Abstract
Much is known about myogenic mechanisms in circular muscle (CM) in the GI tract, but less is known about longitudinal muscle (LM). Two Ca2+ signalling behaviors occur in LM, localized intracellular waves not causing contractions and intercellular waves leading to excitation-contraction (E-C) coupling. An Ano1 channel antagonist inhibited intercellular Ca2+ waves and LM contractions. Ano1 channels are expressed by interstitial cells of Cajal (ICC) but not by smooth muscle cells (SMCs). We investigated Ca2+ signalling in a novel population of ICC that lies along the subserosal surface of LM (ICC-SS) in mice expressing GCaMP6f in ICC. ICC-SS fired stochastic localized Ca2+ transients. Such events have been linked to activation of Ano1 channels in ICC. Ca2+ transients in ICC-SS occurred by release from stores most likely via IP3 receptors. This activity relied on influx via store-operated Ca2+ entry and Orai channels. No voltage-dependent mechanism that synchronized Ca2+ transients in a single cell or between cells was found. Nitrergic agonists inhibited Ca2+ transients in ICC-SS, and stimulation of intrinsic nerves activated nitrergic responses in ICC-SS. Cessation of stimulation resulted in significant enhancement of Ca2+ transients when compared to the pre-stimulus activity. No evidence of innervation by excitatory, cholinergic motor neurons was found. Our data suggest that ICC-SS contribute to regulation of LM motor activity. Spontaneous Ca2+ transients activate Ano1 channels in ICC-SS. Resulting depolarization conducts to SMCs, depolarizing membrane potential, activating L-type Ca2+ channels and initiating contraction. Rhythmic electrical and mechanical behaviors of LM are an emergent property of SMCs and ICC-SS.
Keywords: optogenetics, Ca2+ imaging, c-Kit, Ca2+ stores, colon, SIP syncytium, gastrointestinal motility
Introduction
Circular muscle (CM) contractions contribute to propulsive contractions in the colon, but the role of longitudinal muscle (LM) is less well understood. Previous studies have shown that smooth muscle cells (SMCs) in LM are coupled to contraction via the occurrence of action potentials generated by activation of L-type (dihydropyridine-sensitive) Ca2+ channels (El‐Sharkawy, 1983; Chow & Huizinga, 1987; Smith et al., 1987a; Liu & Huizinga, 1993; Spencer et al., 2002). Spontaneous transient depolarizations (STDs) and action potentials occur spontaneously in LM strips devoid of myenteric ganglia and CM layers. Propagation of action potentials appears to be confined within bundles of cells running in the longitudinal axis, and propagation rarely occurs more than 1 mm in the circumferential direction (Spencer et al., 2002).
Intracellular Ca2+ transients occur spontaneously in longitudinal SMCs (LSMCs) due to Ca2+ release from stores (Hennig et al., 2002). These events are localized, asynchronous in adjacent cells, and are not coupled to contractile events. It is likely that the localized, intracellular transients couple to activation of large-conductance Ca2+-activated K+ (BK) channels, as in many types of SMCs (Wellman & Nelson, 2003), and BK channel antagonists have been shown to increase contractions and action potential firing in LSMCs (Thornbury et al., 1992; Carl et al., 1995). Intercellular Ca2+ waves in LSMC bundles have also been observed. These events are fast and of much greater amplitude than localized, intracellular transients, and likely result from action potential firing (Hennig et al., 2002). Intercellular Ca2+ waves, which along with action potentials can occur quite rhythmically (Spencer et al., 2002), are coupled to LM contractions. Whether an external pacemaker drives LSMC intercellular Ca2+ waves and contractions, as is the case for SMCs in the small bowel and stomach (Sanders et al., 2014), or whether LSMCs manifest intrinsic pacemaker capability are unanswered questions.
Previous morphological examinations have reported 4 distinct types of interstitial cells of Cajal (ICC) in colonic muscles of several species (Berezin et al., 1990; Christensen et al., 1992; Rumessen et al., 1993; Ishikawa & Komuro, 1996; Burns et al., 1997; Faussone-Pellegrini & Thuneberg, 1999; Aranishi et al., 2009; Blair et al., 2012a). A group of cells generating slow waves exists at the submucosal surface (ICC-SM) of the CM (Smith et al., 1987b; Serio et al., 1991; Yoneda et al., 2004); intramuscular ICC (ICC-IM) are intermingled with motor nerve terminals and are coupled electrically to SMCs and interstitial cells distinguished by expression of platelet-derived-growth-factor-receptor-alpha (PDGFRα, aka SIP syncytium; (Sanders et al., 2012). A network of multi-polar ICC lies in the myenteric region between the CM and the LM also displays pacemaker activity (ICC-MY (Smith et al., 1987b)); and a final population lies along the serosal surface (ICC-SS) of the LM (Toma et al., 1999; Vanderwinden et al., 2000; Aranishi et al., 2009; Blair et al., 2012a; Rumessen et al., 2013). ICC-SS were found both in taenia coli and in the inter-taenia regions in colons of cynomolgous monkeys (Blair et al., 2012a). It is known from these previous studies that ICC-SS are present along the subserosal surface of the proximal colon (Vanderwinden et al., 2000; Aranishi et al., 2009; Rumessen et al., 2013). Aside from their morphology and anatomical localization, virtually nothing is currently known about the physiological function or neural regulation of ICC-SS. We hypothesized that these cells participate in pacing and neural regulation of LM.
ICC-SS are stellate, multi-processed cells that are distributed within the connective tissue beneath the mesothelium. The cells are oriented along the axis of the LM muscle fibers in the proximal portion of murine, guinea-pig and primate colons, forming an electrical network via gap junctions between the cells (Burns et al., 1997; Vanderwinden et al., 2000; Aranishi et al., 2009) (Blair et al., 2012a). In the present study, mice were generated by crossing iCre-Kit mice with GCaMP6ffl/fl mice to generate ICC-specific expression of the Ca2+ sensor, GCaMP6f. Video records of Ca2+ transients in ICC-SS were collected via fluorescence microscopy to better understand the role of these cells in regulating LSMCs. ICC-SS express the Ca2+-activated Cl− conductance, Ano1, selectively (Gomez-Pinilla et al., 2009; Blair et al., 2012a), and evaluating the specific properties and mechanisms of Ca2+ transients, shown in other ICC to regulate the activation of Ano1 (Zhu et al., 2015; Baker et al., 2016; Drumm et al., 2017, 2018b, 2019b, 2019c), provides a novel means of investigating the role ICC-SS in LM motility.
Methods
Ethical Approval
All animals used and the protocols performed throughout this study were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All procedures were approved by the Institutional Animal Use and Care Committee at the University of Nevada, Reno. All experiments conform to the principles and regulations for reporting animal experiments as described by Grundy (2015).
Animals
Ai95 (RCL-GCaMP6f)-D (GCaMP6ffl/fl mice) and their wild-type siblings (C57BL/6) were purchased from the Jackson Laboratory (Bar Harbor, MN, USA). c-Kit+/Cre-ERT2 (Kit-Cre mice) were gifted from Dr. Dieter Saur of the Technical University Munich, Germany. Mice expressing an inducible Cre Recombinase driven by the smooth muscle heavy chain promoter (SmMHC‐Cre mice) were generated by Dr. Stefan Offermann (Max‐ Planck‐ Institute, Germany) and gifted to our lab by Dr. James Stull of Southwestern University (TX, USA).
Tamoxifen preparation and administration
GCaMP6ffl/fl mice were crossed with either Kit-Cre mice or SmHC-Cre mice, as outlined above, and the resulting offspring are referred to as Kit-Cre-GCaMP6f or SmHC-Cre-GCaMP6f mice throughout the manuscript. The mice were injected with tamoxifen at 6–8 weeks of age to induce Cre Recombinase and activate expression of GCaMP6f. Tamoxifen (Sigma T5648; 80mg) was dissolved in 800 μL of ethanol (Pharmco-Aaper 200 Proof - Absolute, Anhydrous) by vortexing for 20 minutes. Then 3.2 ml of Safflower (generic) was added to create solutions of 20 mg/ml, which were then sonicated for 30 minutes prior to injection.
Mice were injected (Intraperitoneal injection; IP) with 0.1 ml of tamoxifen solution (2 mg tamoxifen) for three consecutive days. Mice were used for experiments 10 days after the initial injection. Expression of GCaMP6f was confirmed by genotyping and imaging. Mice were anaesthetized by inhalation with isoflurane (Baxter, Deerfield, IL, USA) and killed by cervical dislocation before excision of tissues.
Tissue preparation
Abdomens were opened and the entire colon was removed and placed in Krebs-Ringer bicarbonate (KRB) solution. A segment of proximal colon was opened along the mesenteric border and intra-luminal contents were washed away with KRB solution. The mucosa and submucosa were removed by sharp dissection.
Calcium imaging
Colonic muscle strips were pinned with the LM layer facing upward to the bottom of a 60 mm dish coated with Sylgard elastomer (Dow Corning, Midland, MI). The dish was perfused with warmed KRB solution (37°C) for 60 mins prior to the beginning of experiments. Following this equilibration period, Ca2+ imaging was performed on cells in situ with an Eclipse E600FN microscope (Nikon Inc., Melville, NY, USA) equipped with a 60× 1.0 CFI Fluor lens (Nikon instruments INC, NY, USA). GCaMP6f was excited at 488 nm (T.I.L.L. Polychrome IV, Grafelfing, Germany). The pixel size using this acquisition configuration was 0.225 μm. Image sequences were collected at 33 fps with TILLvisION software (T.I.L.L. Photonics GmbH, Grafelfing, Germany). Movement artefacts were stabilized digitally with custom made Volumetry software prior to analysis of Ca2+ transients. For experiments involving pharmacological treatments, control video sequences were collected for 20–30 sec, and then KRB solution containing the drug concentration to be tested was perfused into the bath for 12–15 mins before another 20–30 sec period of imaging was performed. As reported previously, imaging GCaMP for 20–30 s of consecutive recordings did not lead to a decrease in Ca2+ transients (Drumm et al., 2018a).
Analysis of Ca2+ transients
Ca2+ release/entry events in colonic cells were imaged and analyzed as described previously (Drumm et al., 2019a). Briefly, movies of Ca2+ events were converted to a stack of TIFF (tagged image file format) images and imported into custom software (Volumetry G8c, GW Hennig) for initial processing. Whole-cell ROIs were created to generate spatio-temporal maps (STMs) of Ca2+ transients in individual cells within a FOV. These STMs were imported as TIFF files into Image J (version1.52a, National Institutes of Health, MD, USA, http://rsbweb.nih.gov/ij) for post hoc analysis. Basal fluorescence was acquired from regions of cells that displayed the most uniform and least intense fluorescence (F0). Then fluorescence values throughout the rest of the cell were divided by the F0 value to calibrate the STM for the amplitudes of Ca2+ transients as F/F0. Ca2+ event amplitude, duration and spread were then calculated from the STM. Ca2+ transient frequency was expressed as the number of events fired per cell per minute (min−1). The amplitude of Ca2+ transients was expressed as ΔF/F0, the duration of Ca2+ transients was expressed as full duration at half maximum amplitude (FDHM) and the spatial spread of Ca2+ transients was expressed as μm of cell propagated per Ca2+ transient. Ca2+ transients were included in measurements if their peak amplitude reached > 20% of maximum during control period.
In some examples Ca2+ transients observed in colonic cells were quantified using particle (PTCL) analysis, as described previously (Drumm et al., 2017, 2018b, 2019b). Briefly, movies were firstly imported into Volumetry G8d and motion stabilized to minimize residual motion artifacts. A differential (Δt = ± 66–70 ms) and Gaussian filter (1.5 × 1.5μm, StdDev 1.0) was applied to accurately distinguish Ca2+ transients from the background. A particle analysis routine was applied by using of a flood-fill algorithm, which marked the structure of all adjoining pixels that had intensities above the threshold. Ca2+ transient PTCLs were brighter and larger than noise particles. The threshold at which noise particles emerged and reduced the average particle size was thresholded and then valid Ca2+ PTCLs, which were above this threshold, were then saved as a coordinate based PTCL movie. To identify Ca2+ firing sites, only those particles that did not overlap with any particles in the previous frame but overlapped with particles in the next 70 ms were considered Ca2+ firing sites.
Contractile recordings
LM muscle strips (5–8mm in length and ~2mm in diameter) were cut parallel to the LM fibers from the proximal colon of wildtype mice, attached to a stable mount and to a Gould strain gauge and immersed in jacketed 15 ml tissue baths. The LM muscles were continuously oxygenated and maintained in KRB solution at 37oC. LM tissues were stretched to an initial tension of 5 mN, and experiments began after 60 mins of equilibration. Isometric contractions were recorded using AcqKnowledge software (3.9.1; Biopac Systems, Goleta, CA) and then their area under the curve (AUC), frequency, amplitude and duration were quantified using pClamp 9.0. Contractions at least 10% of maximum during control periods were measured for quantification.
Nerve stimulation
Neural responses were evoked either in Ca2+ imaging experiments or isometric tension recording experiments by electrical field stimulation (EFS). EFS was delivered by two parallel platinum electrodes on either side of a colon muscle sheet (imaging) or a LM strip (tension). EFS was evoked a by Grass stimulator S48 (Quincy MA, USA), at 0.3ms pulse duration and 100 V. Responses to EFS were abolished by tetrodotoxin (1 μM; data not shown).
Drugs and solutions
Tissues used for imaging and electrophysiological experiments were perfused with KRB solution containing (mmol/L): NaCl, 120.35; KCl, 5.9; NaHCO3, 15.5; NaH2PO4, 1.2; MgCl2, 1.2; CaCl2, 2.5; and glucose, 11.5. KRB solution was bubbled with a mixture of 97% O2 – 3% CO2 and warmed to 37 ± 0.2 °C. For experiments using 0 mM [Ca2+]o, CaCl2 was excluded from the KRB solution and 0.5 mM EGTA was added, resulting in a Ca2+ free KRB solution. 2-aminoethyl-diphenylborinate (2-APB), atropine, caffeine, cyclopiazonic acid (CPA), L-NNA, pinacidil and nicardipine were purchased from Sigma-Aldrich (St Louis, MO, USA). Tetrodotoxin (TTX), Ani 9, thapsigargin, ODQ, ryanodine, SKF 96365, U73343 and U73122 were purchased from Tocris Bioscience (Ellisville, Missouri, USA). GSK 7975A was purchased from Aobious. Xestospongin C was purchased from Cayman Chemical. All drugs were dissolved as recommended by the manufacturers and then diluted to the desired concentrations with KRB solution.
Statistics
Data is represented as mean ± standard deviation (S.D.). Statistical analysis was performed using paired student’s t-tests (comparing two paired groups) or a one-way ANOVA with a Tukey post hoc test as appropriate (comparing three paired groups). In all statistical analyses, P<0.05 was taken as significant. Probabilities < 0.05 are represented by a single asterisk (*), probabilities < 0.01 are represented by two asterisks (**), probabilities < 0.001 are represented by three asterisks (***) and probabilities < 0.0001 are represented by four asterisks (****). Throughout the text, “n” refers to the number of animals included in datasets, and “c” refers to the number of individual cells analyzed within a given dataset.
Results
Ca2+ signaling patterns in LSMCs
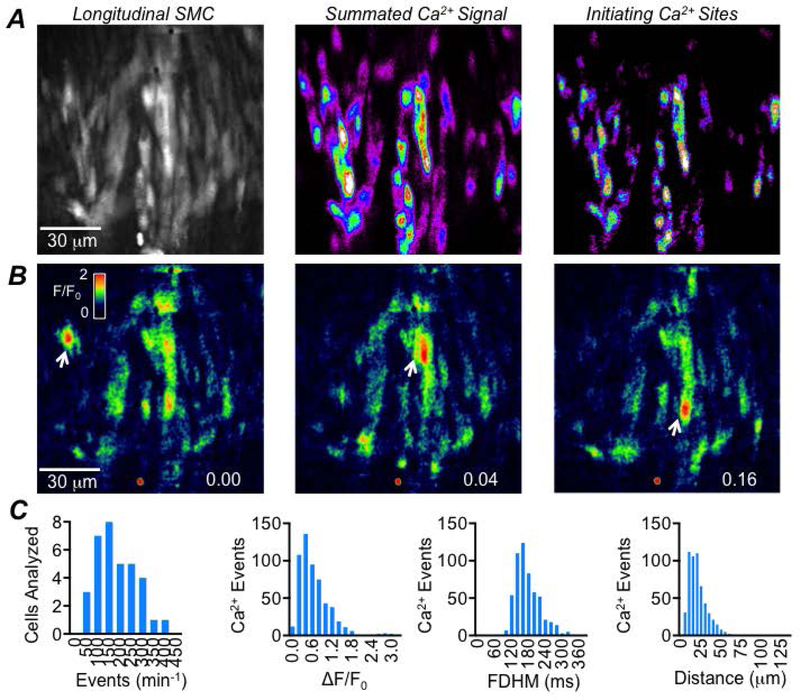
We firstly examined basal Ca2+ signalling in LSMCs in proximal colons from SmHC-Cre-GCaMP6f mice by performing in situ imaging of Ca2+ transients. We confirmed two distinct patterns of Ca2+ signalling in LSMCs; intracellular Ca2+ waves and intercellular Ca2+ waves, as described previously (Hennig et al., 2002). Intracellular Ca2+ waves were imaged using a 60x objective (Fig. 1A–B), and occurred asynchronously between cells and were not associated with contraction of the LM. LSMC intracellular Ca2+ waves had an average frequency of 180.7 ± 89.6 min−1, amplitude of 0.65 ± 0.47 ΔF/F0, a FDHM of 171.1 ± 45 ms and an average spatial spread of 21.1 ± 12.35 μm (c=34, n=5). The distribution of all values for frequency, amplitude, duration and spatial spread of LSMC intracellular Ca2+ waves is displayed as histograms in Fig. 1C.
Fig. 1: LSMC intracellular Ca2+ waves.
A Raw image of LSMCs (left panel) recorded in situ from the proximal colon of a SmHC-Cre-GCaMP6f mouse (60x objective; scale bar pertains to all panels in A). The middle panel shows summed intracellular Ca2+ waves occurring during a 20 sec recording. The panel on the right shows the initiation sites from which intracellular Ca2+ waves originated from in this FOV. B Time-lapse images showing the occurrence of intracellular Ca2+ waves over 0.16 sec with the firing of these events indicated by the white arrows. C Frequency histograms showing the range of values of intracellular Ca2+ wave frequency, amplitude, duration and spatial spread in LSMC, c=34, n=5.
The spatio-temporal maps (STMs) in Fig. 2A demonstrate that the firing of LSMC intracellular Ca2+ waves did not rely on extracellular Ca2+ influx via L-type Ca2+ channels, as application of the Cav1.2 channel antagonist, nicardipine (1 μM), had no effect on the frequency (P=0.94), amplitude (P=0.62), duration (P=0.08) or spatial spread (P=0.12) of the events (Fig. 2B, paired student t-tests, c=14, n=5). Similarly, incubation with a selective and potent antagonist of the Ca2+-activated-Cl− channel Ano1, Ani 9 (Fig. 2C, 1 μM, a potent antagonist of Ano1 channels that is >18 times more potent than T16Ainh-A01 or MONNA (Seo et al., 2016)) also had no effect on LSMC intracellular Ca2+ wave frequency (P=0.8), amplitude (P=0.15), duration (P=0.98) or spatial spread (P=0.39) (Fig. 2D, paired student t-tests, n=10, n=5), suggesting that the generation of these Ca2+ events was not reliant on the activation of Ano1 channels in colonic ICC. However, all intracellular Ca2+ waves observed in LSMCs were abolished when intracellular Ca2+ stores were disrupted with the sarcoplasmic/endoplasmic reticulum (ER) Ca2+-ATPase (SERCA) pump inhibitor cyclopiazonic acid (CPA, 10 μM, Fig. 2E–F, P<0.0001, paired student t-test, c=10, n=5), suggesting, as previously described, that intracellular Ca2+ waves in LSMCs originate from Ca2+ release mechanisms in the ER.
Fig. 2: LSMC intracellular Ca2+ waves do not rely on voltage-dependent Ca2+ entry or Ano1 channels.
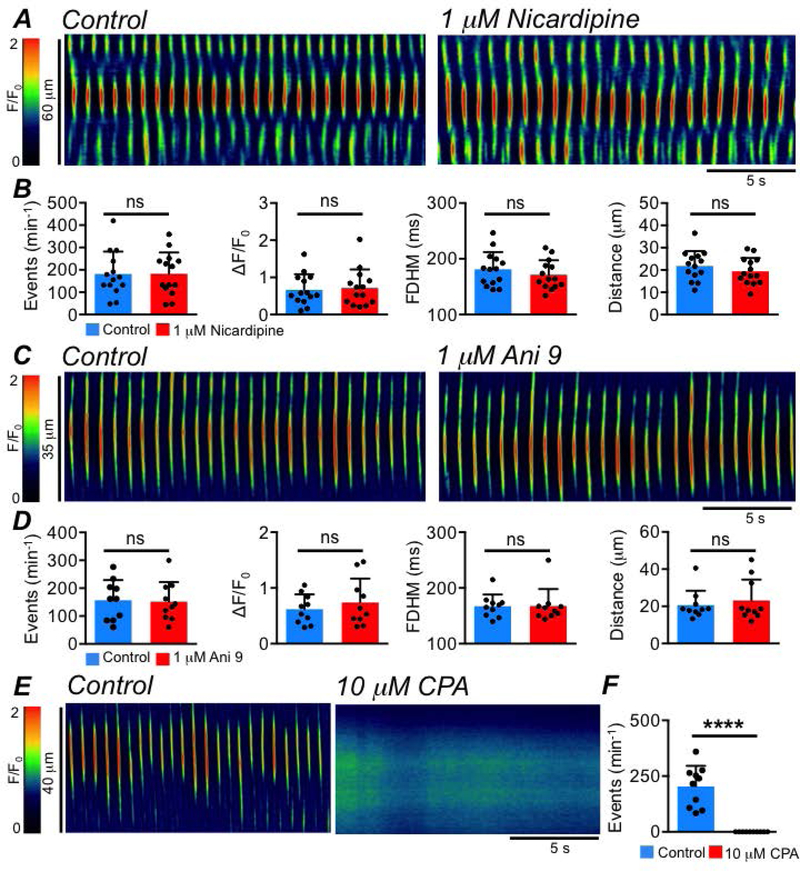
A STMs of intracellular Ca2+ waves in an LSMC recorded in situ, showing the effects of the Cav1.2 channel antagonist, nicardipine (1 μM). B Summary effects of nicardipine on LSMC intracellular Ca2+ waves. Control values: Frequency: 181.3 ± 100.3 min−1; Amplitude: 0.7 ± 0.41 ΔF/F0; FDHM: 181.3 ± 30.7 ms; Spread: 21.85 ± 6.7 μm, c=14, n=5. Nicardipine values: Frequency: 182.4 ± 95.7 min−1; Amplitude: 0.7 ± 0.5 ΔF/F0; FDHM: 173.3 ± 26 ms; Spread: 19.4 ± 6 μm, c=14, n=5. C STMs of intracellular Ca2+ waves occurring in a LSMC recorded in situ, showing the effect of the Ano1 channel antagonist Ani 9 (1 μM). D Summary effects of Ani 9 on LSMC intracellular Ca2+ waves. Control values: Frequency: 156.6 ± 72.4 min−1; Amplitude: 0.6 ± 0.3 ΔF/F0; FDHM: 167.4 ± 20.9 ms; Spread: 20.67 ± 7.7 μm, c=10, n=5. Ani 9 values: Frequency: 151.8 ± 70.7 min−1; Amplitude: 0.7 ± 0.4 ΔF/F0; FDHM: 167.7 ± 30.3 ms; Spread: 23.1 ± 11.3 μm, c=10, n=5. E STMs of intracellular Ca2+ waves occurring in a LSMC recorded in situ, showing the effect of the SERCA pump inhibitor CPA (10 μM). F Summary effect of CPA on LSMC intracellular Ca2+ wave frequency, c=10, n=5.
The second pattern of Ca2+ signalling observed in LSMCs was intercellular Ca2+ waves. Intercellular Ca2+ waves spread rapidly from cell to cell, propagating across the LM when imaged at low power (10–20x, Fig. 3A). The intercellular Ca2+ waves occurred in rapid bursts, and were associated with contractions of the LM. STMs of these events could be drawn by reconstructing an x, t plot across the entire field of view (FOV) of the LM (Fig. 3B). In contrast to intracellular Ca2+ waves, intercellular Ca2+ waves were sensitive to nicardipine (1 μM, Fig. 3C), which reduced their frequency from 7.1 ± 2.1 to 1.7 ± 1.3 min−1 (Fig. 3D, P=0.008, paired student t-test, n=4). These data suggested that intercellular Ca2+ waves and contractions of LM depended on Ca2+ influx via L-type Ca2+ channels. We tested the hypothesis that activation of Ano1 channels in ICC may be responsible for increased excitability of LSMCs, making it possible to reach the threshold for activation of L-type Ca2+ channels. Ani 9 (1 μM) significantly reduced the frequency of intercellular Ca2+ waves from 6.1 ± 1.7 to 2.1 ± 2.9 min−1 (Fig. 3E–F, P=0.015, paired student t-test, n=7). Another approach to examine the role of ICC in generating LSMC intercellular Ca2+ waves was to inhibit store-operated-Ca2+ entry (SOCE) in ICC. SOCE has been demonstrated to be critical for the maintenance of pacemaker activity and stochastic Ca2+ release and Ano1 channel activation in other classes of ICC in the small intestine (Zheng et al., 2018) and colon (Drumm et al., 2019b). SOCE in ICC is mediated by interactions between the ER membrane protein STIM and the plasma membrane bound Ca2+ influx channels of the Orai family (Zheng et al., 2018). We inhibited SOCE in ICC using a selective antagonist of Orai channels, GSK 7975A, and found that at 1 μM LSMC intercellular Ca2+ waves were significantly reduced in frequency from 5.1 ± 2.1 to 2.6 ± 1.8 min−1 (Fig. 3G–H, P=0.03, one way ANOVA, Tukey post hoc test, n=5). Further reduction in frequency was observed was observed with 10 μM GSK 7975A (i.e. to 1.4 ± 1.2 min−1 (Fig. 3H, P=0.017, one way ANOVA, Tukey post hoc test, n=5).
Fig. 3: LSMC intercellular Ca2+ waves.
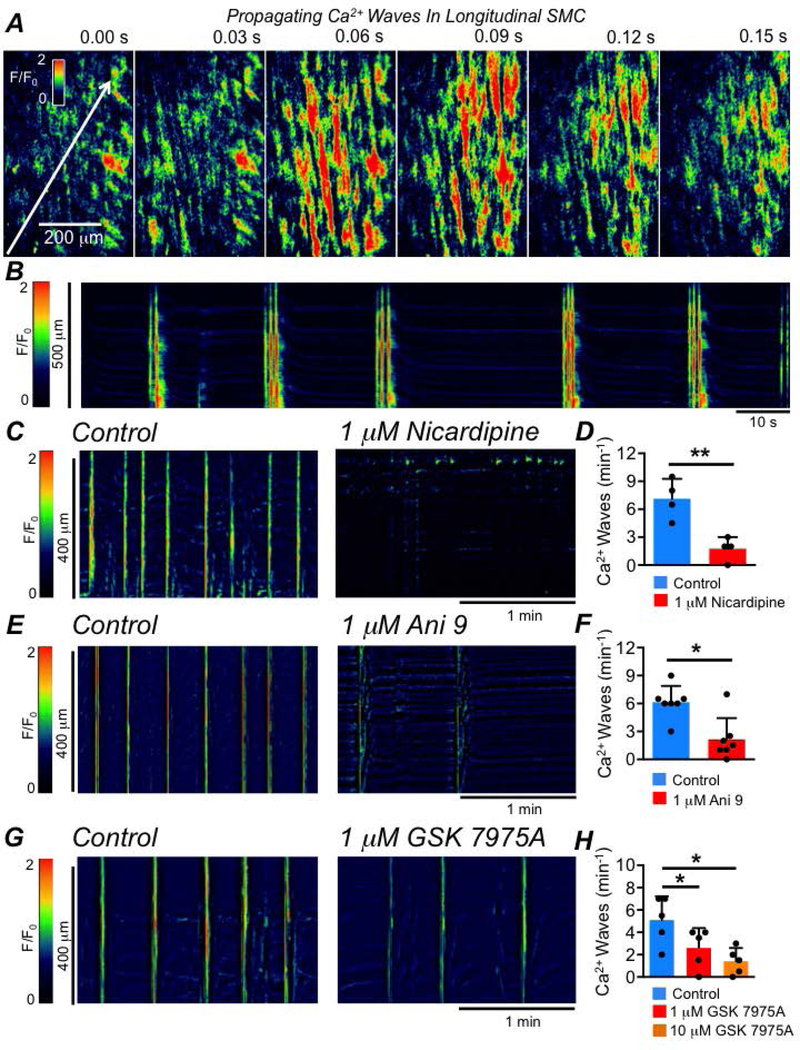
A Time-lapse images showing intercellular Ca2+ waves in the LM of the proximal colon of a SmHC-Cre-GCaMP6f mouse (20x objective; scale bar in the far left panel pertains to all panels). The white arrow shows the direction of the propagation of the intercellular Ca2+ wave in the far left panel. B STM of intercellular Ca2+ waves occurring in an LM sheet recorded in situ. C STMs of intercellular Ca2+ waves occurring in the LM recorded in situ and the inhibitory effect of nicardipine (1 μM). D Summary data showing the effect of nicardipine on the frequency of LSMC intercellular Ca2+ waves, n=4. E STMs of intercellular Ca2+ waves in LM recorded in situ and the effect of Ani 9 (1 μM). F Summary data showing the effect of Ani 9 on the frequency of LSMC intercellular Ca2+ waves, n=7. G STMs of intercellular Ca2+ waves in the LM recorded in situ, showing the effect of the Orai channel antagonist GSK 7975A (1 μM). H Summary data showing the effect of GSK 7975A (1 & 10 μM) on the frequency of LSMC intercellular Ca2+ waves, n=5.
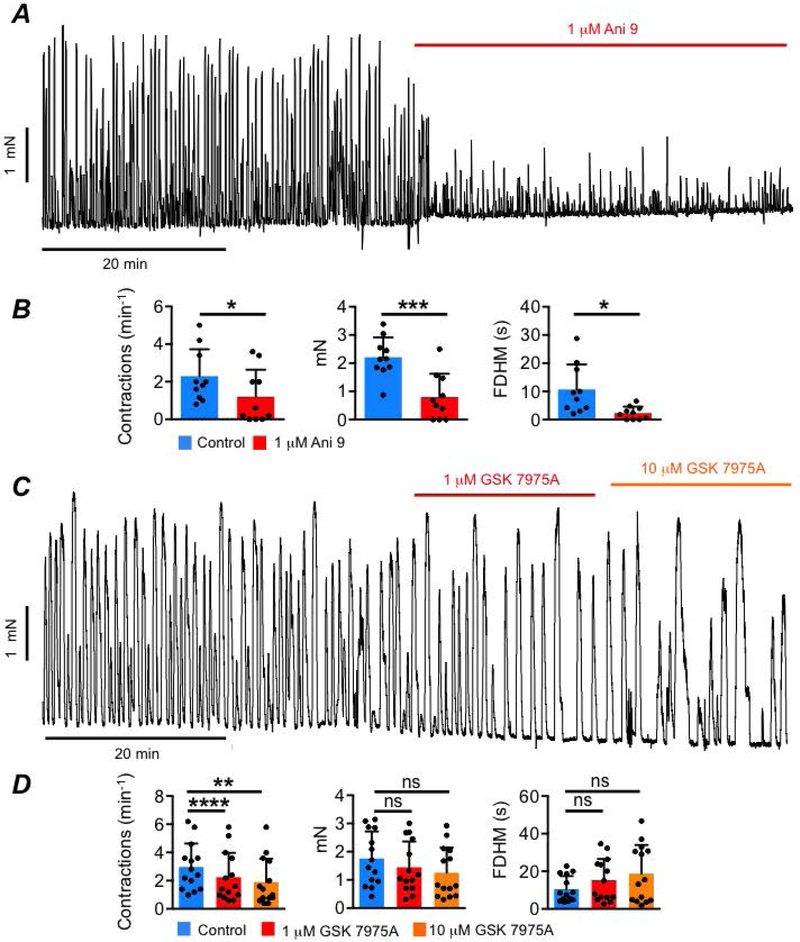
We further investigated a role of ICC in generating normal LM contractions by performing isometric tension recordings of LM strips from the mouse proximal colon. Mouse colonic LM strips exhibited spontaneous rhythmic contractions (Fig. 4A) and the frequency of contractions was reduced from 2.2 ± 1.4 to 1.2 ± 1.4 min−1 by Ani 9 (1 μM, Fig. 4A–B, P=0.02, paired student t-test, n=5). Ani 9 also reduced the amplitude of LM contractions from 2.2 ± 0.7 to 0.8 ± 0.8 mN (Fig. 4B, P= 0.0006, paired student t-test, n=5), and duration of contractions was reduced from 10.7 ± 8.8 to 2.3 ± 2.2 s (Fig. 4B, P=0.02, paired student t-test, n=5). GSK 7975A (1 μM) led to a reduction in LM contraction frequency from 2.9 ± 1.7 to 2.2 ± 1.7 min−1 (Fig. 4C–D, P=0.0001, one way ANOVA, Tukey post hoc test, n=6) and this was further reduced to 1.9 ± 1.65 min−1 by 10 μM GSK 7975A (Fig. 4C–D, P=0.0032, one way ANOVA, Tukey post hoc test, n=6). While there was a trend of a reduced amplitude and increased duration of LM contractions by GSK 7975A, these changes did not reach statistical significance (Fig. 4D, Amplitude: P=0.34 (1 μM), P=0.15 (10 μM), one way ANOVA, Tukey post hoc test, n=6; Duration: P=0.13 (1 μM), P=0.053 (10 μM), one way ANOVA, Tukey post hoc test, n=6). These observations suggest that Ca2+ store loading and Ano1 channels are required for maintenance of L-type Ca2+ channel activation and excitation-contraction coupling in LM.
Fig. 4: LM contractions rely on Ano1 channels and SOCE.
A Mouse LM contractions and the effects of Ani 9 (1 μM). B Summary data for the effects of Ani 9 on LM contraction frequency, amplitude and duration, n=5. C LM contractions and the effects of GSK 7975A (1 &10 μM). D Summary data for the effects of GSK 7975A on LM contraction frequency, amplitude and duration, n=6.
ICC-SS run in parallel with LM bundles and are electrically connected to LSMCs by gap junctions (Vanderwinden et al., 2000; Aranishi et al., 2009; Rumessen et al., 2013). Like other classes of ICC in the GI tract, ICC-SS display exclusive expression of Ano1 channels (Gomez-Pinilla et al., 2009; Blair et al., 2012a). Thus, ICC-SS are in a prime location and express a key ion channel that appears to regulate the excitability of the LM layer in the proximal colon. We hypothesized that if ICC-SS regulate LM activity through activation of Ano1 channels, they must exhibit intracellular Ca2+ signalling behaviours. We investigated this possibility by imaging Ca2+ signaling in ICC-SS using Kit-Cre-GCaMP6f mice.
Basal Ca2+ signaling in ICC-SS
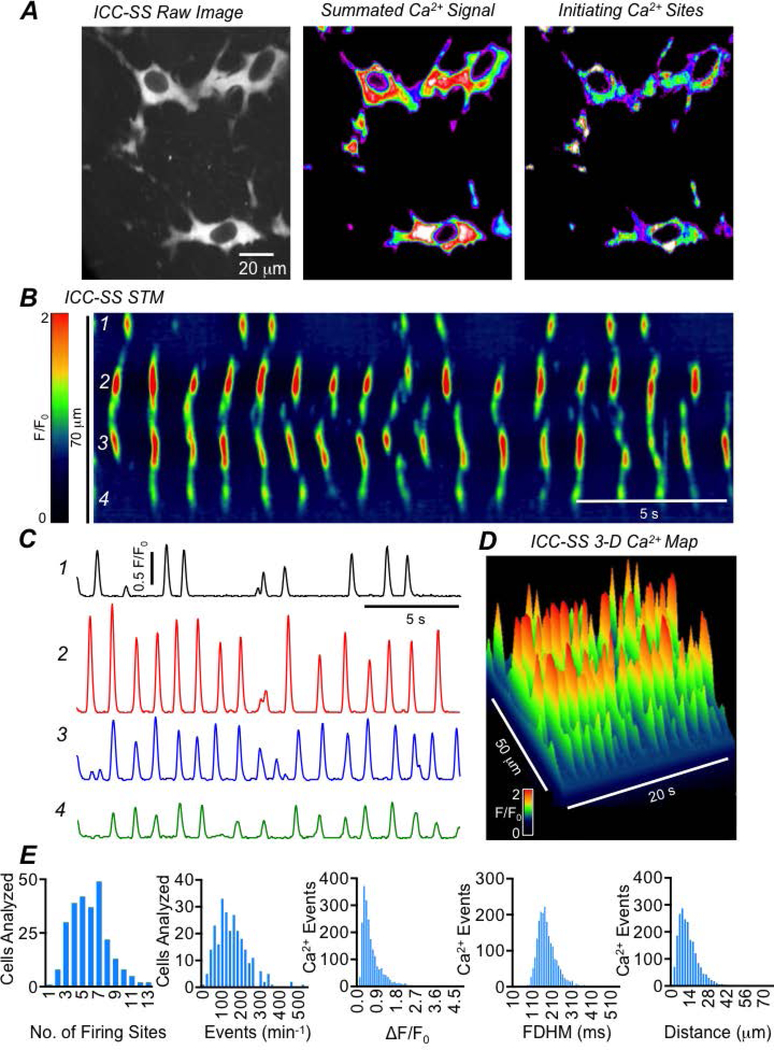
In accordance with previous morphological studies (Vanderwinden et al., 2000; Aranishi et al., 2009; Blair et al., 2012a; Rumessen et al., 2013), we found that ICC-SS in the mouse colon were stellate in shape with a large ovoid soma and multiple processes (Fig. 5A). We imaged these cells along the serosal surface of the proximal colon from Kit-Cre-GCaMP6f mice. ICC-SS were spontaneously active, firing hundreds of Ca2+ transients per minute. Fig. 5A illustrates a typical example of ICC-SS in the proximal colon (left panel) with Ca2+ transients summated over a 20 sec recording (middle panel) and areas of initiation of Ca2+ transients (right panel). To analyze Ca2+ transients in ICC-SS, we employed spatio-temporal maps (STMs), as described previously (Drumm et al., 2019a). One such STM from a cell recorded in situ is shown in Fig. 5B. The STM is colour coded to illustrate more intense fluorescence as warm colours (red, orange) and less intense fluorescence as cold colours (blue, black). ICC-SS were highly active, expressing many Ca2+ firing sites, and each typically generating multiple transients throughout recording periods. Four such sites are indicated on the STM in Fig. 5B, and activities of each site are plotted as traces in Fig. 5C. The STM is also plotted as a 3D graph in Fig. 5D. From this example it is clear that ICC-SS exhibit Ca2+ transients from multiple initiation sites with a range of firing frequencies, amplitudes, durations and spatial spread. On average, an ICC-SS fired Ca2+ transients from at least 6 different sites during 20 sec recording periods, although some cells displayed as many as 13 firing sites during this time frame (Fig. 5E, c=165, n=33). The average firing frequency of Ca2+ transients in ICC-SS was relatively high, 149.5 ± 82 min−1, although in some cases this could be as low as 6 min−1 and as high as 510 min−1 (Fig. 5E, c=165, n=33). The mean amplitude of Ca2+ transients in ICC-SS was 0.65 ± 0.5 ΔF/F0, the average FDHM was 181.8 ± 53.1 ms and the average spatial spread was 12.7 ± 8.2 μm (Fig. 5E, c=165, n=33).
Fig. 5: ICC-SS exhibit dynamic Ca2+ signaling.
A Left panel shows ICC-SS in the proximal colon of a Kit-Cre-GCaMP6f mouse in situ (60x objective). The middle panel shows summed Ca2+ signals in the ICC-SS during a 20 sec recording. Right panel shows an accumulation map of Ca2+ transients and indicates sites of Ca2+ transient initiation during a 20 sec recording period. B STM of Ca2+ transients in a single ICC-SS recorded in situ. Four different firing sites are indicated and their activity plotted as traces in panel C. D 3-D representation of the STM shown in panel B. E Frequency histograms showing the range of values of ICC-SS Ca2+ transient firing site number, frequency, amplitude, duration and spatial spread, c=165, n=33.
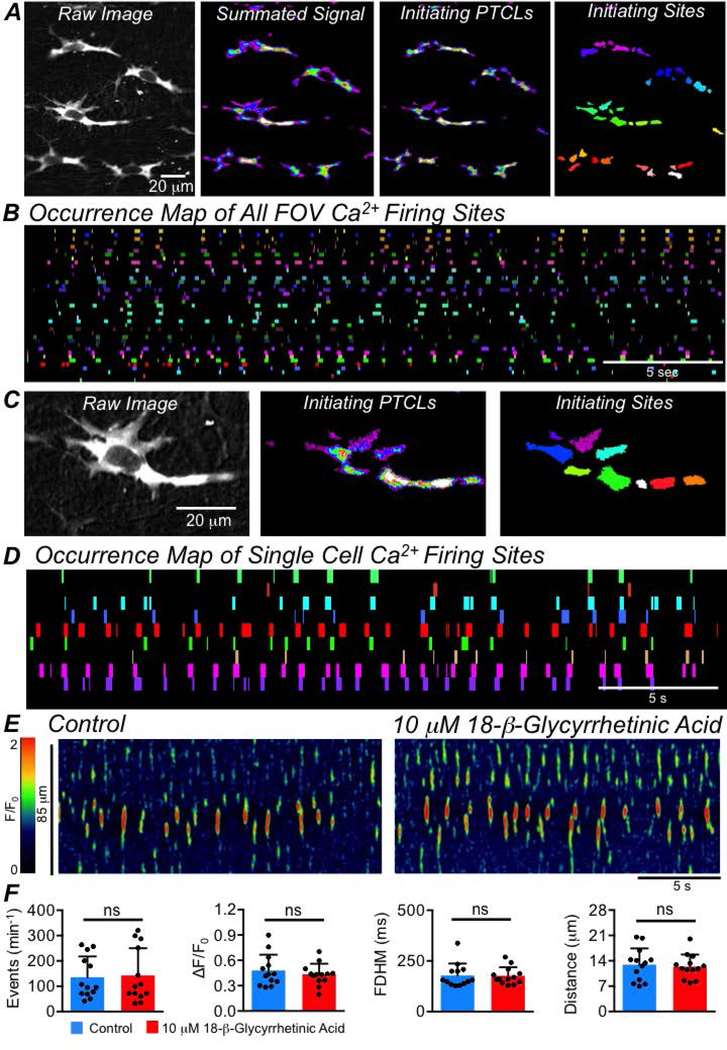
Ca2+ transients in ICC-SS were not entrained, forming FOV-wide Ca2+ waves across multiple cells. We examined this further, as some have implied that Ca2+ transients in ICC must be coupled and entrained to achieve significant influence on GI motility (Van Helden et al., 2000; Imtiaz et al., 2002; van Helden & Imtiaz, 2003). Fig. 6A shows a FOV containing 5 ICC-SS (far left panel). All 5 cells exhibited spontaneous Ca2+ transients, and the summed signal in these cells over a 30 sec recording is shown in Fig. 6A. Using PTCL analysis (see Methods), PTCL maps were created illustrating initiating sites from which Ca2+ transients originated (Fig. 6A) and individual Ca2+ firing sites were colour coded (Fig. 6A, far right panel). Dozens of firing sites were observed in this example, and their activities were plotted as an occurrence map, with firing at each site occupying an individual coloured ‘lane’ and plotted against time (Fig. 6B). From such analysis, no obvious coordination or entrainment of ICC-SS firing sites was evident to indicate network-wide rhythmic Ca2+ signalling. Close examination of the occurrence maps showed that many firing sites displayed quite regular patterns, but the activities of other firing sites in the FOV did not appear to influence or be influenced by this activity.
Fig. 6: ICC-SS Ca2+ signaling is not entrained or coupled.
A Far left panel shows a raw image of multiple ICC-SS in a FOV. The panel to the right shows summed Ca2+ transients in the ICC-SS during a 20 sec recording period. The next panel shows an accumulation map of Ca2+ transient initiation sites during 20 sec (initiating PTCLs). The far right panel shows colour coded Ca2+ firing sites from which Ca2+ transients were initiated. B Occurrence map of Ca2+ firing sites shown in the field of view (FOV) in the far right panel of A. Ca2+ firing sites are placed into individual colour coded lanes and plotted against time. C The far left panel is a raw image of a single ICC-SS taken from an in situ recording. The middle panel shows an accumulation map of Ca2+ transient initiation sites during a 20 sec recording. The far right panel shows colour coded Ca2+ firing sites where Ca2+ transients were initiated. D Occurrence map of all Ca2+ firing sites in a single ICC-SS shown in C. The Ca2+ firing sites are placed into individual colour coded lanes and plotted against time. E STMs showing the effect of the gap junction inhibitor 18 β-glycyrrhetinic acid (18 β-GA, 10 μM) on Ca2+ transients in ICC-SS. F Summary data showing the effect of 18 β-GA on ICC Ca2+ transient frequency, amplitude, duration and spatial spread, c=13, n=4.
We then applied this analysis to the activities of single cells. For example, Fig. 6C shows a representative single ICC-SS in the FOV shown in Fig. 6A, and the Ca2+ firing sites within this cell were identified, as described above (Fig. 6A–B). 9 Ca2+ firing sites were identified in this ICC-SS (Fig. 6C), and their firing patterns were plotted as an occurrence map in Fig. 6D. All 9 firing sites were spontaneously active, but the activity of any one firing site showed no apparent influence on the activities of neighbouring sites within the same cell. Thus, while some firing sites in ICC-SS display intrinsic rhythmicity; firing sites in a single cell or within multiple cells in a FOV were not entrained to produce coupled oscillations. A further demonstration of the lack of coupling was that the gap junction inhibitor 18 β-glycyrrhetinic acid (18 β-GA, 10 μM), had no effect on Ca2+ transients in ICC-SS (Fig. 6E), and no significant effects on the frequency (P=0.68), amplitude (P=0.29), duration (P=0.86) or spatial spread (P=0.54) of Ca2+ transients were observed (Fig. 6F, paired student t-tests, c=13, n=4).
Sources of Ca2+ required for ICC-SS activity
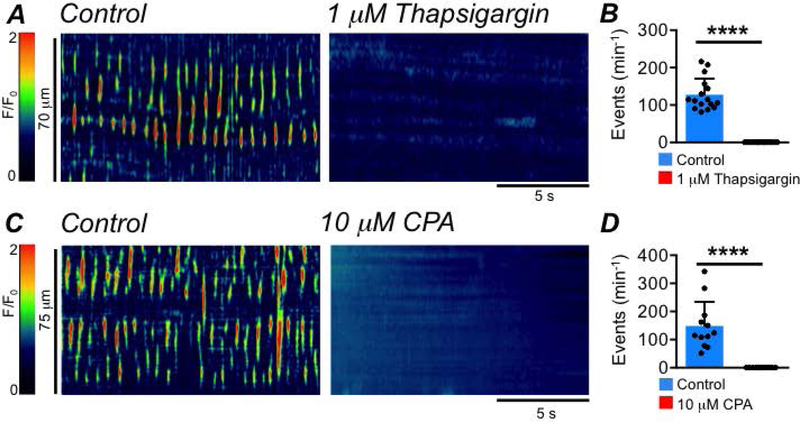
Ca2+ release from the ER is a fundamental behavior of ICC that is linked to activation of Ano1 channels and required for the regulatory functions of ICC (Zhu et al., 2015; Baker et al., 2016; Drumm et al., 2017, 2019b). Ca2+ release from the ER also appeared to be essential for the activity of ICC-SS, as incubation with SERCA pump inhibitors, thapsigargin (1 μM; Fig. 7A–B, c=16, n=5) or CPA (10 μM; Fig. 7C–D, c=12, n=4) abolished Ca2+ transients in ICC-SS (Fig. 7B,D, P<0.0001, paired student t-tests).
Fig. 7: SERCA pump inhibitors abolish ICC-SS Ca2+ transients.
A STMs showing the effect of the SERCA pump inhibitor, thapsigargin (1 μM), on ICC-SS Ca2+ transients. B Summary effect of thapsigargin on ICC-SS Ca2+ transient frequency, c=16, n=5. C STMs showing the effect of the SERCA pump inhibitor, CPA, on ICC-SS Ca2+ transients. D Summary effect of CPA on ICC-SS Ca2+ transient frequency, c=12, n=4.
ICC-SS were also reliant on Ca2+ influx from the external environment for the maintenance of Ca2+ transients, as incubating colon tissues with Ca2+ free solution (containing 0.5 mM EGTA) led to a significant reduction in the firing frequency of Ca2+ transients from 121.9 ± 60.9 to 56.9 ± 45.8 min−1 after just 4 mins (Fig. 8A–B, P=0.0002, one way ANOVA, Tukey post hoc test, c=21, n=5). There was also a reduction in the duration of Ca2+ transients from 189.1 ± 40.2 ms to 98.2 ± 80.8 ms after 10 mins (Fig. 8B, P=0.011, one way ANOVA, Tukey post hoc test, c=21, n=5). The spatial spread of Ca2+ transients was reduced after 6 mins from 12.2 ± 4.2 to 8.1 ± 5.6 μm (Fig. 8B, P=0.0035, one way ANOVA, Tukey post hoc test, c=21, n=5). Conversely, increasing the external Ca2+ concentration from 2.5 mM to 5 mM increased the activity of ICC-SS (Fig. 8C). While there was no effect on the amplitude (P=0.97), duration (P=0.27) or spatial spread (P=0.39), increasing external Ca2+ increased the firing frequency of Ca2+ transients from 187.9 ± 96.6 to 223.5 ± 91.1 min−1 (Fig. 8D, P=0.0011, paired student t-tests, c=16, n=5).
Fig. 8: The frequency of ICC-SS Ca2+ transients is modulated by Ca2+ influx.
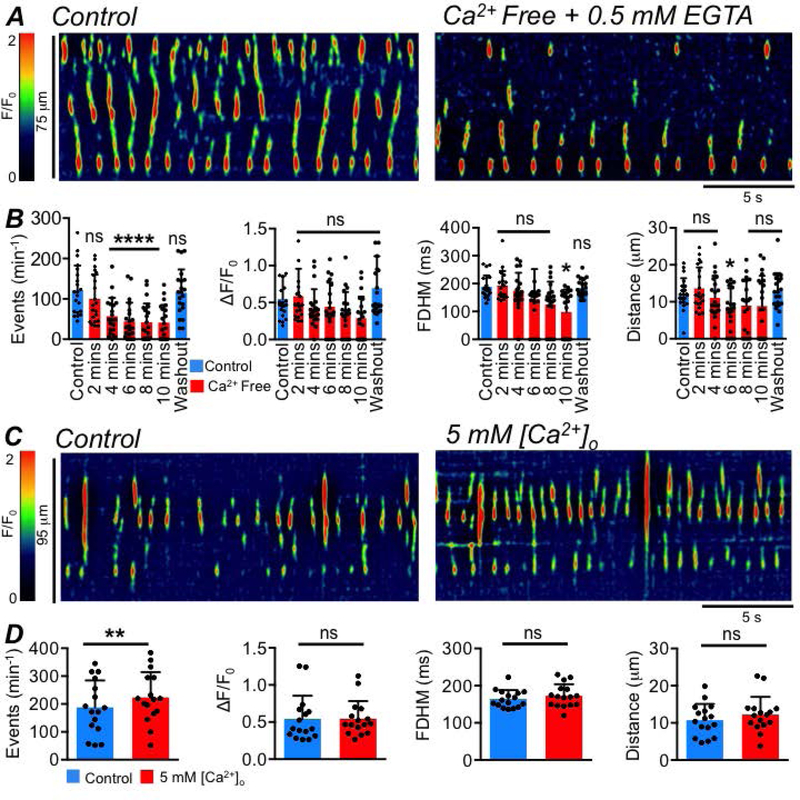
A STMs showing the effect of Ca2+ free solution (buffered with 0.5mM EGTA) on ICC-SS Ca2+ transients after 4 minutes exposure to the Ca2+ free conditions. B Summary data showing the effect of Ca2+ free solution (0.5mM EGTA) on ICC Ca2+ transient frequency, amplitude, duration and spatial spread after 2–10 min exposures, c=21, n=5. All statistical comparisons are against control. C STMs showing the effect of 5 mM external Ca2+ solution on ICC-SS Ca2+ transients. D Summary data showing the effect of 5 mM external Ca2+ solution on ICC Ca2+ transient frequency, amplitude, duration and spatial spread, c=16, n=5.
The specific Ca2+ release channels responsible for generating Ca2+ transients in ICC-SS were examined. Ryanodine (100 μM) had no significant effects on the frequency (P=0.1), amplitude (P=0.73), duration (P=0.13) or spatial spread (P=0.26) of Ca2+ transients in ICC-SS (Fig. 9A–B, paired student t-tests, c=8, n=3). The role of IP3Rs was examined by testing the effects of a phospholipase C inhibitor (U73122) to decrease IP3 production (Fig. 9C) and an inactive analogue of this drug (U73343). The inactive analogue, U73343 (10 μM), had no effect on ICC-SS Ca2+ transients (Fig. 9D, P=0.061, one way ANOVA, Tukey post hoc test, c=9, n=3), but U73122 (10 μM) reduced the firing frequency of Ca2+ transients from 87 ± 60.2 to 45.7 ± 63.3 min−1 (Fig. 9D, P=0.047, one way ANOVA, Tukey post hoc test, c=9, n=3). U73122 also inhibited the spatial spread of Ca2+ transients from 12.9 ± 6.8 to 4.8 ± 5.3 μm (Fig. 9D, P=0.016, one way ANOVA, Tukey post hoc test, c=9, n=3).
Fig. 9: ER channels contributing to Ca2+ release in ICC-SS.
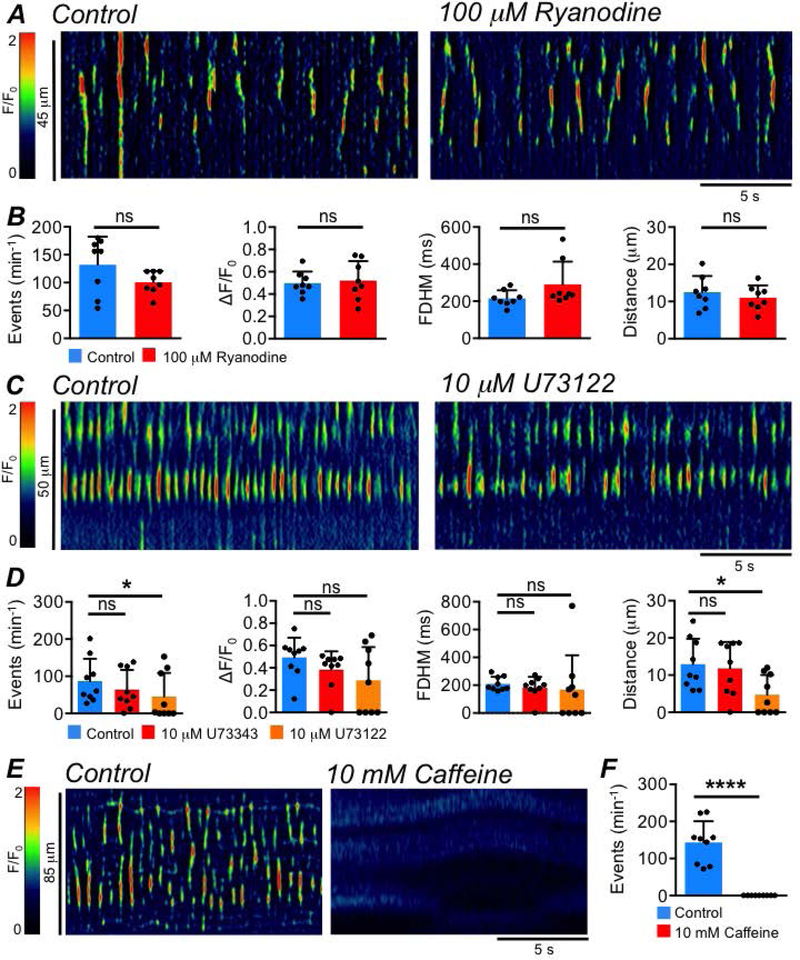
A STMs showing the effect of the ryanodine receptor antagonist, ryanodine (100 μM), on ICC-SS Ca2+ transients. B Summary data showing the effect of ryanodine on ICC Ca2+ transient frequency, amplitude, duration and spatial spread, c=8, n=3. C STMs showing the effect of the PLC inhibitor, U73122 (10 μM), on ICC-SS Ca2+ transients. D Summary data showing the effect of U73122 and its inactive analogue, U73343 (10 μM), on ICC Ca2+ transient frequency, amplitude, duration and spatial spread, c=9, n=3. E STMs showing the effect of caffeine (10 mM) on ICC-SS Ca2+ transients. F Summary effect of caffeine on ICC-SS Ca2+ transient frequency, c=9, n=5.
Xestospongin C (XeC, 10 μM), a direct antagonist of IP3 receptors (IP3Rs) had mixed effects. Across multiple batches of XeC, the drug had dramatic inhibitory effects in some experiments, excitatory effects in others and no effect in others still (data not shown). Recently, the efficacy of XeC as a selective inhibitor of IP3Rs has been questioned (Saleem et al., 2014) and off target effects other than IP3R inhibition have been reported for this agent (Sergeant et al., 2001). Experiments performed on isolated isoforms of the IP3R (IP3R1–3) in expressed systems and on IP3R dependent Ca2+ signaling in vascular endothelial cells have demonstrated that caffeine is an effective antagonist of IP3R1 (dominant isoform in ICC (Baker et al., 2016; Drumm et al., 2017, 2019b)) at concentrations of 10 mM (Saleem et al., 2014; Heathcote et al., 2019). While caffeine is also known to be a modulator of RyRs, the lack of effect of the RyR antagonist on ICC-SS Ca2+ signals (Fig. 9A–B) led us to test caffeine as an antagonist of IP3R1 with any effects on RyRs presumably being minimal. As shown in Fig. 9E–F, incubation with 10 mM caffeine abolished all Ca2+ transients in ICC-SS (Fig. 9F, P<0.0001, paired student t-test, c=9, n=5).
The high frequency firing of Ca2+ transients in ICC-SS begs the questions of how this activity is sustained. This activity clearly requires a mechanism to reinitiate IP3-mediated Ca2+ release from ER stores during long periods of multiple Ca2+ release events. In ICC from other organs, Ca2+ release may be sustained by regular influx of Ca2+ through a voltage-dependent pathway (Park et al., 2006; Bayguinov et al., 2007; Sanders et al., 2014) or by a voltage dependent generation of IP3 (van Helden et al., 2000; Imtiaz et al., 2002; van Helden & Imtiaz, 2003). We tested the role of these voltage-dependent mechanisms in ICC-SS by hyperpolarizing colonic muscles by activation of KATP channels. KATP channels contribute to setting the resting membrane potential in the colon (Koh et al., 1998) and are highly expressed in colonic SMC, but this conductance is not resolved in ICC (Koh et al., 1998; Huang et al., 2018). We reasoned that activation of KATP channels with pinacidil, which hyperpolarizes colonic muscles (Koh et al., 1998), would reduce either voltage dependent Ca2+ influx or voltage-dependent IP3 production. Pinacidil (10 μM; given in the presence of 1 μM tetrodotoxin (TTX; Fig. 10A) to prevent interference from possible voltage-dependent effects on neurotransmitter release) had no effect on Ca2+ transient frequency (P=0.06), amplitude (P=0.7), duration (P=0.9) or spatial spread (P=0.2), (Fig. 10B, paired student t-tests, c=21, n=5). Similarly, inhibiting Ca2+ influx via L-type Ca2+ channels with nicardipine (1 μM) had no effect on ICC-SS Ca2+ transient frequency (P=0.17), amplitude (P=0.48), duration (P=0.57) or spread (P=0.4) (Fig. 10C–D, paired student t-tests, c=13, n=5).
Fig. 10: Ca2+ transients in ICC-SS are voltage-independent.
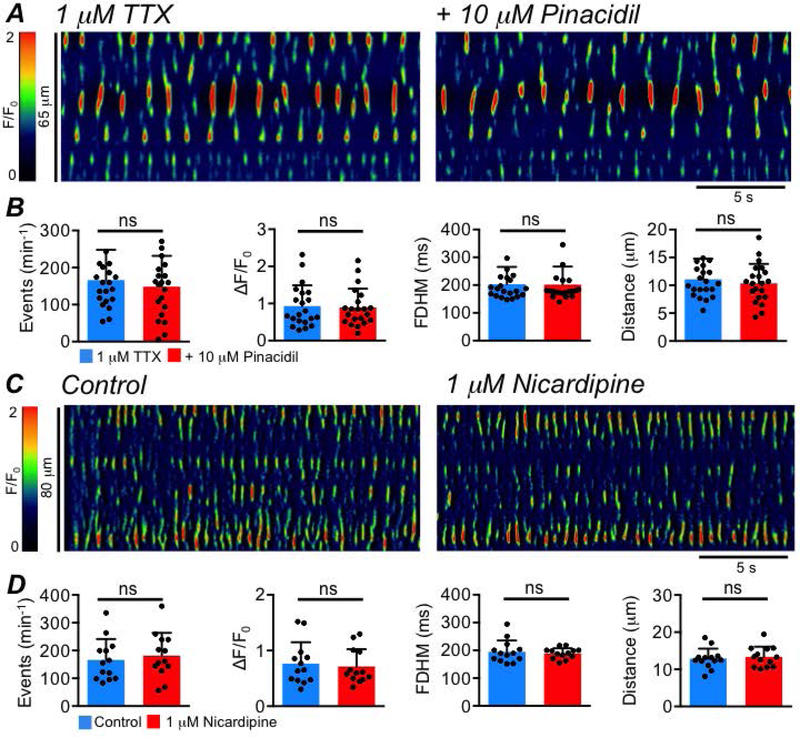
A STMs showing the effect of the KATP channel agonist, pinacidil (10 μM), on ICC-SS Ca2+ transients, in the presence of 1 μM TTX. B Summary data showing the effect of pinacidil on ICC Ca2+ transient frequency, amplitude, duration and spatial spread, c=21, n=5. C STMs showing the effect of nicardipine (1 μM), on ICC-SS Ca2+ transients. D Summary data showing the effect of nicardipine on Ca2+ transient frequency, amplitude, duration and spatial spread, c=13, n=5.
Role of SOCE in sustaining ICC-SS activity
The data above suggested that ICC-SS do not require voltage dependent Ca2+ influx for the generation of spontaneous activity. However, we also demonstrated that a Ca2+ influx pathway must exist to sustain Ca2+ release in these cells, as spontaneous activity was acutely dependent on extracellular Ca2+. SOCE has been shown to be an important mechanism for store refilling and maintaining Ca2+ release in several types of muscles (Trebak et al., 2013), including smooth muscles (Gibson et al., 1998; Drumm et al., 2018a).
SKF 96365 (10 μM), a broadly selective SOCE antagonist, inhibited the frequency, amplitude and spatial spread of Ca2+ transients in ICC-SS (Fig. 11A); the frequency of Ca2+ transients was reduced from 216.9 ± 100.6 min−1 to 139.1 ± 53.5 min−1 (Fig. 11B, P=0.0017, paired student t-test, c=14, n=5), amplitudes were reduced from 0.5 ± 0.2 ΔF/F0 in control to 0.4 ± 0.1 ΔF/F0 by SKF 96365 (Fig. 11B, P=0.013, paired student t-test, c=14, n=5). SKF 96365 decreased the spatial spread of Ca2+ transients from 11.3 ± 2.6 to 9.1 ± 3.2 μm (Fig. 11B, P=0.014, paired student t-test, c=14, n=5), but did not affect Ca2+ transient duration (Fig. 11B, P=0.64, paired student t-test, c=14, n=5). SOCE is mediated by interactions between the stromal interaction molecular (STIM) proteins and Orai Ca2+ influx channels (Trebak & Putney, 2017). We took advantage of the dual effects of 2-aminoethoxydiphenyl borate (2-APB) on Orai channels and STIM proteins to further elucidate the role of SOCE in sustaining Ca2+ transients in ICC-SS. Low concentrations of 2-APB (5–10 μM) increase the conductance of Orai channels, while higher concentrations (50–100 μM) inhibit Orai (Peinelt et al., 2008; Yamashita et al., 2010; Xu et al., 2016; Ali et al., 2017). 2-APB (10 μM) increased the frequency of ICC-SS Ca2+ transients from 177.2 ± 83.9 min−1 to 227.5 ± 74.9 min−1 (Fig. 11C–D, P=0.006, one way ANOVA, Tukey post hoc test, c=19, n=6), and 50 μM 2-APB reduced the firing frequency to 63.9 ± 57.6 min−1 (Fig. 11D, P<0.0001, one way ANOVA, Tukey post hoc test, c=19, n=6). 2-APB (10 μM) also increased spatial spread from 10.7 ± 4.5 to 14.5 ± 5.1 μm (Fig. 11D, P=0.039, one way ANOVA, Tukey post hoc test, c=19, n=6).
Fig. 11: Ca2+ transients in ICC-SS are modulated by SOCE.
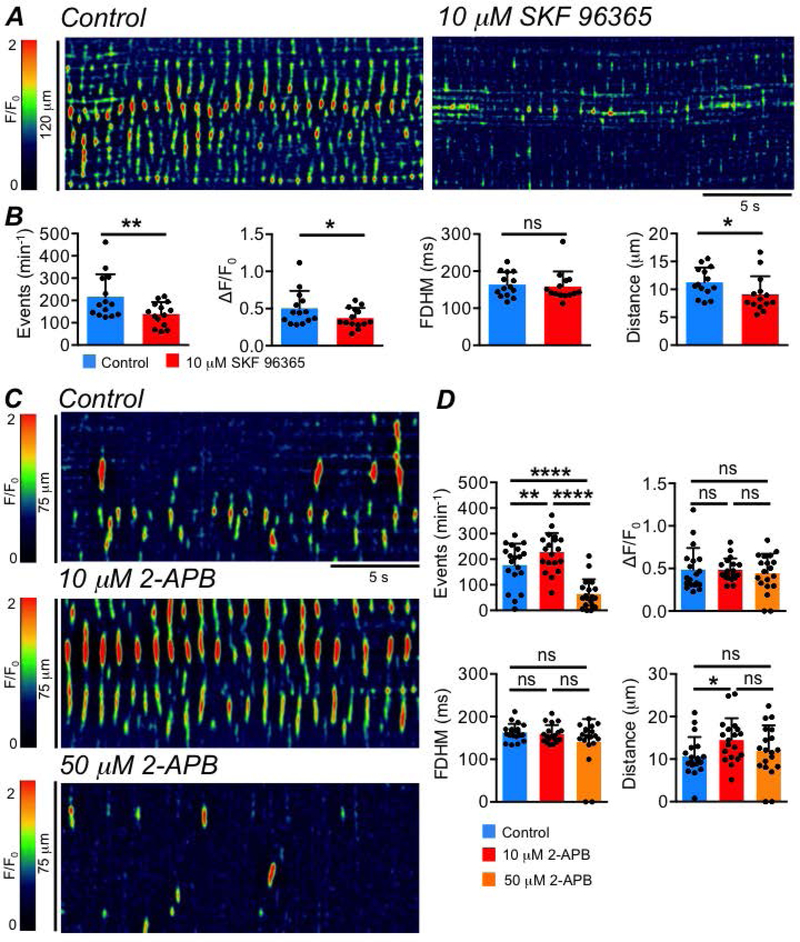
A STMs showing the effect of the broadly selective SOCE inhibitor SKF 96365 (10 μM), on ICC-SS Ca2+ transients. B Summary data showing the effect of SKF 96365 on ICC Ca2+ transient frequency, amplitude, duration and spatial spread, c=14, n=5. C STMs showing the effect of the different concentrations of 2-APB (10 & 50 μM) on ICC-SS Ca2+ transients. D Summary data showing the effect of 2 concentrations of 2-APB on ICC Ca2+ transient frequency, amplitude, duration and spatial spread, c=19, n=6.
GSK 7975A, a selective antagonist of Orai channels, had dramatic inhibitory effects on ICC-SS Ca2+ transients (Fig. 12A). GSK 7975A (1μM) reduced the firing frequency of Ca2+ transients from 164.3 ± 62.9 min−1 to 92.65 ± 55.9 min−1 (Fig. 12B, P=0.006, one way ANOVA, Tukey post hoc test, c=17, n=5), and frequency was further reduced to 26.3 ± 33 min−1 by 10 μM GSK 7975A (Fig. 12B, P<0.0001, one way ANOVA, Tukey post hoc test, c=17, n=5). 10 μM GSK 7975A also significantly reduced the duration (P=0.046) and spatial spread (P=0.0014) of Ca2+ transients (Fig. 12B, one way ANOVA, Tukey post hoc test, c=17, n=5). GSK 7975A (1μM) also reduced the stimulatory effects of 10 μM 2-APB on ICC-SS Ca2+ transients (Fig. 12C). For example, as shown in Fig. 12D, 2-APB (10 μM) increased the frequency of ICC-SS Ca2+ transients from 49.7 ± 84.3 to 82.3 ± 89.1 min−1 (Fig. 12D, P=0.033, one way ANOVA, Tukey post hoc test, c=10, n=3) and this was then reduced to 23.3 ± 34.5 min−1 by GSK 7975A (Fig. 12D, P=0.032, one way ANOVA, Tukey post hoc test, c=10, n=3).
Fig. 12: ICC-SS Ca2+ transients are sustained by Ca2+ influx via Orai channels.
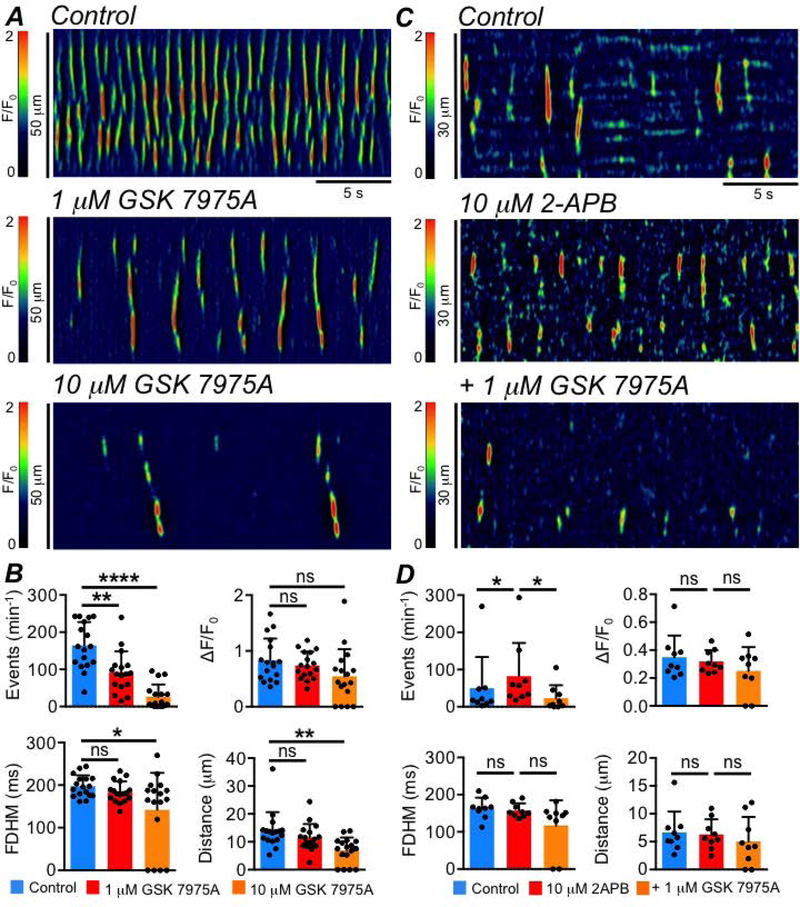
A STMs showing the effect of the Orai channel inhibitor GSK 7975A (1 & 10 μM), on ICC-SS Ca2+ transients. B Summary data showing the effect of GSK 7975A, on ICC-SS Ca2+ transient frequency, amplitude, duration and spatial spread, c=17, n=5. C STMs showing that the stimulatory effect of 2-APB (10 μM) on ICC-SS Ca2+ transients was reversed by GSK 7975A (1 μM). D Summary data showing the effect of GSK 7975A on ICC-SS Ca2+ transient frequency, amplitude, duration and spatial spread in the presence of 2-APB (10 μM), c=10, n=3.
Neural responsiveness of ICC-SS
Some types of ICC are known to transduce inputs from enteric motor neurons in the stomach (Burns et al., 1996; Ward et al., 2000, 2006; Beckett et al., 2002; Sung et al., 2018), small intestine (Ward et al., 2006; Baker et al., 2018a, 2018b) and colon (Wang et al., 2000; Drumm et al., 2019c). Previous studies have found anatomical associations between ICC-SS and nNOS+ neurons in primates (Blair et al., 2012b). We tested responses of ICC-SS to enteric nerve stimulation by observing the effect of neurotransmitter agonists and electrical field stimulation (EFS) on ICC-SS Ca2+ transients.
In contrast to ICC-IM in the proximal colon (Drumm et al., 2019c), we found no evidence that ICC-SS undergo tonic inhibition from nitrergic neurons, as basal Ca2+ transient activity was unaffected by either TTX (1 μM, Fig. 13A–B; Frequency (P=0.053), Amplitude (P=0.44), Duration (P=0.48), Spatial Spread (P=0.25), paired student t-tests, c=18, n=5) or the nNOS synthase inhibitor Nω-nitro-L-arginine (L-NNA, 100 μM, Fig. 13C–D, Frequency (P=0.074), Amplitude (P=0.77), Duration (P=0.096), Spatial Spread (P=0.55), paired student t-tests, c=15, n=5).
Fig. 13: ICC-SS do not undergo tonic neural inhibition.
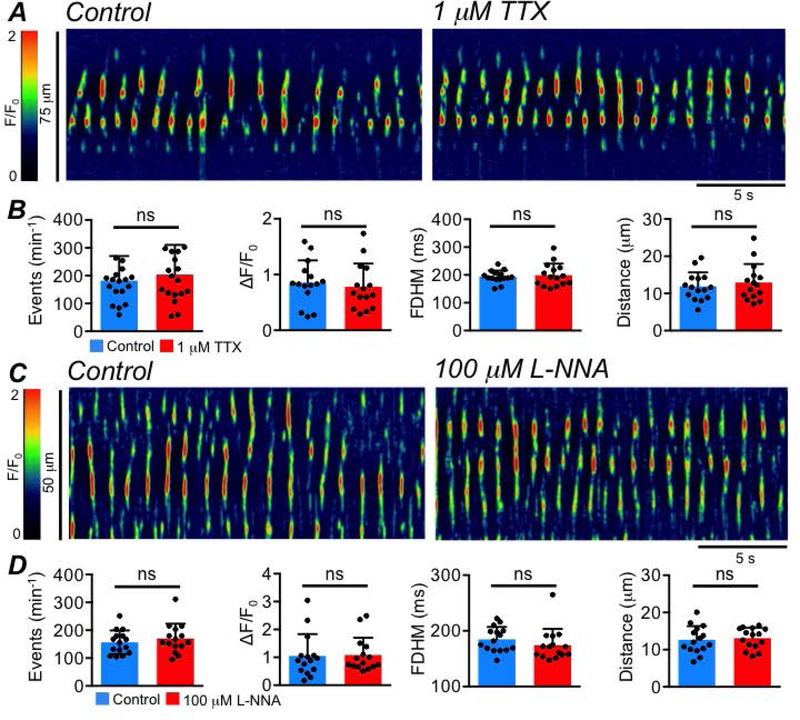
A STMs showing the effect of TTX (1 μM), on ICC-SS Ca2+ transients. B Summary effects of TTX on ICC-SS Ca2+ transient frequency, amplitude, duration and spatial spread, c=18, n=5. C STMs showing the effect of the nNOS synthase inhibitor L-NNA (100 μM) on ICC-SS Ca2+ transients. D Summary effects of L-NNA on ICC-SS Ca2+ transient frequency, amplitude, duration and spatial spread, c=15, n=5.
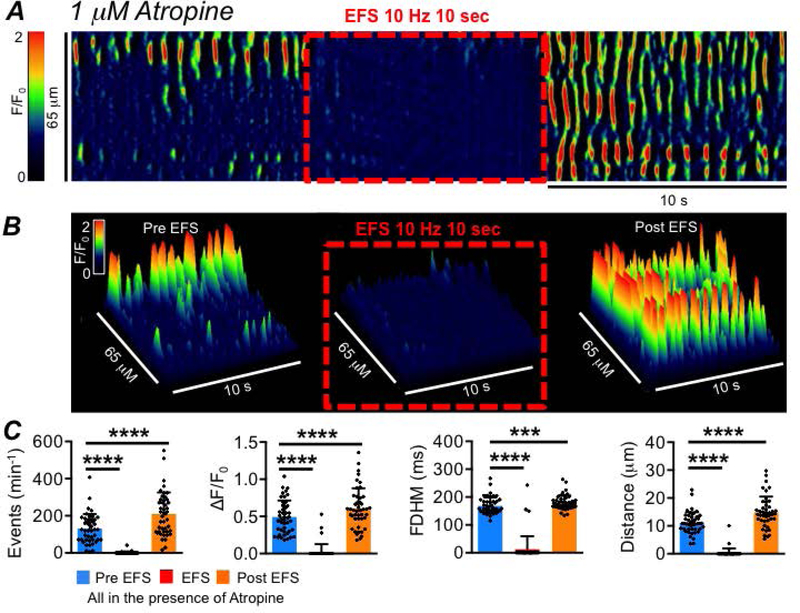
ICC-SS were imaged in situ before, during and after EFS (10 Hz; 10 sec) in the presence of atropine (1 μM; Fig. 14A–B). At initiation of EFS, an immediate cessation of Ca2+ transient firing occurred and lasted for the duration of stimulation (Fig. 14A–B). EFS reduced the firing frequency of Ca2+ transients from 130.8 ± 78.6 to 1.3 ± 6.5 min−1 (Fig. 14C, P<0.0001, one way ANOVA, Tukey post hoc test, c=45, n=10). The amplitude, duration and spatial spread were reduced from 0.5 ± 0.2 to 0.03 ± 0.1 ΔF/F0 (Fig. 14C, P<0.0001, one way ANOVA, Tukey post hoc test, c=45, n=10); from 168.5 ± 30 to 12.2 ± 47.4 ms (Fig. 14C, P<0.0001, one way ANOVA, Tukey post hoc test, c=45, n=10) and from 11.1 ± 3.8 to 0.3 ± 1.6 μm (Fig. 14C, P<0.0001, one way ANOVA, Tukey post hoc test, c=45, n=10), respectively. Upon cessation of EFS, a post-stimulus enhancement (‘rebound’) in Ca2+ transients was observed (Fig. 14A–C), and the frequency (P<0.0001), amplitude (P<0.0001), duration (P=0.0004) and spatial spread (P<0.0001) increased, as compared to pre-EFS values (Fig. 14C, one way ANOVA, Tukey post hoc test, c=45, n=10).
Fig. 14: Inhibitory innervation of ICC-SS.
A STM of the effect of electrical field stimulation (EFS, 10 Hz, 10 sec) on ICC-SS Ca2+ transients in the presence of the muscarinic receptor antagonist atropine (1 μM). B 3-D plots of the effect of EFS (10 Hz, 10 sec) on ICC-SS Ca2+ transients in the presence of atropine. C Summary data of EFS effects in the presence of atropine on the frequency, amplitude, duration and spatial spread of Ca2+ transients, comparing EFS and post EFS periods to pre EFS, c=45, n=10.
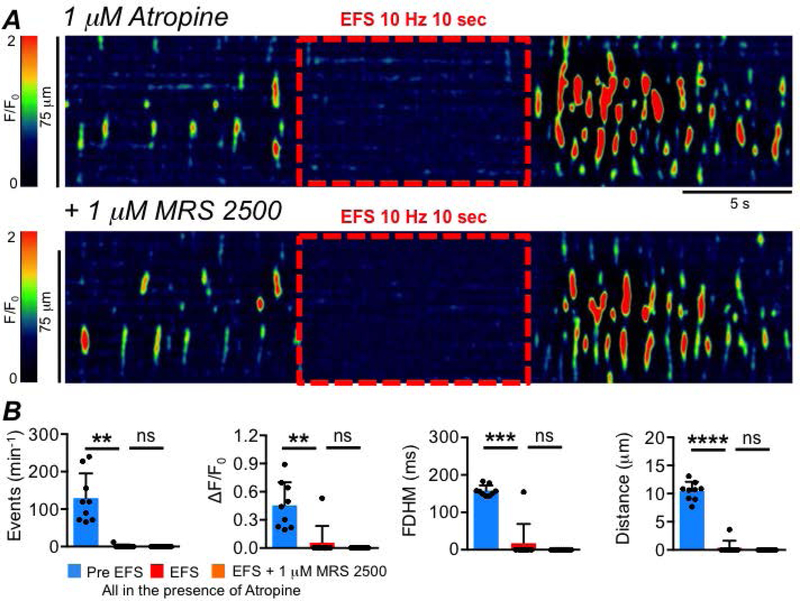
These data suggest that ICC-SS are innervated by inhibitory motor neurons. We tested whether the inhibitory neurotransmitter could be nitric oxide (NO), as is the case in many regions of the GI tract (Bult et al., 1990; Sanders & Ward, 2019). Ca2+ transients in ICC-SS were abolished when muscles were treated with an NO donor, DEA-NONOate (10 μM; Fig. 15A–B, P=0.0001, paired student t-test, c=12, n=4). The inhibitory effects of EFS on Ca2+ transients were blocked by pre-incubation with L-NNA (Fig. 15C–D, P<0.0001, one way ANOVA, Tukey post hoc tests, c=18, n=6). The soluble guanylate cyclase (downstream receptor for NO) activator (sGC, 1 μM Bay 58–2667, Fig. 16A) also abolished Ca2+ transients in ICC-SS (Fig. 16A–B, P<0.0001, paired student t-test, c=11, n=4), and ODQ (10 μM) an inhibitor of sGC, blocked the inhibitory effects of EFS on Ca2+ transients (Fig. 16C–D, P<0.0001, one way ANOVA, Tukey post hoc tests, c=15, n=7). Inhibiting purinergic neurotransmission in the proximal colon by antagonizing P2Y1 receptors with MRS 2500 (1 μM, Fig. 17A) had no effect on EFS induced inhibitory responses on ICC-SS (Fig. 17B; Frequency (P=0.59), Amplitude (P=0.59), Duration (P=0.59), Spatial Spread (P=0.59), one way ANOVA, Tukey post hoc tests, c=9, n=3).
Fig. 15: Inhibitory neural effects on ICC-SS Ca2+ transients are due to NO.
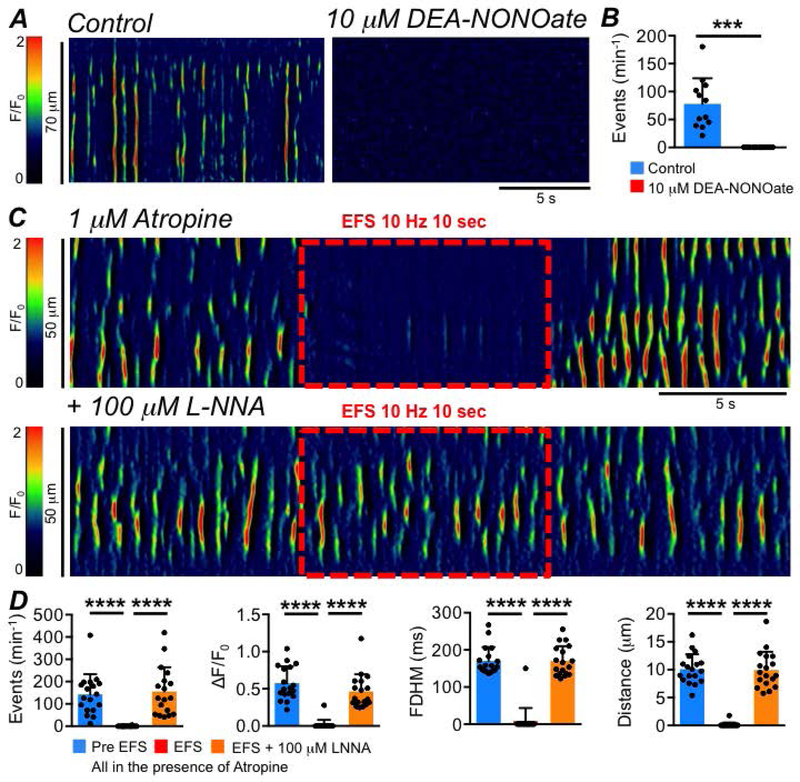
A STMs showing the effect of the NO donor DEA-NONOate (10 μM) on ICC-SS Ca2+ transients. B Summary effect of DEA-NONOate on ICC-SS Ca2+ transient frequency, c=12, n=4. C STMs of the effect of EFS (10 Hz, 10 sec) on ICC-SS Ca2+ transients in the presence of atropine (1 μM) before and after addition of L-NNA (100 μM). D Summary effects of EFS in the presence of atropine on the frequency, amplitude, duration and spatial spread of Ca2+ transients, comparing Pre EFS period to the EFS period and comparing the EFS period to EFS + LNNA, c=18, n=6.
Fig. 16: Inhibitory neural effects on ICC-SS Ca2+ transients are due to sGC.
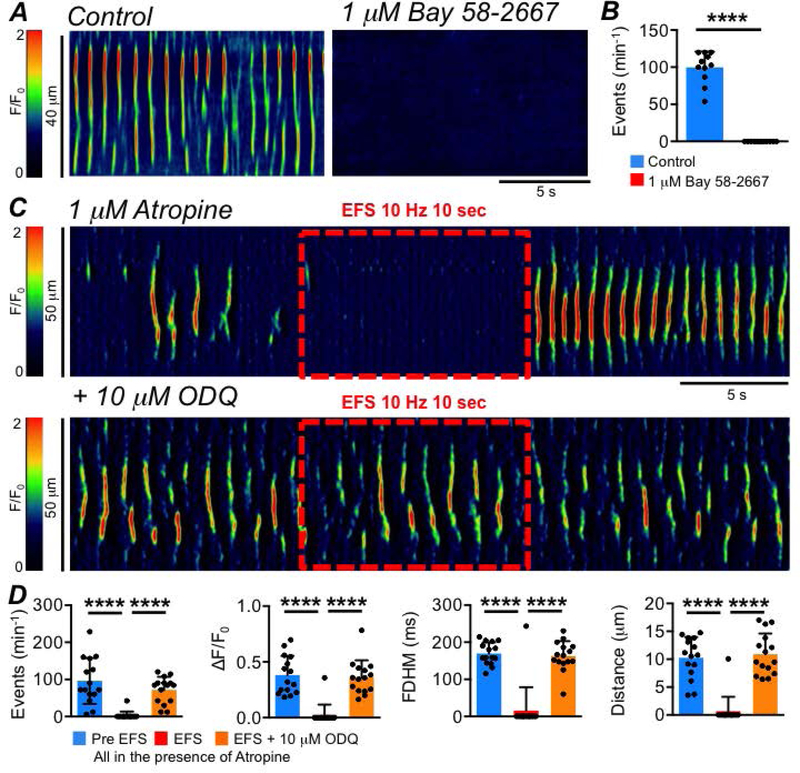
A STMs showing the effect of the sGC activator Bay 58–2667 (1 μM) on ICC-SS Ca2+ transients. B Summary effect of Bay 58–2667 on ICC-SS Ca2+ transient frequency, c=11, n=4. C STMs of the effect of EFS (10 Hz, 10 sec) on ICC-SS Ca2+ transients in the presence of atropine (1 μM) before and after addition of the sGC inhibitor ODQ (10 μM). D Summary effects of EFS in the presence of atropine on the frequency, amplitude, duration and spatial spread of Ca2+ transients, comparing activity before and during EFS and comparing the effects of EFS and the effects of EFS + ODQ, c=15, n=7.
Fig. 17: Purinergic neurotransmission is not involved in inhibitory neural responses in ICC-SS.
A STMs of the effect of EFS (10 Hz, 10 sec) on ICC-SS Ca2+ transients in the presence of atropine (1 μM) before and after addition of the P2Y1 receptor antagonist MRS 2500 (1 μM). B Summary effects of EFS in the presence of atropine on the frequency, amplitude, duration and spatial spread of Ca2+ transients, comparing Pre EFS period to the EFS period and comparing the EFS period to EFS + MRS 2500, c=9, n=3.
We also tested the effects of excitatory neurotransmission on ICC-SS. The cholinergic receptor agonist carbachol (CCh, 1 μM, given in the presence of TTX) failed to yield any change in Ca2+ transient frequency (P=0.17), amplitude (P=0.51), duration (P=0.6) or spatial spread (P=0.2), (Fig. 18A–B, paired student t-tests, c=12, n=4). Furthermore, we imaged ICC-SS in situ before, during and after EFS (10 Hz; 10 sec) in the presence of L-NNA (100 μM) and MRS 2500 (1 μM) to block inhibitory nitrergic and purinergic neurotransmission (Fig. 18C). In stark contrast to the observations above under conditions favoring inhibitory neurotransmission, we found that EFS under these conditions produced no change in the frequency (P=0.7), amplitude (P=0.94), duration (P=0.63) or spatial spread (P=0.96) of ICC-SS Ca2+ transients (Fig. 18D, one way ANOVA, Tukey post hoc tests, c=13, n=5).
Fig. 18: Excitatory neurotransmission does not affect ICC-SS Ca2+ transients.
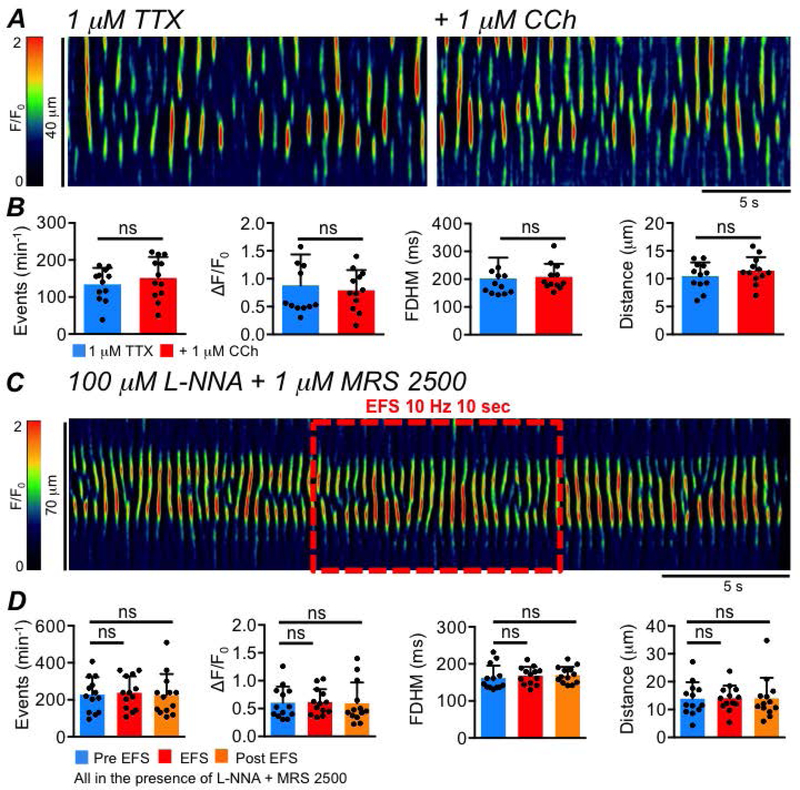
A STMs showing the effect of the cholinergic agonist, carbachol (CCh, 1 μM), on ICC-SS Ca2+ transients, in the presence of 1 μM TTX. B Summary data showing the effect of CCh on ICC Ca2+ transient frequency, amplitude, duration and spatial spread, c=12, n=4. C STMs of the effect of EFS (10 Hz, 10 sec) on ICC-SS Ca2+ transients in the presence of L-NNA (100 μM) and MRS 2500 (1 μM). D Summary effects of EFS in the presence of L-NNA + MRS 2500 on the frequency, amplitude, duration and spatial spread of ICC-SS Ca2+ transients, c=13, n=5.
We also tested the hypothesis that ‘rebound’ excitation in ICC-SS after inhibitory EFS is responsible for the responses manifest in contractions of LM. A strong increase in Ca2+ transients in ICC-SS after cessation of EFS should enhance openings of Ano1, and the depolarization, conducted to LSMC should result in increased Ca2+ action potential firing and contraction.
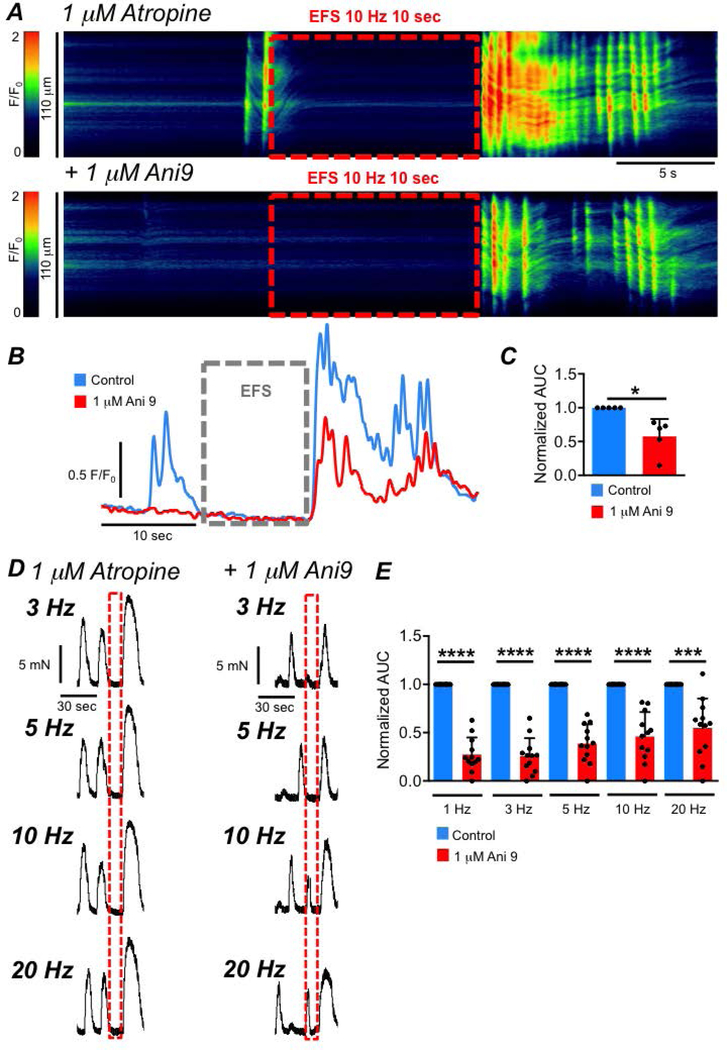
‘Rebound’ responses in LSMCs were explored using two approaches: Ca2+ imaging of LSMCs in muscles of SmHC-Cre-GCaMP6f mice and contractile experiments. EFS in the presence of atropine resulted in inhibition of Ca2+ transients during stimulation and ‘rebound’ excitation manifested as an intense burst of high amplitude intercellular Ca2+ waves upon cessation of EFS (Fig. 19A). Ani 9 (1 μM) caused significant a reduction in the ‘rebound’ response (Fig. 19A–C), and normalized area under the curve (AUC) of the post-EFS Ca2+ response was reduced from 1.0 to 0.57 ± 0.26 (Fig. 19C, paired student t-test, P=0.02, n=5). In contractile experiments, EFS (in the presence of atropine) was applied to LM strips at 1, 3, 5, 10 and 20 Hz (10 s train). ‘Rebound’ contractions were observed at each frequency tested (Fig. 19D, n=5), and these responses were significantly reduced by Ani9 (1 μM) at all frequencies (Fig. 19D–E, n=5). The AUC of ‘rebound’ contraction was reduced by Ani 9 from 1 to 0.3 ± 0.2 at 1 Hz, (Fig. 19E, P<0.0001, n=5), 0.26 ± 0.19 at 3 Hz (Fig. 19E, P<0.0001, n=5), 0.4 ± 0.2 at 5 Hz (Fig. 19E, P<0.0001, n=5), 0.46 ± 0.25 (Fig. 19E, P<0.0001, n=5) at 10 Hz and 0.5 ± 0.3 at 20 Hz (Fig. 19E, P=0.003, n=5).
Fig. 19: Activation of Ano1 channels contributes to LM post EFS ‘rebound’ excitation.
A STMs showing effects of EFS (10 Hz, 10 sec) on intercellular Ca2+ waves in LSMC in the presence of atropine (1 μM) before and after addition of Ani 9 (1 μM) recorded with a 20x objective. Responses are recorded in situ from a SmHC-Cre-GCaMP6f mouse. B Traces of the Ca2+ activity from the STMs in A, showing the decrease in ‘rebound’ excitation after addition of Ani 9. C Summary data showing the effects of Ani 9 on ‘rebound’ excitation in LSMC, measured as AUC from traces such as those shown in B, n=5. D Isometric tension recordings showing ‘rebound’ contractions of proximal colon LM following EFS (3, 5, 10, 20Hz, 10 sec) in the presence of atropine, before and after addition of Ani 9 (1 μM). E Summary data showing the effect of Ani 9 on ‘rebound’ contractions of proximal colon LM post EFS in the presence of atropine, n=5.
Discussion
This study investigated the behavior and functions of a novel class of ICC in the colon and how LM is regulated by ICC-SS. LSMCs generated 2 types of Ca2+ waves, as reported previously (Hennig et al., 2002). Intracellular Ca2+ transients were stochastic in nature, consisted of localized waves and were not coupled to contractions. Intercellular Ca2+ waves were more intense and spread within bundles of LSMCs causing contraction. Unlike intracellular Ca2+ waves, intercellular Ca2+ waves in LSMCs and LM contractions were inhibited by an Ano1 channel antagonist and by a pharmacological antagonist of SOCE. Previous studies described morphological features of ICC-SS and showed that these cells are arranged along LM bundles (Burns et al., 1997; Toma et al., 1999; Vanderwinden et al., 2000; Aranishi et al., 2009; Blair et al., 2012a; Rumessen et al., 2013), however no information about their function(s) was provided previously. The present study hypothesized that due to the cellular expression of Ca2+ signalling pathways, ion channel expression and anatomical connectivity of ICC-SS and LSMCs in the proximal colon, ICC-SS could be involved in driving the firing of intercellular Ca2+ waves and contractions in LSMCs. ICC-SS, like ICC in other regions of the GI tract, express Ano1 (Gomez-Pinilla et al., 2009; Blair et al., 2012a). Ca2+ transients are likely coupled to activation of Ano1, a Ca2+-activated Cl− channel, in ICC-SS. We characterized the properties of Ca2+ transients in ICC-SS using an optogenetic approach. Ca2+ transients in ICC-SS were very sensitive to agents that modulate SOCE and specifically by a blocker of Orai channels (GSK 7975A). Antagonists of Ano1 or block of Ca2+ transients in ICC-SS reduced intercellular Ca2+ waves and contractions of LM, suggesting that ICC-SS provide a pacemaker-like input to LM. Ca2+ transients in ICC-SS were also inhibited by nitrergic agonists and during EFS of intrinsic enteric motor neurons via a nitrergic mechanism. A strong post-stimulus increase in Ca2+ transients (rebound response) followed periods of nitrergic motor neuron stimulation that appears to be responsible, at least in part, for post-stimulus contractions of LM. No innervation by enteric excitatory neurons or responsiveness to excitatory motor neurotransmitters was detected in ICC-SS.
Ca2+ transients occurred in ICC-SS spontaneously in a stochastic manner and were unaffected by TTX or L-NNA, suggesting that these cells are not under the influence of tonic inhibition, as was observed previously in intramuscular ICC (ICC-IM) of the CM layer (Drumm et al., 2019c). ICC-SS form an apparent network with electrical connectivity provided by gap junctions between cells (Vanderwinden et al., 2000; Aranishi et al., 2009), but Ca2+ transients were not coordinated or ‘entrained’, as sometimes suggested by investigators that have viewed ICC as coupled oscillators (Huizinga et al., 2014). Even within single ICC-SS, Ca2+ transients occurred independently of events originating from other initiation sites in the same cell. We also found that the gap junction uncoupler, 18β-glycyrrhetinic acid, which dramatically affects Ca2+ signalling in coupled ICC-MY of the small intestine (Park et al., 2006), did not affect firing of ICC-SS Ca2+ transients, suggesting no influence of electrical coupling on release of Ca2+ from stores. In pacemaker ICC from the small intestine, cell-to-cell coordination of Ca2+ firing occurs via Ca2+ entry through voltage-dependent Ca2+ channels (Drumm et al., 2017). Lack of coordination between Ca2+ transients in ICC-SS suggests that the depolarizing influence of Ca2+ transients and activation of Ano1 channels does not activate voltage-dependent Ca2+ channels in ICC-SS, perhaps due to low expression of these channels. This behavior is reminiscent of the behavior of ICC-DMP (Baker et al., 2016) in the small intestine and ICC-IM in the colon (Drumm et al., 2019b).
If ICC-SS provide pacemaker input to the LM, this activity takes a different form than activities of ICC-MY in the stomach and small intestine, as ICC-MY provide classical pacemaker-like activity setting the frequency and duration of the excitable events responsible for the phasic contractions that underlie peristalsis and segmentation. Due to the apparent lack of a coordinating, voltage-dependent conductance, Ca2+ events in ICC-SS occur independently in a stochastic manner, but summation of depolarizing currents activated in ICC-SS may drive membrane potentials of LSMCs into a range where action potential generation occurs. The voltage-dependent conductances in LSMCs may develop this activity into a rhythmic pattern. A model has shown that driving membrane potential of SMCs into a certain range of potentials, can induce voltage-dependent conductances expressed by SMCs to develop a rhythmic output (Lang et al., 1992). Thus, while ICC-SS may not directly pace the rhythmicity of LSMCs (i.e. providing the timing for excitable events in the muscle) they may contribute to pacemaker activity by conditioning membrane potential to support rhythmic generation of muscle action potentials. Thus, electrical and mechanical rhythmicity may be an emergent property of ICC-SS and LSMC functioning within an integrated cellular network.
The responsiveness of ICC-SS to enteric neurotransmission was somewhat surprising based on prior morphological reports. Sparse innervation was observed in the subserosal layer of mice and guinea pig proximal colon, and few nNOS+ neurons were observed (Vanderwinden et al., 2000; Aranishi et al., 2009). However, ICC-SS were found to be associated with nNOS+ neurons at the subserosal surface of the taenia coli in primates (Blair et al., 2012b). Our study provided evidence of nitrergic innervation of ICC-SS, as EFS in the presence of atropine caused profound inhibition of Ca2+ transients, an effect that was mimicked by application of an exogenous NO donor and sGC agonist and reversed by nNOS synthase and sGC antagonists. In contrast, we observed no evidence suggesting that ICC-SS receive purinergic, cholinergic or excitatory peptidergic neural inputs. Longitudinal muscles clearly receive excitatory neural input, however, and this input might be mediated directly by direct innervation of LSMCs.
It is of historical interest to note that post-stimulus excitatory responses (called ‘rebound’ excitation) was first characterized in colonic longitudinal muscles (Bennett, 1966; Burnstock et al., 1966). Rebound excitation was elicited immediately after a period of inhibitory neurotransmission and persisted in the presence of atropine. Mechanisms to explain rebound excitation included anode break, where hyperpolarization during inhibitory neurotransmission removes inactivation of channels responsible for action potentials (in this case L-type Ca2+ channels). Cessation of the inhibitory period, repolarization and the increased availability of L-type Ca2+ channels can result in enhanced action potentials firing. This may be the case for single stimuli, which are predominantly due to purinergic hyperpolarization. However, nitrergic responses are elicited by multiple stimuli (Gallego et al., 2008), and rebound excitation in LM may result from the dramatic increase in Ca2+ transients in ICC-SS. The post-stimulus increase in ICC-SS Ca2+ transients failed to develop in the presence of L-NNA or ODQ, which inhibited nitrergic neurotransmission. Rebound excitation, in the form of bursts of LSMCs intercellular Ca2+ waves and large amplitude LM contractions after EFS, was reduced by ~ 50% by an Ano1 channel antagonist.
A major conclusion about the role of ICC-SS in regulating LM is derived from the use of 2 reagents, an Ano1 antagonist (Ani 9) and an Orai antagonist (GSK 7975A). In the case of Ano1 antagonists, caution is required because all of the compounds we have tested have non-specific effects on L-type Ca2+ channels at concentrations above 5 μM. However, we found significant reduction in intercellular Ca2+ waves and contractions of LM in response to Ani 9 (1 μM), a dose effective in blocking Ano1 currents, but without effects on the responses of colonic muscles to elevated external K+ (Drumm et al., 2019c). SMCs In the small intestine do not exhibit SOCE properties and SOCE currents could only be resolved in intestinal ICC (Zheng et al., 2018), however it remains unknown if this also holds true in the colon. The other cells that comprise the SIP synticum in the colon (SMCs and PDGFRα+ cells) also express Ca2+ dependent conductances, and activation of these conductances may also rely on SOCE and Ca2+ release. However, in colonic SMCs and PDGFRα+ cells, Ca2+ release is coupled to the activation of outward K+ currents that stabilize membrane potentials or hyperpolarize colonic muscles (BK for SMCs (Thornbury et al., 1992; Hennig et al., 2002) and SK in PDGFRα+ cells (Kurahashi et al., 2011)). Thus, one would predict that inhibiting SOCE in these cells would lead to an increase in muscle excitability. However, we found that GSK 797A decreased LM contractions and intercellular Ca2+ waves, suggesting that the dominant effect of inhibiting SOCE was to disrupt Ca2+ handling in ICC.
A common theme is emerging about the powerful inhibitory regulation imposed by nitrergic stimuli on Ca2+ release events in ICC (Baker et al., 2018a; Drumm et al., 2019c). At present, we know that these effects are mediated by generation of cGMP, but steps down-stream from generation of the 2nd messenger have not been clarified. Previous studies have linked cGMP-dependent protein kinase G (PKG) to inhibition of IP3 dependent Ca2+ release in SMCs, interstitial cells and endothelial cells (Murthy & Zhou, 2003; Sergeant et al., 2006; Borysova & Burdyga, 2015). This mechanism utilizes direct phosphorylation of IP3Rs by PKG to inhibit IP3 receptors and reduce their open probability (Murthy & Zhou, 2003; Borysova & Burdyga, 2015). A signaling molecule activated by PKG is inositol triphosphate receptor (IP3R)-associated cGMP-kinase substrate (IRAG; encoded by Mrvi1). IRAG co-precipitates with IP3Rs and is essential for the cGMP-dependent inhibition of Ca2+ release in cultured human colonic SMCs and for the regulation of IP3Rs more generally (Schlossmann et al., 2000; Fritsch et al., 2004; Zhang et al., 2011). The molecular elements of this pathway, IP3Rs, PKG and IRAG are expressed in colonic and small intestinal ICC (Chen et al., 2007; Baker et al., 2016, 2018a; Drumm et al., 2017, 2019b; Lee et al., 2017), so it is possible that a mechanism similar to that in SMCs provides inhibitory regulation of Ca2+ release in ICC. While Ca2+ release may be inhibited during nitrergic stimulation, Ca2+ uptake into stores or Ca2+ through influx channels may be unimpeded. Thus, during nitrergic stimulation Ca2+ stores may be overloaded, and the burst of Ca2+ transients at cessation of nitrergic input may result from the increased Ca2+ load in stores. Increased store load may increase the activity of luminal Ca2+ binding sites within the ER of ICC, increasing the sensitivity and open probability of IP3Rs, as occurs for the activity of RyRs in cardiac and SMCs under store overload conditions (Cheng et al., 1993; Györke & Györke, 1998; Dargan et al., 2004; Györke et al., 2017; Ríos, 2017). Store overload may initiate enhanced Ca2+ release when nitrergic stimulation ceases and the inhibitory drive on IP3Rs is relieved. Conversely, the period of nitrergic inhibition may allow for an increased refractory time for cytosolic IP3Rs themselves on the ER membrane, which are activated and deactivated by Ca2+ in a bell-shaped manner (Berridge et al., 2000; Berridge, 2009). Thus, a long period of limited Ca2+ release would increase the recovery time of IP3Rs, and allow them to be ‘primed’ for greater release when the inhibitory influence of NO is removed compared to their recovery states under basal conditions, when continual rhythmic Ca2+ release leads to a somewhat constant refractory state for at least some IP3Rs.
Shortcomings of the present study include a lack of evaluation of key genes in ICC-SS that encode the necessary elements of the proposed pacemaker mechanism and cell-to-cell communication apparatus that would be required for ICC-SS to control activation of LM and to mediate neural responses to LM. Prior morphological studies showed that ICC-SS formed close associations with LSMCs and peg-and-socket junctions were also noted (Vanderwinden et al., 2000; Rumessen et al., 2013). Functional evidence, however: i) inhibition of intercellular Ca2+ transients in LM cells by Ano1 channel and Orai antagonists; ii) inhibition of contractions of LM by an Ano1 antagonist; iii) alterations in frequency and duration of LM contractions by an Orai antagonist, suggest that communication occurs between ICC-SS and LSMCs. We found it very difficult to disperse sufficient numbers of ICC-SS unequivocally from serosal tissues that would be necessary for sorting and gene expression assays. Thus, a clear picture of the molecular apparatus to accomplish Ca2+ entry and communication between cells has not yet been determined. While Ca2+ transients were recorded from ICC-SS and LSMCs, these recordings were made from different muscles. Simultaneous recordings of Ca2+ transients in both cell layers or recordings of Ca2+ transients in ICC-SS while recording action potentials in LSMCs would provide a tighter correlation between activities. Cellular studies are also desirable to show that Ca2+ transients are coupled to activation of spontaneous transient inward currents (STICs), however such measurements have not been accomplished to date with any type of ICC. It is not entirely clear how stochastic firing of Ca2+ transients in ICC-SS can result in the rhythmic firing of intercellular Ca2+ transients in LSCMs in intact muscles. This is likely to be a complicated outcome of the kinetic features of Ca2+ release channels, uptake mechanisms and ion channel refractoriness. Modeling of Ca2+ release events in ICC-SS and integration of this activity with the electrophysiological properties of LSMCs may provide a better understanding of this emergent behavior.
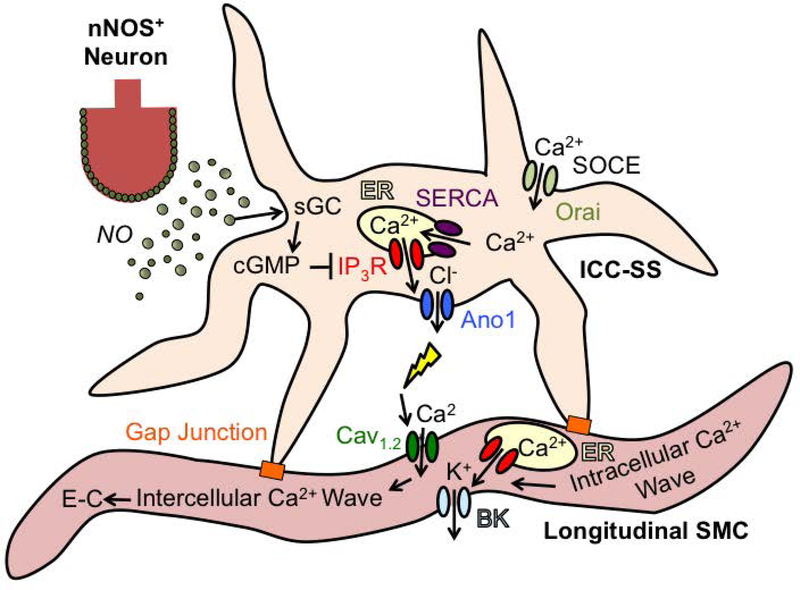
In summary, we have confirmed that the LM of the proximal colon exhibits two distinct patterns of Ca2+ signaling. Intracellular Ca2+ waves do not elicit contractions and are probably coupled to activation of BK channels in LSMCs. Intercellular Ca2+ waves propagate through LM bundles, initiate contractions and are inhibited by antagonists of Ano1 and Orai channels. ICC-SS are in anatomical close proximity to LM bundles, express Ano1 channels, exhibit stochastic, Ca2+ transients that originate from Ca2+ release from ER stores, and are sustained by store refilling via Orai channels (SOCE). By activating Ano1 channels, Ca2+ transients in ICC-SS increase the excitability of electrically coupled LSMCs. ICC-SS are innervated by nitrergic nerves and the NO-sGC pathway provides powerful inhibitory regulation of Ca2+ signalling in ICC-SS. A large post-stimulus increase in Ca2+ transients leads to LSMC depolarization and a robust series of action potentials, intercellular Ca2+ waves and post-stimulus ‘rebound’ contraction. A diagram depicting the major features of this model is shown in Fig. 20.
Fig. 20: Proposed regulatory influence of ICC-SS on longitudinal muscle excitability.
Colonic ICC-SS fire Ca2+ transients from ER stores via IP3Rs. Maintaining Ca2+ transients requires ER refilling by SOCE (via Orai channels) and SERCA. Ca2+ release activates Ano1 and Cl− efflux, leading to ICC-SS depolarization. Depolarization is conducted to LSMCs via gap junctions and increases the open probability of voltage-dependent Cav1.2 channels, leading to action potentials, Ca2+ influx and rapidly propagating Ca2+ waves and excitation-contraction coupling (EC) in LSMC bundles. NO released from nNOS+ enteric inhibitory motor neurons modulates the activity of ICC-SS and LSMC by activating sGC and inhibiting Ca2+ release in ICC-SS. LSMC also fire intracellular Ca2+ waves that are not affected by Ano1 and Cav1.2 channels and are not linked to E-C coupling. These likely represent Ca2+ signals that activate large K+ channels (BK) in SMCs that serve to maintain negative resting potentials in LSMCs.
Supplementary Material
Key Points:
Rhythmic action potentials and intercellular Ca2+ waves are generated in smooth muscle cells of colonic longitudinal muscles (LSMC).
Longitudinal muscle excitability is tuned by input from subserosal ICC (ICC-SS), a population of ICC with previously unknown function.
ICC-SS express Ano1 channels and generate spontaneous Ca2+ transients in a stochastic manner.
Release of Ca2+ and activation of Ano1 channels causes depolarization of ICC-SS and LSMC, leading to activation of L-type Ca2+ channels, action potentials, intercellular Ca2+ waves and contractions in LSMC.
Nitrergic neural inputs regulate the Ca2+ events in ICC-SS.
Pacemaker activity in longitudinal muscle is an emergent property due to integrated processes in ICC-SS and LSMC.
Acknowledgements
The authors would like to thank Nancy Horowitz for maintenance and breeding of mice and Dr. Caroline Cobine for the use of the contractile apparatus.
Funding: This project was supported by R01 DK-120759 from the NIDDK that supported the primary experiments.
Abbreviations
- CM
Circular Muscle
- FOV
Field of view
- GI
Gastrointestinal
- ICC
Interstitial Cells of Cajal
- ICC-DMP
Interstitial cells of Cajal at the level of the deep muscular plexus
- ICC-MY
Interstitial cells of Cajal at the level of the myenteric plexus
- ICC-IM
Intramuscular interstitial cells of Cajal
- ICC-SS
Sub-serosal interstitial cells of Cajal
- GCaMP
Genetically encoded Ca2+ indicator composed of a single GFP
- IP3R
Inositol triphosphate receptor
- KRB
Krebs Ringer Bicarbonate
- LM
Longitudinal Muscle
- PDGFRα
Platelet derived growth factor receptor α
- PTCL
Ca2+ transient particle
- ROI
Region of interest
- SERCA
Sarco/endoplasmic reticulum Ca2+-ATPase
- SIP syncytium
Electrical syncytium formed by smooth muscle cells, ICC and PDGFRα+ cells in GI muscles
- SMC
Smooth muscle cell
- SOCE
Store-operated-Ca2+ Entry
- TTX
Tetrodotoxin
Footnotes
Competing interests: None
References
- Ali S, Xu T & Xu X (2017). CRAC channel gating and its modulation by STIM1 and 2-aminoethoxydiphenyl borate. J Physiol 595, 3085–3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aranishi H, Kunisawa Y & Komuro T (2009). Characterization of interstitial cells of Cajal in the subserosal layer of the guinea-pig colon. Cell Tissue Res 335, 323–329. [DOI] [PubMed] [Google Scholar]
- Baker SA, Drumm BT, Cobine CA, Keef KD & Sanders KM (2018a). Inhibitory neural regulation of the Ca2+ transients in intramuscular interstitial cells of cajal in the small intestine. Front Physiol 9, 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker SA, Drumm BT, Saur D, Hennig GW, Ward SM & Sanders KM (2016). Spontaneous Ca(2+) transients in interstitial cells of Cajal located within the deep muscular plexus of the murine small intestine. J Physiol 594, 3317–3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker SA, Drumm BT, Skowronek KE, Rembetski BE, Peri LE, Hennig GW, Perrino BA & Sanders KM (2018b). Excitatory neuronal responses of Ca2+ transients in interstitial cells of cajal in the small intestine. eNeuro 5, ENEURO.0080-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayguinov O, Ward SM, Kenyon JL & Sanders KM (2007). Voltage-gated {Ca}2+ currents are necessary for slow-wave propagation in the canine gastric antrum. Am J Physiol Cell Physiol 293, C1645--59. [DOI] [PubMed] [Google Scholar]
- Beckett EAH, Horiguchi K, Khoyi M, Sanders KM & Ward SM (2002). Loss of enteric motor neurotransmission in the gastric fundus of Sl/Sldmice. J Physiol 543, 871–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett MR (1966). Rebound excitation of the smooth muscle cells of the guinea‐pig taenia coli after stimulation of intramural inhibitory nerves. J Physiol 185, 124–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berezin I, Huizinga JD & Daniel EE (1990). Structural characterization of interstitial cells of {Cajal} in myenteric plexus and muscle layers of canine colon. Can J Physiol Pharmacol 68, 1419–1431. [DOI] [PubMed] [Google Scholar]
- Berridge MJ (2009). Inositol trisphosphate and calcium signalling mechanisms. Biochim Biophys Acta - Mol Cell Res 1793, 933–940. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Lipp P & Bootman MD (2000). The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol 1, 11–21. [DOI] [PubMed] [Google Scholar]
- Blair PJ, Bayguinov Y, Sanders KM & Ward SM (2012a). Interstitial cells in the primate gastrointestinal tract. Cell Tissue Res 350, 199–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair PJ, Bayguinov Y, Sanders KM & Ward SM (2012b). Relationship between enteric neurons and interstitial cells in the primate gastrointestinal tract. Neurogastroenterol Motil; DOI: 10.1111/j.1365-2982.2012.01975.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borysova L & Burdyga T (2015). Evidence that NO/cGMP/PKG signalling cascade mediates endothelium dependent inhibition of IP3R mediated Ca2+oscillations in myocytes and pericytes of ureteric microvascular network in situ. Cell Calcium 58, 535–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bult H, Boeckxstaens GE, Pelckmans PA, Jordaens FH, Maercke YMV & Herman AG (1990). Nitric oxide as an inhibitory non-adrenergic non-cholinergic neurotransmitter. Nature 345, 346–347. [DOI] [PubMed] [Google Scholar]
- Burns AJ, Herbert TM, Ward SM & Sanders KM (1997). Interstitial cells of Cajal in the guinea-pig gastrointestinal tract as revealed by c-Kit immunohistochemistry. Cell Tissue Res 290, 11–20. [DOI] [PubMed] [Google Scholar]
- Burns AJ, Lomax AE, Torihashi S, Sanders KM & Ward SM (1996). Interstitial cells of Cajal mediate inhibitory neurotransmission in the stomach. Proc Natl Acad Sci U S A 93, 12008–12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G, Campbell G & Rand MJ (1966). The inhibitory innervation of the taenia of the guinea‐pig caecum. J Physiol 182, 504–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carl A, Bayguinov O, Shuttleworth CWR, Ward SM & Sanders KM (1995). Role of Ca2+-activated K+ channels in electrical activity of longitudinal and circular muscle layers of canine colon. Am J Physiol - Cell Physiol 268, C619–627. [DOI] [PubMed] [Google Scholar]
- Chen H, Ördög T, Chen J, Young DL, Bardsley MR, Redelman D, Ward SM & Sanders KM (2007). Differential gene expression in functional classes of interstitial cells of Cajal in murine small intestine. Physiol Genomics 31, 492–509. [DOI] [PubMed] [Google Scholar]
- Cheng H, Lederer WJ & Cannell MB (1993). Calcium sparks: Elementary events underlying excitation-contraction coupling in heart muscle. Science (80- ) 262, 740–744. [DOI] [PubMed] [Google Scholar]
- Chow E & Huizinga JD (1987). Myogenic electrical control activity in longitudinal muscle of human and dog colon. J Physiol 392, 21–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen J, Rick GA & Lowe LS (1992). Distributions of interstitial cells of Cajal in stomach and colon of cat, dog, ferret, opossum, rat, guinea pig and rabbit. J Auton Nerv Syst 37, 47–56. [DOI] [PubMed] [Google Scholar]
- Dargan SL, Schwaller B & Parker I (2004). Spatiotemporal patterning of IP3-mediated Ca2+ signals in Xenopus oocytes by Ca2+-binding proteins. J Physiol 556, 447–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drumm BT, Hennig GW, Baker SA & Sanders KM (2019a). Applications of Spatio-temporal Mapping and Particle Analysis Techniques to Quantify Intracellular Ca2+ Signaling In Situ. J Vis Exp 143, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drumm BT, Hennig GW, Battersby MJ, Cunningham EK, Sung TS, Ward SM, Sanders KM & Baker SA (2017). Clustering of Ca2+ transients in interstitial cells of Cajal defines slow wave duration. J Gen Physiol 149, 703–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drumm BT, Hwang SJ, Baker SA, Ward SM & Sanders KM (2019b). Ca2+ signalling behaviours of intramuscular interstitial cells of Cajal in the murine colon. J Physiol 597, 3587–3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drumm BT, Rembetski BE, Baker SA & Sanders KM (2019c). Tonic inhibition of murine proximal colon is due to nitrergic suppression of Ca 2+ signaling in interstitial cells of Cajal. Sci Rep 9, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drumm BT, Rembetski BE, Cobine CA, Baker SA, Sergeant GP, Hollywood MA, Thornbury KD & Sanders KM (2018a). Ca 2+ signalling in mouse urethral smooth muscle in situ: role of Ca 2+ stores and Ca 2+ influx mechanisms. J Physiol 596, 1433–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drumm BT, Sung TS, Zheng H, Baker SA, Koh SD & Sanders KM (2018b). The effects of mitochondrial inhibitors on Ca2+signalling and electrical conductances required for pacemaking in interstitial cells of Cajal in the mouse small intestine. Cell Calcium 72, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El‐Sharkawy TY (1983). Electrical activities of the muscle layers of the canine colon. J Physiol 342, 67–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faussone-Pellegrini MS & Thuneberg L (1999). Guide to the identification of interstitial cells of Cajal. Microsc Res Tech 47, 248–266. [DOI] [PubMed] [Google Scholar]
- Fritsch RM, Saur D, Kurjak M, Oesterle D, Schlossmann J, Geiselhöringer A, Hofmann F & Allescher HD (2004). InsP3R-associated cGMP Kinase Substrate (IRAG) Is Essential for Nitric Oxide-induced Inhibition of Calcium Signaling in Human Colonic Smooth Muscle. J Biol Chem 279, 12551–12559. [DOI] [PubMed] [Google Scholar]
- Gallego D, Gil V, Aleu J, Aulí M, Clavé P & Jiménez M (2008). Purinergic and nitrergic junction potential in the human colon. Am J Physiol - Gastrointest Liver Physiol 295, G522–533. [DOI] [PubMed] [Google Scholar]
- Gibson A, McFadzean I, Wallace P & Wayman CP (1998). Capacitative Ca2+entry and the regulation of smooth muscle tone. Trends Pharmacol Sci 19, 266–269. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla PJ, Gibbons SJ, Bardsley MR, Lorincz A, Pozo MJ, Pasricha PJ, Van de Rijn M, West RB, Sarr MG, Kendrick ML, Cima RR, Dozois EJ, Larson DW, Ordog T & Farrugia G (2009). Ano1 is a selective marker of interstitial cells of Cajal in the human and mouse gastrointestinal tract. Am J Physiol Gastrointest Liver Physiol 296, G1370–G1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy D (2015). Principles and standards for reporting animal experiments in The Journal of Physiology and Experimental Physiology. Exp Physiol 100, 755–758. [DOI] [PubMed] [Google Scholar]
- Györke I & Györke S (1998). Regulation of the cardiac ryanodine receptor channel by luminal Ca2+ involves luminal Ca2+ sensing sites. Biophys J 75, 2801–2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Györke S, Belevych AE, Liu B, Kubasov I V., Carnes CA & Radwanski PB (2017). The role of luminal Ca regulation in Ca signaling refractoriness and cardiac arrhythmogenesis. J Gen Physiol 49, 877–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heathcote HR, Lee MD, Zhang X, Saunter CD, Wilson C & McCarron JG (2019). Endothelial TRPV4 channels modulate vascular tone by Ca 2+ ‐induced Ca 2+ release at inositol 1,4,5‐trisphosphate receptors. Br J Pharmacol 176, 3297–3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Helden DF & Imtiaz MS (2003). Ca2+phase waves: A basis for cellular pacemaking and longrange synchronicity in the guinea-pig gastric pylorus. J Physiol 548, 271–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Helden DF, Imtiaz MS, Nurgaliyeva K, von der Weid P & Dosen PJ (2000). Role of calcium stores and membrane voltage in the generation of slow wave action potentials in guinea-pig gastric pylorus. J Physiol 524 Pt 1, 245–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Helden DF, Imtiaz MS, Nurgaliyeva K, Von Der Weid PY & Dosen PJ (2000). Role of calcium stores and membrane voltage in the generation of slow wave action potentials in guinea-pig gastric pylorus. J Physiol 524, 245–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennig GW, Smith CB, O’Shea DM & Smith TK (2002). Patterns of intracellular and intercellular Ca2+ waves in the longitudinal muscle layer of the murine large intestine in vitro. J Physiol 233–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Lee SH, Lu H, Sanders KM & Koh SD (2018). Molecular and functional characterization of inwardly rectifying K + currents in murine proximal colon. J Physiol 596, 379–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizinga JD et al. (2014). The origin of segmentation motor activity in the intestine. Nat Commun 5, 3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imtiaz MS, Smith DW & Van Helden DF (2002). A theoretical model of slow wave regulation using voltage-dependent synthesis of inositol 1,4,5-trisphosphate. Biophys J 83, 1877–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa K & Komuro T (1996). Characterization of the interstitial cells associated with the submuscular plexus of the guinea-pig colon. Anat Embryol (Berl) 194, 49–55. [DOI] [PubMed] [Google Scholar]
- Koh SD, Bradley KK, Rae MG, Keef KD, Horowitz B & Sanders KM (1998). Basal activation of ATP-sensitive potassium channels in murine colonic smooth muscle cell. Biophys J 75, 1793–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurahashi M, Zheng H, Dwyer L, Ward SM, Don Koh S & Sanders KM (2011). A functional role for the “fibroblast-like cells” in gastrointestinal smooth muscles. J Physiol 589, 697–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang RJ, Rattray-Wood C & Holman ME (1992). A simple mathematical model of spontaneous electrical activity in a single smooth muscle cell. In Japanese Journal of Pharmacology, p. 58 Suppl 2:371. [PubMed] [Google Scholar]
- Lee MY, Ha SE, Park C, Park PJ, Fuchs R, Wei L, Jorgensen BG, Redelman D, Ward SM, Sanders KM & Ro S (2017). Transcriptome of interstitial cells of Cajal reveals unique and selective gene signatures. PLoS One; DOI: 10.1371/journal.pone.0176031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu LW & Huizinga JD (1993). Electrical coupling of circular muscle to longitudinal muscle and interstitial cells of Cajal in canine colon. J Physiol 470, 445–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy KS & Zhou H (2003). Selective phosphorylation of the IP 3 R-I in vivo by cGMP-dependent protein kinase in smooth muscle. Am J Physiol - Gastrointest Liver Physiol 284, G221–G230. [DOI] [PubMed] [Google Scholar]
- Park KJ, Hennig GW, Lee H-T, Spencer NJ, Ward SM, Smith TK & Sanders KM (2006). Spatial and temporal mapping of pacemaker activity in interstitial cells of Cajal in mouse ileum in situ. Am J Physiol Cell Physiol 290, C1411-27. [DOI] [PubMed] [Google Scholar]
- Peinelt C, Lis A, Beck A, Fleig A & Penner R (2008). 2-Aminoethoxydiphenyl borate directly facilitates and indirectly inhibits STIM1-dependent gating of CRAC channels. J Physiol 586, 3061–3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ríos E (2017). Perspectives on “Control of Ca release from within the cardiac sarcoplasmic reticulum.” J Gen Physiol 149, 833–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumessen JJ, Peters S & Thuneberg L (1993). Light- and electron microscopical studies of interstitial cells of Cajal and muscle cells at the submucosal border of human colon. Lab Investig 68, 481–495. [PubMed] [Google Scholar]
- Rumessen JJ, Vanderwinden JM, Hansen A & Horn T (2013). Ultrastructure of interstitial cells in subserosa of human colon. Cells Tissues Organs 197, 322–332. [DOI] [PubMed] [Google Scholar]
- Saleem H, Tovey SC, Molinski TF & Taylor CW (2014). Interactions of antagonists with subtypes of inositol 1,4,5-trisphosphate (IP3) receptor. Br J Pharmacol 171, 3298–3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders KM, Koh SD, Ro S & Ward SM (2012). Regulation of gastrointestinal motility-insights from smooth muscle biology. Nat Rev Gastroenterol Hepatol 9, 633–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders KM & Ward SM (2019). Nitric oxide and its role as a non-adrenergic, non-cholinergic inhibitory neurotransmitter in the gastrointestinal tract. Br J Pharmacol 176, 212–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders KM, Ward SM & Koh SD (2014). Interstitial cells: Regulators of smooth muscle function. Physiol Rev 94, 859–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlossmann J, Ammendola A, Ashman K, Zong X, Huber A, Neubauer G, Wang G-X, Allescher H-D, Korth M, Wilm M, Hofmann F & Ruth P (2000). Regulation of intracellular calcium by a signalling complex of IRAG, IP3 receptor and cGMP kinase Iβ. Nature 404, 197–201. [DOI] [PubMed] [Google Scholar]
- Seo Y, Lee HK, Park J, Jeon DK, Jo S, Jo M & Namkung W (2016). Ani9, a novel potent small-molecule ANO1 inhibitor with negligible effect on ANO2. PLoS One; DOI: 10.1371/journal.pone.0155771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergeant GP, Hollywood MA, McCloskey KD, McHale NG & Thornbury KD (2001). Role of IP(3) in modulation of spontaneous activity in pacemaker cells of rabbit urethra. AmJPhysiol Cell Physiol 280, C1349–C1356. [DOI] [PubMed] [Google Scholar]
- Sergeant GP, Johnston L, McHale NG, Thornbury KD & Hollywood MA (2006). Activation of the cGMP/PKG pathway inhibits electrical activity in rabbit urethral interstitial cells of Cajal by reducing the spatial spread of Ca2+waves. J Physiol 574, 167–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serio R, Barajas-Lopez C, Daniel EE, Berezin I & Huizinga JD (1991). Slow-wave activity in colon: Role of network of submucosal interstitial cells of Cajal. Am J Physiol - Gastrointest Liver Physiol 260, G636–645. [DOI] [PubMed] [Google Scholar]
- Smith TK, Reed JB & Sanders KM (1987a). Interaction of two electrical pacemakers in muscularis of canine proximal colon. Am J Physiol Physiol 252, C290–299. [DOI] [PubMed] [Google Scholar]
- Smith TK, Reed JB & Sanders KM (1987b). Origin and propagation of electrical slow waves in circular muscle of canine proximal colon. Am J Physiol - Cell Physiol 252, C215–224. [DOI] [PubMed] [Google Scholar]
- Spencer NJ, Hennig GW & Smith TK (2002). Electrical rhythmicity and spread of action potentials in longitudinal muscle of guinea pig distal colon. Am J Physiol - Gastrointest Liver Physiol 282, G904–917. [DOI] [PubMed] [Google Scholar]
- Sung TS, Hwang SJ, Koh SD, Bayguinov Y, Peri LE, Blair PJ, Webb TI, Pardo DM, Rock JR, Sanders KM & Ward SM (2018). The cells and conductance mediating cholinergic neurotransmission in the murine proximal stomach. J Physiol 596, 1549–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornbury KD, Ward SM & Sanders KM (1992). Outward currents in longitudinal colonic muscle cells contribute to spiking electrical behavior. Am J Physiol - Cell Physiol 263, C237–245. [DOI] [PubMed] [Google Scholar]
- Toma H, Nakamura KI, Kuraoka A, Tanaka M & Kawabuchi M (1999). Three-dimensional structures of c-Kit-positive cellular networks in the guinea pig small intestine and colon. Cell Tissue Res 295, 425–436. [DOI] [PubMed] [Google Scholar]
- Trebak M & Putney JW (2017). ORAI Calcium Channels. Physiology 32, 332–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trebak M, Zhang W, Ruhle B, Henkel MM, González-Cobos JC, Motiani RK, Stolwijk JA, Newton RL & Zhang X (2013). What Role for Store-Operated Ca2+Entry in Muscle? Microcirculation 20, 330–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderwinden JM, Rumessen JJ, Bernex F, Schiffmann SN & Panthier JJ (2000). Distribution and ultrastructure of interstitial cells of Cajal in the mouse colon, using antibodies to Kit and Kit(W-lacZ) mice. Cell Tissue Res 302, 155–170. [DOI] [PubMed] [Google Scholar]
- Wang XY, Sanders KM & Ward SM (2000). Relationship between interstitial cells of Cajal and enteric motor neurons in the murine proximal colon. Cell Tissue Res 302, 331–342. [DOI] [PubMed] [Google Scholar]
- Ward SM, Beckett EAH, Wang XY, Baker F, Khoyi M & Sanders KM (2000). Interstitial cells of Cajal mediate cholinergic neurotransmission from enteric motor neurons. J Neurosci 20, 1393–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SM, McLaren GJ & Sanders KM (2006). Interstitial cells of Cajal in the deep muscular plexus mediate enteric motor neurotransmission in the mouse small intestine. J Physiol 573, 147–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellman GC & Nelson MT (2003). Signaling between SR and plasmalemma in smooth muscle: Sparks and the activation of Ca2+-sensitive ion channels. Cell Calcium 34, 211–229. [DOI] [PubMed] [Google Scholar]
- Xu X, Ali S, Li Y, Yu H, Zhang M, Lu J & Xu T (2016). 2-Aminoethoxydiphenyl Borate Potentiates CRAC Current by Directly Dilating the Pore of Open Orai1. Sci Rep; DOI: 10.1038/srep29304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita M, Somasundaram A & Prakriya M (2010). Competitive modulation of CRAC channel gating by STIM1 and 2-aminoethyldiphenyl borate (2-APB). J Biol Chem; DOI: 10.1074/jbc.M110.189035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneda S, Fukui H & Takaki M (2004). Pacemaker activity from submucosal interstitial cells of Cajal drives high-frequency and low-amplitude circular muscle contractions in the mouse proximal colon. Neurogastroenterol Motil 16, 621–627. [DOI] [PubMed] [Google Scholar]
- Zhang S, Fritz N, Ibarra C & Uhlén P (2011). Inositol 1,4,5-trisphosphate receptor subtype-specific regulation of calcium oscillations. Neurochem Res 36, 1175–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Drumm BT, Earley S, Sung TS, Koh SD & Sanders KM (2018). SOCE mediated by STIM and Orai is essential for pacemaker activity in the interstitial cells of cajal in the gastrointestinal tract. Sci Signal; DOI: 10.1126/scisignal.aaq0918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu MH, Sung TS, O’Driscoll K, Koh SD & Sanders KM (2015). Intracellular Ca(2+) release from endoplasmic reticulum regulates slow wave currents and pacemaker activity of interstitial cells of Cajal. Am J Physiol Cell Physiol 308, C608–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.