Abstract
Hydrogen sulfide (H2S) is a biologically-relevant molecule, and recent efforts have focused on developing small molecular donors that deliver H2S on demand. Acid-activated donors have garnered significant interest due to the potential application of such systems in myocardial ischemia injury or for suppressing tumor growth. In this work, we report a new strategy for tuning H2S delivery to a specific pH window. Specifically, we utilize self-immolative thiocarbamates with an imine-derived triggering group. After imine hydrolysis, the self-immolative decomposition releases carbonyl sulfide (COS), which is quickly hydrolyzed to H2S by carbonic anhydrase. Although acid-mediated hydrolysis results in imine cleavage, environments that are too acidic result in protonation of the aniline intermediate and results in inhibition of COS/H2S release. Taken together, this mechanism enables access to donor motifs that are only activated within specific pH windows. Here we demonstrate the design, preparation, and pH evaluation of a series of imine-based COS/H2S donor motifs, which we anticipate will have utility in investigating H2S in acidic microenvironments.
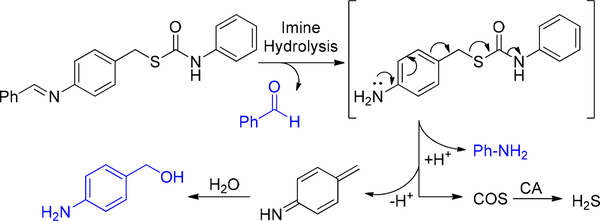
Graphical Abstract

Introduction
Hydrogen sulfide (H2S) is an important biological signaling molecule and the most recent addition to the gasotransmitter family alongside nitric oxide and carbon monoxide.1 Endogenous H2S production is primarily attributed to four main enzymes including cystathionine β-synthase (CBS), cystathionine γ-lyase (CSE), 3-mercaptopyruvate sulfur transferase (3-MST), and cysteine aminotransferase (CAT) through cysteine and homocysteine catabolism. H2S can also be generated through non-enzymatic pathways such as the thiol-mediated release of H2S from allium- and garlic-derived polysulfides.2 Once generated, H2S is involved in different physiological processes, including KATP channel activation3 and antioxidant and antiapoptotic signaling.4 In efforts to modulate H2S levels in different systems, most studies have used inorganic sulfide salts, such as sodium sulfide (Na2S) and sodium hydrosulfide (NaSH), as exogenous H2S sources. Although these sulfide sources increase endogenous H2S levels, the large and immediate dose can result in unwanted toxicity and sulfide oxidation.5 To better understand and leverage H2S levels in various biological systems, a variety of chemical tools have been developed in the last decade and include CSE and CBS inhibitors, H2S donors, and activity-based H2S probes for H2S detection.6,7,8
Of the wide array of available H2S donors, one common strategy to engineer H2S release is to leverage water- or acid-mediated hydrolysis. For example, GYY4137 is one of the most widely used synthetic H2S donors and releases H2S slowly upon hydrolysis in water at physiological pH (Figure 1a).9 Similarly, deprotection of certain thioacetals10–11 generates an unstable gem-dithiol intermediate that is hydrolyzed through an acid-mediated mechanism to release H2S. In more recent examples, JK donors, which are based on the phosphorothioate core of GYY4137, were developed and designed to undergo an intramolecular cyclization upon protonation of the P-S moiety.12 Highlighting potential applications of pH-activated donors, the JK family of donors showed cytoprotective effects in cell models of oxidative damage and cardioprotective effects in an in vivo mouse model of myocardial ischemia-reperfusion injury. More broadly, mildly-acidic pH environments are found during ischemia injury, within the extracellular environment of cancerous cells, and in certain subcellular compartments, such as the lysosome. These acidic environments, when taken in combination with prior work showing the beneficial effects of H2S in myocardial ischemia-reperfusion injury13 and the ability to induce cell cycle arrest to suppress tumor growth14 suggest potential application for acid-activated H2S donors. Aligned with these potential opportunities, a key need remains developing chemistry that enables access to donors that can be activated in specific pH ranges rather than just at increasing rates at more acidic pH. Such a strategy could be useful in developing design strategies for oral administration of hydrolysis-based H2S donor motifs.
Figure 1.
(a) Representative examples of current acid-labile H2S donors. (b) Design of pH-dependent COS/H2S release from caged thiocarbamate scaffolds.
To address this challenge, we viewed that triggerable caged thiocarbamates could be modified to develop donor motifs activated within a specific pH range. Caged thiocarbamates have recently emerged as a highly tunable class of donors that undergo a triggered, self-immolative elimination to release carbonyl sulfide (COS), which is rapidly hydrolyzed to H2S by the ubiquitous enzyme carbonic anhydrase (CA).15,16–17 Examples of triggers employed within this scaffold include reactive oxygen species,18–20 esterases,21–23 light,24–26 and cysteine.27 We envisioned that using a pH sensitive group, such as an imine, as the trigger could be used to develop acid-triggered COS/H2S donors (Figure 1b). Here we demonstrate that use of an imine trigger, when coupled to the mechanism of 1,6-elimination required in the self-immolative thiocarbamates, provides donors that respond within a specific pH window. This new class of donors improves on the pH activation specificity of current acid-labile donors by providing H2S delivery within a specific pH range. We expect this pH activation specificity will be useful in different applications requiring compound stability in strongly acidic or basic environments prior to H2S release.
Results and Discussion
Donor design
To develop donor motifs that are activated within a specific pH range, we chose to use an imine as the acid-sensitive trigger because imines are readily hydrolyzed under acidic conditions and have been used previously to initiate the 1,6-benzyl elimination of carbamate-containing prodrugs.28 In our designed system, imine cleavage would generate a p-aminobenzylcarbamothioate intermediate, which would undergo a subsequent 1,6-elimination to release COS (Scheme 1). Although imine hydrolysis is acid-mediated, if the solution is too acidic, then the resultant aniline intermediate will be protonated, which will inhibit the 1,6-elimination and should decrease the rate of COS release. Similarly, the imine should be stable under basic conditions and not release COS. Combining these parameters, we expected that the efficiency of the imine-based donor would peak at pH values between the pKa of the iminium (pKa ~5–7) and anilinium ion (pKa ~4.6). In contrast to other available acid- or hydrolysis-based donors, this would result in a pH window for activation rather than a direct dependence of release rate with pH.
Scheme 1.
Proposed pH-dependent COS/H2S-Release pathway: Basic conditions decrease the rate of imine hydrolysis. Strongly acidic conditions protonate the aniline intermediate and prevent COS release.
Synthesis
To test our hypothesis that the acid-triggered thiocarbamate donors undergo a pH-dependent release, we prepared a pH sensitive imine-containing thiocarbamate donor. Treatment of 4-aminobenzyl alcohol with benzaldehyde in the presence of acetic acid formed 4-(benzylideneamino)benzyl alcohol, which was coupled with phenyl isothiocyanate in the presence of sodium hydride to obtain the O-alkyl thiocarbamate isomer (Scheme 2a). Although analogous O-alkyl thiocarbamate donors have been prepared and are stable under ambient conditions, we found that the O-alkyl thiocarbamate with the imine trigger isomerized to the S-alkyl pH-sensitive thiocarbamate (S-pHTCM) isomer both in solution and also in the solid state (Figure S1). Thione-thiol isomerization is well known to occur in thiocarbamates via the Newman-Kwart rearrangement,29,30 but typically requires high temperatures or catalysts.31,32 Similarly, benzylic Newman-Kwart rearrangements have recently been reported to occur at elevated temperatures.33 We hypothesize the electron-donating imine substituent aids in the stabilization of the benzylic carbocation intermediate formed in the thiocarbamate rearrangement and thus may facilitate this isomerization under more mild conditions.
Scheme 2.
(a) Synthetic scheme for acid-triggered thiocarbamate COS/H2S donor (S-pHTCM), (b) carbamate control compound (pHCM), and (c) triggerless control compound (S-TCM).
In addition to S-pHTCM, the corresponding pH-sensitive carbamate (pHCM) and triggerless S-alkyl thiocarbamate (S-TCM) were prepared as control compounds to confirm that the COS/H2S release is triggered by imine hydrolysis of the thiocarbamate. In contrast to the model thiocarbamate, pHCM should undergo the same imine hydrolysis to release CO2 instead of COS while generating the same byproducts as S-pHTCM. In the absence of the imine trigger, S-TCM is not expected to decompose and release COS.
Measurement of H2S-release
To evaluate COS/H2S release from this series of compounds, we used the colorimetric methylene blue (MB) assay.34 We measured COS/H2S release from S-pHTCM (50 μM) across a range of pH values (4.0–8.0) in the presence of CA (25 μg/mL) at 37 °C. We confirmed CA activity at pH 5.5 using a p-nitrophenyl acetate assay (see Figure S2). To span this range of pH, we used sodium citrate buffered and phosphate buffered saline (PBS) solutions. We observed no buffer dependence when evaluating H2S release from S-pHTCM in citrate and PBS buffer at pH 6.0 as shown in Figure S3. Consistent with our expectations, we observed more efficient COS/H2S release in weakly-acidic conditions from pH 4.6 to 6.5 than from pH values outside of this range (Figure 2). The rates of H2S release have a similar pH dependence, but because the rate limiting step of the reaction may change as a function of pH and not all the H2S release curves peaked at the same levels, we chose to measure H2S concentration at 5 hours for these investigations (Figure S4). Further supporting our hypothesis, we found that the inflection points of the pH response curve (4.3 and 7.3) matched the expected pKa values of the iminium and the anilinium ions (Figure S5). Taken together, these data support the mechanism of COS/H2S release outlined in Scheme 1.
Figure 2.
(a) COS/H2S release from S-pHTCM (50 μM) at pH 4.0 – 8.0 containing CA (25 μg/mL). (b) pH curve of H2S concentration at 5 h. Experiments were performed in quadruplicate with results expressed as mean ± S.D. (n = 4).
To confirm that H2S release from S-pHTCM requires both imine hydrolysis and the thiocarbamate moiety, we measured the H2S release from the pHCM and S-TCM control compounds at pH 5.5. Under identical conditions as those for S-pHTCM we failed to observe H2S generation from either pHCM or S-TCM (Figure 3). In the absence of CA, S-pHTCM showed a significantly reduced rate of H2S release (Figure S6). To further support the proposed release mechanism, we monitored the reaction by HPLC analysis (Figure S7 and S8). Consistent with the proposed 1,6-elimination mechanism, we observed benzaldehyde, aniline, and 4-aminobenzyl alcohol during the course of the reaction (Scheme 3).
Figure 3.
H2S release from S-pHTCM and triggerless (S-TCM) and carbamate (pHCM) control compounds (50 μM) in citrate buffer (10 mM, pH 5.5) containing CA (25 μg/mL) at 37 °C. Experiments were performed in quadruplicate with results expressed as mean ± S.D. (n = 4)
Scheme 3.
Proposed mechanism of acid-triggered COS/H2S release from caged-thiocarbamate donors with byproducts of self-immolation.
We next investigated the tolerance of the acid-mediated cleavage and self-immolation to the presence of different biological nucleophiles by measuring H2S release from S-pHTCM at pH 5.5 in the presence of different analytes. We did not observe a significant change in donor efficiency in the presence of 250 μM of GSH, Cys, Lys, Ser, Hcy, Gly, or GSSG, which demonstrates the compatibility of our approach with common nucleophiles and reactive species (Figure 4). In the presence of higher levels of GSH (5 mM), however, we did observe modest inhibition. We do not view this as a significant problem, however, because about 90% of GSH is localized in the cytosol, about 10% is localized in the mitochondria and the endoplasmic reticulum, leaving negligible GSH levels in acidic cellular compartments.35 To further investigate whether this observed inhibition was observed at physiological pH values, we repeated the measurement at pH 7.4 in the presence of 5 mM GSH and did not observe a significant difference in efficiency in the absence or presence of GSH (Figure S9). These data suggest that high concentrations of GSH should not interfere with application of this triggering motif in normal cellular environments.
Figure 4.
COS/H2S release at 5 h from S-pHTCM (50 μM) in citrate (pH 5.5) in the presence of CA (25 μg/mL) and various analytes (250 μM unless otherwise noted): no analyte, GSH (5 mM), GSH, Cys, Lys, Ser, Hcy, Gly, and GSSG. Experiments were performed in triplicate with results expressed as mean ± S.D. (n = 3).
Although our primary goal was to develop chemistry that enabled donor response within a specific pH range, we also wanted to demonstrate the feasibility of appending the developed motif to commonly-used subcellular targeting groups that direct compounds to acidic subcellular compartments. To demonstrate this compatibility, we prepared a thiocarbamate donor (Lyso-pHTCM), as well as the associated control compounds (Lyso-pHCM and Lyso-TCM), with an aminoethyl-morpholine group, which has been used previously to direct compounds to the lysosome. We measured the H2S release efficiency from the compounds and found that Lyso-pHTCM showed a 33% H2S release efficiency over 5 h and that the control compounds did not release H2S (Figure 5). Although beyond the scope of the present investigations, we anticipate that these compounds may be of use in investigating the role of lysosomal H2S delivery in various contexts.
Figure 5.
(a) H2S release comparison of Lyso-pHTCM and S-pHTCM. (b) H2S release from Lyso-pHTCM and related control compounds. All experiments performed in quadruplicate in citrate buffer (pH 5.5, 10 mM) containing CA (25 μg/mL) at 37 °C. Results expressed as a mean ± S.D. (n = 4).
Conclusions
Based on the broad utility of hydrolysis- and acid-sensitive H2S donors, we developed a new strategy that enables for the efficiency of H2S release to be tuned to specific pH ranges. This response profile is in contrast to currently-available acid-mediated H2S donor motifs. By including an acid-sensitive imine group on a caged thiocarbamate, we demonstrated that the acid-mediated imine hydrolysis in combination with inhibitory protonation of aniline product after hydrolysis resulted in an operative pH response range of 4.6 – 6.5. The imine-based donor also shows good tolerance to a wide array of biological nucleophiles at physiologically-relevant concentrations. We also demonstrated the modularity of our approach by attaching a common lysosomal targeting group to the donor motif and demonstrated that H2S release is still efficient in this targeted construct. Overall, we anticipate that this approach will provide new opportunities to tune donor motifs to respond to specific pH windows associated with subcellular organelles and that the developed chemistry will enable specific pH windows to be targeted to other acidic microenvironments.
Experimental Section
Materials and methods
Reagents were purchased from Sigma-Aldrich, Tokyo Chemical Industry (TCI), Fisher Scientific, and VWR. Column chromatography was performed with 230–400 mesh silica gel. Deuterated solvents were purchased from Cambridge Isotope Laboratories. 1H and 13C{1H} NMR spectra were recorded on a Bruker 500 MHz or Varian Inova 500 MHz instrument. Chemical shifts are reported relative to residual protic solvent resonances. Air-free experimental procedures were performed in an Innovative Atmospheres N2-filled glove box or using Schlenk technique. UV-Vis spectra were acquired on an Agilent Cary 60 UV-Vis spectrometer. Mass spectrometric data was acquired by the University of Illinois, Urbana Champaign MS Facility, or on a Xevo Waters ESI LC/MS instrument.
General Procedure for H2S Detection
In an N2-filled glovebox, scintillation vials with septa caps were filled with 20 mL of degassed buffer (citrate/PBS, pH 4.0–8.0, 10 mM). All stock solutions were prepared in the glovebox with degassed solvents. The 10 mM donor stock solution was prepared in DMSO, and a 10 mg/mL carbonic anhydrase (CA) stock solution was prepared in Millipore H2O. Each scintillation was charged with 50 μL of the CA stock solution to achieve a final concentration of 25 μg/mL. The solutions were thermally equilibrated to 37 °C while stirring for 20 min. During this time, methylene blue cocktail solutions (0.3 mL) were prepared in 1.5 mL disposable cuvettes. The methylene blue cocktail solutions contained 60 μL of 1% (w/v) Zn(OAc)2, 120 μL of 30 mM FeCl3 in 1.2 M HCl, and 120 μL of 20 mM N,N-dimethyl-p-phenylene diamine in 7.2 M HCl. To each solution, 100 μL of the 10 mM donor stock solution was added to reach a final concentration of 50 μM. Immediately after donor addition at t = 0 min, a 0.3 mL reaction aliquot was removed and added to a methylene blue cocktail solution. This process was repeated at 5, 10, 15, 30, 60, 90, 120, 180, 240, and 300 min time points. The reaction aliquots added to the methylene blue cocktail solutions were mixed thoroughly and incubated for 1 h at room temperature in the dark, after which the absorbance values at 670 nm were measured.
MB Assay Calibration Curve
The methylene blue cocktail solution (0.5 mL) and PBS pH 7.4 buffer (0.5 mL) was added to 1.5 mL disposable cuvettes. A NaSH stock solution (100 mM) was prepared in degassed Millipore H2O under an inert atmosphere and diluted to 1 mM. Immediately after dilution, aliquots of the NaSH stock solution were added to the methylene blue solutions to reach final concentrations of 10, 20, 30, 40, and 50 μM. The solutions were mixed and incubated at room temperature for 1 h, after which the absorbance values at 670 nm were measured.
Syntheses
4-(Benzylideneamino)benzyl alcohol
(Modified from previous report.)28 4-Aminobenzyl alcohol (0.50 g, 4.1 mmol) and benzaldehyde (0.82 mL, 8.2 mmol) were added to anhydrous THF (15 mL) in the presence of MgSO4 (~1g). Glacial AcOH was then added dropwise (~4 drops) and the resultant reaction mixture was refluxed for 2 h. The reaction was then quenched with a solution of saturated NaHCO3 (30 mL) and extracted with EtOAc (3 × 15mL). The organic layers were combined, washed with deionized H2O (30 mL) and brine (30 mL), and dried over MgSO4. The drying agent was removed by filtration, and the organic solvent was removed under reduced pressure. The product was isolated and purified by column chromatography using EtOAc/Hexanes (10–100% gradient; 5% NEt3) to yield a yellow solid (343 mg, 68% yield). 1H NMR (500 MHz, DMSO-d6) δ: 8.63 (s, 1H), 8.02 – 7.87 (m, 2H), 7.59 – 7.48 (m, 3H), 7.36 (d, J = 8.2 Hz, 2H), 7.24 (d, J = 8.2 Hz, 2H), 5.18 (t, J = 5.7 Hz, 1H), 4.51 (d, J = 5.7 Hz, 2H).13C{1H} NMR (126 MHz, DMSO-d6) δ: 160.1, 149.9, 140.5, 136.1, 131.4, 128.8, 128.6, 127.3, 120.8, 62.6. HRMS (ASAP TOF) (m/z): [M + H]+ cacld for C14H14NO, 212.1075; found, 212.1090.
S-4-(Benzylideneamino)benzyl phenylcarbamothioate (S-pHTCM)
4-(Benzylideneamino)-benzyl alcohol (60.0 mg, 0.284 mmol) and phenyl isothiocyanate (41 μL, 0.34 mmol) were combined in anhydrous THF (15 mL). The reaction mixture was cooled to 0 °C before adding NaH (18 mg, 0.75 mmol). The reaction mixture was then stirred at room temperature for 2 h. The solvent was removed under reduced pressure, and the crude mixture was purified by column chromatography with EtOAc/Hexanes (4–34% gradient; 5% NEt3). The product was isolated as a yellow solid, which isomerizes from the O-alkyl to S-alkyl isomer over 3 days in the solid state (73 mg, 74% yield). 1H NMR (500 MHz, DMSO-d6) δ: 10.35 (s, 1H), 8.62 (s, 1H), 8.02–7.82 (m, 2H), 7.59–7.44 (m, 5H), 7.41 (d, J = 8.2 Hz, 2H), 7.31 (dd, J = 8.4, 7.2 Hz, 2H), 7.23 (d, J = 8.2 Hz, 2H), 7.05 (t, J = 7.4 Hz, 1H), 4.19 (s, 2H). 13C{1H} NMR (126 MHz, DMSO-d6) δ: 164.3, 160.6, 150.3, 138.9, 136.6, 136.0, 131.5, 129.6, 128.9, 128.8, 128.6, 123.4, 121.1, 119.0, 32.6. HRMS (ES+ TOF) (m/z): [M + H]+ cacld for C21H19N2OS, 347.1218; found, 347.1212.
4-(Benzylideneamino)benzyl phenylcarbamate (pHCM)
4-(Benzylideneamino)benzyl alcohol (61 mg, 0.28 mmol) and phenyl isocyanate (31 μL, 0.28 mmol) were added to anhydrous THF (15 mL). The reaction mixture was cooled to 0 °C before addition of NEt3 (0.50 mL, 3.6 mmol), after which the reaction mixture was refluxed for 2 h. The reaction was quenched with brine (30 mL) and extracted with EtOAc (3 × 15 mL). The organic layers were combined, washed with deionized H2O (30 mL) and brine (30 mL), and dried over MgSO4. The drying agent was removed by filtration and the solvent was removed under reduced pressure. The crude mixture was purified by column chromatography with EtOAc/Hexanes (4–34% gradient; 5% NEt3) to afford the product as a white solid (41 mg, 44%). 1H NMR (500 MHz, DMSO-d6) δ: 9.76 (s, 1H), 8.63 (s, 1H), 7.94 (dd, J = 7.3, 2.2 Hz, 2H), 7.63–7.37 (m, 7H), 7.37–7.20 (m, 4H), 6.99 (t, J = 7.4 Hz, 1H), 5.17 (s, 2H). 13C{1H} NMR (126 MHz, DMSO-d6) δ: 161.0, 153.4, 151.3, 139.1, 136.0, 134.3, 131.6, 129.3, 128.8, 128.8, 128.7, 122.4, 121.1, 118.2, 65.5. HRMS (ES+ TOF) (m/z): [M + H]+ cacld for C21H19N2O2, 331.1447; found, 331.1435.
S-Benzyl phenylcarbamothioate (S-TCM)
Benzyl mercaptan (61 μL, 0.52 mmol) and phenyl isocyanate (58 μL, 0.51 mmol) were combined in anhydrous THF (15 mL). The reaction mixture was cooled to 0 °C before adding NEt3 (0.1 mL), after which the reaction was stirred at room temperature for 1 h. The reaction was then quenched with brine (30 mL) and extracted with EtOAc (3×15mL). The organic layers were combined and dried over MgSO4. The organic layer was filtered, and the solvent was removed under reduced pressure. The product was isolated by column chromatography using EtOAc/Hexanes (4–34% gradient; 5% NEt3) to afford the product as a white solid (120 mg, 92% yield). 1H NMR (500 MHz, DMSO-d6) δ: 10.33 (s, 1H), 7.55–7.46 (m, 2H), 7.39 – 7.20 (m, 7H), 7.05 (t, J = 7.4 Hz, 1H), 4.15 (s, 2H). 13C{1H} NMR (126 MHz, DMSO-d6) δ: 164.3, 138.9, 138.7, 128.9, 128.7, 128.4, 127.0, 123.4, 119.0, 32.9. HRMS (ASAP TOF) (m/z): [M + H]+ cacld for C14H14NOS, 244.0796; found, 244.0791.
4-(2-Aminoethyl)morpholine phenyl isothiocyanate
4-(2-Morpholinoethyl) aniline (61 mg, 0.30 mmol) was added to anhydrous CH2Cl2 (20 mL). The reaction mixture was cooled to °C, then a solution of 1,1′-thiocarbonyldiimidazole (53 mg, 0.30 mmol) in anhydrous CH2Cl2 (5 mL) was added dropwise. The reaction was stirred at room temperature for 2 h, after which the solvent was removed under reduced pressure. The crude mixture was purified by column chromatography with MeOH/CH2Cl2 (2–20% gradient) and isolated as a yellow oil (180 mg, 86% yield). 1H NMR (500 MHz, CDCl3) δ 7.22 – 7.11 (m, 4H), 3.73 (t, J = 4.6 Hz, 4H), 2.79 (dd, J = 9.7, 6.3 Hz, 2H), 2.57 (dd, J = 9.6, 6.4 Hz, 2H), 2.50 (t, J = 4.6 Hz, 4H). 13C{1H} NMR (126 MHz, CDCl3) δ 140.0, 135.0, 130.0, 129.3, 125.9, 67.1, 60.5, 53.8, 33.1. HRMS (ASAP TOF) (m/z): [M + H]+ cacld for C13H17N2OS, 249.1062; found, 249.1078.
S-4-(Benzylideneamino)benzyl(4-(2-morpholinoethyl)phenyl) carbamothioate (Lyso-pHTCM)
4-(Benzylideneamino)benzyl alcohol (100 mg, 0.473 mmol) and morpholine isothiocyanate (118 mg, 0.473 mmol) were added to anhydrous THF (20 mL). The reaction mixture was cooled to 0 °C before addition of NaH (18 mg, 0.75 mmol) and then stirred for 10 h at room temperature. The solvent was removed under reduced pressure. The crude mixture was purified by column chromatography with EtOAc/Hexanes (8–66% gradient; 5% NEt3) to afford the product as a white solid that isomerizes from the O-alkyl to S-alkyl isomer over 4 days (77 mg, 36%).1H NMR (500 MHz, DMSO-d6) δ: 10.26 (s, 1H), 8.61 (s, 1H), 7.95–7.89 (m, 2H), 7.56–7.48 (m, 3H), 7.43–7.37 (m, 4H), 7.22 (d, 2H), 7.15 (d, 2H), 4.17 (s, 2H), 3.56 (d, J = 5.0 Hz, 4H), 2.67 (t, J = 7.7 Hz, 2H), 2.46 (t, 1H), 2.40 (s, 4H). 13C{1H} NMR (126 MHz, DMSO-d6) δ: 164.6, 161.0, 150.7, 137.3, 137.1, 136.5, 136.0, 132.0, 130.1, 129.5, 129.3, 129.1, 121.6, 119.6, 66.7, 60.5, 53.7, 33.1, 32.3. HRMS (ES+ TOF) (m/z): [M + H]+ cacld for C27H30N3O2S, 460.2059; found, 460.2076.
S-4-(Benzylideneamino)benzyl(4-(2-morpholinoethyl)phenyl) carbamate (Lyso-pHCM)
4-(2-Morpholinoethyl) aniline (61 mg, 0.30 mmol) and triphosgene (41 mg, 0.36 mmol) were combined in anhydrous CH2Cl2 (15 mL). The reaction was cooled to 0 °C and NEt3 (0.2 mL, 5 equiv.) was added. After stirring at 0 °C for 2 h, the reaction mixture was purged with N2 before adding a solution of phenyl imine benzyl alcohol (74 mg, 0.35 mmol) in anhydrous CH2Cl2 (5 mL) dropwise. The reaction was stirred at room temperature for 2 h, after which the solvent was removed under reduced pressure. The crude mixture was purified by column chromatography with EtOAc/Hexanes (12–100% gradient; 5% NEt3) to yield the product as a white solid (70.1 mg, 54% yield). 1H NMR (500 MHz, DMSO-d6) δ: 9.66 (s, 1H), 8.63 (s, 1H), 7.98–7.90 (m, 2H), 7.57–7.50 (m, 3H), 7.50–7.44 (m, 2H), 7.37 (d, J = 8.1 Hz, 2H), 7.33–7.26 (m, 2H), 7.17–7.09 (m, 2H), 5.15 (s, 2H), 3.56 (t, J = 4.7 Hz, 4H), 2.66 (dd, J = 9.3, 6.4 Hz, 2H), 2.46 (dd, J = 9.2, 6.5 Hz, 2H), 2.40 (s, 4H). 13C{1H} NMR (126 MHz, DMSO-d6) δ: 160.9, 153.4, 151.2, 136.9, 135.9, 134.4, 134.3, 131.6, 129.2, 128.9, 128.8, 128.7, 121.0, 118.2, 66.2, 65.4, 60.2, 53.3, 31.8. HRMS (ASAP_TOF) (m/z): [M + H]+ cacld for C27H30N3O3, 444.2287; found, 444.2282.
S-Benzyl(4-(2-morpholinoethyl)phenyl) carbamothioate (Lyso-TCM)
4-(2-Morpholinoethyl) aniline (61 mg, 0.29 mmol) and triphosgene (41 mg, 0.35 mmol) were combined in anhydrous CH2Cl2 (15 mL). The reaction mixture was cooled to 0 °C and NEt3 (0.20 mL, 1.5 mmol) was added. The reaction mixture was warmed from 0 °C to room temperature and stirred overnight, after which it was purged with N2 before adding benzyl mercaptan (41 μL, 0.35 mmol) dropwise. The reaction mixture was stirred at room temperature for 10 h. The solvent was removed under reduced pressure, and the product was purified by column chromatography with MeOH/CH2Cl2 2–20% gradient to afford the product as a yellow solid (41 mg, 39% yield). 1H NMR (500 MHz, DMSO-d6) δ 10.25 (s, 1H), 7.43–7.28 (m, 6H), 7.27–7.21 (m, 1H), 7.18–7.12 (m, 2H), 4.14 (s, 2H), 3.56 (t, J = 4.7 Hz, 4H), 2.67 (t, 2H), 2.47 (t, J = 8.0 Hz, 1H), 2.40 (s, 4H). 13C: NMR (126 MHz, DMSO-d6) δ: 164.6, 139.3, 137.3, 129.5, 129.2, 128.9, 127.4, 119.5, 66.7, 60.5, 55.4, 53.7, 40.5, 33.4, 32.3. HRMS (ES+ TOF) (m/z): [M + H]+ cacld for C20H25N2O2S, 357.1637; found, 357.1641.
Supplementary Material
ACKNOWLEDGMENT
This work was supported by the NIH (MDP; R01GM113030), NSF/GRFP (AKG; DGE-1842486), and the Dreyfus Foundation. The NMR and MS facilities in CAMCOR are supported through NSF awards CHE-0923589 and CHE-1625529.
Footnotes
ASSOCIATED CONTENT
Supporting Information
H2S release data, HPLC data, and NMR Spectra.
References
- 1.Wang R Physiological Implications of Hydrogen Sulfide: A Whiff Exploration that Blossomed. Physiol. Rev 2012, 92, 791–896. [DOI] [PubMed] [Google Scholar]
- 2.Benavides GA; Squadrito GL; Mills RW; Patel HD; Isbell TS; Patel RP; Darley-Usmar VM; Doeller JE; Kraus DW Hydrogen sulfide mediates the vasoactivity of garlic. Proc. Natl. Acad. Sci. USA 2007, 104, 17977–17982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao W; Zhang J; Lu Y; Wang R The vasorelaxant effect of H2S as a novel endogenous gaseous KATP channel opener. EMBO J. 2001, 20, 6008–6016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calvert JW; Jha S; Gundewar S; Elrod JW; Ramachandran A; Pattillo CB; Kevil CG; Lefer DJ Hydrogen sulfide mediates cardioprotection through Nrf2 signaling. Circulation Res. 2009, 105, 365–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whiteman M; Li L; Rose P; Tan C-H; Parkinson DB; Moore PK The effect of hydrogen sulfide donors on lipopolysaccharide-induced formation of inflammatory mediators in macrophages. Antioxid. Redox Signal 2010, 12, 1147–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao Y; Biggs TD; Xian M Hydrogen sulfide (H2S) releasing agents: chemistry and biological applications. Chem. Commun 2014, 50, 11788–11805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hartle MD; Pluth MD A practical guide to working with H2S at the interface of chemistry and biology. Chem. Soc. Rev 2016, 45, 6108–6117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Powell CR; Dillon KM; Matson JB A review of hydrogen sulfide (H2S) donors: Chemistry and potential therapeutic applications. Biochem. Pharmacol 2018, 149, 110–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li L; Whiteman M; Guan YY; Neo KL; Cheng Y; Lee SW; Zhao Y; Baskar R; Tan CH; Moore PK Characterization of a novel, water-soluble hydrogen sulfide - Releasing molecule (GYY4137): New insights into the biology of hydrogen sulfide. Circulation 2008, 117, 2351–2360. [DOI] [PubMed] [Google Scholar]
- 10.Devarie-Baez NO; Bagdon PE; Peng B; Zhao Y; Park C-M; Xian M Light-induced hydrogen sulfide release from “caged” gem-dithiols. Org. Lett 2013, 15, 2786–2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shukla P; Khodade VS; SharathChandra M; Chauhan P; Mishra S; Siddaramappa S; Pradeep BE; Singh A; Chakrapani H “On demand” redox buffering by H2S contributes to antibiotic resistance revealed by a bacteria-specific H2S donor. Chem. Sci 2017, 8, 4967–4972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kang JM; Li Z; Organ CL; Park CM; Yang CT; Pacheco A; Wang DF; Lefer DJ; Xian M pH-Controlled Hydrogen Sulfide Release for Myocardial Ischemia-Reperfusion Injury. J. Am. Chem. Soc 2016, 138, 6336–6339. [DOI] [PubMed] [Google Scholar]
- 13.Elrod JW; Calvert JW; Morrison J; Doeller JE; Kraus DW; Tao L; Jiao XY; Scalia R; Kiss L; Szabo C; Kimura H; Chow CW; Lefer DJ Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proc. Natl. Acad. Sci. USA 2007, 104, 15560–15565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu D; Si W; Wang M; Lv S; Ji A; Li Y Hydrogen sulfide in cancer: friend or foe? Nitric Oxide 2015, 50, 38–45. [DOI] [PubMed] [Google Scholar]
- 15.Steiger AK; Pardue S; Kevil CG; Pluth MD Self-Immolative Thiocarbamates Provide Access to Triggered H2S Donors and Analyte Replacement Fluorescent Probes. J. Am. Chem. Soc 2016, 138, 7256–7259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Powell CR; Foster JC; Okyere B; Theus MH; Matson JB Therapeutic delivery of H2S via COS: small molecule and polymeric donors with benign byproducts. J. Am. Chem. Soc 2016, 138, 13477–13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steiger AK; Zhao Y; Pluth MD Emerging Roles of Carbonyl Sulfide in Chemical Biology: Sulfide Transporter or Gasotransmitter? Antioxid. Redox Signal 2018, 28, 1516–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao Y; Pluth MD Hydrogen Sulfide Donors Activated by Reactive Oxygen Species. Angew. Chem. Int. Ed 2016, 55, 14638–14642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao Y; Henthorn HA; Pluth MD Kinetic insights into hydrogen sulfide delivery from caged-carbonyl sulfide isomeric donor platforms. J. Am. Chem. Soc 2017, 139, 16365–16376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chauhan P; Jos S; Chakrapani H Reactive Oxygen Species-Triggered Tunable Hydrogen Sulfide Release. Org. Lett. 2018, 20, 3766–3770. [DOI] [PubMed] [Google Scholar]
- 21.Chauhan P; Bora P; Ravikumar G; Jos S; Chakrapani H Esterase Activated Carbonyl Sulfide/Hydrogen Sulfide (H2S) Donors. Org. Lett 2017, 19, 62–65. [DOI] [PubMed] [Google Scholar]
- 22.Steiger AK; Marcatti M; Szabo C; Szczesny B; Pluth MD Inhibition of Mitochondrial Bioenergetics by Esterase-Triggered COS/H2S Donors. ACS Chem. Biol 2017, 12, 2117–2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levinn CM; Steiger AK; Pluth MD Esterase-Triggered Self-Immolative Thiocarbamates Provide Insights into COS Cytotoxicity. ACS Chem. Biol 2019, 14, 170–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao Y; Bolton SG; Pluth MD Light-Activated COS/H2S Donation from Photocaged Thiocarbamates. Org. Lett 2017, 19, 2278–2281. [DOI] [PubMed] [Google Scholar]
- 25.Sharma AK; Nair M; Chauhan P; Gupta K; Saini DK; Chakrapani H Visible-Light-Triggered Uncaging of Carbonyl Sulfide for Hydrogen Sulfide (H2S) Release. Org. Lett 2017, 19, 4822–4825. [DOI] [PubMed] [Google Scholar]
- 26.tacko P; Muchová L; Vítek L; Klán P Visible to NIR light photoactivation of hydrogen sulfide for biological targeting. Org. Lett 2018, 20, 4907–4911. [DOI] [PubMed] [Google Scholar]
- 27.Zhao Y; Steiger AK; Pluth MD Cysteine-activated hydrogen sulfide (H2S) delivery through caged carbonyl sulfide (COS) donor motifs. Chem. Commun 2018, 54, 4951–4954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Müller IA; Kratz F; Jung M; Warnecke A Schiff bases derived from p-aminobenzyl alcohol as trigger groups for pH-dependent prodrug activation. Tetrahedron Lett. 2010, 51, 4371–4374. [Google Scholar]
- 29.Lloyd-Jones GC; Moseley JD; Renny JS Mechanism and application of the Newman-Kwart O→ S rearrangement of O-aryl thiocarbamates. Synthesis 2008, 2008, 661–689. [Google Scholar]
- 30.Zonta C; De Lucchi O; Volpicelli R; Cotarca L, Thione–Thiol Rearrangement: Miyazaki–Newman–Kwart Rearrangement and Others In Sulfur-Mediated Rearrangements II, Springer: 2006; pp 131–161. [DOI] [PubMed] [Google Scholar]
- 31.Perkowski AJ; Cruz CL; Nicewicz DA Ambient-Temperature Newman-Kwart Rearrangement Mediated by Organic Photoredox Catalysis. J. Am. Chem. Soc 2015, 137, 15684–15687. [DOI] [PubMed] [Google Scholar]
- 32.Pedersen SK; Ulfkjaer A; Newman MN; Yogarasa S; Petersen AU; Solling TI; Pittelkow M Inverting the Selectivity of the Newman-Kwart Rearrangement via One Electron Oxidation at Room Temperature. J. Org. Chem 2018, 83, 12000–12006. [DOI] [PubMed] [Google Scholar]
- 33.Eriksen K; Ulfkjaer A; Solling TI; Pittelkow M Benzylic Thio and Seleno Newman-Kwart Rearrangements. J. Org. Chem. 2018, 83, 10786–10797. [DOI] [PubMed] [Google Scholar]
- 34. The MB assay is generally not suitable for use with acid-labile H2S donors because of the strongly acidic conditions required during the assay, but we expected that such strongly acidic conditions would protonate the aniline intermediate and prevent release of COS/H2S.
- 35.Lu SC Regulation of hepatic glutathione synthesis: current concepts and controversies. FASEB J. 1999, 13, 1169–1183. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.