Abstract
Introduction:
Long-chain acyl-CoA synthetases (ACSLs) are implicated as regulators of oxidation and storage of fatty acids within skeletal muscle; however, to what extent diet and exercise alter skeletal muscle ACSLs remains poorly understood.
Purpose:
To determine effects of diet and exercise training on skeletal muscle ACSLs and examine relationships between ACSL1 and ACSL6 and fat oxidation and fat storage, respectively.
Methods:
Male C57BL/6J mice consumed a 60% high-fat diet (HFD) for 12 weeks to induce obesity compared with low-fat diet (LFD). At week 4, mice began aerobic exercise (EX-Tr) or remained sedentary (SED) for 8 weeks. At week 12, protein abundance of 5 known ACSL isoforms and mRNA expression for ACSL1 and ACSL6 were measured in gastrocnemius muscle, as was skeletal muscle lipid content. Fat oxidation was measured using metabolic cage indirect calorimetry at week 10.
Results:
Of 5 known ACSL isoforms, 4 were detected at the protein level. HFD resulted in greater, yet non-significant, ACSL1 protein abundance (+18%, P=0.13 vs. LFD), greater ACSL6 (+107%, P<0.01 vs. LFD), and no difference in ACSL4 or ACSL5. Exercise training resulted in greater ACSL6 protein abundance in LFD mice (P=0.05 LFD EX-Tr vs. SED) while ACSL4 was lower following exercise training compared with sedentary, regardless of diet. Under fasted conditions, skeletal muscle ACSL1 protein abundance was not related to measures of whole-body fat oxidation. Conversely, skeletal muscle ACSL6 protein abundance was positively correlated with intramyocellular lipid content (P<0.01, r2=0.22).
Conclusion:
We present evidence that ACSL isoforms 1, 4 and 6 may undergo regulation by HFD and/or exercise training. We further conclude increased skeletal muscle ACSL6 may facilitate increased intramyocellular fat storage during HFD-induced obesity.
Keywords: Long-chain acyl-CoA synthetase, lipid metabolism, skeletal muscle, obesity, aerobic exercise training
Introduction
There is significant interest in understanding regulation of fat oxidation and fat storage in skeletal muscle, including diverse contexts such as exercise performance and the prevention of metabolic disease (1). Both obesity and aerobic exercise training influence skeletal muscle lipid metabolism, including oxidation and storage of fat (2-5). However, how fatty acids are partitioned toward oxidation or storage within skeletal muscle and to what extent such regulatory factors may be influenced by obesity and aerobic exercise training remain largely unanswered questions of interest.
Fatty acids entering skeletal muscle are activated by long-chain acyl-CoA synthetases (ACSLs) to form long-chain acyl-CoA prior to undergoing oxidation or being used for synthesis of triacylglycerols (i.e., storage) (6,7). There are 5 known ACSL isoforms that vary in expression, function, and localization among tissues (7-9). Transcript levels suggest that ACSL isoforms 1, 3, and 6 may be expressed in higher abundance than isoforms 4 and 5 in skeletal muscle (10). However, comprehensive investigation into protein abundance of known ACSL isoforms in skeletal muscle, including to what extent skeletal muscle ACSLs may be altered by diet and exercise interventions, is currently lacking. Such investigations are warranted in light of mounting evidence indicating various ACSL isoforms may serve distinct, critical roles in determining the fate of fatty acids within skeletal muscle.
Mice with skeletal muscle specific knockout of ACSL1 exhibit metabolic inflexibility, attenuated fat oxidation at rest, and exercise intolerance compared with wild type animals (11). Exercising the knockout mice resulted in accumulation of long-chain acyl-CoA in skeletal muscle and hypoglycemia at half the distance covered compared with wild type mice, suggesting compensatory reliance on glucose oxidation due to impaired fat oxidation (11). Prolonged fasting (i.e., 48 hours) has also been shown to increase skeletal muscle mRNA expression and protein abundance of ACSL1 (10), which coincides with increased rates of fat oxidation, indicating a potential role for ACSL1 to facilitate oxidation of fatty acids. Equally compelling evidence suggests skeletal muscle ACSL6 facilitates storage of fatty acids (12). ACSL6 mRNA expression is upregulated in response to a high fat meal (i.e., when fatty acid storage is elevated) and downregulated in response to a prolonged fast (i.e., when fat storage is minimal) (12). Knockdown of ACSL6 in cultured muscle cells attenuated lipid storage, as evidenced by decreased lipid droplet size and reduced content of triacylglycerols. Conversely, overexpression of ACSL6 increased accumulation of phospholipids and decreased fat oxidation (12). Taken together, these findings indicate ACSL1 and ACSL6 may have distinct roles in skeletal muscle fat oxidation and fat storage, respectively.
Despite marked changes in skeletal muscle lipid metabolism in response to high fat diet induced obesity or aerobic exercise training (2,5,13,14), to what extent such interventions influence skeletal muscle ACSL isoforms remains unknown. Therefore, the first purpose of this study was to identify the effects of diet-induced obesity and aerobic exercise training on skeletal muscle ACSL isoforms. The second purpose was to investigate relationships between skeletal muscle ACSL1 and ACSL6 and the oxidation or storage of lipids. Herein we demonstrate 4 of the 5 known ACSL isoforms were readily detected in skeletal muscle at the protein level, with isoform-specific differences in response to diet and exercise intervention. Additionally, we present evidence that diet-induced changes in skeletal muscle ACSL6 protein are related to intramyocellular lipid content.
Methods
Study Design
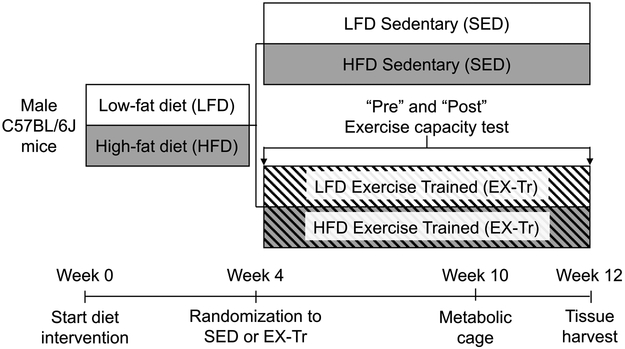
This study was approved by the Animal Care and Use Committee at Oregon State University (#4788). Twelve-week-old male C57BL/6J mice (Jackson Laboratories) were housed 3-5 per cage under 12-hour light-dark cycle at 22°C conditions with free access to food and water. As depicted in Figure 1, mice consumed either a low-fat diet (D12450J; Research Diets) or high-fat diet (D12492; Research Diets) for 12 weeks. The percentage of total kilocalories from fat/carbohydrate/protein for LFD was 10/70/20 and for HFD was 60/20/20. At week 4 of the diet intervention, mice either remained sedentary (SED) or began graded aerobic exercise training (EX-Tr). Aerobic exercise training was performed on a motorized treadmill for 50 minutes per day, 5 days per week. Mice were acclimated to the motorized treadmill (Panlab, Harvard Apparatus, Holliston, MA, USA) for one week with low speed (0-12m/min) for 15 minutes. Exercise then gradually progressed in intensity and duration from 10m/min at 0% incline for 30 minutes to 17m/min for 50 minutes at 10% incline which comprised the last 12 days of exercise training. A graded exercise test was performed after acclimation and at the end of the training period to assess exercise capacity. The belt speed started at 6m/min and 0% incline for 5 minutes then increased by 3m/min and 5% incline every 2 minutes until 18m/min and 15% incline was reached. Speed was then increased by 1-2 m/min every minute until exhaustion. Mice were removed from the treadmill when they refused to run despite sitting on the shock grid continuously for 5 seconds. In-cage metabolic assessment (Promethion, Sable Systems Int.) was performed at week 10 along with body composition measures using dual-energy x-ray absorptiometry (GE Lunar, PIXImus2). Following an acclimation period of 24 hours, in-cage substrate oxidation during fed and fasted conditions were assessed in 12-hour cycles and following a 3-5 hour fast, respectively, using indirect calorimetry (see Fat Oxidation, below, for calculations). At week 12, mice were anesthetized by sodium pentobarbital overdose following a 4 hour fast, 36 hours after their last bout of exercise or remaining sedentary. Tissues were collected, flash frozen in liquid nitrogen, and stored at −80°C until further analysis (n=10 mice/condition). Importantly, tissue analysis was completed using mixed gastrocnemius muscle (isolated independent from soleus and plantaris muscles) as an ideal representative muscle due to its mixed fiber type (15), previously reported ACSL mRNA expression (16), and recruitment during treadmill exercise training (17).
Figure 1: Study Design.
C57Bl/6J mice consumed either a low-fat diet (LFD) or high-fat diet (HFD) for 12 weeks. The percentage of total kilocalories from fat/carbohydrate/protein for LFD was 10/70/20 and for HFD was 60/20/20. At week 4 of the diet intervention, mice either remained sedentary (SED) or began graded aerobic exercise training (EX-Tr). Aerobic exercise training was performed on a motorized treadmill for 50 minutes per day, 5 days per week. Mice were acclimated to the motorized treadmill (Panlab, Harvard Apparatus, Holliston, MA, USA) for one week with low speed (0-12m/min) for 15 minutes. Exercise then gradually progressed in intensity and duration from 10m/min at 0% incline for 30 minutes to 17m/min for 50 minutes at 10% incline which comprised the last 12 days of exercise training. A graded exercise test was performed after acclimation and at the end of the training period to assess exercise capacity. The belt speed started at 6m/min and 0% incline for 5 minutes then increased by 3m/min and 5% incline every 2 minutes until 18m/min and 15% incline was reached. Speed was then increased by 1-2 m/min every minute until exhaustion. Mice were removed from the treadmill when they refused to run despite sitting on the shock grid continuously for 5 seconds. In-cage metabolic assessment (Promethion, Sable Systems Int.) was performed at week 10 along with body composition measures using dual-energy x-ray absorptiometry (GE Lunar, PIXImus2). Following an acclimation period of 24 hours, in-cage substrate oxidation during fed and fasted conditions were assessed in 12-hour cycles and following a 3-5 hour fast, respectively, using indirect calorimetry (see Fat Oxidation, below, for calculations). At week 12, mice were anesthetized by sodium pentobarbital overdose following a 4 hour fast, 36 hours after their last bout of exercise or remaining sedentary. Tissues were collected, flash frozen in liquid nitrogen, and stored at −80°C until further analysis (n=10 mice/condition).
Western Blotting
Gastrocnemius muscle (~30 mg) was homogenized as described previously (2). Homogenates rotated at 4ºC for 20 minutes and then centrifuged at 10,000 × g for 10 minutes at 4°C. The supernatant was stored at −80°C until further analysis. Approximately 35 μg protein was separated on bis-tris gels and transferred to nitrocellulose membranes. Each gel was loaded with the same internal control sample in 2 lanes, the average density of both lanes was used to normalize band density between gels. Ponceau staining of membranes was used to verify equal loading and transfer of protein. Membranes were blocked in 5% bovine serum albumin in tris-buffered saline with tween (TBST) and incubated in primary antibodies at 4°C. Following primary incubation, membranes were washed in TBST and incubated in secondary antibody diluted in blocking buffer at room temperature. Images were generated using infrared detection (LI-COR Odyssey). Primary antibodies used included ACSL1 (product no. 4047, Cell Signaling Technology), ACSL3 (product no. 166374, Santa Cruz), ACSL4 (product no. PA5-27137, Invitrogen), ACSL5 (product no. 365478, Santa Cruz) and ACSL6 (product no. PA5-30465, Invitrogen) diluted 1:1000. Primary antibodies for ACSL isoforms 1, 4, and 5 have been previously verified by knockdown and/or overexpression models (18,19). Primary antibodies for ACSL3 and 6 have not been verified by knockdown or overexpression; however, internal testing by the manufacturer verified expression of ACSL3 in mouse muscle cell line (C2C12) and ACSL6 in mouse heart lysate. The secondary antibodies used were anti-rabbit-700 (product no. 926-68071) and anti-mouse-800 (product no. 926-32212) from LICOR diluted 1:10,000.
Quantitative Polymerase Chain Reaction (qPCR)
Sequence information for target genes are listed in Table 1. Total mRNA was measured as described previously (13). Briefly, mRNA was extracted from gastrocnemius muscle (~20 mg) to determine transcriptional activation of skeletal muscle ACSL isoforms 1 and 6. mRNA concentration and contamination was determined by spectrophotometry (NanoDrop, ThermoFisher Scientific), with 1 μg mRNA reversed transcribed to cDNA. qPCR was performed in triplicate in 384 well clear plates using Sybr® Green reagents (ThermoFisher Scientific), ~20 ng cDNA and 100 nM primers in 20 μl reaction volumes. Thermocycler conditions were 10 minutes at 60°C, 40 cycles of 15 seconds denaturing (95°C) and 60 seconds annealing/extension (60°C) followed by a melt curve. Relative quantification was performed using a 7-point standard curve that spanned 3 log dilutions. Amplification efficiencies were similar between target and reference genes, which were analyzed on separate plates along with no template controls. Target genes were normalized by dividing the average of two reference genes (HSP90 and Cyclophilin) analyzed on separate plates. Melt curves revealed single peaks for each primer set.
Table 1.
Primer sequence information
| Name | Gene Name | Reference Sequence | Sequence |
|---|---|---|---|
| Long-chain acyl-coenzyme a synthetase 1 | ACSL1 | NM_007981.3 | F: GGATTCAGGTGTCAAATAATGG R: GAGAGTTCAGCTTTGTTCAC |
| Long-chain acyl-coenzyme a synthetase 6 | ACSL6 | NC_000077.6 | Purchased from Bio-Rad Assay ID: qMmuCED0044795 |
| Heat shock protein 90ab1 | Hsp90 | NM_008302 | F: CATCATGGACAGCTGTGACG R: AGTTCTCCTTGTCCTCAGCC |
| Cyclophilin A | Ppia | NM_008907.1 | F: CTTCTTGCTGGTCTTGCCATTCCT R: GGATGGCAAGCATGTGGTCTTTG |
F, forward; R, reverse
Fat Oxidation
Previous evidence demonstrates a 48 hour fast alters skeletal muscle ACSL1 mRNA expression and protein abundance (11). We therefore measured substrate oxidation following 3-5 hour fast to closely mimic the conditions in which skeletal muscle was harvested and ACSL protein abundance and mRNA expression were measured (i.e., following a 4-hour fast). Rates of whole-body fat oxidation were calculated from in-cage VO2 and VCO2 measures using the equations of Frayn (20) and normalized to body weight. Importantly our measures of fat oxidation are whole-body, however skeletal muscle comprises ~40% of total body mass and previous reports indicate skeletal muscle is a principal determinant of energy expenditure (21).
Intramyocellular Lipid (IMCL)
IMCL was measured in ~20 mg of gastrocnemius muscle as previously described with minor modifications (22,23). Skeletal muscle was homogenized 2 × 30 seconds in 3 mL of 2:1 chloroform:methanol, with 2.7 mL of homogenate transferred to clean 13mm glass culture tubes. Following an overnight extraction at 4ºC, homogenates were centrifuged for at 3,000 x g for 15 minutes to separate phases and then 1.2 mL of the lower organic phase was dried under N2 gas. Lipids were resuspended and saponified in 4% ethanol-KOH solution at 75°C for 25 minutes and fatty acids were pelleted by the addition of MgSO4 (0.15 M) and centrifugation at 3,000 x g for 15 minutes. Glycerol concentration was determined in the supernatant using colorimetric Infinity Triglyceride Reagent (ThermoFisher Scientific). Total IMCL was calculated from glycerol concentration as:
Total glycerol was calculated by multiplying the concentration by the final volume and accounting for lipid-containing volumes left behind during transfer steps. Total glycerol was then normalized to tissue wet weight to yield total mg IMCL content per g of tissue.
Statistical Analysis
The effect of aerobic exercise training on exercise capacity was analyzed by paired, two-tailed Student’s t-tests. Independent effects of diet and exercise training on outcome variables were analyzed by unpaired, two-tailed Student’s t-tests. More specifically, the effect of diet was evaluated by comparing HFD vs. LFD in the sedentary condition. Effects of exercise training were analyzed within each diet condition by comparing exercise trained mice with sedentary mice. This approach was employed to limit statistical analysis to comparisons of interest. Relationships between ACSL1 and ACSL6 and fat oxidation and fat storage, respectively, were analyzed by Pearson’s correlational analysis. Statistical significance was set as P≤0.05. Statistical analysis and generation of figures were performed using Prism version 6 (GraphPad Software). Data are presented as mean and standard deviation, with individual data points shown, in accordance with recommendations for the field (24).
Results
Effects of HFD and aerobic exercise training on animal characteristics
Animal characteristics are provided in Table 2. Sedentary mice consuming HFD had 67% greater total body mass compared with LFD mice (P<0.01), which was largely attributable to greater fat mass and modest yet significantly greater lean mass (both P<0.01, HFD vs. LFD). Aerobic exercise training resulted in lower total body mass during HFD (P<0.01, HFD Ex-Tr vs. HFD SED), due to lower fat mass (P=0.03) and no difference in lean mass (P=0.73). In LFD mice, aerobic exercise training did not alter body mass or composition. In both LFD and HFD mice, aerobic exercise training increased time to exhaustion during a maximal exercise test (P<0.01 Pre EX-Tr vs. Post EX-Tr in both diet conditions), providing evidence of a robust and effective training stimulus.
Table 2.
Mouse body composition and exercise capacity
| SED | EX-Tr | |||
|---|---|---|---|---|
| LFD | HFD | LFD | HFD | |
| Total mass (g) | 24.0 ± 2.2 | 40.2 ± 2.5* | 24.9 ± 2.0 | 35.6 ± 2.4# |
| Lean mass (g) | 20.0 ± 1.7 | 24.0 ± 1.7* | 20.7 ± 1.6 | 22.4 ± 1.7 |
| Fat mass(g) | 4.0 ± 0.9 | 16.2 ± 1.6* | 4.1 ± 0.7 | 13.2 ± 2.6# |
| LFD | HFD | |||
| Pre EX-Tr | Post EX-Tr | Pre EX-Tr | Post EX-Tr | |
| Time to exhaustion (min) | 13.1 ± 1.2 | 15.8 ± 1.6† | 10.2 ± 2.0* | 13.0 ± 2.1† |
Data are presented as mean ± standard deviation.
P<0.05 for HFD vs. LFD within the sedentary condition.
P<0.05 for EX-Tr vs. SED within diet.
P<0.05 for paired Post EX-Tr vs. Pre EX-Tr.
Effects of HFD and aerobic exercise training on skeletal muscle ACSL isoform protein abundance
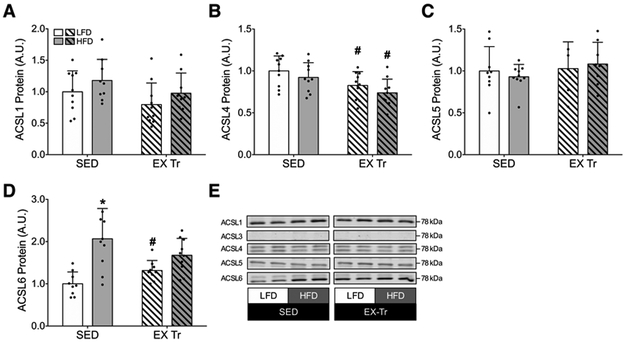
We successfully detected 4 of the 5 ACSL isoforms in gastrocnemius muscle via western blotting, including ACSL isoforms 1, 4, 5 and 6 (Figure 2); ACSL3 protein abundance was below limits of detection (data not shown). HFD resulted in greater abundance of ACSL6, a tendency for greater abundance of ACSL1, and no effect on protein abundance of isoforms 4 and 5 (Figure 2). Aerobic exercise training also resulted in greater abundance of ACSL6 in LFD mice (P=0.05, LFD EX-Tr vs. LFD SED, Figure 2D). Regardless of diet, exercise training resulted in modest yet significantly lower abundance of ACSL4 protein (P<0.05, Ex-Tr vs. SED in both diet conditions) with no effect on ACSL isoforms 1 and 5 (Figure 2). Together these data indicate diet and exercise serve as isoform-specific regulators of skeletal muscle ACSL protein abundance. Because ACSL1 and 6 have been implicated as key determinants of fat oxidation and fat storage (12,18), we further investigated their regulation and relationship to measures of fat oxidation and IMCL content.
Figure 2: Skeletal muscle ACSL isoform protein abundance following diet-induced obesity and aerobic exercise training.
ACSL isoform protein abundance in gastrocnemius muscle following either a 12-week low-fat (LFD) or high-fat diet (HFD) in sedentary (SED) and aerobic exercise trained (EX-Tr) mice. Total protein abundance for A) ACSL1, B) ACSL4, C) ACSL5, D) ACSL6, E) with representative western blot images shown. Each representative image was spliced from the same blot to remove groups not used in this study. The effect of diet was analyzed by unpaired students t-test comparing HFD to LFD among sedentary mice. The effect of exercise training was analyzed by unpaired students t-test comparing EX-Tr to SED within each diet condition. Data are presented as mean and standard deviation with individual data points shown. *P≤0.05 vs. LFD, #P≤0.05 vs. SED. n=10 for all conditions.
Fasting whole-body fat oxidation is not related to skeletal muscle ACSL1 protein abundance
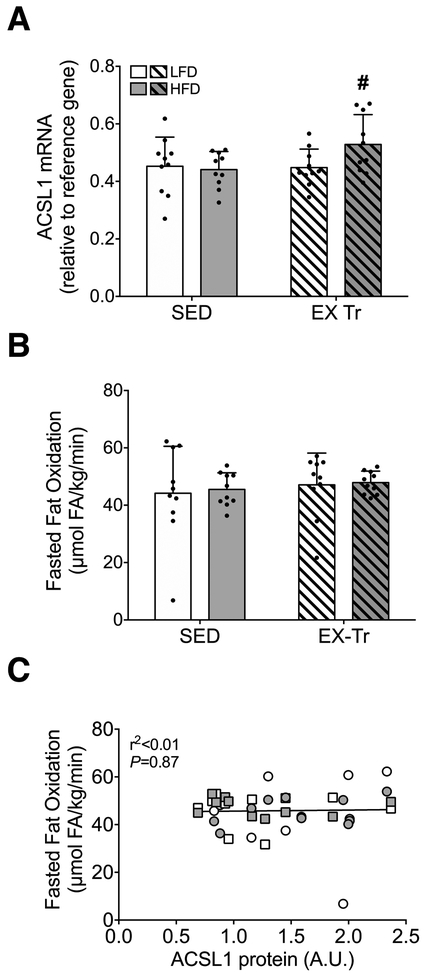
We further explored possible regulation of skeletal muscle ACSL1 by diet and exercise training via measurement of mRNA expression. ACSL1 mRNA expression was not different as a function of diet; however, exercise training did result in a modest yet significantly greater expression of skeletal muscle ACSL1 mRNA in HFD mice (Figure 3A). We next investigated the relationship between skeletal muscle ACSL1 protein abundance and in vivo rates fat oxidation. Importantly, fasting is known to increase ACSL1 mRNA and protein abundance (10). For this reason, ACSL1 protein abundance was related to rates of fat oxidation measured following a 3-5 hour fast to closely mimic physiologic conditions during which tissues were harvested (i.e., to control for any potential effect of fasting on ACSL protein abundance). Following a 4-hour fast, rates of whole-body fat oxidation were similar among all conditions (Figure 3B), likely reflecting the increased fasting-induced reliance upon fat oxidation. Under such conditions, rates of whole-body fat oxidation were not related to skeletal muscle ACSL1 protein abundance (Figure 3C).
Figure 3: Skeletal muscle ACSL1 mRNA expression and relationship to whole-body fat oxidation.
A) ACSL1 mRNA expression following either a 12-week low-fat (LFD) or high-fat diet (HFD) in sedentary (SED) and aerobic exercise trained (EX-Tr) mice. B) Whole-body fat oxidation calculated from VO2 and VCO2 and normalized to body weight following a 4-hour fast during the light cycle to closely mimic physiologic conditions during which tissues were harvested. The effect of diet was analyzed by unpaired students t-test comparing HFD to LFD among sedentary mice. The effect of exercise training was analyzed by unpaired students t-test comparing EX-Tr to SED within each diet condition. Data are presented as mean and standard deviation with individual data points shown. C) Correlation analysis measuring the relationship of skeletal muscle ACSL1 protein abundance and whole-body fat oxidation following a 4 hour fast. LFD SED, white circle; HFD SED, gray circle; LFD EX-Tr, white square; HFD EX-Tr, gray square. Data are presented as individual data points. *P≤0.05 vs. LFD, #P≤0.05 vs. SED. n=10 for all conditions.
Intramyocellular lipid content is positively related to skeletal muscle ACSL6 protein abundance
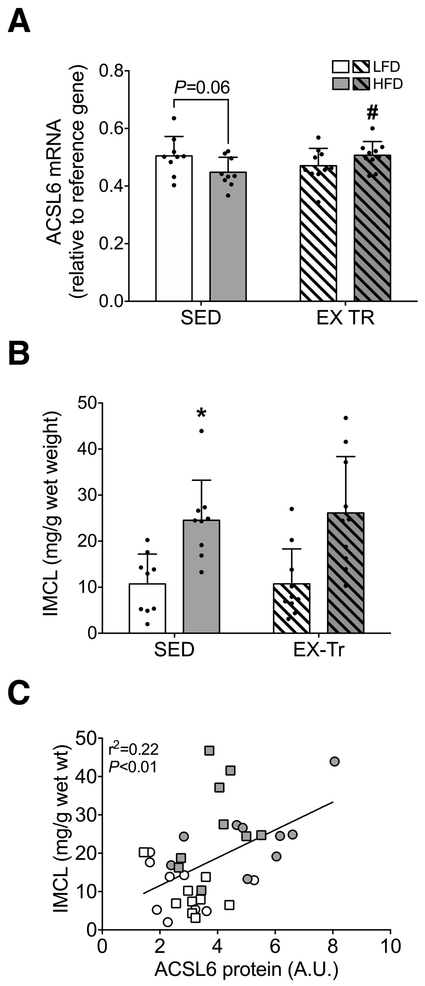
We measured skeletal muscle ACSL6 mRNA expression to investigate potential diet and/or exercise training-related regulation at the transcript level. ACSL6 mRNA expression tended to be lower in HFD compared with LFD mice (P= 0.06, Figure 4A), perhaps reflecting increased translation to support markedly greater ACSL6 protein abundance. Exercise training resulted in a small yet significantly greater skeletal muscle ACSL6 mRNA expression within HFD (Figure 4A). We next investigated the relationship between ACSL6 protein abundance and intramyocellular lipid content (IMCL). IMCL content was significantly greater in HFD compared with LFD in SED mice (P<0.01, Figure 4B). Exercise training did not alter IMCL content regardless of diet (Figure 4B); however, intramyocellular lipid was positively associated with greater ACSL6 protein abundance among all mice (P<0.01, r2=0.22, Figure 4C), indicating ACSL6 may serve to facilitate lipid storage in skeletal muscle.
Figure 4: Skeletal muscle ACSL6 mRNA expression and relationship to IMCL content.
A) ACSL6 mRNA expression following either a 12-week low-fat (LFD) or high-fat diet (HFD) in sedentary (SED) and aerobic exercise trained (EX-Tr) mice. B) Intramyocellular lipid (IMCL) content measured in gastrocnemius muscle. The effect of diet was analyzed by unpaired students t-test comparing HFD to LFD among sedentary mice. The effect of exercise training was analyzed by unpaired students t-test comparing EX-Tr to SED within each diet condition. Data are presented as mean and standard deviation with individual data points shown. C) Correlation analysis measuring the relationship of skeletal muscle ACSL6 protein abundance and IMCL content. LFD SED, white circle; HFD SED, gray circle; LFD EX-Tr, white square; HFD EX-Tr, gray square. Data are presented as individual data points. *P≤0.05 vs. LFD, #P≤0.05 vs. SED. n=10 for all conditions.
Discussion
The purpose of this study was to identify the independent roles of HFD-induced obesity and aerobic exercise training on skeletal muscle ACSLs and to examine relationships of ACSL1 and ACSL6 to oxidation and storage of lipids, respectively. We readily detected skeletal muscle protein abundance for ACSL isoforms 1, 4, 5, and 6 in gastrocnemius muscle of mice, whereas ACSL3 was below limits of detection. Skeletal muscle ACSL6 protein abundance was significantly greater in high-fat compared with low-fat fed mice, and ACSL4 protein abundance was lower following exercise training compared with remaining sedentary, regardless of diet. ACSL1 protein abundance tended to be greater with HFD, whereas ACSL5 was minimally influenced by either intervention. Skeletal muscle ACSL1 protein abundance was not related to measures of whole-body fat oxidation following a 4 hour fast; however, skeletal muscle ACSL6 protein abundance was positively related to intramyocellular lipid content, indicating ACSL6 may facilitate accumulation of lipids in skeletal muscle.
There is growing interest in ACSLs for their potentially critical roles as regulators of lipid metabolism. ACSLs activate fatty acids to form fatty acyl-CoA prior to undergoing further metabolism (6,7), with isoform-specific function likely determined by subcellular localization and substrate specificity. For example, in hepatocytes, ACSL1 localizes to both the endoplasmic reticulum (ER) and to the mitochondria whereas ACSL3 is localized to the ER and lipid droplets (25). Emerging evidence indicates ACSL4 may have specificity for polyunsaturated fatty acids (26). Combined with varying levels of expression, ACSLs may therefore help to determine the metabolic fate of intracellular fatty acids. Nonetheless, this information is largely lacking for skeletal muscle, highlighting the need physiological characterizations studies such as this. We have demonstrated isoform-specific effects of diet and exercise training interventions to alter ACSL protein content, which may contribute to changes in skeletal muscle lipid metabolism. Unexpectedly, ACSL3 was below the level of detection in our samples. ACSL3 mRNA is expressed, albeit at relatively low levels, in skeletal muscle of rodents (10). We also detected ACSL3 protein in C2C12 and L6 myotubes, using the same antibody employed here (data not shown). It is thus possible, if not likely, that ACSL3 is expressed in skeletal muscle and was not detected here due to technical limitation.
We anticipated both HFD and exercise training may result in greater ACSL1 given that peroxisome proliferator-activated receptor alpha (PPARα) is a known regulator of ACSL1 (27,28), and can be increased in response to both HFD (29) and exercise training (30,31). However, we observed no significant differences in ACSL1 protein abundance with HFD or exercise training (Figure 2A). Our findings are consistent with a previous report demonstrating minimal impact of PPARα deletion on known PPARα targets within skeletal muscle of mice (32), suggesting more complex and potentially PPARα-independent regulation of ACSL1 in skeletal muscle. We also anticipated ACSL1 protein abundance may be related to fat oxidation given previous reports using knockout models demonstrating ACSL1 is critical for normal skeletal muscle fat oxidation (11). However, we observed no relationship between skeletal muscle ACSL1 protein abundance and whole-body fat oxidation measured during the fasted state (Figure 3C). One possible explanation for this finding is that whole-body measures of fat oxidation are, of course, not specific to skeletal muscle. However, skeletal muscle represents a significant proportion of whole-body resting energy expenditure and therefore fat oxidation (21). Perhaps more important is that during the fasting state there is increased reliance upon fatty acids as a source of fuel, as evidenced by the similarly elevated rates of fat oxidation measured among all of our experimental conditions (Figure 3B). Increased delivery of fatty acid to skeletal muscle (33) and lowered hormonal stimulus (i.e., insulin) to stimulate oxidation of fat during the fasting state (34) may therefore limit the role of ACSL1 as a critical determinant of the rate of fat oxidation. Further studies are needed to clarify the functional role of ACSL1 as a regulator of skeletal muscle fat oxidation during the physiologic transition from fed to fasting states (10), as novel and perhaps targetable regulators of skeletal muscle fat oxidation may have significant therapeutic potential.
Our model of high fat feeding is well-characterized by obesity, hyperinsulinemia and increased skeletal muscle lipid content (35-37). In light of previous findings demonstrating insulin-induced expression of ACSL6 (38), we anticipated ACSL6 may be upregulated by high fat feeding. Indeed, HFD resulted in significantly greater ACSL6 protein abundance when compared with LFD (Figure 2E). We also found a positive relationship between skeletal muscle ACSL6 protein abundance and lipid content (Figure 4C). We interpret these findings as support for the role for ACSL6 in facilitating diet-induced lipid storage within skeletal muscle. Our findings are in agreement with those of Teodoro et al. demonstrating ACSL6 is linked to fat storage in vitro (12). Importantly, aerobic exercise training can increase skeletal muscle lipid content, particularly triacylglycerols (4). We therefore anticipated exercise training might also increase skeletal muscle ACSL6 protein abundance and IMCL content. In agreement, exercise training resulted in greater ACSL6 protein compared with remaining sedentary among low fat fed mice (Figure 2E); however, IMCL content was unchanged following exercise training (Figure 4B). This may be due, in part, to increased rates of fat oxidation measured in LFD mice after exercise training in the fed state (data not shown), which may have lowered the fatty acid available for lipid storage. Furthermore, our measure of IMCL content may be limited in sensitivity to detect small but perhaps important differences in lipid content. Collectively, we interpret our findings to indicate a role for ACSL6 in accumulation of skeletal muscle lipid in response to obesity and perhaps also in response to aerobic exercise training.
The significance of exercise training-related changes in skeletal muscle ACSL4 is unclear. ACSL4 is highly expressed in the adrenal gland, ovary, testis, and brain, where it demonstrates preference for polyunsaturated fatty acids (26). Some evidence suggests this isoform may facilitate synthesis of polyunsaturated phospholipids (39). For example, overexpression of ACSL4 in human arterial smooth muscle cells results in greater incorporation of arachidonic acid into phospholipids and triacylglycerols (40). However, because ACSL isoform function varies by tissue, manipulation of ACSL4 protein abundance in skeletal muscle may be necessary in order to determine its role and the significance of its downregulation with exercise training. For instance, manipulation of ACSL5 by overexpression within skeletal muscle in vitro resulted in greater measures of fat oxidation (19). These findings suggest ACSL5 may direct fatty acids toward oxidation; however, we observed no change in total protein abundance in response to HFD or aerobic exercise training.
An important consideration is that our measures of ACSL isoform protein abundance may not reflect ACSL activity. Exercise has been shown to increase total skeletal muscle ACSL activity measured ex vivo (41), though the specific contribution of individual ACSL isoforms is unknown. Furthermore, proteomic analysis has revealed numerous phosphorylation and acetylation sites on ACSL1 (42); however, specific antibodies are unavailable and the functional consequence of such post translational modifications remain largely unknown (42). Therefore, the function and regulation of post translational modification among ACSL isoforms in response to diet-induced obesity and aerobic exercise training remain areas of interest. Another important consideration is the extent to which ACSL expression and regulation may differ by fiber type and therefore among skeletal muscles. A previous report demonstrated similar levels of mRNA expression of ACSL isoforms between gastrocnemius and soleus muscles (10); though, mRNA expression is not necessarily indicative of protein abundance Because of known differences in skeletal muscle lipid metabolism as a function of fiber type (43,44), muscle-specific regulation of ACSLs remains another area of interest for future directions.
In conclusion, protein abundance of ACSL isoforms 1, 4, 5, and 6 were readily detected in mouse gastrocnemius muscle. High fat feeding and exercise training resulted in isoform-specific effects on ACSLs whereby ACSL6 was greater in response HFD-induced obesity and following exercise training in low-fat fed mice, and ACSL4 protein abundance was lower in response to exercise training regardless of diet. Furthermore, we identified skeletal muscle ACSL6 protein abundance as being positively related to intramuscular lipid content, indicating potential contribution to regulation of lipid storage. ACSLs remain of interest as potentially critical determinants of skeletal muscle lipid metabolism.
Acknowledgements
We thank Donald Jump, Ph.D. at Oregon State University for his generous primer donations for polymerase chain reaction analysis. The results of the present investigation do not constitute endorsement by the American College of Sports Medicine. We declare that the results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation.
Grants
This project was partly supported by the American College of Sports Medicine Northwest Regional Student Research Grant awarded to H.D.S. The mouse project was supported by DK103829 from the National Institutes of Health awarded to M.M.R. S.A.N. is supported by KL2TR002370 as part of the Oregon Clinical & Translational Research Institute Clinical Translational Science Award UL1TR002371 from the National Institutes of Health. H.D.S. and S.E.E. are supported by fellowships from Oregon State University.
Footnotes
Disclosures
The authors have no conflict of interest to declare.
References
- 1.Kiens B Skeletal muscle lipid metabolism in exercise and insulin resistance. Physiol Rev. 2006. January;86(1):205–43. [DOI] [PubMed] [Google Scholar]
- 2.Newsom SA, Miller BF, Hamilton KL, Ehrlicher SE, Stierwalt HD, Robinson MM. Long-term rates of mitochondrial protein synthesis are increased in mouse skeletal muscle with high-fat feeding regardless of insulin-sensitizing treatment. Am J Physiol Endocrinol Metab. 2017. November 1;313(5):E552–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perreault L, Newsom SA, Strauss A, Kerege A, Kahn DE, Harrison KA, et al. Intracellular localization of diacylglycerols and sphingolipids influences insulin sensitivity and mitochondrial function in human skeletal muscle JCI Insight. American Society for Clinical Investigation; 2018. February 8;3(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goodpaster BH, He J, Watkins S, Kelley DE. Skeletal muscle lipid content and insulin resistance: evidence for a paradox in endurance-trained athletes. J Clin Endocrinol Metab. 2001. December;86(12):5755–61. [DOI] [PubMed] [Google Scholar]
- 5.Spina RJ, Chi MM, Hopkins MG, Nemeth PM, Lowry OH, Holloszy JO. Mitochondrial enzymes increase in muscle in response to 7–10 days of cycle exercise. J Appl Physiol. 1996. June;80(6):2250–4. [DOI] [PubMed] [Google Scholar]
- 6.Coleman RA, Lewin TM, Muoio DM. Physiological and nutritional regulation of enzymes of triacylglycerol synthesis. Annu Rev Nutr. 2000;20(1):77–103. [DOI] [PubMed] [Google Scholar]
- 7.Watt MJ, Hoy AJ. Lipid metabolism in skeletal muscle: generation of adaptive and maladaptive intracellular signals for cellular function Am J Physiol Endocrinol Metab. American Physiological Society; Bethesda, MD; 2012. June 1;302(11):E1315–28. [DOI] [PubMed] [Google Scholar]
- 8.Grevengoed TJ, Klett EL, Coleman RA. Acyl-CoA Metabolism and Partitioning. Annu Rev Nutr. 2014;34(1):1–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adeva-Andany MM, Carneiro-Freire N, Seco-Filgueira M, Fernández-Fernández C, Mouriño-Bayolo D. Mitochondrial β-oxidation of saturated fatty acids in humans. Mitochondrion. 2018. March 15; 10.1016/j.mito.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 10.Mashek DG, Li LO, Coleman RA. Rat long-chain acyl-CoA synthetase mRNA, protein, and activity vary in tissue distribution and in response to diet J Lipid Res. American Society for Biochemistry and Molecular Biology; 2006. September;47(9):2004–10. [DOI] [PubMed] [Google Scholar]
- 11.Li LO, Grevengoed TJ, Paul DS, Ilkayeva O, Koves TR, Pascual F, et al. Compartmentalized acyl-CoA metabolism in skeletal muscle regulates systemic glucose homeostasis Diabetes. American Diabetes Association; 2015. January;64(1):23–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Teodoro BG, Sampaio IH, Bomfim LHM, Queiroz AL, Silveira LR, Souza AO, et al. Long-chain acyl-CoA synthetase 6 regulates lipid synthesis and mitochondrial oxidative capacity in human and rat skeletal muscle. The Journal of Physiology. 2017. February 1;595(3):677–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dasari S, Newsom SA, Ehrlicher SE, Stierwalt HD, Robinson MM. Remodeling of skeletal muscle mitochondrial proteome with high-fat diet involves greater changes to β-oxidation than electron transfer proteins in mice. Am J Physiol Endocrinol Metab. 2018. May 29;10(4):46–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holloszy JO. Biochemical adaptations in muscle. Effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle. Journal of Biological Chemistry. 1967. May 10;242(9):2278–82. [PubMed] [Google Scholar]
- 15.Augusto V, Padovani CR, Campos GER. Skeletal muscle fiber types in C57BL6J mice. Braz J morphol Sci. 2004;21(2):89–94. [Google Scholar]
- 16.Mashek DG, Li LO, Coleman RA. Long-chain acyl-CoA synthetases and fatty acid channeling. Future Lipidol. 2007. August;2(4):465–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davidson SR, Burnett M, Hoffman-Goetz L. Training effects in mice after long-term voluntary exercise. Medicine and Science in Sports and Exercise. 2006. February;38(2):250–5. [DOI] [PubMed] [Google Scholar]
- 18.Li LO, Mashek DG, An J, Doughman SD, Newgard CB, Coleman RA. Overexpression of rat long chain acyl-CoA synthetase 1 alters fatty acid metabolism in rat primary hepatocytes Journal of Biological Chemistry. American Society for Biochemistry and Molecular Biology; 2006;281(48):37246–55. [DOI] [PubMed] [Google Scholar]
- 19.Kwak H-B, Woodlief TL, Green TD, Cox JH, Hickner RC, Neufer PD, et al. Overexpression of Long-Chain Acyl-CoA Synthetase 5 Increases Fatty Acid Oxidation and Free Radical Formation While Attenuating Insulin Signaling in Primary Human Skeletal Myotubes Int J Environ Res Public Health. Multidisciplinary Digital Publishing Institute; 2019. March 31;16(7):1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frayn KN. Calculation of substrate oxidation rates in vivo from gaseous exchange. Journal of Applied Physiology. 1983. August 1;2:628–34. [DOI] [PubMed] [Google Scholar]
- 21.Zurlo F, Larson K, Bogardus C, Ravussin E. Skeletal muscle metabolism is a major determinant of resting energy expenditure J Clin Invest. American Society for Clinical Investigation; 1990. November;86(5):1423–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frayn KN, Maycock PF. Skeletal muscle triacylglycerol in the rat: methods for sampling and measurement, and studies of biological variability. J Lipid Res. 1980. January;21(1):139–44. [PubMed] [Google Scholar]
- 23.Newsom SA, Schenk S, Thomas KM, Harber MP, Knuth ND, Goldenberg N, et al. Energy deficit after exercise augments lipid mobilization but does not contribute to the exercise-induced increase in insulin sensitivity J Appl Physiol. American Physiological Society; Bethesda, MD; 2010. March;108(3):554–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Curran-Everett D Explorations in statistics: standard deviations and standard errors. Adv Physiol Educ. 2008. September;32(3):203–8. [DOI] [PubMed] [Google Scholar]
- 25.Fujimoto Y, Itabe H, Kinoshita T, Homma KJ, Onoduka J, Mori M, et al. Involvement of ACSL in local synthesis of neutral lipids in cytoplasmic lipid droplets in human hepatocyte HuH7 J Lipid Res. American Society for Biochemistry and Molecular Biology; 2007. June 1;48(6):1280–92. [DOI] [PubMed] [Google Scholar]
- 26.Kang M-J, Fujino T, Sasano H, Minekura H, Yabuki N, Nagura H, et al. A novel arachidonate-preferring acyl-CoA synthetase is present in steroidogenic cells of the rat adrenal, ovary, and testis PNAS. National Academy of Sciences; 1997. April 1;94(7):2880–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schoonjans K, Watanabe M, Suzuki H, Mahfoudi A, Krey G, Wahli W, et al. Induction of the acyl-coenzyme A synthetase gene by fibrates and fatty acids is mediated by a peroxisome proliferator response element in the C promoter. Journal of Biological Chemistry. 1995. August 18;270(33):19269–76. [DOI] [PubMed] [Google Scholar]
- 28.Martin G, Schoonjans K, Lefebvre AM, Staels B, Auwerx J. Coordinate regulation of the expression of the fatty acid transport protein and acyl-CoA synthetase genes by PPARalpha and PPARgamma activators. Journal of Biological Chemistry. 1997. November 7;272(45):28210–7. [DOI] [PubMed] [Google Scholar]
- 29.Patsouris D, Reddy JK, Müller M, Kersten S. Peroxisome proliferator-activated receptor alpha mediates the effects of high-fat diet on hepatic gene expression. Endocrinology. 2006. March;147(3):1508–16. [DOI] [PubMed] [Google Scholar]
- 30.Horowitz JF, Leone TC, Feng W, Kelly DP, Klein S. Effect of endurance training on lipid metabolism in women: a potential role for PPARalpha in the metabolic response to training Am J Physiol Endocrinol Metab. American Physiological Society; Bethesda, MD; 2000. August;279(2):E348–55. [DOI] [PubMed] [Google Scholar]
- 31.Cresci S, Wright LD, Spratt JA, Briggs FN, Kelly DP. Activation of a novel metabolic gene regulatory pathway by chronic stimulation of skeletal muscle Am J Physiol, Cell Physiol. American Physiological Society; Bethesda, MD; 1996. May;270(5):C1413–20. [DOI] [PubMed] [Google Scholar]
- 32.Muoio DM, MacLean PS, Lang DB, Li S, Houmard JA, Way JM, et al. Fatty acid homeostasis and induction of lipid regulatory genes in skeletal muscles of peroxisome proliferator-activated receptor (PPAR) alpha knock-out mice. Evidence for compensatory regulation by PPAR delta. Journal of Biological Chemistry. 2002. July 19;277(29):26089–97. [DOI] [PubMed] [Google Scholar]
- 33.Romijn JA, Coyle EF, Sidossis LS, Gastaldelli A, Horowitz JF, Endert E, et al. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration Am J Physiol. American Physiological Society; Bethesda, MD; 1993. September;265(3 Pt 1):E380–91. [DOI] [PubMed] [Google Scholar]
- 34.Kelley DE, Goodpaster B, Wing RR, Simoneau JA. Skeletal muscle fatty acid metabolism in association with insulin resistance, obesity, and weight loss. Am J Physiol. 1999. December;277(6):E1130–41. [DOI] [PubMed] [Google Scholar]
- 35.Krssak M, Petersen KF, Dresner A, DiPietro L, Vogel SM, Rothman DL, et al. Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: a H-1 NMR spectroscopy study. Diabetologia. 1999. January;42(1):113–6. [DOI] [PubMed] [Google Scholar]
- 36.Pan DA, Lillioja S, Kriketos AD, Milner MR, Baur LA, Bogardus C, et al. Skeletal muscle triglyceride levels are inversely related to insulin action. Diabetes. 1997. June;46(6):983–8. [DOI] [PubMed] [Google Scholar]
- 37.Perseghin G, Scifo P, De Cobelli F, Pagliato E, Battezzati A, Arcelloni C, et al. Intramyocellular triglyceride content is a determinant of in vivo insulin resistance in humans: a 1H-13C nuclear magnetic resonance spectroscopy assessment in offspring of type 2 diabetic parents. Diabetes. 1999. August;48(8):1600–6. [DOI] [PubMed] [Google Scholar]
- 38.Durgan DJ, Smith JK, Hotze MA, Egbejimi O, Cuthbert KD, Zaha VG, et al. Distinct transcriptional regulation of long-chain acyl-CoA synthetase isoforms and cytosolic thioesterase 1 in the rodent heart by fatty acids and insulin Am J Physiol Heart Circ Physiol. American Physiological Society; 2006. June;290(6):H2480–97. [DOI] [PubMed] [Google Scholar]
- 39.Golovko Mikhail Y, Rosenberger Thad A, Færgeman Nils J, Feddersen Søren, Cole Nelson B, Pribill Ingrid, et al. Acyl-CoA Synthetase Activity Links Wild-Type but Not Mutant α-Synuclein to Brain Arachidonate Metabolism Biochemistry. American Chemical Society; 2006. May 13;(45):6956–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Golej DL, Askari B, Kramer F, Barnhart S, Vivekanandan-Giri A, Pennathur S, et al. Long-chain acyl-CoA synthetase 4 modulates prostaglandin E₂ release from human arterial smooth muscle cells J Lipid Res. American Society for Biochemistry and Molecular Biology; 2011. April;52(4):782–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cortright RN, Sandhoff KM, Basilio JL, Berggren JR, Hickner RC, Hulver MW, et al. Skeletal Muscle Fat Oxidation Is Increased in African‐American and White Women after 10 days of Endurance Exercise Training Obesity. John Wiley & Sons, Ltd; 2006. July 1;14(7):1201–10. [DOI] [PubMed] [Google Scholar]
- 42.Frahm JL, Li LO, Grevengoed TJ, Coleman RA. Phosphorylation and Acetylation of Acyl-CoA Synthetase- I. J Proteomics Bioinform. 2011. July 22;4(7):129–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Essén B, Jansson E, Henriksson J, Taylor AW, Saltin B. Metabolic characteristics of fibre types in human skeletal muscle Acta Physiol Scand. John Wiley & Sons, Ltd; (10.1111); 1975. October;95(2):153–65. [DOI] [PubMed] [Google Scholar]
- 44.He J, Watkins S, Kelley DE. Skeletal Muscle Lipid Content and Oxidative Enzyme Activity in Relation to Muscle Fiber Type in Type 2 Diabetes and Obesity Diabetes. American Diabetes Association; 2001. April 1;50(4):817–23. [DOI] [PubMed] [Google Scholar]