Abstract
Rationale
Female cigarette smokers tend to show greater cessation failure compared to males. Variables that contribute to the maintenance of smoking, including stress and craving, may differentially impact male and female smokers. Novel pharmacotherapies, such as oxytocin, may attenuate stress reactivity and craving in smokers, but work in this area is limited.
Objectives
This study assessed the influence of gender and oxytocin on stress reactivity, craving, and smoking in a randomized, placebo-controlled laboratory relapse paradigm.
Methods
Male and female adult cigarette smokers (ages 18–45) were enrolled (women oversampled 2:1) and completed a laboratory session, in which intranasal oxytocin or placebo was administered followed by a laboratory social stress task. The role of gender and oxytocin were assessed on measures of stress reactivity, cigarette craving, latency to smoke in a resistance task, subjective responses to smoking, and ad-libitum smoking.
Results
Participants (N=144) had a mean age of 31, were 63% female and 56% White. Following stress induction, female smokers evidenced greater subjective stress than males, though males demonstrated greater neuroendocrine reactivity and smoking intensity than females. No gender differences were demonstrated for craving. Oxytocin did not attenuate any aspect of stress reactivity, craving, smoking, or subjective responses to smoking compared to placebo.
Conclusions
Gender differences in stress reactivity were shown in the hypothesized direction, but oxytocin appeared to exert little impact on subjective or behavioral metrics. Results highlight the complex relationship between gender, stress, and smoking, as well as the implications for oxytocin as a potential pharmacotherapy for smoking cessation.
Keywords: smoking, relapse, stress, cue reactivity, craving, cortisol, oxytocin, gender, sex
Introduction
Despite declining rates of tobacco use, approximately 15% of adults in the United States (US) are cigarette smokers and disparities in smoking exist based on various socioeconomic characteristics (Jamal et al. 2016; Jamal et al. 2018). Though the majority of current smokers endorse a desire to quit and report recent quit attempts, few are able to achieve long-term abstinence (Babb et al. 2017). While rates of smoking in women are currently lower than in men in the US (Jamal et al. 2018), there is evidence that women have more difficulty with achieving cessation (Japuntich et al. 2011; Weinberger et al. 2014; Wetter et al. 1999). Additionally, sex and gender differences have been demonstrated with respect to: 1) the efficacy of various pharmacotherapies to promote cessation (McKee et al. 2015b; Perkins and Scott 2008; Piper et al. 2010; Scharf and Shiffman 2004; Smith et al. 2016; Smith et al. 2015; Smith et al. 2017; Walker et al. 2016), and 2) risk of relapse (Piper et al. 2010; Potter 2014; Smith et al. 2003; Wu et al. 2016).
Numerous factors influence the maintenance of smoking and relapse. Chief among those factors are stress (Richards et al. 2011) and cigarette craving (Killen and Fortmann 1997). Acute or chronic stressors are commonly associated with or predictive of relapse (Baer et al. 1989; Brandon 1994; Lunden et al. 2018; McKee et al. 2003; Piper et al. 2004; Shiffman et al. 2002) and quit rates are lower among smokers experiencing serious psychological distress (Hagman et al. 2008; Sung et al. 2011). However, many studies demonstrating a stress-relapse association rely on retrospective and self-reported recollection of stressors, rather than prospective and comprehensive assessments of stress. In a human laboratory paradigm of smoking relapse, stress was elicited through a personalized imagery induction task and was measured via physiological indicators. Stress induction was shown to decrease the ability to resist smoking, as well as increase smoking intensity (i.e., number of puffs) and satisfaction from smoking (McKee et al. 2011). Likewise, craving has been shown to be elicited by stress induction paradigms in the laboratory (Buchmann et al. 2010; Niaura et al. 2002; Perkins and Grobe 1992; Tiffany and Drobes 1990).
Stress reactivity and craving have been shown to vary among male and female smokers. Compared to male smokers, female smokers report higher levels of cigarette craving, subjective/self-reported stress, and negative affect following stressful cue presentations in the natural environment (Tomko et al. In Press; Wray et al. 2015), and are more likely to endorse stress relief as a motive for smoking (Fidler and West 2009). Sex differences have also been demonstrated in neuroendocrine responses to stress (i.e., cortisol), such that males have a greater cortisol response to stress compared to females (not specific to smokers) (Kudielka and Kirschbaum 2005; Liu et al. 2017). Thus, stress reactivity (subjective and physiological) may play an important role in relapse by reducing the ability to resist smoking, potentially due to increases in craving, and therefore leading to increased smoking behavior and/or relapse. When considering this smoking/relapse trajectory initiated as a result of acute or chronic stress exposure, pharmacotherapies that target heightened stress, craving, and smoking are advantageous to explore, particularly among female smokers given prior evidence of gender differences.
Oxytocin is a neuropeptide that has been implicated as a potential pharmacotherapy for substance use disorders that may target stress-specific pathways (Lee and Weerts 2016), and therefore, may be particularly effective for female smokers. Endogenous oxytocin has an established role in mating, childbirth, lactation, pro-social effects, and anxiolytic effects (Francis et al. 2002; Ludwig and Leng 2006; MacDonald and MacDonald 2010; Williams et al. 1994; Witt et al. 1992). Preclinical literature has shown a reduction in self-administration and drug reinstatement due to oxytocin administration (Baracz et al. 2016; Carson et al. 2010; Peters et al. 2017). Acute intranasal administration of oxytocin reduces subjective and neuroendocrine stress response (de Oliveira et al. 2012; Heinrichs et al. 2003; Kosfeld et al. 2005), has been shown to reduce cannabis craving in response to stress induction among cannabis-dependent adults (McRae-Clark et al. 2013), and appears to differentially impact males and females (Flanagan et al. 2018; Reed et al. 2019). However, oxytocin results are mixed. One recent study of cannabis users found that oxytocin contributed to greater stress reactivity in women, counter to previous literature and hypothesized directionality (Reed et al. 2019). Specific to smoking, it has been shown that intranasal oxytocin reduced cue-induced cigarette craving in daily smokers (Miller et al. 2016); however, two recent studies did not demonstrate any reductions in stress or cigarette craving due to oxytocin administration, though one study showed reductions in cigarette consumption and desire to smoke (Van Hedger et al. 2018; Van Hedger et al. 2019). Studies assessing the effects of oxytocin through fully-powered laboratory designs are particularly needed to control for nicotine deprivation, stress induction, and oxytocin dosing while capturing detailed metrics of subjective, neuroendocrine, and behavioral measures of stress and smoking (cortisol, time to smoke, smoking intensity, and enjoyment). Therefore, the goal of the current study was to explore oxytocin and gender on stress reactivity, craving, and smoking in a laboratory relapse paradigm.
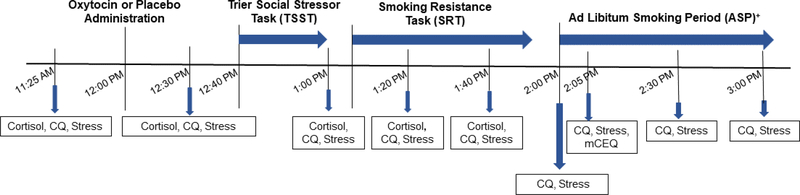
The present study is a subcomponent of a larger study that included a 14-day monitoring period of smoking cue and stress reactivity assessments in the natural environment (Clinicaltrials.gov ID: ). This 14-day period was followed by a laboratory session, which examined the influence of gender and oxytocin on measures of subjective and neuroendocrine stress reactivity, cigarette craving, latency to smoke (proxy of relapse), ad-libitum smoking intensity (via smoking topography), and subjective responses to smoking after overnight abstinence among daily smokers. This laboratory session, depicted in Figure 1, is the focus of the present report. We hypothesized that: 1) compared to males, female smokers would have greater subjective stress, craving, and shorter latency to smoke, but less neuroendocrine reactivity to a laboratory stressor, and 2) oxytocin would attenuate subjective stress, craving, neuroendocrine responses, and latency to smoke compared to placebo in both male and female smokers. Though not hypothesized a priori, we secondarily explored measures of smoking intensity, subjective responses to smoking, and interactions between gender and medication condition.
Figure 1.
Timeline of procedures and measures collected during the laboratory session (Day 15).
Figure Key: CQ=Craving Questionnaire; TSST=Trier Social Stress Task; SRT=Smoking Resistance Task; ASP=Ad libitum Smoking Period; mCEQ=Modified Cigarette Evaluation Questionnaire.
+ During the Ad Libitum Smoking Period, assessments were conducted immediately following the first cigarette (typically +5–10 minutes after the start of the ASP).
Methods
Participants
Male and female adult cigarette smokers (ages 18–45; N=178), who were not seeking treatment, were recruited from the community in Charleston, South Carolina (US) from March 2013 through May 2017. Women were oversampled 2:1 in order to have sufficient power to assess the impact of ovarian hormones and gender on stress reactivity and craving in the natural environment (aim of the parent study), which is not presented here (results forthcoming). To qualify for the study, all participants had to: 1) smoke an average of 5 cigarettes/day for at least 6 months, 2) submit a breath carbon monoxide (CO) sample at screening of at least 5 parts per million (ppm), which is based on recommendations in the literature indicating non-smoking cut-off values between 3–6 ppm (Cropsey et al. 2014; Perkins et al. 2013), 3) if female, had to be post menarche and pre-menopausal with regular menstrual cycles (defined as menses occurring every 25–35 days for the past three months), and 4) if female, had to be at least 3 months post-delivery/breast feeding. Exclusion criteria included: 1) any serious or unstable medical or psychiatric condition, 2) meeting criteria for post-traumatic stress disorder and exhibiting symptoms in the past month, which was assessed based on the Diagnostic and Statistical Manual of Mental Disorders (DSM) IV criteria (First 1994) and administered via the MINI International Neuropsychiatric Interview (Sheehan et al. 1998), 3) any medication that may interfere with psychophysiological (e.g., heart rate) monitoring, 4) current substance dependence in the past month based on criteria from the Diagnostic and Statistical Manual IV (First 1994), 5) current use of other tobacco/nicotine products, 6) females who were pregnant, breast feeding, post hysterectomy, or taking hormonal contraceptives or hormone replacement, and 7) males who were post orchiectomy. Study procedures were approved by the Institutional Review Board at the Medical University of South Carolina, and the study was registered on clinicaltrials.gov (). Additional demographic and clinical characteristics of the sample, as well as behavioral results from the 14-day naturalistic phase of the study have been published elsewhere (Tomko et al. In Press; Wray et al. 2015).
Procedures
The laboratory session (Day 15) was scheduled immediately after the 14-day monitoring phase. Prior to the laboratory session, participants were instructed to abstain from smoking for at least 12 hours, verified via expired breath CO (< 6 ppm or a 25–50% reduction from average CO values collected at in-person visits). Participants were also required to abstain from alcohol and other drug use for two days. This was biochemically verified through a 6-panel instant-read urine drug screen and alcohol breathalyzer, both performed on the morning of the laboratory session before any study procedures.
Laboratory session procedures and measures collected are shown in Figure 1. At approximately 12:00 pm, participants received an intranasal dose of either 40 IUs of oxytocin or a matched placebo nasal spray under the direct supervision of research staff. Oxytocin dosing was based on previous work using similar doses of 40 IUs in human laboratory studies (Ditzen et al. 2009; McRae-Clark et al. 2013), though varying doses have been studied in the literature (Leng and Ludwig 2016). The most common adverse events experienced with oxytocin include; lightheadedness, drowsiness, headache, increased calmness, increased energy, nasal irritation, dry mouth/throat. Medication was prepared by Investigational Drug Services at the Medical University of South Carolina. The investigational pharmacy generated randomization assignments and masked all medication bottles and packaging to maintain the blind for staff and participants. There was a 40-minute delay from the time of medication administration to the start of the social stress task. Timing of procedures was based on previous work with human laboratory paradigms suggesting this is a suitable delay (Heinrichs et al. 2003; McRae-Clark et al. 2013). Prior to medication administration, participant access to personal items, mobile phones, and magazines was restricted. The session room was intentionally barren to avoid distractions or delay of any study procedures for the remainder of the session. The social stressor used in this study was the Trier Social Stress Test (TSST; Kirschbaum et al. 1993). The TSST is a 15-minute procedure that includes preparation for speech (5 minutes), public speaking (5 minutes), and mental arithmetic tasks (5 minutes). The speech and arithmetic components are completed in front of an audience of three confederates unknown to the participant. The Smoking Resistance Task (SRT) began after the first post-TSST assessment was completed. The SRT was modeled from McKee and colleagues (McKee 2009; McKee et al. 2011; McKee et al. 2012). Cigarettes and a lighter were presented to the participant and they were told they could begin smoking at any time, but for every 5 minutes they resisted smoking, they would be compensated $1.50, for a maximum of $15 over 50 minutes. The SRT ended when the participant decided to smoke, or 50 minutes had elapsed. The Ad-libitum Smoking Period (ASP) then began and lasted for 60 minutes. Assessments were collected at 20 and 40 minutes post-TSST (regardless of the occurrence of smoking and termination of the SRT phase). During the ASP, participants were permitted to smoke up to eight cigarettes, each through a smoking topography device (CReSS Pocket, Borgwaldt KC, Inc.).
Measures
Screening/Baseline Assessments
Demographics, smoking history and current tobacco use were collected at the screening visit. Nicotine dependence was assessed via the Fagerstrom Test for Nicotine Dependence (Heatherton et al. 1991). Female participants completed a menstrual history diary to assess the timing and duration of menses over the past 90 days prior to the screening visit.
Self-Report Laboratory Assessments
The 4-item Craving Questionnaire (CQ; Carter and Tiffany 2001) was administered throughout the laboratory session (1–5 point Likert scale; 1=Strongly Disagree; 5=Strongly Agree; Items summed for total score ranging from 4–20). A single subjective stress item (5-point Likert scale; 1=Not at all; 5=Extremely) was derived from a cue and stress reactivity assessment used in the 14-day monitoring phase of this study (Warthen and Tiffany 2009) and asked how stressed the participant felt at that time. The Modified Cigarette Evaluation Questionnaire (mCEQ; Cappelleri et al. 2007) was administered after completion of the first cigarette smoked to capture subjective responses to smoking. The mCEQ is a 12-item instrument (7-point Likert scale; 1=Not at all; 7=Extremely) comprised of five sub-scales: smoking satisfaction, psychological reward, aversion, enjoyment of respiratory tract sensations, and craving reduction. A mean mCEQ score was also calculated from all 12 items. Finally, a Penetration of the Blind Questionnaire was conducted to assess the patient’s perception of their assigned medication.
Neuroendocrine Assays
Cortisol levels (ug/dL) were assessed via salivary samples collected at five time points during the laboratory session.
Smoking
Measures collected from the SRT included latency to smoke (0–50 minutes). During the ASP, smoking topography measures were collected for each cigarette (0–8 cigarettes possible) and total number of cigarettes smoked. Topography measures included: number of puffs taken, mean puff volume (ml), mean puff duration (ms), mean flow rate (ml/s; measure of speed and intensity of inhalation), peak flow rate (ml/s), mean time of peak flow rate (ms), and inter-puff interval (ms).
Statistical analyses
Baseline demographics and pre-TSST clinical characteristics were tabulated for the study cohort as well as by treatment assignment and gender. To evaluate baseline differences between treatment groups and gender a Wilcoxon Rank-sum test was used for continuous measures and Pearson Chi-Square test was used for categorical and ordinal measures.
Response to the TSST
Generalized linear mixed models were developed to assess overall treatment and gender effects, as well as the corresponding interaction effects on self-reported craving, self-reported stress and neuroendocrine reactivity following the TSST. Assumptions of residual normality were checked for each model using QQ plots and appropriate transformations were used if necessary (e.g., log10 for cortisol). Model-based estimates and associated standard errors were tabulated and compared across treatment groups and gender at each post-medication measure. Adjusted models were developed using a stepwise forward selection process with an intention to reduce bias and collinearity among covariates. Changes in the effects of treatment and gender on outcomes over time were tested using interaction terms (Treatment × time, Gender × time). All post-treatment measures were included in the model development (post-treatment/pre-TSST, post-TSST+0 minutes, TSST+20 minutes, TSST+40 minutes). Group level means were used to assess initial response to the TSST (immediately pre- to immediately post-TSST) and post-TSST treatment group and gender differences. Treatment efficacy models contained the main effect of treatment assignment (oxytocin or placebo), as well as measurement time, baseline outcome levels, and gender. Due to the SRT running concurrent with the post-TSST follow-up assessments (Figure 1), participants were able to smoke their first cigarette prior to completion of the TSST follow-up time points, which likely impacted their ratings of subjective stress and craving. Stress and craving measures taken after smoking occurred in the ASP were excluded from the TSST analysis. Additionally, peak TSST response variables (from pre-treatment baseline) were also calculated and examined using similar regression models.
Smoking Resistance Task
Cox Proportional Hazards regression models were developed to assess treatment and gender differences on time to first cigarette during the SRT. In the primary model, treatment and gender effects, as well as possible differential treatment effects across gender were examined. Additionally, the association between peak TSST responses and the time to smoke during the SRT were examined. Following completion of the first cigarette smoked, subjective responses to smoking were measured using the mCEQ and compared across treatment condition and gender.
Smoking Topography
For the analysis of smoking topography, data from the first cigarette smoked in the laboratory were included in statistical models. Topography data were available for 134 of the 144 participants (93%). Outliers were identified first by isolating values that were three standard deviations from the mean for individual puffs for a participant. Possible outliers were then inspected to determine if they were a result of randomly-occurring device malfunction and removed if so. Approximately 1.1% of smoking topography data were considered outliers, and 0.4% were excluded due to device error. Separate multivariate analysis of variance (MANOVA) tests were used to evaluate the global effect of gender and treatment on smoking topography. If indicated, post hoc analysis of variance (ANOVA) tests were used to detect significant between-group differences for topography variables. Results are presented as model-based means and associated standard errors unless otherwise noted. No correction for multiple testing was made and all comparisons are conducted at the α<0.05 level. All analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC, USA) and SPSS version 25 (IBM Corp. Released 2017. IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp.).
Results
Demographics and smoking characteristics
The final sample included in this report consisted of 144 participants (81% of those enrolled in study procedures) who qualified for and completed the Day 15 laboratory session (n=72 Oxytocin; n=72 Placebo). The average age at screening of the 144 participants was 31 (SD=7.4) years, 56% were White and 63% were female. There were largely no demographic or smoking characteristic treatment group differences at screening or pre-medication for subjective or neuroendocrine measures (Table 1; all ps>.05). Participants randomized to placebo reported more years of regular smoking than participants randomized to oxytocin [14.9 (SD=7.8) vs. 12.2 (7.2); p=.047]. Although there were group level differences at study entry, years of regular smoking was not associated with craving or self-reported stress responses to the TSST [β=0.005, SE=0.029, p=.88 and β=0.005, SE=0.022, p=.81, respectively], or latency to smoke in the SRT [HR=1.02 (95% CI=0.98–1.05), p=.44], nor did it modify treatment effects (treatment × yrs of regular smoking interactions, all ps>.40). However, greater years of regular smoking was possibly associated with decreased cortisol levels following the TSST [β=−.003, SE=0.002, p=.06] but did not moderate treatment effects [treatment × yrs of regular smoking interaction, p=.97]. Participants who were enrolled in the study but failed to complete the Day 15 laboratory session had similar smoking characteristics to those who completed the laboratory session (age at regular smoking, years of regular smoking, cigarettes per day, nicotine dependence) as well as similar gender and race distribution. However, those who completed all study procedures were slightly older than those who did not (31.0 (SD=7.4) vs. 28.1 (6.8); p=.043).
Table 1.
Demographics and clinical characteristics of the overall study sample completing laboratory day procedures and separated by treatment assignment and further separated by gender.
| Screening Visit | Overall (N=144) | Placebo |
Oxytocin |
||||
|---|---|---|---|---|---|---|---|
| All Participants (n=72) | Female (n=46) | Male (n=26) | All Participants (n=72) | Female (n=45) | Male (n=27) | ||
| Age | 31.0 (7.4) | 31.6 (7.7) | 32.1 (7.5) | 30.7 (8.0) | 30.4 (7.1) | 31.5 (6.7) | 28.6 (7.4) |
| Female % (n) | 63.2 (91) | 63.9 (46) | 62.5 (45) | ||||
| White % (n) | 56.3 (81) | 55.6 (40) | 60.9 (28) | 46.2 (12) | 56.9 (41) | 66.7 (30) | 40.7 (11)† |
| Education % (n) | |||||||
| < HS | 12.5 (18) | 15.3 (11) | 15.2 (7) | 15.4 (4) | 9.7 (7) | 11.1 (5) | 7.4 (2) |
| HS or Some College | 72.9 (105) | 75.0 (54) | 76.1 (35) | 73.1 (19) | 70.8 (51) | 64.4 (29) | 81.5 (22) |
| College Graduate | 14.6 (21) | 9.7 (7) | 8.7 (4) | 11.5 (3) | 19.4 (14) | 24.4 (11) | 11.1 (3) |
| Age of Regular Smoking | 17.5 (4.6) | 17.1 (5.0) | 16.3 (3.2) | 18.5 (7.1) | 17.9 (4.0) | 17.7 (3.1) | 18.3 (5.3) |
| Years of Regular Smoking | 13.5 (7.6) | 14.9 (7.8) | 15.6 (7.6) | 13.5 (8.2) | 12.2 (7.2)* | 13.5 (7.2) | 9.9 (6.8)† |
| 30-day CPD Average | 15.7 (8.9) | 16.6 (10.4) | 15.5 (7.3) | 18.5 (14.4) | 14.8 (7.1) | 15.7 (7.7) | 13.3 (5.6) |
| FTND Total Score | 4.7 (2.1) | 4.8 (2.0) | 15.0 (5.3) | 16.7 (9.6) | 4.7 (2.3) | 15.3 (8.0) | 13.4 (5.1) |
| Pre-Medication (Day 15) | |||||||
| Stress (1–5 scale) | 2.0 (1.2) | 2.0 (1.2) | 2.2 (1.4) | 1.7 (0.7) | 2.0 (1.2) | 1.8 (1.1) | 2.3 (1.4) |
| Craving Total (4–20 scale) | 15.6 (4.5) | 16.2 (4.4) | 16.8 (4.1) | 15.0 (4.7) | 15.0 (4.6) | 15.2 (4.6) | 14.7 (4.7) |
| Cortisol (ug/dL) | 0.26 (0.15) | 0.24 (0.14) | 0.23 (0.15) | 0.28 (0.12)† | 0.28 (0.17) | 0.26 (0.15) | 0.32 (0.18) |
Table Key: HS=High school; CPD=Cigarettes per day; FTND=Fagerstrom Test for Nicotine Dependence.
p<.05 vs. placebo
p<05 vs. female
At screening, female participants reported greater years of regular smoking [Female 14.6 (7.4) vs. Male 11.7 (7.6); p=.026], primarily driven by significant gender differences in the oxytocin treatment group [Female 13.5 (7.2) vs. Male 9.9 (6.8); p=.05]. Similarly, female participants randomized to the oxytocin group were more likely to be White [Female 63.5% vs. Male 43.4%; p=.018] than males in the oxytocin group; this difference is not significant in the participants randomized to the placebo group [Female 60.9% vs. Male 46.2%; p=.32]. Additionally, female participants had lower cortisol levels on the morning of the laboratory session [Female 0.24 (0.15) ug/dL vs. Male 0.30 (0.15) ug/dL; p=.007].
Gender effects on stress, craving, and smoking (Hypothesis #1)
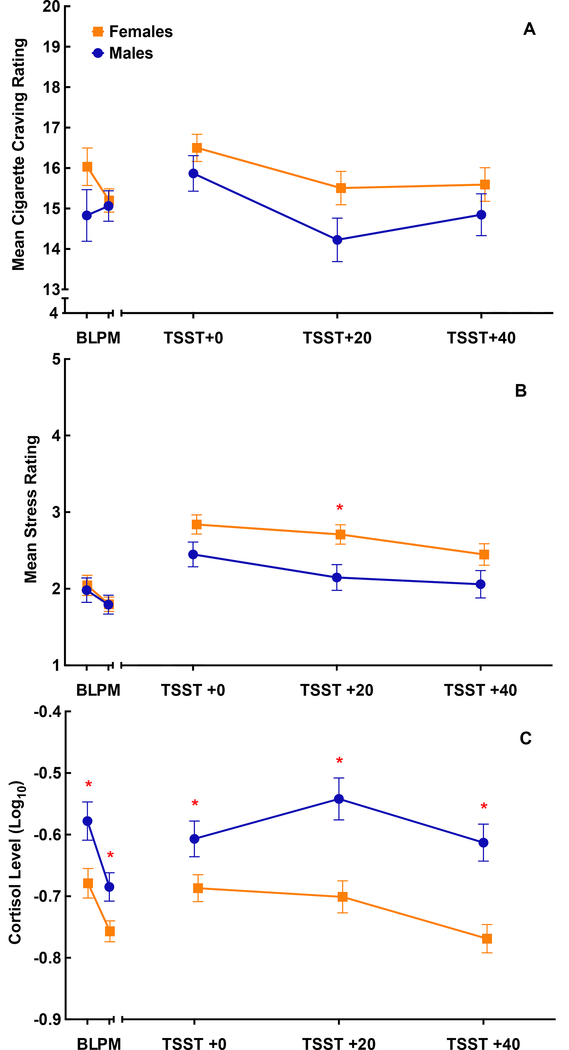
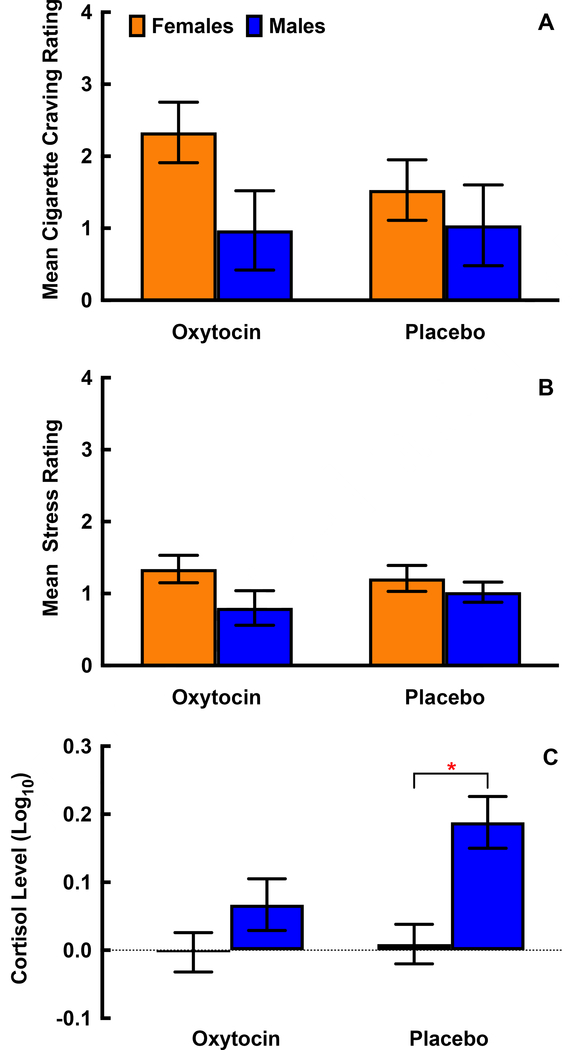
Self-reported craving, self-reported stress, and salivary cortisol responses prior to and following the TSST are shown in Figure 2, separated by gender. Peak responses (greatest change at any post-TSST time point subtracted from pre-treatment baseline), separated by gender and treatment condition are shown in Figure 3. For all study participants, there was a significant increase in initial craving [Δ=1.12 (0.22); p<.001], stress [Δ=0.85 (0.10); p<.001], and (log10) cortisol [Δ=0.07 (0.02); p<.001] to the TSST. There were no gender differences in mean craving response [Female 15.8 (0.3) vs. Male 14.8 (0.4); p=.06, Figure 2a] or peak craving response [Female: Δ=1.94 (0.30) vs. Male: Δ=0.99 (0.39); p=.06; Figure 3a] following the TSST. Although numeric differences across gender appear to be present in the oxytocin group as compared to placebo, gender did not significantly modify the treatment effect on peak craving response (F1,139=0.8; p=.38). When examined by treatment assignment, the gender difference in the oxytocin and placebo groups failed to achieve statistical significance [Oxytocin: Δ=1.37 (0.69); p=.0503; Placebo: Δ=0.49 (0.70); p=.49]. Though females and males self-reported similar stress ratings both prior to [Females; Mean (SEM) = 2.0 (0.1) vs. Males 2.0 (0.2); p=.98] and following medication administration [Females; 1.8 (0.1) vs. Males 1.8 (0.1); p=.97], females reported a greater mean stress response immediately following the TSST than males [Females: Δ=2.6 (0.1) vs. Males: Δ=2.2 (0.1); p=.013 Figure 2b]. Similarly, peak stress response was greater in females than males [Females: Δ=1.3 (0.1) vs. Males: Δ=0.8 (0.2); p=.039; Figure 3b]. There was no evidence of differential gender effects across treatment assignment (F1,139=0.3; p=.57). When examined by treatment assignment, the gender difference in the oxytocin and placebo groups failed to achieve statistical significance [Oxytocin: Δ=0.55 (0.29); p=.063; Placebo: Δ=0.31 (0.30); p=.30]. Cortisol in male participants was significantly higher at all time points as compared to females [Females Δ=2.6 (0.1) vs. Males: Δ=2.2 (0.1); p<.001 Figure 2c]. Male participants had a significant positive peak response in cortisol following the TSST [Δ=0.13 (0.03); p<.001] that was not present in female participants [Δ<0.01 (0.02); p=.88; gender difference p<.001; Figure 3c]. This gender difference was driven by significant effects in the placebo group [Δ=0.17 (0.05); p<.001] that were attenuated in the oxytocin group [Δ=0.07 (0.05); p=.15].
Figure 2.
Mean cigarette craving (range of 4–20; shown in Panel A), self-reported stress (range of 1–5; shown in Panel B), and cortisol (ug/dL; shown in Panel C) response shown prior to and following the Trier Social Stress Task (TSST) for male (n=53) and female (n=91) participants. Responses are displayed at baseline (BL), post-medication (PM), which included oxytocin (40 IUs) or matched placebo administration, immediately following the TSST (TSST +0), 20 minutes following the TSST (TSST +20), and 40 minutes following the TSST (TSST +40). Data are shown as the model-based means and standard errors. Data are shown adjusted for A) treatment condition, time, treatment by time interaction, baseline cigarette craving and gender, B) treatment condition, time, treatment by time interaction, baseline stress, gender and gender by time interaction, C) treatment condition, time, treatment by time interaction, baseline cortisol, gender and gender by time interaction. *Indicates p<.05 between males and females at that specific time point.
Figure 3.
Peak responses (from baseline) are shown for cigarette craving (A), self-reported stress (B), and cortisol (C) to the Trier Social Stress Task (TSST) following oxytocin or placebo administration in male (n=53) and female (n=91) participants. Data are shown as the model-based means and standard errors. Data are shown adjusted for A) treatment condition, baseline craving and gender, B) treatment condition, baseline stress, gender, C) treatment condition, baseline cortisol, gender and gender by treatment interaction. *Indicates p<.05 between male and female participants within a randomized treatment condition.
During the SRT, 12.5% of participants began smoking immediately [n=18; Female n=9 vs. Male n=9; p=.22], while 66% refrained from smoking during the entire 50-minute period [n=95; Female n=56 vs. Male n=39; p=.14]. Gender was not a significant predictor of time to first cigarette [Male vs. Female HR (95% CI) = 1.5 (0.8–2.8); p=.20] and there was no difference in the number of cigarettes smoked during the ASP by gender [Female 2.3 (0.1) vs. Male 2.2 (0.1); p=.40]. Smoking topography significantly differed by gender [(Wilks’ Δ=0.80, F7,126=4.28, p<.001, η2=0.19]. Compared to females, males demonstrated greater mean puff volume [F1,132=6.19, p=.01, η2=0.05], puff duration [F1,132=11.9, p=.001, η2=0.08], and time to peak flow rate [F1,132=18.7, p<.001, η2=0.12], as well as shorter inter-puff intervals [F1,132=6.1, p=.02, η2=0.04]. Number of puffs on the first cigarette, mean flow rate, and peak flow rate did not differ by gender [all ps>.35]. Neither craving, stress, nor cortisol response to the TSST was associated with latency to smoke [all ps>.60] for males or females. Subjective responses to smoking measured via the mCEQ did not differ by gender [adjusted for baseline mCEQ: Female 4.15 (0.12) vs. Male 4.00 (0.16); p=.45], nor did any of the mCEQ subscales differ by gender [all ps>.13].
Oxytocin effects on stress, craving, and smoking (Hypothesis #2)
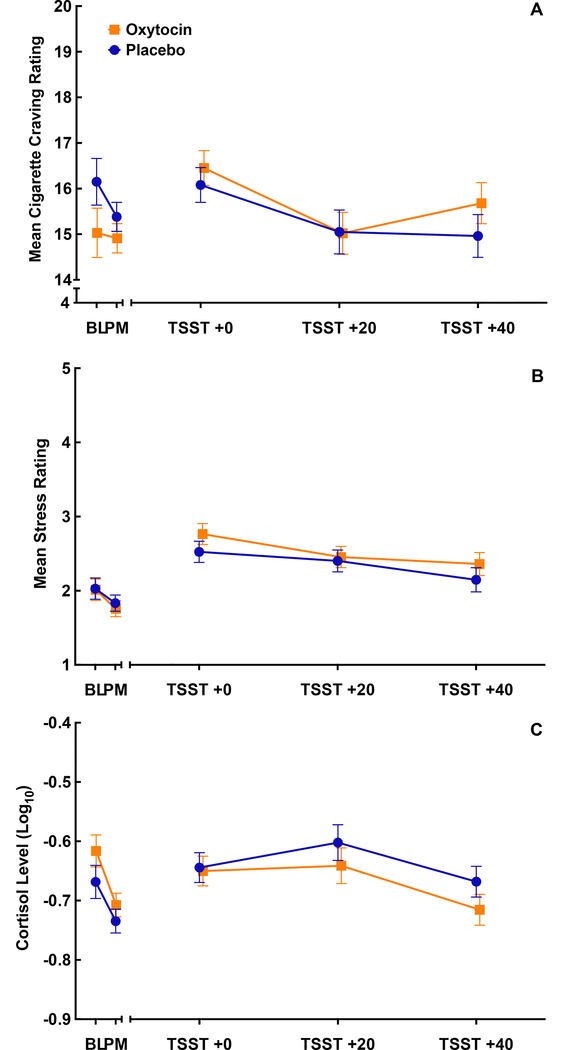
Craving, self-reported stress, and cortisol responses prior to and following the TSST are shown in Figure 4, separated by medication condition. There was no effect of oxytocin on mean self-reported craving response following the TSST [Placebo 15.2 (0.4) vs. Oxytocin 15.5 (0.4); p=.51, Figure 4a], self-reported stress response [Placebo 2.6 (0.1) vs. Oxytocin 2.2 (0.1); p=.28, Figure 4b], or cortisol [Placebo −0.72 (0.02) vs. Oxytocin −0.59 (0.03); p=.73, Figure 4c]. There was no significant treatment difference in peak craving response [Placebo: Δ=0.85 (0.25) vs. Oxytocin: Δ=1.31 (0.25); p=.19] or peak stress response [Placebo: Δ=1.1 (0.2) vs. Oxytocin: Δ=1.1 (0.2); p=.99]. Although there were no differences in the overall cortisol response following medication administration, there was a moderate attenuation in the peak response between oxytocin and placebo [log10 cortisol Placebo: Δ=0.10 (0.02) vs. Oxytocin: Δ=0.03 (0.02); p=.049]. Despite gender differences in response to the TSST, gender did not modify the effect of oxytocin on mean response outcome measures [longitudinal models ps>.30 and peak response models ps>.10].
Figure 4.
Mean cigarette craving (A), self-reported stress (B), and cortisol (C) response prior to and following the Trier Social Stress Task (TSST) for participants randomized to the oxytocin (n=72) or placebo condition (n=72). Data are shown as the model-based means and standard errors. Data are shown adjusted for A) treatment condition, time, treatment by time interaction, baseline cigarette craving and gender, B) treatment condition, time, treatment by time interaction, baseline stress, gender and gender by time interaction, and C) treatment condition, time, treatment by time interaction, baseline cortisol, gender and gender by time interaction.
Medication group was not a significant predictor of time to first cigarette [oxytocin vs. placebo HR (95% CI) = 1.2 (0.7–2.1); p=.55]. Among participants who began smoking immediately, there were no differences between medication groups [n=18; Placebo n=8 vs. Oxytocin n=10; p=.61] or among those who resisted smoking for the full 50 minutes [n=95; Placebo n=46 vs. Oxytocin n=49; p=.60]. There was no difference in the number of cigarettes smoked between treatment groups [Placebo 2.1 (0.1) vs. Oxytocin 2.3 (0.1); p=.28]. Smoking topography did not significantly differ based on medication administration (Wilks’ λ=0.91, F7,126 =1.73, p=. 11, η2=0.09). Finally, the mean mCEQ score did not differ between treatment assignment [Placebo 4.99 (0.14) vs. Oxytocin 4.15 (0.14); p=0.41], nor did any of the mCEQ subscales differ between oxytocin or placebo groups [all ps>.29].
On the day of the laboratory session, 28 adverse events (AEs) were reported. Of those 28, 17 (61%) were deemed unrelated to study procedures or medication administration. Of the 11 AEs deemed possibly related, the most common were headache and lightheadness and were mild to moderate in severity. Seven of the 11 AEs (64%) were experienced by participants randomized to oxytocin. Only 54% of participants correctly identified their medication condition at the end of the laboratory session, suggesting that the blind was effective.
Discussion
This study assessed the influence of gender and oxytocin on subjective and physiological stress reactivity, craving, latency to smoke, smoking intensity, and subjective responses to smoking in a laboratory relapse paradigm following stress induction. Following stress induction, female smokers demonstrated higher overall subjective stress responses and peak changes in stress compared to males, even though pre-TSST stress levels did not differ between males and females. Despite this, overall and peak cortisol response following TSST was significantly higher in males, indicating greater neuroendocrine reactivity in males compared to females. Males also demonstrated greater smoking intensity compared to females. However, no gender differences were found on measures of cigarette craving, latency to smoke, or subjective ratings of smoking.
Our findings demonstrating gender differences in smokers’ subjective stress reactivity are consistent with gender differences found in stress reactivity in the natural environment as part of the first phase of this study (Tomko et al. In Press; Wray et al. 2015). Our findings are also consistent with studies demonstrating a disparity between males and females in their neuroendocrine responses resulting from TSST administration (Back et al. 2008; Gilmore et al. 2019; Liu et al. 2017). Though our results support the hypothesis focused on stress reactivity and indicate that smoking may be differentially maintained based on gender, the demonstrated gender differences did not contribute to discernable differences in craving or latency to smoke. Subjective stress reactivity, as well as neuroendocrine reactivity is likely to impact craving and smoking/relapse and has been shown to be a predictor of smoking in a laboratory relapse paradigm (McKee et al. 2011). However, within the context of the current study, this relationship was not demonstrated. Also, neuroendocrine reactivity, specifically cortisol, can be affected or blunted among a certain sub-set of the population (Carpenter et al. 2007; Raison and Miller 2003), during different phases of the menstrual cycle (Nakajima et al. 2019), and has been shown to vary based on sex (al’Absi et al. 2015). Although beyond the scope of the current analysis, there may have been gender differences in the prevalence of mental health disorders or stressful life events that may partially explain the discrepancy between female smokers’ increased subjective reports of stress and their blunted neuroendocrine responses.
Oxytocin was examined in the current study based on its proposed role in stress modulation as part of substance use and relapse (Lee and Weerts 2016) and promising preliminary results among cannabis users (McRae-Clark et al. 2013). Contrary to our hypothesis, oxytocin had no effect on craving, subjective stress, cortisol response, latency to smoke, smoking topography, or subjective responses to smoking. Further, there was not a significant modifying effect of gender, suggesting that oxytocin did not exert gender-specific effects in our study. These results mark a departure from our hypothesis but contribute to a growing and mixed literature on the influence of oxytocin on smoking. One study found an effect of oxytocin on cigarette craving (Miller et al. 2016), while another found no effect on subjective responses (Van Hedger et al. 2018), and a third study found an effect of oxytocin on reduced cigarette consumption (Van Hedger et al. 2019). There appears to be divergent results in the literature based on subjective compared to behavioral metrics of smoking, though our results showed that oxytocin did not impact either subjective or behavioral measures of smoking. However, this is an area that requires further investigation, especially given that responses to oxytocin may vary based on specific characteristics or history of the individual, such as childhood adverse experiences (Flanagan et al. 2018).
Behavioral metrics of smoking in the laboratory are also subject to limitations that must be considered. Latency to smoke during the SRT (i.e., the ability to resist smoking) is considered a proxy for relapse vulnerability. This laboratory model has been used previously (McKee et al. 2015a; Verplaetse et al. 2017) and has been shown to differ across male and female smokers (Weinberger and McKee 2012). The lack of effect observed here could have been the result of task parameters. The target is to delay smoking for 50% of the interval (25 minutes) to achieve sufficient variability in latency to smoke to detect effects (i.e., medication, gender, etc.). However, the majority of participants (66%) in our study resisted smoking for the full 50 minutes, and as such, any gender or medication effects may have been obscured by the lack of variability in time to smoke. In a recent study of parametric manipulations of this task, compensation for resisting smoking was lower (Oberleitner et al. 2018), suggesting the compensation provided in the current study ($1.50 for each 5 minutes, $15 total) was too high to see sufficient variability.
Finally, smoking topography during the ad-libitum smoking period showed that males demonstrated greater smoking intensity than females, which is consistent with prior literature (Perkins et al. 2012) and may be due to physiological differences or pharmacological effects of nicotine (Benowitz and Hatsukami 1998; Perkins 1996; Perkins et al. 2002). However, there was no difference in treatment groups on measures of smoking topography, which is inconsistent with prior literature (Van Hedger et al. 2019). The laboratory environment may have affected smoking metrics and rate in the current study and future work should explore methods of collecting cigarette consumption data across several hours or days, while potentially exploring chronic dosing of oxytocin, rather than acute dosing, as was done in the current study.
Limitations
This study has several important limitations to consider. One limitation was the timing of the SRT immediately following the TSST. About 13% of participants smoked immediately upon starting the SRT, which influenced craving and subjective stress values at subsequent post-TSST time points (TSST +20 and TSST +40). These data were removed, but the participants who were not able to resist smoking for even a few minutes likely represent a particularly stress responsive group of smokers. However, measures collected at the initial post-TSST time point (TSST +0) included all participants, prior to the SRT, when pronounced oxytocin effects would be expected to be present. Second, payment during the SRT for resisting smoking was a maximum of $15, which may have been too salient to obtain the needed variability on this task. Third, oxytocin dose selection and timing of administration was based on previous human laboratory work (Ditzen et al. 2009; Heinrichs et al. 2003; Leng and Ludwig 2016; McRae-Clark et al. 2013), though we acknowledge that the selected dose and timing may have adversely affected results. Oxytocin dosing was conducted in the laboratory once, which may not be sufficient for detecting an impact on our outcome measures (Sippel et al. 2017), and we did not collect any absorption information for oxytocin in study participants. Further, stress induction closer to the time of oxytocin administration may have yielded more pronounced effects on stress reactivity and craving due to peak oxytocin effects at that time (Tanaka et al. 2018). Fourth, although the moderating effect of gender on treatment was insignificant for study outcomes, these should be interpreted in light of limited statistical power to test these effects. The moderating relationship of gender with oxytocin treatment on peak cortisol response was particularly compelling in men (Figure 3c), but did not reach statistical significance (p=.10), indicating that oxytocin may blunt the heightened cortisol response seen in male smokers. Fifth, all study participants were nicotine deprived during the laboratory session. Previous work has shown that overnight abstinence and acute stressors may increase subjective craving and stress; however, smoking may have differential effects on subjective distress caused by abstinence versus acute stress (Perkins et al. 2010). Because all participants in the current study were subject to both overnight abstinence and stress induction via the TSST, it is not possible to determine whether abstinence or stress induction played a larger role in the current results. Sixth, menstrual cycle and ovarian hormones may have played a role in stress reactivity (subjective and physiological), craving, and smoking metrics, as has been shown in previous literature (Espin et al. 2019; Nakajima et al. 2019). However, the inclusion of ovarian hormone influence was outside the scope of the current report and will be described in sufficient detail elsewhere. Seventh, cortisol measures were only collected during the laboratory session and therefore, we cannot disentangle naturally occurring fluctuations during the day from changes due to study manipulations. Finally, while the study protocol was brief, participant burden was moderately high and the laboratory session had to be completed on a week day. This likely created barriers for certain participants and may have contributed to selection bias that could limit the generalizability of study results.
Conclusions
The current study had two major findings. First, females experienced increased subjective stress following TSST compared to male participants, and lower neuroendocrine cortisol response. Second, oxytocin was not shown to be beneficial in decreasing subjective stress, craving, neuroendocrine response, or the delay of smoking. Though oxytocin did not appear to have beneficial impact in the context of the present study, future work should pursue its suitability as a pharmacotherapy for tobacco use, potentially utilizing longer dosing regimens or prolonged assessments of stress reactivity, craving, and smoking in the natural environment. Stress reactivity and its influence on craving and smoking may manifest differently among treatment-seeking smokers during real-world cessation attempts, and laboratory results may not necessarily translate well to cessation outcomes in the natural environment. Stressors may be experienced rapidly and unexpectedly during a quit attempt and this may be especially detrimental to female smokers attempting to quit. It is reasonable to hypothesize that medications that directly interfere with this relationship and reduce reactivity to stressors would be beneficial for smokers. Further research on oxytocin is needed, especially given recent studies showing nuanced effects of the drug based on varying phenotypic profiles and histories (Flanagan et al. 2018; Jarnecke et al. 2018; Sippel et al. 2017). Gender differences in smoking maintenance and cessation are complex and multi-factorial, thus requiring additional research targeting those factors, which may need to be gender-specific to be maximally efficacious.
Acknowledgments
We would like to thank the study participants for their time and effort in completing study procedures. We would also like to thank the medical and research staff at the Medical University of South Carolina; specifically, Lori Ann Ueberroth, Laruen Beech, Jessica Hinton, Jaclyn Condo, Patrick Cato, Casy Johnson, Christine Horne, Danielle Paquette, Priscilla Muldrow, Elhaam Borhanian and Elizabeth Kryway.
Funding: This study was supported by National Institutes of Health grants from the National Institute on Drug Abuse (NIDA P50DA016511) and National Center for Advancing Translational Sciences (NCATS UL1TR001450). Effort to complete this project and manuscript was provided by grants from the National Institute of Drug Abuse (NIDA U01DA031779, NIDA R01DA042114, NIDA K01DA036739, NIDA T32DA035200, and NIDA R25 DA020537) and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD K12 HD055885).
Footnotes
Disclosures: KMG and MJC have provided consultation for Pfizer, Inc. All other authors have no disclosures to declare.
This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- al’Absi M, Nakajima M, Allen S, Lemieux A, Hatsukami D (2015) Sex differences in hormonal responses to stress and smoking relapse: a prospective examination Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco 17:382–389 doi: 10.1093/ntr/ntu340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babb S, Malarcher A, Schauer G, Asman K, Jamal A (2017) Quitting Smoking Among Adults - United States, 2000–2015 MMWR Morb Mortal Wkly Rep 65:1457–1464 doi: 10.15585/mmwr.mm6552a1 [DOI] [PubMed] [Google Scholar]
- Back SE et al. (2008) Effects of gender and cigarette smoking on reactivity to psychological and pharmacological stress provocation Psychoneuroendocrinology 33:560–568 doi: 10.1016/j.psyneuen.2008.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer JS, Kamarck T, Lichtenstein E, Ransom CC Jr, (1989) Prediction of smoking relapse: analyses of temptations and transgressions after initial cessation J Consult Clin Psychol 57:623–627 [DOI] [PubMed] [Google Scholar]
- Baracz SJ, Everett NA, McGregor IS, Cornish JL (2016) Oxytocin in the nucleus accumbens core reduces reinstatement of methamphetamine-seeking behaviour in rats Addict Biol 21:316–325 doi: 10.1111/adb.12198 [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Hatsukami D (1998) Gender differences in the pharmacology of nicotine addiction Addict Biol 3:383–404 doi: 10.1080/13556219871930 [DOI] [PubMed] [Google Scholar]
- Brandon TH (1994) Negative affect as motivation to smoke Curr Dir Psychol Sci 3:33–37 [Google Scholar]
- Buchmann AF, Laucht M, Schmid B, Wiedemann K, Mann K, Zimmermann US (2010) Cigarette craving increases after a psychosocial stress test and is related to cortisol stress response but not to dependence scores in daily smokers J Psychopharmacol 24:247–255 doi: 10.1177/0269881108095716 [DOI] [PubMed] [Google Scholar]
- Cappelleri JC, Bushmakin AG, Baker CL, Merikle E, Olufade AO, Gilbert DG (2007) Confirmatory factor analyses and reliability of the modified cigarette evaluation questionnaire Addict Behav 32:912–923 doi: 10.1016/j.addbeh.2006.06.028 [DOI] [PubMed] [Google Scholar]
- Carpenter LL et al. (2007) Decreased adrenocorticotropic hormone and cortisol responses to stress in healthy adults reporting significant childhood maltreatment Biol Psychiatry 62:1080–1087 doi: 10.1016/j.biopsych.2007.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson DS, Cornish JL, Guastella AJ, Hunt GE, McGregor IS (2010) Oxytocin decreases methamphetamine self-administration, methamphetamine hyperactivity, and relapse to methamphetamine-seeking behaviour in rats Neuropharmacology 58:38–43 doi: 10.1016/j.neuropharm.2009.06.018 [DOI] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST (2001) The cue-availability paradigm: the effects of cigarette availability on cue reactivity in smokers Exp Clin Psychopharmacol 9:183–190 [DOI] [PubMed] [Google Scholar]
- Cropsey KL, Trent LR, Clark CB, Stevens EN, Lahti AC, Hendricks PS (2014) How Low Should You Go? Determining the Optimal Cutoff for Exhaled Carbon Monoxide to Confirm Smoking Abstinence Using Cotinine as Reference Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco doi: 10.1093/ntr/ntu085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira DC, Zuardi AW, Graeff FG, Queiroz RH, Crippa JA (2012) Anxiolytic-like effect of oxytocin in the simulated public speaking test J Psychopharmacol 26:497–504 doi: 10.1177/0269881111400642 [DOI] [PubMed] [Google Scholar]
- Ditzen B, Schaer M, Gabriel B, Bodenmann G, Ehlert U, Heinrichs M (2009) Intranasal oxytocin increases positive communication and reduces cortisol levels during couple conflict Biol Psychiatry 65:728–731 doi: 10.1016/j.biopsych.2008.10.011 [DOI] [PubMed] [Google Scholar]
- Espin L, Villada C, Hidalgo V, Salvador A (2019) Effects of sex and menstrual cycle phase on cardiac response and alpha- amylase levels in psychosocial stress Biol Psychol 140:141–148 doi: 10.1016/j.biopsycho.2018.12.002 [DOI] [PubMed] [Google Scholar]
- Fidler JA, West R (2009) Self-perceived smoking motives and their correlates in a general population sample Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco 11:1182–1188 doi: 10.1093/ntr/ntp120 [DOI] [PubMed] [Google Scholar]
- First MS R; Gibbon M; Williams JBW (1994) Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. (SCID-I/P). Biometrics Research, New York State Psychiatric Institute, New York. [Google Scholar]
- Flanagan JC, Fischer MS, Nietert PJ, Back SE, Maria MM, Snead A, Brady KT (2018) Effects of oxytocin on cortisol reactivity and conflict resolution behaviors among couples with substance misuse Psychiatry Res 260:346–352 doi: 10.1016/j.psychres.2017.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis DD, Young LJ, Meaney MJ, Insel TR (2002) Naturally occurring differences in maternal care are associated with the expression of oxytocin and vasopressin (V1a) receptors: gender differences J Neuroendocrinol 14:349–353 [DOI] [PubMed] [Google Scholar]
- Gilmore AK et al. (2019) Gender differences in subjective stress and neuroendocrine response to a stress task among individuals with opioid dependence: A pilot study Addict Behav 92:148–154 doi: 10.1016/j.addbeh.2018.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagman BT, Delnevo CD, Hrywna M, Williams JM (2008) Tobacco use among those with serious psychological distress: results from the national survey of drug use and health, 2002 Addict Behav 33:582–592 doi: 10.1016/j.addbeh.2007.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO (1991) The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire Br J Addict 86:1119–1127 [DOI] [PubMed] [Google Scholar]
- Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U (2003) Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress Biol Psychiatry 54:1389–1398 [DOI] [PubMed] [Google Scholar]
- Jamal A, King BA, Neff LJ, Whitmill J, Babb SD, Graffunder CM (2016) Current Cigarette Smoking Among Adults - United States, 2005–2015 MMWR Morb Mortal Wkly Rep 65:1205–1211 doi: 10.15585/mmwr.mm6544a2 [DOI] [PubMed] [Google Scholar]
- Jamal A, Phillips E, Gentzke AS, Homa DM, Babb SD, King BA, Neff LJ (2018) Current Cigarette Smoking Among Adults - United States, 2016 MMWR Morb Mortal Wkly Rep 67:53–59 doi: 10.15585/mmwr.mm6702a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Japuntich SJ, Leventhal AM, Piper ME, Bolt DM, Roberts LJ, Fiore MC, Baker TB (2011) Smoker characteristics and smoking-cessation milestones Am J Prev Med 40:286–294 doi: 10.1016/j.amepre.2010.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarnecke AM, Barden E, Back SE, Brady KT, Flanagan JC (2018) Intimate partner violence moderates the association between oxytocin and reactivity to dyadic conflict among couples Psychiatry Res 270:404–411 doi: 10.1016/j.psychres.2018.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killen JD, Fortmann SP (1997) Craving is associated with smoking relapse: findings from three prospective studies Exp Clin Psychopharmacol 5:137–142 [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH (1993) The ‘Trier Social Stress Test’--a tool for investigating psychobiological stress responses in a laboratory setting Neuropsychobiology 28:76–81 doi: 119004 [DOI] [PubMed] [Google Scholar]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E (2005) Oxytocin increases trust in humans Nature 435:673–676 doi: 10.1038/nature03701 [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Kirschbaum C (2005) Sex differences in HPA axis responses to stress: a review Biol Psychol 69:113–132 doi: 10.1016/j.biopsycho.2004.11.009 [DOI] [PubMed] [Google Scholar]
- Lee MR, Weerts EM (2016) Oxytocin for the treatment of drug and alcohol use disorders Behav Pharmacol 27:640–648 doi: 10.1097/fbp.0000000000000258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng G, Ludwig M (2016) Intranasal Oxytocin: Myths and Delusions Biol Psychiatry 79:243–250 doi: 10.1016/j.biopsych.2015.05.003 [DOI] [PubMed] [Google Scholar]
- Liu JJW, Ein N, Peck K, Huang V, Pruessner JC, Vickers K (2017) Sex differences in salivary cortisol reactivity to the Trier Social Stress Test (TSST): A meta-analysis Psychoneuroendocrinology 82:26–37 doi: 10.1016/j.psyneuen.2017.04.007 [DOI] [PubMed] [Google Scholar]
- Ludwig M, Leng G (2006) Dendritic peptide release and peptide-dependent behaviours Nat Rev Neurosci 7:126–136 doi: 10.1038/nrn1845 [DOI] [PubMed] [Google Scholar]
- Lunden SE, Pittman JC, Prashad N, Malhotra R, Sheffer CE (2018) Cognitive, Behavioral, and Situational Influences on Relapse to Smoking After Group Treatment for Tobacco Dependence Front Psychol 9:2756 doi: 10.3389/fpsyg.2018.02756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald K, MacDonald TM (2010) The peptide that binds: A systematic review of Oxytocin and its prosocial effects in humans Harv Rev Psychiatry 18:1–21 doi: 10.3109/10673220903523615 [DOI] [PubMed] [Google Scholar]
- McKee SA (2009) Developing human laboratory models of smoking lapse behavior for medication screening Addict Biol 14:99–107 doi: 10.1111/j.1369-1600.2008.00135.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Maciejewski PK, Falba T, Mazure CM (2003) Sex differences in the effects of stressful life events on changes in smoking status Addiction 98:847–855 [DOI] [PubMed] [Google Scholar]
- McKee SA et al. (2015a) A translational investigation targeting stress-reactivity and prefrontal cognitive control with guanfacine for smoking cessation J Psychopharmacol 29:300–311 doi: 10.1177/0269881114562091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Sinha R, Weinberger AH, Sofuoglu M, Harrison EL, Lavery M, Wanzer J (2011) Stress decreases the ability to resist smoking and potentiates smoking intensity and reward Journal of psychopharmacology (Oxford, England) 25:490–502 doi: 10.1177/0269881110376694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Smith PH, Kaufman M, Mazure CM, Weinberger AH (2015b) Sex Differences in Varenicline Efficacy for Smoking Cessation: A Meta-Analysis Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco doi: 10.1093/ntr/ntv207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Weinberger AH, Shi J, Tetrault J, Coppola S (2012) Developing and validating a human laboratory model to screen medications for smoking cessation Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco 14:1362–1371 doi: 10.1093/ntr/nts090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae-Clark AL, Baker NL, Maria MM, Brady KT (2013) Effect of oxytocin on craving and stress response in marijuana-dependent individuals: a pilot study Psychopharmacology (Berl) 228:623–631 doi: 10.1007/s00213-013-3062-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MA, Bershad A, King AC, Lee R, de Wit H (2016) Intranasal oxytocin dampens cue-elicited cigarette craving in daily smokers: a pilot study Behav Pharmacol 27:697–703 doi: 10.1097/fbp.0000000000000260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima M, Allen S, al’Absi M (2019) Influences of the Menstrual Phase on Cortisol Response to Stress in Nicotine Dependent Women: A Preliminary Examination Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco 21:617–622 doi: 10.1093/ntr/nty071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niaura R, Shadel WG, Britt DM, Abrams DB (2002) Response to social stress, urge to smoke, and smoking cessation Addict Behav 27:241–250 [DOI] [PubMed] [Google Scholar]
- Oberleitner LMS, Moore KE, Verplaetse T, Roberts W, McKee SA (2018) Developing a laboratory model of smoking lapse targeting stress and brief nicotine deprivation Exp Clin Psychopharmacol 26:244–250 doi: 10.1037/pha0000187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA (1996) Sex differences in nicotine versus nonnicotine reinforcement as determinants of tobacco smoking Exp Clin Psychopharmacol 4:166–177 doi: 10.1037/1064-1297.4.2.166 [DOI] [Google Scholar]
- Perkins KA, Grobe JE (1992) Increased desire to smoke during acute stress Br J Addict 87:1037–1040 [DOI] [PubMed] [Google Scholar]
- Perkins KA, Jacobs L, Sanders M, Caggiula AR (2002) Sex differences in the subjective and reinforcing effects of cigarette nicotine dose Psychopharmacology (Berl) 163:194–201 doi: 10.1007/s00213-002-1168-1 [DOI] [PubMed] [Google Scholar]
- Perkins KA, Karelitz JL, Conklin CA, Sayette MA, Giedgowd GE (2010) Acute negative affect relief from smoking depends on the affect situation and measure but not on nicotine Biol Psychiatry 67:707–714 doi: 10.1016/j.biopsych.2009.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Karelitz JL, Giedgowd GE, Conklin CA (2012) The reliability of puff topography and subjective responses during ad lib smoking of a single cigarette Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco 14:490–494 doi: 10.1093/ntr/ntr150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Karelitz JL, Jao NC (2013) Optimal carbon monoxide criteria to confirm 24-hr smoking abstinence Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco 15:978–982 doi: 10.1093/ntr/nts205; 10.1093/ntr/nts205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Scott J (2008) Sex differences in long-term smoking cessation rates due to nicotine patch Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco 10:1245–1250 doi: 10.1080/14622200802097506 [DOI] [PubMed] [Google Scholar]
- Peters ST, Bowen MT, Bohrer K, McGregor IS, Neumann ID (2017) Oxytocin inhibits ethanol consumption and ethanol-induced dopamine release in the nucleus accumbens Addict Biol 22:702–711 doi: 10.1111/adb.12362 [DOI] [PubMed] [Google Scholar]
- Piper ME, Cook JW, Schlam TR, Jorenby DE, Smith SS, Bolt DM, Loh WY (2010) Gender, race, and education differences in abstinence rates among participants in two randomized smoking cessation trials Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco 12:647–657 doi: 10.1093/ntr/ntq067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper ME, Piasecki TM, Federman EB, Bolt DM, Smith SS, Fiore MC, Baker TB (2004) A multiple motives approach to tobacco dependence: the Wisconsin Inventory of Smoking Dependence Motives (WISDM-68) J Consult Clin Psychol 72:139–154 doi: 10.1037/0022-006x.72.2.139 [DOI] [PubMed] [Google Scholar]
- Potter AS (2014) Smoking cessation in men and women Am J Psychiatry 171:1148–1150 doi: 10.1176/appi.ajp.2014.14081032 [DOI] [PubMed] [Google Scholar]
- Raison CL, Miller AH (2003) When not enough is too much: the role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders Am J Psychiatry 160:1554–1565 doi: 10.1176/appi.ajp.160.9.1554 [DOI] [PubMed] [Google Scholar]
- Reed SC, Haney M, Manubay J, Campagna BR, Reed B, Foltin RW, Evans SM (2019) Sex differences in stress reactivity after intranasal oxytocin in recreational cannabis users Pharmacol Biochem Behav 176:72–82 doi: 10.1016/j.pbb.2018.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JM, Stipelman BA, Bornovalova MA, Daughters Sb, Sinha R, Lejuez CW (2011) Biological mechanisms underlying the relationship between stress and smoking: state of the science and directions for future work Biol Psychol 88:1–12 doi: 10.1016/j.biopsycho.2011.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharf D, Shiffman S (2004) Are there gender differences in smoking cessation, with and without bupropion? Pooled- and meta-analyses of clinical trials of Bupropion SR Addiction 99:1462–1469 doi: 10.1111/j.1360-0443.2004.00845.x [DOI] [PubMed] [Google Scholar]
- Sheehan DV et al. (1998) The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10 J Clin Psychiatry 59 Suppl 20:22–33;quiz 34–57 [PubMed] [Google Scholar]
- Shiffman S et al. (2002) Immediate antecedents of cigarette smoking: an analysis from ecological momentary assessment J Abnorm Psychol 111:531–545 [DOI] [PubMed] [Google Scholar]
- Sippel LM, Allington CE, Pietrzak RH, Harpaz-Rotem I, Mayes LC, Olff M (2017) Oxytocin and Stress-related Disorders: Neurobiological Mechanisms and Treatment Opportunities Chronic Stress (Thousand Oaks) 1 doi: 10.1177/2470547016687996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PH, Bessette AJ, Weinberger AH, Sheffer CE, McKee SA (2016) Sex/gender differences in smoking cessation: A review Prev Med 92:135–140 doi: 10.1016/j.ypmed.2016.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PH et al. (2015) Gender differences in medication use and cigarette smoking cessation: results from the International Tobacco Control Four Country Survey Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco 17:463–472 doi: 10.1093/ntr/ntu212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PH, Weinberger AH, Zhang J, Emme E, Mazure CM, McKee SA (2017) Sex Differences in Smoking Cessation Pharmacotherapy Comparative Efficacy: A Network Meta-analysis Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco 19:273–281 doi: 10.1093/ntr/ntw144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SS et al. (2003) Targeting smokers at increased risk for relapse: treating women and those with a history of depression Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco 5:99–109 [DOI] [PubMed] [Google Scholar]
- Sung HY, Prochaska JJ, Ong MK, Shi Y, Max W (2011) Cigarette smoking and serious psychological distress: a population-based study of California adults Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco 13:1183–1192 doi: 10.1093/ntr/ntr148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka A et al. (2018) Delivery of Oxytocin to the Brain for the Treatment of Autism Spectrum Disorder by Nasal Application Mol Pharm 15:1105–1111 doi: 10.1021/acs.molpharmaceut.7b00991 [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Drobes DJ (1990) Imagery and smoking urges: the manipulation of affective content Addict Behav 15:531–539 [DOI] [PubMed] [Google Scholar]
- Tomko RL et al. (In Press) Sex Differences in Subjective and Behavioral Responses to Stressful and Smoking Cues Presented in the Natural Environment of Smokers Nicotine & Tobacco Research Epub ahead of print [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hedger K, Bershad AK, Lee R, de Wit H (2018) Effects of intranasal oxytocin on stress-induced cigarette craving in daily smokers Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco doi: 10.1093/ntr/nty159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hedger K, Kushner MJ, Lee R, de Wit H (2019) Oxytocin Reduces Cigarette Consumption in Daily Smokers Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco 21:799–804 doi: 10.1093/ntr/nty080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verplaetse TL et al. (2017) Effect of doxazosin on stress reactivity and the ability to resist smoking J Psychopharmacol 31:830–840 doi: 10.1177/0269881117699603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker NJ, van Woerden HC, Kiparoglou V, Yang Y, Robinson H, Croghan E (2016) Gender difference and effect of pharmacotherapy: findings from a smoking cessation service BMC Public Health 16:1038 doi: 10.1186/s12889-016-3672-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warthen MW, Tiffany ST (2009) Evaluation of cue reactivity in the natural environment of smokers using ecological momentary assessment Exp Clin Psychopharmacol 17:70–77 doi: 10.1037/a0015617; 10.1037/a0015617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger AH, McKee SA (2012) Gender differences in smoking following an implicit mood induction Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco 14:621–625 doi: 10.1093/ntr/ntr198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger AH, Pilver CE, Mazure CM, McKee SA (2014) Stability of smoking status in the US population: a longitudinal investigation Addiction 109:1541–1553 doi: 10.1111/add.12647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetter DW, Kenford SL, Smith SS, Fiore MC, Jorenby DE, Baker TB (1999) Gender differences in smoking cessation J Consult Clin Psychol 67:555–562 [DOI] [PubMed] [Google Scholar]
- Williams JR, Insel TR, Harbaugh CR, Carter CS (1994) Oxytocin administered centrally facilitates formation of a partner preference in female prairie voles (Microtus ochrogaster) J Neuroendocrinol 6:247–250 [DOI] [PubMed] [Google Scholar]
- Witt DM, Winslow JT, Insel TR (1992) Enhanced social interactions in rats following chronic, centrally infused oxytocin Pharmacol Biochem Behav 43:855–861 [DOI] [PubMed] [Google Scholar]
- Wray JM, Gray KM, McClure EA, Carpenter MJ, Tiffany ST, Saladin ME (2015) Gender differences in responses to cues presented in the natural environment of cigarette smokers Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco 17:438–442 doi: 10.1093/ntr/ntu248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu PC, Hsueh KC, Mar GY, Hsueh SC, Tu MS, McRobbie H, Hajek P (2016) Gender Differences in Outcome of an Attempt to Stop Smoking Among Smokers Attending a Smoking Cessation Clinic in Taiwan: 3-Year Follow-Up Study Eval Health Prof 39:317–325 doi: 10.1177/0163278715616439 [DOI] [PubMed] [Google Scholar]