Abstract
Background
In the present study, we present our experience for surgical excision for cardiac masses and to analyze survival characteristics of these patients.
Methods
Between January 2004 and December 2015, a total of 131 patients (88 females, 43 males; mean age 49.4±16.2 years; range, 1.2 months to 81 years) with primary cardiac tumors who underwent surgery in our center were included in this study. Demographic and other patient-related data were retrospectively reviewed from medical records of our center.
Results
All benign tumors were completely resected, whereas only palliative procedures were performed for malignant tumors. Pathology results revealed 88.5% (n=116) benign and 11.5% (n=15) malignant tumors. Tumors were most frequently located in the left atrium (76.3%, n=100), followed by the right atrium (11.5%, n=15), and the right ventricle (5.3%, n=7). Among all patients, 116 (88.5%) survived, while late mortality was seen in 15 patients (11.5%). The mean survival was 130.6±4.5 months. The latest mortality was observed at 124 months, whereby the cumulative survival rate was 79.2%. There was a statistically significant relationship between mortality and pathological characteristics of the tumor, and malignant cases had significantly higher mortality rates (p=0.002).
Conclusion
Surgical resection of primary cardiac tumors can be performed with low morbidity and mortality rates. Although survival rates in benign tumors are satisfactory, patients with malignant tumors have poor prognosis. The main clinical predic
Keywords: Cardiac mass, mortality, myxoma, primary cardiac tumor, survival
Introduction
Primary cardiac tumors are rare diseases with reports of prevalence from autopsy series ranging from 0.001 to 0.3%.[1-3] Three-quarters of the tumors are benign, with nearly 50% of which are myxomas, whereas angiosarcoma is the most common primary malignant cardiac tumor.[4,5] Metastatic tumors of the heart are seen much more frequently than tumors originating from the heart.
King[6] published the first report of a primary cardiac tumor in 1845, while the first surgical excision for one was performed after a century by Bahnson and Newman.[7] Literature on both management and outcome is, however, quite limited due to the rarity of cardiac tumors and small-scale surgical series. While surgical intervention in case of benign tumors appears to be curative, survival of malignant tumors still remains dismal.
In the present study, we aimed to present our experience and to analyze survival characteristics of patients undergoing surgical excision for cardiac masses.
Patients and Methods
Between January 2004 and December 2015, a total of 131 patients (88 females, 43 males; mean age 49.4±16.2 years; range, 1.2 months to 81 years) with primary tumors of the heart underwent surgery in our center. These cases constituted 1.05% of all 13,819 open heart operations performed during the same period in our center. Metastatic cardiac tumors were excluded from the study. The study protocol was approved by the Dr. Siyami Ersek Thoracic and Cardiovascular Surgery Training and Research Hospital Ethics Committee. The study was conducted in accordance with the principles of the Declaration of Helsinki. Transthoracic echocardiography (TTE) and computed tomography (CT) were used for diagnosis in all patients. Surgical approach to all patients was through median sternotomy and on cardiopulmonary bypass with aortic and bicaval venous cannulation. Cardiac protection was achieved with moderate systemic hypothermia, deep topical hypothermia, and intermittent hyperkalemic antegrade blood cardioplegia. Approach to tumor resection was through right or/and left atriotomy or ventriculotomy depending on the tumor location. Concomitant procedures included mitral valve replacement (MVR) in 18 patients (11.3%), coronary artery bypass grafting (CABG) in 13 patients (8.1%), and Glenn shunt in a two-year-old case with pulmonary stenosis (0.6%).
Demographic and other patient-related data were retrospectively reviewed from medical records of our center. Details regarding follow-up and survival data of these patients were obtained from subsequent clinic visits and telephone follow-ups. Missing surveillance data were completed using the nationwide database of the Ministry of Interior General Directorate of Civil Registration and Nationality.
Statistical analysis
Statistical analyses were performed using the Number Cruncher Statistical System (NCSS) 2007 statistical software program (NCSS LLC, Kaysville, Utah, USA). Descriptive statistics were expressed in mean ± standard deviation (SD), number and frequency (%). The Mann-Whitney U test was used for comparison of descriptive data and quantitative data which were abnormally distributed. The Yates's Continuity Correction and Fisher-Freeman-Halton test were used to compare the qualitative data. The Kaplan-Meier survival analysis was used for survival analysis. A p value of <0.05 was considered statistically significant with 95% confidence interval.
Results
The mean length of hospital stay was 13.7±8.4 (range, 1 to 58) days. The mean follow-up was 62.2±44.0 (range, 3 to 149) months. The mean EuroSCORE was 2.6±3.1 (range, 0.9 to 30.6) (Table 1).
Table 1. Patient characteristics.
| n | % | Mean±SD | Median | Min-Max | |
| Age (year) | 49.4±16.2 | 51 | 0,10-81 | ||
| Length of hospital stay (days) | 13.7±8.4 | 11 | 1-58 | ||
| Follow-up duration (months) | 62.2±44.0 | 50 | 3-149 | ||
| EuroSCORE | 2.6±3.1 | 1,8 | 0.9-30.6 | ||
| Gender | |||||
| Male | 43 | 32,8 | |||
| Female | 88 | 67,2 | |||
| Long-term mortality | 15 | 11,5 | |||
| Pathology | |||||
| Benign | 116 | 88,5 | |||
| Malign | 15 | 11,5 | |||
| Localization | |||||
| Left atrium | 100 | 76,3 | |||
| Right atrium | 15 | 11,5 | |||
| Right ventricle | 7 | 5,3 | |||
| Left ventricle | 4 | 3,1 | |||
| Mitral valve | 2 | 1,5 | |||
| Pericardium | 2 | 1,5 | |||
| Bilateral atrial | 1 | 0,8 | |||
| SD: Standard deviation; Min: Minimum; Max: Maximum. | |||||
The pathology results revealed benign masses in 88.5% (n=116) and malignant masses in 11.5% (n=15). Tumors were most frequently located in the left atrium (76.3%, n=100), followed by the right atrium (11.5%, n=15), right ventricle (5.3%, n=7), left ventricle (3.1%, n=4), mitral valve (1.5%, n=2), pericardium (1.5%, n=2), and both atria (0.8%, n=1).
No in-hospital mortality was observed in any of the cases, while long-term mortality was found to be 11.5% (n=15).
A total of 20.6% (n=27) of the patients required patch closure, 7.6% (n=10) required MVR, and 6.9% (n=9) required CABG + tumor resection. Comorbidities included diabetes in 6.1% (n=8), hypertension in 22.1% (n=29), chronic obstructive pulmonary disease (COPD) in 3.8% (n=5), hyperlipidemia in 2.3% (n=3), and chronic renal failure in 3.1% (n=4) patients (Table 2).
Table 2. Comorbidities of patients.
| n | % | |
| Additional surgical procedure | ||
| Patch closure | 27 | 20,6 |
| Mitral valve replacement | 10 | 7,6 |
| Coronary artery bypass greft | 9 | 6,9 |
| Diabetes | 8 | 6,1 |
| Hypertension | 29 | 22,1 |
| Chronic obstructive pulmonary disease | 5 | 3,8 |
| Hyperlipidemia | 3 | 2,3 |
| Chronic renal failure | 4 | 3,1 |
| Atrial fibrillation | 23 | 17,6 |
| Emboli | 11 | 8,4 |
| Previous cardiac surgery | 8 | 6,1 |
| Intra-aortic blood pressure monitorization | 1 | 0,8 |
| Nodal rhythm | 3 | 2,3 |
| Recurrence | 4 | 3,1 |
Atrial fibrillation was present in 17.6% (n=23), embolism in 8.4% (n=11), previous cardiac surgery in 6.1% (n=8), intra-aortic balloon pump in 0.8% (n=1), nodal rhythm in 2.3% (n=3), and recurrence in 3.1% (n=4). Four of the patients with a history of previous cardiac surgery were operated for myxoma and had recurrence. The rest of the previous operations were for MVR with tricuspid annuloplasty in one, aortic and MVR in one, MVR in one, and mitral valve repair in one patient.
Among 131 surgeries performed, 116 patients (88.5%) survived with 15 late mortalities. The mean survival was 130.6±4.5 months. The latest mortality was observed at 124 months, while the cumulative survival rate was 79.2% with a standard error of 5.8% (Figure 1).
Figure 1. Kaplan-Meier survival analysis (long-term mortality).
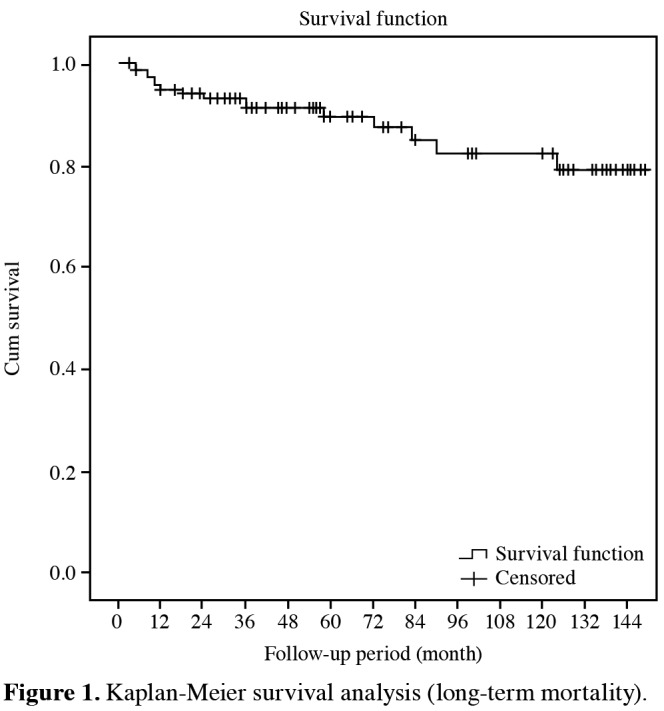
No statistically significant differences were found in age and gender distributions of patients with and without long-term mortality (p>0.05). However, the length of hospital stay (p=0.004) and EuroSCORE (p=0.011) were statistically higher in the patients with long-term mortality (Table 3).
Table 3. Evaluation of long-term mortality.
| Long term mortality (+) | Long term mortality (-) | ||||||||||
| n | % | Mean±SD | Median | Min-Max | n | % | Mean±SD | Median | Min-Max | p | |
| Age (year) | 50.8±24.5 | 60 | 0,10-81 | 49.2±14.9 | 51 | 0,33-76 | 0.327§ | ||||
| Length of hospital stay (days) | 20.5±13.3 | 17 | 8-58 | 12.9±7.2 | 11 | 1-43 | 0.004**§ | ||||
| EuroSCORE | 3.3±2.3 | 2,6 | 1.2-8.3 | 2.4±3.2 | 1,6 | 0.9-30.6 | 0.011*§ | ||||
| Gender | 0.804† | ||||||||||
| Male | 4 | 9,3 | 39 | 90,7 | |||||||
| Female | 11 | 12,5 | 77 | 87,5 | |||||||
| Pathology | 0.002**‡ | ||||||||||
| Benign | 9 | 7,8 | 107 | 92,2 | |||||||
| Malign | 6 | 40,0 | 9 | 60,0 | |||||||
| Localization | 0.003**¶ | ||||||||||
| Left atrium | 7 | 7,0 | 93 | 93,0 | |||||||
| Right atrium | 1 | 6,7 | 14 | 93,3 | |||||||
| Right ventricle | 3 | 42,9 | 4 | 57,1 | |||||||
| Left ventricle | 2 | 50,0 | 2 | 50,0 | |||||||
| Mitral valve | 1 | 50,0 | 1 | 50,0 | |||||||
| Pericardium | 1 | 50,0 | 1 | 50,0 | |||||||
| Bilateral atrial | 0 | 0 | 1 | 100 | |||||||
| SD: Standard deviation; Min: Minimum; Max: Maximum; § Mann-Whitney U test; † Yates’s corrected chi-square test; ‡ Fisher’s exact test; ¶ Fisher-Freeman-Halton test; * p<0.05; ** p<0.0. | |||||||||||
A statistically significant relationship was observed between mortality and pathological characteristics of the tumor, and malignant cases had significantly higher mortality rates (p=0.002).
In addition, we found a significant relationship between mortality and tumor localization (p=0.003; p<0.01). Mortality was lower in cases with tumors of the left and right atrium, while it was higher for the tumors localized in the left ventricle.
Discussion
Despite the increase in available diagnostic methods, cardiac tumors are still very rarely make large series of surgery for primary cardiac tumors, and the effective analysis of survival is still challenging. Compared to the previous series (0.4 to 0.85%),[8,9] the rate of patients who were operated due to cardiac masses in our series was found to be higher (1.1%). The fact that our center is a tertiary center where the patients are referred for treatment makes it possible to analyze more cases. In our study, there was no all-cause in-hospital mortality, and the rate of late mortality (>30 day) was 11.5% (n=15/131).
Metastatic cardiac tumors are 20 to 40 times more common than tumors originating from the heart.[10,11] Three-quarters of the primary cardiac tumors are benign with nearly 50% of which are myxomas, whereas rhabdomyosarcomas are the most common type in children.[4,5] Our case series is compatible with previously published series in which the majority of primary heart tumors were benign and most of them were myxomas.[12] Also, pathology results revealed 88.5% (n=116) benign and 11.5% (n=15) malignant tumors.
Improvements in radiological imaging methods have facilitated the diagnosis of cardiac masses. Transesophageal echocardiography (TEE) may be required for more comprehensive assessment of the relationship of mass with surrounding tissue and valve competence, whereas TTE is used as the initial diagnostic method of cardiac masses.[13,14] In addition, CT and magnetic resonance imaging (MRI) are wellestablished to assess the location, size, morphology, depiction of the great vessels, pericardium, and associated extracardiac structures.[15,16] In our study, we performed TTE and CT during the preoperative evaluation for all patients, and TEE was performed for the patients in whom TTE was insufficient for assessing the intracardiac location of the mass and relationship between the associated structures, such as valve and septum.
There is no consensus on surgical approach to intracardiac masses. Some authors have recommended both right and left atriotomy to evaluate all four chambers during operation.[17] In our series, left atriotomy was performed in 101 patients (87%) and right in 15 patients (13%). A biatrial incision was required in only one patient to evaluate all four chambers due to the lack of clear evaluation of the relationship between the mass and the cardiac chambers on TEE. The histopathological diagnosis of this patient was evaluated as an angiosarcoma. We believe that visual examination of all four cardiac chambers is unnecessary, if a detailed examination of the TEE is performed.
Another controversial issue concerns excision of the mass. Although some authors have suggested extensive excision and patch repair, others have argued that a simple excision is sufficient.[18-20] We performed patch repair in 28 patients in our series due to the need for wide excision. Many hypotheses have been proposed on the causes of recurrence, such as multifocality, intraoperative seeding, and malignant transformation.[21,22] Based on our own experience, we observed no causes related with recurrence.
In the present study, we found a statistically significant relationship between the location of the cardiac mass and long-term mortality. Long-term mortality in the patients with a left ventricular mass was significantly higher (p<0.01). We also found this rate to be significantly higher in malignant and metastatic cases (p<0.01). Histology and location of tumor are the most significant clinical predictors of long-term mortality. In our series, the patients with tumors located in the left ventricle had higher mortality rates. Among four cases with left ventricular tumors, two had late mortality, both of which were myxomas. In addition, the duration of hospital stay and EuroSCORE values were significantly higher in the patients with longterm mortality (p<0.01). However, there was no statistically significant difference in the mean age and gender distribution of the patients with and without long-term mortality (p>0.05).
In conclusion, based on our study results, surgical resection of primary cardiac tumors can be performed with low morbidity and mortality rates. Although survival rates in benign tumors are satisfactory, patients with malignant tumors have poor prognosis. Histology and location of the tumor, duration of hospital stay, and EuroSCORE are the most significant clinical predictors of long-term mortality. Furthermore, a consensus on the management of primary cardiac tumors can be reached by reviewing experiences of many centers with cardiac tumors.
Footnotes
Conflict of Interest: The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
Financial Disclosure: The authors received no financial support for the research and/or authorship of this article.
References
- 1.Patel J, Sheppard MN. Pathological study of primary cardiac and pericardial tumours in a specialist UK Centre: surgical and autopsy series. Cardiovasc Pathol. 2010;19:343–352. doi: 10.1016/j.carpath.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 2.Burke AP, Virmani R. Tumors of the heart and great vessels. In: Rosai j, Sobin LH., editors. Atlas of Tumor Pathology. Washington: Armed Forces Institute of Pathology; 1996. pp. 1–11. [Google Scholar]
- 3.Centofanti P, Di Rosa E, Deorsola L, Dato GM, Patanè F, La Torre M, et al. Primary cardiac tumors: early and late results of surgical treatment in 91 patients. Ann Thorac Surg. 1999;68:1236–1241. doi: 10.1016/s0003-4975(99)00700-6. [DOI] [PubMed] [Google Scholar]
- 4.McAllister HA Jr, Hall RJ, Cooley DA. Tumors of the heart and pericardium. Curr Probl Cardiol. 1999;24:57–116. [PubMed] [Google Scholar]
- 5.Neragi-Miandoab S, Kim J, Vlahakes GJ. Malignant tumours of the heart: a review of tumour type, diagnosis and therapy. Clin Oncol (R Coll Radiol) 2007;19:748–756. doi: 10.1016/j.clon.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 6.King TW. On simple vascular growths in the left auricle of the heart. Lancet. 1845;46:428–429. [Google Scholar]
- 7.Bahnson HT, Newman EV. Diagnosis and surgical removal of intracavitary myxoma of the right atrium. Bull Johns Hopkins Hosp. 1953;93:150–163. [PubMed] [Google Scholar]
- 8.Dell'amore A, Albertini A, Lamarra M. Twenty years experience in oncologic surgery for primary cardiac tumors. G Chir. 2013;34:106–111. [PMC free article] [PubMed] [Google Scholar]
- 9.Strecker T, Rösch J, Weyand M, Agaimy A. Primary and metastatic cardiac tumors: imaging characteristics, surgical treatment, and histopathological spectrum: a 10-year-experience at a German heart center. Cardiovasc Pathol. 2012;21:436–443. doi: 10.1016/j.carpath.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 10.Hanfling SM. Metastatic cancer to the heart. Review of the literature and report of 127 cases. Circulation. 1960;22:474–483. doi: 10.1161/01.cir.22.3.474. [DOI] [PubMed] [Google Scholar]
- 11.Simpson L, Kumar SK, Okuno SH, Schaff HV, Porrata LF, Buckner JC, et al. Malignant primary cardiac tumors: review of a single institution experience. Cancer. 2008;112:2440–2446. doi: 10.1002/cncr.23459. [DOI] [PubMed] [Google Scholar]
- 12.Kuroczynski W, Peivandi AA, Ewald P, Pruefer D, Heinemann M, Vahl CF. Cardiac myxomas: short- and long-term followup. Cardiol J. 2009;16:447–454. [PubMed] [Google Scholar]
- 13.Obeid AI, Marvasti M, Parker F, Rosenberg J. Comparison of transthoracic and transesophageal echocardiography in diagnosis of left atrial myxoma. Am J Cardiol. 1989;63:1006–1008. doi: 10.1016/0002-9149(89)90162-8. [DOI] [PubMed] [Google Scholar]
- 14.Cohen MV. Left atrial myxoma: echocardiographic identification. J Med Soc N J. 1979;76:213–215. [PubMed] [Google Scholar]
- 15.Dawson WB, Mayo JR, Müller NL. Computed tomography of cardiac and pericardial tumors. Can Assoc Radiol J. 1990;41:270–275. [PubMed] [Google Scholar]
- 16.Freedberg RS, Kronzon I, Rumancik WM, Liebeskind D. The contribution of magnetic resonance imaging to the evaluation of intracardiac tumors diagnosed by echocardiography. Circulation. 1988;77:96–103. doi: 10.1161/01.cir.77.1.96. [DOI] [PubMed] [Google Scholar]
- 17.Marvasti MA, Obeid AI, Potts JL, Parker FB. Approach in the management of atrial myxoma with long-term follow-up. Ann Thorac Surg. 1984;38:53–58. doi: 10.1016/s0003-4975(10)62186-8. [DOI] [PubMed] [Google Scholar]
- 18.Gerbode F, Kerth WJ, Hill JD. Surgical management of tumors of the heart. Surgery. 1967;61:94–101. [PubMed] [Google Scholar]
- 19.Attar S, Lee YC, Singleton R, Scherlis L, David R, McLaughlin JS. Cardiac myxoma. Ann Thorac Surg. 1980;29:397–405. doi: 10.1016/s0003-4975(10)61667-0. [DOI] [PubMed] [Google Scholar]
- 20.Melo J, Ahmad A, Chapman R, Wood J, Starr A. Primary tumors of the heart: a rewarding challenge. Am Surg. 1979;45:681–683. [PubMed] [Google Scholar]
- 21.Waller DA, Ettles DF, Saunders NR, Williams G. Recurrent cardiac myxoma: the surgical implications of two distinct groups of patients. Thorac Cardiovasc Surg. 1989;37:226–230. doi: 10.1055/s-2007-1020322. [DOI] [PubMed] [Google Scholar]
- 22.McCarthy PM, Piehler JM, Schaff HV, Pluth JR, Orszulak TA, Vidaillet HJ Jr, et al. The significance of multiple, recurrent, and "complex" cardiac myxomas. J Thorac Cardiovasc Surg. 1986;91:389–396. [PubMed] [Google Scholar]


