Abstract
This study was undertaken to evaluate growth-promoting effects of Pluronic F-68 (PF-68) on recalcitrant MR 219 rice callus. Our study shows that calli grown on Murashige and Skoog medium supplemented with 0.04% PF-68 significantly increased callus proliferation by 58.80% (fresh weight) and 23.98% (dry weight) while root formation from callus was enhanced by 28.57%. Enhanced callus proliferation was supported by biochemical analysis, whereby highest amount of soluble sugar (1.77 mg/mL) and protein (0.17 mg/mL) contents were recorded in calli grown on 0.04% PF-68. Furthermore, enhanced expression of sucrose synthase (2.65-folds) and NADH-dependent glutamate synthase (1.86-folds) genes in calli grown on 0.04% PF-68 also correlates with enhanced callus proliferation. In contrast, high concentration of PF-68 (0.10%) recorded highest amount of phenolic (0.74 mg/mL), flavonoid (0.08 mg/mL), and hydrogen peroxide content (0.06 mg/mL) as compared to other treatment groups indicates activation of plant defence mechanism towards stress. Similarly, high expression of 4-coumarate:CoA ligase 3 (1.28-folds), chalcone-flavonone isomerase (1.65-folds) and ascorbate peroxidase (1.61-folds) genes were observed in calli grown on 0.10% PF-68 further supports increasing stress caused by the high concentration of PF-68. Taken together, our study revealed that optimum concentration of PF-68 could improve recalcitrant rice callus proliferation via enhanced sugar metabolism and amino acid biosynthesis which are crucial towards plant growth and development. However, at high concentration, PF-68 induces stress in plant which enhance the production of secondary metabolite to maintain cellular homeostasis.
Electronic supplementary material
The online version of this article (10.1007/s13205-020-2118-5) contains supplementary material, which is available to authorized users.
Keywords: Callus growth, Pluronic F-68, Recalcitrant indica cv. MR 219, Stress response
Introduction
MR 219 cultivar is one of the most widely cultivated rice cultivars in Malaysia because of its desirable traits such as high yield, increased grain weight, and resistance towards leaf blight disease (Liew et al. 2012). Nevertheless, MR 219 cultivar is highly sensitive towards environmental stresses such as drought and salinity (Tan et al. 2017). Therefore, to overcome such limitations and to produce a more superior rice cultivar, constant improvement through genetic manipulation is required (Kok et al. 2018). To date, genetic manipulation in MR 219 cultivar remains difficult because of its recalcitrant trait towards in vitro regeneration responses (Tan et al. 2017). Similar to many recalcitrant cultivars, MR 219 cultivar suffers from poor callus proliferation, low regeneration efficiency and long regeneration period (Amandeep-Kaur et al. 2014). Hence, improvement on MR 219 cultivar growth performance is required to ensure the success of genetic manipulation on MR 219 cultivar (Low et al. 2018).
In general, rice callus proliferation is a complex metabolic process that requires various essential components such as carbohydrates, amino acids, and lipids (Stitt et al. 2010; Yap and Lai 2017). Besides, exogenous plant growth hormones and additives are also crucial to ensure the success of callus growth and differentiation (Abiri et al. 2017). Commonly used plant growth hormones for callus proliferation in rice includes 2,4-dichlorophenoxyacetic acid (2,4-d),1-naphthaleneacetic acid (NAA), indole-3-acetic acid (IAA), benzylaminopurine (BAP), thidiazuron (TDZ), and kinetin (Shahravari et al. 2010; Wani et al. 2011; Abiri et al. 2017). Supplementation of 2,4-d and kinetin were reported to be commonly used for callus proliferation in MR 219 cultivar (Abiri et al. 2017). Nonetheless, to achieve a desirable amount of callus in a short period of time remains to be a challenge for recalcitrant rice cultivar like MR 219.
Apart from plant growth hormones, rice callus growth is also highly influenced by the presence of additives such as lignosulfonate (Wan Abdullah et al. in press), nitrite (Wang et al. 2014), silicon (He et al. 2013) and many others. Pluronic F-68 (PF-68) is a non-ionic, co-polymer surfactant which has been utilized as an additive in both animal and in vitro plant cultures (Meier et al. 1999; Barbulescu et al. 2011). In animal cell suspension culture, PF-68 has been supplemented to protect and repair damaged cells from constant sparging and agitation (Meier et al. 1999). Moreover, PF-68 was also found to enhance plasma membrane permeability in animal cells (Shelat et al. 2013). Meanwhile, in plant tissue culture, PF-68 was reported to increase shoot regeneration in Citrus sinensis (Curtis and Mirkov 2012), Pyrus communis (Dashti et al. 2012), Ricinus communis (Kulathuran and Narayanasamy 2015) and Abelmoschus esculentus (Irshad et al. 2018). More importantly, PF-68 was shown to successfully improve shoot regeneration of recalcitrant Brassica napus embryos (Barbulescu et al. 2011), suggesting that PF-68 could be a good candidate for plant cell growth and regeneration improvement of recalcitrant cultivar.
To date, there are no reported studies on the growth-promoting effects of PF-68 on recalcitrant rice cultivar. Besides, the underlying growth promoting mechanism of PF-68 in plant cell remains largely unknown. Hence, our study aimed to enhance callus proliferation of recalcitrant MR 219 rice cultivar through supplementation of PF-68. In addition, biochemical assays and gene expression analysis were also performed to shed light on the possible mechanism of PF-68 in promoting rice callus growth.
Materials and methods
Plant materials
The seeds of recalcitrant MR 219 cultivar were obtained from Malaysian Agricultural Research and Development Institute (MARDI), Seberang Perai, Penang, Malaysia.
PF-68 preparation
Analytical grade of PF-68 (10%) (GIBCO) was purchased from Thermo Fisher Scientific, USA.
Seed sterilization and callus induction
Surface sterilization of the seeds was performed according to the described protocol (Lim and Lai 2017) with slight modifications. First, the seeds were de-husked and surface-sterilized with 70% (v/v) ethanol for 1 min and 50% (v/v) Clorox for 30 min. Subsequently, the seeds were washed with distilled water to remove remaining residues of ethanol and air-dried on filter paper. The sterile seeds were then stored at 4 °C until further use.
In callus induction, the sterile seeds were transferred into our previously established callus induction medium containing Gamborg’s B5 basal medium (Gamborg et al. 1968) supplemented with 10 g/L maltose, 0.1 g/L l-glutamine, 0.1 g/L l-asparagine, 0.1 g/L l-arginine, 10 mg/L NAA and 1 mg/L 2,4-d, pH 5.8 (Low et al. 2019). The seeds were placed in the dark at 25 °C for 3 weeks.
Optimisation of PF-68 treatment
The callus induced from the seedlings were standardised to 0.05 g prior to transfer onto the proliferation medium. The proliferation medium was comprised of Murashige and Skoog (MS) medium (Murashige and Skoog 1962) supplemented with 30 g/L sucrose, 0.4 g/L casein hydrolysate, 2 mg/L 2,4-D and 0.5 mg/L kinetin and different PF-68 concentrations (0.02, 0.04, 0.06, 0.08 and 0.10% w/v). The proliferation medium without PF-68 was used as a negative control. The callus was allowed to proliferate in the dark at 25 °C. After 3 weeks, data were recorded based on callus morphological changes such as callus structure, colour and root-like structure. Callus with root-like structure was scored as one regardless of the numbers of root. The fresh weight (FW) of the callus was measured and its dry weight (DW) was recorded after been dried at 50 °C in an oven for 4 days. The measurement was done in triplicates with each replicate contained 20 calli for each treatment.
Sample preparation for biochemical assays and gene expression analysis
To study the effect of PF-68 on callus growth, samples from control treatment (without PF-68), optimum concentration (0.04% PF-68) and high concentration (0.10% PF-68) were used. Optimum concentration was selected to study the growth-promoting effects of PF-68 on callus growth. Meanwhile, high concentration was chosen to evaluate the effects of PF-68 at high concentration on plant cells. The calli was ground into fine powder using liquid nitrogen and stored at − 80 °C until further analysis.
Determination of total soluble sugar (TSS) content
TSS was measured using the phenol–sulphuric acid method (Terzi et al. 2014). Approximately 0.25 g of powdered calli were homogenized in 3 mL of 80% (v/v) ethanol and centrifuged at 880×g for 20 min. The pellet was discarded, and the supernatant was mixed with 5% (v/v) phenol and concentrated sulphuric acid. The absorbance of the samples was measured at 565 nm using a spectrophotometer (Implen GmbH, Germany). Three technical replicates and three biological replicates were performed on each sample.
Determination of total protein content
The protein content of the callus was determined using Bradford assay (Kruger 1994). Approximately 0.1 g powdered calli were mixed with 900 μL of 50 mM of ammonium bicarbonate (ABC) and 100 μL of 50 mM phenylmethylsulfonyl fluoride (PMSF). Subsequently, the mixture was sonicated on ice at 20 amplitude for 10 cycles (10 s sonication and 20 s rest) using a Q55 Sonicator (Qsonica, USA) followed by centrifugation at 10,000×g at 4 °C for 30 min. Acetone precipitation was carried out according to Jiang et al. (2004). Acetone was added into the supernatant with the ratio of 4:1 80% (v/v) acetone to supernatant and incubated at − 20 °C overnight. After centrifugation at 10 000 x g at 4 °C for an hour, the pellet was dissolved in 500 μL of 50 mM ABC and 50 mM PMSF and the protein content were measured at 595 nm using Bradford assay (Kruger 1994). Three technical replicates on three biological replicates were performed for each sample.
Determination of total phenolic content (TPC)
The TPC of the sample was determined through colorimetric assay using Folin-Ciocalteu (FC) method adapted from Singleton and Rossi (1965). Approximately 0.2 g of powdered calli was mixed with 10 mL of 50% (v/v) methanol and the sample was incubated in a water bath at 90 °C for an hour. Subsequently, 100 µL of the sample mixture was mixed with 1.15 mL of 10% (v/v) FC reagents and the mixture was incubated at 25 °C. After 5 min, 400 µL of 20% (v/v) sodium bicarbonate was added into the mixture and distilled water was added to give the final volume of 2 mL. The mixture was allowed to incubate for 30 min at 25 °C. The absorbance of the blue-colour mixture was measured at 750 nm against methanol as a blank. Three technical replicates on three biological replicates were performed on each sample.
Determination of total flavonoid content (TFC)
The TFC was determined using modified aluminium chloride colorimetric method adapted from Ahmad et al. (2016) using catechin as a standard. Approximately 0.2 g of powdered calli was mixed with 10 mL of 50% (v/v) methanol and the sample was incubated in a water bath at 90 °C for an hour. Subsequently, 250 µL of the extract was mixed with 75 µL of 5% (w/v) AlCl3, 500 µL NaOH and 1.25 mL distilled water. The mixture was centrifuged at 13,000×g for 15 min and incubated in the dark for 30 min at 25 °C. The absorbance of each sample was measured at 510 nm with a spectrophotometer. Three technical replicates on three biological replicates were performed on each sample.
Determination of hydrogen peroxide content
Hydrogen peroxide (H2O2) scavenging activity was measured following the method described by Velikova et al. (2000). Approximately 0.2 g of powdered calli was added to 750 μL of 0.1% trichloroacetic acid in an ice bath. The mixture was centrifuged at 14,000×g for 15 min at 4 °C. Subsequently, 20 μL of the supernatant was added to 250 μL 10 mM potassium phosphate buffer (pH 7.0) and 750 μL 1 M potassium iodide. The mixture was allowed to incubate for 30 min at 25 °C. The absorbance of the mixture was measured at 390 nm. Three technical replicates on three biological replicates were performed on each sample.
Real-time polymerase chain reaction (PCR) analysis
Total RNA was isolated from the powdered calli incubated in three treatments (0%, 0.04%, 0.10% PF-68) using RNeasy Plant Mini Kit (Qiagen, Germany) following protocol described in Lai and Takehisa (2013). First-strand cDNA was synthesized from 1 μg of isolated total RNA using QuantiNova Reverse Transcription Kit (Qiagen, Germany). The primers were designed (Supplementary Table S1) using Primer-Blast from National Center for Biotechnology Information (NCBI) and synthesized by Integrated DNA Technologies (IDT, USA). Real-time PCR was performed with Bio-Rad CFX96 system (Bio-Rad, US) with QuantiNova SYBR Green PCR (Qiagen, Germany) following protocol described in Lai et al. (2011a). The PCR reaction conditions used were as follows: 95 °C for 30 s followed by 40 cycles of 95 °C for 5 s and 60 °C for 5 s. The experiment was performed on three technical replicates with three biological replicates for each sample. The data were analyzed using Bio-rad CFX Manager 3.1 software. The relative expression levels (2−ΔΔCT) were calculated according to Livak’s method (Livak and Schmittgen 2001). The reference genes used in this study were rice cyclophilin (OsCYC) and ubiquitin 5 (OsUBQ5).
Statistical analysis
All data presented were the average ± standard error mean (SEM) of three biological replicates. The data were analyzed using one-way analysis of variance (ANOVA) at the significant level of p < 0.05 in Dunnet’s test using Statistical Package for the Social Sciences (SPSS) version 20 (IBM, 2016).
Results
This study showed supplementation of PF-68 increased callus FW and DW compared to the control (non-treated callus). Optimum callus proliferation was observed on callus grown on 0.04% PF-68, with the most significant weight increment (P < 0.05) by 58.80% and 23.98% in FW and DW, respectively (Fig. 1a).
Fig. 1.
Data obtained from callus proliferation incubated with different concentrations of PF-68 for 3 weeks. a Mean fresh and dry weights recorded on 3 weeks old calli; b observation made on callus morphology after incubated in different concentrations of PF-68; c representative figure of control callus at week 3; d representative figure of callus grown on 0.04% PF-68 at week 3; e representative figure of callus grown on 0.10% PF-68 at week 3; f representative figure of black callus at week 3; g representative figure of callus with root-like structure at week 3. Data shows mean of three biological replicates. Asterisk indicates statistical significance difference at p < 0.05 in Dunnet’s test. Scale bars represent 0.5 cm. Error bars represent standard error mean
In Fig. 1b, most of the calli grown on PF-68 were compact, dry and yellowish-white in colour, which resemble embryogenic cells (Fig. 1c, d). However, the number of yellowish-white calli decreases with increasing PF-68 concentrations (Fig. 1b). The highest number of yellowish-white calli (90.00%) was recorded in the control group followed by 0.04% PF-68 (80.00%) and 0.10% PF-68 (53.33%) (Fig. 1b). Besides, as the concentration of PF-68 increased, increasing the frequency of brown (Fig. 1e) and black (Fig. 1f) calli with friable callus was observed. The highest number of brown and black calli recorded in 0.10% PF-68 were 38.33% and 8.33%, respectively (Fig. 1b). Moreover, supplementation of PF-68 also increased the numbers of callus with root-like structure (Fig. 1g). Medium supplemented with 0.04% PF-68 recorded the highest number of calli with root-like structure induced (45.00%), followed by 0.10% PF-68 (36.67%) and the control (35.00%) (Fig. 1b).
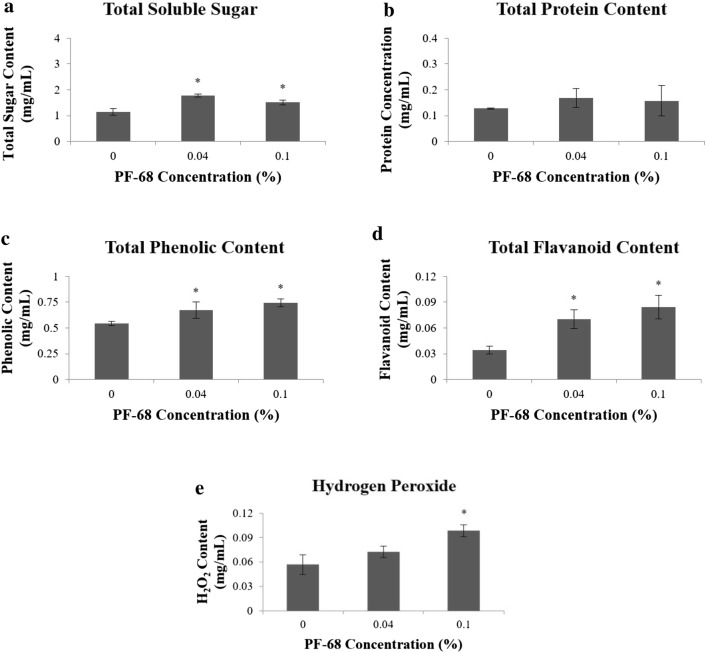
Significant increase of total sugar content was recorded in the calli grown on 0.04% PF-68 (1.77 mg/mL) and 0.10% PF-68 (1.51 mg/mL), as compared to control (1.14 mg/mL) (Fig. 2a). Similar trend was also observed in total protein content, whereby an increment in protein content was recorded in calli grown on 0.04% PF-68 (0.17 mg/mL) and 0.10% PF-68 (0.16 mg/mL), as compared to control (0.13 mg/mL) (Fig. 2b). On the other hand, the highest total phenolic content was recorded in calli grown on 0.10% PF-68 (0.74 mg/mL), followed by 0.04% PF-68 (0.67 mg/mL) and control (0.54 mg/mL) (Fig. 2c). Similarly, a significant increment of total flavonoid content was recorded in calli grown on 0.04% PF-68 (0.07 mg/mL) and 0.10% PF-68 (0.08 mg/mL), as compared to control (0.03 mg/mL) (Fig. 2d). In H2O2 content, a significant increment was observed in calli grown on 0.04% PF-68 (0.07 mg/mL) and 0.10% PF-68 (0.10 mg/mL), as compared to control (0.06 mg/mL) (Fig. 2e).
Fig. 2.
Data obtained from respective biochemical assays performed on three different concentrations of PF-68. a Total soluble sugar in callus grown on control, 0.04% PF-68 and 0.10% of PF-68, b total protein content in callus grown on control, 0.04% PF-68 and 0.10% of PF-68, c total phenolic content in callus grown on control, 0.04% PF-68 and 0.10% of PF-68, d total flavonoid content in callus grown on control, 0.04% PF-68 and 0.10% of PF-68, and e H2O2 in callus grown on control, 0.04% PF-68 and 0.10% of PF-68. Data show the mean of three biological replicates. Asterisk indicates statistically significant at p < 0.05 in Dunnet’s test. Error bars represent standard error mean
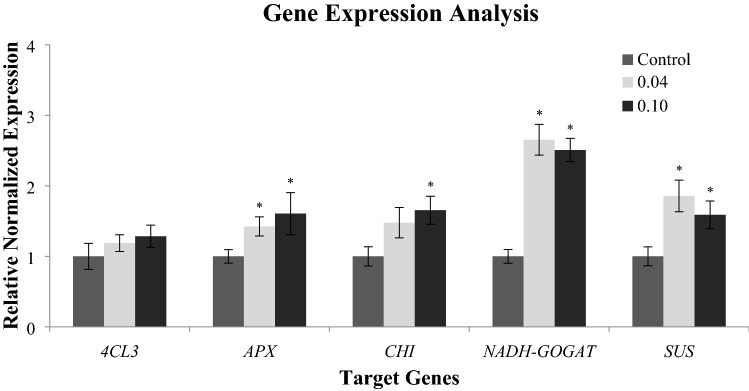
Gene expression analysis via real-time PCR was performed in which five different target genes involved for respective biochemical assays were chosen (Fig. 3). Gene expression analysis showed a significant increase of sucrose synthase (OsSUS) transcript in calli grown on 0.04% PF-68 (2.65-folds) and 0.10% PF-68 (2.51-folds). Likewise, a significant increment of NADH-dependent glutamate synthase (OsNADH-GOGAT) gene expression was detected in calli grown on 0.04% PF-68 (1.86-folds) and 0.10% PF-68 (1.59-folds). High expression of OsSUS and OsNADH-GOGAT genes recorded in 0.04% PF-68 treatment was also in accordance with the increased in total soluble sugar and total protein (Fig. 3). In line with the raised of phenolic content detected in calli grown on 0.04% PF-68 and 0.10% PF-68 mediums (Fig. 3), increase in transcription levels of 4-coumarate:CoA ligase (Os4CL) 3 was found in 0.04% PF-68 (1.12-folds) and 0.10% PF-68 (1.28-folds). Similarly, an enhancement in chalcone–flavonone isomerase (OsCHI) expression up to 1.45 folds was detected in 0.04% PF-68 treatment and increment up to 1.65-folds was recorded in 0.10% PF-68 treatment. The expression level of OsCHI was found to be coherent with total flavonoid content in both 0.04% PF-68 and 0.10% PF-68 treatments (Fig. 3). The expression level of ascorbate peroxidase (OsAPX) was observed to be correlated with increased in H2O2 content in calli grown on 0.04% PF-68 and 0.10% PF-68. An increase in OsAPX transcript up to 1.42-folds and 1.61-folds was detected in calli grown on 0.04% PF-68 and 0.10% PF-68 treatments, respectively.
Fig. 3.
Normalised relative gene expression of selected genes (4CL3, APX, CHI, NADH-GOGAT, and SUS) involved in respective biochemical assays in callus grown on control, 0.04% PF-68 and 0.10% of PF-68. Data show the mean of three biological replicates. The selected genes were normalized with OsCYC and OsUBQ5. Asterisk indicates statistically significant at p < 0.05 in Dunnet’s test. Error bars represent standard error mean
Discussion
Previously, PF-68 was used as an additive in improving shoot regeneration protocol in several plant species (Curtis and Mirkov 2012; Kulathuran and Narayanasamy 2015; Irshad et al. 2018). Addition of PF-68 in shoot regeneration medium had been reported to be more efficient in enhancing growth as compared to additives such as silver nitrate and gibberellic acid (Yildirim and Turker 2014; Irshad et al. 2018). For instance, supplementation of PF-68 is able to enhance shoot proliferation by 90.00% as compared to silver nitrate (40.00%) and gibberellic acid (80%) (Yildirim and Turker 2014; Irshad et al. 2018). Despite its great potential to be used as plant growth enhancer, applications of PF-68 in improving plant growth, in particular the recalcitrant species, are limited.
Our study demonstrated that supplementation of 0.04% PF-68 in callus proliferation medium significantly enhanced the MR 219 callus growth as shown by the increment of callus FW and DW (Fig. 1a). Previously, studies have reported that presence of PF-68 enhanced plasma membrane permeability in animal cell culture (Shelat et al. 2013). Therefore, it was hypothesized that supplementation of PF-68 at low concentration may also enhance plant growth by increasing plasma membrane permeability similar to its counterpart (Irshad et al. 2018). This in turn will increase the uptake of nutrients, phytohormones, and oxygen in plant cells to support its growth (Irshad et al. 2018). However, too high concentration of PF-68 may cause detrimental and irreversible changes to the plasma membrane that will eventually retard cell growth (Curtis and Mirkov 2012). Our results obtained were in agreement with the hypothesis proposed, whereby supplementation of PF-68 at low or optimum concentration was able to enhance callus proliferation without any detrimental effects. In contrast, at higher PF-68 concentrations (> 0.04% PF-68), calli FW and DW decreased steadily, followed by the increased appearances of the callus browning and darkening incidents (Fig. 1b). This implies that, at higher concentration, PF-68 will inhibit callus growth, mainly due to the increase of stress level (Fig. 1a). Therefore, growth-promoting effect of PF-68 is concentration dependent and proper optimisation is required to maximize its usage as a plant growth additive.
Embryogenic callus is usually identified based on several key morphologies such as compact structure, dry and yellowish-white (Zuraida et al. 2012; Lai et al. 2014). Meanwhile, non-embryogenic callus was observed to be friable, watery and yellowish to brownish in colour (Mostafiz and Wagiran 2018). Interestingly, our study showed that most of the calli grown on 0.04% PF-68 proliferation medium resemble the embryogenic callus with compact, dry and yellowish-white morphology. Production of embryogenic callus is crucial towards successful regeneration as it has the ability to regenerate into whole plant. In contrast, at high concentration of PF-68, an increase in the frequency of brown and black calli with friable callus which resembles non-embryogenic calli were observed. Production of non-embryogenic callus is unfavourable as non-embryogenic callus loses its embryogenic capability to regenerate into whole plant (Lai et al. 2011b). This phenomenon could be caused by increasing stress induced from the high concentration of PF-68 (Mostafiz and Wagiran 2018).
Besides, supplementation of 0.04% PF-68 improved the numbers of callus with root-like structure (increase of 28.57%) as compared to the control. Root formation in callus or rhizogenesis is a type of organogenesis in which callus tissue undergoes adventitious root formation. One of the factors which contribute to increase number of root formation in callus is interactions between auxin and cytokinin (Low et al. 2019). Previous study had shown that high concentration of auxin was able to induce rhizogenesis from callus (Sugimoto et al. 2011). In our study, auxin concentration supplemented in each treatment was fixed. Therefore, the number of callus with root-like structure should be similar across different concentration of PF-68. However, supplementation of 0.04% PF-68 significantly induced more root formation in callus (Fig. 1b) which suggests that the presence of PF-68 stimulates the production of endogenous auxin in plant. In plant, the presence of auxin was found to regulate WUSCHEL-Related Homeobox (WOX) transcription factor family (Zhao et al. 2009). During callus proliferation, high expression of WOX1 and WOX4 genes were observed to play a role in root formation and callus proliferation, respectively (Lu et al. 2019). Hence, supplementation of PF-68 may improve callus proliferation through enhanced endogenous auxin production and expression of WOXs gene, which alter the ratio of auxin and cytokinin. This eventually led to higher number of root formation observed on callus grown on 0.04% PF-68.
To further comprehend the possible role of PF-68 in callus proliferation, biochemical assays were performed on the selected concentrations. Based on Fig. 2a, calli grown on 0.04% PF-68 was found to contain the highest sugar content. In general, soluble sugar accumulation plays an important role in sugar sensing and plant development as high sugar content promotes growth and carbohydrate storage (Eveland and Jackson 2012). Similarly, the highest upregulation of OsSUS transcript was detected in calli grown on 0.04% PF-68. Previously, SUS had been reported to play an important role in sucrose metabolism that contributed to the increased of plant cell growth (Stein and Granot 2019). Moreover, overexpression of SUS genes in transgenic tobacco plants enhanced photosynthesis activity and sugar production in plant (Nguyen et al. 2015). Our results show that through supplementation of PF-68, sugar metabolism in callus was enhanced. This in turn, releases carbon and energy required for plant growth and development, which eventually enhances callus proliferation.
In Fig. 2b, calli grown on 0.04% PF-68 contained the highest protein content. One of the factors which contributes to protein accumulation in plant is the availability of nitrogen. Several studies demonstrated that protein content increases significantly with the increase of nitrogen supply and plant density (Rafiq et al. 2010; Yang et al. 2016). Besides, Lehmeier et al. (2013) also reported that deficiency in nitrogen supply reduces the growth of leaves by half. In addition, highest expression of OsNADH-GOGAT was recorded in calli grown on 0.04% PF-68. NADH-GOGAT is known to play a vital role in nitrogen assimilation using NADH as an electron carrier and it is highly expressed in roots (Konishi et al. 2014). Overexpression of NADH-GOGAT in transgenic plant was shown to enhance total carbon and nitrogen content, and increased dry weight of the plant (Chichkova et al. 2001). In our study, the application of PF-68 was able to enhance expression of OsNADH-GOGAT gene which could increase availability of nitrogen content in plant. Increased of nitrogen content could be used as a source of building blocks for amino acid and subsequently enhanced protein biosynthesis which are crucial for plant growth (Rafiq et al. 2010).
Our results showed that calli grown on 0.10% PF-68 contained high phenolic and flavonoid content as compared to control and 0.04% PF-68 (Fig. 2c, d). Phenolic and flavonoid compounds are the largest group of secondary metabolites in plant, and accumulation of phenolic compounds is often correlated with plant’s stress response (Park et al. 2018). Similarly, the highest upregulation of Os4CL3 and OsCHI transcripts was recorded in calli grown on 0.10% PF-68 (Fig. 3). In phenylpropanoid metabolism, 4CL gene is a key rate-limiting enzyme which is involved in the synthesis of phenolic secondary metabolites precursors required for flavonoids and lignin biosynthesis (Li et al. 2015). Studies found that suppression of 4CL3 gene caused reduction in flavonoid and lignin biosynthesis contents which are crucial for plant defence mechanism (Li et al. 2015). On the other hand, modulation of CHI expression had been reported to be crucial for the accumulation of flavonoids in plants which function as an antioxidant compound (Park et al. 2018). Based on our findings, secondary metabolites biosynthesis was enhanced as a means of defence mechanism towards increasing oxidative stresses caused by the high concentration of PF-68 (Munene et al. 2017). Our findings revealed that application of high PF-68 concentrations induced secondary metabolite biosynthesis in plant cell. Increase in secondary metabolites biosynthesis is possibly due to increase in stress induced by PF-68. At optimum concentration, the induction of stress could still be cope by callus through activation of plant defence mechanism via secondary metabolites biosynthesis to regulate cellular homeostasis (Isah 2019). However, at high concentration of PF-68 (0.10%), higher amount of stress induced causes the plant unable to maintain cellular homeostasis which eventually induced cell death and increased in number of brown and black calli (Jones and Saxena 2013).
Reactive oxygen species (ROS) is one of the by-products of aerobic metabolism. H2O2 is one of the most important relative stable non-radical ROS (Sofo et al. 2015). At low concentration, H2O2 serves as important signalling molecules to regulate biological and physiological processes (Wang et al. 2016). However, accumulation of H2O2 may cause damage towards macromolecules and excessive damages towards plant cells which will eventually trigger hypersensitive response and plant cell death (Konieczny et al. 2014). The excessive production of H2O2 is often facilitated by increasing stress response (Konieczny et al. 2014). To maintain a steady level of cellular H2O2, H2O2-scavenger mechanism such as catalases and APX are produced in plants (Chou et al. 2012). Upon stress induction, expression of APX plays an important role in H2O2-scavenging and plant defence (Chou et al. 2012; Sofo et al. 2015). In our study, plant defence mechanism was activated as the expression of OsAPX gene (Fig. 3) was enhanced to reduce the high concentration of H2O2 recorded in calli grown on 0.10% PF-68 (Fig. 2f). This demonstrates that the application of high concentration of PF-68 induces stress response in calli.
Conclusion
Taken together, PF-68 has successfully enhanced callus proliferation of MR 219 cultivar. PF-68 improved callus growth via increment of sugar metabolism and amino acid biosynthesis, which are prerequisites for plant growth. However, at high concentration, PF-68 also induces stress response in plant with the increase in secondary metabolites biosynthesis. Therefore, growth-promoting effects of PF68 is concentration dependent and optimisation of PF-68 is required for different plant species.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to acknowledge the Graduate Research Fellowship and Putra Grants (GP-IPS/2017/9572000) from Universiti Putra Malaysia for the research funds.
Authors contributions
KSL conceived and designed the experiments; ADXK and WMAZWA performed the experiments; NPT, JOA, RS, CYW and KSL contributed materials/reagents/analysis tools/funding acquisition; ADXK and WMAZWA wrote the manuscript. All authors have read, contributed and approved the manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest in the publication.
References
- Abiri R, Maziah M, Shaharuddin NA, Yusof ZNB, Atabaki N, Hanafi MM, Sahebi M, Azizi P, Kalhori N, Valdiani A. Enhancing somatic embryogenesis of Malaysian rice cultivar MR 219 using adjuvant materials in a high-efficiency protocol. Int J Sci Environ Technol. 2017;14:1091–1108. [Google Scholar]
- Ahmad N, Rab A, Ahmad N. Light-induced biochemical variations in secondary metabolite production and antioxidant activity in callus cultures of Stevia rebaudiana (Bert) J Photochem Photobiol B. 2016;154:51–56. doi: 10.1016/j.jphotobiol.2015.11.015. [DOI] [PubMed] [Google Scholar]
- Amandeep-Kaur SKSA, Eep K, Kaur G, Cheema GS. Genetic transformation of rice: problems, progress and prospects. Rice Res. 2014;3:1–10. [Google Scholar]
- Barbulescu DM, Burton WA, Salisbury PA. Pluronic F-68: an answer for shoot regeneration recalcitrance in microspore-derived Brassica napus embryos. Vitro Cell Dev Plant. 2011;47:282–288. [Google Scholar]
- Chichkova S, Arellano J, Vance CP, Hernández G. Transgenic tobacco plants that overexpress alfalfa NADH-glutamate synthase have higher carbon and nitrogen content. J Exp Bot. 2001;52(364):2079–2087. doi: 10.1093/jexbot/52.364.2079. [DOI] [PubMed] [Google Scholar]
- Chou TS, Chao YY, Kao CH. Involvement of hydrogen peroxide in heat shock-and cadmium-induced expression of ascorbate peroxidase and glutathione reductase in leaves of rice seedlings. J Plant Physiol. 2012;169(1):478–486. doi: 10.1016/j.jplph.2011.11.012. [DOI] [PubMed] [Google Scholar]
- Curtis IS, Mirkov TE. Influence of surfactants on growth and regeneration from mature internodal stem segments of sweet orange (Citrus sinensis) cv. Hamlin. Plant Cell Tissue Organ Cult. 2012;108:345–352. [Google Scholar]
- Dashti S, Habashi AA, Azghandi AV, Abdollahi H, Chamani M, Dashti S. Effects of Pluronic F-68 on regeneration and rooting of two pear cultivars (Pyrus communis cvs dar gazi and bartlett) Int J Sci Basic Appl Res. 2012;3:190–196. [Google Scholar]
- Eveland AL, Jackson DP. Sugars, signalling, and plant development. J Exp Bot. 2012;63(9):3367–3377. doi: 10.1093/jxb/err379. [DOI] [PubMed] [Google Scholar]
- Gamborg OL, Miller R, Ojima K. Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res. 1968;50(1):151–158. doi: 10.1016/0014-4827(68)90403-5. [DOI] [PubMed] [Google Scholar]
- He C, Wang L, Liu J, Liu X, Li X, Ma J, Lin Y, Xu F. Evidence for ‘silicon’ within the cell walls of suspension-cultured rice cells. New Phytol. 2013;2013:700–709. doi: 10.1111/nph.12401. [DOI] [PubMed] [Google Scholar]
- Irshad M, Rizwan HM, Debnath B, Anwar M, Li M, Liu S, He B, Qiu D. Ascorbic acid controls lethal browning and Pluronic f-68 promotes high-frequency multiple shoot regeneration from cotyledonary node explant of okra (Abelmoschus esculentus L.) HortScience. 2018;53:183–190. [Google Scholar]
- Isah T. Stress and defense responses in plant secondary metabolites production. Biol Res. 2019;52:39. doi: 10.1186/s40659-019-0246-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, He L, Fountoulakis M. Comparison of protein precipitation methods for sample preparation prior to proteomic analysis. J Chromatogr A. 2004;1023:317–320. doi: 10.1016/j.chroma.2003.10.029. [DOI] [PubMed] [Google Scholar]
- Jones Andrew Maxwell Phineas, Saxena Praveen Kumar. Inhibition of Phenylpropanoid Biosynthesis in Artemisia annua L.: A Novel Approach to Reduce Oxidative Browning in Plant Tissue Culture. PLoS ONE. 2013;8(10):e76802. doi: 10.1371/journal.pone.0076802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok ADX, Low LY, Zetty-Norhana BY, Rogayah S, Wee CY, Lai KS. Iron biofortification of rice: progress and prospects. In: Shah F, Khan ZH, Iqbal A, editors. Rice crop—current developments. Norderstedt: IntechOpen; 2018. pp. 25–44. [Google Scholar]
- Konieczny R, Banaś AK, Surówka E, Michalec Z, Miszalski Z, Libik-Konieczny M. Pattern of antioxidant enzyme activities and hydrogen peroxide content during developmental stages of rhizogenesis from hypocotyl explants of Mesembryanthemum crystallinum L. Plant Cell Rep. 2014;33(1):165–177. doi: 10.1007/s00299-013-1520-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi N, Ishiyama K, Matsuoka K, Maru I, Hayakawa T, Yamaya T, Kojima S. NADH-dependent glutamate synthase plays a crucial role in assimilating ammonium in the Arabidopsis root. Physiol Plant. 2014;152(1):138–151. doi: 10.1111/ppl.12177. [DOI] [PubMed] [Google Scholar]
- Kruger NJ. The Bradford method for protein quantitation. Basic Protein Peptide Protocols. 1994;32:9–15. doi: 10.1385/0-89603-268-X:9. [DOI] [PubMed] [Google Scholar]
- Kulathuran GK, Narayanasamy J. Evaluation of Pluronic F68 and PGR’s for high frequency somatic embryogenesis and plant regeneration in castor (Ricinus communis L.) through solid culture. Int J Curr Biotechnol. 2015;3(8):1–10. [Google Scholar]
- Lai KS, Takehisa M. Isolation and characterization of an Arabidopsis thaliana self-incompatibility mutant induced by heavy-ion beam irradiation. Acta Biol Cracov Bot. 2013;55(2):146–152. [Google Scholar]
- Lai KS, Puad A, Yusoff K, Mahmood M. An efficient protocol for particle bombardment-mediated transformation of Centella asiatica. Acta Physiol Plant. 2011;33(1):2547–2552. [Google Scholar]
- Lai KS, Yusoff K, Mahmood M. Extracellular matrix as the early structural marker for Centella asiatica embryogenic tissues. Biol Plant. 2011;55(3):549–553. [Google Scholar]
- Lai KS, Yusoff K, Mahmood M. Morphological and physiological characterization of embryogenic and non-embryogenic tissues of Centella asiatica. Plant Tissue Cult Biotechnol. 2014;24(1):125–129. [Google Scholar]
- Lehmeier CA, Wild M, Schnyder H. Nitrogen stress affects the turnover and size of nitrogen pools supplying leaf growth in a grass. Plant Physiol. 2013;162(4):2095–2105. doi: 10.1104/pp.113.219311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Kim JI, Pysh L, Chapple C. Four isoforms of Arabidopsis 4-Coumarate:CoA Ligase have overlapping yet distinct roles in phenylpropanoid metabolism. Plant Physiol. 2015;169(4):2409–2421. doi: 10.1104/pp.15.00838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew YA, Syed Omar SR, Husni MHA, Zainal AMA, Nur Ashikin PA. Effects of foliar applied copper and boron on fungal diseases and rice yield on cultivar MR219. Pertanika J Trop Agric Sci. 2012;35(2):339–349. [Google Scholar]
- Lim YY, Lai KS. Generation of transgenic rice expressing cyclotide precursor Oldenlandia affinis kalata B1 protein. J Anim Plant Sci. 2017;27(2):667–671. [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Low LY, Yang SK, Kok DXA, Ong-Abdullah J, Tan NP, Lai KS. Transgenic plants: gene constructs, vector and transformation method. In: Çiftçi YO, editor. New visions in plant science. Norderstedt: IntechOpen; 2018. pp. 41–61. [Google Scholar]
- Low LY, Ong-Abdullah J, Wee CY, Sekeli R, Tan CK, Low JY, Lai KS. Effects of lignosulfonates on callus proliferation and shoot induction of recalcitrant Indica rice. Sains Malays. 2019;48(1):7–13. [Google Scholar]
- Lu Y, Liu ZY, Lyu ML, Yuan Y, Wu BH. Characterization of JsWOX1 and JsWOX4 during callus and root induction in the shrub species Jasminum sambac. Plants. 2019;8(4):79. doi: 10.3390/plants8040079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier SJ, Hatton TA, Wang DIC. Cell death from bursting bubbles: role of cell attachment to rising bubbles in sparged reactors. Biotechnol Bioeng. 1999;62:468–478. [PubMed] [Google Scholar]
- Mostafiz SB, Wagiran A. Efficient callus induction and regeneration in selected indica rice. Agronomy. 2018;8:77. [Google Scholar]
- Munene R, Changamu E, Korir N, Gweyi J. Effects of different nitrogen forms on growth, phenolics, flavonoids and antioxidant activity in Amaranth species. Trop Plant Res. 2017;4(1):81–89. [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plantarum. 1962;15(3):473–497. [Google Scholar]
- Nguyen QA, Luan S, Wi SG, Bae HH, Lee DS, Bae HJ. Pronounced phenotypic changes in transgenic tobacco plants overexpressing sucrose synthase may reveal a novel sugar signaling pathway. Front Plant Sci. 2015;6:1216. doi: 10.3389/fpls.2015.01216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SH, Lee CW, Cho SM, Lee HS, Park H, Lee JE, Lee JH. Crystal structure and enzymatic properties of chalcone isomerase from the Antarctic vascular plant Deschampsia antarctica Desv. PLoS ONE. 2018;13(2):e0192415. doi: 10.1371/journal.pone.0192415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafiq M, Ali A, Malik M, Hussain M. Effect of fertilizer levels and plant densities on yield and protein contents of autumn planted maize. Pak J Agric Sci. 2010;47(3):201–208. [Google Scholar]
- Shahravari E, Maheran AA, Siti Nor Akmar A, Hanafi MM. The effect of plant growth regulators on optimization of tissue culture system in Malaysian upland rice. Afr J Biotecnol. 2010;9(14):2089–2094. [Google Scholar]
- Shelat PB, Plant LD, Wang JC, Lee E, Marks JD. The membrane-active tri-block copolymer Pluronic F-68 profoundly rescues rat hippocampal neurons from oxygen–glucose deprivation-induced death through early inhibition of apoptosis. J Neurosci. 2013;33(30):12287–12299. doi: 10.1523/JNEUROSCI.5731-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Viticult. 1965;16:144–158. [Google Scholar]
- Sofo A, Scopa A, Nuzzaci M, Vitti A. Ascorbate peroxidase and catalase activities and their genetic regulation in plants subjected to drought and salinity stresses. Int J Mol Sci. 2015;16(1):13561–13578. doi: 10.3390/ijms160613561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein O, Granot D. An overview of sucrose synthases in plants. Front Plant Sci. 2019;10:95. doi: 10.3389/fpls.2019.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt M, Sulpice R, Keurentjes J. Metabolic networks: how to identify key components in the regulation of metabolism and growth. Plant Physiol. 2010;152(2):428–444. doi: 10.1104/pp.109.150821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto K, Gordon SP, Meyerowitz EM. Regeneration in plants and animals: dedifferentiation, transdifferentiation, or just differentiation? Trends Cell Biol. 2011;21(4):212–218. doi: 10.1016/j.tcb.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Tan LW, Rahman AZ, Goh HH, Hwang DJ, Ismail I, Zainal Z. Production of transgenic rice (indica 1 cv. MR 219) overexpressing Abp57 gene through Agrobacterium-mediated transformation. Sains Malays. 2017;46:703–711. [Google Scholar]
- Terzi R, Kadioglu A, Kalaycioglu E, Saglam A. Hydrogen peroxide pretreatment induces osmotic stress tolerance by influencing osmolyte and abscisic acid levels in maize leaves. J Plant Interact. 2014;9(1):559–565. [Google Scholar]
- Velikova V, Yordanov I, Edreva A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. Plant Sci. 2000;151(1):59–66. [Google Scholar]
- Wan Abdullah WMAN, Tan NP, Low LY, Ong-Abdullah J, Wee CY, Taib AZM, Lai KS (in press) Calcium lignosulfonate improves proliferation of recalcitrant indica rice callus via modulation of auxin biosynthesis and enhancement of nutrient absorption. Front Plant Sci [DOI] [PubMed]
- Wang X, Li Y, Fang G, Zhao QC, Li XM, Gong HY, Li YS. Nitrite promotes the growth and decreases the lignin content of indica rice calli: a comprehensive transcriptome analysis of nitrite-responsive genes during in vitro culture of rice. PLoS ONE. 2014;9(4):e95105. doi: 10.1371/journal.pone.0095105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CH, Yu J, Cai YX, Zhu PP, Liu CY, Zhao AC, Lü RH, Li MJ, Xu FX, Yu MD. Characterization and functional analysis of 4-coumarate:CoA ligase genes in mulberry. PLoS ONE. 2016;11(5):e0155814. doi: 10.1371/journal.pone.0155814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wani SH, Sanghera GS, Gosal SS. An efficient and reproducible method for regeneration of whole plants from mature seeds of a high yielding Indica rice (Oryza sativa L.) variety PAU 201. New Biotechnol. 2011;28(4):418–422. doi: 10.1016/j.nbt.2011.02.006. [DOI] [PubMed] [Google Scholar]
- Yang HK, Meng YL, Chen BL, Zhang XY, Wang YH, Zhao WQ, Zhou ZG. How integrated management strategies promote protein quality of cotton embryos: high levels of soil available N, N assimilation and protein accumulation rate. Front Plant Sci. 2016;7(1):1118. doi: 10.3389/fpls.2016.01118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap WS, Lai KS. Biochemical properties of twelve Malaysia rice cultivars in relation to yield potential. J Agric Biol Sci. 2017;11(4):137–143. [Google Scholar]
- Yildirim AB, Turker AU. Effects of regeneration enhancers on micropropagation of Fragaria vesca L. and phenolic content comparison of field-grown and in vitro-grown plant materials by liquid chromatography-electrospray tandem mass spectrometry (LC-ESI-MS/MS) Sci Hortic. 2014;169:169–178. [Google Scholar]
- Zhao Y, Hu YF, Dai MQ, Huang LM, Zhou DX. The WUSCHEL-related homeobox gene WOX11 is required to activate shoot-borne crown root development in rice. Plant Cell. 2009;21(3):736–748. doi: 10.1105/tpc.108.061655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuraida AR, Zulkifli AS, Habibuddin H, Naziah B. Regeneration of Malaysian rice variety MR 219 via somatic embryogenesis. J Trop Agric Food Sci. 2012;39(2):167–177. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.