Abstract
Objective
Isolated focal dystonia can spread to muscles beyond the initially affected body region, but risk of spread has not been evaluated in a prospective manner. Furthermore, body regions at risk for spread and the clinical factors associated with spread risk are not well characterised. We sought here to prospectively characterise risk of spread in recently diagnosed adult-onset isolated focal dystonia patients.
Methods
Patients enrolled in the Dystonia Coalition with isolated dystonia affecting only the neck, upper face, hand or larynx at onset of symptoms were included. Timing of follow-up visits was based on a sliding scale depending on symptom onset and ranged from 1 to 4 years. Descriptive statistics, Kaplan-Meier survival curves and Cox proportional hazard regression models were used to assess clinical characteristics associated with dystonia spread.
Results
487 enrolled participants (68.3% women; mean age: 55.6±12.2 years) met our inclusion/exclusion criteria. Spread was observed in 50% of blepharospasm, 8% of cervical dystonia, 17% of hand dystonia and 16% of laryngeal dystonia cases. Most common regions for first spread were the oromandibular region (42.2%) and neck (22.4%) for blepharospasm, hand (3.5%) for cervical dystonia and neck for hand (12.8%) and laryngeal (15.8%) dystonia. Increased spread risk was associated with a positive family history (HR=2.18, p=0.012) and self-reported alcohol responsiveness (HR=2.59, p=0.009).
Conclusions
Initial body region affected in isolated focal dystonia has differential risk and patterns of spread. Genetic factors likely influence the risk of spread. These findings can aid clinical prognostication and inform future investigations into potential disease-modifying treatments.
Introduction
Dystonia is a group of movement disorders characterised by sustained or intermittent involuntary muscle contractions that lead to abnormal and often repetitive movements, postures or both.1 Isolated dystonia disorders are not accompanied by other neurological abnormalities except for tremor, and have no known cause except for relatively rare gene mutations.2 The most common form of isolated dystonia is adult-onset focal dystonia that primarily affects one body region, such as the upper face, neck, limb or larynx.3 At onset, dystonia typically affects only one body region, but with time dystonia can spread to involve other contiguous or distant regions of the body and lead to greater disability dependent on what body region or regions become involved.4–13
Investigating the progression of motor symptom severity in focal dystonia is a complicated endeavour due to a number of factors including the uncertain linearity of rating scales across different affected body regions. Additionally, the common use of botulinum toxin injections to treat focal dystonia introduces variability when assessing motor severity and waiting for toxin to completely wear off to determine the underlying severity of dystonia is not feasible in most cases. These reasons among others have prompted dystonia investigators to focus on spread of dystonia, which is a more easily identifiable and objective measure of increased dystonia involvement than severity in a particular body region. This type of objective measure could be a valuable outcome measure for a clinical trial of a disease-modifying intervention.
Despite shared epidemiological, aetiological and pathophysiological features across focal dystonia phenotypes,14 15 some notable differences occur.16 For example, retrospective data suggest that blepharospasm (BSP) has a higher rate of spread than other forms of focal dystonia including cervical dystonia (CD), hand dystonia and laryngeal dystonia.4 8 10 12 Other clinical factors besides the initial affected body site may also contribute to risk of spread. For example, age at onset and positive family history have been reported to relate to risk of spread,5 8 10 but other studies have not found such relationships.6 12 17 Tremor also may increase or decrease the risk of spread in patients with CD.9 10 Whether the presence of a sensory trick (geste antagoniste) or alcohol responsiveness contribute to risk of spread is not known.
While prior studies have advanced our understanding of spread associated with different presentations of adult-onset focal dystonia, most of these studies have been small, from single centres or geographic regions, and retrospective. In the current study, we sought to prospectively characterise and assess the risk of spread in recently diagnosed isolated focal dystonia patients in a large, multicentre, international cohort. A better understanding of the risk of spread in focal dystonia subtypes, characterisation of the sites of spread and identification of clinical features contributing to increased risk of spread could enhance our understanding of the aetiology and pathophysiology underlying different phenotypes. In addition, this knowledge could help clinicians more effectively treat and determine prognosis of patients who present with focal dystonia and provide critical data for clinical trials testing disease-modifying therapies to reduce risk of spread.
Methods
Study participants
Participant data were acquired from the ongoing Natural History Project database of the Dystonia Coalition (www.rarediseasesnetwork.org/dystonia/), a multicentre, prospective, cross-sectional study of people with isolated, idiopathic dystonia. Participants were enrolled across over 30 clinical sites in the USA, Canada, France, Germany and Italy. Study exclusion criteria included the presence of comorbidities that could confound diagnosis or evaluation (other than tremor) and medical or neurological disorders that could preclude completing the neurological examination protocol and questionnaires. Subjects receiving botulinum toxin injections were enrolled at least 2 months after their last injection. To remove potential genetic confounds and enhance accuracy of our results, we excluded participants if dystonia onset occurred younger than 18 years of age or if a causative gene had ever been identified (genetic testing was not part of Dystonia Coalition Natural History Project). The follow-up visit schedule was based on a sliding scale depending on the timing of symptom onset: 0 to 3 years ago—1 year from current visit; 3 to 5 years ago—2 years from current visit; 5 to 7 years ago—3 years from current visit; >7 years ago—4 years from current visit. Due to the prospective nature of this study and to limit recall bias, we excluded participants who had dystonia affecting any body region for more than 5 years. This study includes participants enrolled in the Natural History Project between 12 January 2011 and 14 December 2018.
Standard protocol approvals, registrations and patient consents
The study was approved by the internal review boards (IRBs) of all participating clinical sites. All subjects gave written informed consent for participation in the study following the principles of the Declaration of Helsinki.
Dystonia spread and characteristics
All participants completed intake forms that included documenting all body regions affected by dystonia currently or in the past, along with the age of onset of dystonia for each body region listed. Focal dystonia subtypes were categorised according to their initial site of dystonia. For sample size reasons, our statistical analyses were limited to the four mostly commonly affected initial body regions and included the neck (CD), upper face (BSP), hand and larynx. Participants with more than one site of dystonia involvement at enrolment were removed from further analyses. Spread was defined as documentation by Dystonia Coalition investigators of dystonia affecting a body region other than the initial site of dystonia. The time to spread was estimated using the age of the participant at the follow-up visit and the reported age of dystonia onset (see Statistical analysis section below). Body regions of spread included in our analysis were the neck, upper face, hand, larynx, oromandibular (lower face, jaw and tongue), upper arm (not including shoulder), trunk, pelvis, upper leg and foot. If the presence of any tremor (regular or irregular/jerky) was documented on examination, the investigator was asked to note whether the patient’s dystonia was dominated by tremor more than tonic or twisting movements. The age of onset of dystonia and the presence of a family history of dystonia, effective sensory trick and alcohol responsiveness were acquired through self-report on a history intake assessment form.
Statistical analysis
Dystonia subject data were analysed to determine time from age of onset to time of first spread to a different site. Time to spread was calculated from integer (non-fraction) years of age at which dystonia onset at a site was identified by a Dystonia Coalition investigator, but if spread occurred at a second time point while the patient was the same age then spread was considered to have occurred at a 6-month mark. Subjects with no recorded spread of dystonia during the time in the study were censored (considered observed only for the time to last observation) at the last observation time. The times to first observed spread for the focal dystonia subtypes were compared with Kaplan-Meier curves and Cox proportional hazards models. Log-rank and Peto tests were performed on the Kaplan-Meier curves to test for differences in the curves among the initial onset sites. The explanatory variables in the Cox proportional hazards models included: initial onset site, family history of dystonia (yes, no or unknown), age of onset, sex (female or male), sensory trick (yes or no), tremor dominant (yes or no) and alcohol response (yes, no or unknown). Linear combinations of the estimated model parameters were used to construct pairwise comparisons among the levels of categorical variables, and to perform omnibus tests. HRs, 95% CIs and p-values for the variables and contrasts were calculated. Testing of the proportional hazard assumption was performed. Exploratory analyses produced frequency tables for location of dystonia spread and distinguished between location of first spread and location of subsequent spread. All statistics were performed in SAS V.9.4 and StataMP 13. The significance level for group comparisons and the Cox proportional hazard analysis was defined as a p<0.05.
Data availability statement
An overview of the type of data that have been collected by the investigators of the Dystonia Coalition can be found at the publicly available site: http://clinportalquery.wustl.edu/pentaho/Home?userid=DYSTONIA_OPENI&password=DYSTONIA. Deidentified individual participant data from this study will be shared upon reasonable request from a qualified investigator after the Dystonia Coalition grants permission and proper IRB approval is obtained.
Results
Participants
Data from a total of 1320 isolated focal dystonia patients enrolled in the Natural History Project were reviewed. Of these, we removed 31 for an age of onset less than 18 years or insufficient documentation of age of onset, 9 for identification of a causative gene and 524 for dystonia duration greater than 5 years at the time of first observation. Additionally, we removed data from 199 patients who had more than one body site affected at onset and 70 patients who had an onset site other than the neck, upper face, hand or larynx. Thus, a total of 487 isolated focal dystonia participants met our inclusion and exclusion criteria. Of these, 286 had initial onset of CD, 116 had initial onset of BSP, 47 had initial onset of dystonia affecting muscles of the hand and 38 had initial onset of dystonia affecting the laryngeal muscles. Demographic information and clinical severity for all the included participants at their initial enrolment visit is listed in table 1.
Table 1.
Participant demographics at their initial visit
| All (n=487) |
Cervical (n=286, 58.7%) | Blepharospasm (n=116, 30.8%) | Hand (n=47, 9.7%) |
Laryngeal (n=38, 7.8%) |
|
| Age, mean (SD), years | 55.6 (12.2) | 54.2 (12.4) | 59.2 (10.4) | 52.9 (12.8) | 58.3 (12.9) |
| Age at onset, mean (SD), years | 53.0 (12.0) | 51.6 (12.2) | 56.5 (10.3) | 50.1 (12.5) | 55.9 (12.5) |
| Sex, F : M, n (%) | 332 (68.3):154 (31.7) | 192 (67.1):94 (32.9) | 81 (70.4):34 (29.6) | 32 (68.1):15 (31.9) | 27 (71.1):11 (28.9) |
| Race, n (%) | |||||
| White | 428 (87.9) | 267 (93.4) | 94 (81.0) | 39 (83.0) | 28 (73.7) |
| Black | 29 (6.0) | 6 (2.1) | 12 (10.3) | 5 (10.6) | 6 (15.8) |
| Asian | 13 (2.7) | 6 (2.1) | 5 (4.3) | 1 (2.1) | 1 (2.6) |
| American Indian | 2 (0.4) | 1 (0.4) | 0 (0) | 0 (0) | 1 (2.6) |
| Unknown/other | 15 (3.1) | 6 (2.1) | 5 (4.3) | 2 (4.3) | 2 (5.3) |
| Ethnicity, n (%) | |||||
| Non-Hispanic/Latino | 392 (80.5) | 232 (81.1) | 92 (79.3) | 34 (72.3) | 34 (89.5) |
| Hispanic or Latino | 22 (4.6) | 11 (3.9) | 7 (6.0) | 2 (4.3) | 2 (5.3) |
| Unknown/other | 73 (15.0) | 43 (15.0) | 17 (14.7) | 11 (23.4) | 2 (5.3) |
| Disease duration, years | 2.7±1.5 | 2.6±1.5 | 2.8±1.4 | 2.8±1.5 | 2.4±1.5 |
| GDRS, mean (SD) | 7.8 (5.5) | 7.2 (4.2) | 10.6 (7.5) | 6.1 (6.1) | 6.2 (3.2) |
| BFMS, mean (SD) | 6.7 (4.8) | 6.1 (3.5) | 9.4 (6.5) | 5.6 (5.9) | 4.6 (3.4) |
| Family history, n (%) | |||||
| Negative | 399 (81.9) | 232 (81.1) | 98 (84.5) | 39 (83.0) | 30 (79.0) |
| Positive | 59 (12.1) | 38 (13.3) | 12 (10.3) | 5 (10.6) | 4 (10.5) |
| Unknown | 29 (6.0) | 16 (5.6) | 6 (5.2) | 3 (6.4) | 4 (10.5) |
| Tremor dominant, n (%) | 56 (11.6) | 41 (14.4) | 1 (0.9) | 8 (17.0) | 6 (15.8) |
| Effective sensory trick, n (%) | 288 (59.1) | 199 (69.6) | 64 (55.2) | 19 (40.4) | 6 (15.8) |
| EtOH responsive, n (%) | |||||
| No | 212 (43.5) | 115 (40.2) | 60 (51.7) | 16 (34.0) | 21 (55.3) |
| Yes | 67 (13.8) | 51 (17.8) | 7 (6.0) | 3 (6.4) | 6 (15.8) |
| Unknown | 208 (42.7) | 120 (42.0) | 49 (42.2) | 28 (59.6) | 11 (29.0) |
BFMS, Burke-Fahn-Marsden Scale; EtOH, ethyl alcohol; GDRS, Global Dystonia Rating Scale.
Spread risk by dystonia subtype
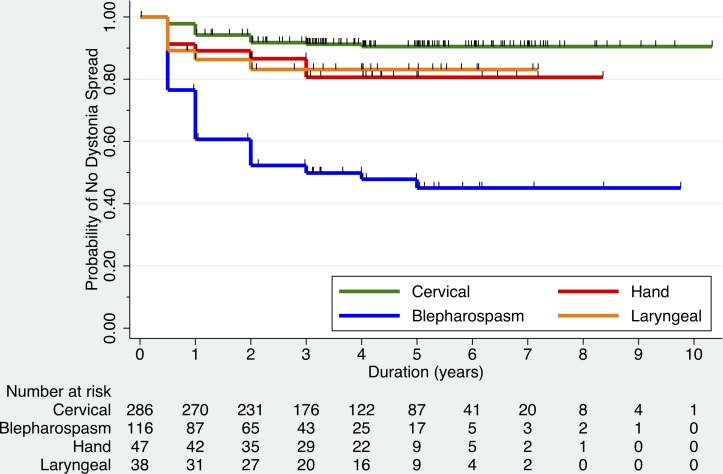
Median and maximum disease durations at the time of last observation were 3.6 and 10.3 years for CD, 3.9 and 9.7 years for BSP, 4.0 and 8.8 years for hand dystonia and 4.0 and 7.2 years for laryngeal dystonia. The total percentage of subjects who spread during observation was 8 for those with CD, 50 for those with BSP, 17 for those with hand dystonia and 16 for those with laryngeal dystonia. Figure 1 shows the results of the Kaplan-Meier survival analysis of the time of spread of dystonia based on initial onset site and age of onset. Characteristics of where dystonia spread first or spread at any time during enrolment for each subgroup of dystonia onset site are shown in table 2. There were no instances where dystonia spread to the pelvis or upper leg. Subjects with CD who had spread most frequently had spread to the hand (45.8%), those with BSP who spread most frequently spread to the oromandibular region (84.5%) and neck (58.6%) and those with hand and laryngeal dystonia who spread most frequently spread to the neck (75% and 100%, respectively).
Figure 1.
Kaplan-Meier survival analysis plot showing the probability of no dystonia spread over time for recently diagnosed isolated focal dystonia patients. Patients are grouped by site of initial dystonia onset: neck (cervical), upper face (blepharospasm), hand and larynx, and numbers of patients at risk of spread at each time point are shown. Tick marks represent when a patient was censored during follow-up.
Table 2.
Dystonia spread characteristics based on onset site
| Cervical (n=286) |
Blepharospasm (n=116) | Hand (n=47) |
Laryngeal (n=38) |
|
| Spread, n (%) | 24 (8.4) | 58 (50.0) | 8 (17.0) | 6 (15.8) |
| No spread, n (%) | 262 (91.6) | 58 (50.0) | 39 (83.0) | 32 (84.2) |
| Time to first spread, mean (SD), years | 3.5 (2.1) | 2.3 (1.9) | 3.3 (2.0) | 3.1 (2.1) |
| First spread, body region, n (% of those who spread) | ||||
| Cervical | – | 26 (44.8) | 6 (75.0) | 6 (100) |
| Blepharospasm | 5 (20.8) | – | 0 (0) | 1 (16.7) |
| Hand | 10 (41.7) | 2 (3.4) | – | 0 (0) |
| Laryngeal | 5 (20.8) | 2 (3.4) | 1 (12.5) | – |
| Oromandibular | 4 (16.7) | 49 (84.5) | 0 (0) | 1 (16.7) |
| Upper arm | 6 (25.0) | 0 (0) | 1 (12.5) | 0 (0) |
| Trunk | 0 (0) | 1 (1.7) | 0 (0) | 0 (0) |
| Foot | 1 (4.2) | 0 (0) | 0 (0) | 0 (0) |
| Any spread, body region, n (% of those who spread) | ||||
| Cervical | – | 34 (58.6) | 6 (75.0) | 6 (100) |
| Blepharospasm | 5 (20.8) | – | 0 (0) | 1 (16.7) |
| Hand | 11 (45.8) | 7 (12.1) | – | 2 (33.3) |
| Laryngeal | 6 (25.0) | 5 (8.6) | 1 (12.5) | – |
| Oromandibular | 5 (20.8) | 49 (84.5) | 0 (0) | 1 (16.7) |
| Tongue | 1 (4.2) | 5 (8.6) | 0 (0) | 0 (0) |
| Upper arm | 6 (25.0) | 2 (3.4) | 1 (12.5) | 0 (0) |
| Trunk | 0 (0) | 2 (3.4) | 0 (0) | 0 (0) |
| Foot | 1 (4.2) | 1 (1.7) | 0 (0) | 0 (0) |
Clinical features associated with dystonia spread risk
Using a Cox model analysis, those subjects with BSP onset had a significantly greater risk of spread than the other three sites of onset (table 3). Family history and alcohol responsiveness were the only other additional clinical features associated with a significant increased risk of spread.
Table 3.
Cox model analysis of potential explanatory variables for risk of dystonia spread
| Contrast | HR | 95% CI | P value |
| Onset site | |||
| BSP versus cervical | 10.980 | 6.459 to 18.667 | <0.001* |
| BSP versus hand | 5.154 | 2.338 to 11.361 | <0.001* |
| BSP versus laryngeal | 5.161 | 2.062 to 12.919 | <0.001* |
| Cervical versus hand | 0.469 | 0.207 to 1.063 | 0.089 |
| Cervical versus laryngeal | 0.470 | 0.182 to 1.213 | 0.143 |
| Laryngeal versus hand | 0.999 | 0.333 to 2.995 | 0.998 |
| Sex (male versus female) | 1.120 | 0.716 to 1.751 | 0.623 |
| Age of onset (10 years) | 1.091 | 0.898 to 1.325 | 0.378 |
| Family history (yes versus no) | 2.184 | 1.243 to 3.837 | 0.012* |
| Tremor dominant (yes versus no) | 1.977 | 1.006 to 3.883 | 0.061 |
| Sensory trick (yes versus no) | 1.026 | 0.653 to 1.612 | 0.913 |
| EtOH responsive (yes versus no) | 2.590 | 1.316 to 5.097 | 0.009* |
Results of the Cox proportional hazard model for time to first spread. Subjects without an observed spread were censored at the last observed dystonia duration time. Three observations were omitted because of missing covariates or time or censoring information. HRs greater than 1 indicate increased chance of dystonia spread, while HRs less than 1 indicate decreased chance. Testing of the proportional hazard assumption found no definite evidence of deviation from the assumption.
*Significant at p<0.05.
BSP, blepharospasm; EtOH, ethyl alcohol.
Sample size estimation for hypothetical clinical study
Using our calculated HRs for risk of dystonia spread, we estimated the sample sizes that would be needed in a hypothetical clinical study of newly diagnosed focal dystonia patients to test a therapeutic agent’s ability to reduce the risk of spread or to distinguish other factors that may influence spread. Assuming equal size and variance across the two groups, a sample size of only 59 BSP subjects per group would be needed to distinguish a 50% reduction in spread over 2 years with 80% power (table 4). In CD, however, a sample size of 498 subjects per group would be needed to distinguish a 50% reduction in spread over 2 years with 80% power. To distinguish a 30% reduction in spread over 2 years with 80% power, sample sizes ranging from 178 to 2194 subjects per each group would be needed depending on the initial onset site of dystonia.
Table 4.
Sample size estimates for distinguishing posited reductions in dystonia spread cumulative probability over 2 years by onset site
| Power | Cervical | Blepharospasm | Hand | Laryngeal |
| 50% reduction | ||||
| 80 | 498 (996) | 59 (118) | 293 (586) | 227 (454) |
| 90 | 666 (1332) | 79 (158) | 393 (786) | 303 (606) |
| 30% reduction | ||||
| 80 | 1639 (3278) | 178 (356) | 959 (1918) | 737 (1474) |
| 90 | 2194 (4388) | 238 (476) | 1283 (2566) | 986 (1972) |
Values are shown as size of treated/untreated groups and total sample size in parentheses under assumption of equal allocation across groups.
Discussion
This is the largest prospectively followed cohort of recently diagnosed adult-onset isolated focal dystonia patients in whom spread has been carefully characterised. Our findings confirm that the initial site of onset in isolated focal dystonia is strongly related to the risk of spread and to the body regions subsequently affected by dystonia with BSP at onset exhibiting more than twice the risk of spread compared with those with cervical, hand or laryngeal dystonia. We further found that patients with BSP most commonly spread to the oromandibular region and neck, those with CD most commonly spread to the hand and those with hand or laryngeal dystonia most commonly spread to the neck. Additionally, our findings support that a positive family history and alcohol responsiveness are potentially associated with an increased risk of spread.
Our findings confirm retrospective studies,4 6 12 and a previous smaller prospective study of focal dystonia patients.8 We also confirm that spread occurs most often within the first several years, and that subsequent body parts affected vary depending on the site of focal dystonia onset.4 6 11 17 In contrast to previous studies, however, we found a lower risk of spread in those with CD onset compared with prior estimates that range from 12% to 38%.4 6 10 12 The lower spread risk in our study may be attributable to recall bias in retrospective studies in patients who may have initially been affected by BSP but had subclinical or non-bothersome symptoms. Another possibility is that neck muscles that have an effect on the shoulder, upper arm and torso (such as the levator scapulae, scalene and trapezius muscles) may have been reported as new sites of dystonia involvement leading to higher rates of spread in some studies. Although we also found a lower risk of spread for patients with laryngeal dystonia onset than reported in a recent large registry study,13 our estimates of spread risk in laryngeal and hand dystonia are similar to prior retrospective studies.4 6 8 10 The difference in spread risk among the dystonia phenotypes may indicate that the underlying pathogenesis differs across the distinct forms of focal dystonia.
The risk of spread in isolated focal dystonia across the different phenotypes did not relate to sex or age of onset in our analysis. Some studies have suggested that age at onset relates to risk of spread, but this relationship is more pronounced in those presenting with dystonia before age 20 where the probability of an underlying genetic aetiology is high.8 Our inclusion of only those with focal dystonia starting after age 18 may explain why we did not see an association between onset age and risk of dystonia spread. In keeping with this idea, we found that a positive family history of dystonia related to an increased risk of spread (HR=2.18, p=0.012). This is in line with a smaller retrospective study involving patients with BSP, CD and hand dystonia in which family history predicted spread of dystonia.10 Familial isolated dystonia has previously been found to have a higher tendency to spread than sporadic cases.5 Thus, our findings suggest that the risk of spread in adult-onset focal dystonia may be more dependent on genetic factors that are not influenced by the age or sex of the individual.
Clinical features including tremor predominant dystonia, effectiveness of sensory tricks and alcohol responsivity were evaluated for their potential association with risk of dystonia spread. The presence of tremor related to an increased risk of spread in a recent long-term follow-up study of different primary focal dystonia patients,10 whereas in a separate retrospective study tremor did not relate to risk of spread in patients with CD.9 Our results suggest the presence of tremor-dominant dystonia is associated with an increased risk of spread, but this relationship did not reach our significance threshold (HR=1.98, p=0.061). In a prior retrospective analysis of Dystonia Coalition data, we found that sensory tricks occur less commonly in patients with CD who had involvement of dystonia beyond the neck,11 but in the current prospective study of CD and other phenotypes of focal dystonia we did not find an association between the presence of a sensory trick and risk of dystonia spread. Alcohol responsiveness was the only other clinical feature that associated with an increased risk of spread (HR=2.59, p=0.009). We recently reported that alcohol responsiveness in isolated focal dystonia correlated with a family history of a movement disorder.18 Together with our current results, this suggests that patients with an underlying genetic contribution to their dystonia may be more likely to spread and exhibit a response to alcohol.
The ability to slow or halt progression of isolated focal dystonia in patients is of great clinical interest. As our understanding of the neurobiological abnormalities underlying isolated dystonia advances, novel disease-modifying therapies are being developed. Our prospective data here permit calculations of the estimated sample sizes that will be required in a clinical trial to demonstrate efficacy of such a potential therapy in reducing spread in patients presenting with BSP, CD, hand or laryngeal dystonia. Development of a therapy that reduces the risk of dystonia spread could reduce suffering from the disability, pain, psychiatric symptoms and impaired quality of life associated with different focal dystonia phenotypes.19–24 Alternatively, our prospective data could permit calculations for power analyses of any study that wants to compare the risk of spread across two focal dystonia subgroups, for example, determining whether selected epigenetic phenomena influences spread risk. Importantly, our sample size estimates support that disease-modifying clinical trials and studies of factors that influence disease progression in isolated focal dystonia are feasible, with the fewest participants needed for studies targeting disease modification of BSP.
Although our study leveraged an international, multicentred study and is the largest of its kind, it is nevertheless limited by small sample sizes of hand and laryngeal dystonia groups. Our study is also weighted toward subjects who were evaluated at specialty tertiary centres and possibly have more severe forms of dystonia, potentially affecting the baseline demographics of study participants and limiting the generalisability of our results. Additionally, because we excluded patients who had evidence on examination of dystonia affecting more than one body region at the baseline visit, and included patients presenting with hand dystonia irrespective of their particular form of dystonia (eg, writer’s cramp, musician’s dystonia, etc), the demographics of our patient cohort may differ from the findings of other studies reporting epidemiology of isolated focal dystonia. As the symptoms of isolated focal dystonia at onset can be mild and may precede diagnosis by years,25 recall bias is another potential limitation in our study. Similarly, recall bias should be considered for family history of dystonia and response of dystonia to alcohol, as these were not independently confirmed. Finally, the overall length of follow-up limits our study. Additional prospective assessments are needed to better define the risk and pattern of dystonia spread over longer periods of time and more rigorously assess the clinical and demographic characteristics associated with spread risk such as the non-motor features of dystonia including pain, anxiety and depression. Furthermore, additional investigations are needed to determine if dystonia spread is correlated with measures of disability and quality of life.
In summary, we found that patients with BSP experience spread much more frequently than those with cervical, hand and laryngeal dystonia, and that the majority of those who spread did so within the first several years after diagnosis. CD most commonly spreads to the upper extremity and BSP most commonly spreads to the oromandibular region. Those presenting with hand and laryngeal dystonia, as well as those with BSP at onset, frequently spread to the neck muscles. Additionally, a positive family history and self-reported alcohol responsiveness were associated with an increased risk of dystonia spread. Our findings highlight some of the similarities and differences in spread characteristics based on the initially affected muscles in those who develop isolated adult-onset focal dystonia. This knowledge can be used to help appraise clinical prognosis, guide investigations into the pathogenesis of dystonia spread and inform future investigation into disease-modifying therapies.
Acknowledgments
We thank Gamze Kilic Berkmen, PhD, for her assistance with managing and accessing the Dystonia Coalition database.
Footnotes
Correction notice: This paper has been corrected since it was published online first. Author name
'Emmanuel Rose' has been corrected to 'Emmanuel Roze'
Contributors: BDB and CLG: Project conception, design, organisation and execution; recruitment and clinical assessment of subjects; statistical analysis design and oversight; manuscript drafting and revision. SHS: Statistical analysis design and execution. SPR, SAN, JJ, NB, PA, RLB, AJE, JAV, CK, TB, SL, SGR, MV, CB, ER: Recruitment and clinical assessment of subjects; manuscript review and critique. HAJ and JSP: Project conception and design; recruitment and clinical assessment of subjects, manuscript review and critique.
Funding: This work was supported by the Dystonia Coalition, which receives the majority of its support through National Institutes of Health grant U54 TR0001456 (formerly NS065701) from the Office of Rare Diseases Research in the National Center for Advancing Translational Science and National Institute of Neurological Disorders and Stroke).
Competing interests: BDB: The author has received research grant support from the Dana Foundation, NIH (NIH/NCATS Colorado CTSI Grant Number KL2 TR001080), Dystonia Coalition (receives the majority of its support through NIH grant NS065701 from the Office of Rare Diseases Research in the National Center for Advancing Translational Science and National Institute of Neurological Disorders and Stroke), and from Mary Rossick Kern and Jerome H. Kern. The author also is a member of the medical advisory boards for the Benign Essential Blepharospasm Research Foundation and the National Spasmodic Torticollis Association. CLG: The author has received research grant support from the Dystonia Medical Research Foundation. SPR: Has received research grant support from NIH (NCRR/NCATS, UNM CTSC KL2 1TR001448-01 and UL1TR001449) and Dystonia Coalition Projects (NIH/NINDS/ORDR) and has received publishing royalties from Springer. Dr. Pirio Richardson serves on the Scientific Advisory Board for the Benign Essential Blepharospasm Research Foundation. SHS, SAN, JAV, SL, MV, CB and ER: The author has no relevant financial disclosures to report. JJ: Has received a travel grant of the Movement Disorder Society. NB: Has received grant support from DFG (BR4328.2-1, GRK1957), the Collaborative Center for X-linked Dystonia-Parkinsonism and the Else-Kröner Fresenius-Stifung (HA17_2017), has received speaker’s honoraria from Grünenthal, UCB and Teva, and has received non-financial support from Bayer. PA: Has received honoraria from US world Meds, Acadia, and Lundbeck. RLB: Serves as an associate editor for Neurology: Clinical Practice; performs botulinum toxin injections at the University of Rochester (30% effort); serves/has served on scientific advisory board for Allergan, Ipsen, Merz and Revance; receives research support from Vaccinex, Fox Foundation, and Revance; has received research support from NIH (NINDS, ORDR): Dystonia Coalition Projects, Site PI; holds stock options in VisualDx; and has served as an expert witness in legal proceedings including malpractice, not involving commercial entities. AJE: Has received research grants from the NIH, Great Lakes Neurotechnologies and the Michael J Fox Foundation; personal compensation as a consultant/scientific advisory board member for Abbvie, Adamas, Acadia, Acorda, Neuroderm, Impax, Sunovion, Lundbeck, Osmotica Pharmaceutical, and USWorldMeds; publishing royalties from Lippincott Williams & Wilkins, Cambridge University Press, and Springer; and honoraria from USWorldMeds, Lundbeck, Acadia, Sunovion, the American Academy of Neurology, and the Movement Disorders Society. CK: has been supported by the German Research Foundation (FOR 2488) and serves as a medical advisor to Biogen and Centogene. TB: receives funding from the German Research Foundation (FOR 2698), has received honoraria from Merz Pharmaceuticals, Allergan and Ipsen Pharma, and serves as a medical advisor to Merz Pharmaceuticals and Allergan. SGR: Has received research support from the NIH/NINDS, is a reviewer for UpToDate, and has received honoraria from the Movement Disorders Society, and served on a Data Safety Monitoring Board for Enterin. HAJ: Has active or recent grant support from the US government (National Institutes of Health), private philanthropic organizations (the Benign Essential Blepharospasm Research Foundation, Cure Dystonia Now), academically-oriented institutions (the Dystonia Study Group), and industry (Cavion Therapeutics, Ipsen Pharmaceuticals, Retrophin Inc.). Dr. Jinnah has also served on advisory boards or as a consultant for CoA Therapeutics and Retrophin Inc. He has received honoraria or stipends for lectures or administrative work from the American Academy of Neurology, the American Neurological Association, the Dystonia Medical Research Foundation, the International Neurotoxin Society, the International Parkinson’s Disease and Movement Disorders Society, The Parkinson’s Disease Foundation, and Tyler’s Hope for a Cure. Dr. Jinnah serves on the Scientific Advisory Boards for several private foundations including the Benign Essential Blepharospasm Research Foundation, Cure Dystonia Now, the Dystonia Medical Research Foundation, and Tyler's Hope for a Cure. He also is principle investigator for the Dystonia Coalition, which receives the majority of its support through NIH grant TR001456 from the Office of Rare Diseases Research at the National Center for Advancing Translational Sciences, and previously NS065701 from the National Institutes of Neurological Disorders and Stroke. The Dystonia Coalition has received additional material or administrative support from industry sponsors (Allergan Inc. and Merz Pharmaceuticals) as well as private foundations (The American Dystonia Society, Beat Dystonia, The Benign Essential Blepharospasm Foundation, Cure Dystonia Now, Dystonia Europe, Dystonia Inc., Dystonia Ireland, The Dystonia Medical Research Foundation, The Foundation for Dystonia Research, The National Spasmodic Dysphonia Association, and The National Spasmodic Torticollis Association). JSP: Has received research grant support from NIH (NCRR/NCATS, UNM CTSC KL2 1TR001448-01 and UL1TR001449) and Dystonia Coalition Projects (NIH/NINDS/ORDR) and has received publishing royalties from Springer.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available in a public, open access repository. Data are available upon reasonable request.
References
- 1. Albanese A, Bhatia K, Bressman SB, et al. . Phenomenology and classification of dystonia: a consensus update. Mov Disord 2013;28:863–73. 10.1002/mds.25475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bressman SB. Dystonia genotypes, phenotypes, and classification. Adv Neurol 2004;94:101–7. [PubMed] [Google Scholar]
- 3. Defazio G. Epidemiology of Primary and Secondary Dystonia : Stacy MA, Handbook of dystonia. New York, NY: Informa Healthcare USA, Inc, 2007: 11–20. [Google Scholar]
- 4. Weiss EM, Hershey T, Karimi M, et al. . Relative risk of spread of symptoms among the focal onset primary dystonias. Mov Disord 2006;21:1175–81. 10.1002/mds.20919 [DOI] [PubMed] [Google Scholar]
- 5. Elia AE, Filippini G, Bentivoglio AR, et al. . Onset and progression of primary torsion dystonia in sporadic and familial cases. Eur J Neurol 2006;13:1083–8. 10.1111/j.1468-1331.2006.01387.x [DOI] [PubMed] [Google Scholar]
- 6. Abbruzzese G, Berardelli A, Girlanda P, et al. . Long-Term assessment of the risk of spread in primary late-onset focal dystonia. J Neurol Neurosurg Psychiatry 2008;79:392–6. 10.1136/jnnp.2007.124594 [DOI] [PubMed] [Google Scholar]
- 7. Defazio G, Esposito M, Abbruzzese G, et al. . The Italian dystonia registry: rationale, design and preliminary findings. Neurol Sci 2017;38:819–25. 10.1007/s10072-017-2839-3 [DOI] [PubMed] [Google Scholar]
- 8. Svetel M, Pekmezović T, Jović J, et al. . Spread of primary dystonia in relation to initially affected region. J Neurol 2007;254:879–83. 10.1007/s00415-006-0457-8 [DOI] [PubMed] [Google Scholar]
- 9. Godeiro-Junior C, Felício AC, Aguiar PMdeC, et al. . Retrocollis, anterocollis or head tremor may predict the spreading of dystonic movements in primary cervical dystonia. Arq Neuropsiquiatr 2009;67:402–6. 10.1590/S0004-282X2009000300006 [DOI] [PubMed] [Google Scholar]
- 10. Svetel M, Pekmezovic T, Tomic A, et al. . The spread of primary late-onset focal dystonia in a long-term follow up study. Clin Neurol Neurosurg 2015;132:41–3. 10.1016/j.clineuro.2015.02.015 [DOI] [PubMed] [Google Scholar]
- 11. Norris SA, Jinnah HA, Espay AJ, et al. . Clinical and demographic characteristics related to onset site and spread of cervical dystonia. Mov Disord 2016;31:1874–82. 10.1002/mds.26817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Martino D, Berardelli A, Abbruzzese G, et al. . Age at onset and symptom spread in primary adult-onset blepharospasm and cervical dystonia. Mov Disord 2012;27:1447–50. 10.1002/mds.25088 [DOI] [PubMed] [Google Scholar]
- 13. Esposito M, Fabbrini G, Ferrazzano G, et al. . Spread of dystonia in patients with idiopathic adult-onset laryngeal dystonia. Eur J Neurol 2018;25:1341–4. 10.1111/ene.13731 [DOI] [PubMed] [Google Scholar]
- 14. Quartarone A, Hallett M. Emerging concepts in the physiological basis of dystonia. Mov Disord 2013;28:958–67. 10.1002/mds.25532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Defazio G, Berardelli A, Hallett M. Do primary adult-onset focal dystonias share aetiological factors? Brain 2007;130:1183–93. 10.1093/brain/awl355 [DOI] [PubMed] [Google Scholar]
- 16. Jinnah HA, Berardelli A, Comella C, et al. . The focal dystonias: current views and challenges for future research. Mov Disord 2013;28:926–43. 10.1002/mds.25567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Defazio G, Berardelli A, Abbruzzese G, et al. . Risk factors for spread of primary adult onset blepharospasm: a multicentre investigation of the Italian movement disorders Study Group. J Neurol Neurosurg Psychiatry 1999;67:613–9. 10.1136/jnnp.67.5.613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Junker J, Brandt V, Berman BD, et al. . Predictors of alcohol responsiveness in dystonia. Neurology 2018;91:e2020–6. 10.1212/WNL.0000000000006551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tomic S, Petkovic I, Pucic T, et al. . Cervical dystonia and quality of life. Acta Neurol Belg 2016;116:589–92. 10.1007/s13760-016-0634-1 [DOI] [PubMed] [Google Scholar]
- 20. Pekmezovic T, Svetel M, Ivanovic N, et al. . Quality of life in patients with focal dystonia. Clin Neurol Neurosurg 2009;111:161–4. 10.1016/j.clineuro.2008.09.023 [DOI] [PubMed] [Google Scholar]
- 21. Girach A, Vinagre Aragon A, Zis P. Quality of life in idiopathic dystonia: a systematic review. J Neurol 2019;266:2897–906. 10.1007/s00415-018-9119-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Müller J, Kemmler G, Wissel J, et al. . The impact of blepharospasm and cervical dystonia on health-related quality of life and depression. J Neurol 2002;249:842–6. 10.1007/s00415-002-0733-1 [DOI] [PubMed] [Google Scholar]
- 23. Avenali M, De Icco R, Tinazzi M, et al. . Pain in focal dystonias - A focused review to address an important component of the disease. Parkinsonism Relat Disord 2018;54:17–24. 10.1016/j.parkreldis.2018.04.030 [DOI] [PubMed] [Google Scholar]
- 24. Berman BD, Junker J, Shelton E, et al. . Psychiatric associations of adult-onset focal dystonia phenotypes. J Neurol Neurosurg Psychiatry 2017;88:595–602. 10.1136/jnnp-2016-315461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bertram KL, Williams DR. Delays to the diagnosis of cervical dystonia. J Clin Neurosci 2016;25:62–4. 10.1016/j.jocn.2015.05.054 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
An overview of the type of data that have been collected by the investigators of the Dystonia Coalition can be found at the publicly available site: http://clinportalquery.wustl.edu/pentaho/Home?userid=DYSTONIA_OPENI&password=DYSTONIA. Deidentified individual participant data from this study will be shared upon reasonable request from a qualified investigator after the Dystonia Coalition grants permission and proper IRB approval is obtained.