Abstract
Plant growth-promoting rhizobacteria (PGPR) are known for growth promotion and mitigating environmental stresses. Here, we examined the propitiousness of three indigenous salt-tolerant PGPR, i.e., Bacillus subtilis (NBRI 28B), B. subtilis (NBRI 33 N), and B. safensis (NBRI 12 M) for plant growth promotion and salt stress amelioration in Zea mays. Results of the in vitro plant growth-promoting attribute revealed NBRI 12 M demonstrated the highest values at 1 M salt (NaCl) concentration. Furthermore, the greenhouse experiment using three Bacillus strains confirmed plant growth-promoting and salt stress-ameliorating ability, through colonizing successfully and mitigating the adverse effects of ethylene by modulating 1-aminocyclopropane-1-carboxylic acid (ACC) accumulation, ACC-oxidase (ACO), and ACC-synthase (ACS) activities under salt stress. Bacillus sp. inoculation has also induced plant response for defense enzymes, chlorophyll, proline and soluble sugar under salt stress. Among three Bacillus strains, NBRI 12 M not only demonstrated higher values for plant growth-promoting (PGP) attributes but also the same was observed in the greenhouse experiment. Thus, the outcomes of this comparative study represent for the first time that salt-tolerant Bacillus strains exhibiting multiple PGP attributes under salt stress along with high rhizosphere competence can alleviate salt stress by reducing the stress ethylene level in the host plant.
Electronic supplementary material
The online version of this article (10.1007/s13205-020-2104-y) contains supplementary material, which is available to authorized users.
Keywords: PGPR, Defense enzymes, PGP attributes, Rhizosphere colonization
Introduction
Land degradation due to soil salinization is a serious risk to agriculture. By the year 2050, more than 50% of cultivated land predicted to be affected by salinity (Panta et al. 2014). Moreover, salt stress possibly contributes most to reductions in crop production worldwide among various abiotic stresses (Zörb et al. 2018). Salt stress causes leaf curling and yellowing, decreased growth rate, and reduced plant height, due to inhibited cell division and elongation. Salt stress is not only limited to stunting plant growth alone, but it also has severe impacts on plant physiology modifications. Perhaps, salt stress can lead to increased reactive oxygen species (ROS) production, such as hydrogen peroxide, superoxide radicals, and hydroxyl free radicals, resulting in lipid peroxidation and induction of oxidative stress in plants (Zörb et al. 2018). Salt stress also promotes the endogenous ethylene level of the plant known as “stress ethylene” which inhibits leaf growth, cell division, and root elongation (Glick 2014; Li et al. 2015; Dubois et al. 2018). Therefore, an extensive investigation is required to improve salt tolerance in crop plants against soil salinity.
Recently, the beneficial microbes have been used to mitigate the harmful effects caused by salt stress along with enhancing the growth of crops. The use of the plant growth-promoting rhizobacteria (PGPR) can be an important strategy for improving agriculture in saline soils (Tewari and Arora 2014). The bacterial genera such as Acetobacter, Azospirillum, Azotobacter, Bacillus, Burkholderia, Klebsiella, Pseudomonas, and Serratia have been reported as PGPR (Verma et al. 2019). Among the above-mentioned beneficial PGPR, the Bacillus genera have been recognized as the dominant community, due to their survivability under varied biotic and abiotic environmental stresses environments (Kang et al. 2015; Radhakrishnan et al. 2017). They generally possess multifarious growth-promoting properties such as nitrogen fixation, producing siderophore, dissolving mineral insoluble phosphate, phytohormones production such as IAA, and synthesizing ACC deaminase enzymes (Wang et al. 2018; Misra et al. 2017; Douriet-Gámez et al. 2018). ACC deaminase utilizes ACC, a precursor for ethylene biosynthesis in plants, thus reducing the concentration of stress ethylene under stress conditions and promoting growth and development (Penrose and Glick 2003). Moreover, under saline stress, Bacillus spp. produce exopolysaccharides (EPS) which not only helps in preventing the entry of toxic ions, but also regulates the ionic balance along with water transport in plant tissues (Radhakrishnan et al. 2017). The presence of the above-mentioned properties makes Bacillus spp. to ameliorate the detrimental effects of salt stress on crops by inducing physiological changes related to water transport, nutrient uptake and the activation of the defense system. Therefore, Bacillus spp. confirm their beneficial effects on plants for improving growth and crop yield.
South Western Semi-Arid zone, North Eastern Plain zone, and Western Plain zone of Uttar Pradesh, India are amongst the low rainfall receiving agroclimatic zones (Agriculture Department, Uttar Pradesh; Lata 2019). Therefore, these locations are more prone to water stress condition and the problem of soil salinization is more intensified. Indigenous microorganisms that occur in native habitats are supposed to share a strategy for resisting high salt concentration and have developed a manifold adaptation for sustaining their active population while coping up with the local environmental stress (Laditi et al. 2012; Abiala et al. 2015; Misra et al. 2017). Therefore, screening of highly effective halotolerant indigenous PGPR is imperative to sustain the agricultural production. Therefore, the present study was aimed to understand the involvement of native salt stress-tolerant Bacillus spp. with ACC deaminase activity in modulating the ethylene biosynthesis for salt stress mitigation and growth promotion in maize.
Materials and methods
Estimation of PGP and other attributes of Bacillus spp.
Plant growth-promoting and salt-tolerant Bacillus subtilis NBRI 28B (NBRI 28B), Bacillus subtilis NBRI 33 N (NBRI 33 N), and Bacillus safensis NBRI 12 M (NBRI 12 M) (GenBank accession no. KX495304, KX495258, and KX495277) were isolated from the arable soil of South Western Semi-Arid zone, North Eastern Plain zone, and Western Plain zone of Uttar Pradesh, India, respectively (Misra et al. 2017). These strains were selected owing to have different isolation sources, multiple PGP attributes, and abiotic stress tolerance for further studies.
In the present study, these bacterial strains were subjected for evaluation of their ability for having varied PGP traits such as phosphate solubilization, indole acetic acid (IAA) production, biofilm formation, and ACC deaminase activity under control (0 M NaCl) as well as salt (0.5 M and 1 M NaCl) stress condition. To estimate the solubilization of phosphate by bacterial isolates, the NBRIP medium was used as a substrate. The solubilized phosphate was then quantified by Molybdate Blue Method (Nautiyal 1999). In the case of quantification of auxin production, the respective bacterial strains were inoculated in NB (Nutrient Broth) supplemented with tryptophan as a substrate and after incubation, the activity was estimated by addition of orthophosphoric acid followed by Salkowski’s reagent (Bric et al. 1991). In the case of biofilm formation, the bacterial inoculum was stained with 0.1% crystal violet followed by washing with 95% ethanol. After washing, OD was measured at 590 nm using a spectrophotometer (EVOLUTION201, ThermoScientific, USA) (Srivastava et al. 2008). ACC deaminase activity of salt-tolerant bacterial strains was estimated by the method mentioned by Penrose and Glick (2003), with slight modifications. In short, overnight-grown bacterial strains were inoculated in NFb (nitrogen free) medium supplemented with ACC (Sigma Chemical Co., USA) as the substitute for the nitrogen source. The ability of the bacteria to utilize ACC was verified by comparing it with the control having the same isolate grown in absentia of nitrogen source. The bacterial strains were also tested for their ability to produce exo-polysaccharide (EPS) and alginate using the phenol–sulphuric acid method (Titus et al. 1995) and carbazole–boric sulfuric acid method (Mishra et al. 2012), respectively. The initial inoculum of selected bacterial strains for these tests was approximately 7.5 × 107 CFU ml−1. Bacterial strains were further characterized qualitatively for demonstrating N2 fixation using a nitrogen-free medium containing bromothymol blue (BTB) as an indicator (Gothwal et al. 2007). The change in color to blue was marked as nitrogen fixation in the solid culture conditions. Several biochemical activities of bacterial strains were also determined using the KB003 Hi25TM identification kit (HiMedia, India). In the present study, bacterial strains were subjected to evaluation of their stress-tolerant ability at different concentrations of salt stress (NaCl; 0.5 M and 1 M). It is determined by counting the respective colony-forming unit (Log10 CFU ml−1) up to 10 days (Mishra et al. 2017). For this, each bacterial strain with three replicates each was grown in a sterile test tube containing 5 mL nutrient broth medium altered with 0.5 M and 1 M NaCl (w/v). The bacterial strains under mentioned salt concentrations were grown in the nutrient medium at 30 °C with the continuous shaking of 180 rpm. As a control, the same three bacteria were grown in nutrient broth medium.
Assessment of Bacillus spp. for plant growth promotion under greenhouse condition
Following the characterization of bacterial strains on the basis of PGP and salt stress tolerance in vitro, the bacterial strains were also tested for growth promotion and salt stress amelioration using maize (Zea mays) as a model plant in pot conditions. Experiments were conducted in a completely randomized block design comprising 12 replicates in pots (15 cm in diameter) containing 2.0 mm sieved unsterilized garden soil (2.0 kg per pot) (pH 8.20; electrical conductivity, 308 μS cm−1) of CSIR-National Botanical Research Institute, Lucknow, India (latitude/longitude 11° 24′ N/ 79° 44′ E). For the same, maize (Zea mays “Maharaja”) seeds were treated with bacteria as described by the method of Nautiyal (1997). For the control condition, seeds were treated with water. Plants were allowed to grow under greenhouse condition and the treatments for host plant with respect to concerned bacterial strains were as follows: control (uninoculated), salt (100 mM NaCl), Bacillus subtilis (NBRI 28B); Bacillus subtilis (NBRI 33 N); Bacillus safensis (NBRI 12 M), and NBRI 28B + S; NBRI 33 N + S; NBRI 12 M + S (bacterial strains with 100 mM NaCl). Soil moisture content in each treatment was maintained to 20% either with water (control and salt treatments) or 48 h grown bacterial culture (NBRI 28B; NBRI 33 N; NBRI 12 M) (~ 108‒9 CFU ml−1) in bacteria (NBRI 12 M; NBRI 28B; NBRI 33 N) and bacteria with salt (NBRI 12 M + S; NBRI 28B + S; NBRI 33 N + S) treatments. The stress of salt (100 mM salt (NaCl)) was given in salt-stressed set as described by Nautiyal et al. (2013). Plants in all treatments were harvested after 30 days of sowing for evaluating growth parameters, biochemical changes, modulation in defense enzymes, and ethylene emission analysis.
Evaluation of plant vegetative parameters, biochemical, and defense enzymes
Vegetative parameters comprising shoot and root length, number of leaves per plant, plant fresh and dry weight were determined using three replicates of maize plant from each treatment.
Chlorophyll (total) and carotenoid content in the leaves were determined by the method described by Arnon (1949). The proline content of the leaves was determined using the method of Bates et al. (1973). Sugar content in the leaves was estimated as per the protocol of Dubois et al. (1956).
Estimation of antioxidants from leaf samples was done using established standard protocols. Briefly, 500 mg of leaves (three replicates from each treatment) were crushed in 1 ml of extraction buffer (100 mM potassium phosphate buffer (pH 7.0) containing 0.1 mM EDTA and 1% polyvinylpyrrolidone [w/v] at 4 °C). The homogenate was centrifuged at 15,000×g for 15 min at 4 °C and the supernatants were stored at − 80 °C. SOD (EC 1.15.1.1) activity was measured as previously described (Beauchamp and Fridovich 1971). Furthermore, a 3 ml mixture was prepared to contain 100 μl of enzyme extract, 13 mM methionine, 75 μM nitrobluetetrazolium (NBT), 40 mM phosphate buffer (pH 7.8), 0.1 mM EDTA, and 02 μM riboflavin in that order. In the case of blank, the mixture containing enzyme extract kept in the dark, whereas those without the protein (control) and with the enzyme (treatments) maintained in the light. The reaction was started by switching on the light for 30 min. The absorbance was measured at 560 nm. The activity of SOD is the measurement of NBT reduction by mixture devoid of protein under light minus NBT reduction by the mixture containing protein. One unit of activity is the quantum of enzyme required to inhibit half of the initial NBT reduction under the light and expressed as units mg−1 protein g−1 FW. CAT (EC 1.11.1.6) activity was determined as described (Aebi 1984). The rate of decline in absorbance was measured during 3 min at 240 nm in the reaction mixture consisting of 50 mM sodium phosphate buffer (pH 7.0), 20 mM H2O2 and 100 μl of enzyme extract. For control, the mixture considered was 50 mM sodium phosphate buffer (pH 7.0) and 100 μl of protein, whereas those for blank were 50 mM sodium phosphate buffer (pH 7.0) and 20 mM H2O2. The expression of enzyme activity was in H2O2 (μmoles) separated in min mg−1 FW. The ascorbate peroxidase (APX; EC 1.11.1.3) was assayed by estimating the reduction in absorbance (290 nm; an absorbance coefficient 2.8 mM−1 cm−1) for the rate of oxidation of ascorbate (Nakano and Asada 1981). In short, the reaction mixture consisted of 50 mM phosphate buffer (pH 7.0), 0.1 mM H2O2, 0.5 mM sodium ascorbate, 0.1 mM EDTA. The reaction was initiated by adding either enzyme extract (treatments) or hydrogen peroxide (control). The decline in absorbance was recorded from 10 to 30 s after the start of the reaction. The expression of the enzyme activity was in μmol of ascorbate oxidized min−1 g−1 FW. Guaiacol peroxidase (EC 1.11.1.7) activity was determined (Hemeda and Klein 1990) colorimetrically at 470 nm using 1% guaiacol (v/v; as a donor), 0.3% H2O2 (as a substrate) and 50 mM phosphate buffer (pH 6.6). The protein was added to initiate the reaction, whereas ethanol was added to serve as a control. The oxidation of guaiacol (extinction coefficient 26.6 mM−1 cm−1) was monitored with an increase in absorbance. The activity of the enzyme was expressed in terms of μmol of guaiacol oxidized min−1 g−1 FW. The polyphenol oxidase (EC 1.10.3.1) assay was performed (Patra and Mishra 1979) with the mixture consisting of 300 μmol phosphate buffer (pH 6.8), 5 μmol pyrogallol, and enzyme extract for 5 min. 5% (v/v) H2SO4 was used to stop the reaction followed by centrifugation for 15 min at 3000 × g. Enzyme activity was measured as an increase in the amount of purpurogallin formed in the reaction at 420 nm. The expression of the polyphenol oxidase activity was in terms of µg PPO mg−1 protein g−1 FW.
Ethylene production analysis
Ethylene emissions from maize plants were estimated using the protocol of Madhaiyan et al. (2007) with slight modification. Leaves were collected from maize plants under different treatments and were placed in 120 ml vials and were sealed with a rubber septum for 4 h. Air sample (1 ml) present in the headspace was injected into a Gas Chromatography machine (Thermo Scientific, USA) having a Poropak-Q column and flame ionization detector. The quantity of ethylene emission was expressed as nmol of ethylene h−1 g dry weight−1 and compared to the pure ethylene standard curve.
Evaluation of ACC level in plant tissue
ACC concentration in plant tissue was determined using the method established by Siddikee et al. (2012). One gram of maize leaf sample was immediately frozen in liquid nitrogen and crushed. Furthermore, ACC was extracted using methanol (80%) containing butylated hydroxyl toluene (BHT; 2 mg l−1) as an antioxidant and incubated for 45 min at room temperature. Sample suspension was then centrifuged at 2000×g at 20 °C for 15 min and was transferred in methanol and was again centrifuged. The resulting supernatant was evaporated to dryness using a rotatory evaporator. Residues were dissolved in distilled water and the upper aqueous phase was extracted using dichloromethane and was further mixed with HgCl2 (80 mmol) in test tubes and were sealed with a rubber septum. Afterward, a 0.2 ml NaOCl solution was added into the tubes, mixed while shaking, and incubated for 8 min. One ml of the gaseous portion was sampled and assayed for ethylene using gas chromatography (GC).
Estimation of enzyme activity involved in the ethylene biosynthetic pathway
Measurement of in vitro ACS and ACO activity were performed from the protein extracts as described by Siddikee et al. (2012). ACS activity was estimated from enzyme extract obtained by homogenizing one gram of leaf sample in 100 mmol Na-phosphate (pH 9.0) containing 5 mM pyridoxal phosphate (PLP), the mmol 2-mercaptoethanol (2-ME), 1 mmol EDTA, and 10% glycerol in the presence of 1 g polyvinylpolypyrrolidone (PVPP). Ammonium sulfate was supplemented to the enzyme extract for precipitation which was further dissolved in a solution containing 100 mmol Na-phosphate (pH 7.8), 5 mM pyridoxal phosphate (PLP), 0.5 mmol 2-ME, and 10% glycerol. All the steps related to enzyme preparation were performed at 4 °C. ACS activity was estimated by incubating the enzyme solution with 100 mmol 2-[4-(2-hydroxyethyl)-1-piperazinyl] ethanesulfonic acid (HEPES)–KOH (pH 8.5), 5 mmol PLP, 100 mmol S-adenosyl-l-methionine (SAM), and test chemicals at given concentrations in a total volume of 400 ml. After incubation of 15 min at 30 °C, the quantity of ACC formed was estimated as described above. For quantifying the ACO activity, frozen plant tissues were ground in liquid nitrogen and homogenized in extraction buffer containing 100 mmol Tris–HCl (pH 7.2), 10% (w/v) glycerol, and 30 mmol sodium ascorbate. The homogenate obtained was centrifuged at 15,000 × g for 15 min at 4 °C. Enzyme activity was estimated by incubating supernatant at 30 °C for 15 min in 10 ml screw-cap tubes fitted with a Teflon-coated septum in presence of 50 mmol FeSO4, 1 mmol ACC, and 5% (v/v) CO2. After the incubation period, the quantity of ethylene released into the headspace was determined by GC.
Tracking of Bacillus spp. in the rhizosphere of maize
To demonstrate the rhizosphere competence ability of bacterial strains on plant roots grown in non-sterilized soils, a spontaneous rifampicin-resistant (RifR) strain of NBRI 28B, NBRI 33 N, and NBRI 12 M was isolated on NA plates, comprising 250 µg rifampicin ml−1 (Sigma Chemical Co., St. Louis, USA) as described earlier (Nautiyal 1997). One gram of root of maize plant was thoroughly cleaned with tap water to remove all loosely adhered soil particles, followed by washing with sterile 0.85% NaCl (w/v) and crushed with a mortar and pestle. The heterogeneous bacterial population in the maize rhizosphere was recovered by serial dilution plating of the homogenate on NA plates and NA plates supplemented with 50 µg rifampicin ml−1. Average colonization (Log10 CFU/g root dry weight) of bacterial strains in the rhizosphere was estimated from three plants at the time of harvesting. No natural population of RifR bacteria was detected when root homogenates of uninoculated controls were plated from non-sterilized soils.
Statistical analysis
First, means were tested for homogeneity of variance to evaluate the variation among obtained values. Furthermore, these means were compared by analysis of variance (ANOVA), followed by the Duncan test to determine significance (p ≤ 0.05). Pearson’s coefficient was also calculated for possible correlation between ACC deaminase activity of Bacillus strains, components of the ethylene biosynthetic pathway and dry weight of plant under salt stress.
Results
Estimation of PGP and other attributes of Bacillus spp.
The selected three PGPR in this study were initially evaluated for qualitative estimation of nitrogen-fixing ability. It was observed that out of three PGPR only NBRI 12 M has the ability to fix nitrogen by changing the color of the medium from yellow to blue (Fig. S1). Moreover, considering biochemical properties, all the PGPR strains found to be positive for having different biochemical activities. However, none of the PGPR found positive for malonate activity (Table S1). NBRI 12 M and NBRI 28B exhibited most of the biochemical properties such as ONPG, sucrose, mannitol, glucose, arabinose, and trehalose (Table S1).
Concerning phosphate solubilization, we observed all the three PGPR exhibiting significant differences under normal and salt stress condition. NBRI 12 M recorded to have the highest phosphate solubilizing activity while the least phosphate solubilization was observed in NBRI 33 N under all the conditions (Table 1). Moreover, NBRI 33 N demonstrated the highest (7.79%) reduction in 0.5 M NaCl stress, while NBRI 12 M showed least (4.34%) reduction when compared to normal condition. Phosphate solubilization by each PGPR demonstrated a significant difference and was reduced remarkably under 1 M NaCl stress condition. There is a reduction in phosphate solubilization capability of three PGPR (NBRI 28B; 39.58%, NBRI 33 N; 70.64%, NBRI 12 M; 11.68%) when compared with normal condition.
Table 1.
Plant growth-promoting (PGP) attributes of Bacillus spp. under different salt (NaCl) stress (0 M, 0.5 M, and 1 M) condition
| Plant growth-promoting traits | P-Solubilization1 | IAA2 | ACC deaminase3 | Biofilm4 | EPS5 | Alg6 |
|---|---|---|---|---|---|---|
| NBRI 28B | ||||||
| Control | 28.88 ± 0.19c | 14.28 ± 0.12b | 2.73 ± 0.03a | 2.63 ± 0.00a | 649.13 ± 5.04a | 423.04 ± 0.70a |
| 0.5 M NaCl | 27.39 ± 0.16b | 14.11 ± 0.18b | 2.74 ± 0.07a | 2.66 ± 0.00b | 706.33 ± 1.52b | 524.48 ± 1.25b |
| 1 M NaCl | 20.70 ± 0.35a | 12.81 ± 0.14a | 2.77 ± 0.14b | 2.66 ± 0.00b | 909.35 ± 1.63c | 631.32 ± 1.12c |
| NBRI 33 N | ||||||
| Control | 27.58 ± 0.15c | 19.65 ± 0.26c | 3.06 ± 0.06a | 2.52 ± 0.00a | 255.01 ± 1.32a | 332.32 ± 0.85a |
| 0.5 M NaCl | 25.58 ± 0.11b | 17.80 ± 0.08b | 3.15 ± 0.00b | 2.58 ± 0.00b | 272.63 ± 1.52b | 391.84 ± 1.53c |
| 1 M NaCl | 16.16 ± 0.24a | 14.21 ± 0.12a | 3.16 ± 0.05b | 2.62 ± 0.00c | 567.75 ± 1.63c | 398.24 ± 0.58c |
| NBRI 12 M | ||||||
| Control | 37.48 ± 0.16c | 16.33 ± 0.06b | 3.82 ± 0.01a | 0.90 ± 0.00a | 843.26 ± 2.91a | 561.44 ± 0.97a |
| 0.5 M NaCl | 35.92 ± 0.19b | 16.19 ± 0.06b | 4.11 ± 0.10b | 1.68 ± 0.01b | 924.30 ± 1.13b | 575.68 ± 1.62b |
| 1 M NaCl | 33.56 ± 0.15a | 14.98 ± 0.12a | 4.12 ± 0.11b | 2.73 ± 0.00c | 1786.20 ± 9.09c | 845.12 ± 0.70c |
PGP attributes are expressed as mean values of three replicates ± SE which were compared by analysis of variance (ANOVA), followed by the Duncan test. Statistically significant differences were then determined at p ≤ 0.05, using the SPSS ver 20.0 and represented by different letters. Values in the rows with the same letter are not significantly different (p ≤ 0.05) by the Duncan test
1Phosphate (P) solubilization is expressed as μg ml−1
2Indole acetic acid (IAA) production, and
3ACC deaminase activity is expressed as nmol α-Ketobutyrate mg protein−1 h−1
4Biofilm was measured at an optical density (O.D) at 590 nm
5Exopolysaccharide (EPS) production, and
6Alginate (Alg) production is expressed as μg ml−1
In the case of IAA production, all three PGPR differed significantly among themselves under normal as well as salt stress condition (Table 1). NBRI 33 N reported being the most effective IAA producing PGPR, while NBRI 28B recorded to have the least IAA producing ability under normal and salt (0.5 M NaCl) stress condition. However, under 1.0 M NaCl condition, NBRI 12 M found to maintain its IAA producing ability at highest and retarded by only 8.28% while NBRI 33 N exhibited maximum declination of 27.67% when compared to normal condition.
Furthermore, all three PGPR strains were subjected to evaluating ACC deaminase activity under control and salt stress conditions. NBRI 12 M exhibited maximum, while NBRI 28B showed minimum ACC deaminase activity under normal and salt stress condition (Table 1). The maximum (7.28%) enhancement for this activity was observed in NBRI 12 M, while NBRI 28B found to be least (1.44%) enhanced under 1 M NaCl condition when compared to normal condition. All PGPR strains showed significant differences for the same under both normal as well as salt stress condition (Table 1).
Regarding biofilm formation under control and salt stress conditions, we observed that the formation of biofilm by all three PGPR was significantly different among themselves under normal and salt stress condition (Table 1). NBRI 28B demonstrated maximum biofilm formation capability followed by NBRI 33 N, while NBRI 12 M demonstrated lowest biofilm formation capability under the control condition (Table 1). Surprisingly, under salt (1 M NaCl) stress condition NBRI 12 M found to have the maximum capability to form biofilm and exhibited 67.03% more biofilm formation than normal condition. NBRI 28B have shown least (1.12%) enhancement in the biofilm-forming capacity as compared to normal condition.
Upon considering exo-polysaccharide (EPS) production all three PGPR strains demonstrated significant variation among themselves under normal and salt stress condition. NBRI 12 M exhibited maximum EPS production while NBRI 33 N accounted least in each condition (Table 1). Unexpectedly, under 0.5 M NaCl stress condition, we observed an enhancement in EPS production by all three PGPR strain (NBRI 28B; 8.07%, NBRI 33 N; 6.25%, NBRI 12 M; 8.76%). However, the increasing trend for EPS production was not only found to be consistent but also more intensed in 1 M NaCl stress condition by all the three PGPR strain (NBRI 28B; 28.60%, NBRI 33 N; 55.02%, NBRI 12 M; 52.79%) when compared with the non-stress condition (Table 1).
Similarly, with regards to alginate production ability, NBRI 12 M has demonstrated significantly higher alginate production capacity while NBRI 33 N recorded to have the least alginate production under control and salt stress condition (Table 1). Also, NBRI 12 M observed to have maximum enhancement (33.60%) for alginate production under 1 M NaCl when compared to the normal condition than the other two PGPR strains.
Salt-tolerance ability of Bacillus spp.
These potential PGPR strains were subsequently tested for their salt stress tolerance ability. At 0.5 and 1.0 M NaCl, NBRI 28B showed survival till day 10 with 6.24 Log10 CFU ml−1 and 5.26 Log10 CFU ml−1, respectively (Fig. 1). While NBRI 33 N survived with 5.77 Log10CFU ml−1 and 5.25 Log10 CFU ml−1 till day 10 under 0.5 and 1.0 M NaCl, respectively (Fig. 1). Moreover, NBRI 12 M has demonstrated its ability to withstand 0.5 and 1.0 M NaCl with 6.54 Log10CFU ml−1 and 5.58 Log10 CFU ml−1, respectively (Fig. 1).
Fig. 1.
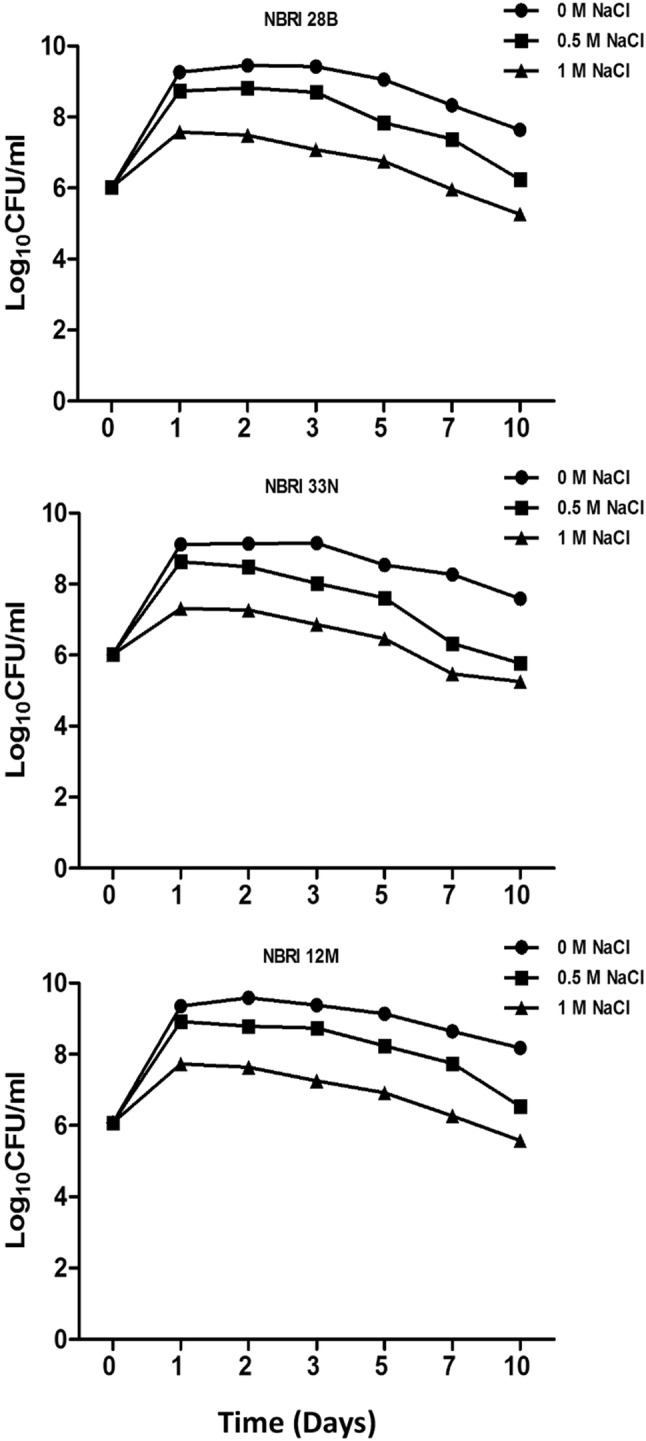
Temporal growth assessment of Bacillus spp. under different salt stress conditions. 0 M NaCl represents control for the experiment under the mentioned stress condition. Error bars are the standard error of the means (n = 3)
In general, NBRI 12 M identified as a potential PGPR to resist salt stress till the 10th day in comparison to rest three PGPR strain. Also, NBRI 12 M demonstrated least temporal fluctuations in colony count (4.85% lesser at day 1; 7.31% lesser at day 3; 10.80% lesser at day 5; 11.47% lesser at day 7) in comparison to that of other three PGPR strains under normal and 0.5 M NaCl salt stress condition. Similarly, it also showed an analogous trend for colony count (20.94% lesser at day 1; 29.35% lesser at day 3; 31.85% lesser at day 5; 38.01% lesser at day 7) under normal and 0 M NaCl salt stress condition.
Effect of Bacillus spp. inoculation on plant growth promotion and salt stress amelioration under greenhouse conditions
Effect of Bacillus spp. inoculation on maize vegetative parameters
The maize (Zea mays) crop plant was used as a host for determining growth promotion in the presence and absence of three PGPR strains under both normal and salt stress condition. Results demonstrated that inoculation with Bacillus spp. have significantly enhanced the overall plant vegetative properties as compared to the respective uninoculated controls under both normal and salt-stressed condition (Table 2, Fig. S2). Among the three PGPR, NBRI 12 M demonstrated significantly higher growth promotion in all the concerned vegetative parameters when compared with control plants (Table 2). However, NBRI 33 N accounted for significant lower values for shoot length and plant fresh weight. NBRI 33 N showed non-significant enhancement for root length, number of leaves and plant dry weight when compared with NBRI 28B under normal condition (Table 2). NBRI 12 M has significantly increased the shoot and root length, number of leaves, plant fresh and dry weight by 38.20%, 44.29%, 38.33%, 46.75%, 37.80% respectively under salt stress.
Table 2.
Measurement of maize vegetative parameters along with survival and competence in the rhizosphere of maize by Bacillus spp.
| Treatment | Plant growth parameters | Microbial population (Log10 CFU g−1) | |||||
|---|---|---|---|---|---|---|---|
| Shoot length (cm) | Root length (cm) | No. of leaves | Fresh plant wt. (g) | Dry plant wt. (g) | Heterogenous | Rifampicin resistant | |
| Control | 42.07 ± 1.67b | 24.13 ± 0.55c | 3.67 ± 0.33b | 5.58 ± 0.04b | 0.69 ± 0.02b | 7.62 ± 0.04 | 0 |
| Salt (100 mM NaCl) | 30.73 ± 0.52a | 17.27 ± 0.49a | 2.67 ± 0.33a | 3.86 ± 0.03a | 0.51 ± 0.03a | 5.66 ± 0.05 | 0 |
| NBRI 28B | 57.30 ± 0.76e | 27.50 ± 0.83d | 4.33 ± 0.33bc | 6.92 ± 0.03f | 0.78 ± 0.02 cd | 8.44 ± 0.07 | 7.06 ± 0.04 |
| NBRI 28B + S | 41.17 ± 0.75b | 26.07 ± 0.43d | 4.00 ± 0.00b | 6.31 ± 0.02d | 0.70 ± 0.01b | 7.05 ± 0.04 | 6.85 ± 0.06 |
| NBRI 33 N | 49.70 ± 0.84d | 27.50 ± 0.42d | 4.33 ± 0.33bc | 6.50 ± 0.03e | 0.74 ± 0.02bc | 8.74 ± 0.08 | 7.66 ± 0.05 |
| NBRI 33 N + S | 45.67 ± 1.21c | 21.07 ± 0.50b | 4.33 ± 0.33bc | 6.11 ± 0.03c | 0.71 ± 0.02b | 7.32 ± 0.05 | 6.88 ± 0.05 |
| NBRI 12 M | 55.73 ± 0.61e | 36.23 ± 0.18f | 5.00 ± 0.00c | 8.23 ± 0.03 h | 0.95 ± 0.01e | 8.77 ± 0.06 | 7.94 ± 0.07 |
| NBRI 12 M + S | 49.73 ± 0.97d | 31.00 ± 0.47e | 4.33 ± 0.33bc | 7.25 ± 0.02 g | 0.82 ± 0.01d | 7.53 ± 0.07 | 7.23 ± 0.08 |
Parameters are expressed as mean values of three replicates ± SE which were compared by analysis of variance (ANOVA), followed by the Duncan test. Statistically significant differences (signified by different letters) were then determined at p ≤ 0.05, using the software SPSS ver 20.0. Values in the columns with the same letter are not significantly different (p ≤ 0.05) by the Duncan test. Four different treatments refer to control (uninoculated; no salt); salt (uninoculated; 100 mM NaCl); NBRI 28B, NBRI 33 N, and NBRI 12 M (Zea mays treated with NBRI 28B, NBRI 33 N, and NBRI 12 M); and NBRI 28B + S, NBRI 33 N + S, and 12 M + S (Zea mays treated with NBRI 28B, NBRI 33 N, and NBRI 12 M under salt stress condition)
Effect of Bacillus spp. inoculation on chlorophyll (total), proline, and soluble sugar content of maize
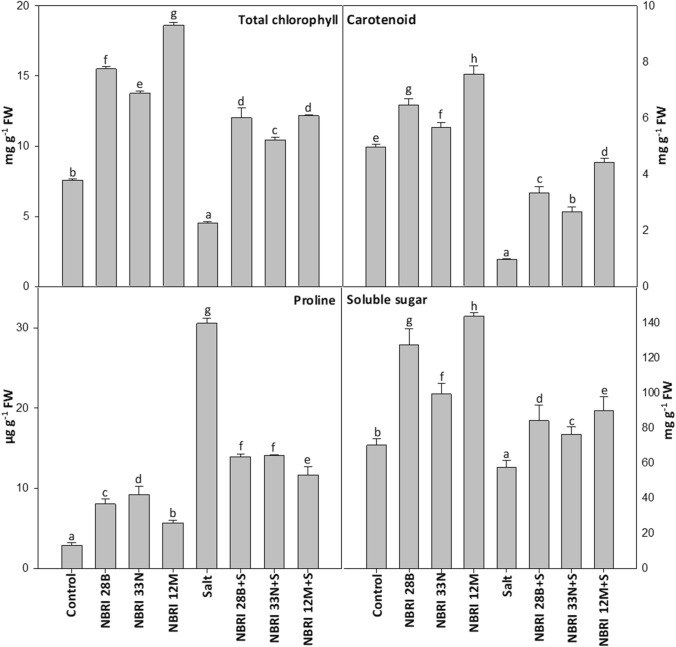
The response of Bacillus spp. treatment towards non-enzymatic properties of maize crop under normal and salt stress condition was determined by performing biochemical assays namely chlorophyll (total), carotenoid, proline, and soluble sugar. Inoculation of all the three PGPR has accounted for a significant increase in chlorophyll (total), carotenoid, and soluble sugar content when compared to uninoculated normal and salt control (Fig. 2). However, NBRI 12 M demonstrated maximum ability to enhance total chlorophyll, carotenoid, and soluble sugar content under salt stress condition by 62.60%, 78.15%, and 35.98%, respectively. Our results also showed a significant decrease in proline accumulation in PGPR-treated plants when compared to salt (control)-treated plants (Fig. 2). Moreover, NBRI 12 M inoculated plants accumulated 61.87% less proline, while NBRI 28B and NBRI 33 N decreased the proline accumulation in plants only by 54.44% and 53.83%, respectively.
Fig. 2.
Chlorophyll, proline, and soluble sugar in maize leaves. The values shown here are the mean of three replicates. Errors bars represent standard errors. Different letters above the bars represent significant differences according to the analysis of variance (ANOVA), followed by the Duncan test (p ≤ 0.05) applied using the software SPSS ver 20.0
Effect of Bacillus spp. inoculation on defense enzymes of maize
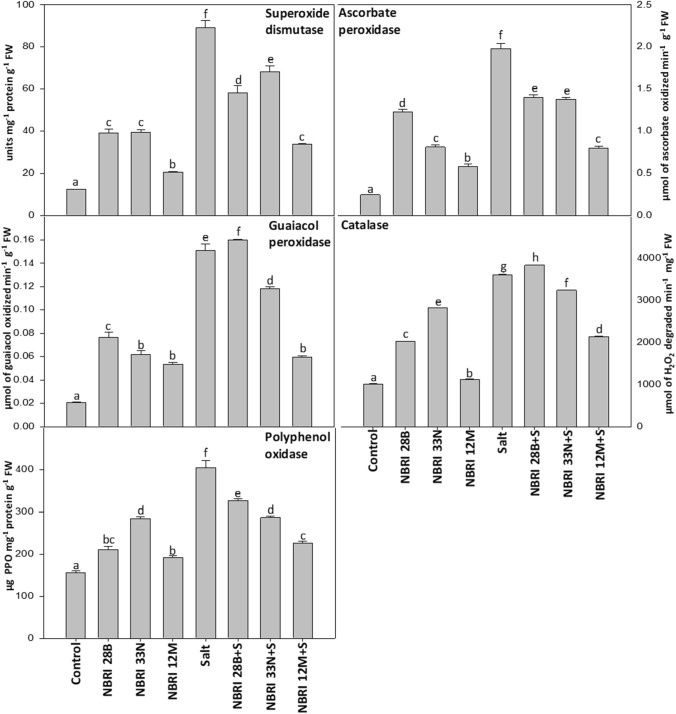
To evaluate the quenching of oxidative stress in maize plants, we performed defense enzyme assay comprising superoxide dismutase (SOD), ascorbate peroxidase (APX), guaiacol peroxidase (GPX), catalase (CAT), and polyphenol oxidase (PPO). All antioxidant enzymes were observed to be significantly maximum in salt (control)-treated plants (Fig. 3). Inoculation of all the three PGPR strains has significantly lowered the activity of defense enzymes under salt stress except NBRI 28B which demonstrated increased values for GPX and CAT, activity. Under salt stress, NBRI 12 M has exhibited a maximum reduction in the defense enzymatic activity by 62.08%, 59.93%, 60.40%, 40.70%, and 43.93% for SOD, APX, GPX, CAT, and PPO, respectively, on comparison with the only salt (control)-treated plants (Fig. 3). Moreover, NBRI 28B treatment exhibited the least decrement by 34.75% for SOD; 29.29% for APX; and 19.25% for PPO activity in maize plants under salt stress condition (Fig. 3).
Fig. 3.
Defense enzyme activities in maize leaves. The values shown here are the mean of three replicates. Errors bars represent standard errors. Different letters above the bars represent significant differences according to the analysis of variance (ANOVA), followed by the Duncan test (p ≤ 0.05) applied using the software SPSS ver 20.0
Effect of Bacillus spp. inoculation on ethylene production, ACC content, ACS, and ACO activities
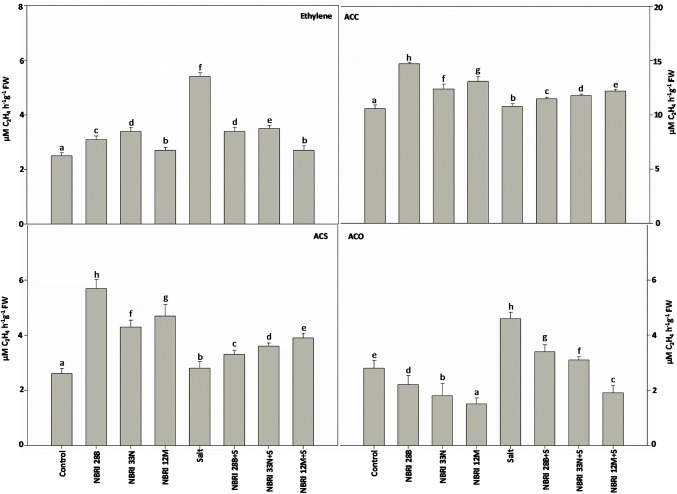
Ethylene production was increased significantly in maize plants by 26.47, 19.35, and 7.40% in NBRI 33 N, NBRI 28B, and NBRI 12 M inoculated maize plants, respectively, as compared to control under normal condition. Ethylene production was maximum in uninoculated plants receiving salt treatment. However, inoculation of NBRI 12 M, NBRI 28B, and NBRI 33 N ameliorated the ethylene emission by 50, 37.04, and 35.18%, respectively, as compared to alone salt inoculated plants (Fig. 4).
Fig. 4.
Effect of Bacillus spp. inoculation on ethylene biosynthesis in maize plants. The values shown here are the mean of three replicates. Errors bars represent standard errors. Different letters above the bars represent significant differences according to the analysis of variance (ANOVA), followed by the Duncan test (p ≤ 0.05) applied using the software SPSS ver 20.0
The ACC concentration in maize plants demonstrated significant enhancement in NBRI 28B, NBRI 12 M, and NBRI 33 N by 27.89, 19.08 and 14.51%, respectively, under normal condition in comparison to control treatment (Fig. 4). In addition, maize plants inoculated with NBRI 12 M showed maximum (11.47%), while NBRI 28B exhibited minimum enhancement (6.08%) in ACC content under salt stress condition (Fig. 4).
ACC synthase is an important enzyme in ethylene biosynthesis which catalyzes the conversion of SAM to ACC. In maize plants under normal condition, ACS activity recorded to be maximum in NBRI 28B and showed 54.38% enhancement as compared to control. Significant reduction of ACS activity was observed in salt stress condition but plants inoculated with NBRI 12 M, NBRI 33 N, and NBRI 28B demonstrated enhanced ACS activity by 28.20, 22.22, and15.15%, respectively, as compared to alone salt-treated plants (Fig. 4).
ACC-oxidase enzyme, which plays a crucial role in the ethylene biosynthetic pathway by converting ACC into ethylene. ACO activity was significantly reduced in PGPR-treated maize plants as compared to control under normal condition. NBRI 12 M-treated maize plants showed minimum while plants inoculated with NBRI 28B exhibited maximum activity. Stress caused due to salt in maize plants significantly enhanced the ACO activity in all the treatments. Inoculation of NBRI 12 M, NBRI 33 N, and NBRI 28B has significantly reduced the ACO activity by 58.69, 32.60, and 26.08%, respectively, as compared to alone salt-treated plants (Fig. 4).
Correlation between ACC deaminase activity, components of ethylene biosynthetic pathway, and dry weight of plant under salt stress condition
The correlation pattern between the ACC deaminase activity of Bacillus strains and components of the ethylene biosynthetic pathway was evaluated and it was found that most of the parameters are positively correlated except ACO and ethylene (Table 3). Moreover, ACC had a significant (p ≤ 0.05) negative correlation with the ethylene content of the plant under salt stress condition. A similar trend was exhibited in the case of ACS but non-significant. Ethylene showed a significant (p ≤ 0.05) negative correlation with dry weight of the plant under control condition (Table 3).
Table 3.
Pearson’s correlation based on modulation of ethylene biosynthetic pathway and dry weight of plant by ACC deaminase producing Bacillus strains under salt stress
| ACC deaminase | ACS | ACC | ACO | Ethylene | Dry wt. (salt) | Dry wt. (control) | |
|---|---|---|---|---|---|---|---|
| ACC deaminase | 1 | ||||||
| ACS | 0.968 | 1.000 | |||||
| ACC | 0.676 | 0.470 | 1.000 | ||||
| ACO | − 0.870 | − 0.718 | − 0.952 | 1.000 | |||
| Ethylene | − 0.781 | − 0.600 | − 0.988a | 0.988a | 1.000 | ||
| Dry wt. (salt) | 0.927 | 0.803 | 0.904 | − 0.991a | − 0.959 | 1.000 | |
| Dry wt. (control) | 0.802 | 0.626 | 0.983 | − 0.992a | − 0.999a | 0.968 | 1 |
aCorrelation is significant at the 0.05 level
Colonization of Bacillus spp. in the maize rhizosphere
Simultaneously, we observed the colonization ability of NBRI 28B, NBRI 33 N, and NBRI 12 M along with the heterogeneous bacterial population in maize rhizosphere under normal as well as salt stress condition. NBRI 12 M showed maximum colonizing and survival ability in maize rhizosphere with 7.94 Log10 CFU g−1 and 7.23 Log10 CFU g−1 under normal and stress condition (Table 2). However, under salt stress, NBRI 28B exhibited minimum survival capacity with 6.85 Log10 CFU g−1 (Table 2). Moreover, the heterogeneous bacterial population was found to be in the range of 7.05–8.77 Log10 CFU g−1 in maize rhizosphere under both normal as well as salt stress condition. However, salt stress has reduced the heterogeneous bacterial population to a maximum of 5.66 Log10 CFU g−1 (Table 2).
Discussion
Sustainable agriculture demands for increased crop production with amelioration of abiotic stresses. This can be achieved by the successful introduction of potential abiotic stress-tolerant and plant growth-promoting rhizobacteria. Keeping this challenge in mind, the present study is focused on accessing the potential of three Bacillus spp. on growth promotion and salt stress amelioration of maize. In the present study, we have studied the influence of three Bacillus spp., two strains closely related to B. subtilis from South Western Semi-Arid and North Eastern Plain zone each, while one strain closely related to B. safensis from Western Plain zone. Bacillus spp. has been known well for their stress tolerance and plant growth promotion but a comparative study with PGPR possessing multiple PGP attributes under salt stress condition has rarely been performed to enhance plant growth promotion and salt stress amelioration by mitigating ethylene stress (Misra et al. 2017; Radhakrishnan et al. 2017; Etesami and Beattie 2018).
The PGPR can influence plant growth directly or indirectly through several mechanisms (Glick 2014; Redmile-Gordon et al. 2014; Abiala et al. 2015; Gouda et al. 2018). Among PGPR, Bacillus spp. are known very well for their abundance in extreme conditions but their comparative analysis under a stressed environment is still scanty (Radhakrishnan et al. 2017). The advantage of using Bacillus strain as a PGPR for plant growth promotion under salt stress condition may be attributed to their ability to withstand extreme conditions in soil by producing endospores (Chakraborty et al. 2013). Earlier, several researchers have estimated PGP attributes of Bacillus spp. under salt stress condition (Goswami et al. 2014; Misra et al. 2017; Khan et al. 2016). Our findings demonstrated that NBRI 12 M showed better PGP attributes and tolerance towards salt stress than NBRI 28B and NBRI 33 N. Earlier reports demonstrating characterization of B. safensis under salt stress were scarce, however, its propitiousness for having PGP attributes have been well established by several researchers under normal condition (Chakraborty et al. 2013; Sarkar et al. 2018).
In our study, we have demonstrated that inoculation of salt-tolerant Bacillus spp. having ACC deaminase activity has significantly increased plant vegetative and biochemical parameters such as chlorophyll (total), carotenoid, and soluble sugar content which found to be in congruence with the findings of earlier studies (Ullah and Bano 2015; Vurukonda et al. 2016; Li and Jiang 2017; Misra et al. 2019). In addition, similar to previous reports, we also observed that proline accumulation was reduced significantly in plants receiving bacterial treatment under salt stress condition (Singh and Jha 2016; Tiwari et al. 2016; Curá et al. 2017; Misra et al. 2019). Among the three, NBRI 12 M (B. safensis) was more effective for improving plant vegetative properties suggesting its potential role in salt stress mitigation and growth promotion.
Proline accumulation in the plant is an important process playing a major role in maintaining the osmotic balance between intracellular and extracellular space while minimizing the damage caused by salt stress (Lei et al. 2016). Our results of PGPR-mediated reduced accumulation of proline in PGPR-treated plants under salt stress were found to be in corroboration with several previous reports (Singh and Jha 2016; Tiwari et al. 2016; Fukami et al. 2018). The possible reason behind such results could be that the exopolysaccharides (EPS) produced by PGPR may not only sequester the toxic ions but also promote the formation of biofilm on root surfaces of plant, thus restricting excess Na+ influx into roots and making it unreachable to plants under saline conditions (Ashraf et al. 2004; Upadhyay et al. 2011; Dodd and Perez-Alfocea 2012; Choudhary et al. 2016; Habib et al. 2016).
Antioxidant enzymes play an important role in plants under salt stress by controlling ROS production. The results of the present study demonstrated that the amount of antioxidative enzymes was significantly reduced in PGPR inoculated maize plants under salt stress (Fig. 3). Notwithstanding, all the three Bacillus strains did not correspond equally in plant growth promotion and salt stress amelioration under the greenhouse experiment. For instance, NBRI 12 M (B. safensis) demonstrated maximum reduction for antioxidant enzymes in comparison to NBRI 28B (B. subtilis) and NBRI 33 N (B. subtilis). Our findings were found to be in corroboration with previous studies stating that plants under different abiotic stresses exhibit high ROS quenching activity, while PGPR treatment tends to reduce the antioxidant activities (Vardharajula et al. 2011; Kang et al. 2014; Tiwari et al. 2016; Misra et al. 2019). Moreover, contrary to our results, several studies have reported that PGPR treatment under salt stress had increased the defense enzymatic activity in the host plants (Ullah and Bano 2015; Habib et al. 2016). However, the response mechanisms related to increased defense enzymes production are likely to incur a high cost to plants productivity. In addition, Kang et al. (2014) have established that the reduction in the activity of defense enzymes may suggest that PGPR-treated plants experience a low level of stress. Hence, NBRI 12 M could provide a beneficial effect in regulating plant integrity without losing its fitness under salt stress.
In general, salt stress strongly correlated with increased ethylene production (Madhaiyan et al. 2006; Yim et al. 2013, 2014). In the present study, salt-tolerant Bacillus strains with the ability to produce ACC deaminase activity regulated the ethylene level in plants under salt stress condition and resulted in plant growth promotion. Earlier reports have also demonstrated the role of PGPR in ameliorating salt stress through modulating the ethylene level (Siddikee et al. 2011; Bal et al. 2012; Misra et al. 2017).
Another crucial enzyme in ethylene biosynthesis is ACO. In the present study, maize plants under salt stress have demonstrated enhanced ACO activity as compared to plants under normal condition. In the presented study, application of PGPR resulted in a significant reduction in ACO activity, NBRI 12 M-treated maize plants have exhibited maximum reduction which is consistent with the low ethylene production under salt stress. Previous studies have also demonstrated that under high salinity, ethylene level increased due to enhanced ACO activity (Kukreja et al. 2005; Peng et al. 2014). However, Chen et al. have observed decreased ACO activity in wheat plants under salt stress condition (Chen et al. 2014). Thus, the results of the present study might suggest a reason for controlling ethylene biosynthesis in plants as a response towards adaption to salinity stress.
Growth and colonization of NBRI 28B, NBRI 33 N, and NBRI 12 M in the rhizosphere of maize were observed under normal and salt stress condition. Along with the native heterotrophic bacterial population, NBRI 12 M has effectively established itself to colonize and compete with the same concentration under both normal as well as salt stress condition. Our findings were supported by previous reports estimating the rhizosphere colonizing capability of PGPR in different host crops (Chauhan and Nautiyal 2010; Mishra et al. 2011; Mendis et al. 2018; Misra et al. 2019).
Conclusion
The role of stress-tolerant Bacillus strains having multifaceted PGP attributes under salt stress is indispensable for the confrontation of problems associated with salt stress. Our comparative study for salt-tolerant ACC deaminase producing bacterial strains exhibiting multiple PGP attributes under normal and salt stress condition revealed NBRI 12 M as a potent PGPR representing the Western Plain zone of UP, India. Moreover, PGPR possessing ACC deaminase activity able to modulate ethylene biosynthesis which ascertains their role in ameliorating salt stress and enhancing plant growth under salt stress. The present study will provide an advantage towards sustainable food crop development in areas facing abiotic stress such as salinity.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors acknowledge the Director, CSIR National Botanical Research Institute for providing facilities and support during the study.
Author contributions
PSC conceived and co-ordinated the research. SM conducted experiments and analyzed the data. PSC and SM wrote the manuscript. Both authors read and approved the manuscript.
Funding
The authors acknowledge the financial assistance from the CSIR Network project MLP022 and In-house project OLP105.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
References
- Abiala MA, Odebode AC, Hsu SF, Blackwood CB. Phytobeneficial properties of bacteria isolated from the rhizosphere of maize in southwestern Nigerian soils. Appl Environ Microbiol. 2015;81:4736–4743. doi: 10.1128/AEM.00570-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Arnon DI. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949;24:1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashraf M, Hasnain S, Berge O, Mahmood T. Inoculating wheat seedlings with exopolysaccharide-producing bacteria restricts sodium uptake and stimulates plant growth under salt stress. Biol Fertil Soils. 2004;40:157–162. [Google Scholar]
- Bal HB, Nayak L, Das S, Adhya TK. Isolation of ACC deaminase producing PGPR from rice rhizosphere and evaluating their plant growth-promoting activity under salt Stress. Plant Soil. 2012;355:1011–1014. [Google Scholar]
- Bates LS, Waldren RP, Teare ID. Rapid determination of free proline for water-stress studies. Plant Soil. 1973;39:205–207. [Google Scholar]
- Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971;44:276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Bric JM, Bostock RM, Silverstone SE. Rapid in situ assay for indole acetic acid production by bacteria immobilized on a nitrocellulose membrane. Appl Environ Microbiol. 1991;57:535–538. doi: 10.1128/aem.57.2.535-538.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty U, Chakraborty BN, Chakraborty AP, Dey PL. Water stress amelioration and plant growth promotion in wheat plants by osmotic stress tolerant bacteria. World J Microbiol Biotechnol. 2013;29:789–803. doi: 10.1007/s11274-012-1234-8. [DOI] [PubMed] [Google Scholar]
- Chauhan PS. NautiyalCS (2010) The purB gene controls rhizosphere colonization by Pantoea agglomerans. Lett Appl Microbiol. 2010;50(2):205–210. doi: 10.1111/j.1472-765X.2009.02779.x. [DOI] [PubMed] [Google Scholar]
- Chen D, Ma X, Li C, Zhang W, Xia G, Wang M. A wheat aminocyclopropane-1-carboxylate oxidase gene, TaACO1, negatively regulates salinity stress in Arabidopsis thaliana. Plant Cell Rep. 2014;33:1815–1827. doi: 10.1007/s00299-014-1659-7. [DOI] [PubMed] [Google Scholar]
- Choudhary DK, Kasotia A, Jain S, Vaishnav A, Kumari S, Sharma KP, Varma A. Bacterial-mediated tolerance and resistance to plants under abiotic and biotic stresses. J Plant Growth Regul. 2016;35:276–300. [Google Scholar]
- Curá JA, Franz DR, Filosofía JE, Balestrasse KB, Burgueño LE. Inoculation with Azospirillum sp. and Herbaspirillum sp. bacteria increases the tolerance of maize to drought stress. Microorganisms. 2017;5:1–16. doi: 10.3390/microorganisms5030041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd IC, Perez-Alfocea F. Microbial amelioration of crop salinity stress. J Exp Bot. 2012;63:3415–3428. doi: 10.1093/jxb/ers033. [DOI] [PubMed] [Google Scholar]
- Douriet-Gámez NR, Maldonado-Mendoza IE, Ibarra-Laclette E, Blom J, Calderón-Vázquez CL. Genomic analysis of Bacillus sp. strain B25, a biocontrol agent of maize pathogen Fusarium verticillioides. Curr Microbiol. 2018;75:247–255. doi: 10.1007/s00284-017-1372-1. [DOI] [PubMed] [Google Scholar]
- DuBois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350–356. [Google Scholar]
- Dubois M, Broeck LV, Inzé D. The pivotal role of ethylene in plant growth. Trends Plant Sci. 2018;23:311–323. doi: 10.1016/j.tplants.2018.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etesami H, Beattie GA. Mining halophytes for plant growth promoting halotolerant bacteria to enhance the salinity tolerance of non-halophytic crops. Front Microbiol. 2018;9:148. doi: 10.3389/fmicb.2018.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukami J, Cerezini P, Hungria M. Azospirillum: benefits that go far beyond biological nitrogen fixation. AMB Express. 2018;8:73. doi: 10.1186/s13568-018-0608-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick BR. Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol Res. 2014;169:30–39. doi: 10.1016/j.micres.2013.09.009. [DOI] [PubMed] [Google Scholar]
- Goswami D, Dhandhukia P, Patel P, Thakker JN. Screening of PGPR from saline desert of Kutch: growth promotion in Arachis hypogea by Bacillus licheniformis A2. Microbiol Res. 2014;169:66–75. doi: 10.1016/j.micres.2013.07.004. [DOI] [PubMed] [Google Scholar]
- Gothwal RK, Nigam VK, Mohan MK, Saamal D, Gosh P. Screening of nitrogen fixer from rhizospheric bacterial isolates associated with important desert plants. AEER. 2007;6:101–109. [Google Scholar]
- Gouda S, Kerry RG, Das G, Paramithiotis S, Shin S, Patra JK. Revitalization of plant growth promoting rhizobacteria for sustainable development in agriculture. Microbiol Res. 2018;206:131–140. doi: 10.1016/j.micres.2017.08.016. [DOI] [PubMed] [Google Scholar]
- Habib SH, Kausar H, Saud HM. Plant growth-promoting rhizobacteria enhance salinity stress tolerance in okra through ROS-scavenging enzymes. Biomed Res Int. 2016;2016:6284547. doi: 10.1155/2016/6284547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemeda HM, Klein BP. Effects of naturally occurring antioxidants on peroxidase activity of vegetable extracts. J Food Sci. 1990;55:184–185. [Google Scholar]
- Kang SM, Khan AL, Waqas M, You Y, Kim J, Kim J, Hamayun M, Lee I. Plant growth promoting rhizobacteria reduce adverse effects of salinity and osmotic stress by regulating phytohormones and antioxidants in Cucumis sativus. J Plant Interact. 2014;9:673–682. [Google Scholar]
- Kang SM, Radhakrishnan R, Lee KE, You YH, Ko JH, Kim JH, Lee IJ. Mechanism of plant growth promotion elicited by Bacillus sp. LKE15 in oriental melon. Acta Agric Scand Sect B Soil Plant Sci. 2015;65:637–647. [Google Scholar]
- Khan A, Sirajuddin ZXQ, Javed MT, Khan KS, Bano A, Shen RF, Masood S. Bacillus pumilus, enhances tolerance in rice (Oryza sativa, L.) to combined stresses of NaCl and high boron due to limited uptake of Na+ Environ Exp Bot. 2016;124:120–129. [Google Scholar]
- Kukreja S, Nandwal A, Kumar N, Sharma S, Unvi V, Sharma P. Plant water status, H2O2 scavenging enzymes, ethylene evolution and membrane integrity of Cicer arietinum roots as affected by salinity. Biol Plant. 2005;49:305–308. [Google Scholar]
- Laditi MA, Nwoke OC, Jemo M, Abaidoo RC, Ogunjobi AA. Evaluation of microbial inoculants as biofertilizers for the improvement of growth and yield of soybean and maize crops in savanna soils. Afr J Agric Res. 2012;7:405–413. [Google Scholar]
- Lata S. Measurement of agricultural productivity and water productivity of crops. In: Nusser M, editor. Irrigation water management for agricultural development in Uttar Pradesh, India. Advances in Asian human-environmental research. Berlin: Springer; 2019. [Google Scholar]
- Lei P, Xu Z, Liang J, Luo X, Zhang Y, Feng X. Poly (γ-glutamic acid) enhanced tolerance to salt stress by promoting proline accumulation in Brassica napus L. Plant Growth Regul. 2016;78:233. [Google Scholar]
- Li HQ, Jiang XW. Inoculation with plant growth-promoting bacteria (PGPB) improves salt tolerance of maize seedling. Russ J Plant Physiol. 2017;64:235–241. [Google Scholar]
- Li J, Xu H, Liu W, Zhang X, Lu Y. Ethylene inhibits root elongation during alkaline stress through AUXIN1 and associated changes in auxin accumulation. Plant Physiol. 2015;168:1777–1791. doi: 10.1104/pp.15.00523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhaiyan M, Poonguzhali S, Ryu J, Sa T. Regulation of ethylene levels in canola (Brassica campestris) by 1-aminocyclopropane-1-carboxylate deaminase-containing Methylobacterium fujisawaense. Planta. 2006;224:268–278. doi: 10.1007/s00425-005-0211-y. [DOI] [PubMed] [Google Scholar]
- Madhaiyan M, Poonguzhali S, Sa T. Characterization of 1-aminocyclopropane- 1-carboxylate (ACC) deaminase containing Methylobacterium oryzae and interactions with auxins and ACC regulation of ethylene in canola (Brassica campestris) Planta. 2007;226:867–876. doi: 10.1007/s00425-007-0532-0. [DOI] [PubMed] [Google Scholar]
- Mendis HC, Thomas VP, Schwientek P, Salamzade R, Chien JT, Waidyarathne P, Kloepper J, De La Fuente L. Strain-specific quantification of root colonization by plant growth promoting rhizobacteria Bacillus firmus I-1582 and Bacillus amyloliquefaciens QST713 in non-sterile soil and field conditions. PloS One. 2018;13:2. doi: 10.1371/journal.pone.0193119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra A, Chauhan PS, Chaudhry V, Tripathi M, Nautiyal CS. Rhizosphere competent Pantoea agglomerans enhances maize (Zea mays) and chickpea (Cicer arietinum L.) growth, without altering the rhizosphere functional diversity. Antonie Van Leeuwenhoek. 2011;100:405–413. doi: 10.1007/s10482-011-9596-8. [DOI] [PubMed] [Google Scholar]
- Mishra S, Mishra A, Chauhan PS, Mishra SK, Kumari M, Niranjan A, Nautiyal CS. Pseudomonas putida NBRIC19 dihydrolipoamide succinyltransferase (SucB) gene controls degradation of toxic allelochemicals produced by Parthenium hysterophorus. J Appl Microbiol. 2012;112:793–808. doi: 10.1111/j.1365-2672.2012.05256.x. [DOI] [PubMed] [Google Scholar]
- Mishra SK, Khan MH, Misra S, Kant VK, Khare P, Srivastava S, Chauhan PS. Characterization of Pseudomonas spp. and Ochrobactrum sp. isolated from volcanic soil. Antonie Van Leeuwenhoek. 2017;110:253–270. doi: 10.1007/s10482-016-0796-0. [DOI] [PubMed] [Google Scholar]
- Misra S, Dixit VK, Khan MH, Mishra SK, Dviwedi G, Yadav S, Lehri A, Chauhan PS. Exploitation of agro-climatic environment for selection of 1-aminocyclopropane-1-carboxylic acid (ACC) deaminase producing salt-tolerant indigenous plant growth promoting rhizobacteria. Microbiol Res. 2017;205:25–34. doi: 10.1016/j.micres.2017.08.007. [DOI] [PubMed] [Google Scholar]
- Misra S, Dixit VK, Mishra SK, Chauhan PS. Demonstrating the potential of abiotic stress-tolerant Jeotgalicoccus huakuii NBRI 13E for plant growth promotion and salt stress amelioration. Ann Microbiol. 2019;69:419–434. [Google Scholar]
- Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in Spinach chloroplasts. Plant Cell Physiol. 1981;22:867–880. [Google Scholar]
- Nautiyal CS. A method for selection and characterization of rhizosphere-competent bacteria of chickpea. Curr Microbiol. 1997;34:12–17. doi: 10.1007/s002849900136. [DOI] [PubMed] [Google Scholar]
- Nautiyal CS. An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol Lett. 1999;170:265–270. doi: 10.1111/j.1574-6968.1999.tb13383.x. [DOI] [PubMed] [Google Scholar]
- Nautiyal CS, Srivastava S, Chauhan PS, Seem K, Mishra A, Sopory SK. Plant growth-promoting bacteria Bacillus amyloliquefaciens NBRISN13 modulates gene expression profile of leaf and rhizosphere community in rice during salt stress. Plant Physiol Biochem. 2013;66:1–9. doi: 10.1016/j.plaphy.2013.01.020. [DOI] [PubMed] [Google Scholar]
- Panta S, Flowers TJ, Lane P, Doyle R, Haros G, Shabala S. Halophyte agriculture: success stories. Environ Exp Bot. 2014;107:71–83. [Google Scholar]
- Patra HK, Mishra M. Pyrophosphatase, peroxidase and polyphenol oxidase activities during leaf development and senescence. Plant Physiol. 1979;63:318–323. doi: 10.1104/pp.63.2.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Z, He S, Gong W, Sun J, Pan Z, Xu F, Lu Y, Du X. Comprehensive analysis of differentially expressed genes and transcriptional regulation induced by salt stress in two contrasting cotton genotypes. BMC Genomics. 2014;15:760. doi: 10.1186/1471-2164-15-760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penrose DM, Glick BR. Methods for isolating and characterizing ACC deaminase containing plant growth promoting rhizobacteria. Physiol Plant. 2003;118:10–15. doi: 10.1034/j.1399-3054.2003.00086.x. [DOI] [PubMed] [Google Scholar]
- Radhakrishnan R, Hashem A, Abd-Allah EF. Bacillus: a biological tool for crop improvement through bio-molecular changes in adverse environments. Front Physiol. 2017;8:667. doi: 10.3389/fphys.2017.00667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redmile-Gordon MA, Brookes PC, Evershed RP, Goulding KWT, Hirsch PR. Measuring the soil-microbial interface: extraction of extracellular polymeric substances (EPS) from soil biofilms. Soil Biol Biochem. 2014;72:163–171. [Google Scholar]
- Sarkar J, Chakraborty B, Chakraborty UJ. Plant growth promoting rhizobacteria protect wheat plants against temperature stress through antioxidant signalling and reducing chloroplast and membrane injury. Plant Growth Regul. 2018;37:1396. [Google Scholar]
- Siddikee MA, Glick BR, Chauhan PS, Yim WJ, Sa T. Enhancement of growth and salt tolerance of red pepper seedlings (Capsicum annuum L.) by regulating stress ethylene synthesis with halotolerant bacteria containing 1-aminocyclopropane-1-carboxylic acid deaminase activity. Plant Physiol Biochem. 2011;49:427–434. doi: 10.1016/j.plaphy.2011.01.015. [DOI] [PubMed] [Google Scholar]
- Siddikee MA, Chauhan PS, Sa T. Regulation of ethylene biosynthesis under salt stress in red pepper (Capsicum annuum L.) by 1-aminocyclopropane- 1-carboxylic acid (ACC) deaminase-producing halotolerant bacteria. J Plant Growth Regul. 2012;31:265–272. [Google Scholar]
- Singh RP, Jha PN. The multifarious PGPR Serratia marcescens CDP-13 augments induced systemic resistance and enhanced salinity tolerance of wheat (Triticum aestivum L.) PLoS ONE. 2016;11:e0155026. doi: 10.1371/journal.pone.0155026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S, Yadav A, Seem K, Mishra S, Chaudhary V, Nautiyal CS. Effect of high temperature on Pseudomonas putida NBRI0987 biofilm formation and expression of stress sigma factor RpoS. Curr Microbiol. 2008;56:453–457. doi: 10.1007/s00284-008-9105-0. [DOI] [PubMed] [Google Scholar]
- Tewari S, Arora NK. Multifunctional exopolysaccharides from Pseudomonas aeruginosa PF23 involved in plant growth stimulation, biocontrol and stress amelioration in sunflower under saline conditions. Curr Microbiol. 2014;69:484–494. doi: 10.1007/s00284-014-0612-x. [DOI] [PubMed] [Google Scholar]
- Titus S, Gasnkar N, Srivastava KB, Karande AA. Exopolymer production by a fouling marine bacterium Pseudomonas alcaligenes. Indian J Mar Sci. 1995;24:45–48. [Google Scholar]
- Tiwari S, Lata C, Chauhan PS, Nautiyal CS. Pseudomonas putida attunes morphophysiological, biochemical and molecular responses in Cicer arietinum L. during drought stress and recovery. Plant Physiol Biochem. 2016;99:108–117. doi: 10.1016/j.plaphy.2015.11.001. [DOI] [PubMed] [Google Scholar]
- Ullah S, Bano A. Isolation of plant-growth-promoting rhizobacteria from rhizospheric soil of halophytes and their impact on maize (Zea mays L.) under induced soil salinity. Can J Microbiol. 2015;61:307–313. doi: 10.1139/cjm-2014-0668. [DOI] [PubMed] [Google Scholar]
- Upadhyay SK, Singh JS, Singh DP. Exopolysaccharide-producing plant growth-promoting rhizobacteria under salinity condition. Pedosphere. 2011;21:214–222. [Google Scholar]
- Vardharajula S, Ali SZ, Grover M, Reddy G, Bandi V. Drought tolerant plant growth promoting Bacillus spp: effect on growth, osmolytes, and antioxidant status of maize under drought stress. J Plant Interact. 2011;6:1–14. [Google Scholar]
- Verma M, Mishra J, Arora NK. Plant growth-promoting rhizobacteria: diversity and applications. In: Sobti R, Arora N, Kothari R, editors. Environmental biotechnology: for sustainable future. Singapore: Springer; 2019. [Google Scholar]
- Vurukonda SSKP, Vardharajula S, Shrivastava M, Ali SKZ. Enhancement of drought stress tolerance in crops by plant growth promoting rhizobacteria. Microbiol Res. 2016;184:13–24. doi: 10.1016/j.micres.2015.12.003. [DOI] [PubMed] [Google Scholar]
- Wang W, Wu Z, He Y, Huang Y, Li X, Ye B. Plant growth promotion and alleviation of salinity stress in Capsicum annuum L. by Bacillus isolated from saline soil in Xinjiang. Ecotoxicol Environ Saf. 2018;164:520–529. doi: 10.1016/j.ecoenv.2018.08.070. [DOI] [PubMed] [Google Scholar]
- Yim WJ, Sundaram S, Kim KY, Lee G, Sa T. Ethylene emission and PR protein synthesis in ACC deaminase producing Methylobacterium spp. inoculated tomato plants (Lycopersicon esculentum Mill.) challenged with Ralstonia solanacearum under greenhouse conditions. Plant Physiol Biochem. 2013;67:95–104. doi: 10.1016/j.plaphy.2013.03.002. [DOI] [PubMed] [Google Scholar]
- Yim WJ, Kim KY, Lee YW, Sundaram SP, Lee Y, Sa T. Real time expression of ACC oxidase and PR-protein genes mediated by Methylobacterium spp. in tomato plants challenged with Xanthomonas campestris pv. Vesicatoria. J Plant Physiol. 2014;171:1064–1075. doi: 10.1016/j.jplph.2014.03.009. [DOI] [PubMed] [Google Scholar]
- Zörb C, Geilfus CM, Dietz KJ. Salinity and crop yield. Plant Biol. 2018;21:31–38. doi: 10.1111/plb.12884. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.