RNA binding proteins. Some circRNAs are also shown to have translation potential to form peptides. The expression and abundance of circRNAs seem to be spatiotemporally maintained in a normal brain. Altered expression of circRNAs is also thought to mediate several disorders, including brain-tumor growth, and acute and chronic neurodegenerative disorders by affecting mechanisms such as angiogenesis, neuronal plasticity, autophagy, apoptosis, and inflammation. This review discusses the involvement of various circRNAs in brain development and CNS diseases. A better understanding of the circRNA function will help to develop novel therapeutic strategies to treat CNS complications.
Keywords: CircRNAs, miRNAs, Brain, Development, Cancer, Acute and chronic neurodegeneration
Graphical Abstract
1. Introduction
Mammalian genome is pervasively active and produces many classes of noncoding RNAs (ncRNAs) in addition to protein-coding mRNAs. Intriguingly, at any stage in time, the larger proportion of the transcriptional output (>90%) is made up of ncRNAs. Recent studies have shown that the ncRNAs are diverse in size, ranging from small RNAs like microRNAs (miRNAs) and piwi-interacting RNAs (piRNAs) which are <32 nucleotides in length, to large long noncoding RNAs (lncRNAs) which can be up to 5,000 nucleotides in length. The precise functions of many ncRNAs are still being discovered; several of them have been identified to control transcription and translation, and thus regulate various biological processes during growth, development, and disease progression (Chandran et al., 2017; Czech and Hannon, 2011; Dharap et al., 2009).
With well-organized regulatory checks and balances, the cellular system exists to prevent abnormalities in normal functions. For this purpose, miRNAs were considered as the guardians of the genome, but what regulate miRNAs is not known. Natural RNA circles called circular RNAs (circRNAs) were reported to function as miRNA sponges to effectively control their levels (Hansen et al., 2013). CircRNAs, which are an elusive class of ncRNAs, are formed by a back-splicing process as from the same set of precursor RNAs which forms protein-coding mRNAs by canonical splicing (Jeck and Sharpless, 2014; Jeck et al., 2013; Memczak et al., 2013; Salzman et al., 2012). As they are covalently closed continuous loops that lack defined 5’ caps and 3’ poly-A tails, making them resistant to RNase R, an enzyme with 3’-to-5’ exonuclease activity that effectively digests nearly all linear RNA species. Thus, due to the lack of free end, circRNAs are incredibly stable with a half-life of more than 48 hours compared to the corresponding linear RNAs (Jeck et al., 2013; Zeng et al., 2017). For several years, circRNAs were considered functionally irrelevant, cryptic viral RNAs, or storage forms of mRNAs; alternatively, they were thought to be merely splicing by-products with low abundance or transcriptional noise, due to splicing of longer mRNAs transcripts (Hsu and Coca-Prados, 1979; Sanger et al., 1976). However, with the advances in the next-generation sequencing (NGS) and circRNA specific bioinformatics analysis, they are recognized to be ubiquitously present in eukaryotic cells, plants, yeast, and viruses (Arnberg et al., 1980; Hansen et al., 2013; Hsu and Coca-Prados, 1979; Kos et al., 1986; Sanger et al., 1976).
When a protein-coding gene is transcribed under physiological conditions, the resulting precursor mRNA (pre-mRNA) undergoes canonical splicing during which the introns are cleaved, and the 3’ end of one exon is joined with the 5’ end of an adjacent exon to produce a mature mRNA. However, in some occasions, back-splicing of pre-mRNA can result in exon scrambling (the downstream donor end of the spliced product covalently binds to the upstream splice acceptor site) to form a circRNA (Ashwal-Fluss et al., 2014; Conn et al., 2015; Hansen et al., 2011; Legnini et al., 2017a; Meng et al., 2016; Starke et al., 2015). The exon-scrambling phenomenon was discovered when the spliced nonpolyadenylated exons were observed to be not always paired sequentially in order of their position in genomic DNA for the transcript of tumor suppressor gene, Deleted in Colorectal Cancer (DCC) (Nigro et al., 1991). Subsequently, this pattern was also found for other transcripts such as human mixed-lineage leukemia (MLL), human E26 transformation-specific sequence-1 (ETS-1) and mouse locus sex-determining region Y (SRY) gene. The prevalence of this pattern indicates that exon scrambling might be a process that mimics partial genomic duplication resulting in the formation of excised circles (Bailleul, 1996; Caldas et al., 1998; Capel et al., 1993).
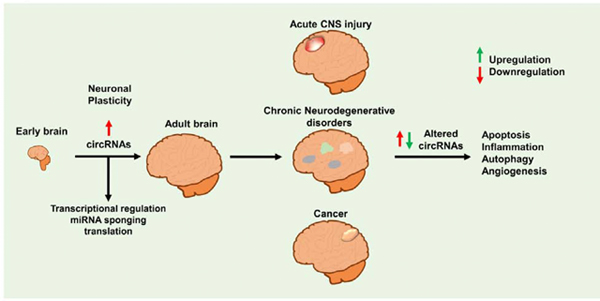
Recent studies have indicated that circRNAs are not the outcome of splicing errors and might be products of a well-regulated process that is potentially associated with normal physiology (Hansen et al., 2013; Memczak et al., 2013). Moreover, accumulating evidence also indicates that circRNAs are dynamically expressed and spatiotemporally regulated in tissue-specific and the development-dependent manner in the brain (Mahmoudi and Cairns, 2019; Memczak et al., 2013; Rybak-Wolf et al., 2015). These characteristics of circRNAs thus seem to be essential for normal biological functions but also could lead to disease progression if their levels are altered. This review describes the formation of circRNAs, their putative functions, role in brain development and aging, and involvement in brain cancer, acute central nervous system (CNS) injury and chronic neurodegeneration. Such an effort is needed to consolidate the present knowledge on circRNAs and define their significance for developing new approaches to treat CNS complications.
2. CircRNA Biogenesis
CircRNAs can be exon-derived, intron-derived, or both exon- and intron-derived; the exon-derived circRNAs are more abundant than the other two subtypes. The circRNA biogenesis follows specific mechanisms mediated by the spliceosomal machinery or by group I and II ribozymes (Chen and Schuman, 2016). During the canonical splicing of pre-RNAs to form mature mRNAs, a lariat with an unusual 2’,5’-phosphodiester bond linkage will be hydrolyzed by the debranching enzyme (DBR1) to eliminate introns sequentially. Exon-derived circRNA formation also involves these steps, but back-splicing leads to lariat-driven and intron-pairing driven circularization (Fig. 1) (Chen and Schuman, 2016; Memczak et al., 2013; Rybak-Wolf et al., 2015). However, the frequency of back-splicing events is low and less efficient compared to canonical splicing. In lariat-driven circularization, pre-mRNA is subjected to partial splicing that results in the skipping of one or more exons, due to the proximity of the exon-donor site and the acceptor site of the different exon on the same loci. It leads to the formation of circRNA intermediates with exons and introns, which will be further processed by canonical splicing machinery to produce exon-derived circRNAs. In the intron-pairing driven circularization model, introns consisting of cis-elements such as inverted Alu repeat sequences pair with each other to bring the downstream donor and upstream acceptor sites into close proximity, leading to circularization of exons (Chen and Schuman; Liang and Wilusz, 2014; Szabo et al., 2015; Zhang et al., 2016a). Importantly, if introns flanking an exon contain abundant inverted Alu repeats, they will be circularized (Chen and Schuman; Liang and Wilusz, 2014; Szabo et al., 2015; Zhang et al., 2016a). Recent evidence suggests that not all complementary sequences on either side of exons and introns necessarily promote circularization (He et al., 2017; Wang et al., 2014). In addition, RNA binding proteins (RBPs) such as muscleblind (Mbl), quaking, double-stranded RNA editing enzyme- adenosine deaminase acting-on RNA (ADAR), and the nuclear helicase DHX9 also control circRNA biogenesis (Aktas et al., 2017; Ashwal-Fluss et al., 2014; Conn et al., 2015; Ivanov et al., 2015). Mbl promotes RNA circularization at a second exon of the primary RNA transcript by interacting with the flanking introns (Ashwal-Fluss et al., 2014). Quaking promote circularization by binding to its consensus sequences in the adjacent introns in the pre-RNA (Ivanov et al., 2015). While Mbl and quaking promote circularization, ADAR prevents circularization by changing the adenine to inosine, and thereby reduces potential RNA complementarity between flanking introns by pairing inosine with guanosine. This pairing repels the downstream donor site and upstream acceptor sites, thereby preventing circRNA formation (Conn et al., 2015). Similarly, DHX9 also negatively regulates circRNA biogenesis by binding specifically to inverted-repeat Alu elements (Aktas et al., 2017). Overall, pre-mRNAs with stable 3’ ends are usually subjected to back-splicing rather than nascent RNA transcripts (He et al., 2017; Liang and Wilusz, 2014).
Fig. 1. Biogenesis of circRNAs.
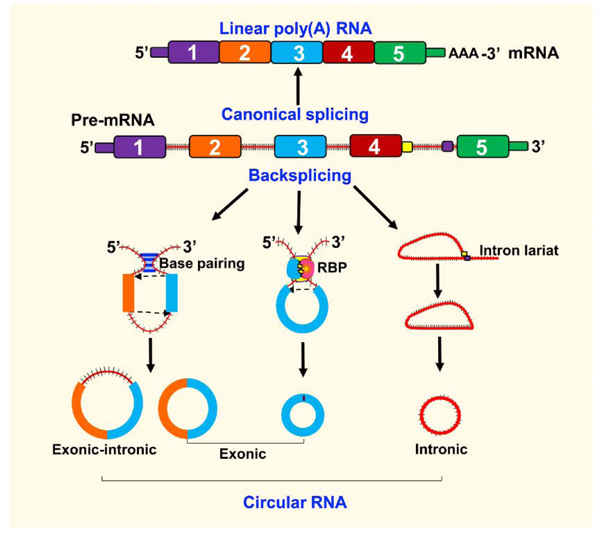
CircRNAs are formed from either exons, introns or both exon-intron by back-splicing events and spliceosomal machinery. On the contrary, canonical splicing forms a mature mRNA after removal of introns. Various processes that form circRNAs are conventional back-splicing driven, intron-pairing-driven circularization, and lariat-driven circularization. The downstream donor and upstream acceptor sites are brought into close proximity during circularization by exon-containing lariats, direct base pairing between cis-acting regulatory elements containing reverse complementary sequences (Alu repeats) and flanking introns, trans-acting factors, such as RNA-binding proteins (RBPs, QKI, MBL) and intron lariats that escape the usual intron debranching and degradation.
During canonical splicing of precursor RNA to form mRNA, introns are removed by complementary binding of the consensus sequences, such as GU-rich regions and Cregions near the branching points at the 5’ and 3’ ends of the introns. However, due to two rounds of trans-esterification between the exon and intron branch points, a circular lariat with 2’,5’-phosphodiester bond linkage can be formed which will be linearized by the debranching enzyme and degraded (Fig. 1). However, if the lariat contains a conserved 7-nucleotide GU-rich motif at the 5′ splicing site and the 11-nucleotide C-rich motif at the 3′-branch site, it escapes the action of the debranching enzyme leading to the formation of an intron-derived circRNA (Ashwal-Fluss et al., 2014; He et al., 2017; Kopczynski and Muskavitch, 1992; Qian et al., 1992; Zhang et al., 2013). Furthermore, RBPs prevent linearization by binding near the unusual 2’−5’ link, allowing intron-derived circRNAs to stay stable.
In some instances, un-spliced introns are retained and persist as exon-intron-derived circRNAs during the formation of exon-derived circRNAs (Fig. 1) (Salzman et al., 2012). Exon-consisting lariat precursors have also been identified in yeast following splice-site mutagenesis of the DBR1, suggesting that exon-intron-derived circRNAs are universal (Barrett et al., 2015).
The majority of circRNAs are exon-derived and are highly conserved (Diederichs, 2014; Jeck and Sharpless, 2014; Memczak et al., 2013; Salzman et al., 2012). It has been shown that circRNAs exhibit high conservation between mammals based on the orthologous co-ordinates and having splice sites within two nucleotides (Rybak-Wolf et al., 2015). A recent study also discovered that among a total of 104,388, 96,675, and 82,321 circRNAs in human, macaque, and mouse, respectively, 70,186 were evolutionarily conserved (Ji et al., 2019). Notably, the regions of DNA that encode exon-derived circRNAs have been reported to be more conserved than the exon-flanking DNA (Legnini et al., 2017b; Rybak-Wolf et al., 2015). Although transcribed from the coding regions of genes, majority of them do not translate any proteins due to the lack of open reading frames (ORFs) and internal ribosome entry sites (IRESs) (Legnini et al., 2017a; Pamudurti et al., 2017b; Yang et al., 2017a).
3. Detecting circRNAs
One of the reasons that eluded the detection and identification of circRNAs for long was the lack of sensitive and competent methods that could capture all the transcriptional and posttranscriptional events. Fortunately, with the advent of high throughput sequencing, it became easier to identify various types of RNAs in a cell (Fig. 2). Especially, deep sequencing with longer reads and improved bioinformatics algorithms enabled the curation of new RNA species. Surprisingly, it was found that several fragments mapped to the same gene in some instances can be arranged in the opposite order (Salzman et al., 2012). As circRNAs lack poly(A) tails, they eluded detection for several years as most sequencing studies used RNAs that were isolated by their poly(A) tails. This was addressed by analysing the sequencing reads of splice junctions formed by an acceptor splice site of an exon at a 5′ end and a donor site at a downstream 3′ end (Memczak et al., 2013). The specificity of circRNA detection was further increased by treating the RNA preparations with RNase R that digests the linear RNAs, but not circRNAs (Jeck and Sharpless, 2014; Memczak et al., 2013). In addition, identifying reads from ribosomal RNA-depleted RNA sequencing with backsplicing improved the specificity of circRNA detection to single-nucleotide resolution (Jeck and Sharpless, 2014; Memczak et al., 2013). Furthermore, circRNA microarrays that use junction-specific probes enabled systematic profiling of the circRNA expression patters in linear RNA-depleted samples (Li et al., 2019b; Mehta et al., 2017). Using this approach Li et al. integrated 87,935 human circRNAs sequences to identify ~80,000 circRNAs expressed in the cervical tumors (Li et al., 2019b). Thus, RNA-seq and/or circRNA microarray analysis with RNA preparations in which linear RNAs and ribosomal RNAs were depleted combined with stringent algorithms refined the circRNA identification and detection in mammals (Pandey et al., 2020).
Fig. 2. Methods to detect circRNAs.
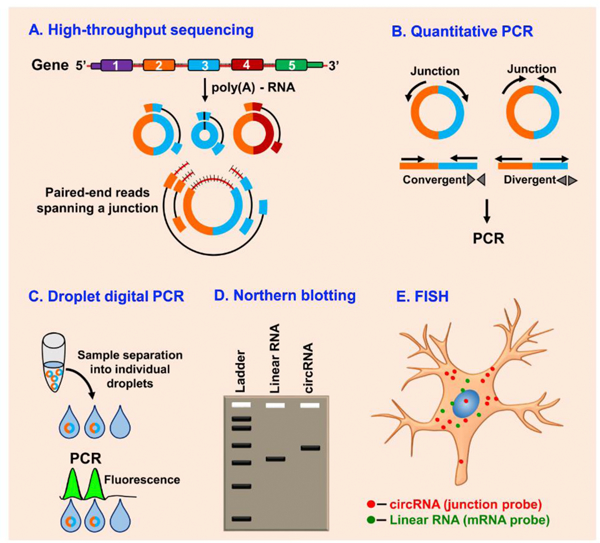
Coupled with the bioinformatics algorithms, deep sequencing and longer reads of RNAs digested with RNase R and poly(A) depleted RNAs increases the specificity of circRNAs detection (A). PCR analysis with convergent and divergent primers in RNase R-treated RNA preparations can be used to detect circRNAs (B). Droplet digital PCR quantifies absolute circRNA levels based on the single droplet molecules (C). Northern blotting can accurately detect and confirm an exact circRNA in a gel using probes that are designed to target both the circular and the linear transcript or only the circRNA with backsplice junction probe (D). Finally, RNA fluorescence in situ hybridization (RNA-FISH) coupled with high-resolution microscopy using probes flanking the junction sites can determine the distribution and abundance of circRNAs (E).
PCR with convergent and divergent primers is another widely used method for circRNA identification (Fig. 2). Convergent primers are conventional primers that face toward each other that are routinely used to amplify linear DNA or RNA. These can be used for detecting circRNAs in linear RNA and ribosomal RNA depleted samples. Whereas, divergent primers which face away from each other and designed to span the circRNA junctions can be used to detect circRNAs without depleting linear or ribosomal RNAs. However, challenges like low abundance and formation of concatamers by rolling circle amplification during reverse transcription might prevent the accuracy of circRNA quantification by PCR. But, these hindrances can be overcome by using high-throughput droplet digital PCR (ddPCR) that can precisely measure the absolute nucleic acid levels even at low abundance by using the ratio of positive and negative droplets (Fig. 2) (Chen et al., 2018b; Hindson et al., 2013).
Northern blot analysis with either long probes covering an entire circRNA or short probes flanking the splice junction sites can also be used to detect circRNAs (Fig. 2) (Schneider et al., 2018). In the gels used for Northern blot analysis, an exonic circRNA migrates at a slower rate than a linear RNA of equal length because of the limited pore size of the gel (Schneider et al., 2018; Tabak et al., 1988). RNA fluorescence in situ hybridization (RNA-FISH) coupled with high-resolution microscopy using probes that are designed to flank the junction sites is a powerful method to locate the distribution and abundance of circRNAs in the cell (Zirkel and Papantonis, 2018). Further, single-molecule RNA-FISH that uses multiple singly labeled oligonucleotide probes (Stellaris® RNA FISH) can also be used to determine the subcellular localization and absolute quantification of RNA molecules in individual cells (Fig. 2) (Kocks et al., 2018; Piwecka et al., 2017).
4. Putative functions of circRNAs
As circRNAs are formed from many linear RNA precursors in archaea to mammals, they are thought to be functionally relevant (Danan et al., 2012; Jeck et al., 2013; Memczak et al., 2013; Salzman et al., 2012). CircRNAs are also shown to be functionally diverse (Fig. 3). Exonic circular RNAs are mainly cytosolic and thought to act as “decoys” that sponge miRNAs and RBPs, possibly to inhibit their interaction with the target mRNAs or to transport them between cell types (Chu et al., 2015; Hansen et al., 2013; Memczak et al., 2013; You et al., 2015; Zhang et al., 2013). In mammalian neural tissue, the typical miRNA sponging activity was shown for several circRNAs, including cerebellar degeneration -related autoantigen 1 (CDR1), antisense (CDR1as aka ciRS-7) (Memczak et al., 2013); sex determining region Y (SRY) circRNA circSRY (Hansen et al., 2013); and homeodomain interacting protein kinase 3 (HIPK3) circRNA circHIPK3 (Zheng et al., 2016). Interestingly, bioinformatics showed that not all circRNAs contain a sufficient number of binding sites for miRNAs and the typical miRNA sponging function seems to be is unique to only a handful of circRNAs (Jeck and Sharpless, 2014; Memczak et al., 2013; You et al., 2015). However, that does not seem to be the case, as several circRNAs were also shown to sponge miRNAs even with one binding site (Duan et al., 2018; Lei et al., 2019; Li and Diao, 2019b; Wang et al., 2018a; Wu et al., 2019). In addition to sequestering miRNAs, studies also showed that multiple circRNAs that are localized to the nucleus have little affinity for miRNA target sites. These circRNAs contain either introns or both exon and intron, including ci-ankrd52, circEIF3J, and circPAIP2, and control transcription mainly by interacting with the RNA polymerase II (Pol II) and U1 small nuclear ribonucleoprotein particle (U1snRNP) complex (Li et al., 2015; Zhang et al., 2013).
Fig. 3. Functions of circRNAs.
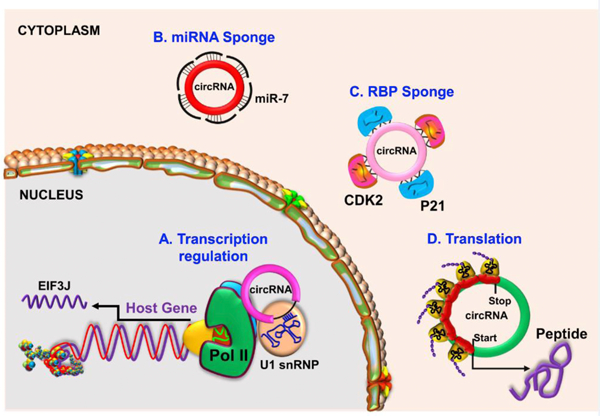
CircRNas plays diverse roles by regulating transcription (circEIF3J and circPAIP2) of their host genes, sponging miRNAs (CDR1as, SRY and HIPK3) and thereby derepressing miRNA-target mRNA translation, sponging RNA binding proteins (RBPs) such as CDK2 and P21, and some circRNAs also show translation potential (circMbl and circ-ZNF609) to form peptides.
Despite being originated from the coding regions of the genes, exon-derived circRNAs are unable to translate into proteins since the ORFs in them are generally truncated. However, recent studies suggest that some exon-derived circRNAs could be translated into proteins or peptides owing to the presence of an IRES and the start codon AUG (Yang et al., 2017b). In support of this theory, a cloned circRNA with GFP+ exons with IRESs under the cytomegalovirus promoter was shown to be translated into GFP protein (Wang et al., 2015). Accumulating evidence now demonstrates that multiple circRNAs are translated under in vivo conditions by associating with polyribosomes (Legnini et al., 2017b; Pamudurti et al., 2017a).
Intriguingly, adenosine in several RNAs is known to undergo methylation to form N6-methyladenosine (m6A) at a specific consensus motif (RRm6ACH) (Chen et al., 2019b; Liu et al., 2014; Yang et al., 2017b; Zhou et al., 2017), mediated by a complex that consists of methyltransferase-like 3 (METTL3), METTL14, and Wilm’s tumor 1-associating protein (Kobayashi et al., 2018; Selberg et al., 2019; Yang et al., 2018c). Recent evidence indicates that m6A modification is not limited to mRNAs, but also abundant and widespread on circRNAs that are often derived from exons not methylated in mRNAs in a cell type-specific manner (Zhou et al., 2017). This epitranscriptomic modification is thought to promote the translation of circRNAs in a cap-independent manner using short sequences containing m6A sites as IRESs (Wang et al., 2015; Yang et al., 2017b).
By profiling polysomes associated with circRNAs, it was shown that m6A-driven circRNA translation is prevalent throughout the human genome (Yang et al., 2017b). Human myoblasts and myotubes showed widespread differences in the expression pattern of circRNAs as a function of the stage of differentiation in Duchenne muscular dystrophy patients (Legnini et al., 2017b). Mainly, circ-ZNF609 was observed to plays a role in myoblast differentiation by producing a protein. Moreover, when siRNAs that selectively targeted the exon junctions but not the parent mRNA of circRNA was used to knockdown circ-ZNF609, the protein coded by the circ-ZNF609 was not formed (Legnini et al., 2017b). The protein forming potential of circRNAs can be confirmed by translating ribosome affinity purification assay, which is used to detect circRNAs associated with ribosomes. One such example circRNA that associates with the polysome is circMbl, and it was reported to translate to a ~10 kDa protein in D. melanogaster (Pamudurti et al., 2017a). These studies thus suggest that many circRNAs can potentially produce a protein or a peptide; however, their functional significance needs to be identified.
5. CircRNAs in brain development and aging
In the mammalian brain, ~20% of the protein-coding genes are known to produce circRNAs (You et al., 2015). The latest research shows that circRNAs are present in all rat tissues but enriched in brain tissue, where their levels continuously increase from 2 weeks to 104 weeks of age (Mahmoudi and Cairns, 2019). Likewise, circRNAs were revealed to be more abundant in the brain than in any other organ, including the heart, liver, and lungs in adult mice (You et al., 2015). Additionally, research in the pigs also showed that circRNAs are highly expressed in the brain, and their levels in various brain structures are developmentally heterogeneous (Veno et al., 2015). The abundance of circRNAs in the brain could be due to the host genes that are exclusively expressed in the brain or their relative contribution to form circRNAs is higher in the brain than any other organs. These studies thereby indicate that the mammalian brain is unique among all other organs owing in part to the abundance of circRNAs (Rybak-Wolf et al., 2015; You et al., 2015). Moreover, the abundance seems to depend on the circRNA’s formation and functions.
Several recent studies indicate that circRNAs play an essential role in regulating CNS development (Fig. 4). Many brain-enriched circRNAs were observed to be associated with neurotransmitter function, neuron maturation, and synaptic activity (Mahmoudi and Cairns, 2019). For example, a circRNA derived from Foxo3 gene (circFoxo3) was shown to bind and impair cyclin-dependent kinase 2 (CDK2) activity, leading to disrupted cell cycle progression (Du et al., 2016). Besides, a circular RNA called circHomer 1 that originated from the Homer Scaffolding Protein 1 pre-RNA (Homer 1 is a member of the Homer family of dendritic proteins) is thought to modulate some of the structural changes at synapse during neuronal plasticity and development (You et al., 2015). Many synaptically-enriched circRNAs are also reported to be derived from the genes of transformed growth factor (TGF-β) pathway, Wnt signaling pathway, and axon guidance (van Rossum et al., 2016; Veno et al., 2015; You et al., 2015; Zhang et al., 2016b). All of these studies implicate circRNAs in the neuronal plasticity.
Fig. 4. Mechanisms regulated by circRNAs during brain development and disease conditions.
CircRNAs are not just abundant, but they continually increase from embryonic to adult stage in the brain to regulates functions related to neuronal plasticity. Additionally, their altered levels are engaged in brain diseases, and degeneration by various mechanisms, including angiogenesis, autophagy, apoptosis, tumorigenesis, and inflammation.
Furthermore, studies showed that 58% of cerebral circRNAs are developmentally regulated, while only 2% of their linear isoforms have shown this trend (Mahmoudi and Cairns, 2019). A cerebral cortex transcriptomic analysis study using three-month-old and eight-year-old pigs showed the circRNAs expression in the brain is dependent on developmental age (Chen et al., 2019a). This study also showed that >80% of mRNAs, miRNAs, and lncRNAs, but <22% of circRNAs, are expressed at both ages. Interestingly, many developmentally regulated circRNAs also showed sexual dimorphism in the brain and were observed to target aging-associated mRNAs (Mahmoudi and Cairns, 2019). Thus, circRNAs might dictate the development and aging process by changing the expression and availability of specific mRNAs.
In the human brain, on an average, at least three circRNAs are formed from annotated exons of the individual gene, whereas more than 2,000 genes form ten or more circRNA isoforms. Thus, the majority of the exon-derived circRNAs preferentially contain coding sequences and 5’ UTR exons (Rybak-Wolf et al., 2015). The circRNA expression profiles are distinct in various regions of the brain. The prefrontal cortex, olfactory bulb, cerebellum, and hippocampus were shown to express region-specific sets of circRNAs in the mouse brain (Rybak-Wolf et al., 2015). Interestingly, evidence also suggests that several of the circRNAs formed from the coding regions of the genes are expressed at a higher level than their canonical/linear isoforms, thereby indicating the existence of cell type-specific mechanisms of production and/or degradation of circRNAs (Rybak-Wolf et al., 2015). The abundance of circRNAs in the brain was thought to be linked to synaptogenesis since an abrupt increase in circRNA expression levels occurs during the transition from P10 to P30 in mice (You et al., 2015). A similar increase in circRNA abundance was also reported in humans, where ~6,000 circRNAs were detected during embryonic development, and their number increased significantly in fetal (~89,000) and adult (~65,000) brains (Chen et al., 2018a; Rybak-Wolf et al., 2015; Szabo et al., 2015). The synaptogenic function of circRNAs was also supported by their host genes that are particularly enriched in synaptosomes and related to synaptic function (You et al., 2015). In situ hybridization studies showed that several of the synaptic function-related circRNAs including circHomer1, circNlgn1, circElavl1, cricKlhl1, circDscam, circGigyf2, circRmst, and circNbea are abundantly present in the dendritic arbor (You et al., 2015). It is also evidenced that mouse neural stem cells at various stages of differentiation showed a set of differentially expressed circRNAs coexpressed with development-related mRNAs (Yang et al., 2018a). In addition to changes in circRNA expression profiles as a function of development, aging was also shown to alter circRNA levels in the Drosophila CNS (Westholm et al., 2014).
The abundance and/or the distribution of circRNAs can change with exposure to radiation during development. A recent study showed that the abundance of the circular transcript variants of Pvt1, Ano3, Sec14l5, and Rnf169 (p53 target genes) continuously increased in the mouse brain from embryonic day 12 until adulthood, while the increase in the respective linear RNAs ceased by postnatal day 30 (Mfossa et al., 2019). Moreover, the mRNA expression of p53 target genes peaked before 6h, while the circRNAs formed form the same genes reached the highest expression at 12h or later in the embryonic brain and primary cortical neurons exposed to ionizing radiation (Mfossa et al., 2019). Overall, these findings suggest that circRNAs are dynamically expressed in a development-stage specific manner in the brain.
6. CircRNAs and brain cancer
Glioma progression is mediated by cell proliferation, invasion, migration, and apoptosis. Angiogenesis and the secretion of various pro-angiogenic growth factors modulate these processes, but circRNAs also play a vital role. It is supported by many studies that showed a strong correlation between circRNA expression and glioma progression (Fig. 4)(Bian et al., 2018; Duan et al., 2018; Hu and Zhang, 2019; Li et al., 2018b; Li and Diao, 2019a; Xie, 2018). Notably, several circRNAs, including circSMARCA5, circHIPK3, circMTO1, circTTBK2, circ007628, and circMMP9, are known to promote glioma progression by sponging miRNAs and thereby targeting their respective host genes and/or down-stream genes (Barbagallo et al., 2019).
It was shown that increased levels of circSMARCA5 promote angiogenesis and glioma progression by sponging serine and arginine-rich splicing factor 1 (SRSF1) mRNA, and thus regulating VEGF-A mRNA (Barbagallo et al., 2019). VEGF-A is a potent inducer of angiogenesis that leads to the progression of solid tumors. Similarly, circ_002136 (circCDK11A_001), which is formed through the looping of linear cyclin-dependent kinase 11A transcript variant 1 (CDK11A-VT1), seems to regulate the expression of SOX13. SOX13, in turn, is known to controls the expression of the spondin-2 gene that is recognized for tumor aggressiveness (He et al., 2019; Jin et al., 2017). It has been shown that circ_002136 but not the linear CDK11A is highly expressed in glioma cells together with SOX13. Increased circ_002136 level sponges miR-138–5p and prevents the repression of SOX13 (He et al., 2019). SOX13 thus induce the expression of spondin-2 and thereby promoting glioma angiogenesis (He et al., 2019). Likewise, circRNA circZNF292 (cZNF292) induced by hypoxia down-regulates various components of the Wnt/β-catenin signaling pathway, including cyclin A, CDK2, p-CDK2, β-catenin, p-STAT3 (Tyr705), p-STAT5 (Tyr694), and proline-rich protein 11 (PRR11) (Yang et al., 2016). All these factors play a crucial role in cellular proliferation, migration, invasion, and angiogenesis during glioma progression. When circZNF292 was silenced in glioma cell lines (U87MG and U251), the expression of cyclin A, CDK2, p-CDK2, β-catenin, p-STAT3 (Tyr705), p-STAT5 (Tyr694), and PRR11 was decreased, indicating that circZNF292 is crucial for glioma proliferation and tube formation (Boeckel et al., 2015; Yang et al., 2016). Furthermore, another circRNA called circSHKBP1 (circ_0000936) that is highly abundant in human endothelial cells was also shown to regulate angiogenesis through miR-544a/FOXP1 and miR-379/FOXP2 (He et al., 2018; Salzman et al., 2013). Knockdown of circSHKBP1 upregulated the expression of miR-544a/miR-379 and reduced the expressions of their target mRNAs FOXP1/FOXP2. It is suggested that FOXP1/FOXP2 levels are positively associated with various types of tumors. Therefore, knockdown of circSHKBP1 suppresses the expression of FOXP1/FOXP2 by miR-544a/miR-379, which thereby prevents cell viability, migration, and tube formation in glioma (He et al., 2018). Hence, all these above-listed studies point out that circRNAs are the dominant regulator of angiogenesis during glioma progression.
In addition to promoting angiogenesis and proliferation of the glioma cells, several circRNAs were also shown to play a potential regulatory role in the viability, migration, and invasion of tumor cells. For example, circHIPK3 promotes invasiveness of tumor cells by silencing miR-124–3p and thus derepressing its target STAT3 (Hu and Zhang, 2019). Similarly, sponging of miR-124 by circMMP9 was also shown to promote migration and invasion of glioma cells through cyclin-dependent kinase 4 and Aurora kinase A, both of which are the targets of miR-124 (Wang et al., 2018a). Likewise, circ_007628 induces glioma oncogenesis by sponging miR-181a and thus increasing the expression of its target SIRT1 (Lei et al., 2019). Similarly, circITCH downregulation promotes both migration and invasion of glioma cells and is also associated with poor prognosis of glioma patients (Li et al., 2018a). By contrast, overexpression of circITCH suppressed cell proliferation and prevented apoptosis by sponging miR-214 and thus allowing the expression of miR-214 target ubiquitin-protein ligase E3. Furthermore, both circITCH and circZNF292 seems to target the Wnt/β-catenin pathway to induces glioma progression (Li et al., 2018a; Yang et al., 2016).
Additionally, circTTBK2 was shown to target miR-217 and the downstream hepatocyte nuclear factor-1β (HNF1β)/Derlin-1 pathway. HNF1β is a homeobox transcription factor that facilitates glucose uptake and glycolytic activity (Okamoto et al., 2015; Yu et al., 2015), and Derlin-1 prevents endoplasmic reticulum stress-induced apoptosis (Wang et al., 2008). Intriguingly, circTTBK2 is induced in glioma and its enhanced expression prevented cell apoptosis and thereby promoted proliferation, migration, and invasion of glioma cells by targeting HNF1β/Derlin-1 pathway (Zheng et al., 2017). Another circRNA called circ_0046701, which is induced in gliomas, sequester miR-143–3p and thereby derepress miR-143–3p target integrin subunit beta 8 (ITGB8). ITGB8 could promote glioma cell proliferation and invasion (Li et al., 2018b). However, when silenced, circ_0046701 prevented cell proliferation and invasion by suppressing ITGB8 in glioma.
A recent study also showed that levels of CDR1 and circRNA circ_0001946 were downregulated, while miR-671 (which target CDR1 and circ_0001946) was upregulated in glioblastoma; these changes were thought to promote glioblastoma growth (Li and Diao, 2019b). When overexpressed, circ_0001946 induced apoptosis and reduced the proliferation, migration, and invasion of glioblastoma cells by sponging miR-671 and derepressing its target CDR1 (Li and Diao, 2019b). Likewise, the miRNA miR-630 promotes chemoresistance of glioblastoma to temozolomide, whereas circMTO1 induction sponges miR-630 and thereby reverse the chemoresistance of glioblastoma to temozolomide (Rao et al., 2018).
Although the translation of circRNAs is not a dominant event, circPINTexon2 formed from the exon 2 of LINC-PINT gene was shown to translate an 87 amino acid tumor-suppressive peptide (PINT87aa) (Zhang et al., 2018a). PINT87aa is expressed at high levels in tissues of the brain, liver, kidney, and stomach, and at a lower level in tissues of the breast, intestine, thyroid, and pancreas (Zhang et al., 2018a). PINT87aa interacts with PAF1 and inhibits the transcriptional elongation of PAF1 downstream oncogenes, including CPEB1, SOX-2, and c-Myc (Zhang et al., 2018a). Down-regulation of circPINTexon2 and PINT87aa in brain tumors negatively impacts the glioma clinical prognosis (Zhang et al., 2018a). The circRNA circFBXW7 formed by circularization of the transcript of exon 3 and exon 4 of the FBXW7 gene encodes a 185 aa protein called FBXW7–185aa which is abundantly expressed in the healthy human brain, but reduced in the gliomas (Yang et al., 2018b). When overexpressed in glioma cells, circFBXW7 (but not the linear FBXW7), inhibited proliferation and cell cycle acceleration, whereas silencing of FBXW7 promoted glioma malignancy. Moreover, intracranial implantation of circFBXW7 overexpressing cells increased the life span of mice. These functions of circFBXW7 are mediated by FBXW7–185aa, which destabilizes c-Myc through USP28dependent ubiquitination (Yang et al., 2018b). All of these studies indicate that circRNAs regulate glioma growth and invasion by sponging miRNAs and preventing the suppression of miRNA targets. Thus, circRNAs acts as a competitive endogenous RNA (ceRNA) to control miRNA availability and function during glioma progression.
7. CircRNAs in secondary brain damage following acute CNS insults
Acute CNS insults, including spinal cord injury (SCI), traumatic brain injury (TBI), and stroke, are leading causes of death and long-term disability in humans. These insults can occur in both sexes and at various ages. Acute CNS insults impair motor functions, cognitive functions, and neuropsychiatric functions in affected individuals. Decades of human and animal studies have not identified any viable therapeutic targets that can be modulated to prevent neuronal death and neurologic dysfunction after any acute CNS injury. Many studies have shown that acute CNS insults result in significant alterations in the ncRNA profiles and functions (Dharap et al., 2009; Dharap et al., 2011, 2012; Gaudet et al., 2016; Mehta et al., 2015; Mehta et al., 2017; Meissner et al., 2016; Paim et al., 2019; Qin et al., 2018; Redell et al., 2010; Sabirzhanov et al., 2016; Shi et al., 2019; Zhang et al., 2019; Zhao et al., 2018).
Recent studies showed that SCI significantly alters the expression profiles of circRNAs in the adult rat brain (Qin et al., 2018; Zhou et al., 2019). Gene Ontology (GO) analysis showed that circRNAs altered after SCI might modulate pathways associated with AMP-activated protein kinase signaling and peroxisomes (Qin et al., 2018), and might also participate in the post-SCI secondary damage via circRNA-targeted miRNA-mRNA axis (Zhou et al., 2019). Recent studies showed that TBI also alters circRNA profiles in the cortex and hippocampus of adult rodents, and the altered circRNAs might be involved in modulating inflammation, cell death, and repair directly or indirectly through circRNA/miRNA interaction (Fig. 4))(Jiang et al., 2019b; Xie et al., 2018). In addition to being present inside the cell, a new study showed that various circRNAs are differentially present in the exosomes isolated from the brain of a mouse after TBI. There is some possibility that altered circRNAs play a role in synaptic plasticity (Zhao et al., 2018). However, the functional significance of any of the circRNAs altered after traumatic injuries to CNS has not yet been evaluated in detail.
Neonatal hypoxia-ischemia (HI) is a frequent problem that arises when blood supply to the fetus is interrupted at gestational week 36 or later. HI leads to hypoxic-ischemic encephalopathy, a condition that is characterized by long-term motor, sensory, and cognitive disabilities. A recent study showed that in a rat model of HI, expression of many circRNAs was significantly altered, and bioinformatics analysis of circRNA/mRNA networks predicted that these RNAs might be involved in brain damage as well as neural degeneration (Jiang et al., 2019a). Induction of periventricular white matter damage in rats, which is a characteristic pathology of HI, also differentially induced the expression of many circRNAs that influence mechanisms such as glutamatergic synaptic function and the VEGF signaling (Zhu et al., 2018).
Stroke is the leading cause of disability in the adult population all over the world. Many mechanisms, including ionic imbalance, edema, inflammation, oxidative stress, endoplasmic reticulum stress, and apoptosis, are thought to promote post-stroke secondary brain damage synergistically (Mehta et al., 2007). Several studies recently showed that stroke rapidly alters the expression profiles of various classes of ncRNAs, including miRNAs, ncRNAs, transcribed ultraconserved regions (T-UCRs), and piRNAs (Dharap et al., 2009; Dharap et al., 2011, 2012). This research also demonstrated that altered ncRNA function leads to a compromised translation and transcription process and thereby modulates the post-ischemic functional outcome (Dharap et al., 2013a; Dharap et al., 2013b; Kim et al., 2018; Mehta et al., 2015; Pandi et al., 2013). We recently reported that transient focal cerebral ischemia in adult mice induces significant changes in the expression of many circRNAs in a sustained manner between 6h to 1 day of reperfusion (Mehta et al., 2017). Another study also reported a change in the expression profile of many circRNAs at two days after reperfusion in the mouse brain following focal ischemia (Liu et al., 2017b). A third recent study also reported altered expression of many circRNAs in the ischemic infarct area of rats four days after focal cerebral ischemia (Duan et al., 2019).
Interestingly, all of these studies show that the majority of circRNAs altered after stroke are coded from the exonic regions of the protein-coding genes (Duan et al., 2019; Liu et al., 2017b; Mehta et al., 2017). However, it is unclear if the altered circRNA levels in the post-stroke brain are due to an altered preference to form circRNAs from linear RNAs, and if any of these circRNAs regulate parent RNA translation after stroke. At this time, the functional significance of altered circRNAs in post-ischemic pathophysiology is not known. However, bioinformatics analysis has shown that circRNAs altered after stroke might modulate mitogen-activated protein kinase signaling, Rap1 signaling, Hippo signaling, autophagy, and endocytosis all of which are known to be associated with cell survival and/or death pathways (Duan et al., 2019; Liu et al., 2017b; Mehta et al., 2017). A recent study showed that expression of circRNA TLK1 (circTLK1) was increased in the mouse brain and plasma after transient MCAO, as well as in the plasma of patients with acute ischemic stroke (Wu et al., 2019). CircTLK1 is formed from exons 2 and 3 of the TLK1 gene, and the expression of TLK1 linear mRNA was decreased in the ischemic brain. Interestingly, when circTLK1 was knocked down, ischemic mice showed decreased apoptosis, alleviated neurological deficits, reduced infarct volume, and improved somatosensory functions (Wu et al., 2019). These effects of circTLK1 were mediated by 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)-inducible poly (ADP-ribose) polymerase (TIPARP), a member of the poly-ADP-ribose polymerase (PARP) family that is known to participate in DNA repair and cell death (Han et al., 2018). The mRNA level of TIPARP was also increased, while miRNA miR-335–3p, which binds to 3’-UTR of TIPARP, was decreased in the brain after transient MCAO. Knockdown of TIPARP resulted in reduced neurological deficits, smaller infarct volume, lowered brain atrophy volume, curtailed mortality, and improved motor function after transient MCAO (Wu et al., 2019). Moreover, decreased levels of miR-335–3p were the result of sponging by circTLK1, suggesting that the circTLK1/miR-335–3p axis worsens ischemic injury through TIPARP (Wu et al., 2019).
Similarly, another study also identified that TIPARP could be regulated by circRNAs circHECTD1 whose expression in significant increased in the ischemic mouse brain, as well as in the plasma of patients with acute ischemic stroke (Han et al., 2018). CircHECTD1 sponges miR-142, which targets TIPARP and promotes astrocyte activation and autophagy (Han et al., 2018). This study further observed that circHECTD1 knockdown leads to the loss of miR-142 sponging and thus derepresses TIPARP, resulting in curtailed autophagy and improved post-ischemic functional outcome (Han et al., 2018). Together, these studies suggest that circTLK1 and circHECTD1 seem to regulate post-ischemic neurologic outcome by targeting TIPARP through sponging miR-335–3p and miR-142, respectively.
Furthermore, a recent study showed that ectopic expression of miR-29b suppresses neuronal apoptosis when exposed to conditioned medium from OGD-subjected and miR-29b mimic transfected microglia (Wang et al., 2019). Our lab previously showed that post-stroke downregulation of miR-29c promotes ischemic brain damage by derepressing its target DNA methyltransferase 3a (Pandi et al., 2013). Intriguingly, miR-29b could inhibit JNK2/STAT3-signaling by inducing SOCS-1, a very potent member of the suppressors of the cytokine-signaling family of proteins involved in the immune response (Wang et al., 2019). JNK2/STAT3-signaling is known to regulate IL-1β production in microglial cells following OGD (Wang et al., 2019). When hippocampal neurons were cultured in a microglial-conditioned medium containing IL-1β, there was significant neuronal apoptosis. Interestingly, miR-29b is a target of circRNA circPTK2, which is also induced following OGD. Therefore, overexpression of circPTK2 suppressed miR-29b and induced apoptosis. Conversely, silencing of circPTK2 preserved miR-29b levels and promoted SOCS-1 mRNA and protein expression. Additionally, circPTK2 silencing inhibited JAK2/STAT3 activation and IL-1β production and thereby prevented neuronal apoptosis (Wang et al., 2019).
Down-regulation of some circRNAs after stroke was also shown to promote ischemic brain damage. For example, circDLGAP4 functions as an endogenous miR-143 sponge; in the post-ischemic mouse brain, circDLGAP4 was downregulated, leading to upregulation of miR-143 (Bai et al., 2018). Plasma from acute stroke patients showed decreased levels of circDLGAP4 (Bai et al., 2018). Increased levels of miR-143 promoted Evans blue extravasation by negatively regulating the expression of tight junction proteins, including claudin-5, occludin, and ZO-1 in the control mice subjected to cerebral ischemia. By contrast, silencing of miR-143 or overexpression of circDLGAP4 ameliorated this change and improved cerebrovascular integrity by preserving the levels of tight junction proteins in mice after transient cerebral ischemia. Furthermore, miR-143 was shown to disrupt the endothelial-mesenchymal transition responsible for maintaining BBB integrity (Bai et al., 2018). This study demonstrated that endothelial cells express mesenchymal cell markers such as Col I, Col III, and α-SMA upon downregulation of circDLGAP4 in mice subjected to transient focal ischemia (Bai et al., 2018). Overexpression of circDLGAP4 or silencing of miR-143 curtailed the increases in Col I, Col III, and α-SMA expression, suggesting that overexpression of circDLGAP4 protects the post-stroke brain by curtailing miR-143-mediated endothelial-mesenchymal transition and BBB disruption (Bai et al., 2018).
8. CircRNAs in chronic neurodegenerative diseases
8.1. Alzheimer′s disease:
Alzheimer′s disease (AD) is one of the most prevalent, irreversible, and progressive forms of dementia in the elderly population; it is caused by a combination of genetic, lifestyle, and environmental factors. Over the years, amyloid-β (Aβ) and tau proteins have been reported to play critical roles in AD pathogenesis. More specifically, the amyloid precursor protein (APP), which is converted to Aβ protein by β- and γ- secretases, clusters together to form toxic amyloid plaques and kill neurons by disrupting intracellular communication. Moreover, tau protein also accumulates to form neurofibrillary tangles that are toxic to neurons. Recent reports suggest that circRNA play a vital role in the development of AD. Notably, the accumulation of circRNAs in the cortex and hippocampus of aged mice seemed to promote an age-related decline in neural function, leading to potential susceptibility to age-related neurodegenerative diseases (Gruner et al., 2016). In a mouse AD model, treatment with Panax notoginseng saponins, which is known to curtail the pathological progress of AD, led the altered expression of several circRNAs that were suggested to modulate AD pathology (Huang et al., 2018). Moreover, dysregulation of hundreds of circRNAs was also shown in the hippocampus of an AD rat model (Wang et al., 2018b).
A recent study identified 17 circRNAs that are derived from the Aβ coding region of the APP gene (Mo et al., 2018). One of those called Aβ circRNA was observed to contain an ORF and has the potential to be translated to form Aβ-related peptide. This seems to be instrumental in increasing the levels of Aβ and Aβ plaques (Mo et al., 2018). In addition to the aforementioned reason, Aβ circRNA was also shown to regulate glycogen synthase kinase-3β (GSK-3β) levels. GSK-3β is known to phosphorylate tau and promotes its aggregation into neurofibrillary tangles (Rankin et al., 2007). Thus, Aβ circRNA also plays a vital role in AD pathogenesis by regulating GSK-3β and tau phosphorylation (Mo et al., 2018).
Additionally, CDR1as (ciRS-7), which is an abundant circRNA in the human brain, was shown to be downregulated in the brains of AD patients (Lukiw, 2013). CDR1as has >70 binding sites for the miRNA miR-7 and thus acts as a miR-7 sponge (Hansen et al., 2013; Piwecka et al., 2017). Ubiquitin protein ligase A (UBE2A), which catalyzes the proteolytic clearing of toxic amyloid peptides in the AD brain, is a robust target of miR-7 (Zhao et al., 2016). It was shown that sporadic AD is associated with a misregulated CDR1as-miR-7UBE2A system (Zhao et al., 2016). Additionally, CDR1as is also shown to prevent NF-κB translation. NF-κB, which one of the other targets of miR-7, regulates the expression of ubiquitin carboxyl-terminal hydrolase L1 (UCHL1) (Shi et al., 2017; Zhao et al., 2015). UCHL1 is known to control the processing of APP and the degradation of β site APP -cleaving enzyme 1 (BACE1) (Zhang et al., 2012). Therefore, CDR1as-dependent inhibition of NF-κB translation derepresses UCHL1 and thereby allowing the degradation of APP and BACE1 (Shi et al., 2017). Moreover, upon activation by tumor necrosis factor -α (TNF-α), NF-κB negatively regulates the transcription of the UCHL1 gene by binding to its response sequences within the promoter. Interestingly, overexpression of CDR1as elevates the NF-κB inhibited UCHL1 promoter activity and thereby increases the mRNA and protein levels of UCHL1 (Shi et al., 2017). By contrast, a similar effect was not observed when NF-κB was inhibited with BAY117082, a specific inhibitor of NF-κB (Shi et al., 2017). Furthermore, it was also shown that overexpression of CDR1as resulted in decreased protein levels of NF-κB (p65) without altering the NF-κB mRNA levels (Shi et al., 2017). Taken together, these studies suggest that circRNAs epigenetically control the gene expression leading to AD pathogenesis.
8.2. Parkinson’s disease:
Parkinson’s disease (PD) is a progressive neurodegenerative disorder associated with dopaminergic neuronal death in the substantia nigra that controls motor function. PD patients thus experience tremors, bradykinesia, limb rigidity, and balance problems. Presence of Lewy bodies that contain α-synuclein (α-Syn) protein aggregates is a hallmark of PD. Many ncRNAs, including miRNAs, lncRNAs and circRNAs, are known to play a role in PD progression (Idda et al., 2018; Kraus et al., 2017; Liu et al., 2016; Majidinia et al., 2016; Zhou et al., 2018). Recent studies suggest that α-Syn is a major target of miR-7 (Junn et al., 2009; Kim et al., 2018). In the substantia nigra of PD patients, miR-7 levels were reported to be decreased, which correlates with α-Syn accumulation and aggregation (McMillan et al., 2017). Interestingly, CDR1as is predominantly expressed in excitatory neurons where it regulates miR-7 stability and/or transport, and hence a loss of CDR1as leads to miR-7 deregulation, thus affecting the sensorimotor gating in PD (Piwecka et al., 2017). Another circRNA called circZip-2 (derived from the Zip-2 gene) indirectly regulates α-Syn levels. Zip-2 is a bZIP transcription factor family member and mediates immune responses. CircZip-2 expression was detected in wild-type and a transgenic strain (NL5901) of C. elegans that expresses human α-synuclein. It was shown that circZip-2 expression decreased in PD. Interestingly, when Zip-2 was silenced with RNAi, it reduced α-Syn aggregation and reactive oxygen species (ROS) generation in a C. elegans PD model (Kumar et al., 2018). These results indicate the possibility of competition between circRNA and its parental gene for their expression (Kumar et al., 2018).
8.3. Amyotrophic lateral sclerosis:
Amyotrophic lateral sclerosis (ALS) is a progressive neurodegenerative disease that causes a selective motor-neuron loss in the brainstem and spinal cord, specifically as they control voluntary muscle movement. The cause of ALS in most patients is unknown, but mutations in the fused-in sarcoma (FUS) gene and its involvement in familial ALS was demonstrated in previous studies (Kwiatkowski et al., 2009; Vance et al., 2009). The normal FUS is a DNA and RNA-binding protein localized in the nucleus where it plays various roles, including DNA repair, transcription, splicing, and translation (Lagier-Tourenne et al., 2012). The mutant FUS forms toxic ribonucleoprotein inclusion bodies, however, and accumulates in the cytoplasm (Sharma et al., 2016; Yang et al., 2015). FUS regulates circRNA biogenesis in motor neurons (MNs) by binding to the exon-intron junctions. When FUS was depleted with RNAi in neural crest-derived neuroblasts (N2A), or with MNs derived from FUS knockout mice, many circRNAs were down-regulated (Errichelli et al., 2017; Verheijen and Pasterkamp, 2017).
Similarly, TAR DNA binding protein 43 (TDP-43), which is a nuclear RNA- and DNA-binding protein, regulates transcription, and RNA splicing. However, when mislocalized to the cytoplasm, TDP-43 forms toxic inclusions that cause sporadic ALS (Lagier-Tourenne et al., 2012; Mackenzie et al., 2010). The toxicity of TDP-43 inclusions can be reduced by inhibiting DBR1, an enzyme responsible for linearizing the circular intronic lariat during splicing events (Armakola et al., 2012). Suppression of DBR1, therefore, facilitates the accumulation of circular intronic lariats and sequestration of TDP-43. Although definitive evidence is lacking about whether these circRNAs play any role in regulating the progression of ALS due to FUS deletion/mutation or DBRI inhibition, these studies indicate the possibility of circRNAs playing a role in ALS.
8.4. Multiple sclerosis:
Multiple sclerosis (MS) is a relapsing or progressive immune-mediated, inflammatory, a demyelinating disease characterized by the loss of oligodendrocytes (Dutta and Trapp, 2014). Due to the heterogeneous nature of this disease, the treatment and prognosis of MS are quite challenging. It has been proposed that various ncRNAs, including miRNAs and lncRNAs, play a role in regulating gene expression and outcomes in MS (Cardamone et al., 2018; Du et al., 2009). A recent study showed that >400 circRNAs are differentially expressed in the leucocytes of MS patients with the majority of them being down-regulated (Iparraguirre et al., 2017). The interplay between various ncRNAs could be significant in regulating the MS-associated alternative splicing events. For instance, lncRNAs upregulated in MS patients can modulate the expression of splicing regulatory genes, thereby affecting the global splicing and back-splicing processes (Cardamone et al., 2018). In particular, a lncRNA called MALAT1 was shown to modulate back-splicing and expression of 49 circRNAs, thus contributing to the heterogeneity in the pathogenesis of MS (Cardamone et al., 2018).
8.5. Multiple system atrophy:
Multiple system atrophy (MSA) is a rare, sporadic, and rapidly progressive neurodegenerative disorder that affects the involuntary functions of the body, including breathing and blood pressure. Many of the symptoms of MSA, including slow movement, rigid muscles, and poor balance, are similar to PD (Brisinda et al., 2014; Lipp et al., 2009; Yamasaki et al., 2019). Moreover, pathological α-Syn accumulation in the brains of MSA patients seems to play a leading role in MSA disease progression (Prusiner et al., 2015; Yamasaki et al., 2019). Altered expression of miRNAs in the cerebellum, serum, and cerebrospinal fluid, and long intergenic ncRNAs in the frontal cortex of MSA patients has been reported (Kume et al., 2018; Lee et al., 2015; Marques et al., 2017; Mills et al., 2016). In an MSA mouse model, early changes in miRNA-mRNAs interactions were thought to precede the clinical onset of the disease (Schafferer et al., 2016). Intriguingly, circRNAs IQCK, MAP4K3, EFCAB11, DTNA, and MCTP1 were overexpressed, whereas their linear transcripts were not altered in the frontal cortex of MSA patients (Chen et al., 2016). This difference also suggests that in specific pathologies, expression of circRNAs and their parent linear RNAs is controlled independently (Rybak-Wolf et al., 2015; Szabo et al., 2015). Notably, there appear to be circRNA hotspots from which more than one circRNAs are produced, and 21 such genes in the MSA transcriptome were reported to individually form over 10 circRNAs (Chen et al., 2016). However, the precise role of circRNAs in MSA pathology is yet to be identified.
9. CircRNAs and other CNS disorders:
The circRNAs have also been shown to play a role in methamphetamine addiction (Li et al., 2019a). In human postmortem schizophrenia brain samples, levels of many circRNAs were observed to be lower than in healthy controls (Mahmoudi et al., 2019), although the significance of these changes is not yet known. A recent study showed that high-fat, diet-induced diabetes significantly alters the circRNA expression profiles in the brain cortex of adult mice; more importantly, they found a correlated expression of circRNAs and their linear counterpart mRNAs, indicating the transcriptional control (Yoon et al., 2019). In addition to these findings, circRNA levels were reported to be altered in the brains of patients with psychiatric disorders such as a major depressive disorder (MDD) (Cui et al., 2016; Zhang et al., 2018b). For example, levels of hsa_circRNA_103636 were significantly altered after 8weeks of antidepressant treatment in MDD patients (Cui et al., 2016). Furthermore, circDYM (which is formed from the exons 4, 5, and 6 of the DYM gene and acts as a miR9 sponge) was significantly decreased in the blood of patients with MDD and chronic unpredictable stress (Zhang et al., 2018b). CircDYM levels were also decreased in the blood of a lipopolysaccharide-induced depression-like mouse model (Zhang et al., 2018b). The increased expression of miR-9 is linked to microglial activation and inflammation (Yao et al., 2014). When overexpressed, circDYM ameliorated depression-like behavior by sponging miR-9 and inhibiting microglial activation (Zhang et al., 2018b). These effects of circDYM were mediated in part by HECTD1, which is repressed by miR-9 and derepressed by circDYM overexpression (Zhang et al., 2018b).
10. Advances and potential challenges in circRNA-based therapies
Altered levels of circRNAs during development or disease conditions can change the functional dynamics of the cell by regulating gene expression. Accumulated evidence suggests that manipulation of circRNAs by knockdown, overexpression, and gain- and loss-of-function mutations are potentially beneficial in alleviating the effects of a disease (Santer et al., 2019). Specific circRNAs can be knocked-down with siRNAs and adenovirus or lentivirus encoded shRNAs (Du et al., 2017; Holdt et al., 2016; Liu et al., 2017a; Shan et al., 2017). One of the challenges of siRNA/shRNA-mediated circRNA knockdown is the ability to target backsplice junction sites to avoid the nonspecific targeting of linear host RNAs. An interesting much needed future strategy in circRNA based therapies is to develop methods to prevent formation of a specific circRNA in addition to knocking-down an already formed mature circRNA (Holdt et al., 2018). A recent study used CRISPR/Cas9 genome-editing to delete circRNA CDR1as locus to understand the functional and biological relevance of CDR1as in the mammalian brain (Piwecka et al., 2017). Using a similar approach, circGCN1L1 expression was abolished without affecting the transcription of the linear GCN1L1mRNA by excising the intronic complement sequence of the circGCN1L1-flanking introns (Zhang et al., 2016b). The overexpression of a specific circRNA can be achieved with an adenoviral or a lentiviral vector carrying the circRNA sequence (Bai et al., 2018; Hansen et al., 2013). US FDA recently approved Zolgensma, which is an adenovirus-based gene therapy to correct bi-allelic mutations in survival motor neuron 1 (SMN1) gene and LUXTURNA (voretigene neparvovec-rzyl) to deliver a normal copy of the RPE65 gene to correct a biallelic RPE65 mutation in patients with retinal dystrophy (Domenger and Grimm, 2019; Keeler and Flotte, 2019). Hence, gene-therapy based approaches to modulate circRNAs are therapeutically feasible in future. Delivering a synthetic functional RNA circle to a cell with a goal to increase specific circRNA levels is also a viable future approach. Natural linear RNAs can be produced by in vitro transcription followed by chemical or enzymatic ring closure. With this strategy, the linear precursors can be entropically disfavored if the circle size is large (Muller and Appel, 2017; Petkovic and Muller, 2018). However, there are several challenges that need to be addressed before circRNAs are ready for the therapy, including the generation, stability, delivery and potential side effects; nevertheless, the development of circRNA-based therapies gives an opportunity to resolve the circRNA dysregulation and the resulting detrimental changes during neurological diseases.
One clinically-relevant aspect of targeted gene therapy is the cell type-specific expression and biological activity of ncRNAs. The circRNAs are found to be more predominant than linear RNAs for many mammalian genomic loci that produce both (Salzman et al., 2013).
Further, the splicing pattern of genes to form circRNAs and linear RNAs is well-orchestrated to produce specific ratios of the two in certain cell-types (Salzman et al., 2013). Thus, to develop cell-specific gene therapy, it is crucial to determine the transcriptome complexity of individual cells to produce a circRNA and a linear RNA from a gene locus. To answer this issue, a wide variety of single-cell RNA-seq methods, including Smart-seq, CEL-Seq, Quartz-Seq and single-cell universal poly(A)-independent RNA sequencing (SUPeR-seq) have been developed (Fan et al., 2015; Tang et al., 2009). Using this approach, Verboom et al have recently detected circRNAs in addition to linear RNA biotypes (Verboom et al., 2019). At this time it is still in its infancy, but accumulating evidence indicates that there is a possibility to engineer lentiviral vectors to modulate circRNAs in a cell-specific manner (Kasaraneni et al., 2018; Yang et al., 2009; Yang et al., 2006; Zhou and Buchholz, 2013).
11. Conclusions
CircRNAs are incipient ncRNAs with potential regulatory properties. CircRNAs are expressed in all tissues but are more abundant in CNS. Recent studies suggest that circRNAs are produced from several neuronal-specific genes, indicating their possible involvement in brain development and synaptic plasticity. CircRNA perturbation might be linked to neurodegenerative diseases and secondary brain damage following acute CNS injuries. A further understanding of the functions of circRNAs and their interaction with other classes of ncRNAs and transcriptional/translational mechanisms will be valuable for identifying new therapeutic paradigms to treat CNS complications.
Supplementary Material
Highlights.
Circular RNAs (circRNAs) are vastly conserved noncoding RNAs formed by back-splicing and the covalent fusion of RNA free ends into natural circles.
They are highly abundant, dynamically expressed, and spatiotemporally regulated in the healthy brain.
Their brain expression steadily increases from embryonic to the adult stage.
The putative functions of circRNAs are transcription regulation, sequestration of miRNAs and ribonucleoproteins, and translation to peptides.
Altered levels of circRNAs mediate brain diseases and degeneration.
Acknowledgments:
The study was partly supported by the United States National Institute of Health Grants RO1 NS099531, NS 109459 and NS101960, and UW ICTR Pilot award AAH1544. The authors wish to thank Dr. Gopal Pandi and Mr. Anil Chokkalla for help with literature review.
Abbreviations
- AD
Alzheimer′s disease
- ADAR
adenosine deaminase acting-on RNA
- ALS
Amyotrophic lateral sclerosis
- APP
amyloid precursor protein
- α-Syn
α-synuclein
- Aβ
amyloid-β
- BACE1
β-site APP-cleaving enzyme 1
- BBB
blood-brain barrier
- CDK2
cyclin-dependent kinase 2
- CDR1
cerebellar degeneration-related autoantigen 1
- CDR1as
CDR1, antisense
- circRNAs
circular RNAs
- CNS
central nervous system
- DBR1
debranching enzyme
- DCC
Deleted in Colorectal Cancer
- ddPCR
droplet digital PCR
- DHX9
DExD/H-box helicase 9
- ETS-1
E26 transformation–specific sequence-1
- FUS
fused in sarcoma
- GO
Gene Ontology
- GSK-3β
glycogen synthase kinase-3β
- HI
hypoxia-ischemia
- HIPK3
homeodomain interacting protein kinase 3
- HNF1β
hepatocyte nuclear factor-1β
- IRES
internal ribosome entry site
- lncRNAs
long noncoding RNAs
- m6A
N6-methyladenosine
- MALAT1
Metastasis Associated Lung Adenocarcinoma Transcript 1
- Mbl
muscleblind
- MCAO
middle cerebral artery occlusion
- MDD
major depressive disorder
- METTL3
methyltransferase-like 3
- miRNAs
microRNAs
- MLL
mixed-lineage leukemia
- MNs
motor neurons
- MS
Multiple sclerosis
- MSA
Multiple system atrophy
- ncRNAs
noncoding RNAs
- OGD
oxygen glucose deprivation
- ORFs
open reading frames
- PD
Parkinson’s disease
- PINT87aa
87 amino acid tumor-suppressive peptide
- PAF1
polymerase associated factor
- pre mRNA
precursor mRNA
- Pol II
polymerase II
- PRR11
proline-rich protein 11
- RBPs
RNA binding proteins
- RNA-FISH
RNA fluorescence in situ hybridization
- ROS
reactive oxygen species
- SCI
spinal cord injury
- SIRT1
Sirtuin 1
- SMN1
survival motor neuron 1
- SOX13
SRY-Box 13
- SRSF1
serine and arginine-rich splicing factor 1
- SRY
sex-determining region Y
- TBI
traumatic brain injury
- TDP-43
TAR DNA binding protein 43
- TGF-β
transformed growth factor
- TIPARP
2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD)-inducible poly (ADP-ribose) polymerase
- TNF-α
tumor necrosis factor-α
- T-UCRs
transcribed ultraconserved regions
- UBE2A
Ubiquitin protein ligase A
- UCHL1
Ubiquitin C-Terminal Hydrolase L1
- UTR
untranslated region
- U1snRNP
U1 small nuclear ribonucleoprotein particle
- VEGF-A
Vascular endothelial growth factor A
- ZO-1
Zonula occludens-1
Footnotes
Conflict of Interest- Authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aktas T, Avsar Ilik I, Maticzka D, Bhardwaj V, Pessoa Rodrigues C, Mittler G, Manke T, Backofen R, Akhtar A, 2017. DHX9 suppresses RNA processing defects originating from the Alu invasion of the human genome. Nature 544, 115119. [DOI] [PubMed] [Google Scholar]
- Armakola M, Higgins MJ, Figley MD, Barmada SJ, Scarborough EA, Diaz Z, Fang X, Shorter J, Krogan NJ, Finkbeiner S, Farese RV Jr., Gitler AD, 2012. Inhibition of RNA lariat debranching enzyme suppresses TDP-43 toxicity in ALS disease models. Nat Genet 44, 1302–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnberg AC, Van Ommen GJ, Grivell LA, Van Bruggen EF, Borst P, 1980. Some yeast mitochondrial RNAs are circular. Cell 19, 313–319. [DOI] [PubMed] [Google Scholar]
- Ashwal-Fluss R, Meyer M, Pamudurti NR, Ivanov A, Bartok O, Hanan M, Evantal N, Memczak S, Rajewsky N, Kadener S, 2014. circRNA biogenesis competes with pre-mRNA splicing. Mol Cell 56, 55–66. [DOI] [PubMed] [Google Scholar]
- Bai Y, Zhang Y, Han B, Yang L, Chen X, Huang R, Wu F, Chao J, Liu P, Hu G, Zhang JH, Yao H, 2018. Circular RNA DLGAP4 Ameliorates Ischemic Stroke Outcomes by Targeting miR-143 to Regulate Endothelial-Mesenchymal Transition Associated with Blood-Brain Barrier Integrity. J Neurosci 38, 32–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailleul B, 1996. During in vivo maturation of eukaryotic nuclear mRNA, splicing yields excised exon circles. Nucleic Acids Res 24, 1015–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbagallo D., Caponnetto A., Brex D., Mirabella F., Barbagallo C., Lauretta G., Morrone A., Cert F., Broggi G., Caltabiano R., Barbagallo GM., SpinaPurrello V., Ragusa M., Di Pietro C., Hanse TB., Purrell M., 2019. CircSMARCA5 Regulates VEGFA mRNA Splicing and Angiogenesis in Glioblastoma Multiforme Through the Binding of SRSF1. Cancers 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett SP, Wang PL, Salzman J, 2015. Circular RNA biogenesis can proceed through an exon-containing lariat precursor. Elife 4, e07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian A, Wang Y, Liu J, Wang X, Liu D, Jiang J, Ding L, Hui X, 2018. Circular RNA Complement Factor H (CFH) Promotes Glioma Progression by Sponging miR-149 and Regulating AKT1. Med Sci Monit 24, 5704–5712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeckel JN, Jae N, Heumuller AW, Chen W, Boon RA, Stellos K, Zeiher AM, John D, Uchida S, Dimmeler S, 2015. Identification and Characterization of Hypoxia-Regulated Endothelial Circular RNA. Circ Res 117, 884–890. [DOI] [PubMed] [Google Scholar]
- Brisinda D, Sorbo AR, Di Giacopo R, Venuti A, Bentivoglio AR, Fenici R, 2014. Cardiovascular autonomic nervous system evaluation in Parkinson disease and multiple system atrophy. J Neurol Sci 336, 197–202. [DOI] [PubMed] [Google Scholar]
- Caldas C, So CW, MacGregor A, Ford AM, McDonald B, Chan LC, Wiedemann LM, 1998. Exon scrambling of MLL transcripts occur commonly and mimic partial genomic duplication of the gene. Gene 208, 167–176. [DOI] [PubMed] [Google Scholar]
- Capel B, Swain A, Nicolis S, Hacker A, Walter M, Koopman P, Goodfellow P, Lovell-Badge R, 1993. Circular transcripts of the testis-determining gene Sry in adult mouse testis. Cell 73, 1019–1030. [DOI] [PubMed] [Google Scholar]
- Cardamone G, Paraboschi EM, Solda G, Cantoni C, Supino D, Piccio L, Duga S, Asselta R, 2018. Not only cancer: the long non-coding RNA MALAT1 affects the repertoire of alternatively spliced transcripts and circular RNAs in multiple sclerosis. Hum Mol Genet. [DOI] [PubMed] [Google Scholar]
- Chandran R, Mehta SL, Vemuganti R, 2017. Non-coding RNAs and neuroprotection after acute CNS injuries. Neurochemistry international, DOI: 10.1016/j.neuint.2017.1001.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BJ, Huang S, Janitz M, 2018a. Changes in circular RNA expression patterns during human foetal brain development. Genomics. [DOI] [PubMed] [Google Scholar]
- Chen BJ, Mills JD, Takenaka K, Bliim N, Halliday GM, Janitz M, 2016. Characterization of circular RNAs landscape in multiple system atrophy brain. J Neurochem 139, 485–496. [DOI] [PubMed] [Google Scholar]
- Chen D-F., Zhang L-J., Tan K., Jing Q., 2018b. Application of droplet digital PCR in quantitative detection of the cell-free circulating circRNAs. Biotechnology & Biotechnological Equipment 32, 116–123. [Google Scholar]
- Chen J, Zou Q, Lv D, Raza MA, Wang X, Li P, Chen Y, Xi X, Wen A, Zhu L, Tang G, Li M, Li X, Jiang Y, 2019a. Comprehensive transcriptional profiling of porcine brain aging. Gene 693, 1–9. [DOI] [PubMed] [Google Scholar]
- Chen W, Schuman E, Circular RNAs in Brain and Other Tissues: A Functional Enigma. Trends in Neurosciences 39, 597–604. [DOI] [PubMed] [Google Scholar]
- Chen W, Schuman E, 2016. Circular RNAs in Brain and Other Tissues: A Functional Enigma. Trends Neurosci 39, 597–604. [DOI] [PubMed] [Google Scholar]
- Chen YG, Chen R, Ahmad S, Verma R, Kasturi SP, Amaya L, Broughton JP, Kim J, Cadena C, Pulendran B, Hur S, Chang HY, 2019b. N6-Methyladenosine Modification Controls Circular RNA Immunity. Mol Cell 76, 96109.e109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu C, Yuan C, Liu X, Yao L, Zhu J, He J, Kwon SW, Huang X, 2015. Phenylobacterium kunshanense sp. nov., isolated from the sludge of a pesticide manufacturing factory. Int J Syst Evol Microbiol 65, 325–330. [DOI] [PubMed] [Google Scholar]
- Conn SJ, Pillman KA, Toubia J, Conn VM, Salmanidis M, Phillips CA, Roslan S, Schreiber AW, Gregory PA, Goodall GJ, 2015. The RNA binding protein quaking regulates formation of circRNAs. Cell 160, 1125–1134. [DOI] [PubMed] [Google Scholar]
- Cui X, Niu W, Kong L, He M, Jiang K, Chen S, Zhong A, Li W, Lu J, Zhang L, 2016. hsa_circRNA_103636: potential novel diagnostic and therapeutic biomarker in Major depressive disorder. Biomark Med 10, 943–952. [DOI] [PubMed] [Google Scholar]
- Czech B, Hannon GJ, 2011. Small RNA sorting: matchmaking for Argonautes. Nature reviews. Genetics 12, 19–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danan M, Schwartz S, Edelheit S, Sorek R, 2012. Transcriptome-wide discovery of circular RNAs in Archaea. Nucleic acids research 40, 3131–3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharap A, Bowen K, Place R, Li LC, Vemuganti R, 2009. Transient focal ischemia induces extensive temporal changes in rat cerebral microRNAome. J Cereb Blood Flow Metab 29, 675–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharap A, Nakka VP, Vemuganti R, 2011. Altered expression of PIWI RNA in the rat brain after transient focal ischemia. Stroke 42, 1105–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharap A, Nakka VP, Vemuganti R, 2012. Effect of focal ischemia on long noncoding RNAs. Stroke 43, 2800–2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharap A., Pokrzywa C., Murali S., Pandi G., Vemuganti R., 2013a. MicroRNA miR324–3p induces promoter-mediated expression of RelA gene. PLoS One 8, e79467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharap A., Pokrzywa C, Vemuganti R, 2013b. Increased binding of stroke-induced long non-coding RNAs to the transcriptional corepressors Sin3A and coREST. ASN neuro 5, 283–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diederichs S, 2014. The four dimensions of noncoding RNA conservation. Trends Genet 30, 121–123. [DOI] [PubMed] [Google Scholar]
- Domenger C, Grimm D, 2019. Next-generation AAV vectors - don’t judge a virus (only) by its cover. Hum Mol Genet. [DOI] [PubMed] [Google Scholar]
- Du C, Liu C, Kang J, Zhao G, Ye Z, Huang S, Li Z, Wu Z, Pei G, 2009. MicroRNA miR-326 regulates TH-17 differentiation and is associated with the pathogenesis of multiple sclerosis. Nat Immunol 10, 1252–1259. [DOI] [PubMed] [Google Scholar]
- Du WW, Yang W, Chen Y, Wu ZK, Foster FS, Yang Z, Li X, Yang BB, 2017. Foxo3 circular RNA promotes cardiac senescence by modulating multiple factors associated with stress and senescence responses. Eur Heart J 38, 1402–1412. [DOI] [PubMed] [Google Scholar]
- Duan X, Li L, Gan J, Peng C, Wang X, Chen W, Peng D, 2019. Identification and functional analysis of circular RNAs induced in rats by middle cerebral artery occlusion. Gene 701, 139–145. [DOI] [PubMed] [Google Scholar]
- Duan X, Liu D, Wang Y, Chen Z, 2018. Circular RNA hsa_circ_0074362 Promotes Glioma Cell Proliferation, Migration, and Invasion by Attenuating the Inhibition of miR-1236–3p on HOXB7 Expression. DNA Cell Biol 37, 917–924. [DOI] [PubMed] [Google Scholar]
- Dutta R, Trapp BD, 2014. Relapsing and progressive forms of multiple sclerosis: insights from pathology. Current opinion in neurology 27, 271–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Errichelli L., Dini Modigliani S., Laneve P., Colantoni A., Legnini I., Capauto D., Rosa A., De Santis R., Scarfo R., Peruzzi G., Lu L., Caffarelli E., Shneider NA., Morlando M., Bozzoni I., 2017. FUS affects circular RNA expression in murine embryonic stem cell-derived motor neurons. Nat Commun 8, 14741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X, Zhang X, Wu X, Guo H, Hu Y, Tang F, Huang Y, 2015. Single-cell RNA-seq transcriptome analysis of linear and circular RNAs in mouse preimplantation embryos. Genome Biol 16, 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudet AD, Mandrekar-Colucci S, Hall JC, Sweet DR, Schmitt PJ, Xu X, Guan Z, Mo X, Guerau-de-Arellano M, Popovich PG, 2016. miR-155 Deletion in Mice Overcomes Neuron-Intrinsic and Neuron-Extrinsic Barriers to Spinal Cord Repair. J Neurosci 36, 8516–8532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruner H, Cortes-Lopez M, Cooper DA, Bauer M, Miura P, 2016. CircRNA accumulation in the aging mouse brain. Sci Rep 6, 38907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han B, Zhang Y, Zhang Y, Bai Y, Chen X, Huang R, Wu F, Leng S, Chao J, Zhang JH, Hu G, Yao H, 2018. Novel insight into circular RNA HECTD1 in astrocyte activation via autophagy by targeting MIR142-TIPARP: implications for cerebral ischemic stroke. Autophagy 14, 1164–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen TB, Jensen TI, Clausen BH, Bramsen JB, Finsen B, Damgaard CK, Kjems J, 2013. Natural RNA circles function as efficient microRNA sponges. Nature 495, 384–388. [DOI] [PubMed] [Google Scholar]
- Hansen TB, Wiklund ED, Bramsen JB, Villadsen SB, Statham AL, Clark SJ, Kjems J, 2011. miRNA-dependent gene silencing involving Ago2-mediated cleavage of a circular antisense RNA. The EMBO journal 30, 4414–4422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Xie Q, Xu H, Li J, Li Y, 2017. Circular RNAs and cancer. Cancer Lett 396, 138–144. [DOI] [PubMed] [Google Scholar]
- He Q, Zhao L, Liu Y, Liu X, Zheng J, Yu H, Cai H, Ma J, Liu L, Wang P, Li Z, Xue Y, 2018. circ-SHKBP1 Regulates the Angiogenesis of U87 Glioma-Exposed Endothelial Cells through miR-544a/FOXP1 and miR-379/FOXP2 Pathways. Molecular therapy. Nucleic acids 10, 331–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z, Ruan X, Liu X, Zheng J, Liu Y, Liu L, Ma J, Shao L, Wang D, Shen S, Yang C, Xue Y, 2019. FUS/circ_002136/miR-138–5p/SOX13 feedback loop regulates angiogenesis in Glioma. J Exp Clin Cancer Res 38, 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindson CM, Chevillet JR, Briggs HA, Gallichotte EN, Ruf IK, Hindson BJ, Vessella RL, Tewari M, 2013. Absolute quantification by droplet digital PCR versus analog real-time PCR. Nature methods 10, 1003–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdt LM, Kohlmaier A, Teupser D, 2018. Circular RNAs as Therapeutic Agents and Targets. Frontiers in physiology 9, 1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdt LM., Stahringer A., Sass K., Pichler G., Kulak NA., Wilfert W., Kohlmaier A., Herbst A., Northoff BH., Nicolaou A., Gabel G., Beutner F., Scholz M., Thiery J., Musunuru K., Krohn K., Mann M., Teupser D., 2016. Circular noncoding RNA ANRIL modulates ribosomal RNA maturation and atherosclerosis in humans. Nat Commun 7, 12429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu MT, Coca-Prados M, 1979. Electron microscopic evidence for the circular form of RNA in the cytoplasm of eukaryotic cells. Nature 280, 339–340. [DOI] [PubMed] [Google Scholar]
- Hu D, Zhang Y, 2019. Circular RNA HIPK3 promotes glioma progression by binding to miR-124–3p. Gene 690, 81–89. [DOI] [PubMed] [Google Scholar]
- Huang JL, Xu ZH, Yang SM, Yu C, Zhang F, Qin MC, Zhou Y, Zhong ZG, Wu DP, 2018. Identification of Differentially Expressed Profiles of Alzheimer’s Disease Associated Circular RNAs in a Panax Notoginseng Saponins-Treated Alzheimer’s Disease Mouse Model. Computational and structural biotechnology journal 16, 523–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idda ML, Munk R, Abdelmohsen K, Gorospe M, 2018. Noncoding RNAs in Alzheimer’s disease. Wiley Interdiscip Rev RNA 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iparraguirre L, Munoz-Culla M, Prada-Luengo I, Castillo-Trivino T, Olascoaga J, Otaegui D, 2017. Circular RNA profiling reveals that circular RNAs from ANXA2 can be used as new biomarkers for multiple sclerosis. Hum Mol Genet 26, 35643572. [DOI] [PubMed] [Google Scholar]
- Ivanov A, Memczak S, Wyler E, Torti F, Porath HT, Orejuela MR, Piechotta M, Levanon EY, Landthaler M, Dieterich C, Rajewsky N, 2015. Analysis of intron sequences reveals hallmarks of circular RNA biogenesis in animals. Cell Rep 10, 170–177. [DOI] [PubMed] [Google Scholar]
- Jeck WR, Sharpless NE, 2014. Detecting and characterizing circular RNAs. Nat Biotechnol 32, 453–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeck WR, Sorrentino JA, Wang K, Slevin MK, Burd CE, Liu J, Marzluff WF, Sharpless NE, 2013. Circular RNAs are abundant, conserved, and associated with ALU repeats. Rna 19, 141–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji P, Wu W, Chen S, Zheng Y, Zhou L, Zhang J, Cheng H, Yan J, Zhang S, Yang P, Zhao F, 2019. Expanded Expression Landscape and Prioritization of Circular RNAs in Mammals. Cell Rep 26, 3444–3460.e3445. [DOI] [PubMed] [Google Scholar]
- Jiang L, Li H, Fan Z, Zhao R, Xia Z, 2019a. Circular RNA expression profiles in neonatal rats following hypoxic-ischemic brain damage. Int J Mol Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang YJ, Cao SQ, Gao LB, Wang YY, Zhou B, Hu X, Pu Y, Li ZL, Wang Q, Xiao X, Zhao L, Wang S, Liang WB, Zhang L, 2019b. Circular Ribonucleic Acid Expression Profile in Mouse Cortex after Traumatic Brain Injury. Journal of neurotrauma 36, 1018–1028. [DOI] [PubMed] [Google Scholar]
- Jin C, Lin JR, Ma L, Song Y, Shi YX, Jiang P, Dong Y, Li XS, 2017. Elevated spondin-2 expression correlates with progression and prognosis in gastric cancer. Oncotarget 8, 10416–10424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junn E, Lee KW, Jeong BS, Chan TW, Im JY, Mouradian MM, 2009. Repression of alpha-synuclein expression and toxicity by microRNA-7. Proc Natl Acad Sci U S A 106, 13052–13057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasaraneni N, Chamoun-Emanuelli AM, Wright GA, Chen Z, 2018. A simple strategy for retargeting lentiviral vectors to desired cell types via a disulfide-bondforming protein-peptide pair. Sci Rep 8, 10990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeler AM., Flotte TR., 2019. Recombinant Adeno-Associated Virus Gene Therapy in Light of Luxturna (and Zolgensma and Glybera): Where Are We, and How Did We Get Here? Annual review of virology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T, Mehta SL, Morris-Blanco KC, Chokkalla AK, Chelluboina B, Lopez M, Sullivan R, Kim HT, Cook TD, Kim JY, Kim H, Kim C, Vemuganti R, 2018. The microRNA miR-7a-5p ameliorates ischemic brain damage by repressing alpha-synuclein. Science signaling 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M, Ohsugi M, Sasako T, Awazawa M, Umehara T, Iwane A, Kobayashi N, Okazaki Y, Kubota N, Suzuki R, Waki H, Horiuchi K, Hamakubo T, Kodama T, Aoe S, Tobe K, Kadowaki T, Ueki K, 2018. The RNA Methyltransferase Complex of WTAP, METTL3, and METTL14 Regulates Mitotic Clonal Expansion in Adipogenesis. Mol Cell Biol 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocks C, Boltengagen A, Piwecka M, Rybak-Wolf A, Rajewsky N, 2018. SingleMolecule Fluorescence In Situ Hybridization (FISH) of Circular RNA CDR1as. Methods Mol Biol 1724, 77–96. [DOI] [PubMed] [Google Scholar]
- Kopczynski CC, Muskavitch MA, 1992. Introns excised from the Delta primary transcript are localized near sites of Delta transcription. The Journal of cell biology 119, 503–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kos A, Dijkema R, Arnberg AC, van der Meide PH, Schellekens H, 1986. The hepatitis delta (delta) virus possesses a circular RNA. Nature 323, 558–560. [DOI] [PubMed] [Google Scholar]
- Kraus TFJ, Haider M, Spanner J, Steinmaurer M, Dietinger V, Kretzschmar HA, 2017. Altered Long Noncoding RNA Expression Precedes the Course of Parkinson’s Disease-a Preliminary Report. Mol Neurobiol 54, 2869–2877. [DOI] [PubMed] [Google Scholar]
- Kumar L, Shamsuzzama, Jadiya P, Haque R, Shukla S, Nazir A, 2018. Functional Characterization of Novel Circular RNA Molecule, circzip-2 and Its Synthesizing Gene zip-2 in C. elegans Model of Parkinson’s Disease. Mol Neurobiol 55, 6914–6926. [DOI] [PubMed] [Google Scholar]
- Kume K, Iwama H, Deguchi K, Ikeda K, Takata T, Kokudo Y, Kamada M, Fujikawa K, Hirose K, Masugata H, Touge T, Masaki T, 2018. Serum microRNA expression profiling in patients with multiple system atrophy. Mol Med Rep 17, 852–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwiatkowski TJ Jr., Bosco DA, Leclerc AL, Tamrazian E, Vanderburg CR, Russ C, Davis A, Gilchrist J, Kasarskis EJ, Munsat T, Valdmanis P, Rouleau GA, Hosler BA, Cortelli P, de Jong PJ, Yoshinaga Y, Haines JL, Pericak-Vance MA, Yan J, Ticozzi N, Siddique T, McKenna-Yasek D, Sapp PC, Horvitz HR, Landers JE, Brown RH Jr., 2009. Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science 323, 1205–1208. [DOI] [PubMed] [Google Scholar]
- Lagier-Tourenne C, Polymenidou M, Hutt KR, Vu AQ, Baughn M, Huelga SC, Clutario KM, Ling SC, Liang TY, Mazur C, Wancewicz E, Kim AS, Watt A, Freier S, Hicks GG, Donohue JP, Shiue L, Bennett CF, Ravits J, Cleveland DW, Yeo GW, 2012. Divergent roles of ALS-linked proteins FUS/TLS and TDP-43 intersect in processing long pre-mRNAs. Nat Neurosci 15, 1488–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee ST, Chu K, Jung KH, Ban JJ, Im WS, Jo HY, Park JH, Lim JY, Shin JW, Moon J, Lee SK, Kim M, Roh JK, 2015. Altered expression of miR202 in cerebellum of multiple-system atrophy. Mol Neurobiol 51, 180–186. [DOI] [PubMed] [Google Scholar]
- Legnini I., Di Timoteo G., Rossi F., Morlando M., Briganti F., Sthandier O., Fatica A., Santini T., Andronache A., Wade M., Laneve P., Rajewsky N., Bozzoni I., 2017a. Circ-ZNF609 Is a Circular RNA that Can Be Translated and Functions in Myogenesis. Mol Cell 66, 22–37 e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legnini I, Di Timoteo G, Rossi F, Morlando M, Briganti F, Sthandier O, Fatica A, Santini T, Andronache A, Wade M, Laneve P, Rajewsky N, Bozzoni I, 2017b. Circ-ZNF609 Is a Circular RNA that Can Be Translated and Functions in Myogenesis. Mol Cell 66, 22–37.e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei B, Huang Y, Zhou Z, Zhao Y, Thapa AJ, Li W, Cai W, Deng Y, 2019. Circular RNA hsa_circ_0076248 promotes oncogenesis of glioma by sponging miR-181a to modulate SIRT1 expression. J Cell Biochem 120, 6698–6708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Ma K, Sun M, Shi S, 2018a. Identification of the tumor-suppressive function of circular RNA ITCH in glioma cells through sponging miR-214 and promoting linear ITCH expression. American journal of translational research 10, 1373–1386. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Li G, Yang H, Han K, Zhu D, Lun P, Zhao Y, 2018b. A novel circular RNA, hsa_circ_0046701, promotes carcinogenesis by increasing the expression of miR-142–3p target ITGB8 in glioma. Biochem Biophys Res Commun 498, 254–261. [DOI] [PubMed] [Google Scholar]
- Li J, Shi Q, Wang Q, Tan X, Pang K, Liu X, Zhu S, Xi K, Zhang J, Gao Q, Hu Y, Sun J, 2019a. Profiling circular RNA in methamphetamine-treated primary cortical neurons identified novel circRNAs related to methamphetamine addiction. Neurosci Lett 701, 146–153. [DOI] [PubMed] [Google Scholar]
- Li S, Teng S, Xu J, Su G, Zhang Y, Zhao J, Zhang S, Wang H, Qin W, Lu ZJ, Guo Y, Zhu Q, Wang D, 2019b. Microarray is an efficient tool for circRNA profiling. Brief Bioinform 20, 1420–1433. [DOI] [PubMed] [Google Scholar]
- Li X, Diao H, 2019a. Circular RNA circ_0001946 acts as a competing endogenous RNA to inhibit glioblastoma progression by modulating miR-671–5p and CDR1. J Cell Physiol. [DOI] [PubMed] [Google Scholar]
- Li X, Diao H, 2019b. Circular RNA circ_0001946 acts as a competing endogenous RNA to inhibit glioblastoma progression by modulating miR-671–5p and CDR1. J Cell Physiol 234, 13807–13819. [DOI] [PubMed] [Google Scholar]
- Li Z., Huang C., Bao C., Chen L., Lin M., Wang X., Zhong G., Yu B., Hu W., Dai L., Zhu P., Chang Z., Wu Q., Zhao Y., Jia Y., Xu P., Liu H., Shan G., 2015. Exon-intron circular RNAs regulate transcription in the nucleus. Nat Struct Mol Biol 22, 256–264. [DOI] [PubMed] [Google Scholar]
- Liang D, Wilusz JE, 2014. Short intronic repeat sequences facilitate circular RNA production. Genes Dev 28, 2233–2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipp A, Sandroni P, Ahlskog JE, Fealey RD, Kimpinski K, Iodice V, Gehrking TL, Weigand SD, Sletten DM, Gehrking JA, Nickander KK, Singer W, Maraganore DM, Gilman S, Wenning GK, Shults CW, Low PA, 2009. Prospective differentiation of multiple system atrophy from Parkinson disease, with and without autonomic failure. Archives of neurology 66, 742–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Yao MD, Li CP, Shan K, Yang H, Wang JJ, Liu B, Li XM, Yao J, Jiang Q, Yan B, 2017a. Silencing Of Circular RNA-ZNF609 Ameliorates Vascular Endothelial Dysfunction. Theranostics 7, 2863–2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Zhang C, Yang J, Geng X, Du H, Ji X, Zhao H, 2017b. Screening circular RNA expression patterns following focal cerebral ischemia in mice. Oncotarget 8, 86535–86547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Yue Y, Han D, Wang X, Fu Y, Zhang L, Jia G, Yu M, Lu Z, Deng X, Dai Q, Chen W, He C, 2014. A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat Chem Biol 10, 93–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Cui B, Dai ZX, Shi PK, Wang ZH, Guo YY, 2016. Long Non-coding RNA HOTAIR Promotes Parkinson’s Disease Induced by MPTP Through upregulating the Expression of LRRK2. Current neurovascular research 13, 115–120. [DOI] [PubMed] [Google Scholar]
- Lukiw WJ, 2013. Circular RNA (circRNA) in Alzheimer’s disease (AD). Frontiers in genetics 4, 307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie IR, Rademakers R, Neumann M, 2010. TDP-43 and FUS in amyotrophic lateral sclerosis and frontotemporal dementia. The Lancet. Neurology 9, 995–1007. [DOI] [PubMed] [Google Scholar]
- Mahmoudi E, Cairns MJ, 2019. Circular RNAs are temporospatially regulated throughout development and ageing in the rat. Sci Rep 9, 2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoudi E., Fitzsimmons C., Geaghan MP., Shannon Weickert C., Atkins JR., Wang X., Cairns MJ., 2019. Circular RNA biogenesis is decreased in postmortem cortical gray matter in schizophrenia and may alter the bioavailability of associated miRNA. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 44, 1043–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majidinia M, Mihanfar A, Rahbarghazi R, Nourazarian A, Bagca B, Avci CB, 2016. The roles of non-coding RNAs in Parkinson’s disease. Mol Biol Rep 43, 1193–1204. [DOI] [PubMed] [Google Scholar]
- Marques TM, Kuiperij HB, Bruinsma IB, van Rumund A, Aerts MB, Esselink RAJ, Bloem BR, Verbeek MM, 2017. MicroRNAs in Cerebrospinal Fluid as Potential Biomarkers for Parkinson’s Disease and Multiple System Atrophy. Mol Neurobiol 54, 7736–7745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan KJ, Murray TK, Bengoa-Vergniory N, Cordero-Llana O, Cooper J, Buckley A, Wade-Martins R, Uney JB, O’Neill MJ, Wong LF, Caldwell MA, 2017. Loss of MicroRNA-7 Regulation Leads to alpha-Synuclein Accumulation and Dopaminergic Neuronal Loss In Vivo. Mol Ther 25, 2404–2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta SL, Kim T, Vemuganti R, 2015. Long Noncoding RNA FosDT Promotes Ischemic Brain Injury by Interacting with REST-Associated Chromatin-Modifying Proteins. J Neurosci 35, 16443–16449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta SL, Manhas N, Raghubir R, 2007. Molecular targets in cerebral ischemia for developing novel therapeutics. Brain research reviews 54, 34–66. [DOI] [PubMed] [Google Scholar]
- Mehta SL, Pandi G, Vemuganti R, 2017. Circular RNA Expression Profiles Alter Significantly in Mouse Brain After Transient Focal Ischemia. Stroke 48, 2541–2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner L, Gallozzi M, Balbi M, Schwarzmaier S, Tiedt S, Terpolilli NA, Plesnila N, 2016. Temporal Profile of MicroRNA Expression in Contused Cortex after Traumatic Brain Injury in Mice. Journal of neurotrauma 33, 713–720. [DOI] [PubMed] [Google Scholar]
- Memczak S, Jens M, Elefsinioti A, Torti F, Krueger J, Rybak A, Maier L, Mackowiak SD, Gregersen LH, Munschauer M, Loewer A, Ziebold U, Landthaler M, Kocks C, le Noble F, Rajewsky N, 2013. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature 495, 333–338. [DOI] [PubMed] [Google Scholar]
- Meng Z, Lei C, Chen X, Jiang S, 2016. Complete mitochondrial genome sequence of Heliconius melpomene rosina (Insecta: Lepidoptera: Nymphalidae). Mitochondrial DNA A DNA Mapp Seq Anal 27, 3911–3912. [DOI] [PubMed] [Google Scholar]
- Mfossa ACM, Puthenparampil HT, Coolkens A, Baatout S, Benotmane MA, Huylebroeck D, Quintens R, 2019. Exposure to Ionizing Radiation Triggers Prolonged Changes in Circular RNA Abundance in the Embryonic Mouse Brain and Primary Neurons. Cells 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills JD, Ward M, Kim WS, Halliday GM, Janitz M, 2016. Strand-specific RNA-sequencing analysis of multiple system atrophy brain transcriptome. Neuroscience 322, 234–250. [DOI] [PubMed] [Google Scholar]
- Mo D, Cui D, Li X, 2018. The role of Aβ circRNA in Alzheimer′s disease: alternative mechanism of Aβ biogenesis from Aβ circRNA translation. bioRxiv, 260968. [Google Scholar]
- Muller S, Appel B, 2017. In vitro circularization of RNA. RNA Biol 14, 1018–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigro JM, Cho KR, Fearon ER, Kern SE, Ruppert JM, Oliner JD, Kinzler KW, Vogelstein B, 1991. Scrambled exons. Cell 64, 607–613. [DOI] [PubMed] [Google Scholar]
- Okamoto T., Mandai M., Matsumura N., Yamaguchi K., Kondoh H., Amano Y., Baba T., Hamanishi J., Abiko K., Kosaka K., Murphy SK., Mori S., Konishi I., 2015. Hepatocyte nuclear factor-1beta (HNF-1beta) promotes glucose uptake and glycolytic activity in ovarian clear cell carcinoma. Molecular carcinogenesis 54, 35–49. [DOI] [PubMed] [Google Scholar]
- Paim LR, Schreiber R, de Rossi G, Matos-Souza JR, Costa ESAA, Calegari DR, Cheng S, Marques FZ, Sposito AC, Gorla JI, Cliquet A Jr., Nadruz W Jr., 2019. Circulating microRNAs, Vascular Risk, and Physical Activity in Spinal Cord-Injured Subjects. Journal of neurotrauma 36, 845–852. [DOI] [PubMed] [Google Scholar]
- Pamudurti NR, Bartok O, Jens M, Ashwal-Fluss R, Stottmeister C, Ruhe L, Hanan M, Wyler E, Perez-Hernandez D, Ramberger E, Shenzis S, Samson M, Dittmar G, Landthaler M, Chekulaeva M, Rajewsky N, Kadener S, 2017a. Translation of CircRNAs. Mol Cell 66, 9–21.e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamudurti NR, Bartok O, Jens M, Ashwal-Fluss R, Stottmeister C, Ruhe L, Hanan M, Wyler E, Perez-Hernandez D, Ramberger E, Shenzis S, Samson M, Dittmar G, Landthaler M, Chekulaeva M, Rajewsky N, Kadener S, 2017b. Translation of CircRNAs. Mol Cell 66, 9–21 e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey PR, Munk R, Kundu G, De S, Abdelmohsen K, Gorospe M, 2020. Methods for analysis of circular RNAs. Wiley Interdiscip Rev RNA 11, e1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandi G, Nakka VP, Dharap A, Roopra A, Vemuganti R, 2013. MicroRNA miR-29c down-regulation leading to de-repression of its target DNA methyltransferase 3a promotes ischemic brain damage. PLoS One 8, e58039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkovic S, Muller S, 2018. Synthesis and Engineering of Circular RNAs. Methods Mol Biol 1724, 167–180. [DOI] [PubMed] [Google Scholar]
- Piwecka M, Glazar P, Hernandez-Miranda LR, Memczak S, Wolf SA, Ryba-kWolf A, Filipchyk A, Klironomos F, Cerda Jara CA, Fenske P, Trimbuch T, Zywitza V, Plass M, Schreyer L, Ayoub S, Kocks C, Kuhn R, Rosenmund C, Birchmeier C, Rajewsky N, 2017. Loss of a mammalian circular RNA locus causes miRNA deregulation and affects brain function. Science 357. [DOI] [PubMed] [Google Scholar]
- Prusiner SB., Woerman AL., Mordes DA., Watts JC., Rampersaud R., Berry DB., Patel S., Oehler A., Lowe JK., Kravitz SN., Geschwind DH., Glidden DV., Halliday GM., Middleton LT., Gentleman SM., Grinberg LT., Giles K., 2015. Evidence for alpha-synuclein prions causing multiple system atrophy in humans with parkinsonism. Proc Natl Acad Sci U S A 112, E5308–5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian L, Vu MN, Carter M, Wilkinson MF, 1992. A spliced intron accumulates as a lariat in the nucleus of T cells. Nucleic acids research 20, 5345–5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin C, Liu CB, Yang DG, Gao F, Zhang X, Zhang C, Du LJ, Yang ML, Li JJ, 2018. Circular RNA Expression Alteration and Bioinformatics Analysis in Rats After Traumatic Spinal Cord Injury. Frontiers in molecular neuroscience 11, 497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin CA, Sun Q, Gamblin TC, 2007. Tau phosphorylation by GSK-3beta promotes tangle-like filament morphology. Molecular neurodegeneration 2, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao J, Cheng X, Zhu H, Wang L, Liu L, 2018. Circular RNA-0007874 (circMTO1) reverses chemoresistance to temozolomide by acting as a sponge of microRNA630 in glioblastoma. Cell Biol Int. [DOI] [PubMed] [Google Scholar]
- Redell JB, Moore AN, Ward NH 3rd, Hergenroeder GW, Dash PK, 2010. Human traumatic brain injury alters plasma microRNA levels. Journal of neurotrauma 27, 2147–2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybak-Wolf A, Stottmeister C, Glazar P, Jens M, Pino N, Giusti S, Hanan M, Behm M, Bartok O, Ashwal-Fluss R, Herzog M, Schreyer L, Papavasileiou P, Ivanov A, Ohman M, Refojo D, Kadener S, Rajewsky N, 2015. Circular RNAs in the Mammalian Brain Are Highly Abundant, Conserved, and Dynamically Expressed. Mol Cell 58, 870–885. [DOI] [PubMed] [Google Scholar]
- Sabirzhanov B, Stoica BA, Zhao Z, Loane DJ, Wu J, Dorsey SG, Faden AI, 2016. miR-711 upregulation induces neuronal cell death after traumatic brain injury. Cell Death Differ 23, 654–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzman J, Chen RE, Olsen MN, Wang PL, Brown PO, 2013. Cell-type specific features of circular RNA expression. PLoS Genet 9, e1003777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzman J, Gawad C, Wang PL, Lacayo N, Brown PO, 2012. Circular RNAs are the predominant transcript isoform from hundreds of human genes in diverse cell types. PloS one 7, e30733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger HL, Klotz G, Riesner D, Gross HJ, Kleinschmidt AK, 1976. Viroids are single-stranded covalently closed circular RNA molecules existing as highly base-paired rod-like structures. Proc Natl Acad Sci U S A 73, 3852–3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santer L, Bar C, Thum T, 2019. Circular RNAs: A Novel Class of Functional RNA Molecules with a Therapeutic Perspective. Mol Ther 27, 1350–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafferer S, Khurana R, Refolo V, Venezia S, Sturm E, Piatti P, Hechenberger C, Hackl H, Kessler R, Willi M, Gstir R, Krogsdam A, Lusser A, Poewe W, Wenning GK, Huttenhofer A, Stefanova N, 2016. Changes in the miRNA-mRNA Regulatory Network Precede Motor Symptoms in a Mouse Model of Multiple System Atrophy: Clinical Implications. PLoS One 11, e0150705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider T, Schreiner S, Preusser C, Bindereif A, Rossbach O, 2018. Northern Blot Analysis of Circular RNAs. Methods Mol Biol 1724, 119–133. [DOI] [PubMed] [Google Scholar]
- Selberg S., Blokhina D., Aatonen M., Koivisto P., Siltanen A., Mervaala E., Kankuri E., Karelson M., 2019. Discovery of Small Molecules that Activate RNA Methylation through Cooperative Binding to the METTL3–14-WTAP Complex Active Site. Cell Rep 26, 3762–3771.e3765. [DOI] [PubMed] [Google Scholar]
- Shan K, Liu C, Liu BH, Chen X, Dong R, Liu X, Zhang YY, Liu B, Zhang SJ, Wang JJ, Zhang SH, Wu JH, Zhao C, Yan B, 2017. Circular Noncoding RNA HIPK3 Mediates Retinal Vascular Dysfunction in Diabetes Mellitus. Circulation 136, 1629–1642. [DOI] [PubMed] [Google Scholar]
- Sharma A, Lyashchenko AK, Lu L, Nasrabady SE, Elmaleh M, Mendelsohn M, Nemes A, Tapia JC, Mentis GZ, Shneider NA, 2016. ALS-associated mutant FUS induces selective motor neuron degeneration through toxic gain of function. Nat Commun 7, 10465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Z, Chen T, Yao Q, Zheng L, Zhang Z, Wang J, Hu Z, Cui H, Han Y, Han X, Zhang K, Hong W, 2017. The circular RNA ciRS-7 promotes APP and BACE1 degradation in an NF-kappaB-dependent manner. Febs j 284, 10961109. [DOI] [PubMed] [Google Scholar]
- Shi Z, Ning G, Zhang B, Yuan S, Zhou H, Pan B, Li J, Wei Z, Cao F, Kong X, Feng S, 2019. Signatures of altered long noncoding RNAs and messenger RNAs expression in the early acute phase of spinal cord injury. J Cell Physiol 234, 8918–8927. [DOI] [PubMed] [Google Scholar]
- Starke S, Jost I, Rossbach O, Schneider T, Schreiner S, Hung LH, Bindereif A, 2015. Exon circularization requires canonical splice signals. Cell Rep 10, 103111. [DOI] [PubMed] [Google Scholar]
- Szabo L, Morey R, Palpant NJ, Wang PL, Afari N, Jiang C, Parast MM, Murry CE, Laurent LC, Salzman J, 2015. Statistically based splicing detection reveals neural enrichment and tissue-specific induction of circular RNA during human fetal development. Genome Biol 16, 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabak HF., Van der Horst G., Smit J., Winter AJ., Mul Y., Groot Koerkamp MJ., 1988. Discrimination between RNA circles, interlocked RNA circles and lariats using two-dimensional polyacrylamide gel electrophoresis. Nucleic Acids Res 16, 6597–6605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang F, Barbacioru C, Wang Y, Nordman E, Lee C, Xu N, Wang X, Bodeau J, Tuch BB, Siddiqui A, Lao K, Surani MA, 2009. mRNA-Seq wholetranscriptome analysis of a single cell. Nature methods 6, 377–382. [DOI] [PubMed] [Google Scholar]
- van Rossum D, Verheijen BM, Pasterkamp RJ, 2016. Circular RNAs: Novel Regulators of Neuronal Development. Frontiers in molecular neuroscience 9, 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance C, Rogelj B, Hortobagyi T, De Vos KJ, Nishimura AL, Sreedharan J, Hu X, Smith B, Ruddy D, Wright P, Ganesalingam J, Williams KL, Tripathi V, Al-Saraj S, Al-Chalabi A, Leigh PN, Blair IP, Nicholson G, de Belleroche J, Gallo JM, Miller CC, Shaw CE, 2009. Mutations in FUS, an RNA processing protein, cause familial amyotrophic lateral sclerosis type 6. Science 323, 1208–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veno MT, Hansen TB, Veno ST, Clausen BH, Grebing M, Finsen B, Holm IE, Kjems J, 2015. Spatio-temporal regulation of circular RNA expression during porcine embryonic brain development. Genome Biol 16, 245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verboom K, Everaert C, Bolduc N, Livak KJ, Yigit N, Rombaut D, Anckaert J, Lee S, Veno MT, Kjems J, Speleman F, Mestdagh P, Vandesompele J, 2019. SMARTer single cell total RNA sequencing. Nucleic Acids Res 47, e93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verheijen BM, Pasterkamp RJ, 2017. Commentary: FUS affects circular RNA expression in murine embryonic stem cell-derived motor neurons. Frontiers in molecular neuroscience 10, 412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Li Z, Gao J, Liao Q, 2019. Circular RNA circPTK2 regulates oxygen-glucose deprivation-activated microglia-induced hippocampal neuronal apoptosis via miR-29b-SOCS-1-JAK2/STAT3-IL-1beta signaling. Int J Biol Macromol 129, 488–496. [DOI] [PubMed] [Google Scholar]
- Wang J., Hua H., Ran Y., Zhang H., Liu W., Yang Z., Jiang Y., 2008. Derlin-1 is overexpressed in human breast carcinoma and protects cancer cells from endoplasmic reticulum stress-induced apoptosis. Breast cancer research : BCR 10, R7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang PL, Bao Y, Yee MC, Barrett SP, Hogan GJ, Olsen MN, Dinneny JR, Brown PO, Salzman J, 2014. Circular RNA is expressed across the eukaryotic tree of life. PLoS One 9, e90859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Zhang S, Chen X, Li N, Li J, Jia R, Pan Y, Liang H, 2018a. EIF4A3induced circular RNA MMP9 (circMMP9) acts as a sponge of miR-124 and promotes glioblastoma multiforme cell tumorigenesis. Mol Cancer 17, 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zhao BS, Roundtree IA, Lu Z, Han D, Ma H, Weng X, Chen K, Shi H, He C, 2015. N(6)-methyladenosine Modulates Messenger RNA Translation Efficiency. Cell 161, 1388–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Xu P, Chen B, Zhang Z, Zhang C, Zhan Q, Huang S, Xia ZA, Peng W, 2018b. Identifying circRNA-associated-ceRNA networks in the hippocampus of Abeta1–42-induced Alzheimer’s disease-like rats using microarray analysis. Aging (Albany NY) 10, 775–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westholm JO, Miura P, Olson S, Shenker S, Joseph B, Sanfilippo P, Celniker SE, Graveley BR, Lai EC, 2014. Genome-wide analysis of drosophila circular RNAs reveals their structural and sequence properties and age-dependent neural accumulation. Cell Rep 9, 1966–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F, Han B, Wu S, Yang L, Leng S, Li M, Liao J, Wang G, Ye Q, Zhang Y, Chen H, Chen X, Zhong M, Xu Y, Liu Q, Zhang JH, Yao H, 2019. Circular RNA TLK1 aggravates neuronal injury and neurological deficits after ischemic stroke via miR-335–3p/TIPARP. J Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie BS, Wang YQ, Lin Y, Zhao CC, Mao Q, Feng JF, Cao JY, Gao GY, Jiang JY, 2018. Circular RNA Expression Profiles Alter Significantly after Traumatic Brain Injury in Rats. Journal of neurotrauma 35, 1659–1666. [DOI] [PubMed] [Google Scholar]
- Xie G, 2018. Circular RNA hsa-circ-0012129 Promotes Cell Proliferation and Invasion in 30 Cases of Human Glioma and Human Glioma Cell Lines U373, A172, and SHG44, by Targeting MicroRNA-661 (miR-661). Med Sci Monit 24, 2497–2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki TR, Holmes BB, Furman JL, Dhavale DD, Su BW, Song ES, Cairns NJ, Kotzbauer PT, Diamond MI, 2019. Parkinson’s disease and multiple system atrophy have distinct alpha-synuclein seed characteristics. J Biol Chem 294, 1045–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Joo KI, Ziegler L, Wang P, 2009. Cell type-specific targeting with surface-engineered lentiviral vectors co-displaying OKT3 antibody and fusogenic molecule. Pharmaceutical research 26, 1432–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Bailey L, Baltimore D, Wang P, 2006. Targeting lentiviral vectors to specific cell types in vivo. Proc Natl Acad Sci U S A 103, 11479–11484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Zhang J, Kamelgarn M, Niu C, Gal J, Gong W, Zhu H, 2015. Subcellular localization and RNAs determine FUS architecture in different cellular compartments. Hum Mol Genet 24, 5174–5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P, Qiu Z, Jiang Y, Dong L, Yang W, Gu C, Li G, Zhu Y, 2016. Silencing of cZNF292 circular RNA suppresses human glioma tube formation via the Wnt/beta-catenin signaling pathway. Oncotarget 7, 63449–63455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q, Wu J, Zhao J, Xu T, Zhao Z, Song X, Han P, 2018a. Circular RNA expression profiles during the differentiation of mouse neural stem cells. BMC systems biology 12, 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Fan X, Mao M, Song X, Wu P, Zhang Y, Jin Y, Yang Y, Chen LL, Wang Y, Wong CC, Xiao X, Wang Z, 2017a. Extensive translation of circular RNAs driven by N6-methyladenosine. Cell Res 27, 626–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Fan X, Mao M, Song X, Wu P, Zhang Y, Jin Y, Yang Y, Chen LL, Wang Y, Wong CC, Xiao X, Wang Z, 2017b. Extensive translation of circular RNAs driven by N(6)-methyladenosine. Cell Res 27, 626–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Gao X., Zhang M., Yan S., Sun C., Xiao F., Huang N., Yang X., Zhao K., Zho H., Huang S., Xie B., Zhang N., 2018b. Novel Role of FBXW7 Circular RNA in Repressing Glioma Tumorigenesis. J Natl Cancer Inst 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Hsu PJ, Chen YS, Yang YG, 2018c. Dynamic transcriptomic m(6)A decoration: writers, erasers, readers and functions in RNA metabolism. Cell Res 28, 616–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao H, Ma R, Yang L, Hu G, Chen X, Duan M, Kook Y, Niu F, Liao K, Fu M, Hu G, Kolattukudy P, Buch S, 2014. MiR-9 promotes microglial activation by targeting MCPIP1. Nat Commun 5, 4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon G, Cho KA, Song J, Kim YK, 2019. Transcriptomic Analysis of High Fat Diet Fed Mouse Brain Cortex. Frontiers in genetics 10, 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You X, Vlatkovic I, Babic A, Will T, Epstein I, Tushev G, Akbalik G, Wang M, Glock C, Quedenau C, Wang X, Hou J, Liu H, Sun W, Sambandan S, Chen T, Schuman EM, Chen W, 2015. Neural circular RNAs are derived from synaptic genes and regulated by development and plasticity. Nat Neurosci 18, 603–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu DD, Guo SW, Jing YY, Dong YL, Wei LX, 2015. A review on hepatocyte nuclear factor-1beta and tumor. Cell & bioscience 5, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X, Lin W, Guo M, Zou Q, 2017. A comprehensive overview and evaluation of circular RNA detection tools. PLoS Comput Biol 13, e1005420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Deng Y, Luo Y, Zhang S, Zou H, Cai F, Wada K, Song W, 2012. Control of BACE1 degradation and APP processing by ubiquitin carboxyl-terminal hydrolase L1. J Neurochem 120, 1129–1138. [DOI] [PubMed] [Google Scholar]
- Zhang M., Zhao K., Xu X., Yang Y., Yan S., Wei P., Liu H., Xu J., Xiao F., Zhou H., Yang X., Huang N., Liu J., He K., Xie K., Zhang G., Huang S., Zhang N., 2018a. A peptide encoded by circular form of LINC-PINT suppresses oncogenic transcriptional elongation in glioblastoma. Nat Commun 9, 4475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XO, Dong R, Zhang Y, Zhang JL, Luo Z, Zhang J, Chen LL, Yang L, 2016a. Diverse alternative back-splicing and alternative splicing landscape of circular RNAs. Genome Res 26, 1277–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Du L, Bai Y, Han B, He C, Gong L, Huang R, Shen L, Chao J, Liu P, Zhang H, Zhang H, Gu L, Li J, Hu G, Xie C, Zhang Z, Yao H, 2018b. CircDYM ameliorates depressive-like behavior by targeting miR-9 to regulate microglial activation via HSP90 ubiquitination. Molecular psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Wang J, Zhang Y, Wei J, Wu R, Cai H, 2019. Overexpression of long noncoding RNA Malat1 ameliorates traumatic brain injury induced brain edema by inhibiting AQP4 and the NF-kappaB/IL-6 pathway. J Cell Biochem. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Xue W, Li X, Zhang J, Chen S, Zhang JL, Yang L, Chen LL, 2016b. The Biogenesis of Nascent Circular RNAs. Cell Rep 15, 611–624. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhang XO, Chen T, Xiang JF, Yin QF, Xing YH, Zhu S, Yang L, Chen LL, 2013. Circular intronic long noncoding RNAs. Mol Cell 51, 792–806. [DOI] [PubMed] [Google Scholar]
- Zhao RT, Zhou J, Dong XL, Bi CW, Jiang RC, Dong JF, Tian Y, Yuan HJ, Zhang JN, 2018. Circular Ribonucleic Acid Expression Alteration in Exosomes from the Brain Extracellular Space after Traumatic Brain Injury in Mice. Journal of neurotrauma 35, 2056–2066. [DOI] [PubMed] [Google Scholar]
- Zhao XD, Lu YY, Guo H, Xie HH, He LJ, Shen GF, Zhou JF, Li T, Hu SJ, Zhou L, Han YN, Liang SL, Wang X, Wu KC, Shi YQ, Nie YZ, Fan DM, 2015. MicroRNA-7/NF-kappaB signaling regulatory feedback circuit regulates gastric carcinogenesis. J Cell Biol 210, 613–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Alexandrov PN, Jaber V, Lukiw WJ, 2016. Deficiency in the Ubiquitin Conjugating Enzyme UBE2A in Alzheimer’s Disease (AD) is Linked to Deficits in a Natural Circular miRNA-7 Sponge (circRNA; ciRS-7). Genes 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Liu X, Xue Y, Gong W, Ma J, Xi Z, Que Z, Liu Y, 2017. TTBK2 circular RNA promotes glioma malignancy by regulating miR217/HNF1beta/Derlin-1 pathway. J Hematol Oncol 10, 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Q., Bao C., Guo W., Li S., Chen J., Chen B., Luo Y., Lyu D., Li Y., Shi G., Liang L., Gu J., He X., Huang S., 2016. Circular RNA profiling reveals an abundant circHIPK3 that regulates cell growth by sponging multiple miRNAs. Nat Commun 7, 11215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C, Molinie B, Daneshvar K, Pondick JV, Wang J, Van Wittenberghe N, Xing Y, Giallourakis CC, Mullen AC, 2017. Genome-Wide Maps of m6A circRNAs Identify Widespread and Cell-Type-Specific Methylation Patterns that Are Distinct from mRNAs. Cell Rep 20, 2262–2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Buchholz CJ, 2013. Cell type specific gene delivery by lentiviral vectors: New options in immunotherapy. Oncoimmunology 2, e22566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Gu C, Li J, Zhu L, Huang G, Dai J, Huang H, 2018. Aberrantly expressed long noncoding RNAs and genes in Parkinson’s disease. Neuropsychiatric disease and treatment 14, 3219–3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou ZB, Du D, Chen KZ, Deng L, Niu YL, Zhu L, 2019. Differential expression profiles and functional predication of circRNA in traumatic spinal cord injury of rats. Journal of neurotrauma. [DOI] [PubMed] [Google Scholar]
- Zhu L, Zhao R, Huang L, Mo S, Yu Z, Jiang L, Qiao L, 2018. Circular RNA Expression in the Brain of a Neonatal Rat Model of Periventricular White Matter Damage. Cell Physiol Biochem 49, 2264–2276. [DOI] [PubMed] [Google Scholar]
- Zirkel A, Papantonis A, 2018. Detecting Circular RNAs by RNA Fluorescence In Situ Hybridization. Methods Mol Biol 1724, 69–75. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


